Comparative Assessment of Beeswax Alcohol and Coenzyme Q10 (CoQ10) to Prevent Liver Aging, Organ Damage, and Oxidative Stress in Hyperlipidemic Zebrafish Exposed to D-Galactose: A 12-Week Dietary Intervention
Abstract
:1. Introduction
2. Results
2.1. Zebrafish Survivability and Body Weight
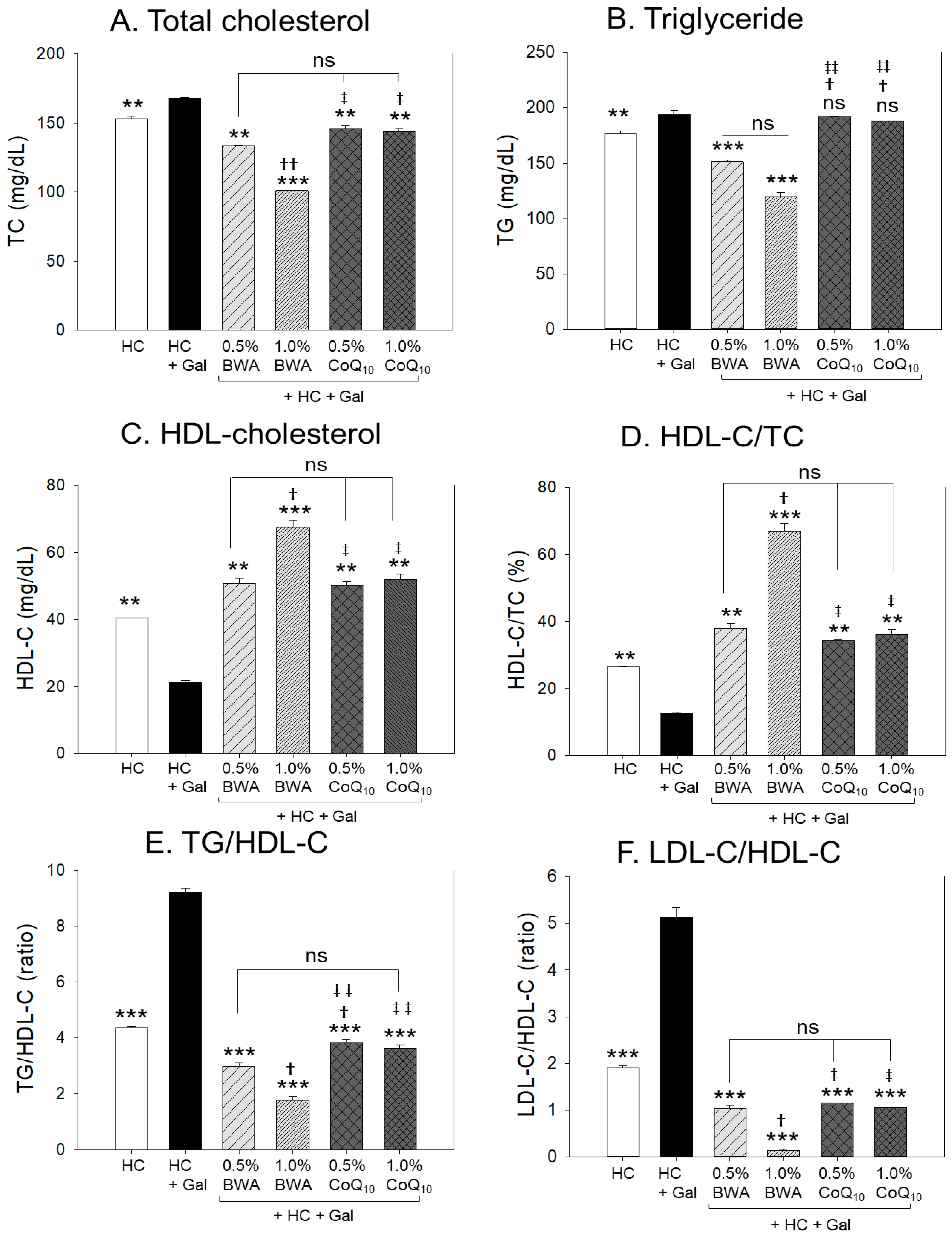
2.2. Change in Lipid Profiles
2.3. Change in Hepatic Damage Parameters
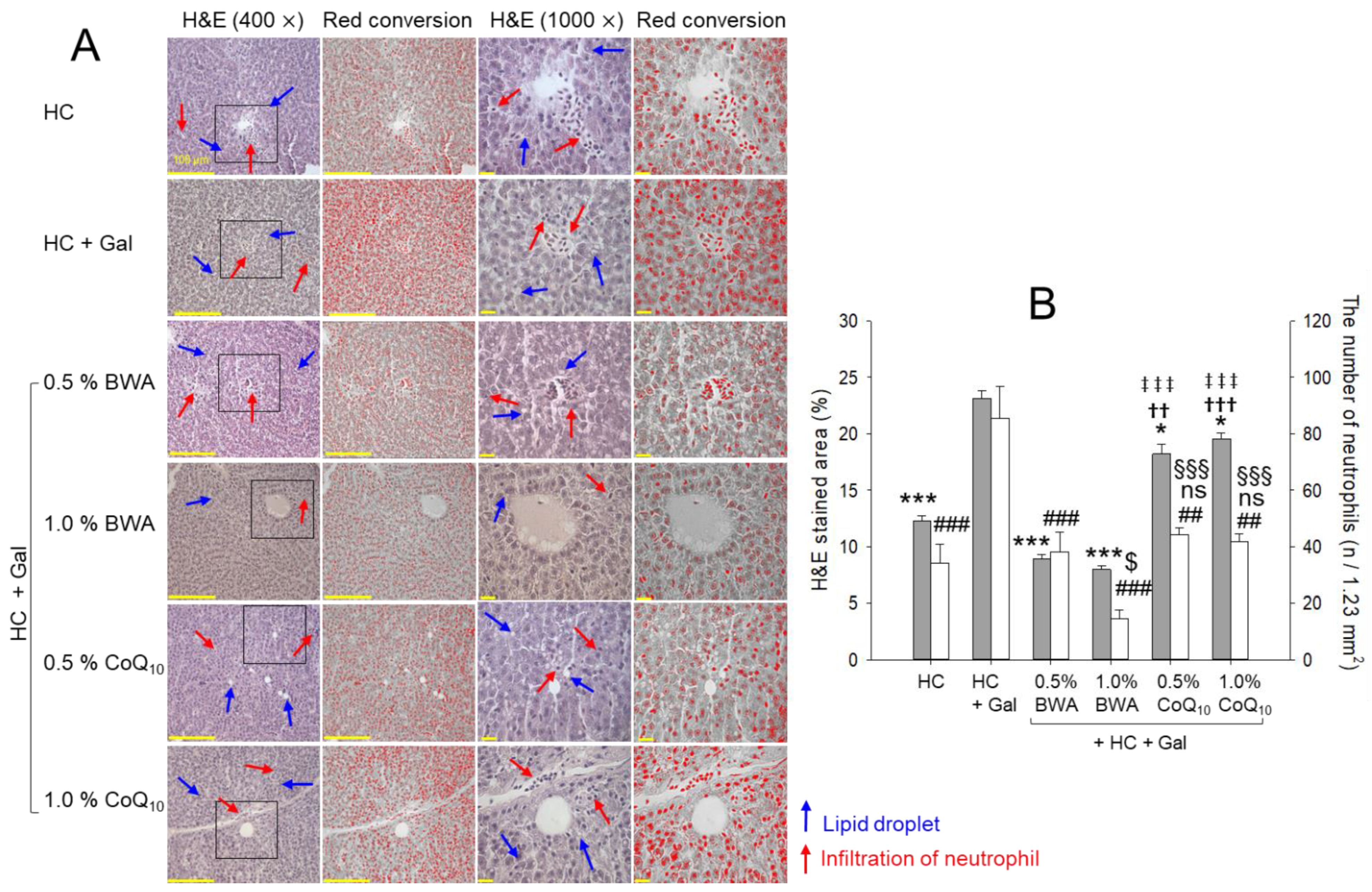
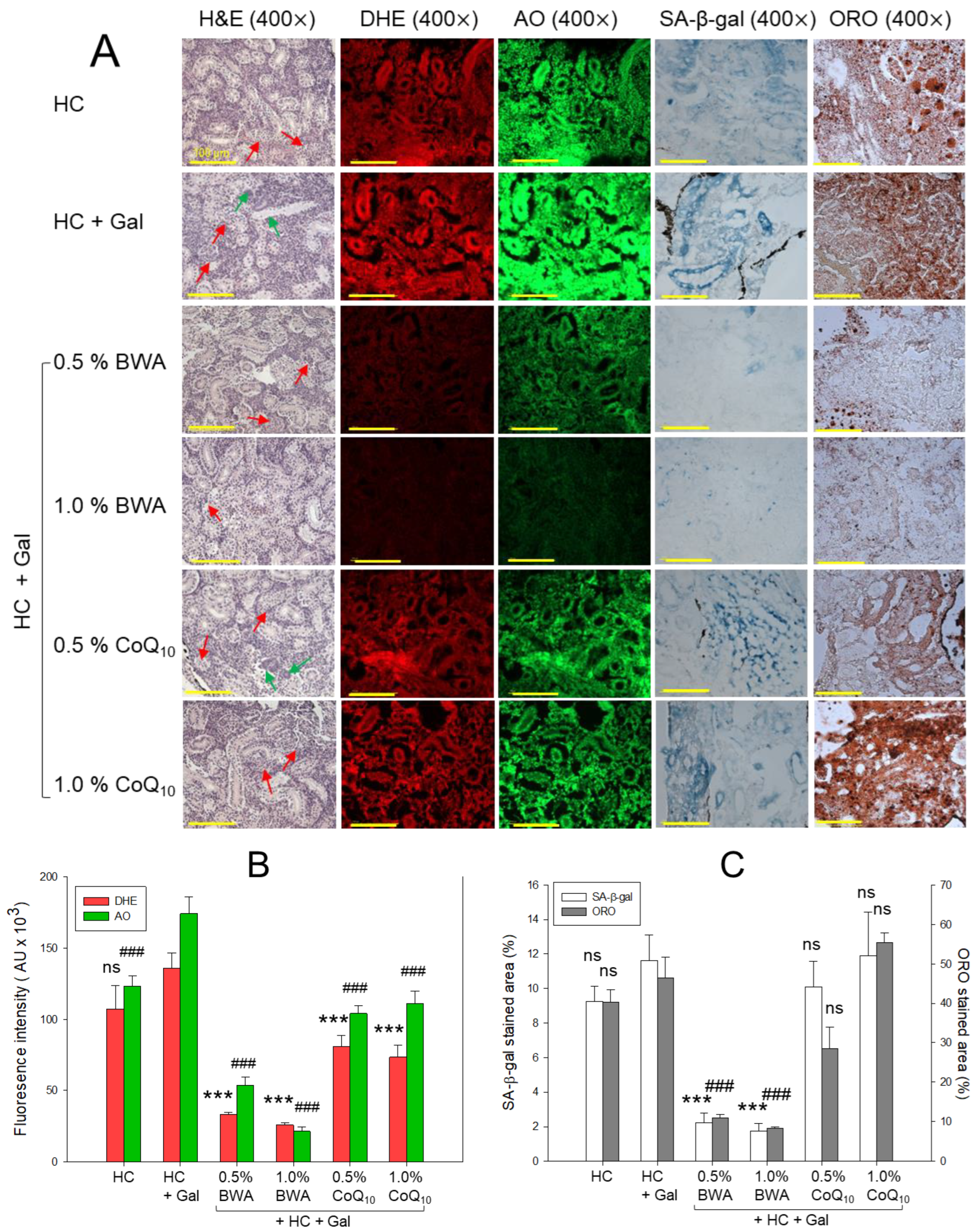
2.4. Histologic Analysis of Liver Tissue
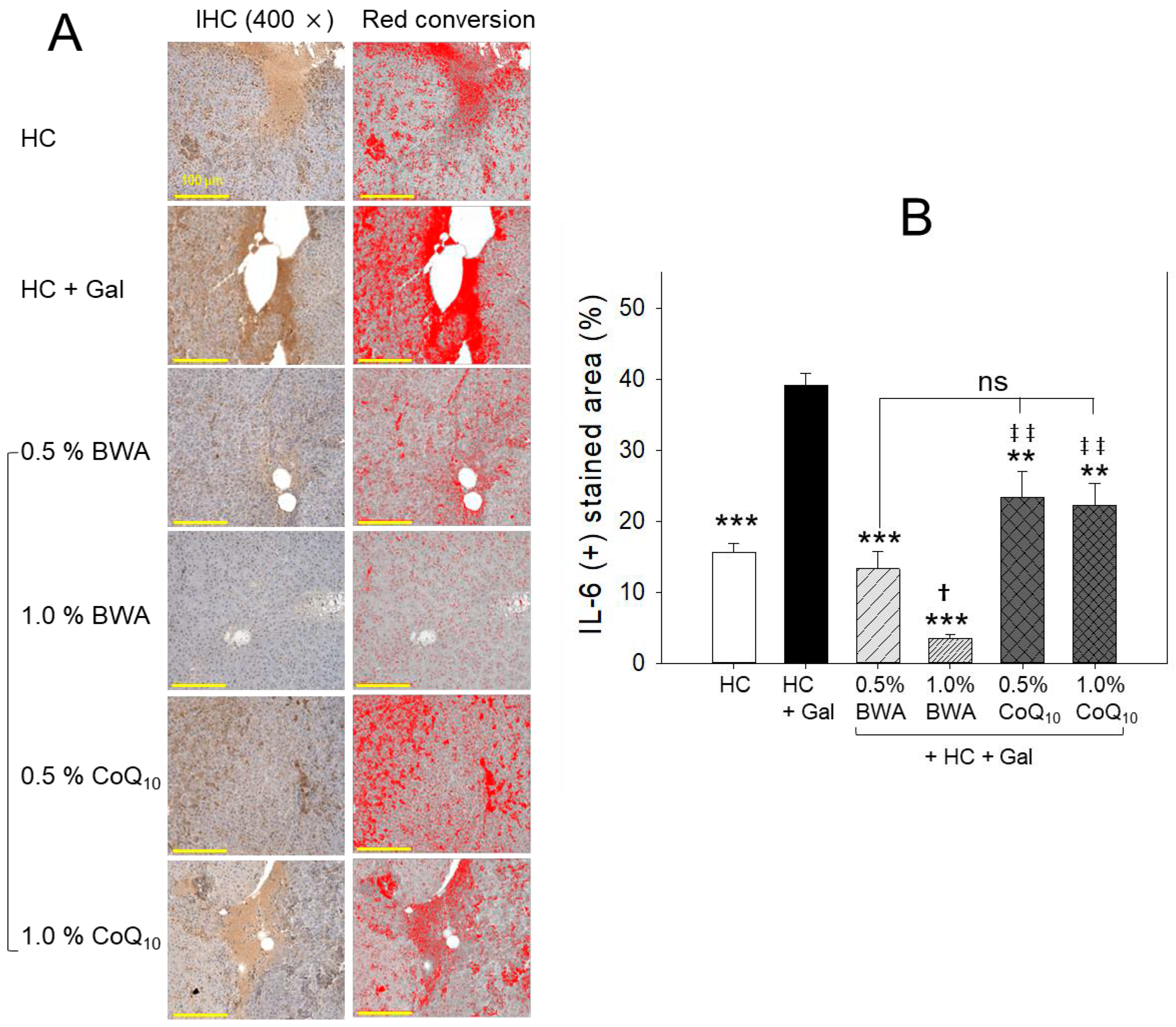
2.5. Interleukin-6 Production in Liver
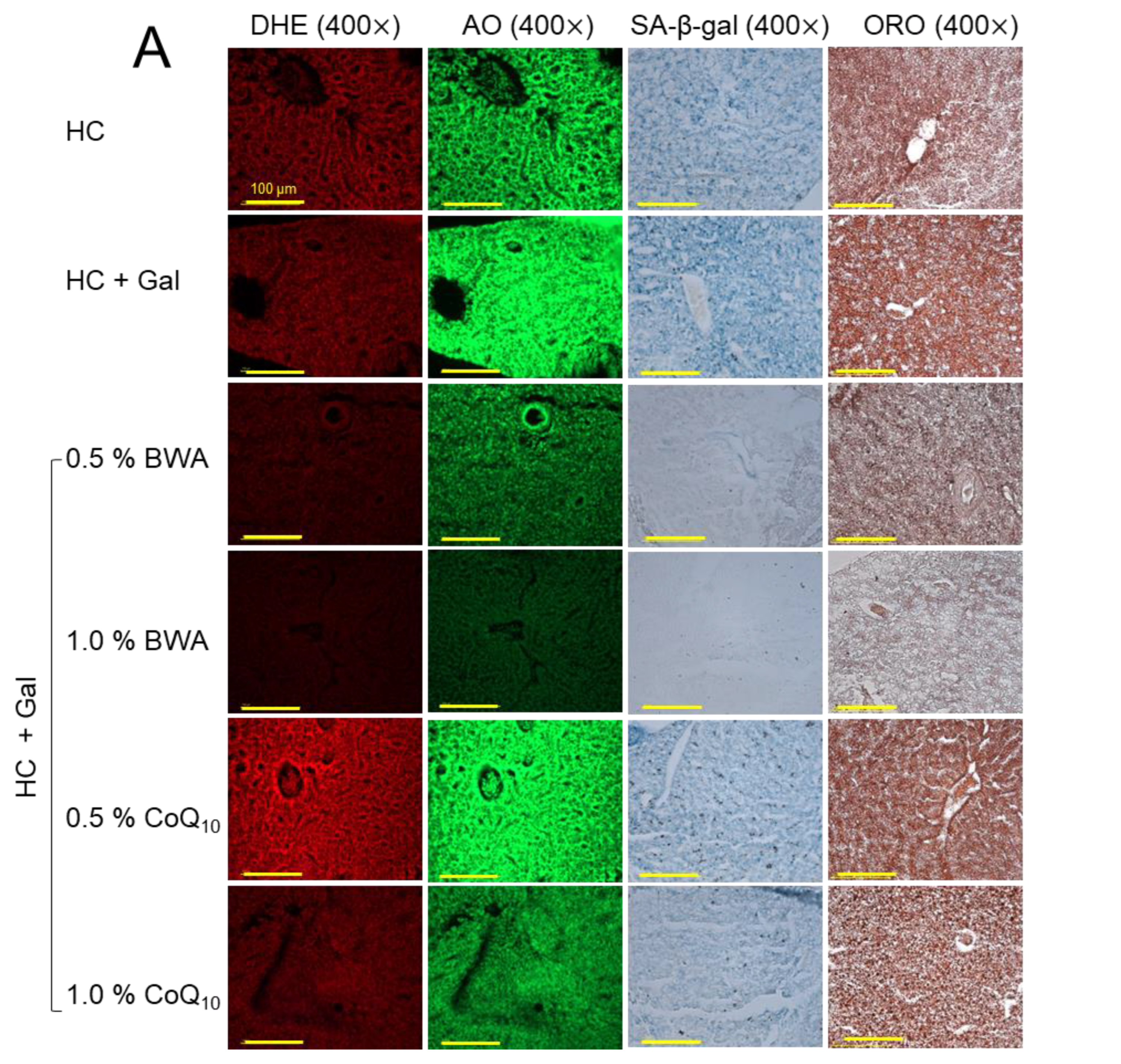
2.6. ROS Production, Apoptosis, Fatty Liver Change, and Cellular Senescence
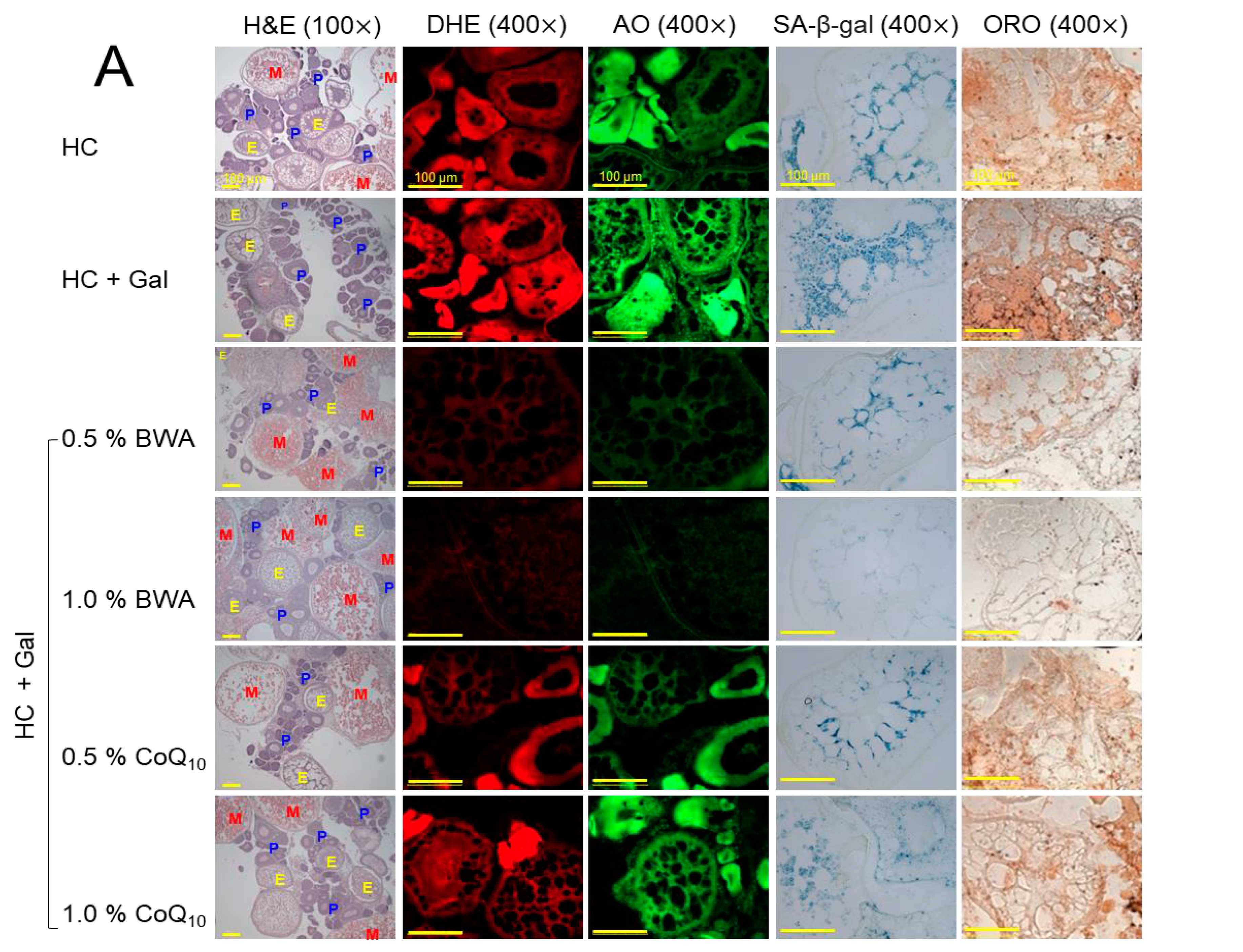
2.7. Histological Analysis of Kidney
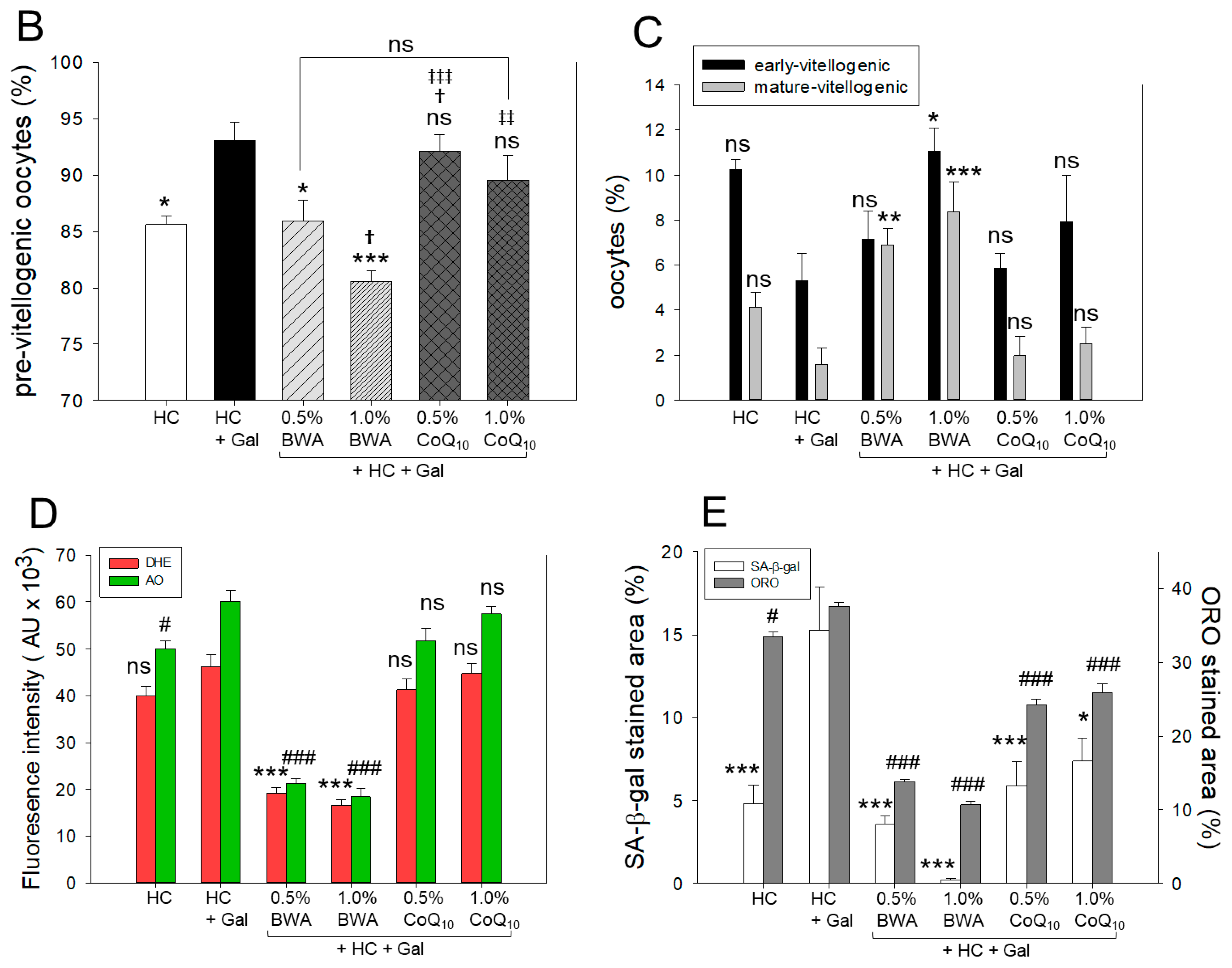
2.8. Examination of Ovarian Tissue
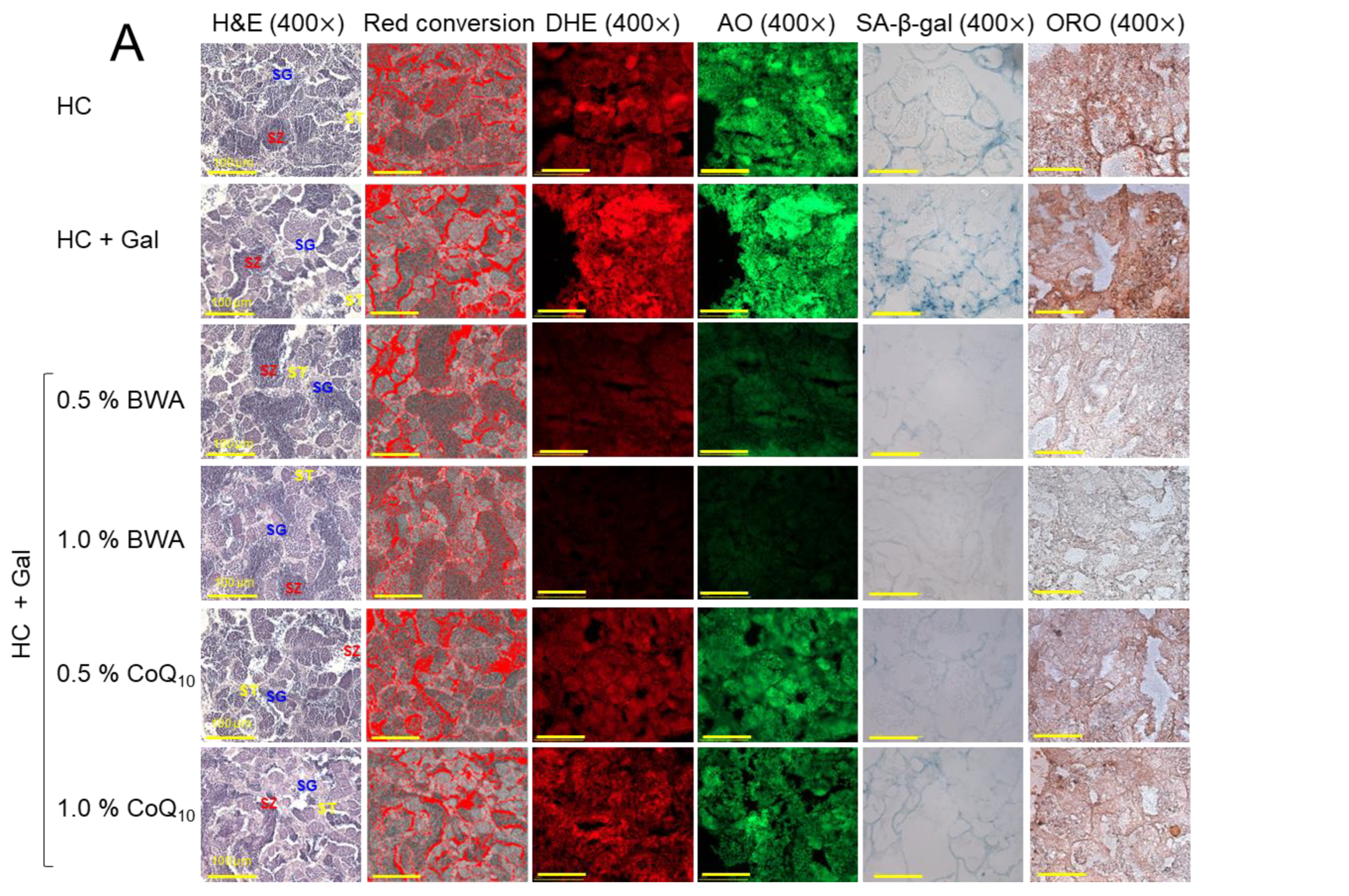
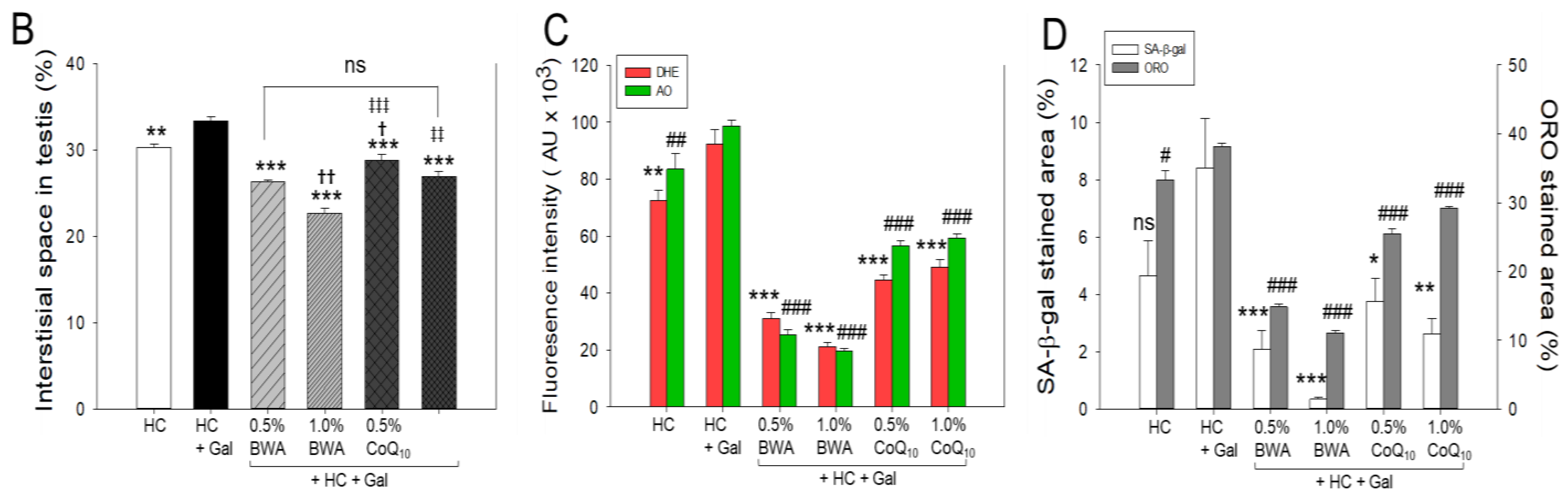
