Comparison of ZnS(Ag) Scintillator and Proportional Counter Tube for Alpha Detection in Thin-Layer Chromatography
Abstract
:1. Introduction
2. Results
Radiochemistry
- Amount of activity required on TLC (1–100 kBq);
- Wait time for the true RCP after TLC development immediately after synthesis;
- Wait time for the true RCP after TLC development 24 h after synthesis;
- Wait time for the true activity after synthesis and purification for calculation of the true RCY;
- Acceptance criteria: Radiochemical purity >90% right after synthesis and TLC development (prospective), >97% 2–5 h after TLC development (retrospective).
3. Discussion
4. Materials and Methods
4.1. Radiochemistry
4.2. Thin-Layer Chromatography
- RCP was determined by TLC either by cutting the developed TLC into two pieces and measuring their activity using a surface contamination monitor (CoMo-170, Nuvia Instruments, Dresden, Germany) or by a TLC scanner;
- For the detection of colloidal 225Ac hydroxide, TLC was performed in 1 M NH4Ac:MeOH 1:1 on silica gel-aluminum sheets for 225Ac-PSMA-I&T and on ITLC-SG for 225Ac-TATE;
- For the detection of free 225Ac, TLC was performed on silica gel-aluminum sheets in 0.1 M citrate buffer (pH 5.0);
- For the detection of colloidal 212Pb hydroxide, TLC was performed in 1 M NH4Ac:MeOH 1:1 on ITLC-SG;
- For the detection of free 212Pb, TLC was performed on ITLC-SG in 0.1 M citrate buffer (pH 5.0);
- RNP and exact volume activity were determined using an HPGe detector (Canberra GmbH, Rüsselsheim, Germany);
- For endotoxin level determination, 10 µL of the product solution was diluted with 990 µL sterile water (1:100) using an EndoSafe PTS (Charles River, Sulzfeld, Germany);
