Application of Mammalian Nudix Enzymes to Capped RNA Analysis
Abstract
:1. Introduction
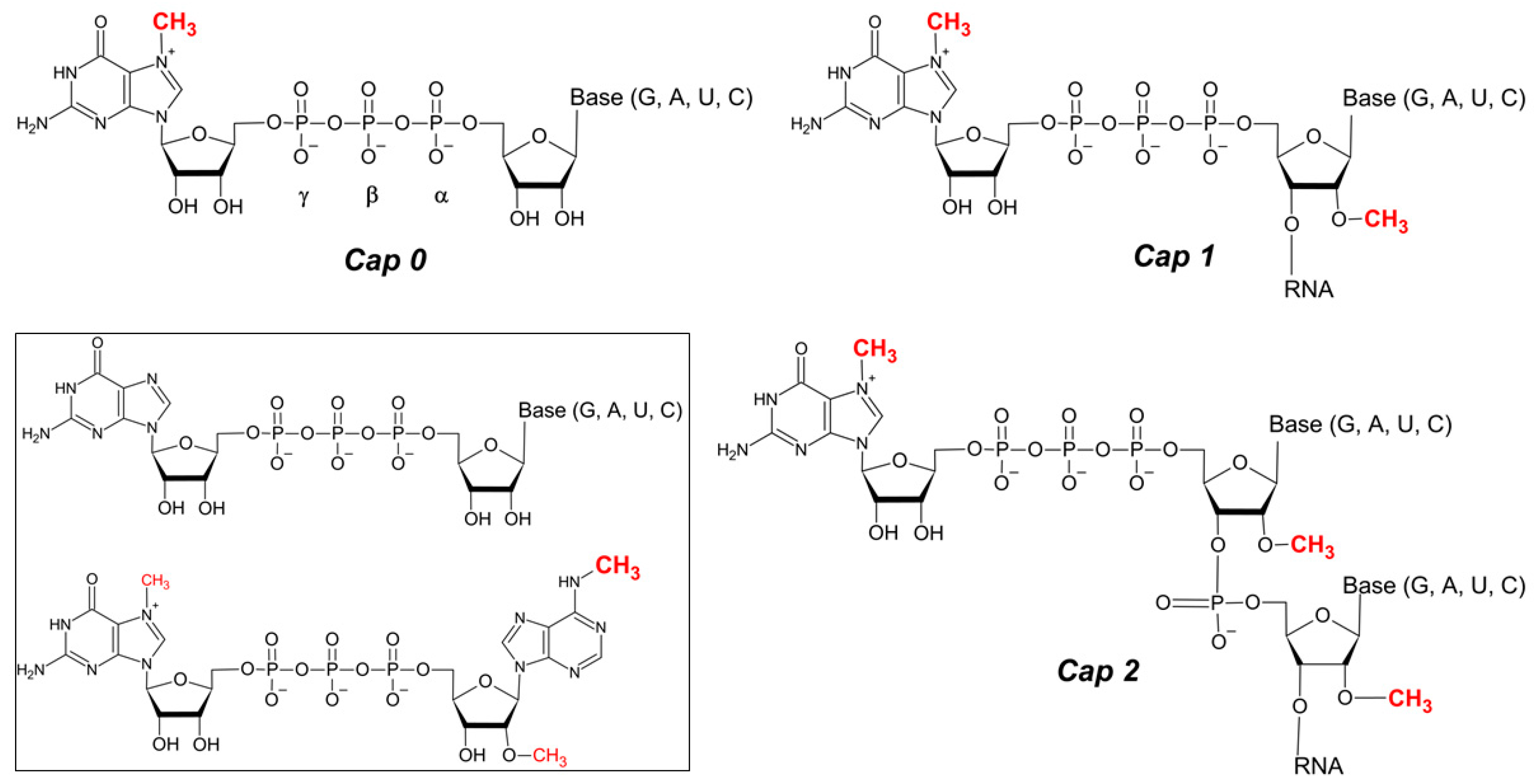
2. Diversity of 5′ RNA Cap Structures—Natural Caps
| Cap Structure | RNA Type | Cell Type | Ref. |
|---|---|---|---|
| m7GpppN1 m7Gpppm2′ON1 m7Gpppm6,2′OA m7Gpppm2′ON12′ON2 | mRNA | Eukaryotic | [8,19,20] |
| m32,2,7GpppN | snRNA (U1, U2, U4, U5), snoRNA primary miRNA (pri-miRNA) selenoprotein encoded transcripts, junD, dhx9 and tgs1 mRNA | Eukaryotic HPFs (human foreskin fibroblasts) HEK293, OSCA-40 canine osteosarcoma, HEK293FT | [20] [34] [22,23,24] |
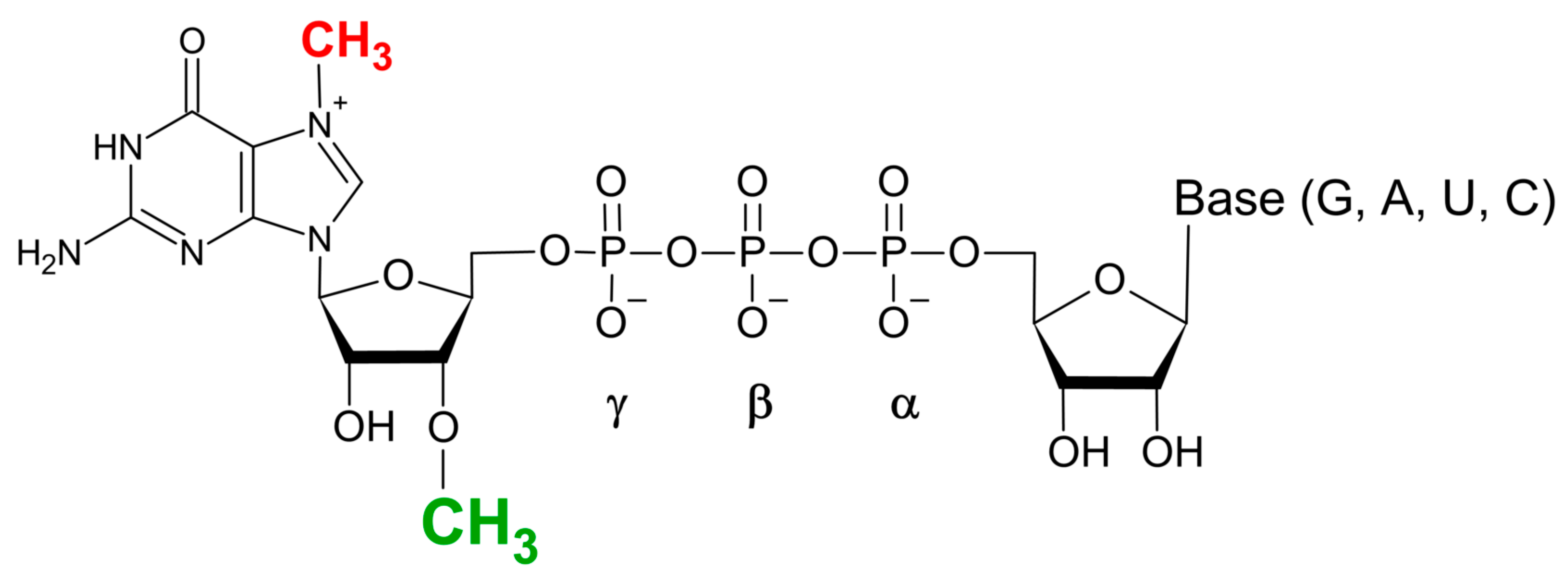
| CH3pppN 5′-gamma-methyl (tri)phosphate cap | U6 and 7SK snRNAs | Eukaryotic | [35,36] |
| (CH3)2pN Alfa-dimethyl-phosphate cap | Precursor RNA of miR-145 (pre-miR-145) | MCF-7 and MDA-MB-231 breast cancer cells | [21] |
| m7GpppA(G) m22,7GpppA(G) m32,2,7GpppA(G) | pre-tRNA | S. cerevisiae HeLa | [25] |
| m7GpppA(G) mGpppC GpppG, GpppA m32,2,7Gp, m22,7Gp, m22,2Gp m1Gp, m2Gp, mCp | Fraction of short RNAs (~20–200 nt), obtained from total RNA preparation | THP-1 (acute monocyte leukemia cell line) | [26] |
| NAD+ (5′ nicotinamide-adenine dinucleotide cap) | mRNA Intronic snoRNA mRNA | S. cerevisiae HEK293T Human (CCRF-SB cell line), mouse (C57BL/6 tissues), S.cervisiae | [28] [29] [37] |
| FAD | mRNA | Human (CCRF-SB cell line), mouse (C57BL/6 tissues), S.cervisiae | [37] |
| dpCoA | Mouse liver RNA | Mouse liver RNA | [32] |
| AppppA | RNA (>200 nt) | HEK293T RBL-2H3 (rat basophilic leukemia cells) | [33] |
| UDP-GlcNAc UDP-Glc | mRNA | Human (CCRF-SB cell line), mouse (C57BL/6 tissues), S.cervisiae | [37] |
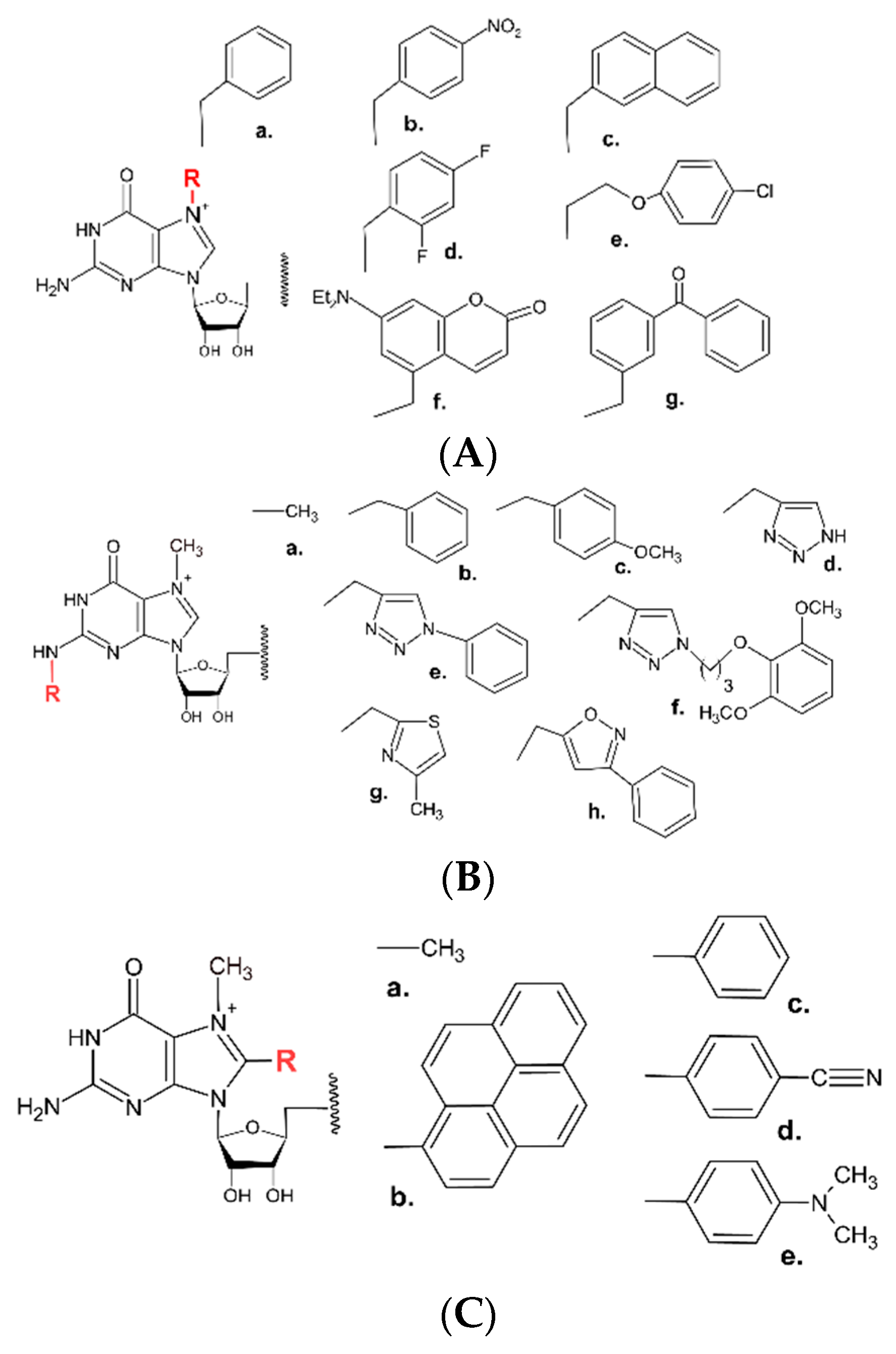
3. Diversity of Synthetic Cap Structures
4. NUDIX Protein Family
5. RNA Decapping with Mammalian Nudt2, Nudt5, Nudt12, Nudt16, and Dcp2
| Dcp2 | Nudt16 | Nudt12 | Nudt2 | |
|---|---|---|---|---|
| m7GpppN | + [105] | |||
| m7GpppNm | + [105] | |||
| m7GpppG | + [92,105,120,136] | +/− [92,93,120] | + [116,130] | + [116] |
| m7GpppA | + [105] | − [120] | ||
| m7GpppGm | + [105] | +/− [120] | ||
| m7GpppAm | + [105] | − [120] | ||
| m7Gpppm6Am | + [105,107] − [19] (transcript dependent) | |||
| m7GpppAmGm | + [103] | |||
| m7Gpppbn6AmG | + [107] | |||
| GpppG | − [14] | + [92,93,120] | + [116,130] | + [33,116] |
| GpppA | + [120] | + [33] | ||
| ApppA | + [33] | |||
| ApppG | − [33] | |||
| GppppG | + [33] | |||
| GppppA | + [33] | |||
| AppppA | + [33] | |||
| AppppG | + [33] | |||
| m27,3′OGpppG | + [58,89,136] | − [92,93,120] | ||
| m27,2′OGpppG | + [38] | |||
| m27,2′OGppppG | + [136] | |||
| m27,2′-OGppCH2ppG | − [136] | |||
| m27,2′-OGppCCl2ppG | − [136] | |||
| m27,2′-OGppCF2ppG | − [136] | |||
| m27,2′-OGpp-tz-C2H4OppG | − [64] | |||
| m27,2′-OGppp-tz-C2H4OpG | + [64] | |||
| m27,2′OGppNHpN | − [56] | |||
| m27,3′OGppCH2pN | − [56,58] | |||
| m27,2′OGppSpG (D1) | −/+ [38] | |||
| m27,2′OGppSpG (D2) | −/+ [38] | |||
| m27,2′OGppBH3pG (D2) | − [38] | |||
| bn2m7GpppG | + [92] | −/+ [92] | ||
| bn2m7GppSpG | − [93] | |||
| bn2m27,3′OGpppG | + [89] | |||
| bn2m27,2′OGpppG | + [89] | |||
| bn2m7GppppG | + [93] | |||
| bn2m7GpppAmG | − [93] | |||
| (4-Cl-bn)2m7GpppG | + [92] | −/+ [92] | ||
| (4-Cl-bn)2m7GppppG | + [93] | |||
| (4-Cl-bn)2m7GpppAmG | − [93] | |||
| (4-OCH3-bn)2m7GpppG | + [92] | −/+ [92] | ||
| (4-OCH3-bn)2m27,3′OGpppN | + [89] | |||
| (4-di(OCH3-bn)-tz)2m7GpppG | + [92] | − [92] | ||
| (4-di(OCH3-bn)-tz-CH2)2m7GpppG | − [93] | |||
| (4-di(OCH3-bn)-tz-(CH2)2)2m7GpppG | +/− [93] | |||
| (4-di(OCH3-bn)-tz-(CH2)4)2m7GpppG | − [93] | |||
| (4-bn-isx)2m7GpppG | + [92] | −/+ [92] | ||
| (4-CH3-th)2m7GpppG | + [92] | −/+ [92] | ||
| 2′-O/3′-O-(FAM-L6N)-m7GpppG | + [77] | + [77] | ||
| m32,2,7GpppG | + [120,129] | + [130] | ||
| m32,2,7GpppA | −/+ [121] |
6. Other RNA Decapping Enzymes
7. Discussion
8. Conclusions
Funding
Conflicts of Interest
References
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T-cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schäfer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef]
- Sahin, U.; Karikó, K.; Türeci, Ö. mRNA-based therapeutics—Developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef]
- Aygün, I.; Barciszewski, J. The forerunners and successful partnerships behind the BioNTech mRNA vaccine. J. Appl. Genet. 2023, 65, 47–55. [Google Scholar] [CrossRef]
- Shi, H.; Chai, P.; Jia, R.; Fan, X. Novel insight into the regulatory roles of diverse RNA modifications: Re-defining the bridge between transcription and translation. Mol. Cancer 2020, 19, 1–17. [Google Scholar] [CrossRef]
- Furuichi, Y. Discovery of m7G-cap in eukaryotic mRNAs. Proc. Jpn. Acad. Ser. B 2015, 91, 394–409. [Google Scholar] [CrossRef]
- Warminski, M.; Mamot, A.; Depaix, A.; Kowalska, J.; Jemielity, J. Chemical Modifications of mRNA Ends for Therapeutic Applications. Accounts Chem. Res. 2023, 56, 2814–2826. [Google Scholar] [CrossRef]
- Kurpiejewski, K.; Jankowska-Anyszka, M.; Grzela, R. N2 modified cap analogues as translation inhibitors and substrates for preparation of therapeutic mRNA. Eur. Biophys. J. 2023, 52, 511–519. [Google Scholar] [CrossRef]
