Hormonal Contraception and Bone Metabolism: Emerging Evidence from a Systematic Review and Meta-Analysis of Studies on Post-Pubertal and Reproductive-Age Women
Abstract
1. Introduction
2. Results
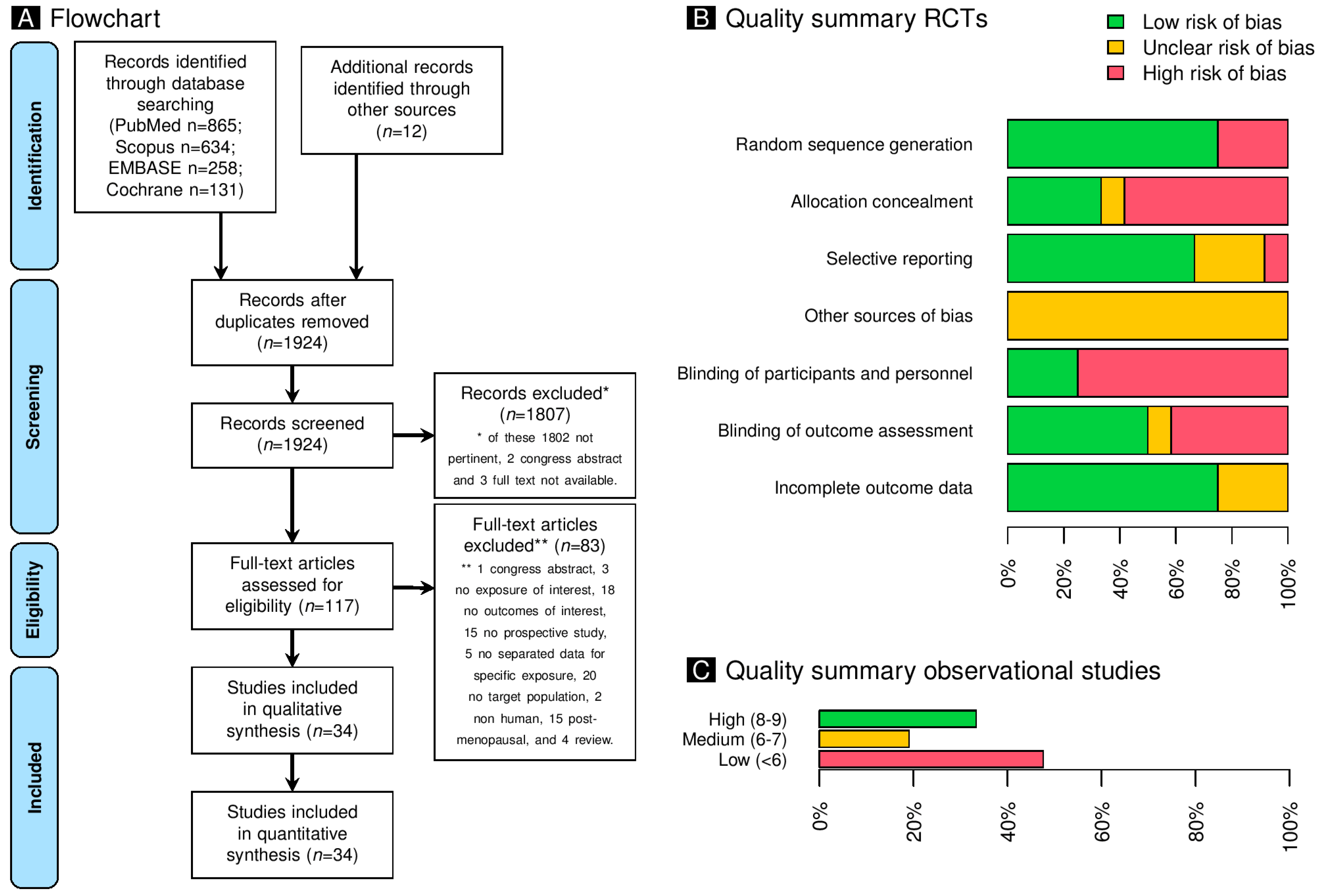
2.1. Study Selection
2.2. Study Characteristics
2.3. Risk of Bias of Included Studies
2.4. Bone Markers: Metabolism in Healthy Women
2.5. Meta-Regressions of the Androgenic Effects of Progestins, the Estrogenic Effect on SHBG, and Bone Markers in Healthy Women
2.6. Calcium Levels and Bone Mineral Density in Healthy Women
2.7. Publication Bias
3. Discussion
3.1. Key Results
3.2. Interpretation and Comparison with the Literature
3.2.1. Bone Turnover in Women ≤21 Years of Age
3.2.2. Bone Turnover in Women >21 Years of Age
3.3. Mechanistic Insights into the Distinct Effects of Estrogens and Progestins on Bone Turnover
3.4. Strengths and Weaknesses
3.5. Relevance and Generalizability of the Findings
3.6. Unanswered Questions and Future Research
4. Materials and Methods
4.1. Study Design and Inclusion Criteria
4.2. Data Source and Search Strategy
4.3. Selection of Relevant Studies and Quality Assessment
4.4. Data Extraction
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bachrach, L.K. Hormonal Contraception and Bone Health in Adolescents. Front. Endocrinol. 2020, 11, 603. [Google Scholar] [CrossRef]
- Teede, H.; Misso, M.; Costello, M.; Dokras, A.; Laven, J. International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome 2018; International PCOS Network: Victoria, Australia, 2018. [Google Scholar]
- Merki-Feld, G.S.; Bitzer, J. Contraception in adolescents with anorexia nervosa. Is there evidence for a negative impact of combined hormonal contraceptives on bone mineral density and the course of the disease? Eur. J. Contracept. Reprod. Health Care 2020, 25, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Morgante, G.; Cappelli, V.; Troìa, L.; De Leo, V. Evaluation of different antiandrogenic progestins on clinical and biochemical variables in polycystic ovary syndrome. Eur. J. Contracept. Reprod. Health Care 2020, 25, 176–181. [Google Scholar] [CrossRef]
- Hadji, P.; Colli, E.; Regidor, P.-A. Bone health in estrogen-free contraception. Osteoporos. Int. 2019, 30, 2391–2400. [Google Scholar] [CrossRef] [PubMed]
- Hee, L.; Kettner, L.O.; Vejtorp, M. Continuous use of oral contraceptives: An overview of effects and side-effects. Acta Obstet. Gynecol. Scand. 2013, 92, 125–136. [Google Scholar] [CrossRef]
- Costa-Paiva, L.; Wender, M.C.O.; Machado, R.B.; Pompei, L.M.; Nahas, E.A.; Nahas-Neto, J.; Del Debbio, S.Y.; Badalotti, M.; Cruz, A.M. Effects of ultra-low dose hormone therapy on biochemical bone turnover markers in postmenopausal women: A randomized, placebo-controlled, double-blind trial. Post. Reprod. Health 2022, 28, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Garnero, P.; Sornay-Rendu, E.; Delmas, P. Decreased bone turnover in oral contraceptive users. Bone 1995, 16, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Scholes, D.; Ichikawa, L.; LaCroix, A.Z.; Spangler, L.; Beasley, J.M.; Reed, S.; Ott, S.M. Oral contraceptive use and bone density in adolescent and young adult women. Contraception 2010, 81, 35–40. [Google Scholar] [CrossRef]
- Walsh, J.S.; Henry, Y.M.; Fatayerji, D.; Eastell, R. Hormonal determinants of bone turnover before and after attainment of peak bone mass. Clin. Endocrinol. 2010, 72, 320–327. [Google Scholar] [CrossRef]
- Banks, E.; Berrington, A.; Casabonne, D. Overview of the relationship between use of progestogen-only contraceptives and bone mineral density. Br. J. Obstet. Gynaecol. 2001, 108, 1214–1221. [Google Scholar]
- Seens, H.; Modarresi, S.; MacDermid, J.C.; Walton, D.M.; Grewal, R. Prevalence of bone fractures among children and adolescents with attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. BMC Pediatr. 2021, 21, 354. [Google Scholar] [CrossRef]
- Caldeirão, T.D.; Orsolini, L.R.; Da Silva, C.C.; Rizzo, A.D.C.B.; Teixeira, A.S.; de Carvalho Nunes, H.R.; Goldberg, T.B.L. Effect of two combinations of low-dose oral contraceptives on adolescent bone mass: A clinical trial with 2 years follow-up. Medicine 2022, 101, e30680. [Google Scholar] [CrossRef] [PubMed]
- Almstedt, H.C.; Cook, M.M.; Bramble, L.F.; Dabir, D.V.; LaBrie, J.W. Oral contraceptive use, bone mineral density, and bone turnover markers over 12 months in college-aged females. J. Bone Miner. Metab. 2020, 38, 544–554. [Google Scholar] [CrossRef]
- Tiedeken, M.; Westhoff, C.L.; Cohen, A.; Cremers, S.; Sitruk-Ware, R.; Blithe, D.L. Bone turnover markers in women participating in a dose-finding trial of a contraceptive vaginal ring releasing Nestorone and estradiol. Contraception 2019, 99, 329–334. [Google Scholar] [CrossRef]
- Rizzo, A.d.C.B.; Goldberg, T.B.L.; Biason, T.P.; Kurokawa, C.S.; da Silva, C.C.; Corrente, J.E.; Nunes, H.R.C. One-year adolescent bone mineral density and bone formation marker changes through the use or lack of use of combined hormonal contraceptives. J. Pediatr. 2019, 95, 567–574. [Google Scholar] [CrossRef]
- Duvan, C.I.; Onaran, Y.; Keskin, E.A.; Yüce, E.; Yanık, B.; Kafali, H.; Turhan, N.O. Effects of the etonogestrel contraceptive implant (Implanon®) on bone metabolism during lactation: A prospective study. J. Fam. Plann. Reprod. Health Care 2017, 43, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Mawet, M.; Maillard, C.; Klipping, C.; Zimmerman, Y.; Foidart, J.-M.; Bennink, H.J.T.C. Unique effects on hepatic function, lipid metabolism, bone and growth endocrine parameters of estetrol in combined oral contraceptives. Eur. J. Contracept. Reprod. Health Care 2015, 20, 463–475. [Google Scholar]
- Di Carlo, C.; Gargano, V.; Sparice, S.; Tommaselli, G.A.; Bifulco, G.; Schettino, D.; Nappi, C. Short-term effects of an oral contraceptive containing oestradiol valerate and dienogest on bone metabolism and bone mineral density: An observational, preliminary study. Eur. J. Contracept. Reprod. Health Care 2013, 18, 388–393. [Google Scholar] [CrossRef]
- Massaro, M.; Di Carlo, C.; Gargano, V.; Formisano, C.; Bifulco, G.; Nappi, C. Effects of the contraceptive patch and the vaginal ring on bone metabolism and bone mineral density: A prospective, controlled, randomized study. Contraception 2010, 81, 209–214. [Google Scholar] [CrossRef]
- Harel, Z.; Riggs, S.; Vaz, R.; Flanagan, P.; Harel, D.; Machan, J.T. Bone accretion in adolescents using the combined estrogen and progestin transdermal contraceptive method Ortho Evra: A pilot study. J. Pediatr. Adolesc. Gynecol. 2010, 23, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Lattakova, M.; Borovsky, M.; Payer, J.; Killinger, Z. Oral contraception usage in relation to bone mineral density and bone turnover in adolescent girls. Eur. J. Contracept. Reprod. Health Care 2009, 14, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Gargano, V.; Massaro, M.; Morra, I.; Formisano, C.; Di Carlo, C.; Nappi, C. Effects of two low-dose combined oral contraceptives containing drospirenone on bone turnover and bone mineral density in young fertile women: A prospective controlled randomized study. Contraception 2008, 78, 10–15. [Google Scholar] [CrossRef]
