Current Insight of Peptide-Based Hydrogels for Chronic Wound Healing Applications: A Concise Review
Abstract
1. Introduction
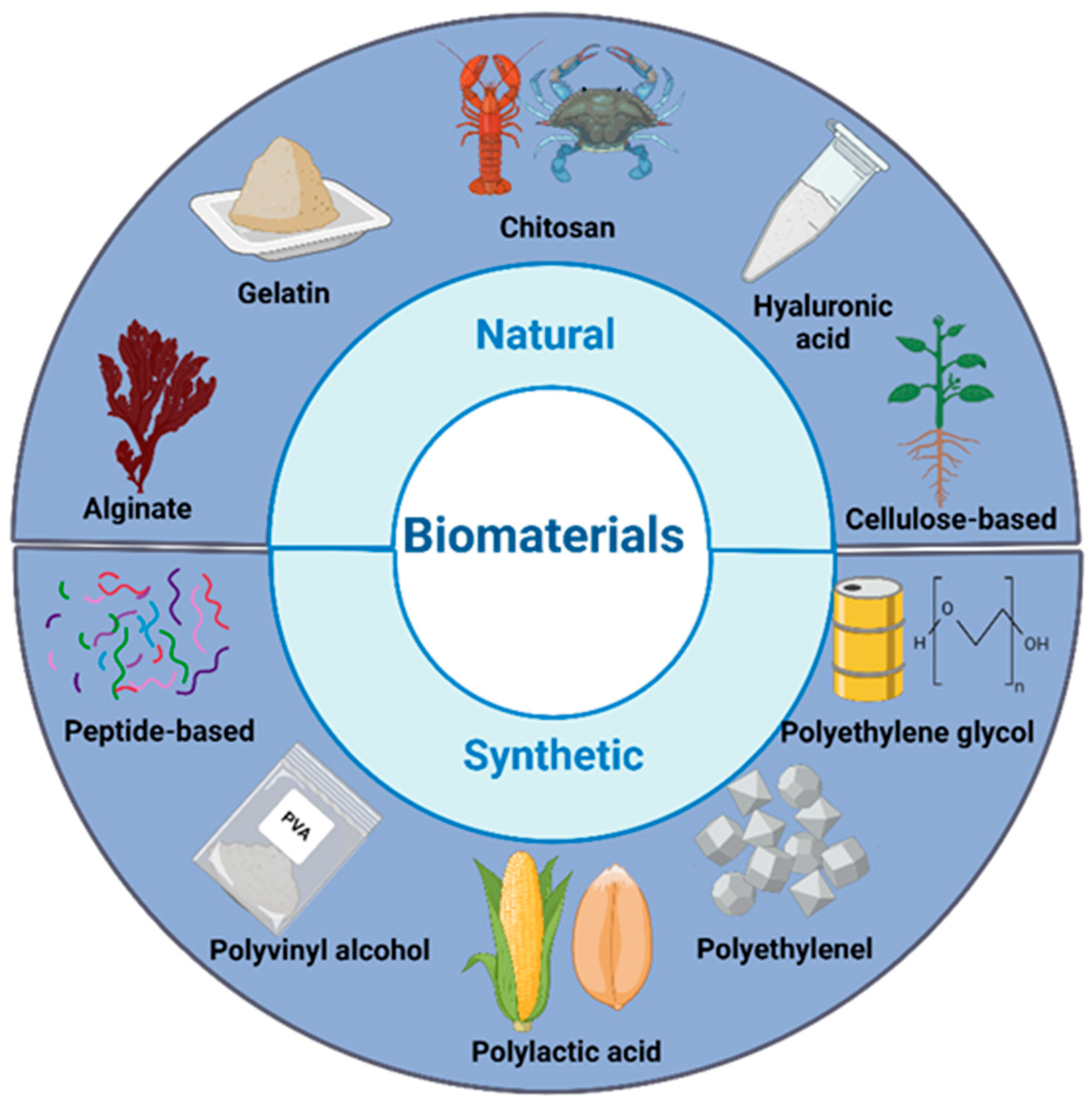
2. Peptide-Based Hydrogels for Chronic Wound Healing
2.1. Peptide Selection: Criteria for Selecting Peptides, Including Sequence Specificity, Functional Motifs, and Bioactivity
Peptide Sequence Specificity
2.2. Self-Assembled Peptide Hydrogels
2.3. Peptide-Based Hydrogels for Wound Healing
| No | Type of Peptide | Name of Peptide | Role in Wound Healing | Reference |
|---|---|---|---|---|
| 1 | AMPs | Pep4 (KRCCPDTCGIKCL) and Pep4M (KRMMPDTMGIKML) | Serve as ideal wound dressing creates a moist environment, prevents infections, absorbs excess fluid, reduces necrosis, and keeps the wound from drying out. | [80] |
| 2 | SAP | RADA16-I- peptide | Provide nanofiber network forms like the extracellular matrix (ECM), providing a good environment for cell growth. | [79,84] |
| 3 | SAP | L-lysine-containing peptides | Provide a strong foundation to speed up burn wound healing in rats by keeping the wound properly hydrated. | [79] |
| 4 | Peptide incorporated into a polymer matrix | Synthetic peptide, ACT1 | Reduce the ulcer size significantly, healed faster the lesion, and the outer layer fully regrew without any negative effects or immune reactions. | [86] |
3. Physicochemical and Mechanical Properties of Peptide-Based Hydrogels
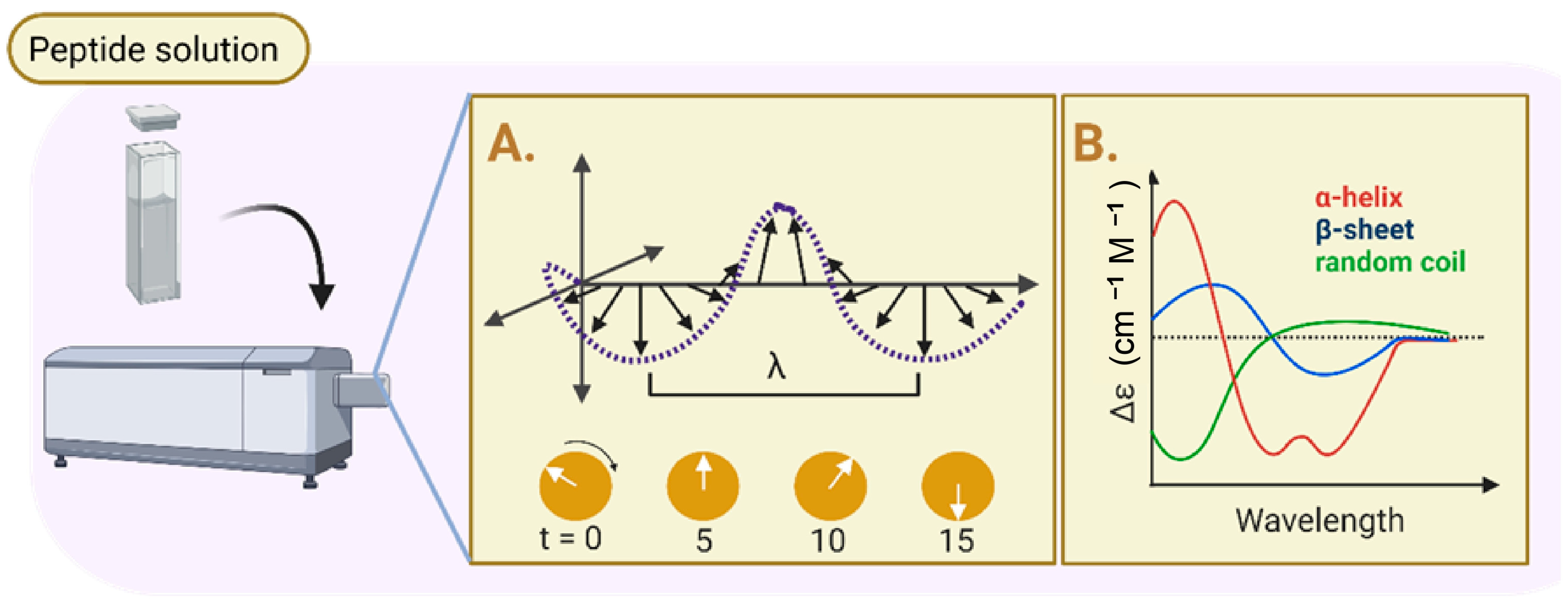
3.1. Physicochemical Properties: Evaluation of Physicochemical Properties of Peptide-Based Hydrogels
| No | Parameters | Biomaterials Used | Result | Conclusion | References |
|---|---|---|---|---|---|
| 1 | FTIR spectroscopy SEM analysis TEM analysis CD analysis Rheology properties | Two synthetic peptide sequence Ac FKFEFKFE-QHREDGS-NH2 (F−Q) and Ac-FKFEFKFE-GRGDS-NH2 (F−G) | FTIR: The presence of absorption peaks in amide I at 1625 cm−1 was higher compared to amide II at 1550 cm−1. SEM and TEM: Every group of peptide hydrogel has a porous, fibrous network structure. Peptide nanofibers made of F-Q, F-G, and EFK8 have the potential to create an intricate network structure with interconnections. CD: The peptide solution exhibited a positive absorption band at 191 nm and a clearly visible negative absorption band at 205 nm. Rheology: The hydrogels were all capable of withstanding the external shear force. Nevertheless, the G′ and G″ values of those four samples did not significantly differ from one another. | These peptide hydrogels have a structure similar to a network of nanofibers with great viscoelasticity. The amide I and amide II areas exhibit a high-frequency absorption band, which suggests that self-assembling nanofibers with parallel β-sheet topologies may potentially develop. | [96] |
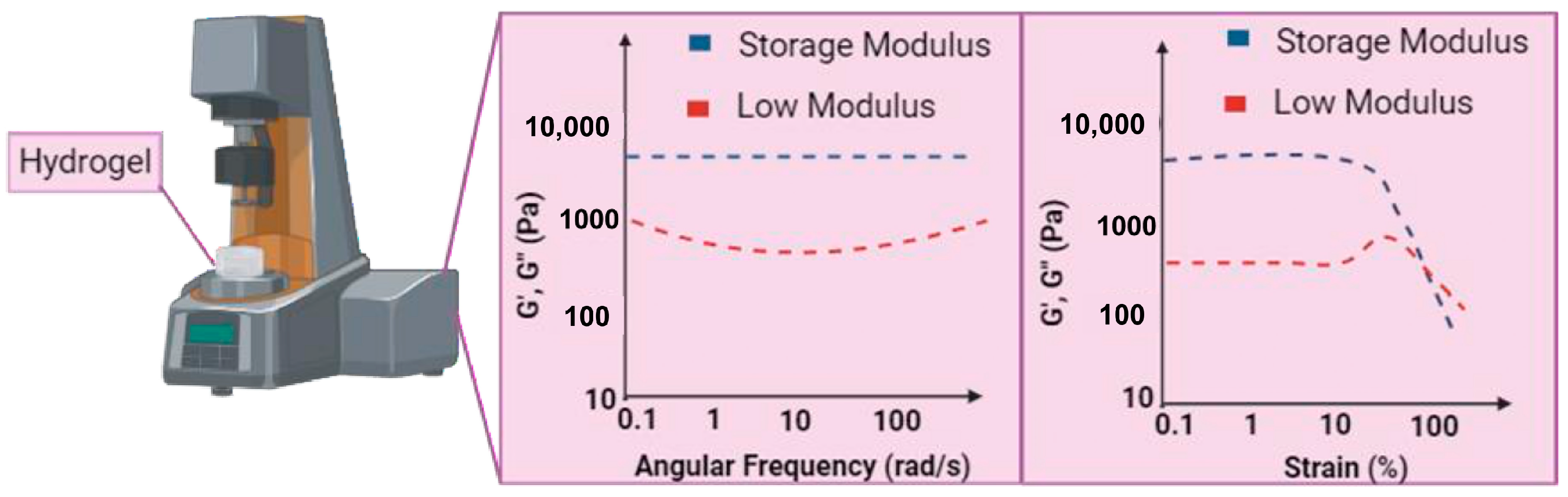
| 2 | FTIR spectroscopy SEM analysis Biodegradability analysis Rheology properties | Lauric acid-peptide conjugate gel, LA-LLys-DPhe-LLys-NH2, loaded with yttrium oxide (Y2O3) nanoparticles (NLG) | FTIR: The amide I peak was detected at 1642 cm−1, which corresponded to stretching vibrations and was attributed to C=O. The amide II peak was identified at 543 cm−1, which corresponded to N−H, and the amide III peak was in the region of 1229−1301 cm−1, which was associated with N−H bending linked to C−N stretching. SEM: The hydrogel based on the peptides NPG and LPG; the SEM pictures showed a nanofibrous structure. Biodegradability: A high rate of degradation in LPG (~62%) over NLG (~45%) in alkaline and comparing pH 8.4 (~45%) to pH 7 (~61%), NLG breakdown less slowly. Rheology: The linear viscoelastic range of peptide conjugate gel was observed at 24.8% while 82.5% for the NP-loaded gel when the range was up to 1% and the crossover point. | Positive charges on peptide chains reduce basicity, which decreases repulsion between peptide chains and improves gel stability. The NPG peptide-based hydrogel has high storage modulus (about 4 KPa) and a notable increase in crossover points. | [92] |
| 3 | SEM analysis TEM analysis HPLC analysis Rheology properties | Amyloid fibril co-assembled peptide (AFCP) | SEM and TEM: The structure of regular peptide hydrogel is mimicked by AFCP hydrogels, which produce a net-like meshwork of fibrils that are wired nanonets. HPLC: The molecular weights are accurate, and they possess more than 95% purity. Rheology: After being crosslinked with Ca2+ and fibrinogen, the rheological stiffness of AFCP hydrogel was shown to be more than ten times enhanced. | The peptide hydrogels have a fibrous network forming inside their structure. The ACFP hydrogel has excellent mechanical properties together with distinct nanonet structures. | [90] |
| 4 | FTIR spectroscopy SEM analysis Swelling study Biodegradability analysis | Chitosan/sodium alginate/velvet antler blood peptides (CS/SA/VBPs) hydrogel (CAVBPH), CS/β-GP/SA/Ca2+ (CAH) | FTIR: The infrared spectrum of CAVBPH revealed clear VBP absorption bands at 1582 cm−1, 1404 cm−1, and 1111 cm−1, indicating that the hydrogel has been loaded with VBPs. SEM: Microscopic analysis revealed that all hydrogels had a highly porous structure, and the addition of VBPs had no effect on the porosity structure. Swelling: The swelling ratios of more than 200% were attained in less than an hour by CAVBPH and CAH due to their rapid water absorption. Biodegradability: As the incubation period increased, the mass residue of the hydrogels gradually decreased; on day 14, the residual weights of CAVBPH and CAH fell from 100% to 59.26% and 56.85%, respectively. | The hydrophilicity and interconnected porous structure of CAVBPH and CAH contribute to their good swelling ratio, making them essential for wound dressing. The diffusion coefficient of VBPs may be improved by the low molecular weight of short-chain peptides and the high porosity of CAVBPH. | [91] |
