Chemical Analysis of Plasma-Activated Culture Media by Ion Chromatography
Abstract
:1. Introduction
- (i)
- Direct plasma treatment: This involves the direct exposure of biological targets (e.g., cells, tissues, or animals) to CAP, resulting in a synergistic effect of all the plasma components mentioned above.
- (ii)
- Indirect plasma treatment: In this method, a liquid (e.g., cell culture medium) is exposed to CAP and then applied to the biological target. In this case, the effect is mainly due to the RONS generated in the activated liquid (PTL: plasma-treated liquid), particularly through its enrichment with long-lived secondary RONS [3].
2. Results and Discussion
2.1. Analysis of Results
- ✓
- Lambda_z (individual estimate of the terminal elimination rate constant);
- ✓
- T½ (half-life);
- ✓
- Tmax (time when the maximum concentration is found);
- ✓
- Cmax (maximum concentration found);
- ✓
- Clast_obs/Cmax (the observed concentration at Tlast, where Tlast is the time of the last sample measurable with the analytical method);
- ✓
- AUC0-t (expressed in µg/mL × min);
- ✓
- AUC0-inf_obs (expressed in µg/mL × min);
- ✓
- AUC0-t/0-inf_obs (expressed in µg/mL × min);
- ✓
- AUMC0-inf_obs (the area under the moment curve from time zero (pre-dose) to the time of the last sample, including after the application of the LOQ rules);
