Gegen Qinlian Decoction Attenuates Colitis-Associated Colorectal Cancer via Suppressing TLR4 Signaling Pathway Based on Network Pharmacology and In Vivo/In Vitro Experimental Validation
Abstract
1. Introduction
2. Results
2.1. UPLC-MS Fingerprint Analysis of GQD
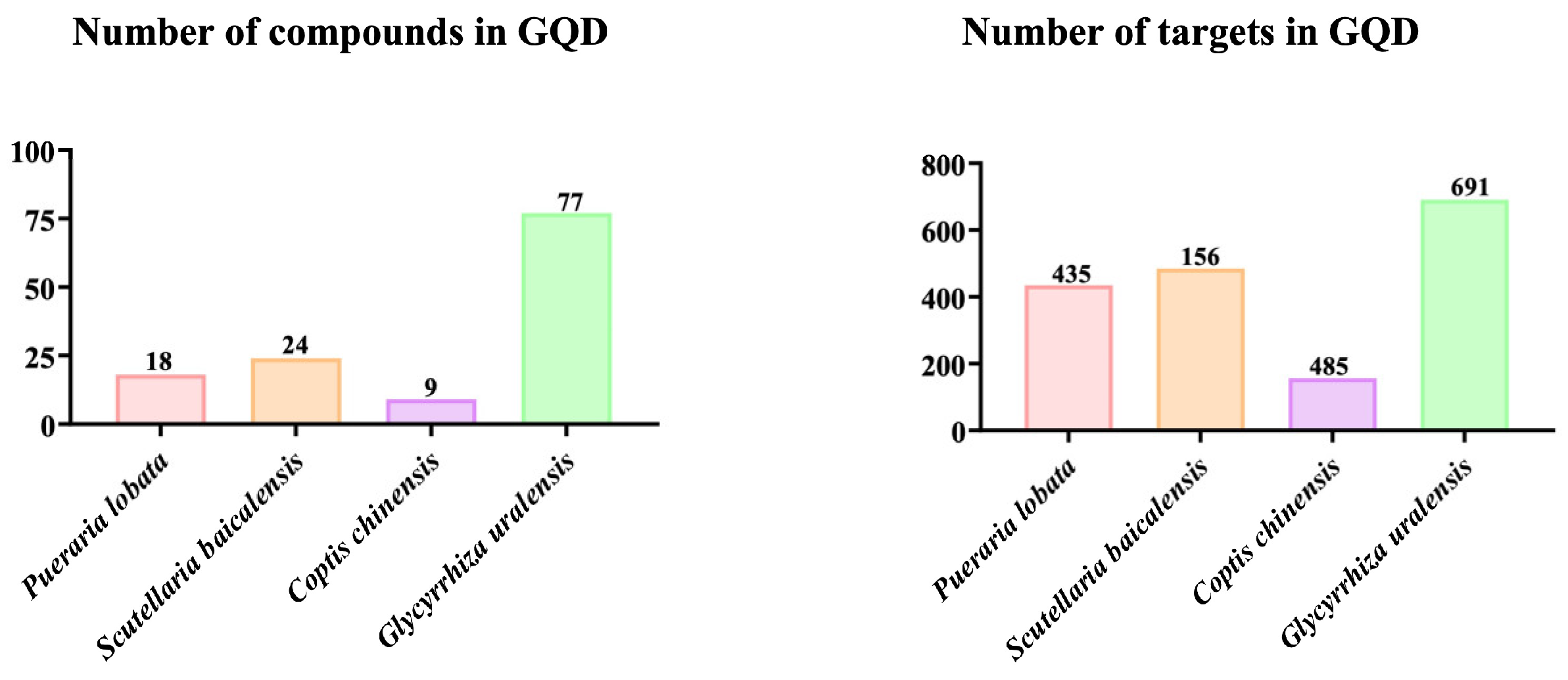
2.2. Active Ingredients and Target Genes of GQD
2.3. Identifying the Intersecting Genes Between GQD and CAC as Well as Creating the Compound-Intersecting Genes-Disease Association Map


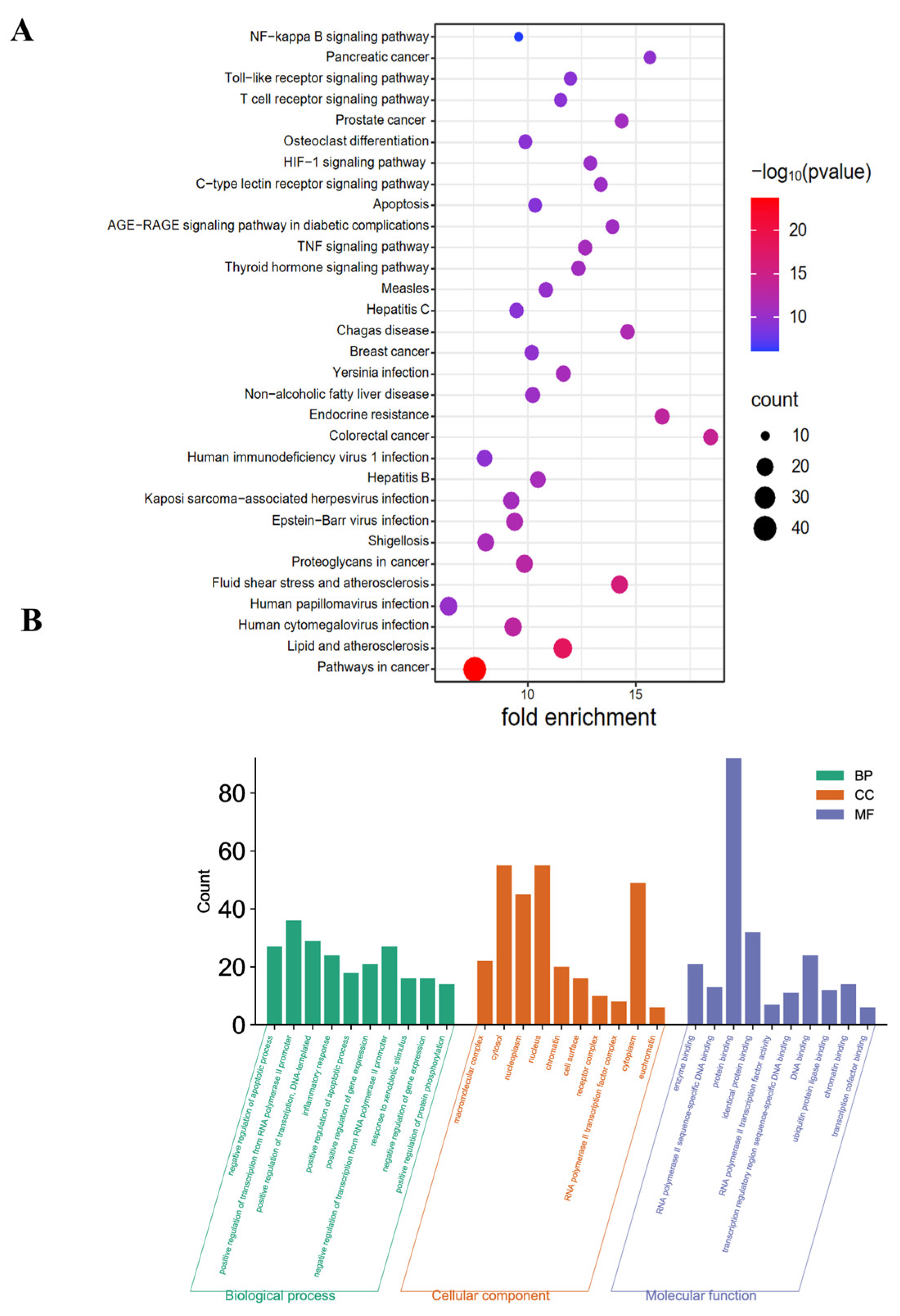
2.4. KEGG and GO Pathway Enrichment Analyses
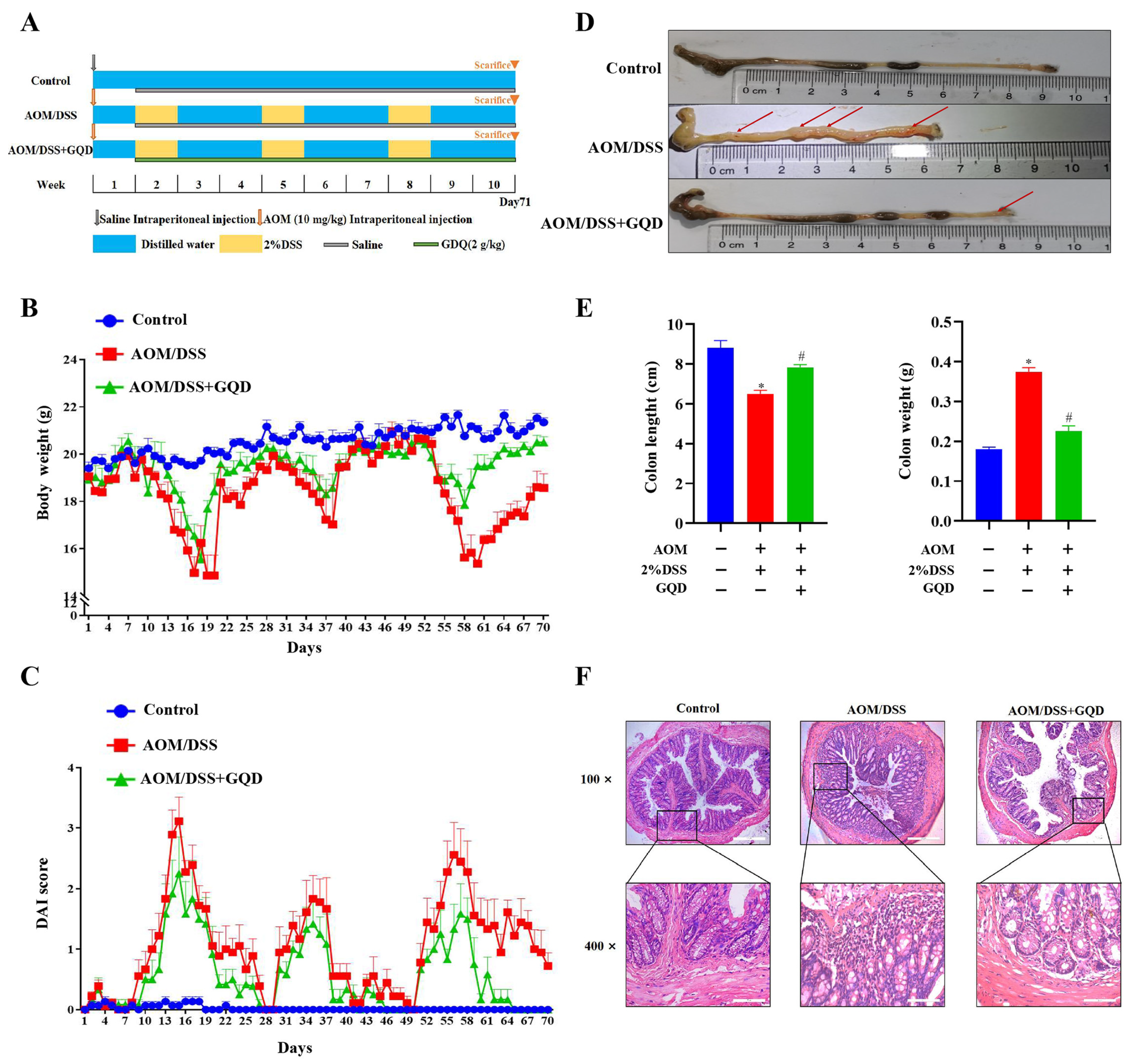
2.5. GQD Attenuated the Symptoms in AOM/DSS-Induced CAC Mice
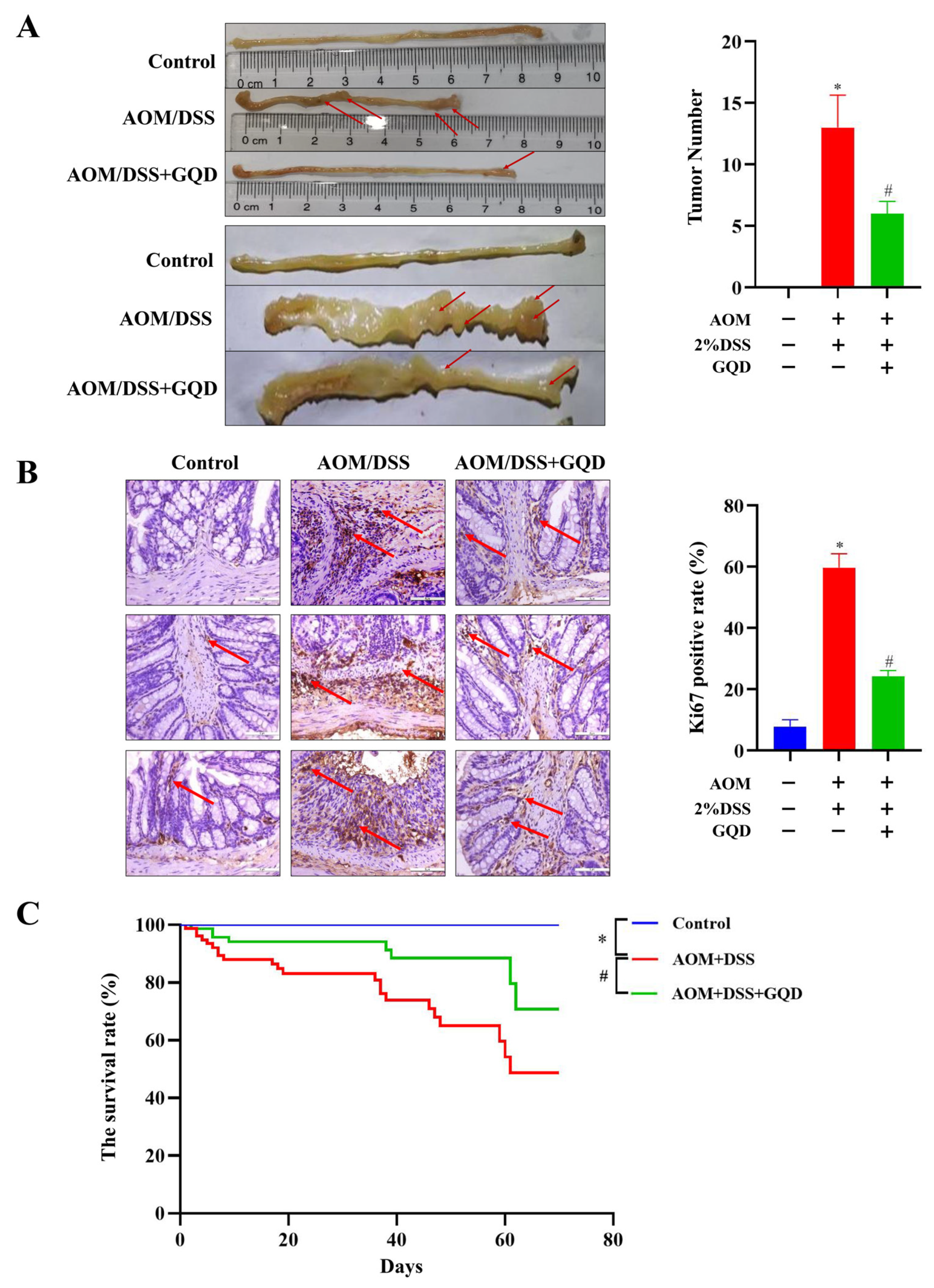
2.6. GQD Reduced the Tumor Incidence and Improved the Survival of AOM/DSS-Induced CAC Mice
2.7. GQD Inhibited Inflammation by Downregulation of the TLR4-Related Signaling Pathways in AOM/DSS-Induced CAC Mice
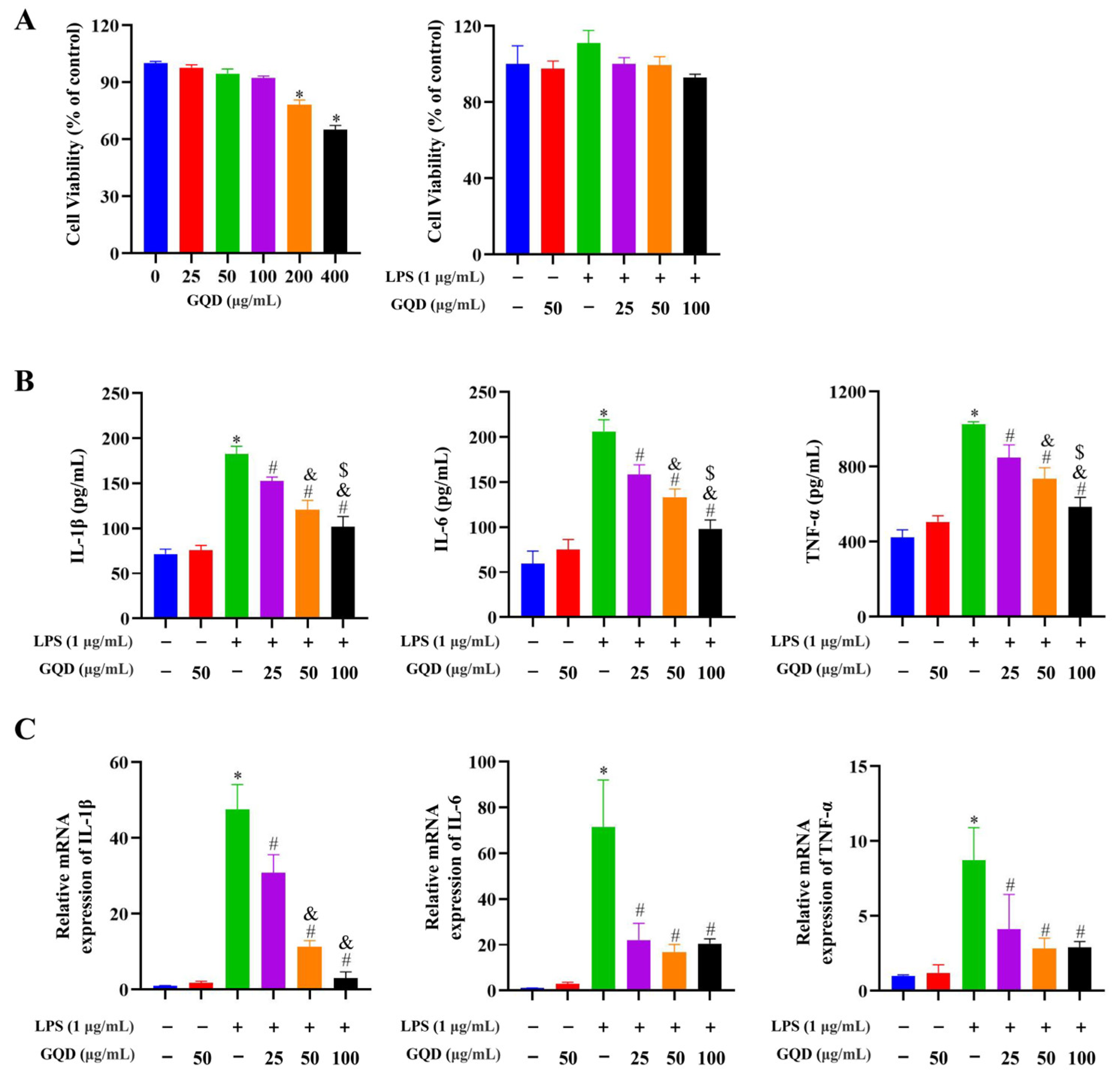
2.8. GQD Reduced the Inflammatory Cytokine Secretion in LPS-Induced RAW264.7 Cells

2.9. GQD Inhibited TLR4-Related Signaling Pathways in LPS-Induced RAW264.7 Cells
2.10. GQD Inhibited Nuclear Translocation of NF-κB, IRF3 in LPS-Induced RAW264.7 Cells
2.11. The Combination of GQD and TAK242 Was Synergistic to Suppress the TLR4-Related Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of GQD Extractions
4.3. Quality Control of GQD Using UPLC-MS
4.4. Collection and Evaluation of GQD Targets and Active Compounds
4.5. Collection of Disease Targets
4.6. Construction of PPI Network Diagram
4.7. Constructing a Compound-Disease-Target-Pathway Network Diagram
4.8. Enrichment Analysis of GO and KEGG Pathways
4.9. Modeling and Intervention of AOM/DSS-Induced CAC in Mice
4.10. DAI Evaluation
4.11. Sample Collection
4.12. Histopathological Analysis
4.13. IHC Analysis
4.14. Cell Culture
4.15. Model of Inflammation In Vitro and GQD Treatment or the Combination of GQD and TAK242
4.16. Cell Viability Assay
4.17. Quantitative Real-Time PCR Determination of Cytokines
4.18. Immunofluorescence Assay
4.19. Detection of Inflammatory Factors via ELISA
4.20. Western Blotting Analysis
4.21. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gara, S.K.; Guntipalli, P.; Marzban, S.; Taqi, M.; Aryal, V.; Khan, Q.U.A.; Shah, S.A.; Akbariromani, H.; Salinger, D.; Diaz-Miret, M. Clinical Outcomes of Ustekinumab in Inflammatory Bowel Disease. Cureus 2023, 15, e46833. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Zheng, C.Q.; Sang, L.X. Mucosal lesions of the upper gastrointestinal tract in patients with ulcerative colitis: A review. World J. Gastroenterol. 2021, 27, 2963–2978. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V., Jr.; Colombel, J.F.; Takeuchi, K.; Gao, X.; Panaccione, R.; Danese, S.; Dubinsky, M.; Schreiber, S.; Ilo, D.; Finney-Hayward, T.; et al. Upadacitinib Therapy Reduces Ulcerative Colitis Symptoms as Early as Day 1 of Induction Treatment. Clin. Gastroenterol. Hepatol. 2023, 21, 2347–2358.e6. [Google Scholar] [CrossRef] [PubMed]
