The Impact of Silver Nanoparticles Functionalized with Spirulina Protein Extract on Rats
Abstract
:1. Introduction
2. Results
2.1. Composition of Spirulina Protein Extract (SPE)
2.2. Characteristics of Silver Nanoparticles Biofunctionalized with Spirulina Protein Extract
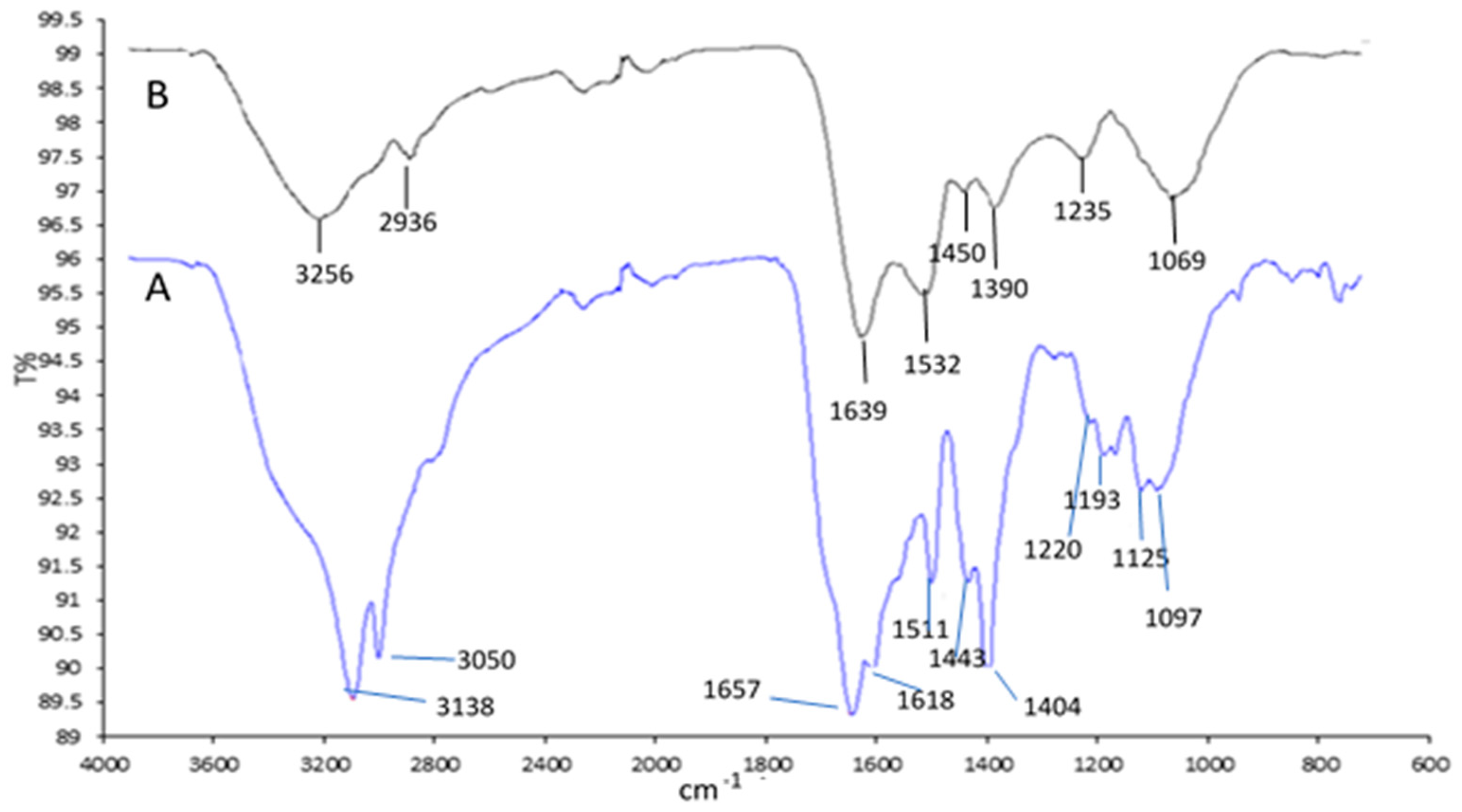
2.2.1. FTIR Spectra
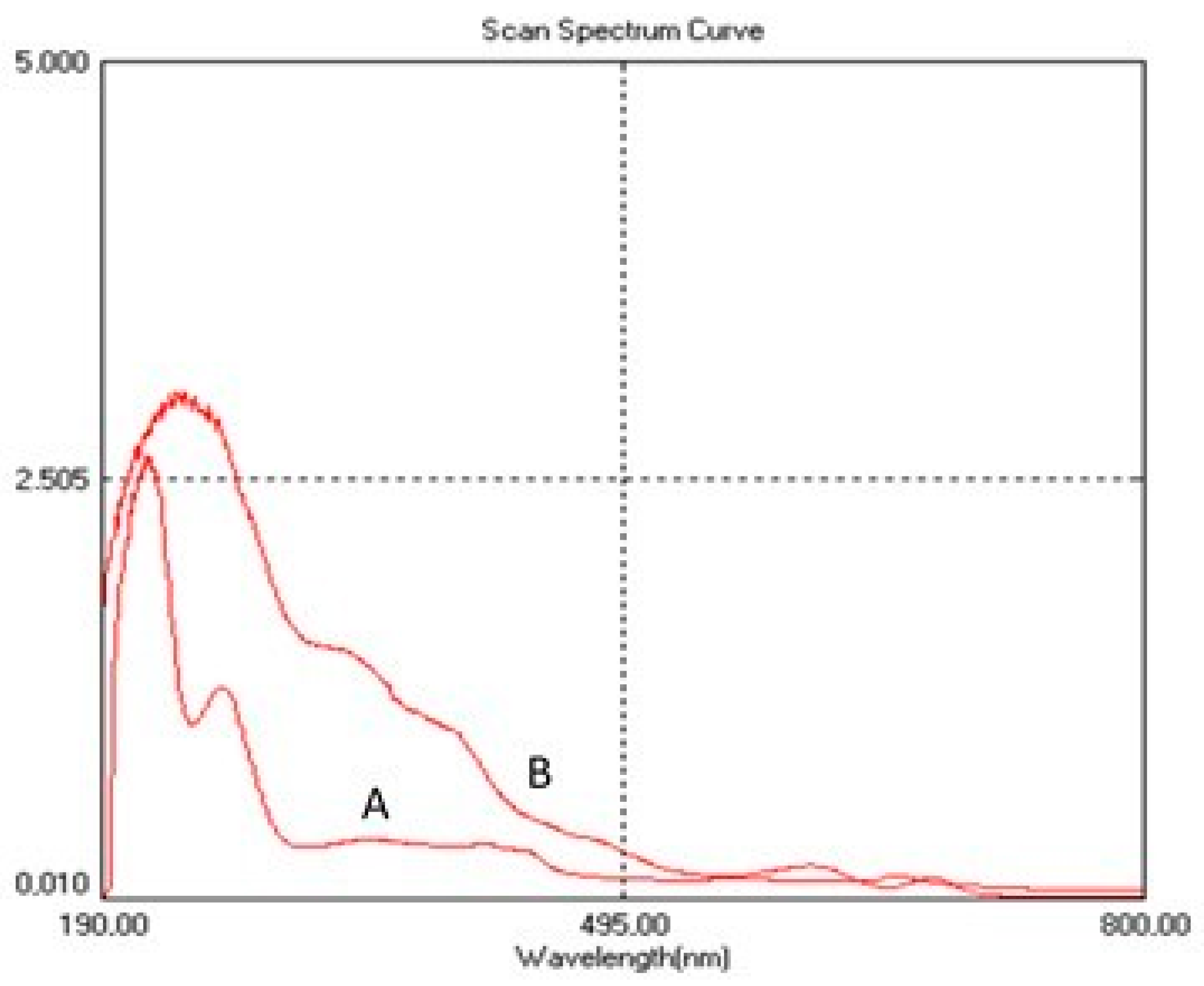
2.2.2. UV-VIS
2.2.3. Antioxidant Activity Stability of SPE and AgNPs-SPE
2.3. Silver Accumulation in Animal Organs
2.4. Hematological and Biochemical Indicators in Animals
2.4.1. Hematological Indicators
2.4.2. Biochemical Indicators
2.4.3. Hematological and Biochemical Indices in Animals after 28 Days of AgNPs Administration and Following the 28-Day Clearance Period
3. Discussion
4. Materials and Methods
4.1. Preparation of Spirulina Protein Extract
4.2. Functionalization of AgNPs with the Formation of the AgNPs-SPE Functional Mixture
4.3. Animals and Experimental Design
4.4. Methods for Characterizing AgNPs-SPE and Detecting Silver
4.4.1. UV-Vis Spectra Recording
4.4.2. Fourier Transform Infrared (FTIR) Analysis
4.4.3. ICP-MS Analysis
4.4.4. Determination of Antioxidant Activity
4.5. Blood Hematology and Biochemistry
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calderón-Jiménez, B.; Johnson, M.E.; Montoro Bustos, A.R.; Murphy, K.E.; Winchester, M.R.; Vega Baudrit, J.R. Silver Nanoparticles: Technological Advances, Societal Impacts, and Metrological Challenges. Front. Chem. 2017, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Keat, C.L.; Aziz, A.; Eid, A.M.; Elmarzugi, N.A. Biosynthesis of nanoparticles and silver nanoparticles. Bioresour. Bioprocess. 2015, 2, 47. [Google Scholar] [CrossRef]
- Liu, S.; Hu, M.; Liu, X.; Liu, X.; Chen, T.; Zhu, Y.; Liang, T.; Xiao, S.; Li, P.; Ma, X. Nanoparticles and Antiviral Vaccines. Vaccines 2024, 12, 30. [Google Scholar] [CrossRef]
- Jangid, H.; Singh, S.; Kashyap, P.; Singh, A.; Kumar, G. Advancing biomedical applications: An in-depth analysis of silver nanoparticles in antimicrobial, anticancer, and wound healing roles. Front. Pharmacol. 2024, 15, 1438227. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Tian, J.; Wong, K.K.; Ho, C.M.; Lok, C.N.; Yu, W.Y.; Che, C.M.; Chiu, J.F.; Tam, P.K. Topical Delivery of Silver Nanoparticles Promotes Wound Healing. ChemMedChem Chem. Enabling Drug Discov. 2007, 2, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.R.; Sampaio, I.; Zucolotto, V. Exploring silver nanoparticles for cancer therapy and diagnosis. Colloids Surf. B Biointerfaces 2022, 210, 112254. [Google Scholar] [CrossRef]
