Neuroprotective Actions of Cannabinoids in the Bovine Isolated Retina: Role of Hydrogen Sulfide
Abstract
:1. Introduction
2. Results
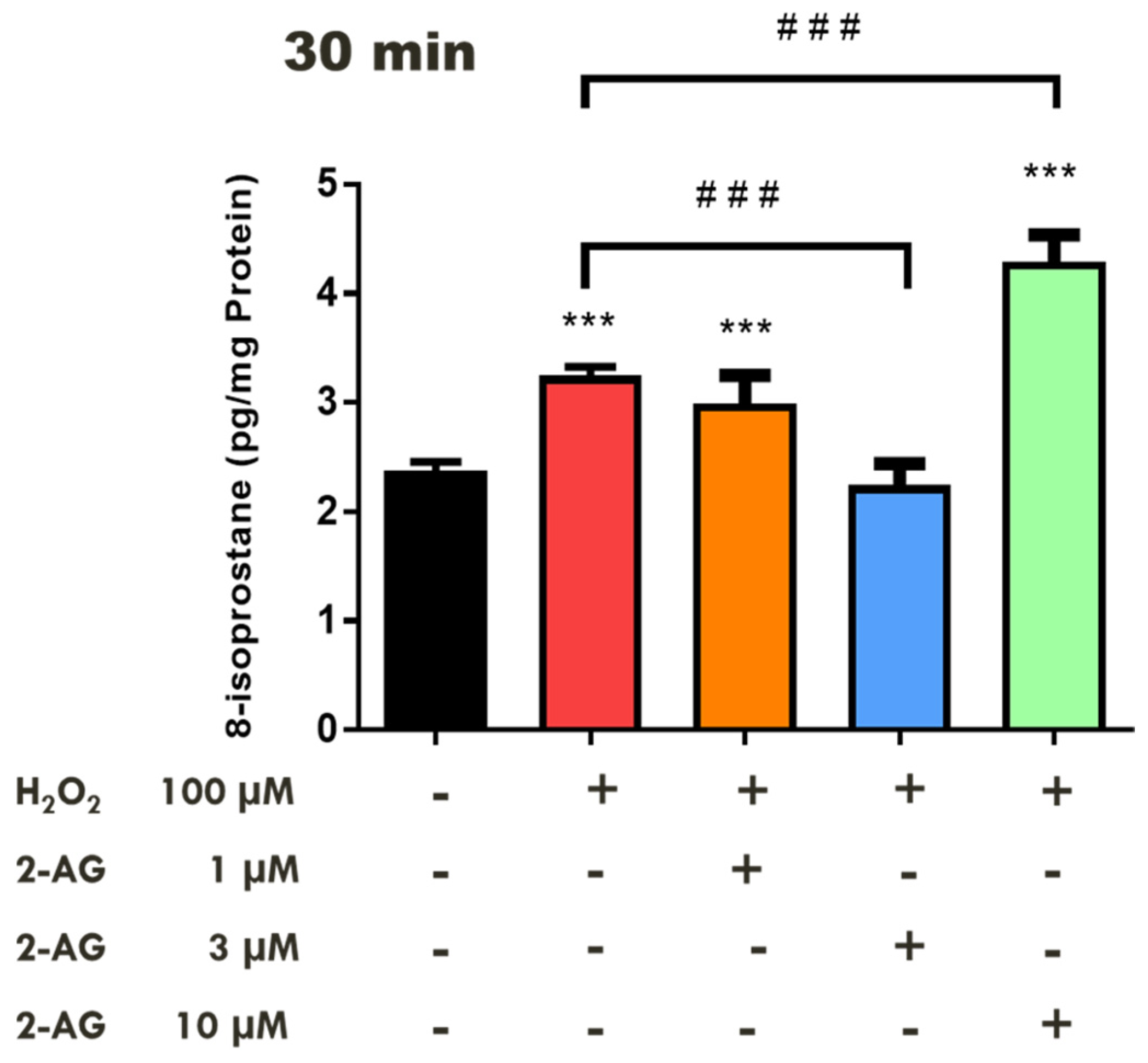
2.1. Effects of Cannabinoids on Lipid Peroxidation in the Bovine Retina
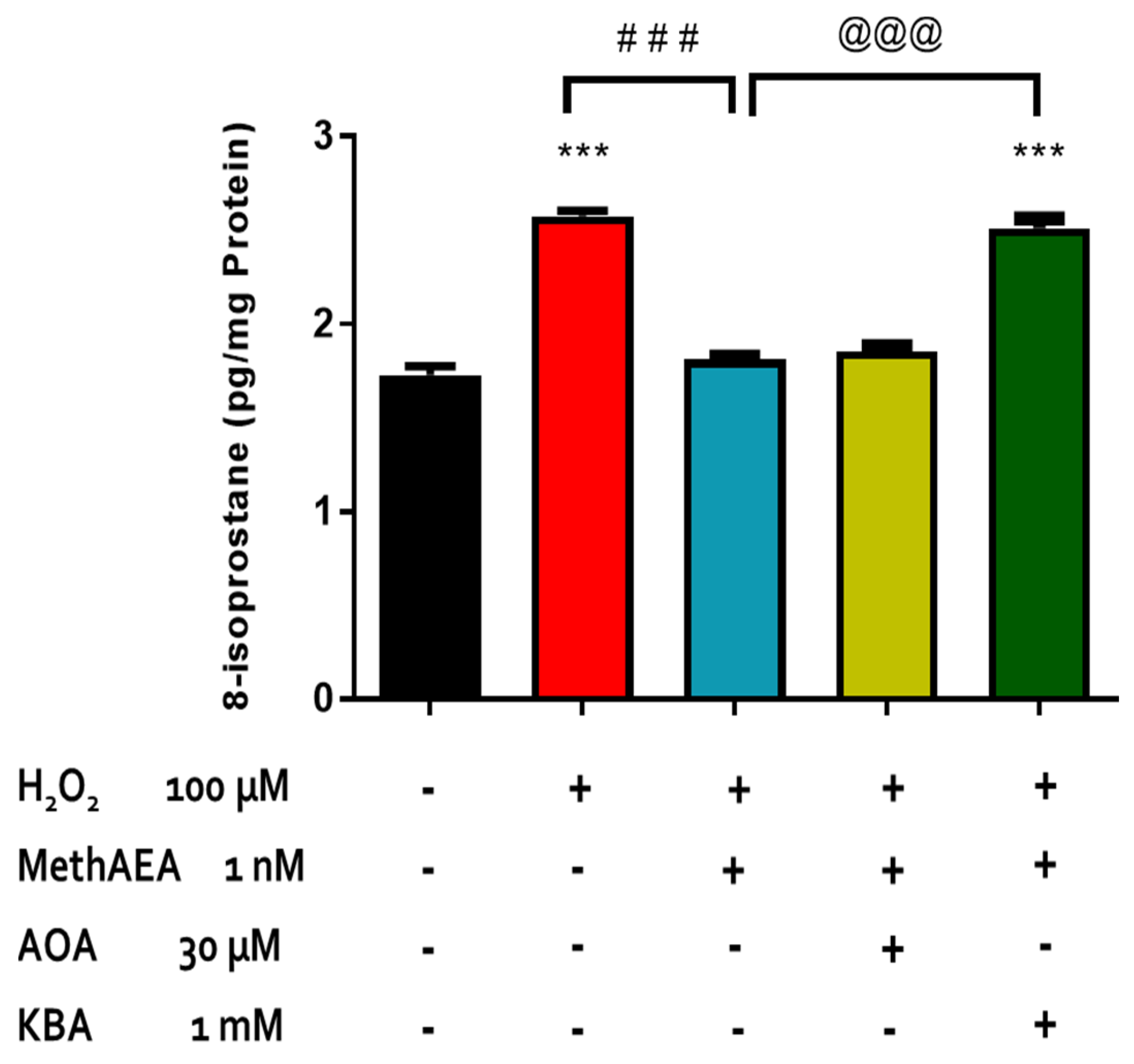
2.2. Involvement of CB1 Receptors and Intramurally Generated H2S in Cannabinoid-Mediated Neuroprotection in the Bovine Retina
3. Discussion
4. Methods
4.1. Chemicals
4.2. Tissue Preparations
4.3. 8-Isoprostane ELISA Assay
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mechoulam, R.; Parker, L.A. The endocannabinoid system and the brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R. The Endocannabinoid System: A Look Back and Ahead. Handb. Exp. Pharmacol. 2015, 231, vii–ix. [Google Scholar] [PubMed]
- Yazulla, S. Endocannabinoids in the retina: From marijuana to neuroprotection. Prog. Retin. Eye Res. 2008, 27, 501–526. [Google Scholar] [CrossRef] [PubMed]
- Katchan, V.; David, P.; Shoenfeld, Y. Cannabinoids and autoimmune diseases: A systematic review. Autoimmun. Rev. 2016, 15, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Kokona, D.; Georgiou, P.C.; Kounenidakis, M.; Kiagiadaki, F.; Thermos, K. Endogenous and Synthetic Cannabinoids as Therapeutics in Retinal Disease. Neural Plast. 2016, 2016, 8373020. [Google Scholar] [CrossRef]
- Nucci, C.; Gasperi, V.; Tartaglione, R.; Cerulli, A.; Terrinoni, A.; Bari, M.; De Simone, C.; Agro, A.F.; Morrone, L.A.; Corasaniti, M.T.; et al. Involvement of the endocannabinoid system in retinal damage after high intraocular pressure-induced ischemia in rats. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2997–3004. [Google Scholar] [CrossRef]
- Njie, Y.F.; He, F.; Qiao, Z.; Song, Z.H. Aqueous humor outflow effects of 2-arachidonylglycerol. Exp. Eye Res. 2008, 87, 106–114. [Google Scholar] [CrossRef]
- Njie, Y.F.; Qiao, Z.; Xiao, Z.; Wang, W.; Song, Z.H. N-arachidonylethanolamide-induced increase in aqueous humor outflow facility. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4528–4534. [Google Scholar] [CrossRef]
- Cairns, E.A.; Baldridge, W.H.; Kelly, M.E. The Endocannabinoid System as a Therapeutic Target in Glaucoma. Neural Plast. 2016, 2016, 9364091. [Google Scholar] [CrossRef]
