Multifunctionality and Possible Medical Application of the BPC 157 Peptide—Literature and Patent Review
Abstract
1. Introduction
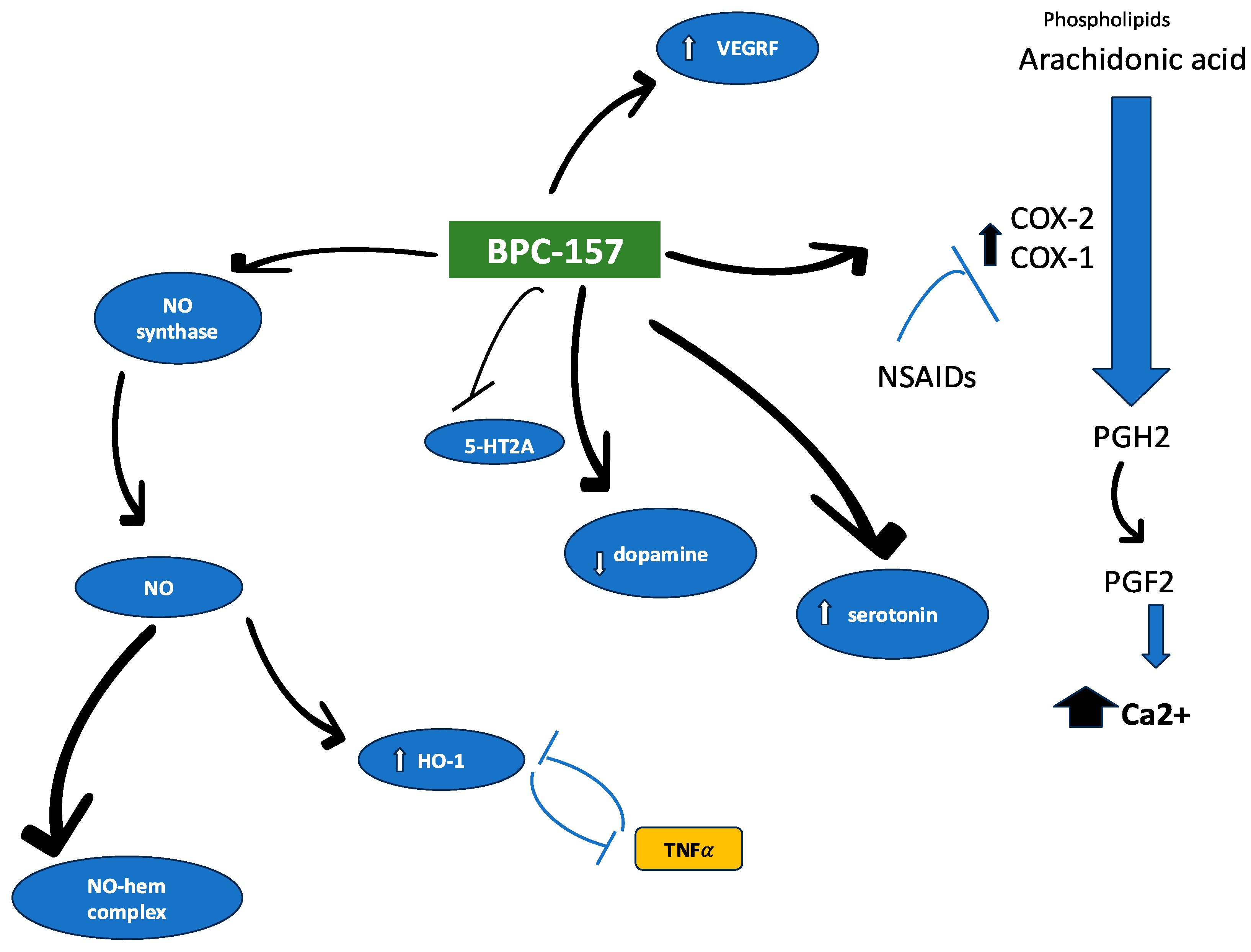
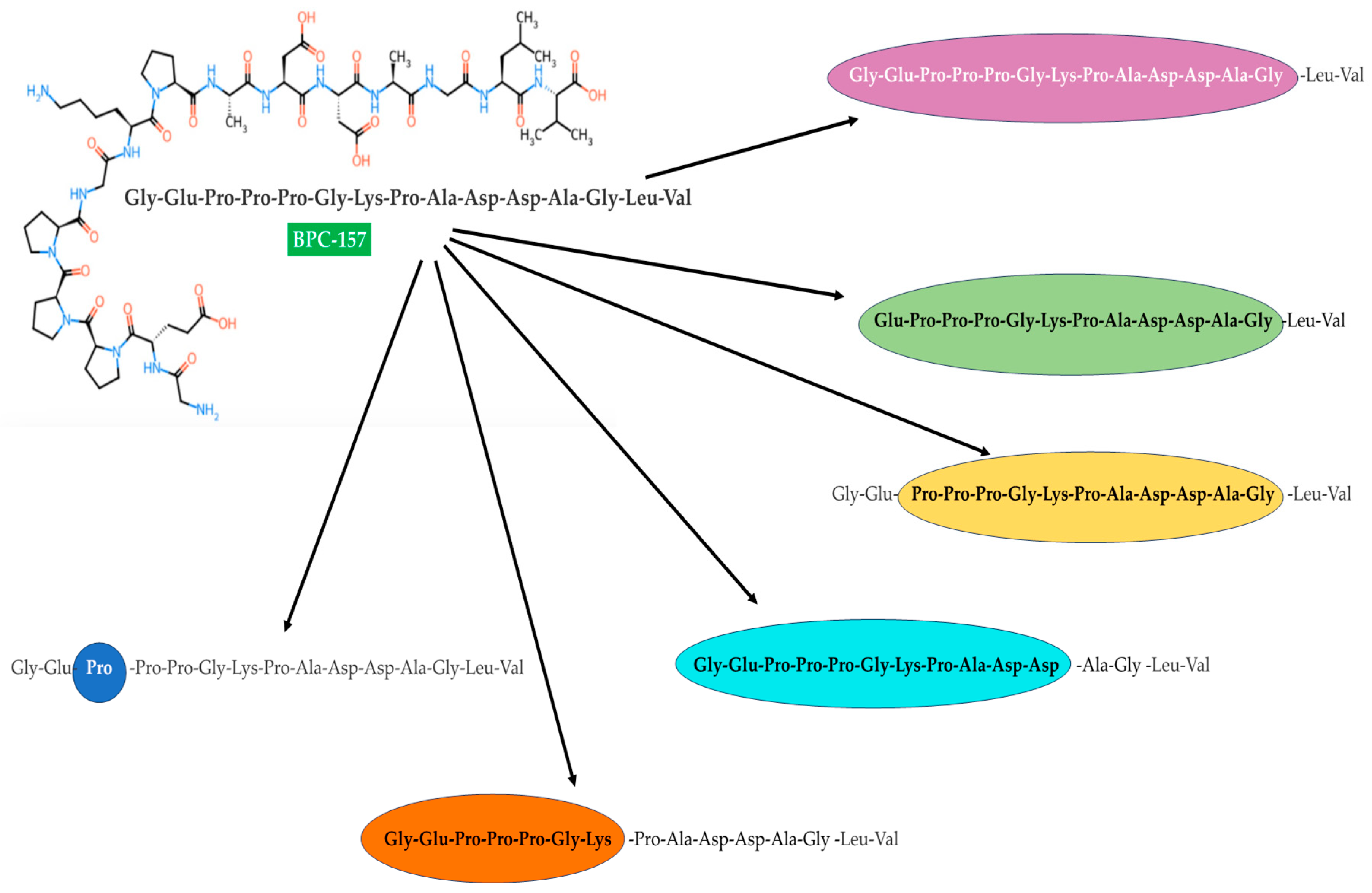
2. BPC 157 Characteristics—Structural Analysis, Targeted Receptors/Molecular Pathways, and ADME Profile
3. Potential Therapeutic Use—Preclinical Studies
3.1. Cancer
3.2. Pain
3.3. Alcohol-Induced Adverse Effects
3.4. Wound Healing and Regeneration
3.5. Neuropsychiatric Disorders
4. BPC 157 in Humans—Clinical Trials and Current Use in Clinical Practice
5. BPC 157 and Probable Toxicity
5.1. Angiogenesis Consequences
5.2. The Role of BPC 157 Metabolites
5.3. BPC 157 Stimulatory Effect on the NO System
6. The Future of BPC 157
| No. | Patent Title | Inventors | Patent Number | Ref. |
|---|---|---|---|---|
| 1 | Fibroblast mediated expansion and augmentation of immune regulatory cells for treatment of acute respiratory distress syndrome (ards) | Ichim, T.; O’Heerin, P. | US 2023/0141224 | [163] |
| 2 | Peptides and adjuvants for augmentation of fibroblast therapy for coronavirus | O’Heeron, P.; Ichim, T. | WO 2021/202031 | [164] |
| 3 | Medical dressing for repairing scars and preparation method thereof | Zhejiang Top Medical Medical Dressing Co., Ltd. | CN 2024/118615479 | [172] |
| 4 | New BPC peptide salts with organo-protective activity, the process for their preparation and their use in therapy | Sikiric, P.; et al. | WO 1998/052973 | [19] |
| 5 | Sublingual semaglutide-BPC 157 combination for weight loss | Bentz, S.; Lucht, A.; Kocher, S. | US 2023/11833189 | [162] |
| 6 | Usefulness of pentadecapeptide for the treatment of multiple sclerosis | Bota, B. | Croatia Patent 2013/1075 | [168] |
| 7 | Formulation and treatment for ophthalmic disorders | Vitti, P.R. | US Patent 2022/0249575 | [171] |
| 8 | Systems and methods for treating persistent pain of neurogenic origin and complex injury | George, D. | WO 2021/252292 | [173] |
| 9 | Pharmaceutical single dosage form for oral delivery of peptides. | Majewski, F | EP 2022/4226918 | [174] |
| 10 | Compositions for improving health | Crisler, M | WO 2024/073762 | [175] |
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Drasar, P.B.; Khripach, V.A. Growing importance of natural products research. Molecules 2019, 25, 6. [Google Scholar] [CrossRef] [PubMed]
- Krzywik, J.; Katarzyńska, J. Renesans peptydów a nowe cele terapeutyczne. Eliksir 2015, 2, 15–22. [Google Scholar]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Sig. Transduct. Target Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef]
- Diao, L.; Meibohm, B. Pharmacokinetics and pharmacokinetic-pharmacodynamic correlations of therapeutic peptides. Clin. Pharmacokinet. 2013, 52, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Hunter, J.C. Problems with peptides—All that glisters is not gold. Trends Neurosci. 1986, 9, 100–102. [Google Scholar] [CrossRef]
- Di, L. Strategic approaches to optimizing peptide ADME properties. AAPS J. 2015, 17, 134–143. [Google Scholar] [CrossRef]
- Al Musaimi, O.; Lombardi, L.; Williams, D.R.; Albericio, F. Strategies for improving Ppeptide stability and delivery. Pharmaceuticals 2022, 15, 1283. [Google Scholar] [CrossRef] [PubMed]
- Boban-Blagaic, A.; Blagaic, V.; Romic, Z.; Jelovac, N.; Dodig, G.; Rucman, R.; Petek, M.; Turkovic, B.; Seiwerth, S.; Sikiric, P. The influence of gastric pentadecapeptide BPC 157 on acute and chronic ethanol administration in mice. The effect of N(G)-nitro-L-arginine methyl ester and L-arginine. Med. Sci. Monit. 2006, 12, BR36–BR45. [Google Scholar] [PubMed]
- Duzel, A.; Vlainic, J.; Antunovic, M.; Malekinusic, D.; Vrdoljak, B.; Samara, M.; Gojkovic, S.; Krezic, I.; Vidovic, T.; Bilic, Z.; et al. Stable gastric pentadecapeptide BPC 157 in the treatment of colitis and ischemia and reperfusion in rats: New insights. World J. Gastroenterol. 2017, 23, 8465–8488. [Google Scholar] [CrossRef]
- Kliček, R.; Kolenc, D.; Šuran, J.; Drmić, D.; Brčić, L.; Aralica, G.; Sever, M.; Holjevac, J.; Radic, B.; Turudic, T.; et al. Stable gastric pentadecapeptide BPC 157 heals cysteamine-colitis and colon-colon-anastomosis and counteracts cuprizone brain injuries and motor disability. J. Physiol. Pharmacol. 2013, 64, 597–612. [Google Scholar] [PubMed]
- Fox, G.; Gabbe, B.; Richardson, M.; Oppy, A.; Page, R.; Edwards, E.; Hau, R.; Ekegren, C.L. Twelve-month outcomes following surgical repair of the Achilles tendon. J. Sci. Med. Sport 2017, 20, e36–e37. [Google Scholar] [CrossRef]
- Fukuta, S.; Oyama, M.; Kavalkovich, K.; Fu, F.; Niyibizi, C. Identification of types II, IX and X collagens at the insertion site of the bovine Achilles tendon. Matrix Biol. 1998, 17, 65–73. [Google Scholar] [CrossRef]
