Uncovering Psychedelics: From Neural Circuits to Therapeutic Applications
Abstract
1. Introduction
2. Molecular and Cellular Targets of Psychedelics
3. Circuitries, Connectivity, and Brain Networks Under Psychedelics’ Effect
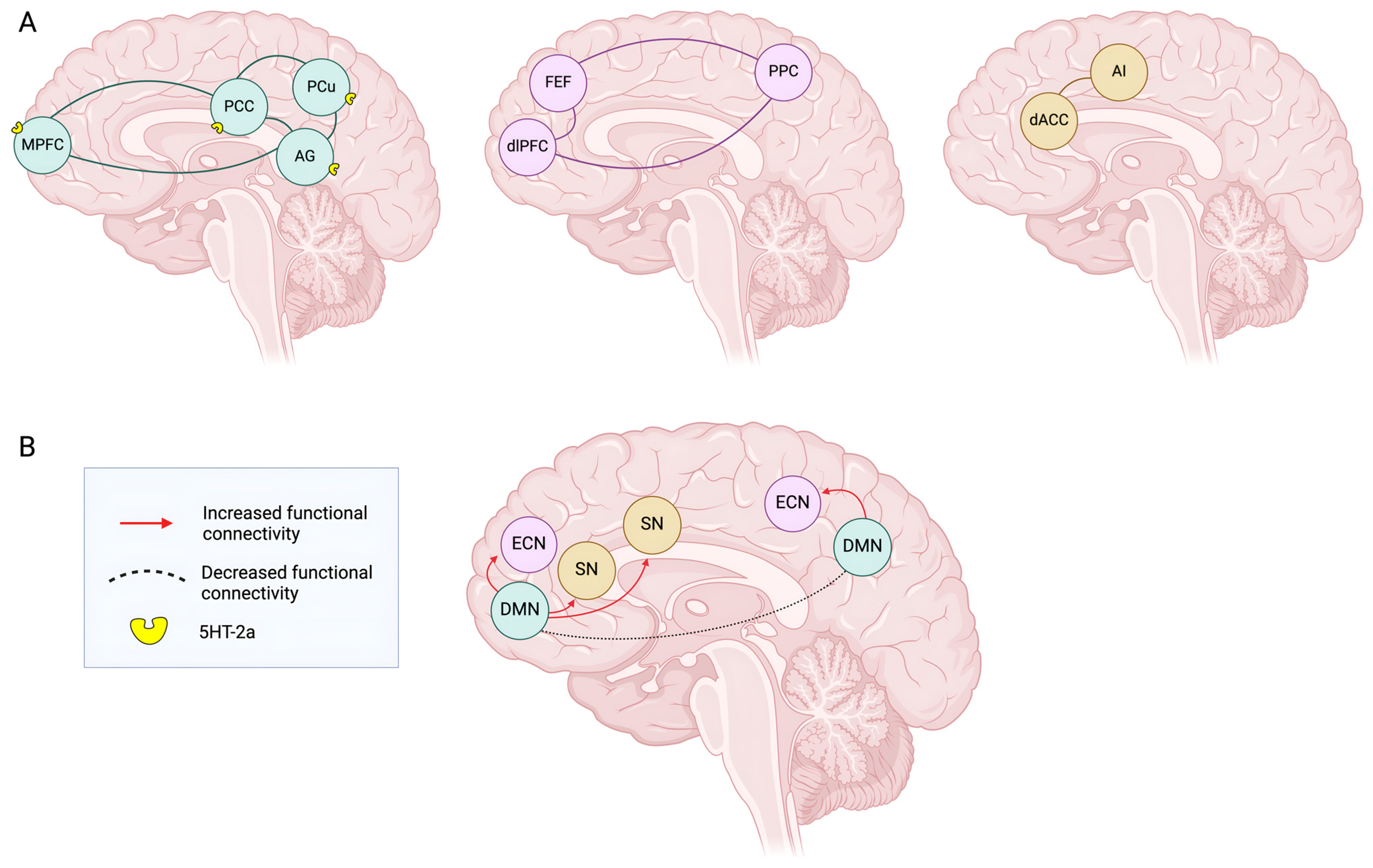
3.1. DMN Model
3.2. CSTC Model
3.3. Relaxed Beliefs Under Psychedelics (REBUS) and the Anarchic Brain Model
4. Overview of Major Completed Clinical Trials of MDMA, Psilocybin, and LSD in PTSD, TRD, and GAD
4.1. Completed Clinical Trials of MDMA in PTSD
4.2. Completed Clinical Trials of Psilocybin in TRD and PTSD
4.3. Overview of Major Completed Clinical Trials of LSD in GAD
5. Conclusions
6. Future Perspectives
7. Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vollenweider, F.X.; Preller, K.H. Psychedelic Drugs: Neurobiology and Potential for Treatment of Psychiatric Disorders. Nat. Rev. Neurosci. 2020, 21, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Heifets, B.D.; Salgado, J.S.; Taylor, M.D.; Hoerbelt, P.; Cardozo Pinto, D.F.; Steinberg, E.E.; Walsh, J.J.; Sze, J.Y.; Malenka, R.C. Distinct Neural Mechanisms for the Prosocial and Rewarding Properties of MDMA. Sci. Transl. Med. 2019, 11, eaaw6435. [Google Scholar] [CrossRef] [PubMed]
- Kishon, R.; Modlin, N.L.; Cycowicz, Y.M.; Mourtada, H.; Wilson, T.; Williamson, V.; Cleare, A.; Rucker, J. A Rapid Narrative Review of the Clinical Evolution of Psychedelic Treatment in Clinical Trials. npj Ment. Health Res. 2024, 3, 33. [Google Scholar] [CrossRef]
- Wang, E.; Mathai, D.S.; Gukasyan, N.; Nayak, S.; Garcia-Romeu, A. Knowledge, Attitudes, and Concerns about Psilocybin and MDMA as Novel Therapies among U.S. Healthcare Professionals. Sci. Rep. 2024, 14, 28022. [Google Scholar] [CrossRef] [PubMed]
- Walther, R.F.E.; van Schie, H.T. ‘Mind-Revealing’ Psychedelic States: Psychological Processes in Subjective Experiences That Drive Positive Change. Psychoactives 2024, 3, 411–436. [Google Scholar] [CrossRef]
- Mitchell, J.M.; Ot’alora, G.M.; van der Kolk, B.; Shannon, S.; Bogenschutz, M.; Gelfand, Y.; Paleos, C.; Nicholas, C.R.; Quevedo, S.; Balliett, B.; et al. MDMA-Assisted Therapy for Moderate to Severe PTSD: A Randomized, Placebo-Controlled Phase 3 Trial. Nat. Med. 2023, 29, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Marks, M.; Cohen, I.G. Psychedelic Therapy: A Roadmap for Wider Acceptance and Utilization. Nat. Med. 2021, 27, 1669–1671. [Google Scholar] [CrossRef] [PubMed]
- Barba, T.; Kettner, H.; Radu, C.; Peill, J.M.; Roseman, L.; Nutt, D.J.; Erritzoe, D.; Carhart-Harris, R.; Giribaldi, B. Psychedelics and Sexual Functioning: A Mixed-Methods Study. Sci. Rep. 2024, 14, 2181. [Google Scholar] [CrossRef] [PubMed]