2.9. Analysis of Testicular Tissue
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Zebrafish Aquaculture
4.3. Preparation of High-Cholesterol Diet Infused with BWA or CoQ10
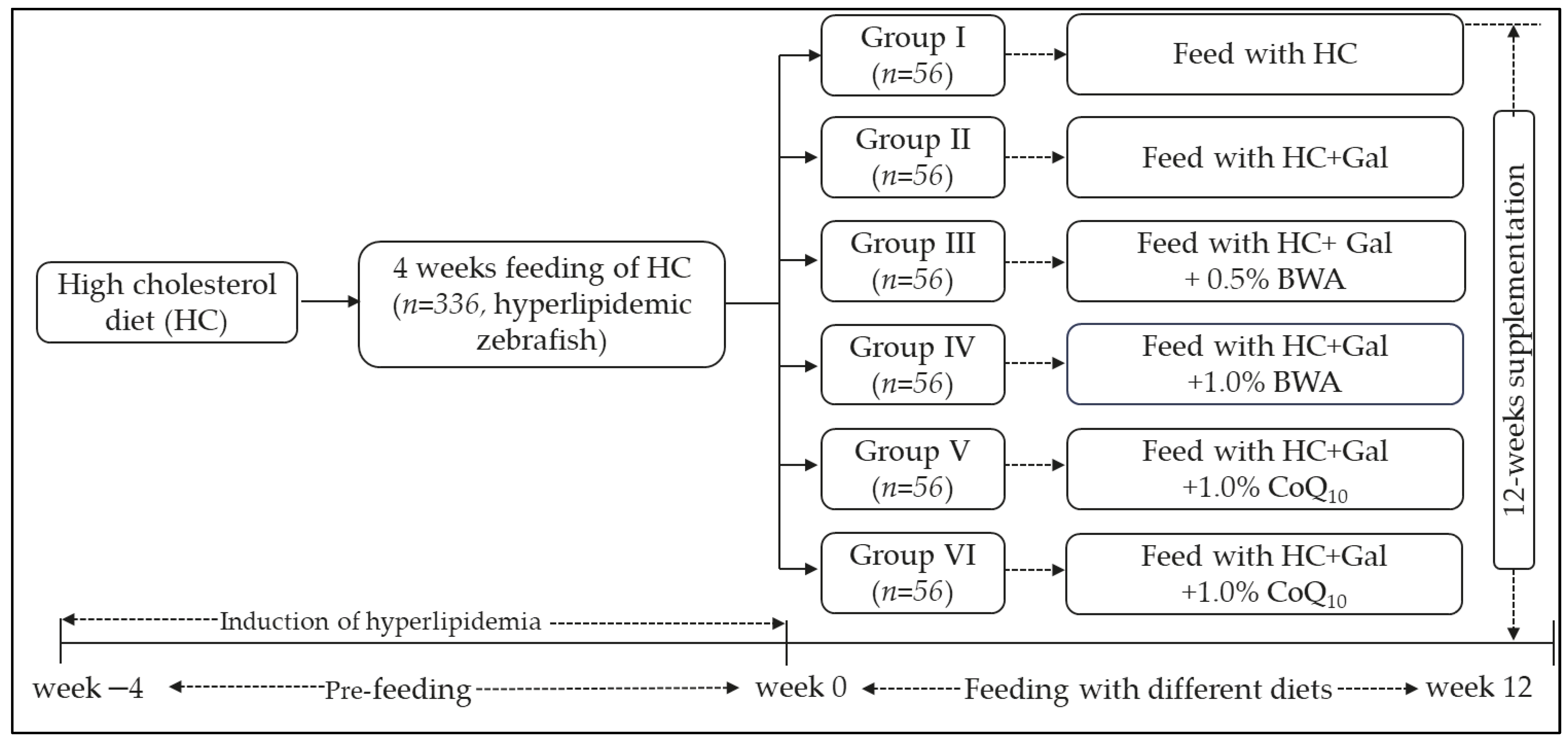
4.4. Zebrafish Fed with Different Diets
4.5. Blood and Tissue Collection
4.6. Plasma Analysis
4.7. Histologic Analysis
4.8. Immunohistochemical (IHC) Staining
4.9. Dihydroethidium (DHE) and Acridine Orange (AO) Staining
4.10. Senescence Staining
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fratini, F.; Cilia, G.; Turchi, B.; Felicioli, A. Beeswax: A minireview of its antimicrobial activity and its application in medicine. Asian Pacif. J. Tropic. Med. 2016, 9, 839–843. [Google Scholar] [CrossRef]
- Bradbear, N. Bees and Their Role in Forest Livelihoods: A Guide to the Services Provided by Bees and the Sustainable Harvesting, Processing and Marketing of Their Products; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009. [Google Scholar]