- Dunckley, T.; Parker, R. The DCP2 protein is required for mRNA decapping in Saccharomyces cerevisiae and contains a functional MutT motif. EMBO J. 1999, 18, 5411–5422. [Google Scholar] [CrossRef]
- Lykke-Andersen, J. Identification of a Human Decapping Complex Associated with hUpf Proteins in Nonsense-Mediated Decay. Mol. Cell. Biol. 2002, 22, 8114–8121. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, E.; Cougot, N.; Meyer, S.; Babajko, S.; Wahle, E.; Séraphin, B. Human Dcp2: A catalytically active mRNA decapping enzyme located in specific cytoplasmic structures. EMBO J. 2002, 21, 6915–6924. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiao, X.; Carr-Schmid, A.; Kiledjian, M. The hDcp2 protein is a mammalian mRNA decapping enzyme. Proc. Natl. Acad. Sci. USA 2002, 99, 12663–12668. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Peterson, B.; Tomasevic, N.; Peculis, B.A. Xenopus U8 snoRNA Binding Protein Is a Conserved Nuclear Decapping Enzyme. Mol. Cell 2004, 13, 817–828. [Google Scholar] [CrossRef]
- Meyer, K.D.; Jaffrey, S.R. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat. Rev. Mol. Cell Biol. 2014, 15, 313–326. [Google Scholar] [CrossRef]
- Zamudio, J.R.; Mittra, B.; Zeiner, G.M.; Feder, M.; Bujnicki, J.M.; Sturm, N.R.; Campbell, D.A. Complete Cap 4 Formation Is Not Required for Viability in Trypanosoma brucei. Eukaryot. Cell 2006, 5, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Murai, R.; Hagiwara, H.; Hoshino, T.; Suyama, K. Preparation of eukaryotic mRNA having differently methylated adenosine at the 5′-terminus and the effect of the methyl group in translation. Nucleic Acids Symp. Ser. 2009, 53, 129–130. [Google Scholar] [CrossRef]
- Mauer, J.; Luo, X.; Blanjoie, A.; Jiao, X.; Grozhik, A.V.; Patil, D.P.; Linder, B.; Pickering, B.F.; Vasseur, J.-J.; Chen, Q.; et al. Reversible methylation of m6Am in the 5′ cap controls mRNA stability. Nature 2017, 541, 371–375. [Google Scholar] [CrossRef]
- Warminski, M.; Sikorski, P.J.; Kowalska, J.; Jemielity, J. Applications of Phosphate Modification and Labeling to Study (m)RNA Caps. Top. Curr. Chem. 2017, 375, 1–29. [Google Scholar] [CrossRef]
- Xhemalce, B.; Robson, S.C.; Kouzarides, T. Human RNA Methyltransferase BCDIN3D Regulates MicroRNA Processing. Cell 2012, 151, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Wurth, L.; Gribling-Burrer, A.-S.; Verheggen, C.; Leichter, M.; Takeuchi, A.; Baudrey, S.; Martin, F.; Krol, A.; Bertrand, E.; Allmang, C. Hypermethylated-capped selenoprotein mRNAs in mammals. Nucleic Acids Res. 2014, 42, 8663–8677. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Fritz, S.E.; Seufzer, B.; Boris-Lawrie, K. The mRNA encoding the JUND tumor suppressor detains nuclear RNA-binding proteins to assemble polysomes that are unaffected by mTOR. J. Biol. Chem. 2020, 295, 7763–7773. [Google Scholar] [CrossRef] [PubMed]
- Zucko, D.; Boris-Lawrie, K. Blocking tri-methylguanosine synthase 1 (TGS1) stops anchorage-independent growth of canine sarcomas. Cancer Gene Ther. 2023, 30, 1274–1284. [Google Scholar] [CrossRef]
- Ohira, T.; Suzuki, T. Precursors of tRNAs are stabilized by methylguanosine cap structures. Nat. Chem. Biol. 2016, 12, 648–655. [Google Scholar] [CrossRef]
- Abdelhamid, R.F.; Plessy, C.; Yamauchi, Y.; Taoka, M.; de Hoon, M.; Gingeras, T.R.; Isobe, T.; Carninci, P. Multiplicity of 5′ Cap Structures Present on Short RNAs. PLoS ONE 2014, 9, e102895. [Google Scholar] [CrossRef]
- Mattay, J. Noncanonical metabolite RNA caps: Classification, quantification, (de)capping, and function. Wiley Interdiscip. Rev. RNA 2022, 13, e1730. [Google Scholar] [CrossRef]
- Walters, R.W.; Matheny, T.; Mizoue, L.S.; Rao, B.S.; Muhlrad, D.; Parker, R. Identification of NAD + capped mRNAs in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2016, 114, 480–485. [Google Scholar] [CrossRef]
- Jiao, X.; Doamekpor, S.K.; Bird, J.G.; Nickels, B.E.; Tong, L.; Hart, R.P.; Kiledjian, M. 5′ End Nicotinamide Adenine Dinucleotide Cap in Human Cells Promotes RNA Decay through DXO-Mediated deNADding. Cell 2017, 168, 1015–1027.e10. [Google Scholar] [CrossRef]
- Kiledjian, M. Eukaryotic RNA 5′-End NAD + Capping and DeNADding. Trends Cell Biol. 2018, 28, 454–464. [Google Scholar] [CrossRef]
- Wiedermannová, J.; Julius, C.; Yuzenkova, Y. The expanding field of non-canonical RNA capping: New enzymes and mechanisms. R. Soc. Open Sci. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Zhang, H.; Zhu, Z.; Ji, F.; He, Z.; Yang, Z.; Xia, Y.; Cai, Z. DpCoA tagSeq: Barcoding dpCoA-Capped RNA for Direct Nanopore Sequencing via Maleimide-Thiol Reaction. Anal. Chem. 2023, 95, 11124–11131. [Google Scholar] [CrossRef] [PubMed]
- Potužník, J.F.; Nešuta, O.; Škríba, A.; Voleníková, B.; Mititelu, M.; Mancini, F.; Serianni, V.; Fernandez, H.; Spustová, K.; Trylčová, J.; et al. Diadenosine Tetraphosphate (Ap4A) Serves as a 5′ RNA Cap in Mammalian Cells. Angew. Chem. Int. Ed. 2023, 63, e202314951. [Google Scholar] [CrossRef]
- Martinez, I.; Hayes, K.E.; Barr, J.A.; Harold, A.D.; Xie, M.; Bukhari, S.I.A.; Vasudevan, S.; Steitz, J.A.; DiMaio, D. An Ex-portin-1-dependent microRNA biogenesis pathway during human cell quiescence. Proc. Natl. Acad. Sci. USA 2017, 114, E4961–E4970. [Google Scholar] [CrossRef]
