Photoantimicrobial and Photoantiviral Textiles: Underestimated Potential
Abstract
:1. Introduction
2. Results
2.1. Textiles
2.2. Light Sources
2.3. Molecules
2.3.1. Porphyrinoids
2.3.2. Keto and Quinone Dyes
2.3.3. Xanthene Dyes
2.3.4. Phenothiazines
2.3.5. Dendrimers and Polymers
2.3.6. Nanoparticles and Inorganic Sensitizers
2.4. Efficacy against Bacteria, Fungi and Viruses
- Type and strain of pathogens;
- Surface-to-volume ratio between the tissue and the pathogen inoculum media;
- The load of the dye per unit of area of the fabric;
- The nature and intensity of the activating light;
- The illumination time;
- Filtering of heat;
- Dark activity;
- Blank/negative control;
- Numerical assessment of inactivation;
- Number of parallel experiments.
- Direct sunlight, 100–1000 J/cm2;
- Bright day, 10–100 J/cm2;
- Shadow outdoor, 1–10 J/cm2;
- Indoor, 0.1–1 J/cm2.
2.5. Dye Load and Stability Tests
2.6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aroso, R.T.; Schaberle, F.A.; Arnaut, L.G.; Pereira, M.M. Photodynamic Disinfection and Its Role in Controlling Infectious Diseases. Photochem. Photobiol. Sci. 2021, 20, 1497–1545. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Y.; Guo, H.; Zhang, K.; Pang, X.; Nie, X.; Zhuge, J.; Wei, Q. Progress in Application of Photodynamic Antibacterial Technology for Textiles. Fangzhi Xuebao/J. Text. Res. 2021, 42, 187–196. [Google Scholar] [CrossRef]
- Cuthbert, T.J.; Ennis, S.; Musolino, S.F.; Buckley, H.L.; Niikura, M.; Wulff, J.E.; Menon, C. Covalent Functionalization of Polypropylene Filters with Diazirine–Photosensitizer Conjugates Producing Visible Light Driven Virus Inactivating Materials. Sci. Rep. 2021, 11, 19029. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-T.; Shou, B.-B.; Yang, L.; Ren, H.-T.; Hu, X.-J.; Lin, J.-H.; Cai, T.; Lou, C.-W. Modification of Traditional Composite Nonwovens with Stable Storage of Light Absorption Transients and Photodynamic Antibacterial Effect. Photochem. Photobiol. 2024. [CrossRef]
- Lee, D.U.; Jeong, S.B.; Lee, B.J.; Park, S.K.; Kim, H.-M.; Shin, J.H.; Lee, S.Y.; Kim, G.; Park, J.; Kim, G.M.; et al. Antimicrobial and Antifouling Effects of Petal-Like Nanostructure by Evaporation-Induced Self-Assembly for Personal Protective Equipment. Small 2024, 20, 2306324. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuan, Q.; Li, M.; Tang, Y. Cationic Conjugated Microporous Polymers Coating for Dual-Modal Antimicrobial Inactivation with Self-Sterilization and Reusability Functions. Adv. Funct. Mater. 2023, 33, 2213440. [Google Scholar] [CrossRef]
- Flores, J.; Blázquez-Moraleja, A.; Bonet-Aracil, M.; Moya, P.; Bosca, F.; Marin, M.L. What Is the Most Effective Percentage of Rose Bengal on Polyamide Fabrics for the Visible-Light Photoinactivation of Gram-Positive Bacteria? J. Environ. Chem. Eng. 2023, 11, 110639. [Google Scholar] [CrossRef]
- Kim, J.R.; Michielsen, S. Photodynamic Activity of Nanostructured Fabrics Grafted with Xanthene and Thiazine Dyes against Opportunistic Fungi. J. Photochem. Photobiol. B Biol. 2015, 150, 50–59. [Google Scholar] [CrossRef]
- Kim, J.R.; Michielsen, S. Synthesis of Antifungal Agents from Xanthene and Thiazine Dyes and Analysis of Their Effects. Nanomaterials 2016, 6, 243. [Google Scholar] [CrossRef]
- Ren, Y.; Yan, B.; Lin, C.; Wang, P.; Zhou, M.; Cui, L.; Yu, Y.; Wang, Q. Multifunctional Textile Constructed via Polyaniline-Mediated Copper Sulfide Nanoparticle Growth for Rapid Photothermal Antibacterial and Antioxidation Applications. ACS Appl. Nano Mater. 2023, 6, 1212–1223. [Google Scholar] [CrossRef]
- Zhang, N.; Xu, Y.; Shi, R.; Zhou, M.; Yu, Y.; Wang, P.; Wang, Q. Protein-Based Coating Strategy for Preparing Durable Sunlight-Driven Rechargeable Antibacterial, Super Hydrophilic, and UV-Resistant Textiles. Int. J. Biol. Macromol. 2024, 258, 128761. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, J.; Li, L.; Wang, X.; Wei, Q.; Ghiladi, R.A.; Wang, Q. Wool/Acrylic Blended Fabrics as Next-Generation Photodynamic Antimicrobial Materials. ACS Appl. Mater. Interfaces 2019, 11, 29557–29568. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, W.; Dong, T.; Lv, Z.; Zheng, S.; Cao, X.; Wei, Q.; Ghiladi, R.A.; Wang, Q. Color-Variable Photodynamic Antimicrobial Wool/Acrylic Blended Fabrics. Materials 2020, 13, 4141. [Google Scholar] [CrossRef]
- European Standard EN 62471:2008; Photobiological Safety of Lamps and Lamp Systems (IEC 62471:2006, modified). Published by European Committee for Electrotechnical Standardization CENELEC: Brussels, Belgium, 2008.
- European Standard EN 12464-1:2021; Light and Lighting—Lighting of Work Places—Part 1: Indoor Work Places. Published by European Committee for Standardization CEN-CENELEC: Brussels, Belgium, 2021.