- Nappi, C.; Sardo, A.D.S.; Greco, E.; Tommaselli, G.A.; Giordano, E.; Guida, M. Effects of an oral contraceptive containing drospirenone on bone turnover and bone mineral density. Obstet. Gynecol. 2005, 105, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Rome, E.; Ziegler, J.; Secic, M.; Bonny, A.; Stager, M.; Lazebnik, R.; Cromer, B.A. Bone biochemical markers in adolescent girls using either depot medroxyprogesterone acetate or an oral contraceptive. J. Pediatr. Adolesc. Gynecol. 2004, 17, 373–377. [Google Scholar] [CrossRef]
- Paoletti, A.M.; Orrù, M.; Lello, S.; Floris, S.; Ranuzzi, F.; Etzi, R.; Zedda, P.; Guerriero, S.; Fratta, S.; Sorge, R.; et al. Short-term variations in bone remodeling markers of an oral contraception formulation containing 3 mg of drospirenone plus 30 microg of ethinyl estradiol: Observational study in young postadolescent women. Contraception 2004, 70, 293–298. [Google Scholar] [CrossRef]
- Endrikat, J.; Mih, E.; Düsterberg, B.; Land, K.; Gerlinger, C.; Schmidt, W.; Felsenberg, D. A 3-year double-blind, randomized, controlled study on the influence of two oral contraceptives containing either 20 microg or 30 microg ethinylestradiol in combination with levonorgestrel on bone mineral density. Contraception 2004, 69, 179–187. [Google Scholar] [CrossRef]
- Nappi, C.; Sardo, A.D.S.; Acunzo, G.; Bifulco, G.; Tommaselli, G.; Guida, M.; Di Carlo, C. Effects of a low-dose and ultra-low-dose combined oral contraceptive use on bone turnover and bone mineral density in young fertile women: A prospective controlled randomized study. Contraception 2003, 67, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, A.M.; Orrù, M.; Floris, S.; Mannias, M.; Vacca, A.M.B.; Ajossa, S.; Guerriero, S.; Melis, G.B. Evidence that treatment with monophasic oral contraceptive formulations containing ethinylestradiol plus gestodene reduces bone resorption in young women. Contraception 2000, 61, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Naessen, T.; Olsson, S.-E.; Gudmundson, J. Differential effects on bone density of progestogen-only methods for contraception in premenopausal women. Contraception 1995, 52, 35–39. [Google Scholar] [CrossRef]
- Mais, V.; Fruzzetti, F.; Ajossa, S.; Paoletti, A.; Guerriero, S.; Melis, G. Bone metabolism in young women taking a monophasic pill containing 20 mcg ethinylestradiol: A prospective study. Contraception 1993, 48, 445–452. [Google Scholar] [CrossRef]
- Roy, S.; Mishell, D.R.; Gray, G.; Dozono-Takano, R.; Brenner, P.F.; Eide, I.; de Quattro, V.; Shaw, S. Comparison of metabolic and clinical effects of four oral contraceptive formulations and a contraceptive vaginal ring. Am. J. Obstet. Gynecol. 1980, 136, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Sadik, W.; Kovacs, L.; Pretnar-Darovec, A.; de Acosta, O.M.; Toddywalla, V.; Dhall, G.; Ng, C.; Holck, S.; Belsey, M.; Pinol, M.; et al. A randomized double-blind study of the effects of two low-dose combined oral contraceptives on biochemical aspects. Report from a seven-centred study. WHO Special Programme of Research, Development and Research Training in Human Reproduction. Task force on Oral Contraceptives. Contraception 1985, 32, 223–236. [Google Scholar]
- Cibula, D.; Skrenkova, J.; Hill, M.; Stepan, J.J. Low-dose estrogen combined oral contraceptives may negatively influence physiological bone mineral density acquisition during adolescence. Eur. J. Endocrinol. 2012, 166, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Kaunitz, A.M.; Miller, P.D.; Rice, V.M.; Ross, D.; McClung, M.R. Bone mineral density in women aged 25–35 years receiving depot medroxyprogesterone acetate: Recovery following discontinuation. Contraception 2006, 74, 90–99. [Google Scholar] [CrossRef]
- Ahrén, T.; Victor, A.; Lithell, H.; Johansson, E.D. Comparison of the metabolic effects of two hormonal contraceptive methods: An oral formulation and a vaginal ring. I. Carbohydrate metabolism and liver function. Contraception 1981, 24, 415–427. [Google Scholar] [CrossRef]
- Amatayakul, K.; Sivassomboon, B.; Singkamani, R. Effects of medroxyprogesterone acetate on serum lipids, protein, glucose tolerance and liver function in Thai women. Contraception 1980, 21, 283–297. [Google Scholar] [CrossRef]
- Hurley, D.L.; Tiegs, R.D.; Barta, J.; Laakso, K.; Heath, H.D. Effects of oral contraceptive and estrogen administration on plasma calcitonin in pre- and postmenopausal women. J. Bone Miner. Res. 1989, 4, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Etzrodt, R.; Klinger, G.; Carol, W. The action of Sequostat in comparison to Sequence Ovosiston on selected metabolic parameters. Zentralbl. Gynakol. 1990, 112, 489–496. [Google Scholar]
- García, C.R.; Wallach, E.E. Liver function studies and progestagen contraception. Review of an intramuscularly administered contraceptive. Fertil. Steril. 1968, 19, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Brügmann, E.; Göretzlehner, G.; Töwe, J.; Rehpenning, W. Liver function tests after a 6-month deposiston therapy. Zentralbl. Gynakol. 1975, 97, 669–673. [Google Scholar] [PubMed]
- Bamji, M.S.; Safaya, S.; Prema, K. Low dose injectable contraceptive norethisterone enanthate 20mg monthly-II. Metabolic side effects. Contraception 1981, 23, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Juarez, J.; Garcia-Latorre, E.A.; Moreno-Hernandez, M.; Moran-Perez, J.F.; Rodriguez-Escobedo, M.A.; Cogque-Hernandez, G.; Julián-Nacer, R.; Hernandez-Giron, X.; Palafox-Gomez, R.; Isordia-Salas, I.; et al. Metabolic effects of the contraceptive skin patch and subdermal contraceptive implant in Mexican women: A prospective study. Reprod. Health 2014, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Orsolini, L.R.; Goldberg, T.B.L.; Caldeirão, T.D.; da Silva, C.C.; Rizzo, A.d.C.B.; Biason, T.P.; Teixeira, A.S.; Nunes, H.R.C. Bone impact after two years of low-dose oral contraceptive use during adolescence. PLoS ONE 2023, 18, e0285885. [Google Scholar] [CrossRef] [PubMed]
- Amatayakul, K.; Sivasomboon, B.; Thanangkul, O. Vitamin and trace mineral metabolism in medroxyprogesterone acetate users. Contraception 1978, 18, 253–269. [Google Scholar] [CrossRef]
- Misra, M.; Katzman, D.; Miller, K.K.; Mendes, N.; Snelgrove, D.; Russell, M.; Goldstein, M.A.; Ebrahimi, S.; Clauss, L.; Weigel, T.; et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J. Bone Miner. Res. 2011, 26, 2430–2438. [Google Scholar] [CrossRef] [PubMed]
- Burns, P.B.; Rohrich, R.J.; Chung, K.C.M. The levels of evidence and their role in evidence-based medicine. Plast. Reconstr. Surg. 2011, 128, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Chew, C.K.; Clarke, B.L. Causes of low peak bone mass in women. Maturitas 2018, 111, 61–68. [Google Scholar] [CrossRef]
- Mora, S.; Pitukcheewanont, P.; Kaufman, F.R.; Nelson, J.C.; Gilsanz, V. Biochemical markers of bone turnover and the volume and the density of bone in children at different stages of sexual development. J. Bone Miner. Res. 1999, 14, 1664–1671. [Google Scholar] [CrossRef]
