Sex-Related Safety Signals of Sotorasib in Non-Small Cell Lung Cancer: A Real-World, Pharmacovigilance Study from the EudraVigilance Database
Abstract
1. Introduction
2. Results
2.1. General Characteristics in the Real-World Population
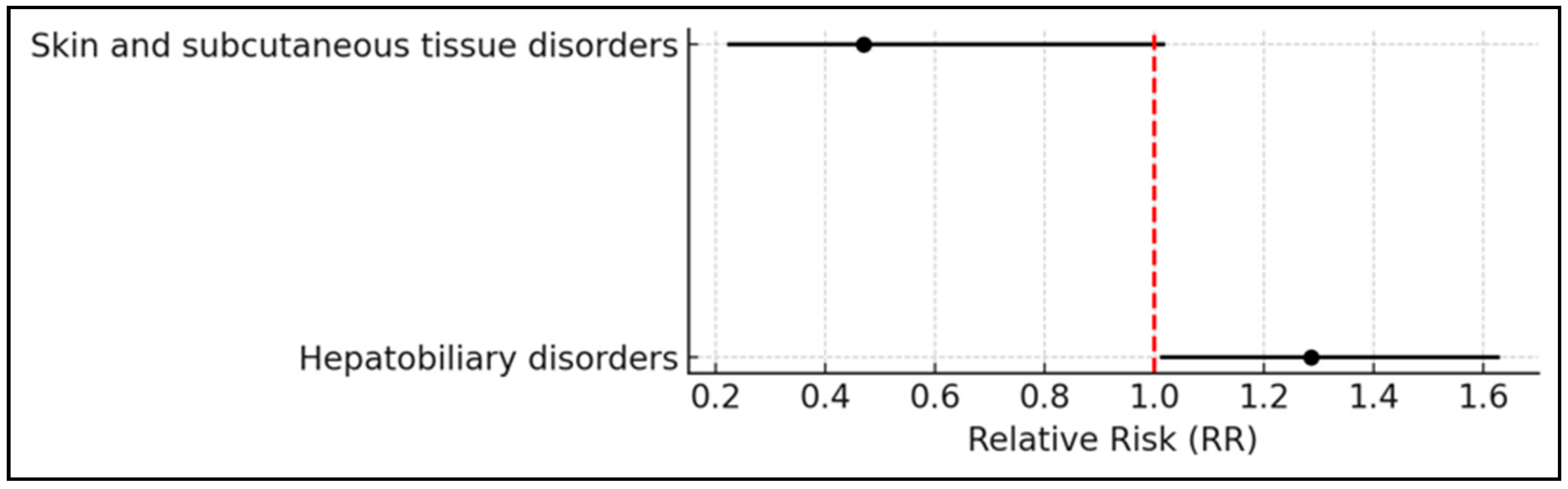
2.2. System Organ Classes Analysis/Assessment
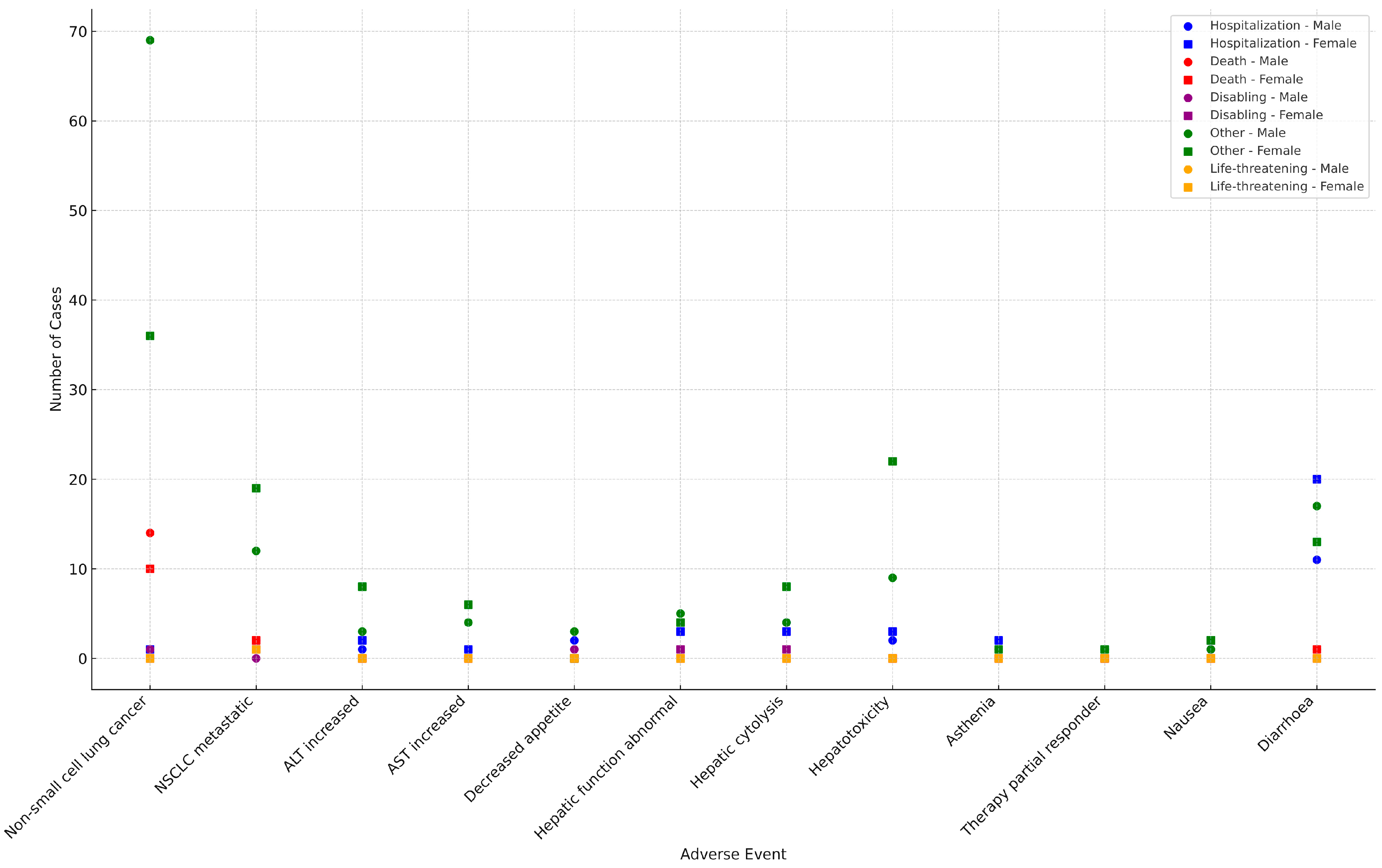
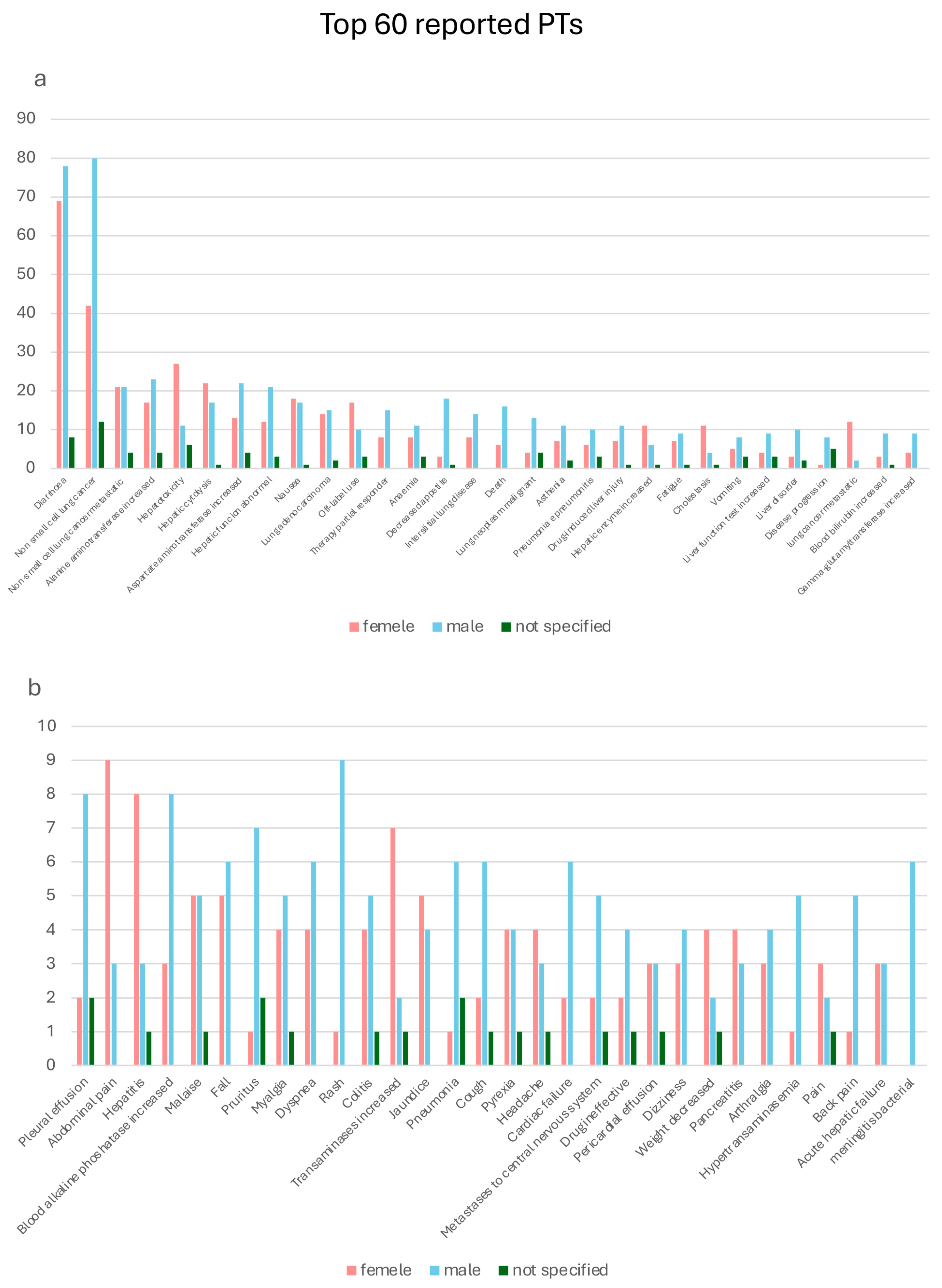
2.3. Preferred Terms Analysis/Assessment
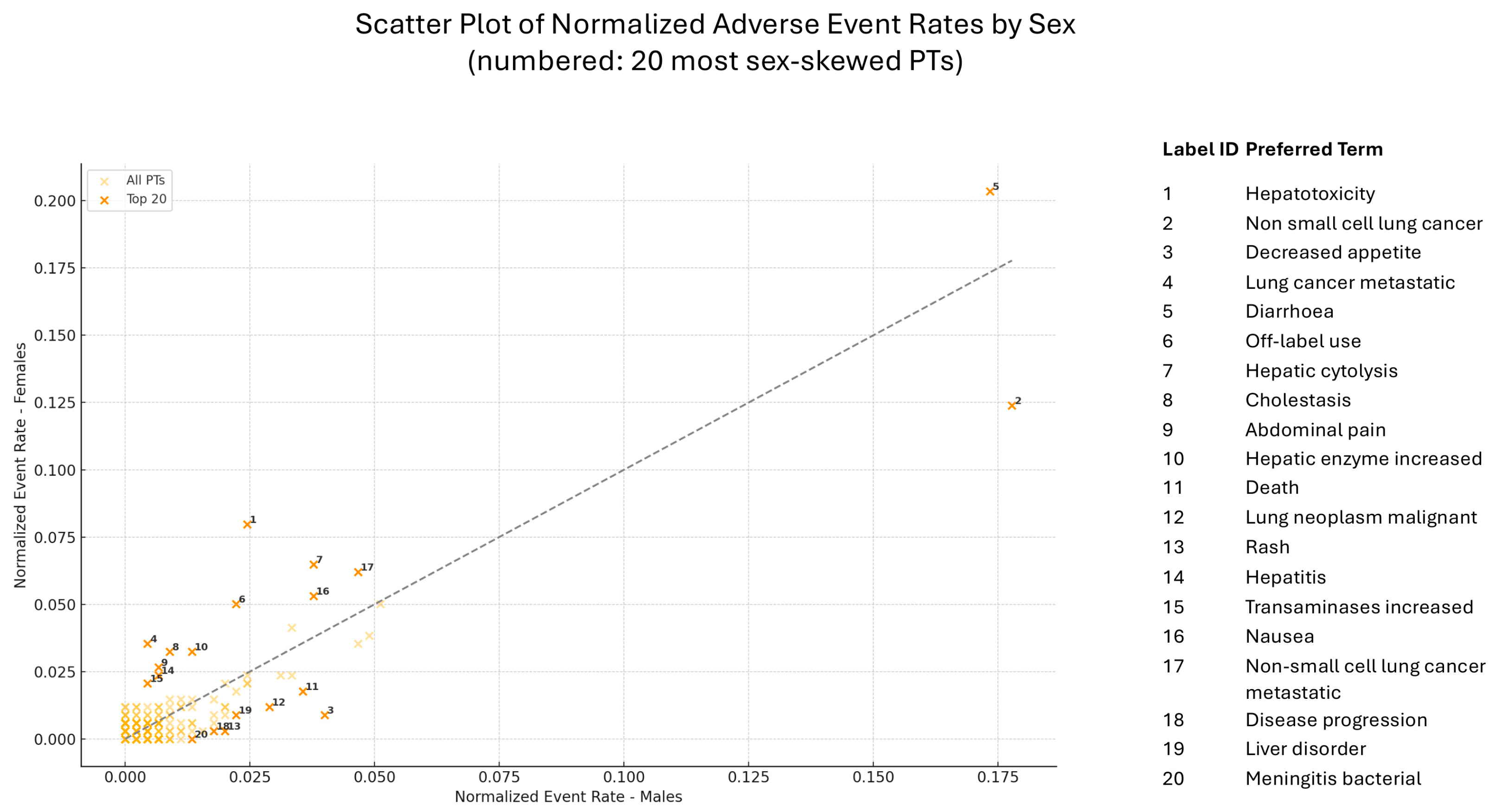
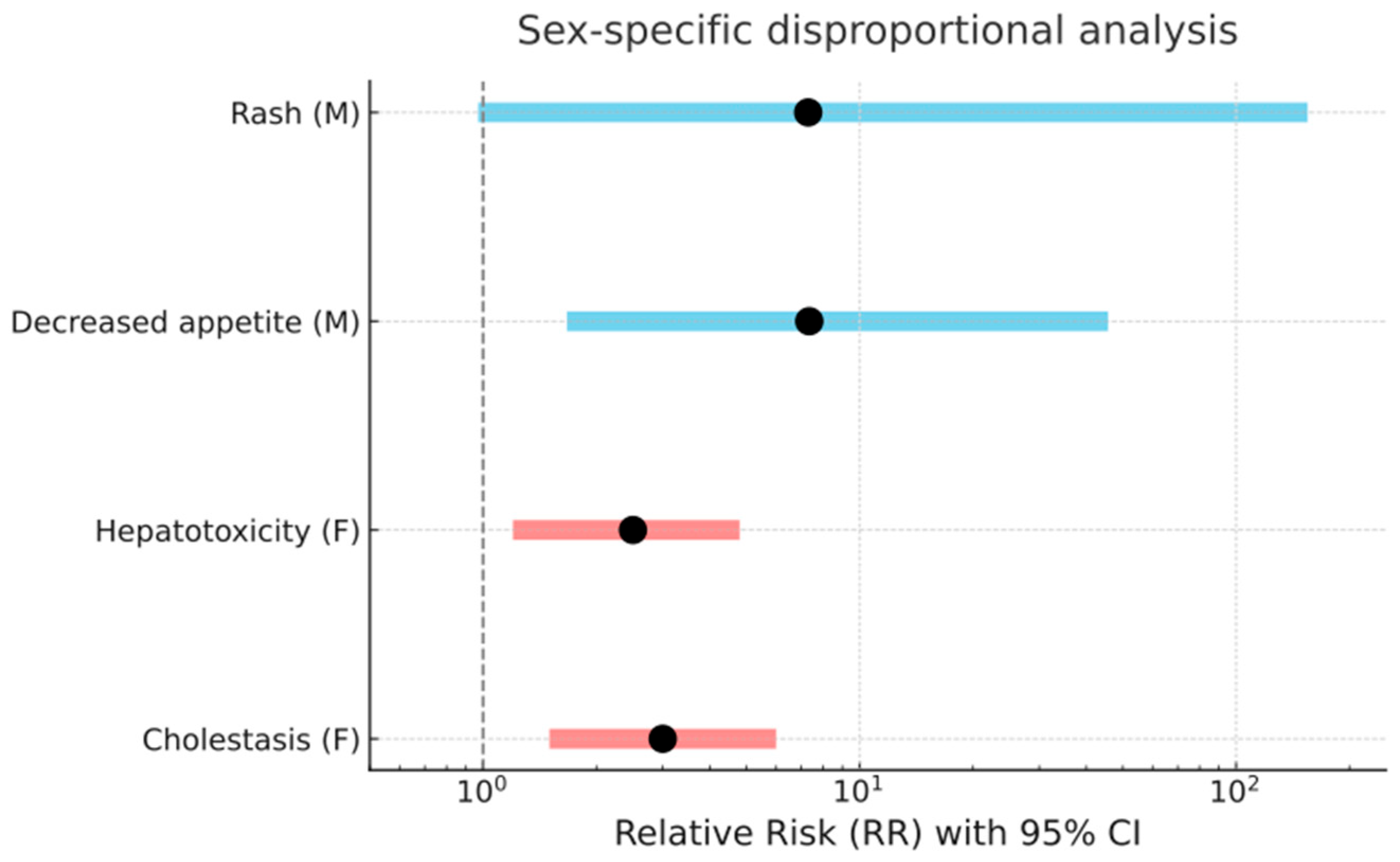
2.4. Comparative Assessment of Safety Signals from RCTs and EV
2.5. Assessment of Onset Time of ADRs
3. Discussion
Strengths and Limitations
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADR | Adverse drug reaction |
| AE | Adverse event |
| ALK | Anaplastic lymphoma kinase |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| CI | Confidence interval |
| CTCAE | Common Terminology Criteria for Adverse Events |
| DILI | Drug-induced liver injury |
| EGFR | Epidermal growth factor receptor |
| EV | EudraVigilance |
| FDA | Food and Drug Administration |
| GTP | Guanosine triphosphate |
| HR | Hazard ratio |
| ICSRS | Individual case safety reports |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| KRAS | Kirsten rat sarcoma |
| LKB1 | Liver kinase B1 |
| MedDRA | Medical Dictionary for Regulatory Activities |
| NGS | Next-generation sequencing |
| NSCLC | Non-small cell lung cancer |
| NTRK | Neurotrophic tyrosine receptor kinase |
| PDGFRA | Platelet-derived growth factor receptor alpha |
| PD-L1 | Programmed death-ligand 1 |
| PFS | Progression free survival |
| PT | Preferred term |
| PTEN | Phosphatase and tensin homolog |
| RR | Relative risk |
| RTC | Randomized clinical trial |
| SOC | System organ class |
| SRS | Spontaneous reporting system |
| STK11 | Serine/threonine kinase 11 |
| TRAE | Treatment-related AE |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Huang, Q.; Li, Y.; Huang, Y.; Wu, J.; Bao, W.; Xue, C.; Li, X.; Dong, S.; Dong, Z.; Hu, S. Advances in Molecular Pathology and Therapy of Non-Small Cell Lung Cancer. Signal Transduct. Target Ther. 2025, 10, 186. [Google Scholar] [CrossRef]
