Bixin Combined with Metformin Ameliorates Insulin Resistance and Antioxidant Defenses in Obese Mice
Abstract
1. Introduction
2. Results
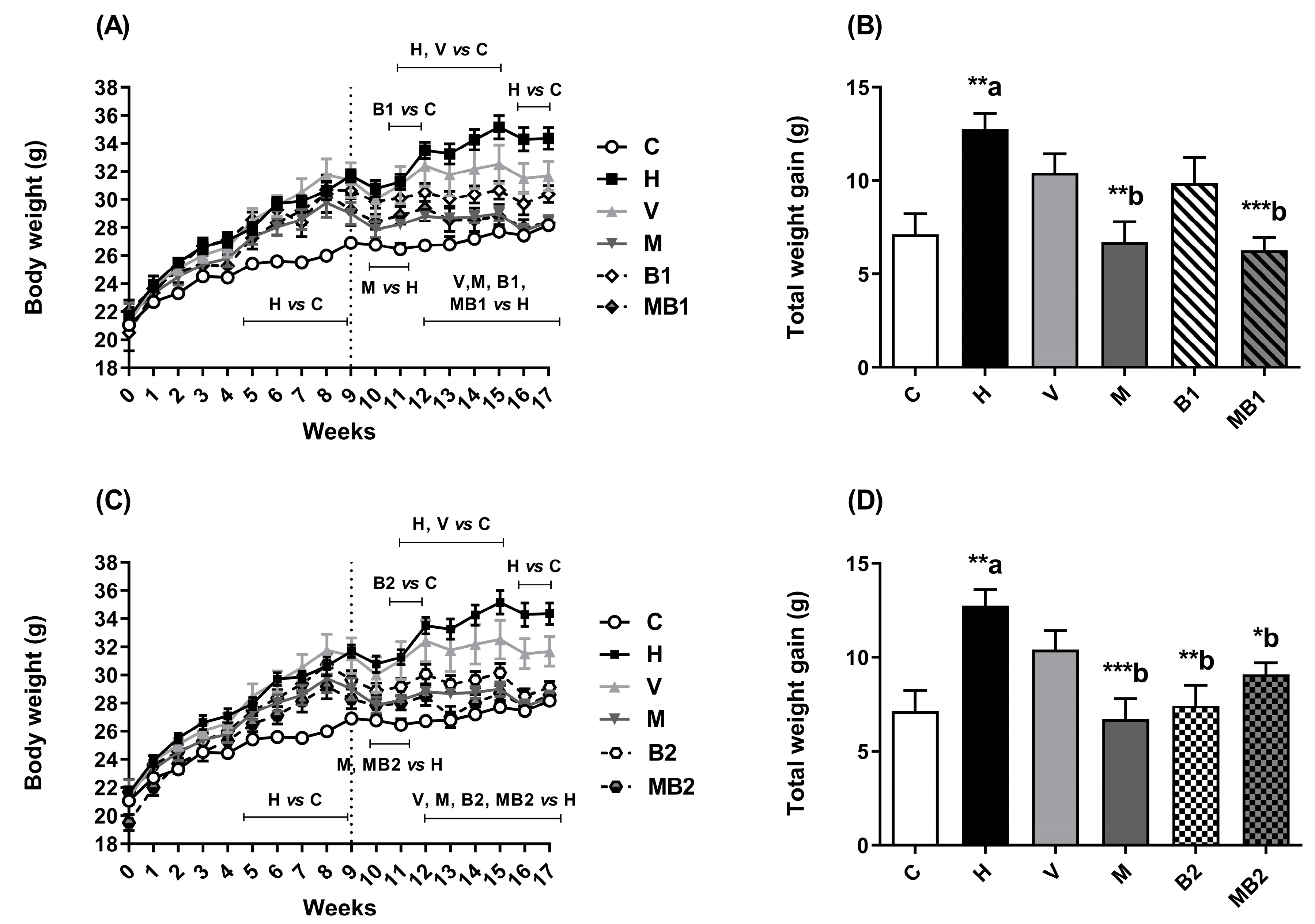
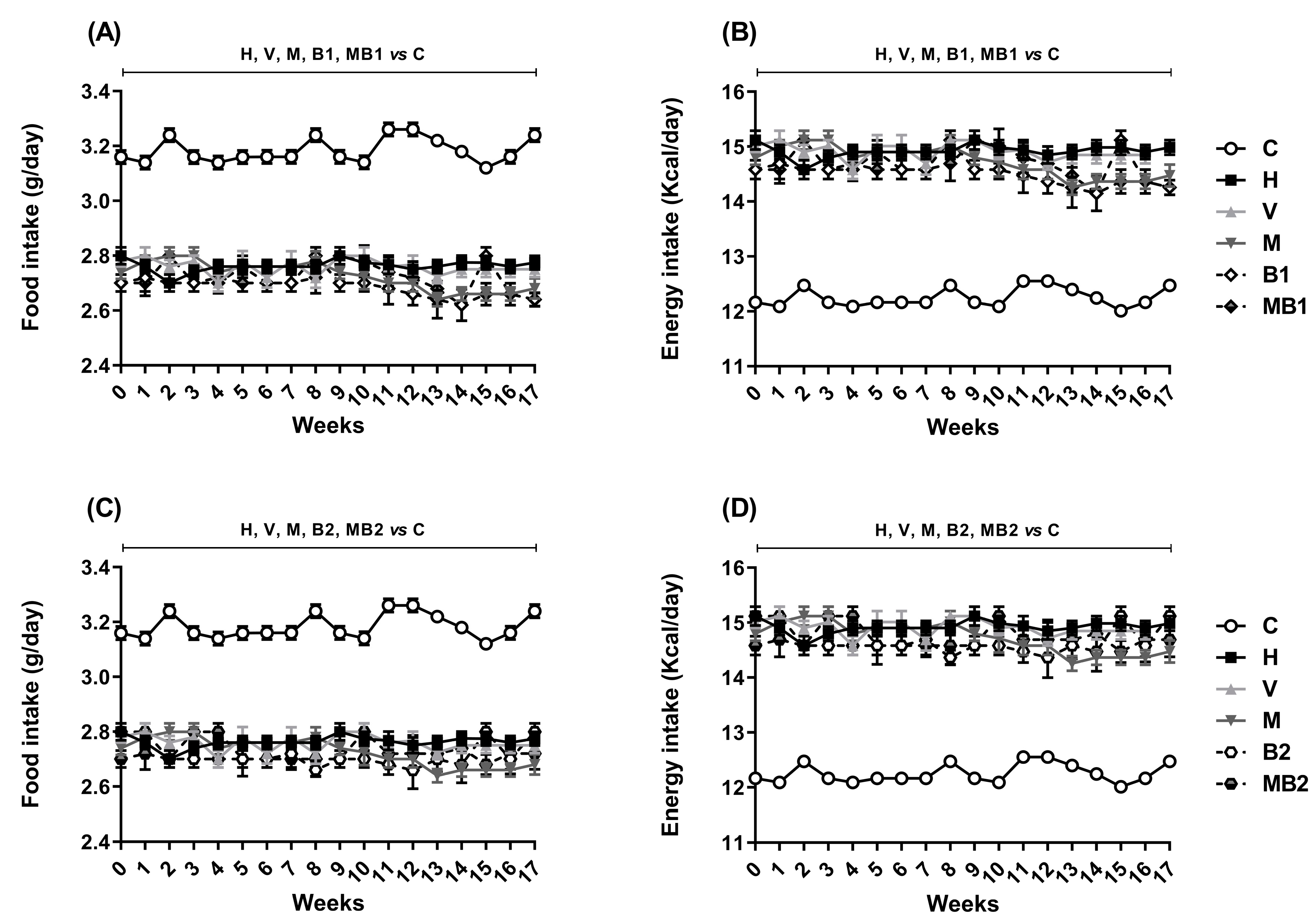
2.1. Physiological and Biochemical Parameters
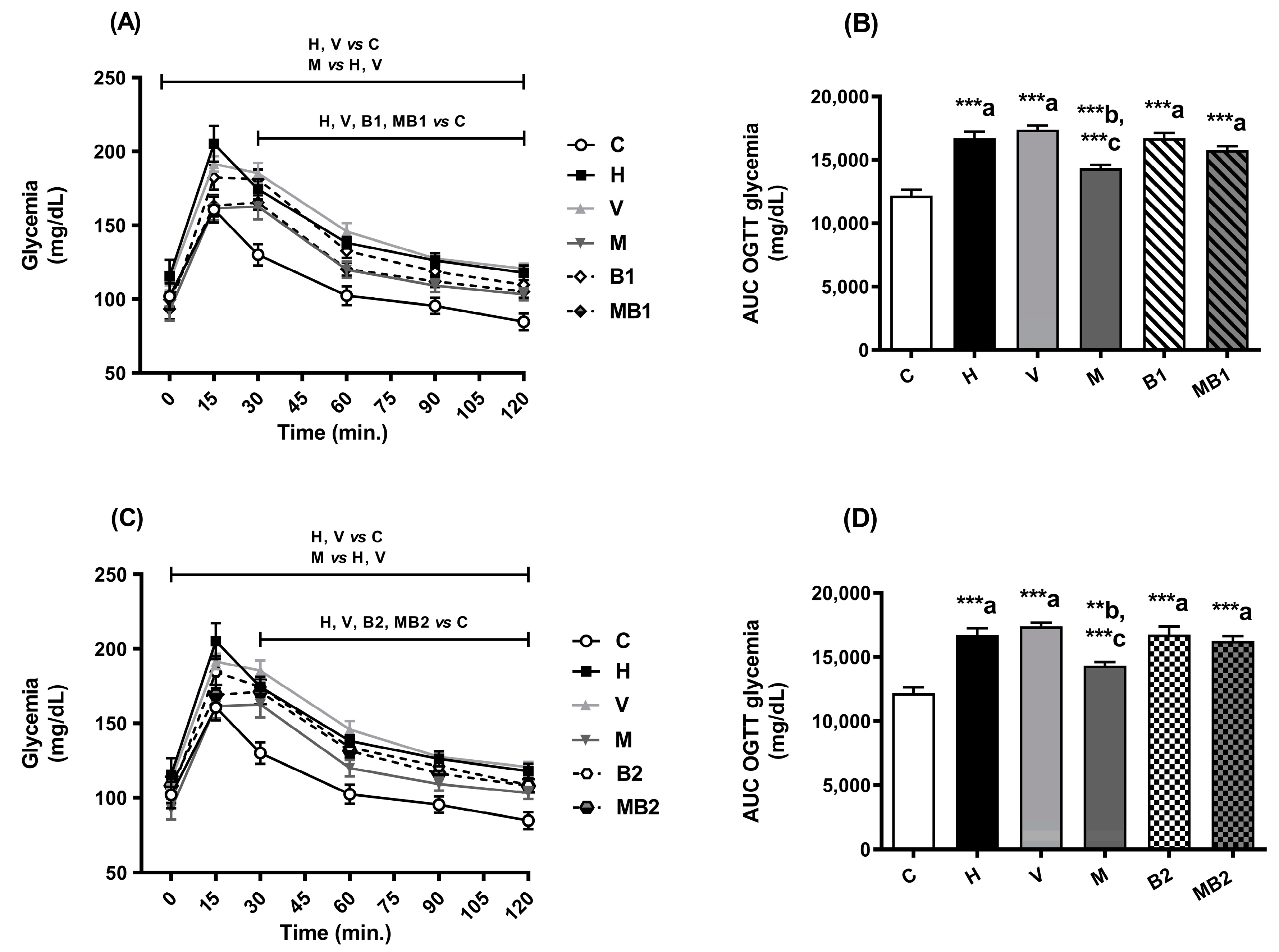
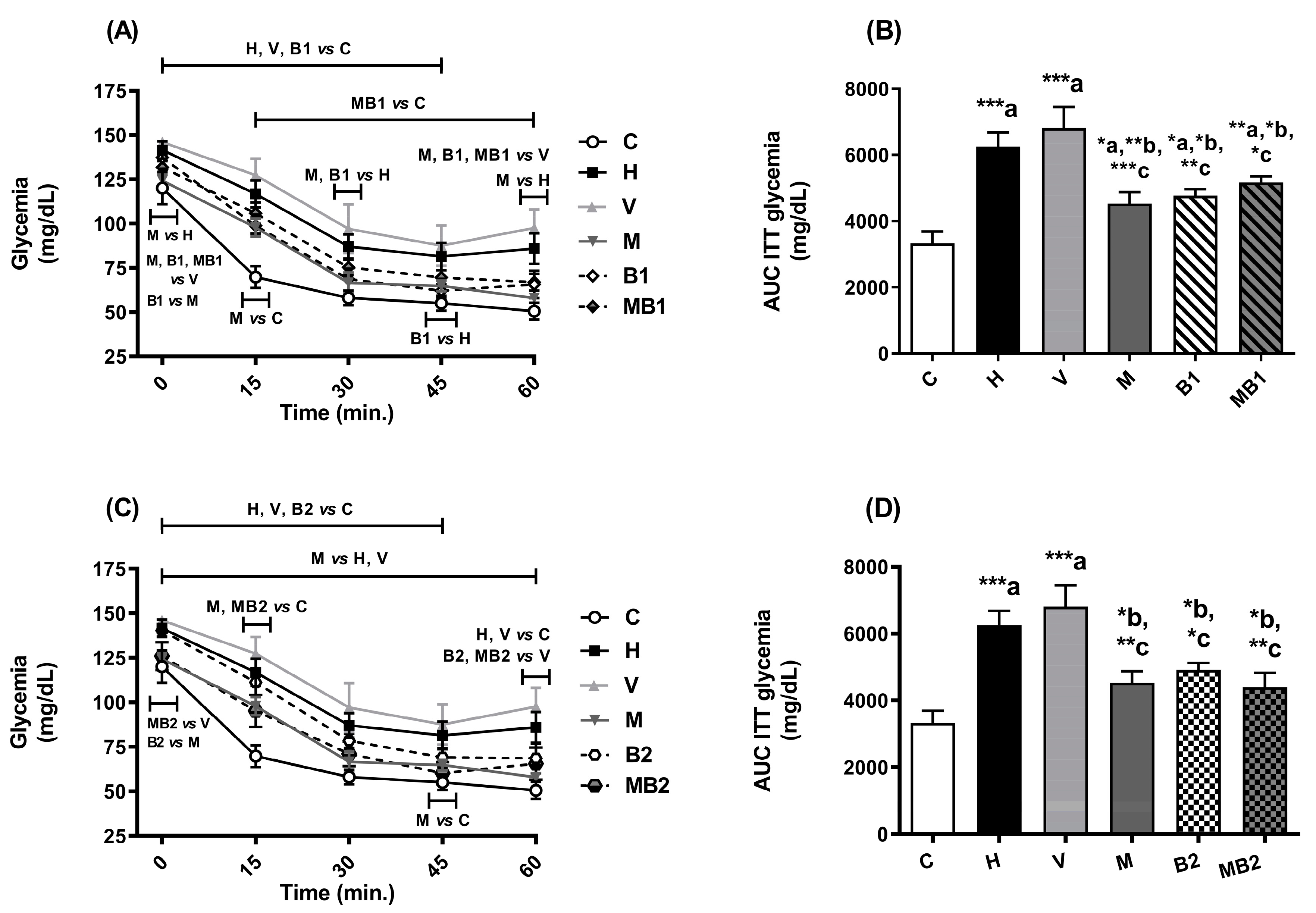
2.2. Glucose Tolerance and Insulin Sensitivity
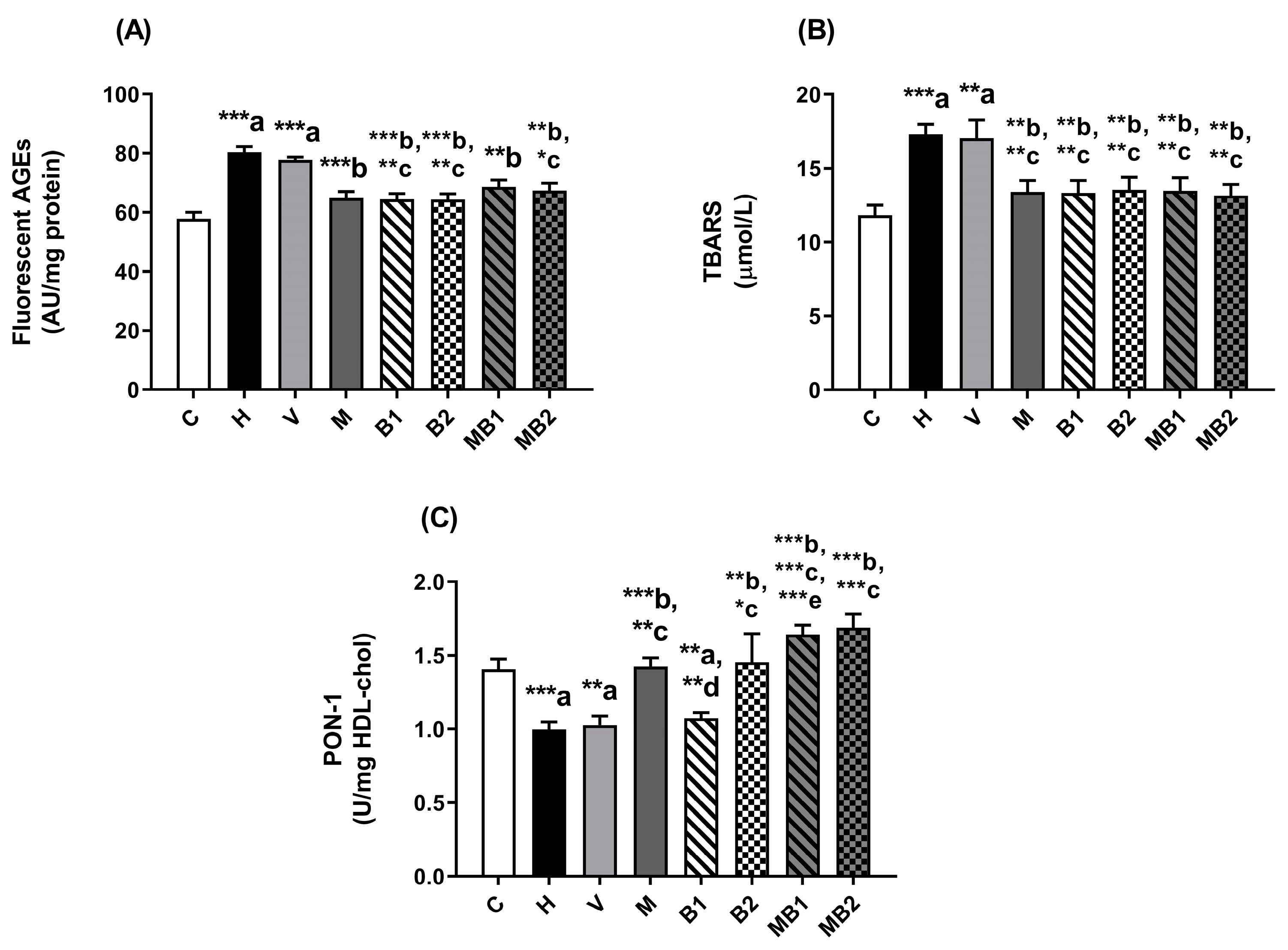
2.3. Biomarkers of Glycoxidative Stress and Antioxidant Defenses in Plasma
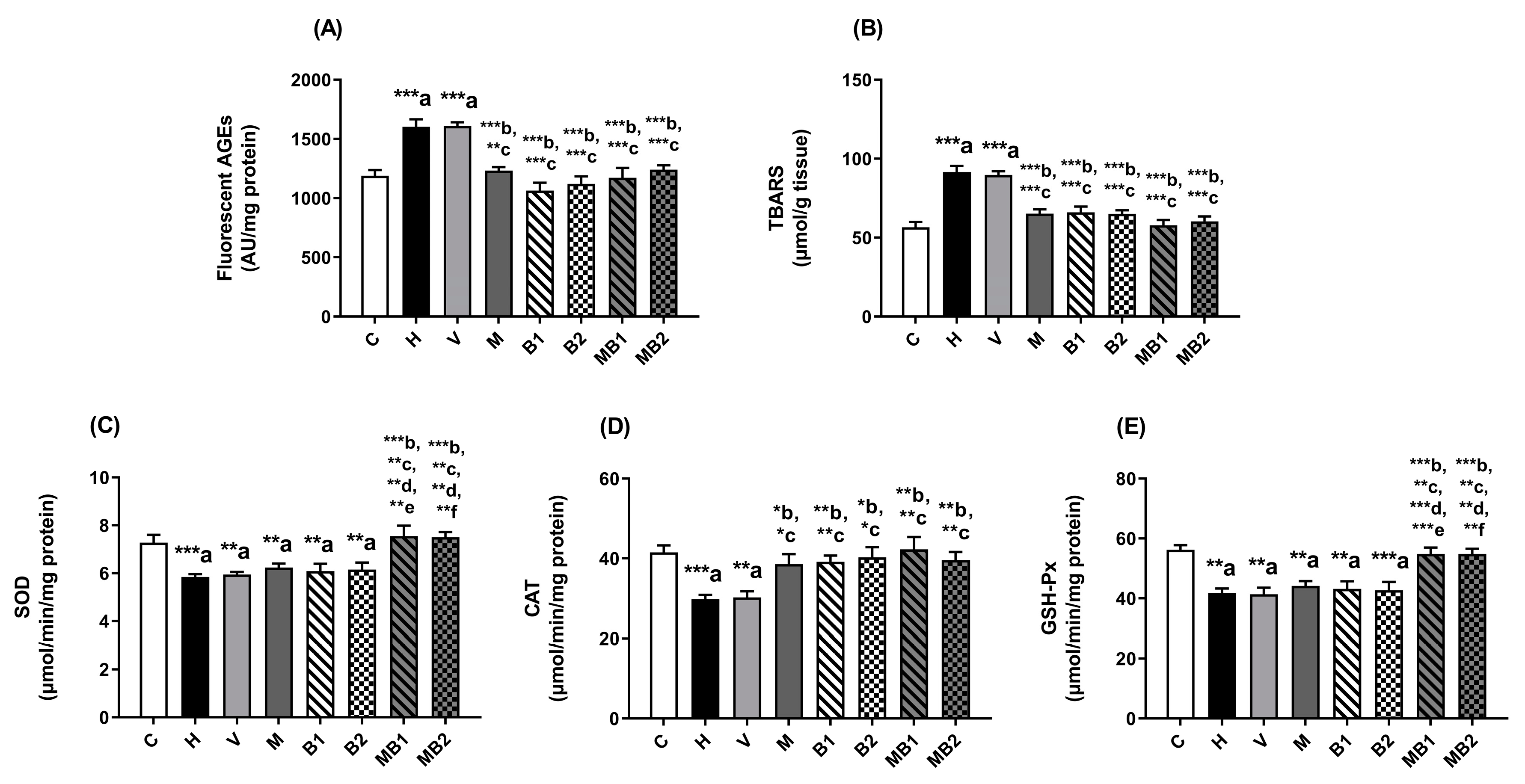
2.4. Biomarkers of Glycoxidative Stress and Antioxidant Defenses in the Liver
2.5. Biomarkers of Glycoxidative Stress and Antioxidant Defenses in the Kidneys
3. Discussion
4. Materials and Methods
4.1. Preparation of the Nanostructured Lipid System
4.2. Animal Model—Induction of Obesity/Insulin Resistance and Experiment Conclusion
4.3. Analysis of Plasma Biochemical Markers
4.4. Analysis of Glycoxidative Stress Biomarkers
4.5. Analysis of Endogenous Antioxidant Defenses
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Obri, A.; Serra, D.; Herrero, L.; Mera, P. The Role of Epigenetics in the Development of Obesity. Biochem. Pharmacol. 2020, 177, 113973. [Google Scholar] [CrossRef]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete Santos, A.; Grune, T.; Simm, A. Role of Advanced Glycation End Products in Cellular Signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef]
- Iacobini, C.; Vitale, M.; Pesce, C.; Pugliese, G.; Menini, S. Diabetic Complications and Oxidative Stress: A 20-Year Voyage Back in Time and Back to the Future. Antioxidants 2021, 10, 727. [Google Scholar] [CrossRef]
- Adak, T.; Samadi, A.; Ünal, A.Z.; Sabuncuoğlu, S. A Reappraisal on Metformin. Regul. Toxicol. Pharmacol. 2018, 92, 324–332. [Google Scholar] [CrossRef]
- Nasri, H.; Rafieian-Kopaei, M. Metformin: Current knowledge. J. Res. Med. Sci. 2014, 19, 658–664, Erratum in: J. Res. Med. Sci. 2024, 29, 6. [Google Scholar] [CrossRef]
