Distribution, Phytochemical Insights, and Cytotoxic Potential of the Sesbania Genus: A Comprehensive Review of Sesbania grandiflora, Sesbania sesban, and Sesbania cannabina
Abstract
:1. Introduction
2. Methodology
3. Sesbania Genus: Selected Species and Their Distribution
3.1. Sesbania Adans
3.2. Sesbania sesban (L.) Merr.
3.3. Sesbania grandiflora (L.) Poir.
3.4. Sesbania cannabina (Retz.) Poir.
4. Sesbania Adans Propagation
4.1. S. sesban
4.2. S. grandiflora
4.3. S. cannabina
5. Sesbania Phytochemistry
5.1. Sesbania grandiflora
5.2. Sesbania sesban
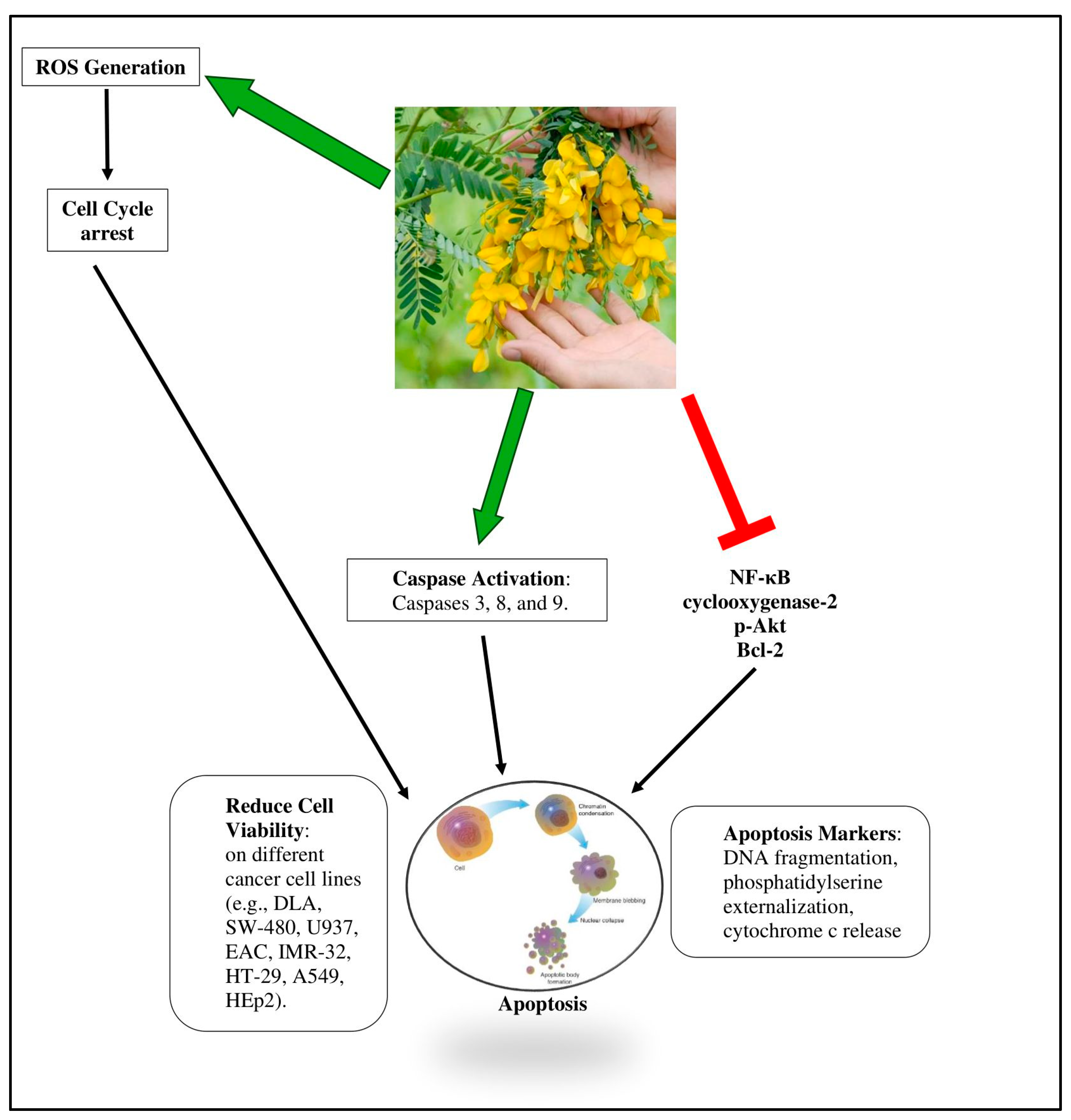
6. Cytotoxic Effect of Sesbania Species
6.1. Cytotoxicity of Sesbania grandiflora
6.2. Cytotoxicity of Sesbania cannabina
6.3. Cytotoxicity of Sesbania sesban
| Plant Species | Part Used | Type of Study | Type of Extract | Results | Reference |
|---|---|---|---|---|---|
| Sesbania grandiflora | Flower | In vitro: human cancer cell lines and two ascites tumor cell lines from mice | Protein fraction: SF2 | Limited the growth of cells and brought about apoptosis by the activation of caspases 3, 8, and 9, cleavage of poly (ADP-ribose) polymerase, and release of cytochrome c, all of which point to the death of cells through apoptosis. | [53] |
| In vivo: Swiss Albino mice bearing Ehrlich ascites carcinoma (EAC) | Ethanol extracts at doses of 100 and 200 mg/kg body weight | Considerable reductions in tumor weight, viable cell count, and volume. | [54] | ||
| Leaf | In vitro: neuroblastoma (IMR-32), and colon (HT-29) cell lines | Extract concentration ranged from 50 to 300 μg/mL | All the extracts have an IC50 of 200 μg/mL against the neuroblastoma (IMR-32) and colon (HT-29) cell lines. With increasing extract concentration cell viability decreased. | [21] | |
| Leaf | In vitro: MCF-7, HepG2, Hep-2, HCT-15, and A549 | Methanolic extract | Extracts activated caspase-3, increased ROS intermediates, as well as decreased the levels of cyclin D1, which caused apoptosis. | [59] | |
| In vitro: U937 cells | Protein fraction: F2 | Extract had an IC50 of 18.6 μg/mL. Cytotoxicity was achieved by decreasing oxygen consumption and increasing reactive oxygen species formation as well as release of cytochrome c, which activates pro-apoptotic proteins. | [56] | ||
| Leaf | In vitro: HEp2 (human larynx carcinoma cell line) | The concentrations of water, ethanol, and acetone extracts utilized in the activity varied from 50 to 300 μg/mL | All of the extracts have an IC50 of 200 μg/mL against HEp2 (human larynx carcinoma cell line) cell lines. | [60] | |
| Sesbania cannabina | Seed | In vitro: A549, Hela, HepG2, and MCF-7 | Water extraction and ethanol precipitation | Owing to their capacity to elevate caspase-12 expression, all fractions dose-dependently suppressed the proliferation of the targeted cells. | [61] |
| Seed coat | In vitro: human lung cancer cell line Hop-62 | Methanolic extract | Extract prevented cell growth by disrupting cell homeostasis as well as changes in cell morphology. | [67] | |
| Stem | In vitro: MCF-2, HeLa, and A549 | Isolates of two novel 2-arylbenzofuran compounds, sesbcanfuran A and B (1 and 2), and six 2-arylbenzofuran derivatives (3–8) | Compounds 1, 2, and 4 are more potent against HeLa, MCF-7, and A549 cells than compounds 3 and 5–8. | [68] | |
| Sesbania sesban | Leaf | In vitro: murine leukemia P-388 cells | Methanol extract and isolated 3-hydroxy-4′,7-dimethoxyflavone | Methanol extract and compound 1 both demonstrated IC50 values of 60.04 μg/mL and 5.40 μg/mL, respectively. | [70] |
| Leaves | In vitro: K562 cell line | Aqueous ethanol extract | Cytotoxicity was accomplished by either inhibiting the Wnt pathway (comp.21, 22) or inhibiting the Smad pathway (comp.22), as well as docking against various targets connected to the K562 cell line. | [69] |