- Efimov, A.; Dagallier, C.; Frochot, C.; Myrzakhmetov, B.; Arnoux, P.; Heinonen, T.; Mannerström, M.; Toimela, T.; Ahmed, Z.; Audibert, J.F.; et al. LASU: An Efficient and Stable Phthalocyanine Dye with Tolerable Safety Profile for Self-Disinfecting Anti-COVID Textiles Activated by Ambient Light. Photodiagnosis Photodyn. Ther. 2024, 45, 103978. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Zhang, Z.; El-Moghazy, A.Y.; Wisuthiphaet, N.; Nitin, N.; Sun, G. Daylight-Induced Antibacterial and Antiviral Cotton Cloth for Offensive Personal Protection. ACS Appl. Mater. Interfaces 2020, 12, 49442–49451. [Google Scholar] [CrossRef]
- Feese, E.; Sadeghifar, H.; Gracz, H.S.; Argyropoulos, D.S.; Ghiladi, R.A. Photobactericidal Porphyrin-Cellulose Nanocrystals: Synthesis, Characterization, and Antimicrobial Properties. Biomacromolecules 2011, 12, 3528–3539. [Google Scholar] [CrossRef]
- Stanley, S.L.; Scholle, F.; Zhu, J.; Lu, Y.; Zhang, X.; Situ, X.; Ghiladi, R.A. Photosensitizer-Embedded Polyacrylonitrile Nanofibers as Antimicrobial Non-Woven Textile. Nanomaterials 2016, 6, 77. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Hu, P.; Wang, D.; Lin, F.; Xue, J.; Chen, Z.; Iqbal, Z.; Huang, M. Dual Antimicrobial Actions on Modified Fabric Leads to Inactivation of Drug-Resistant Bacteria. Dye. Pigment. 2017, 140, 236–243. [Google Scholar] [CrossRef]
- Arenbergerova, M.; Arenberger, P.; Bednar, M.; Kubat, P.; Mosinger, J. Light-Activated Nanofibre Textiles Exert Antibacterial Effects in the Setting of Chronic Wound Healing. Exp. Dermatol. 2012, 21, 619–624. [Google Scholar] [CrossRef]
- Zhuo, J.; Sun, G. Light-Induced Surface Graft Polymerizations Initiated by an Anthraquinone Dye on Cotton Fibers. Carbohydr. Polym. 2014, 112, 158–164. [Google Scholar] [CrossRef]
- Hu, L.; Song, X.; Li, M.; Xie, K.; Hou, A. Scalable Fabrication of Benzophenone/Phthalocyanine-Decorated Photodynamic Cotton Fabrics for Enhanced Dye Degradation and Antibacterial Performance. Cellulose 2023, 30, 4683–4696. [Google Scholar] [CrossRef]
- Oh, K.W.; Choi, H.-M.; Kim, J.M.; Park, J.H.; Park, I.S. A Novel Antimicrobial Photosensitizer: Hydroxyethyl Michler’s Ketone. Text. Res. J. 2014, 84, 808–818. [Google Scholar] [CrossRef]
- Kováčová, M.; Kleinová, A.; Vajďák, J.; Humpolíček, P.; Kubát, P.; Bodík, M.; Marković, Z.; Špitálský, Z. Photodynamic-Active Smart Biocompatible Material for an Antibacterial Surface Coating. J. Photochem. Photobiol. B Biol. 2020, 211, 112012. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, C.; Zhang, N.; Ding, X.; Yu, B.; Xu, F.-J. Polycationic Synergistic Antibacterial Agents with Multiple Functional Components for Efficient Anti-Infective Therapy. Adv. Funct. Mater. 2018, 28, 1706709. [Google Scholar] [CrossRef]
- Wright, T.; Vlok, M.; Shapira, T.; Olmstead, A.D.; Jean, F.; Wolf, M.O. Photodynamic and Contact Killing Polymeric Fabric Coating for Bacteria and SARS-CoV-2. ACS Appl. Mater. Interfaces 2022, 14, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Morsi, R.E.; Gentili, D.; Corticelli, F.; Morandi, V.; Figoli, A.; Russo, F.; Galiano, F.; Gentilomi, G.A.; Bonvicini, F.; Manet, I.; et al. Cellulose Acetate Membranes Loaded with Combinations of Tetraphenylporphyrin, Graphene Oxide and Pluronic F-127 as Responsive Materials with Antibacterial Photodynamic Activity. RSC Adv. 2023, 13, 26550–26562. [Google Scholar] [CrossRef]
- Kurskaya, O.G.; Sharshov, K.A.; Solomatina, M.V.; Voevoda, M.I.; Shestopalov, A.M.; Meerovich, G.A.; Strakhovskaya, M.G. Photodynamic Inactivation of SARS-CoV-2 on Inanimate Surfaces. Laser Phys. Lett. 2022, 19, 115601. [Google Scholar] [CrossRef]
- Nie, X.; Wu, S.; Mensah, A.; Wang, Q.; Huang, F.; Li, D.; Wei, Q. Insight into Light-Driven Antibacterial Cotton Fabrics Decorated by in Situ Growth Strategy. J. Colloid Interface Sci. 2020, 579, 233–242. [Google Scholar] [CrossRef]
- Chen, W.; Wang, W.; Ge, X.; Wei, Q.; Ghiladi, R.A.; Wang, Q. Photooxidation Properties of Photosensitizer/Direct Dye Patterned Polyester/Cotton Fabrics. Fibers Polym. 2018, 19, 1687–1693. [Google Scholar] [CrossRef]
- Mensah, A.; Yajun, C.; Asinyo, B.K.; Howard, E.K.; Huang, J.; Narh, C.; Wei, Q. Singlet Oxygen (1O2) Induced Photodynamic Inactivation of Bacterials with Bioactive Icariin/Beta-Cyclodextrin/Bacterial Cellulose. Polym. Test. 2022, 112, 107600. [Google Scholar] [CrossRef]
- Rahimi, R.; Fayyaz, F.; Rassa, M. The Study of Cellulosic Fabrics Impregnated with Porphyrin Compounds for Use as Photo-Bactericidal Polymers. Mater. Sci. Eng. C 2016, 59, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, V.; Rittmeyer, T.; Correa, R.J.; Brêda, G.C.; Almeida, R.V.; Simões, G.; de França, B.M.; de Azevedo, P.N.; Bello Forero, J.S. Photoactive Cotton Fabric: Synthesis, Characterization and Antibacterial Evaluation of Anthraquinone-Based Dyes Linked to Cellulose. Dye. Pigment. 2019, 161, 16–23. [Google Scholar] [CrossRef]
- Lv, Z.; Wang, Q.; Wang, Y.; Yuan, X.; Xia, X.; Liao, S.; Wei, Q. A Facile and Scalable Strategy for Fabricating Bio-Based Photodynamic Antimicrobial Nonwoven Eco-Textiles. Green Chem. 2024, 26, 2213–2224. [Google Scholar] [CrossRef]
- Lin, J.-H.; Yang, L.; Hu, X.; Peng, H.; Ren, H.; Li, T.-T.; Lou, C.-W. Photodynamic Antibacterial Micro/Nanofiber Composite Membrane with High Efficiency and Low Resistance Filtration Performance for Medical Protective Materials. J. Ind. Eng. Chem. 2023, 128, 184–195. [Google Scholar] [CrossRef]
- Yu, Z.; Deng, C.; Ding, C.; Zhang, X.; Liu, Y.; Liu, C.; Lou, Z.; Seidi, F.; Han, J.; Yong, Q.; et al. Organic-Inorganic Hybrid ZIF-8/MXene/Cellulose-Based Textiles with Improved Antibacterial and Electromagnetic Interference Shielding Performance. Int. J. Biol. Macromol. 2024, 266, 131080. [Google Scholar] [CrossRef]
- Shivalkar, S.; Arshad, F.; Sahoo, A.K.; Sk, M.P. Visible Light-Mediated Photoactivated Sulfur Quantum Dots as Heightened Antibacterial Agents. ACS Omega 2022, 7, 33358–33364. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Dejarnette, S.; Chen, W.; Scholle, F.; Wang, Q.; Ghiladi, R.A. Color-Variable Dual-Dyed Photodynamic Antimicrobial Polyethylene Terephthalate (PET)/Cotton Blended Fabrics. Photochem. Photobiol. Sci. 2023, 22, 1573–1590. [Google Scholar] [CrossRef]
- Lhotáková, Y.; Plíštil, L.; Morávková, A.; Kubát, P.; Lang, K.; Forstová, J.; Mosinger, J. Virucidal Nanofiber Textiles Based on Photosensitized Production of Singlet Oxygen. PLoS ONE 2012, 7, e49226. [Google Scholar] [CrossRef] [PubMed]
- Ringot, C.; Saad, N.; Brégier, F.; Bressollier, P.; Poli, E.; Chaleix, V.; Ouk, T.S.; Sol, V. Antibacterial Activity of a Photosensitive Hybrid Cellulose Fabric. Photochem. Photobiol. Sci. 2018, 17, 1780–1786. [Google Scholar] [CrossRef]
- Song, L.; Sun, L.; Zhao, J.; Wang, X.; Yin, J.; Luan, S.; Ming, W. Synergistic Superhydrophobic and Photodynamic Cotton Textiles with Remarkable Antibacterial Activities. ACS Appl. Bio Mater. 2019, 2, 2756–2765. [Google Scholar] [CrossRef]