| 5 | FTIR spectroscopy SEM analysis TEM analysis Swelling study | Pro-healing peptide (RL-QN15) loaded into hollow silica nanoparticles (HSNs), zinc alginate (ZA) | FTIR: Regardless of the hydrophobic interaction between HSN and RL-QN15, the HSN@RL-QN15 nanocomposites showed lower wave measurements at both positions while the ZA, HSN@RL-QN15/ZA, and Sac spectra did not differ significantly as the crosslinking of zinc ions. SEM: The HSNs in the HSN@RL-QN15 nanocomposites consisted an outer shell and a hollow core, containing RL-QN15. The ZA hydrogels have a sponge-like surface structure and are extremely porous, with an average pore size of 18.3 μm. TEM: The transparency of the HSN@RL-QN15 nanocomposites was lower compared to the HSNs alone. Swelling: The ZA hydrogel had favorable swelling characteristics, exhibiting an equilibrium swelling rate above 1400%. The equilibrium swelling rate of ZA remained unchanged upon the addition of RL-QN15 and HSN@RL-QN15. | For microscopic view, the reduced transparency of HSN@RL-QN15 implies that RL-QN15 is present in the HSNs. The evenly sized nano-composites incorporated in the hybrid hydrogel were the cause of its rough surface. Water is absorbed by the hydrophilic groups in hydrogels, and the micropore structure permits unrestricted water flow in and out. This causes the hydrogels to swell. | [93] |
| 6 | SEM analysis TEM analysis CD analysis HPLC analysis Rheology properties | Self-assembling peptide namely KGH | SEM and TEM: After gelation, the newly formed KGH hydrogel possessed a 3D nanofiber network structure. CD: The KGH peptide exhibited a typical β-sheet secondary structure, with a positive peak at 191 nm and a negative peak about 216 nm. HPLC: The KGH peptide possessed a molecular weight of 1719.02 Da and a purity of more than 95%. Rheology: G′ was greater than G″ for the rheological property, and both G′ and G″ in the KGH solution had low values. | The KGH hydrogel has low elasticity and low viscosity, as demonstrated by its low loss and storage modulus (G′ and G″). | [94] |
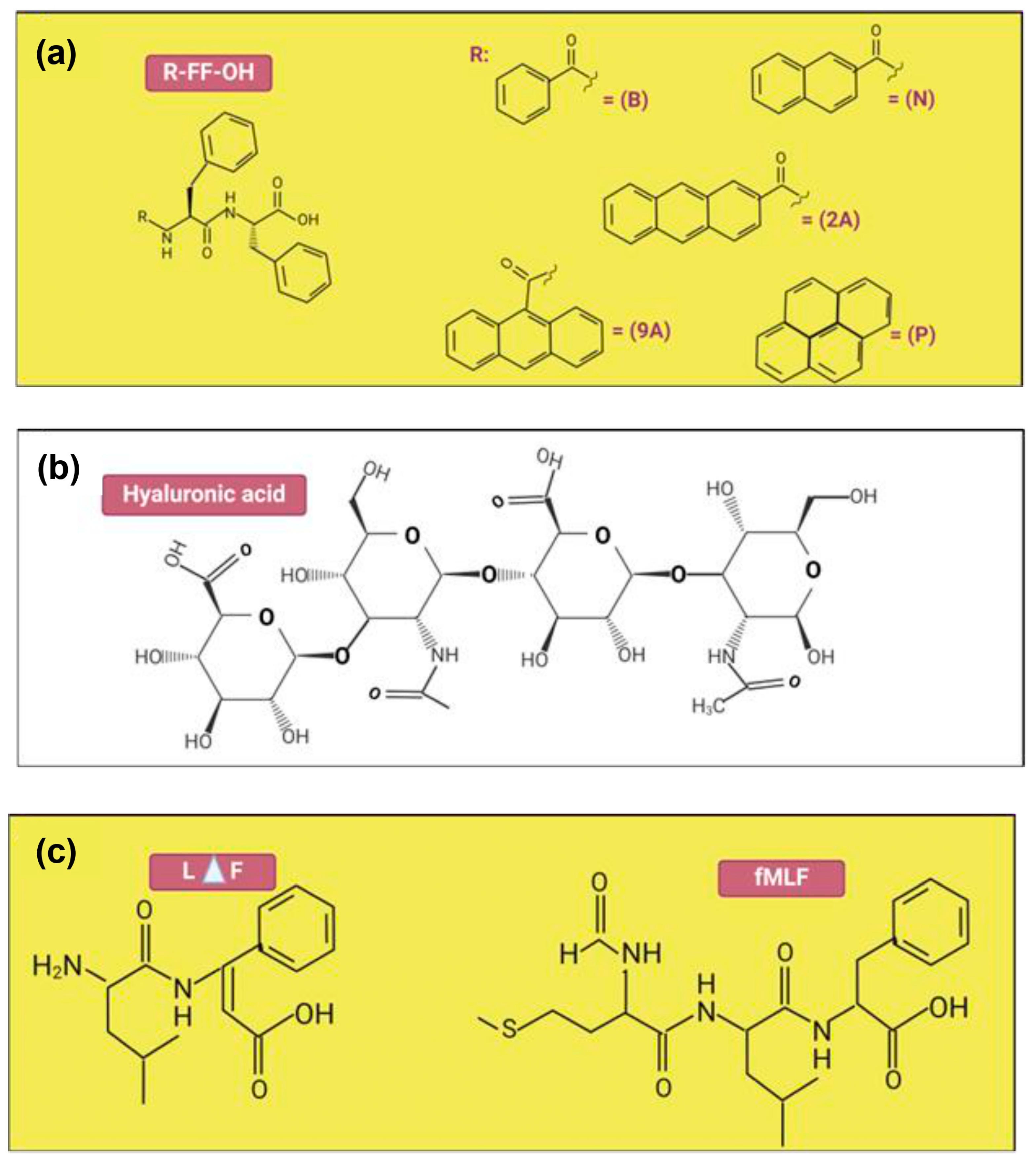
| 7 | SEM analysis TEM analysis Rheology properties | Hyaluronic acid (HA), Diphenylalanine (FF) | SEM: B-FF hydrogel exhibited smooth silky fibers, while the composite hydrogels had clusters of block mass. The SEM pictures of the N-FF hydrogel revealed flaky form structures, whereas the N-FF/HA gels featured microporous structures. TEM: The fibrous networks inside composite hydrogels were physically crosslinked by entangled soft nanofibers which was similar to the peptide hydrogels alone. Rheology: N-FF hydrogels had substantially greater G′ than B-FF and P-FF, while G′ of N-FF/HA and B-FF/HA increased slightly. | The good mechanical properties were obtained for all composite hydrogels. Nevertheless, the N-FF-based hydrogels exhibited significantly better mechanical properties at low concentrations compared to others. | [97] |
| 8 | SEM analysis FTIR analysis CD analysis Rheology properties | Self-assembling peptide namely EAK16 and RADA16 | SEM: Thicker fibers with a few spindle-shaped beads develop when the SAP hydrogel concentration is further increased (20–30% w/v). FTIR: The crosslinked SAPs of FT-IR spectra in the amide I and amide II regions feature three prominent peaks located at 1695, 1630, and 1540 cm−1. CD: The CD spectra of β-forming SAP were expanded using a 7-residue functional motif and a variable-length Gly spacer. Rheology: If SAP hydrogels were crosslinked instead of non-crosslinked, their stiffness would increase from 0.1 to 100 KP. | SEM picture of the crosslinked SAP shows lengthy, tangled nanofibers that are aligned. Both conventional and crosslinked SAPs exhibit cross-β structuration, as confirmed by the results of their FT-IR spectra. | [87] |
| 9 | SEM analysis TEM analysis CD analysis Rheology properties | Self-assembling peptide namely N-formyl-methionyl-leucyl-phenylalanine | SEM: The hydrogels in every group exhibit dense networks of bundles of nanofibers in their ultrastructure. Qualitatively, it appears that the thickness of bundle increases as the fMLF peptide concentration increases. TEM: The presence of fMLF causes the nanofibers that make up the LΔF hydrogel to become thicker. CD: The increases of fMLF content in the mixtures, there were a slight decrease in intensity of the minima at 275 nm. Rheology: LΔF alone has a G′ value of 132 kPa, while LΔF/fMLF (1:1) has a G′ value of 189 kPa which these show that both co-assembled hydrogels are classified as soft hydrogels. | Intermolecular interactions might be the factor responsible for the apparent rise in nanofiber diameter. An increase in the concentration of fMLF content does not result in any apparent secondary structural changes. | [88] |
