Fusion-Negative NTRK Overexpression Exhibit Biological Relevance in Colorectal Cancer: Implications for Prediction of Responses to Kinase Inhibitors
Abstract
1. Introduction
2. Results
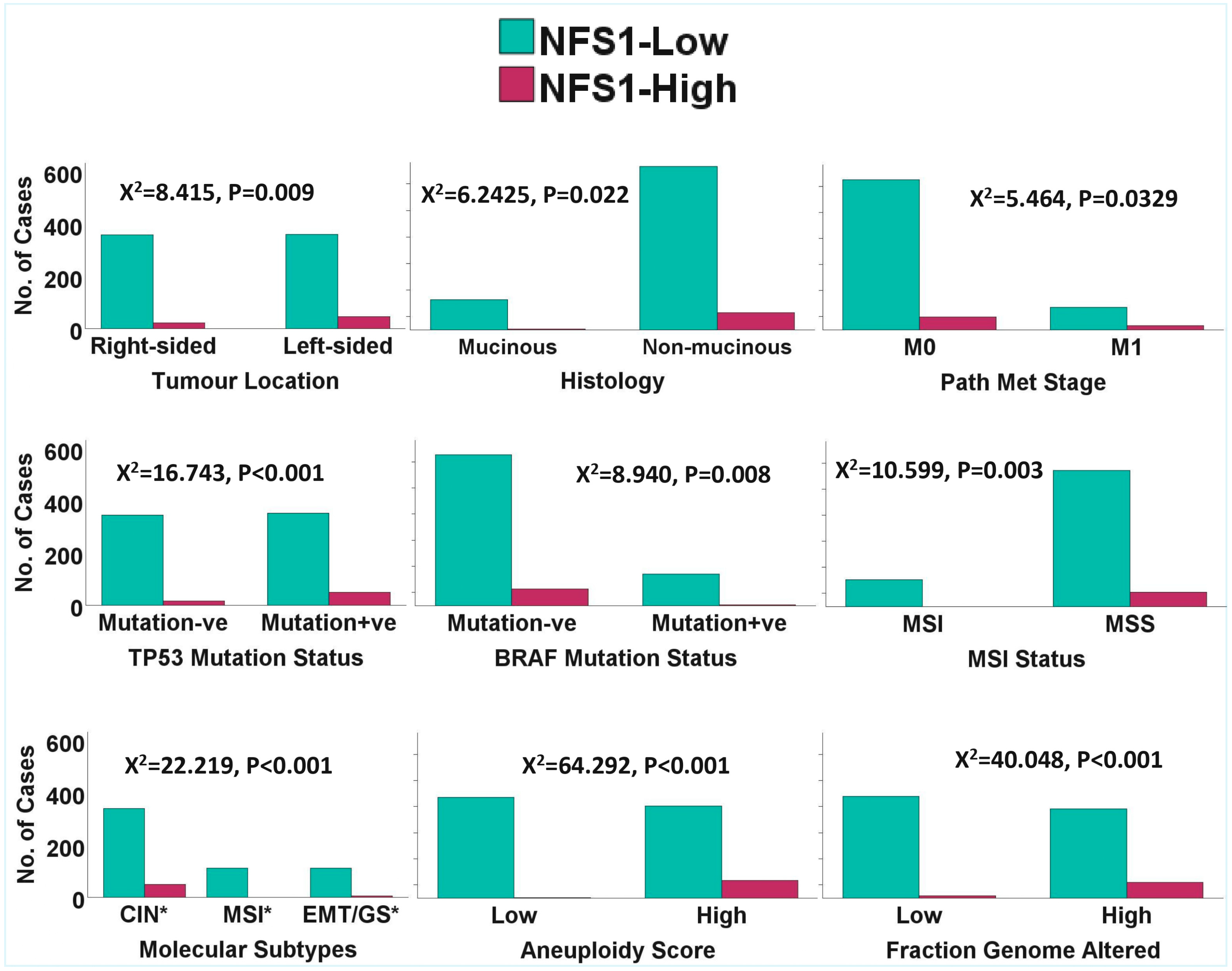
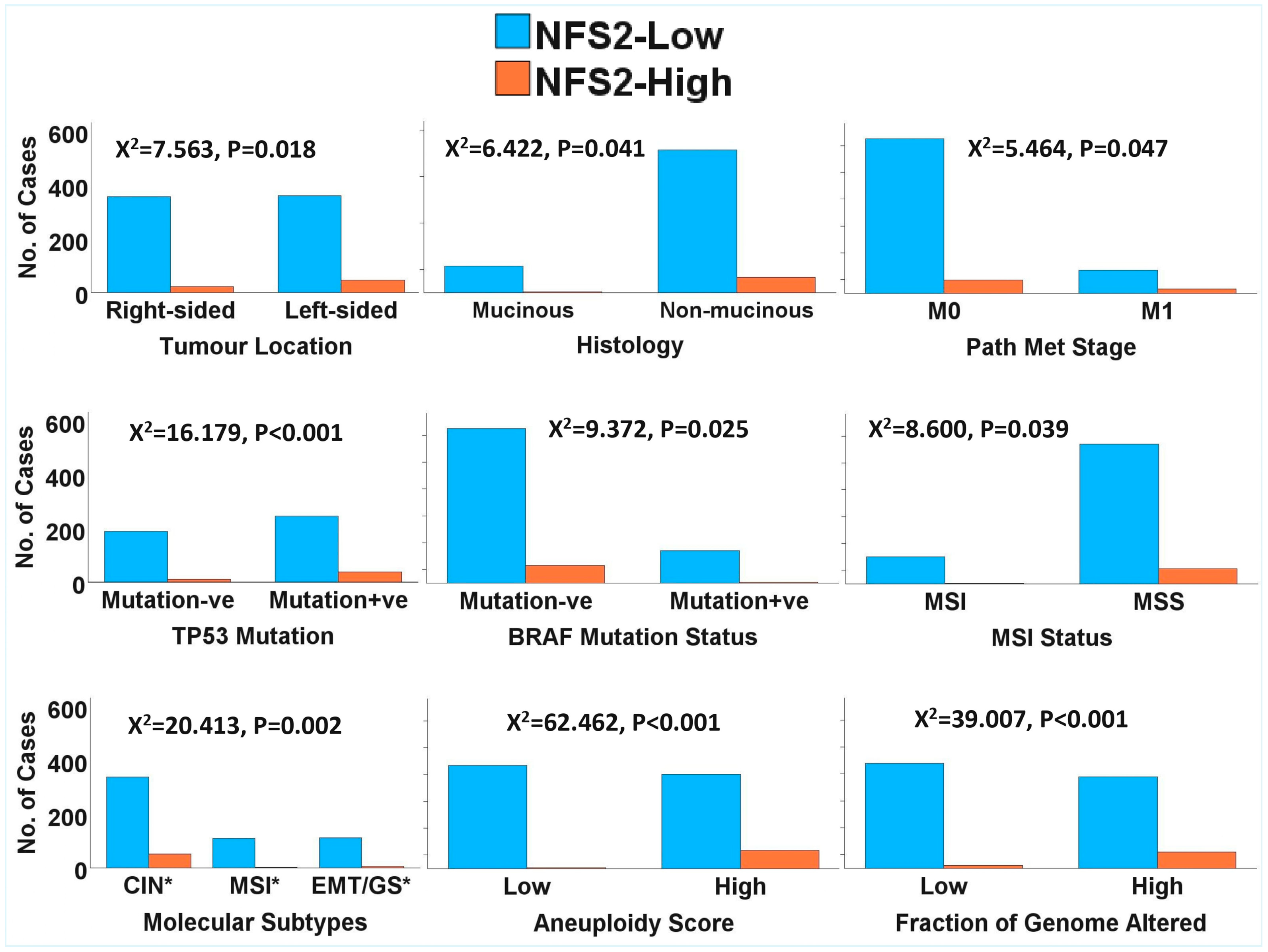
2.1. Clinicopathological and Molecular Correlates of NTRK1/2/3 Expression in CRC
2.2. NTRK1/2/3 Expression Deregulation in CRC
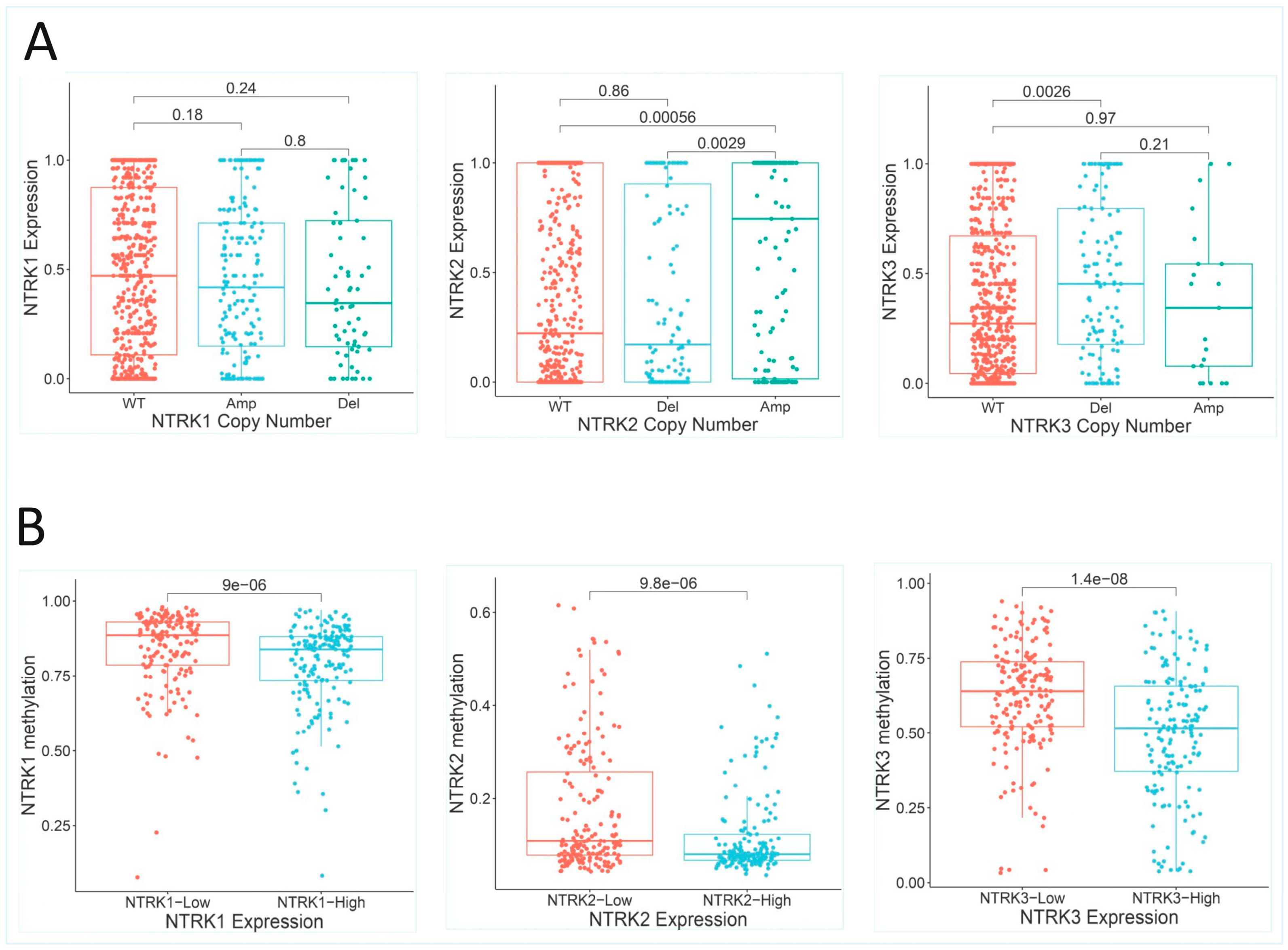
2.2.1. NTRK1/2/3 Copy Number Alterations
2.2.2. Potential NTRK3 Gene Fusion
2.2.3. NTRK1/2/3 Methylation Status
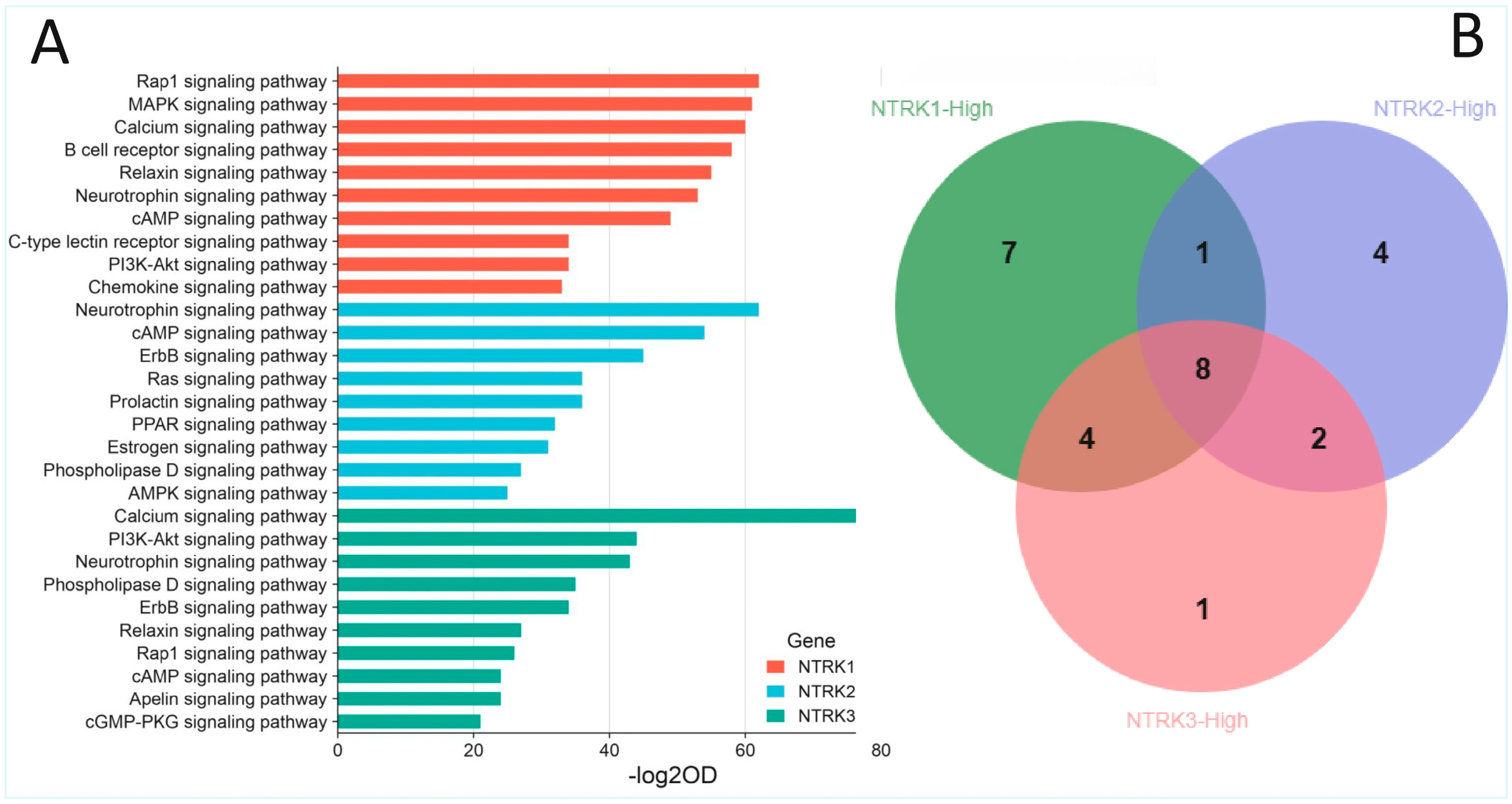
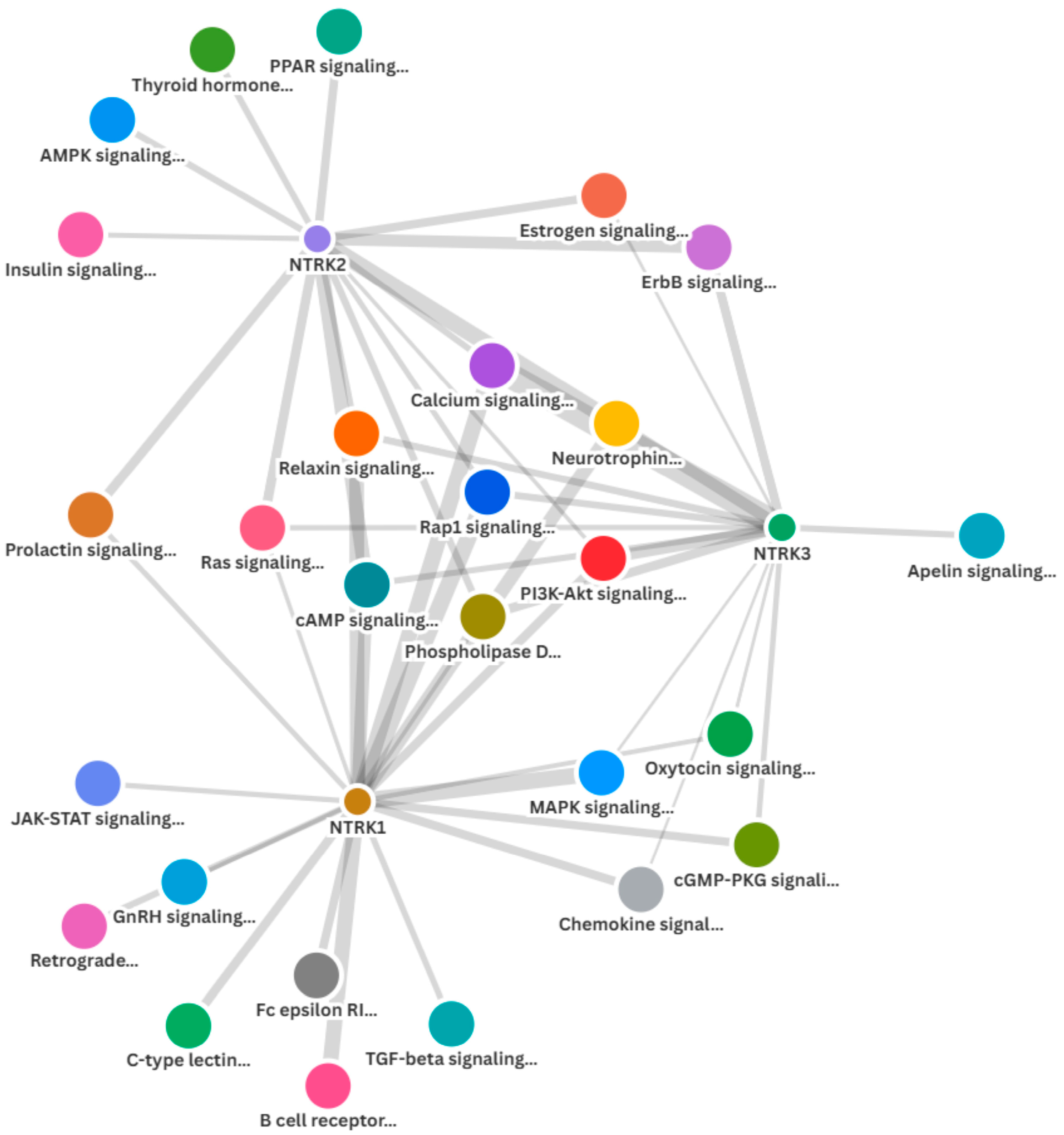
2.3. Enrichment of NTRK1/2/3 and Other Cancer Signalling Pathways in the CRC Cohorts
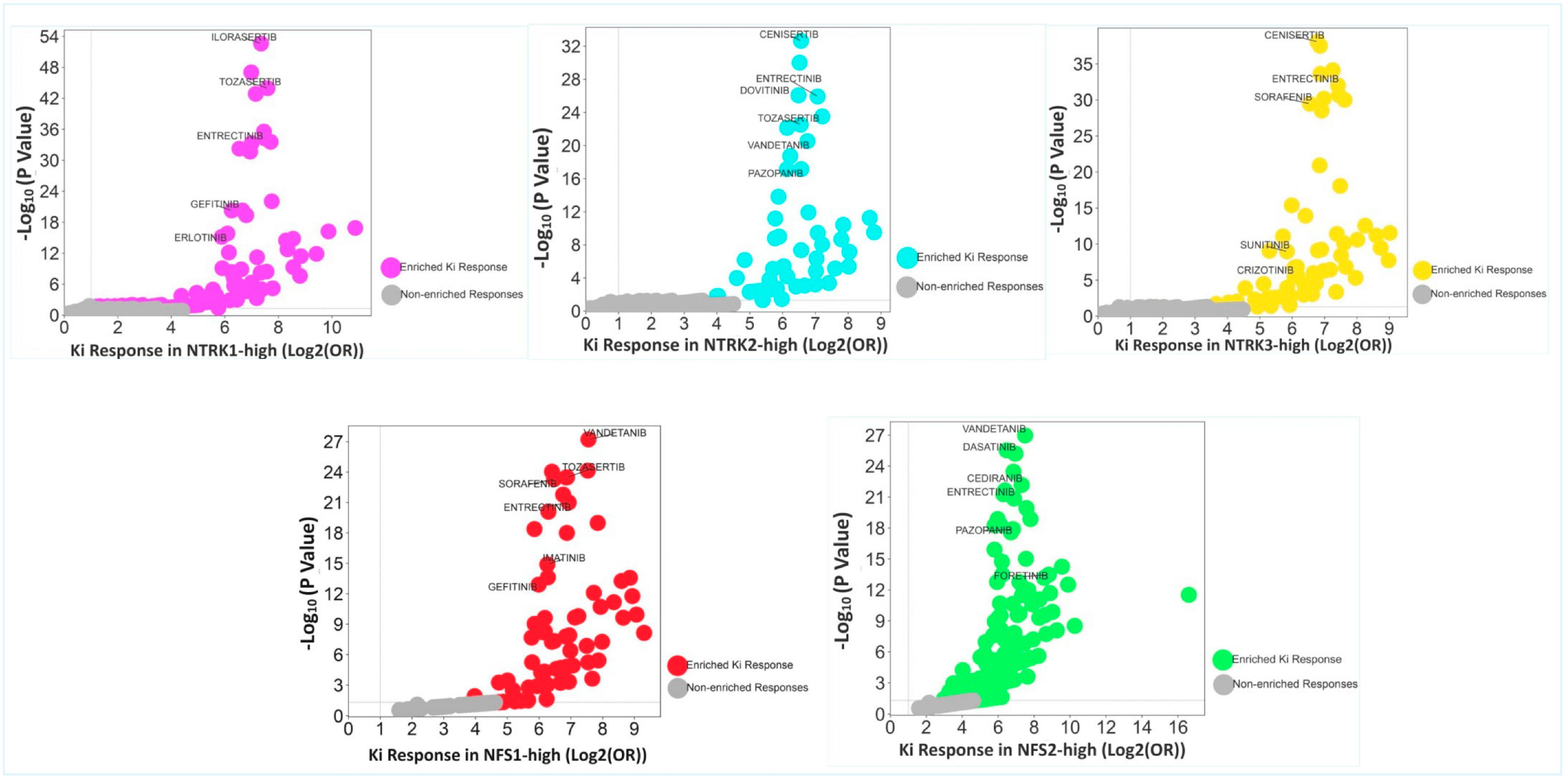
2.4. Enrichment of Responses to Multiple Tyrosine Kinase Inhibitors in the CRC Cohorts
2.5. Enrichment of Kinase Inhibitor Responses in NFS1/2-High Subsets
3. Discussion
4. Materials and Methods
4.1. Study Approach
4.2. Study Cohorts
4.3. Data Retrieval and Processing
4.4. Gene Set Enrichment Analysis with Gene Ontology Enrichment Analyses