- Svečnjak, L.; Chesson, L.A.; Gallina, A.; Maia, M.; Martinello, M.; Mutinelli, F.; Muz, M.N.; Nunes, F.M.; Saucy, F.; Tipple, B.J.; et al. Standard methods for Apis mellifera beeswax research. J. Apic. Res. 2019, 58, 1–108. [Google Scholar] [CrossRef]
- Srisaipet, A.; Phromchan, S.; Jaipaeng, T. Policosanol extraction from beeswax and improvement of the purity. MATEC Web Conf. 2017, 111, 02005. [Google Scholar] [CrossRef]
- Molina, V.; Mas, R.; Carbajal, D. D-002 (beeswax alcohols): Concurrent joint health benefits and gastroprotection. Indian J. Pharm. Sci. 2015, 77, 127–134. [Google Scholar] [CrossRef]
- Menéndez, R.; Amor, A.; González, R.; Jiménez, S.; Mas, R. Inhibition of rat microsomal lipid peroxidation by the oral administration of D002. Braz. J. Med. Biol. Res. 2000, 33, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Lopez, E.; Illnait, J.; Molina, V.; Oyarzabal, A.; Fernandez, L.; Perez, Y.; Mas, R.; Mesa, M.; Fernandez, J.; Mendoza, S. Effects