| 10 | SEM analysis TEM analysis CD analysis Biodegradability analysis Rheology properties | Self-assembling peptide namely RADA16-GGQQLK (QLK) and RADA16-GGLRKKLGKA (LRK) | SEM: More densely packed nanofibrous entanglements and more continuous self-assembling fibers appeared with the QLK peptide post mTG crosslinking. TEM: Similar to natural ECM fibers, the interwoven nanofibers had diameters of of 10–20 nm and pore sizes of 10–200 nm. CD: The β-sheet secondary structure is exhibited by three experimental groups: QLK peptide, QLK peptide with enzymatic crosslinking and QLK peptide combined with LRK peptide and HS. Biodegradability: After 35 days, both groups displayed degardation, with a total degradation of roughly 61% (crosslinked) and 82% (non-crosslinked). Rheology: QLK hydrogel showed a rise in its storage modulus (G′) from 1000 Pa to 5000 Pa, whereas QLK/LRK hydrogel had an increase in G′ from 400 Pa to 2500 Pa. | The mechanical properties of hydrogels were likewise impacted by the transglutaminase: as the concentration of mTG increased, the storage modulus (G′) of hydrogel increased as well. Comparing the crosslinking group to the non-crosslinking group, the crosslinking group’s hydrogel disintegrate rate was slower. | [89] |
3.2. Mechanical Test: Evaluation of Mechanical Properties of Peptide-Based Hydrogels
4. Biocompatibility and Cell Interaction of Peptide-Based Hydrogels Against Cells
Peptide-Based Hydrogels in In Vivo Model Studies
5. Applications of Peptide-Based Hydrogels in Chronic Wound Healing
6. Challenges, Limitations, and Future Perspective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Man, E.; Hoskins, C. Towards advanced wound regeneration. Eur. J. Pharm. Sci. 2020, 149, 105360. [Google Scholar] [CrossRef]
- Rittié, L. Cellular mechanisms of skin repair in humans and other mammals. J. Cell Commun. Signal. 2016, 10, 103–120. [Google Scholar] [CrossRef]
- Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and Impact of Antioxidant Hydrogel in Chronic Wound Healing. Adv. Healthc. Mater. 2020, 9, e1901502. [Google Scholar] [CrossRef] [PubMed]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of acute and chronic wound healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Fadilah, N.I.M.; Phang, S.J.; Kamaruzaman, N.; Salleh, A.; Zawani, M.; Sanyal, A.; Maarof, M.; Fauzi, M.B. Antioxidant Biomaterials in Cutaneous Wound Healing and Tissue Regeneration: A Critical Review. Antioxidants 2023, 12, 787. [Google Scholar] [CrossRef] [PubMed]
- Malone-Povolny, M.J.; Maloney, S.E.; Schoenfisch, M.H. Nitric Oxide Therapy for Diabetic Wound Healing. Adv. Healthc. Mater. 2019, 8, e1801210. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 342, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Flynn, K.; Mahmoud, N.N.; Sharifi, S.; Gould, L.J.; Mahmoudi, M. Chronic Wound Healing Models. ACS Pharmacol. Transl. Sci. 2023, 6, 783–801. [Google Scholar] [CrossRef]
- Andreassi, A.; Bilenchi, R.; Biagioli, M.; D’Aniello, C. Classification and pathophysiology of skin grafts. Clin. Dermatol. 2005, 23, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, F.; Lineaweaver, W.C. Clinical Applications of Allograft Skin in Burn Care. Ann. Plast. Surg. 2020, 84, S158–S160. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.; Soleimani, M.; Rouhani, M.; Noorkhajavi, G.; Aghaei-Zarch, S.M.; Hasannejad-Asl, B.; Bagheri-Mohammadi, S.; Ebrahimi, M.; Keshel, S.H. Xenograft-based skin substitutes: A critical review. J. Drug Deliv. Sci. Technol. 2024, 95, 105613. [Google Scholar] [CrossRef]
- Jaller, J.A.; Herskovitz, I.; Borda, L.J.; Mervis, J.; Darwin, E.; Hirt, P.A.; Lev-Tov, H.; Kirsner, R.S. Evaluation of Donor Site Pain after Fractional Autologous Full-Thickness Skin Grafting. Adv. Wound Care 2018, 7, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Tassiopoulos, A.; Kirsner, R.S. Evaluation and Management of Lower-Extremity Ulcers. N. Engl. J. Med. 2017, 377, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Rowan, M.P.; Cancio, L.C.; Elster, E.A.; Burmeister, D.M.; Rose, L.F.; Natesan, S.; Chan, R.K.; Christy, R.J.; Chung, K.K. Burn wound healing and treatment: Review and advancements. Crit. Care 2015, 19, 243. [Google Scholar] [CrossRef]
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Ramasubbu, D.A.; Smith, V.; Hayden, F.; Cronin, P. Systemic antibiotics for treating malignant wounds. Cochrane Database Syst. Rev. 2017, 2017, CD011609. [Google Scholar] [CrossRef]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern wound dressings: Hydrogel dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef]
- Barillo, D.J.; Barillo, A.R.; Korn, S.; Lam, K.; Attar, P.S. The antimicrobial spectrum of Xeroform®. Burns 2017, 43, 1189–1194. [Google Scholar] [CrossRef]
- Fadilah, N.I.M.; Riha, S.M.; Mazlan, Z.; Wen, A.P.Y.; Hao, L.Q.; Joseph, B.; Maarof, M.; Thomas, S.; Motta, A.; Fauzi, M.B. Functionalised-biomatrix for wound healing and cutaneous regeneration: Future impactful medical products in clinical translation and precision medicine. Front. Bioeng. Biotechnol. 2023, 11, 1160577. [Google Scholar] [CrossRef]
