Phytochemical and Biological Investigations of Crude Extracts of Astragalus pisidicus
Abstract
1. Introduction
2. Results
2.1. Extraction
2.2. Phytochemical Analysis of Extracts
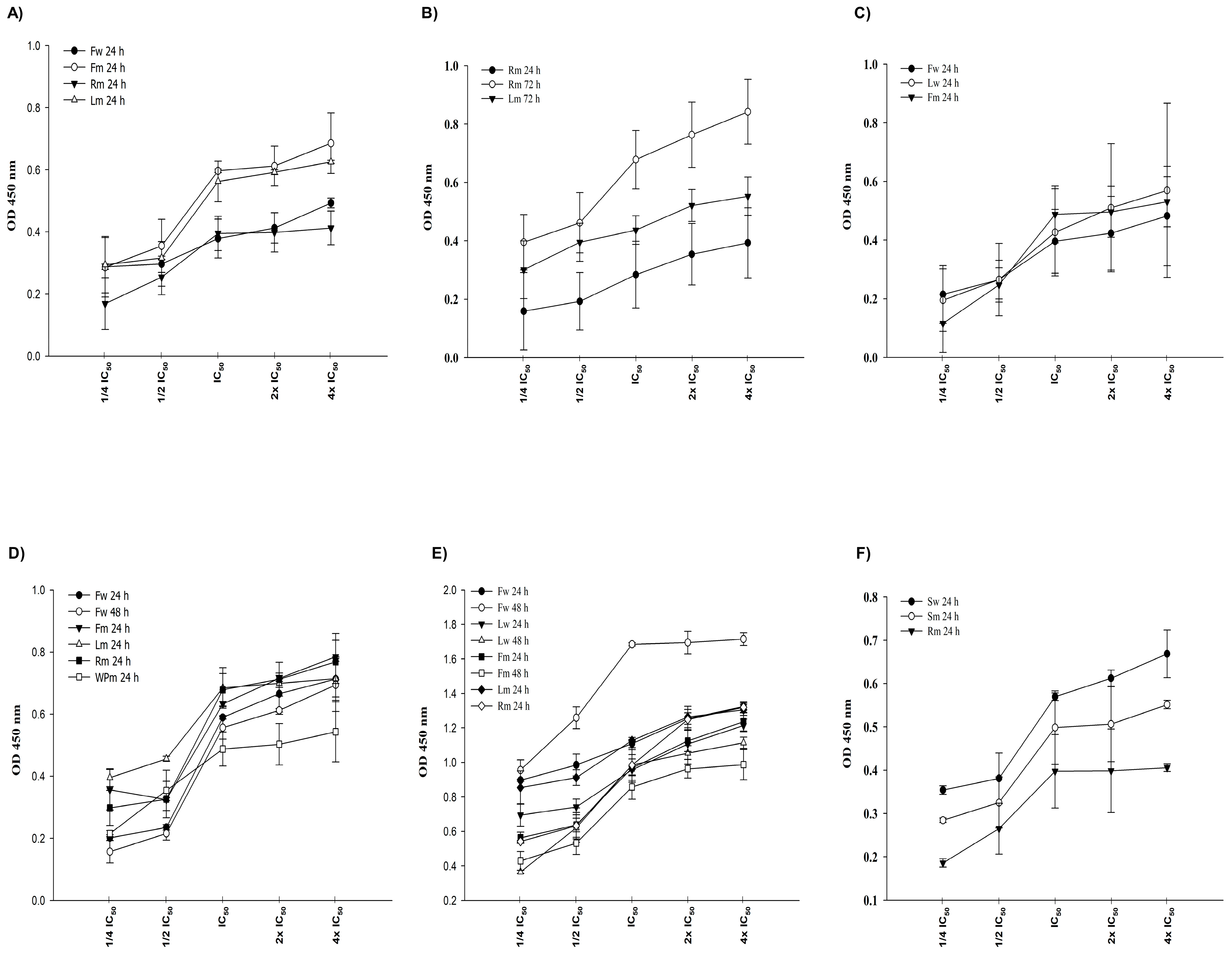
2.3. Cytotoxic Effects of A. pisidicus Extracts
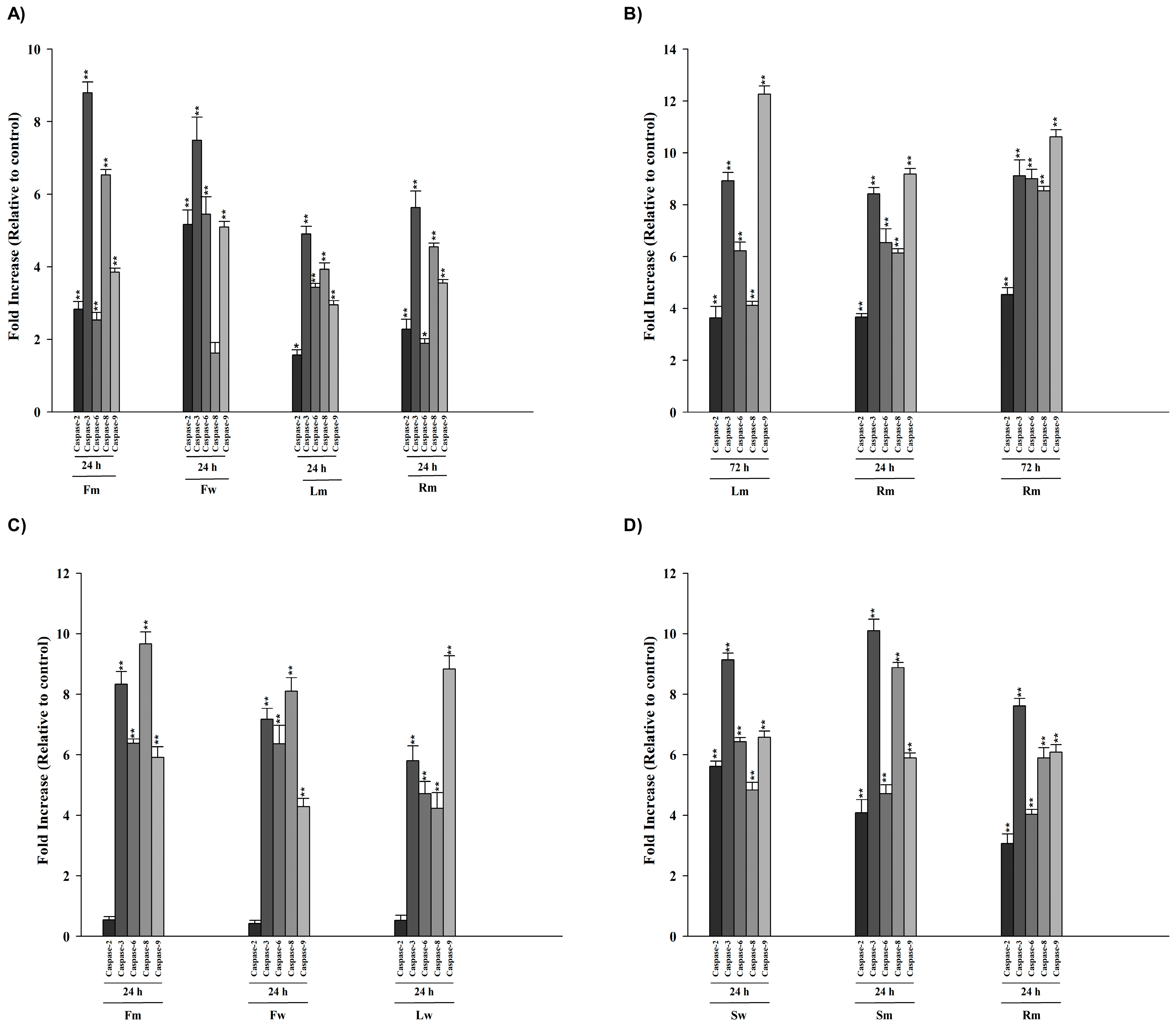
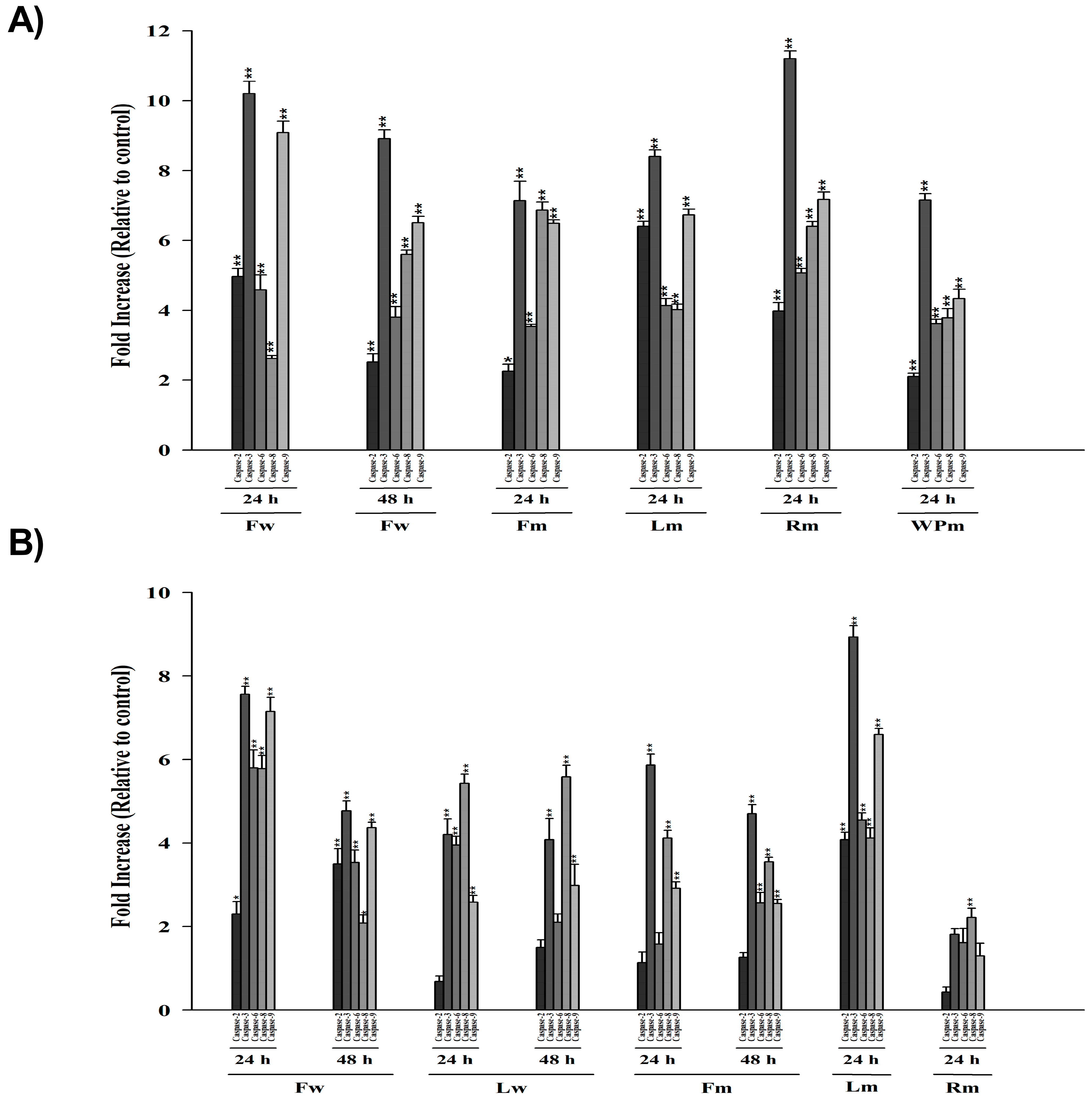
2.4. Colorimetric Protease (Caspase 2, -3, -6, -8, -9) Assay
2.5. Cellular DNA Fragmentation
2.6. Antimicrobial Activity
2.7. Determination of Antioxidant Activities of Extracts
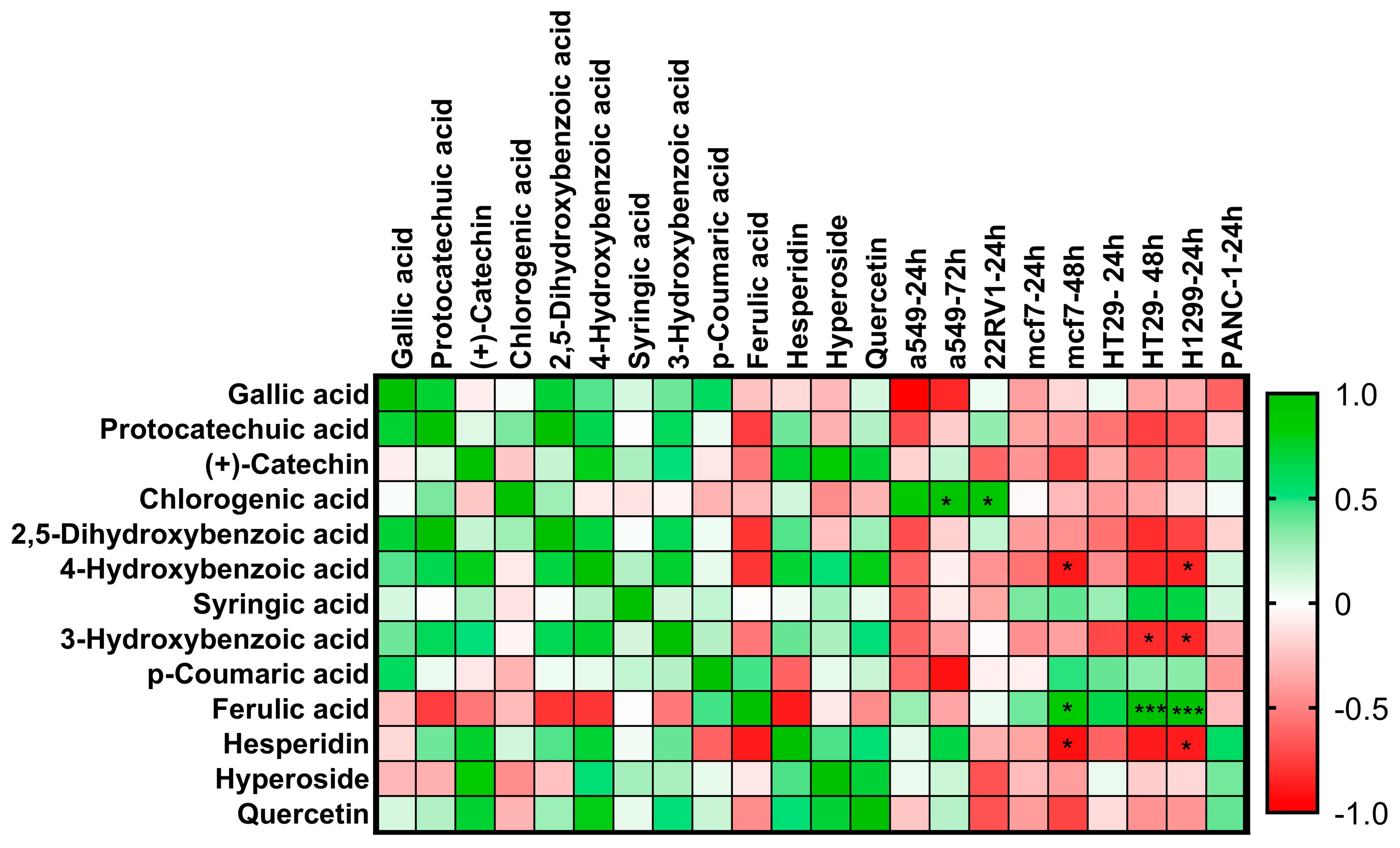
2.8. Pearson Correlation Analysis
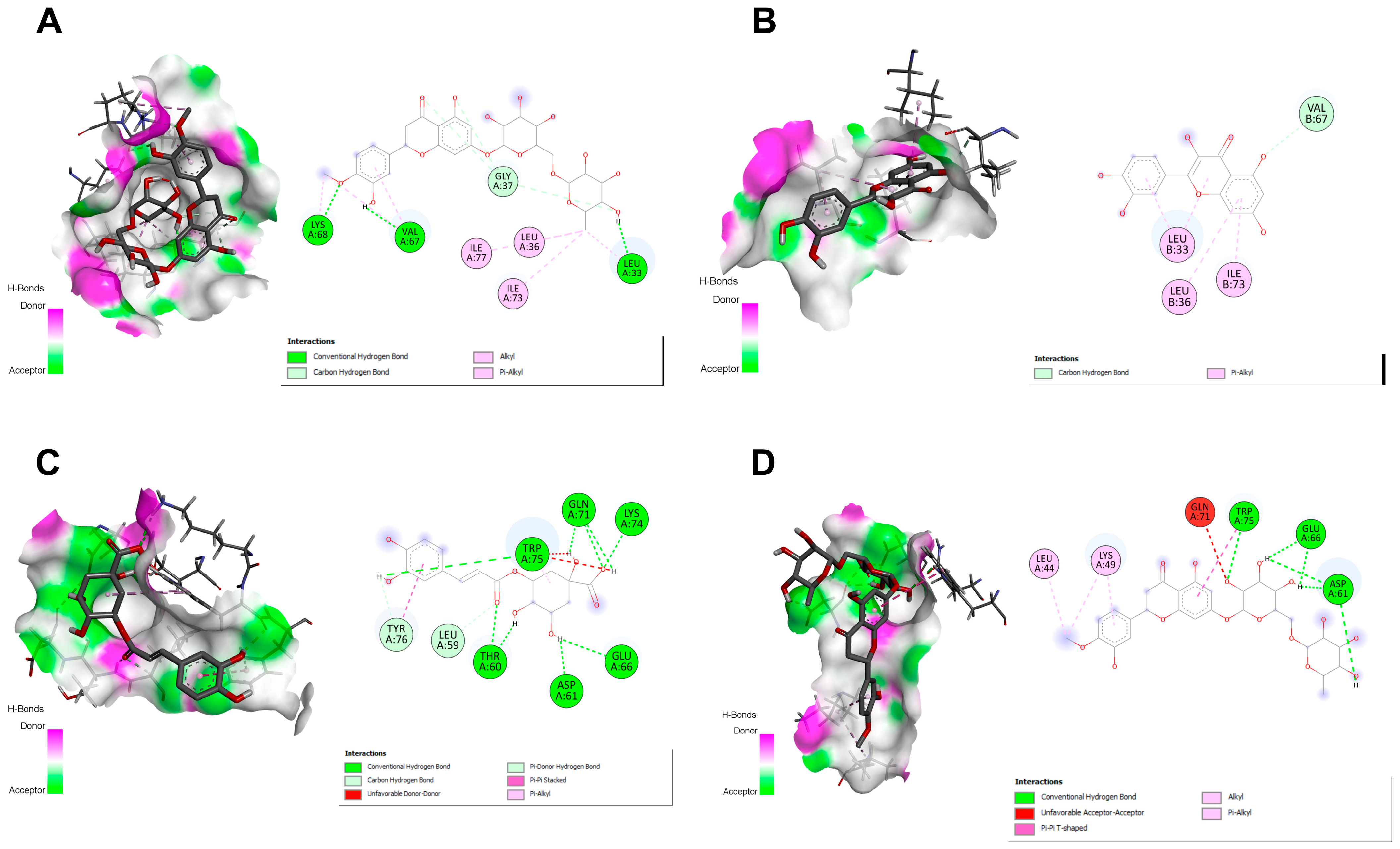
2.9. Docking Studies
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction
4.3. Identification of Phenolic Compounds by LC–ESI–MS/MS
4.4. Cell Culture
4.5. Cell Proliferation (WST-1) Assay
4.6. DNA Fragmentation
4.7. Caspase Assay
4.8. Antimicrobial Activity
4.8.1. Bacterial Strains
4.8.2. Disc Diffusion Method
4.8.3. Broth Microdilution Method
4.9. Antioxidant Activity
4.9.1. Total Phenolic Contents of the Extracts
4.9.2. Determination of Total Flavonoid Contents
4.9.3. DPPH Radical Scavenging Assay
4.9.4. Determination of CUPRAC
4.9.5. Ferric Reducing Antioxidant Power (FRAP)
4.9.6. Determination of Lipid Peroxidation Inhibitory Effect in β-Carotene/Linoleic Acid System
4.9.7. Determination of Total Antioxidant Capacity
4.9.8. Determination of Antioxidant Capacity Equivalent to Trolox
4.10. Pearson Correlation Analysis
4.11. Molecular Docking
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Podlech, D.; Zarre, S.H. Taxonomic Revision of the Genus Astragalus L. (Leguminosae) in the Old World; Vienna Natural History Museum: Vienna, Austria, 2013; Volume 2, ISBN 9783902421760. [Google Scholar]
- Aytac, Z.; Ekici, M. Astragalus L. In Türkiye Bitkileri Listesi (Damarlı Bitkiler); Güner, A., Aslan, S., Ekim, T., Vural, M., Babaç, M., Eds.; Nezahat Gökyiğit Botanik Bahçesi ve Flora Araştırmaları Derneği Yayını: İstanbul, Turkey, 2012; pp. 427–456. [Google Scholar]
- Salehi, B.; Carneiro, J.N.P.; Rocha, J.E.; Coutinho, H.D.M.; Morais Braga, M.F.B.; Sharifi-Rad, J.; Semwal, P.; Painuli, S.; Moujir, L.M.; de Zarate Machado, V.; et al. Astragalus Species: Insights on Its Chemical Composition toward Pharmacological Applications. Phytother. Res. 2021, 35, 2445–2476. [Google Scholar] [CrossRef]
- Altundag, E.; Ozturk, M. Ethnomedicinal Studies on the Plant Resources of East Anatolia, Turkey. Procedia Soc. Behav. Sci. 2011, 19, 756–777. [Google Scholar] [CrossRef]
- Bagheri, S.M.; Keyhani, L.; Heydari, M.; Dashti-R, M.H. Antinociceptive Activity of Astragalus gummifer Gum (Gum Tragacanth) through the Adrenergic System: A in vivo Study in Mice. J. Ayurveda Integr. Med. 2015, 6, 19–23. [Google Scholar] [CrossRef][Green Version]
- Sheik, A.; Kim, K.; Varaprasad, G.L.; Lee, H.; Kim, S.; Kim, E.; Shin, J.-Y.; Oh, S.Y.; Huh, Y.S. The Anti-Cancerous Activity of Adaptogenic Herb Astragalus membranaceus. Phytomedicine 2021, 91, 153698. [Google Scholar] [CrossRef]
- Hong, K.-F.; Liu, P.-Y.; Zhang, W.; Gui, D.-K.; Xu, Y.-H. The Efficacy and Safety of Astragalus as an Adjuvant Treatment for Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. J. Integr. Complement. Med. 2024, 30, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Gao, J.; Jiang, L.; Dai, Y. Astragalus Polysaccharide Ameliorates Renal Inflammatory Responses in a Diabetic Nephropathy by Suppressing the TLR4/NF-ΚB Pathway. Drug Des. Devel. Ther. 2023, 17, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Astragalus, an Ancient Medicinal Root in Traditional Chinese Medicine, a Gift from Silk Road. Int. J. Agric. Biol. Sci. 2019, 3, 27–38. [Google Scholar] [CrossRef]
- Fu, S.; Holla, S.; Zhu, H.; Fu, S.; Liu, K.; Vu, A.; Alhaj, Z.; Huynh, D.; Khaled Soliman, O.; Orengo, I. Fact-Checking Cosmetic Trends: Systematic Review of the Use of Topical Astragalus Derivatives to Treat Dermatologic Conditions. Our Dermatol. Online 2024, 15, 337–344. [Google Scholar] [CrossRef]