- Chen, J.; Matias, I.; Dinh, T.; Lu, T.; Venezia, S.; Nieves, A.; Woodward, D.F.; Di Marzo, V. Finding of endocannabinoids in human eye tissues: Implications for glaucoma. Biochem. Biophys. Res. Commun. 2005, 330, 1062–1067. [Google Scholar] [CrossRef]
- Matias, I.; Wang, J.W.; Moriello, A.S.; Nieves, A.; Woodward, D.F.; Di Marzo, V. Changes in endocannabinoid and palmitoylethanolamide levels in eye tissues of patients with diabetic retinopathy and age-related macular degeneration. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.S.; Moesgaard, B.; Hansen, H.H.; Petersen, G. N-Acylethanolamines and precursor phospholipids—Relation to cell injury. Chem. Phys. Lipids 2000, 108, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Hydrogen sulfide as a physiological mediator: Its function and therapeutic applications. Nihon Yakurigaku Zasshi 2010, 136, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Mikami, Y.; Shibuya, N.; Kimura, Y.; Nagahara, N.; Yamada, M.; Kimura, H. Hydrogen sulfide protects the retina from light-induced degeneration by the modulation of Ca2+ influx. J. Biol. Chem. 2011, 286, 39379–39386. [Google Scholar] [CrossRef]
- Nagai, Y.; Tsugane, M.; Oka, J.; Kimura, H. Hydrogen sulfide induces calcium waves in astrocytes. FASEB J. 2004, 18, 557–559. [Google Scholar] [CrossRef]
- Opere, C.A.; Zheng, W.D.; Zhao, M.; Lee, J.S.; Kulkarni, K.H.; Ohia, S.E. Inhibition of potassium- and ischemia-evoked [3H] D-aspartate release from isolated bovine retina by cannabinoids. Curr. Eye Res. 2006, 31, 645–653. [Google Scholar] [CrossRef]
- Opere, C.A.; Monjok, E.M.; Kulkarni, K.H.; Njie, Y.F.; Ohia, S.E. Regulation of [3H] D-aspartate release from mammalian isolated retinae by hydrogen sulfide. Neurochem. Res. 2009, 34, 1962–1968. [Google Scholar] [CrossRef]
- Telezhkin, V.; Brazier, S.P.; Cayzac, S.; Muller, C.T.; Riccardi, D.; Kemp, P.J. Hydrogen sulfide inhibits human BK(Ca) channels. Adv. Exp. Med. Biol. 2009, 648, 65–72. [Google Scholar]
- Kimura, H. Hydrogen sulfide: Its production, release and functions. Amino Acids 2011, 41, 113–121. [Google Scholar] [CrossRef]