- Takáč, P.; Michalková, R.; Čižmáriková, M.; Bedlovičová, Z.; Balážová, Ľ.; Takáčová, G. The Role of Silver Nanoparticles in the Diagnosis and Treatment of Cancer: Are There Any Perspectives for the Future? Life 2023, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Burdus, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Borowik, A.; Butowska, K.; Konkel, K.; Banasiuk, R.; Derewonko, N.; Wyrzykowski, D.; Davydenko, M.; Cherepanov, V.; Styopkin, V.; Prylutskyy, Y.; et al. The Impact of Surface Functionalization on the Biophysical Properties of Silver Nanoparticles. Nanomaterials 2019, 9, 973. [Google Scholar] [CrossRef]
- Hassanen, E.I.; Khalaf, A.A.; Tohamy, A.F.; Mohammed, E.R.; Farroh, K.Y. Toxicopathological and immunological studies on different concentrations of chitosan-coated silver nanoparticles in rats. Int. J. Nanomed. 2019, 14, 4723–4739. [Google Scholar] [CrossRef] [PubMed]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nanotoday 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Ravindran, A.; Chandran, P.; Khan, S.S. Biofunctionalized silver nanoparticles: Advances and prospects. Colloids Surf. B Biointerfaces 2013, 105, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.A.; Abdullah, M.A. Biosynthesis, Mechanisms, and Biomedical Applications of Silver Nanoparticles. In Functional Bionanomaterials—Nanotechnology in the Life Sciences; Thangadurai, D., Sangeetha, J., Prasad, R., Eds.; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Dinparvar, S.; Bagirova, M.; Allahverdiyev, A.M.; Abamor, E.S.; Safarov, T.; Aydogdu, M.; Aktas, D.J. A nanotechnology-based new approach in the treatment of breast cancer: Biosynthesized silver nanoparticles using Cuminum cyminum L. seed extract. Photochem. Photobiol. B 2020, 208, 111902. [Google Scholar] [CrossRef]
- Gul, A.; Baig, M.N.; Ahmed, D.; Najam, Z.; Aslam, T.; Ali, S. Green synthesis of silver nanoparticles from Spirulina platensis extract: Antibacterial and antioxidant potential. BioNanoScience 2024. [Google Scholar] [CrossRef]
