Synthesis of New Thiazole-Privileged Chalcones as Tubulin Polymerization Inhibitors with Potential Anticancer Activities
Abstract
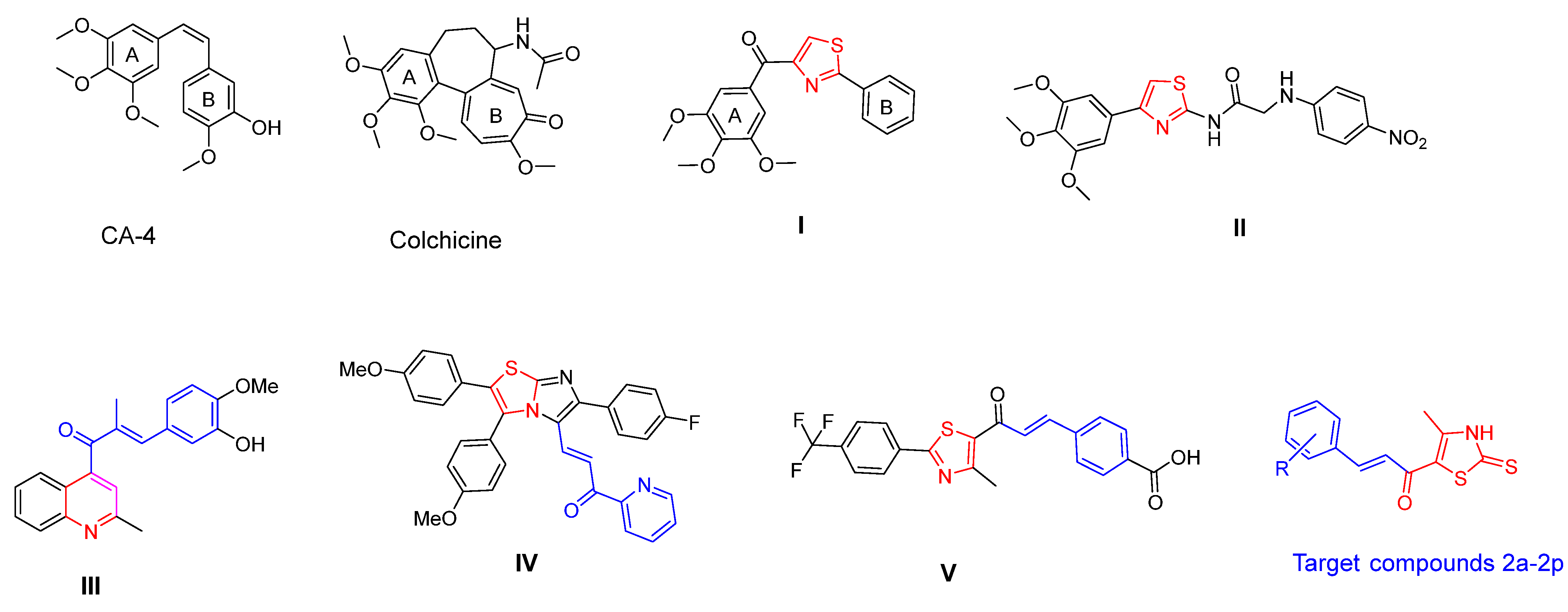
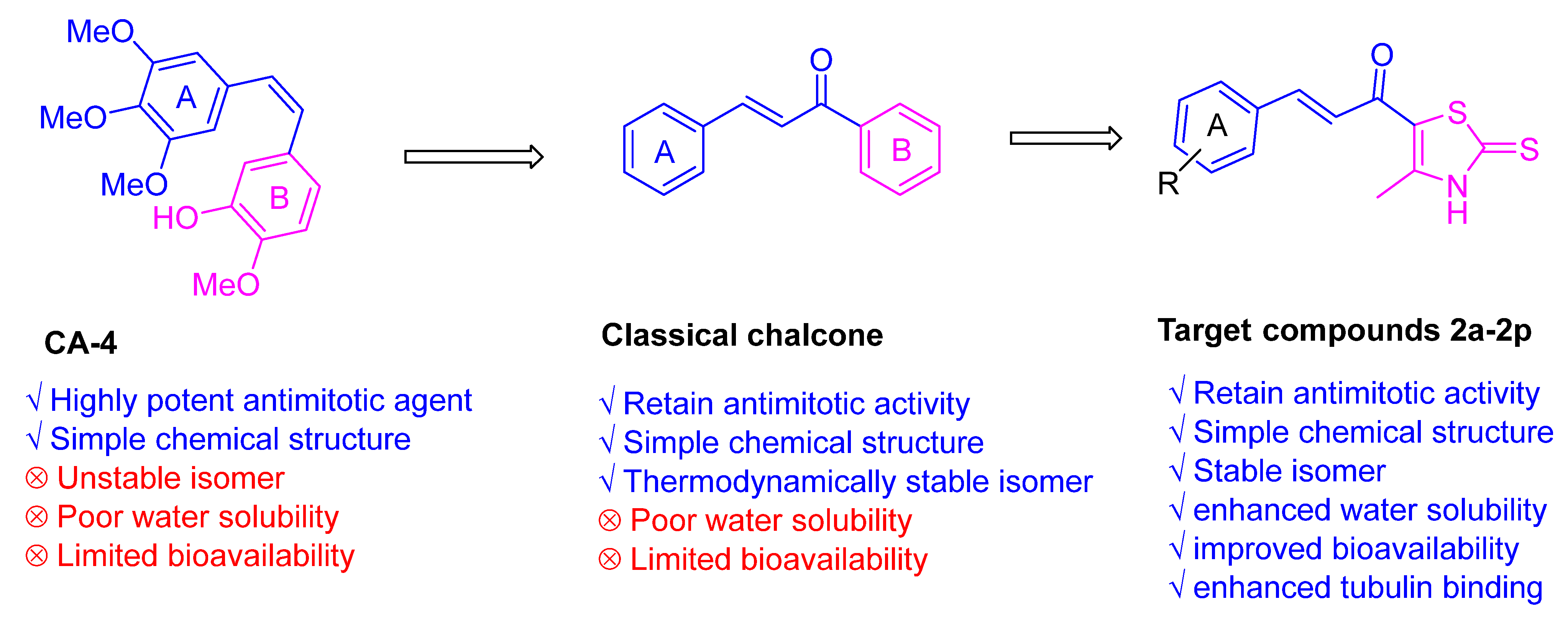
:1. Introduction
2. Results and Discussion
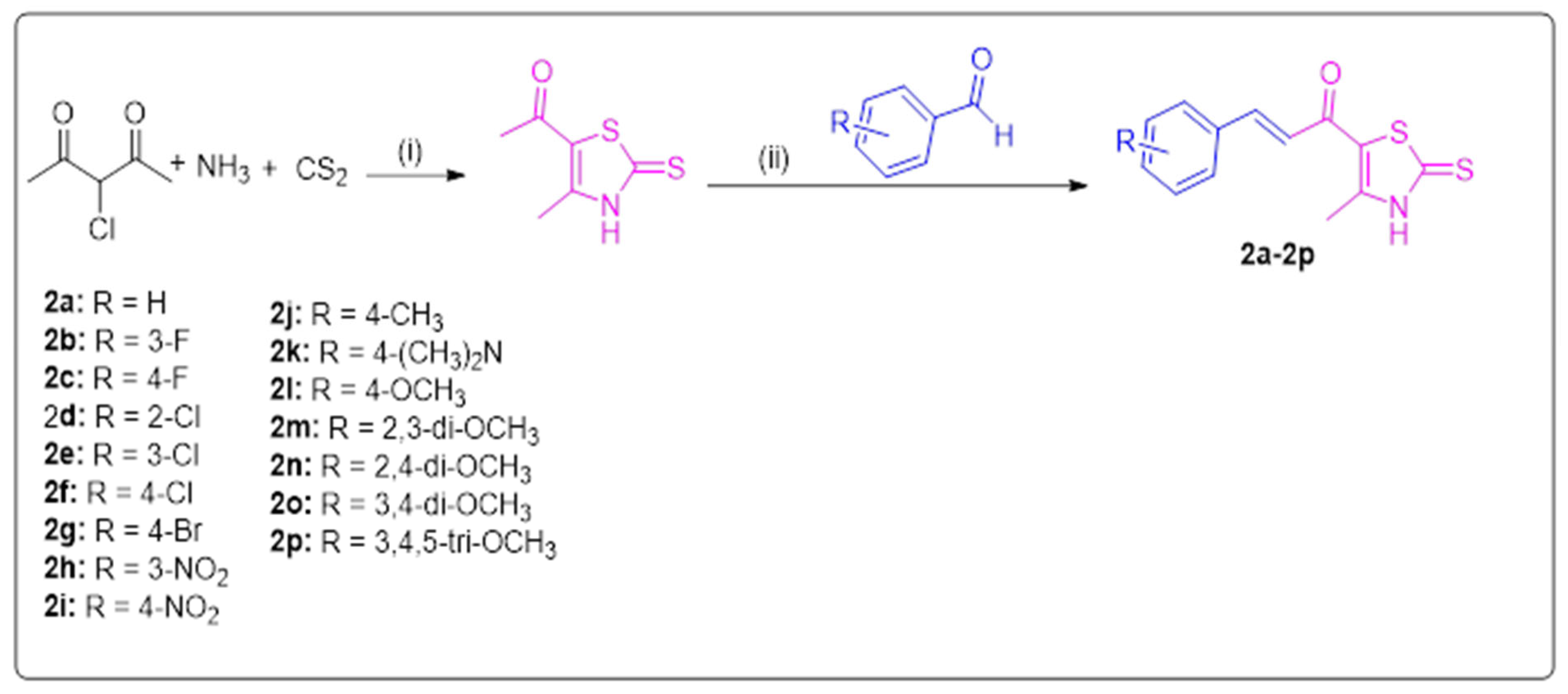
2.1. Chemistry
2.2. Biological Investigation
2.2.1. In Vitro Screening of Anticancer Activity of Thiazole Derivatives 2a–2p at 10 μM
2.2.2. Screening of the Anticancer Activity at Five Doses
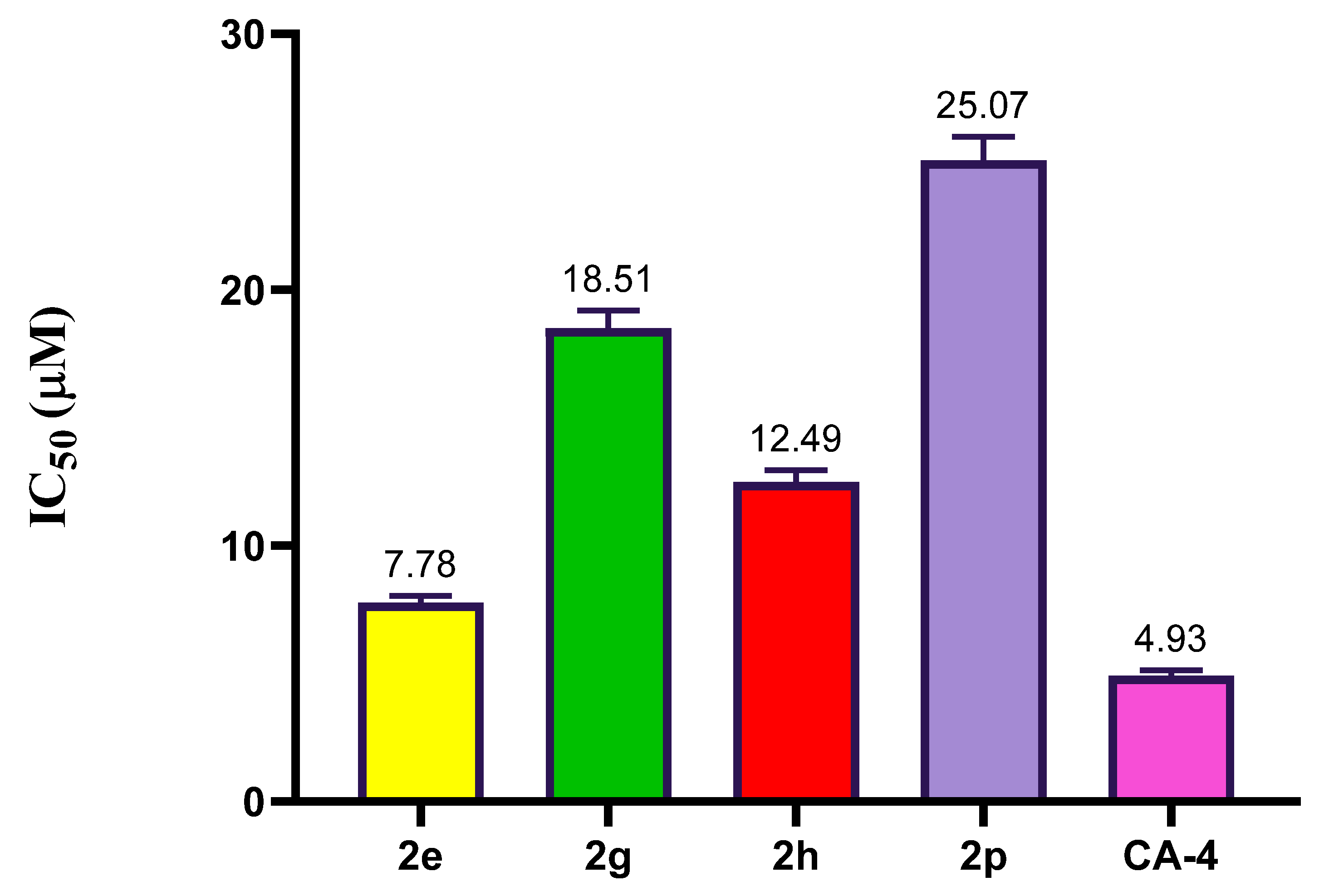
2.2.3. Effect of Compound 2e, 2g, 2h, and 2p on Tubulin Polymerization
2.3. In Silico Studies
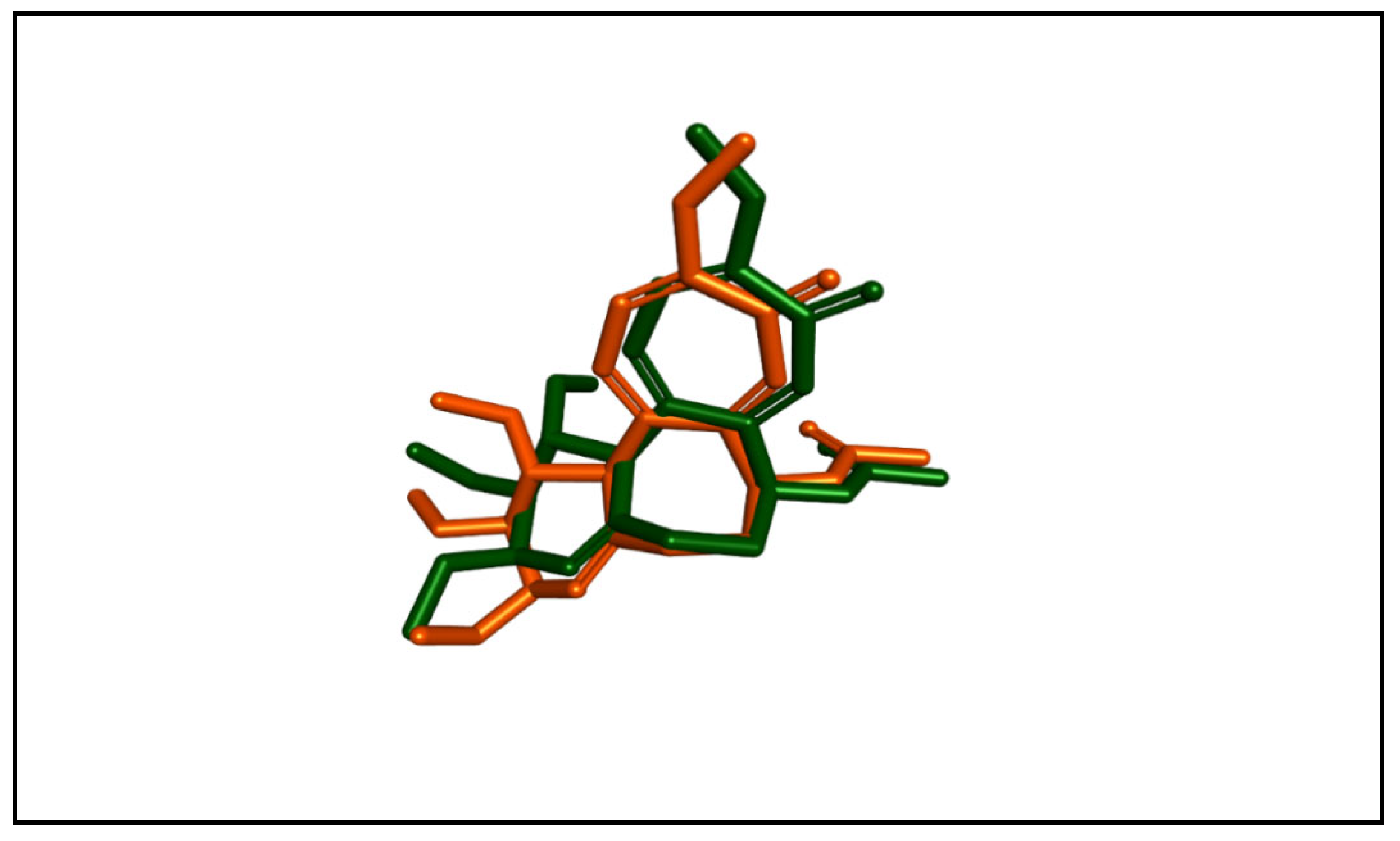
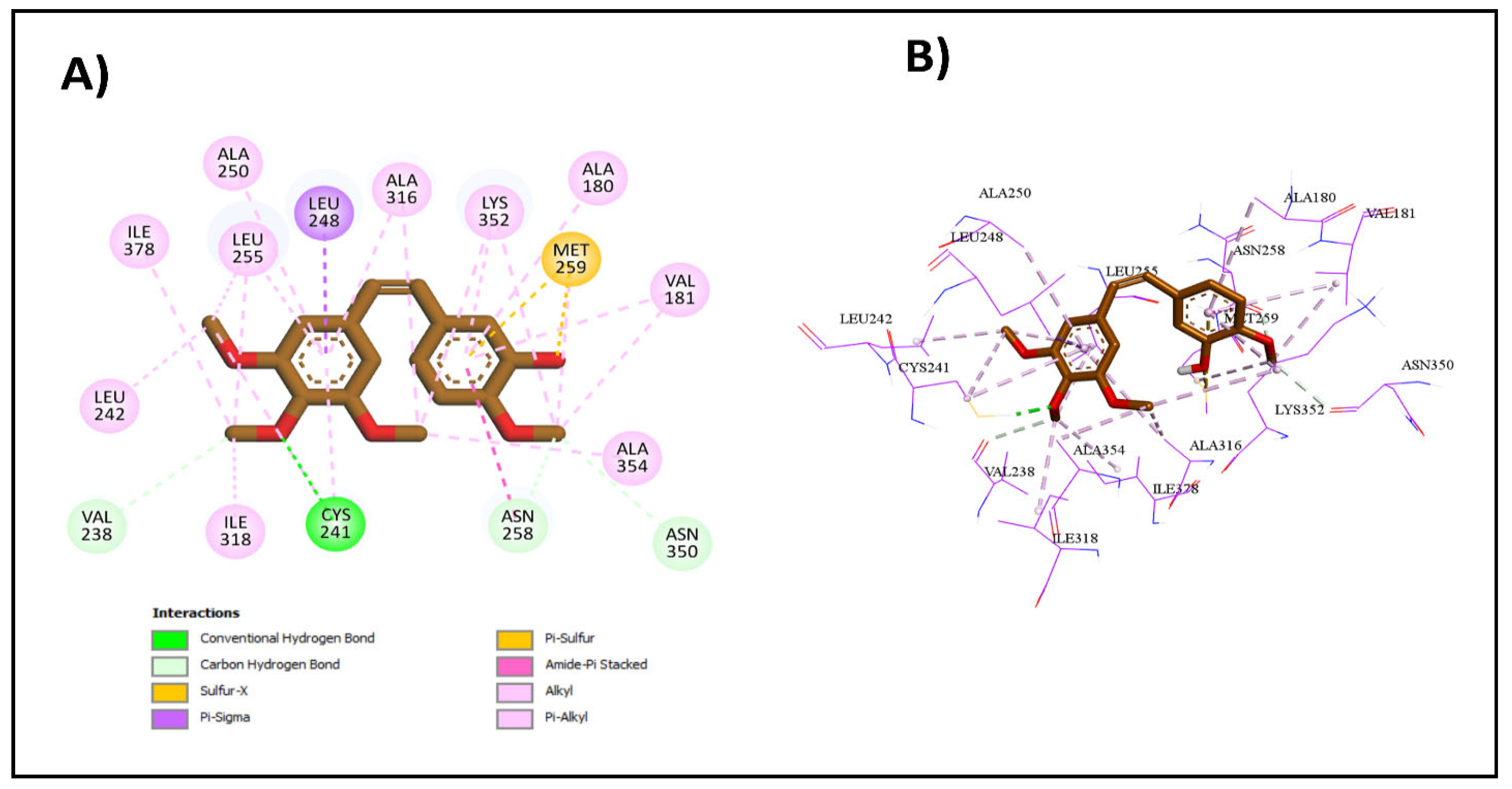
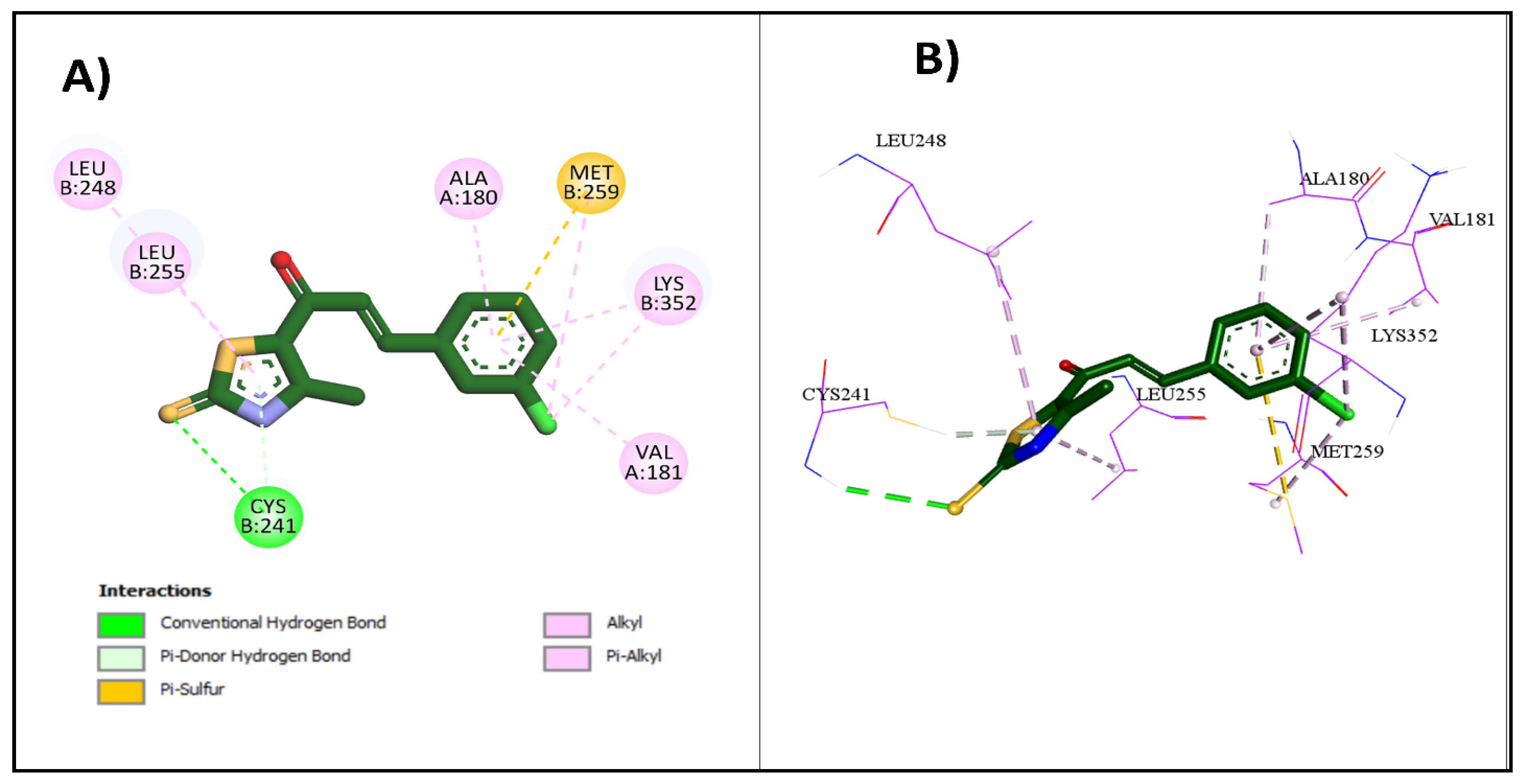
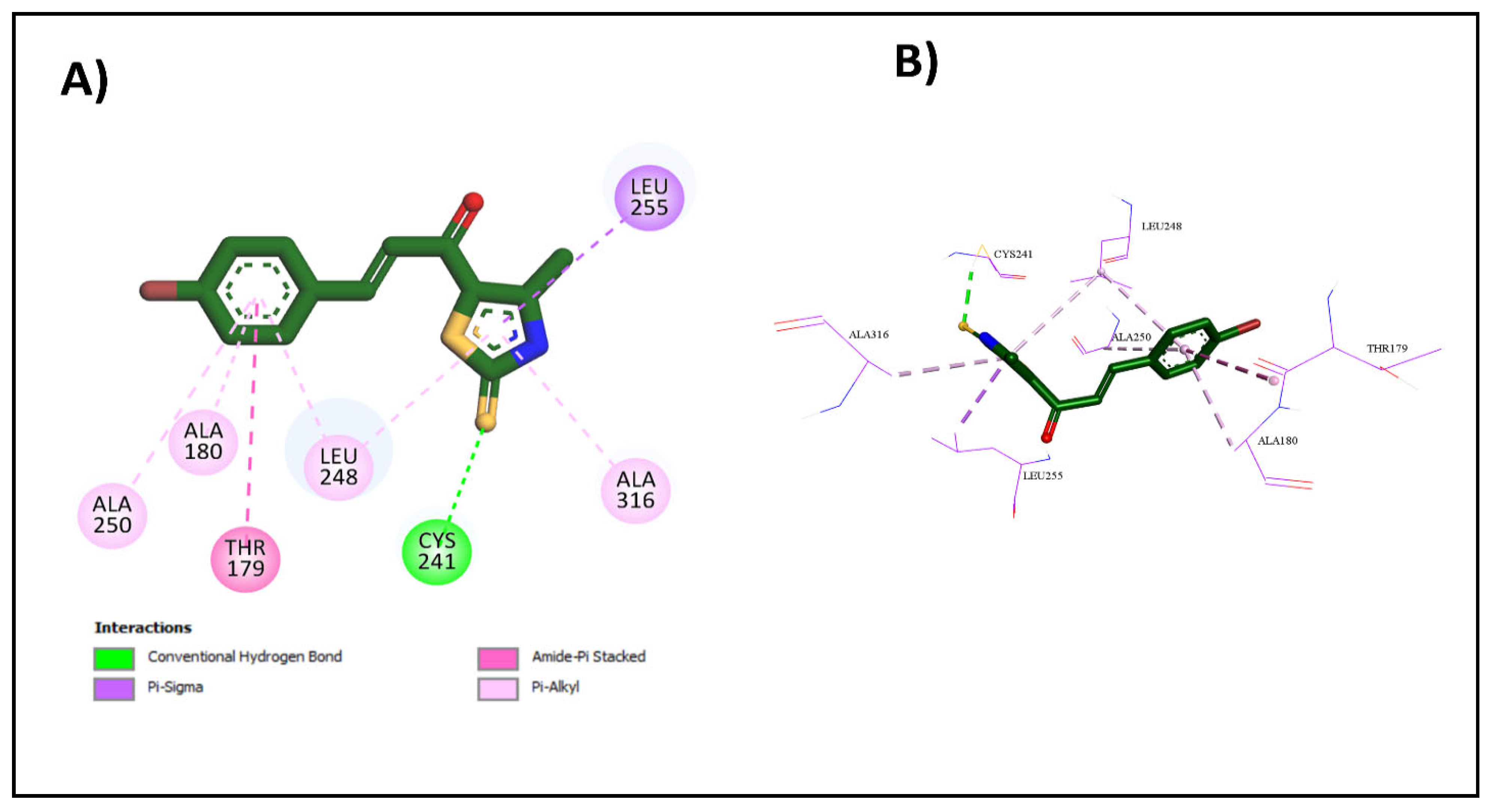
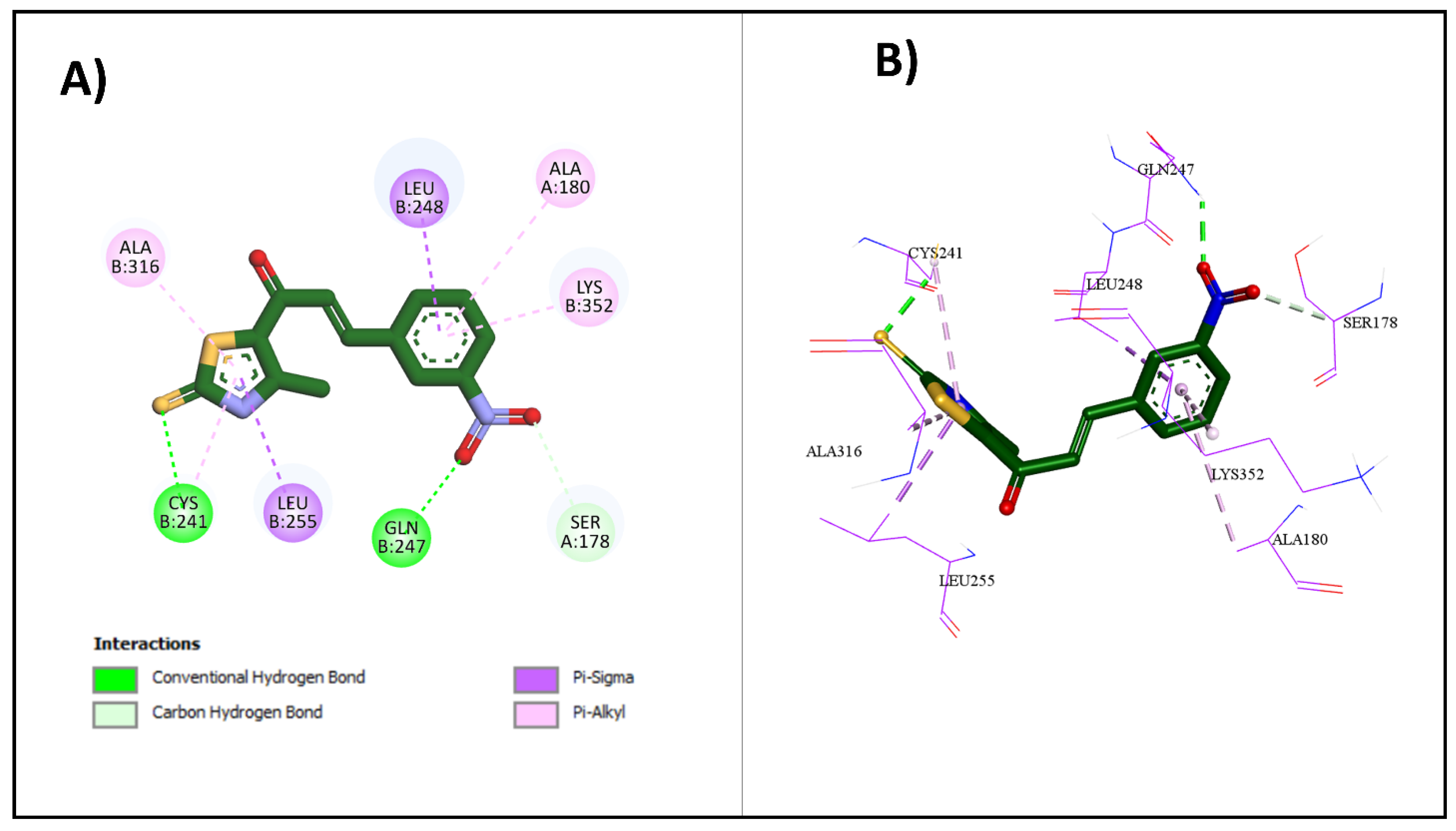
2.3.1. Molecular Docking Studies
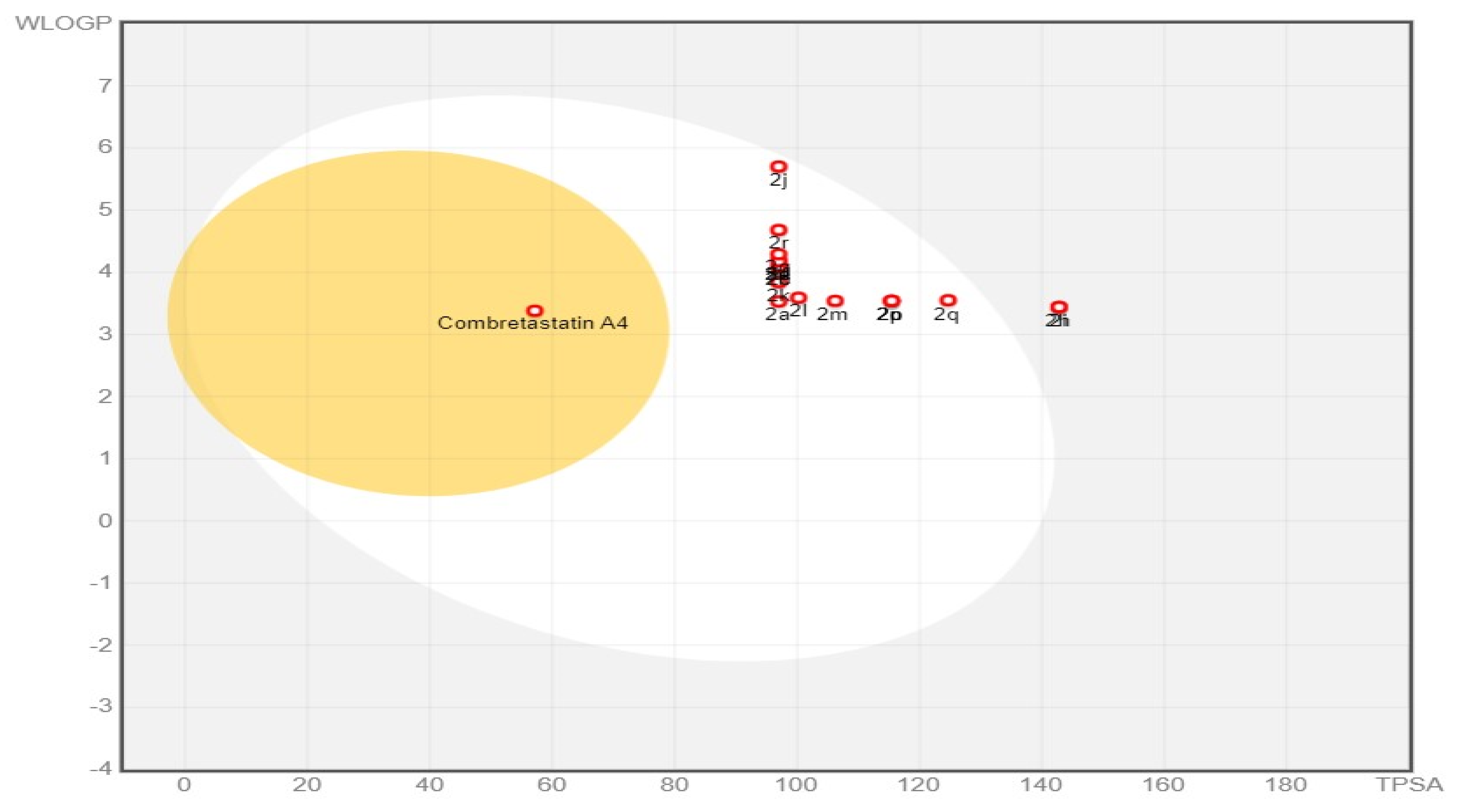
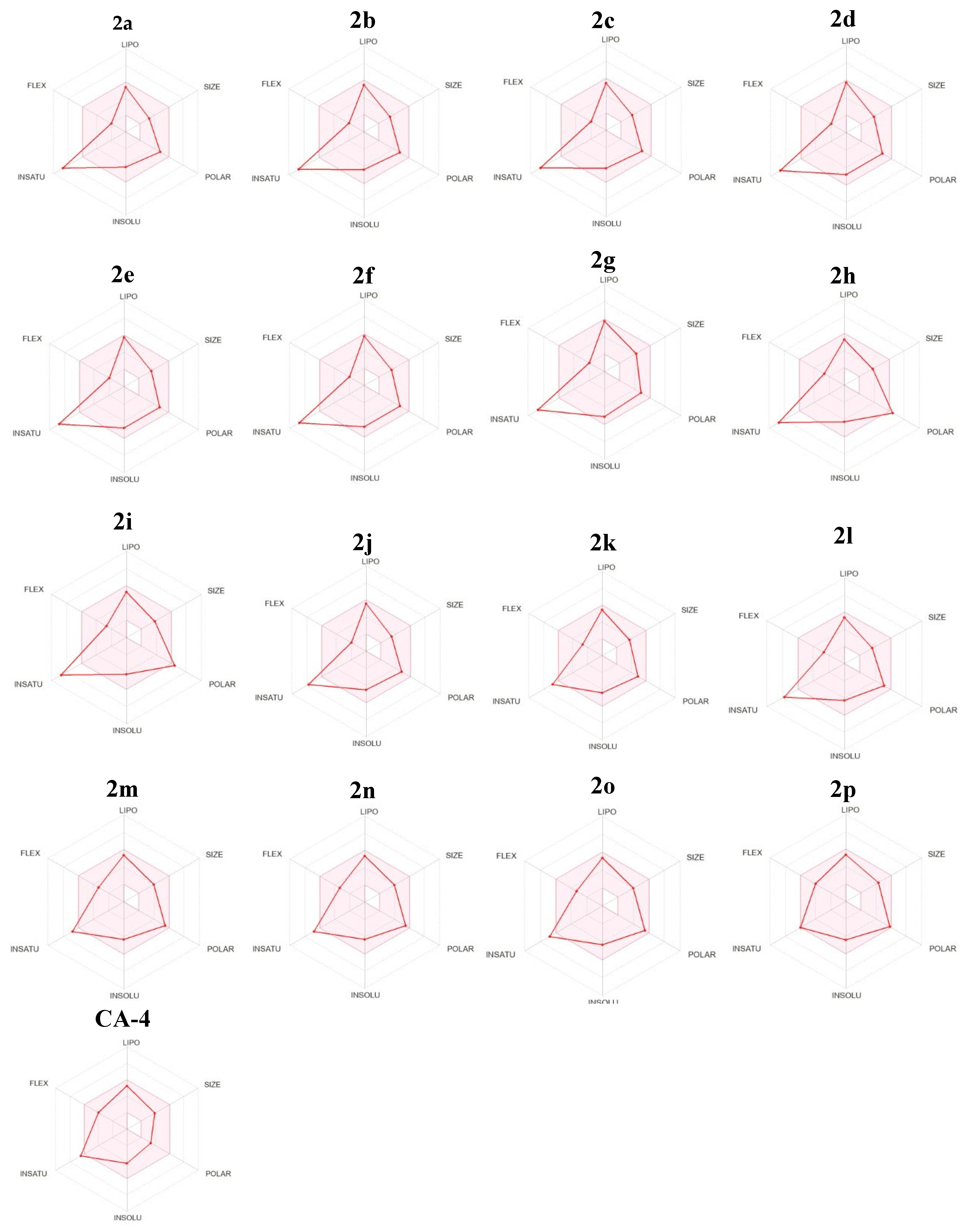
2.3.2. Physicochemical and ADME Prediction
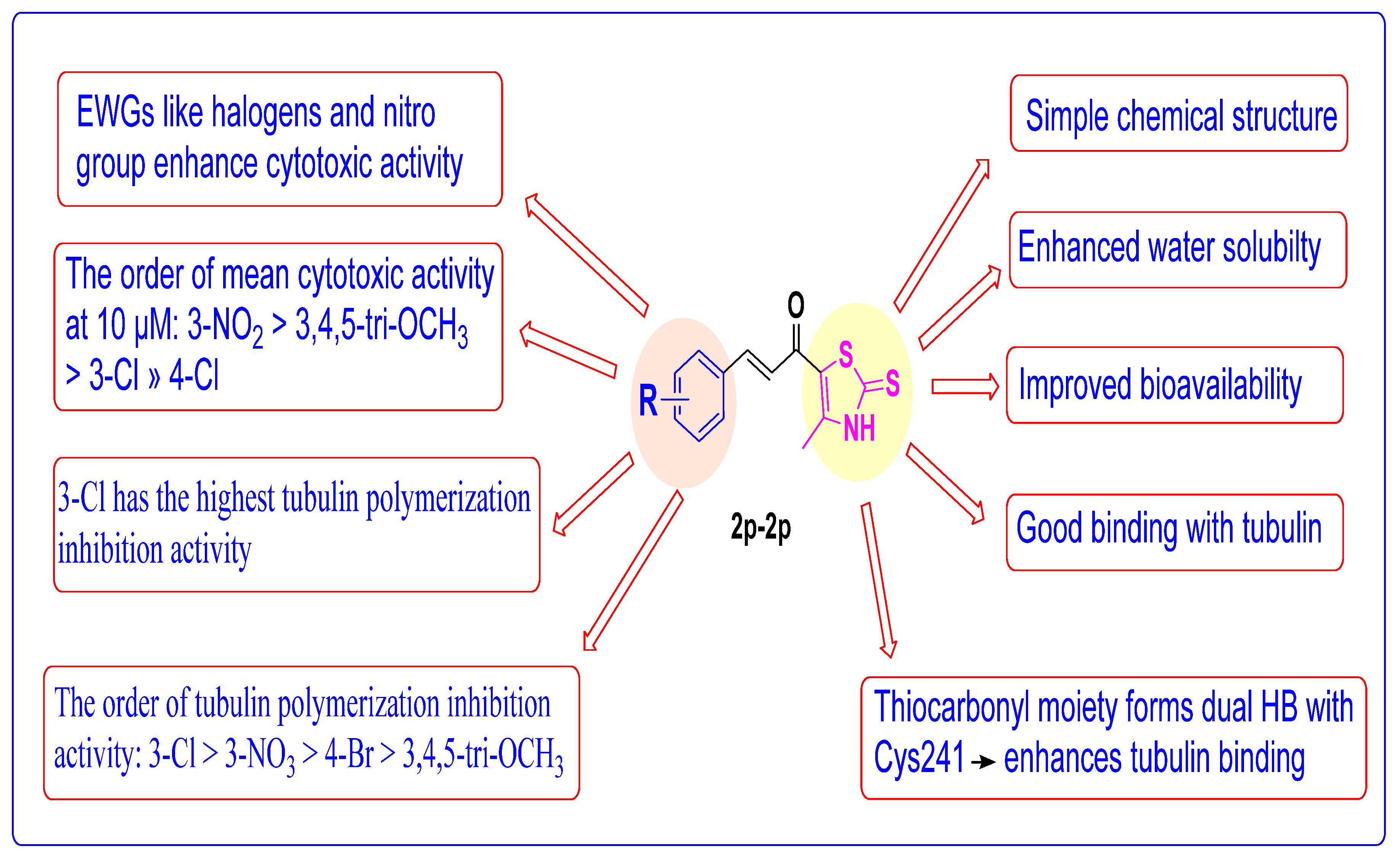
2.4. Structure Activity Relationship (SAR) Studies
3. Experimental Section
3.1. Chemistry
3.1.1. General Procedures for the Synthesis of Derivatives 2a–2p
(E)-1-(4-Methyl-2-Thioxo-2,3-Dihydrothiazol-5-yl)-3-Phenylprop-2-En-1-One 2a
(E)-3-(3-Fluorophenyl)-1-(4-Methyl-2-Thioxo-2,3-Dihydrothiazol-5-yl)Prop-2-En-1-One 2b
(E)-3-(4-Fluorophenyl)-1-(4-Methyl-2-Thioxo-2,3-Dihydrothiazol-5-yl)Prop-2-En-1-One 2c
(E)-3-(2-Chlorophenyl)-1-(4-Methyl-2-Thioxo-2,3-Dihydrothiazol-5-yl)Prop-2-En-1-One 2d
(E)-3-(3-Chlorophenyl)-1-(4-Methyl-2-Thioxo-2,3-Dihydrothiazol-5-yl)Prop-2-En-1-One 2e
(E)-3-(4-Chlorophenyl)-1-(4-Methyl-2-Thioxo-2,3-Dihydrothiazol-5-yl)Prop-2-En-1-One 2f
(E)-3-(4-Bromophenyl)-1-(4-Methyl-2-Thioxo-2,3-Dihydrothiazol-5-yl)Prop-2-En-1-One 2g
(E)-1-(4-Methyl-2-Thioxo-2,3-Dihydrothiazol-5-yl)-3-(3-Nitrophenyl)Prop-2-En-1-One 2h
(E)-1-(4-Methyl-2-Thioxo-2,3-Dihydrothiazol-5-yl)-3-(4-Nitrophenyl)Prop-2-En-1-One 2i
(E)-1-(4-Methyl-2-Thioxo-2,3-Dihydrothiazol-5-yl)-3-(p-Tolyl)Prop-2-En-1-One 2j
(E)-3-(4-(Dimethylamino)Phenyl)-1-(4-Methyl-2-Thioxo-2,3-Dihydrothiazol-5-yl)Prop-2-En-1-One 2k
(E)-3-(4-Methoxyphenyl)-1-(4-Methyl-2-Thioxo-2,3-Dihydrothiazol-5-yl)Prop-2-En-1-One 2l
(E)-3-(2,3-Dimethoxyphenyl)-1-(4-Methyl-2-Thioxo-2,3-Dihydrothiazol-5-yl)Prop-2-En-1-One 2m
(E)-3-(2,4-Dimethoxyphenyl)-1-(4-Methyl-2-Thioxo-2,3-Dihydrothiazol-5-yl)Prop-2-En-1-One 2n
(E)-3-(3,4-Dimethoxyphenyl)-1-(4-Methyl-2-Thioxo-2,3-Dihydrothiazol-5-yl)Prop-2-En-1-One 2o
(E)-1-(4-Methyl-2-Thioxo-2,3-Dihydrothiazol-5-yl)-3-(3,4,5-Trimethoxyphenyl)Prop-2-En-1-One 2p
3.2. Biological Evaluation and In Silico Studies Methodology
3.2.1. Screening of the Anticancer Activity against a Panel of 60 Cell Lines
3.2.2. In Vitro Tubulin Polymerization Inhibition Assay
3.2.3. In Silico Studies
Molecular Docking
In Silico Physicochemical and Pharmacokinetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malik, H.S.; Bilal, A.; Ullah, R.; Iqbal, M.; Khan, S.; Ahmed, I.; Krohn, K.; Saleem, R.S.Z.; Hussain, H.; Faisal, A. Natural and Semisynthetic Chalcones as Dual FLT3 and Microtubule Polymerization Inhibitors. J. Nat. Prod. 2020, 83, 3111–3121. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.H.H.; El-Hafeez, A.A.A.; Ebeid, K.; Mekkawy, A.I.; Abourehab, M.A.S.; Wafa, E.I.; Alhaj-Suliman, S.O.; Salem, A.K.; Ghosh, P.; Abuo-Rahma, G.E.-D.A.; et al. New 1,2,3-triazole linked ciprofloxacin-chalcones induce DNA damage by inhibiting human topoisomerase I&II and tu-bulin polymerization. J. Enzym. Inhib. Med. Chem. 2022, 37, 1346–1363. [Google Scholar] [CrossRef]
- Hassan, A.; Badr, M.; Hassan, H.A.; Abdelhamid, D.; Abuo-Rahma, G.E.A. Novel 4-(piperazin-1-yl)quinolin-2(1H)-one bearing thiazoles with antiproliferative activity through VEGFR-2-TK inhibition. Bioorg. Med. Chem. 2021, 40, 116168. [Google Scholar] [CrossRef]
- Mohammed, H.H.H.; El-Hafeez, A.A.A.; Abbas, S.H.; Abdelhafez, E.-S.M.N.; Abuo-Rahma, G.E.-D.A. New antiproliferative 7-(4-(N-substituted carbamoylmethyl)piperazin-1-yl) derivatives of ciprofloxacin induce cell cycle arrest at G2/M phase. Bioorg. Med. Chem. 2016, 24, 4636–4646. [Google Scholar] [CrossRef]
- Yan, J.; Xu, Y.; Jin, X.; Zhang, Q.; Ouyang, F.; Han, L.; Zhan, M.; Li, X.; Liang, B.; Huang, X. Structure modification and biological evaluation of indole-chalcone derivatives as anti-tumor agents through dual targeting tubulin and TrxR. Eur. J. Med. Chem. 2022, 227, 113897. [Google Scholar] [CrossRef] [PubMed]
- Al-Ostoot, F.H.; Salah, S.; Khamees, H.A.; Khanum, S.A. Tumor angiogenesis: Current challenges and therapeutic opportunities. Cancer Treat. Res. Commun. 2021, 28, 100422. [Google Scholar] [CrossRef]