of D-002 (beeswax alcohols) on lipid peroxidation in middle-aged and older subjects. Lat. Am. J. Pharm. 2008, 27, 695–703. [Google Scholar]
- Rodriguez, I.; Illnait, J.; Molina, V.; Oyarzabal, A.; Fernandez, L.; Mesa, M.; Más, R.; Mendoza, S.; Gámez, R.; Jiménez, S. Comparison of the antioxidant effects of D-002 (beeswax alcohols) and grape seed extract (GSE) on plasma oxidative variables in healthy subjects. Lat. Am. J. Pharm. 2010, 29, 255–262. [Google Scholar]
- Menéndez, R.; Más, R.; Illnait, J.; Pérez, J.; Amor, A.M.; Fernández, J.C.; González, R.M. Effects of D-002 on lipid peroxidation in older subjects. J. Med. Food 2001, 4, 71–77. [Google Scholar] [CrossRef]
- Illnait, J.; Rodríguez, I.; Mendoza, S.; Fernández, Y.; Mas, R.; Miranda, M.; Piñera, J.; Fernández, J.C.; Mesa, M.; Fernández, L.; et al. Effects of D-002, a mixture of high molecular weight beeswax alcohols, on patients with nonalcoholic fatty liver disease. Korean J. Intern. Med. 2013, 28, 439. [Google Scholar] [CrossRef]
