Uncovering the Mechanism of Action of Antiprotozoal Agents: A Survey on Photoaffinity Labeling Strategy
Abstract
:1. Introduction
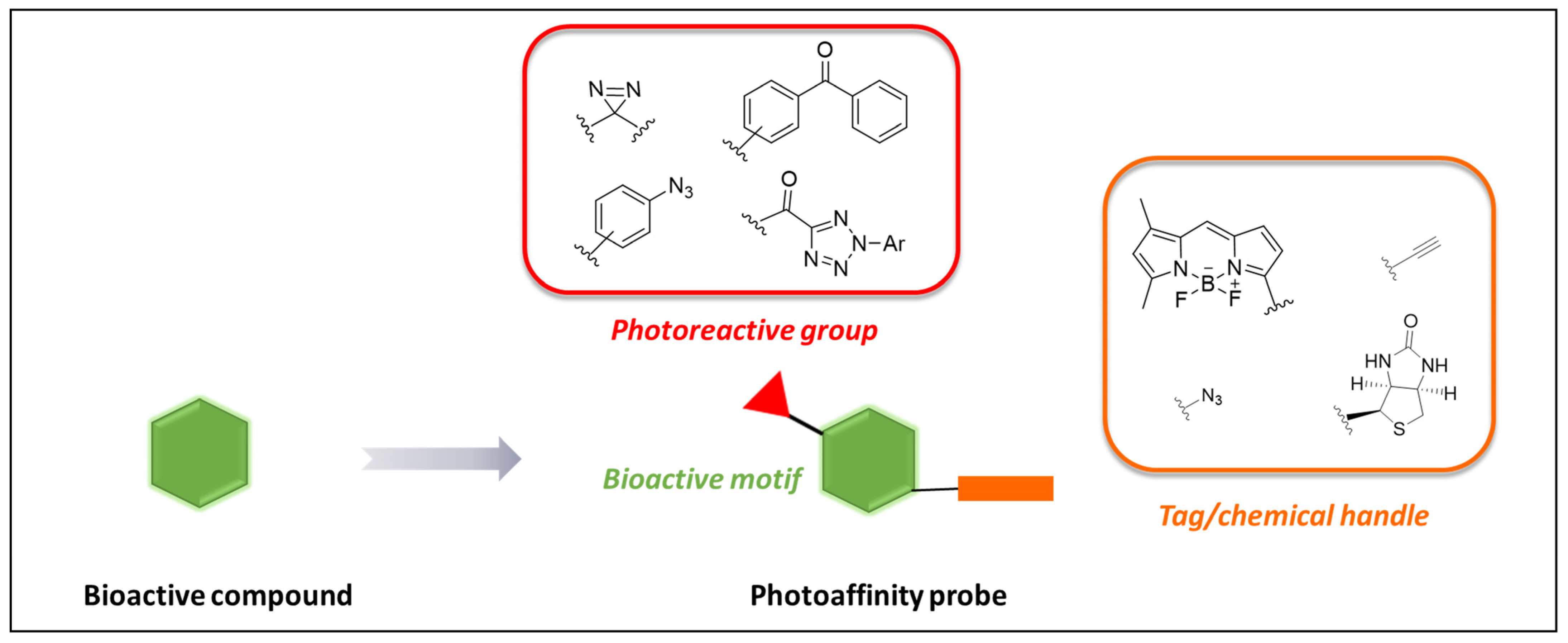
2. Photoaffinity Labeling (PAL)
Probes’ Design Considerations
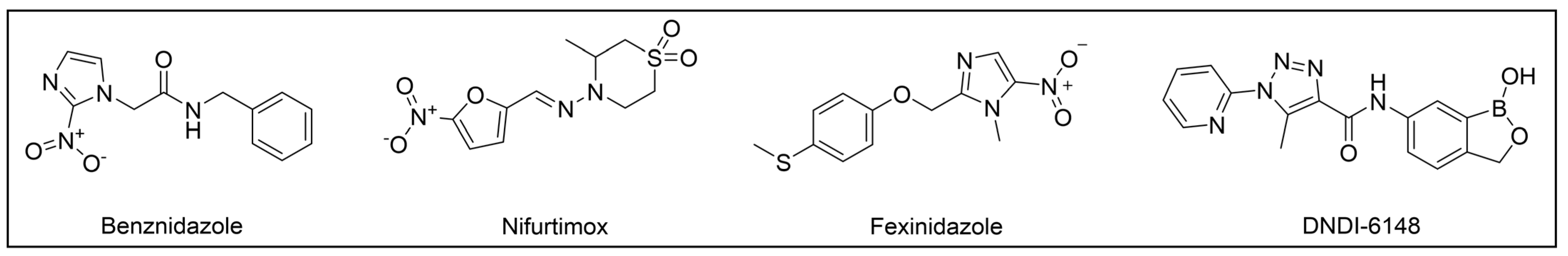
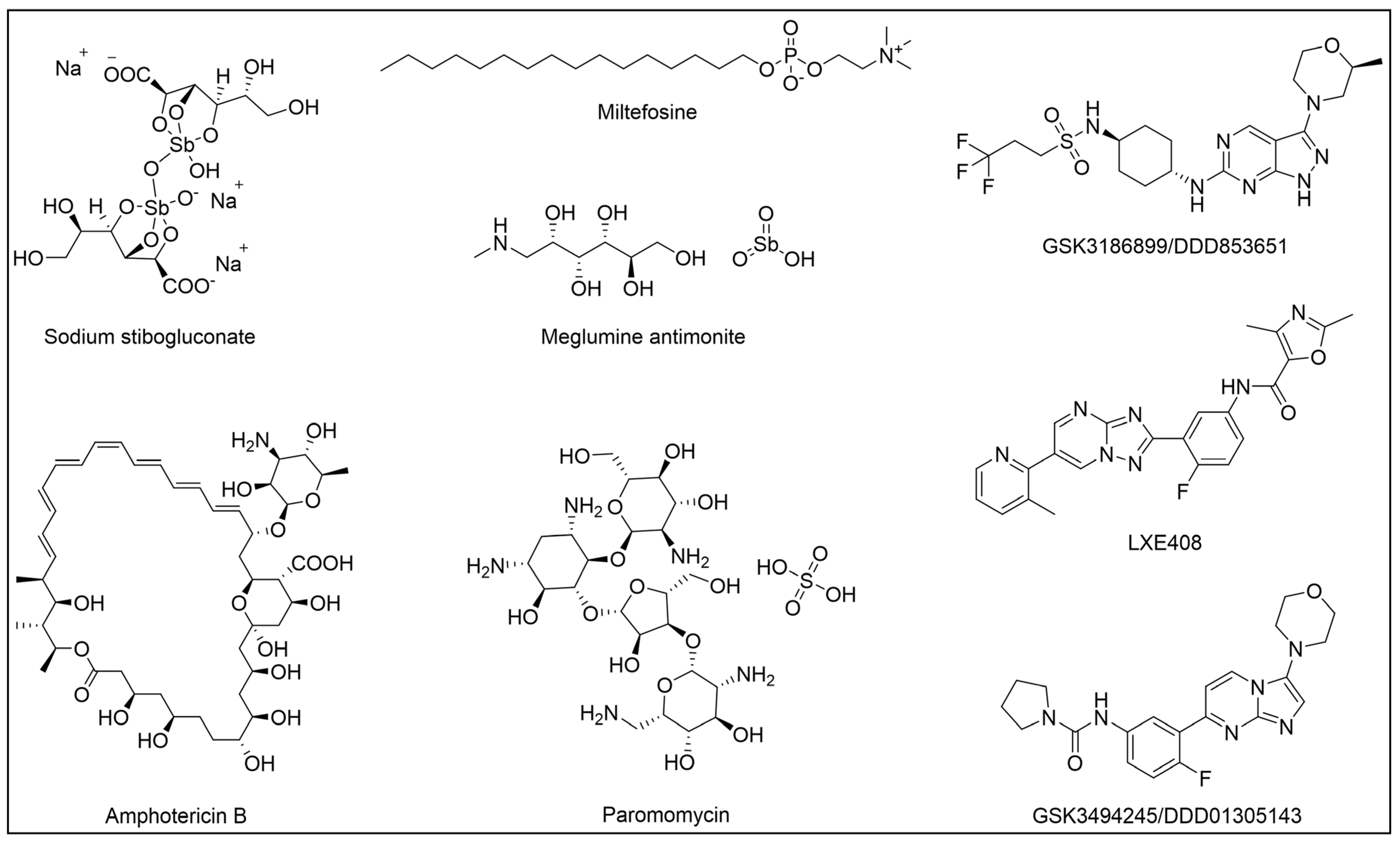
3. Kinetoplastid Diseases (KDs) and Their Therapeutic Options
4. PAL Studies on KDs
4.1. PAL Studies on Trypanosoma Brucei
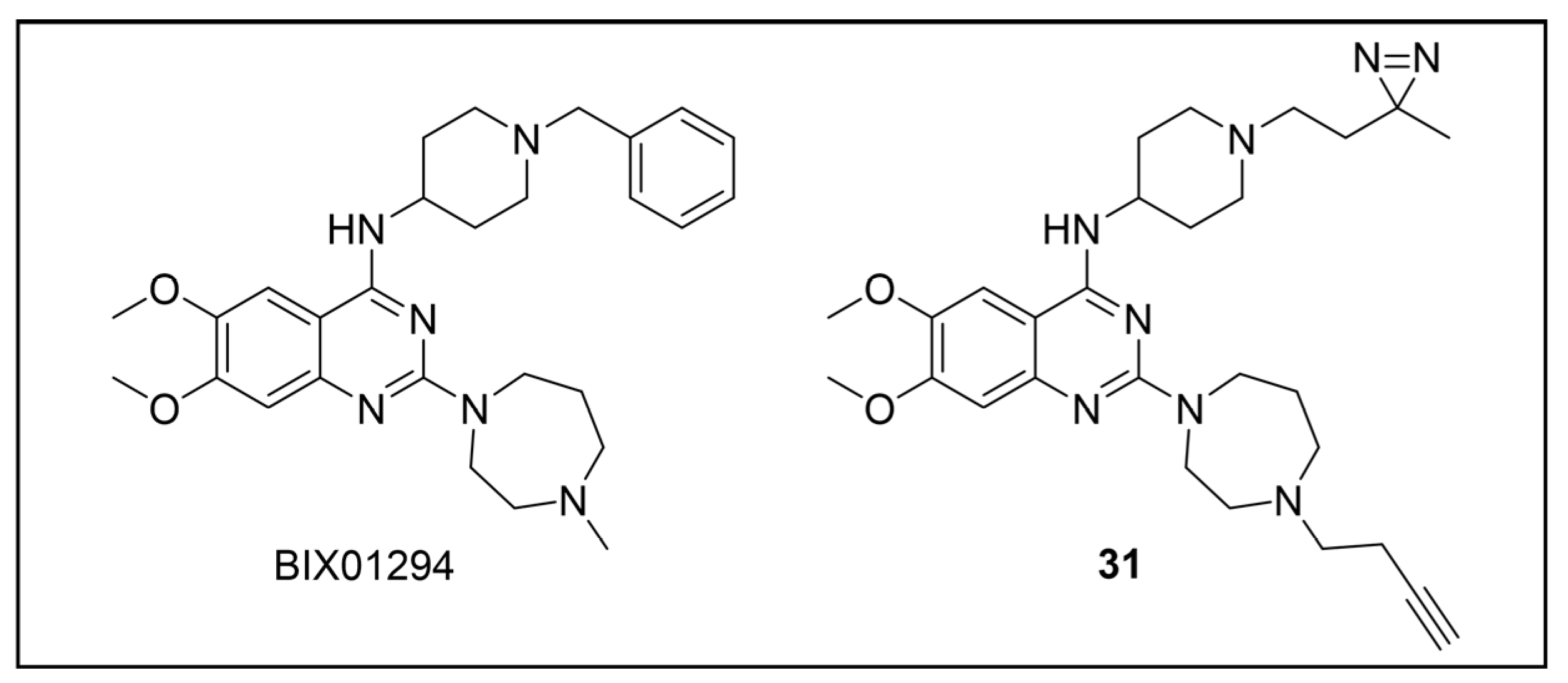
4.2. PAL Studies on Broad-Spectrum Anti-KDs Agents
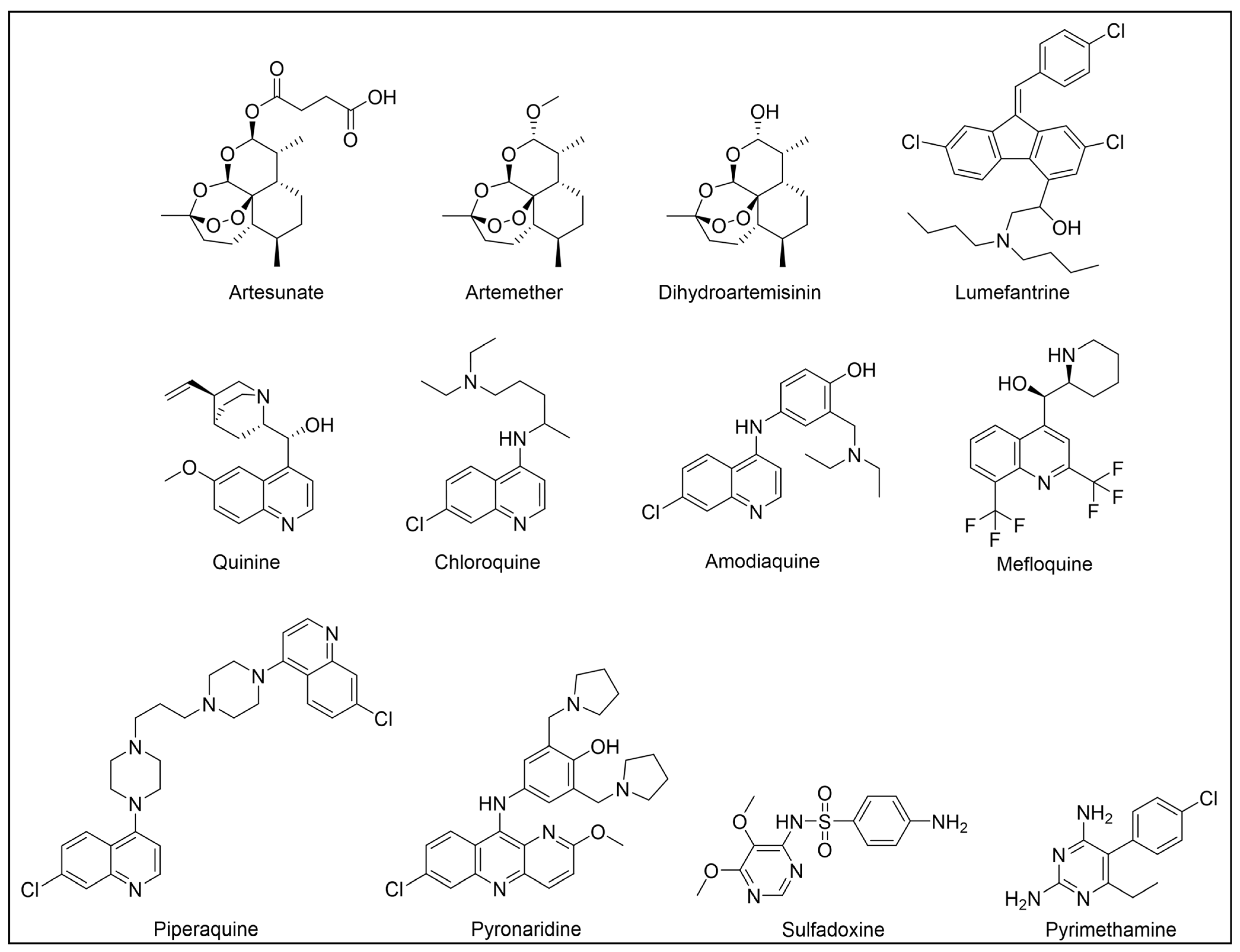
5. Malaria and Therapeutic Options
6. PAL Studies on Malaria
6.1. Artemisinin-Based Probes
6.2. Chloroquine-Based Probes
6.3. Mefloquine-Based Probes
6.4. Plasmodione-Based Probes
6.5. Miscellaneous Probes
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Rycker, M.; Wyllie, S.; Horn, D.; Read, K.D.; Gilbert, I.H. Anti-Trypanosomatid Drug Discovery: Progress and Challenges. Nat. Rev. Microbiol. 2023, 21, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Engels, D.; Huang, F.; Zhou, X.N. Time to Integrate Malaria and Neglected Tropical Diseases Control and Elimination. China CDC Wkly. 2021, 3, 372–374. [Google Scholar] [CrossRef] [PubMed]
- WHO. Neglected Tropical Diseases (NTDs). Available online: https://www.who.int/health-topics/neglected-tropical-diseases#tab=tab_1 (accessed on 11 October 2024).