- Alwhibi, M.S.; Soliman, D.A.; Awad, M.A.; Alangery, A.B.; Al Dehaish, H.; Alwasel, Y.A. Green synthesis of silver nanoparticles: Characterization and its potential biomedical applications. Green Process. Synth. 2021, 10, 412–420. [Google Scholar] [CrossRef]
- Hou, T.; Guo, Y.; Han, W.; Zhou, Y.; Netala, V.R.; Li, H.; Li, H.; Zhang, Z. Exploring the Biomedical Applications of Biosynthesized Silver Nanoparticles Using Perilla frutescens Flavonoid Extract: Antibacterial, Antioxidant, and Cell Toxicity Properties against Colon Cancer Cells. Molecules 2023, 28, 6431. [Google Scholar] [CrossRef]
- Torabian, F.; Rezayat, A.A.; Nour, M.G.; Ghorbanzadeh, A.; Najafi, S.; Sahebkar, A.; Sabouri, Z.; Darroudi, M. Administration of Silver Nanoparticles in Diabetes Mellitus: A Systematic Review and Meta-analysis on Animal Studies. Biol. Trace Elem. Res. 2022, 200, 1699–1709. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Mudila, H.; Gupta, G.; Sharma, A.K.; Kumar, D.; Bakshi, H.A.; Negi, P.; Kapoor, D.N.; Chellappan, D.K.; et al. Emerging trends in clinical implications of bio-conjugated silver nanoparticles in drug delivery. Colloid Interface Sci. Commun. 2020, 35, 100244. [Google Scholar] [CrossRef]
- Ahumada, M.; Suuronen, E.J.; Alarcon, E.I. Biomolecule Silver Nanoparticle-Based Materials for Biomedical Applications. In Handbook of Ecomaterials; Martínez, L., Kharissova, O., Kharisov, B., Eds.; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Buthenia, A.; Hasoon, K.H.; Jawad, I.S.; Mohammed, N.N.; Hussein, K.F.; Al-azawi, M.S.J. Silver nanoparticles conjugated amoxicillin: A promising nano-suspension for overcoming multidrug resistance bacteria and preservation of endotracheal tube. Inorg. Chem. Commun. 2024, 165, 112456. [Google Scholar] [CrossRef]
- Madhyastha, H.; Madhyastha, R.; Thakur, A.; Kentaro, S.; Dev, A.; Singh, S.; Chandrashekharappa, R.B.; Kumar, H.; Acevedo, O.; Nakajima, Y.; et al. c-Phycocyanin primed silver nano conjugates: Studies on red blood cell stress resilience mechanism. Colloids Surf. B Biointerfaces 2020, 94, 111211. [Google Scholar] [CrossRef] [PubMed]