6.4. Nanoparticles of Sesbania Species and Their Anticancer Use
6.4.1. PEGylated Silver Nanoparticles of Sesbania sesban
6.4.2. Green Synthesized Silver Nanoparticles of S. sesban
7. Discussion
8. Future Aspects for Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKenna, D.J.; Jones, K.; Hughes, K.; Tyler, V.M. Botanical Medicines: The Desk Reference for Major Herbal Supplements, 2nd ed.; Routledge: New York, NY, USA, 2012. [Google Scholar]
- Li, F.-S.; Weng, J.-K. Demystifying traditional herbal medicine with modern approach. Nat. Plants 2017, 3, 1–7. [Google Scholar] [CrossRef]
- Joshi, S.G. Medicinal Plants; Oxford and IBH Publishing: Delhi, India, 2000; Volume 130. [Google Scholar]
- Sreelatha, S.; Padma, P.; Umasankari, E. Evaluation of anticancer activity of ethanol extract of Sesbania grandiflora (Agati Sesban) against Ehrlich ascites carcinoma in Swiss albino mice. J. Ethnopharmacol. 2011, 134, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Naher, U.A.; Choudhury, A.T.; Biswas, J.C.; Panhwar, Q.A.; Kennedy, I.R. Prospects of using leguminous green manuring crop Sesbania rostrata for supplementing fertilizer nitrogen in rice production and control of environmental pollution. J. Plant Nutr. 2020, 43, 285–296. [Google Scholar] [CrossRef]
- Wachiebene, S. Assessment of Feed Resources for Ruminant Production in Northern Region of Ghana; University of Development Studies: Tamale, Ghana, 2021. [Google Scholar]
- Bunma, S.; Balslev, H. A review of the economic botany of Sesbania (Leguminosae). Bot. Rev. 2019, 85, 185–251. [Google Scholar] [CrossRef]
- Zainab, N.; Amna; Khan, A.A.; Azeem, M.A.; Ali, B.; Wang, T.; Shi, F.; Alghanem, S.M.; Hussain Munis, M.F.; Hashem, M. PGPR-mediated plant growth attributes and metal extraction ability of Sesbania sesban L. in industrially contaminated soils. Agronomy 2021, 11, 1820. [Google Scholar] [CrossRef]
- Royal Botanic Gardens, & Kew and Missouri Botanical Garden. Available online: http://www.theplantlist.org/browse/A/Leguminosae/Sesbania/ (accessed on 25 July 2024).
- Farruggia, F.T. Phylogenetic and Monographic Studies of the Pantropical Genus Sesbania adanson (Leguminosae); Arizona State University: Tempe, AZ, USA, 2009. [Google Scholar]
- Compendium, C.I.S. CAB International: Wallingford, UK. 2022. Available online: https://www.cabi.org/tag/isc/ (accessed on 30 August 2021).
- Vijayakumar, P.; Singaravadivelan, A.; Senthilkumar, D.; Vasanthakumar, T.; Ramachandran, M. Effect of Sesbania grandiflora (Agati) Supplementation on Weight Gain of Crossbred Jersey Heifer Calves. Int. J. Econ. Plants 2021, 8, 162–164. [Google Scholar]
- Arfan, N.; Julie, A.; Mohiuddin, A.; Khan, S.; Labu, Z. Medicinal properties of the sesbania grandiflora leaves. Ibnosina J. Med. Biomed. Sci. 2016, 8, 271–277. [Google Scholar] [CrossRef]
- Ansil, P.; Soumya, S.; Shafna, S. Sesbania grandiflora: A Potential Source of Phytopharmaceuticals; AkiNik Publications: Delhi, India, 2022. [Google Scholar]
- Balan, A.P.; Udayan, P.; Predeep, S. Palynotaxonomical Studies on Selected Indian Endemic Legumes; Exceller Books: Predeep, India, 2023. [Google Scholar]
- Momin, R.; Kadam, V. Biochemical analysis of leaves of some medicinal plants of genus Sesbania. J. Ecobiotechnol. 2011, 3, 14–16. [Google Scholar]
- Pandian, A.; Sivalingam, A.M.; Lakshmanan, G.; Semwal, R.; Selvakumari, J.; Semwal, D. Phytochemical analysis and antioxidant activity of the leaf extract of Sesbania grandiflora (L.) Poiret. Curr. Med. Drug Res. 2023, 7, 228. [Google Scholar]
- Heuzé, V.; Tran, G.; Bastianelli, D.; Lebas, F. Sesban (Sesbania sesban). NRA, CIRAD, AFZ and FAO. 2015. Available online: https://www.feedipedia.org/node/253 (accessed on 30 August 2021).