- Van Coeverden, S.C.C.M.; Netelenbos, J.C.; De Ridder, C.M.; Roos, J.C.; Popp-Snijders, C.; Delemarre-van de Waal, H.A. Bone metabolism markers and bone mass in healthy pubertal boys and girls. Clin. Endocrinol. 2002, 57, 107–116. [Google Scholar] [CrossRef]
- Allaway, H.C.; Misra, M.; Southmayd, E.A.; Stone, M.S.; Weaver, C.M.; Petkus, D.L.; De Souza, M.J. Are the Effects of Oral and Vaginal Contraceptives on Bone Formation in Young Women Mediated via the Growth Hormone-IGF-I Axis? Front. Endocrinol. 2020, 11, 334. [Google Scholar] [CrossRef]
- Donangelo, C.M.; Cornes, R.; Sintes, C.; Bezerra, F.F. Combined Oral Contraceptives: Association with Serum 25-Hydroxyvitamin D and Calcium and Bone Homeostasis. J. Womens Health 2024, 33, 805–815. [Google Scholar] [CrossRef]
- Dixit, M.; Poudel, S.B.; Yakar, S. Effects of G.H./IGF axis on bone and cartilage. Mol. Cell. Endocrinol. 2021, 519, 111052. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.A.; Mckay, H.A.; Mirwald, R.L.; Crocker, P.R.E.; Faulkner, R.A. A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: The university of Saskatchewan bone mineral accrual study. J. Bone Miner. Res. 1999, 14, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef]
- Harel, Z.; Gold, M.; Cromer, B.; Bruner, A.; Stager, M.; Bachrach, L.; Wolter, K.; Reid, C.; Hertweck, P.; Nelson, A.; et al. Bone mineral density in postmenarchal adolescent girls in the United States: Associated biopsychosocial variables and bone turnover markers. J. Adolesc. Health 2007, 40, 44–53. [Google Scholar] [CrossRef]
- Blumsohn, A.; Hannon, R.A.; Wrate, R.; Barton, J.; Ai-Dehaimi, A.W.; Colwell, A.; Eastell, R. Biochemical markers of bone turnover in girls during puberty. Clin. Endocrinol. 1994, 40, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Cadogan, J.; Blumsohn, A.; Barker, M.E.; Eastell, R. A longitudinal study of bone gain in pubertal girls: Anthropometric and biochemical correlates. J. Bone Miner. Res. 1998, 13, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.; Goltzman, D.; Langsetmo, L.; Joseph, L.; Jackson, S.; Kreiger, N.; Tenenhouse, A.; Davison, K.S.; Josse, R.G.; Prior, J.C.; et al. Peak bone mass from longitudinal data: Implications for the prevalence, pathophysiology, and diagnosis of osteoporosis. J. Bone Miner. Res. 2010, 25, 1948–1957. [Google Scholar] [CrossRef]
- Riggs, B.L.; Melton, L.J.; Robb, R.A.; Camp, J.J.; Atkinson, E.J.; McDaniel, L.; Amin, S.; Rouleau, P.A.; Khosla, S. A population-based assessment of rates of bone loss at multiple skeletal sites: Evidence for substantial trabecular bone loss in young adult women and men. J. Bone Miner. Res. 2008, 23, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, T.; Taylor, D.S.; Lin, H.M.; Matthews, A.E.; Eggli, D.F.; Legro, R.S. Oral contraceptive use by teenage women does not affect peak bone mass: A longitudinal study. Fertil. Steril. 2000, 74, 734–738. [Google Scholar] [CrossRef]
- White, L.; Losciale, J.M.; Squier, K.; Guy, S.; Scott, A.; Prior, J.C.; Whittaker, J.L. Combined hormonal contraceptive use is not protective against musculoskeletal conditions or injuries: A systematic review with data from 5 million females. Br. J. Sports Med. 2023, 57, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Cagnacci, A.; Bruni, V.; Di Carlo, C.; Fruzzetti, F. How often is oral contraception used for contraception? The need of benefit’s formalisation. Eur. J. Contracept. Reprod. Health Care 2023, 28, 81–82. [Google Scholar] [CrossRef]
- Goshtasebi, A.; Subotic Brajic, T.; Scholes, D.; Beres Lederer Goldberg, T.; Berenson, A.; Prior, J.C. Adolescent use of combined hormonal contraception and peak bone mineral density accrual: A meta-analysis of international prospective controlled studies. Clin. Endocrinol. 2019, 90, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Seibel, M.J. The effects of hormonal contraceptives on bone turnover markers and bone health. Clin. Endocrinol. 2010, 72, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.-W.; Kwon, A.-R.; Kim, D.-H.; Kim, H.-S. Sex hormone binding globulin, free estradiol index, and lipid profiles in girls with precocious puberty. Ann. Pediatr. Endocrinol. Metab. 2013, 18, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Gambacciani, M.; Cappagli, B.; Lazzarini, V.; Ciaponi, M.; Fruzzetti, F.; Genazzani, A.R. Longitudinal evaluation of perimenopausal bone loss: Effects of different low dose oral contraceptive preparations on bone mineral density. Maturitas 2006, 54, 176–180. [Google Scholar] [CrossRef]
- Jackowski, S.A.; Baxter-Jones, A.D.; McLardy, A.J.; Pierson, R.A.; Rodgers, C.D. The associations of exposure to combined hormonal contraceptive use on bone mineral content and areal bone mineral density accrual from adolescence to young adulthood: A longitudinal study. Bone Rep. 2016, 5, e333–e341. [Google Scholar] [CrossRef] [PubMed]
- Prior, J.; Whittaker, J.L.; Scott, A.W. Adolescent combined hormonal contraceptives and surgical repair of anterior cruciate tears: A risky recommendation based on an unproven causal relationship. Phys. Sportsmed. 2019, 47, 240–241. [Google Scholar] [CrossRef]
- Wiren, K.M. Androgen Action in Bone: Basic Cellular and Molecular Aspects. In Osteoporosis; Adler, R.A., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 359–383. [Google Scholar]
- Hsu, S.-H.; Chen, L.-R.; Chen, K.-H. Primary Osteoporosis Induced by Androgen and Estrogen Deficiency: The Molecular and Cellular Perspective on Pathophysiological Mechanisms and Treatments. Int. J. Mol. Sci. 2024, 25, 12139. [Google Scholar] [CrossRef]
- Sfeir, J.G.; Drake, M.T. The Effects of Androgens on Bone Metabolism: Clinical Aspects. In Osteoporosis: Pathophysiology and Clinical Management, Contemporary Endocrinology, 3rd ed.; Leder, B.Z., Wein, M.N., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 259–275. [Google Scholar]
- Sitruk-Ware, R. New progestagens for contraceptive use. Hum. Reprod. Update 2006, 12, 169–178. [Google Scholar] [CrossRef]
- Greenblatt, M.B.; Tsai, J.N.; Wein, M.N. Bone Turnover Markers in the Diagnosis and Monitoring of Metabolic Bone Disease. Clin. Chem. 2017, 63, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Di Medio, L.; Brandi, M.L. Chapter Three-Advances in bone turnover markers. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 105, pp. 101–140. [Google Scholar]
- Eastell, R.; Krege, J.H.; Chen, P.; Glass, E.V.; Reginster, J.-Y. Development of an algorithm for using PINP to monitor treatment of patients with teriparatide. Curr. Med. Res. Opin. 2006, 22, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Gundberg, C.M.; Nishimoto, S.K.; Vitamin, K. Dependent Proteins of Bone and Cartilage. In Dynamics of Bone and Cartilage Metabolism; Elsevier: Amsterdam, The Netherlands, 2006; pp. 55–70. [Google Scholar]
- Delmas, P.D. Biochemical markers of bone turnover: Methodology and clinical use in osteoporosis. Am. J. Med. 1991, 91, 59S–63S. [Google Scholar] [CrossRef] [PubMed]
- Oniszczuk, A.; Kaczmarek, A.; Kaczmarek, M.; Ciałowicz, M.; Arslan, E.; Silva, A.F.; Clemente, F.M.; Murawska-Ciałowicz, E. Sclerostin as a biomarker of physical exercise in osteoporosis: A narrative review. Front. Endocrinol. 2022, 13, 954895. [Google Scholar] [CrossRef]