- The pH was determined using a reflection photometer.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apostolidis, C.; Molinet, R.; McGinley, J.; Abbas, K.; Mollenbeck, J.; Morgenstern, A. Cyclotron production of Ac-225 for targeted alpha therapy. Appl. Radiat. Isot. 2005, 62, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Pruszyński, M.; Walczak, R.; Rodak, M.; Bruchertseifer, F.; Morgenstern, A.; Bilewicz, A. Radiochemical separation of 224Ra from 232U and 228Th sources for 224Ra/212Pb/212Bi generator. Appl. Radiat. Isot. 2021, 172, 109655. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.C.; Schäfer, M.; Bauder-Wüst, U.; Lehnert, W.; Leotta, K.; Morgenstern, A.; Kopka, K.; Haberkorn, U.; Mier, W.; Kratochwil, C. Development and dosimetry of 203Pb/212Pb-labelled PSMA ligands: Bringing "the lead" into PSMA-targeted alpha therapy? Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1081–1091. [Google Scholar] [CrossRef]
- Li, M.; Baumhover, N.J.; Liu, D.; Cagle, B.S.; Boschetti, F.; Paulin, G.; Lee, D.; Dai, Z.; Obot, E.R.; Marks, B.M.; et al. Preclinical evaluation of a lead specific chelator (PSC) conjugated to radiopeptides for 203Pb and 212Pb-Based theranostics. Pharmaceutics 2023, 15, 414. [Google Scholar] [CrossRef]
- Thiele, N.A.; Brown, V.; Kelly, J.M.; Amor-Coarasa, A.; Jermilova, U.; MacMillan, S.N.; Nikolopoulou, A.; Ponnala, S.; Ramogida, C.F.; Robertson, A.K.H.; et al. An Eighteen-Membered Macrocyclic Ligand for Actinium-225 Targeted Alpha Therapy. Angew. Chem. Int. Ed. Engl. 2017, 56, 14712–14717. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.; Quinn, T.P.; Lee, D.; Liu, D.; Kunkel, F.; Zimmerman, B.E.; McAlister, D.; Olewein, K.R.; Menda, Y.; et al. Automated cassette-based production of high specific activity [203/212Pb] peptide-based theranostic radiopharmaceuticals for image-guided radionuclide therapy for cancer. Appl. Radiat. Isot. 2017, 127, 52–60. [Google Scholar] [CrossRef]