- Testa, R.; Bonfigli, A.R.; Prattichizzo, F.; La Sala, L.; De Nigris, V.; Ceriello, A. The “Metabolic Memory” Theory and the Early Treatment of Hyperglycemia in Prevention of Diabetic Complications. Nutrients 2017, 9, 437. [Google Scholar] [CrossRef]
- Enayati, A.; Assadpour, E.; Jafari, S.M. Bixin—Properties and Applications. In Handbook of Food Bioactive Ingredients; Jafari, S.M., Rashidinejad, A., Simal-Gandara, J., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Vilar, D.A.; Vilar, M.S.; de Lima e Moura, T.F.; Raffin, F.N.; de Oliveira, M.R.; Franco, C.F.; de Athayde-Filho, P.F.; Diniz, M.F.; Barbosa-Filho, J.M. Traditional uses, chemical constituents, and biological activities of Bixa orellana L.: A review. Sci. World J. 2014, 2014, 857292. [Google Scholar] [CrossRef]
- Ma, J.Q.; Zhang, Y.J.; Tian, Z.K.; Liu, C.M. Bixin Attenuates Carbon Tetrachloride Induced Oxidative Stress, Inflammation and Fibrosis in Kidney by Regulating the Nrf2/TLR4/MyD88 and PPAR-γ/TGF-Β1/Smad3 Pathway. Int. Immunopharmacol. 2021, 90, 107117. [Google Scholar] [CrossRef]
- Roohbakhsh, A.; Karimi, G.; Iranshahi, M. Carotenoids in the Treatment of Diabetes Mellitus and Its Complications: A Mechanistic Review. Biomed. Pharmacother. 2017, 91, 31–42. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Metformin: Update on mechanisms of action and repurposing potential. Nat. Rev. Endocrinol. 2023, 19, 460–476. [Google Scholar] [CrossRef]
- Shahid-ul-Islam; Rather, L.J.; Mohammad, F. Phytochemistry, Biological Activities and Potential of Annatto in Natural Colorant Production for Industrial Applications—A Review. J. Adv. Res. 2016, 7, 499–514. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioactive-Loaded Nanocarriers for Functional Foods: From Designing to Bioavailability. Curr. Opin. Food Sci. 2020, 33, 21–29. [Google Scholar] [CrossRef]
- Salvi, V.R.; Pawar, P. Nanostructured Lipid Carriers (NLC) System: A Novel Drug Targeting Carrier. J. Drug Deliv. Sci. Technol. 2019, 51, 255–267. [Google Scholar] [CrossRef]