- Rifai, N. Tietz Fundamentals of Clinical Chemistry and Molecular Diagnostics, 8th ed.; Saunders: Philadelphia, PA, USA, 2019. [Google Scholar]
- Kraenzlin, M.E.; Seibel, M.J. Measurement of Biochemical Markers of Bone Resorption. In Dynamics of Bone and Cartilage Metabolism; Elsevier: Amsterdam, The Netherlands, 2006; pp. 541–563. [Google Scholar]
- Risteli, J.; Elomaa, I.; Niemi, S.; Novamo, A.; Risteli, L. Radioimmunoassay for the pyridinoline cross-linked carboxy-terminal telopeptide of type I collagen: A new serum marker of bone collagen degradation. Clin. Chem. 1993, 39, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Bonde, M.; Qvist, P.; Fledelius, C.; Riis, B.J.; Christiansen, C. Immunoassay for quantifying type I collagen degradation products in urine evaluated. Clin. Chem. 1994, 40, 2022–2025. [Google Scholar] [CrossRef]
- Hanson, D.A.; Weis, M.A.; Bollen, A.M.; Maslan, S.L.; Singer, F.R.; Eyre, D.R. A specific immunoassay for monitoring human bone resorption: Quantitation of type I collagen cross-linked N-telopeptides in urine. J. Bone Miner. Res. 1992, 7, 1251–1258. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.; Dinnes, J.; D’Amico, R.; Sowden, A.; Sakarovitch, C.; Song, F.; Petticrew, M.; Altman, D. Evaluating non-randomised intervention studies. Health Technol. Assess. 2003, 7, 1–173. [Google Scholar] [CrossRef] [PubMed]
- Mashchak, C.; Lobo, R.A.; Dozono-Takano, R.; Eggena, P.; Nakamura, R.M.; Brenner, P.F.; Mishell, D.R. Comparison of pharmacodynamic properties of various estrogen formulations. Am. J. Obstet. Gynecol. 1982, 144, 511–518. [Google Scholar] [CrossRef]
- Becker, B.J. Synthesizing standardized mean-change measures. Br. J. Math. Stat. Psychol. 1988, 41, 257–278. [Google Scholar] [CrossRef]
- Londero, A.P.; Gallina, V.; Cremonini, F.; Xholli, A.; Cagnacci, A. Systematic review and meta-analysis of the effects of progestins on depression in post-menopausal women: An evaluation of randomized clinical studies that used validated questionnaires. Maturitas 2024, 189, 108105. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Egger, M.; Smith, G.D. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ 2001, 323, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Stepan, J.; Skrenkova, J.; Hill, M. BMD and biochemical markers of bone turnover in adolescent girls on oral contraceptives with different estrogen content. Bone 2009, 44, S259. [Google Scholar] [CrossRef]
- Caird, L.E.; Reid-Thomas, V.; Hannan, W.J.; Gow, S.; Glasier, A.F. Oral progestogen-only contraception may protect against loss of bone mass in breast-feeding women. Clin. Endocrinol. (Oxf.) 1994, 41, 739–745. [Google Scholar] [CrossRef]
- Svedlund, A.; Pettersson, C.; Tubic, B.; Ellegård, L.; Elfvin, A.; Magnusson, P.; Swolin-Eide, D. Bone mass and biomarkers in young women with anorexia nervosa: A prospective 3-year follow-up study. J. Bone Miner. Metab. 2022, 40, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Carmina, E.; Janni, A.; Lobo, R.A. Physiological estrogen replacement may enhance the effectiveness of the gonadotropin-releasing hormone agonist in the treatment of hirsutism. J. Clin. Endocrinol. Metab. 1994, 78, 126–130. [Google Scholar]
- Polatti, F.; Perotti, F.; Filippa, N.; Gallina, D.; Nappi, R.E. Bone mass and long-term monophasic oral contraceptive treatment in young women. Contraception 1995, 51, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, R.; Eden, S.; von Schoultz, B. Oral contraception affects osteocalcin serum profiles in young women. Osteoporos. Int. 1992, 2, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Sammour, M.B.; Hilal, S.O. Alkaline phosphatase activity of polymorphonulcear leukocytes in relation to oral contraceptives. Am. J. Obstet. Gynecol. 1969, 103, 823–827. [Google Scholar] [CrossRef]
- DeMerre, L.J.; Litofsky, F.S. Alkaline-phosphatase activity during menstruation. Fertil. Steril. 1968, 19, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Sall, N.D.; Sow, A.; Toure, M.; Sarr, G.N.; Seck, I.; Diadhiou, F. [Biochemical profile of Senegalese women on oral contraceptives]. Dakar Med. 1992, 37, 159–162. [Google Scholar]
- Weijers, M.J. Desogestrel, a new progestational compound, and the liver. Arzneimittelforschung 1983, 33, 774–776. [Google Scholar]
- Cosson, M.; Querleu, D.; Donnez, J.; Madelenat, P.; Konincks, P.; Audebert, A.; Manhes, H. Dienogest is as effective as triptorelin in the treatment of endometriosis after laparoscopic surgery: Results of a prospective, multicenter, randomized study. Fertil. Steril. 2002, 77, 684–692. [Google Scholar] [CrossRef]
- Grant, E.C.; Pryse-Davies, J. Effect of oral contraceptives on depressive mood changes and on endometrial monoamine oxidase and phosphatases. Br. Med. J. 1968, 3, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsen, B.; Stilgren, L.S.; Rettmer, E.; Bonnevie-Nielsen, V.; Beck-Nielsen, H. Effects of the natural and artificial menstrual cycle on the production of osteoprotegerin and the bone resorptive cytokines IL-1beta and IL-6. Calcif. Tissue Int. 2003, 72, 18–23. [Google Scholar] [CrossRef]
- Brocklehurst, D.; Wilde, C.E. Evidence for the hormonal regulation of the multimolecular forms of serum alkaline phosphatase. Prog. Clin. Biol. Res. 1984, 166, 277–288. [Google Scholar] [PubMed]
- Skouby, S.O. Laboratory and Clinical Assessment of a New Progestational Compound, Desogestrel: A Phase I Study. Acta Obstet. Gynecol. Scand. 1982, 61, 7–11. [Google Scholar] [CrossRef]
- Brügmann, E.; Göretzlehner, G.; Klie, E.; Schwager, A.; Maass, M. [Liver function studies under the effect of 4 sequential hormonal contraceptives]. Z. Gesamte Inn. Med. 1978, 33, 826–829. [Google Scholar] [PubMed]
- Brügmann, E.; Göretzlehner, G.; Dabels, J.; Töwe, J. [Liver function studies under the influence of hormonal contraceptives (sequential preparations)]. Dtsch. Z. Verdau Stoffwechselkr. 1979, 39, 69–74. [Google Scholar] [PubMed]
- Akpowowo, H.E.; Göretzlehner, G.; Brügmann, E.; Töwe, J. [Liver function tests under the influence of sequential treatment using ethinyl estradiol-norethisterone acetate and ethinyl estradiol-chlormadinone acetate]. Zentralbl. Gynakol. 1976, 98, 1198–1203. [Google Scholar] [PubMed]
- Larsson-Cohn, U. Oral contraception and liver-function tests. Br. Med. J. 1965, 1, 1414–1415. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brügmann, E.; Göretzlehner, G.; Dabels, J.; Töwe, J. Studies on liver function under the influence of oral contraceptives. Int. J. Gynaecol. Obstet. 1979, 16, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Schenker, J.H.; Jungreis, E.; Polishuk, W.Z. Oral contraceptives and serum copper concentration. Obstet. Gynecol. 1971, 37, 233–237. [Google Scholar]
- Klibanski, A.; Biller, B.M.; Schoenfeld, D.A.; Herzog, D.B.; Saxe, V.C. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J. Clin. Endocrinol. Metab. 1995, 80, 898–904. [Google Scholar] [PubMed]
- Coombs, C.V.; O’Leary, T.J.; Tang, J.C.Y.; Fraser, W.D.; Greeves, J.P. Hormonal contraceptive use, bone density and biochemical markers of bone metabolism in British Army recruits. BMJ Mil. Health 2023, 169, 9–16. [Google Scholar] [CrossRef]
