Synthesis and Antiproliferative Effect of 3,4,5-Trimethoxylated Chalcones on Colorectal and Prostatic Cancer Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Activity
2.2.1. Effect of Chalcones on Cell Viability
2.2.2. Effect of Chalcones on Cellular Microtubules
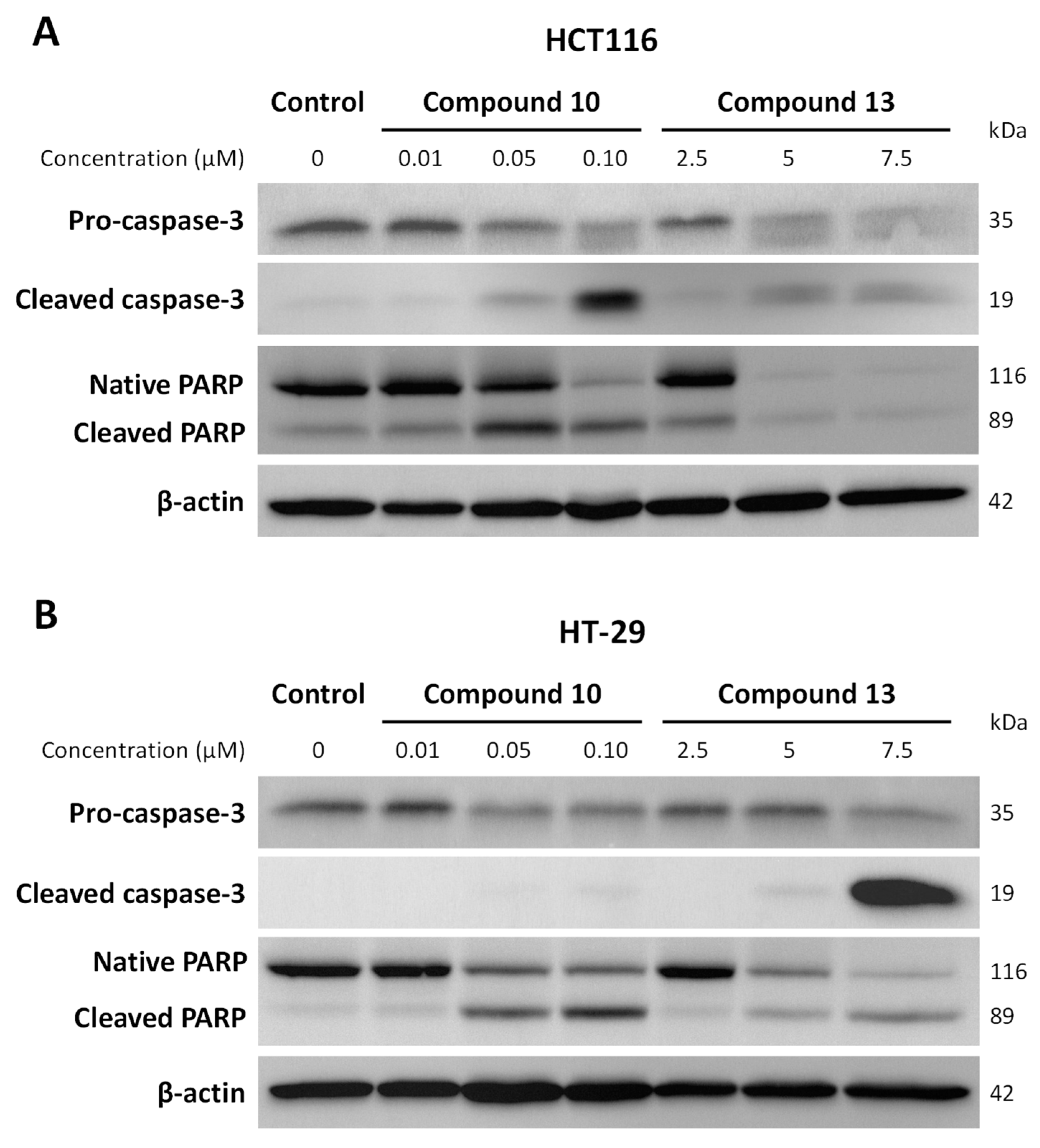
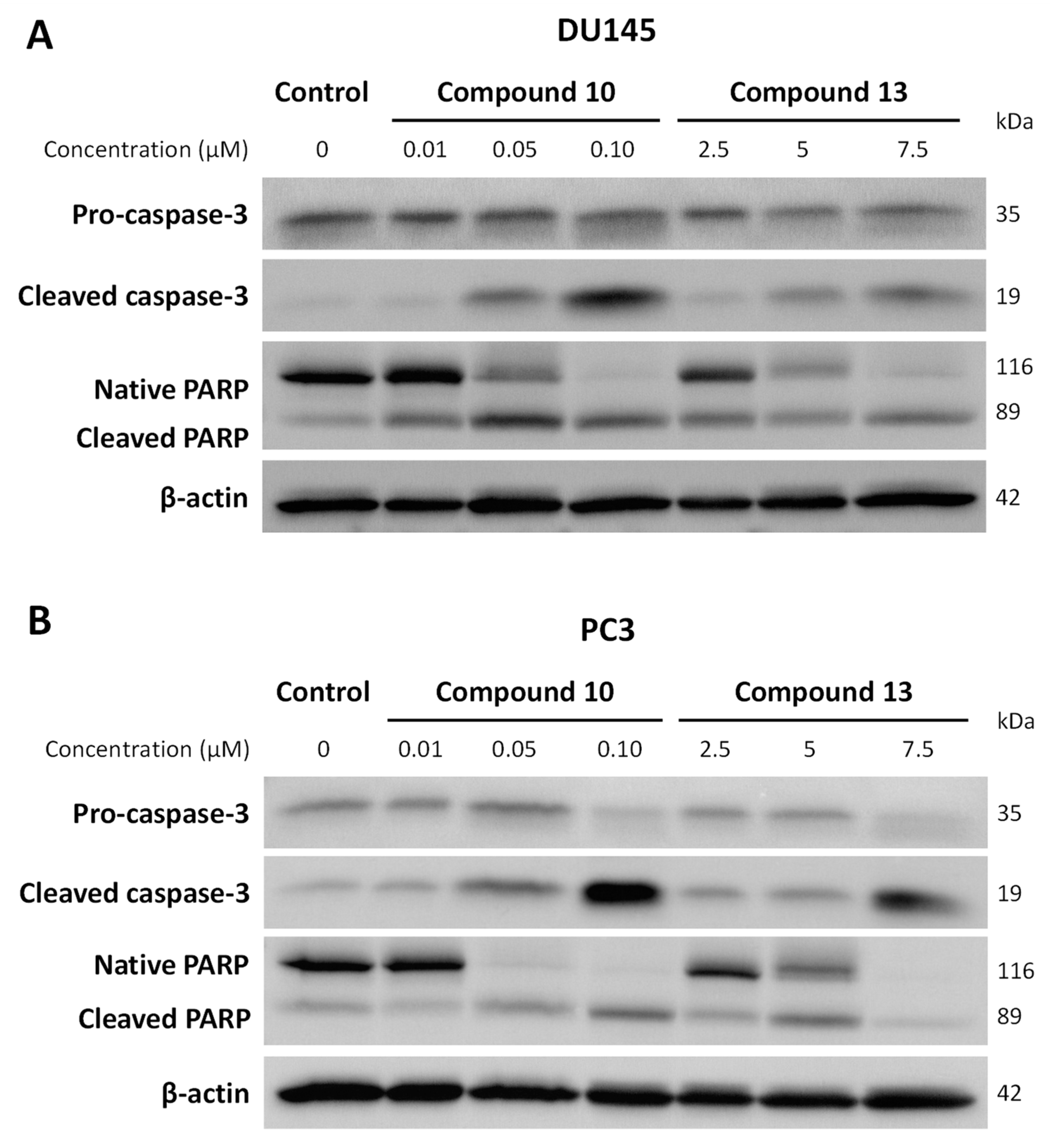
2.2.3. Effect of Chalcones on Apoptosis-Related Proteins Expression
2.3. Structure–Activity Relationship Study
3. Materials and Methods
3.1. Chemical Synthesis
3.1.1. General Chemistry
3.1.2. General Procedure for the Synthesis of Chalcones 1–11 in Basic Conditions


3.1.3. General Procedure for the Synthesis of Chalcones 3 and 9 Using a Protection Step
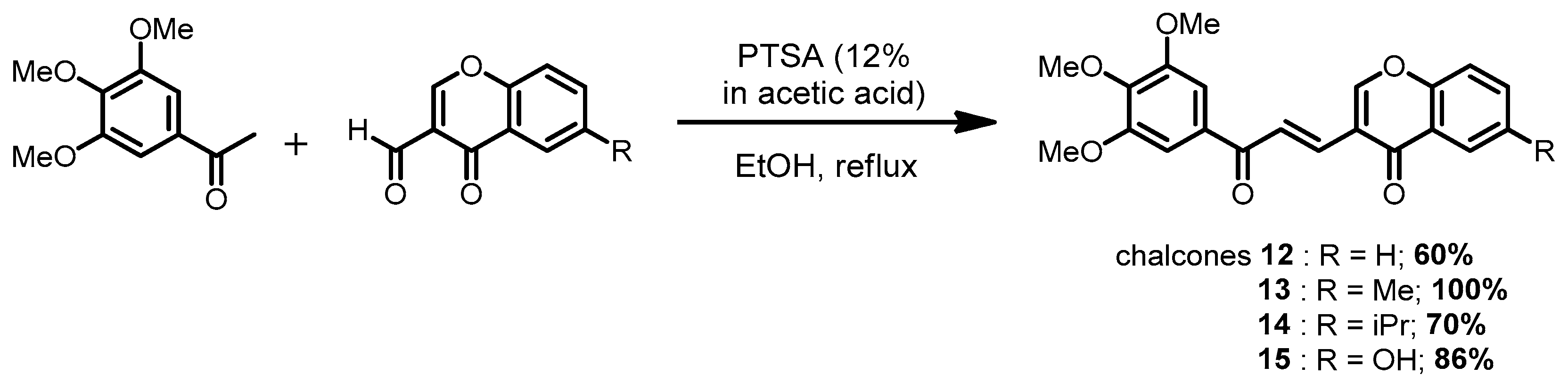
3.1.4. General Procedure for the Synthesis of Chalcones 12–15 in Acidic Conditions
3.2. In Vitro Biological Assays
3.2.1. Materials
3.2.2. Cell Lines and Culture Conditions
3.2.3. Cell Viability
- (a)
- MTT assay: The antiproliferative effect of all compounds was determined using MTT assay (a metabolic activity test). Briefly, cells were seeded in 96-well culture plates at 4 × 103 cells/well for HCT116, PC3, and DU145 cells and 6 × 103 cells/well for HT-29 cells (100 µL/well), and grown for 24 h in culture medium prior to exposure or not to obtain compounds 1–15 with concentrations ranging from 5 nM to 50 μM. After 24 and 48 h of treatment, MTT (5 mg/mL in PBS) was added and incubated for another 3 h. MTT was then removed from the wells before adding 100 μL/well of DMSO. The optical density was detected with a microplate reader (Multiskan FC, Thermoscientific, Bordeaux, France) at 550 nm and cell viability was expressed as a percentage of each treatment condition compared to control cells. IC50 values were calculated for all compounds from the dose–response curve. Data are expressed as the arithmetic means ± standard error of the mean (SEM) of separate experiments. All experiments were performed at least in triplicate.