- Hsieh, M.J.; Liu, H.T.; Wang, C.N.; Huang, H.Y.; Lin, Y.; Ko, Y.S.; Wang, J.S.; Chang, V.H.S.; Pang, J.H.S. Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. J. Mol. Med. 2017, 95, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.A.; Han, Y.M.; An, J.M.; Park, Y.J.; Sikiric, P.; Kim, D.H.; Kwon, K.A.; Kim, Y.J.; Yang, D.; Tchah, H.; et al. BPC157 as potential agent rescuing from cancer cachexia. Curr. Pharm. Des. 2018, 24, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Krivic, A.; Anic, T.; Seiwerth, S.; Huljev, D.; Sikiric, P. Achilles detachment in rat and stable gastric pentadecapeptide BPC 157: Promoted tendon-to-bone healing and opposed corticosteroid aggravation. J. Orthop. Res. 2006, 24, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Lovric-Bencic, M.; Sikiric, P.; Hanzevacki, J.S.; Seiwerth, S.; Rogic, D.; Kusec, V.; Aralica, G.; Konjevoda, P.; Batelja, L.; Blagaic, A.B. Doxorubicine-congestive heart failure-increased big endothelin-1 plasma concentration: Reversal by amlodipine, losartan, and gastric pentadecapeptide BPC157 in rat and mouse. J. Pharmacol. Sci. 2004, 95, 19–26. [Google Scholar] [CrossRef]
- Xu, C.; Sun, L.; Ren, F.; Huang, P.; Tian, Z.; Cui, J.; Zhang, W.; Wang, S.; Zhang, K.; He, L.; et al. Preclinical safety evaluation of body protective compound-157, a potential drug for treating various wounds. Regul. Toxicol. Pharmacol. 2020, 114, 104665. [Google Scholar] [CrossRef]
- Sikiric, P.; Petek, M.; Seiwerth, S.; Turkovic, B.; Grabarevic, Z.; Rotkvic, I.; Mise, S.; Duvnjak, M.; Udovicic, I. New BPC Peptide Salts with Organo-Protective Activity, the Process for Their Preparation and Their Use in Therapy. World Patent 1998/052973, 26 November 1998. [Google Scholar]
- Ilic, S.; Drmic, D.; Zarkovic, K.; Kolenc, D.; Brcic, L.; Radic, B.; Djuzel, V.; Blagaic, A.B.; Romic, Z.; Dzidic, S.; et al. Ibuprofen hepatic encephalopathy, hepatomegaly, gastric lesion and gastric pentadecapeptide BPC 157 in rats. Eur. J. Pharmacol. 2011, 667, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Gojkovic, S.; Krezic, I.; Vranes, H.; Zizek, H.; Drmic, D.; Batelja Vuletic, L.; Milavic, M.; Sikiric, S.; Stilinovic, I.; Simeon, P.; et al. Robert’s intragastric alcohol-induced gastric lesion model as an escalated general peripheral and central syndrome, counteracted by the stable gastric pentadecapeptide BPC 157. Biomedicines 2021, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Lee, H.J.; Sikiric, P.; Hahm, K.B. BPC 157 rescued NSAID-cytotoxicity via stabilizing intestinal permeability and enhancing cytoprotection. Curr. Pharm. Des. 2020, 26, 2971–2981. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Toxicity by NSAIDs. Counteraction by stable gastric pentadecapeptide BPC 157. Curr. Pharm. Des. 2013, 19, 76–83. [Google Scholar] [PubMed]
- Seiwerth, S.; Rucman, R.; Turkovic, B.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Stupnisek, M.; Misic, M.; Vuletic, L.B.; et al. BPC 157 and standard angiogenic growth factors. gastrointestinal tract healing, lessons from tendon, ligament, muscle and bone healing. Curr. Pharm. Des. 2018, 24, 1972–1989. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Rucman, R.; Turkovic, B.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Stupnisek, M.; Misic, M.; Vuletic, L.B.; et al. Novel cytoprotective mediator, stable gastric pentadecapeptide BPC 157. Vascular recruitment and gastrointestinal tract healing. Curr. Pharm. Des. 2018, 24, 1990–2001. [Google Scholar] [CrossRef]
- Boban-Blagaic, A.; Blagaic, V.; Mirt, M.; Jelovac, N.; Dodig, G.; Rucman, R.; Petek, M.; Turkovic, B.; Anic, T.; Dubovecak, M.; et al. Gastric pentadecapeptide BPC 157 effective against serotonin syndrome in rats. Eur. J. Pharmacol. 2005, 512, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Petek, M.; Rucman, R.; Seiwerth, S.; Grabarevic, Z.; Rotkvic, I.; Turkovic, B.; Jagic, V.; Mildner, B.; Duvnjak, M.; et al. A new gastric juice peptide, BPC. An overview of the stomach-stress- organoprotection hypothesis and beneficial effects of BPC. J. Physiol. Paris 1993, 87, 313–327. [Google Scholar]
- Chang, C.-H.; Tsai, W.C.; Lin, M.S.; Hsu, Y.H.; Pang Su, J.H. The promoting effects of petnadecapeptide BPC157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J. Appl. Physiol. 2011, 110, 774–7780. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Boban-Blagaic, A.; Strbe, S.; Beketic Oreskovic, L.; Oreskovic, I.; Sikiric, S.; Staresinic, M.; Sever, M.; Kokot, A.; Jurjevic, I.; et al. The stable gastric pentadecapeptide BPC157 pleiotropic beneficial activity and its possible relations with neurotransmitter activity. Pharmaceuticals 2024, 17, 461. [Google Scholar] [CrossRef]
- Sikiric, P.; Petek, M.; Rucman, R.; Seiwerth, S.; Grabarevic, Z.; Rotkvic, I.; Turkovic, B.; Jagic, V.; Mildner, B.; Duvnjak, M.; et al. The significance of the gastroprotective effect of body protection compound (BPC): Modulation by different procedures. Acta Physiol. Hung. 1992, 80, 89–98. [Google Scholar] [PubMed]
- Pflaum, Z.; Rucman, R. Solid phase peptide synthesis of the fragment BPC157 of human gastric juice protein BPC and its analogues. Acta Chim. Slov. 2005, 52, 34–39. [Google Scholar]
- Staresinic, M.; Japjec, M.; Vranes, H.; Prtoric, A.; Zizek, H.; Krezic, I.; Gojkovic, S.; Smoday, I.M.; Oroz, K.; Staresinic, E.; et al. Stable gastric pentadecapeptide BPC157 and striated, smooth, and heart muscle. Biomedicines 2022, 10, 3221. [Google Scholar] [CrossRef]