- Shadani, S.; Conn, K.; Andrews, Z.B.; Foldi, C.J. Potential Differences in Psychedelic Actions Based on Biological Sex. Endocrinology 2024, 165, bqae083. [Google Scholar] [CrossRef] [PubMed]
- Aqil, M.; Roseman, L. More than Meets the Eye: The Role of Sensory Dimensions in Psychedelic Brain Dynamics, Experience, and Therapeutics. Neuropharmacology 2023, 223, 109300. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Friston, K.J. REBUS and the Anarchic Brain: Toward a Unified Model of the Brain Action of Psychedelics. Pharmacol. Rev. 2019, 71, 316–344. [Google Scholar] [CrossRef]
- Gattuso, J.J.; Perkins, D.; Ruffell, S.; Lawrence, A.J.; Hoyer, D.; Jacobson, L.H.; Timmermann, C.; Castle, D.; Rossell, S.L.; Downey, L.A.; et al. Default Mode Network Modulation by Psychedelics: A Systematic Review. Int. J. Neuropsychopharmacol. 2023, 26, 155–188. [Google Scholar] [CrossRef]
- Doss, M.K.; Madden, M.B.; Gaddis, A.; Nebel, M.B.; Griffiths, R.R.; Mathur, B.N.; Barrett, F.S. Models of Psychedelic Drug Action: Modulation of Cortical-Subcortical Circuits. Brain 2022, 145, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Schindler, E.A.D.; Dave, K.D.; Smolock, E.M.; Aloyo, V.J.; Harvey, J.A. Serotonergic and Dopaminergic Distinctions in the Behavioral Pharmacology of (±)-1-(2,5-Dimethoxy-4-Iodophenyl)-2-Aminopropane (DOI) and Lysergic Acid Diethylamide (LSD). Pharmacol. Biochem. Behav. 2012, 101, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Lizarraga, L.E.; Cholanians, A.B.; Phan, A.V.; Herndon, J.M.; Lau, S.S.; Monks, T.J. Vesicular Monoamine Transporter 2 and the Acute and Long-Term Response to 3,4-(±)-Methylenedioxymethamphetamine. Toxicol. Sci. 2015, 143, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Ballard, E.D.; Zarate, C.A. The Role of Dissociation in Ketamine’s Antidepressant Effects. Nat. Commun. 2020, 11, 6431. [Google Scholar] [CrossRef] [PubMed]
- Mathai, D.S.; Meyer, M.J.; Storch, E.A.; Kosten, T.R. The Relationship between Subjective Effects Induced by a Single Dose of Ketamine and Treatment Response in Patients with Major Depressive Disorder: A Systematic Review. J. Affect. Disord. 2020, 264, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Maggio, R.; Fasciani, I.; Petragnano, F.; Coppolino, M.F.; Scarselli, M.; Rossi, M. Unraveling the Functional Significance of Unstructured Regions in G Protein-Coupled Receptors. Biomolecules 2023, 13, 1431. [Google Scholar] [CrossRef]
- Wallach, J.; Cao, A.B.; Calkins, M.M.; Heim, A.J.; Lanham, J.K.; Bonniwell, E.M.; Hennessey, J.J.; Bock, H.A.; Anderson, E.I.; Sherwood, A.M.; et al. Identification of 5-HT2A Receptor Signaling Pathways Associated with Psychedelic Potential. Nat. Commun. 2023, 14, 8221. [Google Scholar] [CrossRef]