- Prete, V.; Abate, A.C.; Di Pietro, P.; De Lucia, M.; Vecchione, C.; Carrizzo, A. Beneficial Effects of Spirulina Supplementation in the Management of Cardiovascular Diseases. Nutrients 2024, 16, 642. [Google Scholar] [CrossRef] [PubMed]
- Fais, G.; Manca, A.; Bolognesi, F.; Borselli, M.; Concas, A.; Busutti, M.; Broggi, G.; Sanna, P.; Castillo-Aleman, Y.M.; Rivero-Jiménez, R.A.; et al. Wide Range Applications of Spirulina: From Earth to Space Missions. Mar. Drugs 2022, 20, 299. [Google Scholar] [CrossRef]
- Rudi, L.; Zinicovscaia, I.; Cepoi, L.; Chiriac, T.; Peshkova, A.; Cepoi, A.; Grozdov, D. Accumulation and Effect of Silver Nanoparticles Functionalized with Spirulina platensis on Rats. Nanomaterials 2021, 11, 2992. [Google Scholar] [CrossRef]
- Muthusamy, G.; Thangasamy, S.; Raja, M.; Chinnappan, S.; Kandasamy, S. Biosynthesis of silver nanoparticles from Spirulina microalgae and its antibacterial activity. Environ. Sci. Pollut. Res. Int. 2017, 24, 19459–19464. [Google Scholar] [CrossRef]
- Das, B.; Tripathy, S.; Adhikary, J.; Chattopadhyay, S.M.; Debasis, D.; Sandeep, K.D.; Sabyasachi, D.; Aditi, D.; Sankar, K.; Das, D.; et al. Surface modification minimizes the toxicity of silver nanoparticles: An in vitro and in vivo study. J. Biol. Inorg. Chem. 2017, 22, 893–918. [Google Scholar] [CrossRef]
- Meena, J.; Gupta, A.; Ahuja, R.; Singh, M.; Bhaskar, S. Inorganic nanoparticles for natural product delivery: A review. Environ. Chem. Lett. 2020, 18, 2107–2118. [Google Scholar] [CrossRef]
- Sowmya, S.; Suba Sri, M.; Dineshkumar, R. In vitro Therapeutic Effect of Spirulina Extract. Asian J. Biol. Life Sci. 2021, 10, 582–589. [Google Scholar] [CrossRef]
- Karkos, P.D.; Leong, S.C.; Karkos, C.D.; Sivaji, N.; Assimakopoulos, D.A. Spirulina in Clinical Practice: Evidence-Based Human Applications. Evid. Based Complement. Alternat. Med. 2011, 2011, 531053. [Google Scholar] [CrossRef]
- Goel, M.; Sharma, A.; Sharma, B. Recent Advances in Biogenic Silver Nanoparticles for Their Biomedical Applications. Sustain. Chem. 2023, 4, 61–94. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.S.; Song, K.S.; Ryu, H.R.; Sung, J.H.; Park, J.D.; Park, H.M.; Song, N.W.; Shin, B.S.; Marshak, D.; et al. Biopersistence of silver nanoparticles in tissues from Sprague-Dawley rats. Part. Fibre Toxicol. 2013, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xiong, L.; Wang, S.; Wang, J.; Liu, L.; Li, J.; Wan, Z.; Xi, T. Influence of silver nanoparticles on neurons and blood-brain barrier via subcutaneous injection in rats. Appl. Surf. Sci. 2008, 255, 502–504. [Google Scholar] [CrossRef]