- Pei, J.; Gao, X.; Pan, D.; Hua, Y.; He, J.; Liu, Z.; Dana, Y. Advances in the stability challenges of bioactive peptides and improvement strategies. Curr. Res. Food Sci. 2022, 5, 2162–2170. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, Y.; Li, R.; Huang, L.; Wang, Z.; Deng, Q.; Dong, S. N-terminal acetylation of antimicrobial peptide L163 improves its stability against protease degradation. J. Pept. Sci. 2021, 27, e3337. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Zhou, S.; Xu, M.; Zeng, S.; Fan, W.; Yu, L.; Lin, N. The metabolic stability of antimicrobial peptides Ik8 in plasma and liver S9. Appl. Sci. 2021, 11, 11661. [Google Scholar] [CrossRef]
- Grishin, D.V.; Zhdanov, D.D.; Pokrovskaya, M.V.; Sokolov, N.N. D-amino acids in nature, agriculture and biomedicine. Front. Life Sci. 2020, 13, 11–22. [Google Scholar] [CrossRef]
- Xu, S.; Xu, X.; Wang, Z.; Wu, R. A systematic investigation of proteoforms with n-terminal glycine and their dynamics reveals its impacts on protein stability. Angew. Chem. Int. Ed. Engl. 2024, 63, e202315286. [Google Scholar]
- Brandts, J.F.; Halvorson, H.R.; Brennan, M. Consideration of the possibility that the slow step in protein denaturation reactions is due to cis-trans isomerism of proline residues. Biochemistry 1975, 14, 4953–4963. [Google Scholar] [CrossRef]
- Ge, M.; Pan, X.M. The contribution of proline residues to protein stability is associated with isomerization equilibrium in both unfolded and folded states. Extremophiles 2009, 13, 481–489. [Google Scholar] [CrossRef]
- Vanhoof, G.; Goossens, F.; De Meester, I.; Hendriks, D.; Scharpé, S. Proline motifs in peptides and their biological processing. FASEB J. 1995, 9, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Grabarevic, Z.; Tisljar, M.; Artukovic, B.; Bratulic, M.; Dzaja, P.; Seiwerth, S.; Sikiric, P.; Peric, J.; Geres, D.; Kos, J. The influence of BPC 157 on nitric oxide agonist and antagonist induced lesions in broiler chicks. J. Physiol. Paris 1997, 91, 139–149. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr. Pharm. Des. 2014, 20, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Djakovic, Z.; Djakovic, I.; Cesarec, V.; Madzarac, G.; Becejac, T.; Zukanovic, G.; Drmic, D.; Batelja, L.; Sever, A.Z.; Kolenc, D.; et al. Esophagogastric anastomosis in rats: Improved healing by BPC 157 and L-arginine, aggravated by L-NAME. World J. Gastroenterol. 2016, 22, 9127–9140. [Google Scholar] [CrossRef]
- Zemba Cilic, A.; Zemba, M.; Cilic, M.; Strbe, S.; Ilic, S.; Vukojevic, J.; Zoricic, Z.; Filipcic, I.; Kokot, A.; Smoday, I.M.; et al. BPC 157, L-NAME, L-arginine, NO-relation, in the suited rat ketamine models resembling “negative-like” symptoms of schizophrenia. Biomedicines 2022, 10, 1462. [Google Scholar] [CrossRef] [PubMed]
- Kolovrat, M.; Gojkovic, S.; Krezic, I.; Malekinusic, D.; Vrdoljak, B.; Kasnik Kovac, K.; Kralj, T.; Drmic, D.; Barisic, I.; Horvat Pavlov, K.; et al. Pentadecapeptide BPC 157 resolves Pringle maneuver in rats, both ischemia and reperfusion. World J. Hepatol. 2020, 12, 184–206. [Google Scholar] [CrossRef]
- Gojkovic, S.; Krezic, I.; Vrdoljak, B.; Malekinusic, D.; Barisic, I.; Petrovic, A.; Horvat Pavlov, K.; Kolovrat, M.; Duzel, A.; Knezevic, M.; et al. Pentadecapeptide BPC 157 resolves suprahepatic occlusion of the inferior caval vein, Budd-Chiari syndrome model in rats. World J. Gastrointest. Pathophysiol. 2020, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Japjec, M.; Horvat Pavlov, K.; Petrovic, A.; Staresinic, M.; Sebecic, B.; Buljan, M.; Vranes, H.; Giljanovic, A.; Drmic, D.; Japjec, M.; et al. Stable gastric pentadecapeptide BPC 157 as a therapy for the disable myotendinous junctions in rats. Biomedicines 2021, 9, 1547. [Google Scholar] [CrossRef] [PubMed]
- Ahmadinejad, F.; Geir Møller, S.; Hashemzadeh-Chaleshtori, M.; Bidkhori, G.; Jami, M.S. Molecular mechanisms behind free radical scavengers function against oxidative stress. Antioxidants 2017, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Park. Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [PubMed]
- Kargl, C.K.; Jia, Z.; Shera, D.A.; Sullivan, B.P.; Burton, L.C.; Kim, K.H.; Nie, Y.; Hubal, M.J.; Shannahan, J.H.; Kuang, S.; et al. Angiogenic potential of skeletal muscle derived extracellular vesicles differs between oxidative and glycolytic muscle tissue in mice. Sci. Rep. 2023, 13, 18943. [Google Scholar] [CrossRef]
- Verde, C.; Giordano, D.; Bruno, S. NO and heme proteins: Cross-talk between heme and cysteine residues. Antioxidants 2023, 12, 321. [Google Scholar] [CrossRef]
- Vitturi, D.A.; Sun, C.W.; Harper, V.M.; Thrash-Williams, B.; Cantu-Medellin, N.; Chacko, B.K.; Peng, N.; Dai, Y.; Wyss, J.M.; Townes, T.; et al. Antioxidant functions for the hemoglobin beta93 cysteine residue in erythrocytes and in the vascular compartment in vivo. Free Radic. Biol. Med. 2013, 55, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Jelovac, N.; Sikiric, P.; Rucman, R.; Petek, M.; Marovic, A.; Perovic, D.; Seiwerth, S.; Mise, S.; Turkovic, B.; Dodig, G.; et al. Pentadecapeptide BPC 157 attenuates disturbances induced by neuroleptics: The effect on catalepsy and gastric ulcers in mice and rats. Eur. J. Pharmacol. 1999, 379, 19–31. [Google Scholar] [CrossRef]