- Al-Hamashi, A.A.; Koranne, R.; Dlamini, S.; Alqahtani, A.; Karaj, E.; Rashid, M.S.; Knoff, J.R.; Dunworth, M.; Pflum, M.K.H.; Casero, R.A.; et al. A new class of cytotoxic agents targets tubulin and disrupts microtubule dynamics. Bioorg. Chem. 2021, 116, 105297. [Google Scholar] [CrossRef]
- Kirchner, S.; Pianowski, Z. Photopharmacology of Antimitotic Agents. Int. J. Mol. Sci. 2022, 23, 5657. [Google Scholar] [CrossRef] [PubMed]
- Kaul, R.; Risinger, A.L.; Mooberry, S.L. Microtubule-Targeting Drugs: More than Antimitotics. J. Nat. Prod. 2019, 82, 680–685. [Google Scholar] [CrossRef]
- Henriques, A.C.; Ribeiro, D.; Pedrosa, J.; Sarmento, B.; Silva, P.M.A.; Bousbaa, H. Mitosis inhibitors in anticancer therapy: When blocking the exit becomes a solution. Cancer Lett. 2019, 440–441, 64–81. [Google Scholar] [CrossRef]
- Guo, K.; Ma, X.; Li, J.; Zhang, C.; Wu, L. Recent advances in combretastatin A-4 codrugs for cancer therapy. Eur. J. Med. Chem. 2022, 241, 114660. [Google Scholar] [CrossRef]
- Sun, K.; Sun, Z.; Zhao, F.; Shan, G.; Meng, Q. Recent advances in research of colchicine binding site inhibitors and their interaction modes with tubulin. Future Med. Chem. 2021, 13, 839–858. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Miller, D.D.; Li, W. Molecular interactions at the colchicine binding site in tubulin: An X-ray crystallography perspective. Drug Discov. Today 2022, 27, 759–776. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J.; Xiao, M.; Li, W.; Miller, D.D. An Overview of Tubulin Inhibitors That Interact with the Colchicine Binding Site. Pharm. Res. 2012, 29, 2943–2971. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jin, J.; Wu, Y.; Du, B.; Zhang, L.; Lu, D.; Liu, Y.; Chen, X.; Lin, J.; Chen, H.; et al. Fluoroindole chalcone analogues targeting the colchicine binding site of tubulin for colorectal oncotherapy. Eur. J. Med. Chem. 2023, 257, 115540. [Google Scholar] [CrossRef]
- Fang, Y.; Wu, Z.; Xiao, M.; Wei, L.; Li, K.; Tang, Y.; Ye, J.; Xiang, J.; Hu, A. Design, synthesis, and evaluation of new 2-oxoquinoline arylaminothiazole derivatives as potential anticancer agents. Bioorg. Chem. 2021, 106, 104469. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yuan, M.; Kang, Y.; Qin, J.; Zhang, Y.; Duan, Y.; Wang, L.; Yao, Y. Identification of novel non-toxic and anti-angiogenic α-fluorinated chalcones as potent colchicine binding site inhibitors. J. Enzym. Inhib. Med. Chem. 2022, 37, 339–354. [Google Scholar] [CrossRef]
- Wang, X.-F.; Wang, S.-B.; Ohkoshi, E.; Wang, L.-T.; Hamel, E.; Qian, K.; Morris-Natschke, S.L.; Lee, K.-H.; Xie, L. N-Aryl-6-methoxy-1,2,3,4-tetrahydroquinolines: A novel class of antitumor agents targeting the colchicine site on tubulin. Eur. J. Med. Chem. 2013, 67, 196–207. [Google Scholar] [CrossRef]
- Li, L.; Jiang, S.; Li, X.; Liu, Y.; Su, J.; Chen, J. Recent advances in trimethoxyphenyl (TMP) based tubulin inhibitors targeting the colchicine binding site. Eur. J. Med. Chem. 2018, 151, 482–494. [Google Scholar] [CrossRef]
- Fu, D.-J.; Liu, S.-M.; Li, F.-H.; Yang, J.-J.; Li, J. Antiproliferative benzothiazoles incorporating a trimethoxyphenyl scaffold as novel colchicine site tubulin polymerisation inhibitors. J. Enzym. Inhib. Med. Chem. 2020, 35, 1050–1059. [Google Scholar] [CrossRef]
- Li, W.; Xu, F.; Shuai, W.; Sun, H.; Yao, H.; Ma, C.; Xu, S.; Yao, H.; Zhu, Z.; Yang, D.-H.; et al. Discovery of Novel Quinoline–Chalcone Derivatives as Potent Antitumor Agents with Microtubule Polymerization Inhibitory Activity. J. Med. Chem. 2019, 62, 993–1013. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, E.C.; O’Boyle, N.M. Colchicine-Binding Site Inhibitors from Chemistry to Clinic: A Review. Pharmaceuticals 2020, 13, 8. [Google Scholar] [CrossRef]
- Mohammed, H.H.H.; Abbas, S.H.; Hayallah, A.M.; Abuo-Rahma, G.E.-D.A.; Mostafa, Y.A. Novel urea linked ciprofloxacin-chalcone hybrids having antiproliferative topoisomerases I/II inhibitory activities and caspases-mediated apoptosis. Bioorg. Chem. 2021, 106, 104422. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lv, J.; Cheng, S.; Jing, T.; Meng, T.; Huo, D.; Ma, X.; Wen, R. Recent Progresses in Chalcone Derivatives as Potential Anticancer Agents. Anticancer Agents Med. Chem. 2023, 23, 1265–1283. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khan, J.; Dukhyil, A.A.B.; Alarousy, R.M.I.I.; Attah, E.I.; Sharma, T.; Khairnar, S.J.; Bendale, A.R. Chalcone Scaf-folds, Bioprecursors of Flavonoids: Chemistry, Bioactivities, and Pharmacokinetics. Molecules 2021, 26, 7177. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Emami, S. Recent advances of cytotoxic chalconoids targeting tubulin polymerization: Synthesis and biological activity. Eur. J. Med. Chem. 2016, 121, 610–639. [Google Scholar] [CrossRef] [PubMed]