- Scarselli, M.; Annibale, P.; Gerace, C.; Radenovic, A. Enlightening G-Protein-Coupled Receptors on the Plasma Membrane Using Super-Resolution Photoactivated Localization Microscopy. Biochem. Soc. Trans. 2013, 41, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Halberstadt, A.L.; Chatha, M.; Klein, A.K.; Wallach, J.; Brandt, S.D. Correlation between the Potency of Hallucinogens in the Mouse Head-Twitch Response Assay and Their Behavioral and Subjective Effects in Other Species. Neuropharmacology 2020, 167, 107933. [Google Scholar] [CrossRef] [PubMed]
- González-Maeso, J.; Weisstaub, N.V.; Zhou, M.; Chan, P.; Ivic, L.; Ang, R.; Lira, A.; Bradley-Moore, M.; Ge, Y.; Zhou, Q.; et al. Hallucinogens Recruit Specific Cortical 5-HT2A Receptor-Mediated Signaling Pathways to Affect Behavior. Neuron 2007, 53, 439–452. [Google Scholar] [CrossRef]
- Bockaert, J.; Claeysen, S.; Bécamel, C.; Dumuis, A.; Marin, P. Neuronal 5-HT Metabotropic Receptors: Fine-Tuning of Their Structure, Signaling, and Roles in Synaptic Modulation. Cell Tissue Res. 2006, 326, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Soltanzadeh Zarandi, S.; Sood, A.; Paddy, M.R.; et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 2018, 23, 3170–3182. [Google Scholar] [CrossRef] [PubMed]
- Moliner, R.; Girych, M.; Brunello, C.A.; Kovaleva, V.; Biojone, C.; Enkavi, G.; Antenucci, L.; Kot, E.F.; Goncharuk, S.A.; Kaurinkoski, K.; et al. Psychedelics Promote Plasticity by Directly Binding to BDNF Receptor TrkB. Nat. Neurosci. 2023, 26, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, R.; Farré, M.; Roset, P.N.; Pizarro, N.; Abanades, S.; Segura, M.; Segura, J.; Camí, J. Human Pharmacology of MDMA: Pharmacokinetics, Metabolism, and Disposition. Ther. Drug Monit. 2004, 26, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Achat-Mendes, C.; Lynch, L.J.; Sullivan, K.A.; Vallender, E.J.; Miller, G.M. Augmentation of Methamphetamine-Induced Behaviors in Transgenic Mice Lacking the Trace Amine-Associated Receptor 1. Pharmacol. Biochem. Behav. 2012, 101, 201–207. [Google Scholar] [CrossRef]
- Pei, Y.; Asif-Malik, A.; Canales, J.J. Trace Amines and the Trace Amine-Associated Receptor 1: Pharmacology, Neurochemistry, and Clinical Implications. Front. Neurosci. 2016, 10, 148. [Google Scholar] [CrossRef]
- Rein, B.; Raymond, K.; Boustani, C.; Tuy, S.; Zhang, J.; St Laurent, R.; Pomrenze, M.B.; Boroon, P.; Heifets, B.; Smith, M.; et al. MDMA Enhances Empathy-like Behaviors in Mice via 5-HT Release in the Nucleus Accumbens. Sci. Adv. 2024, 10, eadl6554. [Google Scholar] [CrossRef] [PubMed]