- Shahzad, M.; Shabbir, A.; Wojcikowski, K.; Wohlmuth, H.; Gobe, C.G. The Antioxidant Effects of Radix Astragali (Astragalus membranaceus and Related Species) in Protecting Tissues from Injury and Disease. Curr. Drug Targets 2016, 17, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-H.; Bao, Y.-M.; Wei, C.-L.; An, L.-J. Studies of Chemical Constituents and Their Antioxidant Activities from Astragalus mongholicus Bunge. Biomed. Environ. Sci. 2005, 18, 297–301. [Google Scholar] [PubMed]
- Guo, Z.; Lou, Y.; Kong, M.; Luo, Q.; Liu, Z.; Wu, J. A Systematic Review of Phytochemistry, Pharmacology and Pharmacokinetics on Astragali Radix: Implications for Astragali Radix as a Personalized Medicine. Int. J. Mol. Sci. 2019, 20, 1463. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Z.; Huang, L.; Zheng, S.; Wang, D.; Chen, S.; Zhang, H.; Yang, S. Review of the Botanical Characteristics, Phytochemistry, and Pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 2014, 28, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, Z.; Zhang, Z.; Cao, H.; Kong, L.; Ma, W.; Ren, W. A Review of the Botany, Phytochemistry, Traditional Uses, Pharmacology, Toxicology, and Quality Control of the Astragalus memeranaceus. Front. Pharmacol. 2023, 14, 1242318. [Google Scholar] [CrossRef]
- Zheng, Y.; Ren, W.; Zhang, L.; Zhang, Y.; Liu, D.; Liu, Y. A Review of the Pharmacological Action of Astragalus polysaccharide. Front. Pharmacol. 2020, 11, 349. [Google Scholar] [CrossRef]
- Chamberlain, D.F.; Matthews, V.A. Astragalus L. In Flora of Turkey and the East Aegean Islands; Davis, P.H., Ed.; Edinburgh University Press: Edinburgh, Scotland, 1970; Volume 3, pp. 49–254. [Google Scholar]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Kaur, R.; Bhardwaj, A.; Gupta, S. Cancer Treatment Therapies: Traditional to Modern Approaches to Combat Cancers. Mol. Biol. Rep. 2023, 50, 9663–9676. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef] [PubMed]
- Kubczak, M.; Szustka, A.; Rogalińska, M. Molecular Targets of Natural Compounds with Anti-Cancer Properties. Int. J. Mol. Sci. 2021, 22, 13659. [Google Scholar] [CrossRef]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In vitro Antimicrobial Activity of Some Medicinal Plants against Human Pathogenic Bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef]
- Yadav, S.; Kapley, A. Antibiotic Resistance: Global Health Crisis and Metagenomics. Biotechnol. Rep. 2021, 29, e00604. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef] [PubMed]
- Turker, A.U.; Koyluoglu, H. Biological Activities of Some Endemic Plants in Turkey. Rom. Biotechnol. Lett. 2012, 17, 6949–6961. [Google Scholar]
- Turker, A.; Yıldırım, A. Evaluation of Antibacterial and Antitumor Activities of Some Turkish Endemic Plants. Trop. J. Pharm. Res. 2014, 12, 1003. [Google Scholar] [CrossRef]
- Yildirim, A.; Uyar, E.; Turker, A. In vitro Culture of Endemic Astragalus gymnolobus Fischer and Comparison of Its Antibacterial, Antioxidant, and Phenolic Profiles with Field Grown Plants. J. Agr. Sci. Tech. 2020, 22, 815–828. [Google Scholar]
- Aydemir, E.; Odabaş Köse, E.; Yavuz, M.; Kilit, A.C.; Korkut, A.; Özkaya Gül, S.; Sarikurkcu, C.; Celep, M.E.; Göktürk, R.S. Phenolic Compound Profiles, Cytotoxic, Antioxidant, Antimicrobial Potentials and Molecular Docking Studies of Astragalus gymnolobus Methanolic Extracts. Plants 2024, 13, 658. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Sahinler, S.S.; Tepe, B. Astragalus gymnolobus, A. leporinus var. hirsutus, and A. onobrychis: Phytochemical analysis and biological activity. Ind. Crops Prod. 2020, 150, 112366. [Google Scholar] [CrossRef]
- Arumugam, R.; Kirkan, B.; Sarikurkcu, C. Phenolic Profile, Antioxidant and Enzyme Inhibitory Potential of Methanolic Extracts from Different Parts of Astragalus ponticus Pall. S. Afr. J. Bot. 2019, 120, 268–273. [Google Scholar] [CrossRef]
- Hasimi, N.; Ertas, A.; Yılmaz, M.A.; Boğa, M.; Temel, H.; Demirci, S.; Yılmaz-Özden, T.; Yener, I.; Kolak, U. LC-MS/MS and GC-MS Analyses of Three Endemic Astragalus Species from Anatolia towards Their Total Phenolicflavonoid Contents and Biological Activities. Biol. Divers Conserv. 2017, 10, 18–30. [Google Scholar]