- ✓
- MRT0-inf_obs (the mean residence times, based on AUC);
- ✓
- Vz_obs (apparent volume of distribution) expressed in (J/s)/(µg/mL);
- ✓
- Cl_obs (systemic clearance) expressed in (J/s)/(µg/mL)/min;
- ✓
- Vss_obs (volume of distribution at steady state) expressed in (J/s)/(µg/mL).
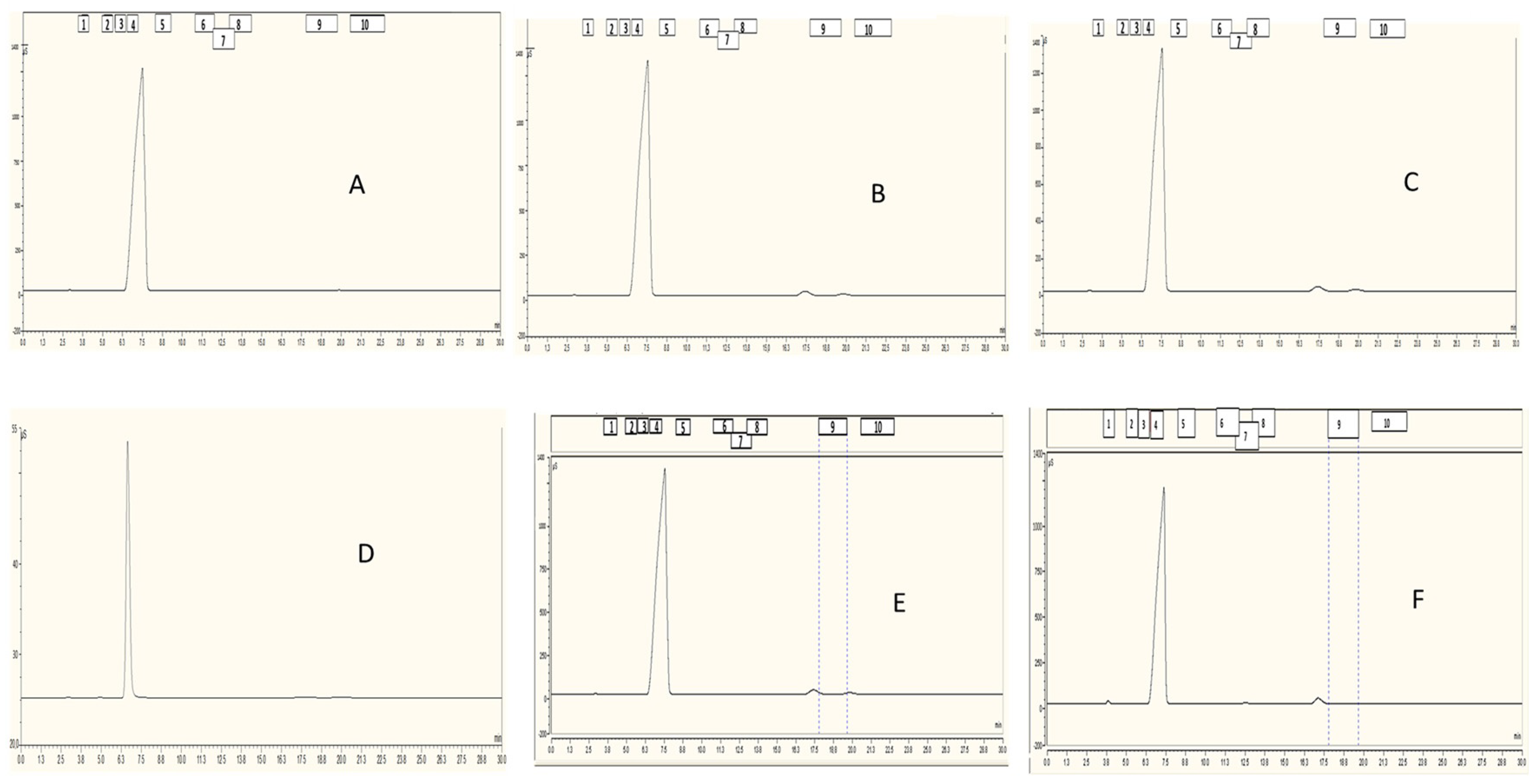
2.2. Results
3. Materials and Methods
3.1. Chemicals and Materials
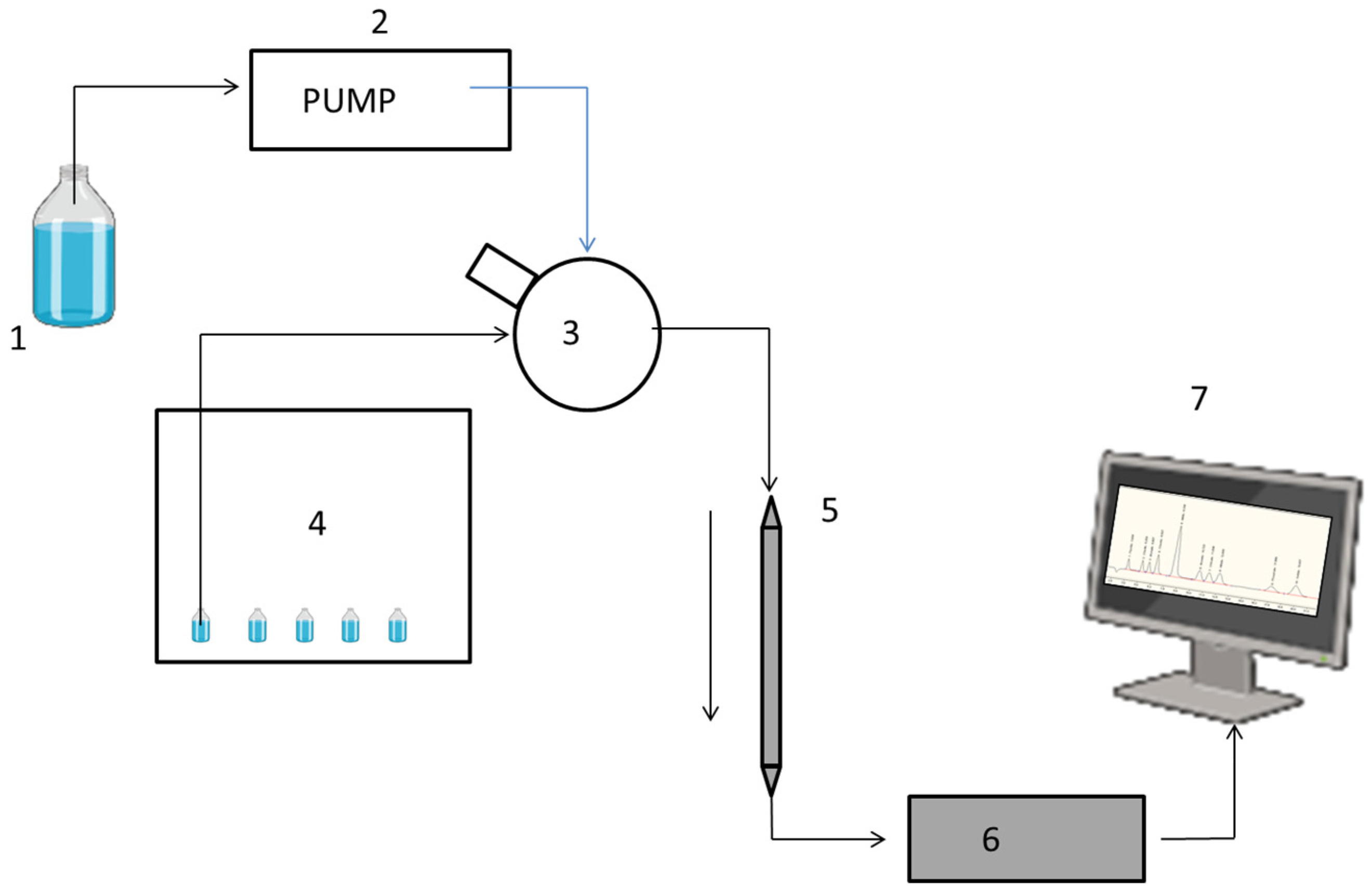
3.2. Equipment
3.3. Standard Solution Preparation
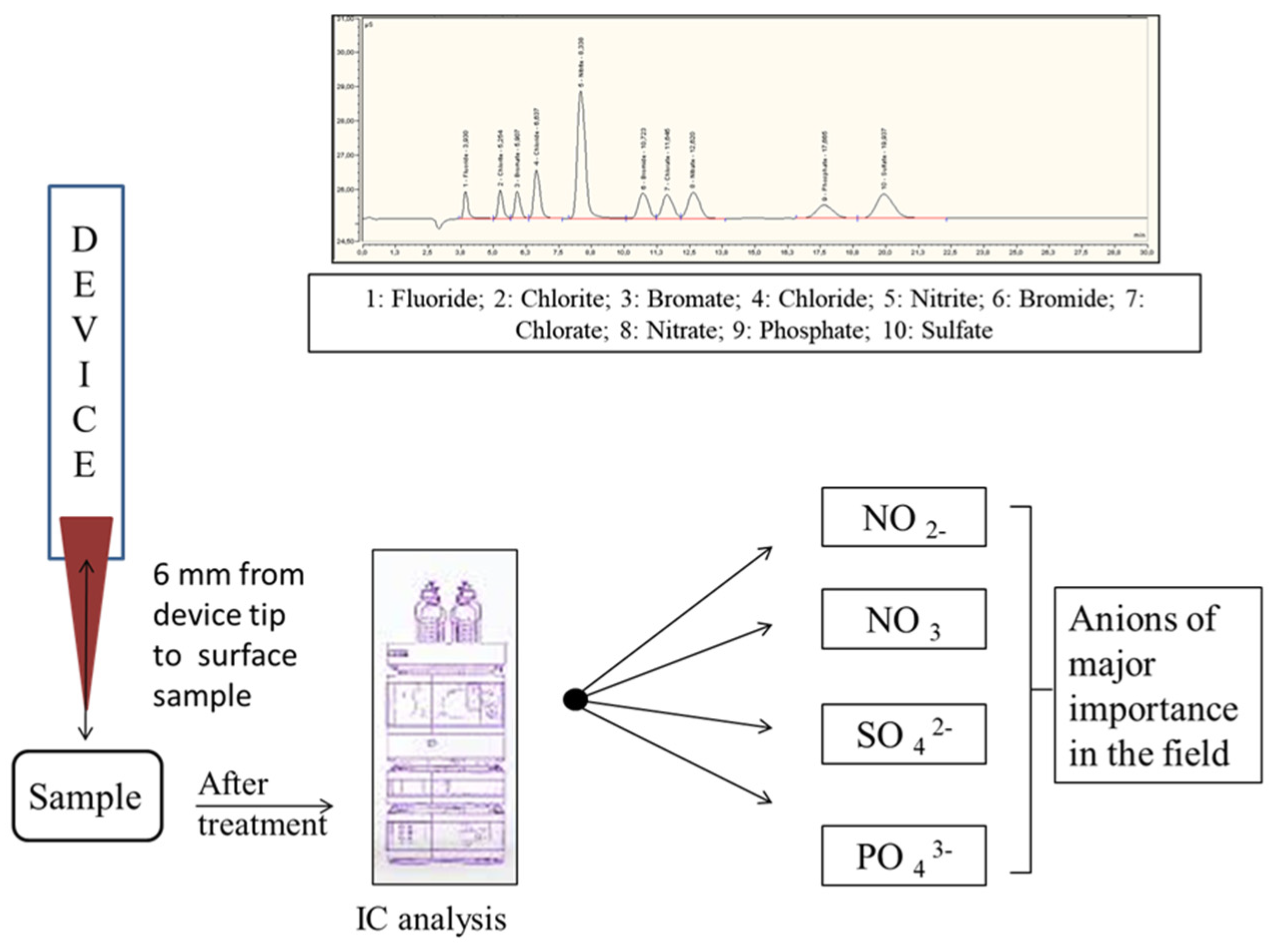
3.4. Sample Preparation
3.5. IC Analysis
3.6. Software
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tornin, J.; Labay, C.; Tampieri, F.; Ginebra, M.P.; Canal, C. Evaluation of the effects of cold atmospheric plasma and plasma-treated liquids in cancer cell cultures. Nat. Protoc. 2021, 16, 2826–2850. [Google Scholar] [CrossRef]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef]
- Veronico, V.; Morelli, S.; Piscioneri, A.; Gristina, R.; Casiello, M.; Favia, P.; Armenise, V.; Fracassi, F.; De Bartolo, L.; Sardella, E. Anticancer Effects of Plasma-Treated Water Solutions from Clinically Approved Infusion Liquids Supplemented with Organic Molecules. ACS Omega 2023, 8, 33723–33736. [Google Scholar] [CrossRef] [PubMed]