- Du, L.L.; Ha, C. Epidemiology and Pathogenesis of Ulcerative Colitis. Gastroenterol. Clin. North. Am. 2020, 49, 643–654. [Google Scholar] [CrossRef]
- Zhang, M.R.; Li, X.P.; Zhang, Q.; Yang, J.H.; Liu, G. Roles of macrophages on ulcerative colitis and colitis-associated colorectal cancer. Front. Immunol. 2023, 14, 1103617. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Luo, C.X.; Zhang, H. Early detection of ulcerative colitis-associated colorectal cancer. Gastroenterol. Rep. 2018, 6, 83–92. [Google Scholar] [CrossRef]
- Wei, X.N.; Liang, J.W.; Liu, J.H.; Dai, Y.G.; Leng, X.H.; Cheng, Y.; Chi, L.L. Anchang Yuyang Decoction inhibits experimental colitis-related carcinogenesis by regulating PPAR signaling pathway and affecting metabolic homeostasis of host and microbiota. J. Ethnopharmacol. 2024, 326, 117995. [Google Scholar] [CrossRef] [PubMed]
- Zisman, T.L.; Bronner, M.P.; Rulyak, S.; Kowdley, K.V.; Saunders, M.; Lee, S.D.; Ko, C.; Kimmey, M.B.; Stevens, A.; Maurer, J.; et al. Prospective study of the progression of low-grade dysplasia in ulcerative colitis using current cancer surveillance guidelines. Inflamm. Bowel Dis. 2012, 18, 2240–2246. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Li, Y.S.; Shao, T.T.; Zhao, Z.; Wang, Y.; Wu, A.W.; Chen, H.; Li, S.L.; Jiang, C.J.; Xu, J.; et al. Integrating analysis reveals microRNA-mediated pathway crosstalk among Crohn’s disease, ulcerative colitis and colorectal cancer. Mol. Biosyst. 2014, 10, 2317–2328. [Google Scholar] [CrossRef]
- Eaden, J.A.; Abrams, K.R.; Mayberry, J.F. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut 2001, 48, 526–535. [Google Scholar] [CrossRef]
- Hirano, T.; Hirayama, D.; Wagatsuma, K.; Yamakawa, T.; Yokoyama, Y.; Nakase, H. Immunological Mechanisms in Inflammation-Associated Colon Carcinogenesis. Int. J. Mol. Sci. 2020, 21, 3062. [Google Scholar] [CrossRef]
- Shah, S.C.; Itzkowitz, S.H. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology 2022, 162, 715–730.e3. [Google Scholar] [CrossRef]
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef]
- Guo, J.; Liao, M.F.; Wang, J. TLR4 signaling in the development of colitis-associated cancer and its possible interplay with microRNA-155. Cell Commun Signal. 2021, 19, 90. [Google Scholar] [CrossRef]
- Velloso, L.A.; Folli, F.; Saad, M.J. TLR4 at the Crossroads of Nutrients, Gut Microbiota, and Metabolic Inflammation. Endocr. Rev. 2015, 36, 245–271. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Ma, L.L.; Zhang, C.; Lin, J.Z.; Han, L.; He, Y.N.; Xie, C.G. Exploring the Mechanism of Indigo Naturalis in the Treatment of Ulcerative Colitis Based on TLR4/MyD88/NF-κB Signaling Pathway and Gut Microbiota. Front. Pharmacol. 2021, 12, 674416. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Kao, C.L.; Liu, C.M. The Cancer Prevention, Anti-Inflammatory and Anti-Oxidation of Bioactive Phytochemicals Targeting the TLR4 Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 2729. [Google Scholar] [CrossRef]