- (b)
- Trypan blue dye exclusion method: Cells were grown for 24 h then treated with compounds 10 and 13 at indicated concentrations. After 48 h of treatment, cells were trypsinized and resuspended in complete medium. Each sample was mixed with Trypan blue solution (0.14% in HBSS, Invitrogen-GIBCO, ThermoFisher, Illkirch, France). Colored (non-viable) and dye-excluding (viable) cells were counted with the LUNA-II automated cell Counter (Logos Biosystems; MC2, Aubière, France). The control was normalized to 100% including viable and dead cells. The results indicated on the histograms were expressed as the percentage of cells compared to the control. IC50 values were determined from the percentages of viable cells compared to the control.
3.2.4. Quantitative Assay of the Cellular Microtubule Content
3.2.5. Immunofluorescence
3.2.6. Protein Extraction and Western Blot Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Semaan, J.; Pinon, A.; Rioux, B.; Hassan, L.; Limami, Y.; Pouget, C.; Fagnère, C.; Sol, V.; Diab-Assaf, M.; Simon, A.; et al. Resistance to 3-HTMC-Induced Apoptosis Through Activation of PI3K/Akt, MEK/ERK, and P38/COX-2/PGE2 Pathways in Human HT-29 and HCT116 Colorectal Cancer Cells. J. Cell. Biochem. 2016, 117, 2875–2885. [Google Scholar] [CrossRef] [PubMed]
- Rioux, B.; Pinon, A.; Gamond, A.; Martin, F.; Laurent, A.; Champavier, Y.; Barette, C.; Liagre, B.; Fagnère, C.; Sol, V.; et al. Synthesis and Biological Evaluation of Chalcone-Polyamine Conjugates as Novel Vectorized Agents in Colorectal and Prostate Cancer Chemotherapy. Eur. J. Med. Chem. 2021, 222, 113586. [Google Scholar] [CrossRef] [PubMed]
- Rioux, B.; Pouget, C.; Fidanzi-Dugas, C.; Gamond, A.; Laurent, A.; Semaan, J.; Pinon, A.; Champavier, Y.; Léger, D.Y.; Liagre, B.; et al. Design and Multi-Step Synthesis of Chalcone-Polyamine Conjugates as Potent Antiproliferative Agents. Bioorg. Med. Chem. Lett. 2017, 27, 4354–4357. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef]
- Syam, S.; Abdelwahab, S.I.; Al-Mamary, M.A.; Mohan, S. Synthesis of Chalcones with Anticancer Activities. Molecules 2012, 17, 6179–6195. [Google Scholar] [CrossRef]