- Kumar, A.; Jaiswal, M. Design and in vitro investigation of nanocomposite hydrogel based in situ spray dressing for chronic wounds and synthesis of silver nanoparticles using green chemistry. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced hydrogels as wound dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Bilici, C.; Can, V.; Nöchel, U.; Behl, M.; Lendlein, A.; Okay, O. Melt-processable shape-memory hydrogels with self-healing ability of high mechanical strength. Macromolecules 2016, 49, 7442–7449. [Google Scholar] [CrossRef]
- Md Fadilah, N.I.; Khairul Nizam, N.A.A.; Fauzi, M.B. Antibacterial compounds-incorporated functional biomaterials for chronic wound healing application via 3D bioprinting: The mechanism of action. Int. J. Bioprinting 2024, 10, 3372. [Google Scholar] [CrossRef]
- Akbar, A.R.; Su, S.; Amjad, B.; Cai, Y.; Lin, L. Effect of Bamboo Viscose on the Wicking and Moisture Management Properties of Gauze. IOP Conf. Ser. Mater. Sci. Eng. 2018, 275, 012042. [Google Scholar] [CrossRef]
- Fadilah, N.I.M.; Ahmat, N.; Hao, L.Q.; Maarof, M.; Rajab, N.F.; Idrus, R.B.H.; Fauzi, M.B. Biological Safety Assessments of High-Purified Ovine Collagen Type I Biomatrix for Future Therapeutic Product: International Organisation for Standardisation (ISO) and Good Laboratory Practice (GLP) Settings. Polymers 2023, 15, 2436. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and properties of gelatin methacryloyl (GelMA) hydrogels and their recent applications in load-bearing tissue. Polymers 2018, 10, 1290. [Google Scholar] [CrossRef] [PubMed]
- Firlar, I.; Altunbek, M.; McCarthy, C.; Ramalingam, M.; Camci-Unal, G. Functional Hydrogels for Treatment of Chronic Wounds. Gels 2022, 8, 127. [Google Scholar] [CrossRef]
- Ko, J.H.; Yin, H.; An, J.; Chung, D.J.; Kim, J.H.; Lee, S.B.; Pyun, D.G. Characterization of cross-linked gelatin nanofibers through electrospinning. Macromol. Res. 2010, 18, 137–143. [Google Scholar] [CrossRef]
- Vhora, I.; Patil, S.; Bhatt, P.; Misra, A. Protein– and Peptide–Drug Conjugates: An Emerging Drug Delivery Technology, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; Volume 98. [Google Scholar]
- Dias, J.R.; Baptista-Silva, S.; de Oliveira, C.M.T.; Sousa, A.; Oliveira, A.L.; Bártolo, P.J.; Granja, P.L. In situ crosslinked electrospun gelatin nanofibers for skin regeneration. Eur. Polym. J. 2017, 95, 161–173. [Google Scholar] [CrossRef]
- Ndlovu, S.P.; Ngece, K.; Alven, S.; Aderibigbe, B.A. Gelatin-based hybrid scaffolds: Promising wound dressings. Polymers 2021, 13, 2959. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Kiick, K.L. Hybrid multicomponent hydrogels for tissue engineering. Macromol. Biosci. 2009, 9, 140–156. [Google Scholar] [CrossRef]
- Sohail, M.; Mudassir; Minhas, M.U.; Khan, S.; Hussain, Z.; de Matas, M.; Shah, S.A.; Khan, S.; Kousar, M.; Ullah, K. Natural and synthetic polymer-based smart biomaterials for management of ulcerative colitis: A review of recent developments and future prospects. Drug Deliv. Transl. Res. 2019, 9, 595–614. [Google Scholar] [CrossRef] [PubMed]
- Fadilah, N.I.M.; Maarof, M.; Motta, A.; Tabata, Y.; Fauzi, M.B. The Discovery and Development of Natural-Based Biomaterials with Demonstrated Wound Healing Properties: A Reliable Approach in Clinical Trials. Biomedicines 2022, 10, 2226. [Google Scholar] [CrossRef]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Tudoroiu, E.E.; Dinu-Pîrvu, C.E.; Kaya, M.G.A.; Popa, L.; Anuța, V.; Prisada, R.M.; Ghica, M.V. An overview of cellulose derivatives-based dressings for wound-healing management. Pharmaceuticals 2021, 14, 1215. [Google Scholar] [CrossRef] [PubMed]
- Abazari, M.F.; Gholizadeh, S.; Karizi, S.Z.; Birgani, N.H.; Abazari, D.; Paknia, S.; Derakhshankhah, H.; Allahyari, Z.; Amini, S.M.; Hamidi, M.; et al. Recent advances in cellulose-based structures as the wound-healing biomaterials: A clinically oriented review. Appl. Sci. 2021, 11, 7769. [Google Scholar] [CrossRef]
- Aderibigbe, B.A.; Buyana, B. Alginate in wound dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-based composite materials for wound dressing application:A mini review. Carbohydr. Polym. 2020, 236, 116025. [Google Scholar] [CrossRef]
- Barnett, S.E.; Varley, S.J. The effects of calcium alginate on wound healing. Ann. R. Coll. Surg. Engl. 1987, 69, 153–155. [Google Scholar]
- Yang, J.S.; Xie, Y.J.; He, W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Price, R.D.; Myers, S.; Leigh, I.M.; Navsaria, H.A. The Role of Hyaluronic Acid in Wound Healing. Am. J. Clin. Dermatol. 2005, 6, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Castrejón-Comas, V.; Alemán, C.; Pérez-Madrigal, M.M. Multifunctional conductive hyaluronic acid hydrogels for wound care and skin regeneration. Biomater. Sci. 2023, 11, 2266–2276. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, Y.; Xu, Y.; Wang, J.; Yu, Y. Modification and crosslinking strategies for hyaluronic acid-based hydrogel biomaterials. Smart Med. 2023, 2, e20230029. [Google Scholar] [CrossRef] [PubMed]
- Pepe, A.; Laezza, A.; Armiento, F.; Bochicchio, B. Chemical Modifications in Hyaluronic Acid-Based Electrospun Scaffolds. Chempluschem 2024, 89, e202300599. [Google Scholar] [CrossRef]
- Salleh, A.; Mustafa, N.; Teow, Y.H.; Fatimah, M.N.; Khairudin, F.A.; Ahmad, I.; Fauzi, M.B. Dual-Layered Approach of Ovine Collagen-Gelatin/Cellulose Hybrid Biomatrix Containing Graphene Oxide-Silver Nanoparticles for Cutaneous Wound Healing: Fabrication, Physicochemical, Cytotoxicity and Antibacterial Characterisation. Biomedicines 2022, 10, 816. [Google Scholar] [CrossRef]