- Sikiric, P.; Jelovac, N.; Jelovac-Gjeldum, A.; Dodig, G.; Staresinic, M.; Anic, T.; Zoricic, I.; Rak, D.; Perovic, D.; Aralica, G.; et al. Pentadecapeptide BPC 157 attenuates chronic amphetamine-induced behavior disturbances. Acta Pharmacol. Sin. 2002, 23, 412–422. [Google Scholar]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and its receptor (vegfr) signaling in angiogenesis: A crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Escribano, M.; Molero, L.; Farré, A.L.; Abarrategui, C.; Carrasco, C.; Garcia-Mendez, A.; Manzarbeitia, F.; Martin, M.J.; Vazquez, M.; Sanchez-Fayos, P.; et al. Aspirin inhibits endothelial nitric oxide synthase (eNOS) and Flk-1 (vascular endothelial growth factor receptor-2) prior to rat colon tumour development. Clin. Sci. 2004, 106, 83–91. [Google Scholar] [CrossRef]
- Toliins, J.P.; Shultz, P.J. Endogenous nitric oxide synthesis determines sensitivity to the pressor effect of salt. Kidney Int. 1994, 46, 230–236. [Google Scholar] [CrossRef]
- Li, H.; Förstermann, U. Nitric oxide in the pathogenesis of vascular disease. J. Pathol. 2000, 190, 244–254. [Google Scholar] [CrossRef]
- Steinert, J.R.; Chernova, T.; Forsythe, I.D. Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist 2010, 16, 435–452. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Feng, D.; Guo, H.; Zhou, Y.; Li, Z.; Zhang, K.; Zhang, W.; Wang, S.; Wang, Z.; Hao, Q.; et al. Pharmacokinetics, distribution, metabolism, and excretion of body-protective compound 157, a potential drug for treating various wounds, in rats and dogs. Front. Pharmacol. 2022, 13, 1026182. [Google Scholar] [CrossRef] [PubMed]
- Veljača, M.; Pavić-Sladoljev, D.; Mildner, B.; Brajša, K.; Krnić, Ž.; Bubenik, M.; Stipanicic, S.; Tabka-Slosic, M.; Brnic, L.; Khan, Z.; et al. Safety, tolerability and pharmacokinetics of PL 14736, a novel agent for treatment of ulcerative colitis, in healthy male volunteers. In Proceedings of the Abstracts of the 6th ESGENA Conference and 10th United European Gastroentherology Week: UEGW: European Journal of Pharmaceutical Sciences (ISSN 0928-0987), Geneva, Switzerland, 20 October 2002; pp. 74–75. [Google Scholar]
- Mallick-Searle, T.; Fillman, M. The pathophysiology, incidence, impact, and treatment of opioid-induced nausea and vomiting. J. Am. Assoc. Nurse Pract. 2017, 29, 704–710. [Google Scholar] [CrossRef]
- Boom, M.; Niesters, M.; Sarton, E.; Aarts, L.; Smith, T.W.; Dahan, A. Non-analgesic effects of opioids: Opioid-induced respiratory depression. Curr. Pharm. Des. 2012, 18, 5994–6004. [Google Scholar] [CrossRef] [PubMed]
- Adriaensen, H.; Vissers, K.; Noorduin, H.; Meert, T. Opioid tolerance and dependence: An inevitable consequence of chronic treatment? Acta Anaesthesiol. Belg. 2003, 54, 37–47. [Google Scholar] [PubMed]
- Garcia Rodriguez, L.A.; Jick, H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet 1994, 343, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Whelton, A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: Physiologic foundations and clinical implications. Am. J. Med. 1999, 106, 13S–24S. [Google Scholar] [CrossRef]
- Dixit, M.; Doan, T.; Kirschner, R.; Dixit, N. Significant acute kidney injury due to non-steroidal anti-inflammatory drugs: Inpatient setting. Pharmaceuticals 2010, 3, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Choi, S.R.; Kim, J.H.; Lee, S.C.; Jeong, J.H.; Lee, T.Y. Antinociceptive Effect of BPC-157 in the formalin-induced pain model. Kosin Med. J. 2021, 36, 1–13. [Google Scholar] [CrossRef]
- Jung, Y.H.; Kim, H.; Kim, H.; Kim, E.; Baik, J.; Kang, H. The anti-nociceptive effect of BPC-157 on the incisional pain model in rats. J. Dent. Anesth. Pain Med. 2022, 22, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Boban Blagaic, A.; Turcic, P.; Blagaic, V.; Dubovecak, M.; Jelovac, N.; Zemba, M.; Radic, B.; Becejac, T.; Rokotov, D.S.; Sikiric, P. Gastric pentadecapeptide BPC 157 counteracts morphine-induced analgesia in mice. J. Physiol. Pharmacol. 2009, 60 (Suppl. S7), 177–181. [Google Scholar]
- Sikiric, P.; Seiwerth, S.; Grabarevic, Z.; Rucman, R.; Petek, M.; Rotkvic, I.; Turkovic, B.; Jagic, V.; Mildner, B.; Duvnjak, M.; et al. Hepatoprotective effect of BPC 157, a 15-amino acid peptide, on liver lesions induced by either restraint stress or bile duct and hepatic artery ligation or CCl4 administration. A comparative study with dopamine agonists and somatostatin. Life Sci. 1993, 53, PL291–PL296. [Google Scholar] [CrossRef]
- Bosch, J.; Kravetz, D.; Rodes, J. Effects of somatostatin on hepatic and systemic hemodynamics in patients with cirrhosis of the liver: Comparison with vasopressin. Gastroenterology 1981, 80, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Del Almeida Barra, C.I. Effect of Bromocriptine in Improving Non-Alcoholic Fatty Liver Disease in Obese Animal Model of Type 2 Diabetes. Ph.D. Thesis, University of Coimbra, Coimbra, Portugal, November 2009. Available online: https://estudogeral.uc.pt/retrieve/221842/Trabalho%20Final%20MIM%20-%20Cátia%20Barra.pdf (accessed on 16 December 2024).
