The Gut Microbiota-Related Antihyperglycemic Effect of Metformin
Abstract
1. Introduction
2. Search Methodology
3. Physiological Gut Microbiota: Composition and Functions
3.1. Composition of Human Gut Microbiota
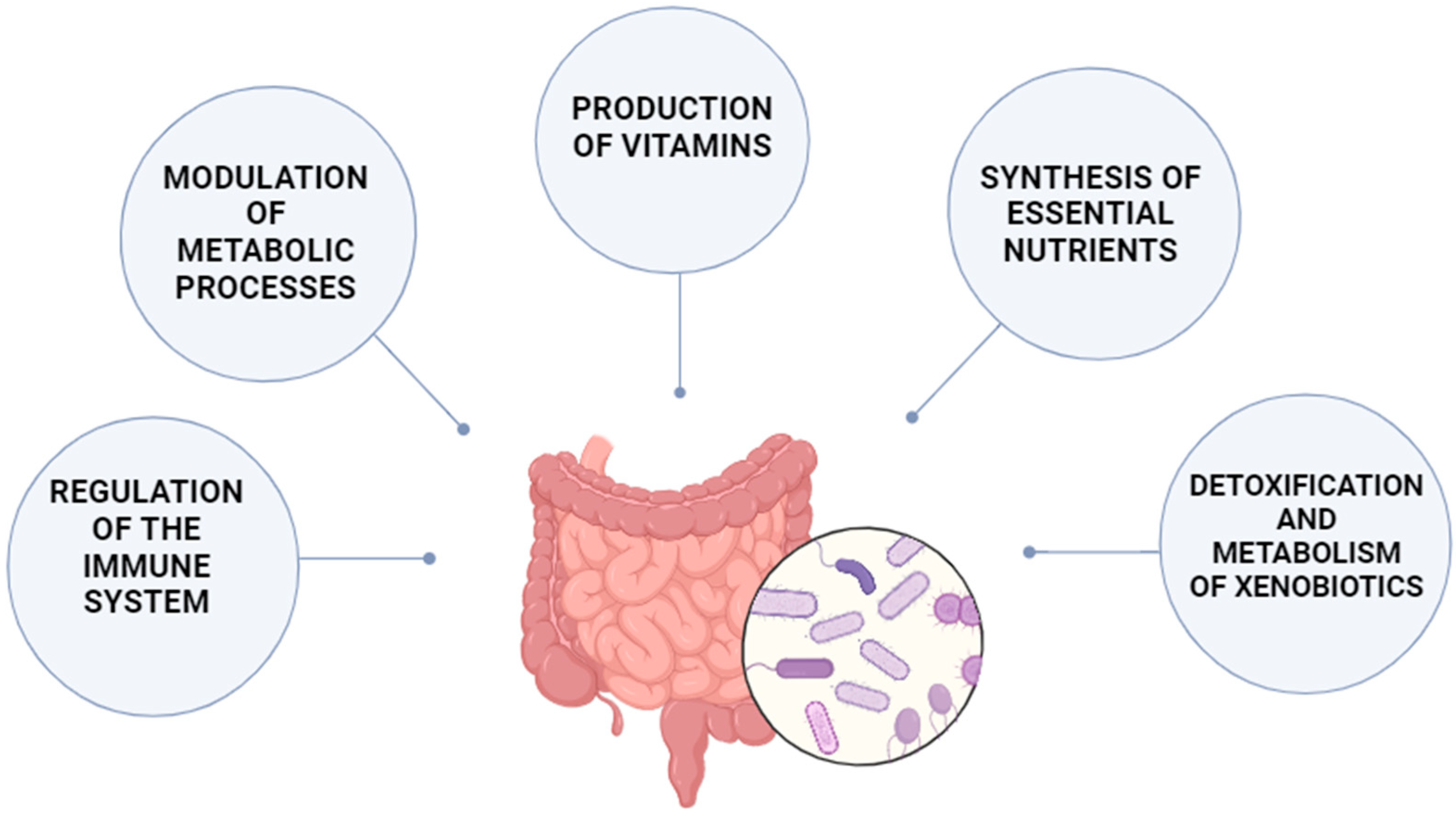
3.2. Microbiota Functions
4. Gut Dysbiosis Is Associated with Insulin Resistance
5. Metformin: Historical Perspectives, Mechanisms, and Gut Interactions
6. Metformin Alters the Composition of the Gut Microbiota
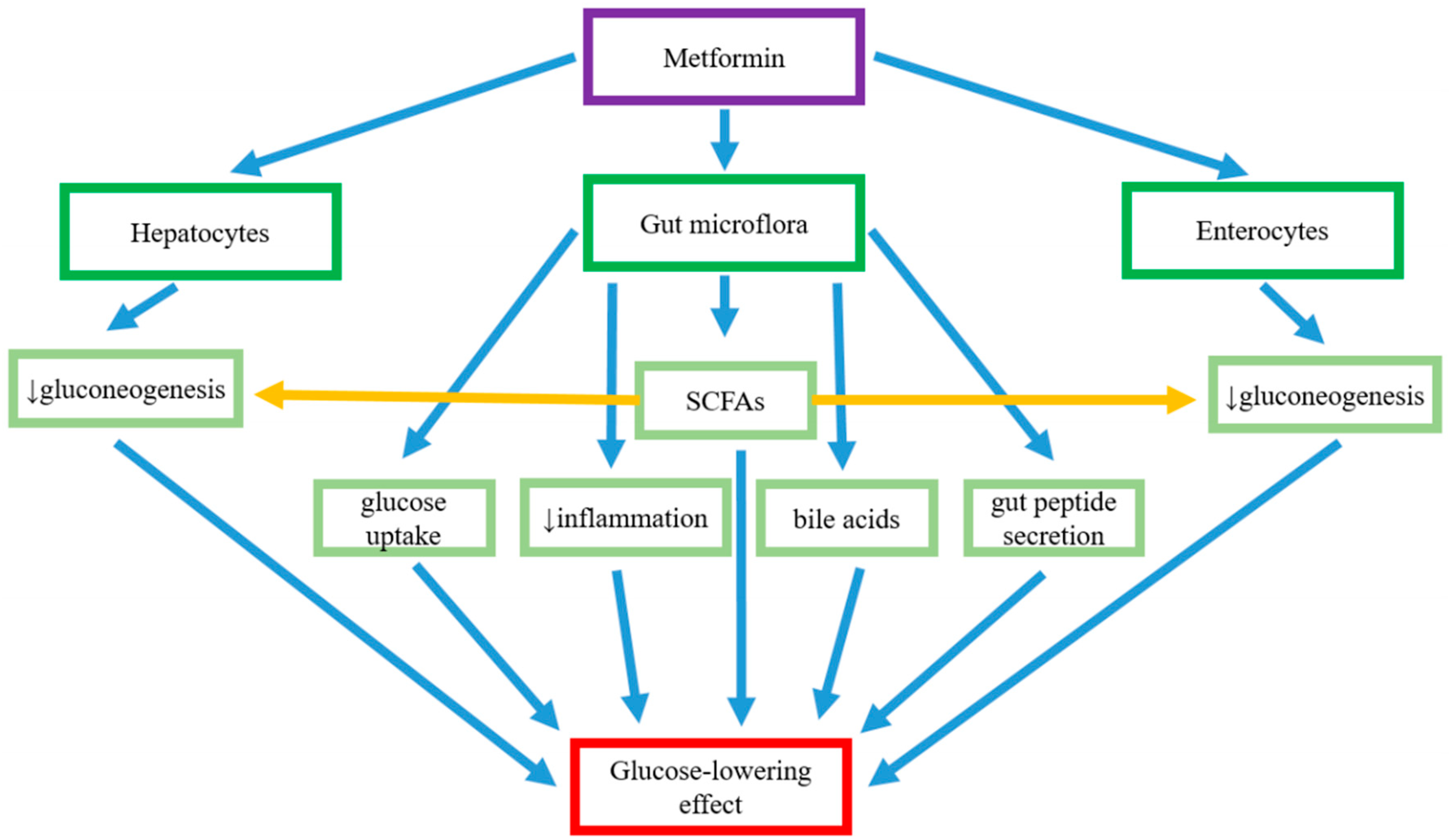
7. Metformin–Gut Interactions Believed to Be Responsible for Its Glucose-Lowering Effect
7.1. Regulation of Glucose Absorption in the Intestine
7.2. The Regulation of Abundance of SCFA-Producing Bacteria
7.3. The Reduction in Gut Permeability
7.4. The Anti-Inflammatory Action Mediated by Gut Microflora and Metformin
7.5. Actions of Metformin on Bile Acid Circulation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Diabetes Data Report 2000–2045. Available online: https://diabetesatlas.org/data/ (accessed on 22 July 2024).