- Saptarshi, S.; Ghosh, A.K. Pharmacological effects of Sesbania sesban Linn: An overview. PharmaTutor 2017, 5, 16–21. [Google Scholar]
- Goswami, S.; Mishra, K.; Singh, R.P.; Singh, P.; Singh, P. Sesbania sesban, a plant with diverse therapeutic benefits: An overview. J Pharma. Res. Edu. 2016, 1, 111–121. [Google Scholar]
- Gomase, P.V. Sesbania sesban Linn: A review on its ethnobotany, phytochemical and pharmacological profile. Asian J. Biomed. Pharm. Sci. 2012, 2, 11. [Google Scholar]
- Zuanny, D.C.; Vilela, B.; Moonlight, P.W.; Särkinen, T.E.; Cardoso, D. expowo: An R package for mining global plant diversity and distribution data. Appl. Plant Sci. 2024, 12, e11609. [Google Scholar] [CrossRef]
- Lewis, G.P.; Schrire, B.; Mackinder, B.; Lock, M. Legumes of the World; Royal Botanic Gardens Kew: London, UK, 2005; Volume 577. [Google Scholar]
- POWO. Plants of The World Online. Available online: https://powo.science.kew.org (accessed on 27 June 2024).
- Betts, J.; Young, R.P.; Hilton-Taylor, C.; Hoffmann, M.; Rodríguez, J.P.; Stuart, S.N.; Milner-Gulland, E. A framework for evaluating the impact of the IUCN Red List of threatened species. Conserv. Biol. 2020, 34, 632–643. [Google Scholar] [CrossRef]
- Ghogue, J.-P. Sesbania sesban. The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/species/185456/13560631 (accessed on 7 June 2024).
- IUCN. The IUCN Red List of Threatened Species. Version 2023-1. Available online: https://www.iucnredlist.org/species/168726/20141760 (accessed on 7 June 2024).
- Chong, K.Y.; Koh, C.; Low, Y.W.; Lua, H.K.; Mustaqim, W.; Sam, Y.Y.; Yee, W. Crudia wrayi. The IUCN Red List of Threatened Species 2023: E.T224976135A224976137; International Union for Conservation of Nature: Gland, Switzerland, 2023. [Google Scholar]
- Oduol, P.A.; Akunda, E. Vegetative Propagation of Sesbania sesban by Cuttings; International Council for Research in Agroforestry (ICRAF): Nairobi, Kenya, 1988. [Google Scholar]
- Orwa, C. Agroforestree Database: A Tree Reference and Selection Guide, Version 4.0. 2009. Available online: https://www.cifor-icraf.org/ (accessed on 11 July 2024).
- Karmakar, P.; Singh, V.; Yadava, R.; Singh, B.; Singh, R.; Kushwaha, M. Agathi [Sesbania grandiflora L. (Agast)]: Current status of production, protection and genetic improvement: IIVR-Regional Research Station, Sargatia, Kushinagar, Uttar Pradesh-274406. In Proceedings of the National Symposium on Vegetable Legumes for Soil and Human Health, Varanasi, India, 12–14 February 2016; pp. 153–161. [Google Scholar]
- Iqbal, N.; Manalil, S.; Chauhan, B.S.; Adkins, S.W. Germination biology of sesbania (Sesbania cannabina): An emerging weed in the Australian cotton agro-environment. Weed Sci. 2019, 67, 68–76. [Google Scholar] [CrossRef]
- Anulika, N.P.; Ignatius, E.O.; Raymond, E.S.; Osasere, O.-I.; Abiola, A.H. The chemistry of natural product: Plant secondary metabolites. Int. J. Technol. Enhanc. Emerg. Eng. Res 2016, 4, 1–9. [Google Scholar]