- Negi, M.; Kaushik, N.; Nguyen, L.N.; Choi, E.H.; Kaushik, N.K. Argon gas plasma-treated physiological solutions stimulate immunogenic cell death and eradicates immunosuppressive CD47 protein in lung carcinoma. Free Radic. Biol. Med. 2023, 201, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Gay-Mimbrera, J.; Garica, M.C.; Isla-Tejera, B.; Rodero-Serrano, A.; Garcia-Nieto, A.V.; Ruano, J. Clinical and Biological Principles of Cold Atmospheric Plasma Application in Skin Cancer. Adv. Ther. 2016, 33, 894–909. [Google Scholar] [CrossRef] [PubMed]
- Sklias, K.; Sousa, J.S.; Girard, P.M. Role of short- and long-lived reactive species on the selectivity and anti-cancer action of plasma treatment in vitro. Cancers 2021, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.B. Reactive species from cold atmospheric plasma: Implications for cancer therapy. Plasma Process. Polym. 2014, 11, 1120–1127. [Google Scholar] [CrossRef]
- Jablonowski, H.; von Woedtke, T. Research on plasma medicine-relevant plasma-liquid interaction: What happened in the past five years? Clin. Plasma Med. 2015, 3, 42–52. [Google Scholar] [CrossRef]
- Locatelli, M.; Kabir, A.; Perrucci, M.; Ulusoy, S.; Ulusoy, H.I.; Ali, I. Green profile tools: Current status and future perspectives. Adv. Sample Prep. 2023, 6, 100068. [Google Scholar] [CrossRef]