- Prkacin, I.; Separovic, J.; Aralicia, G.; Perovic, D.; Gjurasin, M.; Lovric-Bencic, M.; Stancic-Rotkov, D.; Staresinic, M.; Anic, T.; Mikus, D.; et al. Portal hypertension and liver lesions in chronically alcohol drinking rats prevented and reversed by stable gastric pentadecapeptide BPC 157 (PL-10, PLD-116), and propranolol, but not ranitidine. J. Physiol Paris 2001, 95, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Boban-Blagaic, A.; Blagaic, V.; Romic, Z.; Sikiric, P. The influence of gastric pentadecapeptide BPC 157 on acute and chronic ethanol administration in mice. Eur. J. Pharmacol. 2004, 499, 285–290. [Google Scholar] [CrossRef]
- Becejac, T.; Cesarec, V.; Drmic, D.; Hirsl, D.; Madzarac, G.; Djakovic, Z.; Bunjevac, I.; Zenko Sever, A.A.; Sepac, A.; Vuletic, L.B.; et al. An endogenous defensive concept, renewed cytoprotection/adaptive cytoprotection: Intra(per)-oral/intragastric administration of strong alcohol in rat. Involvement of pentadecapeptide BPC 157 and nitric oxide system. J Physiol. Pharmacol. 2018, 69, 429–440. [Google Scholar]
- Petek, M.; Sikiric, P.; Anic, T.; Buljat, G.; Separovic, J.; Stancic-Rokotov, D.; Seiwerth, S.; Grabarevic, Z.; Rucman, R.; Mikus, D.; et al. Pentadecapeptide BPC 157 attenuates gastric lesions induced by alloxan in rats and mice. J. Physiol. Paris 1999, 93, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Mikus, D.; Sikiric, P.; Seiwerth, S.; Petricevic, A.; Aralica, G.; Druzijancic, N.; Rucman, R.; Petek, M.; Pigac, B.; Perovic, D.; et al. Pentadecapeptide BPC 157 cream improves burn-wound healing and attenuates burn-gastric lesions in mice. Burns 2001, 27, 817–827. [Google Scholar] [CrossRef]
- Seiwerth, S.; Milavic, M.; Vukojevic, J.; Gojkovic, S.; Krezic, I.; Vuletic, L.B.; Pavlov, K.H.; Petrovic, A.; Sikiric, S.; Vranes, H.; et al. Stable gastric pentadecapeptide BPC 157 and wound healing. Front. Pharmacol. 2021, 12, 627533. [Google Scholar] [CrossRef]
- Seiwerth, S.; Sikiric, P.; Grabarevic, Z.; Zorici, I.; Hanzevacki, M.; Ljubanovic, D.; Coric, V.; Konjevoda, P.; Petek, M.; Rucman, R.; et al. BPC 157’s effect on healing. J. Physiol. Paris 1997, 91, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Seveljevic-Jaran, D.; Cuzic, S.; Dominis-Kramaric, M.; Glojnaric, I.; Ivetic, V.; Radosevic, S.; Parnham, M.J. Accelerated healing of excisional skin wounds by PL 14736 in alloxan-hyperglycemic rats. Skin Pharmacol. Physiol. 2006, 19, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Tkalcević, V.I.; Cuzić, S.; Brajsa, K.; Mildner, B.; Bokulić, A.; Situm, K.; Perović, D.; Glojnarić, I.; Parnham, M.J. Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur. J. Pharmacol. 2007, 570, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhang, K.; Sun, L.; Xue, X.; Zhang, C.; Shu, Z.; Mu, N.; Gu, J.; Zhang, W.; Wang, Y.; et al. Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des. Devel. Ther. 2015, 9, 2485–2499. [Google Scholar] [CrossRef] [PubMed]
- Bricic, L.; Bricic, I.; Staresinic, M.; Novinscak, T.; Sikiric, P.; Seiweerth, S. Modulatory effect of gastric pentadecapeptide BPC 157 on angiogenesis in muscle and tendon healing. J. Physiol. Phramacol. 2009, 60, 191–196. [Google Scholar]
- Stupnisek, M.; Franjic, S.; Drmic, D.; Hrelec, M.; Kolenc, D.; Radic, B.; Bojic, D.; Vcev, A.; Seiwerth, S.; Sikiric, P. Pentadecapeptide BPC 157 reduces bleeding time and thrombocytopenia after amputation in rats treated with heparin, warfarin or aspirin. Thromb. Res. 2012, 129, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Rasic, D.; Sever, A.Z.; Rasic, F.; Strbe, S.; Rasic, Z.; Djuzel, A.; Duplancic, B.; Blagaic, A.B.; Skrtic, A.; Seiwerth, S.; et al. Stable gastric pentadecapeptide BPC 157 heals established vesicovaginal fistula and counteracts stone formation in rats. Biomedicines 2021, 9, 1206. [Google Scholar] [CrossRef] [PubMed]
- Zivanovic-Posilovic, G.; Balenovic, D.; Barisic, I.; Strinic, D.; Stambolija, V.; Udovicic, M.; Uzun, S.; Drmic, D.; Vlainic, J.; Bencic, M.L.; et al. Stable gastric pentadecapeptide BPC 157 and bupivacaine. Eur. J. Pharmacol. 2016, 793, 56–65. [Google Scholar] [CrossRef]
- Ilic, S.; Drmic, D.; Franjic, S.; Kolenc, D.; Coric, M.; Brcic, L.; Klicek, R.; Radic, B.; Sever, M.; Djuzel, V.; et al. Pentadecapeptid BPC 157 and its effects on a NSAID toxicity model: Diclofenac-induced gastrointestinal, liver, and encephalopathy lesions. Life Sci. 2011, 88, 535–542. [Google Scholar] [CrossRef]
- Konosic, S.; Petricevic, M.; Ivancan, V.; Konosic, L.; Goluza, E.; Krtalic, B.; Drmic, D.; Stupnisek, M.; Seiwerth, S.; Sikiric, P. Intragastric application of Aspirin, Clopidogrel, Cilostazol, and BPC 157 in rats: Platelet aggregation and blood clot. Oxid. Med. Cell. Longev. 2019, 2019, 9084643. [Google Scholar] [CrossRef] [PubMed]
- Katsnelson, B.A.; Privalova, L.I.; Kireyeva, Y.P.; Yeremenko, O.S.; Sutunkova, M.P.; Valamina, I.E.; Varaksin, A.N.; Panov, V.G.; Kazmer, J.I. Combined subchronic fluoride-lead intoxication and its attenuation with the help of a complex of bioprotectors. Med. Lav. 2012, 103, 146–159. [Google Scholar] [PubMed]
- Minigalieva, I.A.; Katsnelson, B.A.; Privalova, L.I.; Sutunkova, M.P.; Gurvich, V.B.; Shur, V.Y.; Shishkina, E.V.; Valamina, I.E.; Makeyev, O.H.; Panov, V.G.; et al. Attenuation of combined nickel (II) oxide and manganese (II, III) oxide nanoparticles’ adverse effects with a complex of bioprotectors. Int. J. Mol. Sci. 2015, 16, 22555–22583. [Google Scholar] [CrossRef] [PubMed]
- Cowen, P.J.; Browning, M. What has serotonin to do with depression? World Psychiatry 2015, 14, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T. Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry 2023, 28, 3243–3256. [Google Scholar] [CrossRef] [PubMed]
- Zarrindast, M.R.; Khakpai, F. The modulatory role of dopamine in anxiety-like behavior. Arch. Iran Med. 2015, 18, 591–603. [Google Scholar]
- Seeman, P.; Kapur, S. Schizophrenia: More dopamine, more D2 receptors. Proc. Natl. Acad. Sci. USA 2000, 97, 7673–7675. [Google Scholar] [CrossRef]
- Gordon, J.A.; Hen, R. The serotonergic system and anxiety. Neuromolecular Med. 2004, 5, 27–40. [Google Scholar] [CrossRef]
- Nuss, P. Anxiety disorders and GABA neurotransmission: A disturbance of modulation. Neuropsychiatr. Dis. Treat. 2015, 11, 165–175. [Google Scholar]