- Velu, G.; Palanichamy, V.; Rajan, A.P. Phytochemical and pharmacological importance of plant secondary metabolites in modern medicine: In Bioorganic Phase in Natural Food: An Overview. In Bioorganic Phase in Natural Food: An Overview; Roopan, S.M., Madhumitha, G., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 135–156. [Google Scholar]
- Patil, P.; Shah, N. Sesbania grandiflora (L.) Pers.(Agati): Its Ethnobotanical Knowledge, Phytochemical Studies, Pharmacological Aspects, and Future Prospects. TMR Integr. Med. 2022, 6, e22032. [Google Scholar] [CrossRef]
- Hussain, A.Z.; Ignatius, A. GC-MS Analysis and Antimicrobial Activity of Acalypha indica Linn; CABI Digital Library: Wallingford, UK, 2010; pp. 3591–3595. [Google Scholar]
- Emitaro, W.O.; Musyimi, D.M.; Opande, G.T.; Odiambo, G. Phytochemical and antimicrobial properties of leaf extracts of Calliandra calothyrsus, Leucaena diversifolia and Sesbania sesban. Bact. Emp. 2020, 3, 20–24. [Google Scholar] [CrossRef]
- Deepthi, K.; Renjith, P.; Habeeb Rahman, K.; Chandramohanakumar, N. A comprehensive review of Sesbania grandiflora (L.) Pers: Traditional uses, phytochemistry and pharmacological properties. Vegetos 2024, 37, 31–40. [Google Scholar] [CrossRef]
- Padmalochana, K.; Rajan, M.D. Antimicrobial activity of Aqueous, Ethanol and Acetone extracts of Sesbania grandiflora leaves and its phytochemical characterization. Int. J. Pharma Sci. Res. 2014, 5, 957–962. [Google Scholar]
- Vinothini, K.; Devi, M.S.; Shalini, V.; Sekar, S.; Semwal, R.B.; Arjun, P.; Semwal, D.K. In vitro micropropagation, total phenolic content and comparative antioxidant activity of different extracts of Sesbania grandiflora (L.) Pers. Curr. Sci. 2017, 113, 1142–1147. [Google Scholar] [CrossRef]
- Noviany, N.; Samadi, A.; Yuliyan, N.; Hadi, S.; Aziz, M.; Purwitasari, N.; Mohamad, S.; Ismail, N.N.; Gable, K.P.; Mahmud, T. Structure characterization and biological activity of 2-arylbenzofurans from an Indonesian plant, Sesbania grandiflora (L.) Pers. Phytochem. Lett. 2020, 35, 211–215. [Google Scholar] [CrossRef]
- Ajitha, B.; Reddy, Y.A.K.; Rajesh, K.; Reddy, P.S. Sesbania grandiflora leaf extract assisted green synthesis of silver nanoparticles: Antimicrobial activity. Mater. Today Proc. 2016, 3, 1977–1984. [Google Scholar] [CrossRef]
- Arthanari, S.; Periyasamy, P. Phenolic composition, antioxidant and anti-fibrotic effects of Sesbania grandiflora L. (Agastya)—An edible medicinal plant. AYU (Int. Q. J. Res. Ayurveda) 2020, 41, 242–249. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Abirami, A.; Nagarani, G.; Sangeethapriya, M. Antioxidant capacity and total phenolic content of aqueous acetone and ethanol extract of edible parts of Moringa oleifera and Sesbania grandiflora. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2014, 8, 1090–1098. [Google Scholar]
- Chandralekha, B.; Kailash, G.; Anuprita, J. Assessment of nutritional and phytochemical properties of Sesbania grandiflora flower and leaves. Pharma Innov. J. 2022, 11, 90–94. [Google Scholar]
- Mohiuddin, A.K. Medicinal and therapeutic values of Sesbania grandiflora. J. Pharm. Sci. Exp. Pharmacol. 2019, 2019, 81–86. [Google Scholar] [CrossRef]