- Pérez, Y.; Oyárzabal, A.; Mas, R.; Molina, V.; Jiménez, S. Protective effect of D-002, a mixture of beeswax alcohols, against indomethacin-induced gastric ulcers and mechanism of action. J. Nat. Med. 2013, 67, 182–189. [Google Scholar] [CrossRef]
- Cho, K.-H.; Baek, S.-H.; Nam, H.-S.; Bahuguna, A.; López-González, L.E.; Rodríguez-Cortina, I.; Illnait-Ferrer, J.; Fernández-Travieso, J.C.; Molina-Cuevas, V.; Pérez-Guerra, Y.; et al. Beeswax alcohol prevents low-density lipoprotein oxidation and demonstrates antioxidant activities in zebrafish embryos and human subjects: A clinical study. Curr. Issues Mol. Biol. 2024, 46, 409–429. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Baek, S.-H.; Nam, H.-S.; Bahuguna, A. Enhancement of antioxidant and anti-glycation properties of beeswax alcohol in reconstituted high-density lipoprotein: Safeguarding against carboxymethyllysine toxicity in zebrafish. Antioxidants 2023, 12, 2116. [Google Scholar] [CrossRef] [PubMed]
- Puig, M.N.; Castaño, S.M.; Ferreiro, R.M.; Clara, M.V.; Hernansez, N.M. Effects of oral administration of D-002 (Beeswax alcohols) on histological and functional outcomes in a Rat model of antigen-induced arthritis: Preliminary Study. Int. J. Pharmacol. Phytochem. Ethnomed. 2016, 5, 60–68. [Google Scholar]
- Illnait, J.; Rodriguez, I.; Molina, V.; Mendoza, S.; Mas, R.; Fernandez, L.; Oyarzabal, A.; Perez, Y.; Mesa, M.; Fernández, J. Effects of D-002 (beeswax alcohols) on gastrointestinal symptoms and oxidative markers in middle-aged and older subjects. Lat. Am. J. Pharm. 2013, 32, 166–174. [Google Scholar]
- Korean Food and Drug Administration (KFDA). Available online: https://www.foodsafetykorea.go.kr/portal/healthyfoodlife/searchHomeHFDetail.do?prdlstReportLedgNo=2023021000330706 (accessed on 16 May 2023).
