Photodynamic Therapy as a Novel Therapeutic Modality Applying Quinizarin-Loaded Nanocapsules and 3D Bioprinting Skin Permeation for Inflammation Treatment
Abstract
:1. Introduction
2. Results
2.1. Physical–Chemical and Two-Dimensional Topography Analysis: Particle Size, PdI, Zeta Potential, and AFM
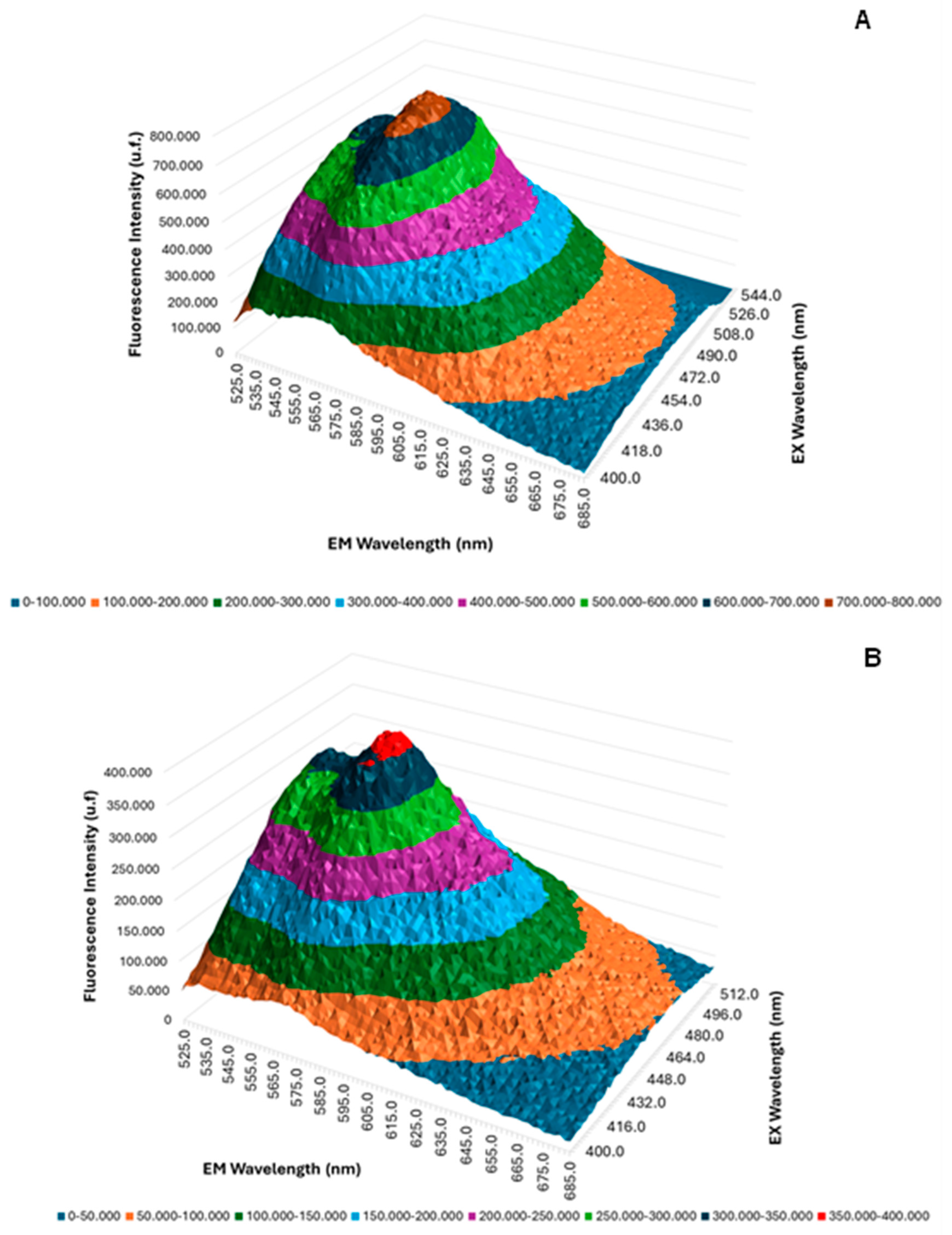
2.2. Three-Dimensional Fluorescence Emission Spectroscopy UV/Vis
2.3. Cytotoxicity Assay Using Resazurin Test—NIH/3T3 Cells
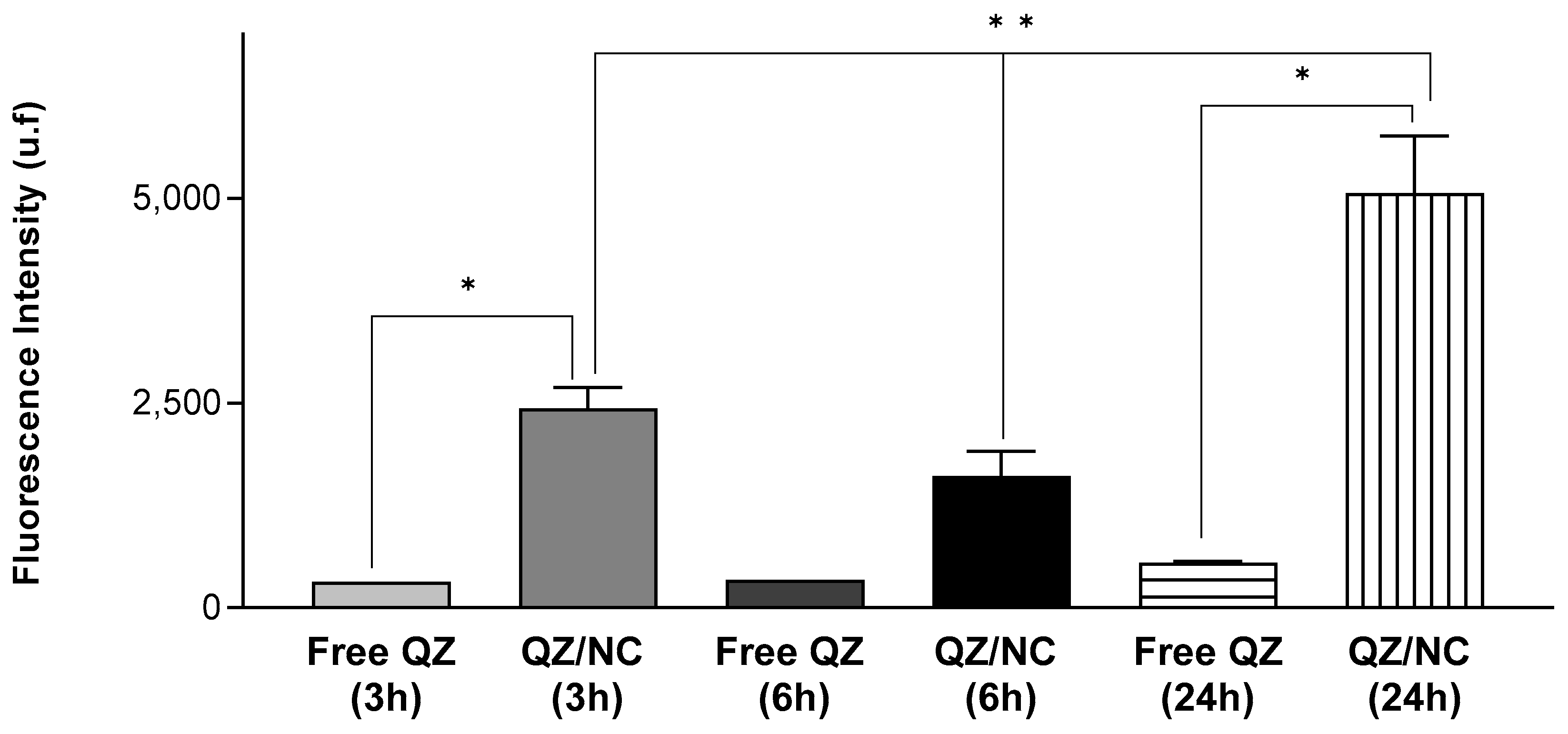
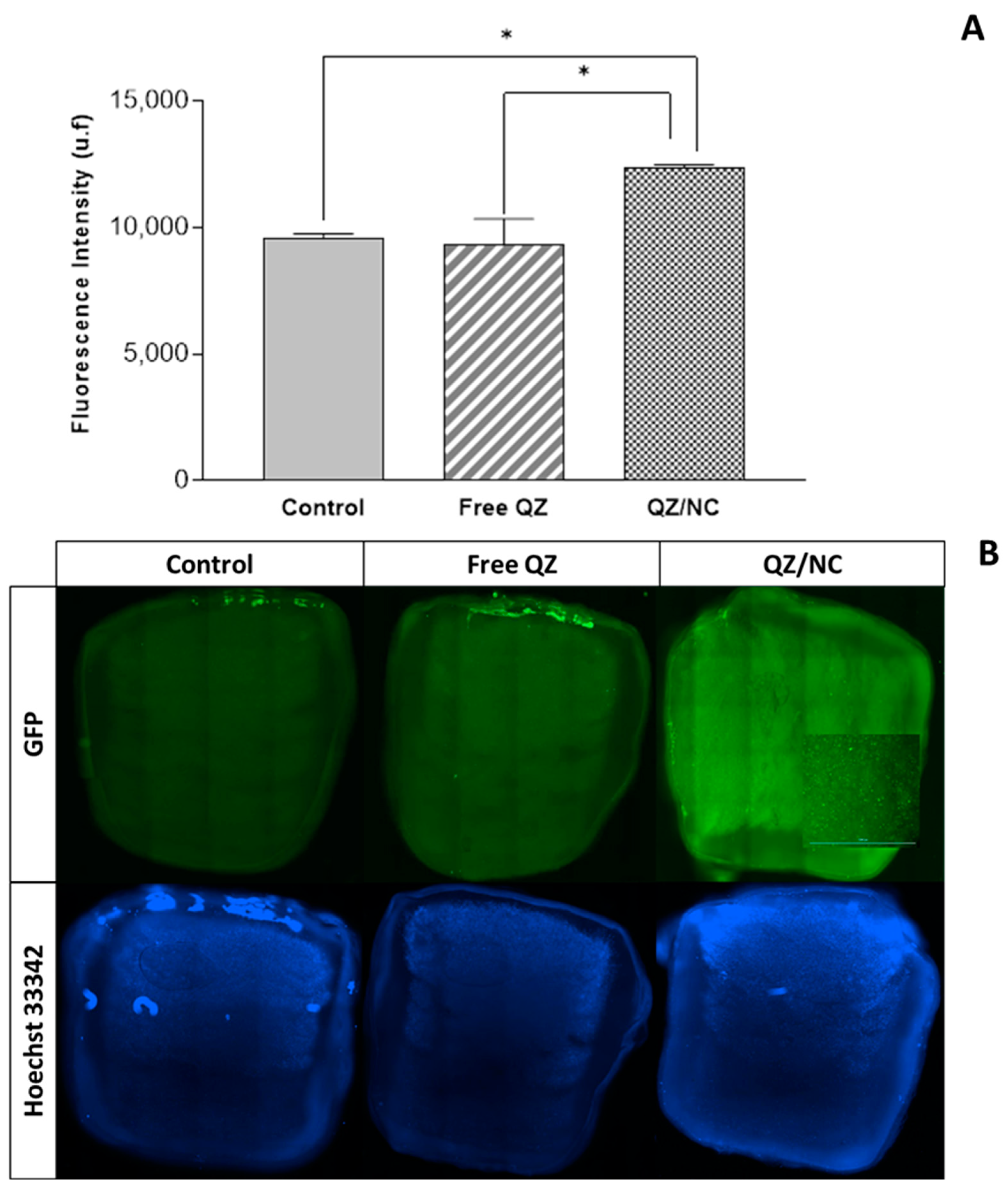
2.4. Cell Internalization and Permeation Study
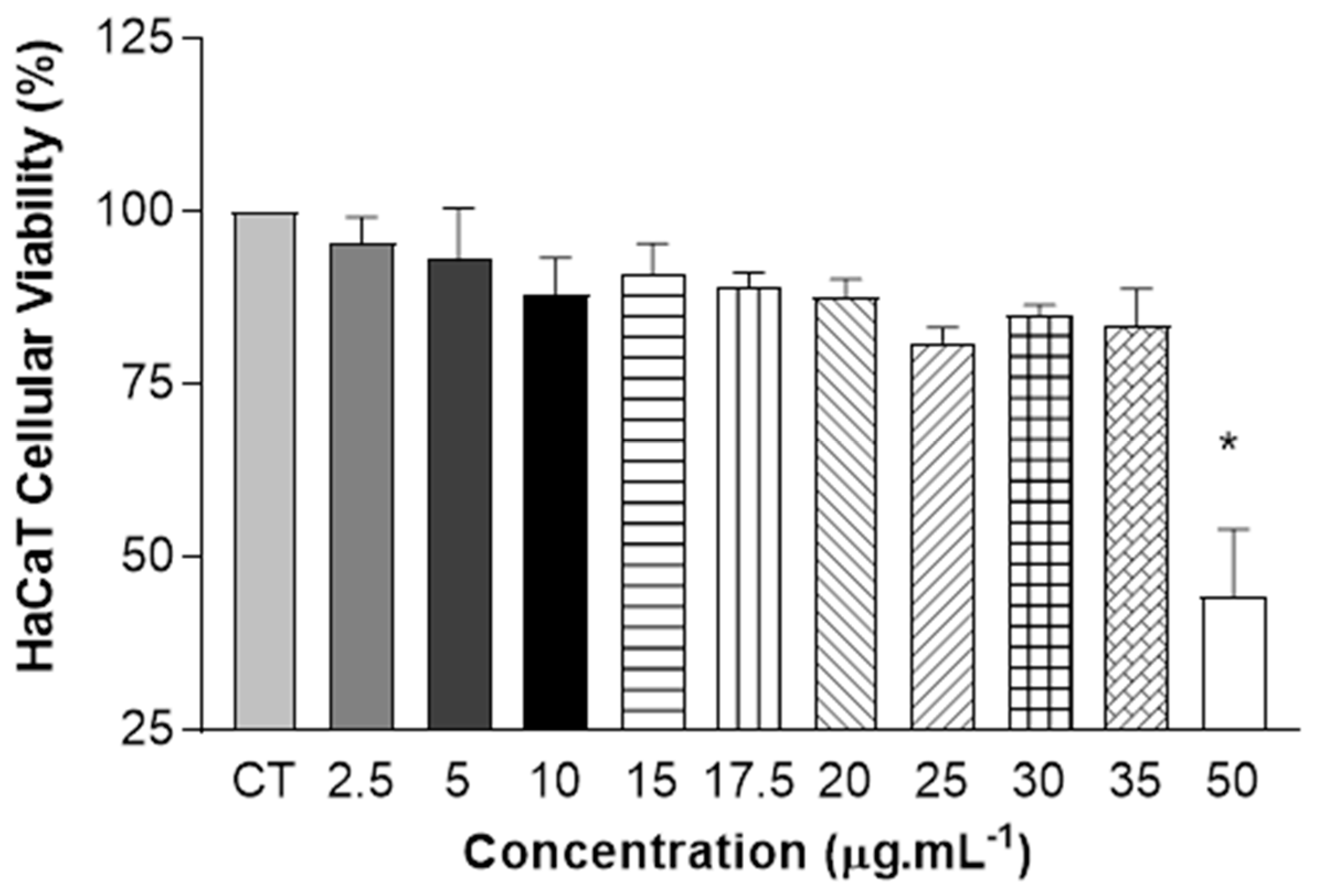
2.5. Cytotoxicity Assay Using Resazurin Test—HaCaT Cells
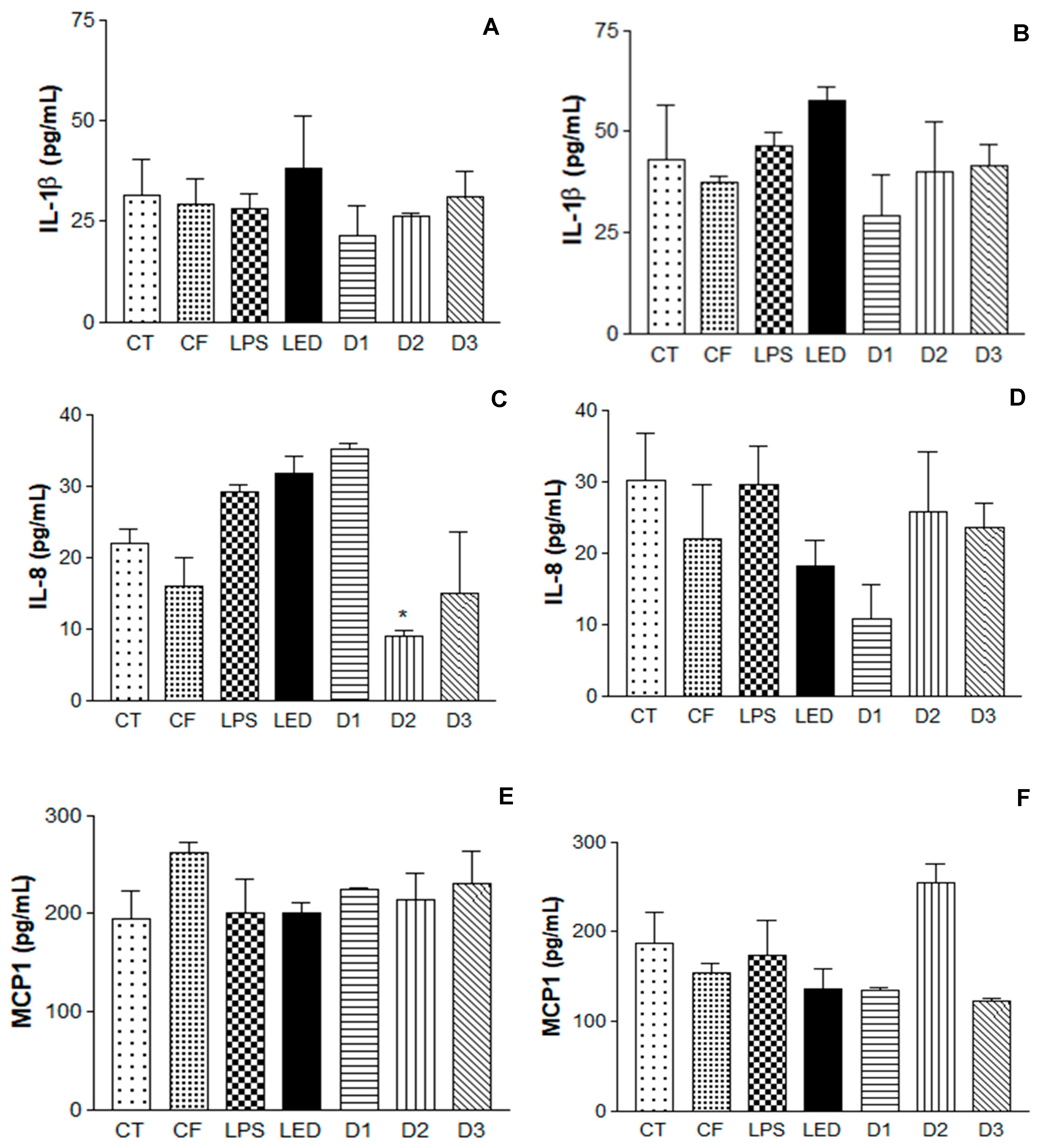
2.6. Induction of Inflammatory Process in Human Keratinocytes with Application of Photodynamic Therapy and Immunoenzymatic Assay
3. Discussion
4. Materials and Methods
4.1. Development of Polymeric Nanocapsules Loaded with Quinizarin
4.2. Characterization of Particle Size, Polydispersity Index (PdI), and Zeta Potential
4.3. Atomic Force Microscopy
4.4. Development of Three-Dimensional Fluorescence Emission Spectroscopy UV/Vis
4.5. Cell Culture
4.6. Cellular Uptake
4.7. Cytotoxicity Assay Using Resazurin Test
4.8. Permeation Study in a New 3D Bioprinted Skin Equivalent: Suspended Layer Additive Manufacturing (SLAM) Method
4.9. Induction of Inflammatory Process in HaCaT Cells and Application of Photodynamic Therapy
4.10. Enzyme-Linked Immunosorbent Assay (ELISA)
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolarsick, P.A.J.B.; Kolarsick, M.A.M.; Goodwin, C.A.-B. Anatomy and physiology of the skin. J. Dermatol. Nurses’ Assoc. 2011, 3, 203–213. [Google Scholar] [CrossRef]
- Lee, T.-A.; Huang, Y.-T.; Hsiao, P.-F.; Chiu, L.-Y.; Chern, S.-R.; Wu, N.-L. Critical roles of irradiance in the regulation of UVB-induced inflammasome activation and skin inflammation in human skin keratinocytes. J. Photochem. Photobiol. B Biol. 2022, 226, 112373. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.-J.; Lee, E.; Yun, S.; Lim, W.; Lim, T.-G. Particulate matter-induced skin inflammation is suppressed by polyphenol-enriched dietary supplement via inhibition of the AhR/ARNT signaling pathway. J. Funct. Foods 2023, 106, 105593. [Google Scholar] [CrossRef]