- Sarikurkcu, C.; Zengin, G. Polyphenol Profile and Biological Activity Comparisons of Different Parts of Astragalus macrocephalus subsp. Finitimus Turk. Biol. 2020, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, F.; Zhang, X.; Li, P.; Zhang, X.; Wu, Z.; Li, D. In vitro Synergistic Antioxidant Activity and Identification of Antioxidant Components from Astragalus membranaceus and Paeonia lactiflora. PLoS ONE 2014, 9, e96780. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.-H.; Choi, D.; Choi, O.-Y.; Cho, K.-A.; Kim, R.; Choi, H.-S.; Cho, H. Effect of Astragalus sinicus L. Seed Extract on Antioxidant Activity. J. Ind. Eng. Chem. 2011, 17, 510–516. [Google Scholar] [CrossRef]
- Ionkova, I.; Shkondrov, A.; Zarev, Y.; Kozuharova, E.; Krasteva, I. Anticancer Secondary Metabolites: From Ethnopharmacology and Identification in Native Complexes to Biotechnological Studies in Species of Genus Astragalus L. and Gloriosa L. Curr. Issues Mol. Biol. 2022, 44, 3884–3904. [Google Scholar] [CrossRef]
- Zhou, L.; Li, M.; Chai, Z.; Zhang, J.; Cao, K.; Deng, L.; Liu, Y.; Jiao, C.; Zou, G.-M.; Wu, J.; et al. Anticancer Effects and Mechanisms of Astragaloside-IV. Oncol. Rep. 2022, 49, 5. [Google Scholar] [CrossRef]
- Yang, Q.; Meng, D.; Zhang, Q.; Wang, J. Advances in Research on the Anti-Tumor Mechanism of Astragalus polysaccharides. Front. Oncol. 2024, 14, 1334915. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, X.; Qin, S.; Yang, Q.; Na, J.; Xue, Z.; Zhong, L. Anticancer Mechanism of Astragalus polysaccharide and Its Application in Cancer Immunotherapy. Pharmaceuticals 2024, 17, 636. [Google Scholar] [CrossRef]
- Yusein-Myashkova, S.; Stoykov, I.; Gospodinov, A.; Ugrinova, I.; Pasheva, E. The Repair. Capacity of Lung Cancer Cell Lines A549 and H1299 Depends on HMGB1 Expression Level and the P53 Status. J. Biochem. 2016, 160, 37–47. [Google Scholar] [CrossRef]
- Nazari, M.; Ghorbani, A.; Hekmat-Doost, A.; Jeddi-Tehrani, M.; Zand, H. Inactivation of Nuclear Factor-ΚB by Citrus Flavanone Hesperidin Contributes to Apoptosis and Chemo-Sensitizing Effect in Ramos Cells. Eur. J. Pharmacol. 2011, 650, 526–533. [Google Scholar] [CrossRef]
- Rakshit, S.; Mandal, L.; Pal, B.C.; Bagchi, J.; Biswas, N.; Chaudhuri, J.; Chowdhury, A.A.; Manna, A.; Chaudhuri, U.; Konar, A.; et al. Involvement of ROS in Chlorogenic Acid-Induced Apoptosis of Bcr-Abl+ CML Cells. Biochem. Pharmacol. 2010, 80, 1662–1675. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Huo, H.; Tang, Q. Hesperidin Inhibits the P53-MDMXInteraction-Induced Apoptosis of Non-Small-Cell Lung Cancer and Enhances the Antitumor Effect of Carboplatin. J. Oncol. 2022, 2022, 5308577. [Google Scholar] [CrossRef]
- Ghorbani, A.; Nazari, M.; Jeddi-Tehrani, M.; Zand, H. The Citrus Flavonoid Hesperidin Induces P53 and Inhibits NF-ΚB Activation in Order to Trigger Apoptosis in NALM-6 Cells: Involvement of PPARγ-Dependent Mechanism. Eur. J. Nutr. 2012, 51, 39–46. [Google Scholar] [CrossRef]
- Verma, S.; Singh, A.; Mishra, A. Quercetin and Taxifolin Completely Break MDM2–P53 Association: Molecular Dynamics Simulation Study. Med. Chem. Res. 2013, 22, 2778–2787. [Google Scholar] [CrossRef]
- Caparica, R.; Rolim Baby, A.; Almeida, T.; Guilherme Costa, J. In vitro Cytotoxicity Assessment of Ferulic, Caffeic and p-Coumaric Acids on Human Renal Cancer Cells. Biomed. Biopharm. Res. J. 2020, 17, 63–74. [Google Scholar] [CrossRef]
- Bakholdina, L.A.; Markova, A.A.; Khlebnikov, A.I.; Sevodin, V.P. Cytotoxicity of New Ferulic-Acid Derivatives on Human Colon Carcinoma (HCT116) Cells. Pharm. Chem. J. 2019, 53, 516–520. [Google Scholar] [CrossRef]