- Jridi, M.; Bardaa, S.; Moalla, D.; Rebaii, T.; Souissi, N.; Sahnoun, Z.; Nasri, M. Microstructure, rheological and wound healing properties of collagen-based gel from cuttlefish skin. Int. J. Biol. Macromol. 2015, 77, 369–374. [Google Scholar] [CrossRef]
- Haghi, A.K.; Oluwafemi, O.S.; Jose, J.P.; Maria, H.J. Composites and Nanocomposites; Apple Academic Press: New York, NY, USA, 2013; ISBN 9781466568761. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef]
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of polymer hydrogels and nanoparticulate systems for biomedical and pharmaceutical applications. Nanomaterials 2015, 5, 2054–2130. [Google Scholar] [CrossRef]
- Bahati, D.; Bricha, M.; El Mabrouk, K. Synthesis, characterization, and in vitro apatite formation of strontium-doped sol-gel-derived bioactive glass nanoparticles for bone regeneration applications. Ceram. Int. 2023, 49, 23020–23034. [Google Scholar] [CrossRef]
- Chelu, M.; Musuc, A.M. Advanced Biomedical Applications of Multifunctional Natural and Synthetic Biomaterials. Processes 2023, 11, 2696. [Google Scholar] [CrossRef]
- Md Fadilah, N.I.; Rahman, M.B.A.; Yusof, L.M.; Mustapha, N.M.; Ahmad, H. The therapeutic effect and in vivo assessment of palmitoyl-gdph on the wound healing process. Pharmaceutics 2021, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wu, C.; Zhang, X.; Bian, W.; Liu, N.; Yin, S.; Yang, M.F.; Luo, M.; Tang, J.; Yang, X. A short peptide potentially promotes the healing of skin wound. Biosci. Rep. 2019, 39, BSR20181734. [Google Scholar] [CrossRef] [PubMed]
- Amantana, A.; Moulton, H.M.; Cate, M.L.; Reddy, M.T.; Whitehead, T.; Hassinger, J.N.; Youngblood, D.S.; Iversen, P.L. Pharmacokinetics, biodistribution, stability and toxicity of a cell-penetrating peptide—Morpholino oligomer conjugate. Bioconjug. Chem. 2007, 18, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Mondal, J.H.; Ahmed, S.; Das, D. Physicochemical analysis of mixed micelles of a viologen surfactant: Extended to water-in-oil (w/o) microemulsion and cucurbit[8]uril-assisted vesicle formation. Langmuir 2014, 30, 8290–8299. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Das, D. Rational Design of Peptide-based Smart Hydrogels for Therapeutic Applications. Front. Chem. 2021, 9, 770102. [Google Scholar] [CrossRef]
- Aggeli, A. Engineering of peptide β-sheet nanotapes. J. Mater. Chem. 1997, 7, 1135–1145. [Google Scholar] [CrossRef]
- Estroff, L.A.; Hamilton, A.D. Water gelation by small organic molecules. Chem. Rev. 2004, 104, 1201–1217. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Li, J.; Chen, C.; Liu, Y. Self-Assembling Peptide-Based Hydrogels for Wound Tissue Repair. Adv. Sci. 2022, 9, e2104165. [Google Scholar] [CrossRef]
- Fadilah, N.I.M.; Ahmad, H.; Abdul Rahman, M.B.; Chia, S.L.; Ng, S.F.; Leong, S.W. Synthesis and in vitro biological evaluations of novel tetrapeptide as therapeutic agent for wound treatment. J. Saudi Chem. Soc. 2020, 24, 606–619. [Google Scholar] [CrossRef]
- Edwards-Gayle, C.J.C.; Hamley, I.W. Self-assembly of bioactive peptides, peptide conjugates, and peptide mimetic materials. Org. Biomol. Chem. 2017, 15, 5867–5876. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Chen, W.T.; Sun, T.; Gao, Y.; Li, L.L.; Wang, H. Recent advances: Peptides and self-assembled peptide-nanosystems for antimicrobial therapy and diagnosis. Biomater. Sci. 2020, 8, 4975–4996. [Google Scholar] [CrossRef]
- Nur Izzah, M.F.; Ahmad, H.; Rahman, M.F.A.; Rahman, N.A. Electrospun poly (vinyl alcohol) nanofibers doped with mesoporous silica nanoparticles for controlled release of hydrophilic model drug. Malaysian J. Anal. Sci. 2019, 23, 212–218. [Google Scholar] [CrossRef]
- Silva, G.A.; Czeisler, C.; Niece, K.L.; Beniash, E.; Harrington, D.A.; Kessler, J.A.; Stupp, S.I. Selective Differentiation of Neural Progenitor Cells by High-Epitope Density Nanofibers. Science 2004, 303, 1352–1355. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Tao, S.; Wang, Q.; Ma, P.Q.; Li, Z.B.; Wu, Y.L.; Li, D.W. Research advances in smart responsive-hydrogel dressings with potential clinical diabetic wound healing properties. Mil. Med. Res. 2023, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Stefanov, I.; Pérez-Rafael, S.; Hoyo, J.; Cailloux, J.; Santana Pérez, O.O.; Hinojosa-Caballero, D.; Tzanov, T. Multifunctional Enzymatically Generated Hydrogels for Chronic Wound Application. Biomacromolecules 2017, 18, 1544–1555. [Google Scholar] [CrossRef]
- Gavel, P.K.; Parmar, H.S.; Tripathi, V.; Kumar, N.; Biswas, A.; Das, A.K. Investigations of Anti-Inflammatory Activity of a Peptide-Based Hydrogel Using Rat Air Pouch Model. ACS Appl. Mater. Interfaces 2019, 11, 2849–2859. [Google Scholar] [CrossRef]
- Gavel, P.K.; Dev, D.; Parmar, H.S.; Bhasin, S.; Das, A.K. Investigations of Peptide-Based Biocompatible Injectable Shape-Memory Hydrogels: Differential Biological Effects on Bacterial and Human Blood Cells. ACS Appl. Mater. Interfaces 2018, 10, 10729–10740. [Google Scholar] [CrossRef] [PubMed]
- Acar, H.; Srivastava, S.; Chung, E.J.; Schnorenberg, M.R.; Barrett, J.C.; LaBelle, J.L.; Tirrell, M. Self-assembling peptide-based building blocks in medical applications. Adv. Drug Deliv. Rev. 2017, 110–111, 65–79. [Google Scholar] [CrossRef]