- Hendriks, L.E.L.; Cortiula, F.; Martins-Branco, D.; Mariamidze, E.; Popat, S.; Reck, M.; ESMO Guidelines Committee. Updated Treatment Recommendations for Systemic Treatment: From the ESMO Oncogene-Addicted Metastatic NSCLC Living Guideline. Ann. Oncol. 2025, 36, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Odintsov, I.; Sholl, L.M. Prognostic and Predictive Biomarkers in Non-Small Cell Lung Carcinoma. Pathology 2024, 56, 192–204. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive Molecular Profiling of Lung Adenocarcinoma. Nature 2014, 511, 543–550. [Google Scholar] [CrossRef]
- Judd, J.; Abdel Karim, N.; Khan, H.; Naqash, A.R.; Baca, Y.; Xiu, J.; VanderWalde, A.M.; Mamdani, H.; Raez, L.E.; Nagasaka, M.; et al. Characterization of KRAS Mutation Subtypes in Non-Small Cell Lung Cancer. Mol. Cancer Ther. 2021, 20, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Prior, I.A.; Hood, F.E.; Hartley, J.L. The Frequency of Ras Mutations in Cancer. Cancer Res. 2020, 80, 2969–2974. [Google Scholar] [CrossRef]
- Riely, G.J.; Kris, M.G.; Rosenbaum, D.; Marks, J.; Li, A.; Chitale, D.A.; Nafa, K.; Riedel, E.R.; Hsu, M.; Pao, W.; et al. Frequency and Distinctive Spectrum of KRAS Mutations in Never Smokers with Lung Adenocarcinoma. Clin. Cancer Res. 2008, 14, 5731–5734. [Google Scholar] [CrossRef]
- Santarpia, M.; Ciappina, G.; Spagnolo, C.C.; Squeri, A.; Passalacqua, M.I.; Aguilar, A.; Gonzalez-Cao, M.; Giovannetti, E.; Silvestris, N.; Rosell, R. Targeted Therapies for KRAS-Mutant Non-Small Cell Lung Cancer: From Preclinical Studies to Clinical Development—A Narrative Review. Transl. Lung Cancer Res. 2023, 12, 346–368. [Google Scholar] [CrossRef]
- Rosell, R.; Codony-Servat, J.; González, J.; Santarpia, M.; Jain, A.; Shivamallu, C.; Wang, Y.; Giménez-Capitán, A.; Molina-Vila, M.A.; Nilsson, J.; et al. KRAS G12C-Mutant Driven Non-Small Cell Lung Cancer (NSCLC). Crit. Rev. Oncol. Hematol. 2024, 195, 104228. [Google Scholar] [CrossRef] [PubMed]
- Soh, J.; Toyooka, S.; Matsuo, K.; Yamamoto, H.; Wistuba, I.I.; Lam, S.; Fong, K.M.; Gazdar, A.F.; Miyoshi, S. Ethnicity Affects EGFR and KRAS Gene Alterations of Lung Adenocarcinoma. Oncol. Lett. 2015, 10, 1775–1782. [Google Scholar] [CrossRef]