- Kesari, C.; Rama, K.; Sedighi, K.; Stenvang, J.; Björkling, F.; Kankala, S.; Thota, N. Synthesis of thiazole linked chalcones and their pyrimidine analogues as anticancer agents. Synth. Commun. 2021, 51, 1406–1416. [Google Scholar] [CrossRef]
- Rana, R.; Kumar, N.; Gulati, H.K.; Sharma, A.; Khanna, A.; Pooja, R.; Badhwar, R.; Dhir, M.; Jyoti, P.M.S.; Singh, J.V.; et al. A comprehensive review on thiazole based conjugates as anti-cancer agents. J. Mol. Struct. 2023, 1292, 136194. [Google Scholar] [CrossRef]
- Kassem, A.F.; Althomali, R.H.; Anwar, M.M.; El-Sofany, W.I. Thiazole moiety: A promising scaffold for anticancer drug discovery. J. Mol. Struct. 2024, 1303, 137510. [Google Scholar] [CrossRef]
- Gümüş, M.; Yakan, M.; Koca, İ. Recent advances of thiazole hybrids in biological applications. Future Med. Chem. 2019, 11, 1979–1998. [Google Scholar] [CrossRef]
- Sun, M.; Xu, Q.; Xu, J.; Wu, Y.; Wang, Y.; Zuo, D.; Guan, Q.; Bao, K.; Wang, J.; Wu, Y.; et al. Synthesis and bioevaluation of N,4-diaryl-1,3-thiazole-2-amines as tubulin inhibitors with potent antiproliferative activity. PLoS ONE 2017, 12, e0174006. [Google Scholar] [CrossRef] [PubMed]
- El-Abd, A.O.; Bayomi, S.M.; El-Damasy, A.K.; Mansour, B.; Abdel-Aziz, N.I.; El-Sherbeny, M.A. Synthesis and Molecular Docking Study of New Thiazole Derivatives as Potential Tubulin Polymerization Inhibitors. ACS Omega 2022, 7, 33599–33613. [Google Scholar] [CrossRef] [PubMed]
- Mezgebe, K.; Melaku, Y.; Mulugeta, E. Synthesis and Pharmacological Activities of Chalcone and Its Derivatives Bearing N-Heterocyclic Scaffolds: A Review. ACS Omega 2023, 8, 19194–19211. [Google Scholar] [CrossRef]
- Mallia, A.; Sloop, J. Advances in the Synthesis of Heteroaromatic Hybrid Chalcones. Molecules 2023, 28, 3201. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Balakrishna, M.; Nayak, V.L.; Shaik, T.B.; Faazil, S.; Nimbarte, V.D. Design and synthesis of imidazo[2,1-b]thiazole-chalcone conjugates: Microtubule-destabilizing agents. ChemMedChem 2014, 9, 2766–2780. [Google Scholar] [CrossRef]
- Mabkhot, Y.N.; Algarni, H.; Alsayari, A.; Muhsinah, A.B.; Kheder, N.A.; Almarhoon, Z.M.; Al-aizari, F.A. Synthesis, X-ray Analysis, Biological Evaluation and Molecular Docking Study of New Thiazoline Deriva-tives. Molecules 2019, 24, 1654. [Google Scholar] [CrossRef]
- Prota, A.E.; Danel, F.; Bachmann, F.; Bargsten, K.; Buey, R.M.; Pohlmann, J.; Reinelt, S.; Lane, H.; Steinmetz, M.O. The Novel Microtubule-Destabilizing Drug BAL27862 Binds to the Colchicine Site of Tubulin with Distinct Ef-fects on Microtubule Organization. J. Mol. Biol. 2014, 426, 1848–1860. [Google Scholar] [CrossRef]
- Gracheva, I.A.; Shchegravina, E.S.; Schmalz, H.-G.; Beletskaya, I.P.; Fedorov, A.Y. Colchicine Alkaloids and Synthetic Analogues: Current Progress and Perspectives. J. Med. Chem. 2020, 63, 10618–10651. [Google Scholar] [CrossRef]
- Hassan, A.; Mubarak, F.A.F.; Shehadi, I.A.; Mosallam, A.M.; Temairk, H.; Badr, M.; Abdelmonsef, A.H. Design and biological evaluation of 3-substituted quinazoline-2,4(1 H, 3 H)-dione derivatives as dual c-Met/VEGFR-2-TK inhibitors. J. Enzym. Inhib. Med. Chem. 2023, 38, 2189578. [Google Scholar] [CrossRef]
- Hassan, A.; Badr, M.; Abdelhamid, D.; Hassan, H.A.; Abourehab, M.A.S.; Abuo-Rahma, G.E.A. Design, synthesis, in vitro antiproliferative evaluation and in silico studies of new VEGFR-2 inhibitors based on 4-piperazinylquinolin-2(1H)-one scaffold. Bioorg. Chem. 2022, 120, 105631. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization of Known Drug Databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of Drug Absorption Using Multivariate Statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Hassan, A.; Mosallam, A.M.; Ibrahim, A.O.A.; Badr, M.; Abdelmonsef, A.H. Novel 3-phenylquinazolin-2,4(1H,3H)-diones as dual VEGFR-2/c-Met-TK inhibitors: Design, synthesis, and biological evaluation. Sci. Rep. 2023, 13, 18567. [Google Scholar] [CrossRef]
- Al-Hakkani, M.F.; Ahmed, N.; Abbas, A.A.; Hassan, M.H.A.; Aziz, H.A.; Elshamsy, A.M.; Khalifa, H.O.; Abdelshakour, M.A.; Saddik, M.S.; Elsayed, M.M.A.; et al. Synthesis, Physicochemical Characterization using a Facile Validated HPLC Quantitation Analysis Method of 4-Chloro-phenylcarbamoyl-methyl Ciprofloxacin and Its Biological Investigations. Int. J. Mol. Sci. 2023, 24, 14818. [Google Scholar] [CrossRef]
| Cell Line Panel/Cell Line Name | Growth Percentage of Thiazole Chalcones 2a–2p | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2a | 2b | 2c | 2d | 2e | 2f | 2g | 2h | 2i | 2j | 2k | 2l | 2m | 2n | 2o | 2p | ||