- Loeschner, K.; Hadrup, N.; Qvortrup, K.; Larsen, A.; Gao, X.; Vogel, U.; Mortensen, A.; Lam, H.R.; Larsen, E.H. Distribution of silver in rats following 28 days of repeated oral exposure to silver nanoparticles or silver acetate. Part. Fibre Toxicol. 2011, 8, 18. [Google Scholar] [CrossRef]
- Sung, J.H.; Ji, J.H.; Park, J.D.; Yoon, J.U.; Kim, D.S.; Jeon, K.S.; Song, M.Y.; Jeong, J.; Han, B.S.; Han, J.H.; et al. Subchronic inhalation toxicity of silver nanoparticles. Toxicol. Sci. 2009, 108, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Park, M.V.; Neigh, A.M.; Vermeulen, J.P.; de la Fonteyne, L.J.; Verharen, H.W.; Briedé, J.J.; van Loveren, H.; de Jong, W.H. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 2011, 32, 9810–9817. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kuang, H.; Zhang, W.; Aguilar, Z.P.; Wei, H.; Xu, H. Comparisons of the biodistribution and toxicological examinations after repeated intravenous administration of silver and gold nanoparticles in mice. Sci. Rep. 2017, 7, 3303. [Google Scholar] [CrossRef]
- Opris, R.; Toma, V.; Olteanu, D.; Baldea, I.; Baciu, A.M.; Lucaci, F.I.; Filip, G.A. Effects of Silver Nanoparticles Functionalized with Cornus Mas L. Extract on Architecture and Apoptosis in Rat Testicle. Nanomedicine 2019, 14, 275–299. [Google Scholar] [CrossRef]
- van der Zande, M.; Vandebriel, R.J.; Van Doren, E.; Kramer, E.; Herrera Rivera, Z.; Serrano-Rojero, C.S.; Gremmer, E.R.; Mast, J.; Peters, R.J.; Hollman, P.C.; et al. Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure. ACS Nano 2012, 6, 7427–7442. [Google Scholar] [CrossRef]
- Wei, Y.; Quan, L.; Zhou, C.; Zhan, Q. Factors relating to the biodistribution & clearance of nanoparticles & their effects on in vivo application. Nanomedicine 2018, 13, 1495–1512. [Google Scholar] [CrossRef]