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From Mechanisms of Action to Therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Andújar-Plata, P.; Pi-Sunyer, X.; Laferrère, B. Metformin Effects Revisited. Diabetes Res. Clin. Pract. 2012, 95, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The Mechanisms of Action of Metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Rojas, L.B.A.; Gomes, M.B. Metformin: An Old but Still the Best Treatment for Type 2 Diabetes. Diabetol. Metab. Syndr. 2013, 5, 6. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Wenclewska, S.; Śliwińska, A. Metabolic Action of Metformin. Pharmaceuticals 2022, 15, 810. [Google Scholar] [CrossRef]
- Coughlan, K.A.; Valentine, R.J.; Ruderman, N.B.; Saha, A.K. AMPK Activation: A Therapeutic Target for Type 2 Diabetes? Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 241–253. [Google Scholar] [CrossRef]
- Caspard, H.; Jabbour, S.; Hammar, N.; Fenici, P.; Sheehan, J.J.; Kosiborod, M. Recent Trends in the Prevalence of Type 2 Diabetes and the Association with Abdominal Obesity Lead to Growing Health Disparities in the USA: An Analysis of the NHANES Surveys from 1999 to 2014. Diabetes Obes. Metab. 2018, 20, 667–671. [Google Scholar] [CrossRef]
- Pearson, E.R. Type 2 Diabetes: A Multifaceted Disease. Diabetologia 2019, 62, 1107–1112. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Cani, P.D.; Everard, A.; Neyrinck, A.M.; Bindels, L.B. Gut Microorganisms as Promising Targets for the Management of Type 2 Diabetes. Diabetologia 2015, 58, 2206–2217. [Google Scholar] [CrossRef]
- Brunkwall, L.; Orho-Melander, M. The Gut Microbiome as a Target for Prevention and Treatment of Hyperglycaemia in Type 2 Diabetes: From Current Human Evidence to Future Possibilities. Diabetologia 2017, 60, 943–951. [Google Scholar] [CrossRef]
- Stepensky, D.; Friedman, M.; Raz, I.; Hoffman, A. Pharmacokinetic-Pharmacodynamic Analysis of the Glucose-Lowering Effect of Metformin in Diabetic Rats Reveals First-Pass Pharmacodynamic Effect. Drug Metab. Dispos. Biol. Fate Chem. 2002, 30, 861–868. [Google Scholar] [CrossRef] [PubMed]
- McCreight, L.J.; Bailey, C.J.; Pearson, E.R. Metformin and the Gastrointestinal Tract. Diabetologia 2016, 59, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J. Metformin and Intestinal Glucose Handling. Diabetes. Metab. Rev. 1995, 11 (Suppl. S1), S23–S32. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Iwata, K.; Murakami, H. Inhibitory Effect of Metformin on Intestinal Glucose Absorption in the Perfused Rat Intestine. Biochem. Pharmacol. 2000, 59, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Wilcock, C.; Bailey, C.J. Reconsideration of Inhibitory Effect of Metformin on Intestinal Glucose Absorption. J. Pharm. Pharmacol. 1991, 43, 120–121. [Google Scholar] [CrossRef]
- Wu, T.; Xie, C.; Wu, H.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Metformin Reduces the Rate of Small Intestinal Glucose Absorption in Type 2 Diabetes. Diabetes Obes. Metab. 2017, 19, 290–293. [Google Scholar] [CrossRef]
- Cabreiro, F.; Au, C.; Leung, K.-Y.; Vergara-Irigaray, N.; Cochemé, H.M.; Noori, T.; Weinkove, D.; Schuster, E.; Greene, N.D.E.; Gems, D. Metformin Retards Aging in C. Elegans by Altering Microbial Folate and Methionine Metabolism. Cell 2013, 153, 228–239. [Google Scholar] [CrossRef]
- Nijhout, H.F.; Reed, M.C.; Budu, P.; Ulrich, C.M. A Mathematical Model of the Folate Cycle: New Insights into Folate Homeostasis. J. Biol. Chem. 2004, 279, 55008–55016. [Google Scholar] [CrossRef]
- Mokgalaboni, K.; Dludla, P.V.; Mkandla, Z.; Mutize, T.; Nyambuya, T.M.; Mxinwa, V.; Nkambule, B.B. Differential Expression of Glycoprotein IV on Monocyte Subsets Following High-Fat Diet Feeding and the Impact of Short-Term Low-Dose Aspirin Treatment. Metab. Open 2020, 7, 100047. [Google Scholar] [CrossRef]
- Oliveira, W.H.; Nunes, A.K.; França, M.E.R.; Santos, L.A.; Lós, D.B.; Rocha, S.W.; Barbosa, K.P.; Rodrigues, G.B.; Peixoto, C.A. Effects of Metformin on Inflammation and Short-Term Memory in Streptozotocin-Induced Diabetic Mice. Brain Res. 2016, 1644, 149–160. [Google Scholar] [CrossRef]
- Almuttairi, R.S. The Effects of Metformin Treatment on Diabetic Albino Rats’ Pancreas, Liver, and Kidney Histology. Arch. Razi Inst. 2023, 78, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Szymczak-Pajor, I.; Drzewoski, J.; Wenclewska, S.; Śliwińska, A. Metformin-Associated Gastrointestinal Adverse Events Are Reduced by Probiotics: A Meta-Analysis. Pharmaceuticals 2024, 17, 898. [Google Scholar] [CrossRef] [PubMed]
- Ursell, L.K.; Clemente, J.C.; Rideout, J.R.; Gevers, D.; Caporaso, J.G.; Knight, R. The Interpersonal and Intrapersonal Diversity of Human-Associated Microbiota in Key Body Sites. J. Allergy Clin. Immunol. 2012, 129, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- Gevers, D.; Knight, R.; Petrosino, J.F.; Huang, K.; McGuire, A.L.; Birren, B.W.; Nelson, K.E.; White, O.; Methé, B.A.; Huttenhower, C. The Human Microbiome Project: A Community Resource for the Healthy Human Microbiome. PLoS Biol. 2012, 10, e1001377. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Niemeyer-van der Kolk, T.; van der Wall, H.E.C.; Balmforth, C.; Van Doorn, M.B.A.; Rissmann, R. A Systematic Literature Review of the Human Skin Microbiome as Biomarker for Dermatological Drug Development. Br. J. Clin. Pharmacol. 2018, 84, 2178–2193. [Google Scholar] [CrossRef]
- Faner, R.; Sibila, O.; Agustí, A.; Bernasconi, E.; Chalmers, J.D.; Huffnagle, G.B.; Manichanh, C.; Molyneaux, P.L.; Paredes, R.; Pérez Brocal, V.; et al. The Microbiome in Respiratory Medicine: Current Challenges and Future Perspectives. Eur. Respir. J. 2017, 49, 1602086. [Google Scholar] [CrossRef]
- Scepanovic, P.; Hodel, F.; Mondot, S.; Partula, V.; Byrd, A.; Hammer, C.; Alanio, C.; Bergstedt, J.; Patin, E.; Touvier, M.; et al. A Comprehensive Assessment of Demographic, Environmental, and Host Genetic Associations with Gut Microbiome Diversity in Healthy Individuals. Microbiome 2019, 7, 130. [Google Scholar] [CrossRef] [PubMed]
- Nayfach, S.; Roux, S.; Seshadri, R.; Udwary, D.; Varghese, N.; Schulz, F.; Wu, D.; Paez-Espino, D.; Chen, I.-M.; Huntemann, M.; et al. A Genomic Catalog of Earth’s Microbiomes. Nat. Biotechnol. 2021, 39, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Nayfach, S.; Boland, M.; Strozzi, F.; Beracochea, M.; Shi, Z.J.; Pollard, K.S.; Sakharova, E.; Parks, D.H.; Hugenholtz, P.; et al. A Unified Catalog of 204,938 Reference Genomes from the Human Gut Microbiome. Nat. Biotechnol. 2021, 39, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and Evolutionary Forces Shaping Microbial Diversity in the Human Intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Whitman, W.B.; Coleman, D.C.; Wiebe, W.J. Prokaryotes: The Unseen Majority. Proc. Natl. Acad. Sci. USA 1998, 95, 6578–6583. [Google Scholar] [CrossRef]
- Berg, R.D. The Indigenous Gastrointestinal Microflora. Trends Microbiol. 1996, 4, 430–435. [Google Scholar] [CrossRef]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of Mammals and Their Gut Microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef]
- Tannock, G.W. Analysis of the Intestinal Microflora: A Renaissance. Antonie Van Leeuwenhoek 1999, 76, 265–278. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Nava, G.M.; Stappenbeck, T.S. Diversity of the Autochthonous Colonic Microbiota. Gut Microbes 2011, 2, 99–104. [Google Scholar] [CrossRef]
- Manfredo Vieira, S.; Hiltensperger, M.; Kumar, V.; Zegarra-Ruiz, D.; Dehner, C.; Khan, N.; Costa, F.R.C.; Tiniakou, E.; Greiling, T.; Ruff, W.; et al. Translocation of a Gut Pathobiont Drives Autoimmunity in Mice and Humans. Science 2018, 359, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.P.; Erb-Downward, J.R.; Huffnagle, G.B. Homeostasis and Its Disruption in the Lung Microbiome. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L1047–L1055. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A. Our Unique Microbial Identity. Genome Biol. 2015, 16, 97. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.E.; Caporaso, J.G.; Henley, J.B.; Rideout, J.R.; Domogala, D.; Chase, J.; Leff, J.W.; Vázquez-Baeza, Y.; Gonzalez, A.; Knight, R.; et al. Temporal Variability Is a Personalized Feature of the Human Microbiome. Genome Biol. 2014, 15, 531. [Google Scholar] [CrossRef]
- Wilmanski, T.; Diener, C.; Rappaport, N.; Patwardhan, S.; Wiedrick, J.; Lapidus, J.; Earls, J.C.; Zimmer, A.; Glusman, G.; Robinson, M.; et al. Gut Microbiome Pattern Reflects Healthy Ageing and Predicts Survival in Humans. Nat. Metab. 2021, 3, 274–286. [Google Scholar] [CrossRef]
- Honda, K.; Littman, D.R. The Microbiome in Infectious Disease and Inflammation. Annu. Rev. Immunol. 2012, 30, 759–795. [Google Scholar] [CrossRef]
- Bouskra, D.; Brézillon, C.; Bérard, M.; Werts, C.; Varona, R.; Boneca, I.G.; Eberl, G. Lymphoid Tissue Genesis Induced by Commensals through NOD1 Regulates Intestinal Homeostasis. Nature 2008, 456, 507–510. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Roberfroid, M.B.; Bornet, F.; Bouley, C.; Cummings, J.H. Colonic Microflora: Nutrition and Health. Summary and Conclusions of an International Life Sciences Institute (ILSI) [Europe] Workshop Held in Barcelona, Spain. Nutr. Rev. 1995, 53, 127–130. [Google Scholar] [CrossRef]