- Loong, H.H.; Du, N.; Cheng, C.; Lin, H.; Guo, J.; Lin, G.; Li, M.; Jiang, T.; Shi, Z.; Cui, Y.; et al. KRAS G12C Mutations in Asia: A Landscape Analysis of 11,951 Chinese Tumor Samples. Transl. Lung Cancer Res. 2020, 9, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Wagner, P.L.; Stiedl, A.C.; Wilbertz, T.; Petersen, K.; Scheble, V.; Menon, R.; Reischl, M.; Mikut, R.; Rubin, M.A.; Fend, F.; et al. Frequency and Clinicopathologic Correlates of KRAS Amplification in Non-Small Cell Lung Carcinoma. Lung Cancer 2011, 74, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.M.; Hwang, D.W.; Ahn, J.S.; Ahn, M.J.; Park, K. Prognostic and Predictive Value of KRAS Mutations in Advanced Non-Small Cell Lung Cancer. PLoS ONE 2013, 8, e64816. [Google Scholar] [CrossRef]
- Sholl, L.M.; Cooper, W.A.; Kerr, K.M.; Tan, D.S.W.; Tsao, M.-S.; Yang, J.C.-H. IASLC Atlas of Molecular Testing for Targeted Therapy in Lung Cancer; International Association for the Study of Lung Cancer: Denver, CO, USA, 2023. [Google Scholar]
- Arbour, K.C.; Jordan, E.; Kim, H.R.; Dienstag, J.; Yu, H.A.; Sanchez-Vega, F.; Lito, P.; Berger, M.; Solit, D.B.; Hellmann, M.; et al. Effects of Co-Occurring Genomic Alterations on Outcomes in Patients with KRAS-Mutant Non-Small Cell Lung Cancer. Clin. Cancer Res. 2018, 24, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Vaclova, T.; Chakraborty, A.; Sherwood, J.; Ross, S.; Carroll, D.; Barrett, J.C.; Downward, J.; de Bruin, E.C. Concomitant KRAS Mutations Attenuate Sensitivity of Non-Small Cell Lung Cancer Cells to KRAS G12C Inhibition. Sci. Rep. 2022, 12, 2699. [Google Scholar] [CrossRef]
- Li, D.; Zhu, X.; Wang, H.; Li, N. Association between PD-L1 Expression and Driven Gene Status in NSCLC: A Meta-Analysis. Eur. J. Surg. Oncol. 2017, 43, 1372–1379. [Google Scholar] [CrossRef]
- Finn, S.P.; Addeo, A.; Dafni, U.; Thunnissen, E.; Bubendorf, L.; Madsen, L.B.; Biernat, W.; Verbeken, E.; Hernandez-Losa, J.; Marchetti, A.; et al. Prognostic Impact of KRAS G12C Mutation in Patients with NSCLC: Results from the European Thoracic Oncology Platform Lungscape Project. J. Thorac. Oncol. 2021, 16, 990–1002. [Google Scholar] [CrossRef]