- Singh, R.; Reddy, R. Gamma-monomethyl phosphate: A cap structure in spliceosomal U6 small nuclear RNA. Proc. Natl. Acad. Sci. USA 1989, 86, 8280–8283. [Google Scholar] [CrossRef]
- Gupta, S.; Busch, R.K.; Singh, R.; Reddy, R. Characterization of U6 small nuclear RNA cap-specific antibodies. Identification of gamma-monomethyl-GTP cap structure in 7SK and several other human small RNAs. J. Biol. Chem. 1990, 265, 19137–19142. [Google Scholar] [CrossRef]
- Wang, J.; Chew, B.L.A.; Lai, Y.; Dong, H.; Xu, L.; Balamkundu, S.; Cai, W.M.; Cui, L.; Liu, C.F.; Fu, X.-Y.; et al. Quantifying the RNA cap epitranscriptome reveals novel caps in cellular and viral RNA. Nucleic Acids Res. 2019, 47, e130. [Google Scholar] [CrossRef] [PubMed]
- Grudzien-Nogalska, E.; Stepinski, J.; Jemielity, J.; Zuberek, J.; Stolarski, R.; Rhoads, R.E.; Darzynkiewicz, E. Synthesis of anti-reverse cap analogs (ARCAs) and their applications in mRNA translation and stability. Methods Enzymol. 2007, 431, 203–227. [Google Scholar] [CrossRef]
- Li, S.; Jia, Y.; Jacobson, B.; McCauley, J.; Kratzke, R.; Bitterman, P.B.; Wagner, C.R. Treatment of Breast and Lung Cancer Cells with a N-7 Benzyl Guanosine Monophosphate Tryptamine Phosphoramidate Pronucleotide (4Ei-1) Results in Chemosensitization to Gemcitabine and Induced eIF4E Proteasomal Degradation. Mol. Pharm. 2013, 10, 523–531. [Google Scholar] [CrossRef]
- Golojuch, S.; Kopcial, M.; Strzelecka, D.; Kasprzyk, R.; Baran, N.; Sikorski, P.J.; Kowalska, J.; Jemielity, J. Exploring tryptamine conjugates as pronucleotides of phosphate-modified 7-methylguanine nucleotides targeting cap-dependent transla-tion. Bioorganic Med. Chem. 2020, 28, 115523. [Google Scholar] [CrossRef]
- Kurpiejewski, K.; Piecyk, K.; Lukaszewicz, M.; Kamel, K.; Chmurski, K.; Kmiecik, S.; Jankowska-Anyszka, M. The Synergistic Effect of N2 and N7 Modifications on the Inhibitory Efficacy of mRNA Cap Analogues. Pharmaceuticals 2024, 17, 632. [Google Scholar] [CrossRef] [PubMed]
- Konarska, M.M.; Padgett, R.A.; Sharp, P.A. Recognition of cap structure in splicing in vitro of mRNA precursors. Cell 1984, 38, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Darzynkiewicz, E.; Stepinski, J.; Ekiel, I.; Jin, Y.; Haber, D.; Sijuwade, T.; Tahara, S. Beta-globin mRNAs capped with m7G, m22.7G or m32.2.7G differ in intrinsic translation efficiency. Nucleic Acids Res. 1988, 16, 8953–8962. [Google Scholar] [CrossRef]
- Yisraeli, J.K.; Melton, D.A. Synthesis of long, capped transcripts in vitro by SP6 and T7 RNA polymerases. Methods Enzymol. 1989, 180, 42–50. [Google Scholar] [CrossRef]
- Coleman, T.M. Superior 5′ homogeneity of RNA from ATP-initiated transcription under the T7 2.5 promoter. Nucleic Acids Res. 2004, 32, 14e. [Google Scholar] [CrossRef]
- Pasquinelli, A.E.; Dahlberg, J.E.; Lund, E. Reverse 5′ caps in RNAs made in vitro by phage RNA polymerases. RNA 1995, 1, 957–967. [Google Scholar]
- Stepinski, J.; Waddell, C.; Stolarski, R.; Darzynkiewicz, E.; Rhoads, R.E. Synthesis and properties of mRNAs containing the novel “anti-reverse” cap analogs 7-methyl(3′-O-methyl)GpppG and 7-methyl (3′-deoxy)GpppG. RNA 2001, 7, 1486–1495. [Google Scholar]
- Jemielity, J.; Fowler, T.; Zuberek, J.; Stepinski, J.; Lewdorowicz, M.; Niedzwiecka, A.; Stolarski, R.; Darzynkiewicz, E.; Rhoads, R.E. Novel “anti-reverse” cap analogs with superior translational properties. RNA 2003, 9, 1108–1122. [Google Scholar] [CrossRef] [PubMed]
- Cai, A.; Jankowska-Anyszka, M.; Centers, A.; Chlebicka, L.; Stepinski, J.; Stolarski, R.; Darzynkiewicz, E.; Rhoads, R.E. Quantitative Assessment of mRNA Cap Analogues as Inhibitors of in Vitro Translation. Biochemistry 1999, 38, 8538–8547. [Google Scholar] [CrossRef]
- Zuberek, J.; Wyslouch-Cieszynska, A.; Niedzwiecka, A.; Dadlez, M.; Stepinski, J.; Augustyniak, W.; Gingras, A.-C.; Zhang, Z.; Burley, S.K.; Sonenberg, N.; et al. Phosphorylation of eIF4E attenuates its interaction with mRNA cap analogs by electrostatic repulsion: Intein-mediated protein ligation strategy to obtain phosphorylated protein. RNA 2003, 9, 52–61. [Google Scholar] [CrossRef]
- Kowalska, J.; Lewdorowicz, M.; Zuberek, J.; Bojarska, E.; Wojcik, J.; Cohen, L.S.; Davis, R.E.; Stepinski, J.; Stolarski, R.; Darzynkiewicz, E.; et al. Synthesis and Properties of Mrna cap Analogs Containing Phosphorothioate Moiety In 5′,5′-Triphosphate Chain. Nucleosides Nucleotides Nucleic Acids 2005, 24, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, J.; Lewdorowicz, M.; Zuberek, J.; Grudzien-Nogalska, E.; Bojarska, E.; Stepinski, J.; Rhoads, R.E.; Darzynkiewicz, E.; Davis, R.E.; Jemielity, J. Synthesis and characterization of mRNA cap analogs containing phosphorothioate substitutions that bind tightly to eIF4E and are resistant to the decapping pyrophosphatase DcpS. RNA 2008, 14, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, J.; Lukaszewicz, M.; Zuberek, J.; Darzynkiewicz, E.; Jemielity, J. Phosphoroselenoate Dinucleotides for Modification of mRNA 5′ End. ChemBioChem 2009, 10, 2469–2473. [Google Scholar] [CrossRef]