- Klicznik, M.; Szenes-Nagy, A.; Campbell, D.; Gratz, I. Taking the lead–how keratinocytes orchestrate skin T cell immunity. Immunol. Lett. 2018, 200, 43–51. [Google Scholar] [CrossRef]
- Gustafsson, A.; Prgomet, Z.; Jankovskaja, S.; Ruzgas, T.; Engblom, J.; Ohlsson, L.; Wingren, A.G. Effect of IFN-γ on the kynurenine/tryptophan ratio in monolayer-cultured keratinocytes and a 3D reconstructed human epidermis model. J. Dermatol. Sci. 2020, 99, 177–184. [Google Scholar] [CrossRef]
- Devos, M.; Mogilenko, D.A.; Fleury, S.; Gilbert, B.; Becquart, C.; Quemener, S.; Dehondt, H.; Tougaard, P.; Staels, B.; Bachert, C.; et al. Keratinocyte expression of A20/TNFAIP3 controls skin inflammation associated with atopic dermatitis and psoriasis. J. Investig. Dermatol. 2019, 139, 135–145. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Zhou, L.; Yuan, X.; Wang, Y.; Deng, Q.; Deng, Z.; Xu, S.; Wang, Q.; Xie, H.; et al. Nav1. 8 in keratinocytes contributes to ROS-mediated inflammation in inflammatory skin diseases. Redox Biol. 2022, 55, 102427. [Google Scholar] [CrossRef]
- Hari, G.; Kishore, A.; Karkala, S.R.P. Treatments for psoriasis: A journey from classical to advanced therapies. How far have we reached? Eur. J. Pharmacol. 2022, 929, 175147. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, X.; Zhang, B.; Kang, H.; Du, L.; Li, M. Nanostructures of an amphiphilic zinc phthalocyanine polymer conjugate for photodynamic therapy of psoriasis. Colloids Surf. B Biointerfaces 2015, 128, 405–409. [Google Scholar] [CrossRef]
- Hazari, S.A.; Kaur, H.; Karwasra, R.; Abourehab, M.A.; Khan, A.A.; Kesharwani, P. An overview of topical lipid-based and polymer-based nanocarriers for treatment of psoriasis. Int. J. Pharm. 2023, 638, 122938. [Google Scholar] [CrossRef]
- Aleem, D.; Tohid, H. Pro-inflammatory cytokines, biomarkers, genetics and the immune system: A mechanistic approach of depression and psoriasis. Rev. Colomb. Psiquiatr. 2018, 47, 177–186. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Chen, X.; Su, Z.; Deng, Y.; Zhao, Q. Khasianine ameliorates psoriasis-like skin inflammation and represses TNF-α/NF-κB axis mediated transactivation of IL-17A and IL-33 in keratinocytes. J. Ethnopharmacol. 2022, 292, 115124. [Google Scholar] [CrossRef]