- Sebastian, M.; Eberhardt, W.E.E.; Hoffknecht, P.; Metzenmacher, M.; Wehler, T.; Kokowski, K.; Alt, J.; Schütte, W.; Büttner, R.; Heukamp, L.C.; et al. KRAS G12C-Mutated Advanced Non-Small Cell Lung Cancer: A Real-World Cohort from the German Prospective, Observational, Nationwide CRISP Registry (AIO-TRK-0315). Lung Cancer 2021, 154, 51–61. [Google Scholar] [CrossRef]
- Santos, E.; Martin-Zanca, D.; Reddy, E.P.; Pierotti, M.A.; Della Porta, G.; Barbacid, M. Malignant Activation of a K-ras Oncogene in Lung Carcinoma but Not in Normal Tissue of the Same Patient. Science 1984, 223, 661–664. [Google Scholar] [CrossRef]
- Canon, J.; Rex, K.; Saiki, A.Y.; Mohr, C.; Cooke, K.; Bagal, D.; Gaida, K.; Holt, T.; Knutson, C.G.; Koppada, N.; et al. The Clinical KRAS(G12C) Inhibitor AMG 510 Drives Anti-Tumour Immunity. Nature 2019, 575, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef]
- Skoulidis, F.; Li, B.T.; de Langen, A.J.; Hong, D.S.; Lena, H.; Wolf, J.; Dy, G.K.; Curioni Fontecedro, A.; Tomasini, P.; Velcheti, V.; et al. Molecular Determinants of Sotorasib Clinical Efficacy in KRASG12C-Mutated Non-Small-Cell Lung Cancer. Nat. Med. 2025, 31, 2755–2767. [Google Scholar] [CrossRef]
- Hong, D.S.; Fakih, M.G.; Strickler, J.H.; Desai, J.; Durm, G.A.; Shapiro, G.I.; Falchook, G.S.; Price, T.J.; Sacher, A.; Denlinger, C.S.; et al. KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. N. Engl. J. Med. 2020, 383, 1207–1217. [Google Scholar] [CrossRef]
- Nakajima, E.C.; Drezner, N.; Li, X.; Mishra-Kalyani, P.S.; Liu, Y.; Zhao, H.; Bi, Y.; Liu, J.; Rahman, A.; Wearne, E.; et al. FDA Approval Summary: Sotorasib for KRAS G12C-Mutated Metastatic NSCLC. Clin. Cancer Res. 2022, 28, 1482–1486. [Google Scholar] [CrossRef]
- de Langen, A.J.; Johnson, M.L.; Mazieres, J.; Dingemans, A.C.; Mountzios, G.; Pless, M.; Wolf, J.; Schuler, M.; Lena, H.; Skoulidis, F.; et al. Sotorasib versus Docetaxel for Previously Treated Non-Small-Cell Lung Cancer with KRASG12C Mutation: A Randomised, Open-Label, Phase 3 Trial. Lancet 2023, 401, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Zucker, I.; Prendergast, B.J. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol. Sex Differ. 2020, 11, 32. [Google Scholar] [CrossRef]