- Nasr Bouzaiene, N.; Kilani Jaziri, S.; Kovacic, H.; Chekir-Ghedira, L.; Ghedira, K.; Luis, J. The Effects of Caffeic, Coumaric and Ferulic Acids on Proliferation, Superoxide Production, Adhesion and Migration of Human Tumor Cells in vitro. Eur. J. Pharmacol. 2015, 766, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Jose Merlin, J.P.; Venkadesh, B.; Hussain, R.; Rajan, S.S. Biochemical Estimations of Multidrug Resistance (Ferulic Acid and Paclitaxel) in Non-Small Cells Lung Carcinoma Cells In vitro. Biomed. Aging Pathol. 2013, 3, 47–50. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.; Guo, S.; Luan, F.; Liu, R.; Zeng, N. Ferulic Acid: A Review of Its Pharmacology, Pharmacokinetics and Derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Wang, T.; Fu, Y.; Yu, T.; Ding, Y.; Nie, H. Ferulic Acid: A Review of Pharmacology, Toxicology, and Therapeutic Effects on Pulmonary Diseases. Int. J. Mol. Sci. 2023, 24, 8011. [Google Scholar] [CrossRef] [PubMed]
- Singh Tuli, H.; Kumar, A.; Ramniwas, S.; Coudhary, R.; Aggarwal, D.; Kumar, M.; Sharma, U.; Chaturvedi Parashar, N.; Haque, S.; Sak, K. Ferulic Acid: A Natural Phenol That Inhibits Neoplastic Events through Modulation of Oncogenic Signaling. Molecules 2022, 27, 7653. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Singh, A.K.; Loka, M.; Pandey, A.K.; Bishayee, A. Ferulic Acid-Mediated Modulation of Apoptotic Signaling Pathways in Cancer. Adv. Protein Chem. Struct. Biol. 2021, 125, 215–257. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Nan, H.; Shi, N.; Hao, W.; Dong, J.; Chen, H. Chlorogenic Acid Inhibits Proliferation in Human Hepatoma Cells by Suppressing Noncanonical NF-ΚB Signaling Pathway and Triggering Mitochondrial Apoptosis. Mol. Biol. Rep. 2021, 48, 2351–2364. [Google Scholar] [CrossRef]
- Yamagata, K.; Izawa, Y.; Onodera, D.; Tagami, M. Chlorogenic Acid Regulates Apoptosis and Stem Cell Marker-Related Gene Expression in A549 Human Lung Cancer Cells. Mol. Cell Biochem. 2018, 441, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Boise, L.; Shanmugam, M. Cancer Metabolism and the Evasion of Apoptotic Cell Death. Cancers 2019, 11, 1144. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Canfield, K.; Feng, W.; Kurokawa, M. Metabolic Regulation of Apoptosis in Cancer. Int. Rev. Cell Mol. Biol. 2016, 327, 43–87. [Google Scholar] [CrossRef] [PubMed]
- Fernald, K.; Kurokawa, M. Evading Apoptosis in Cancer. Trends Cell Biol. 2013, 23, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Majtnerová, P.; Roušar, T. An Overview of Apoptosis Assays Detecting DNA Fragmentation. Mol. Biol. Rep. 2018, 45, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Nagase, H.; Kawane, K.; Mukae, N.; Fukuyama, H. Degradation of Chromosomal DNA during Apoptosis. Cell Death Differ. 2003, 10, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S. Apoptotic DNA Fragmentation. Exp. Cell Res. 2000, 256, 12–18. [Google Scholar] [CrossRef]
- Enari, M.; Sakahira, H.; Yokoyama, H.; Okawa, K.; Iwamatsu, A.; Nagata, S. Erratum: Correction: A Caspase-Activated DNase That Degrades DNA during Apoptosis and Its Inhibitor ICAD. Nature 1998, 393, 396. [Google Scholar] [CrossRef]
- Liu, X.; Zou, H.; Slaughter, C.; Wang, X. DFF, a Heterodimeric Protein That Functions Downstream of Caspase-3 to Trigger DNA Fragmentation during Apoptosis. Cell 1997, 89, 175–184. [Google Scholar] [CrossRef]
- Jaradat, N.; AL-Masri, M.; Zaid, A.N.; Hussien, A. Preliminary Phytochemical Screening and In-Vitro Evaluation of Antioxidant and Antimicrobial Activities for Astragalus pelecinus from Palestine. J. Mat. Environ. Sci. 2017, 8, 1492–1497. [Google Scholar]
- Shahrivari-Baviloliaei, S.; Erdogan Orhan, I.; Abaci Kaplan, N.; Konopacka, A.; Waleron, K.; Plenis, A.; Viapiana, A. Characterization of Phenolic Profile and Biological Properties of Astragalus membranaceus Fisch. Ex Bunge Commercial Samples. Antioxidants 2024, 13, 993. [Google Scholar] [CrossRef] [PubMed]
- Selim, S.; Albaqawi, A. Antimicrobial Activity of Methanol Extract of Anziroat (Astragalus sp.). In Proceedings of the 2nd Int’l Conference on Advances in Environment, Agriculture & Medical Sciences (ICAEAM’15), Antalya, Turkey, 11–12 June 2015; pp. 69–71. [Google Scholar]