- Boushehri, M.A.S.; Lamprecht, A. TLR4-Based Immunotherapeutics in Cancer: A Review of the Achievements and Shortcomings. Mol. Pharm. 2018, 15, 4777–4800. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhou, R.; Wang, H.; Li, W.D.; Pan, M.X.; Yao, X.Q.; Zhan, W.Q.; Yang, S.B.; Xu, L.J.; Ding, Y.Q.; et al. Gut microbiota-stimulated cathepsin K secretion mediates TLR4-dependent M2 macrophage polarization and promotes tumor metastasis in colorectal cancer. Cell Death Differ. 2019, 26, 2447–2463. [Google Scholar]
- Fukata, M.; Shang, L.; Santaolalla, R.; Sotolongo, J.; Pastorini, C.; España, C.; Ungaro, R.; Harpaz, N.; Cooper, H.S.; Elson, G.; et al. Constitutive activation of epithelial TLR4 augments inflammatory responses to mucosal injury and drives colitis-associated tumorigenesis. Inflamm. Bowel Dis. 2010, 17, 1464–1473. [Google Scholar]
- Patel, M.; Horgan, P.G.; McMillan, D.C.; Edwards, J. NF-κB pathways in the development and progression of colorectal cancer. Transl. Res. 2018, 197, 43–56. [Google Scholar] [CrossRef]
- Wan, G.S.; Xie, M.L.; Zhang, X.Y.; Li, M.Y. Chang-wei-qing, a Chinese herbal formula, ameliorates colitis-associated tumour development via inhibiting NF-κB and STAT3 signalling pathway. Pharm. Biol. 2019, 57, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xu, Y.; Ji, W.; An, R. Network Pharmacology-based Strategy for Studying the Mechanism of Gegen Qinlian Decoction for the Treatment of Acute Colitis. CHM 2020. [Google Scholar] [CrossRef]
- Liu, L.X.; Wu, W.; Pang, L.L.; Song, Z.Q.; Shi, C.X.; Yang, G.L.; Zhang, H.Y. Research Progress on Chemical Composition, Pharmacological Action and Clinical Application of Gegen Qinlian Decoction. China J. Chin. Mater. Medica 2022, 40, 147–154. [Google Scholar]
- Xu, B.L.; Zhang, G.J.; Ji, Y.B. Active components alignment of Gegenqinlian decoction protects ulcerative colitis by attenuating inflammatory and oxidative stress. J. Ethnopharmacol. 2015, 162, 253–260. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.X.; Xie, C.Y.; Fan, J.; Lv, J.; Xu, X.J.; Lv, J.; Kuai, W.T.; Jia, Y.T. Gegen Qinlian Decoction enhances immunity and protects intestinal barrier function in colorectal cancer patients via gut microbiota. World J. Gastroenterol. 2020, 26, 7633–7651. [Google Scholar] [CrossRef]
- Lv, J.; Jia, Y.T.; Li, J.; Kuai, W.T.; Li, Y.; Guo, F.; Xu, X.J.; Zhao, Z.L.; Lv, J.; Li, Z.X. Gegen Qinlian Decoction enhances the effect of PD-1 blockade in colorectal cancer with microsatellite stability by remodelling the gut microbiota and the tumour microenvironment. Cell Death Dis. 2019, 10, 415. [Google Scholar] [CrossRef]