- Fadavi, F.; Abdulkhani, A.; Hamzeh, Y.; Bacher, M.; Gorfer, M.; Bandian, D.; Rosenau, T.; Hettegger, H. Photodynamic Antimicrobial Cellulosic Material Through Covalent Linkage of Protoporphyrin IX onto Lyocell Fibers. J. Wood Chem. Technol. 2019, 39, 57–74. [Google Scholar] [CrossRef]
- Bonnett, R.; Evans, R.L.; Galia, A.B.B. Immobilized Photosensitizers: Photosensitizer Films with Microbicidal Effects. In Proceedings of the Photochemotherapy: Photodynamic Therapy and Other Modalities III, San Remo, Italy, 4–6 September 1997; Volume 3191, pp. 79–88. [Google Scholar]
- Ringot, C.; Saad, N.; Granet, R.; Bressollier, P.; Sol, V.; Krausz, P. Meso-Functionalized Aminoporphyrins as Efficient Agents for Photo-Antibacterial Surfaces. J. Porphyr. Phthalocyanines 2010, 14, 925–931. [Google Scholar] [CrossRef]
- Jiang, C.; Scholle, F.; Jin, F.; Wei, Q.; Wang, Q.; Ghiladi, R.A. Chlorophyllin as a Photosensitizer in Photodynamic Antimicrobial Materials. Cellulose 2024, 31, 2475–2491. [Google Scholar] [CrossRef]
- Castriciano, M.A.; Zagami, R.; Casaletto, M.P.; Martel, B.; Trapani, M.; Romeo, A.; Villari, V.; Sciortino, M.T.; Grasso, L.; Guglielmino, S.; et al. Poly(Carboxylic Acid)-Cyclodextrin/Anionic Porphyrin Finished Fabrics as Photosensitizer Releasers for Antimicrobial Photodynamic Therapy. Biomacromolecules 2017, 18, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Wu, S.; Liao, S.; Chen, J.; Huang, F.; Li, W.; Wang, Q.; Wei, Q. Light-Driven Self-Disinfecting Textiles Functionalized by PCN-224 and Ag Nanoparticles. J. Hazard. Mater. 2021, 416, 125786. [Google Scholar] [CrossRef]
- Nie, X.; Wu, S.; Mensah, A.; Wang, Q.; Huang, F.; Wei, Q. FRET as a Novel Strategy to Enhance the Singlet Oxygen Generation of Porphyrinic MOF Decorated Self-Disinfecting Fabrics. Chem. Eng. J. 2020, 395, 125012. [Google Scholar] [CrossRef]
- Nie, X.; Wu, S.; Huang, F.; Wang, Q.; Wei, Q. Smart Textiles with Self-Disinfection and Photothermochromic Effects. ACS Appl. Mater. Interfaces 2021, 13, 2245–2255. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.; Chen, J.; Liu, W.; Zhang, D.; Xu, P.; Dai, T.; Shang, L.; Yang, Y.; Tang, S.; et al. Composite of Silver Nanoparticles and Photosensitizer Leads to Mutual Enhancement of Antimicrobial Efficacy and Promotes Wound Healing. Chem. Eng. J. 2019, 374, 1373–1381. [Google Scholar] [CrossRef]
- Askes, S.H.C.; Reddy, G.U.; Wyrwa, R.; Bonnet, S.; Schiller, A. Red Light-Triggered CO Release from Mn2(CO)10 Using Triplet Sensitization in Polymer Nonwoven Fabrics. J. Am. Chem. Soc. 2017, 139, 15292–15295. [Google Scholar] [CrossRef]
- Rahal, R.; Le Bechec, M.; Guyoneaud, R.; Pigot, T.; Paolacci, H.; Lacombe, S. Bactericidal Activity under UV and Visible Light of Cotton Fabrics Coated with Anthraquinone-Sensitized TiO2. Catal. Today 2013, 209, 134–139. [Google Scholar] [CrossRef]
- Jin, F.; Liao, S.; Wang, Q.; Shen, H.; Jiang, C.; Zhang, J.; Wei, Q.; Ghiladi, R.A. Dual-Functionalized Luminescent/Photodynamic Composite Fabrics: Synergistic Antibacterial Activity for Self-Disinfecting Textiles. Appl. Surf. Sci. 2022, 587, 152737. [Google Scholar] [CrossRef]
- Kim, J.R.; Michielsen, S. Photodynamic Antifungal Activities of Nanostructured Fabrics Grafted with Rose Bengal and Phloxine B against Aspergillus fumigatus. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Youssef, D.; Fekry, O.; Badr, A.; Afify, A.; Hamed, E. A New Perspective on Quantitative Assessment of Photodynamic Therapy Mediated Hydrogel Nanocomposite in Wound Healing Using Objective Biospeckle and Morphological Local-Gradient. Comput. Biol. Med. 2023, 163, 107196. [Google Scholar] [CrossRef] [PubMed]
- Staneva, D.; Vasileva-Tonkova, E.; Grozdanov, P.; Vilhelmova-Ilieva, N.; Nikolova, I.; Grabchev, I. Synthesis and Photophysical Characterisation of 3-Bromo-4-Dimethylamino-1,8-Naphthalimides and Their Evaluation as Agents for Antibacterial Photodynamic Therapy. J. Photochem. Photobiol. A Chem. 2020, 401, 112730. [Google Scholar] [CrossRef]
- Manov, H.; Staneva, D.; Vasileva-Tonkova, E.; Grozdanov, P.; Nikolova, I.; Stoyanov, S.; Grabchev, I. Photosensitive Dendrimers as a Good Alternative to Antimicrobial Photodynamic Therapy of Gram-Negative Bacteria. J. Photochem. Photobiol. A Chem. 2021, 419, 113480. [Google Scholar] [CrossRef]
- Staneva, D.; Atanasova, D.; Nenova, A.; Vasileva-Tonkova, E.; Grabchev, I. Cotton Fabric Modified with a Pamam Dendrimer with Encapsulated Copper Nanoparticles: Antimicrobial Activity. Materials 2021, 14, 7832. [Google Scholar] [CrossRef] [PubMed]
- Staneva, D.; Manov, H.; Vasileva-Tonkova, E.; Kukeva, R.; Stoyanova, R.; Grabchev, I. Enhancing the Antibacterial Activity of PAMAM Dendrimer Modified with 1,8-Naphthalimides and Its Copper Complex via Light Illumination. Polym. Adv. Technol. 2022, 33, 3163–3172. [Google Scholar] [CrossRef]
- Staneva, D.; Said, A.I.; Vasileva-Tonkova, E.; Grabchev, I. Enhanced Photodynamic Efficacy Using 1,8-Naphthalimides: Potential Application in Antibacterial Photodynamic Therapy. Molecules 2022, 27, 5743. [Google Scholar] [CrossRef]
- Liu, W.; Yu, Y.; Cheng, W.; Wang, X.; Zhou, M.; Xu, B.; Wang, P.; Wang, Q. D–A Structured High-Performance Photothermal/Photodynamic Thionin-Synthetic Melanin Nanoparticles for Rapid Bactericidal and Wound Healing Effects. Adv. Healthc. Mater. 2023, 12, 2203303. [Google Scholar] [CrossRef]
- Novikova, E.D.; Pronina, E.V.; Vorotnikov, Y.A.; Adamenko, L.S.; Alekseev, A.Y.; Shestopalov, A.M.; Tsygankova, A.R.; Gusel’nikova, T.Y.; Kubát, P.; Kirakci, K.; et al. Cotton Fabrics Modified with Molybdenum Nanoclusters for Photodynamic Inactivation of Bacteria and Viruses. J. Environ. Chem. Eng. 2023, 11, 110796. [Google Scholar] [CrossRef]
- Li, B.; Wang, D.; Lee, M.M.S.; Wang, W.; Tan, Q.; Zhao, Z.; Tang, B.Z.; Huang, X. Fabrics Attached with Highly Efficient Aggregation-Induced Emission Photosensitizer: Toward Self-Antiviral Personal Protective Equipment. ACS Nano 2021, 15, 13857–13870. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Du, B.; Gao, X.; Huang, Y.; Li, M.; Yu, Z.; Li, J.; Shi, X.; Deng, Y.; Liang, K. Endogenous Oxygen-Evolving Bio-Catalytic Fabrics with Fortified Photonic Disinfection for Invasive Bacteria-Caused Refractory Cutaneous Regeneration. Nano Today 2022, 46, 101595. [Google Scholar] [CrossRef]
- Yu, Z.; Deng, C.; Sun, J.; Zhang, X.; Liu, Y.; Liu, C.; Seidi, F.; Han, J.; Yong, Q.; Xiao, H. Cellulosic Nonwovens Incorporated with Fully Utilized MXene Precursor as Smart Pressure Sensor and Multi-Protection Materials. Adv. Funct. Mater. 2024, 2402707. [Google Scholar] [CrossRef]
- Pinho, E.; Magalhães, L.; Henriques, M.; Oliveira, R. Antimicrobial Activity Assessment of Textiles: Standard Methods Comparison. Ann. Microbiol. 2011, 61, 493–498. [Google Scholar] [CrossRef]
- AATCC TM100-2019; Test Method for Antibacterial Finishes on Textile Materials: Assess. American Association of Textile Chemists and Colorists: Durham, NC, USA, 2019.