- Mascarenhas-Melo, F.; Carvalho, A.; Gonçalves, M.B.S.; Paiva-Santos, A.C.; Veiga, F. Nanocarriers for the topical treatment of psoriasis-pathophysiology, conventional treatments, nanotechnology, regulatory and toxicology. Eur. J. Pharm. Biopharm. 2022, 176, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Tambe, V.S.; Nautiyal, A.; Wairkar, S. Topical lipid nanocarriers for management of psoriasis-an overview. J. Drug Deliv. Sci. Technol. 2021, 64, 102671. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, S.; Li, J.; Chen, M.; Wu, L. The application of physical pretreatment in photodynamic therapy for skin diseases. Lasers Med. Sci. 2021, 36, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Makuch, S.; Dróżdż, M.; Makarec, A.; Ziółkowski, P.; Woźniak, M. An Update on Photodynamic Therapy of Psoriasis—Current Strategies and Nanotechnology as a Future Perspective. Int. J. Mol. Sci. 2022, 23, 9845. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy–mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Agazzi, M.L.; Ballatore, M.B.; Durantini, A.M.; Durantini, E.N.; Tomé, A.C. BODIPYs in antitumoral and antimicrobial photodynamic therapy: An integrating review. J. Photochem. Photobiol. C Photochem. Rev. 2019, 40, 21–48. [Google Scholar] [CrossRef]
- Ree, J.; Kim, J.I.; Lee, C.W.; Lee, J.; Kim, H.J.; Kim, S.C.; Sohng, J.K.; Park, Y.I. Quinizarin suppresses the differentiation of adipocytes and lipogenesis in vitro and in vivo via down-regulation of C/EBP-beta/SREBP pathway. Life Sci. 2021, 287, 120131. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Herbal Drugs as Therapeutic Agents; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Malik, E.M.; Müller, C.E. Anthraquinones as pharmacological tools and drugs. Med. Res. Rev. 2016, 36, 705–748. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, M.B.; Gañan, N.; Comini, L.; Martini, R.; Bottini, S.; Andreatta, A. Experimental measurement and modeling of quinizarin solubility in pressurized hot water. J. Supercrit. Fluids 2017, 125, 1–11. [Google Scholar] [CrossRef]
- Quinti, L.; Allen, N.S.; Edge, M.; Murphy, B.P.; Perotti, A. A study of the strongly fluorescent species formed by the interaction of the dye 1, 4-dihydroxyanthraquinone (quinizarin) with Al (III). J. Photochem. Photobiol. A Chem. 2003, 155, 79–91. [Google Scholar] [CrossRef]