- Mittal, A.; Vashistha, V.K.; Das, D.K. Recent Advances in the Antioxidant Activity and Mechanisms of Chalcone Derivatives: A Computational Review. Free Radic. Res. 2022, 56, 378–397. [Google Scholar] [CrossRef]
- Vijaya Bhaskar Reddy, M.; Hung, H.-Y.; Kuo, P.-C.; Huang, G.-J.; Chan, Y.-Y.; Huang, S.-C.; Wu, S.-J.; Morris-Natschke, S.L.; Lee, K.-H.; Wu, T.-S. Synthesis and Biological Evaluation of Chalcone, Dihydrochalcone, and 1,3-Diarylpropane Analogs as Anti-Inflammatory Agents. Bioorg. Med. Chem. Lett. 2017, 27, 1547–1550. [Google Scholar] [CrossRef]
- Lagu, S.B.; Yejella, R.P.; Bhandare, R.R.; Shaik, A.B. Design, Synthesis, and Antibacterial and Antifungal Activities of Novel Trifluoromethyl and Trifluoromethoxy Substituted Chalcone Derivatives. Pharmaceuticals 2020, 13, 375. [Google Scholar] [CrossRef]
- Elkhalifa, D.; Al-Hashimi, I.; Al Moustafa, A.-E.; Khalil, A. A Comprehensive Review on the Antiviral Activities of Chalcones. J. Drug Target. 2021, 29, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; Loureiro, J.B.; Correia, D.; Palmeira, A.; Pinto, M.M.; Saraiva, L.; Cidade, H. Structure–Activity Relationship Studies of Chalcones and Diarylpentanoids with Antitumor Activity: Potency and Selectivity Optimization. Pharmaceuticals 2023, 16, 1354. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Lee, E.; Baek, K.H.; Kwon, H.B.; Woo, H.; Lee, E.-S.; Kwon, Y.; Na, Y. Chalcones, Inhibitors for Topoisomerase I and Cathepsin B and L, as Potential Anti-Cancer Agents. Bioorg. Med. Chem. Lett. 2013, 23, 3320–3324. [Google Scholar] [CrossRef]
- WalyEldeen, A.A.; Sabet, S.; El-Shorbagy, H.M.; Abdelhamid, I.A.; Ibrahim, S.A. Chalcones: Promising Therapeutic Agents Targeting Key Players and Signaling Pathways Regulating the Hallmarks of Cancer. Chem. Biol. Interact. 2023, 369, 110297. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, T.; Lungu, C.N. Anticancer Activity of Natural and Synthetic Chalcones. Int. J. Mol. Sci. 2021, 22, 11306. [Google Scholar] [CrossRef]