- Shirley, H.; Grifferty, G.; Yates, E.F.; Raykar, N.; Wamai, R.; McClain, C.D. The Connection between Climate Change, Surgical Care and Neglected Tropical Diseases. Ann. Glob. Health 2022, 88, 68. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Antiprotozoal Drugs: Challenges and Opportunities. Expert Opin. Ther. Pat. 2023, 33, 133–136. [Google Scholar] [CrossRef]
- Zekar, L.; Sharman, T. Plasmodium Falciparum Malaria. StatPearls 2023. [Google Scholar]
- World Malaria Report 2023; World Health Organization: Geneva, Switzerland, 2023; Licence: CC BY-NC-SA 3.0 IGO.
- CDC. Malaria’s Impact Worldwide. Available online: https://www.cdc.gov/malaria/php/impact/index.html#:~:text=Nearly%20half%20the%20world’s%20population,clinical%20episodes%2C%20and%20608%2C000%20deaths (accessed on 11 October 2024).
- Rodrigues, J.C.F.; Godinho, J.L.P.; de Souza, W. Biology of Human Pathogenic Trypanosomatids: Epidemiology, Lifecycle and Ultrastructure. Subcell. Biochem. 2014, 74, 1–42. [Google Scholar] [CrossRef]
- WHO. Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 11 October 2024).
- WHO. Chagas Disease (Also Known as American Trypanosomiasis). Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 11 October 2024).
- WHO. Trypanosomiasis, Human African (Sleeping Sickness). Available online: https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness) (accessed on 11 October 2024).
- Rao, S.P.S.; Barrett, M.P.; Dranoff, G.; Faraday, C.J.; Gimpelewicz, C.R.; Hailu, A.; Jones, C.L.; Kelly, J.M.; Lazdins-Helds, J.K.; Mäser, P.; et al. Drug Discovery for Kinetoplastid Diseases: Future Directions. ACS Infect. Dis. 2019, 5, 152–157. [Google Scholar] [CrossRef]
- Santos, S.S.; de Araújo, R.V.; Giarolla, J.; El Seoud, O.; Ferreira, E.I. Searching for Drugs for Chagas Disease, Leishmaniasis and Schistosomiasis: A Review. Int. J. Antimicrob. Agents 2020, 55, 105906. [Google Scholar] [CrossRef]
- Hudu, S.A.; Jimoh, A.O.; Adeshina, K.A.; Otalike, E.G.; Tahir, A.; Hegazy, A.A. An Insight into the Success, Challenges, and Future Perspectives of Eliminating Neglected Tropical Disease. Sci. Afr. 2024, 24, e02165. [Google Scholar] [CrossRef]
- Müller, J.; Hemphill, A. Drug Target Identification in Protozoan Parasites. Expert Opin. Drug Discov. 2016, 11, 815–824. [Google Scholar] [CrossRef]
- De Rycker, M.; Baragaña, B.; Duce, S.L.; Gilbert, I.H. Challenges and Recent Progress in Drug Discovery for Tropical Diseases. Nature 2018, 559, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Saccoliti, F.; Di Santo, R.; Costi, R. Recent Advancement in the Search of Innovative Antiprotozoal Agents Targeting Trypanothione Metabolism. ChemMedChem 2020, 15, 2420–2435. [Google Scholar] [CrossRef] [PubMed]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, W.L.; Amato, R.; van der Pluijm, R.W.; Jacob, C.G.; Quang, H.H.; Thuy-Nhien, N.T.; Hien, T.T.; Hongvanthong, B.; Chindavongsa, K.; Mayxay, M.; et al. Evolution and Expansion of Multidrug-Resistant Malaria in Southeast Asia: A Genomic Epidemiology Study. Lancet Infect. Dis. 2019, 19, 943–951. [Google Scholar] [CrossRef]
- Field, M.C.; Horn, D.; Fairlamb, A.H.; Ferguson, M.A.J.; Gray, D.W.; Read, K.D.; De Rycker, M.; Torrie, L.S.; Wyatt, P.G.; Wyllie, S.; et al. Anti-Trypanosomatid Drug Discovery: An Ongoing Challenge and a Continuing Need. Nat. Rev. Microbiol. 2017, 15, 217–231. [Google Scholar] [CrossRef]
- Rao, S.P.S.; Manjunatha, U.H.; Mikolajczak, S.; Ashigbie, P.G.; Diagana, T.T. Drug Discovery for Parasitic Diseases: Powered by Technology, Enabled by Pharmacology, Informed by Clinical Science. Trends Parasitol. 2023, 39, 260–271. [Google Scholar] [CrossRef]
- DNDi. Available online: https://dndi.org/ (accessed on 11 October 2024).
- Wellcome. Available online: https://wellcome.org/ (accessed on 11 October 2024).
- Global Health Innovative Technology Fund. Fight Neglected Diseases Through Partnerships. Available online: https://www.ghitfund.org/ (accessed on 11 October 2024).
- Lindner, A.K.; Lejon, V.; Barrett, M.P.; Blumberg, L.; Bukachi, S.A.; Chancey, R.J.; Edielu, A.; Matemba, L.; Mesha, T.; Mwanakasale, V.; et al. New WHO Guidelines for Treating Rhodesiense Human African Trypanosomiasis: Expanded Indications for Fexinidazole and Pentamidine. Lancet Infect. Dis. 2024; Epub ahead of print. [Google Scholar] [CrossRef]
- Lindner, A.K.; Lejon, V.; Chappuis, F.; Seixas, J.; Kazumba, L.; Barrett, M.P.; Mwamba, E.; Erphas, O.; Akl, E.A.; Villanueva, G.; et al. New WHO Guidelines for Treatment of Gambiense Human African Trypanosomiasis Including Fexinidazole: Substantial Changes for Clinical Practice. Lancet Infect. Dis. 2020, 20, e38–e46. [Google Scholar] [CrossRef]
- Neau, P.; Hänel, H.; Lameyre, V.; Strub-Wourgaft, N.; Kuykens, L. Innovative Partnerships for the Elimination of Human African Trypanosomiasis and the Development of Fexinidazole. Trop. Med. Infect. Dis. 2020, 5, 17. [Google Scholar] [CrossRef]
- LaMonte, G.M.; Rocamora, F.; Marapana, D.S.; Gnädig, N.F.; Ottilie, S.; Luth, M.R.; Worgall, T.S.; Goldgof, G.M.; Mohunlal, R.; Santha Kumar, T.R.; et al. Pan-Active Imidazolopiperazine Antimalarials Target the Plasmodium Falciparum Intracellular Secretory Pathway. Nat. Commun. 2020, 11, 1780. [Google Scholar] [CrossRef]
- Schmitt, E.K.; Ndayisaba, G.; Yeka, A.; Asante, K.P.; Grobusch, M.P.; Karita, E.; Mugerwa, H.; Asiimwe, S.; Oduro, A.; Fofana, B.; et al. Efficacy of Cipargamin (KAE609) in a Randomized, Phase II Dose-Escalation Study in Adults in Sub-Saharan Africa With Uncomplicated Plasmodium Falciparum Malaria. Clin. Infect. Dis. 2022, 74, 1831–1839. [Google Scholar] [CrossRef]
- Wijnant, G.J.; Croft, S.L.; De La Flor, R.; Alavijeh, M.; Yardley, V.; Braillard, S.; Mowbray, C.; Van Bocxlaer, K. Pharmacokinetics and Pharmacodynamics of the Nitroimidazole DNDI-0690 in Mouse Models of Cutaneous Leishmaniasis. Antimicrob. Agents Chemother. 2019, 63, e00829-19. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.G.; De Rycker, M.; Ajakane, M.; Albrecht, S.; Álvarez-Pedraglio, A.I.; Boesche, M.; Brand, S.; Campbell, L.; Cantizani-Perez, J.; Cleghorn, L.A.T.; et al. Identification of GSK3186899/DDD853651 as a Preclinical Development Candidate for the Treatment of Visceral Leishmaniasis. J. Med. Chem. 2019, 62, 1180–1202. [Google Scholar] [CrossRef] [PubMed]
- Corfu, A.I.; Santarem, N.; Luelmo, S.; Mazza, G.; Greco, A.; Altomare, A.; Ferrario, G.; Nasta, G.; Keminer, O.; Aldini, G.; et al. Discovery of 1,3,4-Oxadiazole Derivatives as Broad-Spectrum Antiparasitic Agents. ACS Infect. Dis. 2024, 10, 2222–2238. [Google Scholar] [CrossRef] [PubMed]
- Fairlamb, A.H.; Wyllie, S. The Critical Role of Mode of Action Studies in Kinetoplastid Drug Discovery. Front. Drug Discov. 2023, 3, 1185679. [Google Scholar] [CrossRef]
- Lu, K.Y.; Mansfield, C.R.; Fitzgerald, M.C.; Derbyshire, E.R. Chemoproteomics for Plasmodium Parasite Drug Target Discovery. Chembiochem 2021, 22, 2591–2599. [Google Scholar] [CrossRef]
- Davis, R.L. Mechanism of Action and Target Identification: A Matter of Timing in Drug Discovery. iScience 2020, 23, 101487. [Google Scholar] [CrossRef]
- Gilbert, I.H. Drug Discovery for Neglected Diseases: Molecular Target-Based and Phenotypic Approaches. J. Med. Chem. 2013, 56, 7719–7726. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, M.; Li, W.; Lei, X. Chemoproteomics, A Broad Avenue to Target Deconvolution. Adv. Sci. 2024, 11, e2305608. [Google Scholar] [CrossRef]
- Vincent, F.; Nueda, A.; Lee, J.; Schenone, M.; Prunotto, M.; Mercola, M. Phenotypic Drug Discovery: Recent Successes, Lessons Learned and New Directions. Nat. Rev. Drug Discov. 2022, 21, 899–914. [Google Scholar] [CrossRef]
- Annang, F.; Pérez-Moreno, G.; García-Hernández, R.; Cordon-Obras, C.; Martín, J.; Tormo, J.R.; Rodríguez, L.; De Pedro, N.; Gómez-Pérez, V.; Valente, M.; et al. High-Throughput Screening Platform for Natural Product-Based Drug Discovery against 3 Neglected Tropical Diseases: Human African Trypanosomiasis, Leishmaniasis, and Chagas Disease. J. Biomol. Screen 2015, 20, 82–91. [Google Scholar] [CrossRef]
- Terstappen, G.C.; Schlüpen, C.; Raggiaschi, R.; Gaviraghi, G. Target Deconvolution Strategies in Drug Discovery. Nat. Rev. Drug Discov. 2007, 6, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Thorne, N.; McKew, J.C. Phenotypic Screens as a Renewed Approach for Drug Discovery. Drug Discov. Today 2013, 18, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Whitebread, S.; Hamon, J.; Bojanic, D.; Urban, L. Keynote Review: In Vitro Safety Pharmacology Profiling: An Essential Tool for Successful Drug Development. Drug Discov. Today 2005, 10, 1421–1433. [Google Scholar] [CrossRef] [PubMed]
- Schenone, M.; Dančík, V.; Wagner, B.K.; Clemons, P.A. Target Identification and Mechanism of Action in Chemical Biology and Drug Discovery. Nat. Chem. Biol. 2013, 9, 232–240. [Google Scholar] [CrossRef]
- Moffat, J.G.; Vincent, F.; Lee, J.A.; Eder, J.; Prunotto, M. Opportunities and Challenges in Phenotypic Drug Discovery: An Industry Perspective. Nat. Rev. Drug Discov. 2017, 16, 531–543. [Google Scholar] [CrossRef]
- Burton, N.R.; Kim, P.; Backus, K.M. Photoaffinity Labelling Strategies for Mapping the Small Molecule-Protein Interactome. Org. Biomol. Chem. 2021, 19, 7792–7809. [Google Scholar] [CrossRef]
- Murale, D.P.; Hong, S.C.; Haque, M.M.; Lee, J.S. Photo-Affinity Labeling (PAL) in Chemical Proteomics: A Handy Tool to Investigate Protein-Protein Interactions (PPIs). Proteome Sci. 2017, 15, 14. [Google Scholar] [CrossRef]