- Albayrak, S.; Kaya, O. Antioxidant, Antimicrobial and Cytotoxic Activities of Endemic Astragalus argaeus Boiss. from Turkey. Hacettepe J. Biol. Chem. 2019, 47, 87–97. [Google Scholar]
- Teyeb, H.; Zanina, N.; Neffati, M.; Douki, W.; Najjar, M.F. Cytotoxic and Antibacterial Activities of Leaf Extracts of Astragalus gombiformis Pomel (Fabaceae) Growing Wild in Tunisia. Turk. J. Biol. 2012, 36, 53–58. [Google Scholar] [CrossRef]
- Keskin, C.; Ozen, H.Ç.; Toker, Z.; Kizil, G.; Kizil, M. Determination of In vitro Antioxidant and Antimicrobial Properties of Shoot and Root Extracts of Astragalus diphtherites FENZL var. diphtherites and Astragalus gymnalopecias RECH. FIL. Obtained by Different Solvents. KSU J. Agric. Nat. 2018, 21, 157–166. [Google Scholar] [CrossRef]
- Wink, M. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Joubert, E. HPLC Quantification of the Dihydrochalcones, Aspalathin and Nothofagin in Rooibos Tea (Aspalathus Linearis) as Affected by Processing. Food Chem. 1996, 55, 403–411. [Google Scholar] [CrossRef]
- Cittan, M.; Çelik, A. Development and Validation of an Analytical Methodology Based on Liquid Chromatography–Electrospray Tandem Mass Spectrometry for the Simultaneous Determination of Phenolic Compounds in Olive Leaf Extract. J. Chromatogr. Sci. 2018, 56, 336–343. [Google Scholar] [CrossRef]
- Yeh, C.-N.; Wu, R.-C.; Cheng, C.-T.; Tsai, C.-Y.; Chang, Y.-R.; Yeh, T.-S.; Wu, T.-H.; Lee, W.-C.; Chiang, K.-C. HO-1 Is a Favorable Prognostic Factor for HBV-HCC Patients Who Underwent Hepatectomy. Cancer Manag. Res. 2018, 10, 6049–6059. [Google Scholar] [CrossRef]
- Kustiati, U.; Ergün, S.; Karnati, S.; Nugrahaningsih, D.A.A.; Kusindarta, D.L.; Wihadmadyatami, H. Ethanolic Extract of Ocimum Sanctum Linn. Inhibits Cell Migration of Human Lung Adenocarcinoma Cells (A549) by Downregulation of Integrin Avβ3, A5β1, and VEGF. Sci. Pharm. 2022, 90, 69. [Google Scholar] [CrossRef]
- Jablonski, R.P.; Kim, S.; Cheresh, P.; Williams, D.B.; Morales-Nebreda, L.; Cheng, Y.; Yeldandi, A.; Bhorade, S.; Pardo, A.; Selman, M.; et al. SIRT3 Deficiency Promotes Lung Fibrosis by Augmenting Alveolar Epithelial Cell Mitochondrial DNA Damage and Apoptosis. FASEB J. 2017, 31, 2520–2532. [Google Scholar] [CrossRef]
- Nikhil, K.; Sharan, S.; Chakraborty, A.; Roy, P. Pterostilbene-Isothiocyanate Conjugate Suppresses Growth of Prostate Cancer Cells Irrespective of Androgen Receptor Status. PLoS ONE 2014, 9, e93335. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard—Twelfth Edition. CLSI document M02-A12; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; CLSI standard M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Miller, H.E. A Simplified Method for the Evaluation of Antioxidants. J. Am. Oil Chem. Soc. 1971, 48, 91. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, W.; Ali, G.; Ashraf, N.; Fatima, I.; Kayani, W.K.; Shaheen, H.; Ghoneim, M.M.; Abdelgawad, M.A.; Khames, A. Efficiency of Multiple Extraction Solvents on Antioxidant, Cytotoxic, and Phytotoxic Potential of Taraxacum officinale (L.) Weber Ex F.H. Wigg. from Poonch Valley, Azad Kashmir, Pakistan. Evid. Based Complement. Altern. Med. 2022, 2022, 5118553. [Google Scholar] [CrossRef]
- Das, P.R.; Darwish, A.G.; Ismail, A.; Haikal, A.M.; Gajjar, P.; Balasubramani, S.P.; Sheikh, M.B.; Tsolova, V.; Soliman, K.F.A.; Sherif, S.M.; et al. Diversity in Blueberry Genotypes and Developmental Stages Enables Discrepancy in the Bioactive Compounds, Metabolites, and Cytotoxicity. Food Chem. 2022, 374, 131632. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Albadari, N.; Du, Y.; Fowler, J.F.; Sang, H.T.; Xian, W.; McKeon, F.; Li, W.; Zhou, J.; Zhang, R. MDM2 Inhibitors for Cancer Therapy: The Past, Present, and Future. Pharmacol. Rev. 2024, 76, 414–453. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Zhang, H.; Liu, T.; Zhou, S.; Du, Y.; Xiong, J.; Yi, S.; Qu, C.-K.; Fu, H.; Zhou, M. Discovery of Dual Inhibitors of MDM2 and XIAP for Cancer Treatment. Cancer Cell 2016, 30, 623–636. [Google Scholar] [CrossRef]