- Gutierrez, R.M.; Romero, R.V. Effects of bixin in high-fat diet-fed-induced fatty liver in C57BL/6J mice. Asian Pac. J. Trop. Biomed. 2016, 6, 1015–1021. [Google Scholar] [CrossRef]
- Lytrivi, M.; Castell, A.L.; Poitout, V.; Cnop, M. Recent Insights into Mechanisms of β-Cell Lipo- and Glucolipotoxicity in Type 2 Diabetes. J. Mol. Biol. 2020, 432, 1514–1534. [Google Scholar] [CrossRef]
- Zhou, K.; Yee, S.W.; Seiser, E.L.; Van Leeuwen, N.; Tavendale, R.; Bennett, A.J.; Groves, C.J.; Coleman, R.L.; Van Der Heijden, A.A.; Beulens, J.W.; et al. Variation in the Glucose Transporter Gene SLC2A2 Is Associated with Glycemic Response to Metformin. Nat. Genet. 2016, 48, 1055–1059. [Google Scholar] [CrossRef]
- Meex, R.C.R.; Watt, M.J. Hepatokines: Linking Nonalcoholic Fatty Liver Disease and Insulin Resistance. Nat. Rev. Endocrinol. 2017, 13, 509–520. [Google Scholar] [CrossRef]
- Benador, I.Y.; Veliova, M.; Liesa, M.; Shirihai, O.S. Mitochondria Bound to Lipid Droplets: Where Mitochondrial Dynamics Regulate Lipid Storage and Utilization. Cell Metab. 2019, 29, 827–835. [Google Scholar] [CrossRef]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef]
- Roehrs, M.; Conte, L.; da Silva, D.T.; Duarte, T.; Maurer, L.H.; de Carvalho, J.A.M.; Moresco, R.N.; Somacal, S.; Emanuelli, T. Annatto Carotenoids Attenuate Oxidative Stress and Inflammatory Response after High-Calorie Meal in Healthy Subjects. Food Res. Int. 2017, 100, 771–779. [Google Scholar] [CrossRef]
- Roehrs, M.; Figueiredo, C.G.; Zanchi, M.M.; Bochi, G.V.; Moresco, R.N.; Quatrin, A.; Somacal, S.; Conte, L.; Emanuelli, T. Bixin and Norbixin Have Opposite Effects on Glycemia, Lipidemia, and Oxidative Stress in Streptozotocin-Induced Diabetic Rats. Int. J. Endocrinol. 2014, 2014, 839095. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Lee, E.J.; Ahmad, K.; Ahmad, S.S.; Lim, J.H.; Choi, I. A Comprehensive Review and Perspective on Natural Sources as Dipeptidyl Peptidase-4 Inhibitors for Management of Diabetes. Pharmaceuticals 2021, 14, 591. [Google Scholar] [CrossRef]