- Homan, R.A.; Lapek, J.D.; Woo, C.M.; Niessen, S.; Jones, L.H.; Parker, C.G. Photoaffinity Labelling with Small Molecules. Nat. Rev. Methods Primers 2024, 4, 30. [Google Scholar] [CrossRef]
- Smith, E.; Collins, I. Photoaffinity Labeling in Target- and Binding-Site Identification. Future Med. Chem. 2015, 7, 159–183. [Google Scholar] [CrossRef]
- Ge, S.S.; Chen, B.; Wu, Y.Y.; Long, Q.S.; Zhao, Y.L.; Wang, P.Y.; Yang, S. Current Advances of Carbene-Mediated Photoaffinity Labeling in Medicinal Chemistry. RSC Adv. 2018, 8, 29428–29454. [Google Scholar] [CrossRef]
- Hill, J.R.; Robertson, A.A.B. Fishing for Drug Targets: A Focus on Diazirine Photoaffinity Probe Synthesis. J. Med. Chem. 2018, 61, 6945–6963. [Google Scholar] [CrossRef] [PubMed]
- West, A.V.; Woo, C.M. Photoaffinity Labeling Chemistries Used to Map Biomolecular Interactions. Isr. J. Chem. 2023, 63, e202200081. [Google Scholar] [CrossRef]
- Karaj, E.; Sindi, S.H.; Viranga Tillekeratne, L.M. Photoaffinity Labeling and Bioorthogonal Ligation: Two Critical Tools for Designing “Fish Hooks” to Scout for Target Proteins. Bioorg. Med. Chem. 2022, 62, 116721. [Google Scholar] [CrossRef]
- Haldón, E.; Nicasio, M.C.; Pérez, P.J. Copper-Catalysed Azide-Alkyne Cycloadditions (CuAAC): An Update. Org. Biomol. Chem. 2015, 13, 9528–9550. [Google Scholar] [CrossRef]
- Meldal, M.; Diness, F. Recent Fascinating Aspects of the CuAAC Click Reaction. Trends Chem. 2020, 2, 569–584. [Google Scholar] [CrossRef]
- Hein, J.E.; Fokin, V.V. Copper-Catalyzed Azide-Alkyne Cycloaddition (CuAAC) and beyond: New Reactivity of Copper(I) Acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef]
- Kardos, J.; Héja, L.; Simon, Á.; Jablonkai, I.; Kovács, R.; Jemnitz, K. Copper Signalling: Causes and Consequences. Cell Commun. Signal. 2018, 16, 71. [Google Scholar] [CrossRef]
- Li, Z.; Wang, D.; Li, L.; Pan, S.; Na, Z.; Tan, C.Y.J.; Yao, S.Q. “Minimalist” Cyclopropene-Containing Photo-Cross-Linkers Suitable for Live-Cell Imaging and Affinity-Based Protein Labeling. J. Am. Chem. Soc. 2014, 136, 9990–9998. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Ma, N.; Tian, J.; Shao, Y.; Zhu, B.; Wong, Y.K.; Liang, Z.; Zou, C.; Wang, J. Target Identification of Natural Medicine with Chemical Proteomics Approach: Probe Synthesis, Target Fishing and Protein Identification. Signal Transduct. Target. Ther. 2020, 5, 72. [Google Scholar] [CrossRef]
- Ha, J.; Park, H.; Park, J.; Park, S.B. Recent Advances in Identifying Protein Targets in Drug Discovery. Cell Chem. Biol. 2021, 28, 394–423. [Google Scholar] [CrossRef]
- Li, Z.; Hao, P.; Li, L.; Tan, C.Y.J.; Cheng, X.; Chen, G.Y.J.; Sze, S.K.; Shen, H.M.; Yao, S.Q. Design and Synthesis of Minimalist Terminal Alkyne-Containing Diazirine Photo-Crosslinkers and Their Incorporation into Kinase Inhibitors for Cell- and Tissue-Based Proteome Profiling. Angew. Chem. Int. Ed. Engl. 2013, 52, 8551–8556. [Google Scholar] [CrossRef] [PubMed]
- Pays, E.; Radwanska, M.; Magez, S. The Pathogenesis of African Trypanosomiasis. Annu. Rev. Pathol. 2023, 18, 19–45. [Google Scholar] [CrossRef] [PubMed]
- Schijman, A.G.; Alonso-Padilla, J.; Britto, C.; Herrera Bernal, C.P. Retrospect, Advances and Challenges in Chagas Disease Diagnosis: A Comprehensive Review. Lancet Reg. Health Am. 2024, 36, 100821. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, S.Y.; Hassan, F.; Shukla, D.; Bala, S.; Faruqui, T.; Akhter, Y.; Khan, A.R.; Nasibullah, M. A Review on Potential Therapeutic Targets for the Treatment of Leishmaniasis. Parasitol. Int. 2024, 100, 102863. [Google Scholar] [CrossRef] [PubMed]
- Van Griensven, J.; Carrillo, E.; López-Vélez, R.; Lynen, L.; Moreno, J. Leishmaniasis in Immunosuppressed Individuals. Clin. Microbiol. Infect. 2014, 20, 286–299. [Google Scholar] [CrossRef]
- Bilbe, G. Infectious Diseases. Overcoming Neglect of Kinetoplastid Diseases. Science 2015, 348, 974–976. [Google Scholar] [CrossRef]
- Requena-Méndez, A.; Aldasoro, E.; de Lazzari, E.; Sicuri, E.; Brown, M.; Moore, D.A.J.; Gascon, J.; Muñoz, J. Prevalence of Chagas Disease in Latin-American Migrants Living in Europe: A Systematic Review and Meta-Analysis. PLoS Neglected Trop. Dis. 2015, 9, e0003540. [Google Scholar] [CrossRef]
- eBioMedicine Leishmania: An Urgent Need for New Treatments. EBioMedicine 2023, 87, 104440. [CrossRef]
- Altamura, F.; Rajesh, R.; Catta-Preta, C.M.C.; Moretti, N.S.; Cestari, I. The Current Drug Discovery Landscape for Trypanosomiasis and Leishmaniasis: Challenges and Strategies to Identify Drug Targets. Drug Dev. Res. 2022, 83, 225–252. [Google Scholar] [CrossRef]
- Pane, S.; Giancola, M.L.; Piselli, P.; Corpolongo, A.; Repetto, E.; Bellagamba, R.; Cimaglia, C.; Carrara, S.; Ghirga, P.; Oliva, A.; et al. Serological Evaluation for Chagas Disease in Migrants from Latin American Countries Resident in Rome, Italy. BMC Infect. Dis. 2018, 18, 212. [Google Scholar] [CrossRef]
- Pérez-Molina, J.A.; Molina, I. Chagas Disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.P.; Kyle, D.E.; Sibley, L.D.; Radke, J.B.; Tarleton, R.L. Protozoan Persister-like Cells and Drug Treatment Failure. Nat. Rev. Microbiol. 2019, 17, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Crespillo-Andújar, C.; Comeche, B.; Hamer, D.H.; Arevalo-Rodriguez, I.; Alvarez-Díaz, N.; Zamora, J.; Pérez-Molina, J.A. Use of Benznidazole to Treat Chronic Chagas Disease: An Updated Systematic Review with a Meta-Analysis. PLoS Neglected Trop. Dis. 2022, 16, e0010386. [Google Scholar] [CrossRef] [PubMed]
- Kourbeli, V.; Chontzopoulou, E.; Moschovou, K.; Pavlos, D.; Mavromoustakos, T.; Papanastasiou, I.P. An Overview on Target-Based Drug Design against Kinetoplastid Protozoan Infections: Human African Trypanosomiasis, Chagas Disease and Leishmaniases. Molecules 2021, 26, 4629. [Google Scholar] [CrossRef]
- Valera-Vera, E.A.; Sayé, M.; Reigada, C.; Miranda, M.R.; Pereira, C.A. In Silico Repositioning of Etidronate as a Potential Inhibitor of the Trypanosoma Cruzi Enolase. J. Mol. Graph. Model. 2020, 95, 107506. [Google Scholar] [CrossRef]
- Bhambra, A.S.; Ruparelia, K.C.; Tan, H.L.; Tasdemir, D.; Burrell-Saward, H.; Yardley, V.; Beresford, K.J.M.; Arroo, R.R.J. Synthesis and Antitrypanosomal Activities of Novel Pyridylchalcones. Eur. J. Med. Chem. 2017, 128, 213–218. [Google Scholar] [CrossRef]
- de VC Sinatti, V.; Baptista, L.P.R.; Alves-Ferreira, M.; Dardenne, L.; da Silva, J.H.M.; Guimarães, A.C. In Silico Identification of Inhibitors of Ribose 5-Phosphate Isomerase from Trypanosoma Cruzi Using Ligand and Structure Based Approaches. J. Mol. Graph. Model. 2017, 77, 168–180. [Google Scholar] [CrossRef]
- Gonzalez, S.N.; Mills, J.J.; Maugeri, D.; Olaya, C.; Laguera, B.L.; Enders, J.R.; Sherman, J.; Rodriguez, A.; Pierce, J.G.; Cazzulo, J.J.; et al. Design, Synthesis, and Evaluation of Substrate—Analogue Inhibitors of Trypanosoma Cruzi Ribose 5-Phosphate Isomerase Type B. Bioorg. Med. Chem. Lett. 2021, 32, 127723. [Google Scholar] [CrossRef]
- Ferreira, D.D.; Mesquita, J.T.; Da Costa Silva, T.A.; Romanelli, M.M.; Da Gama Jaen Batista, D.; Da Silva, C.F.; Da Gama, A.N.S.; Neves, B.J.; Melo-Filho, C.C.; Correia Soeiro, M.D.N.; et al. Efficacy of Sertraline against Trypanosoma Cruzi: An in Vitro and In Silico Study. J. Venom. Anim. Toxins. Incl. Trop. Dis. 2018, 24, 30. [Google Scholar] [CrossRef]
- Matutino Bastos, T.; Mannochio Russo, H.; Silvio Moretti, N.; Schenkman, S.; Marcourt, L.; Gupta, M.P.; Wolfender, J.L.; Ferreira Queiroz, E.; Botelho Pereira Soares, M. Chemical Constituents of Anacardium Occidentale as Inhibitors of Trypanosoma Cruzi Sirtuins. Molecules 2019, 24, 1299. [Google Scholar] [CrossRef]
- Bastos, T.M.; Soares, M.B.P.; Franco, C.H.; Alcântara, L.; Antonini, L.; Sabatino, M.; Mautone, N.; Freitas-Junior, L.H.; Moraes, C.B.; Ragno, R.; et al. Identification of Inhibitors to Trypanosoma Cruzi Sirtuins Based on Compounds Developed to Human Enzymes. Int. J. Mol. Sci. 2020, 21, 3659. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.M.; Kronenberger, T.; Maltarollo, V.G.; Poso, A.; de Moura Gatti, F.; Almeida, V.M.; Marana, S.R.; Lopes, C.D.; Tezuka, D.Y.; de Albuquerque, S.; et al. Trypanosoma Cruzi Sirtuin 2 as a Relevant Druggable Target: New Inhibitors Developed by Computer-Aided Drug Design. Pharmaceuticals 2023, 16, 428. [Google Scholar] [CrossRef] [PubMed]
- Calvet, C.M.; Vieira, D.F.; Choi, J.Y.; Kellar, D.; Cameron, M.D.; Siqueira-Neto, J.L.; Gut, J.; Johnston, J.B.; Lin, L.; Khan, S.; et al. 4-Aminopyridyl-Based CYP51 Inhibitors as Anti-Trypanosoma Cruzi Drug Leads with Improved Pharmacokinetic Profile and in Vivo Potency. J. Med. Chem. 2014, 57, 6989–7005. [Google Scholar] [CrossRef]
- Saccoliti, F.; Madia, V.N.; Tudino, V.; De Leo, A.; Pescatori, L.; Messore, A.; De Vita, D.; Scipione, L.; Brun, R.; Kaiser, M.; et al. Design, Synthesis, and Biological Evaluation of New 1-(Aryl-1 H-Pyrrolyl)(Phenyl)Methyl-1 H-Imidazole Derivatives as Antiprotozoal Agents. J. Med. Chem. 2019, 62, 1330–1347. [Google Scholar] [CrossRef]
- Saccoliti, F.; Madia, V.N.; Tudino, V.; De Leo, A.; Pescatori, L.; Messore, A.; De Vita, D.; Scipione, L.; Brun, R.; Kaiser, M.; et al. Biological Evaluation and Structure-Activity Relationships of Imidazole-Based Compounds as Antiprotozoal Agents. Eur. J. Med. Chem. 2018, 156, 53–60. [Google Scholar] [CrossRef]