- Bell, E.W.; Zhang, Y. DockRMSD: An Open-Source Tool for Atom Mapping and RMSD Calculation of Symmetric Molecules through Graph Isomorphism. J. Cheminform. 2019, 11, 40. [Google Scholar] [CrossRef]
| A. pisidicus | Methanol (%) | Water (%) |
|---|---|---|
| Root | 16.94 | 18.56 |
| Stem | 13.08 | 9.37 |
| Flower | 8.34 | 7.62 |
| Leaf | 20.39 | 19.89 |
| Whole plant | 21.48 | 16.27 |
| Methanol Extract | Water Extract | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Name | Fm | Sm | Rm | Lm | WPm | Fw | Sw | Rw | Lw | WPw |
| Gallic acid | 20.2 ± 0.3 a,x | 18.6 ± 0.2 a,x | 6.62 ± 0.46 c,x | 12.0 ± 0.7 b,x | 19.7 ± 0.1 a,x | 20.3 ± 0.2 a,x | 15.8 ± 0.3 b,y | 9.64 ± 0.03 e,y | 11.1 ± 0.3 d,x | 12.5 ± 0.3 c,y |
| Protocatechuic acid | 192 ± 3 a,x | 146 ± 1 b,x | 87.4 ± 1.5 dx, | 20.1 ± 0.1 e,x | 111 ± 2 c,x | 214 ± 5 a,y | 139 ± 1 b,y | 85.7 ± 0.2 d,x | 18.9 ± 0.3 e,y | 116 ± 1 c,x |
| Gentisic acid | 193 ± 3 a,x | 146 ± 3 b,x | 89.9 ± 1.9 d,x | 20.3 ± 0.2 e,x | 113 ± 1 c,x | 190 ± 3 a,x | 141 ± 4 b,x | 87.2 ± 0.2 d,x | 18.8 ± 0.3 e,y | 120 ± 1 c,y |
| (+)-Catechin | 8.22 ± 0.08 c,x | 275 ± 4 a,x | 151 ± 4 b,x | 9.11 ± 0.17 c | 141 ± 1 b,x | 2.81 ± 0.12 d,y | 174 ± 6 b,y | 151 ± 2 c,x | nd | 188 ± 4 a,y |
| Chlorogenic acid | 2.99 ± 0.07 e,x | 5.48 ± 0.09 d,x | 13.5 ± 0.1 a,x | 6.73 ± 0.17 b,x | 6.12 ± 0.20 c,x | 28.3 ± 0.3 a,y | 5.42 ± 0.44 c,x | 11.5 ± 0.1 b,y | 6.45 ± 0.33 c,x | 10.6 ± 0.3 b,y |
| 3-Hydroxybenzoic acid | 367 ± 4 c,x | 591 ± 3 a,x | 329 ± 3 d,x | 55.7 ± 0.1 e,x | 450 ± 1 b,x | 322 ± 1 c,y | 600 ± 11 a,x | 312 ± 3 c,y | 52.3 ± 1.0 d,y | 458 ± 3 b,x |
| p-Hydroxybenzoic acid | 359 ± 9 c,x | 599 ± 10 a,x | 331 ± 1 d,x | 54.7 ± 1.5 e,x | 451 ± 1 b,x | 326 ± 5 c,y | 589 ± 8 a,x | 310 ± 1 c,y | 50.6 ± 0.7 d,x | 465 ± 5 b,x |
| Syringic acid | 24.4 ± 0.2 b,x | 38.9 ± 1.5 a,x | 25.3 ± 1.7 b,x | 8.0 ± 0.7 c,x | 40.5 ± 2.8 a,x | 28.0 ± 0.8 b,y | 28.7 ± 3.8 b,y | 18.8 ± 1.4 c,x | 52.9 ± 2.3 a,y | 47.8 ± 0.5 a,x |
| p-Coumaric acid | 37.0 ± 1.9 b,x | 45.7 ± 1.0 a,x | 24.0 ± 0.6 c,x | 44.7 ± 2.0 a,x | 39.1 ± 0.5 b,x | 38.0 ± 2.0 bc,x | 43.2 ± 0.3 a,y | 21.4 ± 1.1 d,x | 40.3 ± 1.4 ab,x | 34.2 ± 0.3 c,y |
| Ferulic acid | 18.3 ± 1.6 cd,x | 20.9 ± 0.9 c,x | 11.0 ± 1.0 d,x | 132 ± 4 a,x | 34.0 ± 0. 8 b,x | 16.4 ± 0.1 cd,x | 21.5 ± 1.0 bc,x | 11.1 ± 0.5 d,x | 118 ± 3 a,x | 26.0 ± 0.2 b,x |
| Hesperidin | 9513 ± 79 d,x | 13450 ± 211 b,x | 19174 ± 32 a,x | 142 ± 3 e,x | 11768 ± 36 c,x | 8741 ± 99 d,y | 13544 ± 67 b,x | 17220 ± 268 a,y | 76.3 ± 4.9 c,y | 17400 ± 279 a,y |
| Hyperoside | 331 ± 4 e,x | 1630 ± 20 a,x | 1256 ± 12 c,x | 1021 ± 10 d,x | 1375 ± 3 b,x | 188 ± 3 e,y | 1630 ± 10 a,x | 1101 ± 6 c,y | 847 ± 2 d,y | 1542 ± 29 b,y |
| Quercetin | 25.9 ± 0.1 d,x | 103 ± 1 a,x | 75.2 ± 2.7 c,x | 13.2 ± 0.4 e,x | 85.6 ± 0.2 b,x | 15.1 ± 0.1 d,y | 179 ± 3 a,y | 43.4 ± 0.3 c,y | 4.20 ± 0.02 e,y | 50.8 ± 0.6 b,u |
| Cell | Extract | Hours | IC50 (µg/mL) | Cell | Extract | Hours | IC50 (µg/mL) |
|---|---|---|---|---|---|---|---|
| A549 | Rm | 24 h | 307.160 | HT29 | Lm | 24 h | 162.967 |
| 72 h | 379.765 | Rm | 24 h | 14.89 | |||
| Lm | 24 h | 207.257 | Fm | 24 h | 30.323 | ||
| MCF7 | Rm | 24 h | 540.998 | WPm | 24 h | 292.245 | |
| Lm | 24 h | 32.057 | Lw | 24 h | 258.594 | ||
| Fm | 24 h | 233.635 | 48 h | 28.640 | |||
| 48 h | 440.874 | H1299 | Fm | 24 h | 9.57 | ||
| Lw | 24 h | 972.290 | Lw | 24 h | 242.609 | ||
| 48 h | 998.651 | Fw | 24 h | 25.836 | |||
| Fw | 24 h | 173.904 | PANC1 | Rm | 24 h | 308.23 | |
| 48 h | 174.745 | Sm | 24 h | 9.57 | |||
| 22RV1 | Lm | 24 h | 249.059 | Sw | 24 h | 120.622 | |
| Rm | 24 h | 150.628 | |||||
| Fm | 24 h | 64.25 | |||||
| 48 h | 1048.57 | ||||||
| Fw | 24 h | 646.430 |
| Bacteria | Fm (mm) | Lm (mm) | Sm (mm) | Rm (mm) | WPm (mm) | A (mm) | N (mm) |
|---|---|---|---|---|---|---|---|
| S. aureus ATCC 25923 | 9 | 8 | 9 | - | 10 | 31 (P) | - |
| S. aureus ATCC 29213 | 8 | 8 | 8 | - | 9 | 19 (P) | - |
| S. aureus ATCC 43300 | 8 | 8 | 8 | - | 9 | 16 (FOX) | - |
| S. epidermidis ATCC 12228 | - | - | - | - | - | 21 (VA) | - |
| E. faecalis ATCC 51299 | - | - | - | - | 8 | 14 (VA) | - |