- Papanikolaou, A.; Wang, Q.S.; Delker, D.A.; Rosenberg, D.W. Azoxymethane-induced colon tumors and aberrant crypt foci in mice of different genetic susceptibility. Cancer Lett. 1998, 130, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Dzhalilova, D.; Zolotova, N.; Fokichev, N.; Makarova, O. Murine models of colorectal cancer: The azoxymethane (AOM)/dextran sulfate sodium (DSS) model of colitis-associated cancer. PeerJ 2023, 11, e16159. [Google Scholar] [CrossRef]
- Lin, R.; Piao, M.Y.; Song, Y.; Liu, C.Y. Quercetin Suppresses AOM/DSS-Induced Colon Carcinogenesis through Its Anti-Inflammation Effects in Mice. J. Immunol. Res. 2020, 2020, 9242601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, J.T.; Zhang, Y.L.; Song, Z.; Bian, J.; Yi, H.F.; Ma, Z.C. Identifying neutrophil-associated subtypes in ulcerative colitis and confirming neutrophils promote colitis-associated colorectal cancer. Front. Immunol. 2023, 14, 1095098. [Google Scholar] [CrossRef]
- Tan, G.; Huang, C.Y.; Chen, J.Y.; Zhi, F.C. HMGB1 released from GSDME-mediated pyroptotic epithelial cells participates in the tumorigenesis of colitis-associated colorectal cancer through the ERK1/2 pathway. J. Hematol. Oncol. 2020, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Chronic Inflammation as an Immunological Abnormality and Effectiveness of Exercise. Biomolecules. 2019, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.J.; Yang, C.Y.; Li, P.C.; Zhang, M.L.; Xie, X.Q.; Xie, X.T.; Chen, Y.L.; Wang, Q.; Zhou, L.; Luo, X. Astragaloside IV inhibits AOM/DSS-induced colitis-associated tumorigenesis via activation of PPARγ signaling in mice. Phytomedicine 2023, 121, 155116. [Google Scholar] [PubMed]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar]
- Jiao, H.L.; Ye, Y.P.; Yang, R.W.; Sun, H.Y.; Wang, S.Y.; Wang, Y.X.; Xiao, Z.Y.; He, L.Q.; Cai, J.J.; Wei, W.T.; et al. Downregulation of SAFB Sustains the NF-κB Pathway by Targeting TAK1 during the Progression of Colorectal Cancer. Clin. Cancer Res. 2017, 23, 7108–7118. [Google Scholar]
- Li, N.; Zhou, H.; Wu, H.M.; Wu, Q.Q.; Duan, M.X.; Deng, W.; Tang, Q.Z. STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox. Biol. 2019, 24, 101215. [Google Scholar] [CrossRef] [PubMed]
- Li, R.Y.; Chen, Y.Y.; Shi, M.J.; Xu, X.X.; Zhao, Y.X.; Wu, X.J.; Zhang, Y.B. Gegen Qinlian Decoction alleviates experimental colitis via suppressing TLR4/NF-κB signaling and enhancing antioxidant effect. Phytomedicine 2016, 23, 1012–1020. [Google Scholar] [CrossRef]
- Wang, X.J.; Huang, S.W.; Zhang, M.L.; Su, Y.L.; Pan, Z.F.; Liang, J.J.; Xie, X.Q.; Wang, Q.; Chen, J.Y.; Zhou, L.; et al. Gegen Qinlian Decoction activates AhR/IL-22 to repair intestinal barrier by modulating gut microbiota-related tryptophan metabolism in ulcerative colitis mice. J. Ethnopharmacol. 2023, 302, 115919. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Luan, H.F.; Gao, H.; Wu, X.J.; Zhang, Y.B.; Li, R.Y. Gegen Qinlian Decoction maintains colonic mucosal homeostasis in acute/chronic ulcerative colitis via bidirectionally modulating dysregulated Notch signaling. Phytomedicine 2020, 68, 153182. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Zhou, Y.P.; Zhang, J.J.; Hu, Z.W. The potential target and mechanism of action in the treatment of colorectal cancer. Mod. Dig. Interv. 2022, 27, 1129–1140. [Google Scholar]