- Haddad, P.R. Ion chromatography. Anal. Bioanal. Chem. 2004, 379, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and Pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef] [PubMed]
- di Giacomo, V.; Balaha, M.; Pinti, M.; Di Marcantonio, M.C.; Cela, I.; Acharya, T.R.; Kaushik, N.K.; Choi, E.H.; Mincione, G.; Sala, G.; et al. Cold atmospheric plasma activated media selectively affects human head and neck cancer cell lines. Oral Dis. 2024, in press. [Google Scholar] [CrossRef]
- Acharya, T.R.; Lamichhane, P.; Jaiswal, A.; Amsalu, K.; Hong, Y.J.; Kaushik, N.; Kaushik, N.K.; Choi, E.H. The potential of multicylindrical dielectric barrier discharge plasma for diesel-contaminated soil remediation and biocompatibility assessment. Environ. Res. 2024, 240, 117398. [Google Scholar] [CrossRef] [PubMed]
- Acharya, T.R.; Lamichhane, P.; Jaiswal, A.; Kaushik, N.; Kaushik, N.K. Evaluation of degradation efficacy and toxicity mitigation for 4-nitrophenol using argon and air-mixed argon plasma jets. Chemosphere 2024, 358, 142211. [Google Scholar] [CrossRef] [PubMed]
- FDA. Bioanalytical Method Validation Guidance for Industry. 2018. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry (accessed on 28 January 2025).