- Naqi, M. Comparative of Phytochemical and Antimicrobial of Sesbania grandiflora Leaves Extract. Med. J. Babylon 2014, 11, 667–674. [Google Scholar]
- Saifudin, A.; Forentin, A.M.; Fadhilah, A.; Tirtodiharjo, K.; Melani, W.D.; Widyasari, D.; Saroso, T.A. Bioprospecting for anti-Streptococcus mutans: The activity of 10% Sesbania grandiflora flower extract comparable to erythromycin. Asian Pac. J. Trop. Biomed. 2016, 6, 751–754. [Google Scholar] [CrossRef]
- Brahim Mahamat, O.; Younes, S.; Otchom, B.B.; Franzel, S.; Ouchar Mahamat Hidjazi, A.-D.; Soumaya, E.i. A Review on Medicinal and Ethnomedicinal Uses, Biological Features, and Phytochemical Constituents of Sesbania sesban L. Merr., A Nitrogen-Fixing Plant Native to the Republic of Chad. Sci. World J. 2024, 2024, 1225999. [Google Scholar] [CrossRef]
- Rageeb, M.; Usman, M.; Patil, S.B.; Patil, S.S.; Patil, R.S. Sesbania sesban Linn.: An overview. Int. J. Pharm. Life Sci. 2013, 4, 2644–2648. [Google Scholar]
- Soren, A.D.; Chen, R.P.; Yadav, A.K. In vitro and in vivo anthelmintic study of Sesbania sesban var. bicolor, a traditionally used medicinal plant of Santhal tribe in Assam, India. J. Parasit. Dis. 2021, 45, 1–9. [Google Scholar] [CrossRef]
- Vijaya; Yadav, A.K.; Gogoi, S. In vitro and in vivo anthelmintic efficacy of two pentacyclic triterpenoids, ursolic acid and betulinic acid against mice pinworm, Syphacia obvelata. J. Parasit. Dis. 2018, 42, 144–149. [Google Scholar]
- Santos, F.O.; Cerqueira, A.P.M.; Branco, A.; Batatinha, M.J.M.; Botura, M.B. Anthelmintic activity of plants against gastrointestinal nematodes of goats: A review. Parasitology 2019, 146, 1233–1246. [Google Scholar] [CrossRef]
- Marappan, S.; Manickam, K.; Devi, P.S. Bioactive compounds in Sesbania sesban flower and its Antioxidant and Antimicrobial activity. J. Pharm. Res. 2012, 5, 390–393. [Google Scholar]
- Chaiyasut, C.; Sivamaruthi, B.S.; Pengkumsri, N.; Sirilun, S.; Peerajan, S.; Chaiyasut, K.; Kesika, P. Anthocyanin profile and its antioxidant activity of widely used fruits, vegetables, and flowers in Thailand. Asian J. Pharm. Clin. Res. 2016, 9, 218–224. [Google Scholar] [CrossRef]
- Abdelgawad, S.M.; Hetta, M.H.; Ibrahim, M.A.; Fawzy, G.A.; El-Askary, H.I.; Ross, S.A. Holistic Overview of the Phytoconstituents and Pharmacological Activities of Egyptian Riverhemp [Sesbania sesban (L.) Merr.]: A Review. Nat. Prod. Commun. 2023, 18, 1934578X231160882. [Google Scholar] [CrossRef]
- Boesten, D.M.; den Hartog, G.J.; de Cock, P.; Bosscher, D.; Bonnema, A.; Bast, A. Health effects of erythritol. Nutrafoods 2015, 14, 3–9. [Google Scholar] [CrossRef]
- Fouillaud, M.C.Y.; Venkatachalam, M.; Grondin, I.; Dufossé, L. Anthraquinones. In Phenolic Compounds in Food Charac-terization and Analysis; Nollet, L.M.L., Gutiérrez-Uribe, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 130–170. ISBN 978-1-4987-2296-4. [Google Scholar]