- Pascale, A.; Marchesi, N.; Govoni, S.; Coppola, A.; Gazzaruso, C. The Role of Gut Microbiota in Obesity, Diabetes Mellitus, and Effect of Metformin: New Insights into Old Diseases. Curr. Opin. Pharmacol. 2019, 49, 1–5. [Google Scholar] [CrossRef]
- Human Microbiome Project Consortium. A Framework for Human Microbiome Research. Nature 2012, 486, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Pascale, A.; Marchesi, N.; Marelli, C.; Coppola, A.; Luzi, L.; Govoni, S.; Giustina, A.; Gazzaruso, C. Microbiota and Metabolic Diseases. Endocrine 2018, 61, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Iulia-Suceveanu, A.; Micu, S.I.; Voinea, C.; Manea, M.E.; Catrinoiu, D.; Mazilu, L.; Stoian, A.P.; Parepa, I.; Stoica, R.A.; Suceveanu, A.-P.; et al. Metformin and Its Benefits in Improving Gut Microbiota Disturbances in Diabetes Patients. In Metformin; IntechOpen: London, UK, 2019; ISBN 978-1-83880-428-2. [Google Scholar]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Esteve, E.; Tremaroli, V.; Khan, M.T.; Caesar, R.; Mannerås-Holm, L.; Ståhlman, M.; Olsson, L.M.; Serino, M.; Planas-Fèlix, M.; et al. Metformin Alters the Gut Microbiome of Individuals with Treatment-Naive Type 2 Diabetes, Contributing to the Therapeutic Effects of the Drug. Nat. Med. 2017, 23, 850–858. [Google Scholar] [CrossRef]
- de la Cuesta-Zuluaga, J.; Mueller, N.T.; Corrales-Agudelo, V.; Velásquez-Mejía, E.P.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Metformin Is Associated With Higher Relative Abundance of Mucin-Degrading Akkermansia Muciniphila and Several Short-Chain Fatty Acid-Producing Microbiota in the Gut. Diabetes Care 2017, 40, 54–62. [Google Scholar] [CrossRef]
- Chávez-Carbajal, A.; Pizano-Zárate, M.L.; Hernández-Quiroz, F.; Ortiz-Luna, G.F.; Morales-Hernández, R.M.; De Sales-Millán, A.; Hernández-Trejo, M.; García-Vite, A.; Beltrán-Lagunes, L.; Hoyo-Vadillo, C.; et al. Characterization of the Gut Microbiota of Individuals at Different T2D Stages Reveals a Complex Relationship with the Host. Microorganisms 2020, 8, 94. [Google Scholar] [CrossRef]
- Jocken, J.W.E.; González Hernández, M.A.; Hoebers, N.T.H.; van der Beek, C.M.; Essers, Y.P.G.; Blaak, E.E.; Canfora, E.E. Short-Chain Fatty Acids Differentially Affect Intracellular Lipolysis in a Human White Adipocyte Model. Front. Endocrinol. 2017, 8, 372. [Google Scholar] [CrossRef]
- Martínez Leo, E.E.; Segura Campos, M.R. Effect of Ultra-Processed Diet on Gut Microbiota and Thus Its Role in Neurodegenerative Diseases. Nutr. Burbank Los Angel. Cty. Calif 2020, 71, 110609. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Karaki, S.-I.; Tazoe, H.; Hayashi, H.; Kashiwabara, H.; Tooyama, K.; Suzuki, Y.; Kuwahara, A. Expression of the Short-Chain Fatty Acid Receptor, GPR43, in the Human Colon. J. Mol. Histol. 2008, 39, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-like Peptide-1 Secretion via the G-Protein-Coupled Receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus Barrier, Mucins and Gut Microbiota: The Expected Slimy Partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Cornick, S.; Tawiah, A.; Chadee, K. Roles and Regulation of the Mucus Barrier in the Gut. Tissue Barriers 2015, 3, e982426. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Tanti, J.F.; Gual, P.; Grémeaux, T.; Gonzalez, T.; Barrès, R.; Le Marchand-Brustel, Y. Alteration in Insulin Action: Role of IRS-1 Serine Phosphorylation in the Retroregulation of Insulin Signalling. Ann. Endocrinol. 2004, 65, 43–48. [Google Scholar] [CrossRef]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective Increases of Bifidobacteria in Gut Microflora Improve High-Fat-Diet-Induced Diabetes in Mice through a Mechanism Associated with Endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Ho, S.B. Intestinal Goblet Cells and Mucins in Health and Disease: Recent Insights and Progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Macchione, I.G.; Lopetuso, L.R.; Ianiro, G.; Napoli, M.; Gibiino, G.; Rizzatti, G.; Petito, V.; Gasbarrini, A.; Scaldaferri, F. Akkermansia Muciniphila: Key Player in Metabolic and Gastrointestinal Disorders. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8075–8083. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.V.; Larsson, J.M.H.; Hansson, G.C. The Two Mucus Layers of Colon Are Organized by the MUC2 Mucin, Whereas the Outer Layer Is a Legislator of Host-Microbial Interactions. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4659–4665. [Google Scholar] [CrossRef]
- Shin, N.-R.; Lee, J.-C.; Lee, H.-Y.; Kim, M.-S.; Whon, T.W.; Lee, M.-S.; Bae, J.-W. An Increase in the Akkermansia Spp. Population Induced by Metformin Treatment Improves Glucose Homeostasis in Diet-Induced Obese Mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef]
- Amar, J.; Chabo, C.; Waget, A.; Klopp, P.; Vachoux, C.; Bermúdez-Humarán, L.G.; Smirnova, N.; Bergé, M.; Sulpice, T.; Lahtinen, S.; et al. Intestinal Mucosal Adherence and Translocation of Commensal Bacteria at the Early Onset of Type 2 Diabetes: Molecular Mechanisms and Probiotic Treatment. EMBO Mol. Med. 2011, 3, 559–572. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-Talk between Akkermansia Muciniphila and Intestinal Epithelium Controls Diet-Induced Obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Lefebvre, P.; Cariou, B.; Lien, F.; Kuipers, F.; Staels, B. Role of Bile Acids and Bile Acid Receptors in Metabolic Regulation. Physiol. Rev. 2009, 89, 147–191. [Google Scholar] [CrossRef]
- Twisk, J.; Hoekman, M.F.; Mager, W.H.; Moorman, A.F.; de Boer, P.A.; Scheja, L.; Princen, H.M.; Gebhardt, R. Heterogeneous Expression of Cholesterol 7 Alpha-Hydroxylase and Sterol 27-Hydroxylase Genes in the Rat Liver Lobulus. J. Clin. Investig. 1995, 95, 1235–1243. [Google Scholar] [CrossRef]
- Staels, B.; Fonseca, V.A. Bile Acids and Metabolic Regulation: Mechanisms and Clinical Responses to Bile Acid Sequestration. Diabetes Care 2009, 32 (Suppl. S2), S237–S245. [Google Scholar] [CrossRef]
- Majait, S.; Nieuwdorp, M.; Kemper, M.; Soeters, M. The Black Box Orchestra of Gut Bacteria and Bile Acids: Who Is the Conductor? Int. J. Mol. Sci. 2023, 24, 1816. [Google Scholar] [CrossRef] [PubMed]
- Guzior, D.V.; Quinn, R.A. Review: Microbial Transformations of Human Bile Acids. Microbiome 2021, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, H.; Kolodziejczyk, A.A.; Halstuch, D.; Elinav, E. Bile Acids in Glucose Metabolism in Health and Disease. J. Exp. Med. 2018, 215, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Okpara, E.S.; Hu, W.; Yan, C.; Wang, Y.; Liang, Q.; Chiang, J.Y.L.; Han, S. Interactive Relationships between Intestinal Flora and Bile Acids. Int. J. Mol. Sci. 2022, 23, 8343. [Google Scholar] [CrossRef] [PubMed]
- Vardarli, I.; Arndt, E.; Deacon, C.F.; Holst, J.J.; Nauck, M.A. Effects of Sitagliptin and Metformin Treatment on Incretin Hormone and Insulin Secretory Responses to Oral and “Isoglycemic” Intravenous Glucose. Diabetes 2014, 63, 663–674. [Google Scholar] [CrossRef]
- Jia, B.; Park, D.; Hahn, Y.; Jeon, C.O. Metagenomic Analysis of the Human Microbiome Reveals the Association between the Abundance of Gut Bile Salt Hydrolases and Host Health. Gut Microbes 2020, 11, 1300–1313. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, X.; Cong, B. Advances in the Mechanism of Metformin with Wide-Ranging Effects on Regulation of the Intestinal Microbiota. Front. Microbiol. 2024, 15, 1396031. [Google Scholar] [CrossRef]
- Gorboulev, V.; Schürmann, A.; Vallon, V.; Kipp, H.; Jaschke, A.; Klessen, D.; Friedrich, A.; Scherneck, S.; Rieg, T.; Cunard, R.; et al. Na(+)-D-Glucose Cotransporter SGLT1 Is Pivotal for Intestinal Glucose Absorption and Glucose-Dependent Incretin Secretion. Diabetes 2012, 61, 187–196. [Google Scholar] [CrossRef]
- Röder, P.V.; Geillinger, K.E.; Zietek, T.S.; Thorens, B.; Koepsell, H.; Daniel, H. The Role of SGLT1 and GLUT2 in Intestinal Glucose Transport and Sensing. PLoS ONE 2014, 9, e89977. [Google Scholar] [CrossRef]
- Chiang, J.Y.L. Bile Acid Metabolism and Signaling. Compr. Physiol. 2013, 3, 1191. [Google Scholar] [CrossRef]
- Sadagopan, A.; Mahmoud, A.; Begg, M.; Tarhuni, M.; Fotso, M.; Gonzalez, N.A.; Sanivarapu, R.R.; Osman, U.; Latha Kumar, A.; Mohammed, L. Understanding the Role of the Gut Microbiome in Diabetes and Therapeutics Targeting Leaky Gut: A Systematic Review. Cureus 2023, 15, e41559. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota from Lean Donors Increases Insulin Sensitivity in Individuals with Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef] [PubMed]
- Umirah, F.; Neoh, C.F.; Ramasamy, K.; Lim, S.M. Differential Gut Microbiota Composition between Type 2 Diabetes Mellitus Patients and Healthy Controls: A Systematic Review. Diabetes Res. Clin. Pract. 2021, 173, 108689. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.; Feng, Q.-Q.; Ojo, O.O.; Wang, X.-H. The Role of Dietary Fibre in Modulating Gut Microbiota Dysbiosis in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2020, 12, 3239. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, B.; Yu, D.; Zhu, C. Gut Microbiota: An Important Player in Type 2 Diabetes Mellitus. Front. Cell. Infect. Microbiol. 2022, 12, 834485. [Google Scholar] [CrossRef]
- Jang, H.R.; Lee, H.-Y. Mechanisms Linking Gut Microbial Metabolites to Insulin Resistance. World J. Diabetes 2021, 12, 730–744. [Google Scholar] [CrossRef]