- Osborne, N.N.; Ji, D.; Majid, A.S.A.; Fawcett, R.J.; Sparatore, A.; Del Soldato, P. ACS67, a hydrogen sulfide-releasing derivative of latanoprost acid, attenuates retinal ischemia and oxidative stress to RGC-5 cells in culture. Investig. Ophthalmol. Vis. Sci. 2010, 51, 284–294. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, X.; Zhao, F.; Zhao, P.Q.; Kang, X.L. Cannabinoid receptor 1 blockade protects human retinal pigment epithelial cells from oxidative injury. Mol. Vis. 2013, 19, 357–366. [Google Scholar] [PubMed]
- Krishnan, G.; Chatterjee, N. Endocannabinoids alleviate proinflammatory conditions by modulating innate immune response in muller glia during inflammation. Glia 2012, 60, 1629–1645. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.F.; Wang, J.; Guan, J.; Zhou, L.; Sheng, Y.; Zhao, J. Treatment with hydrogen sulfide alleviates streptozotocin-induced diabetic retinopathy in rats. Br. J. Pharmacol. 2013, 169, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Straiker, A.; Stella, N.; Piomelli, D.; Mackie, K.; Karten, H.J.; Maguire, G. Cannabinoid CB1 receptors and ligands in vertebrate retina: Localization and function of an endogenous signaling system. Proc. Natl. Acad. Sci. USA 1999, 96, 14565–14570. [Google Scholar] [CrossRef] [PubMed]
- Smart, D.; Gunthorpe, M.J.; Jerman, J.C.; Nasir, S.; Gray, J.; Muir, A.I.; Chambers, J.K.; Randall, A.D.; Davis, J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1). Br. J. Pharmacol. 2000, 129, 227–230. [Google Scholar] [CrossRef]
- Kapoor, A.; Thiemermann, C. Hydrogen sulfide, neurogenic inflammation, and cardioprotection: A tale of rotten eggs and vanilloid receptors. Crit. Care Med. 2010, 38, 728–730. [Google Scholar] [CrossRef]
- Abadji, V.; Lin, S.; Taha, G.; Griffin, G.; Stevenson, L.A.; Pertwee, R.G.; Makriyannis, A. (R)-methanandamide: A chiral novel anandamide possessing higher potency and metabolic stability. J. Med. Chem. 1994, 37, 1889–1893. [Google Scholar] [CrossRef]
- Romano, M.R.; Lograno, M.D. Cannabinoid agonists induce relaxation in the bovine ophthalmic artery: Evidences for CB1 receptors, nitric oxide and potassium channels. Br. J. Pharmacol. 2006, 147, 917–925. [Google Scholar] [CrossRef]
- Romano, M.R.; Lograno, M.D. Involvement of the peroxisome proliferator-activated receptor (PPAR) alpha in vascular response of endocannabinoids in the bovine ophthalmic artery. Eur. J. Pharmacol. 2012, 683, 197–203. [Google Scholar] [CrossRef]