- Chhabria, S.; Mathur, S.; Vadakan, S.; Sahoo, D.K.; Mishra, P.; Paital, B. A review on phytochemical and pharmacological facets of tropical ethnomedicinal plants as reformed DPP-IV inhibitors to regulate incretin activity. Front. Endocrinol. 2022, 13, 1027237. [Google Scholar] [CrossRef]
- Singh, A.K.; Yadav, D.; Sharma, N.; Jin, J.O. Dipeptidyl Peptidase (DPP)-IV Inhibitors with Antioxidant Potential Isolated from Natural Sources: A Novel Approach for the Management of Diabetes. Pharmaceuticals 2021, 14, 586. [Google Scholar] [CrossRef]
- Tulipano, G. Integrated or Independent Actions of Metformin in Target Tissues Underlying Its Current Use and New Possible Applications in the Endocrine and Metabolic Disorder Area. Int. J. Mol. Sci. 2021, 22, 13068. [Google Scholar] [CrossRef]
- Kenechukwu, F.C.; Isaac, G.T.; Nnamani, D.O.; Momoh, M.A.; Attama, A.A. Enhanced circulation longevity and pharmacodynamics of metformin from surface-modified nanostructured lipid carriers based on solidified reverse micellar solutions. Heliyon 2022, 8, e09100. [Google Scholar] [CrossRef]
- Guo, W.R.; Liu, J.; Cheng, L.D.; Liu, Z.Y.; Zheng, X.B.; Liang, H.; Xu, F. Metformin Alleviates Steatohepatitis in Diet-Induced Obese Mice in a SIRT1-Dependent Way. Front. Pharmacol. 2021, 12, 704112. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Ren, L.W.; Zhan, P.; Yang, H.Y.; Chai, D.D.; Yu, Z.W. Metformin exerts glucose-lowering action in high-fat fed mice via attenuating endotoxemia and enhancing insulin signaling. Acta Pharmacol. Sin. 2016, 37, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Nanoencapsulation of Carotenoids within Lipid-Based Nanocarriers. J. Control. Release 2019, 298, 38–67. [Google Scholar] [CrossRef] [PubMed]
- Enayati, A.; Rezaei, A.; Falsafi, S.R.; Rostamabadi, H.; Malekjani, N.; Akhavan-Mahdavi, S.; Kharazmi, M.S.; Jafari, S.M. Bixin-loaded colloidal nanodelivery systems, techniques and applications. Food Chem. 2023, 412, 135479. [Google Scholar] [CrossRef]