- Tohyama, Y.; Sikirić, P.; Diksic, M. Effects of Pentadecapeptide BPC157 on regional serotonin synthesis in the rat brain: α-methyl-L-tryptophan autoradiographic measurements. Life Sci. 2004, 76, 345–357. [Google Scholar] [CrossRef]
- Sikiric, P.; Separovic, J.; Buljat, G.; Anic, T.; Stancic-Rokotov, D.; Mikus, D.; Marovic, A.; Prkacin, I.; Duplancic, B.; Zoricic, I.; et al. The antidepressant effect of an antiulcer pentadecapeptide BPC157 in Porsolt’s test and chronic unpredictable stress in rats. A comparison with antidepressants. J. Physiol. Paris 2000, 94, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Calipari, E.S.; Ferris, M.J. Amphetamine mechanisms and actions at the dopamine terminal revisited. J. Neurosci. 2013, 33, 8923–8925. [Google Scholar] [CrossRef] [PubMed]
- Jelovac, N.; Sikiric, P.; Rucman, R.; Petek, M.; Perovic, D.; Konjevoda, P.; Marovic, A.; Seiwerth, S.; Grabarevic, Z.; Sumajstorcic, J.; et al. The effect of a novel pentadecapeptide BPC157 on development of tolerance and physical dependence following repeated administration of diazepam. Chin. J. Physiol. 1999, 42, 171–179. [Google Scholar]
- Rastogi, R.B.; Lapierre, Y.D.; Singhal, R.L. Evidence for the role of brain norepinephrine and dopamine in “rebound” phenomenon seen during withdrawal after repeated exposure to benzodiazepines. J. Psychiatr. Res. 1977, 13, 65–75. [Google Scholar] [CrossRef]
- Lee, E.; Padgett, B. Intra-articular injection of BPC 157 for multiple types of knee pain. Altern. Ther. Health. Med. 2021, 27, 8–13. [Google Scholar] [PubMed]
- PCO-02–Safety and Pharmacokinetics Trial. NCT02637284. Available online: https://clinicaltrials.gov/study/NCT02637284?term=bpc-157&rank=1 (accessed on 24 December 2024).
- Costa, C.; Incio, J.; Soares, R. Angiogenesis and chronic inflammation: Cause or consequence? Angiogenesis 2007, 10, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Sacewicz, I.; Wiktorska, M.; Wysocki, T.; Niewiarowska, J. Mechanizmy angiogenezy nowotworowej. Postępy Hig. Med. Dośw. 2009, 63, 159–168. [Google Scholar]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk. Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Viallard, C.; Larrivée, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, L.; O’Reilly, M.S.; Folkman, J. Dormancy of micrometastases: Balance proliferation and apoptosis in the presence of angiogenesis suppression. Nat. Med. 1995, 1, 149–153. [Google Scholar] [CrossRef]
- Salven, P.; Lymboussaki, A.; Heikkila, P.; Jaaskela-Saari, H.; Enholm, B.; Aase, K.; von Euler, G.; Eriksson, U.; Alitalo, K.; Joensuu, H. Vascular endothelial growth factors VEGF-B and VEGF-C are expressed in human tumors. Am. J. Pathol. 1998, 153, 103–108. [Google Scholar] [CrossRef]
- Molhoek, K.R.; Erdag, G.; Rasamy, J.K.; Murphyg, C.; Deacon, D.; Patterson, J.W.; Slingluff, C.L., Jr.; Brautigan, D.L. VEGFR-2 expression in human melanoma: Revised assessment. Int. J. Cancer 2011, 129, 2807–2815. [Google Scholar] [CrossRef] [PubMed]
- Spannuth, W.A.; Nick, A.M.; Jennings, N.B.; Armaiz-Pena, G.N.; Magala, L.S.; Danes, C.G.; Ling, Y.G.; Merritt, W.M.; Thaker, P.H.; Kamat, A.A.; et al. Functional significance of VEGFR-2 on ovarian cancer cells. Int. J. Cancer 2009, 124, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Capp, C.; Wajner, S.M.; Siqueira, D.R.; Brasil, B.A.; Meurer, L.; Maia, A.L. Increased expression of vascular endothelial growth factor and its receptors, VEGFR-1 and VEGFR-2, in medullary thyroid carcinoma. Thyroid 2010, 20, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Seiwerth, S.; Grabarevic, Z.; Rucman, R.; Petek, M.; Jagic, V.; Turkovic, B.; Rotkvic, I.; Mise, S.; Zoricic, I.; et al. The influence of a novel pentadecapeptide, BPC 157, on NG-nitro-L-arginine methylester and L-arginine effects on stomach mucosa integrity and blood pressure. Eur. J. Pharmacol. 1997, 323, 23–33. [Google Scholar] [CrossRef]
- Morbidelli, L.; Donnini, S.; Ziche, M. Role of nitric oxide in tumor angiogenesis. Cancer Treat. Res. 2004, 117, 155–167. [Google Scholar] [PubMed]
- Fujita, S.; Masago, K.; Hatachi, Y.; Fukuhara, A.; Hata, A.; Kaji, R.; Kim, Y.H.; Mio, T.; Mishima, M.; Katakami, N. Genetic polymorphisms in the endothelial nitric oxide synthase gene correlate with overall survival in advanced non-small-cell lung cancer patients treated with platinum-based doublet chemotherapy. BMC Med. Genet. 2010, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Kashiwagi, S.; Jain, R.K. The role of nitric oxide in tumour progression. Nat. Rev. Cancer 2006, 6, 521–534. [Google Scholar] [CrossRef]
- Girotti, A.W.; Fahey, J.F.; Korytowski, W. Role of nitric oxide in hyper-aggressiveness of tumor cells that survive various anti-cancer therapies. Crit. Rev. Oncol. Hematol. 2022, 179, 103805. [Google Scholar] [CrossRef] [PubMed]
- Luanpitpong, S.; Chanvorachote, P. Nitric oxide and aggressive behavior of lung cancer cells. Anticancer Res. 2015, 35, 4585–4592. [Google Scholar] [PubMed]
- Li, L.; Ameri, A.; Wang, S.; Jansson, K.; Casey, O.; Yang, Q.; Beshiri, M.L.; Fang, L.; Lake, R.G.; Agarwal, S.; et al. EGR1 regulates angiogenic and osteoclastogenic factors in prostate cancer and promotes metastasis. Oncogene 2019, 38, 6241–6255. [Google Scholar] [CrossRef] [PubMed]
- Myung, E.; Park, Y.L.; Kim, N.; Chung, C.Y.; Park, H.B.; Park, H.C.; Myung, D.S.; Kim, J.S.; Cho, S.B.; Lee, W.S.; et al. Expression of early growth response-1 in human gastric cancer and its relationship with tumor cell behaviors and prognosis. Pathol. Res. Pract. 2013, 209, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Fu, C.; Kent, K.C.; Bush, H.; Schulick, A.H.; Kreiger, K.; Collins, T.; McCaffrey, T.A. Elevated Egr-1 in human atherosclerotic cells transcriptionally represses the transforming growth factor-b type II receptor. J. Biol. Chem. 2000, 275, 39039–39047. [Google Scholar] [CrossRef]
- Ghazvini-Boroujerdi, M.; Clark, J.; Narula, N.; Palmatory, E.; Connolly, J.M.; DeFelice, S.; Xu, J.; Jian, B.; Hazelwood, S.; Levy, R.J. Transcription factor Egr-1 in calcific aortic valve disease. J. Heart Valve Dis. 2004, 13, 894–903. [Google Scholar]