- Reissig, F.; Bauer, D.; Zarschler, K.; Novy, Z.; Bendova, K.; Ludik, M.C.; Kopka, K.; Pietzsch, H.J.; Petrik, M.; Mamat, C. Towards targeted alpha therapy with Actinium-225: Chelators for mild condition radiolabeling and targeting PSMA—A proof of concept study. Cancers 2021, 13, 1974. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef]
- Delpassand, E.S.; Tworowska, I.; Esfandiari, R.; Torgue, J.; Hurt, J.; Shafie, A.; Núnez, R. Targeted α-emitter therapy with 212Pb-DOTAMTATE for the treatment of metastatic SSTR-expressing neuroendocrine tumors: First-in-humans dose-escalation clinical trial. J. Nucl. Med. 2022, 63, 1326–1333. [Google Scholar] [CrossRef]
- Graf, F.; Fahrer, J.; Maus, S.; Morgenstern, A.; Bruchertseifer, F.; Venkatachalam, S.; Fottner, C.; Weber, M.M.; Huelsenbeck, J.; Schreckenberger, M.; et al. DNA double strand breaks as predictor of efficacy of the alpha-particle emitter Ac-225 and the electron emitter Lu-177 for somatostatin receptor targeted radiotherapy. PLoS ONE 2014, 9, e88239. [Google Scholar] [CrossRef]
- Roohi, S.; Rizvi, S.K.; Naqvi, S.A.R. 177Lu-DOTATATE Peptide Receptor Radionuclide Therapy: Indigenously Developed Freeze Dried Cold Kit and Biological Response in In-Vitro and In-Vivo Models. Dose Response 2021, 19, 1559325821990147. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.J.B.; Wilson, J.; Andersson, J.D.; Wuest, F. Theranostic Imaging Surrogates for Targeted Alpha Therapy: Progress in Production, Purification, and Applications. Pharmaceuticals 2023, 16, 1622. [Google Scholar] [CrossRef] [PubMed]