- Ardekani, A.; Tabrizi, R.; Maleki, E.; Bagheri Lankarani, K.; Heydari, S.T.; Moradinazar, M.; Akbari, M. Effects of coenzyme Q10 supplementation on lipid profiles and liver enzymes of nonalcoholic fatty liver disease (NAFLD) patients: A systematic review and meta-analysis of randomized controlled trials. Food Sci. Nutr. 2023, 11, 2580–2588. [Google Scholar] [CrossRef]
- Ashkani Esfahani, S.; Esmaeilzadeh, E.; Bagheri, F.; Emami, Y.; Farjam, M. The effect of co-enzyme q10 on acute liver damage in rats, a biochemical and pathological study. Hepat. Mon. 2013, 13, e13685. [Google Scholar] [CrossRef]
- Jiménez-Santos, M.A.; Juárez-Rojop, I.E.; Tovilla-Zárate, C.A.; Espinosa-García, M.T.; Juárez-Oropeza, M.A.; Ramón-Frías, T.; Bermúdez-Ocaña, D.Y.; Díaz-Zagoya, J.C. Coenzyme Q10 supplementation improves metabolic parameters, liver function and mitochondrial respiration in rats with high doses of atorvastatin and a cholesterol-rich diet. Lipids Health Dis. 2014, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Pasha, R.; Moon, T.W. Coenzyme Q10 protects against statin-induced myotoxicity in zebrafish larvae (Danio rerio). Environ. Toxicol. Pharmacol. 2017, 52, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Jaskelioff, M.; Muller, F.L.; Paik, J.-H.; Thomas, E.; Jiang, S.; Adams, A.C.; Sahin, E.; Kost-Alimova, M.; Protopopov, A.; Cadiñanos, J.; et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 2011, 469, 102–106. [Google Scholar] [CrossRef]
- Azman, K.F.; Zakaria, R. D-Galactose-induced accelerated aging model: An overview. Biogerontology 2019, 20, 763–782. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Valko, M.; Alomar, S.Y.; Alwasel, S.H.; Cruz-Martins, N.; Dhanjal, D.S.; Singh, R.; Kuča, K.; Verma, R.; et al. Antioxidative potential of Lactobacillus sp. in ameliorating D-galactose-induced aging. Appl. Microbiol. Biotechnol. 2022, 106, 4831–4843. [Google Scholar] [CrossRef] [PubMed]
- Azman, K.F.; Safdar, A.; Zakaria, R. D-galactose-induced liver aging model: Its underlying mechanisms and potential therapeutic interventions. Exp. Gerontol. 2021, 150, 111372. [Google Scholar] [CrossRef]
- Du, Z.; Yang, Y.; Hu, Y.; Sun, Y.; Zhang, S.; Peng, W.; Zhong, Y.; Huang, X.; Kong, W. A long-term high-fat diet increases oxidative stress, mitochondrial damage and apoptosis in the inner ear of D-galactose-induced aging rats. Hear. Res. 2012, 287, 15–24. [Google Scholar] [CrossRef]
- Cui, X.; Zuo, P.; Zhang, Q.; Li, X.; Hu, Y.; Long, J.; Packer, L.; Liu, J. Chronic systemic D-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: Protective effects of R-α-lipoic acid. J. Neurosci. Res. 2006, 84, 647–654. [Google Scholar] [CrossRef]
- Han, Y.; Zee, S.; Cho, K.-H. Beeswax alcohol and fermented black rice bran synergistically ameliorated hepatic injury and dyslipidemia to exert antioxidant and anti-inflammatory activity in ethanol-supplemented zebrafish. Biomolecules 2023, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Bonvehí, J.S.; Bermejo, F.O. Detection of adulterated commercial Spanish beeswax. Food Chem. 2012, 132, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Aichholz, R.; Lorbeer, E. Investigation of combwax of honeybees with high-temperature gas chromatography and high-temperature gas chromatography–chemical ionization mass spectrometry: I. High-temperature gas chromatography. J. Chromatogr. A 1999, 855, 601–615. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, J.-E.; Nam, H.-S.; Baek, S.-H.; Bahuguna, A. Consumption of policosanol (Raydel®) improves hepatic, renal, and reproductive functions in zebrafish: In vivo comparison study among Cuban, Chinese, and American policosanol. Pharmaceuticals 2023, 17, 66. [Google Scholar] [CrossRef]
- Yoon, Y.; Yoon, J.; Jang, M.Y.; Na, Y.; Ko, Y.; Choi, J.H.; Seok, S.H. High cholesterol diet induces IL-1beta expression in adult but not larval zebrafish. PLoS ONE 2013, 8, e66970. [Google Scholar] [CrossRef]
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish disease models in drug discovery: From preclinical modelling to clinical trials. Nat. Rev. Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef]
- Juan-García, A.; Bind, M.-A.; Engert, F. Larval zebrafish as an in vitro model for evaluating toxicological effects of mycotoxins. Ecotoxicol. Environ. Saf. 2020, 202, 110909. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Liu, C.; Miller, Y.I. Zebrafish models of dyslipidemia: Relevance to atherosclerosis and angiogenesis. Transl. Res. 2014, 163, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.X.; Xu, S.Y.; Wei, W.; Sun, X.X.; Yang, J.; Liu, L.H.; Dong, C. Melatonin reduces memory changes and neural oxidative damage in mice treated with D-galactose. J. Pineal Res. 2002, 32, 173–178. [Google Scholar] [CrossRef]
- Liao, C.H.; Chen, B.H.; Chiang, H.S.; Chen, C.W.; Chen, M.F.; Ke, C.C.; Wang, Y.Y.; Lin, W.N.; Wang, C.C.; Lin, Y.H. Optimizing a male reproductive aging mouse model by D-galactose injection. Int. J. Mol. Sci. 2016, 17, 98. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tian, Z.; Zhao, D.; Liang, Y.; Dai, S.; Liu, M.; Hou, S.; Dong, X.; Zhaxinima; Yang, Y. Effects of coenzyme Q10 supplementation on lipid profiles in adults: A meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2023, 108, 232–249. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, H.; Eghtesadi, S.; Seifirad, S.; Rezaee, N.; Shidfar, F.; Heydari, I.; Golestan, B.; Jazayeri, S. Effects of CoQ10 supplementation on lipid profiles and glycemic control in patients with Type 2 diabetes: A randomized, double blind, placebo-controlled trial. J. Diabetes Metab. Disord. 2014, 13, 81. [Google Scholar] [CrossRef]