- JIS L 1902:2015; Textiles—Determination of Antibacterial Activity and Efficacy of textile Products. Japanese Standard Association: Tokyo, Japan, 2015.
- AATCC TM147-2011 (2016e); Test Method for Antibacterial Activity of Textile Materials: Parallel Streak. American Association of Textile Chemists and Colorists: Durham, NC, USA, 2016.
- ISO 18184:2019; Textiles—Determination of Antiviral Activity of Textile Products. The International Organization for Standardization: Geneve, Switzerland, 2019.
- ISO 22196:2011; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. The International Organization for Standardization: Geneve, Switzerland, 2011.
- ASTM E2149-01; Standard Test Method for Determining the Antimicrobial Activity of Immobilized Antimicrobial Agents Under Dynamic Contact Conditions. ASTM International: West Conshohocken, PA, USA, 2001.
- AATCC 183-2014; Test Method for Transmittance or Blocking of Erythemally Weighted Ultraviolet Radiation through Fabrics. American Association of Textile Chemists and Colorists: Durham, NC, USA, 2014.
- AATCC 107-2017; Colorfastness to Water. American Association of Textile Chemists and Colorists: Durham, NC, USA, 2017.
- AATCC 61-1996; Colorfastness to Laundering, Home, and Commercial: Accelerated. American Association of Textile Chemists and Colorists: Durham, NC, USA, 1996.
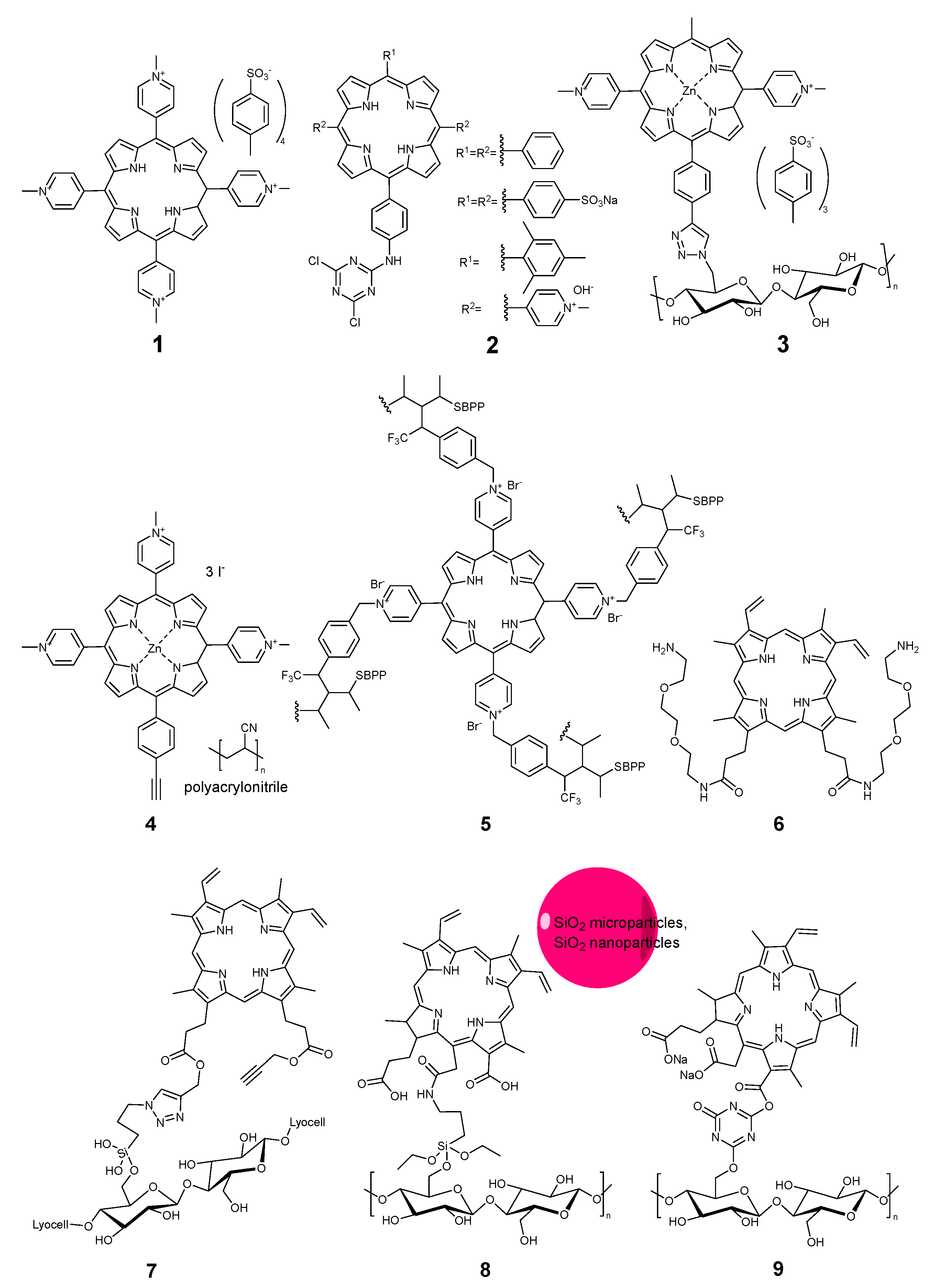
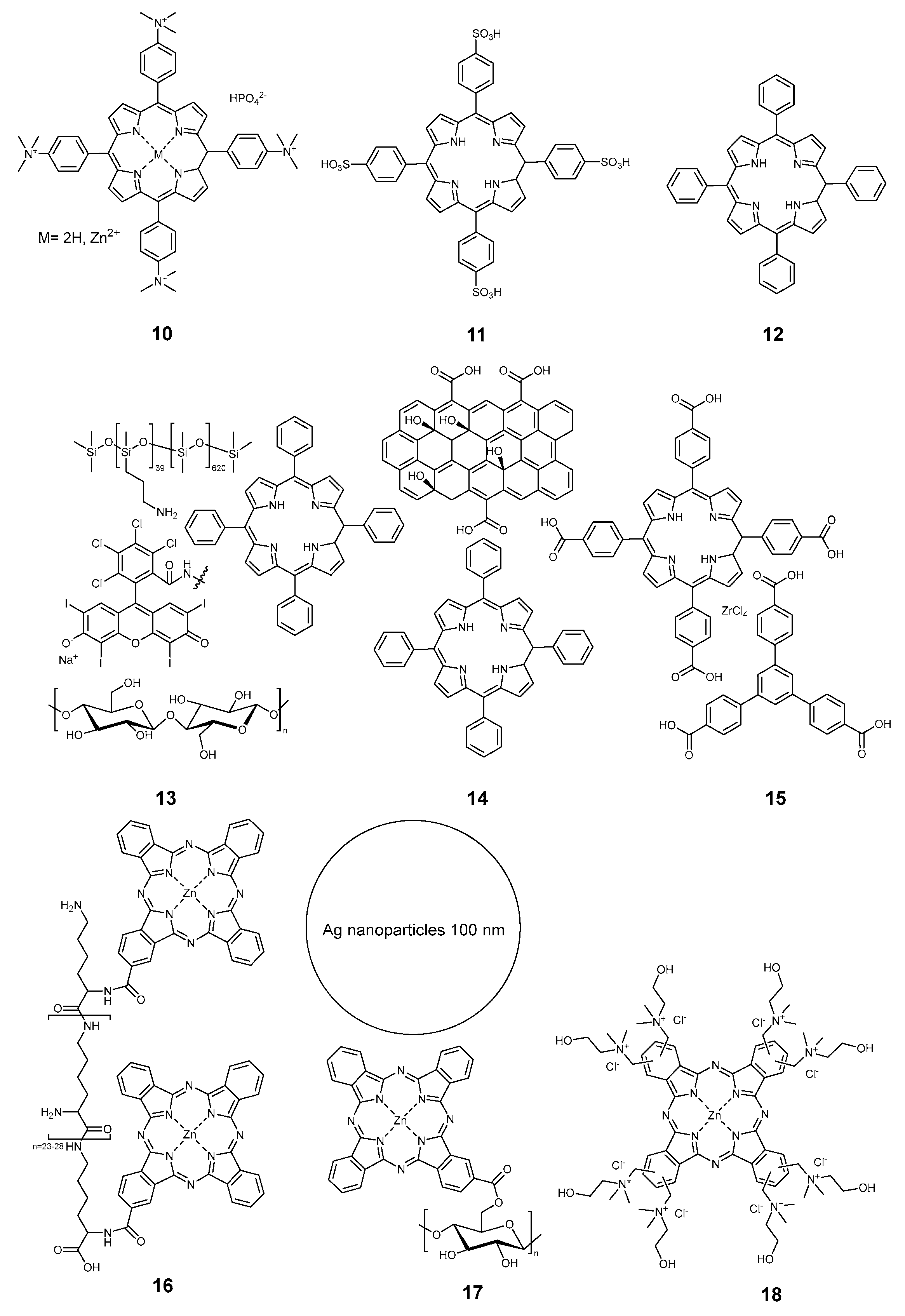
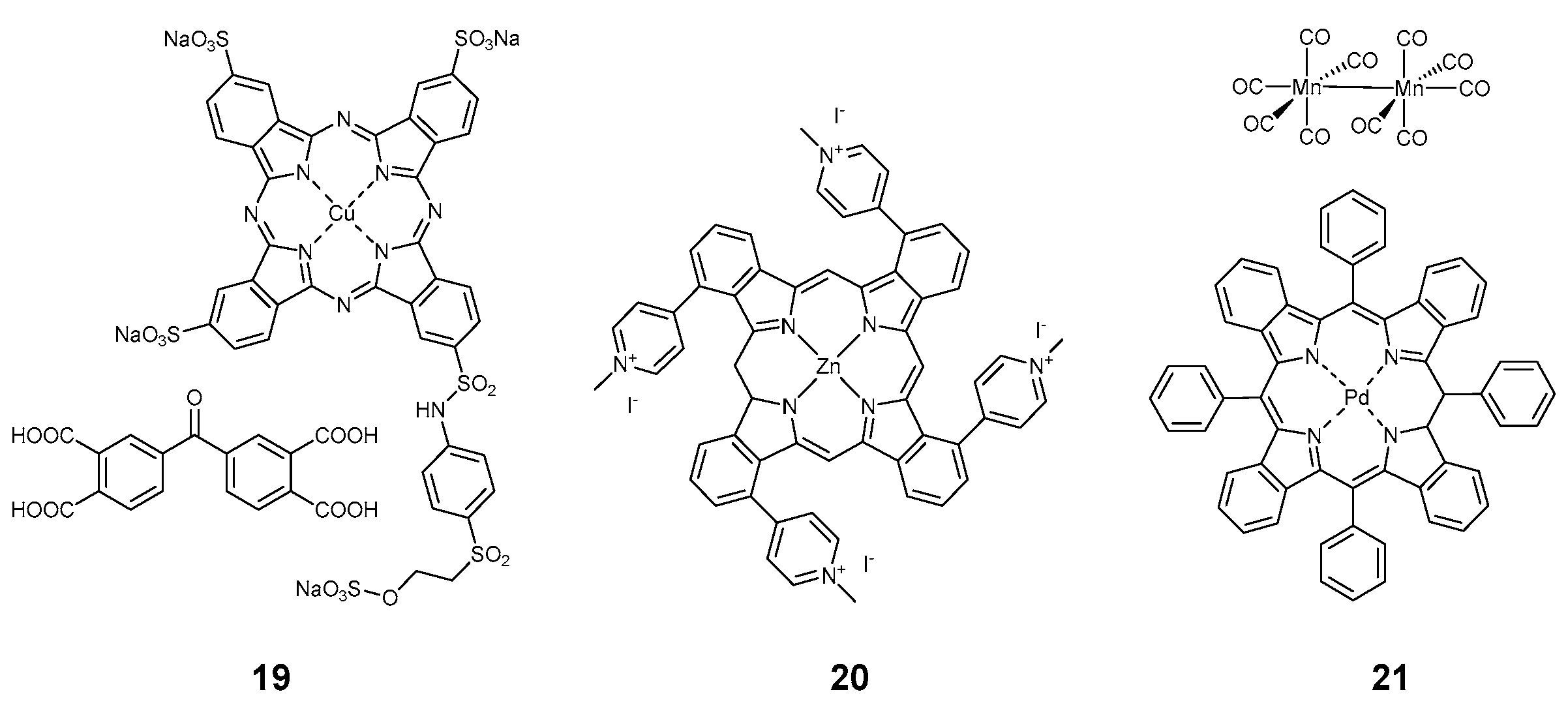
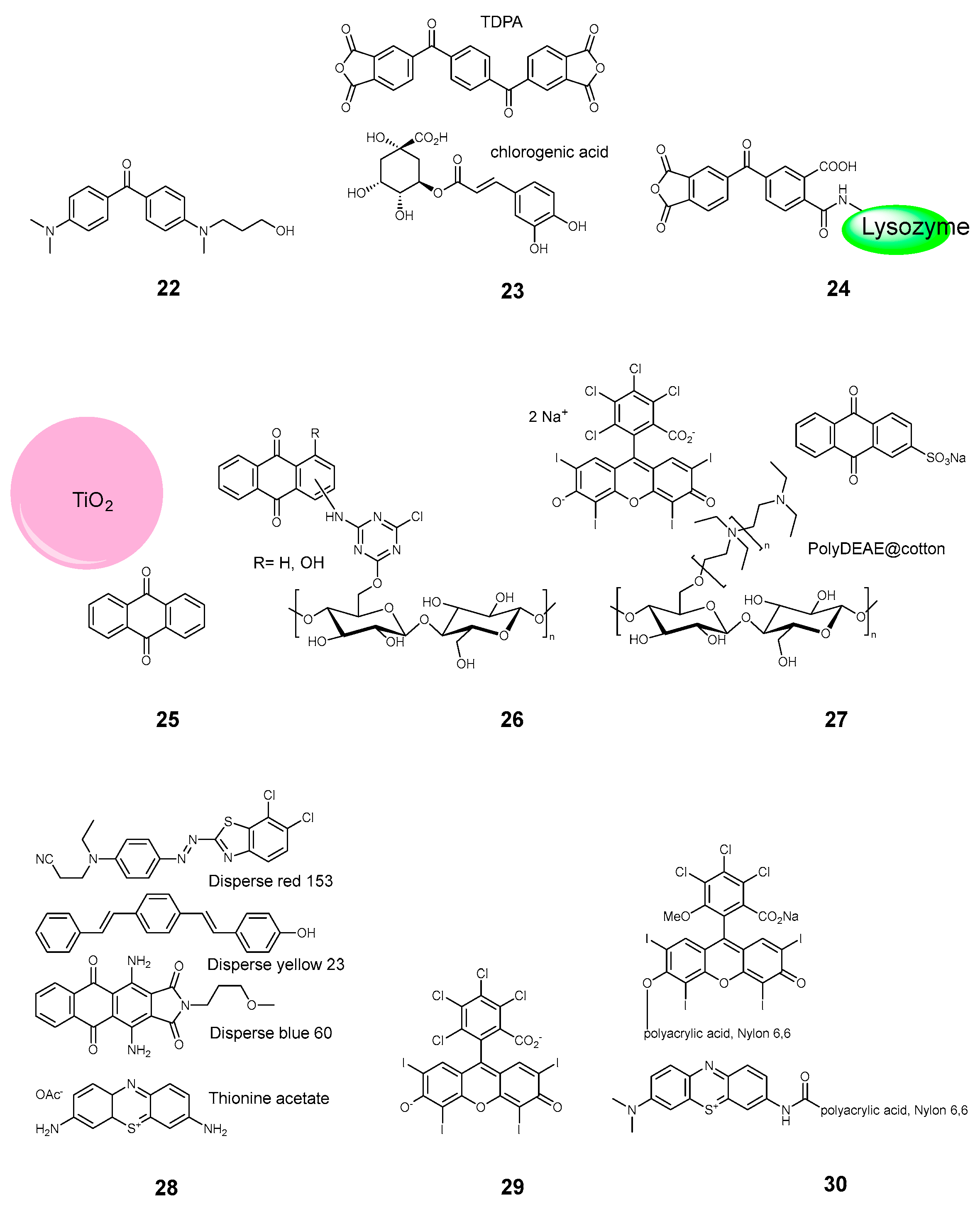
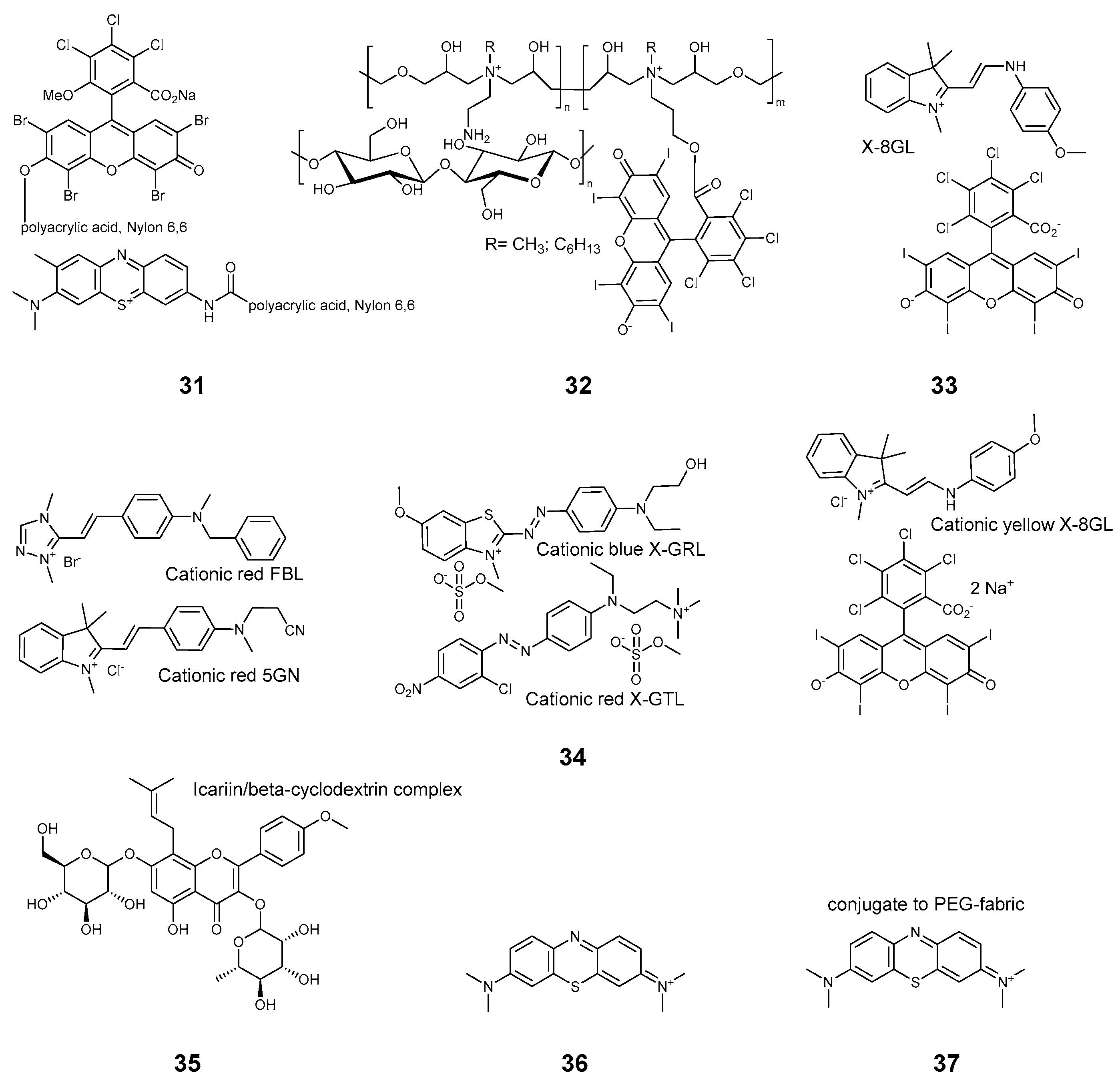
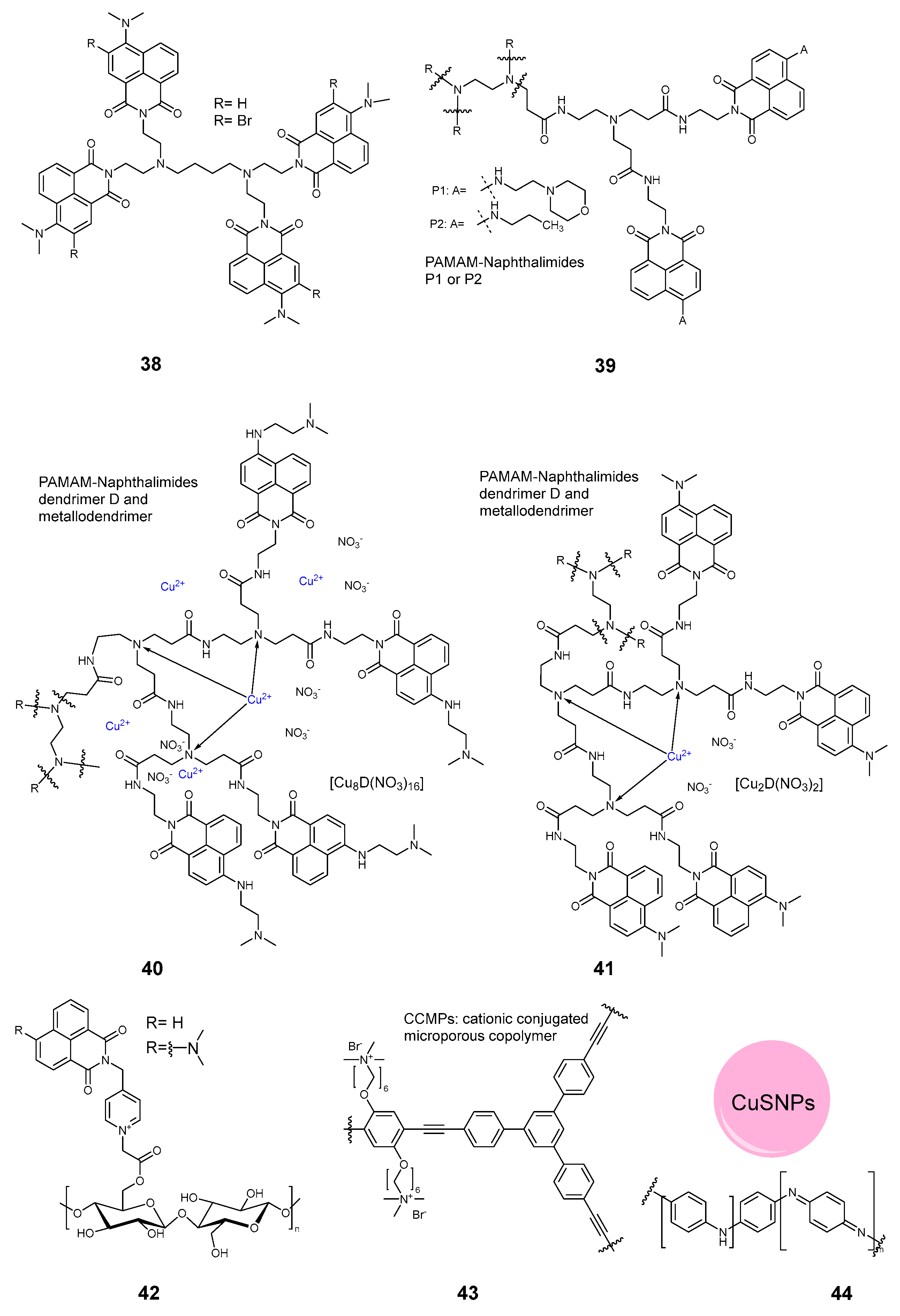

| Compound | Ref. | Staphylococcus aureus | Pseudomonas aeruginosa | Escherichia coli | B. subtilis | MRSA Methicillin-Resistant S. aureus | S. epidermidis | M. smegmatis | E. faecalis | B. cereus | K. pneumoniae | Aspergillus fumigatus | C. albicans | Influenza | SARS-CoV 227 | Non-Enveloped Feline Calicivirus | Autographa Californica Multiple Nuclear Polyhedrosis Virus (AcMNPV), | MHV-A59 | Standard | Light | Time, min | Light Dose, J/cm2 | Mode |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | [45] | 5 | 1440 | 9.5 | surface | ||||||||||||||||||