| Leukemia | CCRF-CEM | 37.22 | 15.42 | −0.21 | 46.79 | 15.19 | 3.18 | −11.02 | 13.78 | −7.25 | 24.6 | 68.52 | 58.98 | 12.41 | 53.72 | 15.53 | 4.92 |
| HL-60(TB) | 49.59 | 12.04 | 12.98 | 53.03 | 25.32 | −0.98 | 13.16 | 5.25 | −5.39 | 61 | 87.05 | 58.48 | 12.74 | 55.84 | 35.6 | 3.95 | |
| K-562 | 50.96 | 22.81 | 20.43 | 47.41 | 16.76 | 7.94 | 13.64 | 13.77 | 12.28 | 34.16 | 47.03 | 56.62 | 27.51 | 48.87 | 32.43 | 8.21 | |
| MOLT-4 | 74.19 | 43.64 | 58.71 | 84.22 | 19.78 | 10.18 | 30.17 | 13.31 | 14.2 | 68.76 | 62.1 | 81.55 | 36.78 | 72.27 | 73.82 | 9.11 | |
| RPMI-8226 | 14.13 | 15.24 | 2.32 | 28.65 | 10.37 | 13.82 | 1.4 | 9.39 | −7.9 | 18.86 | 41.48 | 19.63 | 9.09 | 35.64 | 18.58 | 5.31 | |
| SR | 32.39 | 6.82 | 18.33 | 14.1 | 3.42 | ND | 8.46 | −10.99 | −4.81 | 45.55 | 57.41 | 43.69 | 5.15 | ND | 32.51 | ND | |
| NSCLC | A549/ATCC | 82.31 | 76.38 | 55.96 | 87.57 | 26.49 | 61.14 | 64.07 | 19.51 | 75.71 | 74.27 | 68.06 | 82.86 | 43.09 | 90 | 64.57 | 26.57 |
| EKVX | 73.11 | 68.61 | 51.04 | 35.62 | 34.72 | 40.96 | 62.9 | 0.65 | 74.66 | 55.52 | 67.01 | 84.75 | 54.52 | 56.24 | 54.16 | 23.4 | |
| HOP-62 | ND | 34.77 | ND | 91.65 | 10.45 | −2.73 | 11.94 | 0.68 | 21.63 | 54.1 | 86.25 | ND | 91.75 | 88.82 | 34.07 | 4.61 | |
| HOP-92 | 89.61 | ND | 45.42 | ND | ND | 34.78 | 67.12 | ND | 57.63 | 98.01 | 118.71 | 100.67 | 61.54 | 103.21 | 83.73 | 16.45 | |
| NCI-H226 | 96.48 | 85.65 | 67.1 | 89.61 | 32.73 | 20.49 | 54.51 | −41.9 | 91.38 | 93.76 | 88.28 | 93.62 | 90.78 | 79.73 | 80.5 | 16.43 | |
| NCI-H23 | 77.52 | 71.09 | 62.66 | 91.77 | 18 | 36.43 | 65.01 | −72.77 | 49.2 | 68.54 | 71.13 | 81.76 | 86.47 | 72.87 | 65.13 | 7.27 | |
| NCI-H322M | 66.51 | 84.46 | 63.98 | 106.22 | 50.15 | ND | 44.13 | 28.92 | 50.72 | 62.15 | 81.14 | 92.4 | 62.76 | ND | 63.43 | ND | |
| NCI-H460 | 56.7 | 43.22 | 39.31 | 82.17 | 12.05 | 82.56 | 39.06 | −30.68 | 11.33 | 53.09 | 85.67 | 85.62 | 40.11 | 89.77 | 40.01 | −1.34 | |
| NCI-H522 | 71.74 | 63.86 | 44.19 | 90.13 | 21.99 | 13.54 | 21.1 | −58.81 | 33 | 60.24 | 58.91 | 61.35 | 32.87 | 73.46 | 56.8 | 16.3 | |
| Colon Cancer | COLO 205 | ND | 94.51 | ND | 117.87 | 70.71 | 78.15 | 89.96 | −24.88 | 91.53 | 121.66 | 115.46 | ND | 102.52 | 122.75 | 115.96 | 81.66 |
| HCC-2998 | 91.33 | 58.22 | 27.06 | 132.42 | 13.88 | −21.28 | 3.23 | −86.72 | −11.87 | 77.15 | 95.3 | 100.59 | 79.76 | 80.73 | 41.71 | 19.01 | |
| HCT-116 | 49.38 | 21.2 | 23.71 | 44.58 | 3.28 | 6.98 | 16.57 | −6.25 | −47.76 | 46.74 | 72.22 | 75.95 | 46.54 | 62.3 | 34.55 | 6.81 | |
| HCT-15 | 35 | 19.06 | 14.71 | 57.4 | −22.01 | −19.78 | 5.48 | −36.42 | −62.1 | 38.58 | 64.44 | 50.75 | 32.68 | 45.78 | 31.33 | −3.26 | |
| HT29 | 99.67 | 47.06 | 73.33 | 122.14 | 29.92 | 31.17 | 76.26 | 20.35 | 62.83 | 98.71 | 94.03 | 96.79 | ND | 114.99 | 97.72 | 40.09 | |
| KM12 | 29.04 | 11.91 | 6.85 | 63.81 | −30.72 | 6.77 | 1.17 | −78.4 | −72.47 | 29.81 | 62.65 | 42.17 | 25.26 | 73.4 | 42.25 | 9.52 | |
| SW-620 | 69.11 | 20.94 | 13.76 | 82.04 | 8.6 | 8.02 | 5.7 | −37.8 | −44.79 | 35.87 | 89.86 | 75.28 | 47.89 | 84.93 | 34.92 | 6.92 | |
| CNS Cancer | SF-268 | 69.81 | 82.33 | 58.62 | 80.38 | 51.84 | 67.94 | 46.1 | 27.46 | 47.36 | 86.89 | 99.28 | 81.05 | 54.04 | 89.73 | 67.22 | 18.5 |
| SF-295 | 88.86 | 38.42 | 60.61 | 82.55 | 41.84 | 42.62 | 90.37 | 4.14 | 86.08 | 83.38 | 79.76 | 83.49 | 26.31 | 72.5 | 66.5 | 42.28 | |
| SF-539 | 41.72 | 6.17 | −4.61 | 93.59 | −10.65 | −30.44 | −25.34 | −85.88 | −25.22 | 42.96 | 88.7 | 69.82 | 22.73 | 87.29 | 29.82 | −13.9 | |
| SNB-19 | 66.75 | 44.03 | 33.66 | 85.22 | ND | 15.84 | 32.68 | 10.38 | 41.18 | 65.54 | 71.2 | 75.59 | 37.21 | 77.37 | 61.91 | 19.56 | |
| SNB-75 | 92.71 | ND | 36.49 | ND | 8.74 | ND | 2.52 | ND | 35.98 | 78.06 | 96 | 98.01 | 50.41 | ND | 58.12 | ND | |
| U251 | 56.91 | 17.63 | 14.09 | 63.31 | 12.07 | 11.35 | 15.53 | −75.99 | 13.65 | 60.37 | 67.11 | 62.29 | 20.33 | 68.6 | 33.72 | 14.98 | |
| Melanoma | LOX IMVI | 31.05 | 13.83 | 8.91 | 57.46 | −26.09 | −48.15 | −5.17 | −98.99 | −86.21 | 31.22 | 60.24 | 44.25 | 21.68 | 57.81 | 26.92 | 17.13 |
| MALME-3M | 87.78 | 101.24 | 85.79 | 104.51 | 86.93 | ND | 72.94 | −42.94 | 75.16 | 84.65 | 79.42 | 90.04 | 97.13 | ND | 89.57 | ND | |
| M14 | 77.06 | 78.45 | 49.58 | 82.29 | 40.25 | 34.39 | 74.48 | 1.86 | 69.52 | 83.02 | 83.18 | 87.51 | 53.99 | 75.84 | 82.23 | 15.43 | |
| MDA-MB-435 | 50.1 | 35.94 | 4.67 | 80.75 | 17.26 | 22.51 | 8.26 | −36.95 | 3.76 | 39.01 | 58.35 | 63.89 | 51.86 | 84.73 | 38.89 | 10.98 | |
| SK-MEL-2 | 68.19 | 81.22 | 56.59 | 84.85 | 32.1 | 58.38 | 62.33 | 16.88 | 49.55 | 89.83 | 88.89 | 80.29 | 94.22 | 64.72 | 68.6 | 34.94 | |
| SK-MEL-28 | 85.25 | 93.67 | 50.57 | 101.08 | 44.69 | 45.71 | 64.25 | −47.02 | 23.87 | 92.85 | 92.66 | 101.91 | 70 | 92.8 | 83.19 | 24.24 | |