- Jene, K.; Meyers, D.E.; Prasad, V. The inclusion of women in global oncology drug trials over the past 20 years. JAMA Oncol. 2021, 7, 1569–1570. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, B.C.; Gerard, C.L.; Espinosa da Silva, C. Sex and gender differences in anticancer treatment toxicity: A call for revisiting drug dosing in oncology. Endocrinology 2022, 163, bqac058. [Google Scholar] [CrossRef]
- Triggianese, P.; Novelli, L.; Galdiero, M.R.; Chimenti, M.S.; Conigliaro, P.; Perricone, R.; Perricone, C.; Gerli, R. Immune checkpoint inhibitors-induced autoimmunity: The impact of gender. Autoimmun. Rev. 2020, 19, 102590. [Google Scholar] [CrossRef]
- Rubino, R.; Marini, A.; Roviello, G.; Presotto, E.M.; Desideri, I.; Ciardetti, I.; Brugia, M.; Pimpinelli, N.; Antonuzzo, L.; Mini, E.; et al. Endocrine-related adverse events in a large series of cancer patients treated with anti-PD1 therapy. Endocrine 2021, 74, 172–179. [Google Scholar] [CrossRef]
- Ismail, A.; Kennedy, L.; Francis, H. Sex-dependent differences in cholestasis: Why estrogen signaling may be a key pathophysiological driver. Am. J. Pathol. 2023, 193, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Amacher, D.E. Female gender as a susceptibility factor for drug-induced liver injury. Hum. Exp. Toxicol. 2014, 33, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Bai, X.; Lin, J.; Villegas, A.; Patel, R.; Gadgeel, S.; Leighl, N.; Sabari, J.; Liu, S.V.; Nagasaka, M. Time from Immune Checkpoint Inhibitor to Sotorasib Use Correlates with Risk of Hepatotoxicity in Non-Small Cell Lung Cancer: A Brief Report. Cancer Treat. Res. Commun. 2023, 36, 100743. [Google Scholar] [CrossRef]