- Ahmed, A.; Labu, Z.; Dey, S.; Hira, A.; Howlader, M.; Hossain, M.; Roy, J. Phytochemical screening, antibacterial and cytotoxic activity of different fractions of Xylocarpus mekongensis Bark. Ibnosina J. Med. Biomed. Sci. 2013, 5, 206–213. [Google Scholar] [CrossRef]
- Laladhas, K.P.; Cheriyan, V.T.; Puliappadamba, V.T.; Bava, S.V.; Unnithan, R.G.; Vijayammal, P.L.; Anto, R.J. A novel protein fraction from Sesbania grandiflora shows potential anticancer and chemopreventive efficacy, in vitro and in vivo. J. Cell. Mol. Med. 2010, 14, 636–646. [Google Scholar] [CrossRef]
- Joselin, A.P. The Role of the Apoptosis and Splicing Associated Protein Acinus During Apoptotic Nuclear Changes. Ph.D. Thesis, Institut für Molekulare Medizin, HeinrichHeine-Universität Düsseldorf, Düsseldorf, Germany, 2006. [Google Scholar]
- Roy, R.; Kumar, D.; Chakraborty, B.; Chowdhury, C.; Das, P. Apoptotic and autophagic effects of Sesbania grandiflora flowers in human leukemic cells. PLoS ONE 2013, 8, e71672. [Google Scholar] [CrossRef]
- Ponnanikajamideen, M.; Nagalingam, M.; Vanaja, M.; Malarkodi, C.; Rajeshkumar, S. Anticancer activity of different solvent extracts of Sesbania grandiflora against neuroblastima (imr-32) and colon (ht-29) cell lines. Eur. J. Biomed. Pharm. Sci 2015, 2, 509–517. [Google Scholar]
- Pajaniradje, S.; Mohankumar, K.; Pamidimukkala, R.; Subramanian, S.; Rajagopalan, R. Antiproliferative and apoptotic effects of Sesbania grandiflora leaves in human cancer cells. BioMed Res. Int. 2014, 2014, 474953. [Google Scholar] [CrossRef]
- Padmalochana, K.; Rajan, M.D. In-vitro anticancer activity of different extracts of Sesbania grandiflora against HEP2 cell lines. World J. Pharm. Sci. 2015, 3, 1589–1592. [Google Scholar]
- Zhou, M.; Yang, L.; Yang, S.; Zhao, F.; Xu, L.; Yong, Q. Isolation, characterization and in vitro anticancer activity of an aqueous galactomannan from the seed of Sesbania cannabina. Int. J. Biol. Macromol. 2018, 113, 1241–1247. [Google Scholar] [CrossRef]
- Mehta, N.; Rao, P.; Saini, R. Evaluation of antioxidant and anticancer potential of Sesbania aculeata-a multipurpose legume crop. Ann. Food Sci. Technol. 2019, 20, 109–115. [Google Scholar]
- Fu, X.-J.; Yi, J.-L.; Yang, J.-Y.; Lin, X.-Q.; Huang, W.-H.; Zhou, X.-M. Bioactive 2-arylbenzofurans derivatives from Sesbania cannabina. Phytochem. Lett. 2021, 41, 106–108. [Google Scholar] [CrossRef]
- Abdelgawad, S.M.; Hetta, M.H.; Ibrahim, M.A.; Balachandran, P.; Zhang, J.; Wang, M.; Eldehna, W.M.; Fawzy, G.A.; El-Askary, H.I.; Ross, S.A. Phytochemical investigation of Egyptian Riverhemp: A potential source of antileukemic metabolites. J. Chem. 2022, 2022, 8766625. [Google Scholar] [CrossRef]
- Dianhar, H.; Syah, Y.M.; Mujahidin, D.; Hakim, E.H.; Juliawaty, L.D. A flavone derivative from Sesbania sesban leaves and its cytotoxicity against murine leukemia P-388 cells. In Proceedings of the AIP Conference Proceedings, Bandung, Indonesia, 24 March 2014; pp. 411–413. [Google Scholar]
- Pandian, S.R.K.; Anjanei, D.; Raja, N.L.; Sundar, K. PEGylated silver nanoparticles from Sesbania aegyptiaca exhibit immunomodulatory and anti-cancer activity. Mater. Res. Express 2018, 6, 035402. [Google Scholar] [CrossRef]