- Hu, X.; Cao, Y.; Yin, X.; Zhu, L.; Chen, Y.; Wang, W.; Hu, J. Design and synthesis of various quinizarin derivatives as potential anticancer agents in acute T lymphoblastic leukemia. Bioorganic Med. Chem. 2019, 27, 1362–1369. [Google Scholar] [CrossRef]
- Toader, A.M.; Enache, M. Quinizarin interaction with bile salts micelles as biomimetic model membranes. Rev. Roum. Chim. 2021, 66, 75–80. [Google Scholar] [CrossRef]
- Díaz-Muñoz, M.D.; Turner, M. Uncovering the role of RNA-binding proteins in gene expression in the immune system. Front. Immunol. 2018, 9, 1094. [Google Scholar] [CrossRef]
- Amantino, C.F.; de Baptista-Neto, Á.; Badino, A.C.; Siqueira-Moura, M.P.; Tedesco, A.C.; Primo, F.L. Anthraquinone encapsulation into polymeric nanocapsules as a new drug from biotechnological origin designed for photodynamic therapy. Photodiagnosis Photodyn. Ther. 2020, 31, 101815. [Google Scholar] [CrossRef] [PubMed]
- Abed, E.M.; Hoseini-Alfatemi, S.M.; Sabati, H.; Gaskarei, M.A.K.; Delpasand, K.; Ghasemi, M. Use of nanotechnology in the diagnosis and treatment of coronavirus. J. Curr. Biomed. Rep. 2021, 2, 2717-1906. [Google Scholar]
- Neto, S.F.; Prada, A.L.; Achod, L.D.R.; Torquato, H.F.V.; Lima, C.S.; Paredes-Gamero, E.J.; de Moraes, M.O.S.; Lima, E.S.; Sosa, E.H.; de Souza, T.P.; et al. α-amyrin-loaded nanocapsules produce selective cytotoxic activity in leukemic cells. Biomed. Pharmacother. 2021, 139, 111656. [Google Scholar] [CrossRef]
- Jain, P.; Kathuria, H.; Dubey, N. Advances in 3D bioprinting of tissues/organs for regenerative medicine and in-vitro models. Biomaterials 2022, 287, 121639. [Google Scholar] [CrossRef] [PubMed]
- Aazmi, A.; Zhang, D.; Mazzaglia, C.; Yu, M.; Wang, Z.; Yang, H.; Huang, Y.Y.S.; Ma, L. Biofabrication methods for reconstructing extracellular matrix mimetics. Bioact. Mater. 2024, 31, 475–496. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Singh, Y.P.; Datta, P.; Ozbolat, V.; O’Donnell, A.; Yeo, M.; Ozbolat, I.T. Strategies for 3D bioprinting of spheroids: A comprehensive review. Biomaterials 2022, 291, 121881. [Google Scholar] [CrossRef]