- Kowalska, J.; del Nogal, A.W.; Darzynkiewicz, Z.M.; Buck, J.; Nicola, C.; Kuhn, A.N.; Lukaszewicz, M.; Zuberek, J.; Strenkowska, M.; Ziemniak, M.; et al. Synthesis, properties, and biological activity of boranophosphate analogs of the mRNA cap: Versatile tools for manipulation of therapeutically relevant cap-dependent processes. Nucleic Acids Res. 2014, 42, 10245–10264. [Google Scholar] [CrossRef]
- Grudzien-Nogalska, E.; Jemielity, J.; Kowalska, J.; Darzynkiewicz, E.; Rhoads, R.E. Phosphorothioate cap analogs stabilize mRNA and increase translational efficiency in mammalian cells. RNA 2007, 13, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Slepenkov, S.; Grudzien-Nogalska, E.; Kowalska, J.; Kulis, M.; Zuberek, J.; Lukaszewicz, M.; Darzynkiewicz, E.; Jemielity, J.; Rhoads, R.E. Translation, stability, and resistance to decapping of mRNAs containing caps substituted in the triphosphate chain with BH3, Se, and NH. RNA 2011, 17, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Kalek, M.; Jemielity, J.; Darzynkiewicz, Z.M.; Bojarska, E.; Stepinski, J.; Stolarski, R.; Davis, R.E.; Darzynkiewicz, E. Enzymatically stable 5′ mRNA cap analogs: Synthesis and binding studies with human DcpS decapping enzyme. Bioorganic Med. Chem. 2006, 14, 3223–3230. [Google Scholar] [CrossRef]
- Grudzien, E.; Kalek, M.; Jemielity, J.; Darzynkiewicz, E.; Rhoads, R.E. Differential Inhibition of mRNA Degradation Pathways by Novel Cap Analogs. J. Biol. Cgem. 2006, 281, 1857–1867. [Google Scholar] [CrossRef]
- Rydzik, A.M.; Lukaszewicz, M.; Zuberek, J.; Kowalska, J.; Darzynkiewicz, Z.M.; Darzynkiewicz, E.; Jemielity, J. Synthetic dinucleotide mRNA cap analogs with tetraphosphate 5′,5′ bridge containing methylenebis(phosphonate) modification. Org. Biomol. Chem. 2009, 7, 4763–4776. [Google Scholar] [CrossRef]
- Ziemniak, M.; Kowalska, J.; Lukaszewicz, M.; Zuberek, J.; Wnek, K.; Darzynkiewicz, E.; Jemielity, J. Phosphate-modified analogues of m 7 GTP and m 7 Gppppm 7 G—Synthesis and biochemical properties. Bioorganic Med. Chem. 2015, 23, 5369–5381. [Google Scholar] [CrossRef]
- Strenkowska, M.; Grzela, R.; Majewski, M.; Wnek, K.; Kowalska, J.; Lukaszewicz, M.; Zuberek, J.; Darzynkiewicz, E.; Kuhn, A.N.; Sahin, U.; et al. Cap analogs modified with 1,2-dithiodiphosphate moiety protect mRNA from decapping and enhance its translational potential. Nucleic Acids Res. 2016, 44, 9578–9590. [Google Scholar] [CrossRef] [PubMed]
- Wojtczak, B.A.; Sikorski, P.J.; Fac-Dabrowska, K.; Nowicka, A.; Warminski, M.; Kubacka, D.; Nowak, E.; Nowotny, M.; Kowalska, J.; Jemielity, J. 5′-Phosphorothiolate Dinucleotide Cap Analogues: Reagents for Messenger RNA Modification and Potent Small-Molecular Inhibitors of Decapping Enzymes. J. Am. Chem. Soc. 2018, 140, 5987–5999. [Google Scholar] [CrossRef] [PubMed]
- Walczak, S.; Nowicka, A.; Kubacka, D.; Fac, K.; Wanat, P.; Mroczek, S.; Kowalska, J.; Jemielity, J. A novel route for preparing 5′ cap mimics and capped RNAs: Phosphate-modified cap analogues obtained via click chemistry. Chem. Sci. 2017, 8, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Walczak, S.; Sikorski, P.J.; Kasprzyk, R.; Kowalska, J.; Jemielity, J. Exploring the potential of phosphotriazole 5′ mRNA cap analogues as efficient translation initiators. Org. Biomol. Chem. 2018, 16, 6741–6748. [Google Scholar] [CrossRef]
- Kozarski, M.; Drazkowska, K.; Bednarczyk, M.; Warminski, M.; Jemielity, J.; Kowalska, J. Towards superior mRNA caps accessible by click chemistry: Synthesis and translational properties of triazole-bearing oligonucleotide cap analogs. RSC Adv. 2023, 13, 12809–12824. [Google Scholar] [CrossRef]
- Kore, A.R.; Shanmugasundaram, M.; Charles, I.; Cheng, A.M.; Barta, T.J. Synthesis and application of 2′-fluoro-substituted cap analogs. Bioorganic Med. Chem. Lett. 2007, 17, 5295–5299. [Google Scholar] [CrossRef]
- Kore, A.R.; Charles, I. Synthesis and evaluation of 2′-O-allyl substituted dinucleotide cap analog for mRNA translation. Bioorganic Med. Chem. 2010, 18, 8061–8065. [Google Scholar] [CrossRef]
- Shanmugasundaram, M.; Charles, I.; Kore, A.R. Design, synthesis and biological evaluation of dinucleotide mRNA cap analog containing propargyl moiety. Bioorganic Med. Chem. 2016, 24, 1204–1208. [Google Scholar] [CrossRef]
- Senthilvelan, A.; Vonderfecht, T.; Shanmugasundaram, M.; Potter, J.; Kore, A.R. Click-iT trinucleotide cap analog: Synthesis, mRNA translation, and detection. Bioorganic Med. Chem. 2023, 77, 117128. [Google Scholar] [CrossRef]
- Kore, A.R.; Shanmugasundaram, M.; Vlassov, A.V. Synthesis and application of a new 2′,3′-isopropylidene guanosine substituted cap analog. Bioorganic Med. Chem. Lett. 2008, 18, 4828–4832. [Google Scholar] [CrossRef]
- Warminski, M.; Kowalska, J.; Buck, J.; Zuberek, J.; Lukaszewicz, M.; Nicola, C.; Kuhn, A.N.; Sahin, U.; Darzynkiewicz, E.; Jemielity, J. The synthesis of isopropylidene mRNA cap analogs modified with phosphorothioate moiety and their evaluation as promoters of mRNA translation. Bioorganic Med. Chem. Lett. 2013, 23, 3753–3758. [Google Scholar] [CrossRef] [PubMed]