- Molina, I.; Gómez i Prat, J.; Salvador, F.; Treviño, B.; Sulleiro, E.; Serre, N.; Pou, D.; Roure, S.; Cabezos, J.; Valerio, L.; et al. Randomized Trial of Posaconazole and Benznidazole for Chronic Chagas’ Disease. N. Engl. J. Med. 2014, 370, 1899–1908. [Google Scholar] [CrossRef]
- Torrico, F.; Gascon, J.; Ortiz, L.; Alonso-Vega, C.; Pinazo, M.J.; Schijman, A.; Almeida, I.C.; Alves, F.; Strub-Wourgaft, N.; Ribeiro, I.; et al. Treatment of Adult Chronic Indeterminate Chagas Disease with Benznidazole and Three E1224 Dosing Regimens: A Proof-of-Concept, Randomised, Placebo-Controlled Trial. Lancet Infect. Dis. 2018, 18, 419–430. [Google Scholar] [CrossRef]
- Morillo, C.A.; Waskin, H.; Sosa-Estani, S.; del Carmen Bangher, M.; Cuneo, C.; Milesi, R.; Mallagray, M.; Apt, W.; Beloscar, J.; Gascon, J.; et al. Benznidazole and Posaconazole in Eliminating Parasites in Asymptomatic T. Cruzi Carriers: The STOP-CHAGAS Trial. J. Am. Coll Cardiol. 2017, 69, 939–947. [Google Scholar] [CrossRef]
- Torrico, F.; Gascón, J.; Barreira, F.; Blum, B.; Almeida, I.C.; Alonso-Vega, C.; Barboza, T.; Bilbe, G.; Correia, E.; Garcia, W.; et al. New Regimens of Benznidazole Monotherapy and in Combination with Fosravuconazole for Treatment of Chagas Disease (BENDITA): A Phase 2, Double-Blind, Randomised Trial. Lancet Infect. Dis. 2021, 21, 1129–1140. [Google Scholar] [CrossRef]
- Gabaldón-Figueira, J.C.; Martinez-Peinado, N.; Escabia, E.; Ros-Lucas, A.; Chatelain, E.; Scandale, I.; Gascon, J.; Pinazo, M.-J.; Alonso-Padilla, J. State-of-the-Art in the Drug Discovery Pathway for Chagas Disease: A Framework for Drug Development and Target Validation. Res. Rep. Trop. Med. 2023, 14, 1–19. [Google Scholar] [CrossRef]
- Kratz, J.M.; Gonçalves, K.R.; Romera, L.M.D.; Moraes, C.B.; Bittencourt-Cunha, P.; Schenkman, S.; Chatelain, E.; Sosa-Estani, S. The Translational Challenge in Chagas Disease Drug Development. Mem. Inst. Oswaldo Cruz. 2022, 117, e200501. [Google Scholar] [CrossRef] [PubMed]
- Pinazo, M.J.; Forsyth, C.; Losada, I.; Esteban, E.T.; García-Rodríguez, M.; Villegas, M.L.; Molina, I.; Crespillo-Andújar, C.; Gállego, M.; Ballart, C.; et al. Efficacy and Safety of Fexinidazole for Treatment of Chronic Indeterminate Chagas Disease (FEXI-12): A Multicentre, Randomised, Double-Blind, Phase 2 Trial. Lancet Infect. Dis. 2024, 24, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Mowbray, C.E.; Braillard, S.; Glossop, P.A.; Whitlock, G.A.; Jacobs, R.T.; Speake, J.; Pandi, B.; Nare, B.; Maes, L.; Yardley, V.; et al. DNDI-6148: A Novel Benzoxaborole Preclinical Candidate for the Treatment of Visceral Leishmaniasis. J. Med. Chem. 2021, 64, 16159–16176. [Google Scholar] [CrossRef] [PubMed]
- DNDi. DNDI-6148 Chagas Disease. Available online: https://dndi.org/research-development/portfolio/dndi-6148-chagas/ (accessed on 13 October 2024).
- Pisarski, K. The Global Burden of Disease of Zoonotic Parasitic Diseases: Top 5 Contenders for Priority Consideration. Trop. Med. Infect. Dis. 2019, 4, 44. [Google Scholar] [CrossRef]
- Kapil, S.; Singh, P.K.; Silakari, O. An Update on Small Molecule Strategies Targeting Leishmaniasis. Eur. J. Med. Chem. 2018, 157, 339–367. [Google Scholar] [CrossRef]
- Mansuri, R.; Singh, J.; Diwan, A. An Insight into the Current Perspective and Potential Drug Targets for Visceral Leishmaniasis (VL). Curr. Drug Targets 2020, 21, 1105–1129. [Google Scholar] [CrossRef]
- Freitas, E.O.; Nico, D.; Alves-Silva, M.V.; Morrot, A.; Clinch, K.; Evans, G.B.; Tyler, P.C.; Schramm, V.L.; Palatnik-de-Sousa, C.B. Immucillins ImmA and ImmH Are Effective and Non-Toxic in the Treatment of Experimental Visceral Leishmaniasis. PLoS Neglected Trop. Dis. 2015, 9, e0004297. [Google Scholar] [CrossRef]
- Vieira, P.S.; Souza, T.d.A.C.B.; Honorato, R.V.; Zanphorlin, L.M.; Severiano, K.U.; Rocco, S.A.; de Oliveira, A.H.C.; Cordeiro, A.T.; Oliveira, P.S.L.; de Giuseppe, P.O.; et al. Pyrrole-Indolinone SU11652 Targets the Nucleoside Diphosphate Kinase from Leishmania Parasites. Biochem. Biophys. Res. Commun. 2017, 488, 461–465. [Google Scholar] [CrossRef]
- Azzouz, S.; Lawton, P. In Vitro Effects of Purine and Pyrimidine Analogues on Leishmania Donovani and Leishmania Infantum Promastigotes and Intracellular Amastigotes. Acta Parasitol. 2017, 62, 582–588. [Google Scholar] [CrossRef]
- Stevanović, S.; Perdih, A.; Senćanski, M.; Gliić, S.; Duarte, M.; Tomás, A.M.; Sena, F.V.; Sousa, F.M.; Pereira, M.M.; Solmajer, T. In Silico Discovery of a Substituted 6-Methoxy-Quinalidine with Leishmanicidal Activity in Leishmania Infantum. Molecules 2018, 23, 772. [Google Scholar] [CrossRef]
- Melo, T.S.; Gattass, C.R.; Soares, D.C.; Cunha, M.R.; Ferreira, C.; Tavares, M.T.; Saraiva, E.; Parise-Filho, R.; Braden, H.; Delorenzi, J.C. Oleanolic Acid (OA) as an Antileishmanial Agent: Biological Evaluation and in Silico Mechanistic Insights. Parasitol. Int. 2016, 65, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Battista, T.; Colotti, G.; Ilari, A.; Fiorillo, A. Targeting Trypanothione Reductase, a Key Enzyme in the Redox Trypanosomatid Metabolism, to Develop New Drugs against Leishmaniasis and Trypanosomiases. Molecules 2020, 25, 1924. [Google Scholar] [CrossRef] [PubMed]
- Colotti, G.; Saccoliti, F.; Gramiccia, M.; Di Muccio, T.; Prakash, J.; Yadav, S.; Dubey, V.K.; Vistoli, G.; Battista, T.; Mocci, S.; et al. Structure-Guided Approach to Identify a Novel Class of Anti-Leishmaniasis Diaryl Sulfide Compounds Targeting the Trypanothione Metabolism. Amino Acids 2020, 52, 247–259. [Google Scholar] [CrossRef]
- Saccoliti, F.; Angiulli, G.; Pupo, G.; Pescatori, L.; Madia, V.N.; Messore, A.; Colotti, G.; Fiorillo, A.; Scipione, L.; Gramiccia, M.; et al. Inhibition of Leishmania Infantum Trypanothione Reductase by Diaryl Sulfide Derivatives. J. Enzym. Inhib. Med. Chem. 2017, 32, 304–310. [Google Scholar] [CrossRef]
- Madia, V.N.; Ialongo, D.; Patacchini, E.; Exertier, C.; Antonelli, L.; Colotti, G.; Messore, A.; Tudino, V.; Saccoliti, F.; Scipione, L.; et al. Inhibition of Leishmania Infantum Trypanothione Reductase by New Aminopropanone Derivatives Interacting with the NADPH Binding Site. Molecules 2023, 28, 338. [Google Scholar] [CrossRef]
- Taha, M.; Ismail, N.H.; Ali, M.; Rashid, U.; Imran, S.; Uddin, N.; Khan, K.M. Molecular Hybridization Conceded Exceptionally Potent Quinolinyl-Oxadiazole Hybrids through Phenyl Linked Thiosemicarbazide Antileishmanial Scaffolds: In Silico Validation and SAR Studies. Bioorg. Chem. 2017, 71, 192–200. [Google Scholar] [CrossRef]
- Taha, M.; Ismail, N.H.; Imran, S.; Anouar, E.H.; Selvaraj, M.; Jamil, W.; Ali, M.; Kashif, S.M.; Rahim, F.; Khan, K.M.; et al. Synthesis and Molecular Modelling Studies of Phenyl Linked Oxadiazole-Phenylhydrazone Hybrids as Potent Antileishmanial Agents. Eur. J. Med. Chem. 2017, 126, 1021–1033. [Google Scholar] [CrossRef]
- Sangshetti, J.N.; Kalam Khan, F.A.; Kulkarni, A.A.; Patil, R.H.; Pachpinde, A.M.; Lohar, K.S.; Shinde, D.B. Antileishmanial Activity of Novel Indolyl-Coumarin Hybrids: Design, Synthesis, Biological Evaluation, Molecular Docking Study and In Silico ADME Prediction. Bioorg. Med. Chem. Lett. 2016, 26, 829–835. [Google Scholar] [CrossRef]
- Patil, S.R.; Asrondkar, A.; Patil, V.; Sangshetti, J.N.; Kalam Khan, F.A.; Damale, M.G.; Patil, R.H.; Bobade, A.S.; Shinde, D.B. Antileishmanial Potential of Fused 5-(Pyrazin-2-Yl)-4H-1,2,4-Triazole-3-Thiols: Synthesis, Biological Evaluations and Computational Studies. Bioorg. Med. Chem. Lett. 2017, 27, 3845–3850. [Google Scholar] [CrossRef]
- Raj, S.; Saha, G.; Sasidharan, S.; Dubey, V.K.; Saudagar, P. Biochemical Characterization and Chemical Validation of Leishmania MAP Kinase-3 as a Potential Drug Target. Sci. Rep. 2019, 9, 16209. [Google Scholar] [CrossRef]
- De Jesus, L.C.L.; Soares, R.E.P.; Moreira, V.R.; Pontes, R.L.; Castelo-Branco, P.V.; Ferreira Pereira, S.R. Genistein and Ascorbic Acid Reduce Oxidative Stress-Derived DNA Damage Induced by the Antileishmanial Meglumine Antimoniate. Antimicrob. Agents Chemother. 2018, 62, e00456-18. [Google Scholar] [CrossRef] [PubMed]
- Nagle, A.; Biggart, A.; Be, C.; Srinivas, H.; Hein, A.; Caridha, D.; Sciotti, R.J.; Pybus, B.; Kreishman-Deitrick, M.; Bursulaya, B.; et al. Discovery and Characterization of Clinical Candidate LXE408 as a Kinetoplastid-Selective Proteasome Inhibitor for the Treatment of Leishmaniases. J. Med. Chem. 2020, 63, 10773–10781. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.; Nagle, A.S.; Biggart, A.; Lai, Y.H.; Liang, F.; Davis, L.C.; Barnes, S.W.; Mathison, C.J.N.; Myburgh, E.; Gao, M.Y.; et al. Proteasome Inhibition for Treatment of Leishmaniasis, Chagas Disease and Sleeping Sickness. Nature 2016, 537, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Brand, S.; De Rycker, M.; Zuccotto, F.; Lukac, I.; Dodd, P.G.; Ko, E.J.; Manthri, S.; McGonagle, K.; Osuna-Cabello, M.; et al. Scaffold-Hopping Strategy on a Series of Proteasome Inhibitors Led to a Preclinical Candidate for the Treatment of Visceral Leishmaniasis. J. Med. Chem. 2021, 64, 5905–5930. [Google Scholar] [CrossRef]
- Wyllie, S.; Thomas, M.; Patterson, S.; Crouch, S.; De Rycker, M.; Lowe, R.; Gresham, S.; Urbaniak, M.D.; Otto, T.D.; Stojanovski, L.; et al. Cyclin-Dependent Kinase 12 Is a Drug Target for Visceral Leishmaniasis. Nature 2018, 560, 192–197. [Google Scholar] [CrossRef]
- DNDi. DNDI-6899 (GSK899/DDD853651). Available online: https://dndi.org/research-development/portfolio/dndi-6899/ (accessed on 14 October 2024).