- Usmani, S.; Rasheed, R.; Al Kandari, F.; Marafi, F.; Naqvi, S.A.R. 225Ac prostate-specific membrane antigen posttherapy α imaging: Comparing 2 and 3 photopeaks. Clin. Nucl. Med. 2019, 44, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Pandya, D.N.; Hantgan, R.; Budzevich, M.M.; Kock, N.D.; Morse, D.L.; Batista, I.; Mintz, A.; Li, K.C.; Wadas, T.J. Preliminary Therapy Evaluation of (225)Ac-DOTA-c(RGDyK) Demonstrates that Cerenkov Radiation Derived from (225)Ac Daughter Decay Can Be Detected by Optical Imaging for In Vivo Tumor Visualization. Theranostics 2016, 6, 698–709. [Google Scholar] [CrossRef]
- Beattie, B.J.; Thorek, D.L.; Schmidtlein, C.R.; Pentlow, K.S.; Humm, J.L.; Hielscher, A.H. Quantitative modeling of Cerenkov light production efficiency from medical radionuclides. PLoS ONE 2012, 7, e31402. [Google Scholar] [CrossRef]
- Meredith, R.; Torgue, J.; Shen, S.; Fisher, D.R.; Banaga, E.; Bunch, P.; Morgan, D.; Fan, J.; Straughn, J.M., Jr. Dose escalation and dosimetry of first-in-human alpha radioimmunotherapy with 212Pb-TCMC-trastuzumab. J. Nucl. Med 2014, 55, 1636–1642. [Google Scholar] [CrossRef]
- Müller, D.; Herrmann, H.; Schultz, M.K.; Solbach, C.; Ettrich, T.; Prasad, V. 203Pb-VMT-α-NET scintigraphy of a patient with neuroendocrine tumor. Clin. Nucl. Med. 2023, 48, 54–55. [Google Scholar] [CrossRef]
- Mikalsen, L.T.G.; Kvassheim, M.; Stokke, C. Optimized SPECT Imaging of 224Ra α-particle therapy by 212Pb photon emissions. J. Nucl. Med 2023, 64, 1131–1137. [Google Scholar] [CrossRef]
- Kelly, J.M.; Amor-Coarasa, A.; Sweeney, E.; Wilson, J.J.; Causey, P.W.; Babich, J.W. A suitable time point for quantifying the radiochemical purity of 225Ac-labeled radiopharmaceuticals. EJNMMI Radiopharm. Chem. 2021, 6, 38. [Google Scholar] [CrossRef]
- Pretze, M.; Kunkel, F.; Runge, R.; Freudenberg, R.; Braune, A.; Hartmann, H.; Schwarz, U.; Brogsitter, C.; Kotzerke, J. Ac-EAZY! Towards GMP-compliant module syntheses of 225Ac-labeled peptides for clinical application. Pharmaceuticals 2021, 14, 652. [Google Scholar] [CrossRef]