- Shaarawy, M.; El-Mallah, S.Y.; Seoudi, S.; Hassan, M.; Mohsen, I.A. Effects of the long-term use of depot medroxyprogesterone acetate as hormonal contraceptive on bone mineral density and biochemical markers of bone remodeling. Contraception 2006, 74, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Vanderjagt, D.J.; Sagay, A.S.; Imade, G.E.; Farmer, S.E.; Glew, R.H. Effect of Norplant contraceptive on the bones of Nigerian women as assessed by quantitative ultrasound and serum markers of bone turnover. Contraception 2005, 72, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.M.; Scholes, D.; LaCroix, A.Z.; Ichikawa, L.E.; Yoshida, C.K.; Barlow, W.E. Effects of contraceptive use on bone biochemical markers in young women. J. Clin. Endocrinol. Metab. 2001, 86, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Schiele, F.; Vincent-Viry, M.; Fournier, B.; Starck, M.; Siest, G. Biological effects of eleven combined oral contraceptives on serum triglycerides, gamma-glutamyltransferase, alkaline phosphatase, bilirubin and other biochemical variables. Clin. Chem. Lab. Med. 1998, 36, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Ponthieux, A.; Herbeth, B.; Droesch, S.; Haddy, N.; Lambert, D.; Visvikis, S. Biological determinants of serum ICAM-1, E-selectin, P-selectin and L-selectin levels in healthy subjects: The Stanislas study. Atherosclerosis 2004, 172, 299–308. [Google Scholar] [CrossRef]
- Weinbrenner, T.; Zittermann, A.; Gouni-Berthold, I.; Stehle, P.; Berthold, H.K. Body mass index and disease duration are predictors of disturbed bone turnover in anorexia nervosa. A case-control study. Eur. J. Clin. Nutr. 2003, 57, 1262–1267. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Callegari, E.T.; Garland, S.M.; Gorelik, A.; Chiang, C.Y.; Wark, J.D. Bone turnover marker determinants in young women: Results from the Safe-D study. Ann. Clin. Biochem. 2018, 55, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Maïmoun, L.; Guillaume, S.; Lefebvre, P.; Philibert, P.; Bertet, H.; Picot, M.-C.; Gaspari, L.; Paris, F.; Seneque, M.; Dupuys, A.-M.; et al. Evidence of a link between resting energy expenditure and bone remodelling, glucose homeostasis and adipokine variations in adolescent girls with anorexia nervosa. Osteoporos. Int. 2016, 27, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Campesi, I.; Sanna, M.; Zinellu, A.; Carru, C.; Rubattu, L.; Bulzomi, P.; Seghieri, G.; Tonolo, G.; Palermo, M.; Rosano, G.; et al. Oral contraceptives modify DNA methylation and monocyte-derived macrophage function. Biol. Sex Differ. 2012, 3, 4. [Google Scholar] [CrossRef]
- Guañabens, N.; Filella, X.; Monegal, A.; Gómez-Vaquero, C.; Bonet, M.; Buquet, D.; Casado, E.; Cerdá, D.; Erra, A.; Martinez, S.; et al. Reference intervals for bone turnover markers in Spanish premenopausal women. Clin. Chem. Lab. Med. 2016, 54, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Revilla, M.; Fraile, E.; Aguado, F.; Hermandez, E.R.; Villa, L.F.; Rico, H. Vertebral and metacarpal morphometry as indicators of nutritional improvement. Clin. Rheumatol. 1997, 16, 279–283. [Google Scholar] [CrossRef]
- Simpson, G.R.; Dale, E. Serum levels of phosphorus, magnesium, and calcium in women utilizing combination oral or long-acting injectable progestational contraceptives. Fertil. Steril. 1972, 23, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Seeman, E.; Szmukler, G.I.; Formica, C.; Tsalamandris, C.; Mestrovic, R. Osteoporosis in anorexia nervosa: The influence of peak bone density, bone loss, oral contraceptive use, and exercise. J. Bone Miner. Res. 1992, 7, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Engineer, A.D.; Gupta, V.; Tandon, P. Liver function tests in patients on oral progestogens. J. Obstet. Gynaecol. India 1968, 18, 598–605. [Google Scholar]
- Chieffi, O.; Brogioni, M.; Saltarelli, O.; Pecchioli, S.; Benardi, M. Oestroprogestogen interference on some haematochemical parameters: Glycaemia, lipid metabolism and hepatic functionality (author’s transl). Patol. Clin. Ostet. Ginecol. 1981, 9, 7–17. [Google Scholar] [PubMed]
- Guisado-Cuadrado, I.; Romero-Parra, N.; Elliott-Sale, K.J.; Sale, C.; Díaz, Á.E.; Peinado, A.B. Influence of Menstrual Cycle and Oral Contraceptive Phases on Bone (re)modelling Markers in Response to Interval Running. Calcif. Tissue Int. 2024, 115, 382–392. [Google Scholar] [CrossRef] [PubMed]
- DeMasi, T.; Tsang, M.; Mueller, J.; Giltvedt, K.; Nguyen, T.N.; Kern, M.; Hooshmand, S. Prunes May Blunt Adverse Effects of Oral Contraceptives on Bone Health in Young Adult Women: A Randomized Clinical Trial. Curr. Dev. Nutr. 2024, 8, 104417. [Google Scholar] [CrossRef] [PubMed]
- Resulaj, M.; Polineni, S.; Meenaghan, E.; Eddy, K.; Lee, H.; Fazeli, P.K. Transdermal Estrogen in Women with Anorexia Nervosa: An Exploratory Pilot Study. JBMR Plus 2020, 4, e10251. [Google Scholar] [CrossRef]
- Singhal, V.; Ackerman, K.E.; Bose, A.; Flores, L.P.T.; Lee, H.; Misra, M. Impact of Route of Estrogen Administration on Bone Turnover Markers in Oligoamenorrheic Athletes and Its Mediators. J. Clin. Endocrinol. Metab. 2019, 104, 1449–1458. [Google Scholar] [CrossRef]
- Warren, M.P.; Miller, K.K.; Olson, W.H.; Grinspoon, S.K.; Friedman, A.J. Effects of an oral contraceptive (norgestimate/ethinyl estradiol) on bone mineral density in women with hypothalamic amenorrhea and osteopenia: An open-label extension of a double-blind, placebo-controlled study. Contraception 2005, 72, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Grinspoon, S.K.; Friedman, A.J.; Miller, K.K.; Lippman, J.; Olson, W.H.; Warren, M.P. Effects of a triphasic combination oral contraceptive containing norgestimate/ethinyl estradiol on biochemical markers of bone metabolism in young women with osteopenia secondary to hypothalamic amenorrhea. J. Clin. Endocrinol. Metab. 2003, 88, 3651–3656. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Castelo-Branco, C.; Vicente, J.J.; Pons, F.; Martínez de Osaba, M.J.; Casals, E.; Vanrell, J.A. Bone mineral density in young, hypothalamic oligoamenorrheic women treated with oral contraceptives. J. Reprod. Med. 2001, 46, 875–879. [Google Scholar]
- Gregoriou, O.; Bakas, P.; Konidaris, S.; Papadias, K.; Mathiopoulos, D.; Creatsas, G. The effect of combined oral contraception with or without spironolactone on bone mineral density of hyperandrogenic women. Gynecol. Endocrinol. 2000, 14, 369–373. [Google Scholar] [CrossRef]
- Volpe, A.; Amram, A.; Cagnacci, A.; Battaglia, C. Biochemical aspects of hormonal contraception: Effects on bone metabolism. Eur. J. Contracept. Reprod. Health Care 1997, 2, 123–126. [Google Scholar] [CrossRef]
- Ackerman, K.E.; Singhal, V.; Baskaran, C.; Slattery, M.; Reyes, K.J.C.; Toth, A.; Eddy, K.T.; Bouxsein, M.L.; Lee, H.; Klibanski, A.; et al. Oestrogen replacement improves bone mineral density in oligo-amenorrhoeic athletes: A randomised clinical trial. Br. J. Sports Med. 2019, 53, 229–236. [Google Scholar] [CrossRef]