- Jeon, Y.D.; Lee, J.H.; Lee, Y.M.; Kim, D.K. Puerarin inhibits inflammation and oxidative stress in dextran sulfate sodium-induced colitis mice model. Biomed. Pharmacother. 2020, 124, 109847. [Google Scholar] [CrossRef]
- Yan, S.H.; Chang, J.Y.; Hao, X.H.; Liu, J.; Tan, X.Y.; Geng, Z.R.; Wang, Z.L. Berberine regulates short-chain fatty acid metabolism and alleviates the colitis-associated colorectal tumorigenesis through remodeling intestinal flora. Phytomedicine 2022, 102, 154217. [Google Scholar] [CrossRef]
- Kim, D.H.; Hossain, M.A.; Kang, Y.J.; Jang, J.Y.; Lee, Y.J.; Im, E.; Yoon, J.H.; Kim, H.S.; Chung, H.Y.; Kim, N.D. Baicalein, an active component of Scutellariabaicalensis georgi, induces apoptosis in human colon cancer cells and prevents AOM/DSS-induced colon cancer in mice. Int. J. Oncol. 2013, 43, 1652–1658. [Google Scholar] [CrossRef]
- Shen, J.; Cheng, J.Z.; Zhu, S.G.; Zhao, J.; Ye, Q.Y.; Xu, Y.Y.; Dong, H.L.; Zheng, X.H. Regulating effect of baicalin on IKK/IKB/NF-kB signaling pathway and apoptosis-related proteins in rats with ulcerative colitis. Int. Immunopharmacol. 2019, 73, 193–200. [Google Scholar] [CrossRef]
- Wang, G.F.; Hiramoto, K.C.; Ma, N.; Yoshikawa, N.; Ohnishi, S.; Murata, M.; Kawanishi, S. Glycyrrhizin Attenuates Carcinogenesis by Inhibiting the Inflammatory Response in a Murine Model of Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 2609. [Google Scholar] [CrossRef]
- Liu, Y.R.; Zhao, J.M.; Zhao, Y.L.; Zong, S.M.; Tian, Y.X.; Chen, S.; Li, M.; Liu, H.J.; Zhang, Q.; Jing, X.S.; et al. Therapeutic effects of lentinan on inflammatory bowel disease and colitis-associated cancer. J. Cell Mol. Med. 2019, 23, 750–760. [Google Scholar] [CrossRef]
- Yao, D.B.; Dong, M.; Dai, C.L.; Wu, S.D. Inflammation and Inflammatory Cytokine Contribute to the Initiation and Development of Ulcerative Colitis and Its Associated Cancer. Inflamm. Bowel Dis. 2019, 25, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Fukata, M.; Chen, A.; Vamadevan, A.S.; Cohen, J.; Breglio, K.; Krishnareddy, S.; Hsu, D.; Xu, R.L.; Harpaz, N.; Dannenberg, A.J.; et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology 2007, 133, 1869–1881. [Google Scholar] [CrossRef]
- Fan, C.S.; Chen, C.C.; Chen, L.L.; Chua, K.V.; Hung, H.C.; Hsu, J.T.; Huang, T.S. Extracellular HSP90α Induces MyD88-IRAK Complex-Associated IKKα/β-NF-κB/IRF3 and JAK2/TYK2-STAT-3 Signaling in Macrophages for Tumor-Promoting M2-Polarization. Cells 2022, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.Z.; Su, S.; Zou, H.M.; Guo, Y.; Wang, S.Y.; Li, S.; Luo, M.H.; Wang, Y.Y. Human Cytomegalovirus DNA Polymerase Subunit UL44 Antagonizes Antiviral Immune Responses by Suppressing IRF3- and NF-κB-Mediated Transcription. J. Virol. 2019, 93, e00181-19. [Google Scholar] [CrossRef]