- Speranza, D.; Omero, F.; Cianci, V.; Marafioti, M.; Infurna, C.; Carroccio, P.; Spina, E.; Barbieri, M.A.; Esposito, E.; Silvestris, N.; et al. Comparison Study of the Safety Profile of Olaparib Versus Niraparib: Analysis of Real-World Data from EudraVigilance. Pharmaceuticals 2025, 18, 528. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Pala, L.; Pagan, E.; Corti, C.; Bagnardi, V.; Queirolo, P.; Catania, C.; De Pas, T.; Giaccone, G. Sex-based differences in response to anti-PD-1 or PD-L1 treatment in patients with non-small-cell lung cancer expressing high PD-L1 levels: A systematic review and meta-analysis of randomized clinical trials. ESMO Open 2021, 6, 100251. [Google Scholar] [CrossRef] [PubMed]
- Salvador, M.R.; Monteiro, C.; Pereira, L.; Duarte, A.P. Quality of spontaneous reports of adverse drug reactions sent to a regional pharmacovigilance unit. Int. J. Environ. Res. Public Health 2022, 19, 3754. [Google Scholar] [CrossRef]
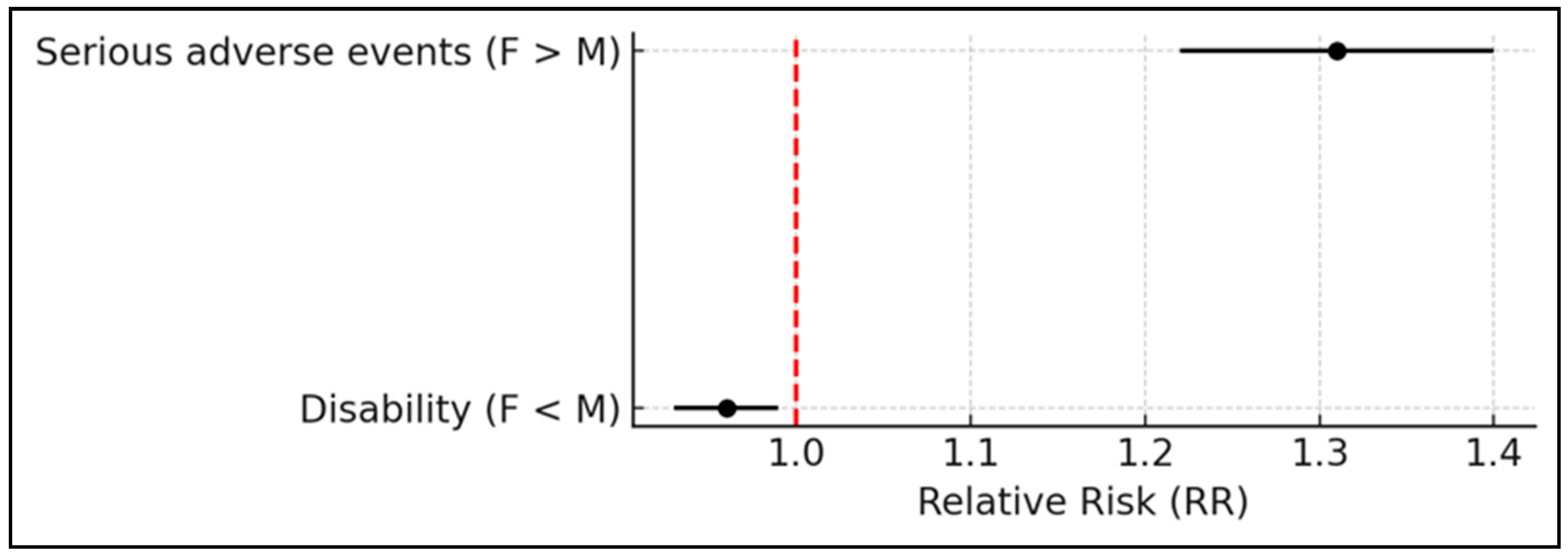
| SOC | Total (%) | Female (n) | Male (n) | Not Specified | RR |
|---|---|---|---|---|---|
| Neoplasms benign, malignant, and unspecified (incl cysts and polyps) | 320 (15.8%) | 116 (13.7%) | 160 (18.9%) | 44 (5.2%) | 0.94 |
| Gastrointestinal disorders | 310 (15.3%) | 141 (16.7%) | 155 (18.3%) | 14 (1.7%) | 1.18 |
| Investigations | 306 (15.1%) | 103 (12.2%) | 153 (18.1%) | 50 (5.9%) | 0.87 |
| General disorders and administration site conditions | 244 (12.1%) | 79 (9.3%) | 122 (14.4%) | 43 (5.1%) | 0.84 |
| Hepatobiliary disorders | 232 (11.5%) | 115 (13.6%) | 116 (13.7%) | 1 (0.1%) | 1.29 |
| Respiratory, thoracic, and mediastinal disorders | 148 (7.3%) | 40 (4.7%) | 74 (8.8%) | 34 (4.0%) | 0.70 |
| Nervous system disorders | 86 (4.3%) | 23 (2.7%) | 43 (5.1%) | 20 (2.4%) | 0.69 |
| Injury, poisoning, and procedural complications | 56 (2.8%) | 29 (3.4%) | 22 (2.6%) | 5 (0.6%) | 1.71 |
| Cardiac disorders | 46 (2.3%) | 16 (1.9%) | 23 (2.7%) | 7 (0.8%) | 0.90 |
| Skin and subcutaneous tissue disorders | 46 (2.3%) | 8 (0.9%) | 23 (2.7%) | 15 (1.8%) | 0.07 |
| Blood and lymphatic system disorders | 44 (2.2%) | 20 (2.4%) | 22 (2.6%) | 2 (0.2%) | 1.18 |
| Musculoskeletal and connective tissue disorders | 42 (2.1%) | 19 (2.2%) | 21 (2.5%) | 2 (0.2%) | 1.17 |
| Infections and infestations | 42 (2.1%) | 18 (2.1%) | 21 (2.5%) | 3 (0.4%) | 1.11 |