- Moakes, R.J.A.; Senior, J.J.; Robinson, T.E.; Chipara, M.; Atansov, A.; Naylor, A.; Metcalfe, A.D.; Smith, A.M.; Grover, L.M. A suspended layer additive manufacturing approach to the bioprinting of tri-layered skin equivalents. APL Bioeng. 2021, 5, 046103. [Google Scholar] [CrossRef]
- Daneshfar, R.; Ashoori, S.; Soulgani, B.S. Interaction of electrolyzed nanomaterials with sandstone and carbonate rock: Experimental study and DLVO theory approach. Geoenergy Sci. Eng. 2023, 230, 212218. [Google Scholar] [CrossRef]
- Logue, B.A.; Manandhar, E. Percent residual accuracy for quantifying goodness-of-fit of linear calibration curves. Talanta 2018, 189, 527–533. [Google Scholar] [CrossRef]
- Pan, Z.; Li, R.H.; Cui, Y.Y.; Wu, X.J.; Zhang, Y.Y.; Wang, Y.T. A simple and quick method to detect adulterated sesame oil using 3D fluorescence spectra. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 245, 118948. [Google Scholar] [CrossRef]
- ISO 10993-5:2009(E); Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. Organisation for Standardisation (ISO): Geneva, Switzerland, 2009.
- Poumay, Y.; Coquette, A. Modelling the human epidermis in vitro: Tools for basic and applied research. Arch. Dermatol. Res. 2007, 298, 361–369. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- El Ayadi, A.; Herndon, D.N.; Finnerty, C.C. Biomarkers in Burn Patient Care. In Total Burn Care; Elsevier: Amsterdam, The Netherlands, 2018; Volume 5, pp. 232–235. [Google Scholar] [CrossRef]
- Gillitzer, R.; Berger, R.; Mielke, V.; Müller, C.; Wolff, K.; Stingl, G. Upper keratinocytes of psoriatic skin lesions express high levels of NAP-1/IL-8 mRNA in situ. J. Investig. Dermatol. 1991, 97, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Amantino, C.F.; Amaral, S.R.D.; Aires-Fernandes, M.; Oliani, S.M.; Tedesco, A.C.; Primo, F.L. Development of 3d skin equivalents for application in photodynamic biostimulation therapy assays using curcumin nanocapsules. Heliyon 2024, 10, e32808. [Google Scholar] [CrossRef]
- Souto, E.B.; Fangueiro, J.F.; Fernandes, A.R.; Cano, A.; Sanchez-Lopez, E.; Garcia, M.L.; Severino, P.; Paganelli, M.O.; Chaud, M.V.; Silva, A.M. Physicochemical and biopharmaceutical aspects influencing skin permeation and role of SLN and NLC for skin drug delivery. Heliyon 2022, 8, e08938. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Potential of nanoparticles as permeation enhancers and targeted delivery options for skin: Advantages and disadvantages. Drug Des. Dev. Ther. 2020, 14, 3271–3289. [Google Scholar] [CrossRef] [PubMed]