- Ducki, S.; Rennison, D.; Woo, M.; Kendall, A.; Chabert, J.F.D.; McGown, A.T.; Lawrence, N.J. Combretastatin-like Chalcones as Inhibitors of Microtubule Polymerization. Part 1: Synthesis and Biological Evaluation of Antivascular Activity. Bioorg. Med. Chem. 2009, 17, 7698–7710. [Google Scholar] [CrossRef]
- Boumendjel, A.; McLeer-Forin, A.; Champelovier, P.; Allegro, D.; Muhammad, D.; Souard, F.; Derouazi, M.; Peyrot, V.; Toussaint, B.; Boutonnat, J. A Novel Chalcone Derivative Which Acts as a Microtubule Depolymerising Agent and an Inhibitor of P-Gp and BCRP in in-Vitro and in-Vivo Glioblastoma Models. BMC Cancer 2009, 9, 242. [Google Scholar] [CrossRef]
- Ducki, S.; Forrest, R.; Hadfield, J.A.; Kendall, A.; Lawrence, N.J.; McGown, A.T.; Rennison, D. Potent Antimitotic and Cell Growth Inhibitory Properties of Substituted Chalcones. Bioorg. Med. Chem. Lett. 1998, 8, 1051–1056. [Google Scholar] [CrossRef]
- Sunil, D.; Kamath, P.R. Indole Based Tubulin Polymerization Inhibitors: An Update on Recent Developments. Mini-Rev. Med. Chem. 2016, 16, 1470–1499. [Google Scholar] [CrossRef]
- Hong, Y.; Zhu, Y.-Y.; He, Q.; Gu, S.-X. Indole Derivatives as Tubulin Polymerization Inhibitors for the Development of Promising Anticancer Agents. Bioorg. Med. Chem. 2022, 55, 116597. [Google Scholar] [CrossRef]
- Liu, X.; Jin, J.; Wu, Y.; Du, B.; Zhang, L.; Lu, D.; Liu, Y.; Chen, X.; Lin, J.; Chen, H.; et al. Fluoroindole Chalcone Analogues Targeting the Colchicine Binding Site of Tubulin for Colorectal Oncotherapy. Eur. J. Med. Chem. 2023, 257, 115540. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Z.N.; Praveen, S.; Musthafa, T.N.M.; Ahmad, A.; Khan, A.U. Thermal Solvent-Free Synthesis of Chromonyl Chalcones, Pyrazolines and Their in Vitro Antibacterial, Antifungal Activities. J. Enzym. Inhib. Med. Chem. 2012, 27, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Butora, G.; Reed, J.W.; Hudlicky, T.; Brammer, L.E.; Higgs, P.I.; Simmons, D.P.; Heard, N.E. Electrochemical versus Anionic Oxygenation of Azathymine Derivatives. J. Am. Chem. Soc. 1997, 119, 7694–7701. [Google Scholar] [CrossRef]