- Tuppurainen, M.; Kröger, H.; Saarikoski, S.; Honkanen, R.; Alhava, E. The effect of previous oral contraceptive use on bone mineral density in perimenopausal women. Osteoporos. Int. 1994, 4, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, P.; Valsamis, J.; Van Perborgh, J.; De Schepper, J.; Van Vliet, G. Comparative study of the changes in insulin-like growth factor-I, procollagen-III N-terminal extension peptide, bone Gla-protein, and bone mineral content in children with Turner’s syndrome treated with recombinant growth hormone. J. Clin. Endocrinol. Metab. 1990, 71, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Suwikrom, S.; Jaisamrarn, U. Comparison of the metabolic effects of oral contraceptive and nonhormonal contraceptive use in women over 40 years old. Contraception 2005, 71, 183–187. [Google Scholar] [CrossRef]
- Grecu, E.O.; Weinshelbaum, A.; Simmons, R. Effective therapy of glucocorticoid-induced osteoporosis with medroxyprogesterone acetate. Calcif. Tissue Int. 1990, 46, 294–299. [Google Scholar] [CrossRef] [PubMed]
- DiVasta, A.D.; Feldman, H.A.; O’Donnell, J.M.; Long, J.; Leonard, M.B.; Gordon, C.M. Impact of Adrenal Hormone Supplementation on Bone Geometry in Growing Teens with Anorexia Nervosa. J. Adolesc. Health 2019, 65, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Falsetti, L.; Galbignani, E. Long-term treatment with the combination ethinylestradiol and cyproterone acetate in polycystic ovary syndrome. Contraception 1990, 42, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Gambacciani, M.; Ciaponi, M.; Cappagli, B.; Benussi, C.; Genazzani, A.R. Longitudinal evaluation of perimenopausal femoral bone loss: Effects of a low-dose oral contraceptive preparation on bone mineral density and metabolism. Osteoporos. Int. 2000, 11, 544–548. [Google Scholar] [CrossRef]
- Gambacciani, M.; Spinetti, A.; Taponeco, F.; Cappagli, B.; Piaggesi, L.; Fioretti, P. Longitudinal evaluation of perimenopausal vertebral bone loss: Effects of a low-dose oral contraceptive preparation on bone mineral density and metabolism. Obstet. Gynecol. 1994, 83, 392–396. [Google Scholar]
- Vexiau, P.; Gueux, B.; Vexiau-Robert, D.; Fiet, J.; Laureaux, C.; Tabuteau, F.; Brerault, J.; Mathieson, J.; Cathelineau, G. Metabolic effects of combined cyproterone acetate and percutaneous 17 beta oestradiol after six and twelve months therapy in 61 patients. Horm. Metab. Res. 1988, 20, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Divasta, A.D.; Feldman, H.A.; Giancaterino, C.; Rosen, C.J.; Leboff, M.S.; Gordon, C.M. The effect of gonadal and adrenal steroid therapy on skeletal health in adolescents and young women with anorexia nervosa. Metabolism 2012, 61, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.M.; Grace, E.; Emans, S.J.; Feldman, H.A.; Goodman, E.; Becker, K.A.; Rosen, C.J.; Gundberg, C.M.; LeBoff, M.S. Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: A randomized trial. J. Clin. Endocrinol. Metab. 2002, 87, 4935–4941. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.M.; Grace, E.; Emans, S.J.; Goodman, E.; Crawford, M.H.; Leboff, M.S. Changes in bone turnover markers and menstrual function after short-term oral DHEA in young women with anorexia nervosa. J. Bone Miner. Res. 1999, 14, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, A.S.; Wyness, S.P.; Ray, J.A.; Willcox, T.L.; Seiter, J.D.; Genzen, J.R. Detection and characterization of estradiol (E2) and unconjugated estriol (uE3) immunoassay interference due to anti-bovine alkaline phosphatase (ALP) antibodies. Pract. Lab. Med. 2019, 17, e00131. [Google Scholar] [CrossRef]
- Iida, J.; Yoshikawa, T.; Miyazaki, K.; Okumura, N.; Takakura, Y. Osteogenic Potential of Estriol-Treated Cultured Bone—In Vitro and In Vivo. KEM 2005, 284–286, 651–654. [Google Scholar] [CrossRef]
- Douxfils, J.; Gaspard, U.; Taziaux, M.; Jost, M.; Bouvy, C.; Lobo, R.A.; Utian, W.H.; Foidart, J.-M. Impact of estetrol (E4) on hemostasis, metabolism and bone turnover in postmenopausal women. Climacteric 2023, 26, 55–63. [Google Scholar] [CrossRef]
- Christiansen, C. Effects of drospirenone/estrogen combinations on bone metabolism. Climacteric 2005, 8 (Suppl. S3), 35–41. [Google Scholar] [CrossRef] [PubMed]
- Nguyên-Pascal, M.L.; Thomas, J.L.; Bergougnoux, L.; Garnero, P.; Drapier-Faure, E.; Delmas, P.D. Nomegestrol acetate may enhance the skeletal effects of estradiol on biochemical markers of bone turnover in menopausal women after a 12-week treatment period. Climacteric 2005, 8, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Ormarsdóttir, S.; Mallmin, H.; Naessén, T.; Petrén-Mallmin, M.; Broomé, U.; Hultcrantz, R.; Lööf, L. An open, randomized, controlled study of transdermal hormone replacement therapy on the rate of bone loss in primary biliary cirrhosis. J. Intern. Med. 2004, 256, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Gutteridge, D.H.; Holzherr, M.L.; Retallack, R.W.; Price, R.I.; Will, R.K.; Dhaliwal, S.S.; Faulkner, D.L.; Stewart, G.O.; Stuckey, B.G.A.; Prince, R.L.; et al. A randomized trial comparing hormone replacement therapy (HRT) and HRT plus calcitriol in the treatment of postmenopausal osteoporosis with vertebral fractures: Benefit of the combination on total body and hip density. Calcif. Tissue Int. 2003, 73, 33–43. [Google Scholar] [CrossRef]
- Paoletti, A.M.; Pilloni, M.; Orrù, M.; Floris, S.; Pistis, M.; Guerriero, S.; Ajossa, S.; Melis, G.B. Efficacy and safety of oral and transdermal hormonal replacement treatment containing levonorgestrel. Maturitas 2002, 42, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Bernard-Poenaru, O.; Roux, C.; Blanqué, R.; Gardner, C.; de Vemejoul, M.C.; Cohen-Solal, M.E. Bone-resorbing cytokines from peripheral blood mononuclear cells after hormone replacement therapy: A longitudinal study. Osteoporos. Int. 2001, 12, 769–776. [Google Scholar] [CrossRef]
- Yoshitake, K.; Yokota, K.; Kasugai, Y.; Kagawa, M.; Sukamoto, T.; Nakamura, T. Effects of 16 weeks of treatment with tibolone on bone mass and bone mechanical and histomorphometric indices in mature ovariectomized rats with established osteopenia on a low-calcium diet. Bone 1999, 25, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.; Eastell, R. Effects of estrogen therapy of postmenopausal women on cytokines measured in peripheral blood. J. Bone Miner. Res. 1998, 13, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Prelević, G.M.; Beljić, T.; Balint-Perić, L.; Petrović, J.; Elliesen, J. Effect of two different progestins (cyproterone acetate and norgestrel), administered in a cyclical estradiol valerate regimen, on markers of bone turnover. Gynecol. Endocrinol. 1994, 8, 209–214. [Google Scholar] [CrossRef]
- Saure, A.; Hirvonen, E.; Tikkanen, M.J.; Viinikka, L.; Ylikorkala, O. A novel oestradiol–desogestrel preparation for hormone replacement therapy: Effects on hormones, lipids, bone, climacteric symptoms and endometrium. Maturitas 1993, 16, 1–12. [Google Scholar] [CrossRef]