- Varenne, F.; Rustique, E.; Botton, J.; Coty, J.-B.; Lanusse, G.; Lahcen, M.A.; Rio, L.; Zandanel, C.; Lemarchand, C.; Germain, M.; et al. Towards quality assessed characterization of nanomaterial: Transfer of validated protocols for size measurement by dynamic light scattering and evaluation of zeta potential by electrophoretic light scattering. Int. J. Pharm. 2017, 528, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Gobo, G.G.; Piva, H.L.; Tedesco, A.C.; Primo, F.L. Novel quinizarin spray-dried nanoparticles for treating melanoma with photodynamic therapy. Mater. Today Commun. 2022, 33, 104882. [Google Scholar] [CrossRef]
- Huerta-García, E.; Márquez-Ramírez, S.G.; Ramos-Godinez, M.d.P.; López-Saavedra, A.; Herrera, L.A.; Parra, A.; Alfaro-Moreno, E.; Gómez, E.O.; López-Marure, R. Internalization of titanium dioxide nanoparticles by glial cells is given at short times and is mainly mediated by actin reorganization-dependent endocytosis. Neurotoxicology 2015, 51, 27–37. [Google Scholar] [CrossRef]
- Batheja, P.; Sheihet, L.; Kohn, J.; Singer, A.J.; Michniak-Kohn, B. Topical drug delivery by a polymeric nanosphere gel: Formulation optimization and in vitro and in vivo skin distribution studies. J. Control. Release 2011, 149, 159–167. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Lima, S.A.C.; Reis, S. Development of methotrexate loaded fucoidan/chitosan nanoparticles with anti-inflammatory potential and enhanced skin permeation. Int. J. Biol. Macromol. 2019, 124, 1115–1122. [Google Scholar] [CrossRef]
- Yang, W.; Bai, X.; Luan, X.; Min, J.; Tian, X.; Li, H.; Li, H.; Sun, W.; Liu, W.; Fan, W.; et al. Delicate regulation of IL-1β-mediated inflammation by cyclophilin A. Cell Rep. 2022, 38, 110513. [Google Scholar] [CrossRef]
- Muñoz-Mata, L.S.; López-Cárdenas, M.T.; Espinosa-Montesinos, A.; Sosa-Delgado, S.M.; Rosales-García, V.H.; Moreno-Lafont, M.C.; Ramón-Gallegos, E. Photodynamic therapy stimulates IL-6 and IL-8 in responding patients with HPV infection associated or not with LSIL. J. Photochem. Photobiol. 2022, 11, 100137. [Google Scholar] [CrossRef]