4.5. Statistical Analyses and Data Visualization
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TCGA | The Cancer Genome Atlas |
| Sidra-LUMC | Sidra-Leiden University Medical Center’s Atlas and Compass of Immune–Cancer–Microbiome |
| GDC | Genome Data Commons |
| GSEA | Gene set enrichment analysis |
| POEA | Pathway ontology enrichment analysis |
| DOEA | Drug ontology enrichment analysis |
| NTRK1/2/3 | NTRK1, NTRK2 and NTRK3 |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Pierantoni, C.; Cosentino, L.; Ricciardiello, L. Molecular Pathways of Colorectal Cancer Development: Mechanisms of Action and Evolution of Main Systemic Therapy Compounds. Dig. Dis. 2024, 42, 319–324. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Bungaro, M.; Garbo, E. NTRK1/2/3: Biology, Detection and Therapy. Precis. Cancer Med. 2024, 7, 3. [Google Scholar] [CrossRef]
- Gutierrez-Herrera, J.; Montero-Fernandez, M.A.; Kokaraki, G.; De Petris, L.; Maia Falcão, R.; Molina-Centelles, M.; Guijarro, R.; Ekman, S.; Ortiz-Villalón, C. NTRK Gene Expression in Non-Small-Cell Lung Cancer. J. Respir. 2025, 5, 2. [Google Scholar] [CrossRef]