- Habibi, N.; Kamaly, N.; Memic, A.; Shafiee, H. Self-assembled peptide-based nanostructures: Smart nanomaterials toward targeted drug delivery. Nano Today 2016, 11, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Stephanopoulos, N.; Ortony, J.H.; Stupp, S.I. Self-assembly for the synthesis of functional biomaterials. Acta Mater. 2013, 61, 912–930. [Google Scholar] [CrossRef] [PubMed]
- Gavel, P.K.; Kumar, N.; Parmar, H.S.; Das, A.K. Evaluation of a Peptide-Based Coassembled Nanofibrous and Thixotropic Hydrogel for Dermal Wound Healing. ACS Appl. Bio Mater. 2020, 3, 3326–3336. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, S.; Guerin, T.; Toth, I.; Stephenson, R.J. Recent advances in self-assembled peptides: Implications for targeted drug delivery and vaccine engineering. Adv. Drug Deliv. Rev. 2017, 110–111, 169–187. [Google Scholar] [CrossRef]
- Ferreira, N.N.; Ferreira, L.M.B.; Cardoso, V.M.O.; Boni, F.I.; Souza, A.L.R.; Gremião, M.P.D. Recent advances in smart hydrogels for biomedical applications: From self-assembly to functional approaches. Eur. Polym. J. 2018, 99, 117–133. [Google Scholar] [CrossRef]
- Barros, S.C.; Martins, J.A.; Marcos, J.C.; Cavaco-Paulo, A. Influence of secretory leukocyte protease inhibitor-based peptides on elastase activity and their incorporation in hyaluronic acid hydrogels for chronic wound therapy. Biopolymers 2012, 98, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Sedighi, M.; Shrestha, N.; Mahmoudi, Z.; Khademi, Z.; Ghasempour, A.; Dehghan, H.; Talebi, S.F.; Toolabi, M.; Chen, B. Multifunctional Self-Assembled Peptide Hydrogels for Biomedical Applications. Polymers 2023, 15, 1160. [Google Scholar] [CrossRef] [PubMed]
- Sirousazar, M.; Forough, M.; Farhadi, K.; Shaabani, Y.; Molaei, R. Hydrogels: Properties, Preparation, Characterization and Biomedical, Applications in Tissue Engineering, Drug, Delivery and Wound Care. In Advanced Healthcare Materials; Wiley Blackwell 6: Hoboken, NJ, USA, 2014; pp. 295–357. ISBN 9781118773598. [Google Scholar] [CrossRef]
- Xie, Z.; Aphale, N.V.; Kadapure, T.D.; Wadajkar, A.S.; Orr, S.; Gyawali, D.; Qian, G.; Nguyen, K.T.; Yang, J. Design of antimicrobial peptides conjugated biodegradable citric acid derived hydrogels for wound healing. J. Biomed. Mater. Res.-Part A 2015, 103, 3907–3918. [Google Scholar] [CrossRef]
- Thapa, R.K.; Diep, D.B.; Tønnesen, H.H. Topical antimicrobial peptide formulations for wound healing: Current developments and future prospects. Acta Biomater. 2020, 103, 52–67. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.H.; Lee, J.S.; Park, C.B. Beta-Sheet-Forming, Self-Assembled Peptide Nanomaterials towards Optical, Energy, and Healthcare Applications. Small 2015, 11, 3623–3640. [Google Scholar] [CrossRef]
- Trier, A.M.; Mack, M.R.; Kim, B.S. The Neuroimmune Axis in Skin Sensation, Inflammation, and Immunity. J. Immunol. 2019, 202, 2829–2835. [Google Scholar] [CrossRef]
- Md Fadilah, N.I.; Mohd Abdul Kader Jailani, M.S.; Badrul Hisham, M.A.I.; Sunthar Raj, N.; Shamsuddin, S.A.; Ng, M.H.; Fauzi, M.B.; Maarof, M. Cell secretomes for wound healing and tissue regeneration: Next generation acellular based tissue engineered products. J. Tissue Eng. 2022, 13, 20417314221114273. [Google Scholar] [CrossRef] [PubMed]
- Grek, C.L.; Prasad, G.M.; Viswanathan, V.; Armstrong, D.G.; Gourdie, R.G.; Ghatnekar, G.S. Topical administration of a connexin43-based peptide augments healing of chronic neuropathic diabetic foot ulcers: A multicenter, randomized trial. Wound Repair Regen. 2015, 23, 203–212. [Google Scholar] [CrossRef]
- Gelain, F.; Luo, Z.; Zhang, S. Self-Assembling Peptide EAK16 and RADA16 Nanofiber Scaffold Hydrogel. Chem. Rev. 2020, 120, 13434–13460. [Google Scholar] [CrossRef]
- Thota, C.K.; Berger, A.A.; Elomaa, L.; Nie, C.X.; Böttcher, C.; Koksch, B. Coassembly Generates Peptide Hydrogel with Wound Dressing Material Properties. ACS OMEGA 2020, 5, 8557–8563. [Google Scholar] [CrossRef]
- Huang, L.C.; Wang, H.C.; Chen, L.H.; Ho, C.Y.; Hsieh, P.H.; Huang, M.Y.; Wu, H.C.; Wang, T.W. Bioinspired Self-assembling Peptide Hydrogel with Proteoglycan-assisted Growth Factor Delivery for Therapeutic Angiogenesis. Theranostics 2019, 9, 7072–7087. [Google Scholar] [CrossRef]
- Yuan, J.Z.; Wang, Y.; Yang, W.G.; Li, X.; Tao, K.S.; He, W.X.; Yan, J. Biomimetic peptide dynamic hydrogel inspired by humanized defensin nanonets as the wound-healing gel coating. Chem. Eng. J. 2023, 470, 144266. [Google Scholar] [CrossRef]
- Hao, M.; Ding, C.; Sun, S.; Peng, X.; Liu, W. Chitosan/Sodium Alginate/Velvet Antler Blood Peptides Hydrogel Promotes Diabetic Wound Healing via Regulating Angiogenesis, Inflammatory Response and Skin Flora. J. Inflamm. Res. 2022, 15, 4921–4938. [Google Scholar] [CrossRef] [PubMed]
- Chawla, V.; Sharma, S.; Singh, Y. Yttrium Oxide Nanoparticle-Loaded, Self-Assembled Peptide Gel with Antibacterial, Anti-Inflammatory, and Proangiogenic Properties for Wound Healing. ACS Biomater. Sci. Eng. 2023, 9, 2647–2662. [Google Scholar] [CrossRef]
- Qin, P.; Tang, J.; Sun, D.D.; Yang, Y.; Liu, N.X.; Li, Y.L.; Fu, Z.; Wang, Y.L.; Li, C.; Li, X.J.; et al. Zn2+ Cross-Linked Alginate Carrying Hollow Silica Nanoparticles Loaded with RL-QN15 Peptides Provides Promising Treatment for Chronic Skin Wounds. ACS Appl. Mater. Interfaces 2022, 14, 29491–29505. [Google Scholar] [CrossRef]