| 3 | [18] | 4 | 3 | 4 | 0.1 | suspension | |||||||||||||||||
| 4 | [19] | 2 | 2 | surface | |||||||||||||||||||
| 5 | [3] | 4 | 30,000 lux | 240 | 432 | surface | |||||||||||||||||
| 6 | [41] | 4 | 0.2 | 9.5 | surface | ||||||||||||||||||
| 7 | [43] | n/d | n/d | 1440 lm 15 W LED | Agar + fibers | ||||||||||||||||||
| 8 | [42] | 1 | 15,500 lux | 180 | Fabric + liquid | ||||||||||||||||||
| 9 | [46] | 5 | 5 | 6 | 3 | 65/80 mW/cm2 | 60 | 288 | surface | ||||||||||||||
| 10 | [33] | 0.5 | 0.2 | 0.1–0.3 | White light 0.36 mW/cm2 | 90 | 1.9 | surface | |||||||||||||||
| 11 | [47] | 4 | 0 | AATCC100 | 50 W Halogen | 10/30 | 5 | surface | |||||||||||||||
| 22 | [24] | 2 | 1.3 | 365 nm, 4 W or 8 W | 120 | surface | |||||||||||||||||
| 12 | [40] | 1.3 | 300 W | 30 | surface | ||||||||||||||||||
| 13 | [27] | 1 | 1 | 1 | 530 nm | 120 | surface | ||||||||||||||||
| 14 | [28] | 0.5 | 1 | Hg 100 W, 43 mW/cm2 | 30 | 77 | surface | ||||||||||||||||
| 15 | [49] | 6 | 6 | 6 | 6 | 500 W Xe 15 cm | 30 | 56 | surface | ||||||||||||||
| 15 | [30] | 6 | 6 | 6 | 6 | 500 W Xe 15 cm | 45 | 56 | surface | ||||||||||||||
| 15 | [50] | 6 | 6 | 500 W Xe 15 cm | 30 | 56 | surface | ||||||||||||||||
| 15 | [48] | 6 | 6 | 500W Xe 15 cm, 31.45 W/cm2 | 30 | 56 | surface | ||||||||||||||||
| 16 | [20] | 2 | 2 | 2 | AATCC100 | 150 mW/cm2 | 10 | 90 | surface | ||||||||||||||
| 17 | [51] | 3 | 3 | 3 | AATCC100 | 660 nm, 75mW/cm2 | 10 | 45 | surface | ||||||||||||||
| 19 | [23] | 1–4 | UVA light | surface | |||||||||||||||||||
| 20 | [16] | 4 | ISO 18184 | LED, 590 lux | 30 | 1 | surface | ||||||||||||||||
| 21 | [52] | 635 nm, 36 mW | 60 | surface | |||||||||||||||||||
| 22 | [24] | 365 nm, 4 W or 8 W | 120 | surface | |||||||||||||||||||
| 23 | [4] | 2 | 2 | surface | |||||||||||||||||||
| 24 | [11] | 3 | 3 | sunlight-driven | surface | ||||||||||||||||||
| 25 | [53] | 3 | 350 or 420 nm | Fabric + liquid | |||||||||||||||||||
| 26 | [34] | 1.3–6 | AATCC100 | 1380 lm 50 W 15cm | surface | ||||||||||||||||||
| 27 | [17] | 3 | 500 W Xe, 420 nm | 60 | surface | ||||||||||||||||||