| SK-MEL-5 | 68.82 | 62.16 | 42.56 | 68.46 | 16.05 | 27.99 | 48.84 | −68.8 | 53.78 | 56.13 | 51.26 | 55.87 | 23.46 | 37.84 | 63.12 | −64.57 | |
| UACC-257 | 76.15 | 83.72 | 64.09 | 87.25 | 52.71 | 66.8 | 69.1 | 9.4 | 63.26 | 80.08 | 64.13 | 67.97 | 35.39 | 84.44 | 71.4 | 42.08 | |
| UACC-62 | 65.26 | 58.32 | 47.27 | 72.88 | 29.97 | 33.88 | 46.84 | −56.71 | 47.53 | 59.43 | 58.71 | 71.85 | 45.46 | 65.16 | 63.85 | −3.11 | |
| Ovarian Cancer | IGROV1 | 81.82 | 32.3 | 51.24 | 91.91 | 33.98 | ND | 37.11 | 10.16 | 16.06 | 83.42 | 82.27 | 89.07 | 73.86 | ND | 69.47 | ND |
| OVCAR-3 | 42.69 | −1.17 | −10.94 | 77.36 | 8.26 | −24.25 | −12.3 | −79.48 | −6 | 14.99 | 82.2 | 66.86 | 24.27 | 91.16 | 3.22 | −25.23 | |
| OVCAR-4 | 76.39 | 79.55 | 48.43 | 90.32 | 31.68 | 76.31 | 49.51 | 13.05 | 49.16 | 59.22 | 51.23 | 72.33 | 62.2 | 52.05 | 47.63 | 15.6 | |
| OVCAR-5 | 128.73 | 88.15 | 119.09 | 116.44 | 36.37 | 50.81 | 115.99 | −37.4 | 109.56 | 138.42 | 142.09 | 144.69 | 81.68 | 120.39 | 131.45 | 6.56 | |
| OVCAR-8 | 65.76 | 60.46 | −13.63 | 85.43 | 10.39 | 4.96 | −34.08 | 13.44 | 7.06 | 17.26 | 83.37 | 76.93 | 44.61 | 81.51 | 34.3 | 4.32 | |
| NCI/ADR-RES | 64.78 | 10.81 | −10.78 | 88.8 | −5.11 | −8.39 | −13.89 | 8.19 | −4.45 | 31 | 56.92 | 76.38 | 30.95 | 48.05 | 26.85 | 0.58 | |
| SK-OV-3 | ND | 110.63 | ND | 118.47 | 59.91 | 60.83 | 96.66 | 44.61 | 107.77 | 102.36 | 92.59 | ND | 85.62 | 100.52 | 76.93 | 31.19 | |
| Renal Cancer | 786-0 | 68.1 | 46.91 | 37.27 | 93.1 | 13.35 | 16.59 | 25.03 | −10.85 | 25.64 | 70.71 | 91.67 | 78.11 | ND | 85.94 | 60 | 21.09 |
| A498 | 105.99 | 106.3 | 115 | 116 | 98.01 | 105.85 | 108.9 | 98.87 | 104.64 | 130.77 | 129.94 | 120.21 | 108.77 | 100.71 | 125.58 | 96.5 | |
| ACHN | 71.77 | 55.96 | 47.17 | 93.42 | 22.9 | 34.61 | 55.01 | −58.35 | 3.88 | 71.22 | 75.67 | 85.64 | 52.53 | 76.96 | 64.88 | 19.58 | |
| CAKI-1 | 52.53 | 37.69 | 31.6 | 68.49 | 23.85 | 53.21 | 32.76 | −56.46 | 31.07 | 43.41 | 68.64 | 59.04 | 41.93 | 82.85 | 42.04 | 28.19 | |
| RXF 393 | 46.35 | 23.17 | 23.24 | 93.84 | 10.66 | 9.78 | 15.96 | −91.92 | −4.35 | 55.42 | 90.79 | 77.53 | 52.45 | 68.86 | 48.08 | 30.28 | |
| SN12C | 66.07 | 32.09 | 24.05 | 80.04 | 12 | 4.62 | 19.22 | −25.64 | 18.63 | 62.33 | 75.73 | 74.87 | 44.99 | 80.95 | 45.37 | 7.3 | |
| TK-10 | 80.33 | ND | 40.37 | ND | ND | 40.37 | 37.82 | ND | 12.9 | 79.78 | 86.81 | 80.89 | 64.47 | 94.1 | 75.43 | 52.99 | |
| UO-31 | 39.33 | 44.34 | 28.13 | 73.69 | 34.33 | 30.44 | 22.38 | −89.63 | −41.32 | 46.79 | 48.26 | 49 | 53.74 | ND | 49.17 | ND | |
| PC | PC-3 | 77.6 | 54.71 | 43.41 | 71.78 | 31.83 | ND | 48.79 | 8.43 | 51.28 | 74.84 | 71.73 | 82.15 | 41.63 | 75.69 | 68.88 | 35.71 |
| DU-145 | 27.95 | 12.86 | 20.32 | 75.95 | 16.1 | 7.29 | 23.9 | 8.55 | 21.39 | 54.64 | 92.79 | 58.53 | 41.98 | 86.87 | 48.1 | 12.86 | |
| Prostate Cancer | MCF7 | 19.79 | 7.97 | 9.48 | 28.93 | 7.9 | 4.86 | 10.52 | −45.42 | 5.51 | 28.33 | 59.99 | 30.84 | 16.65 | 45.98 | 28.86 | 9.35 |
| MDA-MB-231/ATCC | 70.05 | 67.38 | 29.72 | 101.38 | −0.43 | 39.29 | 9.49 | −17.58 | 10.75 | 71.69 | 90.28 | 89.32 | 68.26 | 98.69 | 58.36 | 2.51 | |
| HS 578T | 90.12 | 79.81 | 95.29 | 92.76 | 47.21 | 47.98 | 74.6 | 24.47 | 68.11 | 101.32 | 118 | 96.5 | 49.25 | 89.49 | 84.71 | 3.94 | |
| BT-549 | 68.85 | 65.65 | 23.21 | 76.82 | 2.28 | −14.41 | 19.5 | −14.03 | 20.09 | 56.13 | 82.22 | 75.04 | 70.95 | 59.75 | 18.94 | −11.78 | |
| T-47D | ND | 0.69 | ND | 47.34 | 4.54 | 1.38 | 29.03 | −56.63 | 44.32 | 38.31 | 32.35 | ND | 34.48 | 30.94 | 44.15 | −1.59 | |
| MDA-MB-468 | 20.79 | −22.69 | −4.51 | 30.83 | −7.12 | −21.19 | −4.75 | −82.86 | −18.95 | 11.77 | 35.38 | 15.11 | 41.07 | 19.19 | −0.42 | 3.79 | |
| Mean growth percentage | 64.97 | 47.5 | 36.74 | 79.42 | 22.13 | 23.72 | 34.25 | −21.75 | 25.23 | 63.31 | 77.71 | 74.58 | 49.88 | 75.09 | 55.11 | 14.89 | |
| Cell Line Panel/Cell Line NAME | 2c | 2e | 2f | 2g | 2h | 2i | 2p | CA-4 | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GI50 | LC50 | TGI | GI50 | IC50 | LC50 | TGI | GI50 | IC50 | LC50 | TGI | GI50 | LC50 | TGI | GI50 | IC50 | LC50 | TGI | GI50 | LC50 | TGI | GI50 | IC50 | LC50 | TGI | GI50 | LC50 | TGI | ||
| Leukemia | CCRF-CEM | 3.68 | >100 | >100 | 2.45 | 3.72 | >100 | 7.94 | 2.88 | 3.98 | >100 | 11.22 | 3.06 | >100 | 61.5 | 2.14 | 3.39 | >100 | 6.61 | 4.56 | >100 | >100 | 2.45 | 3.55 | >100 | 8.71 | 0.10 | 89.95 | 5.43 |
| HL-60(TB) | 2.83 | >100 | 32.2 | 2.24 | 4.27 | >100 | 7.08 | 3.55 | 5.89 | >100 | 38.02 | 1.94 | >100 | 9.92 | 1.95 | 3.31 | >100 | 5.75 | 3.05 | >100 | 16.9 | 2.57 | 3.89 | >100 | 11.22 | 0.03 | 59.02 | 0.06 | |
| K-562 | 3.25 | >100 | 48.8 | 3.39 | 4.27 | >100 | >100 | 3.89 | 4.68 | >100 | >100 | 2.73 | >100 | >100 | 3.09 | 3.72 | >100 | >100 | 3.5 | >100 | >100 | 3.02 | 3.72 | >100 | >100 | 0.03 | >100 | 2.09 | |