- Costa, M.C.; Lima, T.F.O.; Arcaro, C.A.; Inacio, M.D.; Batista-Duharte, A.; Carlos, I.Z.; Spolidorio, L.C.; Assis, R.P.; Brunetti, I.L.; Baviera, A.M. Trigonelline and Curcumin Alone, but Not in Combination, Counteract Oxidative Stress and Inflammation and Increase Glycation Product Detoxification in the Liver and Kidney of Mice with High-Fat Diet-Induced Obesity. J. Nutr. Biochem. 2020, 76, 108303. [Google Scholar] [CrossRef]
- Koren-Gluzer, M.; Aviram, M.; Meilin, E.; Hayek, T. The Antioxidant HDL-Associated Paraoxonase-1 (PON1) Attenuates Diabetes Development and Stimulates β-Cell Insulin Release. Atherosclerosis 2011, 219, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Shokri, Y.; Variji, A.; Nosrati, M.; Khonakdar-tarsi, A.; Kianmehr, A.; Kashi, Z.; Bahar, A.; Bagheri, A.; Mahrooz, A. Importance of Paraoxonase 1 (PON1) as an Antioxidant and Antiatherogenic Enzyme in the Cardiovascular Complications of Type 2 Diabetes: Genotypic and Phenotypic Evaluation. Diabetes Res. Clin. Pract. 2020, 1, 108067. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Melnichenko, A.A.; Orekhov, A.N.; Bobryshev, Y.V. Paraoxonase and Atherosclerosis-Related Cardiovascular Diseases. Biochimie 2017, 132, 19–27. [Google Scholar] [CrossRef]
- Assis, R.P.; Arcaro, C.A.; Gutierres, V.O.; Oliveira, J.O.; Costa, P.I.; Baviera, A.M.; Brunetti, I.L. Combined Effects of Curcumin and Lycopene or Bixin in Yoghurt on Inhibition of LDL Oxidation and Increases in HDL and Paraoxonase Levels in Streptozotocin-Diabetic Rats. Int. J. Mol. Sci. 2017, 18, 332. [Google Scholar] [CrossRef]
- Koren-Gluzer, M.; Aviram, M.; Hayek, T. Paraoxonase1 (PON1) Reduces Insulin Resistance in Mice Fed a High-Fat Diet, and Promotes GLUT4 Overexpression in Myocytes, via the IRS-1/Akt Pathway. Atherosclerosis 2013, 229, 71–78. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First Line Defence Antioxidants-Superoxide Dismutase (SOD), Catalase (CAT) and Glutathione Peroxidase (GPX): Their Fundamental Role in the Entire Antioxidant Defence Grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative Stress Mitigation by Antioxidants—An Overview on Their Chemistry and Influences on Health Status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Zhang, H.; Zhang, J.; Li, B.; Zhang, Z.; Tao, S. Bixin Protects against Particle-Induced Long-Term Lung Injury in an NRF2-Dependent Manner. Toxicol. Res. 2018, 7, 258–270. [Google Scholar] [CrossRef]