- Ziemniak, M.; Szabelski, M.; Lukaszewicz, M.; Nowicka, A.; Darzynkiewicz, E.; Rhoads, R.E.; Wieczorek, Z.; Jemielity, J. Synthesis and evaluation of fluorescent cap analogues for mRNA labelling. RSC Adv. 2013, 3, 20943–20958. [Google Scholar] [CrossRef] [PubMed]
- Kore, A.R.; Shanmugasundaram, M.; Charles, I.; Vlassov, A.V.; Barta, T.J. Locked Nucleic Acid (LNA)-Modified Dinucleotide mRNA Cap Analogue: Synthesis, Enzymatic Incorporation, and Utilization. J. Am. Chem. Soc. 2009, 131, 6364–6365. [Google Scholar] [CrossRef] [PubMed]
- Senthilvelan, A.; Vonderfecht, T.; Shanmugasundaram, M.; Pal, I.; Potter, J.; Kore, A.R. Trinucleotide Cap Analogue Bearing a Locked Nucleic Acid Moiety: Synthesis, mRNA Modification, and Translation for Therapeutic Applications. Org. Lett. 2021, 23, 4133–4136. [Google Scholar] [CrossRef]
- Jemielity, J.; Lukaszewicz, M.; Kowalska, J.; Czarnecki, J.; Zuberek, J.; Darzynkiewicz, E. Synthesis of biotin labelled cap analogue–incorporable into mRNA transcripts and promoting cap-dependent translation. Org. Biomol. Chem. 2012, 10, 8570–8574. [Google Scholar] [CrossRef]
- Bednarek, S.; Madan, V.; Sikorski, P.J.; Bartenschlager, R.; Kowalska, J.; Jemielity, J. mRNAs biotinylated within the 5′ cap and protected against decapping: New tools to capture RNA–protein complexes. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20180167. [Google Scholar] [CrossRef]
- Warminski, M.; Sikorski, P.J.; Warminska, Z.; Lukaszewicz, M.; Kropiwnicka, A.; Zuberek, J.; Darzynkiewicz, E.; Kowalska, J.; Jemielity, J. Amino-Functionalized 5′ Cap Analogs as Tools for Site-Specific Sequence-Independent Labeling of mRNA. Bioconjugate Chem. 2017, 28, 1978–1992. [Google Scholar] [CrossRef]
- Warminski, M.; Grab, K.; Szczepanski, K.; Spiewla, T.; Zuberek, J.; Kowalska, J.; Jemielity, J. Photoactivatable mRNA 5′ Cap Analogs for RNA-Protein Crosslinking. Adv. Sci. 2024, e2400994. [Google Scholar] [CrossRef]
- Mamot, A.; Sikorski, P.J.; Warminski, M.; Kowalska, J.; Jemielity, J. Azido-Functionalized 5′ Cap Analogues for the Preparation of Translationally Active mRNAs Suitable for Fluorescent Labeling in Living Cells. Angew. Chem. Int. Ed. 2017, 56, 15628–15632. [Google Scholar] [CrossRef]
- Matsuo, H.; Li, H.; McGuire, A.M.; Fletcher, C.M.; Gingras, A.-C.; Sonenberg, N.; Wagner, G. Structure of translation factor elF4E bound to m7GDP and interaction with 4E-binding protein. Nat. Struct. Mol. Biol. 1997, 4, 717–724. [Google Scholar] [CrossRef]
- Marcotrigiano, J.; Gingras, A.-C.; Sonenberg, N.; Burley, S.K. Cocrystal Structure of the Messenger RNA 5′ Cap-Binding Protein (eIF4E) Bound to 7-methyl-GDP. Cell 1997, 89, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecka, A.; Marcotrigiano, J.; Stepinski, J.; Jankowska-Anyszka, M.; Wyslouch-Cieszynska, A.; Dadlez, M.; Gingras, A.-C.; Mak, P.; Darzynkiewicz, E.; Sonenberg, N.; et al. Biophysical Studies of eIF4E Cap-binding Protein: Recognition of mRNA 5′ Cap Structure and Synthetic Fragments of eIF4G and 4E-BP1 Proteins. J. Mol. Biol. 2002, 319, 615–635. [Google Scholar] [CrossRef] [PubMed]
- Grudzien, E.; Stepinski, J.; Jankowska-Anyszka, M.; Stolarski, R.; Darzynkiewicz, E.; Rhoads, R.E. Novel cap analogs for in vitro synthesis of mRNAs with high translational efficiency. RNA 2004, 10, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Kore, A.R.; Xiao, Z.; Li, M. Synthesis and biological validation of N7-(4-chlorophenoxyethyl) substituted dinucleotide cap analogs for mRNA translation. Bioorganic Med. Chem. 2013, 21, 4570–4574. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, R.; Baranowski, M.R.; Markiewicz, L.; Kubacka, D.; Bednarczyk, M.; Baran, N.; Wojtczak, A.; Sikorski, P.J.; Zuberek, J.; Kowalska, J.; et al. Novel N7-Arylmethyl Substituted Dinucleotide mRNA 5′ cap Analogs: Synthesis and Evaluation as Modulators of Translation. Pharmaceutics 2021, 13, 1941. [Google Scholar] [CrossRef]
- Jia, Y.; Chiu, T.-L.; Amin, E.A.; Polunovsky, V.; Bitterman, P.B.; Wagner, C.R. Design, synthesis and evaluation of analogs of initiation factor 4E (eIF4E) cap-binding antagonist Bn7-GMP. Eur. J. Med. Chem. 2010, 45, 1304–1313. [Google Scholar] [CrossRef]
- Cornelissen, N.V.; Mineikaitė, R.; Erguven, M.; Muthmann, N.; Peters, A.; Bartels, A.; Rentmeister, A. Post-synthetic benzylation of the mRNA 5′ cap via enzymatic cascade reactions. Chem. Sci. 2023, 14, 10962–10970. [Google Scholar] [CrossRef]
- Piecyk, K.; Davis, R.E.; Jankowska-Anyszka, M. Synthesis of N2-modified 7-methylguanosine 5′-monophosphates as nematode translation inhibitors. Bioorganic Med. Chem. 2012, 20, 4781–4789. [Google Scholar] [CrossRef]
- Kocmik, I.; Piecyk, K.; Rudzinska, M.; Niedzwiecka, A.; Darzynkiewicz, E.; Grzela, R.; Jankowska-Anyszka, M. Modified ARCA analogs providing enhanced translational properties of capped mRNAs. Cell Cycle 2018, 17, 1624–1636. [Google Scholar] [CrossRef]
- Piecyk, K.; Lukaszewicz, M.; Darzynkiewicz, E.; Jankowska-Anyszka, M. Triazole-containing monophosphate mRNA cap analogs as effective translation inhibitors. RNA 2014, 20, 1539–1547. [Google Scholar] [CrossRef]
- Piecyk, K.; Lukaszewicz, M.; Kamel, K.; Janowska, M.; Pietrow, P.; Kmiecik, S.; Jankowska-Anyszka, M. Isoxazole-containing 5′ mRNA cap analogues as inhibitors of the translation initiation process. Bioorganic Chem. 2020, 96, 103583. [Google Scholar] [CrossRef] [PubMed]