- Buitrago, M.; Lorenz, K.; Maass, M.; Oberdorf-Maass, S.; Keller, U.; Schmitteckert, E.M.; Ivaschenko, Y.; Lohse, M.J.; Engelhardt, S. The transcriptional repressor NAB1 is a specific regulator of pathological cardiac hypertrophy. Nat. Med. 2005, 11, 837–844. [Google Scholar] [CrossRef]
- Kawahara, N.; Wang, Y.; Mukasa, A.; Furuya, K.; Shimizu, T.; Hamakubo, T.; Aburatani, H.; Kodama, T.; Kirino, T. Genome-wide gene expression analysis for induced ischemic tolerance and delayed neuronal death following transient global ischemia in rats. J. Cereb. Blood Flow Metab. 2004, 24, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Tian, H.; Feng, Y.; Zhu, Y.; Zhang, W. Egr-1 promotes cell proliferation and invasion by increasing β-catenin expression in gastric cancer. Dig. Dis. Sci. 2013, 58, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Wu, X.; Ji, X.; Liang, N.; Li, Z. Early growth response 1 promoted the invasion of glioblastoma multiforme by elevating HMGB1. J. Neurosurg. Sci. 2023, 67, 422–430. [Google Scholar] [CrossRef]
- Baluk, P.; Hashizume, H.; McDonald, D.M. Cellular abnormalities of blood vessels as targets in cancer. Curr. Opin. Genet. Dev. 2005, 15, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.A.; Chang, S.H.; Shih, S.C.; Dvorak, A.M.; Dvorak, H.F. Heterogeneity of the tumor vasculature. Semin. Thromb. Hemost. 2010, 36, 321–331. [Google Scholar] [CrossRef]
- Huang, D.; Lan, H.; Liu, F.; Wang, S.; Chen, X.; Jin, K.; Mou, X. Anti-angiogenesis or pro-angiogenesis for cancer treatment: Focus on drug distribution. Int. J. Clin. Exp. Med. 2015, 8, 8369–8376. [Google Scholar] [PubMed]
- Krishnan, N.; Dickman, M.B.; Becker, D.F. Proline modulates the intracellular redox environment and protects mammalian cells against oxidative stress. Free Radic. Biol. Med. 2008, 44, 671–681. [Google Scholar] [CrossRef]
- Donald, S.P.; Sun, X.Y.; Hu, C.A.; Yu, J.; Mei, J.M.; Valle, D.; Phang, J.M. Proline oxidase, encoded by p53-induced gene-6, catalyzes the generation of proline-dependent reactive oxygen species. Cancer Res. 2001, 61, 1810–1815. [Google Scholar] [PubMed]
- Hancock, C.N.; Liu, W.; Alvord, W.G.; Phang, J.M. Co-regulation of mitochondrial respiration by proline dehydrogenase/oxidase and succinate. Amino Acids. 2016, 48, 859–872. [Google Scholar] [CrossRef]
- Pandhare, J.; Cooper, S.K.; Phang, J.M. Proline oxidase, a proapoptotic gene, is induced by troglitazone: Evidence for both peroxisome proliferator-activated receptor gamma-dependent and -independent mechanisms. J. Biol. Chem. 2006, 281, 2044–2052. [Google Scholar] [CrossRef]
- Cecchini, N.M.; Monteoliva, M.I.; Alvarez, M.E. Proline dehydrogenase is a positive regulator of cell death in different kingdoms. Plant Signal. Behav. 2011, 6, 1195–1197. [Google Scholar] [CrossRef] [PubMed]
- Phang, J.M.; Donald, S.P.; Pandhare, J.; Liu, Y. The metabolism of proline, a stress substrate, modulates carcinogenic pathways. Amino Acids 2008, 35, 681–690. [Google Scholar] [CrossRef]
- Hu, C.-A.A.; Yu, J.; Lin, W.W.; Donald, S.P.; Sun, X.Y.; Almashanu, S. Overexpression of proline oxidase, a p53 induced gene (PIG6) induces reactive oxygen species generation and apoptosis in cancer cells. Proc. Am. Assoc. Cancer Res. 2001, 42, 225. [Google Scholar]
- Liu, Y.; Borchert, G.L.; Surazynski, A.; Hu, C.A.; Phang, J.M. Proline oxidase activates both intrinsic and extrinsic pathways for apoptosis: The role of ROS/superoxides, NFAT and MEK/ERK signaling. Oncogene 2006, 25, 5640–5647. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M.; Roy, R.S. The pathophysiology of superoxide: Roles in inflammation and ischemia. Can. J. Physiol. Pharmacol. 1982, 60, 1346–1352. [Google Scholar] [CrossRef]
- Massaad, C.A.; Pautler, R.G.; Klann, E. Mitochondrial superoxide: A key player in Alzheimer’s disease. Aging 2009, 1, 758–761. [Google Scholar] [CrossRef][Green Version]
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am J Physiol. 1996, 271, C1424–C1437. [Google Scholar] [CrossRef]
- Guzik, T.J.; West, N.E.; Pillai, R.; Taggart, D.P.; Channon, K.M. Nitric oxide modulates superoxide release and peroxynitrite formation in human blood vessels. Hypertension 2002, 39, 1088–1094. [Google Scholar] [CrossRef]
- Guzik, T.J.; West, N.E.; Black, E.; McDonald, D.; Ratnatunga, C.; Pillai, R.; Channon, K.M. Vascular superoxide production by NAD(P)H oxidase: Association with endothelial dysfunction and clinical risk factors. Circ. Res. 2000, 86, e85–e90. [Google Scholar] [CrossRef] [PubMed]
- Baylis, C.; Vallance, P. Nitric oxide and blood pressure: Effects of nitric oxide deficiency. Curr. Opin. Nephrol. Hypertens. 1996, 5, 80–88. [Google Scholar] [CrossRef]
- Tessari, P.; Cecchet, D.; Cosma, A.; Vettore, M.; Coracina, A.; Millioni, R.; Iori, E.; Puricelli, L.; Avogaro, A.; Vedovato, M. Nitric oxide synthesis is reduced in subjects with type 2 diabetes and nephropathy. Diabetes 2010, 59, 2152–2159. [Google Scholar] [CrossRef]
- Foster, M.W.; Hess, D.T.; Stamler, J.S. Protein S-nitrosylation in health and disease: A current perspective. Trends Mol. Med. 2009, 15, 391–404. [Google Scholar] [CrossRef]
- Waheed, S.M.; Ghosh, A.; Chakravarti, R.; Biswas, A.; Haque, M.M.; Panda, K.; Stuehr, D.J. Nitric oxide blocks cellular heme insertion into a broad range of heme proteins. Free Radic. Biol. Med. 2010, 48, 1548–1558. [Google Scholar] [CrossRef]
- Albakri, Q.A.; Stuehr, D.J. Intracellular assembly of inducible NO synthase is limited by nitric oxide-mediated changes in heme insertion and availability. J. Biol. Chem. 1996, 271, 5414–5421. [Google Scholar] [CrossRef] [PubMed]
- Stuehr, D.J.; Biswas, P.; Dai, Y.; Ghosh, A.; Islam, S.; Jayaram, D.T. A natural heme deficiency exists in biology that allows nitric oxide to control heme protein functions by regulating cellular heme distribution. Bioessays 2023, 45, e2300055. [Google Scholar] [CrossRef]
- Liu, C.; Liang, M.C.; Soong, T.W. Nitric Oxide, iron and neurodegeneration. Front. Neurosci. 2019, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.R.; Snyder, B.S.; Beard, J.L.; Fine, R.E.; Mufson, E.J. Regional distribution of iron and iron-regulatory proteins in the brain in aging and Alzheimer’s disease. J. Neurosci. Res. 1992, 31, 327–335. [Google Scholar] [CrossRef]