- Suárez-Valladares, M.J.; Calleja-Antolín, S.M.; de Morales, J.M.G.R.; Prieto, M.A.R.; Vega-Gutierrez, J. Inflammatory response and cytokine levels induced by intralesional photodynamic therapy and 630-nm laser in a case series of basal cell carcinoma. J. Am. Acad. Dermatol. 2018, 79, 580–582. [Google Scholar] [CrossRef]
- Lembo, S.; Capasso, R.; Balato, A.; Cirillo, T.; Flora, F.; Zappia, V.; Balato, N.; Ingrosso, D.; Ayala, F. MCP-1 in psoriatic patients: Effect of biological therapy. J. Dermatol. Treat. 2014, 25, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Akram, Z.; Abduljabbar, T.; Sauro, S.; Daood, U. Effect of photodynamic therapy and laser alone as adjunct to scaling and root planing on gingival crevicular fluid inflammatory proteins in periodontal disease: A systematic review. Photodiagnosis Photodyn. Ther. 2016, 16, 142–153. [Google Scholar] [CrossRef]
- Klaus, F.; Mitchell, K.; Liou, S.C.; Eyler, L.T.; Nguyen, T.T. Chemokine MCP1 is associated with cognitive flexibility in schizophrenia: A preliminary analysis. J. Psychiatr. Res. 2021, 138, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-González, J.; Vosburg, D.A.; Mora-Rodriguez, S.E.; Vázquez, M.A.; Zepeda, L.G.; Gómez, C.V.; Lagunas-Rivera, S. Anthraquinones: Versatile organic photocatalysts. ChemCatChem 2020, 12, 3811–3827. [Google Scholar] [CrossRef]
- Gollnick, K.; Held, S.; Mártire, D.O.; Braslavsky, S.E. Hydroxyanthraquinones as sensitizers of singlet oxygen reactions: Quantum yields of triplet formation and singlet oxygen generation in acetonitrile. J. Photochem. Photobiol. A Chem. 1992, 69, 155–165. [Google Scholar] [CrossRef]
- Mothilal, K.; Inbaraj, J.J.; Gandhidasan, R.; Murugesan, R. Photosensitization with anthraquinone derivatives: Optical and EPR spin trapping studies of photogeneration of reactive oxygen species. J. Photochem. Photobiol. A Chem. 2004, 162, 9–16. [Google Scholar] [CrossRef]
- Sautrot-Ba, P.; Jockusch, S.; Malval, J.-P.; Brezová, V.; Rivard, M.; Abbad-Andaloussi, S.; Blacha-Grzechnik, A.; Versace, D.-L. Quinizarin derivatives as photoinitiators for free-radical and cationic photopolymerizations in the visible spectral range. Macromolecules 2020, 53, 1129–1141. [Google Scholar] [CrossRef]