- den Besten, G.; Bleeker, A.; Gerding, A.; van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.-J.; et al. Short-Chain Fatty Acids Protect Against High-Fat Diet-Induced Obesity via a PPARγ-Dependent Switch From Lipogenesis to Fat Oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Yamashita, H.; Fujisawa, K.; Ito, E.; Idei, S.; Kawaguchi, N.; Kimoto, M.; Hiemori, M.; Tsuji, H. Improvement of Obesity and Glucose Tolerance by Acetate in Type 2 Diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. Biosci. Biotechnol. Biochem. 2007, 71, 1236–1243. [Google Scholar] [CrossRef]
- Kondo, T.; Kishi, M.; Fushimi, T.; Kaga, T. Acetic Acid Upregulates the Expression of Genes for Fatty Acid Oxidation Enzymes in Liver to Suppress Body Fat Accumulation. J. Agric. Food Chem. 2009, 57, 5982–5986. [Google Scholar] [CrossRef]
- Sahuri-Arisoylu, M.; Brody, L.P.; Parkinson, J.R.; Parkes, H.; Navaratnam, N.; Miller, A.D.; Thomas, E.L.; Frost, G.; Bell, J.D. Reprogramming of Hepatic Fat Accumulation and “browning” of Adipose Tissue by the Short-Chain Fatty Acid Acetate. Int. J. Obes. 2016, 40, 955–963. [Google Scholar] [CrossRef]
- Yoshida, H.; Ishii, M.; Akagawa, M. Propionate Suppresses Hepatic Gluconeogenesis via GPR43/AMPK Signaling Pathway. Arch. Biochem. Biophys. 2019, 672, 108057. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Mollica, M.P.; Mattace Raso, G.; Cavaliere, G.; Trinchese, G.; De Filippo, C.; Aceto, S.; Prisco, M.; Pirozzi, C.; Di Guida, F.; Lama, A.; et al. Butyrate Regulates Liver Mitochondrial Function, Efficiency, and Dynamics in Insulin-Resistant Obese Mice. Diabetes 2017, 66, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, G.; Untereiner, A.; Ju, Y.; Wu, L.; Wang, R. Hydrogen Sulfide Impairs Glucose Utilization and Increases Gluconeogenesis in Hepatocytes. Endocrinology 2013, 154, 114–126. [Google Scholar] [CrossRef]
- Hoyles, L.; Fernández-Real, J.-M.; Federici, M.; Serino, M.; Abbott, J.; Charpentier, J.; Heymes, C.; Luque, J.L.; Anthony, E.; Barton, R.H.; et al. Molecular Phenomics and Metagenomics of Hepatic Steatosis in Non-Diabetic Obese Women. Nat. Med. 2018, 24, 1070–1080. [Google Scholar] [CrossRef]
- Gao, X.; Liu, X.; Xu, J.; Xue, C.; Xue, Y.; Wang, Y. Dietary Trimethylamine N-Oxide Exacerbates Impaired Glucose Tolerance in Mice Fed a High Fat Diet. J. Biosci. Bioeng. 2014, 118, 476–481. [Google Scholar] [CrossRef]
- Chen, S.; Henderson, A.; Petriello, M.C.; Romano, K.A.; Gearing, M.; Miao, J.; Schell, M.; Sandoval-Espinola, W.J.; Tao, J.; Sha, B.; et al. Trimethylamine N-Oxide Binds and Activates PERK to Promote Metabolic Dysfunction. Cell Metab. 2019, 30, 1141–1151.e5. [Google Scholar] [CrossRef]
- Yamashita, H.; Maruta, H.; Jozuka, M.; Kimura, R.; Iwabuchi, H.; Yamato, M.; Saito, T.; Fujisawa, K.; Takahashi, Y.; Kimoto, M.; et al. Effects of Acetate on Lipid Metabolism in Muscles and Adipose Tissues of Type 2 Diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. Biosci. Biotechnol. Biochem. 2009, 73, 570–576. [Google Scholar] [CrossRef]
- Houghton, M.J.; Kerimi, A.; Mouly, V.; Tumova, S.; Williamson, G. Gut Microbiome Catabolites as Novel Modulators of Muscle Cell Glucose Metabolism. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 1887–1898. [Google Scholar] [CrossRef]
- Takagaki, A.; Yoshioka, Y.; Yamashita, Y.; Nagano, T.; Ikeda, M.; Hara-Terawaki, A.; Seto, R.; Ashida, H. Effects of Microbial Metabolites of (-)-Epigallocatechin Gallate on Glucose Uptake in L6 Skeletal Muscle Cell and Glucose Tolerance in ICR Mice. Biol. Pharm. Bull. 2019, 42, 212–221. [Google Scholar] [CrossRef]
- Choi, Y.; Kwon, Y.; Kim, D.-K.; Jeon, J.; Jang, S.C.; Wang, T.; Ban, M.; Kim, M.-H.; Jeon, S.G.; Kim, M.-S.; et al. Gut Microbe-Derived Extracellular Vesicles Induce Insulin Resistance, Thereby Impairing Glucose Metabolism in Skeletal Muscle. Sci. Rep. 2015, 5, 15878. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-H.; Nishimura, Y.; Hishikawa, D.; Tsuzuki, H.; Miyahara, H.; Gotoh, C.; Choi, K.-C.; Feng, D.D.; Chen, C.; Lee, H.-G.; et al. Acetate and Propionate Short Chain Fatty Acids Stimulate Adipogenesis via GPCR43. Endocrinology 2005, 146, 5092–5099. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Li, X.; Weiszmann, J.; Wang, P.; Baribault, H.; Chen, J.-L.; Tian, H.; Li, Y. Activation of G Protein-Coupled Receptor 43 in Adipocytes Leads to Inhibition of Lipolysis and Suppression of Plasma Free Fatty Acids. Endocrinology 2008, 149, 4519–4526. [Google Scholar] [CrossRef] [PubMed]
- Hanatani, S.; Motoshima, H.; Takaki, Y.; Kawasaki, S.; Igata, M.; Matsumura, T.; Kondo, T.; Senokuchi, T.; Ishii, N.; Kawashima, J.; et al. Acetate Alters Expression of Genes Involved in Beige Adipogenesis in 3T3-L1 Cells and Obese KK-Ay Mice. J. Clin. Biochem. Nutr. 2016, 59, 207–214. [Google Scholar] [CrossRef]
- Li, Z.; Yi, C.-X.; Katiraei, S.; Kooijman, S.; Zhou, E.; Chung, C.K.; Gao, Y.; van den Heuvel, J.K.; Meijer, O.C.; Berbée, J.F.P.; et al. Butyrate Reduces Appetite and Activates Brown Adipose Tissue via the Gut-Brain Neural Circuit. Gut 2018, 67, 1269–1279. [Google Scholar] [CrossRef]
- Ohira, H.; Fujioka, Y.; Katagiri, C.; Mamoto, R.; Aoyama-Ishikawa, M.; Amako, K.; Izumi, Y.; Nishiumi, S.; Yoshida, M.; Usami, M.; et al. Butyrate Attenuates Inflammation and Lipolysis Generated by the Interaction of Adipocytes and Macrophages. J. Atheroscler. Thromb. 2013, 20, 425–442. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kushiro, M.; Shinohara, K.; Ide, T. Dietary Conjugated Linoleic Acid Reduces Body Fat Mass and Affects Gene Expression of Proteins Regulating Energy Metabolism in Mice. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 133, 395–404. [Google Scholar] [CrossRef]
- Park, Y.; Park, Y. Conjugated Fatty Acids Increase Energy Expenditure in Part by Increasing Voluntary Movement in Mice. Food Chem. 2012, 133, 400–409. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Park, J.-H.; Seok, S.-H.; Baek, M.-W.; Kim, D.-J.; Lee, K.-E.; Paek, K.-S.; Lee, Y.; Park, J.-H. Human Originated Bacteria, Lactobacillus Rhamnosus PL60, Produce Conjugated Linoleic Acid and Show Anti-Obesity Effects in Diet-Induced Obese Mice. Biochim. Biophys. Acta 2006, 1761, 736–744. [Google Scholar] [CrossRef]
- Virtue, A.T.; McCright, S.J.; Wright, J.M.; Jimenez, M.T.; Mowel, W.K.; Kotzin, J.J.; Joannas, L.; Basavappa, M.G.; Spencer, S.P.; Clark, M.L.; et al. The Gut Microbiota Regulates White Adipose Tissue Inflammation and Obesity via a Family of microRNAs. Sci. Transl. Med. 2019, 11, eaav1892. [Google Scholar] [CrossRef]
- Goto, T.; Kim, Y.-I.; Furuzono, T.; Takahashi, N.; Yamakuni, K.; Yang, H.-E.; Li, Y.; Ohue, R.; Nomura, W.; Sugawara, T.; et al. 10-Oxo-12(Z)-Octadecenoic Acid, a Linoleic Acid Metabolite Produced by Gut Lactic Acid Bacteria, Potently Activates PPARγ and Stimulates Adipogenesis. Biochem. Biophys. Res. Commun. 2015, 459, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Furuzono, T.; Yamakuni, K.; Li, Y.; Kim, Y.-I.; Takahashi, H.; Ohue-Kitano, R.; Jheng, H.-F.; Takahashi, N.; Kano, Y.; et al. 10-Oxo-12(Z)-Octadecenoic Acid, a Linoleic Acid Metabolite Produced by Gut Lactic Acid Bacteria, Enhances Energy Metabolism by Activation of TRPV1. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 5036–5048. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Produced Succinate Improves Glucose Homeostasis via Intestinal Gluconeogenesis. Cell Metab. 2016, 24, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Drzewoski, J.; Hanefeld, M. The Current and Potential Therapeutic Use of Metformin-The Good Old Drug. Pharmaceuticals 2021, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J. Metformin: Historical Overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef]
- Blonde, L.; Dipp, S.; Cadena, D. Combination Glucose-Lowering Therapy Plans in T2DM: Case-Based Considerations. Adv. Ther. 2018, 35, 939–965. [Google Scholar] [CrossRef]
- Wilcock, C.; Wyre, N.D.; Bailey, C.J. Subcellular Distribution of Metformin in Rat Liver. J. Pharm. Pharmacol. 1991, 43, 442–444. [Google Scholar] [CrossRef]
- Shu, Y.; Sheardown, S.A.; Brown, C.; Owen, R.P.; Zhang, S.; Castro, R.A.; Ianculescu, A.G.; Yue, L.; Lo, J.C.; Burchard, E.G.; et al. Effect of Genetic Variation in the Organic Cation Transporter 1 (OCT1) on Metformin Action. J. Clin. Investig. 2007, 117, 1422–1431. [Google Scholar] [CrossRef]
- Natali, A.; Ferrannini, E. Effects of Metformin and Thiazolidinediones on Suppression of Hepatic Glucose Production and Stimulation of Glucose Uptake in Type 2 Diabetes: A Systematic Review. Diabetologia 2006, 49, 434–441. [Google Scholar] [CrossRef]
- Rena, G.; Pearson, E.R.; Sakamoto, K. Molecular Mechanism of Action of Metformin: Old or New Insights? Diabetologia 2013, 56, 1898–1906. [Google Scholar] [CrossRef]
- Madiraju, A.K.; Erion, D.M.; Rahimi, Y.; Zhang, X.-M.; Braddock, D.T.; Albright, R.A.; Prigaro, B.J.; Wood, J.L.; Bhanot, S.; MacDonald, M.J.; et al. Metformin Suppresses Gluconeogenesis by Inhibiting Mitochondrial Glycerophosphate Dehydrogenase. Nature 2014, 510, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Hébrard, S.; Leclerc, J.; Zarrinpashneh, E.; Soty, M.; Mithieux, G.; Sakamoto, K.; Andreelli, F.; Viollet, B. Metformin Inhibits Hepatic Gluconeogenesis in Mice Independently of the LKB1/AMPK Pathway via a Decrease in Hepatic Energy State. J. Clin. Investig. 2010, 120, 2355–2369. [Google Scholar] [CrossRef] [PubMed]