- Pretze, M.; Michler, E.; Runge, R.; Wetzig, K.; Tietze, K.; Brandt, F.; Schultz, M.K.; Kotzerke, J. Influence of the Molar Activity of 203/212Pb-PSC-PEG2-TOC on Somatostatin Receptor Type 2-Binding and Cell Uptake. Pharmaceuticals 2023, 16, 1605. [Google Scholar] [CrossRef] [PubMed]
- Hamisu, A.; Khiter, O.; Al-Zhrani, S.; Haridh, W.S.B.; Al-Hadeethi, Y.; Sayyed, M.I.; Tijani, S.A. The use of nanomaterial polymeric materials as ionizing radiation shields. Radiat. Phys. Chem. 2024, 216, 111448. [Google Scholar] [CrossRef]
- Essler, M.; Gartner, F.C.; Neff, F.; Blechert, B.; Senekowitsch-Schmidtke, R.; Bruchertseifer, F.; Morgenstern, A.; Seidl, C. Therapeutic efficacy and toxicity of 225Ac-labelled vs. 213Bi-labelled tumour-homing peptides in a preclinical mouse model of peritoneal carcinomatosis. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 602–612. [Google Scholar] [CrossRef]
- Pretze, M.; Michler, E.; Kästner, D.; Kunkel, F.; Sagastume, E.A.; Schultz, M.K.; Kotzerke, J. Lead-it-EAZY! GMP-compliant production of [212Pb]Pb-PSC-PEG2-TOC. EJNMMI Radiopharm. Chem. 2024, 9, 81. [Google Scholar] [CrossRef] [PubMed]

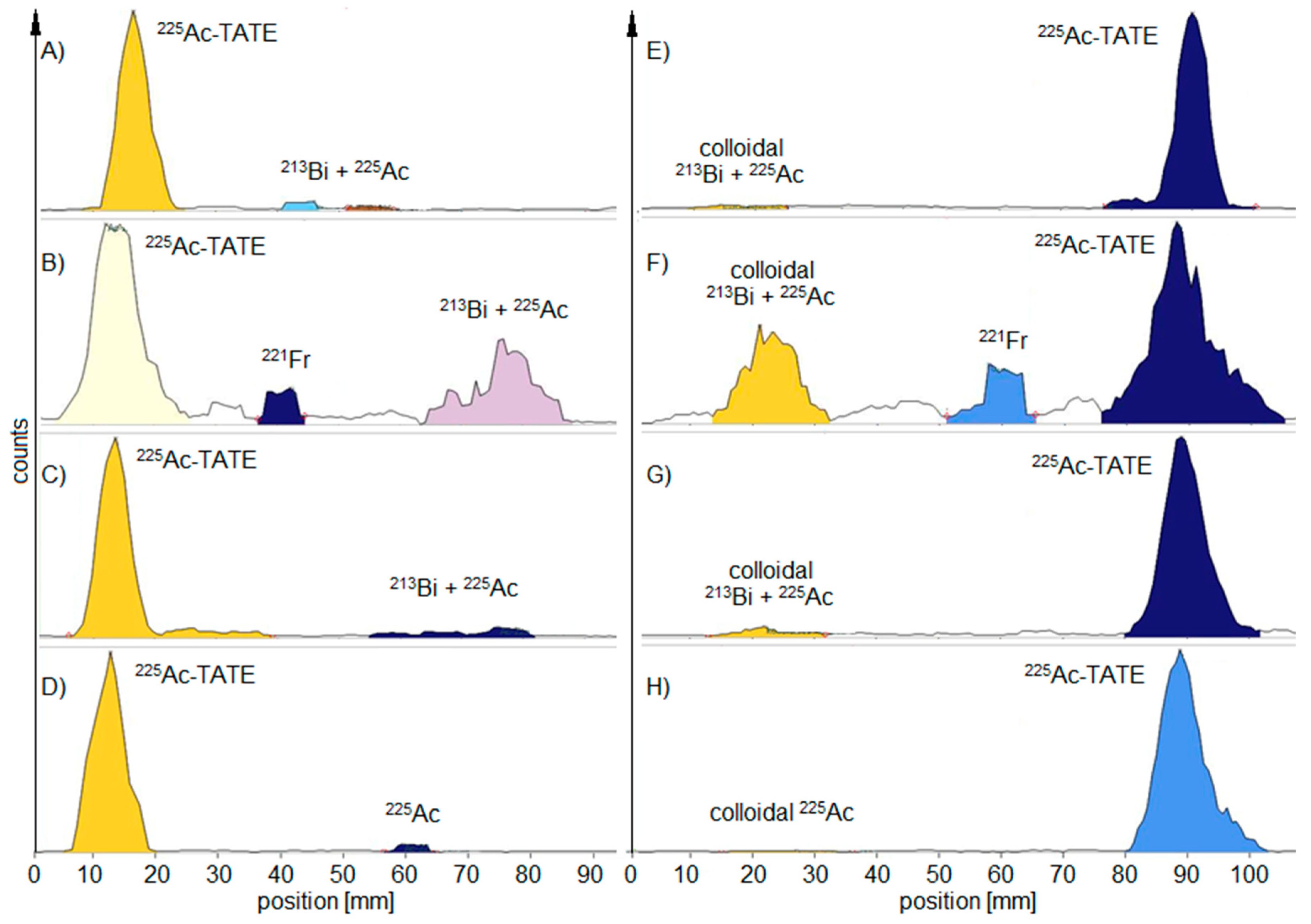


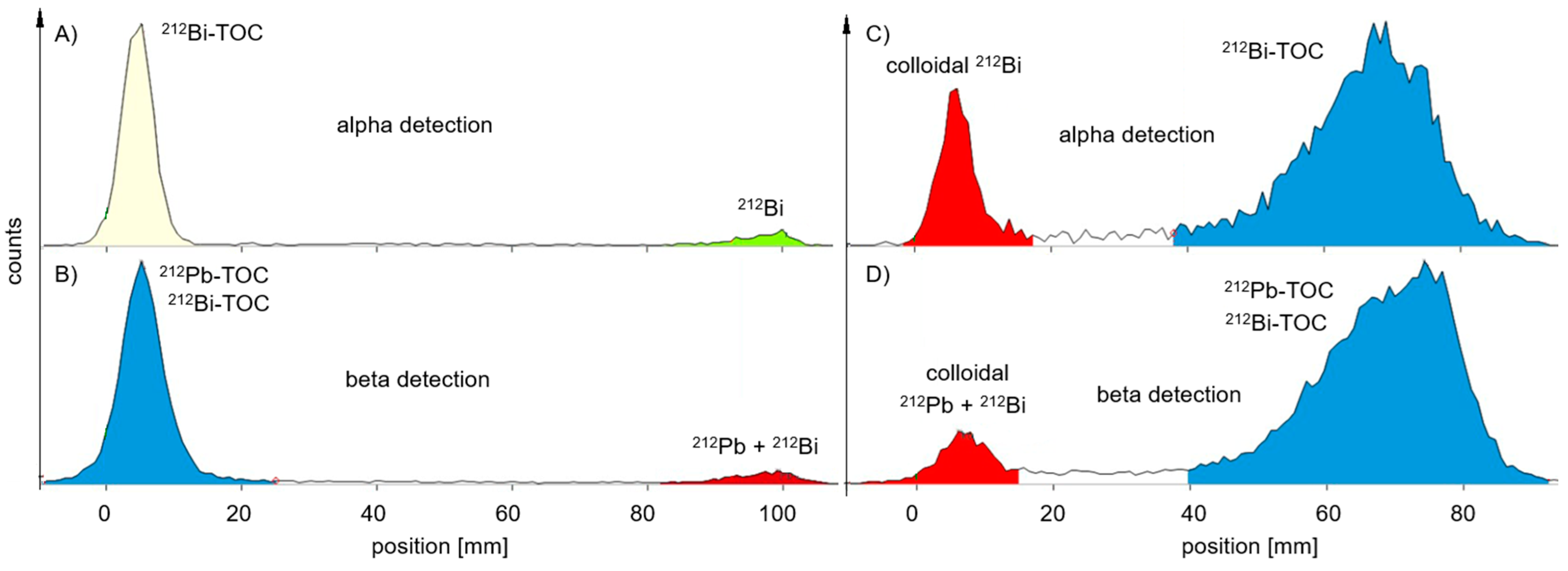
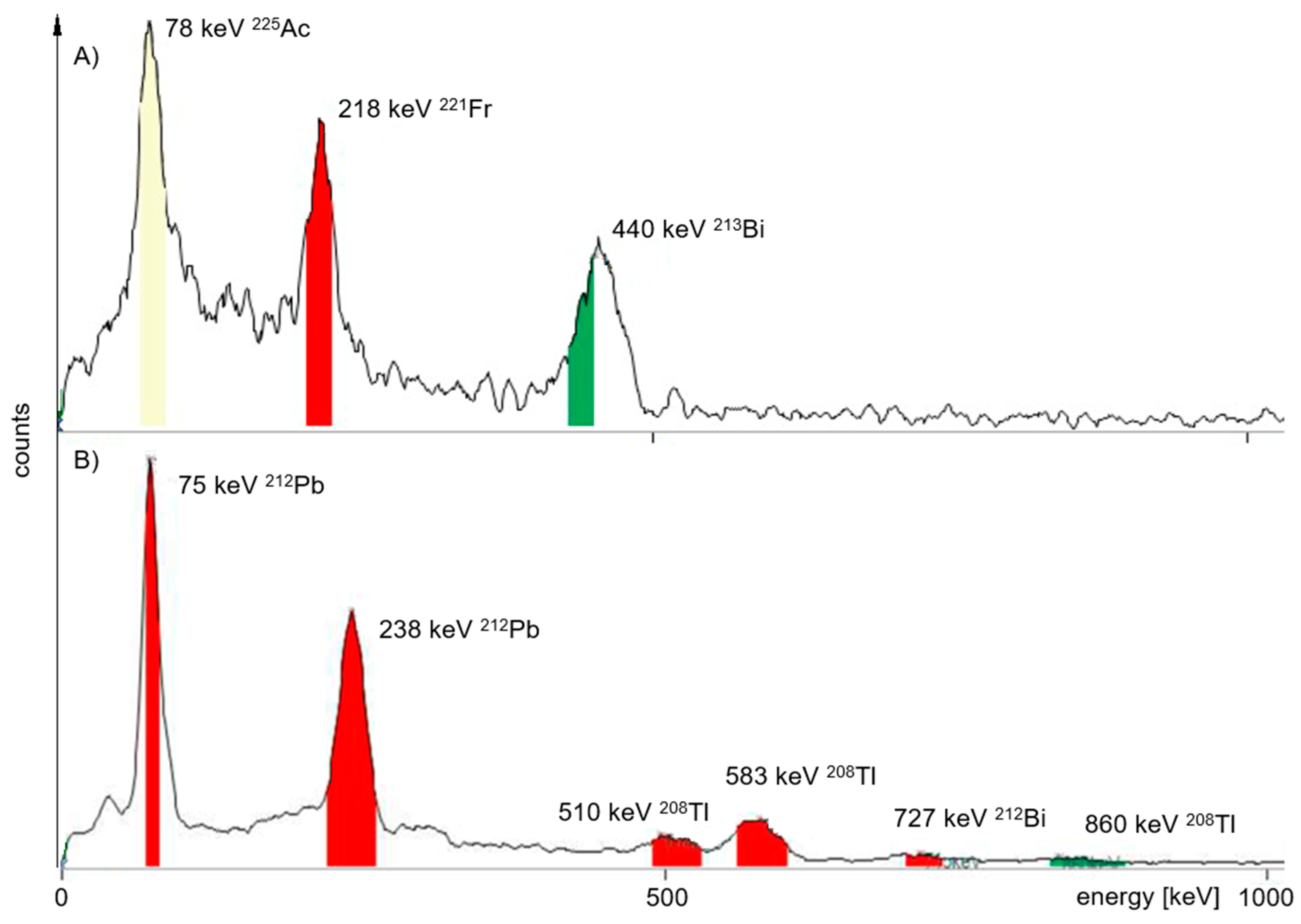
| TLC Development | TLC Measurement | Product Peak (%) MiniScanPRO+ | Product Peak (%) AR-2000 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 225Ac-PSMA-I&T | 225Ac-TATE | 225Ac-PSMA-I&T | 225Ac-TATE | ||||||
| Citrate | NH4Az | Citrate | NH4Az | Citrate | NH4Az | Citrate | NH4Az | ||
| Immediately after synthesis | immediately | 94.7 | 90.4 | 94.1 | 96.6 | 97.6 | 95.5 | 99.6 | 99.2 |
| 2 h later | 98.1 | 99.2 | n.d. * | n.d. | 99.9 | 99.5 | n.d. | n.d. | |
| 24 h after synthesis | immediately | 55.7 | 85.7 | 66.9 | 75.6 | 82.9 | 84.8 | 86.4 | 77.8 |