- Kim, Y.S.; Song, M.Y.; Park, J.D.; Song, K.S.; Ryu, H.R.; Chung, Y.H.; Chang, H.K.; Lee, J.H.; Oh, K.H.; Kelman, B.J.; et al. Subchronic oral toxicity of silver nanoparticles. Part. Fibre Toxicol. 2010, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Tang, S.; Li, S.; Lu, H.; Wang, Y.; Zhao, P.; Li, B.; Zhang, J.; Peng, L. Toxicological evaluation of silver nanoparticles and silver nitrate in rats following 28 days of repeated oral exposure. Environ. Toxicol. 2017, 32, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]


| Amino Acid | Protein Extract from Spirulina (SPE) | |
|---|---|---|
| mg/100 mg Extract | % Total Extract | |
| Alanine | 4.28 | 8.23 |
| Arginine | 5.58 | 10.73 |
| Asparagine | 0.00 | 0.00 |
| Aspartic acid | 5.02 | 9.66 |
| Cysteine | 1.14 | 2.19 |
| Glutamic acid | 9.04 | 17.39 |
| Glutamine | 0.00 | 0.00 |
| Glycine | 2.88 | 5.54 |
| Histidine * | 0.81 | 1.56 |
| Isoleucine * | 0.08 | 0.15 |
| Leucine * | 5.12 | 9.85 |
| Lysine * | 3.16 | 6.08 |
| Methionine * | 0.15 | 0.29 |
| Phenylalanine * | 2.27 | 4.37 |
| Proline | 2.03 | 3.90 |
| Threonine * | 2.59 | 4.98 |
| Tryptophan * | 0.19 | 0.37 |
| Serine | 3.06 | 5.89 |
| Tyrosine | 2.68 | 5.15 |
| Valine * | 1.91 | 3.67 |
| Total | 51.99 | 100.00 |
| Experimental Group | C (−) | AgNPs | ||
| Animal Sex | Male | Female | Male | Female |
| HB g/L | 153.25 ± 5.32 | 160.67 ± 4.04 | 155.0 ± 1.15 | 152.30 ± 2.31 * |
| RBC, 1012/L | 8.73 ± 0.34 | 8.83 ± 0.28 | 8.58 ± 0.21 | 8.14 ± 0.09 * |
| WBC, 109/L | 18.07 ± 2.41 | 15.76 ± 2.51 | 16.75 ± 3.12 | 13.56 ± 0.84 |
| PLT, 109/L | 505.0 ± 101.22 | 626.0 ± 232.0 | 613.50 ± 32.21 * | 633.0 ± 43.30 |
| PMN, % | 31.79 ± 6.80 | 24.33 ± 4.15 | 22.08 ± 6.17 * | 24.0 ± 0.17 |
| LY, % | 56.30 ± 8.12 | 58.87 ± 3.35 | 60.68 ± 3.99 | 61.2 ± 5.02 |
| MON, % | 6.03 ± 1.84 | 9.50 ± 0.46 | 8.38 ± 0.61 * | 6.20 ± 1.25 * |
| EOS, % | 4.45 ± 0.30 | 6.73 ± 1.50 | 8.3 ± 2.55 * | 8.07 ± 1.95 * |
| BAS, % | 0.58 ± 0.33 | 0.47 ± 0.38 | 3.83 ± 0.60 ** | 0.64 ± 0.23 * |
| RET, % | 3.35 ± 0.37 | 3.35 ± 1.81 | 2.92 ± 0.13 * | 4.03 ± 0.44 |
| Experimental Group | C (+) | AgNPs-SPE | ||
| Animal Sex | Male | Female | Male | Female |
| HB g/L | 151.25 ± 5.50 | 151.33 ± 4.51 | 152.75 ± 3.50 | 138.0 ± 10.39 * |
| RBC, 1012/L | 8.11 ± 0.43 | 8.19 ± 0.41 | 8.41 ± 0.13 | 7.87 ± 0.31 * |
| WBC, 109/L | 12.59 ± 3.69 | 12.92 ± 4.08 | 14.24 ± 3.19 | 12.02 ± 2.23 |