- Demaré, S.; Kothari, A.; Calcutt, N.A.; Fernyhough, P. Metformin as a Potential Therapeutic for Neurological Disease: Mobilizing AMPK to Repair the Nervous System. Expert Rev. Neurother. 2021, 21, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Park, K.-G. Metabolic Roles of AMPK and Metformin in Cancer Cells. Mol. Cells 2013, 36, 279–287. [Google Scholar] [CrossRef]
- Luo, T.; Nocon, A.; Fry, J.; Sherban, A.; Rui, X.; Jiang, B.; Xu, X.J.; Han, J.; Yan, Y.; Yang, Q.; et al. AMPK Activation by Metformin Suppresses Abnormal Extracellular Matrix Remodeling in Adipose Tissue and Ameliorates Insulin Resistance in Obesity. Diabetes 2016, 65, 2295–2310. [Google Scholar] [CrossRef]
- Viollet, B.; Guigas, B.; Sanz Garcia, N.; Leclerc, J.; Foretz, M.; Andreelli, F. Cellular and Molecular Mechanisms of Metformin: An Overview. Clin. Sci. Lond. Engl. 2012, 122, 253–270. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMP-Activated Protein Kinase: A Target for Drugs Both Ancient and Modern. Chem. Biol. 2012, 19, 1222–1236. [Google Scholar] [CrossRef]
- Li, J.Z.; Li, Y.R. Cardiovascular Protection by Metformin: Latest Advances in Basic and Clinical Research. Cardiology 2023, 148, 374–384. [Google Scholar] [CrossRef]
- Bu, Y.; Peng, M.; Tang, X.; Xu, X.; Wu, Y.; Chen, A.F.; Yang, X. Protective Effects of Metformin in Various Cardiovascular Diseases: Clinical Evidence and AMPK-dependent Mechanisms. J. Cell. Mol. Med. 2022, 26, 4886. [Google Scholar] [CrossRef]
- Kalender, A.; Selvaraj, A.; Kim, S.Y.; Gulati, P.; Brûlé, S.; Viollet, B.; Kemp, B.E.; Bardeesy, N.; Dennis, P.; Schlager, J.J.; et al. Metformin, Independent of AMPK, Inhibits mTORC1 in a Rag GTPase-Dependent Manner. Cell Metab. 2010, 11, 390–401. [Google Scholar] [CrossRef]
- Evans, J.M.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and Reduced Risk of Cancer in Diabetic Patients. BMJ 2005, 330, 1304–1305. [Google Scholar] [CrossRef]
- Heckman-Stoddard, B.M.; DeCensi, A.; Sahasrabuddhe, V.V.; Ford, L.G. Repurposing Metformin for the Prevention of Cancer and Cancer Recurrence. Diabetologia 2017, 60, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, B.; Aronis, K.N.; Vamvini, M.T.; Shields, K.; Mantzoros, C.S. Metformin and Sulfonylureas in Relation to Cancer Risk in Type II Diabetes Patients: A Meta-Analysis Using Primary Data of Published Studies. Metabolism. 2013, 62, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Guo, Y. Metformin and Its Benefits for Various Diseases. Front. Endocrinol. 2020, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.S.; Gubbi, S.; Barzilai, N. Benefits of Metformin in Attenuating the Hallmarks of Aging. Cell Metab. 2020, 32, 15–30. [Google Scholar] [CrossRef]
- Valencia, W.M.; Palacio, A.; Tamariz, L.; Florez, H. Metformin and Ageing: Improving Ageing Outcomes beyond Glycaemic Control. Diabetologia 2017, 60, 1630–1638. [Google Scholar] [CrossRef]
- Bailey, C.J.; Mynett, K.J.; Page, T. Importance of the Intestine as a Site of Metformin-Stimulated Glucose Utilization. Br. J. Pharmacol. 1994, 112, 671–675. [Google Scholar] [CrossRef]
- Tucker, G.T.; Casey, C.; Phillips, P.J.; Connor, H.; Ward, J.D.; Woods, H.F. Metformin Kinetics in Healthy Subjects and in Patients with Diabetes Mellitus. Br. J. Clin. Pharmacol. 1981, 12, 235–246. [Google Scholar] [CrossRef]
- Bailey, C.J.; Wilcock, C.; Scarpello, J.H.B. Metformin and the Intestine. Diabetologia 2008, 51, 1552–1553. [Google Scholar] [CrossRef]
- Wilcock, C.; Bailey, C.J. Accumulation of Metformin by Tissues of the Normal and Diabetic Mouse. Xenobiotica Fate Foreign Compd. Biol. Syst. 1994, 24, 49–57. [Google Scholar] [CrossRef]
- Jensen, J.B.; Sundelin, E.I.; Jakobsen, S.; Gormsen, L.C.; Munk, O.L.; Frøkiær, J.; Jessen, N. [11C]-Labeled Metformin Distribution in the Liver and Small Intestine Using Dynamic Positron Emission Tomography in Mice Demonstrates Tissue-Specific Transporter Dependency. Diabetes 2016, 65, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Dujic, T.; Zhou, K.; Donnelly, L.A.; Tavendale, R.; Palmer, C.N.A.; Pearson, E.R. Association of Organic Cation Transporter 1 With Intolerance to Metformin in Type 2 Diabetes: A GoDARTS Study. Diabetes 2015, 64, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Paldánius, P.M.; Proot, P.; Chiang, Y.; Stumvoll, M.; Del Prato, S.; VERIFY study group. Glycaemic Durability of an Early Combination Therapy with Vildagliptin and Metformin versus Sequential Metformin Monotherapy in Newly Diagnosed Type 2 Diabetes (VERIFY): A 5-Year, Multicentre, Randomised, Double-Blind Trial. Lancet Lond. Engl. 2019, 394, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Schernthaner, G.; Schernthaner, G.-H. The Right Place for Metformin Today. Diabetes Res. Clin. Pract. 2020, 159, 107946. [Google Scholar] [CrossRef]
- Ahmad, E.; Sargeant, J.A.; Zaccardi, F.; Khunti, K.; Webb, D.R.; Davies, M.J. Where Does Metformin Stand in Modern Day Management of Type 2 Diabetes? Pharmaceuticals 2020, 13, 427. [Google Scholar] [CrossRef]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical Use in Type 2 Diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef]
- Schommers, P.; Thurau, A.; Bultmann-Mellin, I.; Guschlbauer, M.; Klatt, A.R.; Rozman, J.; Klingenspor, M.; de Angelis, M.H.; Alber, J.; Gründemann, D.; et al. Metformin Causes a Futile Intestinal-Hepatic Cycle Which Increases Energy Expenditure and Slows down Development of a Type 2 Diabetes-like State. Mol. Metab. 2017, 6, 737–747. [Google Scholar] [CrossRef]
- Dujic, T.; Zhou, K.; Tavendale, R.; Palmer, C.N.A.; Pearson, E.R. Effect of Serotonin Transporter 5-HTTLPR Polymorphism on Gastrointestinal Intolerance to Metformin: A GoDARTS Study. Diabetes Care 2016, 39, 1896–1901. [Google Scholar] [CrossRef]
- Scarpello, J.H.; Hodgson, E.; Howlett, H.C. Effect of Metformin on Bile Salt Circulation and Intestinal Motility in Type 2 Diabetes Mellitus. Diabet. Med. J. Br. Diabet. Assoc. 1998, 15, 651–656. [Google Scholar] [CrossRef]
- Yee, S.W.; Lin, L.; Merski, M.; Keiser, M.J.; Gupta, A.; Zhang, Y.; Chien, H.-C.; Shoichet, B.K.; Giacomini, K.M. Prediction and Validation of Enzyme and Transporter Off-Targets for Metformin. J. Pharmacokinet. Pharmacodyn. 2015, 42, 463–475. [Google Scholar] [CrossRef]
- McCreight, L.J.; Stage, T.B.; Connelly, P.; Lonergan, M.; Nielsen, F.; Prehn, C.; Adamski, J.; Brøsen, K.; Pearson, E.R. Pharmacokinetics of Metformin in Patients with Gastrointestinal Intolerance. Diabetes Obes. Metab. 2018, 20, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Kanazawa, A.; Ikeda, F.; Yoshihara, T.; Goto, H.; Abe, H.; Komiya, K.; Kawaguchi, M.; Shimizu, T.; Ogihara, T.; et al. Gut Dysbiosis and Detection of “Live Gut Bacteria” in Blood of Japanese Patients with Type 2 Diabetes. Diabetes Care 2014, 37, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut Metagenome in European Women with Normal, Impaired and Diabetic Glucose Control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Barengolts, E.; Green, S.J.; Eisenberg, Y.; Akbar, A.; Reddivari, B.; Layden, B.T.; Dugas, L.; Chlipala, G. Gut Microbiota Varies by Opioid Use, Circulating Leptin and Oxytocin in African American Men with Diabetes and High Burden of Chronic Disease. PLoS ONE 2018, 13, e0194171. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xie, C.; Wang, G.; Wu, Y.; Wu, Q.; Wang, X.; Liu, J.; Deng, Y.; Xia, J.; Chen, B.; et al. Gut Microbiota and Intestinal FXR Mediate the Clinical Benefits of Metformin. Nat. Med. 2018, 24, 1919–1929. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, M.; Yang, J.; Xu, Q.; Liang, C.; Chen, B.; Zhang, J.; Yang, Y.; Wang, H.; Shang, Y.; et al. Response of Gut Microbiota in Type 2 Diabetes to Hypoglycemic Agents. Endocrine 2019, 66, 485–493. [Google Scholar] [CrossRef]
- Duca, F.A.; Côté, C.D.; Rasmussen, B.A.; Zadeh-Tahmasebi, M.; Rutter, G.A.; Filippi, B.M.; Lam, T.K.T. Metformin Activates a Duodenal Ampk-Dependent Pathway to Lower Hepatic Glucose Production in Rats. Nat. Med. 2015, 21, 506–511. [Google Scholar] [CrossRef]
- Maida, A.; Lamont, B.J.; Cao, X.; Drucker, D.J. Metformin Regulates the Incretin Receptor Axis via a Pathway Dependent on Peroxisome Proliferator-Activated Receptor-α in Mice. Diabetologia 2011, 54, 339–349. [Google Scholar] [CrossRef]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V. Probiotic Yogurt Improves Antioxidant Status in Type 2 Diabetic Patients. Nutr. Burbank Los Angel. Cty. Calif 2012, 28, 539–543. [Google Scholar] [CrossRef]
- Balakumar, M.; Prabhu, D.; Sathishkumar, C.; Prabu, P.; Rokana, N.; Kumar, R.; Raghavan, S.; Soundarajan, A.; Grover, S.; Batish, V.K.; et al. Improvement in Glucose Tolerance and Insulin Sensitivity by Probiotic Strains of Indian Gut Origin in High-Fat Diet-Fed C57BL/6J Mice. Eur. J. Nutr. 2018, 57, 279–295. [Google Scholar] [CrossRef]
- El Aidy, S.; Merrifield, C.A.; Derrien, M.; van Baarlen, P.; Hooiveld, G.; Levenez, F.; Doré, J.; Dekker, J.; Holmes, E.; Claus, S.P.; et al. The Gut Microbiota Elicits a Profound Metabolic Reorientation in the Mouse Jejunal Mucosa during Conventionalisation. Gut 2013, 62, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Bauer, P.V.; Duca, F.A.; Waise, T.M.Z.; Rasmussen, B.A.; Abraham, M.A.; Dranse, H.J.; Puri, A.; O’Brien, C.A.; Lam, T.K.T. Metformin Alters Upper Small Intestinal Microbiota That Impact a Glucose-SGLT1-Sensing Glucoregulatory Pathway. Cell Metab. 2018, 27, 101–117.e5. [Google Scholar] [CrossRef] [PubMed]