- Hechtman, J.F. NTRK Insights: Best Practices for Pathologists. Mod. Pathol. 2022, 35, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Solomon, J.P.; Linkov, I.; Rosado, A.; Mullaney, K.; Rosen, E.Y.; Frosina, D.; Jungbluth, A.A.; Zehir, A.; Benayed, R.; Drilon, A.; et al. NTRK Fusion Detection Across Multiple Assays and 33,997 Cases: Diagnostic Implications and Pitfalls. Mod. Pathol. 2020, 33, 38–46. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, X.; Yi, Y.; Wang, X.; Zhu, L.; Shen, Y.; Lin, D.; Wu, C. Tumor Initiation and Early Tumorigenesis: Molecular Mechanisms and Interventional Targets. Signal Transduct. Target. Ther. 2024, 9, 149. [Google Scholar] [CrossRef]
- Lippai, Z.; Papp, G.; Szuhai, K.; Sápi, J.; Dezső, K.; Sápi, Z. NTRK Amplification Occurs Frequently in pan-TRK Immunopositive Dedifferentiated Liposarcomas. Pathol. Oncol. Res. 2025, 30, 1611993. [Google Scholar] [CrossRef]
- Yan, P.; Dai, X.; Zhang, F.; Dong, Y.; Shi, H.; Han, A. NTRK Expression and Its Clinical Significance in Pancreatic Neuroendocrine Tumors. Discov. Oncol. 2025, 16, 1647. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, I.; Heim, S. Interstitial Deletions Generating Fusion Genes. Cancer Genom. Proteom. 2021, 18, 167–196. [Google Scholar] [CrossRef]
- Alshalalfa, M.; Bismar, T.A.; Alhajj, R. Detecting Cancer Outlier Genes with Potential Rearrangement Using Gene Expression Data and Biological Networks. Adv. Bioinform. 2012, 2012, 373506. [Google Scholar] [CrossRef] [PubMed]
- Dell’Orso, G.; Passarella, T.; Cappato, S.; Cappelli, E.; Regis, S.; Maffei, M.; Balbi, M.; Ravera, S.; Di Martino, D.; Viaggi, S.; et al. Chromosomal Deletion Involving ANKRD26 Leads to Expression of a Fusion Protein Responsible for ANKRD26-Related Thrombocytopenia. Int. J. Mol. Sci. 2025, 26, 7330. [Google Scholar] [CrossRef]
- Mariella, E.; Grasso, G.; Miotto, M.; Buzo, K.; Reilly, N.M.; Andrei, P.; Vitiello, P.P.; Crisafulli, G.; Arena, S.; Rospo, G.; et al. Transcriptome-Wide Gene Expression Outlier Analysis Pinpoints Therapeutic Vulnerabilities in Colorectal Cancer. Mol. Oncol. 