- Xu, Z.; Kong, X.Q. Bixin Ameliorates High Fat Diet-Induced Cardiac Injury in Mice through Inflammation and Oxidative Stress Suppression. Biomed. Pharmacother. 2017, 89, 991–1004. [Google Scholar] [CrossRef]
- Luisa-Bonet, M.; Canas, J.A.; Ribot, J.; Palou, A. Carotenoids and Their Conversion Products in the Control of Adipocyte Function, Adiposity and Obesity. Arch. Biochem. Biophys. 2015, 572, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, F.; Ortiz, M.; Valenzuela, R.; Videla, L.A. Long-Chain Polyunsaturated Fatty Acids Regulation of PPARs, Signaling: Relationship to Tissue Development and Aging. Prostaglandins Leukot. Essent. Fat. Acids 2016, 114, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.J.; Holder, J.C. PPAR-γ Agonists: Therapeutic Role in Diabetes, Inflammation and Cancer. Trends Pharmacol. Sci. 2000, 21, 469–474. [Google Scholar] [CrossRef]
- de Freitas, E.S.; da Silva, P.B.; Chorilli, M.; Batista, A.A.; de Oliveira Lopes, E.; da Silva, M.M.; Leite, C.Q.; Pavan, F.R. Nanostructured lipid systems as a strategy to improve the in vitro cytotoxicity of ruthenium(II) compounds. Molecules 2014, 19, 5999–6008. [Google Scholar] [CrossRef]
- Motta, B.P.; Pinheiro, C.G.; Figueiredo, I.D.; Cardoso, F.N.; Oliveira, J.O.; Machado, R.T.A.; da Silva, P.B.; Chorilli, M.; Brunetti, I.L.; Baviera, A.M. Combined Effects of Lycopene and Metformin on Decreasing Oxidative Stress by Triggering Endogenous Antioxidant Defenses in Diet-Induced Obese Mice. Molecules 2022, 27, 8503. [Google Scholar] [CrossRef]
- Zilin, S.; Naifeng, L.; Bicheng, L.; Jiping, W. The determination of AGE-peptides by flow injection assay, a practical marker of diabetic nephropathy. Clin. Chim. Acta 2001, 313, 69–75. [Google Scholar] [CrossRef]