- Rooj, A.K.; Kimura, Y.; Buddington, R.K. Metabolites Produced by Probiotic Lactobacilli Rapidly Increase Glucose Uptake by Caco-2 Cells. BMC Microbiol. 2010, 10, 16. [Google Scholar] [CrossRef]
- Tanaka, T.; Katsuma, S.; Adachi, T.; Koshimizu, T.; Hirasawa, A.; Tsujimoto, G. Free Fatty Acids Induce Cholecystokinin Secretion through GPR120. Naunyn. Schmiedebergs Arch. Pharmacol. 2008, 377, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Fredborg, M.; Theil, P.K.; Jensen, B.B.; Purup, S. G Protein-Coupled Receptor120 (GPR120) Transcription in Intestinal Epithelial Cells Is Significantly Affected by Bacteria Belonging to the Bacteroides, Proteobacteria, and Firmicutes Phyla. J. Anim. Sci. 2012, 90 (Suppl. S4), 10–12. [Google Scholar] [CrossRef]
- Koffert, J.P.; Mikkola, K.; Virtanen, K.A.; Andersson, A.-M.D.; Faxius, L.; Hällsten, K.; Heglind, M.; Guiducci, L.; Pham, T.; Silvola, J.M.U.; et al. Metformin Treatment Significantly Enhances Intestinal Glucose Uptake in Patients with Type 2 Diabetes: Results from a Randomized Clinical Trial. Diabetes Res. Clin. Pract. 2017, 131, 208–216. [Google Scholar] [CrossRef]
- Lee, H.; Ko, G. Effect of Metformin on Metabolic Improvement and Gut Microbiota. Appl. Environ. Microbiol. 2014, 80, 5935–5943. [Google Scholar] [CrossRef]
- Cani, P.D.; de Vos, W.M. Next-Generation Beneficial Microbes: The Case of Akkermansia Muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Xu, J.; Xue, Z.; Zhang, M.; Pang, X.; Zhang, X.; Zhao, L. Modulation of Gut Microbiota by Berberine and Metformin during the Treatment of High-Fat Diet-Induced Obesity in Rats. Sci. Rep. 2015, 5, 14405. [Google Scholar] [CrossRef]
- Zhou, Z.-Y.; Ren, L.-W.; Zhan, P.; Yang, H.-Y.; Chai, D.-D.; Yu, Z.-W. Metformin Exerts Glucose-Lowering Action in High-Fat Fed Mice via Attenuating Endotoxemia and Enhancing Insulin Signaling. Acta Pharmacol. Sin. 2016, 37, 1063–1075. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J.-H.; Yu, T.; Chen, Q.-K. Effects of Berberine and Metformin on Intestinal Inflammation and Gut Microbiome Composition in Db/Db Mice. Biomed. Pharmacother. Biomedecine Pharmacother. 2019, 118, 109131. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, Y.; Kim, J.; An, J.; Lee, S.; Kong, H.; Song, Y.; Lee, C.-K.; Kim, K. Modulation of the Gut Microbiota by Metformin Improves Metabolic Profiles in Aged Obese Mice. Gut Microbes 2018, 9, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Rios-Covian, D.; Arboleya, S.; Hernandez-Barranco, A.M.; Alvarez-Buylla, J.R.; Ruas-Madiedo, P.; Gueimonde, M.; de los Reyes-Gavilan, C.G. Interactions between Bifidobacterium and Bacteroides Species in Cofermentations Are Affected by Carbon Sources, Including Exopolysaccharides Produced by Bifidobacteria. Appl. Environ. Microbiol. 2013, 79, 7518–7524. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liang, L.; Yu, G.; Li, Q. Pumpkin Polysaccharide Modifies the Gut Microbiota during Alleviation of Type 2 Diabetes in Rats. Int. J. Biol. Macromol. 2018, 115, 711–717. [Google Scholar] [CrossRef]
- Ryan, P.M.; Patterson, E.; Carafa, I.; Mandal, R.; Wishart, D.S.; Dinan, T.G.; Cryan, J.F.; Tuohy, K.M.; Stanton, C.; Ross, R.P. Metformin and Dipeptidyl Peptidase-4 Inhibitor Differentially Modulate the Intestinal Microbiota and Plasma Metabolome of Metabolically Dysfunctional Mice. Can. J. Diabetes 2020, 44, 146–155.e2. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Li, J.; Li, T.; Zhang, Y.; Liang, Y.; Mei, Y. Phellinus Linteus Polysaccharide Extract Improves Insulin Resistance by Regulating Gut Microbiota Composition. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 1065–1078. [Google Scholar] [CrossRef]
- Ahmadi, S.; Razazan, A.; Nagpal, R.; Jain, S.; Wang, B.; Mishra, S.P.; Wang, S.; Justice, J.; Ding, J.; McClain, D.A.; et al. Metformin Reduces Aging-Related Leaky Gut and Improves Cognitive Function by Beneficially Modulating Gut Microbiome/Goblet Cell/Mucin Axis. J. Gerontol. A. Biol. Sci. Med. Sci. 2020, 75, e9–e21. [Google Scholar] [CrossRef]
- Hao, Z.; Li, L.; Ning, Z.; Zhang, X.; Mayne, J.; Cheng, K.; Walker, K.; Liu, H.; Figeys, D. Metaproteomics Reveals Growth Phase-Dependent Responses of an In Vitro Gut Microbiota to Metformin. J. Am. Soc. Mass Spectrom. 2020, 31, 1448–1458. [Google Scholar] [CrossRef]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef]
- Zheng, J.; Li, H.; Zhang, X.; Jiang, M.; Luo, C.; Lu, Z.; Xu, Z.; Shi, J. Prebiotic Mannan-Oligosaccharides Augment the Hypoglycemic Effects of Metformin in Correlation with Modulating Gut Microbiota. J. Agric. Food Chem. 2018, 66, 5821–5831. [Google Scholar] [CrossRef]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides Distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235.e5. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, E.; Yin, B.; Fang, D.; Chen, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Effects of Lactobacillus Casei CCFM419 on Insulin Resistance and Gut Microbiota in Type 2 Diabetic Mice. Benef. Microbes 2017, 8, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carrio, J.; Salazar, N.; Margolles, A.; González, S.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Suárez, A. Free Fatty Acids Profiles Are Related to Gut Microbiota Signatures and Short-Chain Fatty Acids. Front. Immunol. 2017, 8, 823. [Google Scholar] [CrossRef] [PubMed]
- Slouha, E.; Rezazadah, A.; Farahbod, K.; Gerts, A.; Clunes, L.A.; Kollias, T.F. Type-2 Diabetes Mellitus and the Gut Microbiota: Systematic Review. Cureus 2023, 15, e49740. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing A Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Zhang, S.; Wang, C.; Mao, Z.; Huo, W.; Yang, T.; Li, Y.; Xing, W.; Li, L. Association of the Short-Chain Fatty Acid Levels and Dietary Quality with Type 2 Diabetes: A Case-Control Study Based on Henan Rural Cohort. Br. J. Nutr. 2024, 131, 1668–1677. [Google Scholar] [CrossRef]
- Zhao, L.; Lou, H.; Peng, Y.; Chen, S.; Fan, L.; Li, X. Elevated Levels of Circulating Short-Chain Fatty Acids and Bile Acids in Type 2 Diabetes Are Linked to Gut Barrier Disruption and Disordered Gut Microbiota. Diabetes Res. Clin. Pract. 2020, 169, 108418. [Google Scholar] [CrossRef]
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; Prifti, E.; Vieira-Silva, S.; Gudmundsdottir, V.; Pedersen, H.K.; et al. Disentangling Type 2 Diabetes and Metformin Treatment Signatures in the Human Gut Microbiota. Nature 2015, 528, 262–266. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, N. Effects of Metformin on the Gut Microbiota in Obesity and Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 5003–5014. [Google Scholar] [CrossRef]
- Huang, F.; Nilholm, C.; Roth, B.; Linninge, C.; Höglund, P.; Nyman, M.; Ohlsson, B. Anthropometric and Metabolic Improvements in Human Type 2 Diabetes after Introduction of an Okinawan-Based Nordic Diet Are Not Associated with Changes in Microbial Diversity or SCFA Concentrations. Int. J. Food Sci. Nutr. 2018, 69, 729–740. [Google Scholar] [CrossRef]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-Based Metagenomics Analysis Reveals Markers for Gut Microbiome Composition and Diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.T.; Differding, M.K.; Zhang, M.; Maruthur, N.M.; Juraschek, S.P.; Miller, E.R.; Appel, L.J.; Yeh, H.-C. Metformin Affects Gut Microbiome Composition and Function and Circulating Short-Chain Fatty Acids: A Randomized Trial. Diabetes Care 2021, 44, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Hernández-Arriaga, A.; Kehm, R.; Sánchez, V.; Jin, C.J.; Nier, A.; Baumann, A.; Camarinha-Silva, A.; Bergheim, I. Metformin Attenuates the Onset of Non-Alcoholic Fatty Liver Disease and Affects Intestinal Microbiota and Barrier in Small Intestine. Sci. Rep. 2019, 9, 6668. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Chen, J.; Meng, Y.; Yang, J.; Cui, Q.; Zhou, Y. Metformin Alters Gut Microbiota of Healthy Mice: Implication for Its Potential Role in Gut Microbiota Homeostasis. Front. Microbiol. 2018, 9, 1336. [Google Scholar] [CrossRef]
- Rosario, D.; Benfeitas, R.; Bidkhori, G.; Zhang, C.; Uhlen, M.; Shoaie, S.; Mardinoglu, A. Understanding the Representative Gut Microbiota Dysbiosis in Metformin-Treated Type 2 Diabetes Patients Using Genome-Scale Metabolic Modeling. Front. Physiol. 2018, 9, 775. [Google Scholar] [CrossRef]
- Belzer, C.; de Vos, W.M. Microbes Inside--from Diversity to Function: The Case of Akkermansia. ISME J. 2012, 6, 1449–1458. [Google Scholar] [CrossRef]
- An, H.; He, L. Current Understanding of Metformin Effect on the Control of Hyperglycemia in Diabetes. J. Endocrinol. 2016, 228, R97–R106. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, M.-J. AMP-Activated Protein Kinase: A Therapeutic Target in Intestinal Diseases. Open Biol. 2017, 7, 170104. [Google Scholar] [CrossRef]
- Wu, W.; Wang, S.; Liu, Q.; Shan, T.; Wang, Y. Metformin Protects against LPS-Induced Intestinal Barrier Dysfunction by Activating AMPK Pathway. Mol. Pharm. 2018, 15, 3272–3284. [Google Scholar] [CrossRef]