- Lou, P.; Liu, S.; Wang, Y.; Pan, C.; Xu, X.; Zhao, M.; Liao, G.; Yang, G.; Yuan, Y.; Li, L.; et al. Injectable self-assembling peptide nanofiber hydrogel as a bioactive 3D platform to promote chronic wound tissue regeneration. Acta Biomater. 2021, 135, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Shaik, M.I.; Sarbon, N.M. A Review on Purification and Characterization of Anti-proliferative Peptides Derived from Fish Protein Hydrolysate. Food Rev. Int. 2022, 38, 1389–1409. [Google Scholar] [CrossRef]
- Li, J.; Ma, X.G.; Wu, D.G.; Su, Z.W.; Su, H.; Liu, Z.X.; Chen, Y.; Yu, B. Hybrid Self-Assembled Peptide Hydrogels Promote Skin Wound Healing in Diabetic Mice. ACS Appl. Polym. Mater. 2024, 6, 1929–1943. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Xiong, Y.; Wu, Y.H.; Yang, F.; Guo, Y.; Chen, Z.L.; Gao, L.Q.; Deng, W.B. Ultrashort Peptides and Hyaluronic Acid-Based Injectable Composite Hydrogels for Sustained Drug Release and Chronic Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2021, 13, 58329–58339. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between structure and rheology of hydrogels for various applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Han, R.; Tang, K.; Zhao, S.; Ding, C.; Luo, X. Biocompatible peptide hydrogels with excellent antibacterial and catalytic properties for electrochemical sensing application. Anal. Chim. Acta 2021, 1154, 338295. [Google Scholar] [CrossRef] [PubMed]
- Zulkiflee, I.; Fauzi, M.B. Gelatin-polyvinyl alcohol film for tissue engineering: A concise review. Biomedicines 2021, 9, 979. [Google Scholar] [CrossRef]
- Hoque, J.; Prakash, R.G.; Paramanandham, K.; Shome, B.R.; Haldar, J. Biocompatible injectable hydrogel with potent wound healing and antibacterial properties. Mol. Pharm. 2017, 14, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, J.H.; Kim, S.H.; Jung, Y. Skin Regeneration with Self-Assembled Peptide Hydrogels Conjugated with Substance P in a Diabetic Rat Model. Tissue Eng. Part A 2017, 24, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Stern, D.; Cui, H.G. Crafting Polymeric and Peptidic Hydrogels for Improved Wound Healing. Adv. Healthc. Mater. 2019, 8, e1900104. [Google Scholar] [CrossRef] [PubMed]
- Rijal, N.P.; Narmoneva, D.A. Biomaterials for Diabetic Wound-Healing Therapies; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128164136. [Google Scholar]
- Carrejo, N.C.; Moore, A.N.; Silva, T.L.L.; Leach, D.G.; Li, I.C.; Walker, D.R.; Hartgerink, J.D. Multidomain Peptide Hydrogel Accelerates Healing of Full-Thickness Wounds in Diabetic Mice. ACS Biomater. Sci. Eng. 2018, 4, 1386–1396. [Google Scholar] [CrossRef]
- Kang, H.J.; Chen, N.; Dash, B.C.; Hsia, H.C.; Berthiaume, F. Self-Assembled Nanomaterials for Chronic Skin Wound Healing. Adv. Wound Care 2021, 10, 221–233. [Google Scholar] [CrossRef]
- López-Gutierrez, J.; Ramos-Payán, R.; Ayala-Ham, A.; Romero-Quintana, J.G.; Castillo-Ureta, H.; Villegas-Mercado, C.; Bermúdez, M.; Sanchez-Schmitz, G.; Aguilar-Medina, M. Biofunctionalization of hydrogel-based scaffolds for vascular tissue regeneration. Front. Mater. 2023, 10, 1168616. [Google Scholar] [CrossRef]
- Xiao, Y.; Reis, L.A.; Feric, N.; Knee, E.J.; Gu, J.H.; Cao, S.W.; Laschinger, C.; Londono, C.; Antolovich, J.; McGuigan, A.P.; et al. Diabetic wound regeneration using peptide-modified hydrogels to target re-epithelialization. Proc. Natl. Acad. Sci. USA 2016, 113, E5792–E5801. [Google Scholar] [CrossRef]
- Su, Y.; Wang, H.; Mishra, B.; Lakshmaiah Narayana, J.; Jiang, J.; Reilly, D.A.; Hollins, R.R.; Carlson, M.A.; Wang, G.; Xie, J. Nanofiber Dressings Topically Delivering Molecularly Engineered Human Cathelicidin Peptides for the Treatment of Biofilms in Chronic Wounds. Mol. Pharm. 2019, 16, 2011–2020. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Mahajan, A.; Patel, K.; Syed, S.; Acevedo-Jake, A.M.; Kumar, V.A. Materials and Cytokines in the Healing of Diabetic Foot Ulcers. Adv. Ther. 2021, 4, 2100075. [Google Scholar] [CrossRef]
- Gao, Y.F.; Li, Z.; Huang, J.; Zhao, M.; Wu, J. In situ formation of injectable hydrogels for chronic wound healing. J. Mater. Chem. B 2020, 8, 8768–8780. [Google Scholar] [CrossRef] [PubMed]
- Mandla, S.; Huyer, L.D.; Wang, Y.F.; Radisic, M. Macrophage Polarization with Angiopoietin-1 Peptide QHREDGS. ACS Biomater. Sci. Eng. 2019, 5, 4542–4550. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Duan, J.; Ma, G.; Zhang, W.; Wang, Q.; Hu, Z. Enzymatic crosslinking to fabricate antioxidant peptide-based supramolecular hydrogel for improving cutaneous wound healing. J. Mater. Chem. B 2019, 7, 2220–2225. [Google Scholar] [CrossRef]
- Balaji, S.; Vaikunth, S.S.; Lang, S.A.; Sheikh, A.Q.; Lim, F.Y.; Crombleholme, T.M.; Narmoneva, D.A. Tissue-engineered provisional matrix as a novel approach to enhance diabetic wound healing. Wound Repair Regen. 2012, 20, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Nidadavolu, L.S.; Stern, D.; Lin, R.; Wang, Y.Z.; Li, Y.; Wu, Y.Q.; Marin, S.; Antonio, M.J.; Yenokyan, G.; Boronina, T.; et al. Valsartan nano-filaments alter mitochondrial energetics and promote faster healing in diabetic rat wounds. Wound Repair Regen. 2021, 29, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Li, L.C.; Zhu, Z.Y.; Liu, S.Y.; Zhao, Y.Q.; Yu, M.S. Scorpion Venom Active Polypeptide May Be a New External Drug of Diabetic Ulcer. Evid.-Based Complement. Altern. Med. 2017, 2017, 5161565. [Google Scholar] [CrossRef]