| 28 | [39] | 4 | 4 | 4 | 500 W Xe 65 mW/cm2 | 60 | 234 | surface | |||||||||||||||
| 29 | [7] | 5 | 6.75 mW/cm2 | 20 | 8 | Fabric + liquid | |||||||||||||||||
| 29 | [55] | 5 | 4 | Xe 35 mW/cm2 | 60 | 126 | surface | ||||||||||||||||
| 30 | [56] | 6 | 16,000 lux | 300 | 288 | surface | |||||||||||||||||
| 30 | [8] | 0.2–0.3 | ASTM E2149-01 [73] | 15,500 lux | 180 | 167 | Fabric + liquid | ||||||||||||||||
| 31 | [9] | 0.2–0.3 | ASTM E2149-01 | 15,500 lux | 180 | 167 | Fabric + liquid | ||||||||||||||||
| 32 | [26] | 3 | 3 | 520 nm | 30 | 60 | Fabric + liquid | ||||||||||||||||
| 33 | [12] | 4 | 4 | 500 W Xe 12 cm | 60 | surface | |||||||||||||||||
| 34 | [13] | 3 | Xe 500 W, 12 cm | 60 | surface | ||||||||||||||||||
| 35 | [32] | 6 | 6 | Xe lamp, 5 mW/cm2 | 60 | 18 | surface | ||||||||||||||||
| 36 | [31] | 3 | 500 W Xe 20 cm | 30 | Fabric + liquid | ||||||||||||||||||
| 37 | [56] | 520 nm–25 mW | surface | ||||||||||||||||||||
| 38 | [57] | 1 | 1 | HL 8325, 25 W, 1230 lm | 60 | surface | |||||||||||||||||
| 39 | [58] | 3 | sunlight | 60 | surface | ||||||||||||||||||
| 40 | [59] | 1 | HL 8325, 25 W, 1230 lm | 60 | surface | ||||||||||||||||||
| 41 | [60] | 1.5 | sunlight | 1080 | 648 | Fabric + liquid | |||||||||||||||||
| 42 | [61] | sunlight | 1080 | 648 | Fabric + liquid | ||||||||||||||||||
| 43 | [6] | 2 | 2 | Xe 500 W, 12 cm | 30 | surface | |||||||||||||||||
| 44 | [10] | 4 | 4 | AATCC 183-2014 [74] | Simulated daylight | 5 | 200 | surface | |||||||||||||||
| 45 | [38] | 0.5–1 | 0.1–0.2 | Sunlight | 360 | 216 | surface, Agar | ||||||||||||||||
| 46 | [62] | 1 | 1 | 808 nm, 2.0 W/cm2 and 660nm | 600 | 1200 | surface | ||||||||||||||||
| 47 | [63] | 4 | 2 | 4 | 1.7 | White light 45 mW/cm2 | 20 | 54 | surface | ||||||||||||||
| 48 | [64] | 5 | White light 3 mW/cm2 | 8 | 1.44 | surface | |||||||||||||||||
| 49 | [65] | 4 | Xe 500 W, 12 cm | 60 | surface | ||||||||||||||||||
| 50 | [66] | 3 | 3 | 808 nm, 300 mW/cm2 | 300 | 90 | surface | ||||||||||||||||
| 51 | [5] | 4 | White light, 9.5 mW/cm2 | 60 | 34 | surface | |||||||||||||||||
| 52 | [25] | 2–3 | ISO 22196 | LED 50 W, 470 nm, 50 cm | 60 | surface | |||||||||||||||||