| Vascular disorders | 26 (1.3%) | 5 (0.6%) | 13 (1.5%) | 8 (0.9%) | 0.50 |
| Renal and urinary disorders | 23 (1.1%) | 13 (1.5%) | 10 (1.2%) | 0 (0%) | 1.69 |
| Psychiatric disorders | 11 (0.5%) | 8 (0.9%) | 3 (0.4%) | 0 (0%) | 3.46 |
| Ear and labyrinth disorders | 10 (0.5%) | 4 (0.5%) | 5 (0.6%) | 1 (0.1%) | 1.04 |
| Surgical and medical procedures | 10 (0.5%) | 3 (0.4%) | 5 (0.6%) | 2 (0.2%) | 0.78 |
| Eye disorders | 5 (0.2%) | 4 (0.5%) | 1 (0.1%) | 0 (0%) | 5.19 |
| Immune system disorders | 5 (0.2%) | 3 (0.4%) | 2 (0.2%) | 0 (0%) | 1.95 |
| Metabolism and nutrition disorders | 4 (0.2%) | 2 (0.2%) | 2 (0.2%) | 0 (0%) | 1.10 |
| Congenital familiar and genetic disorders | 4 (0.2%) | 0 (0%) | 2 (0.2%) | 2 (0.2%) | - |
| Endocrine disorders | 2 (0.1%) | 1 (0.1%) | 1 (0.1%) | 0 (0%) | 1.04 |
| Reproductive system and breast disorders | 1 (0.1%) | 1 (0.1%) | 0 (0%) | 0 (0%) | 1.23 |
| TRAE | Grade 3 | Grade 4 |
|---|---|---|
| Alanine aminotransferase increase | 4.7% | 0.8% |
| Diarrhea | 3.9% | |
| Anemia | 3.1% | |
| Aspartate aminotransferase increase | 2.3% | |
| Blood alkaline phosphatase increase | 1.6% | |
| Hepatitis | 0.8% | |
| Decreased lymphocyte count | 0.8% | |
| Gamma-glutamyl transferase increase | 0.8% | |
| Hyponatremia | 0.8% |
| TRAE | Any Grade | Grade ≥ 3 |
|---|---|---|
| Diarrhea | 31.7% | 4.0% |
| Nausea | 19% | 0.0% |
| Alanine aminotransferase increase | 15.1% | 6.3% |
| Aspartate aminotransferase increase | 15.1% | 5.6% |
| Fatigue | 11.1% | 0.0% |
| Vomiting | 7.9% | 0.0% |
| Gamma-glutamyl transferase increase | 2.4% | 2.4% |
| Hepatotoxic event | 0.8% | 0.8% |
| Dyspnea | 1.6% | 0.8% |
| TRAE | Any Grade | Grade ≥ 3 |
|---|---|---|
| Diarrhea | 34% | 12% |
| Fatigue | 7% | 1% |
| Nausea | 14% | 1% |
| Decreased appetite | 11% | 2% |
| Alanine aminotransferase increased | 10% | 8% |
| Aspartate aminotransferase increased | 10% | 5% |
| TRAE | Phase 1 CodeBreaK 100 Trial | Phase 2 CodeBreaK 100 Trial | CodeBreaK 200 Clinical Trial | Real-World Evidence |
|---|---|---|---|---|
| Alanine aminotransferase increase | 11.6% | 15.1% | 10% | 2.3% |
| Anemia | 13.2% | NA | 3% | 1.2% |