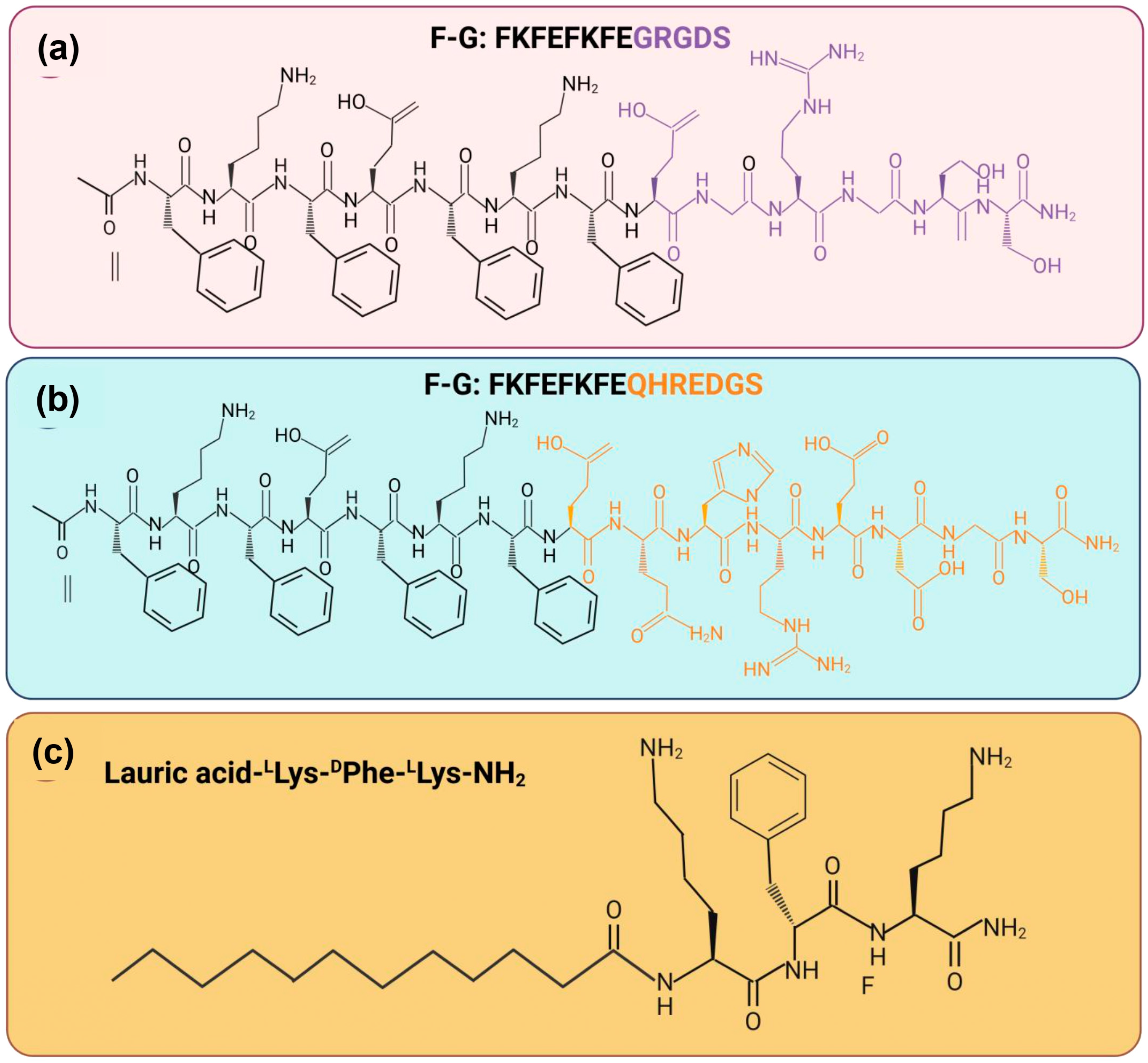

| No | Type of Peptide Hydrogel | In Vitro | In Vivo | Ref. |
|---|---|---|---|---|
| 1 | Self-assembling peptide hydrogel (SAP) | The Ac-FKFEFKFE-QHREDGS-NH2 (F−Q)/Ac-FKFEFKFE-GRGDS-NH2 (F−G) hydrogels exhibit excellence biocompatibility as determined by CCK-8 kit, resulting in an increased number of endothelial cells which led to highest cell viability at day 4 and 7. | No cytotoxicity effects in internal organs of mice that received peptide hydrogel. Greater cell proliferation and re-epithelialization were observed in the F-Q/F-G group, leading to nearly full closure of wound as well as appearance of new hair follicles at healing site. | [96] |
| 2 | Functionalized peptide hydrogel | CAH and CAVBPH shows 81.29% and 86.74% cell vitality, respectively, when the concentration of peptide was 5000 μg/mL. More than 75% cell viability was obtained, indicates that these peptide hydrogels have good biosafety and minimum toxicity. | On day 15, the AVBPH group had the highest rate of wound healing, at 96.55%, indicating the best treatment effectiveness. Pathological staining demonstrated CAVBPH’s positive impact on collagen deposition, fibroblast proliferation, angiogenesis, re-epithelialization, and inflammation regulation. | [91] |
| 3 | Crosslinked peptide hydrogel | The effects of the hydrogels ZA, RL-QN15/ZA, and HSN@RL-QN15/ZA (500 μg/mL) may promote keratinocyte migration and proliferation of cells. the viability of cell proliferation was demonstrated by ZA and HSN@RL~QN15/ZA, with the maximum values of 132.61 ± 8.74 and 159.74 ± 15.29%, respectively. | Hematoxylin and mouse mortality were not detected when HSN@RL-QN15/ZA hydrogel was applied topically to dorsal skin wounds. Instead, angiogenesis was regulated, inflammation was decreased, and the creation of granulation tissue and re-epithelialization was expedited, leading to a quick healing response. | [93] |
| 4 | Self-assembling peptide hydrogel (SAP) | The KGH hydrogel promoted the production of growth factors and extracellular matrix (ECM) proteins in skin keratinocytes, supported the formation of cell spheroids, and produced a 3D microenvironment for skin cells. | Up to seven days following injection, the KGH hydrogel remained in the wounds and effectively accelerated wound closure by around 20% as compared to the control groups. The result was achieved by boosting angiogenesis, cell proliferation, granulation tissue development, and extracellular matrix deposition/ remodelling. | [94] |
| 5 | Self-assembling peptide hydrogel (SAP) | The R+SP group was discovered to have a greater contribution to the development of keratinocytes in wounded skin, as evidenced by the robust expression of cytokeratin 14 and involucrin positive cells in the wound after three weeks. | The wound area s with R+SP and R+R-SP groups were primarily filled with regenerated tissue, gradually shrank after receiving treatment with SAP and substance P. These findings suggest that substance P in peptide hydrogel speeds up wound healing. | [102] |
| 6 | Self-assembling peptide hydrogel (SAP) | SAP that included stromal cell-derived factor-1 (SDF1-ELP), enhanced endothelial cells migration, proliferation, and vascularization. | Greater vascular endothelial cells (CD31+ cells), quicker wound closure, and much thicker epidermis and dermis were observed when SDF1-ELP in fibrin gel was introduced to in vivo diabetic wounds in mice as opposed to free SDF1 and other control groups. | [106] |
| 7 | Multidomain peptide hydrogel (MDP) | K2 hydrogel exhibited biocompatibility, which allowed for rapid cellular infiltration to promote granulation tissue formation, angiogenesis, and hair follicle regeneration as well as act as a degradable matrix to facilitate cell infiltration including fibroblasts. | When tested as a dressing in a diabetic rat wound model, the K2 hydrogel groups demonstrated a significantly greater healing rate with almost complete closure. This composite hydrogel also showed no cytotoxicity and promoted fibroblast differentiation. | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nizam, A.A.K.; Masri, S.; Fadilah, N.I.M.; Maarof, M.; Fauzi, M.B. Current Insight of Peptide-Based Hydrogels for Chronic Wound Healing Applications: A Concise Review. Pharmaceuticals 2025, 18, 58. https://doi.org/10.3390/ph18010058
Nizam AAK, Masri S, Fadilah NIM, Maarof M, Fauzi MB. Current Insight of Peptide-Based Hydrogels for Chronic Wound Healing Applications: A Concise Review. Pharmaceuticals. 2025; 18(1):58. https://doi.org/10.3390/ph18010058
Chicago/Turabian StyleNizam, Aifa Asyhira Khairul, Syafira Masri, Nur Izzah Md Fadilah, Manira Maarof, and Mh Busra Fauzi. 2025. "Current Insight of Peptide-Based Hydrogels for Chronic Wound Healing Applications: A Concise Review" Pharmaceuticals 18, no. 1: 58. https://doi.org/10.3390/ph18010058
APA StyleNizam, A. A. K., Masri, S., Fadilah, N. I. M., Maarof, M., & Fauzi, M. B. (2025). Current Insight of Peptide-Based Hydrogels for Chronic Wound Healing Applications: A Concise Review. Pharmaceuticals, 18(1), 58. https://doi.org/10.3390/ph18010058