| 53 | [35] | 4 | Xe lamp 60,000/8000/800 lux | surface |
| Lighting Conditions | lux | mW/cm2 | Time | Light Dose J/cm2 | Light Dose Range |
|---|---|---|---|---|---|
| Direct sunlight | 100,000 | 100 | 30 min | 180 | 100–1000 |
| Bright day | 10,000 | 10 | 30 min | 18 | 10–100 |
| Shadow outdoors | 1000 | 1 | 30 min | 1,8 | 1–10 |
| Indoors | 500 | 0,5 | 30 min | 0,9 | 0.1–1 |
| Comp | Ref. | Dye Load | Dye Load-Activity | Morphology | Mechanical Properties | Rub-Fastness | Thermal Stability | Light-Fastness | Washing Stability |
|---|---|---|---|---|---|---|---|---|---|
| 1 | [44] | ||||||||
| 2 | [45] | 0.4–0.6 mg/cm2 | washed | ||||||
| 3 | [18] | 0.16 umol/mg | tested | TGA, 320°C | washed | ||||
| 4 | [19] | 3.9% owf | tested | SEM | TGA, 300 °C | washed | |||
| 5 | [3] | SEM | washed | ||||||
| 6 | [41] | 1.5% | SEM | TGA, 300 °C | washed | ||||
| 7 | [43] | 12% | SEM | washed | |||||
| 8 | [42] | 20–30% | SEM | TGA, 300 °C | |||||
| 9 | [46] | 0.4–5 ug/mg | SEM | TGA, 300 °C | 1 log loss in 2 h | washed | |||
| 10 | [33] | 0.003–0.3% | tested | SEM | TGA, 300 °C | washed | |||
| 11 | [47] | 0.02 mg/cm2 | SEM | release studied | |||||
| 12 | [21] | ||||||||
| 12 | [40] | 1% | SEM | ||||||
| 13 | [27] | 1.5% | SEM | tested | bleaches out | ||||
| 14 | [28] | 0.1% | tested | SEM | |||||
| 15 | [30] | 9.1% | SEM | washed | |||||
| 15 | [48] | SEM | 1–2 log loss in 5–10 wash cycles | ||||||
| 15 | [49] | 14% | SEM/TEM | washed | |||||
| 15 | [50] | 0.4 mg/cm2 | SEM | tested | TGA, 300 °C | 30 min | 1 log loss in 1–2 washes | ||
| 16 | [20] | 0.2% owf | SEM | tested | TGA, 300 °C | 30 min | washed | ||
| 17 | [51] | 3% | SEM | tested | tested | TGA | 1–2 log loss in 2–4 h | tested | |
| 18 | [29] | ||||||||
| 19 | [23] | 0.5–8% | tested | 4 cycles | |||||
| 20 | [16] | 0.15% | 24 h stable | 5 washes stable | |||||
| 21 | [52] | 0.04% | |||||||
| 22 | [24] | SEM | TGA | 2 h UV | |||||
| 23 | [4] | rechargeable | SEM | TGA | |||||
| 24 | [11] | SEM | 0–5–2 log loss 1–5 washes, | ||||||
| 25 | [53] | ||||||||