- Wyllie, S.; Brand, S.; Thomas, M.; De Rycker, M.; wa Chung, C.; Pena, I.; Bingham, R.P.; Bueren-Calabuig, J.A.; Cantizani, J.; Cebrian, D.; et al. Preclinical Candidate for the Treatment of Visceral Leishmaniasis That Acts through Proteasome Inhibition. Proc. Natl. Acad. Sci. USA 2019, 116, 9318–9323. [Google Scholar] [CrossRef]
- DNDi. GSK245 (DDD1305143). Available online: https://dndi.org/research-development/portfolio/gsk245/ (accessed on 14 October 2024).
- DNDi. LXE408 Novartis. Available online: https://dndi.org/research-development/portfolio/novartis-lxe408/ (accessed on 14 October 2024).
- DNDi. DNDI-6148. Available online: https://dndi.org/research-development/portfolio/dndi-6148/ (accessed on 14 October 2024).
- DNDi. DNDI-0690. Available online: https://dndi.org/research-development/portfolio/dndi-0690/ (accessed on 14 October 2024).
- Büscher, P.; Cecchi, G.; Jamonneau, V.; Priotto, G. Human African Trypanosomiasis. Lancet 2017, 390, 2397–2409. [Google Scholar] [CrossRef]
- Franco, J.R.; Priotto, G.; Paone, M.; Cecchi, G.; Ebeja, A.K.; Simarro, P.P.; Sankara, D.; Metwally, S.B.A.; Argaw, D.D. The Elimination of Human African Trypanosomiasis: Monitoring Progress towards the 2021-2030 WHO Road Map Targets. PLoS Neglected Trop. Dis. 2024, 18, e0012111. [Google Scholar] [CrossRef]
- Barrett, M.P.; Priotto, G.; Franco, J.R.; Lejon, V.; Lindner, A.K. Elimination of Human African Trypanosomiasis: The Long Last Mile. PLoS Neglected Trop. Dis. 2024, 18, e0012091. [Google Scholar] [CrossRef]
- Brun, R.; Blum, J.; Chappuis, F.; Burri, C. Human African Trypanosomiasis. Lancet 2010, 375, 148–159. [Google Scholar] [CrossRef]
- Kande Betu Ku Mesu, V.; Mutombo Kalonji, W.; Bardonneau, C.; Valverde Mordt, O.; Ngolo Tete, D.; Blesson, S.; Simon, F.; Delhomme, S.; Bernhard, S.; Mahenzi Mbembo, H.; et al. Oral Fexinidazole for Stage 1 or Early Stage 2 African Trypanosoma Brucei Gambiense Trypanosomiasis: A Prospective, Multicentre, Open-Label, Cohort Study. Lancet Glob. Health 2021, 9, e999–e1008. [Google Scholar] [CrossRef] [PubMed]
- DNDi. Fexinidazole for T.b. Gambiense. Available online: https://dndi.org/researchdevelopment/portfolio/fexinidazole/ (accessed on 15 October 2024).
- DNDi. Fexinidazole for T.b. Rhodesiense. Available online: https://dndi.org/research-development/portfolio/fexinidazole-tb-rhodesiense/ (accessed on 15 October 2024).
- DNDi. WHO Recommends First All-Oral Treatment for Acute Sleeping Sickness Found in Eastern and Southern Africa. Available online: https://dndi.org/press-releases/2024/who-recommends-first-all-oral-treatment-for-acute-sleeping-sickness-found-in-eastern-and-southern-africa/ (accessed on 15 October 2024).
- WHO. Guidelines for the Treatment of Human African Trypanosomiasis. Available online: https://www.who.int/publications/i/item/9789240096035 (accessed on 15 October 2024).
- Drugs.com. Fexinidazole: Package Insert / Prescribing Info. Available online: https://www.drugs.com/pro/fexinidazole.html (accessed on 18 December 2024).
- Betu Kumeso, V.K.; Kalonji, W.M.; Rembry, S.; Valverde Mordt, O.; Ngolo Tete, D.; Prêtre, A.; Delhomme, S.; Ilunga Wa Kyhi, M.; Camara, M.; Catusse, J.; et al. Efficacy and Safety of Acoziborole in Patients with Human African Trypanosomiasis Caused by Trypanosoma Brucei Gambiense: A Multicentre, Open-Label, Single-Arm, Phase 2/3 Trial. Lancet Infect. Dis. 2023, 23, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Hoet, S.; Opperdoes, F.; Brun, R.; Quetin-Leclercq, J. Natural Products Active against African Trypanosomes: A Step towards New Drugs. Nat. Prod. Rep. 2004, 21, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Naß, J.; Efferth, T. The Activity of Artemisia Spp. and Their Constituents against Trypanosomiasis. Phytomedicine 2018, 47, 184–191. [Google Scholar] [CrossRef]
- Eckstein-Ludwig, U.; Webb, R.J.; Van Goethem, I.D.A.; East, J.M.; Lee, A.G.; Kimura, M.; O’Neill, P.M.; Bray, P.G.; Ward, S.A.; Krishna, S. Artemisinins Target the SERCA of Plasmodium Falciparum. Nature 2003, 424, 957–961. [Google Scholar] [CrossRef]
- Woodrow, C.J.; Krishna, S. Antimalarial Drugs: Recent Advances in Molecular Determinants of Resistance and Their Clinical Significance. Cell. Mol. Life Sci. 2006, 63, 1586–1596. [Google Scholar] [CrossRef]
- Konziase, B. Biotinylated Probes of Artemisinin with Labeling Affinity Toward Trypanosoma Brucei Brucei Target Proteins. Anal. Biochem. 2015, 482, 25–31. [Google Scholar] [CrossRef]
- Konziase, B. Synthesis of Biotinylated Probes of Artemisinin for Affinity Labeling. Data Brief 2015, 4, 66–74. [Google Scholar] [CrossRef]
- Brunner, J.; Senn, H.; Richards, F.M. 3-Trifluoromethyl-3-Phenyldiazirine. A New Carbene Generating Group for Photolabeling Reagents. J. Biol. Chem. 1980, 255, 3313–3318. [Google Scholar] [CrossRef]
- O’Neill, P.M.; Barton, V.E.; Ward, S.A. The Molecular Mechanism of Action of Artemisinin--the Debate Continues. Molecules 2010, 15, 1705–1721. [Google Scholar] [CrossRef]
- Bermejo, A.; Figadère, B.; Zafra-Polo, M.C.; Barrachina, I.; Estornell, E.; Cortes, D. Acetogenins from Annonaceae: Recent Progress in Isolation, Synthesis and Mechanisms of Action. Nat. Prod. Rep. 2005, 22, 269–303. [Google Scholar] [CrossRef] [PubMed]
- Mangal, M.; Imran Khan, M.; Mohan Agarwal, S. Acetogenins as Potential Anticancer Agents. Anticancer. Agents Med. Chem. 2015, 16, 138–159. [Google Scholar] [CrossRef] [PubMed]
- Degli Esposti, M.; Ghelli, A.; Ratta, M.; Cortes, D.; Estornell, E. Natural Substances (Acetogenins) from the Family Annonaceae Are Powerful Inhibitors of Mitochondrial NADH Dehydrogenase (Complex I). Biochem J. 1994, 301 Pt 1, 161–167. [Google Scholar] [CrossRef]
- Kakutani, N.; Murai, M.; Sakiyama, N.; Miyoshi, H. Exploring the Binding Site of Delta(Lac)-Acetogenin in Bovine Heart Mitochondrial NADH-Ubiquinone Oxidoreductase. Biochemistry 2010, 49, 4794–4803. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Kuwabara, K.; Nakato, H.; Tanaka, A.; Iwamura, H.; Miyoshi, H. Definition of Crucial Structural Factors of Acetogenins, Potent Inhibitors of Mitochondrial Complex I. Biochim. Et Biophys. Acta (BBA)-Bioenergetics 2000, 1460, 302–310. [Google Scholar] [CrossRef]
- Kojima, N.; Abe, M.; Suga, Y.; Ohtsuki, K.; Tanaka, T.; Iwasaki, H.; Yamashita, M.; Miyoshi, H. Critical Role of a Methyl Group on the γ-Lactone Ring of Annonaceous Acetogenins in the Potent Inhibition of Mitochondrial Complex I. Bioorg. Med. Chem. Lett. 2013, 23, 1217–1219. [Google Scholar] [CrossRef]
- Florence, G.J.; Morris, J.C.; Murray, R.G.; Vanga, R.R.; Osler, J.D.; Smith, T.K. Total Synthesis, Stereochemical Assignment, and Biological Activity of Chamuvarinin and Structural Analogues. Chemistry 2013, 19, 8309–8320. [Google Scholar] [CrossRef]
- Florence, G.J.; Fraser, A.L.; Gould, E.R.; King, E.F.B.; Menzies, S.K.; Morris, J.C.; Tulloch, L.B.; Smith, T.K. Non-Natural Acetogenin Analogues as Potent Trypanosoma Brucei Inhibitors. ChemMedChem 2014, 9, 2548–2556. [Google Scholar] [CrossRef]
- Verner, Z.; Čermáková, P.; Škodová, I.; Kriegová, E.; Horváth, A.; Lukeš, J. Complex I (NADH:Ubiquinone Oxidoreductase) Is Active in but Non-Essential for Procyclic Trypanosoma Brucei. Mol. Biochem. Parasitol. 2011, 175, 196–200. [Google Scholar] [CrossRef]
- Surve, S.; Heestand, M.; Panicucci, B.; Schnaufer, A.; Parsons, M. Enigmatic Presence of Mitochondrial Complex I in Trypanosoma Brucei Bloodstream Forms. Eukaryot. Cell 2012, 11, 183–193. [Google Scholar] [CrossRef]
- Tulloch, L.B.; Menzies, S.K.; Fraser, A.L.; Gould, E.R.; King, E.F.; Zacharova, M.K.; Florence, G.J.; Smith, T.K. Photo-Affinity Labelling and Biochemical Analyses Identify the Target of Trypanocidal Simplified Natural Product Analogues. PLoS Neglected Trop. Dis. 2017, 11, e0005886. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.G.; Gahura, O.; Leslie, A.G.W.; Zíková, A.; Walker, J.E. ATP Synthase from Trypanosoma Brucei Has an Elaborated Canonical F1-Domain and Conventional Catalytic Sites. Proc. Natl. Acad. Sci. USA 2018, 115, 2102–2107. [Google Scholar] [CrossRef] [PubMed]