- Grzela, R.; Piecyk, K.; Stankiewicz-Drogon, A.; Pietrow, P.; Lukaszewicz, M.; Kurpiejewski, K.; Darzynkiewicz, E.; Jankowska-Anyszka, M. N2 modified dinucleotide cap analogs as a potent tool for mRNA engineering. RNA 2023, 29, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Kurpiejewski, K.; Stankiewicz-Drogon, A.; Piecyk, K.; Rajkowska, E.; Skrzypczyk, P.; Geng, J.; Darzynkiewicz, E.; Grzela, R.; Jankowska-Anyszka, M. The potential of N2-modified cap analogues for precise genetic manipulation through mRNA engineering. Front. Mol. Biosci. 2024, 10, 1269028. [Google Scholar] [CrossRef]
- Klöcker, N.; Weissenboeck, F.P.; van Dülmen, M.; Špaček, P.; Hüwel, S.; Rentmeister, A. Photocaged 5′ cap analogues for optical control of mRNA translation in cells. Nat. Chem. 2022, 14, 905–913. [Google Scholar] [CrossRef]
- Weissenboeck, F.P.; Klöcker, N.; Špaček, P.; Hüwel, S.; Rentmeister, A. Stabilized 5′ Cap Analogue for Optochemical Activation of mRNA Translation. ACS Omega 2024, 9, 12810–12816. [Google Scholar] [CrossRef]
- Inagaki, M.; Abe, N.; Li, Z.; Nakashima, Y.; Acharyya, S.; Ogawa, K.; Kawaguchi, D.; Hiraoka, H.; Banno, A.; Meng, Z.; et al. Cap analogs with a hydrophobic photocleavable tag enable facile purification of fully capped mRNA with various cap structures. Nat. Commun. 2023, 14, 1–17. [Google Scholar] [CrossRef]
- Anhäuser, L.; Klöcker, N.; Muttach, F.; Mäsing, F.; Špaček, P.; Studer, A.; Rentmeister, A. A Benzophenone-Based Photocaging Strategy for the N7 Position of Guanosine. Angew. Chem. Int. Ed. 2019, 59, 3161–3165. [Google Scholar] [CrossRef]
- Bollu, A.; Schepers, H.; Klöcker, N.; Erguven, M.; Lawrence-Dörner, A.; Rentmeister, A. Visible Light Activates Coumarin-Caged mRNA for Cytoplasmic Cap Methylation in Cells. Chem.–A Eur. J. 2023, 30, e202303174. [Google Scholar] [CrossRef] [PubMed]
- Wojtczak, B.A.; Bednarczyk, M.; Sikorski, P.J.; Wojtczak, A.; Surynt, P.; Kowalska, J.; Jemielity, J. Synthesis and Evaluation of Diguanosine Cap Analogs Modified at the C8-Position by Suzuki–Miyaura Cross-Coupling: Discovery of 7-Methylguanosine-Based Molecular Rotors. J. Org. Chem. 2023, 88, 6827–6846. [Google Scholar] [CrossRef]
- Hyde, J.L.; Diamond, M.S. Innate immune restriction and antagonism of viral RNA lacking 2′-O methylation. Virology 2015, 479-480, 66–74. [Google Scholar] [CrossRef]
- Schuberth-Wagner, C.; Ludwig, J.; Bruder, A.K.; Herzner, A.-M.; Zillinger, T.; Goldeck, M.; Schmidt, T.; Schmid-Burgk, J.L.; Kerber, R.; Wolter, S.; et al. A Conserved Histidine in the RNA Sensor RIG-I Controls Immune Tolerance to N1-2′O-Methylated Self RNA. Immunity 2015, 43, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Despic, V.; Jaffrey, S.R. mRNA ageing shapes the Cap2 methylome in mammalian mRNA. Nature 2023, 614, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Drazkowska, K.; Tomecki, R.; Warminski, M.; Baran, N.; Cysewski, D.; Depaix, A.; Kasprzyk, R.; Kowalska, J.; Jemielity, J.; Sikorski, P.J. 2′-O-Methylation of the second transcribed nucleotide within the mRNA 5′ cap impacts the protein production level in a cell-specific manner and contributes to RNA immune evasion. Nucleic Acids Res. 2022, 50, 9051–9071. [Google Scholar] [CrossRef]
- Henderson, J.M.; Ujita, A.; Hill, E.; Yousif-Rosales, S.; Smith, C.; Ko, N.; McReynolds, T.; Cabral, C.R.; Escamilla-Powers, J.R.; Houston, M.E. Cap 1 Messenger RNA Synthesis with Co-transcriptional CleanCap® Analog by In Vitro Transcription. Curr. Protoc. 2021, 1, e39. [Google Scholar] [CrossRef] [PubMed]
- Sikorski, P.J.; Warminski, M.; Kubacka, D.; Ratajczak, T.; Nowis, D.; Kowalska, J.; Jemielity, J. The identity and methylation status of the first transcribed nucleotide in eukaryotic mRNA 5′ cap modulates protein expression in living cells. Nucleic Acids Res. 2020, 48, 1607–1626. [Google Scholar] [CrossRef]
- Wojtczak, A.; Kasprzyk, R.; Warmiński, M.; Ubych, K.; Kubacka, D.; Sikorski, P.J.; Jemielity, J.; Kowalska, J. Evaluation of carboxyfluorescein-labeled 7-methylguanine nucleotides as probes for studying cap-binding proteins by fluorescence anisotropy. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Warminski, M.; Trepkowska, E.; Smietanski, M.; Sikorski, P.J.; Baranowski, M.R.; Bednarczyk, M.; Kedzierska, H.; Majewski, B.; Mamot, A.; Papiernik, D.; et al. Trinucleotide mRNA Cap Analogue N6-Benzylated at the Site of Posttranscriptional m6Am Mark Facilitates mRNA Purification and Confers Superior Translational Properties In Vitro and In Vivo. J. Am. Chem. Soc. 2024, 146, 8149–8163. [Google Scholar] [CrossRef]
- Mlynarska-Cieslak, A.; Depaix, A.; Grudzien-Nogalska, E.; Sikorski, P.J.; Warminski, M.; Kiledjian, M.; Jemielity, J.; Kowalska, J. Nicotinamide-Containing Di- and Trinucleotides as Chemical Tools for Studies of NAD-Capped RNAs. Org. Lett. 2018, 20, 7650–7655. [Google Scholar] [CrossRef]
- Depaix, A.; Grudzien-Nogalska, E.; Fedorczyk, B.; Kiledjian, M.; Jemielity, J.; Kowalska, J. Preparation of RNAs with non-canonical 5′ ends using novel di- and trinucleotide reagents for co-transcriptional capping. Front. Mol. Biosci. 2022, 9, 854170. [Google Scholar] [CrossRef]