| E. faecalis ATCC 29212 | - | - | - | - | 8 | 20 (VA) | - |
| S. pyogenes ATCC 19615 | 11 | 9 | 10 | - | 12 | 42 (P) | - |
| Extract | TP (mg GAE/g) | TF (mg QE/g) | TOAC (mg AAE/g) | TEAC (µM TE/g) |
|---|---|---|---|---|
| Fm | 97.95 ± 11.53 | 14.37 ± 2.18 | 31 ± 1.37 | 168 ± 5.37 |
| Fw | 120.17 ± 7.96 | 14.37 ± 3.38 | 51 ± 5.07 | 154 ± 3.38 |
| Rm | 220.84 ± 9.80 | 38.78 ± 3.62 | 94 ± 7.14 | 248 ± 8.31 |
| Rw | 235.06 ± 3.45 | 34.61 ± 2.49 | 96 ± 8.13 | 234 ± 3.79 |
| Sm | 167.79 ± 10.07 | 23.54 ± 4.39 | 68 ± 3.68 | 201 ± 3.96 |
| Sw | 134.17 ± 6.59 | 20.73 ± 4.71 | 47 ± 2.19 | 197 ± 1.67 |
| Lm | 336.37 ± 9.998 | 70.14 ± 4.18 | 291 ± 4.49 | 700 9.31 |
| Lw | 364.42 ± 10.37 | 51.46 ± 6.37 | 294 ± 10.17 | 649 ± 7.19 |
| WPm | 317.68 ± 4.87 | 51.43 ± 5.07 | 276 ± 3.86 | 537 ± 4.13 |
| WPw | 304.94 ± 13.23 | 41. 62 ± 4.91 | 274 ± 4.89 | 499 ± 8.07 |
| Extract | DPPH (EC 50 µg/mL) | FRAP (mM FeSO4/g) | CUPRAC (mg AAE/g) | β-Caroten (% Activity of 1 mg/mL) |
|---|---|---|---|---|
| Fm | 744 ± 9.32 | 0.34 ± 0.05 | 22 ± 0.87 | 37.91 ± 1.34 |
| Fw | 768 ± 4.3 | 0.67 ± 0.06 | 24 ± 3.61 | 37.28 ± 3.64 |
| Rm | 384 ± 7.34 | 1.43 ± 0. 09 | 49 ± 3.42 | 76.84 ± 2.16 |
| Rw | 367 ± 5.63 | 1.30 ± 0.04 | 44 ± 4.27 | 71.37 ± 2.51 |
| Sm | 717 ± 15.57 | 0.87 ± 0.07 | 34 ± 3.95 | 48.88 ± 0.48 |
| Sw | 709 ± 16.38 | 0.93 ± 0.03 | 33 ± 4.10 | 43.19 ± 1.89 |
| Lm | 119 ± 10.11 | 4.19 ± 0.08 | 140 ± 7.36 | 88.47 ± 2.62 |
| Lw | 124 ± 8.39 | 3.67 ± 0.05 | 128 ± 4.58 | 91.37 ±1.73 |
| WPm | 187 ± 9.80 | 3.52 ± 0.1 | 138 ± 2.08 | 83.63 ± 3.18 |
| WPw | 146 ± 10.37 | 3.34 ± 0.06 | 125 ± 6.17 | 81.19 ± 2.85 |
| Compound | Binding Affinity (kcal/mol) | PubChem CID | ||
|---|---|---|---|---|
| MDM2 | XIAP | |||
| Hesperidin | −7.2 | −6.6 | 10621 | |
| Quercetin | −6.9 | −6.3 | 5280343 | |
| Hyperoside | −6.8 | −5.6 | 5281643 | |
| Catechin | −6.7 | −6.4 | 9064 | |
| Chlorogenic acid | −6.6 | −6.7 | 1794427 | |
| Ferulic acid | −5.5 | −5.5 | 445858 | |
| p-Coumaric acid | −5.4 | −4.9 | 637542 | |
| Gentisic acid | −5.0 | −4.9 | 3469 | |
| 3-Hydroxybenzoic acid | −5.0 | −4.5 | 7420 | |
| Syringic acid | −4.9 | −4.8 | 10742 | |
| Gallic acid | −4.7 | −4.6 | 370 | |
| Protocatechuic acid | −4.7 | −4.5 | 72 | |
| p-Hydroxybenzoic acid | −4.6 | −4.4 | 135 | |
| Co-crystallized ligands | −8.6 a | −8.4 b | 56591324 a | 134611691 b |
| Target Compounds | Rt (min) | Precursor Ion | MRM1 (CE, V) | MRM2 (CE, V) |
|---|---|---|---|---|
| Compounds analyzed by NI mode | ||||
| Gallic acid | 8.891 | 168.9 [M − H]− | 125.0 (10) | – |
| Protocatechuic acid | 10.818 | 152.9 [M − H]− | 108.9 (12) | – |
| 3,4-Dihydroxyphenylacetic acid | 11.224 | 167.0 [M − H]− | 123.0 (2) | – |
| (+)-Catechin | 11.369 | 289.0 [M − H]− | 245.0 (6) | 202.9 (12) |
| Pyrocatechol | 11.506 | 109.0 [M − H]− | 90.6 (18) | 52.9 (16) |
| 2,5-Dihydroxybenzoic acid | 12.412 | 152.9 [M − H]− | 109.0 (10) | – |
| 4-Hydroxybenzoic acid | 12.439 | 136.9 [M − H]− | 93.1 (14) | – |
| Caffeic acid | 12.841 | 179.0 [M − H]− | 135.0 (12) | – |
| Syringic acid | 12.963 | 196.9 [M − H]− | 181.9 (8) | 152.8 (6) |
| 3-Hydroxybenzoic acid | 13.259 | 137.0 [M − H]− | 93.0 (6) | – |
| Vanillin | 13.397 | 151.0 [M − H]− | 136.0 (10) | – |
| Verbascoside | 13.589 | 623.0 [M − H]− | 461.0 (26) | 160.8 (36) |
| Taxifolin | 13.909 | 303.0 [M − H]− | 285.1 (2) | 125.0 (14) |
| Sinapic acid | 13.992 | 222.9 [M − H]− | 207.9 (6) | 163.8 (6) |
| p-Coumaric acid | 14.022 | 162.9 [M − H]− | 119.0 (12) | – |
| Ferulic acid | 14.120 | 193.0 [M − H]− | 177.8 (8) | 134.0 (12) |
| Luteolin 7-glucoside | 14.266 | 447.1 [M − H]− | 285.0 (24) | – |
| Rosmarinic acid | 14.600 | 359.0 [M − H]− | 196.9 (10) | 160.9 (10) |
| 2-Hydroxycinnamic acid | 15.031 | 162.9 [M − H]− | 119.1 (10) | – |
| Pinoresinol | 15.118 | 357.0 [M − H]− | 151.0 (12) | 135.7 (34) |
| Eriodictyol | 15.247 | 287.0 [M − H]− | 151.0 (4) | 134.9 (22) |
| Quercetin | 15.668 | 301.0 [M − H]− | 178.6 (10) | 151.0 (16) |
| Kaempferol | 16.236 | 285.0 [M − H]− | 242.8 (16) | 229.1 (18) |