- Siqueira-Moura, M.P.; Primo, F.L.; Espreafico, E.M.; Tedesco, A.C. Development, characterization, and photocytotoxicity assessment on human mel-anoma of chloroaluminum phthalocyanine nanocapsules. Mater. Sci. Eng. C 2013, 33, 1744–1752. [Google Scholar] [CrossRef]
- Cardoso, L.P.; de Sousa, S.O.; Gusson-Zanetoni, J.P.; Silva, L.L.d.M.M.; Frigieri, B.M.; Henrique, T.; Tajara, E.H.; Oliani, S.M.; Rodrigues-Lisoni, F.C. Piperine Reduces Neoplastic Progression in Cervical Cancer Cells by Downregulating the Cyclooxygenase 2 Pathway. Pharmaceuticals 2023, 16, 103. [Google Scholar] [CrossRef]
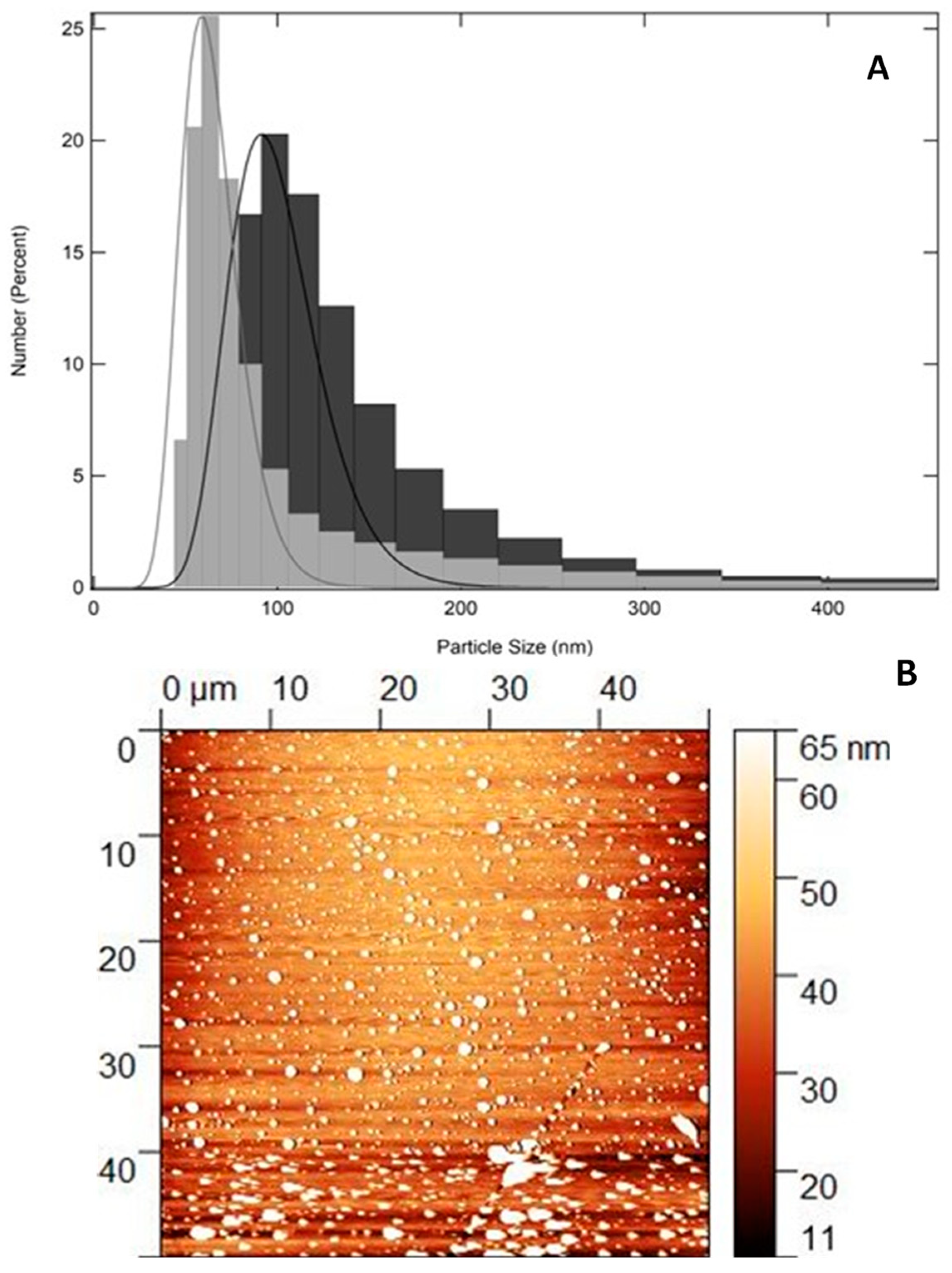
| Nanomaterial | Average Particle Size (nm) | PdI | Zeta Potential (mV) |
|---|---|---|---|
| QZ/NC | 103.9 ± 34.5 | 0.4 ± 0.03 | −31.8 ± 0.723 |
| Unloaded/NC | 137.2 ± 46.1 | 0.5 ± 0.06 | −35.1 ± 1.107 |
| Sample/Concentration (µg.mL−1) | 3T3—Cellular Viability (%) | Significance Level (* p < 0.05, ** p < 0.001) |
|---|---|---|
| Control | 100 | - |
| Unloaded Nanocapsule | 81.43 | - |
| Free QZ 50 | 100.06 | - |
| Free QZ 70 | 91.30 | - |
| QZ/NC 2.5 | 98.32 | - |
| QZ/NC 5.0 | 100.92 | - |
| QZ/NC 15.0 | 96.69 | - |
| QZ/NC 25.0 | 69.82 | * |
| QZ/NC 50 | 9.66 | ** |
| QZ/NC 70 | 9.02 | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Amaral, S.R.; Amantino, C.F.; Atanasov, A.; Sousa, S.; Moakes, R.; Oliani, S.M.; Grover, L.M.; Primo, F.L. Photodynamic Therapy as a Novel Therapeutic Modality Applying Quinizarin-Loaded Nanocapsules and 3D Bioprinting Skin Permeation for Inflammation Treatment. Pharmaceuticals 2024, 17, 1169. https://doi.org/10.3390/ph17091169
do Amaral SR, Amantino CF, Atanasov A, Sousa S, Moakes R, Oliani SM, Grover LM, Primo FL. Photodynamic Therapy as a Novel Therapeutic Modality Applying Quinizarin-Loaded Nanocapsules and 3D Bioprinting Skin Permeation for Inflammation Treatment. Pharmaceuticals. 2024; 17(9):1169. https://doi.org/10.3390/ph17091169
Chicago/Turabian Styledo Amaral, Stéphanie R., Camila F. Amantino, Aleksandar Atanasov, Stefanie Sousa, Richard Moakes, Sonia Maria Oliani, Liam M. Grover, and Fernando L. Primo. 2024. "Photodynamic Therapy as a Novel Therapeutic Modality Applying Quinizarin-Loaded Nanocapsules and 3D Bioprinting Skin Permeation for Inflammation Treatment" Pharmaceuticals 17, no. 9: 1169. https://doi.org/10.3390/ph17091169