- Bolt, J.; Sandhu, S.; Mohammadi, A. Effect of coenzyme Q10 supplementation on sarcopenia, frailty, and falls: A scoping review. J. Nutr. Health Aging 2023, 27, 586–592. [Google Scholar] [CrossRef]
- Hou, S.; Tian, Z.; Zhao, D.; Liang, Y.; Dai, S.; Ji, Q.; Fan, Z.; Liu, Z.; Liu, M.; Yang, Y. Efficacy and optimal dose of coenzyme Q10 supplementation on inflammation-related biomarkers: AGRADE-assessed systematic review and updated meta-analysis of randomized controlled trials. Mol. Nutr. Food Res. 2023, 67, 220800. [Google Scholar] [CrossRef]
- Soleimani Damaneh, M.; Fatahi, S.; Aryaeian, N.; Bavi Behbahani, H. The effect of coenzyme Q10 supplementation on liver enzymes: A systematic review and meta-analysis of randomized clinical trials. Food Sci Nutr. 2023, 11, 4912–4925. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Cho, H.-N.; Soh, J.-W.; Jhon, G.J.; Cho, C.-K.; Chung, H.-Y.; Bae, S.; Lee, S.-J.; Lee, Y.-S. Oxidative stress-induced apoptosis is mediated by ERK1/2 phosphorylation. Exp. Cell Res. 2003, 291, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Nousis, L.; Kanavaros, P.; Barbouti, A. Oxidative stress-induced cellular senescence: Is labile iron the connecting link? Antioxidants 2023, 12, 1250. [Google Scholar] [CrossRef]
- Faraonio, R. Oxidative stress and cell senescence process. Antioxidants 2022, 11, 1718. [Google Scholar] [CrossRef] [PubMed]
- Molina, V.; Valdés, S.; Carbajal, D.; Arruzazabala, L.; Menéndez, R.; Más, R. Antioxidant effect of D-002 on gastric mucosa of rats with experimentally induced injury. J. Med. Food 2001, 4, 79–83. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, J.-E.; Bahuguna, A.; Kang, D.-J. Long-term supplementation of ozonated sunflower oil improves dyslipidemia and hepatic inflammation in hyperlipidemic zebrafish: Suppression of oxidative stress and inflammation against carboxymethyllysine toxicity. Antioxidants 2023, 12, 1240. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb. Protoc. 2008, 2008, prot4986. [Google Scholar] [CrossRef]
- Al-Ghamdi, T.H.; Atta, I.S. Efficacy of interleukin-6 in the induction of liver cell proliferation after hemi-hepatectomy: Histopathologic and immunohistochemical study. Int. J. Clin. Exp. Pathol. 2020, 13, 1540–1549. [Google Scholar]
- Owusu-Ansah, E.; Yavari, A.; Mandal, S.; Banerjee, U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat. Genet. 2008, 40, 356–361. [Google Scholar] [CrossRef]
- Umali, J.; Hawkey-Noble, A.; French, C.R. Loss of foxc1 in zebrafish reduces optic nerve size and cell number in the retinal ganglion cell layer. Vis. Res. 2019, 156, 66–72. [Google Scholar] [CrossRef]
- Cho, K.-H.; Bahuguna, A.; Kang, D.-J.; Kim, J.-E. Prolonged supplementation of ozonated sunflower oil bestows an antiaging effect, improves blood lipid profile and spinal deformities, and protects vital organs of zebrafish (Danio rerio) against age-related degeneration: Two-years consumption study. Antioxidants 2024, 13, 123. [Google Scholar] [CrossRef] [PubMed]
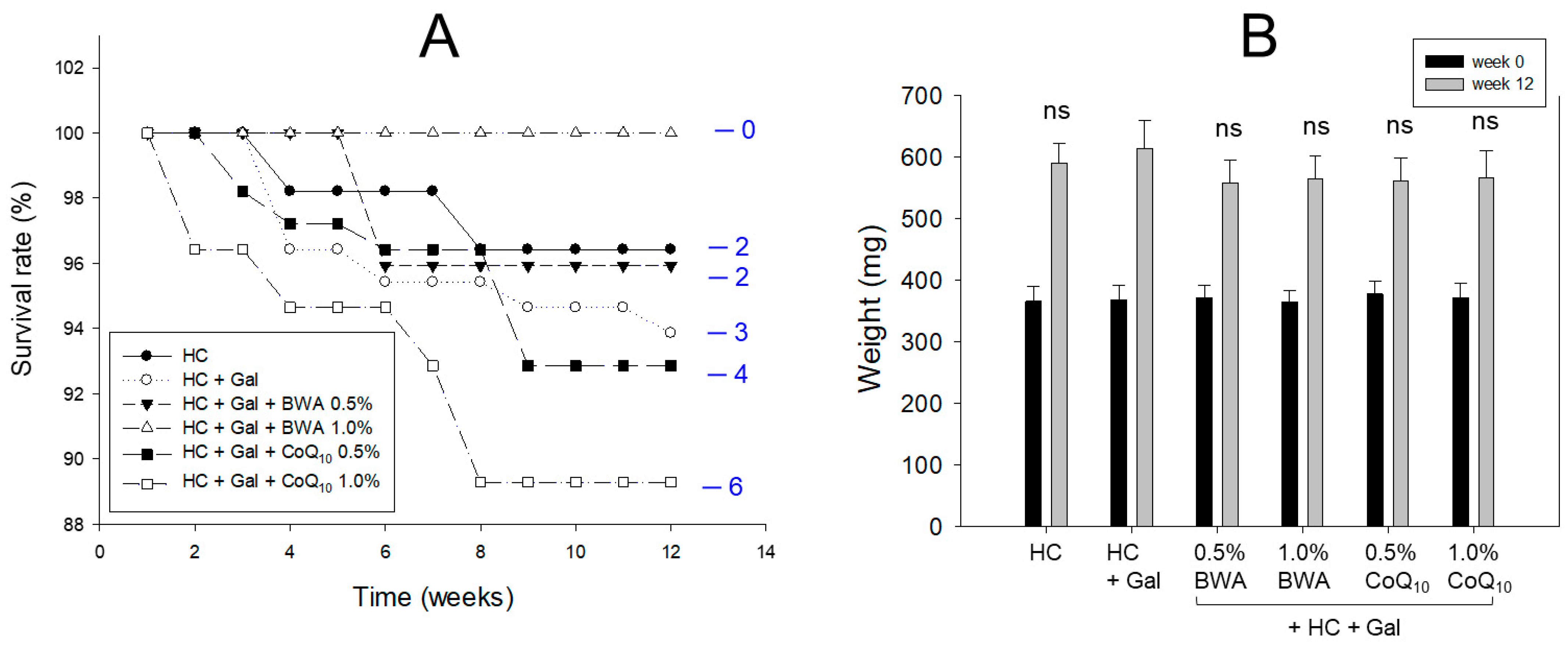


| Survivability and BW of Zebrafish | HC | HC+Gal | HC+Gal +0.5% BWA | HC+Gal +1.0% BWA | HC+Gal +0.5% CoQ10 | HC+Gal +1.0% CoQ10 |
|---|---|---|---|---|---|---|
| Week 0 (n) | 56 | 56 | 56 | 56 | 56 | 56 |
| Week 12 (n) | 54 | 52 | 54 | 56 | 52 | 50 |
| Survivability | 96.4 | 92.9 | 96.4 | 100.0 | 92.9 | 89.3 |
| BW at week 0 | 366.4 ± 24.5 | 368.3 ± 22.9 | 372.3 ± 20.1 | 365.1 ± 17.9 | 378 ± 21.5 | 372.3 ± 22.3 |
| BW at week 12 | 590.9 ± 31.8 | 614.3 ± 45.3 | 558.7 ± 37.4 | 564.5 ± 36.8 | 561.7 ± 37.0 | 565.9 ± 45.0 |
| Net increase in BW (mg) | 224.5 ± 8.7 | 246.1 ± 25.4 | 180.1 ± 19.8 | 212.4 ± 20 | 175.5 ± 16.2 | 164.7 ± 27 |
| Net increase in BW (%) | 161.3 ± 2.3 | 166.8 ± 3.9 | 150.1 ± 3.9 | 154.6 ± 3.0 | 148.6 ± 2.1 | 152.0 ± 4.7 |
| Diet (10 mg) | HC | HC + Gal | HC+Gal + 0.5% BWA | HC+Gal + 1.0% BWA | HC+Gal + 0.5% CoQ10 | HC+Gal + 1.0% CoQ10 |
|---|---|---|---|---|---|---|
| Tetrabits | 9.6 | 8.6 | 8.55 | 8.5 | 8.55 | 8.5 |
| Galactose | 0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Cholesterol | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| BWA | 0 | 0 | 0.05 | 0.1 | 0 | 0 |
| CoQ10 | 0 | 0 | 0 | 0 | 0.05 | 0.1 |
| Total (mg) | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.-H.; Bahuguna, A.; Kim, J.-E.; Lee, Y.; Lee, S.H. Comparative Assessment of Beeswax Alcohol and Coenzyme Q10 (CoQ10) to Prevent Liver Aging, Organ Damage, and Oxidative Stress in Hyperlipidemic Zebrafish Exposed to D-Galactose: A 12-Week Dietary Intervention. Pharmaceuticals 2024, 17, 1250. https://doi.org/10.3390/ph17091250
Cho K-H, Bahuguna A, Kim J-E, Lee Y, Lee SH. Comparative Assessment of Beeswax Alcohol and Coenzyme Q10 (CoQ10) to Prevent Liver Aging, Organ Damage, and Oxidative Stress in Hyperlipidemic Zebrafish Exposed to D-Galactose: A 12-Week Dietary Intervention. Pharmaceuticals. 2024; 17(9):1250. https://doi.org/10.3390/ph17091250
Chicago/Turabian StyleCho, Kyung-Hyun, Ashutosh Bahuguna, Ji-Eun Kim, Yunki Lee, and Sang Hyuk Lee. 2024. "Comparative Assessment of Beeswax Alcohol and Coenzyme Q10 (CoQ10) to Prevent Liver Aging, Organ Damage, and Oxidative Stress in Hyperlipidemic Zebrafish Exposed to D-Galactose: A 12-Week Dietary Intervention" Pharmaceuticals 17, no. 9: 1250. https://doi.org/10.3390/ph17091250
APA StyleCho, K. -H., Bahuguna, A., Kim, J. -E., Lee, Y., & Lee, S. H. (2024). Comparative Assessment of Beeswax Alcohol and Coenzyme Q10 (CoQ10) to Prevent Liver Aging, Organ Damage, and Oxidative Stress in Hyperlipidemic Zebrafish Exposed to D-Galactose: A 12-Week Dietary Intervention. Pharmaceuticals, 17(9), 1250. https://doi.org/10.3390/ph17091250