- Njie-Mbye, Y.F.; Kulkarni-Chitnis, M.; Opere, C.A.; Barrett, A.; Ohia, S.E. Lipid peroxidation: Pathophysiological and pharmacological implications in the eye. Front. Physiol. 2013, 4, 366. [Google Scholar] [CrossRef]
- Kulkarni-Chitnis, M.; Njie-Mbye, Y.F.; Mitchell, L.; Robinson, J.; Whiteman, M.; Wood, M.E.; Opere, C.A.; Ohia, S.E. Inhibitory action of novel hydrogen sulfide donors on bovine isolated posterior ciliary arteries. Exp. Eye Res. 2015, 134, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Ohia, S.E.; Bush, L.; Robinson, J.; Opere, C.; Njie-Mbye, Y.F. A Neuroprotective Action of Cannabinoids in The Bovine Isolated Retina: Role of Hydrogen Sulfide. In Proceedings of the Annual Meeting of the Japanese Pharmacological Society WCP2018 (The 18th World Congress of Basic and Clinical Pharmacology), Kyoto, Japan, 1–6 July 2018; Japanese Pharmacological Society: Tokyo, Japan, 2018; p. PO2-1. [Google Scholar]
- Bush, L.; Robinson, J.; Okolie, A.; Muili, F.; Opere, C.A.; Whiteman, M.; Ohia, S.E.; Mbye, Y.F.N. Neuroprotective Actions of Hydrogen Sulfide-Releasing Compounds in Isolated Bovine Retinae. Pharmaceuticals 2024, 17, 1311. [Google Scholar] [CrossRef]
- El-Remessy, A.B.; Khalil, I.E.; Matragoon, S.; Abou-Mohamed, G.; Tsai, N.J.; Roon, P.; Caldwell, R.B.; Caldwell, R.W.; Green, K.; Liou, G.I. Neuroprotective effect of (-)Delta9-tetrahydrocannabinol and cannabidiol in N- methyl-D-aspartate-induced retinal neurotoxicity: Involvement of peroxynitrite. Am. J. Pathol. 2003, 163, 1997–2008. [Google Scholar] [CrossRef] [PubMed]
- Parolini, M.; Binelli, A. Oxidative and genetic responses induced by Delta-9-tetrahydrocannabinol (Delta-9-THC) to Dreissena polymorpha. Sci. Total Environ. 2014, 468–469, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Rangel-López, E.; Colín-González, A.L.; Paz-Loyola, A.L.; Pinzón, E.; Torres, I.; Serratos, I.N.; Castellanos, P.; Wajner, M.; Souza, D.O.; Santamaría, A. Cannabinoid receptor agonists reduce the short-term mitochondrial dysfunction and oxidative stress linked to excitotoxicity in the rat brain. Neuroscience 2015, 285, 97–106. [Google Scholar] [CrossRef]