- Lukasiewicz, K.; Baker, J.J.; Zuo, Y.; Lu, J. Serotonergic Psychedelics in Neural Plasticity. Front. Mol. Neurosci. 2021, 14, 748359. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, L.E.; Andrews, A.M.; Olson, D.E. Dark Classics in Chemical Neuroscience: 3,4-Methylenedioxymethamphetamine. ACS Chem. Neurosci. 2018, 9, 2408–2427. [Google Scholar] [CrossRef]
- Hake, H.S.; Davis, J.K.P.; Wood, R.R.; Tanner, M.K.; Loetz, E.C.; Sanchez, A.; Ostrovskyy, M.; Oleson, E.B.; Grigsby, J.; Doblin, R.; et al. 3,4-Methylenedioxymethamphetamine (MDMA) Impairs the Extinction and Reconsolidation of Fear Memory in Rats. Physiol. Behav. 2019, 199, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Erritzoe, D.; Williams, T.; Stone, J.M.; Reed, L.J.; Colasanti, A.; Tyacke, R.J.; Leech, R.; Malizia, A.L.; Murphy, K.; et al. Neural Correlates of the Psychedelic State as Determined by FMRI Studies with Psilocybin. Proc. Natl. Acad. Sci. USA 2012, 109, 2138–2143. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.K.; Fisher, P.M.; Burmester, D.; Dyssegaard, A.; Stenbæk, D.S.; Kristiansen, S.; Johansen, S.S.; Lehel, S.; Linnet, K.; Svarer, C.; et al. Psychedelic Effects of Psilocybin Correlate with Serotonin 2A Receptor Occupancy and Plasma Psilocin Levels. Neuropsychopharmacology 2019, 44, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; Brändle, R.; Liechti, M.E.; Borgwardt, S. Neuroimaging of Chronic MDMA (“ecstasy”) Effects: A Meta-Analysis. Neurosci. Biobehav. Rev. 2019, 96, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, C.; Roseman, L.; Haridas, S.; Rosas, F.E.; Luan, L.; Kettner, H.; Martell, J.; Erritzoe, D.; Tagliazucchi, E.; Pallavicini, C.; et al. Human Brain Effects of DMT Assessed via EEG-FMRI. Proc. Natl. Acad. Sci. USA 2023, 120, e2218949120. [Google Scholar] [CrossRef] [PubMed]
- Tófoli, L.F.; de Araujo, D.B. Treating Addiction: Perspectives from EEG and Imaging Studies on Psychedelics. Int. Rev. Neurobiol. 2016, 129, 157–185. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Muthukumaraswamy, S.; Roseman, L.; Kaelen, M.; Droog, W.; Murphy, K.; Tagliazucchi, E.; Schenberg, E.E.; Nest, T.; Orban, C.; et al. Neural Correlates of the LSD Experience Revealed by Multimodal Neuroimaging. Proc. Natl. Acad. Sci. USA 2016, 113, 4853–4858. [Google Scholar] [CrossRef] [PubMed]
- Daws, R.E.; Timmermann, C.; Giribaldi, B.; Sexton, J.D.; Wall, M.B.; Erritzoe, D.; Roseman, L.; Nutt, D.; Carhart-Harris, R. Increased Global Integration in the Brain after Psilocybin Therapy for Depression. Nat. Med. 2022, 28, 844–851. [Google Scholar] [CrossRef]