- Lackey, D.E.; Olefsky, J.M. Regulation of Metabolism by the Innate Immune System. Nat. Rev. Endocrinol. 2016, 12, 15–28. [Google Scholar] [CrossRef]
- Brestoff, J.R.; Artis, D. Immune Regulation of Metabolic Homeostasis in Health and Disease. Cell 2015, 161, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Osborn, O.; Olefsky, J.M. The Cellular and Signaling Networks Linking the Immune System and Metabolism in Disease. Nat. Med. 2012, 18, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Lee, S.H.; Yang, E.-J.; Kim, E.-K.; Kim, J.-K.; Shin, D.-Y.; Cho, M.-L. Metformin Ameliorates Inflammatory Bowel Disease by Suppression of the STAT3 Signaling Pathway and Regulation of the between Th17/Treg Balance. PLoS ONE 2015, 10, e0135858. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-N.; Wang, X.; Zeng, Q.-T.; Feng, Y.-B.; Cheng, X.; Mao, X.-B.; Wang, T.-H.; Deng, H.-P. Metformin Inhibits Nuclear Factor kappaB Activation and Decreases Serum High-Sensitivity C-Reactive Protein Level in Experimental Atherogenesis of Rabbits. Heart Vessels 2009, 24, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.-L.; Chiang, S.-H.; Hsueh, C.-H.; Liang, Y.-J.; Chen, Y.-J.; Lai, L.-P. Metformin Inhibits TNF-Alpha-Induced IkappaB Kinase Phosphorylation, IkappaB-Alpha Degradation and IL-6 Production in Endothelial Cells through PI3K-Dependent AMPK Phosphorylation. Int. J. Cardiol. 2009, 134, 169–175. [Google Scholar] [CrossRef]
- Liu, G.; Bei, J.; Liang, L.; Yu, G.; Li, L.; Li, Q. Stachyose Improves Inflammation through Modulating Gut Microbiota of High-Fat Diet/Streptozotocin-Induced Type 2 Diabetes in Rats. Mol. Nutr. Food Res. 2018, 62, e1700954. [Google Scholar] [CrossRef]
- Wang, J.-H.; Bose, S.; Shin, N.R.; Chin, Y.-W.; Choi, Y.H.; Kim, H. Pharmaceutical Impact of Houttuynia Cordata and Metformin Combination on High-Fat-Diet-Induced Metabolic Disorders: Link to Intestinal Microbiota and Metabolic Endotoxemia. Front. Endocrinol. 2018, 9, 620. [Google Scholar] [CrossRef]
- Hansen, C.H.F.; Holm, T.L.; Krych, Ł.; Andresen, L.; Nielsen, D.S.; Rune, I.; Hansen, A.K.; Skov, S. Gut Microbiota Regulates NKG2D Ligand Expression on Intestinal Epithelial Cells. Eur. J. Immunol. 2013, 43, 447–457. [Google Scholar] [CrossRef]
- Png, C.W.; Lindén, S.K.; Gilshenan, K.S.; Zoetendal, E.G.; McSweeney, C.S.; Sly, L.I.; McGuckin, M.A.; Florin, T.H.J. Mucolytic Bacteria with Increased Prevalence in IBD Mucosa Augment in Vitro Utilization of Mucin by Other Bacteria. Am. J. Gastroenterol. 2010, 105, 2420–2428. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia Muciniphila in Overweight and Obese Human Volunteers: A Proof-of-Concept Exploratory Study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Pryor, R.; Norvaisas, P.; Marinos, G.; Best, L.; Thingholm, L.B.; Quintaneiro, L.M.; De Haes, W.; Esser, D.; Waschina, S.; Lujan, C.; et al. Host-Microbe-Drug-Nutrient Screen Identifies Bacterial Effectors of Metformin Therapy. Cell 2019, 178, 1299–1312.e29. [Google Scholar] [CrossRef] [PubMed]
- Weigert, C.; Hennige, A.M.; Brodbeck, K.; Häring, H.U.; Schleicher, E.D. Interleukin-6 Acts as Insulin Sensitizer on Glycogen Synthesis in Human Skeletal Muscle Cells by Phosphorylation of Ser473 of Akt. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E251–E257. [Google Scholar] [CrossRef] [PubMed]
- Rotter, V.; Nagaev, I.; Smith, U. Interleukin-6 (IL-6) Induces Insulin Resistance in 3T3-L1 Adipocytes and Is, like IL-8 and Tumor Necrosis Factor-Alpha, Overexpressed in Human Fat Cells from Insulin-Resistant Subjects. J. Biol. Chem. 2003, 278, 45777–45784. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-S.; Ban, M.; Choi, E.-J.; Moon, H.-G.; Jeon, J.-S.; Kim, D.-K.; Park, S.-K.; Jeon, S.G.; Roh, T.-Y.; Myung, S.-J.; et al. Extracellular Vesicles Derived from Gut Microbiota, Especially Akkermansia Muciniphila, Protect the Progression of Dextran Sulfate Sodium-Induced Colitis. PLoS ONE 2013, 8, e76520. [Google Scholar] [CrossRef]
- Wang, J.-H.; Bose, S.; Lim, S.-K.; Ansari, A.; Chin, Y.-W.; Choi, H.S.; Kim, H. Houttuynia Cordata Facilitates Metformin on Ameliorating Insulin Resistance Associated with Gut Microbiota Alteration in OLETF Rats. Genes 2017, 8, 239. [Google Scholar] [CrossRef]
- Bharti, A.C.; Aggarwal, B.B. Nuclear Factor-Kappa B and Cancer: Its Role in Prevention and Therapy. Biochem. Pharmacol. 2002, 64, 883–888. [Google Scholar] [CrossRef]
- Cui, H.-X.; Zhang, L.-S.; Luo, Y.; Yuan, K.; Huang, Z.-Y.; Guo, Y. A Purified Anthraquinone-Glycoside Preparation From Rhubarb Ameliorates Type 2 Diabetes Mellitus by Modulating the Gut Microbiota and Reducing Inflammation. Front. Microbiol. 2019, 10, 1423. [Google Scholar] [CrossRef]
- Kinoshita, M.; Suzuki, Y.; Saito, Y. Butyrate Reduces Colonic Paracellular Permeability by Enhancing PPARgamma Activation. Biochem. Biophys. Res. Commun. 2002, 293, 827–831. [Google Scholar] [CrossRef]
- Inan, M.S.; Rasoulpour, R.J.; Yin, L.; Hubbard, A.K.; Rosenberg, D.W.; Giardina, C. The Luminal Short-Chain Fatty Acid Butyrate Modulates NF-kappaB Activity in a Human Colonic Epithelial Cell Line. Gastroenterology 2000, 118, 724–734. [Google Scholar] [CrossRef]
- Carter, D.; Howlett, H.C.S.; Wiernsperger, N.F.; Bailey, C.J. Differential Effects of Metformin on Bile Salt Absorption from the Jejunum and Ileum. Diabetes Obes. Metab. 2003, 5, 120–125. [Google Scholar] [CrossRef]
- Napolitano, A.; Miller, S.; Nicholls, A.W.; Baker, D.; Van Horn, S.; Thomas, E.; Rajpal, D.; Spivak, A.; Brown, J.R.; Nunez, D.J. Novel Gut-Based Pharmacology of Metformin in Patients with Type 2 Diabetes Mellitus. PLoS ONE 2014, 9, e100778. [Google Scholar] [CrossRef] [PubMed]
- Caspary, W.F.; Creutzfeldt, W. Inhibition of Bile Salt Absorption by Blood-Sugar Lowering Biguanides. Diabetologia 1975, 11, 113–117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meng, X.-M.; Ma, X.-X.; Tian, Y.-L.; Jiang, Q.; Wang, L.-L.; Shi, R.; Ding, L.; Pang, S.-G. Metformin Improves the Glucose and Lipid Metabolism via Influencing the Level of Serum Total Bile Acids in Rats with Streptozotocin-Induced Type 2 Diabetes Mellitus. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2232–2237. [Google Scholar] [PubMed]
- Watanabe, M.; Horai, Y.; Houten, S.M.; Morimoto, K.; Sugizaki, T.; Arita, E.; Mataki, C.; Sato, H.; Tanigawara, Y.; Schoonjans, K.; et al. Lowering Bile Acid Pool Size with a Synthetic Farnesoid X Receptor (FXR) Agonist Induces Obesity and Diabetes through Reduced Energy Expenditure. J. Biol. Chem. 2011, 286, 26913–26920. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, M.-S.; Daoudi, M.; Prawitt, J.; Ducastel, S.; Touche, V.; Sayin, S.I.; Perino, A.; Brighton, C.A.; Sebti, Y.; Kluza, J.; et al. Farnesoid X Receptor Inhibits Glucagon-like Peptide-1 Production by Enteroendocrine L Cells. Nat. Commun. 2015, 6, 7629. [Google Scholar] [CrossRef]
- Li, F.; Jiang, C.; Krausz, K.W.; Li, Y.; Albert, I.; Hao, H.; Fabre, K.M.; Mitchell, J.B.; Patterson, A.D.; Gonzalez, F.J. Microbiome Remodelling Leads to Inhibition of Intestinal Farnesoid X Receptor Signalling and Decreased Obesity. Nat. Commun. 2013, 4, 2384. [Google Scholar] [CrossRef]
- Zhang, Y.; Lee, F.Y.; Barrera, G.; Lee, H.; Vales, C.; Gonzalez, F.J.; Willson, T.M.; Edwards, P.A. Activation of the Nuclear Receptor FXR Improves Hyperglycemia and Hyperlipidemia in Diabetic Mice. Proc. Natl. Acad. Sci. USA 2006, 103, 1006–1011. [Google Scholar] [CrossRef]
- Ma, K.; Saha, P.K.; Chan, L.; Moore, D.D. Farnesoid X Receptor Is Essential for Normal Glucose Homeostasis. J. Clin. Investig. 2006, 116, 1102–1109. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.-U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut Microbiota Regulates Bile Acid Metabolism by Reducing the Levels of Tauro-Beta-Muricholic Acid, a Naturally Occurring FXR Antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef]
- Cariou, B.; van Harmelen, K.; Duran-Sandoval, D.; van Dijk, T.H.; Grefhorst, A.; Abdelkarim, M.; Caron, S.; Torpier, G.; Fruchart, J.-C.; Gonzalez, F.J.; et al. The Farnesoid X Receptor Modulates Adiposity and Peripheral Insulin Sensitivity in Mice. J. Biol. Chem. 2006, 281, 11039–11049. [Google Scholar] [CrossRef]
- Pathak, P.; Xie, C.; Nichols, R.G.; Ferrell, J.M.; Boehme, S.; Krausz, K.W.; Patterson, A.D.; Gonzalez, F.J.; Chiang, J.Y.L. Intestine Farnesoid X Receptor Agonist and the Gut Microbiota Activate G-Protein Bile Acid Receptor-1 Signaling to Improve Metabolism. Hepatol. Baltim. Md 2018, 68, 1574–1588. [Google Scholar] [CrossRef] [PubMed]
- Bauer, P.V.; Duca, F.A.; Waise, T.M.Z.; Dranse, H.J.; Rasmussen, B.A.; Puri, A.; Rasti, M.; O’Brien, C.A.; Lam, T.K.T. Lactobacillus Gasseri in the Upper Small Intestine Impacts an ACSL3-Dependent Fatty Acid-Sensing Pathway Regulating Whole-Body Glucose Homeostasis. Cell Metab. 2018, 27, 572–587.e6. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.Y.; Mashek, M.T.; Mashek, D.G. Suppression of Long Chain Acyl-CoA Synthetase 3 Decreases Hepatic de Novo Fatty Acid Synthesis through Decreased Transcriptional Activity. J. Biol. Chem. 2009, 284, 30474–30483. [Google Scholar] [CrossRef] [PubMed]
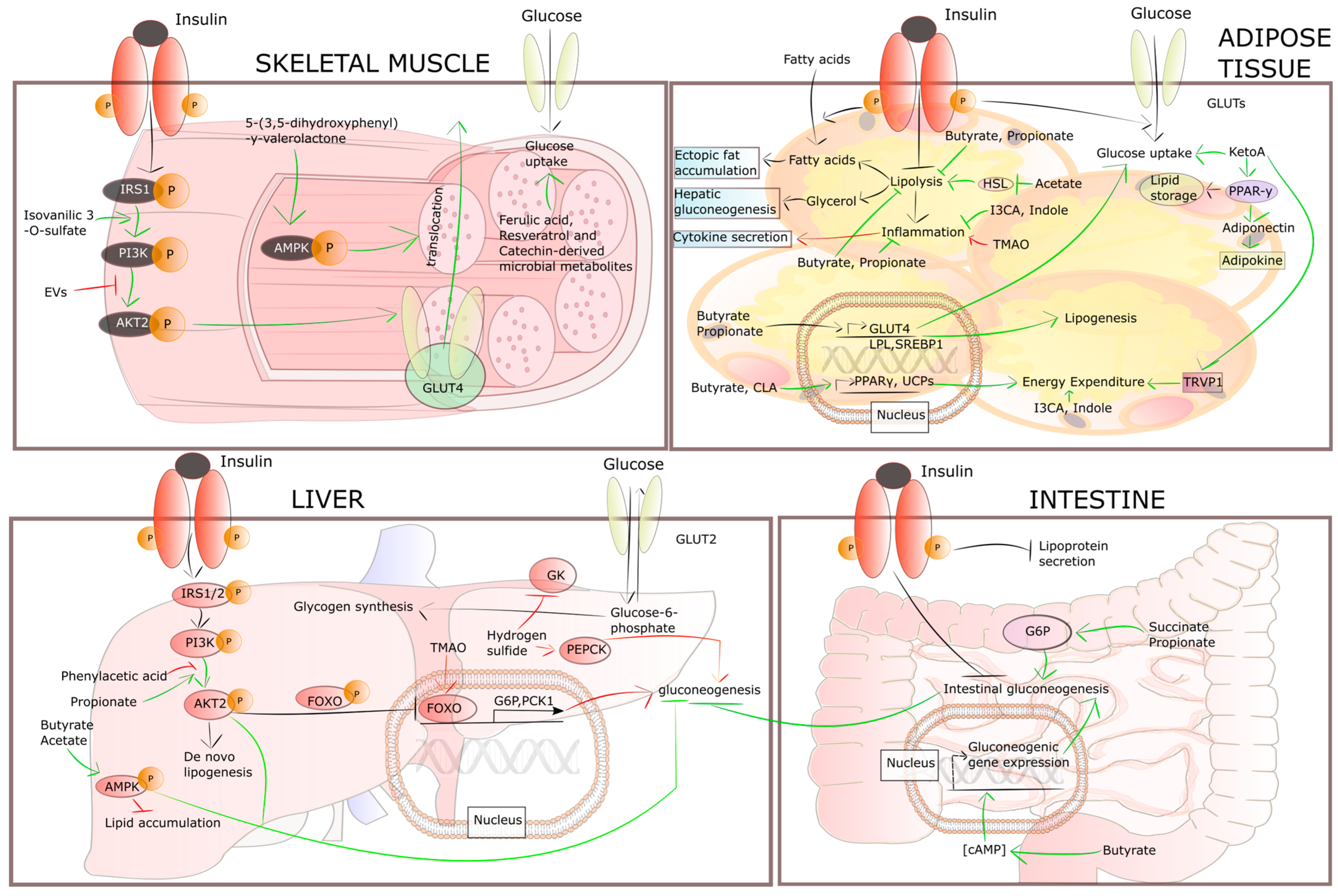
| Metabolite (Bacterial Source) | Cell Types/Target Organ/Tissue | Effects | Ref. |
|---|---|---|---|
| Acetate [Bifidobacteria, Bacteroidetes and Lactobacillus] | Hepatocytes | Decreased lipogenesis/increased lipid oxidation | [99,100,101,102] |