- Jellinger, K.; Paulus, W.; Grundke-Iqbal, I.; Riederer, P.; Youdim, M.B. Brain iron and ferritin in Parkinson’s and Alzheimer’s diseases. J. Neural Transm. Park. Dis. Dement. Sect. 1990, 2, 2327–2340. [Google Scholar] [CrossRef]
- Brown, G.C. Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Lett. 1995, 369, 136–139. [Google Scholar] [CrossRef]
- Brown, G.C.; Cooper, C.E. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal cytochrome oxidase respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994, 356, 295–298. [Google Scholar] [CrossRef]
- Poderoso, J.J.; Carreras, M.C.; Lisdero, C.; Riobo, N.; Schopfer, F.; Boveris, A. Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch. Biochem. Biophys. 1996, 328, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Clementi, E.; Brown, G.C.; Feelisch, M.; Moncada, S. Persistent inhibition of cell respiration by nitric oxide: Crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc. Natl. Acad. Sci. USA 1998, 95, 7631–7636. [Google Scholar] [CrossRef]
- Cooper, C.E. Nitric oxide and iron proteins. Biochim. Biophys. Acta (BBA) Bioenerg. 1999, 1411, 290–309. [Google Scholar] [CrossRef]
- Salgo, M.G.; Stone, K.; Squadrito, G.L.; Battista, J.R.; Pryor, W.A. Peroxynitrite causes DNA nikc in plasmid pBR322. Communication 1995, 210, 1025–1030. [Google Scholar]
- Ischiropoulos, H.; Al-Mehdi, A.B. Peroxynitrite-mediated oxidative protein modification. FEBS Lett. 1995, 364, 279–282. [Google Scholar] [CrossRef]
- Torreilles, F.; Salman-Tabcheb, S.; Guerin, M.C.; Torreilles, J. Neurodegenerative disorders: The role of peroxynitrite. Brain Res. Rev. 1999, 30, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Bentz, S.; Lucht, A.; Kocher, S. Sublingual Semaglutide-BPC 157 Combination for Weight Loss. US Patent 2023/11833189, 6 July 2023. [Google Scholar]
- Ichim, T.; O’Heeron, P. Fibroblast Mediated Expansion and Augmentation of Immune Regulatory Cells for Treatment of Acute Respiratory Syndrome (ARDS). US Patent 2023/0141224, 11 May 2023. [Google Scholar]
- O’Heeron, P.; Ichim, T. Peptides and Adjuvants for Augmentation of Fibroblast Therapy for Coronavirus. World Patent 2021/202031, 7 October 2021. [Google Scholar]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef]
- Zambon, M.; Vincent, J.L. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest 2008, 133, 1120–1127. [Google Scholar] [CrossRef]
- Wilson, K.C.; Saukkonen, J.J. Acute respiratory failure from abused substances. J. Intensive Care Med. 2004, 19, 183–193. [Google Scholar] [CrossRef]
- Bota, B. Usefulness of Pentadecapeptide for the Treatment of Multiple Sclerosis. Croatia Patent 2013/1075, 22 May 2015. [Google Scholar]
- Pucak, M.L.; Carroll, K.A.; Kerr, D.A.; Kaplin, A.I. Neuropsychiatric manifestations of depression in multiple sclerosis: Neuroinflammatory, neuroendocrine, and neurotrophic mechanisms in the pathogenesis of immune-mediated depression. Dialogues Clin. Neurosci. 2007, 9, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Braley, T.J.; Chervin, R.D. Fatigue in multiple sclerosis: Mechanisms, evaluation, and treatment. Sleep 2010, 33, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Vitti, P.R. Formulation and Treatment for Ophthalmic Disorders. US Patent 2022/0249575, 11 August 2022. [Google Scholar]
- Zhejiang Top Medical Medical Dressing Co., Ltd. Medical Dressing for Repairing Scars and Preparation Method Thereof. Chinese Patent 2024/118615479, 10 September 2024. [Google Scholar]
- George, D. Systems and Methods for Treating Persistent Pain of Neurogenic Origin and Complex Injury. World Patent 2021/252292, 16 December 2021. [Google Scholar]
- Majewski, F. Pharmaceutical Single Dosage Form for Rral Delivery of Peptides. EP 2022/4226918, 16 August 2023. [Google Scholar]
- Crisler, M. Compositions for improving health. World Patent WO 2024/073762, 2 May 2024. [Google Scholar]
| Preclinical Model | Dose Regiment | Species | Outcomes Efficacy | Ref. |
|---|---|---|---|---|
| Aloxan-induced diabetic wound | 10, 100, 500, and 1000 g/0.05 g of carbopol gel daily for 5 days (s.c.) | Male Wistar Han rats | Promotion of mature collagen content in granulation tissue | [82] |
| Excisional, non-occulated full-thickness wound | 100 μg/wound once daily for 18 days | Genetically diabetic female C57BL/KsJ db+/db+ mice | Immediate closure of wounds; impact on the organization of collagen; stimulation of granulation tissue formation | [83] |
| Thermal (flame) burn-induced wound | Topically 1 μg/1 g of vehicle (commercial neutral cream) | NMRI-Hannover male mice | Improvement in all parameters of burn healing (i.e., less edema, reduction in the number of inflammatory cells, advanced formation of dermal reticulin and collagen fibers) | [79] |
| Skin alkali burn | 200–800 ng/mL of the drug applied topically twice every day for 18 days | Male Sprague-Dawley rats | Fast wound closure (i.e., impact on the organization of granulation tissue formation, re-epithelialization, and dermal remodeling | [84] |
| Muscle crush injury model | 10 μg/kg i.p. once a day for 13 days | Male Wistar Albino rats | Increased blood vessel formation | [85] |
| Animal’s tail amputation | 10 μg/kg or 10 ng/kg i.p. before amputation | Male Wistar Albino rats | Reduction in bleeding time and blood loss vs. saline-treated animals; attenuation of acute thrombocytopenia | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Józwiak, M.; Bauer, M.; Kamysz, W.; Kleczkowska, P. Multifunctionality and Possible Medical Application of the BPC 157 Peptide—Literature and Patent Review. Pharmaceuticals 2025, 18, 185. https://doi.org/10.3390/ph18020185
Józwiak M, Bauer M, Kamysz W, Kleczkowska P. Multifunctionality and Possible Medical Application of the BPC 157 Peptide—Literature and Patent Review. Pharmaceuticals. 2025; 18(2):185. https://doi.org/10.3390/ph18020185
Chicago/Turabian StyleJózwiak, Michalina, Marta Bauer, Wojciech Kamysz, and Patrycja Kleczkowska. 2025. "Multifunctionality and Possible Medical Application of the BPC 157 Peptide—Literature and Patent Review" Pharmaceuticals 18, no. 2: 185. https://doi.org/10.3390/ph18020185
APA StyleJózwiak, M., Bauer, M., Kamysz, W., & Kleczkowska, P. (2025). Multifunctionality and Possible Medical Application of the BPC 157 Peptide—Literature and Patent Review. Pharmaceuticals, 18(2), 185. https://doi.org/10.3390/ph18020185