- Preller, K.H.; Burt, J.B.; Ji, J.L.; Schleifer, C.H.; Adkinson, B.D.; Stämpfli, P.; Seifritz, E.; Repovs, G.; Krystal, J.H.; Murray, J.D.; et al. Changes in Global and Thalamic Brain Connectivity in LSD-Induced Altered States of Consciousness Are Attributable to the 5-HT2A Receptor. Elife 2018, 7, 35082. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; Lenz, C.; Dolder, P.; Lang, U.; Schmidt, A.; Liechti, M.; Borgwardt, S. Increased Thalamic Resting-State Connectivity as a Core Driver of LSD-Induced Hallucinations. Acta Psychiatr. Scand. 2017, 136, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Preller, K.H.; Duerler, P.; Burt, J.B.; Ji, J.L.; Adkinson, B.; Stämpfli, P.; Seifritz, E.; Repovš, G.; Krystal, J.H.; Murray, J.D.; et al. Psilocybin Induces Time-Dependent Changes in Global Functional Connectivity. Biol. Psychiatry 2020, 88, 197–207. [Google Scholar] [CrossRef]
- Beliveau, V.; Ganz, M.; Feng, L.; Ozenne, B.; Højgaard, L.; Fisher, P.M.; Svarer, C.; Greve, D.N.; Knudsen, G.M. A High-Resolution In Vivo Atlas of the Human Brain’s Serotonin System. J. Neurosci. 2017, 37, 120–128. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, D.E.-W.; Madsen, M.K.; Stenbæk, D.S.; Kristiansen, S.; Ozenne, B.; Jensen, P.S.; Knudsen, G.M.; Fisher, P.M. Lasting Effects of a Single Psilocybin Dose on Resting-State Functional Connectivity in Healthy Individuals. J. Psychopharmacol. 2022, 36, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, M.; Shulman, G.L. Control of Goal-Directed and Stimulus-Driven Attention in the Brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Schimmelpfennig, J.; Topczewski, J.; Zajkowski, W.; Jankowiak-Siuda, K. The Role of the Salience Network in Cognitive and Affective Deficits. Front. Hum. Neurosci. 2023, 17, 1133367. [Google Scholar] [CrossRef] [PubMed]
- James, M.H.; McNally, G.P.; Li, X. Editorial: Role of the Thalamus in Motivated Behavior. Front. Behav. Neurosci. 2021, 15, 720592. [Google Scholar] [CrossRef] [PubMed]
- Quednow, B.B.; Kometer, M.; Geyer, M.A.; Vollenweider, F.X. Psilocybin-Induced Deficits in Automatic and Controlled Inhibition Are Attenuated by Ketanserin in Healthy Human Volunteers. Neuropsychopharmacology 2012, 37, 630–640. [Google Scholar] [CrossRef]
- Preller, K.H.; Razi, A.; Zeidman, P.; Stämpfli, P.; Friston, K.J.; Vollenweider, F.X. Effective Connectivity Changes in LSD-Induced Altered States of Consciousness in Humans. Proc. Natl. Acad. Sci. USA 2019, 116, 2743–2748. [Google Scholar] [CrossRef]
- Riba, J.; Rodríguez-Fornells, A.; Barbanoj, M. Effects of Ayahuasca on Sensory and Sensorimotor Gating in Humans as Measured by P50 Suppression and Prepulse Inhibition of the Startle Reflex, Respectively. Psychopharmacology 2002, 165, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, F. Positron Emission Tomography and Fluorodeoxyglucose Studies of Metabolic Hyperfrontality and Psychopathology in the Psilocybin