- Beltowski, J. Endogenous hydrogen sulfide in perivascular adipose tissue: Role in the regulation of vascular tone in physiology and pathology. Can. J. Physiol. Pharmacol. 2013, 91, 889–898. [Google Scholar] [CrossRef]
- Wallace, J.L.; Flannigan, K.L.; McKnight, W.; Wang, L.; Ferraz, J.G.; Tuitt, D. Pro-resolution, protective and anti-nociceptive effects of a cannabis extract in the rat gastrointestinal tract. J. Physiol. Pharmacol. 2013, 64, 167–175. [Google Scholar]
- Castany, S.; Carcole, M.; Leanez, S.; Pol, O. The role of carbon monoxide on the anti-nociceptive effects and expression of cannabinoid 2 receptors during painful diabetic neuropathy in mice. Psychopharmacology 2016, 233, 2209–2219. [Google Scholar] [CrossRef]
- Lipina, C.; Hundal, H.S. The endocannabinoid system: ‘NO’ longer anonymous in the control of nitrergic signalling? J. Mol. Cell Biol. 2017, 9, 91–103. [Google Scholar] [CrossRef]
- Pacher, P.; Batkai, S.; Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar]
- Sarzani, R. Endocannabinoids, blood pressure and the human heart. J. Neuroendocrinol. 2008, 20 (Suppl. S1), 58–62. [Google Scholar] [CrossRef] [PubMed]
- Schwitzer, T.; Schwan, R.; Angioi-Duprez, K.; Giersch, A.; Laprevote, V. The Endocannabinoid System in the Retina: From Physiology to Practical and Therapeutic Applications. Neural Plast. 2016, 2016, 2916732. [Google Scholar] [CrossRef]
- Paloczi, J.; Varga, Z.V.; Hasko, G.; Pacher, P. Neuroprotection in Oxidative Stress-Related Neurodegenerative Diseases: Role of Endocannabinoid System Modulation. Antioxid. Redox Signal. 2017, 29, 75–108. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.E.; Younts, T.J.; Chavez, A.E.; Hashimotodani, Y. Endocannabinoid signaling and synaptic function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Hervera, A.; Leanez, S.; Motterlini, R.; Pol, O. Treatment with carbon monoxide-releasing molecules and an HO-1 inducer enhances the effects and expression of micro-opioid receptors during neuropathic pain. Anesthesiology 2013, 118, 1180–1197. [Google Scholar] [CrossRef]
- Carcole, M.; Castany, S.; Leanez, S.; Pol, O. Treatment with a heme oxygenase 1 inducer enhances the antinociceptive effects of micro-opioid, delta- opioid, and cannabinoid 2 receptors during inflammatory pain. J. Pharmacol. Exp. Ther. 2014, 351, 224–232. [Google Scholar] [CrossRef]