2024, 18, 1460–1485. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.W.; Ou, Q.; Wu, X.; Nagasaka, M.; Shao, Y.; Ou, S.I.; Yang, Y. NTRK Fusion Positive Colorectal Cancer Is a Unique Subset of CRC with High TMB and Microsatellite Instability. Cancer Med. 2022, 11, 2541–2549. [Google Scholar] [CrossRef]
- Murphy, D.A.; Ely, H.A.; Shoemaker, R.; Boomer, A.; Culver, B.P.; Hoskins, I.; Haimes, J.D.; Walters, R.D.; Fernandez, D.; Stahl, J.A.; et al. Detecting Gene Rearrangements in Patient Populations Through a 2-Step Diagnostic Test Comprised of Rapid IHC Enrichment Followed by Sensitive Next-Generation Sequencing. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 513–523. [Google Scholar] [CrossRef]
- Doostmohammadi, A.; Jooya, H.; Ghorbanian, K.; Gohari, S.; Dadashpour, M. Potentials and Future Perspectives of Multi-Target Drugs in Cancer Treatment: The Next Generation Anti-Cancer Agents. Cell Commun. Signal. 2024, 22, 228. [Google Scholar] [CrossRef] [PubMed]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination Therapy in Combating Cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Alfahed, A. PARP Inhibition in Colorectal Cancer—A Comparison of Potential Predictive Biomarkers for Therapy. Pharmaceuticals 2025, 18, 905. [Google Scholar] [CrossRef]
- Liu, J.; Lichtenberg, T.; Hoadley, K.A.; Poisson, L.M.; Lazar, A.J.; Cherniack, A.D.; Kovatich, A.J.; Benz, C.C.; Levine, D.A.; Lee, A.V.; et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018, 173, 400–416.e11. [Google Scholar] [CrossRef]
- Roelands, J.; Kuppen, P.J.K.; Ahmed, E.I.; Mall, R.; Masoodi, T.; Singh, P.; Monaco, G.; Raynaud, C.; de Miranda, N.F.C.C.; Ferraro, L.; et al. An Integrated Tumor, Immune and Microbiome Atlas of Colon Cancer. Nat. Med. 2023, 29, 1273–1286. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) Hallmark Gene Set Collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]
- Maleki, F.; Ovens, K.; Hogan, D.J.; Kusalik, A.J. Gene Set Analysis: Challenges, Opportunities, and Future Research. Front. Genet. 2020, 11, 654. [Google Scholar] [CrossRef] [PubMed]