- Nohara, A.; Umetani, T.; Sanno, Y. Studies on Antianaphylactic Agents—I: A Facile Synthesis of 4-Oxo-4H-1-Benzopyran-3-Carboxaldehydes by Vilsmeier Reagents. Tetrahedron 1974, 30, 3553–3561. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, N.M.; Akamatsu, K.; Kusaka, E.; Harada, H.; Ito, T. Synthesis and Biological Evaluation of Indolyl Chalcones as Antitumor Agents. Bioorg. Med. Chem. Lett. 2010, 20, 3916–3919. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, M.; Huang, R.; Hu, T.; Jing, Y.; Huang, X.; Hu, W.; Cao, G.; Wang, H. Novel Indole–Chalcone Derivative-Ligated Platinum(IV) Prodrugs Attenuate Cisplatin Resistance in Lung Cancer through ROS/ER Stress and Mitochondrial Dysfunction. J. Med. Chem. 2023, 66, 4868–4887. [Google Scholar] [CrossRef]
- Laisne, M.-C.; Michallet, S.; Lafanechère, L. Characterization of Microtubule Destabilizing Drugs: A Quantitative Cell-Based Assay That Bridges the Gap between Tubulin Based- and Cytotoxicity Assays. Cancers 2021, 13, 5226. [Google Scholar] [CrossRef]
- Rioux, B.; Pouget, C.; Ndong-Ntoutoume, G.M.A.; Granet, R.; Gamond, A.; Laurent, A.; Pinon, A.; Champavier, Y.; Liagre, B.; Fagnère, C.; et al. Enhancement of Hydrosolubility and in Vitro Antiproliferative Properties of Chalcones Following Encapsulation into β-Cyclodextrin/Cellulose-Nanocrystal Complexes. Bioorg. Med. Chem. Lett. 2019, 29, 1895–1898. [Google Scholar] [CrossRef]




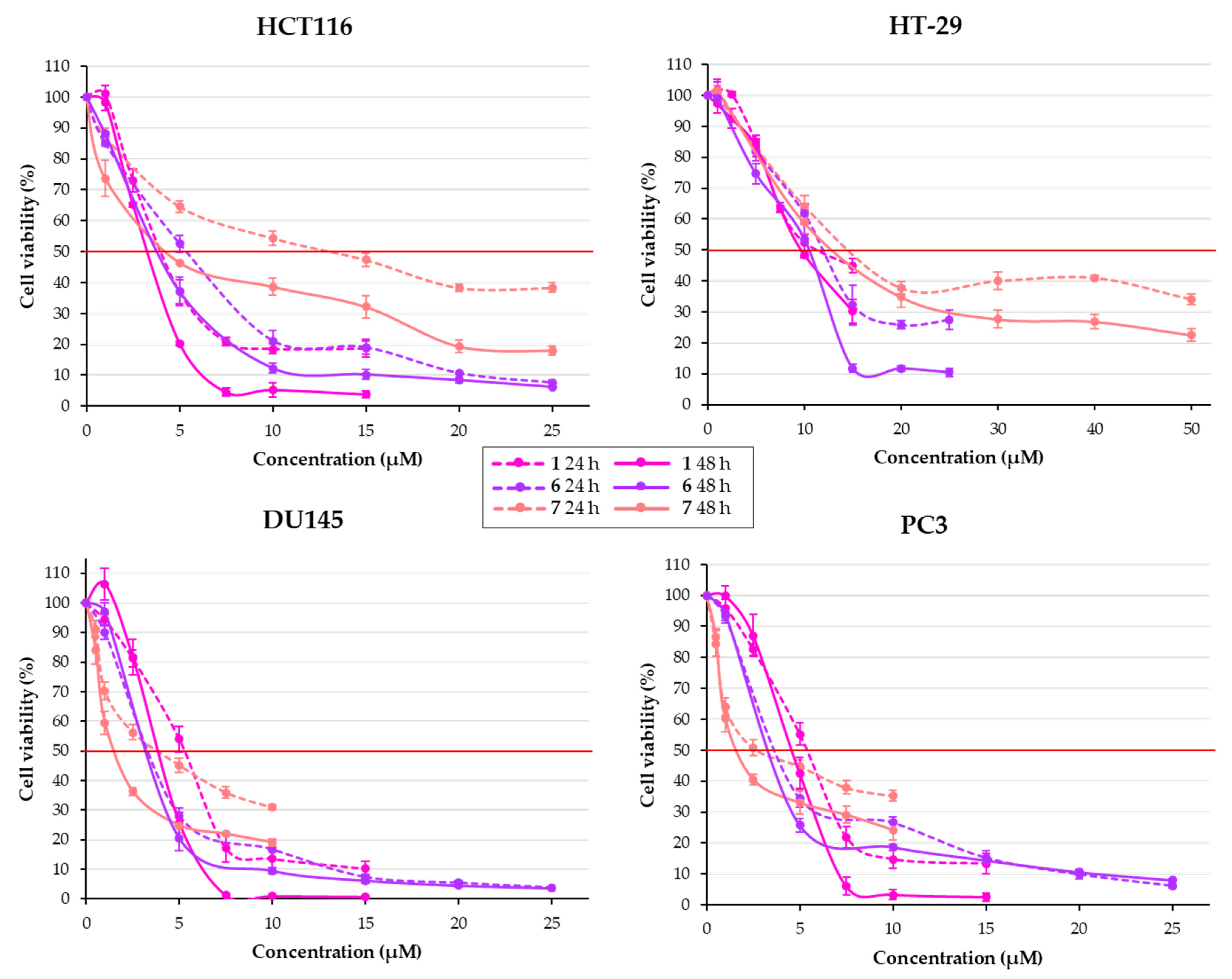
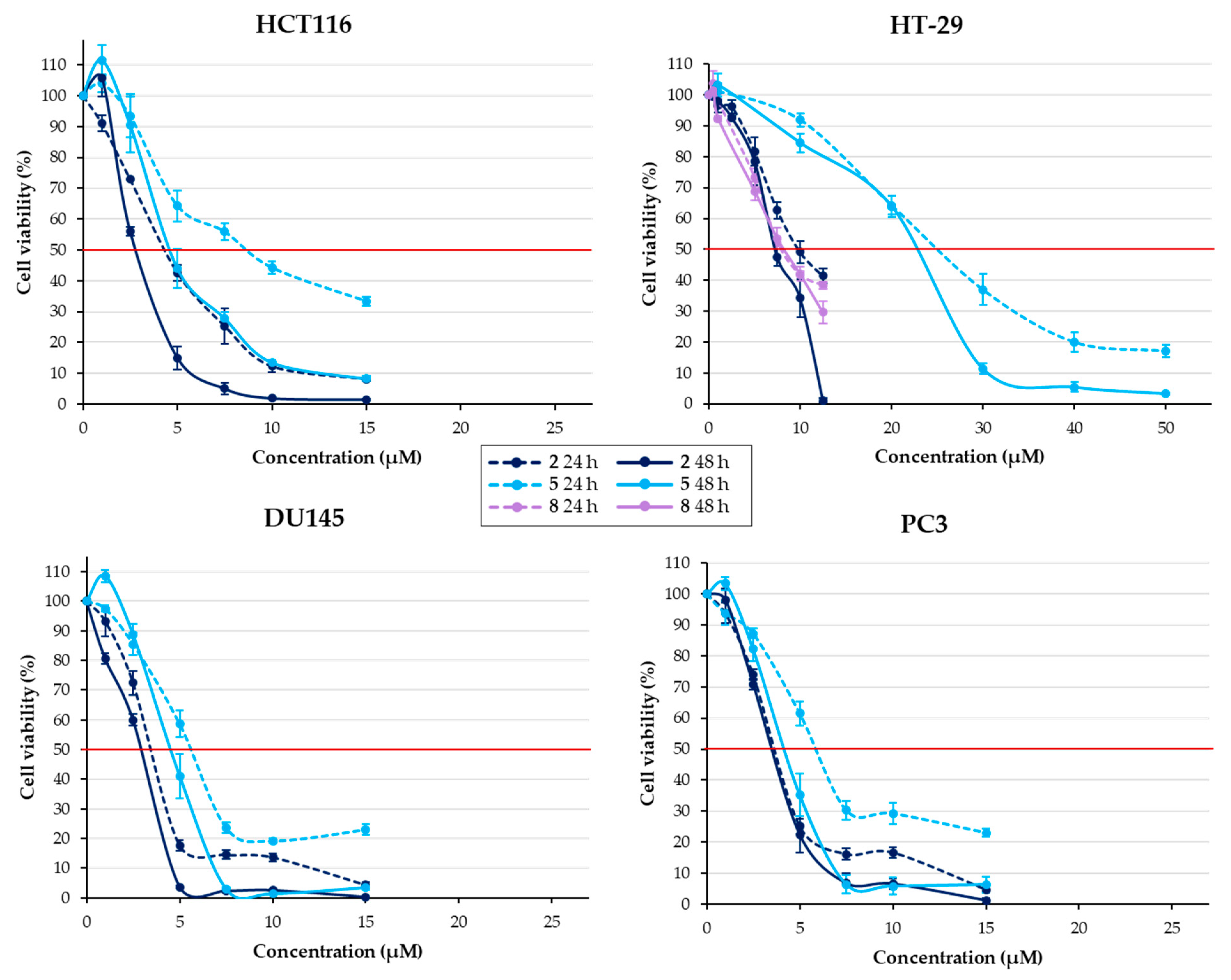
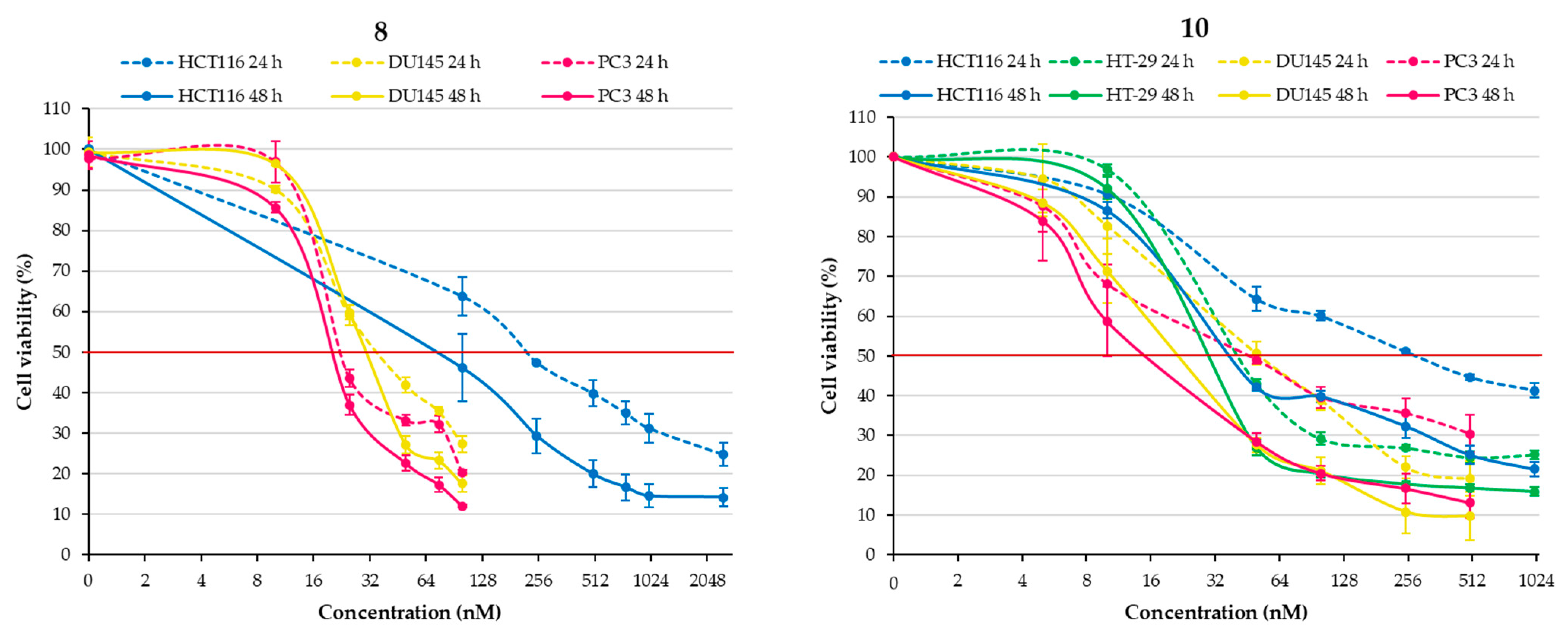
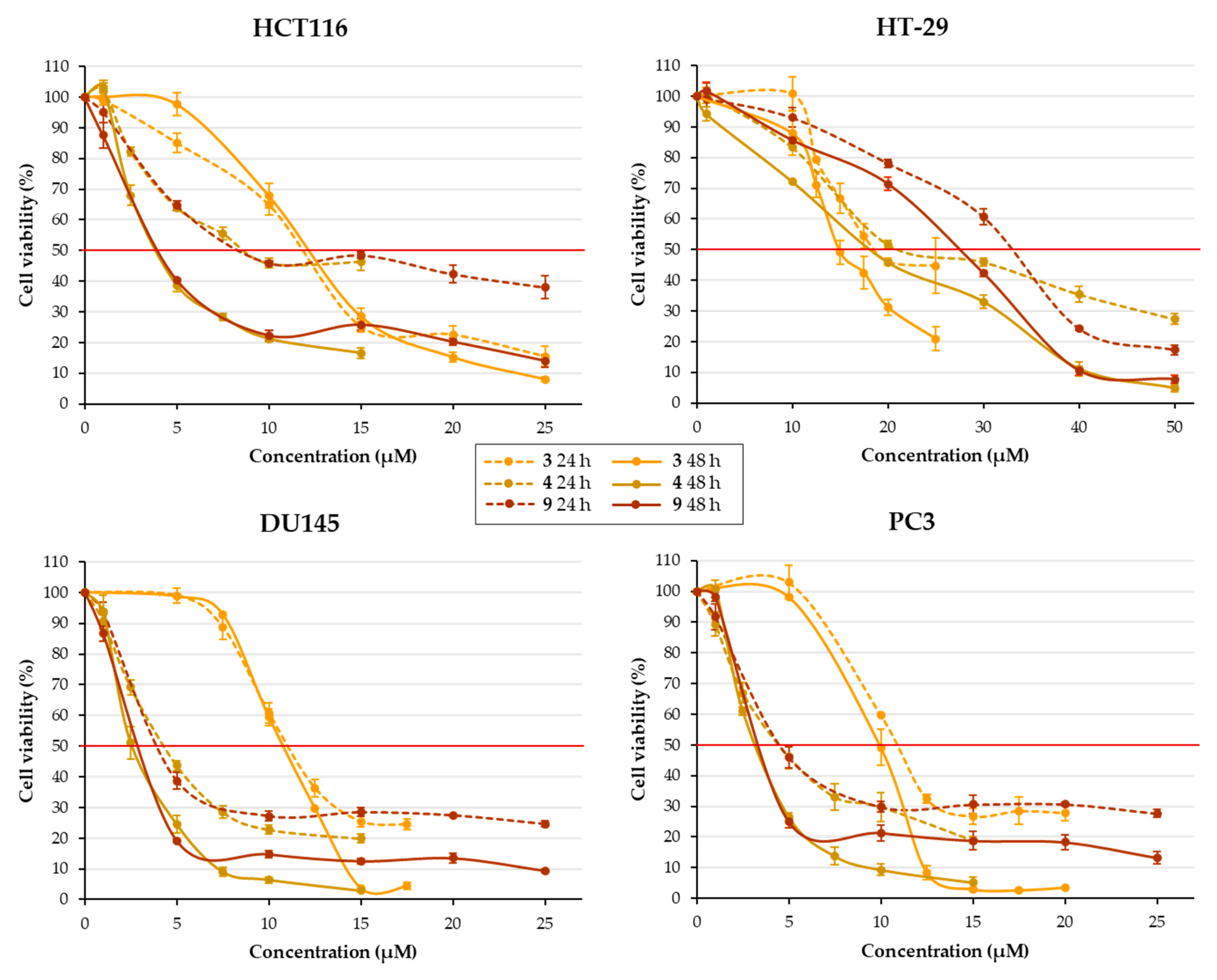



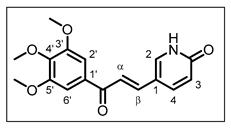
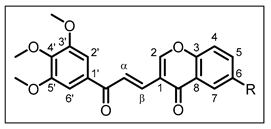
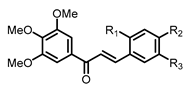
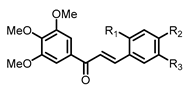
| Structure | Substitution | n° | HCT116 | HT-29 | ||||
|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | 24 h | 48 h | 24 h | 48 h | ||
 | OH | H | H | 1 | 4.54 ± 0.65 | 3.27 ± 0.11 | 10.83 ± 0.57 | 9.65 ± 0.12 |
| H | H | OH | 2 | 4.23 ± 0.11 | 2.66 ± 0.10 | 9.87 ± 0.44 | 7.34 ± 0.22 | |
| H | OH | H | 3 | 11.62 ± 0.06 | 12.14 ± 0.44 | 17.91 ± 0.69 | 15.10 ± 0.46 | |
| OMe | OH | H | 4 | 8.79 ± 0.39 | 3.98 ± 0.22 | 21.26 ± 0.94 | 17.71 ± 0.29 | |