- Zíková, A.; Schnaufer, A.; Dalley, R.A.; Panigrahi, A.K.; Stuart, K.D. The F(0)F(1)-ATP Synthase Complex Contains Novel Subunits and Is Essential for Procyclic Trypanosoma Brucei. PLoS Pathog. 2009, 5, e1000436. [Google Scholar] [CrossRef]
- Schnaufer, A.; Clark-Walker, G.D.; Steinberg, A.G.; Stuart, K. The F1-ATP Synthase Complex in Bloodstream Stage Trypanosomes Has an Unusual and Essential Function. EMBO J. 2005, 24, 4029–4040. [Google Scholar] [CrossRef]
- Šubrtová, K.; Panicucci, B.; Zíková, A. ATPaseTb2, a Unique Membrane-Bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes. PLoS Pathog. 2015, 11, e1004660. [Google Scholar] [CrossRef]
- NOLAN, D.P.; VOORHEIS, H.P. The Mitochondrion in Bloodstream Forms of Trypanosoma Brucei Is Energized by the Electrogenic Pumping of Protons Catalysed by the F1F0-ATPase. Eur. J. Biochem. 1992, 209, 207–216. [Google Scholar] [CrossRef]
- Brown, S.V.; Hosking, P.; Li, J.; Williams, N. ATP Synthase Is Responsible for Maintaining Mitochondrial Membrane Potential in Bloodstream Form Trypanosoma Brucei. Eukaryot. Cell 2006, 5, 45–53. [Google Scholar] [CrossRef]
- Kovalevich, J.; Cornec, A.S.; Yao, Y.; James, M.; Crowe, A.; Lee, V.M.Y.; John, T.Q.; Smith, A.B.; Ballatore, C.; Brunden, K.R. Characterization of Brain-Penetrant Pyrimidine-Containing Molecules with Differential Microtubule-Stabilizing Activities Developed as Potential Therapeutic Agents for Alzheimer’s Disease and Related Tauopathies. J. Pharmacol. Exp. Ther. 2016, 357, 432–450. [Google Scholar] [CrossRef]
- Alle, T.; Varricchio, C.; Yao, Y.; Lucero, B.; Nzou, G.; Demuro, S.; Muench, M.; Vuong, K.D.; Oukoloff, K.; Cornec, A.S.; et al. Microtubule-Stabilizing 1,2,4-Triazolo [1,5-a]Pyrimidines as Candidate Therapeutics for Neurodegenerative Disease: Matched Molecular Pair Analyses and Computational Studies Reveal New Structure-Activity Insights. J. Med. Chem. 2023, 66, 435–459. [Google Scholar] [CrossRef]
- Oukoloff, K.; Nzou, G.; Varricchio, C.; Lucero, B.; Alle, T.; Kovalevich, J.; Monti, L.; Cornec, A.S.; Yao, Y.; James, M.J.; et al. Evaluation of the Structure-Activity Relationship of Microtubule-Targeting 1,2,4-Triazolo[1,5-a]Pyrimidines Identifies New Candidates for Neurodegenerative Tauopathies. J. Med. Chem. 2021, 64, 1073–1102. [Google Scholar] [CrossRef]
- Monti, L.; Wang, S.C.; Oukoloff, K.; Smith, A.B.; Brunden, K.R.; Caffrey, C.R.; Ballatore, C. Brain-Penetrant Triazolopyrimidine and Phenylpyrimidine Microtubule Stabilizers as Potential Leads to Treat Human African Trypanosomiasis. ChemMedChem 2018, 13, 1751–1754. [Google Scholar] [CrossRef] [PubMed]
- Monti, L.; Liu, L.J.; Varricchio, C.; Lucero, B.; Alle, T.; Yang, W.; Bem-Shalom, I.; Gilson, M.; Brunden, K.R.; Brancale, A.; et al. Structure-Activity Relationships, Tolerability and Efficacy of Microtubule-Active 1,2,4-Triazolo[1,5-a]Pyrimidines as Potential Candidates to Treat Human African Trypanosomiasis. ChemMedChem 2023, 18, e202300193. [Google Scholar] [CrossRef] [PubMed]
- Lucero, B.; Francisco, K.R.; Varricchio, C.; Liu, L.J.; Yao, Y.; Brancale, A.; Brunden, K.R.; Caffrey, C.R.; Ballatore, C. Design, Synthesis, and Evaluation of An Anti-Trypanosomal [1,2,4]Triazolo[1,5-a]Pyrimidine Probe for Photoaffinity Labeling Studies. ChemMedChem 2024, 19, e202300656. [Google Scholar] [CrossRef] [PubMed]
- Oukoloff, K.; Kovalevich, J.; Cornec, A.S.; Yao, Y.; Owyang, Z.A.; James, M.; Trojanowski, J.Q.; Lee, V.M.Y.; Smith, A.B.; Brunden, K.R.; et al. Design, Synthesis and Evaluation of Photoactivatable Derivatives of Microtubule (MT)-Active [1,2,4]Triazolo[1,5-a]Pyrimidines. Bioorg. Med. Chem. Lett. 2018, 28, 2180–2183. [Google Scholar] [CrossRef]
- Fraser, A.L.; Menzies, S.K.; King, E.F.B.; Tulloch, L.B.; Gould, E.R.; Zacharova, M.K.; Smith, T.K.; Florence, G.J. Design and Synthesis of Broad Spectrum Trypanosomatid Selective Inhibitors. ACS Infect. Dis. 2018, 4, 560–567. [Google Scholar] [CrossRef]
- Taipale, M.; Jarosz, D.F.; Lindquist, S. HSP90 at the Hub of Protein Homeostasis: Emerging Mechanistic Insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 515–528. [Google Scholar] [CrossRef]
- Park, H.K.; Yoon, N.G.; Lee, J.E.; Hu, S.; Yoon, S.; Kim, S.Y.; Hong, J.H.; Nam, D.; Chae, Y.C.; Park, J.B.; et al. Unleashing the Full Potential of Hsp90 Inhibitors as Cancer Therapeutics through Simultaneous Inactivation of Hsp90, Grp94, and TRAP1. Exp. Mol. Med. 2020, 52, 79–91. [Google Scholar] [CrossRef]
- Santos, D.M.; Petersen, A.L.O.A.; Celes, F.S.; Borges, V.M.; Veras, P.S.T.; de Oliveira, C.I. Chemotherapeutic Potential of 17-AAG against Cutaneous Leishmaniasis Caused by Leishmania (Viannia) Braziliensis. PLoS Neglected Trop. Dis. 2014, 8, e3275. [Google Scholar] [CrossRef]
- Graefe, S.E.B.; Wiesgigl, M.; Gaworski, I.; Macdonald, A.; Clos, J. Inhibition of HSP90 in Trypanosoma Cruzi Induces a Stress Response but No Stage Differentiation. Eukaryot. Cell 2002, 1, 936–943. [Google Scholar] [CrossRef]
- Wang, T.; Mäser, P.; Picard, D. Inhibition of Plasmodium Falciparum Hsp90 Contributes to the Antimalarial Activities of Aminoalcohol-Carbazoles. J. Med. Chem. 2016, 59, 6344–6352. [Google Scholar] [CrossRef]
- Wiesgigl, M.; Clos, J. Heat Shock Protein 90 Homeostasis Controls Stage Differentiation in Leishmania Donovani. Mol. Biol. Cell 2001, 12, 3307–3316. [Google Scholar] [CrossRef] [PubMed]
- Hombach-Barrigah, A.; Bartsch, K.; Smirlis, D.; Rosenqvist, H.; MacDonald, A.; Dingli, F.; Loew, D.; Späth, G.F.; Rachidi, N.; Wiese, M.; et al. Leishmania Donovani 90 KD Heat Shock Protein—Impact of Phosphosites on Parasite Fitness, Infectivity and Casein Kinase Affinity. Sci. Rep. 2019, 9, 5074. [Google Scholar] [CrossRef] [PubMed]
- Kalesh, K.; Sundriyal, S.; Perera, H.; Cobb, S.L.; Denny, P.W. Quantitative Proteomics Reveals That Hsp90 Inhibition Dynamically Regulates Global Protein Synthesis in Leishmania Mexicana. mSystems 2021, 6, e00089-21. [Google Scholar] [CrossRef] [PubMed]
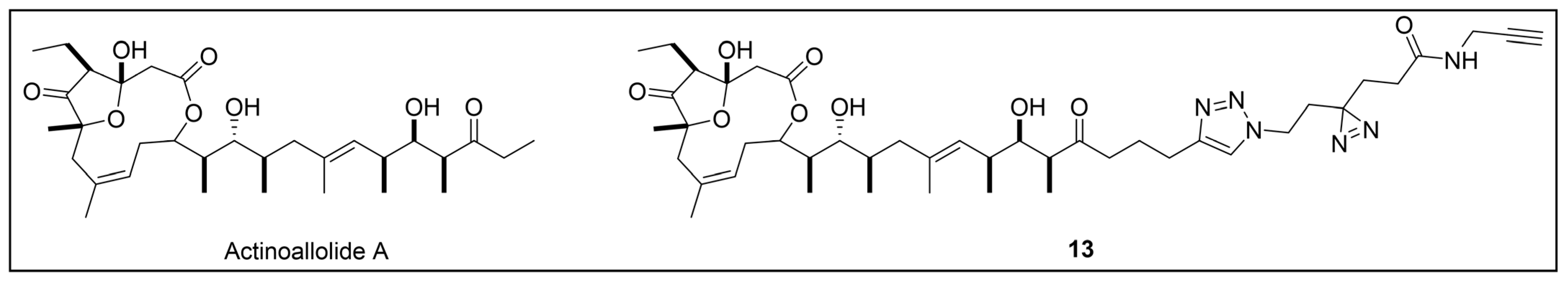
- Inahashi, Y.; Iwatsuki, M.; Ishiyama, A.; Matsumoto, A.; Hirose, T.; Oshita, J.; Sunazuka, T.; Panbangred, W.; Takahashi, Y.; Kaiser, M.; et al. Actinoallolides A-E, New Anti-Trypanosomal Macrolides, Produced by an Endophytic Actinomycete, Actinoallomurus Fulvus MK10-036. Org. Lett. 2015, 17, 864–867. [Google Scholar] [CrossRef] [PubMed]
- Anketell, M.J.; Sharrock, T.M.; Paterson, I. Total Synthesis of the Actinoallolides and a Designed Photoaffinity Probe for Target Identification. Org. Biomol. Chem. 2020, 18, 8109–8118. [Google Scholar] [CrossRef]
- Poespoprodjo, J.R.; Douglas, N.M.; Ansong, D.; Kho, S.; Anstey, N.M. Malaria. Lancet 2023, 402, 2328–2345. [Google Scholar] [CrossRef]
- Siqueira-Neto, J.L.; Wicht, K.J.; Chibale, K.; Burrows, J.N.; Fidock, D.A.; Winzeler, E.A. Antimalarial Drug Discovery: Progress and Approaches. Nat. Rev. Drug Discov. 2023, 22, 807–826. [Google Scholar] [CrossRef]
- Stickles, A.M.; Smilkstein, M.J.; Morrisey, J.M.; Li, Y.; Forquer, I.P.; Kelly, J.X.; Pou, S.; Winter, R.W.; Nilsen, A.; Vaidya, A.B.; et al. Atovaquone and ELQ-300 Combination Therapy as a Novel Dual-Site Cytochrome Bc1 Inhibition Strategy for Malaria. Antimicrob. Agents Chemother. 2016, 60, 4853. [Google Scholar] [CrossRef]
- De Villiers, K.A.; Egan, T.J. Heme Detoxification in the Malaria Parasite: A Target for Antimalarial Drug Development. Acc. Chem. Res. 2021, 54, 2649–2659. [Google Scholar] [CrossRef]
- Heinberg, A.; Kirkman, L. The Molecular Basis of Antifolate Resistance in Plasmodium Falciparum: Looking beyond Point Mutations. Ann. N. Y. Acad. Sci. 2015, 1342, 10–18. [Google Scholar] [CrossRef]
- Chitnumsub, P.; Jaruwat, A.; Talawanich, Y.; Noytanom, K.; Liwnaree, B.; Poen, S.; Yuthavong, Y. The Structure of Plasmodium Falciparum Hydroxymethyldihydropterin Pyrophosphokinase-Dihydropteroate Synthase Reveals the Basis of Sulfa Resistance. FEBS J. 2020, 287, 3273–3297. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Edwards, R.L.; Ball, H.; Johnson, C.; Haymond, A.; Girma, M.; Manikkam, M.; Brothers, R.C.; McKay, K.T.; Arnett, S.D.; et al. MEPicides: α,β-Unsaturated Fosmidomycin Analogues as DXR Inhibitors against Malaria. J. Med. Chem. 2018, 61, 8847–8858. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.L.; Zocco, D.; Sundrud, M.S.; Hendrick, M.; Edenius, M.; Yum, J.; Kim, Y.J.; Lee, H.K.; Cortese, J.F.; Wirth, D.F.; et al. Halofuginone and Other Febrifugine Derivatives Inhibit Prolyl-TRNA Synthetase. Nat. Chem. Biol. 2012, 8, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Dharia, N.V.; Plouffe, D.; Bopp, S.E.R.; González-Páez, G.E.; Lucas, C.; Salas, C.; Soberon, V.; Bursulaya, B.; Kochel, T.J.; Bacon, D.J.; et al. Genome Scanning of Amazonian Plasmodium Falciparum Shows Subtelomeric Instability and Clindamycin-Resistant Parasites. Genome Res. 2010, 20, 1534. [Google Scholar] [CrossRef]