- Honda, K.; Taniguchi, T. IRFs: Master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 2006, 6, 644–658. [Google Scholar] [CrossRef]
- Zhao, T.J.; Yang, L.; Sun, Q.; Arguello, M.; Ballard, D.W.; Hiscott, J.; Lin, R.T. The NEMO adaptor bridges the nuclear factor-kappaB and interferon regulatory factor signaling pathways. Nat. Immunol. 2007, 8, 592–600. [Google Scholar] [CrossRef]
- Aziz, N.; Son, Y.J.; Cho, J.Y. Thymoquinone Suppresses IRF-3-Mediated Expression of Type I Interferons via Suppression of TBK1. Int J Mol Sci. 2018, 19, 1355. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, S.; Beg, A.A. Defining emerging roles for NF-κB in antivirus responses: Revisiting the interferon-β enhanceosome paradigm. PLoSPathog. 2011, 7, e1002165. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Guo, F.F.; Wang, Y.; Li, C.; Zhang, X.L.; Li, H.; Diao, L.H.; Gu, J.Y.; Wang, W.; Li, D.; et al. BATMAN-TCM: A Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine. Sci. Rep. 2016, 6, 21146. [Google Scholar] [CrossRef]
- Chen, X.N.; Yang, Z.; Du, L.; Guan, Y.X.; Li, Y.F.; Liu, C.G. Study on the active ingredients and mechanism of action of Jiaotai Pill in the treatment of type 2 diabetes based on network pharmacology: A review. Medicine 2023, 102, e33317. [Google Scholar] [CrossRef]
- Yuan, M.; Sun, G.D.; Liu, H.S.; Huo, J.H.; Wang, W.M. Therapeutic material basis of Daqinglong Decoction: Based on serum pharmacochemistry and network pharmacology. Zhongguo Zhong Yao Za Zhi. 2022, 47, 3876–3886. [Google Scholar]
- Lin, G.Z.; Jiang, H.; Zhang, Z.H.; Ning, L.; Zhang, W.B.; Peng, L.F.; Xu, S.; Sun, W.; Tao, S.; Zhang, T.; et al. Molecular mechanism of NR4A1/MDM2/P53 signaling pathway regulation inducing ferroptosis in renal tubular epithelial cells involved in the progression of renal ischemia-reperfusion injury. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166968. [Google Scholar] [CrossRef]
- Li, X.; Wei, S.Z.; Niu, S.Q.; Ma, X.; Li, H.T.; Jing, M.Y.; Zhao, Y.L. Network pharmacology prediction and molecular docking-based strategy to explore the potential mechanism of Huanglian Jiedu Decoction against sepsis. Comput. Biol. Med. 2022, 144, 105389. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Miao, F.Z.; Xin, R.J.; Tai, Z.G.; Pan, H.J.; Huang, H.; Yu, J.X.; Chen, Z.J.; Zhu, Q.G. Combining network pharmacology, molecular docking, molecular dynamics simulation, and experimental verification to examine the efficacy and immunoregulation mechanism of FHB granules on vitiligo. Front. Immunol. 2023, 14, 1194823. [Google Scholar] [CrossRef]