- Pokupec, R.; Kalauz, M.; Turk, N.; Turk, Z. Advanced glycation endproducts in human diabetic and non-diabetic cataractous lenses. Graefe’s Arch. Clin. Exp. Ophthalmol. 2003, 241, 378–384. [Google Scholar] [CrossRef]
- Kohn, H.I.; Liversedge, M. On a new aerobic metabolite whose production by brain is inhibited by apomorphine, emetine, ergotamine, epinephrine, and menadione. J. Pharmacol. Exp. Ther. 1944, 82, 292–300. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Beers, R.F., Jr.; Sizer, I.W. A spectrophotometric method of measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Rush, J.W.; Sandiford, S.D. Plasma glutathione peroxidase in healthy young adults: Influence of gender and physical activity. Clin. Biochem. 2003, 36, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]

| Groups | C | H | V | M | B1 | B2 | MB1 | MB2 |
|---|---|---|---|---|---|---|---|---|
| Liver | 59.5 ± 1.0 | 64.1 ± 1.9 | 58.3 ± 2.2 | 57.4 ± 1.8 | 57.2 ± 1.6 | 57.5 ± 1.8 | 55.0 ± 1.8 b | 57.9 ± 2.2 |
| Kidneys | 9.2 ± 0.6 | 10.4 ± 0.7 | 9.7 ± 0.7 | 9.1 ± 0.6 | 9.4 ± 0.8 | 9.7 ± 0.7 | 9.1 ± 0.4 | 6.7 ± 0.3 |
| Heart | 7.0 ± 0.2 | 7.4 ± 0.1 | 7.1 ± 0.2 | 7.1 ± 0.2 | 7.3 ± 0.2 | 7.1 ± 0.2 | 7.1 ± 0.2 | 7.1 ± 0.2 |
| Muscles (gastrocnemius) | 8.1 ± 0.1 | 8.4 ± 0.2 | 8.1 ± 0.1 | 8.1 ± 0.1 | 8.1 ± 0.1 | 8.2 ± 0.1 | 8.1 ± 0.1 | 8.1 ± 0.1 |
| WAT (epididymal) | 26.7 ± 1.5 | 66.4 ± 5.3 a | 54.6 ± 3.8 a,b | 41.2 ± 5.0 b | 37.4 ± 2.9 b | 43.3 ± 4.0 b | 44.2 ± 4.8 a,b | 42.1 ± 3.2 b |
| BAT (interscapular) | 4.1 ± 0.2 | 3.8 ± 0.3 | 3.5 ± 0.2 | 3.0 ± 0.2 a | 2.9 ± 0.1 a | 3.2 ± 0.2 a | 3.2 ± 0.2 a | 3.1 ± 0.2 a |
| Groups | C | H | V | M | B1 | B2 | MB1 | MB2 |
|---|---|---|---|---|---|---|---|---|
| Triglycerides (mg/dL) | 53.4 ± 7.1 | 41.3 ± 8.4 a | 41.6 ± 5.5 a | 38.6 ± 10.1 a | 38.1 ± 8.0 a | 39.8 ± 5.3 a | 39.4 ± 10.5 a | 39.5 ± 6.2 a |
| Total Cholesterol (mg/dL) | 98.3 ± 3.3 | 135.6 ± 15.8 a | 131.2 ± 19.4 a | 107.3 ± 14.1 b | 113.3 ± 9.6 b | 112.3 ± 14.1 b | 108.5 ± 8.7 b | 116.0 ± 9.4 b |
| HDL-Cholesterol (mg/dL) | 79.3 ± 2.5 | 108.3 ± 15.1 a | 99.8 ± 7.5 a | 89.7 ± 14.4 b | 94.2 ± 2.8 | 94.0 ± 8.3 | 90.1 ± 6.6 b | 90.8 ± 12.2 b |