- Weber, F.; Motzkus, N.A.; Brandl, L.; Möhler, M.; Alempijevic, A.; Jäschke, A. Identification and in vitro characterization of UDP-GlcNAc-RNA cap-modifying and decapping enzymes. Nucleic Acids Res. 2024, 52, 5438–5450. [Google Scholar] [CrossRef]
- McLennan, A.G. The Nudix hydrolase superfamily. Cell. Mol. Life Sci. 2006, 63, 123–143. [Google Scholar] [CrossRef] [PubMed]
- McLennan, A.G. Substrate ambiguity among the nudix hydrolases: Biologically significant, evolutionary remnant, or both? Cell. Mol. Life Sci. 2013, 70, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Bessman, M.J.; Frick, D.N.; O’Handley, S.F. The MutT Proteins or “Nudix” Hydrolases, a Family of Versatile, Widely Distributed, “Housecleaning” Enzymes. J. Biol. Chem. 1996, 271, 25059–25062. [Google Scholar] [CrossRef] [PubMed]
- Mildvan, A.; Xia, Z.; Azurmendi, H.; Saraswat, V.; Legler, P.; Massiah, M.; Gabelli, S.; Bianchet, M.; Kang, L.-W.; Amzel, L. Structures and mechanisms of Nudix hydrolases. Arch. Biochem. Biophys. 2005, 433, 129–143. [Google Scholar] [CrossRef]
- Nakamura, T.; Yamagata, Y. Visualization of mutagenic nucleotide processing by Escherichia coli MutT, a Nudix hydrolase. Proc. Natl. Acad. Sci. USA 2022, 119, e2203118119. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-G.; Bail, S.; Kiledjian, M. Multiple Nudix family proteins possess mRNA decapping activity. RNA 2013, 19, 390–399. [Google Scholar] [CrossRef]
- Grudzien-Nogalska, E.; Kiledjian, M. New insights into decapping enzymes and selective mRNA decay. Wiley Interdiscip. Rev. RNA 2016, 8, e1379. [Google Scholar] [CrossRef]
- Grudzien-Nogalska, E.; Jiao, X.; Song, M.-G.; Hart, R.P.; Kiledjian, M. Nudt3 is an mRNA decapping enzyme that modulates cell migration. RNA 2016, 22, 773–781. [Google Scholar] [CrossRef]
- Song, M.-G.; Li, Y.; Kiledjian, M. Multiple mRNA Decapping Enzymes in Mammalian Cells. Mol. Cell 2010, 40, 423–432. [Google Scholar] [CrossRef]
- Grzela, R.; Nasilowska, K.; Lukaszewicz, M.; Tyras, M.; Stepinski, J.; Jankowska-Anyszka, M.; Bojarska, E.; Darzynkiewicz, E.; Grzela, R.; Nasilowska, K.; et al. Hydrolytic activity of human Nudt16 enzyme on dinucleotide cap analogs and short capped oligonucleotides. RNA 2018, 24, 633–642. [Google Scholar] [CrossRef]
- Wojtczak, B.A.; Warminski, M.; Kowalska, J.; Lukaszewicz, M.; Honcharenko, M.; Smith, C.I.E.; Strömberg, R.; Darzynkiewicz, E.; Jemielity, J. Clickable trimethylguanosine cap analogs modified within the triphosphate bridge: Synthesis, conjugation to RNA and susceptibility to degradation. RSC Adv. 2016, 6, 8317–8328. [Google Scholar] [CrossRef]
- Garneau, N.L.; Wilusz, J.; Wilusz, C.J. The highways and byways of mRNA decay. Nat. Rev. Mol. Cell Biol. 2007, 8, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Floor, S.N.; Jones, B.N.; Hernandez, G.A.; Gross, J.D. A split active site couples cap recognition by Dcp2 to activation. Nat. Struct. Mol. Biol. 2010, 17, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Cho, H.; Liu, Z.; Bowler, M.W.; Piao, S.; Parker, R.; Kim, Y.K.; Song, H. Structural Basis of the PNRC2-Mediated Link between mRNA Surveillance and Decapping. Structure 2012, 20, 2025–2037. [Google Scholar] [CrossRef]
- Li, Y.; Song, M.; Kiledjian, M. Differential utilization of decapping enzymes in mammalian mRNA decay pathways. RNA 2011, 17, 419–428. [Google Scholar] [CrossRef]
- Li, Y.; Dai, J.; Song, M.; Fitzgerald-Bocarsly, P.; Kiledjian, M. Dcp2 Decapping Protein Modulates mRNA Stability of the Critical Interferon Regulatory Factor (IRF) IRF-7. Mol. Cell. Biol. 2012, 32, 1164–1172. [Google Scholar] [CrossRef]
- Boulias, K.; Toczydłowska-Socha, D.; Hawley, B.R.; Liberman, N.; Takashima, K.; Zaccara, S.; Guez, T.; Vasseur, J.-J.; Debart, F.; Aravind, L.; et al. Identification of the m6Am Methyltransferase PCIF1 Reveals the Location and Functions of m6Am in the Transcriptome. Mol. Cell 2019, 75, 631–643.e8. [Google Scholar] [CrossRef]
- Lu, G.; Zhang, J.; Li, Y.; Li, Z.; Zhang, N.; Xu, X.; Wang, T.; Guan, Z.; Gao, G.F.; Yan, J. hNUDT16: A universal decapping enzyme for small nucleolar RNA and cytoplasmic mRNA. Protein Cell 2011, 2, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Zytek, M.; Kowalska, J.; Lukaszewicz, M.; Wojtczak, B.A.; Zuberek, J.; Ferenc-Mrozek, A.; Darzynkiewicz, E.; Niedzwiecka, A.; Jemielity, J. Towards novel efficient and stable nuclear import signals: Synthesis and properties of trimethylguanosine cap analogs modified within the 5′,5′-triphosphate bridge. Org. Biomol. Chem. 2014, 12, 9184–9199. [Google Scholar] [CrossRef]
- Lukaszewicz, M.; Ferenc-Mrozek, A.; Bojarska, E.; Stelmach, J.; Stepinski, J.; Darzynkiewicz, E. Contribution of Nudt12 enzyme to differentially methylated dinucleotides of 5′RNA cap structure. Biochim. Biophys. Acta (BBA) Gen. Subj. 2023, 1867, 130400. [Google Scholar] [CrossRef]