- Zhang, W.T.; Tian, W.Q.; Wang, Y.F.; Jin, X.J.; Guo, H.; Wang, Y.W.; Tang, Y.P.; Yao, X.J. Explore the mechanism and substance basis of Mahuang FuziXixin Decoction for the treatment of lung cancer based on network pharmacology and molecular docking. Comput. Biol. Med. 2022, 151, 106293. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xing, B.; Liu, X.; Jiang, X.W.; Lu, H.Y.; Xu, Z.H.; Yang, Y.; Wu, Q.; Yao, D.; Zhang, Y.S.; et al. Network pharmacology-based research uncovers cold resistance and thermogenesis mechanism of Cinnamomum cassia. Fitoterapia 2021, 149, 104824. [Google Scholar]
- Yan, S.; Shi, Q.C.; Ma, H.T.; Xu, Q. Study on mechanism of Zhenwu Decoction in treatment of heart failure based on network pharmacology: A review. Medicine 2023, 102, e36073. [Google Scholar] [CrossRef]
- Qi, S.C.; Liang, X.Y.; Wang, Z.J.; Jin, H.R.; Zou, L.Q.; Yang, J.L. Potential Mechanism of Tibetan Medicine Liuwei Muxiang Pills against Colorectal Cancer: Network Pharmacology and Bioinformatics Analyses. Pharmaceuticals 2024, 17, 429. [Google Scholar] [CrossRef]
- Du, H.J.; Zhang, L.Y.; Sun, H.X.; Zheng, S.Q.; Zhang, H.Y.; Yuan, S.J.; Zhou, J.Y.; Fang, Z.H.; Song, J.P.; Mei, M.X.; et al. Exploring the Underlying Mechanisms of Qingxing Granules Treating H1N1 Influenza Based on Network Pharmacology and Experimental Validation. Pharmaceuticals 2024, 17, 731. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Wu, C.N.; Shen, Z.F.; Liu, S.L.; Zou, X.; Qian, J.; Wu, Z.F.; Huan, X.K.; Mu, B.X.; Ye, N.Y.; et al. Yiqi Huayu Jiedu Decoction inhibits liver metastasis of colorectal cancer via enhancing natural killer cells function. J. Ethnopharmacol. 2024, 318, 116915. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, Y.; Wang, W.T.; He, Y.; Zhong, H.; Zhou, X.X.; Chen, Y.; Cai, X.J.; Liu, L.Q. Mechanisms underlying the therapeutic effects of Qingfeiyin in treating acute lung injury based on GEO datasets, network pharmacology and molecular docking. Comput. Biol. Med. 2022, 145, 105454. [Google Scholar] [CrossRef]
- Chen, S.N.; Li, B.; Chen, L.; Jiang, H.L. Uncovering the mechanism of resveratrol in the treatment of diabetic kidney disease based on network pharmacology, molecular docking, and experimental validation. J. Transl. Med. 2023, 21, 380. [Google Scholar] [CrossRef]
- Snider, A.J.; Bialkowska, A.B.; Ghaleb, A.M.; Yang, V.W.; Obeid, L.M.; Hannun, Y.A. Murine Model for Colitis-Associated Cancer of the Colon. Methods Mol. Biol. 2016, 1438, 245–254. [Google Scholar] [PubMed]
- Wirtz, S.; Neufert, C.; Weigmann, B.; Neurath, M.F. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007, 2, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Neufert, C.; Becker, C.; Neurath, M.F. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat. Protoc. 2007, 2, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kohno, H.; Suzuki, R.; Yamada, Y.; Sugie, S.; Mori, H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003, 94, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.Y.; Zhao, C.Y.; Xu, Y.Y.; Lin, H.W.; Jia, B.B.; Huang, B.; Lin, S.; Chen, D.X.; Jia, P.Z.; Wang, M.L.; et al. Qingda granule alleviates cerebral ischemia/reperfusion injury by inhibiting TLR4/NF-κB/NLRP3 signaling in microglia. J. Ethnopharmacol. 2024, 324, 117712. [Google Scholar] [CrossRef]