- Schurman, L.D.; Lichtman, A.H. Endocannabinoids: A Promising Impact for Traumatic Brain Injury. Front. Pharmacol. 2017, 8, 69. [Google Scholar] [CrossRef]
- Amenta, P.S.; Jallo, J.I.; Tuma, R.F.; Elliott, M.B. A cannabinoid type 2 receptor agonist attenuates blood-brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. J. Neurosci. Res. 2012, 90, 2293–2305. [Google Scholar] [CrossRef]
- Lepicier, P.; Bouchard, J.F.; Lagneux, C.; Lamontagne, D. Endocannabinoids protect the rat isolated heart against ischaemia. Br. J. Pharmacol. 2003, 139, 805–815. [Google Scholar] [CrossRef]
- Cernak, I.; Vink, R.; Natale, J.; Stoica, B.; Lea PMt Movsesyan, V.; Ahmed, F.; Knoblach, S.M.; Fricke, S.T.; Faden, A.I. The “dark side” of endocannabinoids: A neurotoxic role for anandamide. J. Cereb. Blood Flow. Metab. 2004, 24, 564–578. [Google Scholar] [CrossRef]
- Movsesyan, V.A.; Stoica, B.A.; Yakovlev, A.G.; Knoblach, S.M.; Lea PMt Cernak, I.; Vink, R.; Faden, A.I. Anandamide-induced cell death in primary neuronal cultures: Role of calpain and caspase pathways. Cell Death Differ. 2004, 11, 1121–1132. [Google Scholar] [CrossRef]
- Kello, M.; Mikes, J.; Jendzelovsky, R.; Koval, J.; Fedorocko, P. PUFAs enhance oxidative stress and apoptosis in tumour cells exposed to hypericin-mediated PDT. Photochem. Photobiol. Sci. 2010, 9, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Norris, S.E.; Mitchell, T.W.; Else, P.L. Phospholipid peroxidation: Lack of effect of fatty acid pairing. Lipids 2012, 47, 451–460. [Google Scholar] [CrossRef]
- Navarrete, C.M.; Fiebich, B.L.; de Vinuesa, A.G.; Hess, S.; de Oliveira, A.C.; Candelario-Jalil, E.; Caballero, F.J.; Calzado, M.A.; Munoz, E. Opposite effects of anandamide and N-arachidonoyl dopamine in the regulation of prostaglandin E and 8-iso-PGF formation in primary glial cells. J. Neurochem. 2009, 109, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Kim, J.E.; Oh, S.M.; Cha, W.J.; Hah, J.H.; Sung, M.W. Anticancer effects of anandamide on head and neck squamous cell carcinoma cells via the production of receptor-independent reactive oxygen species. Head Neck 2015, 37, 1187–1192. [Google Scholar] [CrossRef]