| 2 h later | 91.3 | 94.3 | 90.0 | 94.8 | 96.5 | 94.3 | 96.5 | 97.5 | |
| 5 h later | 98.1 | 96.6 | n.d. | n.d. | 98.4 | 96.1 | n.d. | n.d. | |
| 24 h later | 98.7 | 97.5 | 96.7 | 98.9 | 98.4 | 96.3 | 98.9 | 99.4 | |
| TLC Development | TLC Measurement Time | Product Peak [%] MiniScanPRO+ | Product Peak [%] AR-2000 | ||
|---|---|---|---|---|---|
| 212Pb-VMT-α-NET | 212Pb-VMT-α-NET | ||||
| Citrate | NH4Az | Citrate | NH4Az | ||
| entry | immediately | 95.5 | 96.9 | 93.1 | 94.7 |
| 6 h later | 95.3 | 94.8 | 96.5 | 90.0 | |
| 18 h later | 95.8 | 96.8 | 96.7 | 94.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pretze, M.; Wendrich, J.; Hartmann, H.; Freudenberg, R.; Bundschuh, R.A.; Kotzerke, J.; Michler, E. Comparison of ZnS(Ag) Scintillator and Proportional Counter Tube for Alpha Detection in Thin-Layer Chromatography. Pharmaceuticals 2025, 18, 26. https://doi.org/10.3390/ph18010026
Pretze M, Wendrich J, Hartmann H, Freudenberg R, Bundschuh RA, Kotzerke J, Michler E. Comparison of ZnS(Ag) Scintillator and Proportional Counter Tube for Alpha Detection in Thin-Layer Chromatography. Pharmaceuticals. 2025; 18(1):26. https://doi.org/10.3390/ph18010026
Chicago/Turabian StylePretze, Marc, Jan Wendrich, Holger Hartmann, Robert Freudenberg, Ralph A. Bundschuh, Jörg Kotzerke, and Enrico Michler. 2025. "Comparison of ZnS(Ag) Scintillator and Proportional Counter Tube for Alpha Detection in Thin-Layer Chromatography" Pharmaceuticals 18, no. 1: 26. https://doi.org/10.3390/ph18010026
APA StylePretze, M., Wendrich, J., Hartmann, H., Freudenberg, R., Bundschuh, R. A., Kotzerke, J., & Michler, E. (2025). Comparison of ZnS(Ag) Scintillator and Proportional Counter Tube for Alpha Detection in Thin-Layer Chromatography. Pharmaceuticals, 18(1), 26. https://doi.org/10.3390/ph18010026