| Compounds analyzed by PI mode | ||||
| Chlorogenic acid | 11.802 | 355.0 [M + H]+ | 163.0 (10) | – |
| (−)-Epicatechin | 12.458 | 291.0 [M + H]+ | 139.1 (12) | 122.9 (36) |
| Hesperidin | 14.412 | 611.1 [M + H]+ | 449.2 (4) | 303.0 (20) |
| Hyperoside | 14.506 | 465.1 [M + H]+ | 303.1 (8) | – |
| Apigenin 7-glucoside | 14.781 | 433.1 [M + H]+ | 271.0 (18) | – |
| Luteolin | 15.923 | 287.0 [M + H]+ | 153.1 (34) | 135.1 (36) |
| Apigenin | 16.382 | 271.0 [M + H]+ | 153.0 (34) | 119.1 (36) |
| Linearity and Sensitivity Characteristics | |||||
|---|---|---|---|---|---|
| Compounds | Range (μg/L) | Linear Equation | R2 | LOD (μg/L) | LOQ (μg/L) |
| Gallic acid | 5–500 | y = 4.82x − 26.48 | 0.9988 | 1.46 | 4.88 |
| Protocatechuic acid | 2.5–500 | y = 5.65x − 9.99 | 0.9990 | 1.17 | 3.88 |
| 3,4-Dihydroxyphenylacetic acid | 5–500 | y = 5.13x − 12.39 | 0.9990 | 1.35 | 4.51 |
| (+)-Catechin | 10–500 | y = 1.45x + 1.95 | 0.9974 | 3.96 | 13.20 |
| Pyrocatechol | 25–400 | y = 0.11x − 0.52 | 0.9916 | 9.62 | 32.08 |
| Chlorogenic acid | 1–500 | y = 12.14x + 32.34 | 0.9995 | 0.55 | 1.82 |
| 2,5-Dihydroxybenzoic acid | 5–500 | y = 3.79x − 14.12 | 0.9980 | 2.12 | 7.08 |
| 4-Hydroxybenzoic acid | 5–500 | y = 7.62x + 22.79 | 0.9996 | 1.72 | 5.72 |
| (−)-Epicatechin | 5–500 | y = 9.11x − 9.99 | 0.9971 | 1.85 | 6.18 |
| Caffeic acid | 5–500 | y = 11.09x + 16.73 | 0.9997 | 3.15 | 10.50 |
| Syringic acid | 10–500 | y = 0.74x − 1.54 | 0.9975 | 3.75 | 12.50 |
| 3-Hydroxybenzoic acid | 5–500 | y = 3.69x − 12.29 | 0.9991 | 1.86 | 6.20 |
| Vanillin | 50–500 | y = 2.02x + 135.49 | 0.9926 | 15.23 | 50.77 |
| Verbascoside | 2.5–500 | y = 8.59x − 28.05 | 0.9988 | 0.82 | 2.75 |
| Taxifolin | 5–500 | y = 12.32x + 9.98 | 0.9993 | 1.82 | 6.05 |
| Sinapic acid | 5–500 | y = 2.09x − 6.79 | 0.9974 | 2.64 | 8.78 |
| p-Coumaric acid | 5–500 | y = 17.51x + 53.73 | 0.9997 | 1.93 | 6.44 |
| Ferulic acid | 5–500 | y = 3.32x − 4.30 | 0.9992 | 1.43 | 4.76 |
| Luteolin 7-glucoside | 1–500 | y = 45.25x + 156.48 | 0.9996 | 0.45 | 1.51 |
| Hesperidin | 5–500 | y = 5.98x + 0.42 | 0.9993 | 1.73 | 5.77 |
| Hyperoside | 2.5–500 | y = 16.32x − 1.26 | 0.9998 | 0.99 | 3.31 |
| Rosmarinic acid | 1–500 | y = 9.82x − 17.98 | 0.9989 | 0.57 | 1.89 |
| Apigenin 7-glucoside | 1–500 | y = 21.33x − 31.69 | 0.9983 | 0.41 | 1.35 |
| 2-Hydroxycinnamic acid | 1–500 | y = 16.72x − 26.94 | 0.9996 | 0.61 | 2.03 |
| Pinoresinol | 10–500 | y = 0.80x − 2.69 | 0.9966 | 3.94 | 13.12 |
| Eriodictyol | 2.5–500 | y = 14.24x − 0.50 | 0.9998 | 0.80 | 2.68 |
| Quercetin | 5–500 | y = 14.68x − 18.25 | 0.9997 | 1.23 | 4.10 |
| Luteolin | 5–500 | y = 8.96x + 26.80 | 0.9992 | 1.34 | 4.46 |
| Kaempferol | 10–500 | y = 0.82x − 3.06 | 0.9959 | 3.30 | 10.99 |
| Apigenin | 2.5–500 | y = 11.29x + 38.05 | 0.9987 | 0.96 | 3.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydemir, E.; Odabaş Köse, E.; Özkaya Gül, S.; Korkut, A.; Kilit, A.C.; Celep, M.E.; Yavuz, M.; Göktürk, R.S.; Sarikurkcu, C. Phytochemical and Biological Investigations of Crude Extracts of Astragalus pisidicus. Pharmaceuticals 2025, 18, 10. https://doi.org/10.3390/ph18010010
Aydemir E, Odabaş Köse E, Özkaya Gül S, Korkut A, Kilit AC, Celep ME, Yavuz M, Göktürk RS, Sarikurkcu C. Phytochemical and Biological Investigations of Crude Extracts of Astragalus pisidicus. Pharmaceuticals. 2025; 18(1):10. https://doi.org/10.3390/ph18010010
Chicago/Turabian StyleAydemir, Esra, Elif Odabaş Köse, Serap Özkaya Gül, Alaaddin Korkut, A. Cansu Kilit, Mehmet Engin Celep, Mustafa Yavuz, R. Süleyman Göktürk, and Cengiz Sarikurkcu. 2025. "Phytochemical and Biological Investigations of Crude Extracts of Astragalus pisidicus" Pharmaceuticals 18, no. 1: 10. https://doi.org/10.3390/ph18010010
APA StyleAydemir, E., Odabaş Köse, E., Özkaya Gül, S., Korkut, A., Kilit, A. C., Celep, M. E., Yavuz, M., Göktürk, R. S., & Sarikurkcu, C. (2025). Phytochemical and Biological Investigations of Crude Extracts of Astragalus pisidicus. Pharmaceuticals, 18(1), 10. https://doi.org/10.3390/ph18010010