- Bélard, S.; Ramharter, M. DSM265: A Novel Drug for Single-Dose Cure of Plasmodium Falciparum Malaria. Lancet Infect. Dis. 2018, 18, 819–820. [Google Scholar] [CrossRef]
- Pippione, A.C.; Sainas, S.; Goyal, P.; Fritzson, I.; Cassiano, G.C.; Giraudo, A.; Giorgis, M.; Tavella, T.A.; Bagnati, R.; Rolando, B.; et al. Hydroxyazole Scaffold-Based Plasmodium Falciparum Dihydroorotate Dehydrogenase Inhibitors: Synthesis, Biological Evaluation and X-Ray Structural Studies. Eur. J. Med. Chem. 2019, 163, 266–280. [Google Scholar] [CrossRef]
- Palmer, M.J.; Deng, X.; Watts, S.; Krilov, G.; Gerasyuto, A.; Kokkonda, S.; El Mazouni, F.; White, J.; White, K.L.; Striepen, J.; et al. Potent Antimalarials with Development Potential Identified by Structure-Guided Computational Optimization of a Pyrrole-Based Dihydroorotate Dehydrogenase Inhibitor Series. J. Med. Chem. 2021, 64, 6085–6136. [Google Scholar] [CrossRef]
- Ashton, T.D.; Dans, M.G.; Favuzza, P.; Ngo, A.; Lehane, A.M.; Zhang, X.; Qiu, D.; Chandra Maity, B.; De, N.; Schindler, K.A.; et al. Optimization of 2,3-Dihydroquinazolinone-3-Carboxamides as Antimalarials Targeting PfATP4. J. Med. Chem. 2023, 66, 3540–3565. [Google Scholar] [CrossRef]
- Sinxadi, P.; Donini, C.; Johnstone, H.; Langdon, G.; Wiesner, L.; Allen, E.; Duparc, S.; Chalon, S.; McCarthy, J.S.; Lorch, U.; et al. Safety, Tolerability, Pharmacokinetics, and Antimalarial Activity of the Novel Plasmodium Phosphatidylinositol 4-Kinase Inhibitor MMV390048 in Healthy Volunteers. Antimicrob. Agents Chemother. 2020, 64, e01896-19. [Google Scholar] [CrossRef]
- McCarthy, J.S.; Yalkinoglu, Ö.; Odedra, A.; Webster, R.; Oeuvray, C.; Tappert, A.; Bezuidenhout, D.; Giddins, M.J.; Dhingra, S.K.; Fidock, D.A.; et al. Safety, Pharmacokinetics, and Antimalarial Activity of the Novel Plasmodium Eukaryotic Translation Elongation Factor 2 Inhibitor M5717: A First-in-Human, Randomised, Placebo-Controlled, Double-Blind, Single Ascending Dose Study and Volunteer Infection Study. Lancet Infect. Dis. 2021, 21, 1713–1724. [Google Scholar] [CrossRef]
- Ng, C.L.; Siciliano, G.; Lee, M.C.S.; de Almeida, M.J.; Corey, V.C.; Bopp, S.E.; Bertuccini, L.; Wittlin, S.; Kasdin, R.G.; Le Bihan, A.; et al. CRISPR-Cas9-Modified Pfmdr1 Protects Plasmodium Falciparum Asexual Blood Stages and Gametocytes against a Class of Piperazine-Containing Compounds but Potentiates Artemisinin-Based Combination Therapy Partner Drugs. Mol. Microbiol. 2016, 101, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Meshnick, S.R. Artemisinin: Mechanisms of Action, Resistance and Toxicity. Int. J. Parasitol. 2002, 32, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Bridgford, J.L.; Xie, S.C.; Cobbold, S.A.; Pasaje, C.F.A.; Herrmann, S.; Yang, T.; Gillett, D.L.; Dick, L.R.; Ralph, S.A.; Dogovski, C.; et al. Artemisinin Kills Malaria Parasites by Damaging Proteins and Inhibiting the Proteasome. Nat. Commun. 2018, 9, 3801. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Balta, V.A.; West, R.; Newlin, K.N.; Miljanić, O.S.; Sullivan, D.J.; Vekilov, P.G.; Rimer, J.D. A Second Mechanism Employed by Artemisinins to Suppress Plasmodium Falciparum Hinges on Inhibition of Hematin Crystallization. J. Biol. Chem. 2021, 296, 100123. [Google Scholar] [CrossRef]
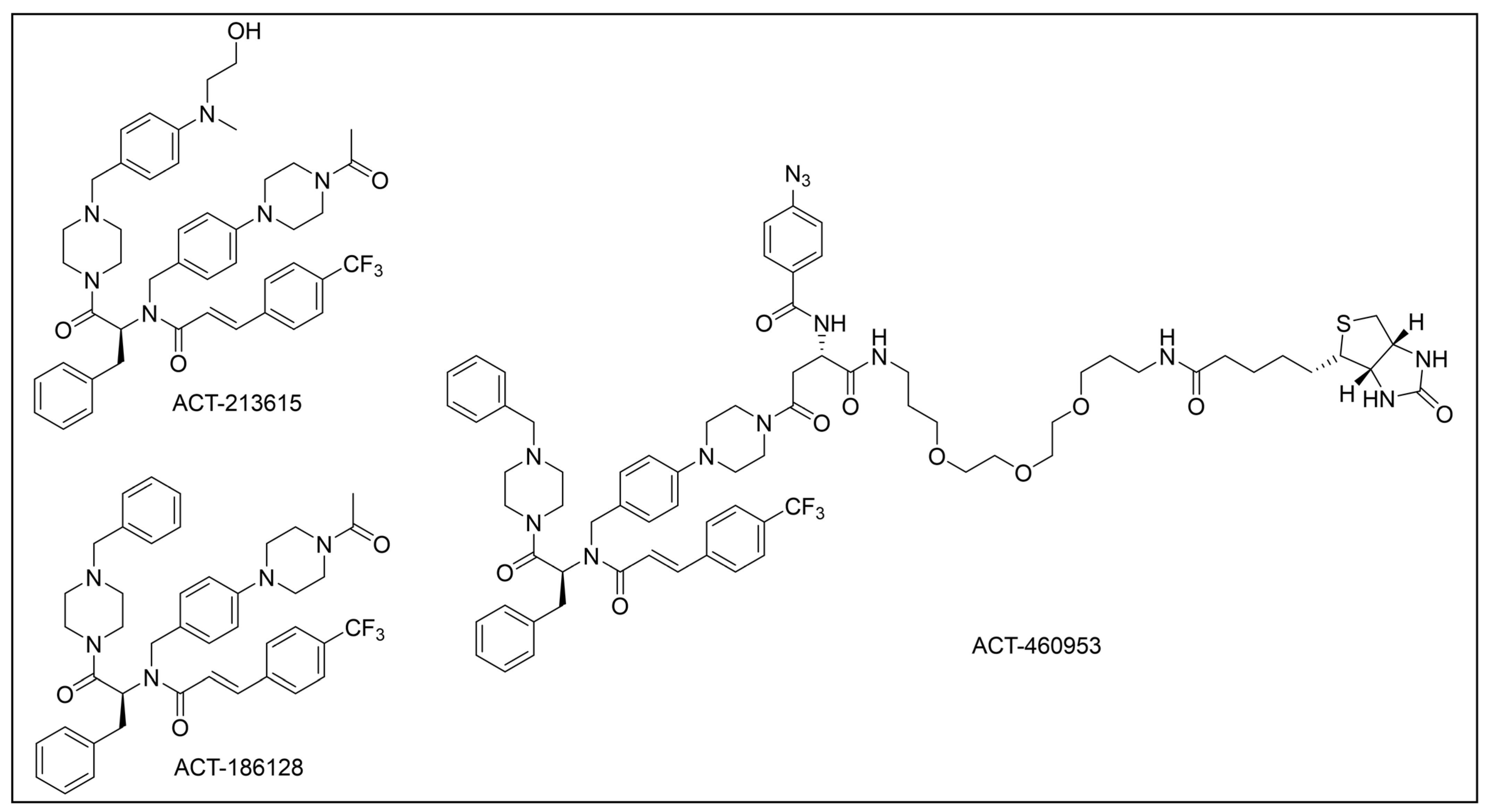
- Ismail, H.M.; Barton, V.; Phanchana, M.; Charoensutthivarakul, S.; Wong, M.H.L.; Hemingway, J.; Biagini, G.A.; O’Neill, P.M.; Ward, S.A. Artemisinin Activity-Based Probes Identify Multiple Molecular Targets within the Asexual Stage of the Malaria Parasites Plasmodium Falciparum 3D7. Proc. Natl. Acad. Sci. USA 2016, 113, 2080–2085. [Google Scholar] [CrossRef]
- Gao, P.; Chen, J.; Sun, P.; Wang, J.; Tang, H.; Xia, F.; Gu, L.; Zhang, H.; Wang, C.; Wong, Y.K.; et al. Chemical Proteomic Profiling with Photoaffinity Labeling Strategy Identifies Antimalarial Targets of Artemisinin. Chin. Chem. Lett. 2023, 34, 108296. [Google Scholar] [CrossRef]
- Gao, P.; Wang, J.; Qiu, C.; Zhang, H.; Wang, C.; Zhang, Y.; Sun, P.; Chen, H.; Wong, Y.K.; Chen, J.; et al. Photoaffinity Probe-Based Antimalarial Target Identification of Artemisinin in the Intraerythrocytic Developmental Cycle of Plasmodium Falciparum. iMeta 2024, 3, e176. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, H.; Yang, Y.; Chen, Z.S.; Zou, C.; Zhang, J. Chloroquine against Malaria, Cancers and Viral Diseases. Drug Discov. Today 2020, 25, 2012. [Google Scholar] [CrossRef]
- Foley, M.; Deady, L.W.; Ng, K.; Cowman, A.F.; Tilley, L. Photoaffinity Labeling of Chloroquine-Binding Proteins in Plasmodium Falciparum. J. Biol. Chem. 1994, 269, 6955–6961. [Google Scholar] [CrossRef]
- Menting, J.G.T.; Tilley, L.; Deady, L.W.; Ng, K.; Simpson, R.J.; Cowman, A.F.; Foley, M. The Antimalarial Drug, Chloroquine, Interacts with Lactate Dehydrogenase from Plasmodium Falciparum. Mo.l Biochem. Parasitol. 1997, 88, 215–224. [Google Scholar] [CrossRef]
- Gao, P.; Liu, Y.Q.; Xiao, W.; Xia, F.; Chen, J.Y.; Gu, L.W.; Yang, F.; Zheng, L.H.; Zhang, J.Z.; Zhang, Q.; et al. Identification of Antimalarial Targets of Chloroquine by a Combined Deconvolution Strategy of ABPP and MS-CETSA. Mil. Med. Res. 2022, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Lekostaj, J.K.; Natarajan, J.K.; Paguio, M.F.; Wolf, C.; Roepe, P.D. Photoaffinity Labeling of the Plasmodium Falciparum Chloroquine Resistance Transporter with a Novel Perfluorophenylazido Chloroquine. Biochemistry 2008, 47, 10394–10406. [Google Scholar] [CrossRef] [PubMed]
- Kapuku, B.; Bohle, D.S. Synthesis and Photolysis Properties of a New Chloroquine Photoaffinity Probe. Molecules 2024, 29, 1084. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.; Bai, X.C.; Sleebs, B.E.; Triglia, T.; Brown, A.; Thompson, J.K.; Jackson, K.E.; Hanssen, E.; Marapana, D.S.; Fernandez, I.S.; et al. Mefloquine Targets the Plasmodium Falciparum 80S Ribosome to Inhibit Protein Synthesis. Nat. Microbiol. 2017, 2, 17031. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ghosh, D.K.; Ali, J.; Ranjan, A. Characterization of Lipid Binding Properties of Plasmodium Falciparum Acyl-Coenzyme A Binding Proteins and Their Competitive Inhibition by Mefloquine. ACS Chem. Biol. 2019, 14, 901–915. [Google Scholar] [CrossRef]
- Ghosh, D.K.; Kumar, A.; Ranjan, A. Cellular Targets of Mefloquine. Toxicology 2021, 464, 152995. [Google Scholar] [CrossRef]
- Desneves, J.; Thorn, G.; Berman, A.; Galatis, D.; La Greca, N.; Sinding, J.; Foley, M.; Deady, L.W.; Cowman, A.F.; Tilley, L. Photoaffinity Labeling of Mefloquine-Binding Proteins in Human Serum, Uninfected Erythrocytes and Plasmodium Falciparum-Infected Erythrocytes. Mol. Biochem. Parasitol. 1996, 82, 181–194. [Google Scholar] [CrossRef]
- Cichocki, B.A.; Khobragade, V.; Donzel, M.; Cotos, L.; Blandin, S.; Schaeffer-Reiss, C.; Cianférani, S.; Strub, J.M.; Elhabiri, M.; Davioud-Charvet, E. A Class of Valuable (Pro-)Activity-Based Protein Profiling Probes: Application to the Redox-Active Antiplasmodial Agent, Plasmodione. JACS Au 2021, 1, 669–689. [Google Scholar] [CrossRef]
- Iacobucci, I.; Monaco, V.; Hovasse, A.; Dupouy, B.; Keumoe, R.; Cichocki, B.; Elhabiri, M.; Meunier, B.; Strub, J.M.; Monti, M.; et al. Proteomic Profiling of Antimalarial Plasmodione Using 3-Benz(o)Ylmenadione Affinity-Based Probes. Chembiochem 2024, 25, e202400187. [Google Scholar] [CrossRef]
- Müller, T.; Johann, L.; Jannack, B.; Brückner, M.; Lanfranchi, D.A.; Bauer, H.; Sanchez, C.; Yardley, V.; Deregnaucourt, C.; Schrével, J.; et al. Glutathione Reductase-Catalyzed Cascade of Redox Reactions to Bioactivate Potent Antimalarial 1,4-Naphthoquinones—A New Strategy to Combat Malarial Parasites. J. Am. Chem. Soc. 2011, 133, 11557–11571. [Google Scholar] [CrossRef]