| OMe | H | OH | 5 | 8.72 ± 0.41 | 4.92 ± 0.49 | 25.19 ± 1.28 | 22.11 ± 0.48 | |
| OH | H | OMe | 6 | 5.27 ± 0.37 | 3.44 ± 0.42 | 12.31 ± 0.37 | 10.55 ± 0.62 | |
| OH | OMe | H | 7 | 13.38 ± 0.21 | 3.99 ± 0.16 | 14.43 ± 0.65 | 11.37 ± 0.68 | |
| H | OMe | OH | 8 | 0.22 ± 0.01 | 0.11 ± 0.02 | 8.03 ± 0.18 | 8.11 ± 0.50 | |
| H | OH | OMe | 9 | 8.58 ± 0.28 | 3.48 ± 0.19 | 32.60 ± 0.51 | 26.79 ± 0.40 | |
 | / | / | / | 10 | 0.282 ± 0.012 | 0.039 ± 0.001 | 0.041 ± 0.001 | 0.028 ± 0.001 |
 | / | / | / | 11 | 25.18 ± 0.48 | 16.58 ± 0.79 | 35.56 ± 0.94 | 23.61 ± 0.37 |
| Structure | Substitution | n° | DU145 | PC3 | ||||
|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | 24 h | 48 h | 24 h | 48 h | ||
 | OH | H | H | 1 | 5.33 ± 0.42 | 3.60 ± 0.10 | 5.04 ± 0.29 | 4.23 ± 0.35 |
| H | H | OH | 2 | 3.47 ± 0.24 | 2.97 ± 0.15 | 3.76 ± 0.28 | 3.44 ± 0.11 | |
| H | OH | H | 3 | 11.26 ± 0.48 | 11.16 ± 0.44 | 10.94 ± 0.10 | 9.85 ± 0.43 | |
| OMe | OH | H | 4 | 4.20 ± 0.20 | 2.69 ± 0.22 | 4.80 ± 0.48 | 3.05 ± 0.12 | |
| OMe | H | OH | 5 | 5.50 ± 0.26 | 4.37 ± 0.30 | 5.54 ± 0.31 | 4.00 ± 0.30 | |
| OH | H | OMe | 6 | 2.84 ± 0.14 | 2.70 ± 0.10 | 3.31 ± 0.22 | 2.81 ± 0.16 | |
| OH | OMe | H | 7 | 2.99 ± 0.33 | 1.33 ± 0.16 | 2.57 ± 0.44 | 1.47 ± 0.27 | |
| H | OMe | OH | 8 | 0.036 ± 0.002 | 0.031 ± 0.001 | 0.021 ± 0.001 | 0.020 ± 0.001 | |
| H | OH | OMe | 9 | 3.75 ± 0.15 | 2.41 ± 0.08 | 4.42 ± 0.24 | 2.97 ± 0.13 | |
 | / | / | / | 10 | 0.051 ± 0.006 | 0.020 ± 0.002 | 0.044 ± 0.006 | 0.017 ± 0.005 |
 | / | / | / | 11 | 22.30 ± 0.76 | 15.60 ± 0.84 | 24.47 ± 0.64 | 17.72 ± 0.55 |
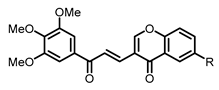
| Structure | Substitution | n° | HCT116 | HT-29 | ||
|---|---|---|---|---|---|---|
| R | 24 h | 48 h | 24 h | 48 h | ||
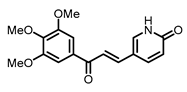
 | H | 12 | 14.20 ± 0.41 | 13.15 ± 0.40 | 13.59 ± 0.30 | 13.25 ± 0.42 |
| Me | 13 | 2.86 ± 0.20 | 2.59 ± 0.12 | 3.88 ± 0.08 | 3.10 ± 0.17 | |
| iPr | 14 | 15.28 ± 0.52 | 13.33 ± 0.30 | 22.86 ± 0.72 | 17.00 ± 1.41 | |
| OH | 15 | 5.84 ± 0.28 | 14.06 ± 0.27 | > 50 | > 50 | |
| Structure | Substitution | n° | DU145 | PC3 | ||