- Chrabąszczewska, M.; Winiewska-Szajewska, M.; Ostrowska, N.; Bojarska, E.; Stępiński, J.; Mancewicz, Ł.; Łukaszewicz, M.; Trylska, J.; Taube, M.; Kozak, M.; et al. Insight into the Binding and Hydrolytic Preferences of hNudt16 Based on Nucleotide Diphosphate Substrates. Int. J. Mol. Sci. 2021, 22, 10929. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Grudzien-Nogalska, E.; Hamilton, K.; Jiao, X.; Yang, J.; Tong, L.; Kiledjian, M. Mammalian Nudix proteins cleave nucleotide metabolite caps on RNAs. Nucleic Acids Res. 2020, 48, 6788–6798. [Google Scholar] [CrossRef]
- Grudzien-Nogalska, E.; Wu, Y.; Jiao, X.; Cui, H.; Mateyak, M.K.; Hart, R.P.; Tong, L.; Kiledjian, M. Structural and mechanistic basis of mammalian Nudt12 RNA deNADding. Nat. Chem. Biol. 2019, 15, 575–582. [Google Scholar] [CrossRef]
- Wu, H.; Li, L.; Chen, K.-M.; Homolka, D.; Gos, P.; Fleury-Olela, F.; McCarthy, A.A.; Pillai, R.S. Decapping Enzyme NUDT12 Partners with BLMH for Cytoplasmic Surveillance of NAD-Capped RNAs. Cell Rep. 2019, 29, 4422–4434.e13. [Google Scholar] [CrossRef]
- Laudenbach, B.T.; Krey, K.; Emslander, Q.; Andersen, L.L.; Reim, A.; Scaturro, P.; Mundigl, S.; Dächert, C.; Manske, K.; Moser, M.; et al. NUDT2 initiates viral RNA degradation by removal of 5′-phosphates. Nat. Commun. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Rydzik, A.M.; Warminski, M.; Sikorski, P.J.; Baranowski, M.R.; Walczak, S.; Kowalska, J.; Zuberek, J.; Lukaszewicz, M.; Nowak, E.; Claridge, T.D.W.; et al. mRNA cap analogues substituted in the tetraphosphate chain with CX2: Identification of O-to-CCl2 as the first bridging modification that confers resistance to decapping without impairing translation. Nucleic Acids Res. 2017, 45, 8661–8675. [Google Scholar] [CrossRef] [PubMed]
- Page, B.D.G.; Valerie, N.C.K.; Wright, R.H.G.; Wallner, O.; Isaksson, R.; Carter, M.; Rudd, S.G.; Loseva, O.; Jemth, A.-S.; Almlöf, I.; et al. Targeted NUDT5 inhibitors block hormone signaling in breast cancer cells. Nat. Commun. 2018, 9, 250. [Google Scholar] [CrossRef] [PubMed]
- Mititelu, M.-B.; Hudeček, O.; Gozdek, A.; Benoni, R.; Nešuta, O.; Krasnodębski, S.; Kufel, J.; Cahová, H. Arabidopsis thaliana NudiXes have RNA-decapping activity. RSC Chem. Biol. 2023, 4, 223–228. [Google Scholar] [CrossRef]
- Kramer, S.; McLennan, A.G. The complex enzymology of mRNA decapping: Enzymes of four classes cleave pyrophosphate bonds. Wiley Interdiscip. Rev. RNA 2018, 10, e1511. [Google Scholar] [CrossRef]
- Deana, A.; Celesnik, H.; Belasco, J.G. The bacterial enzyme RppH triggers messenger RNA degradation by 5′ pyrophosphate removal. Nature 2008, 451, 355–358. [Google Scholar] [CrossRef]
- Cahová, H.; Winz, M.-L.; Höfer, K.; Nübel, G.; Jäschke, A. NAD captureSeq indicates NAD as a bacterial cap for a subset of regulatory RNAs. Nature 2015, 519, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Chang, J.H.; Kilic, T.; Tong, L.; Kiledjian, M. A Mammalian Pre-mRNA 5′ End Capping Quality Control Mechanism and an Unexpected Link of Capping to Pre-mRNA Processing. Mol. Cell 2013, 50, 104–115. [Google Scholar] [CrossRef]
- Picard-Jean, F.; Brand, C.; Tremblay-Létourneau, M.; Allaire, A.; Beaudoin, M.C.; Boudreault, S.; Duval, C.; Rainville-Sirois, J.; Robert, F.; Pelletier, J.; et al. 2′-O-methylation of the mRNA cap protects RNAs from decapping and degradation by DXO. PLoS ONE 2018, 13, e0193804. [Google Scholar] [CrossRef]
- Doamekpor, S.K.; Grudzien-Nogalska, E.; Mlynarska-Cieslak, A.; Kowalska, J.; Kiledjian, M.; Tong, L. DXO/Rai1 enzymes remove 5′-end FAD and dephospho-CoA caps on RNAs. Nucleic Acids Res. 2020, 48, 6136–6148. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-T.; Bercovich, N.; Loh, B.; Jonas, S.; Izaurralde, E. The activation of the decapping enzyme DCP2 by DCP1 occurs on the EDC4 scaffold and involves a conserved loop in DCP1. Nucleic Acids Res. 2014, 42, 5217–5233. [Google Scholar] [CrossRef]
- Borbolis, F.; Syntichaki, P. Biological implications of decapping: Beyond bulk mRNA decay. FEBS J. 2021, 289, 1457–1475. [Google Scholar] [CrossRef]
- Luo, Y.; Schofield, J.A.; Simon, M.D.; Slavoff, S.A. Global Profiling of Cellular Substrates of Human Dcp2. Biochemistry 2020, 59, 4176–4188. [Google Scholar] [CrossRef]
- Luo, Y.; Schofield, J.A.; Na, Z.; Hann, T.; Simon, M.D.; Slavoff, S.A. Discovery of cellular substrates of human RNA-decapping enzyme DCP2 using a stapled bicyclic peptide inhibitor. Cell Chem. Biol. 2020, 28, 463–474.e7. [Google Scholar] [CrossRef]
- Vvedenskaya, I.O.; Nickels, B.E. CapZyme-Seq: A 5′-RNA-Seq Method for Differential Detection and Quantitation of NAD-Capped and Uncapped 5′-Triphosphate RNA. STAR Protoc. 2020, 1, 100002. [Google Scholar] [CrossRef]




| NAD Cap | FAD Cap | dpCoA Cap | UDP-GlcNAc Cap | |
|---|---|---|---|---|
| Nudt16 | + | + | + | |
| Nudt12 | + | + | ||
| Nudt5 | + | |||
| Nudt2 | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukaszewicz, M. Application of Mammalian Nudix Enzymes to Capped RNA Analysis. Pharmaceuticals 2024, 17, 1195. https://doi.org/10.3390/ph17091195
Lukaszewicz M. Application of Mammalian Nudix Enzymes to Capped RNA Analysis. Pharmaceuticals. 2024; 17(9):1195. https://doi.org/10.3390/ph17091195
Chicago/Turabian StyleLukaszewicz, Maciej. 2024. "Application of Mammalian Nudix Enzymes to Capped RNA Analysis" Pharmaceuticals 17, no. 9: 1195. https://doi.org/10.3390/ph17091195