- Jarvinen, T.; Pate, D.W.; Laine, K. Cannabinoids in the treatment of glaucoma. Pharmacol. Ther. 2002, 95, 203–220. [Google Scholar] [CrossRef]
- Wang, M.T.; Danesh-Meyer, H.V. Cannabinoids and the eye. Surv. Ophthalmol. 2021, 66, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Payne, S. Eicosanoids in bovine retinal microcirculation. J. Ocul. Pharmacol. Ther. 1997, 13, 139–149. [Google Scholar] [CrossRef] [PubMed]
- LeDay, A.M.; Kulkarni, K.H.; Opere, C.A.; Ohia, S.E. Arachidonic acid metabolites and peroxide-induced inhibition of [3H]D-aspartate release from bovine isolated retinae. Curr. Eye Res. 2004, 28, 367–372. [Google Scholar] [CrossRef]
- Matsuda, K.; Ohnishi, K.; Misaka, E.; Yamazaki, M. Decrease of urinary prostaglandin E2 and prostaglandin F2 alpha excretion by nonsteroidal anti-inflammatory drugs in rats. Relationship to anti-inflammatory activity. Biochem. Pharmacol. 1983, 32, 1347–1352. [Google Scholar] [CrossRef]
- Zhan, G.-L.; Ohia, S.; Camras, C.; Ohia, E.; Wang, Y. Superior cervical ganglionectomy-induced lowering of intraocular pressure in rabbits: Role of prostaglandins and neuropeptide Y. Gen. Pharmacol. Vasc. Syst. 1999, 32, 189–194. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bush, L.; Okolie, A.; Robinson, J.; Muili, F.; Opere, C.A.; Ohia, S.E.; Njie Mbye, Y.F. Neuroprotective Actions of Cannabinoids in the Bovine Isolated Retina: Role of Hydrogen Sulfide. Pharmaceuticals 2025, 18, 117. https://doi.org/10.3390/ph18010117
Bush L, Okolie A, Robinson J, Muili F, Opere CA, Ohia SE, Njie Mbye YF. Neuroprotective Actions of Cannabinoids in the Bovine Isolated Retina: Role of Hydrogen Sulfide. Pharmaceuticals. 2025; 18(1):117. https://doi.org/10.3390/ph18010117
Chicago/Turabian StyleBush, Leah, Anthonia Okolie, Jenaye Robinson, Fatima Muili, Catherine A. Opere, Sunny E. Ohia, and Ya Fatou Njie Mbye. 2025. "Neuroprotective Actions of Cannabinoids in the Bovine Isolated Retina: Role of Hydrogen Sulfide" Pharmaceuticals 18, no. 1: 117. https://doi.org/10.3390/ph18010117
APA StyleBush, L., Okolie, A., Robinson, J., Muili, F., Opere, C. A., Ohia, S. E., & Njie Mbye, Y. F. (2025). Neuroprotective Actions of Cannabinoids in the Bovine Isolated Retina: Role of Hydrogen Sulfide. Pharmaceuticals, 18(1), 117. https://doi.org/10.3390/ph18010117