| PLT, 109/L | 523.25 ± 116.25 | 672.0 ± 77.09 | 490.75 ± 125.65 | 643.33 ± 198.94 |
| PMN, % | 25.08 ± 9.41 | 24.77 ± 1.46 | 26.63 ± 2.55 | 35.67 ± 9.58 * |
| LY, % | 56.40 ± 11.97 | 62.47 ± 2.63 | 53.83 ± 1.99 | 52.17 ± 5.83 |
| MON, % | 6.70 ± 3.11 | 8.23 ± 1.75 | 7.62 ± 1.60 | 8.70 ± 1.73 |
| EOS, % | 5.56 ± 0.82 | 7.23 ± 2.29 | 5.38 ± 0.37 | 5.10 ± 0.52 |
| BAS, % | 0.68 ± 0.10 | 0.30 ± 0.10 | 3.30 ± 1.41 * | 0.67 ± 0.29 * |
| RET, % | 3.43 ± 0.45 | 3.97 ± 1.19 | 4.13 ± 0.25 * | 7.54 ± 0.76 * |
| Experiment Group | C (−) | AgNPs | ||
| Animal Sex | Male | Female | Male | Female |
| Prot, g/L | 58.55 ± 7.09 | 59.53 ± 4.51 | 60.75 ± 3.72 | 57.43 ± 3.27 |
| Glu, mmol/L | 4.93 ± 0.35 | 6.22 ± 0.40 | 5.26 ± 0.64 | 5.76 ± 0.75 |
| CREA, µM/L | 92.76 ± 20.03 | 84.97 ± 4.04 | 161.40 ± 19.13 * | 170.30 ± 16.8 ** |
| Urea mg/dL | 29.35 ± 8.76 | 28.62 ± 6.81 | 28.91 ± 4.69 | 26.57 ± 4.41 |
| ALT, U/L | 173.6 ± 9.08 | 103.45 ± 8.60 | 237.45 ± 25.63 * | 201.83 ± 47.89 * |
| AST, U/L | 3.57 ± 0.86 | 3.57 ± 0.12 | 4.63 ± 0.13 * | 7.23 ± 1.79 * |
| Experiment Group | C (+) | AgNPs-SPE | ||
| Animal Sex | Male | Female | Male | Female |
| Prot, g/L | 66.13 ± 2.29 | 61.57 ± 9.74 | 60.73 ± 3.55 | 57.33 ± 9.67 |
| Glu, mmol/L | 6.33 ± 0.51 | 5.26 ± 0.91 | 5.86 ± 0.76 | 3.76 ± 1.22 * |
| CREA, µM/L | 105.60 ± 4.16 | 94.60 ± 4.30 | 176.4 ± 4.16 ** | 168.90 ± 4.21 ** |
| Urea mg/dL | 24.75 ± 6.95 | 26.57 ± 5.63 | 27.60 ± 4.61 | 27.16 ± 5.33 |
| ALT, U/L | 197.45 ± 39.07 | 168.07 ± 67.32 | 178,68 ± 12.23 | 227.50 ± 1.66 ** |
| AST, U/L | 3.07 ± 2.22 | 2.53 ± 1.79 | 4.83 ± 5.24 * | 5.13 ± 1.01 * |
| Indices | C (−) | AgNPs | AgNPs CET | C (+) | AgNPs-SPE | AgNPs-SPE CET |
|---|---|---|---|---|---|---|
| HB g/L | 156.43 ± 5.94 | 153.86 ± 2.11 | 154.00 ± 1.87 | 145.57 ± 7.14 | 146.43 ± 10.2 | 150.0 ± 1.41 |
| RBC, 1012/L | 8.77 ± 0.30 | 8.39 ± 0.28 | 8.41 ± 0.29 | 8.15 ± 0.38 | 8.18 ± 0.35 | 8.05 ± 0.50 |
| WBC, 109/L | 17.08 ± 2.55 | 15.38 ± 2.83 * | 14.84 ± 2.68 | 12.73 ± 3.52 | 13.29 ± 2.86 | 15.70 ± 2.08 |
| PLT, 109/L | 556.8 ± 165.6 | 623.5 ± 37.7 | 621.4 ± 35.5 | 587.0 ± 122.7 | 556.1 ± 163.0 | 472.0 ± 159.8 |
| PMN, % | 28.59 ± 6.69 | 23.0 ± 3.18 * | 23.66 ± 4.84 | 22.37 ± 7.51 | 30.5 ± 7.56 * | 23.36 ± 4.20 * |