| MOLT-4 | 16.7 | >100 | 64.3 | 2.82 | 4.27 | >100 | >100 | 14.45 | 24.55 | >100 | 79.43 | 3.57 | >100 | 36.6 | 3.47 | 5.01 | >100 | 30.2 | 3.79 | >100 | >100 | 2.88 | 4.17 | >100 | 22.91 | 0.10 | 78.34 | 3.72 | |
| RPMI-8226 | 2.85 | >100 | >100 | 2.04 | 3.72 | >100 | 5.5 | 2.4 | 4.68 | >100 | 8.32 | 2.29 | >100 | >100 | 2.04 | 3.72 | >100 | 6.31 | 3.55 | >100 | >100 | 2.19 | 4.47 | >100 | 9.12 | 0.15 | 96.61 | 6.50 | |
| SR | 3.19 | >100 | >100 | 2.63 | 3.89 | >100 | >100 | 3.55 | 5.37 | >100 | 56.23 | 2.83 | >100 | 23.2 | 2.75 | 3.89 | >100 | >100 | 2.86 | >100 | >100 | 2.34 | 3.8 | >100 | 16.22 | 0.11 | >100 | 75.34 | |
| NSCLC | A549/ATCC | 12.4 | 57.7 | 26.8 | 4.79 | 7.41 | >100 | 47.86 | 10.72 | 16.22 | 52.48 | 23.99 | 11.7 | 55.3 | 25.4 | 3.24 | 4.47 | 54.95 | 12.02 | 17 | 69 | 34.2 | 3.55 | 5.01 | 44.67 | 13.8 | 0.07 | 96.38 | 76.56 |
| EKVX | 12.8 | 64.6 | 28.7 | 10.47 | 23.99 | 95.5 | 31.62 | 12.88 | 23.99 | 52.48 | 26.3 | 10.6 | 51 | 23.3 | 5.75 | 15.14 | 46.77 | 19.5 | 16.4 | 58.1 | 30.9 | 4.07 | 10.96 | >100 | 22.91 | 0.36 | >100 | 82.04 | |
| HOP-62 | 3.83 | 48.7 | 16.2 | 2.57 | 4.68 | 74.13 | 6.31 | 5.37 | 12.02 | 72.44 | 22.91 | 3.94 | 61.2 | 14 | 3.98 | 6.76 | 40.74 | 12.3 | 6.43 | 67.7 | 23.2 | 3.16 | 5.37 | >100 | 16.22 | 0.18 | 89.13 | 2.53 | |
| HOP-92 | 3.35 | 56 | 15.8 | 3.55 | 79.43 | >100 | 30.9 | 11.48 | 30.2 | 63.1 | 26.92 | 6.44 | 58.8 | 22.5 | 3.89 | 22.39 | 44.67 | 16.6 | 12.2 | 64.4 | 28 | 2.95 | 44.67 | >100 | 19.95 | 0.22 | >100 | 36.31 | |
| NCI-H226 | 5.27 | >100 | 27.6 | 4.37 | 29.51 | >100 | 38.9 | 13.8 | 36.31 | >100 | 38.02 | 4.13 | >100 | 25.4 | 11.22 | 33.11 | >100 | 38.02 | 15.6 | >100 | 42.1 | 3.63 | 32.36 | >100 | 36.31 | 0.67 | 96.16 | 48.98 | |
| NCI-H23 | 10.6 | 49.9 | 23 | 2.09 | 3.63 | 12.88 | 4.68 | 14.79 | 26.92 | 58.88 | 29.51 | 8.23 | 48 | 21.3 | 5.37 | 10 | 45.71 | 18.2 | 12.2 | 52.6 | 25.3 | 2.95 | 5.25 | 44.67 | 10.47 | 0.02 | 96.16 | 0.40 | |
| NCI-H322M | 4.1 | 44.8 | 17.9 | 6.03 | 16.22 | 85.11 | 26.3 | 10.23 | 19.95 | 51.29 | 22.91 | 3.76 | 41.3 | 15.2 | 8.71 | 17.38 | 50.12 | 21.88 | 11 | 54.6 | 24.5 | 4.57 | 14.13 | 51.29 | 18.62 | 0.07 | >100 | 74.82 | |
| NCI-H460 | 15.2 | 83.3 | 35.6 | 2.75 | 3.55 | 45.71 | 7.59 | 12.02 | 19.05 | 69.18 | 28.84 | 14.3 | 80.9 | 34.1 | 3.63 | 4.27 | 54.95 | 13.8 | 15.9 | 72.5 | 34 | 2.14 | 3.31 | 44.67 | 5.62 | 0.03 | >100 | 66.83 | |
| NCI-H522 | 8.63 | 46.8 | 21.2 | 4.57 | 11.48 | 45.71 | 17.38 | 11.75 | 22.39 | 52.48 | 25.12 | 3.37 | 41.2 | 14.3 | 5.01 | 11.75 | 46.77 | 17.78 | 9.37 | 51.5 | 22.5 | 3.16 | 7.59 | 40.74 | 12.3 | 0.03 | 88.51 | 3.46 | |
| Colon Cancer | COLO 205 | 15.4 | 80.4 | 35.2 | 3.55 | 5.25 | 51.29 | 14.45 | 19.05 | 33.88 | 74.13 | 38.02 | 13.9 | 80 | 33.3 | 5.75 | 8.71 | 51.29 | 19.05 | 15.9 | >100 | 41.1 | 16.22 | 28.84 | 70.79 | 33.88 | 2.49 | >100 | 43.25 |
| HCC-2998 | 3.46 | 36.5 | 13.2 | 2.57 | 4.37 | 28.18 | 7.76 | ND | ND | ND | ND | 2.72 | 31.6 | 9.62 | 2 | 3.63 | 11.75 | 4.57 | 9.37 | 47 | 21.5 | 5.13 | 11.75 | 44.67 | 17.38 | 0.05 | 26.85 | 1.22 | |
| HCT-116 | 3.99 | 42.5 | 15.2 | 1.82 | 2.95 | 7.41 | 3.63 | 6.61 | 7.94 | 74.13 | 24.55 | 3.82 | 40.9 | 13.1 | 2.88 | 3.31 | >100 | >100 | 2.78 | 44.3 | 7.89 | 3.16 | 3.63 | 69.18 | 14.45 | 0.03 | >100 | 0.22 | |
| HCT-15 | 3.27 | 43.7 | 14.2 | 2.09 | 3.55 | 46.77 | 5.89 | 4.07 | 5.37 | 48.98 | 16.6 | 2.66 | 36.4 | 10 | 2.14 | 3.47 | 31.62 | 7.76 | 2.46 | 33.7 | 7.86 | 3.24 | 4.17 | 74.13 | 15.49 | 0.03 | >100 | 9.51 | |
| HT29 | 10.6 | 52.4 | 23.6 | 2.24 | 3.55 | 12.59 | 4.9 | 6.76 | 9.55 | 53.7 | 20.89 | 5.09 | 57.8 | 19.3 | 5.5 | 7.24 | 60.26 | 19.95 | 10.7 | 68.7 | 27.1 | 3.47 | 4.57 | >100 | 13.49 | 0.66 | 38.73 | 5.51 | |
| KM12 | 4.28 | 41 | 15.4 | 1.95 | 3.39 | 7.76 | 3.89 | 4.17 | 8.13 | 45.71 | 16.6 | 3.27 | 35.9 | 11 | 3.63 | 5.37 | 42.66 | 13.18 | 3.8 | 40 | 13.6 | 3.24 | 6.46 | 42.66 | 13.8 | 0.05 | 91.41 | 0.76 | |
| SW-620 | 3.37 | 49.3 | 15 | 1.91 | 3.16 | 7.59 | 3.8 | 3.09 | 4.37 | 38.9 | 11.22 | 2.82 | 41.6 | 11.7 | 3.39 | 4.07 | 45.71 | 10.96 | 3.59 | 44.2 | 13.9 | 2.57 | 3.72 | 46.77 | 9.77 | 0.03 | >100 | 86.30 | |
| CNS Cancer | SF-268 | 13 | 63.1 | 28.7 | 3.72 | 7.59 | 97.72 | 16.98 | 12.02 | 24.55 | 64.57 | 28.18 | 13.5 | 64.2 | 29.5 | 10.47 | 19.05 | 57.54 | 24.55 | 15.6 | 67.3 | 32.4 | 2.63 | 6.46 | 54.95 | 11.22 | 0.13 | 91.62 | 49.66 |
| SF-295 | 12.5 | 52.9 | 25.7 | 4.37 | 11.48 | 43.65 | 16.98 | 15.85 | 28.84 | 54.95 | 29.51 | 12.7 | 52.7 | 25.8 | 3.63 | 8.71 | 39.81 | 14.79 | 16.1 | 56.5 | 30.2 | 5.5 | 14.45 | 66.07 | 21.38 | 0.07 | >100 | 1.28 | |
| SF-539 | 2.94 | 35.5 | 10.8 | 2 | 3.63 | 8.32 | 4.07 | 6.92 | 16.22 | 47.86 | 20.42 | 2 | 12.8 | 4.67 | 2.19 | 3.98 | 12.88 | 4.9 | 2.31 | 27.2 | 7.02 | 2.19 | 4.47 | 20.89 | 5.5 | 0.04 | 86.90 | 0.20 | |