- Zhao, F.; Maren, N.A.; Kosentka, P.Z.; Liao, Y.Y.; Lu, H.; Duduit, J.R.; Huang, D.; Ashrafi, H.; Zhao, T.; Huerta, A.I.; et al. An optimized protocol for stepwise optimization of real-time RT-PCR analysis. Hortic. Res. 2021, 8, 179. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, M.H.; Wang, H.D.; Shi, G.P.; Chen, B.Q.; Chen, T.; Yuan, X.M.; Zhu, P.; Zhou, J.Y.; Wang, Q.; et al. Wu Mei Wan attenuates CAC by regulating gut microbiota and the NF-kB/IL6-STAT3 signaling pathway. Biomed. Pharmacother. 2020, 125, 109982. [Google Scholar] [CrossRef]
- Jia, P.Z.; Chen, D.X.; Zhu, Y.; Wang, M.L.; Zeng, J.W.; Zhang, L.; Cai, Q.Y.; Lian, D.W.; Zhao, C.Y.; Xu, Y.; et al. Liensinine improves AngII-induced vascular remodeling via MAPK/TGF-β1/Smad2/3 signaling. J. Ethnopharmacol. 2023, 317, 116768. [Google Scholar] [CrossRef]



| Genes | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| GAPDH | ACGGCAAGTTCAACGGCACAG | GAAGACGCCAGTAGACTCCACGAC |
| IL-1β | GAAATGCCACCTTTTGACAGTG | TGGATGCTCTCATCAGGACAG |
| IL-6 | CTGCAAGAGACTTCCATCCAG | AGTGGTATAGACAGGTCTGTTGG |
| TNF-α | GCCGATGGGTTGTACCTTGT | TCTTGACGGCAGAGAGGAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Cai, Q.; Zhao, C.; Zhang, W.; Xu, X.; Lin, H.; Lin, Y.; Chen, D.; Lin, S.; Jia, P.; et al. Gegen Qinlian Decoction Attenuates Colitis-Associated Colorectal Cancer via Suppressing TLR4 Signaling Pathway Based on Network Pharmacology and In Vivo/In Vitro Experimental Validation. Pharmaceuticals 2025, 18, 12. https://doi.org/10.3390/ph18010012
Xu Y, Cai Q, Zhao C, Zhang W, Xu X, Lin H, Lin Y, Chen D, Lin S, Jia P, et al. Gegen Qinlian Decoction Attenuates Colitis-Associated Colorectal Cancer via Suppressing TLR4 Signaling Pathway Based on Network Pharmacology and In Vivo/In Vitro Experimental Validation. Pharmaceuticals. 2025; 18(1):12. https://doi.org/10.3390/ph18010012
Chicago/Turabian StyleXu, Yaoyao, Qiaoyan Cai, Chunyu Zhao, Weixiang Zhang, Xinting Xu, Haowei Lin, Yuxing Lin, Daxin Chen, Shan Lin, Peizhi Jia, and et al. 2025. "Gegen Qinlian Decoction Attenuates Colitis-Associated Colorectal Cancer via Suppressing TLR4 Signaling Pathway Based on Network Pharmacology and In Vivo/In Vitro Experimental Validation" Pharmaceuticals 18, no. 1: 12. https://doi.org/10.3390/ph18010012
APA StyleXu, Y., Cai, Q., Zhao, C., Zhang, W., Xu, X., Lin, H., Lin, Y., Chen, D., Lin, S., Jia, P., Wang, M., Zhang, L., & Lin, W. (2025). Gegen Qinlian Decoction Attenuates Colitis-Associated Colorectal Cancer via Suppressing TLR4 Signaling Pathway Based on Network Pharmacology and In Vivo/In Vitro Experimental Validation. Pharmaceuticals, 18(1), 12. https://doi.org/10.3390/ph18010012