- Tarallo, P.; Henny, J.; Fournier, B.; Siest, G. Plasma osteocalcin: Biological variations and reference limits. Scand. J. Clin. Lab. Investig. 1990, 50, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Leis, D.; Zach, H.; Kohler, P. [Comparative high rising dose study of oral 17-alpha-ethinylestradiol (EE2), estriol (E3), and parenteral 16-alpha-17-beta-estrioldihemisuccinate (E3-suc) in their effects on serum levels of glutamate transaminase (GOT), pyruvate transaminase (GPT), leucine amino peptidase (LAP), alkaline phosphatase (AP), and bilirubin in 30 hysterectomized and ovarectomized women (author’s transl)]. Arch. Gynecol. 1978, 226, 333–339. [Google Scholar]
- Marslew, U.; Riis, B.J.; Christiansen, C. Desogestrel in hormone replacement therapy: Long-term effects on bone, calcium and lipid metabolism, climacteric symptoms, and bleeding. Eur. J. Clin. Investig. 1991, 21, 601–607. [Google Scholar] [CrossRef]
- Agostino, H.; Di Meglio, G. Low-dose oral contraceptives in adolescents: How low can you go? J. Pediatr. Adolesc. Gynecol. 2010, 23, 195–201. [Google Scholar] [CrossRef]
- Rocca, M.L.; Palumbo, A.R.; Bitonti, G.; Brisinda, C.; DI Carlo, C. Bone health and hormonal contraception. Minerva Obstet. Gynecol. 2021, 73, 678–696. [Google Scholar] [CrossRef]
- Wei, S.; Winzenberg, T.; Laslett, L.L.; Venn, A.; Jones, G. Oral contraceptive use and bone. Curr. Osteoporos. Rep. 2011, 9, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Beral, V.; Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 2003, 362, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Pasqualini, J.R.; Paris, J.; Sitruk-Ware, R.; Chetrite, G.; Botella, J. Progestins and breast cancer. J. Steroid Biochem. Mol. Biol. 1998, 65, 225–235. [Google Scholar] [CrossRef]
- de Lignieres, B.; Dennerstein, L.; Backstrom, T. Influence of route of administration on progesterone metabolism. Maturitas 1995, 21, 251–257. [Google Scholar] [CrossRef]
- Cagnacci, A.; Arangino, S.; Baldassari, F.; Alessandrini, C.; Landi, S.; Volpe, A. A comparison of the central effects of different progestins used in hormone replacement therapy. Maturitas 2004, 48, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Cagnacci, A.; Biasioli, A. The Effect of Hormonal Contraceptives on Metabolism. In Female and Male Contraception; Meriggiola, M.C., Gemzell-Danielsson, K., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 299–317. [Google Scholar]
- Grandi, G.; Napolitano, A.; Cagnacci, A. Metabolic impact of combined hormonal contraceptives containing estradiol. Expert Opin. Drug Metab. Toxicol. 2016, 12, 779–787. [Google Scholar] [CrossRef]
- Cagnacci, A.; Zanin, R.; Cannoletta, M.; Generali, M.; Caretto, S.; Volpe, A. Menopause, estrogens, progestins, or their combination on body weight and anthropometric measures. Fertil. Steril. 2007, 88, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Schindler, A.E.; Campagnoli, C.; Druckmann, R.; Huber, J.; Pasqualini, J.R.; Schweppe, K.W.; Thijssen, J.H. Classification and pharmacology of progestins. Maturitas 2008, 61, 171–180. [Google Scholar] [CrossRef] [PubMed]


| Study | Condition | Country | Study Period | Allocation | Age Range (Min–Max) |
|---|---|---|---|---|---|
| Orsolini 2023 [44] | Healthy | Brazil | 2014–2020 | Unknown | 12–20 |
| Caldeirão 2022 [13] | Healthy | Brazil | 2014–2020 | Unknown | 15–20 |
| Almstedt 2020 [14] | Healthy | USA | 2016–2017 | Systematic allocation | 18–20 |
| Tiedeken 2019 [15] | Healthy | USA | 2012–2013 | Random allocation | 18–39 |
| Rizzo 2019 [16] | Healthy | Brazil | 2011–2017 | Systematic allocation | 12–20 |
| Iltemir Duvan 2017 [17] | Breast feeding | Turkey | 2009–2011 | Systematic allocation | 24–38 |
| Mawet 2015 [18] | Healthy | Netherlands | 2009 | Random allocation | 18–35 |
| Hernandez-Juarez 2014 [43] | Healthy | Mexico | 2011–2013 | Systematic allocation | 18–35 |
| Di Carlo 2013 [19] | Healthy | Italy | 2011 | Single arm | 21–34 |
| Cibula 2012 [34] | Healthy | Czech Republic | 2006–2008 | Random allocation | 15–20 |
| Misra 2011 [46] | Healthy/amenorrhea | Multinational | 2009–2010 | Systematic and random allocation | 12–18 |
| Massaro 2010 [20] | Healthy | Italy | 2008 | Random allocation | 23–34 |
| Harel 2010 [21] | Healthy | USA | 2008–2009 | Systematic allocation | 12–21 |
| Lattakova 2009 [22] | Healthy | Slovakia | 2007–2008 | Systematic allocation | 16–19 |
| Gargano 2008 [23] | Healthy | Italy | 2006 | Random allocation | 21–34 |
| Kaunitz 2006 [35] | Healthy | USA | 1997–2004 | Unknown | 25–35 |
| Nappi 2005 [24] | Healthy | Italy | 2002–2003 | Random allocation | 22–34 |
| Paoletti 2004 [26] | Healthy | Italy | 2003 | Systematic allocation | 20–30 |
| Endrikat 2004 [27] | Healthy | Germany | 1997–2001 | Random allocation | 20–38 |
| Rome 2004 [25] | Healthy | USA | 2002–2003 | Systematic allocation | 12–18 |
| Nappi 2003 [28] | Healthy | Italy | 2000–2002 | Random allocation | 22–34 |
| Paoletti 2000 [29] | Healthy | Italy | 1997–1999 | Systematic and random allocation | 22–30 |
| Naessen 1995 [30] | Healthy | Sweden | 1993–1994 | Random allocation | 20–45 |
| Mais 1993 [31] | Healthy | Italy | 1991–1992 | Single arm | 20–30 |
| Etzrodt 1990 [39] | Healthy | Germany | 1987–1988 | Unknown | 18–35 |
| Hurley 1989 [38] | Healthy | USA | 1987–1988 | Systematic allocation | 21–29 |
| Sadik 1985 [33] | Healthy | Multinational | 1983–1984 | Systematic and random allocation | 18–39 |
| Ahrén 1981 [36] | Healthy | Sweden | 1979–1980 | Unknown | 18–35 |
| Bamji 1981 [42] | Healthy | India | 1978–1979 | Single arm | Unknown |
| Roy 1980 [32] | Healthy | USA | 1976–1978 | Systematic and random allocation | 17–33 |
| Amatayakul 1980 [37] | Healthy | Thailand | 1976–1977 | Single arm | 18–29 |
| Amatayakul 1978 [45] | Healthy | Thailand | 1976–1977 | Single arm | 18–29 |
| Brügmann 1975 [41] | Healthy | Germany | 1973–1974 | Single arm | 17–38 |
| García 1968 [40] | Healthy | USA | 1966–1968 | Single arm | Unknown |
| Variable | SMC (CI.95)/p-Value (all) | SMC (CI.95)/p-Value (≤21) | SMC (CI.95)/p-Value (>21) |
|---|---|---|---|
| Estrogens | |||
| Bone-formation markers | |||
| OC | <0.001 (*) | 0.944 (*) | <0.001 (*) |
| E2V | −0.10 (−0.47/0.27) | --- | −0.10 (−0.47/0.27) |
| E4 | −0.43 (−0.76/−0.10) | --- | −0.43 (−0.76/−0.10) |
| EE | −0.54 (−0.64/−0.43) | −0.63 (−0.77/−0.49) | −0.42 (−0.61/−0.24) |
| Only progestin | 0.55 (0.07/1.04) | --- | 0.55 (0.07/1.04) |
| Controls | −0.12 (−0.35/0.10) | −0.65 (−1.06/−0.23) | 0.01 (−0.20/0.21) |
| ALP (bone) | 0.501 (*) | 0.321 (*) | 0.949 (*) |
| EE | −0.39 (−0.67/−0.11) | −0.55 (−0.86/−0.24) | −0.06 (−0.47/0.35) |
| Only progestin | 0.13 (−0.14/0.40) | 0.13 (−0.14/0.40) | --- |
| Controls | −0.40 (−0.93/0.14) | −0.56 (−1.27/0.16) | −0.03 (−0.42/0.35) |
| ALP (all) | 0.134 (*) | 0.321 (*) | 0.208 (*) |
| E2 | −0.41 (−0.78/−0.04) | --- | −0.41 (−0.78/−0.04) |
| E2EN | −0.23 (−0.63/0.16) | --- | −0.23 (−0.63/0.16) |
| EE | −0.46 (−0.62/−0.30) | −0.55 (−0.86/−0.24) | −0.42 (−0.60/−0.24) |
| EEME | −0.61 (−1.47/0.25) | --- | −0.61 (−1.47/0.25) |
| EES | 0.00 (−0.46/0.46) | --- | 0.00 (−0.46/0.46) |
| Only progestin | 0.02 (−0.23/0.27) | 0.13 (−0.14/0.40) | −0.02 (−0.33/0.30) |
| Controls | −0.29 (−0.70/0.11) | −0.56 (−1.27/0.16) | −0.05 (−0.19/0.09) |
| PINP | 0.001 (*) | 0.083 (*) | --- |
| E2 | 0.12 (−0.10/0.33) | --- | 0.12 (−0.10/0.33) |
| EE | −0.55 (−0.83/−0.26) | −0.55 (−0.83/−0.26) | --- |