| Creatinine (mg/dL) | 0.16 ± 0.003 | 0.15 ± 0.002 | 0.16 ± 0.004 | 0.15 ± 0.003 | 0.16 ± 0.011 | 0.16 ± 0.003 | 0.16 ± 0.003 | 0.15 ± 0.003 |
| Uric Acid (mg/dL) | 0.4 ± 0.02 | 0.4 ± 0.02 | 0.3 ± 0.03 | 0.3 ± 0.03 | 0.4 ± 0.09 | 0.4 ± 0.04 | 0.4 ± 0.04 | 0.4 ± 0.07 |
| Albumin (g/dL) | 2.1 ± 0.02 | 2.1 ± 0.03 | 2.1 ± 0.03 | 2.1 ± 0.04 | 2.1 ± 0.03 | 2.1 ± 0.02 | 2.1 ± 0.03 | 2.1 ± 0.03 |
| ALT (U/L) | 24.8 ± 2.3 | 28.4 ± 4.4 | 27.3 ± 2.4 | 21.4 ± 2.4 | 24.4 ± 1.6 | 21.5 ± 3.1 | 24.1 ± 2.5 | 23.0 ± 4.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, C.G.; Motta, B.P.; Oliveira, J.O.; Cardoso, F.N.; Figueiredo, I.D.; Machado, R.T.A.; da Silva, P.B.; Chorilli, M.; Brunetti, I.L.; Baviera, A.M. Bixin Combined with Metformin Ameliorates Insulin Resistance and Antioxidant Defenses in Obese Mice. Pharmaceuticals 2024, 17, 1202. https://doi.org/10.3390/ph17091202
Pinheiro CG, Motta BP, Oliveira JO, Cardoso FN, Figueiredo ID, Machado RTA, da Silva PB, Chorilli M, Brunetti IL, Baviera AM. Bixin Combined with Metformin Ameliorates Insulin Resistance and Antioxidant Defenses in Obese Mice. Pharmaceuticals. 2024; 17(9):1202. https://doi.org/10.3390/ph17091202
Chicago/Turabian StylePinheiro, Camila Graça, Bruno Pereira Motta, Juliana Oriel Oliveira, Felipe Nunes Cardoso, Ingrid Delbone Figueiredo, Rachel Temperani Amaral Machado, Patrícia Bento da Silva, Marlus Chorilli, Iguatemy Lourenço Brunetti, and Amanda Martins Baviera. 2024. "Bixin Combined with Metformin Ameliorates Insulin Resistance and Antioxidant Defenses in Obese Mice" Pharmaceuticals 17, no. 9: 1202. https://doi.org/10.3390/ph17091202
APA StylePinheiro, C. G., Motta, B. P., Oliveira, J. O., Cardoso, F. N., Figueiredo, I. D., Machado, R. T. A., da Silva, P. B., Chorilli, M., Brunetti, I. L., & Baviera, A. M. (2024). Bixin Combined with Metformin Ameliorates Insulin Resistance and Antioxidant Defenses in Obese Mice. Pharmaceuticals, 17(9), 1202. https://doi.org/10.3390/ph17091202