- Kuchekar, A.; Desai, R.; Gawade, A. Antimitotic Activity of Green Synthesized Silver Nanoparticles by Seed Extract of Sesbania Sesban. Mater. Int. 2022, 4, 4. [Google Scholar]
- Chen, Y.; Gibson, S.B. Is mitochondrial generation of reactive oxygen species a trigger for autophagy? Autophagy 2008, 4, 246–248. [Google Scholar] [CrossRef]


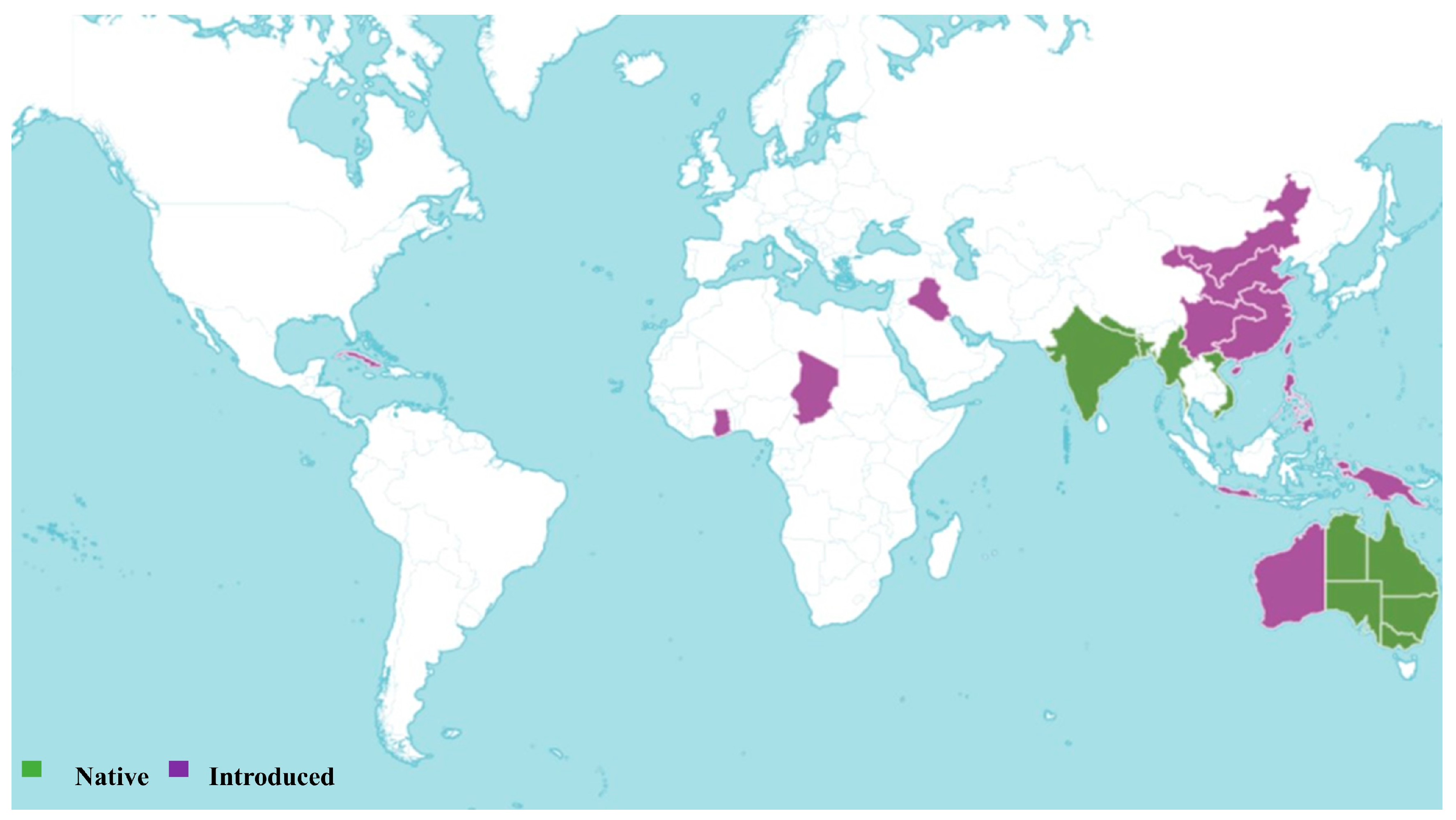
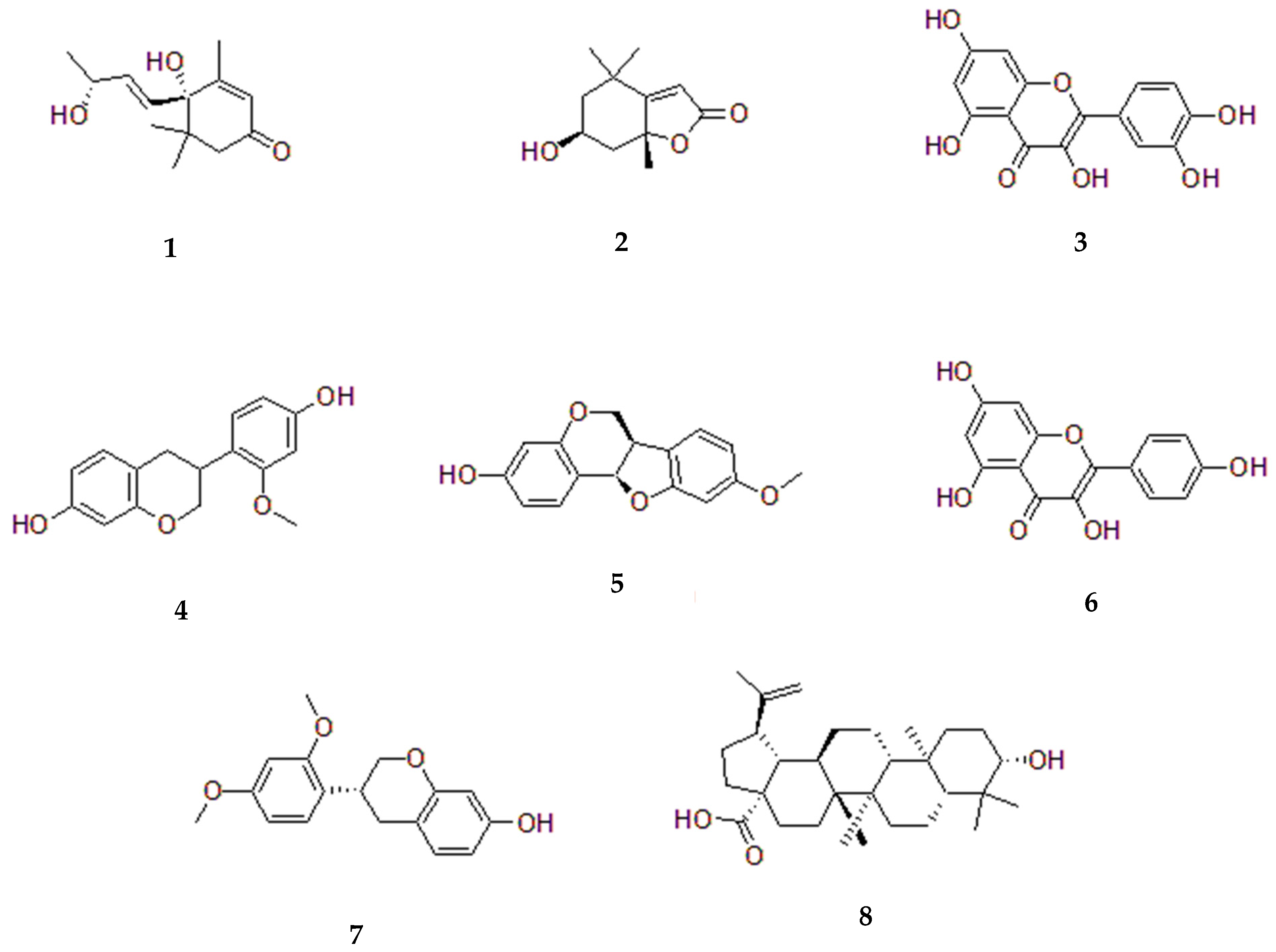
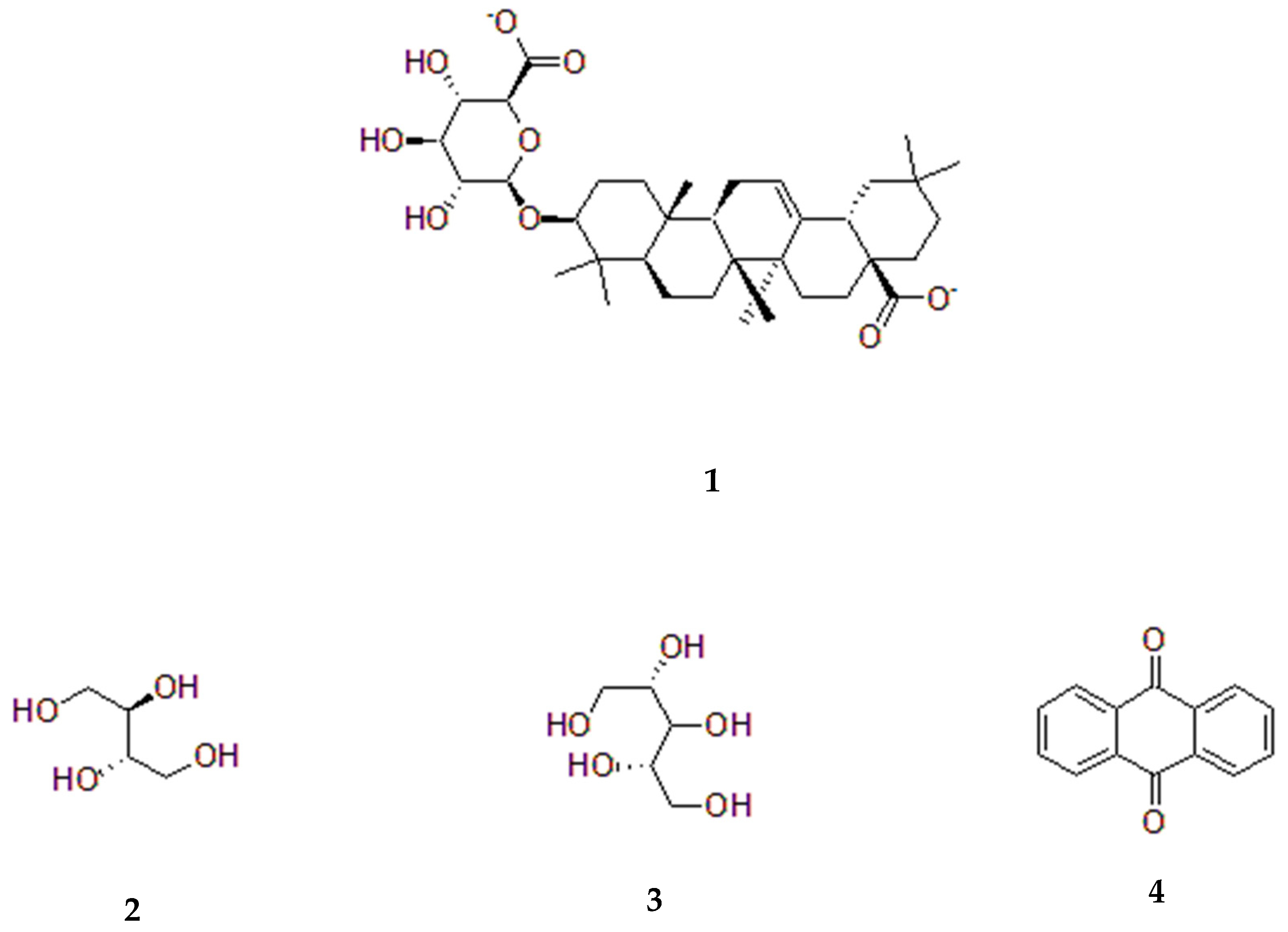

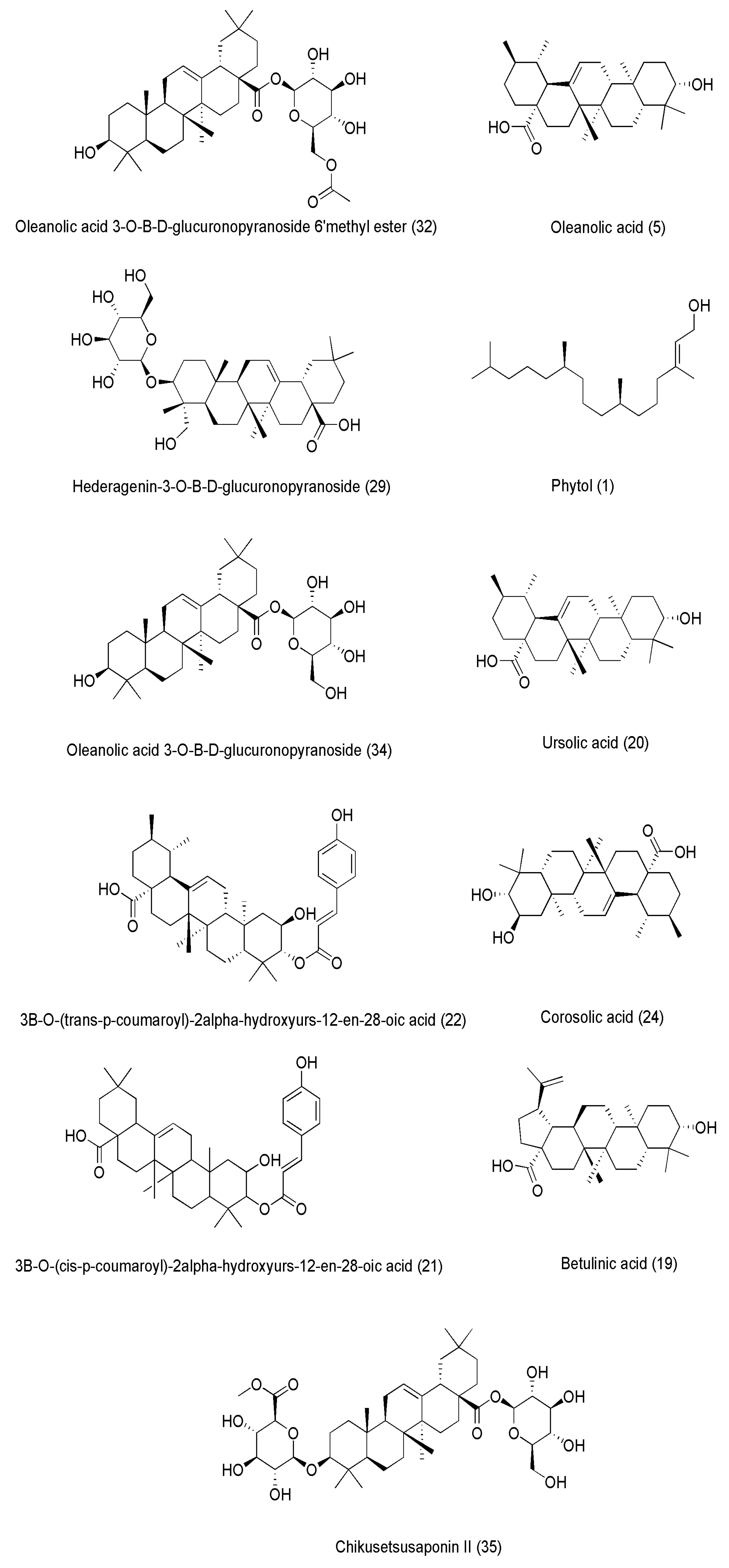
| Species | IUCN Status | Last Assessed | Scope of Assessment | Year Published | Current Population Trend |
|---|---|---|---|---|---|
| Sesbania sesban | Least Concern (LC) | 2 April 2019 | Global | 2020 | Stable |
| Sesbania grandiflora | Data Deficient (DD) | 28 February 2023 | Global | 2023 | Unknown |
| Sesbania cannabina | Least Concern (LC) | 21 July 2010 | Global | 2012 | Stable |
| Plant Part | Solvent Fraction | Phytochemicals | References |
|---|---|---|---|
| Bark | Ethanol | Saponins (5%), tannins (3%), triterpenes (2%), and 2 arylbenzofurans (Sesbagrandiforian A and B) | [41,43] |
| Ethyl acetate | Gallic acid (1.5%) | ||
| Root | Ethanol | Alkaloids (4%), plant sterols/beta-sitosterol (2%), campesterol (1%), and stigmasterol (1%); glycosidic structures: glycosidic saponins (3%), bioflavonoids (2%), steroidal compounds (2%), and triterpenes (2%) | [43] |
| Seed | Ethanol | Leucocyanidin (2%), cyanidin (1.5%), saponins (3%), sesbanimide (1%) | [40] |
| 10% sodium hydroxide | Galactomannan (4%) | ||
| Acetone | Esterase B (1%) and esterase C (1%) | ||
| Hexane | Plant sterol/β-sitosterol (2%) and vitamin E (1%) | ||
| Leaf | Ethanol | Alkaloids (3%), amino acids/proteins (4%), starch (5%), reducing and non-reducing sugars (4%), glycosides (3%), phenols (2%), saponins (3%), tannins (2%), terpenoids (2%), flavonoids such as quercetin and kaempferol (2%) | [36,37] |
| Ethanol–water | Polyphenols (3%), carotenoids (2%), flavonoids (2%), and favanones (1.5%) | [42] | |
| 50% and 70% aqueous ethanolic solution | Amino acids/proteins (4%), starch/carbohydrates (5%), calcium (1%), phenolic compounds (2%), and ascorbic acid (1%) | [43] | |
| Benzine | Starch/carbohydrates (5%), phenolic compounds (2%), amino acids/proteins (4%), steroids (2%), saponins (3%), tannins (2%), terpenoids (2%), flavonoids (2%), plant sterol/β-sitosterol (1%), and anthraquinone (1%) | [47] | |
| Ethyl acetate/methanol/chloroform | Polyphenols (3%), alkaloids (3%), flavonoids (2%), favanones (1.5%), amino acids/proteins (4%), starch/carbohydrates (5%), reducing sugar (2%), saponins (3%), and tannins (2%) | [13,16,42,47] | |
| Water | Alkaloids (3%), amino acids/proteins (4%), starch/carbohydrates (5%), glycosides/cyanogenic glycoside (1%), phenolic compounds (2%), and reducing sugars (2%) | [43] | |