| Controls | −0.20 (−0.50/0.10) | −0.20 (−0.50/0.10) | --- |
| Bone-resorption markers | |||
| DPD | <0.001 (*) | 0.436 (*) | <0.001 (*) |
| E2V | −1.57 (−2.13/−1.02) | --- | −1.57 (−2.13/−1.02) |
| EE | −0.33 (−0.43/−0.22) | −0.07 (−0.22/0.08) | −0.58 (−0.73/−0.42) |
| Only progestin | −0.07 (−0.34/0.20) | −0.07 (−0.34/0.20) | --- |
| Controls | −0.02 (−0.14/0.10) | 0.07 (−0.09/0.23) | −0.13 (−0.31/0.05) |
| PYD | 0.003 (*) | --- | 0.003 (*) |
| E2V | −0.69 (−1.10/−0.27) | --- | −0.69 (−1.10/−0.27) |
| EE | −0.37 (−0.53/−0.21) | --- | −0.37 (−0.53/−0.21) |
| Controls | −0.05 (−0.23/0.13) | --- | −0.05 (−0.23/0.13) |
| NTX | 0.084 (*) | 0.149 (*) | --- |
| EE | −0.43 (−0.74/−0.12) | −0.45 (−1.37/0.47) | −0.43 (−0.76/−0.10) |
| Controls | −1.65 (−3.00/−0.30) | −1.65 (−3.00/−0.30) | --- |
| CTX | 0.02 (*) | 0.908 (*) | <0.001 (*) |
| E2 | 0.25 (0.02/0.48) | --- | 0.25 (0.02/0.48) |
| E4 | −0.41 (−0.63/−0.20) | --- | −0.41 (−0.63/−0.20) |
| EE | −0.38 (−0.69/−0.07) | −0.26 (−0.48/−0.03) | −1.33 (−1.93/−0.73) |
| Controls | −0.23 (−0.56/0.10) | −0.23 (−0.56/0.10) | --- |
| Progestins | |||
| Bone-formation markers | |||
| OC | 0.005 (*) | 0.628 (*) | 0.004 (*) |
| Controls | −0.12 (−0.35/0.10) | −0.65 (−1.06/−0.23) | 0.01 (−0.20/0.21) |
| Anti-androgenic | −0.61 (−0.79/−0.43) | −0.69 (−0.87/−0.50) | −0.59 (−0.88/−0.30) |
| Neutral | −0.23 (−1.10/0.65) | −1.11 (−2.22/0.01) | 0.07 (−0.82/0.97) |
| Pro-androgenic | −0.31 (−0.47/−0.15) | −0.54 (−0.75/−0.33) | −0.18 (−0.36/−0.01) |
| ALP (bone) | 0.059 (*) | 0.085 (*) | 0.084 (*) |
| Controls | −0.40 (−0.93/0.14) | −0.56 (−1.27/0.16) | −0.03 (−0.42/0.35) |
| Anti-androgenic | −0.79 (−1.15/−0.44) | −0.97 (−1.20/−0.73) | −0.42 (−0.81/−0.04) |
| Neutral | 0.02 (−0.43/0.47) | 0.02 (−0.43/0.47) | |
| Pro-androgenic | −0.16 (−0.37/0.05) | −0.27 (−0.48/−0.06) | 0.14 (−0.18/0.46) |
| ALP (all) | 0.087 (*) | 0.085 (*) | 0.360 (*) |
| Controls | −0.29 (−0.70/0.11) | −0.56 (−1.27/0.16) | −0.05 (−0.19/0.09) |
| Anti-androgenic | −0.64 (−1.03/−0.26) | −0.97 (−1.20/−0.73) | −0.31 (−0.57/−0.04) |
| Neutral | −0.02 (−0.23/0.20) | 0.02 (−0.43/0.47) | −0.12 (−0.41/0.17) |
| Pro-androgenic | −0.35 (−0.49/−0.21) | −0.27 (−0.48/−0.06) | −0.36 (−0.54/−0.19) |
| PINP | 0.001 (*) | 0.083 (*) | --- |
| Controls | −0.20 (−0.50/0.10) | −0.20 (−0.50/0.10) | --- |
| Neutral | 0.12 (−0.10/0.33) | 0.12 (−0.10/0.33) | |
| Pro-androgenic | −0.55 (−0.83/−0.26) | −0.55 (−0.83/−0.26) | --- |
| Bone-resorption markers | |||
| DPD | <0.001 (*) | 0.436 (*) | <0.001 (*) |
| Controls | −0.02 (−0.14/0.10) | 0.07 (−0.09/0.23) | −0.13 (−0.31/0.05) |
| Anti-androgenic | −0.84 (−1.05/−0.63) | --- | −0.84 (−1.05/−0.63) |
| Neutral | −0.21 (−0.46/0.03) | −0.07 (−0.34/0.20) | −0.88 (−1.46/−0.30) |
| Pro-androgenic | −0.19 (−0.31/−0.06) | −0.07 (−0.22/0.08) | −0.41 (−0.63/−0.20) |
| PYD | <0.001 (*) | --- | <0.001 (*) |
| Controls | −0.05 (−0.23/0.13) | --- | −0.05 (−0.23/0.13) |
| Anti-androgenic | −0.56 (−0.75/−0.37) | --- | −0.56 (−0.75/−0.37) |
| Neutral | −0.63 (−1.17/−0.10) | --- | −0.63 (−1.17/−0.10) |
| Pro-androgenic | −0.19 (−0.40/0.02) | --- | −0.19 (−0.40/0.02) |
| NTX | 0.225 (*) | 0.149 (*) | --- |
| Controls | −1.65 (−3.00/−0.30) | −1.65 (−3.00/−0.30) | --- |
| Neutral | −0.45 (−1.37/0.47) | −0.45 (−1.37/0.47) | --- |
| Pro-androgenic | −0.43 (−0.76/−0.10) | −0.43 (−0.76/−0.10) | |
| CTX | 0.020 (*) | 0.978 (*) | 0.001 (*) |
| Controls | −0.23 (−0.56/0.10) | −0.23 (−0.56/0.10) | --- |
| Anti-androgenic | −0.47 (−0.84/−0.10) | −0.22 (−0.50/0.06) | −0.68 (−1.27/−0.10) |
| Neutral | 0.25 (0.02/0.48) | --- | 0.25 (0.02/0.48) |
| Pro-androgenic | −0.33 (−0.57/−0.09) | −0.28 (−0.61/0.06) | −0.43 (−0.78/−0.09) |
| Variable | SMC (CI.95)/p-Value (all) | SMC (CI.95)/p-Value (≤21) | SMC (CI.95)/p-Value (>21) |
|---|---|---|---|
| Estrogens | |||
| Bone-formation markers (all) | <0.001 (*) | 0.027 (*) | 0.001 (*) |
| Controls | −0.17 (−0.33/−0.02) | −0.43 (−0.75/−0.11) | −0.03 (−0.15/0.08) |
| E2 | −0.04 (−0.28/0.20) | --- | −0.04 (−0.28/0.20) |
| E2V | −0.10 (−0.47/0.27) | --- | −0.10 (−0.47/0.27) |
| E2EN | −0.23 (−0.63/0.16) | --- | −0.23 (−0.63/0.16) |
| E4 | −0.43 (−0.76/−0.10) | --- | −0.43 (−0.76/−0.10) |
| EE | −0.49 (−0.59/−0.39) | −0.58 (−0.72/−0.44) | −0.42 (−0.54/−0.29) |
| EEME | −0.61 (−1.47/0.25) | --- | −0.61 (−1.47/0.25) |
| EES | 0.00 (−0.46/0.46) | --- | 0.00 (−0.46/0.46) |
| Only progestin | 0.11 (−0.13/0.36) | 0.13 (−0.14/0.40) | 0.12 (−0.18/0.42) |
| Bone-resorption markers (all) | <0.001 (*) | 0.833 (*) | <0.001 (*) |
| Controls | −0.07 (−0.16/0.02) | −0.21 (−0.52/0.10) | −0.09 (−0.22/0.04) |
| E2 | 0.25 (0.03/0.47) | --- | 0.25 (0.02/0.48) |
| E2V | −1.00 (−1.33/−0.67) | --- | −1.11 (−1.98/−0.24) |
| E4 | −0.41 (−0.63/−0.20) | --- | −0.41 (−0.63/−0.20) |
| EE | −0.33 (−0.40/−0.26) | −0.22 (−0.40/−0.04) | −0.49 (−0.62/−0.37) |
| Only progestin | −0.07 (−0.34/0.20) | −0.07 (−0.34/0.20) | --- |
| Progestins | |||
| Bone-formation markers (all) | <0.001 (*) | 0.010 (*) | <0.001 (*) |
| Controls | −0.17 (−0.33/−0.02) | −0.43 (−0.75/−0.11) | −0.03 (−0.15/0.08) |
| Anti-androgenic | −0.63 (−0.79/−0.46) | −0.80 (−0.94/−0.65) | −0.51 (−0.74/−0.29) |
| Neutral | 0.02 (−0.11/0.16) | −0.31 (−1.02/0.40) | 0.02 (−0.14/0.18) |
| Pro-androgenic | −0.34 (−0.44/−0.25) | −0.42 (−0.58/−0.27) | −0.31 (−0.44/−0.18) |
| Bone-resorption markers (all) | <0.001 (*) | 0.993 (*) | <0.001 (*) |
| Controls | −0.07 (−0.16/0.02) | −0.21 (−0.52/0.10) | −0.09 (−0.22/0.04) |
| Anti-androgenic | −0.54 (−0.65/−0.44) | −0.22 (−0.50/0.06) | −0.70 (−0.89/−0.51) |
| Neutral | −0.03 (−0.18/0.13) | −0.10 (−0.36/0.16) | −0.12 (−0.61/0.37) |
| Pro-androgenic | −0.24 (−0.32/−0.15) | −0.22 (−0.48/0.04) | −0.34 (−0.47/−0.22) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tassi, A.; Londero, A.P.; Xholli, A.; Lanzolla, G.; Bertozzi, S.; Savelli, L.; Prefumo, F.; Cagnacci, A. Hormonal Contraception and Bone Metabolism: Emerging Evidence from a Systematic Review and Meta-Analysis of Studies on Post-Pubertal and Reproductive-Age Women. Pharmaceuticals 2025, 18, 61. https://doi.org/10.3390/ph18010061
Tassi A, Londero AP, Xholli A, Lanzolla G, Bertozzi S, Savelli L, Prefumo F, Cagnacci A. Hormonal Contraception and Bone Metabolism: Emerging Evidence from a Systematic Review and Meta-Analysis of Studies on Post-Pubertal and Reproductive-Age Women. Pharmaceuticals. 2025; 18(1):61. https://doi.org/10.3390/ph18010061
Chicago/Turabian StyleTassi, Alice, Ambrogio P Londero, Anjeza Xholli, Giulia Lanzolla, Serena Bertozzi, Luca Savelli, Federico Prefumo, and Angelo Cagnacci. 2025. "Hormonal Contraception and Bone Metabolism: Emerging Evidence from a Systematic Review and Meta-Analysis of Studies on Post-Pubertal and Reproductive-Age Women" Pharmaceuticals 18, no. 1: 61. https://doi.org/10.3390/ph18010061
APA StyleTassi, A., Londero, A. P., Xholli, A., Lanzolla, G., Bertozzi, S., Savelli, L., Prefumo, F., & Cagnacci, A. (2025). Hormonal Contraception and Bone Metabolism: Emerging Evidence from a Systematic Review and Meta-Analysis of Studies on Post-Pubertal and Reproductive-Age Women. Pharmaceuticals, 18(1), 61. https://doi.org/10.3390/ph18010061