| Propionate [Propionibacterium sp., Clostridium sp., Megasphaera sp., Propionibacterium shermanii, Bacteroides species (i.e., Bacteroides fragilis and Bacteroides eggerthii), Veillonella species and Acidaminococcus species] | Suppressed gluconeogenesis/decreased lipogenesis/increased lipid oxidation | [99,103] | |
| Butyrate [the Clostridium cluster of the phylum Firmicutes i.e., Eubacterium, Subdoligranulum, Faecalibacterium, Coprococcus, Anaerostipes, Roseburia and Anaerobutyricum; Butyricimonas spp., Allobaculum, Subdoligranulum] | Decreased lipogenesis/increased lipid oxidaion | [99,104,105] | |
| Hydrogen sulfide [Desulfobulbus, Desulfobacter, Desulfovibrio and Desulfomonas] | Stimulated gluconeogenesis/decreased glucogen synthesis | [106] | |
| Phenylacetic acid [Bacteroides spp.] | Increased lipogenesis (induced the accumulation of hepatic triglycerides) | [107] | |
| Trimethylamine N-oxide (TMAO) [Firmicutes and Proteobacteria] | Increased gluconeogenic gene expression (increased gluconeogenesis) | [108,109] | |
| Acetate [Bifidobacteria, Bacteroidetes and Lactobacillus] | Myocytes | Increased lipid oxidation | [110] |
| Butyrate [the Clostridium cluster of the phylum Firmicutes i.e., Eubacterium, Subdoligranulum, Faecalibacterium, Coprococcus, Anaerostipes, Roseburia and Anaerobutyricum; Butyricimonas spp., Allobaculum, Subdoligranulum] | Increased lipid oxidation | [103] | |
| Ferulic acid 4-O-sulfate and Dihydroferulic acid 4-O-sulfate (and Trans-resveratrol 4′-O-glucuro-nide, Trans-resveratol 3-O-sulfate) | Increased glucose uptake | [111] | |
| Isovanillic acid 3-O-sulfate | Increased glucose uptake in myotubes | [111] | |
| 4-Hydroxy-5-(3,4,5-trihydroxyphenyl) valeric acid, 4-hydroxy-5-(3,4,5-trihydroxyphenyl)-γ-valerolactone and 5-(3-hydroxyphenyl) valeric acid [Clostridia, Megasphaera massiliensis] | Promoted 2-deoxy-glucose uptake in myotubes (increased glucose uptake) | [112] | |
| Metabolites derived from extracellular vesicles (EVs) [Pseudomonas aeruginosa, Helicobacter pylori and Salmonella typhimurium and gram-positive bacteria, like Staphylococcus aureus, Bacillus subtilis and Bacillus anthracis] | Impaired glucose uptake by decreasing insulin-dependent GLUT4 translocation | [113] | |
| Acetate [Bifidobacteria, Bacteroidetes and Lactobacillus] | Adipocytes | Stimulated adipogenesis/inhibited lipolysis/increased browning | [59,102,114,115,116] |
| Propionate [Propionibacterium sp., Clostridium sp., Megasphaera sp., Propionibacterium shermanii, Bacteroides species (i.e., Bacteroides fragilis and Bacteroides eggerthii), Veillonella species and Acidaminococcus species] | Increased adipogenesis/inhibited lipolysis | [114,115] | |
| Butyrate [Eubacterium, Subdoligranulum, Faecalibacterium, Coprococcus, Anaerostipes, Roseburia and Anaerobutyricum] | Suppressed lipolysis and inflammatory response/improved inflammation/increased thermogenesis | [104,117,118] | |
| Conjugated linoleic acid (CLA) [Propionibacterium, Bifidobacterium and some lactic acid bacteria (i.e., Lactobacillus plantarum)] | Enhanced energy expenditure (increased UCP genes expression) | [119,120,121] | |
| Indole (and Indole-3-carboxylic acid [I3CA]) [Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Pseudomonas, Bacillus] | Decreased inflammation/improved insulin sensitivity | [122] | |
| 10-oxo-12(Z)-Octadecenoic acid (KetoA) [gut lactic acid bacteria such as Lactobacilli, Lactococci] | Induced adipocyte differentiation/increased the production of adiponectin (induced adipogenesis, increased thermogenesis) | [123,124] | |
| Conjugated linoleic acid (CLA) [Propionibacterium, Bifidobacterium and some lactic acid bacteria (i.e., Lactobacillus plantarum)] | Increased energy expenditure | [119,121] | |
| Trimethylamine N-oxide (TMAO) [Firmicutes and Proteobacteria] | Increased gluconeogenesis (increased gluconeogenic gene expression) | [108,109] | |
| Propionate [Propionibacterium sp., Clostridium sp., Megasphaera sp., Propionibacterium shermanii, Bacteroides species (i.e., Bacteroides fragilis and Bacteroides eggerthii), Veillonella species and Acidaminococcus species] | Enterocytes | Promoted intestinal gluconeogenesis | [67] |
| Butyrate [the Clostridium cluster of the phylum Firmicutes i.e., Eubacterium, Subdoligranulum, Faecalibacterium, Coprococcus, Anaerostipes, Roseburia and Anaerobutyricum; Butyricimonas spp., Allobaculum, Subdoligranulum] | Promoted gluconeogenesis in enterocytes | [67] | |
| Succinate [Anaerobiospirillum succiniciproducens, Actinobacillus succinogenes, E. coli, Corynebacterium glutamicum, Mannheimia succiniciproducens, Parabacteroides] | Activated intestinal gluconeogenesis/ Improved glucose tolerance and insulin sensitivity | [125] |
| Population | Gut Microbiota | Biochemical and HbA1c; FPG Alterations | References |
|---|---|---|---|
| Spanish Met+ (n = 22) Met− (n = 18) Sex (M/F) Met+ (n = 8/14) Met− (n = 9/9) Age Met+ (52.6 ± 2.0) Met− (54.9 ± 1.9) | Phylum: Firmicutes ↑, Proteobacteria ↑ Genus: Actinetobacter ↑, Pseudomonas ↑, Escherichia ↑, Enterobacter ↑, Salmonella ↑, Alkaliphilus ↓, Intestinibacter ↓, Klebsiella ↓ Species: Akkermansia muciniphila ↑, Bifidobacterium adolescentis ↑ | Butyrate and propionate (in men) ↑, Plasma bile acids ↑, HbA1c-NA FPG-NA | [56] |
| Colombian Met+ (n = 14) Met− (n = 14) Sex (M/F) Met+ (n = 9/5) Met− (n = 7/7) Age Met+ (50 ± 10) Met− (44 ± 9) | Phylum: Firmicutes ↑, Family: Prevotellaceae ↑, Veillonellaceae ↑, Ruminococcaceae ↓, Barnesiellaceae ↓, Clostridiaceae ↓ Genus: Prevotella ↑, Oscilospira ↓, Bacteroides ↑, Megasphaera ↑ | NA HbA1c ↓ FPG ↓ | [57] |
| Mexican Met+ (n = 14) Met− (n = 14) Sex (M/F) Met+ (n = 2/12) Met− (n = 7/7) Age Met+ (48.1 ± 4.6) Met− (48.1 ± 4.7) | Order: Bacteroidales ↑ Phylum: Proteobacteria ↑, Bacteroidetes ↑, Actinobacteria ↑ Family: Coribacteraceae ↑ Genus: Sutterela spp. ↓, Pelomonas spp. ↑ | SCFA production ↑, Gut peptides production, HbA1c ↓ FPG ↓ | [58] |
| Japanese Met+ (n = 17) Met− (n = 33) | Family: Enterobacteriaceae ↑ Genus: Staphylococcus ↑ Species: Clostridium coccoides ↓, Lactobacillus plantarum ↑, Lactobacillus reuteri ↑ | NA HbA1c ↓ FPG ↓ | [164] |
| Swedish Met+ (n = 20) Met− (n = 33) | Family: Enterobacteriaceae ↑ Genus: Clostridium ↓, Escherichia ↑, Shigella ↑, Klebsiella ↑, Salmonella ↑, Eubacterium ↓, | NA HbA1c-NA FPG-NA | [165] |
| USA Met+ (n = 19) Met− (n = 11) Age Met+ (58.2 ± 4.5) Met− (57.5 ± 6.6) | Phylum: Firmicutes ↑ Genus: Parabacteroides ↑, Catenibacterium ↑ Species: Bifidobacterium ↑ | NA HbA1c ↓ FPG ↓ | [166] |
| Chinese Met+ (n = 22) Met− (n = 22) | Genus: Bacteroides ↓ Species: Bacteroides intestinalis ↓, Bacteroides dorei ↓, Bacteroides fragilis ↓, Bacteroides caccae ↓ | GUDCA ↑, Conjugated Secondary bile acids ↑, HbA1c-NA FPG-NA | [167] |
| Population | Gut Microbiome | Biochemical and HbA1c; FPG Alterations | References |
|---|---|---|---|
| Swedish T2DM (n = 53) ND (n = 43) Age T2DM (70.5 ± 0.1) ND (70.3 ± 0.1) | Genus: Clostridium ↓ Species: Clostridium botulinum ↓, Clostridium baijerinckii ↓, Roseburia ↓, Eubacterium eligens ↓, Lactobacillus↑, Lactobacillus gasseri ↑, Streptococcus mutans ↑ | C-peptide ↑ HbA1c-NA FPG-NA | [165] |
| Colombian T2DM (n = 14) ND (n = 84) Sex (M/F) T2DM (n = 9/5) ND (n = 48/36) Age T2DM (50 ± 10) ND (47 ± 9) | Order: Clostridiales ↓ Phylum: Firmicutes↑, Actinobacteria↑ Genus: Prevotella↑, Bacteroides↑, Oscillospira ↓, Butyrivibrio↑, Megasphaera↑ Family: Veillonellaceae↑ Species: Bifidobacterium bifidum ↑, Clostridium celatum ↓ Class: Mollicutes↑ | NA HbA1c ↑ FPG ↑ | [57] |
| Mexican T2DM (n = 14) ND (n = 76) Sex (M/F) T2DM (n = 2/12) ND (n = 26/50) Age T2DM (48.1 ± 4.6) ND (48 ± 5.4) | Phylum: Bacteroidetes↑, Proteobacteria↑ Family: Alcaligenaceae ↑ | NA HbA1c ↑ FPG ↑ | [58] |
| Japanese T2DM (n = 17) ND (n = 50) Age T2DM (62.5 ± 10.8) ND (60.2 ± 12.9) | Genus: Prevotella ↓, Lactobacillus ↑ Species: Clostridium coccoides ↓, Lactobacillus plantarum ↑, Lactobacillus reuteri ↑, Atopobium ↓ | Acetic acid ↓, Propionic acid ↓, Fecal organic acids ↓, HbA1c ↑ FPG ↑ | [164] |
| Chinese T2DM (n = 51) ND (n = 26) Sex (M/F) T2DM (n = 28/23) ND (n = 14/12) Age T2DM (58.1 ± 9.4) ND (56.4 ± 10.6) | Phylum: Actinobacteria ↓ Family: Turicibacteraceae ↑, Enterobacteriaceae ↓, Spirochaetaceae ↑ Genus: Fusobacterium ↑, Turicibacter ↑ | NA HbA1c ↑ FPG-NA | [168] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymczak-Pajor, I.; Drzewoski, J.; Kozłowska, M.; Krekora, J.; Śliwińska, A. The Gut Microbiota-Related Antihyperglycemic Effect of Metformin. Pharmaceuticals 2025, 18, 55. https://doi.org/10.3390/ph18010055
Szymczak-Pajor I, Drzewoski J, Kozłowska M, Krekora J, Śliwińska A. The Gut Microbiota-Related Antihyperglycemic Effect of Metformin. Pharmaceuticals. 2025; 18(1):55. https://doi.org/10.3390/ph18010055
Chicago/Turabian StyleSzymczak-Pajor, Izabela, Józef Drzewoski, Małgorzata Kozłowska, Jan Krekora, and Agnieszka Śliwińska. 2025. "The Gut Microbiota-Related Antihyperglycemic Effect of Metformin" Pharmaceuticals 18, no. 1: 55. https://doi.org/10.3390/ph18010055
APA StyleSzymczak-Pajor, I., Drzewoski, J., Kozłowska, M., Krekora, J., & Śliwińska, A. (2025). The Gut Microbiota-Related Antihyperglycemic Effect of Metformin. Pharmaceuticals, 18(1), 55. https://doi.org/10.3390/ph18010055