| SNB-19 | 4.87 | 43.1 | 17.8 | 7.76 | 16.98 | 48.98 | 21.38 | 12.02 | 22.39 | 50.12 | 24.55 | 3.47 | 37.7 | 13.4 | 4.9 | 11.75 | 41.69 | 16.98 | 5.42 | 43.2 | 18.3 | 5.01 | 13.18 | 43.65 | 17.78 | 0.04 | >100 | 15.85 | |
| SNB-75 | ND | ND | ND | 1.66 | 3.8 | 7.76 | 3.63 | 2.19 | 8.51 | 28.84 | 6.76 | ND | ND | ND | 2.29 | 8.51 | 27.54 | 7.41 | ND | ND | ND | 2.19 | 7.59 | 28.84 | 6.17 | 0.83 | 82.41 | 24.72 | |
| U251 | 3.63 | 41.9 | 15.1 | 1.91 | 3.31 | 7.24 | 3.72 | 3.39 | 4.79 | 40.74 | 13.18 | 2.87 | 39.7 | 11.7 | 1.74 | 3.09 | 5.89 | 3.24 | 4.1 | 53 | 17 | 3.02 | 4.17 | 43.65 | 12.02 | 0.04 | 98.86 | 13.21 | |
| Melanoma | LOX IMVI | 3.26 | 36.6 | 12.7 | 1.66 | 2.88 | 5.75 | 3.09 | 3.39 | 4.47 | 38.02 | 12.02 | 2.8 | 33.7 | 10.2 | 1.58 | 2.75 | 6.03 | 3.09 | 1.85 | 8.91 | 4.06 | 3.24 | 4.47 | 38.9 | 12.02 | 0.01 | >100 | 12.74 |
| MALME-3M | 12.4 | 60.9 | 27.5 | 2.24 | 5.13 | 50.12 | 5.75 | 14.45 | 30.9 | 75.86 | 33.11 | 12.5 | 67.2 | 29 | 12.3 | 24.55 | 54.95 | 26.3 | 13.7 | 70.8 | 31.1 | 5.75 | 17.78 | 54.95 | 20.42 | 0.44 | >100 | 70.31 | |
| M14 | 6.97 | 56.7 | 22.2 | 2.88 | 4.17 | >100 | 9.55 | 14.13 | 24.55 | 72.44 | 32.36 | 9.02 | 54.4 | 22.9 | 7.08 | 11.75 | 83.18 | 25.7 | 12.2 | 66.3 | 28.4 | 3.24 | 5.13 | 72.44 | 13.18 | 0.14 | 88.31 | 0.24 | |
| MDA-MB-435 | 4.24 | 48.7 | 17.5 | 2.09 | 3.55 | 16.98 | 5.01 | 5.5 | 10.96 | 48.98 | 19.05 | 4.12 | 45.1 | 16.7 | 4.57 | 7.41 | 44.67 | 16.98 | 7.39 | 50.9 | 21.1 | 2.57 | 4.47 | >100 | 10.47 | 0.03 | 95.06 | 0.08 | |
| SK-MEL-2 | 11.6 | 50.1 | 24.1 | 10.47 | 29.51 | 70.79 | 27.54 | 13.8 | 27.54 | 56.23 | 28.18 | 5.87 | 47.9 | 19.5 | 12.59 | 26.92 | 53.7 | 26.3 | 13.6 | 62 | 29 | 3.09 | 10.96 | 42.66 | 11.48 | 8.20 | >100 | 60.53 | |
| SK-MEL-28 | 11.8 | 51.4 | 24.7 | 2.14 | 4.07 | 13.49 | 4.9 | 14.45 | 26.3 | 53.7 | 28.18 | 11.9 | 50.2 | 24.5 | 5.5 | 13.18 | 44.67 | 18.2 | 13.7 | 52.5 | 26.8 | 4.07 | 9.12 | 57.54 | 17.38 | 3.16 | 90.78 | 82.41 | |
| SK-MEL-5 | 6.77 | 44.2 | 19.5 | 4.79 | 9.12 | 41.69 | 16.98 | 11.22 | 20.42 | 48.98 | 23.44 | 5.82 | 43 | 18.4 | 13.18 | 22.91 | 51.29 | 26.3 | 12 | 49.7 | 24.4 | 1.58 | 3.31 | 10 | 3.98 | 0.01 | 94.19 | 53.70 | |
| UACC-257 | 12.6 | 56.6 | 26.7 | 3.63 | 9.77 | 63.1 | 14.79 | 13.8 | 26.92 | 56.23 | 27.54 | 11.3 | 51.9 | 24.2 | 3.39 | 10 | 38.02 | 13.49 | 15.1 | 61.4 | 30.4 | 5.13 | 18.62 | 75.86 | 21.88 | 4.95 | 1.97 | 0.01 | |
| UACC-62 | 5.3 | 45.7 | 19.3 | 2.34 | 4.17 | 34.67 | 8.71 | 9.77 | 16.98 | 48.98 | 22.39 | 5.17 | 44.2 | 18.6 | 3.55 | 7.94 | 41.69 | 15.85 | 10.5 | 48.1 | 22.4 | 2.82 | 5.37 | 42.66 | 12.3 | 0.01 | >100 | 80.54 | |
| Ovarian Cancer | IGROV1 | 4.93 | 62.3 | 19.7 | 2.45 | 4.17 | >100 | 6.46 | 13.18 | 25.7 | 93.33 | 34.67 | 3.29 | 53.4 | 11.9 | 3.02 | 4.9 | 47.86 | 12.3 | 4.43 | 50.8 | 14.9 | 3.31 | 5.75 | >100 | 13.8 | 0.06 | >100 | 63.53 |
| OVCAR-3 | 2.42 | 24.5 | 6.81 | 2.29 | 4.17 | 22.91 | 6.03 | 2.82 | 6.46 | 33.11 | 10.72 | 1.88 | 9.7 | 4.27 | 2.4 | 4.37 | 23.99 | 6.46 | 2.22 | 16.6 | 5.28 | 2.45 | 5.25 | 25.7 | 6.92 | 0.04 | 93.54 | 61.66 | |
| OVCAR-4 | 6.03 | 44.7 | 19.6 | 4.47 | 12.88 | >100 | 41.69 | 13.8 | 25.12 | 54.95 | 27.54 | 10.7 | 48.5 | 22.8 | 10.96 | 20.42 | 51.29 | 23.99 | 14.9 | 53.8 | 28.3 | 2.82 | 6.61 | 51.29 | 12.59 | 0.26 | 20.23 | 0.16 | |
| OVCAR-5 | 15.6 | 57.7 | 30 | 5.25 | 15.49 | 57.54 | 19.95 | 17.78 | 32.36 | 60.26 | 33.11 | 15.4 | 57.3 | 29.7 | 3.63 | 9.12 | 38.9 | 13.8 | 17.8 | 58.2 | 32.2 | 3.8 | 12.3 | 43.65 | 15.14 | 0.18 | >100 | 83.18 | |
| OVCAR-8 | 3.99 | 50.9 | 16.2 | 2.69 | 3.89 | >100 | 7.59 | 8.51 | 14.45 | 54.95 | 22.91 | 2.86 | 37.1 | 8.77 | 3.31 | 4.57 | 38.02 | 11.75 | 7.73 | 65.2 | 23.9 | 2.14 | 3.55 | 45.71 | 5.5 | 0.03 | 90.78 | 78.52 | |
| NCI/ADR-RES | 5.92 | 85 | 24.9 | 2.88 | 5.01 | >100 | >100 | 3.72 | 7.08 | 77.62 | 17.38 | 6.04 | 73.4 | 23.4 | 2.51 | 4.27 | >100 | 5.75 | 14.9 | 82.8 | 35.2 | 2.4 | 4.37 | >100 | 7.41 | 0.06 | 90.36 | 39.17 | |
| SK-OV-3 | 11 | 61.8 | 26.1 | 17.38 | 51.29 | >100 | >100 | 15.85 | 30.2 | 66.07 | 32.36 | 13.8 | 74.9 | 32.2 | 5.89 | 15.14 | 45.71 | 18.62 | 18.6 | 76.9 | 37.8 | 7.41 | 46.77 | >100 | >100 | 28.44 | 89.54 | 0.36 | |
| Renal Cancer | 786-0 | 4.59 | 47.1 | 17.6 | 2.51 | 4.07 | 37.15 | 6.31 | 4.68 | 7.76 | 58.88 | 20.42 | 3.33 | 41.8 | 13.3 | 3.98 | 6.31 | 50.12 | 15.85 | 3.99 | 54 | 17.6 | 4.57 | 6.61 | 97.72 | 20.89 | 0.02 | >100 | >100 |
| A498 | 13.6 | 52.6 | 26.8 | 19.5 | 47.86 | 91.2 | 42.66 | 15.85 | 30.9 | 56.23 | 29.51 | 13.5 | 51.8 | 26.5 | 14.79 | 29.51 | 54.95 | 28.18 | 15.5 | 55.3 | 29.3 | 11.48 | 43.65 | >100 | 34.67 | 0.05 | >100 | 25.47 | |