| LY, % | 57.40 ± 6.21 | 60.9 ± 4.06 | 59.80 ± 4.40 | 62.00 ± 9.19 | 53.11 ± 3.76 | 58.74 ± 4.82 |
| MON, % | 7.56 ± 2.33 | 7.44 ± 1.42 | 8.04 ± 1.61 | 7.36 ± 2.55 | 8.08 ± 1.62 | 8.01 ± 2.02 |
| EOS, % | 5.43 ± 1.61 | 8.20 ± 2.23 * | 7.85 ± 2.42 | 6.33 ± 1.67 | 5.24 ± 0.44 | 7.07 ± 1.23 |
| BAS, % | 0.53 ± 0.33 | 2.23 ± 0.42 * | 0.64 ± 0.42 | 0.51 ± 0.22 | 2.17 ± 1.73 * | 0.8 ± 0.77 |
| RET, % | 3.35 ± 1.08 | 3.39 ± 0.65 | 3.33 ± 0.60 | 3.66 ± 0.81 | 5.84 ± 0.50 * | 4.26 ± 0.17 |
| Indices | C (−) | AgNPs | AgNPs CET | C (+) | AgNPs-SPE | AgNPs-SPE CET |
|---|---|---|---|---|---|---|
| Prot, g/L | 58.97 ± 5.68 | 59.34 ± 3.70 | 68.87 ± 3.50 | 64.17 ± 6.47 | 59.27 ± 6.38 | 62.46 ± 4.04 |
| Glu, mmol/L | 5.49 ± 0.77 | 5.47 ± 0.68 | 5.49 ± 0.41 | 5.87 ± 0.86 | 4.96 ± 1.34 | 5.65 ± 0.47 |
| CREA, µM/L | 86.43 ± 14.9 | 165.2 ± 17.10 ** | 69.33 ± 3.71 ** | 100.93 ± 7.77 | 172.77 ± 5.92 * | 68.04 ± 3.92 * |
| Urea, mg/dL | 29.03 ± 5.69 | 27.01 ± 4.36 | 31.17 ± 0.36 | 25.53 ± 5.97 | 27.41 ± 4.49 | 33.72 ± 0.36 |
| ALT, U/L | 162.4 ± 39.6 | 222.0 ± 45.0 ** | 188.2 ± 13.5 * | 184.9 ± 50.2 | 199.6 ± 27.0 * | 208.6 ± 10.6 |
| AST, U/L | 3.57 ± 2.31 | 5.74 ± 1.74 * | 5.43 ± 0.80 | 2.84 ± 1.90 | 4.96 ± 2.56 | 7.03 ± 0.68 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudi, L.; Zinicovscaia, I.; Cepoi, L.; Chiriac, T.; Grozdov, D.; Kravtsova, A. The Impact of Silver Nanoparticles Functionalized with Spirulina Protein Extract on Rats. Pharmaceuticals 2024, 17, 1247. https://doi.org/10.3390/ph17091247
Rudi L, Zinicovscaia I, Cepoi L, Chiriac T, Grozdov D, Kravtsova A. The Impact of Silver Nanoparticles Functionalized with Spirulina Protein Extract on Rats. Pharmaceuticals. 2024; 17(9):1247. https://doi.org/10.3390/ph17091247
Chicago/Turabian StyleRudi, Ludmila, Inga Zinicovscaia, Liliana Cepoi, Tatiana Chiriac, Dmitrii Grozdov, and Alexandra Kravtsova. 2024. "The Impact of Silver Nanoparticles Functionalized with Spirulina Protein Extract on Rats" Pharmaceuticals 17, no. 9: 1247. https://doi.org/10.3390/ph17091247
APA StyleRudi, L., Zinicovscaia, I., Cepoi, L., Chiriac, T., Grozdov, D., & Kravtsova, A. (2024). The Impact of Silver Nanoparticles Functionalized with Spirulina Protein Extract on Rats. Pharmaceuticals, 17(9), 1247. https://doi.org/10.3390/ph17091247