- Rouillard, A.D.; Gundersen, G.W.; Fernandez, N.F.; Wang, Z.; Monteiro, C.D.; McDermott, M.G.; Ma’ayan, A. The Harmonizome: A Collection of Processed Datasets Gathered to Serve and Mine Knowledge about Genes and Proteins. Database 2016, 2016, baw100. [Google Scholar] [CrossRef]
- Diamant, I.; Clarke, D.J.B.; Evangelista, J.E.; Lingam, N.; Ma’ayan, A. Harmonizome 3.0: Integrated Knowledge about Genes and Proteins from Diverse Multi-Omics Resources. Nucleic Acids Res. 2025, 53, D1016–D1028. [Google Scholar] [CrossRef]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef]
- Yoo, M.; Shin, J.; Kim, J.; Ryall, K.A.; Lee, K.; Lee, S.; Jeon, M.; Kang, J.; Tan, A.C. DSigDB: Drug Signatures Database for Gene Set Analysis. Bioinformatics 2015, 31, 3069–3071. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A Free Online Platform for Data Visualization and Graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef] [PubMed]
- Flourish. Available online: https://flourish.studio/product/data-visualization/ (accessed on 15 September 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfahed, A. Fusion-Negative NTRK Overexpression Exhibit Biological Relevance in Colorectal Cancer: Implications for Prediction of Responses to Kinase Inhibitors. Pharmaceuticals 2025, 18, 1562. https://doi.org/10.3390/ph18101562
Alfahed A. Fusion-Negative NTRK Overexpression Exhibit Biological Relevance in Colorectal Cancer: Implications for Prediction of Responses to Kinase Inhibitors. Pharmaceuticals. 2025; 18(10):1562. https://doi.org/10.3390/ph18101562
Chicago/Turabian StyleAlfahed, Abdulaziz. 2025. "Fusion-Negative NTRK Overexpression Exhibit Biological Relevance in Colorectal Cancer: Implications for Prediction of Responses to Kinase Inhibitors" Pharmaceuticals 18, no. 10: 1562. https://doi.org/10.3390/ph18101562
APA StyleAlfahed, A. (2025). Fusion-Negative NTRK Overexpression Exhibit Biological Relevance in Colorectal Cancer: Implications for Prediction of Responses to Kinase Inhibitors. Pharmaceuticals, 18(10), 1562. https://doi.org/10.3390/ph18101562