| 26 | [34] | 0.2–9.6 umol/g | SEM | TGA, 300 °C | 48 whash cycles | ||||
| 27 | [17] | 2.5% | tested | SEM | TGA, 300 °C | 2 log loss for AQ in 7 days | 3 log for AQ in 3 wash cycles | ||
| 28 | [39] | 0.23–0.33% | SEM | tested | TGA, 270°C | 1–2 logs loss in 12 h | 1–2 log loss upon washing | ||
| 29 | [7] | 0.5, 1, 3% owf | tested | ||||||
| 29 | [54] | SEM | tested | tested | TGA | 2 logs loss in 5 h | |||
| 29 | [55] | 30–80 umol/L | SEM | ||||||
| 30 | [8] | SEM | |||||||
| 30 | [56] | ||||||||
| 31 | [9] | SEM | |||||||
| 32 | [26] | ||||||||
| 33 | [12] | 1–3% | SEM | tested | tested | 1–2 logs loss in 2–4 h | tested | ||
| 34 | [13] | 1% | |||||||
| 35 | [32] | SEM | DSC | ||||||
| 36 | [31] | 1–3% | tested | ||||||
| 38 | [57] | 0.17–0.19% | |||||||
| 39 | [58] | 0.02% | tested | ||||||
| 40 | [59] | 0.5–1% | SEM | ||||||
| 41 | [60] | 0.02% | |||||||
| 42 | [61] | 0.02% | |||||||
| 43 | [6] | SEM | |||||||
| 44 | [10] | SEM | TGA | ||||||
| 45 | [38] | 24–36 mg/cm2 | SEM | ||||||
| 46 | [62] | SEM | |||||||
| 47 | [63] | 0.05–0.3% | SEM | wash stable | |||||
| 48 | [64] | 43–76 ug/cm2 | SEM | 5–14 days stable | 100 washes stable | ||||
| 49 | [65] | 33% | SEM | ||||||
| 50 | [66] | SEM | |||||||
| 51 | [5] | SEM | |||||||
| 52 | [25] | 2% | AFM | ||||||
| 53 | [35] | 1% | tested | 1–2 log loss in 12 h | 1–2 log loss in 7 washes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efimov, A.; Mordon, S. Photoantimicrobial and Photoantiviral Textiles: Underestimated Potential. Pharmaceuticals 2024, 17, 1164. https://doi.org/10.3390/ph17091164
Efimov A, Mordon S. Photoantimicrobial and Photoantiviral Textiles: Underestimated Potential. Pharmaceuticals. 2024; 17(9):1164. https://doi.org/10.3390/ph17091164
Chicago/Turabian StyleEfimov, Alexander, and Serge Mordon. 2024. "Photoantimicrobial and Photoantiviral Textiles: Underestimated Potential" Pharmaceuticals 17, no. 9: 1164. https://doi.org/10.3390/ph17091164
APA StyleEfimov, A., & Mordon, S. (2024). Photoantimicrobial and Photoantiviral Textiles: Underestimated Potential. Pharmaceuticals, 17(9), 1164. https://doi.org/10.3390/ph17091164