| Flower | Ethanol, 70% aqueous ethanolic solution | Kaempferol (1%), grandifloral (1%), amino acids/cystine (1%), isoleucine (1%), starch/carbohydrates (5%), glycosides (3%), steroids (2%), ascorbic acid (1%), tannins (2%), alkaloids (3%), flavonoids (2%) | [47,48] |
| Methanol, ethyl acetate, water | Flavonoids (2%), tannins (2%), alkaloids (3%), anthraquinone (1%), glycosides (3%) |
| Plant Part | Solvent Fraction | Phytochemicals | References |
|---|---|---|---|
| Bark | Methanol, chloroform, petroleum ether | Alkaloids: 3%, carbohydrates: 5%, glycosides: 2%, phenols: 1.5%, saponins: 2%, plant sterols: 1% | [49,60] |
| Ethanol ether (diethyl ether) chloroform | Alkaloids: 3%, carbohydrates: 5%, steroids: 2%, flavonoids: 1.5%, saponins: 2%, tannins: 1.5% | ||
| Water | Reducing sugars: 4%, sugar alcohols: 1.5% | ||
| Root | Ethyl acetate and n-butanol-saturated extracts | Triterpenoids: 2% | [20] |
| Wood | Petroleum ether and chloroform ethyl acetate | Sterols: 2%, triterpenes: 1.5%, flavonoids: 1% | [52] |
| Leaves | Methanol, chloroform, and petroleum ether at 60–80° | Alkaloids: 3%, flavonoids: 2%, amino acids/proteins: 4%, fats: 2%, saponins: 2%, glycosides: 2%, plant sterols: 1% | [52] |
| Water | Triterpenoids: 2%, carbohydrates: 5%, amino acids/proteins: 4%, tannins: 2%, saponins: 2%, glycosides: 2%, campesterol: 1%, cholesterol: 1% | [51,53] | |
| Flower | Methanol and acidified methanol | Anthocyanins: 2%, phenols: 1.5%, flavonoids: 2% | [20,55] |
| Blossoms | Water | Glucosides: 2% | [50] |
| Pollen and dust tubes | Water | Keto-acids: 1.5% | [21] |
| Lignin | Water | Phenylpropanoid: 2%, flavonoids: 2% | [21,50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhtar, F.A.; Ahmed, M.; Al Dhanhani, A.S.; Elbehairi, S.E.I.; Alfaifi, M.Y.; Shati, A.A.; Fakhry, A.M. Distribution, Phytochemical Insights, and Cytotoxic Potential of the Sesbania Genus: A Comprehensive Review of Sesbania grandiflora, Sesbania sesban, and Sesbania cannabina. Pharmaceuticals 2025, 18, 64. https://doi.org/10.3390/ph18010064
Mokhtar FA, Ahmed M, Al Dhanhani AS, Elbehairi SEI, Alfaifi MY, Shati AA, Fakhry AM. Distribution, Phytochemical Insights, and Cytotoxic Potential of the Sesbania Genus: A Comprehensive Review of Sesbania grandiflora, Sesbania sesban, and Sesbania cannabina. Pharmaceuticals. 2025; 18(1):64. https://doi.org/10.3390/ph18010064
Chicago/Turabian StyleMokhtar, Fatma Alzahraa, Mariam Ahmed, Aishah Saeed Al Dhanhani, Serag Eldin I. Elbehairi, Mohammad Y. Alfaifi, Ali A. Shati, and Amal M. Fakhry. 2025. "Distribution, Phytochemical Insights, and Cytotoxic Potential of the Sesbania Genus: A Comprehensive Review of Sesbania grandiflora, Sesbania sesban, and Sesbania cannabina" Pharmaceuticals 18, no. 1: 64. https://doi.org/10.3390/ph18010064
APA StyleMokhtar, F. A., Ahmed, M., Al Dhanhani, A. S., Elbehairi, S. E. I., Alfaifi, M. Y., Shati, A. A., & Fakhry, A. M. (2025). Distribution, Phytochemical Insights, and Cytotoxic Potential of the Sesbania Genus: A Comprehensive Review of Sesbania grandiflora, Sesbania sesban, and Sesbania cannabina. Pharmaceuticals, 18(1), 64. https://doi.org/10.3390/ph18010064