Model of Psychosis. Neuropsychopharmacology 1997, 16, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Gouzoulis-Mayfrank, E.; Schreckenberger, M.; Sabri, O.; Arning, C.; Thelen, B.; Spitzer, M.; Kovar, K.A.; Hermle, L.; Büll, U.; Sass, H. Neurometabolic Effects of Psilocybin, 3,4-Methylenedioxyethylamphetamine (MDE) and d-Methamphetamine in Healthy Volunteers. A Double-Blind, Placebo-Controlled PET Study with [18F]FDG. Neuropsychopharmacology 1999, 20, 565–581. [Google Scholar] [CrossRef]
- Noorani, T.; Alderson-Day, B. Spotlight Commentary: REBUS and the Anarchic Brain. Neurosci. Conscious. 2020, 2020, niaa007. [Google Scholar] [CrossRef] [PubMed]
- Decurtis, M.; Pare, D. The Rhinal Cortices: A Wall of Inhibition between the Neocortex and the Hippocampus. Prog. Neurobiol. 2004, 74, 101–110. [Google Scholar] [CrossRef]
- van Elk, M.; Yaden, D.B. Pharmacological, Neural, and Psychological Mechanisms Underlying Psychedelics: A Critical Review. Neurosci. Biobehav. Rev. 2022, 140, 104793. [Google Scholar] [CrossRef]
- Cofré, R.; Herzog, R.; Mediano, P.A.M.; Piccinini, J.; Rosas, F.E.; Sanz Perl, Y.; Tagliazucchi, E. Whole-Brain Models to Explore Altered States of Consciousness from the Bottom Up. Brain Sci. 2020, 10, 626. [Google Scholar] [CrossRef]
- Mu, C.; Dang, X.; Luo, X.-J. Mendelian Randomization Analyses Reveal Causal Relationships between Brain Functional Networks and Risk of Psychiatric Disorders. Nat. Hum. Behav. 2024, 8, 1417–1428. [Google Scholar] [CrossRef]
- Nkrumah, R.O.; Demirakca, T.; von Schröder, C.; Zehirlioglu, L.; Valencia, N.; Grauduszus, Y.; Vollstädt-Klein, S.; Schmahl, C.; Ende, G. Brain Connectivity Disruptions in PTSD Related to Early Adversity: A Multimodal Neuroimaging Study. Eur. J. Psychotraumatol 2024, 15, 2430925. [Google Scholar] [CrossRef] [PubMed]
- Grieco, S.F.; Castrén, E.; Knudsen, G.M.; Kwan, A.C.; Olson, D.E.; Zuo, Y.; Holmes, T.C.; Xu, X. Psychedelics and Neural Plasticity: Therapeutic Implications. J. Neurosci. 2022, 42, 8439–8449. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.S.; Subramanian, S.; Perry, D.; Kay, B.P.; Gordon, E.M.; Laumann, T.O.; Reneau, T.R.; Metcalf, N.V.; Chacko, R.V.; Gratton, C.; et al. Psilocybin Desynchronizes the Human Brain. Nature 2024, 632, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Burback, L.; Winkler, O.; Xu, L.; Dennett, L.; Vermetten, E.; Greenshaw, A.; Li, X.-M.; Milne, M.; Wang, F.; et al. Alterations in Brain Network Connectivity and Subjective Experience Induced by Psychedelics: A Scoping Review. Front. Psychiatry 2024, 15, 1386321. [Google Scholar] [CrossRef]
- ClinicalTrials.Gov. Available online: https://clinicaltrials.gov (accessed on 18 December 2024).