| Aspartate aminotransferase increase | 13.2% | 15.1% | 10% | 2.1% |
| Blood alkaline phosphatase increase | NA | NA | 7% | 0.6% |
| Decreased appetite | 14.7% | NA | 11% | 1.2% |
| Decreased lymphocyte count | NA | 2.4% | NA | 0.1% |
| Diarrhea | 29.5% | 31.7% | 34% | 8.2% |
| Dyspnea | 16.3% | 1.6% | NA | 0.5% |
| Fatigue | 23.3% | 11.1% | 7% | 0.9% |
| Gamma-glutamyl transferase increase | NA | 2.4% | NA | 0.7% |
| Hepatitis | NA | NA | NA | 0.6% |
| Hepatotoxic event | NA | 0.8% | NA | 2.3% |
| Hyponatremia | NA | NA | NA | 0.1% |
| Nausea | 20.9% | 19% | 14% | 1.9% |
| Vomiting | 17.8% | 7.9% | 5% | 0.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speranza, D.; Marafioti, M.; Musarra, M.; Cianci, V.; Omero, F.; Spagnolo, C.C.; Calabrò, M.; Silvestris, N.; Irrera, N.; Santarpia, M. Sex-Related Safety Signals of Sotorasib in Non-Small Cell Lung Cancer: A Real-World, Pharmacovigilance Study from the EudraVigilance Database. Pharmaceuticals 2025, 18, 1574. https://doi.org/10.3390/ph18101574
Speranza D, Marafioti M, Musarra M, Cianci V, Omero F, Spagnolo CC, Calabrò M, Silvestris N, Irrera N, Santarpia M. Sex-Related Safety Signals of Sotorasib in Non-Small Cell Lung Cancer: A Real-World, Pharmacovigilance Study from the EudraVigilance Database. Pharmaceuticals. 2025; 18(10):1574. https://doi.org/10.3390/ph18101574
Chicago/Turabian StyleSperanza, Desirèe, Mariapia Marafioti, Martina Musarra, Vincenzo Cianci, Fausto Omero, Calogera Claudia Spagnolo, Marco Calabrò, Nicola Silvestris, Natasha Irrera, and Mariacarmela Santarpia. 2025. "Sex-Related Safety Signals of Sotorasib in Non-Small Cell Lung Cancer: A Real-World, Pharmacovigilance Study from the EudraVigilance Database" Pharmaceuticals 18, no. 10: 1574. https://doi.org/10.3390/ph18101574
APA StyleSperanza, D., Marafioti, M., Musarra, M., Cianci, V., Omero, F., Spagnolo, C. C., Calabrò, M., Silvestris, N., Irrera, N., & Santarpia, M. (2025). Sex-Related Safety Signals of Sotorasib in Non-Small Cell Lung Cancer: A Real-World, Pharmacovigilance Study from the EudraVigilance Database. Pharmaceuticals, 18(10), 1574. https://doi.org/10.3390/ph18101574