| ACHN | 7.02 | 44.3 | 19.6 | 2.29 | 3.72 | 21.38 | 6.03 | 13.8 | 23.99 | 51.29 | 26.92 | 7.67 | 45.4 | 20.3 | 4.07 | 6.17 | 38.9 | 14.79 | 11.3 | 48.4 | 23.3 | 3.89 | 6.46 | 54.95 | 16.6 | 0.01 | >100 | 0.72 | |
| CAKI-1 | 6.89 | 45.4 | 20.2 | 4.68 | 15.49 | 69.18 | 20.89 | 4.47 | 8.71 | 43.65 | 17.38 | 10 | 47.2 | 21.8 | 10.47 | 20.42 | 51.29 | 22.91 | 13.9 | 52.7 | 27.1 | 2.57 | 4.68 | 45.71 | 13.18 | 0.04 | 97.05 | 8.22 | |
| RXF 393 | 3.02 | 37.2 | 13 | 1.91 | 5.13 | 10 | 4.37 | 5.89 | 23.99 | 48.98 | 19.95 | 2.58 | 33 | 9.43 | 10.23 | 24.55 | 47.86 | 22.39 | 4.88 | 44.8 | 18.6 | 2.51 | 11.22 | 30.9 | 7.08 | 0.89 | >100 | 13.87 | |
| SN12C | 3.95 | 46.7 | 17 | 2 | 3.55 | 8.91 | 4.27 | 4.9 | 10.72 | 43.65 | 18.2 | 3.15 | 36.8 | 13.1 | 2.69 | 4.37 | 33.11 | 10.23 | 6.06 | 44.7 | 19.3 | 2.29 | 3.89 | 46.77 | 9.55 | 0.01 | 91.62 | 82.04 | |
| TK-10 | 12.5 | 50.7 | 25.2 | 19.5 | 45.71 | >100 | 47.86 | 15.14 | 28.18 | 54.95 | 28.84 | 3.04 | 35.2 | 11.8 | 11.22 | 22.91 | 50.12 | 23.44 | 12.4 | 51.3 | 25.3 | 5.01 | 16.22 | 47.86 | 17.78 | 0.57 | >100 | 11.51 | |
| UO-31 | 2.88 | 41.2 | 15.7 | 1.55 | 2.88 | 6.03 | 3.09 | 4.07 | 11.22 | 47.86 | 18.2 | 2.46 | 39.7 | 13.5 | 3.89 | 10.72 | 43.65 | 16.98 | 4.86 | 46.3 | 18.8 | 3.02 | 7.76 | 46.77 | 15.49 | 0.19 | >100 | 4.16 | |
| PC | PC-3 | 5.82 | >100 | 29.7 | 2.88 | 4.9 | >100 | 13.8 | 5.37 | 9.77 | 81.28 | 23.44 | 4.68 | >100 | 26 | 4.07 | 8.13 | 56.23 | 17.78 | 10.3 | >100 | 37.3 | 5.01 | 8.91 | >100 | 41.69 | 0.03 | >100 | 51.40 |
| DU-145 | 4.32 | 39.9 | 15.7 | 3.24 | 4.79 | 42.66 | 11.48 | 4.9 | 8.91 | 42.66 | 17.38 | 3.52 | 35.3 | 12 | 2.4 | 3.89 | 23.44 | 6.17 | 3.41 | 34.5 | 11 | 3.24 | 5.75 | 69.18 | 15.49 | 0.01 | >100 | 78.89 | |
| Breast Cancer | MCF7 | 2.65 | 37 | 11.4 | 3.31 | 8.13 | 37.15 | 12.3 | 3.16 | 4.68 | 43.65 | 13.18 | 2.57 | 38.7 | 10.6 | 2.75 | 6.31 | 38.02 | 9.77 | 2.67 | 51.3 | 11.7 | 3.02 | 4.27 | 45.71 | 10.72 | 0.04 | >100 | >100 |
| MDA-MB-231/ATCC | 15.7 | 66.1 | 32.2 | 2.45 | 6.17 | 39.81 | 6.92 | 16.22 | 30.9 | 61.66 | 31.62 | 10.9 | 50.9 | 23.5 | 5.25 | 16.22 | 44.67 | 17.78 | 12 | 51.5 | 24.8 | 3.24 | 10 | >100 | 13.18 | 0.01 | 91.62 | 73.62 | |
| HS 578T | 13.2 | >100 | 40.2 | 3.09 | >100 | >100 | 85.11 | 21.38 | >100 | >100 | 77.62 | 10.4 | >100 | 33 | 17.38 | 67.61 | >100 | 52.48 | 13 | 80.9 | 32.4 | 2.88 | >100 | >100 | 39.81 | 0.15 | >100 | 0.22 | |
| BT-549 | 2.34 | 33.1 | 8.27 | 2.24 | 5.89 | 28.18 | 6.17 | 5.75 | 20.89 | 57.54 | 20.42 | 2.6 | 30.6 | 7.28 | 3.8 | 13.49 | 43.65 | 14.79 | 5.04 | 48.5 | 19.3 | 2.95 | 11.75 | 50.12 | 10.72 | 0.01 | 99.54 | 8.09 | |
| T-47D | 3.94 | >100 | 28.8 | 3.39 | 7.94 | >100 | 20.89 | 3.47 | 14.13 | 81.28 | 19.05 | 2.77 | 61.7 | 11.8 | 2 | 4.9 | 66.07 | 7.08 | 4.96 | >100 | 25.7 | 2.19 | 7.24 | >100 | 13.18 | 47.97 | >100 | 2.04 | |
| MDA-MB-468 | 1.93 | 25.3 | 6.46 | 3.8 | 12.59 | 46.77 | 15.85 | 2.51 | 12.02 | 30.2 | 8.32 | 1.79 | 27.2 | 6.77 | 2.95 | 7.76 | 40.74 | 10.72 | 2.2 | 31.4 | 8 | 1.55 | 5.75 | 17.78 | 4.47 | ND | ND | ND | |
| Compound | Binding Affinity (kcal/mol) | Classical Hydrogen Bonding | Non-Classical Hydrogen Bonding | Hydrophobic Interactions |
|---|---|---|---|---|
| CA4 | −9.2 | Cys241 | Asn258, Asn350, Val238 | Met259, Ala316, Ala354, Val181, Lys352, Cys241, Leu242, Leu255, Ile318, Ile378, Ala180, Leu248, Ala250, Asn258 |
| 2e | −8.6 | Cys241 | Cys241 | Met259, Lys352, Leu248, Leu255, Ala180, Val181, Lys352 |
| 2g | −7.3 | Cys241 | N/A | Thr179, Ala180, Leu248, Leu255, Ala316, Ala250 |
| 2h | −8.9 | Cys241, Gln247 | Ser178 | Cys241, Leu255, Ala316, Ala180, Leu248, Lys352 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashem, H.; Hassan, A.; Abdelmagid, W.M.; Habib, A.G.K.; Abdel-Aal, M.A.A.; Elshamsy, A.M.; El Zawily, A.; Radwan, I.T.; Bräse, S.; Abdel-Samea, A.S.; et al. Synthesis of New Thiazole-Privileged Chalcones as Tubulin Polymerization Inhibitors with Potential Anticancer Activities. Pharmaceuticals 2024, 17, 1154. https://doi.org/10.3390/ph17091154
Hashem H, Hassan A, Abdelmagid WM, Habib AGK, Abdel-Aal MAA, Elshamsy AM, El Zawily A, Radwan IT, Bräse S, Abdel-Samea AS, et al. Synthesis of New Thiazole-Privileged Chalcones as Tubulin Polymerization Inhibitors with Potential Anticancer Activities. Pharmaceuticals. 2024; 17(9):1154. https://doi.org/10.3390/ph17091154
Chicago/Turabian StyleHashem, Hamada, Abdelfattah Hassan, Walid M. Abdelmagid, Ahmed G. K. Habib, Mohamed A. A. Abdel-Aal, Ali M. Elshamsy, Amr El Zawily, Ibrahim Taha Radwan, Stefan Bräse, Ahmed S. Abdel-Samea, and et al. 2024. "Synthesis of New Thiazole-Privileged Chalcones as Tubulin Polymerization Inhibitors with Potential Anticancer Activities" Pharmaceuticals 17, no. 9: 1154. https://doi.org/10.3390/ph17091154