- Ehrhardt, K.; Deregnaucourt, C.; Goetz, A.A.; Tzanova, T.; Gallo, V.; Arese, P.; Pradines, B.; Adjalley, S.H.; Bagrel, D.; Blandin, S.; et al. The Redox Cycler Plasmodione Is a Fast-Acting Antimalarial Lead Compound with Pronounced Activity against Sexual and Early Asexual Blood-Stage Parasites. Antimicrob. Agents Chemother. 2016, 60, 5146–5158. [Google Scholar] [CrossRef] [PubMed]
- Brunner, R.; Aissaoui, H.; Boss, C.; Bozdech, Z.; Brun, R.; Corminboeuf, O.; Delahaye, S.; Fischli, C.; Heidmann, B.; Kaiser, M.; et al. Identification of a New Chemical Class of Antimalarials. J. Infect. Dis. 2012, 206, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Brunner, R.; Ng, C.L.; Aissaoui, H.; Akabas, M.H.; Boss, C.; Brun, R.; Callaghan, P.S.; Corminboeuf, O.; Fidock, D.A.; Frame, I.J.; et al. UV-Triggered Affinity Capture Identifies Interactions between the Plasmodium Falciparum Multidrug Resistance Protein 1 (PfMDR1) and Antimalarial Agents in Live Parasitized Cells. J. Biol. Chem. 2013, 288, 22576–22583. [Google Scholar] [CrossRef] [PubMed]
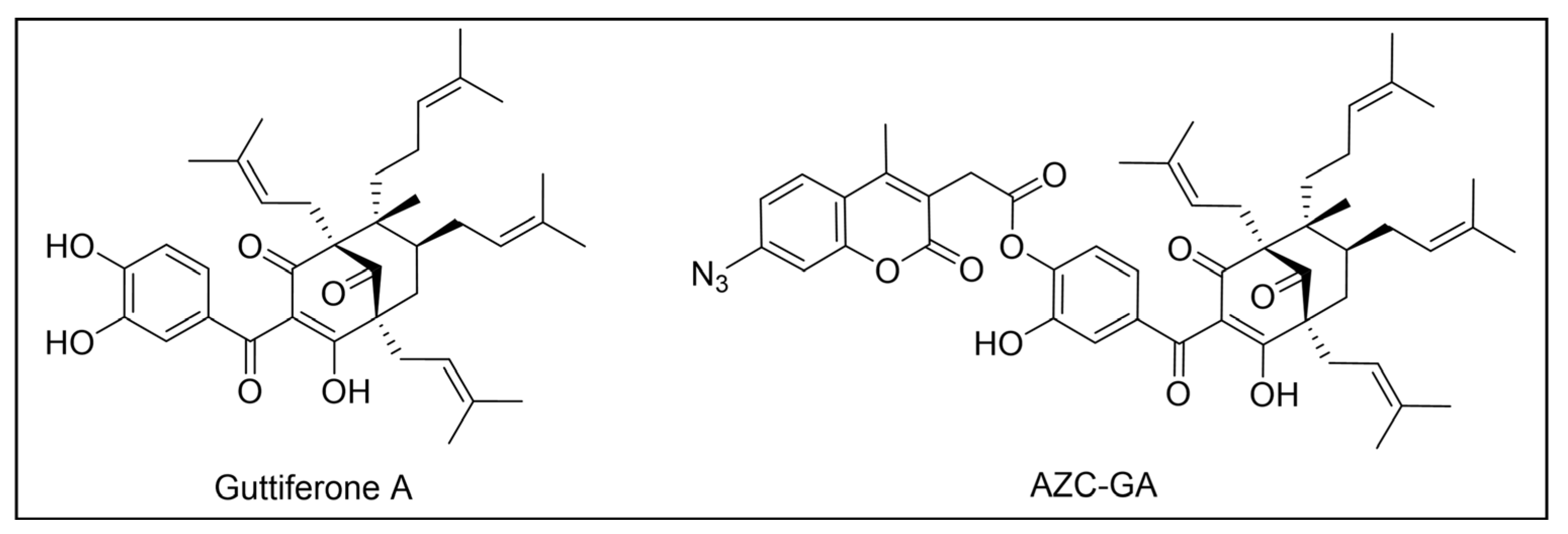
- Monzote, L.; Cuesta-Rubio, O.; Matheeussen, A.; Van Assche, T.; Maes, L.; Cos, P. Antimicrobial Evaluation of the Polyisoprenylated Benzophenones Nemorosone and Guttiferone A. Phytother. Res. 2011, 25, 458–462. [Google Scholar] [CrossRef]
- Fromentin, Y.; Gaboriaud-Kolar, N.; Lenta, B.N.; Wansi, J.D.; Buisson, D.; Mouray, E.; Grellier, P.; Loiseau, P.M.; Lallemand, M.C.; Michel, S. Synthesis of Novel Guttiferone A Derivatives: In-Vitro Evaluation toward Plasmodium Falciparum, Trypanosoma Brucei and Leishmania Donovani. Eur. J. Med. Chem. 2013, 65, 284–294. [Google Scholar] [CrossRef]
- Duval, R.; Cottet, K.; Blaud, M.; Merckx, A.; Houzé, S.; Grellier, P.; Lallemand, M.C.; Michel, S. A Photoalkylative Fluorogenic Probe of Guttiferone A for Live Cell Imaging and Proteome Labeling in Plasmodium Falciparum. Molecules 2020, 25, 5139. [Google Scholar] [CrossRef]
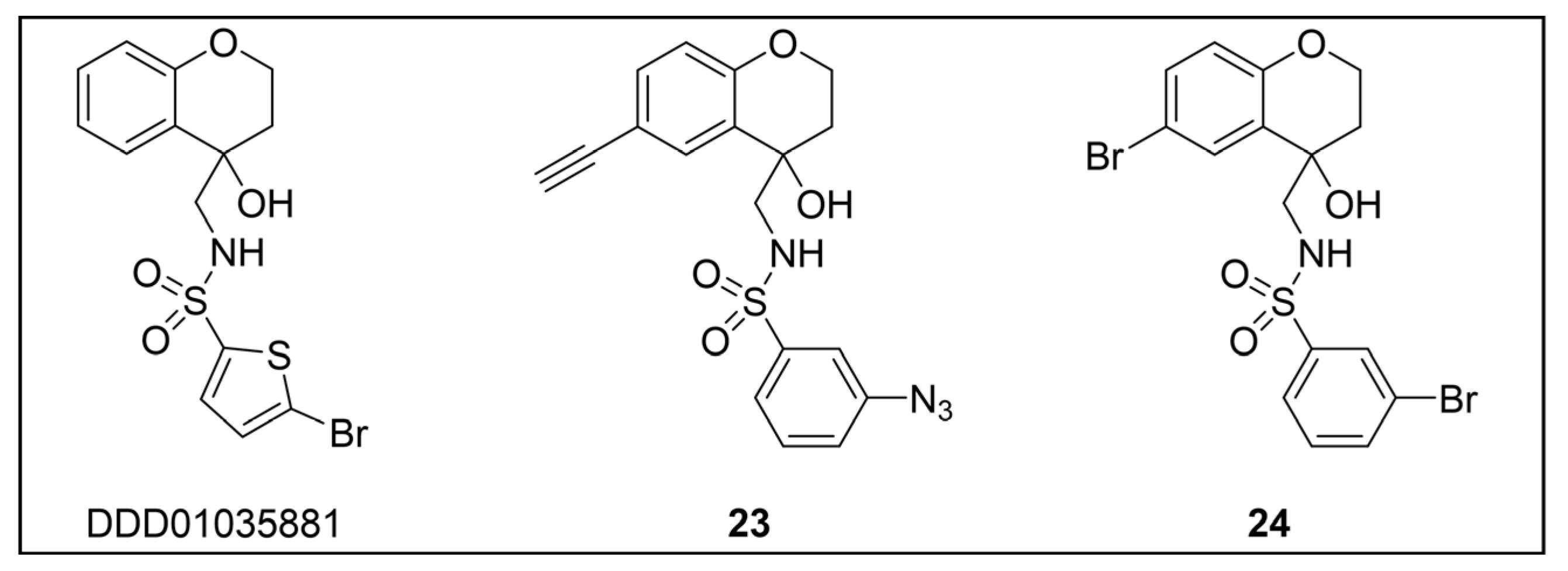
- Rueda-Zubiaurre, A.; Yahiya, S.; Fischer, O.J.; Hu, X.; Saunders, C.N.; Sharma, S.; Straschil, U.; Shen, J.; Tate, E.W.; Delves, M.J.; et al. Structure-Activity Relationship Studies of a Novel Class of Transmission Blocking Antimalarials Targeting Male Gametes. J. Med. Chem. 2020, 63, 2240–2262. [Google Scholar] [CrossRef]
- Yahiya, S.; Saunders, C.N.; Hassan, S.; Straschil, U.; Fischer, O.J.; Rueda-Zubiaurre, A.; Haase, S.; Vizcay-Barrena, G.; Famodimu, M.T.; Jordan, S.; et al. A Novel Class of Sulphonamides Potently Block Malaria Transmission by Targeting a Plasmodium Vacuole Membrane Protein. DMM Dis. Models Mech. 2023, 16, dmm049950. [Google Scholar] [CrossRef]
- Pino, P.; Caldelari, R.; Mukherjee, B.; Vahokoski, J.; Klages, N.; Maco, B.; Collins, C.R.; Blackman, M.J.; Kursula, I.; Heussler, V.; et al. A Multistage Antimalarial Targets the Plasmepsins IX and X Essential for Invasion and Egress. Science 2017, 358, 522–528. [Google Scholar] [CrossRef]
- Lisauskaitė, M.; Nixon, G.L.; Woodley, C.M.; Berry, N.G.; Coninckx, A.; Qie, L.C.; Leung, S.C.; Taramelli, D.; Basilico, N.; Parapini, S.; et al. Design, Synthesis and Modelling of Photoreactive Chemical Probes for Investigating Target Engagement of Plasmepsin IX and X in Plasmodium Falciparum. RSC Chem. Biol. 2024, 5, 19–29. [Google Scholar] [CrossRef]
- Sundriyal, S.; Malmquist, N.A.; Caron, J.; Blundell, S.; Liu, F.; Chen, X.; Srimongkolpithak, N.; Jin, J.; Charman, S.A.; Scherf, A.; et al. Development of Diaminoquinazoline Histone Lysine Methyltransferase Inhibitors as Potent Blood-Stage Antimalarial Compounds. ChemMedChem 2014, 9, 2360–2373. [Google Scholar] [CrossRef] [PubMed]
- Malmquist, N.A.; Moss, T.A.; Mecheri, S.; Scherf, A.; Fuchter, M.J. Small-Molecule Histone Methyltransferase Inhibitors Display Rapid Antimalarial Activity Against All Blood Stage Forms in Plasmodium Falciparum. Proc. Natl. Acad. Sci. USA 2012, 109, 16708–16713. [Google Scholar] [CrossRef] [PubMed]
- Lubin, A.S.; Rueda-Zubiaurre, A.; Matthews, H.; Baumann, H.; Fisher, F.R.; Morales-Sanfrutos, J.; Hadavizadeh, K.S.; Nardella, F.; Tate, E.W.; Baum, J.; et al. Development of a Photo-Cross-Linkable Diaminoquinazoline Inhibitor for Target Identification in Plasmodium Falciparum. ACS Infect. Dis. 2018, 4, 523–530. [Google Scholar] [CrossRef] [PubMed]
- François, G.; Bringmann, G.; Dochez, C.; Schneider, C.; Timperman, G.; Assi, L.A. Activities of Extracts and Naphthylisoquinoline Alkaloids from Triphyophyllum Peltatum, Ancistrocladus Abbreviatus and Ancistrocladus Barteri Against Plasmodium Berghei (Anka Strain) In Vitro. J. Ethnopharmacol. 1995, 46, 115–120. [Google Scholar] [CrossRef] [PubMed]
- François, G.; Bringmann, G.; Phillipson, J.D.; Assi, L.A.; Dochez, C.; Rübenacker, M.; Schneider, C.; Wéry, M.; Warhurst, D.C.; Kirby, G.C. Activity of Extracts and Naphthylisoquinoline Alkaloids from Triphyophyllum Peltatum, Ancistrocladus Abbreviatus and A. Barteri Against Plasmodium Falciparum In Vitro. Phytochemistry 1994, 35, 1461–1464. [Google Scholar] [CrossRef]
- François, G.; Timperman, G.; Eling, W.; Aké Assi, L.; Holenz, J.; Bringmann, G. Naphthylisoquinoline Alkaloids against Malaria: Evaluation of the Curative Potentials of Dioncophylline C and Dioncopeltine A against Plasmodium Berghei In Vivo. Antimicrob. Agents Chemother. 1997, 41, 2533. [Google Scholar] [CrossRef]
- Bringmann, G.; Gampe, C.M.; Reichert, Y.; Bruhn, T.; Faber, J.H.; Mikyna, M.; Reichert, M.; Leippe, M.; Brun, R.; Gelhaus, C. Synthesis and Pharmacological Evaluation of Fluorescent and Photoactivatable Analogues of Antiplasmodial Naphthylisoquinolines. J. Med. Chem. 2007, 50, 6104–6115. [Google Scholar] [CrossRef]
- Hamzé, A.; Rubi, E.; Arnal, P.; Boisbrun, M.; Carcel, C.; Salom-Roig, X.; Maynadier, M.; Wein, S.; Vial, H.; Calas, M. Mono- and Bis-Thiazolium Salts Have Potent Antimalarial Activity. J. Med. Chem. 2005, 48, 3639–3643. [Google Scholar] [CrossRef]
- Vial, H.J.; Wein, S.; Farenc, C.; Kocken, C.; Nicolas, O.; Ancelin, M.L.; Bressolle, F.; Thomas, A.; Calas, M. Prodrugs of Bisthiazolium Salts Are Orally Potent Antimalarials. Proc. Natl. Acad. Sci. USA 2004, 101, 15458–15463. [Google Scholar] [CrossRef]
- Wein, S.; Maynadier, M.; Bordat, Y.; Perez, J.; Maheshwari, S.; Bette-Bobillo, P.; Tran Van Ba, C.; Penarete-Vargas, D.; Fraisse, L.; Cerdan, R.; et al. Transport and Pharmacodynamics of Albitiazolium, an Antimalarial Drug Candidate. Br. J. Pharmacol. 2012, 166, 2263–2276. [Google Scholar] [CrossRef]
- Penarete-Vargas, D.M.; Boisson, A.; Urbach, S.; Chantelauze, H.; Peyrottes, S.; Fraisse, L.; Vial, H.J. A Chemical Proteomics Approach for the Search of Pharmacological Targets of the Antimalarial Clinical Candidate Albitiazolium in Plasmodium Falciparum Using Photocrosslinking and Click Chemistry. PLoS ONE 2014, 9, e113918. [Google Scholar] [CrossRef]













Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giraudo, A.; Bolchi, C.; Pallavicini, M.; Di Santo, R.; Costi, R.; Saccoliti, F. Uncovering the Mechanism of Action of Antiprotozoal Agents: A Survey on Photoaffinity Labeling Strategy. Pharmaceuticals 2025, 18, 28. https://doi.org/10.3390/ph18010028
Giraudo A, Bolchi C, Pallavicini M, Di Santo R, Costi R, Saccoliti F. Uncovering the Mechanism of Action of Antiprotozoal Agents: A Survey on Photoaffinity Labeling Strategy. Pharmaceuticals. 2025; 18(1):28. https://doi.org/10.3390/ph18010028
Chicago/Turabian StyleGiraudo, Alessandro, Cristiano Bolchi, Marco Pallavicini, Roberto Di Santo, Roberta Costi, and Francesco Saccoliti. 2025. "Uncovering the Mechanism of Action of Antiprotozoal Agents: A Survey on Photoaffinity Labeling Strategy" Pharmaceuticals 18, no. 1: 28. https://doi.org/10.3390/ph18010028
APA StyleGiraudo, A., Bolchi, C., Pallavicini, M., Di Santo, R., Costi, R., & Saccoliti, F. (2025). Uncovering the Mechanism of Action of Antiprotozoal Agents: A Survey on Photoaffinity Labeling Strategy. Pharmaceuticals, 18(1), 28. https://doi.org/10.3390/ph18010028