- Mitchell, J.M.; Bogenschutz, M.; Lilienstein, A.; Harrison, C.; Kleiman, S.; Parker-Guilbert, K.; Ot’alora, G.M.; Garas, W.; Paleos, C.; Gorman, I.; et al. MDMA-Assisted Therapy for Severe PTSD: A Randomized, Double-Blind, Placebo-Controlled Phase 3 Study. Nat. Med. 2021, 27, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, G.M.; Croal, M.; Feifel, D.; Kelly, J.R.; Marwood, L.; Mistry, S.; O’Keane, V.; Peck, S.K.; Simmons, H.; Sisa, C.; et al. Psilocybin for Treatment Resistant Depression in Patients Taking a Concomitant SSRI Medication. Neuropsychopharmacology 2023, 48, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, G.M.; Aaronson, S.T.; Alvarez, O.; Arden, P.C.; Baker, A.; Bennett, J.C.; Bird, C.; Blom, R.E.; Brennan, C.; Brusch, D.; et al. Single-Dose Psilocybin for a Treatment-Resistant Episode of Major Depression. New. Engl. J. Med. 2022, 387, 1637–1648. [Google Scholar] [CrossRef] [PubMed]
- Compass Pathways Announces Durable Improvement in Symptoms Through 12 Weeks in Open-Label Phase 2 Study of COMP360 Psilocybin in Post-Traumatic Stress Disorder. Available online: https://ir.compasspathways.com/News--Events-/news/news-details/2024/Compass-Pathways-announces-durable-improvement-in-symptoms-through-12-weeks-in-open-label-phase-2-study-of-COMP360-psilocybin-in-post-traumatic-stress-disorder/default.aspx (accessed on 18 December 2024).
- MindMed Announces Positive Topline Results from Phase 2b Trial of MM-120 in Generalized Anxiety Disorder: Mind Medicine (MindMed) Inc. (MNMD). Available online: https://ir.mindmed.co/news-events/press-releases/detail/131/mindmed-announces-positive-topline-results-from-phase-2b-trial-of-mm-120-in-generalized-anxiety-disorder (accessed on 18 December 2024).
- MindMed Receives FDA Breakthrough Therapy Designation and Announces Positive 12-Week Durability Data from Phase 2B Study of MM120 for Generalized Anxiety Disorder: Mind Medicine (MindMed) Inc. (MNMD). Available online: https://ir.mindmed.co/news-events/press-releases/detail/137/mindmed-receives-fda-breakthrough-therapy-designation-and-announces-positive-12-week-durability-data-from-phase-2b-study-of-mm120-for-generalized-anxiety-disorder (accessed on 18 December 2024).
- Storm, J.F.; Klink, P.C.; Aru, J.; Senn, W.; Goebel, R.; Pigorini, A.; Avanzini, P.; Vanduffel, W.; Roelfsema, P.R.; Massimini, M.; et al. An Integrative, Multiscale View on Neural Theories of Consciousness. Neuron 2024, 112, 1531–1552. [Google Scholar] [CrossRef]
- Preller, K.H.; Vollenweider, F.X. Phenomenology, Structure, and Dynamic of Psychedelic States. Natl. Libr. Med. 2016, 36, 221–256. [Google Scholar]
- Aru, J.; Suzuki, M.; Larkum, M.E. Cellular Mechanisms of Conscious Processing. Trends Cogn. Sci. 2020, 24, 814–825. [Google Scholar] [CrossRef]
- Dworkin, R.H.; McDermott, M.P.; Nayak, S.M.; Strain, E.C. Psychedelics and Psychotherapy: Is the Whole Greater than the Sum of Its Parts? Clin. Pharmacol. Ther. 2023, 114, 1166–1169. [Google Scholar] [CrossRef]
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbricht, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin Produces Substantial and Sustained Decreases in Depression and Anxiety in Patients with Life-Threatening Cancer: A Randomized Double-Blind Trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.R.; Richards, W.A.; McCann, U.; Jesse, R. Psilocybin Can Occasion Mystical-Type Experiences Having Substantial and Sustained Personal Meaning and Spiritual Significance. Psychopharmacology 2006, 187, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Palitsky, R.; Kaplan, D.M.; Peacock, C.; Zarrabi, A.J.; Maples-Keller, J.L.; Grant, G.H.; Dunlop, B.W.; Raison, C.L. Importance of Integrating Spiritual, Existential, Religious, and Theological Components in Psychedelic-Assisted Therapies. JAMA Psychiatry 2023, 80, 743. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, K.P.; Ng, L.; Erritzoe, D.; Knudsen, G.M.; Nichols, C.D.; Nichols, D.E.; Pani, L.; Soula, A.; Nutt, D. Microdosing Psychedelics: More Questions than Answers? An Overview and Suggestions for Future Research. J. Psychopharmacol. 2019, 33, 1039–1057. [Google Scholar] [CrossRef] [PubMed]
- Sellers, E.M.; Romach, M.K. Psychedelics: Science Sabotaged by Social Media. Neuropharmacology 2023, 227, 109426. [Google Scholar] [CrossRef] [PubMed]
- Rouaud, A.; Calder, A.E.; Hasler, G. Microdosing Psychedelics and the Risk of Cardiac Fibrosis and Valvulopathy: Comparison to Known Cardiotoxins. J. Psychopharmacol. 2024, 38, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Kraehenmann, R. Dreams and Psychedelics: Neurophenomenological Comparison and Therapeutic Implications. Curr. Neuropharmacol. 2017, 15, 1032–1042. [Google Scholar] [CrossRef] [PubMed]

| Phase 3—Interventional Studies | ||||
|---|---|---|---|---|
| Reference ID | Date, Start–End | Aims | Arms and Interventions | Results |
| NCT03537014 (MAPP1) | Nov 2018–Aug 2020 | To assess efficacy and safety of MDMA-assisted therapy (MDMA-AT) vs. placebo in participants with severe PTSD |
|
|
| NCT04077437 (MAPP2) | Sep 2020–Nov 2022 | To evaluate efficacy and safety of MDMA-AT vs. placebo in participants with moderate-to-severe PTSD |
|
|
| Phase 2—Interventional Studies | ||||
|---|---|---|---|---|
| Reference ID | Date, Start–End | Aims | Arms and Interventions | Results |
| NCT03775200 (COMP001) | Mar 2019–Sep 2021 | To evaluate safety and efficacy of psilocybin in TRD |
|
|
| NCT04739865 (COMP003) | Aug 2020–Oct 2021 | To assess safety and efficacy of psilocybin as adjunct therapy in TRD |
|
|
| Phase 2—Interventional Studies | ||||
|---|---|---|---|---|
| Reference ID | Date, Start–End | Aims | Arms and Interventions | Results |
| NCT05312151 (COMP201) | Jun 2022–Feb 2024 | To assess safety and tolerability of COMP360 in PTSD |
|
|
| Phase 2—Interventional Studies | ||||
|---|---|---|---|---|
| Reference ID | Date, Start–End | Aims | Arms and Interventions | Results |
| NCT05407064 (MMED008) | Aug 2022–Nov 2023 | To evaluate effects of four MM-120 (LSD D-tartrate) doses in GAD |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melani, A.; Bonaso, M.; Biso, L.; Zucchini, B.; Conversano, C.; Scarselli, M. Uncovering Psychedelics: From Neural Circuits to Therapeutic Applications. Pharmaceuticals 2025, 18, 130. https://doi.org/10.3390/ph18010130
Melani A, Bonaso M, Biso L, Zucchini B, Conversano C, Scarselli M. Uncovering Psychedelics: From Neural Circuits to Therapeutic Applications. Pharmaceuticals. 2025; 18(1):130. https://doi.org/10.3390/ph18010130
Chicago/Turabian StyleMelani, Alice, Marco Bonaso, Letizia Biso, Benedetta Zucchini, Ciro Conversano, and Marco Scarselli. 2025. "Uncovering Psychedelics: From Neural Circuits to Therapeutic Applications" Pharmaceuticals 18, no. 1: 130. https://doi.org/10.3390/ph18010130
APA StyleMelani, A., Bonaso, M., Biso, L., Zucchini, B., Conversano, C., & Scarselli, M. (2025). Uncovering Psychedelics: From Neural Circuits to Therapeutic Applications. Pharmaceuticals, 18(1), 130. https://doi.org/10.3390/ph18010130