|---|---|---|---|---|---|---|
| R | 24 h | 48 h | 24 h | 48 h | ||
 | H | 12 | 10.76 ± 0.34 | 11.74 ± 0.29 | 11.52 ± 0.28 | 12.17 ± 0.23 |
| Me | 13 | 3.92 ± 0.24 | 3.31 ± 0.09 | 5.18 ± 0.39 | 5.13 ± 0.25 | |
| iPr | 14 | 13.18 ± 0.49 | 12.42 ± 0.20 | 14.11 ± 0.04 | 12.81 ± 0.37 | |
| OH | 15 | 3.88 ± 0.32 | 7.09 ± 0.52 | 6.48 ± 0.40 | 7.60 ± 0.44 | |
| Cell Lines | Compound n° | MTT | Trypan Blue Test |
|---|---|---|---|
| HCT116 | 10 | 0.039 | 0.043 |
| 13 | 2.59 | 3.48 | |
| HT-29 | 10 | 0.028 | 0.021 |
| 13 | 3.10 | 4.05 | |
| DU145 | 10 | 0.020 | 0.040 |
| 13 | 3.31 | 3.98 | |
| PC3 | 10 | 0.017 | 0.032 |
| 13 | 5.13 | 5.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Letulle, C.; Toublet, F.-X.; Pinon, A.; Hba, S.; Laurent, A.; Sol, V.; Fagnère, C.; Rioux, B.; Allais, F.; Michallet, S.; et al. Synthesis and Antiproliferative Effect of 3,4,5-Trimethoxylated Chalcones on Colorectal and Prostatic Cancer Cells. Pharmaceuticals 2024, 17, 1207. https://doi.org/10.3390/ph17091207
Letulle C, Toublet F-X, Pinon A, Hba S, Laurent A, Sol V, Fagnère C, Rioux B, Allais F, Michallet S, et al. Synthesis and Antiproliferative Effect of 3,4,5-Trimethoxylated Chalcones on Colorectal and Prostatic Cancer Cells. Pharmaceuticals. 2024; 17(9):1207. https://doi.org/10.3390/ph17091207
Chicago/Turabian StyleLetulle, Cécile, François-Xavier Toublet, Aline Pinon, Soufyane Hba, Aurélie Laurent, Vincent Sol, Catherine Fagnère, Benjamin Rioux, Florent Allais, Sophie Michallet, and et al. 2024. "Synthesis and Antiproliferative Effect of 3,4,5-Trimethoxylated Chalcones on Colorectal and Prostatic Cancer Cells" Pharmaceuticals 17, no. 9: 1207. https://doi.org/10.3390/ph17091207
APA StyleLetulle, C., Toublet, F.-X., Pinon, A., Hba, S., Laurent, A., Sol, V., Fagnère, C., Rioux, B., Allais, F., Michallet, S., Lafanechère, L., Limami, Y., Oudghiri, M., Othman, M., Daïch, A., Liagre, B., Lawson, A. M., & Pouget, C. (2024). Synthesis and Antiproliferative Effect of 3,4,5-Trimethoxylated Chalcones on Colorectal and Prostatic Cancer Cells. Pharmaceuticals, 17(9), 1207. https://doi.org/10.3390/ph17091207