- European Medicines Agency. ICH M10 on Bioanalytical Method Validation–Scientific Guideline. 2019. Available online: https://www.ema.europa.eu/en/ich-m10-bioanalytical-method-validation-scientific-guideline (accessed on 28 January 2025).
- Ich Official Web Site: Ich. 2023. Available online: https://www.ich.org/fileadmin/Publi%20c_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1_Guideline (accessed on 28 January 2025).
- Zhang, Y.; Du, X.; Shi, Q.; Xiao, W.; Li, H. Precise Control of Glioma Cell Apoptosis Induced by Micro-Plasma-Activated Water (μ-PAW). Micromachines 2022, 13, 2145. [Google Scholar] [CrossRef] [PubMed]
- Yap, Q.; Xu, H.; Zhuang, J.; Cui, D.; Ma, R.; Jiao, Z. Inhibition of Fungal Growth and Aflatoxin B1 Synthesis in Aspergillus flavus by Plasma-Activated Water. Foods 2023, 12, 2490. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.J.; Shackleford, D.M.; Charman, S.A.; Wagstaff, K.M.; Porter, C.J.H.; Jans, D.A. Increased In Vivo Exposure of N-(4-Hydroxyphenyl) Retinamide (4-HPR) to Achieve Plasma Concentrations Effective against Dengue Virus. Pharmaceutics 2023, 15, 1974. [Google Scholar] [CrossRef] [PubMed]

| Analyte | Slope | Intercept | Linearity a | Weighting Factor | r2 | LOD | LOQ |
|---|---|---|---|---|---|---|---|
| Fluoride | 0.1266 | 0.003549 | 0.0275–2.7 | 1/X2 | 0.9985 | 0.001 | 0.0275 |
| Chlorite | 0.04068 | 0.000029236 | 0.101–10 | 0.9973 | 0.034 | 0.1010 | |
| Bromate | 0.02216 | 0.000046963 | 0.202–20 | 0.9984 | 0.067 | 0.2020 | |
| Chloride | 0.1045 | 0.01663 | 0.091–9 | 0.9930 | 0.030 | 0.0910 | |
| Nitrite | 0.2199 | 0.0006067 | 0.152–15 | 0.9997 | 0.050 | 0.1520 | |
| Bromide | 0.0003999 | 0.0002064 | 0.182–18 | 0.9989 | 0.060 | 0.1820 | |
| Chlorate | 0.03462 | 0.0006250 | 0.202–20 | 0.9982 | 0.067 | 0.2020 | |
| Nitrate | 0.04768 | 0.003628 | 0.182–18 | 0.9994 | 0.060 | 0.1820 | |
| Phosphate | 0.02101 | 0.0007420 | 0.273–27 | 0.9985 | 0.011 | 0.2730 | |
| Sulfate | 0.06802 | 0.002854 | 0.182–18 | 0.9976 | 0.060 | 0.1820 |
| Analyte | Fl− | ClO2− | BrO3− | Cl− | NO2− | Br− | ClO3− | NO3− | PO4− | SO4− |
|---|---|---|---|---|---|---|---|---|---|---|
| Conc [µg/mL] | 2.7 | 10 | 18 | 9 | 15 | 18 | 20 | 18 | 27 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Locatelli, M.; Perrucci, M.; Balaha, M.; Acharya, T.-R.; Kaushik, N.-K.; Choi, E.-H.; Rapino, M.; Perrotti, V. Chemical Analysis of Plasma-Activated Culture Media by Ion Chromatography. Pharmaceuticals 2025, 18, 199. https://doi.org/10.3390/ph18020199
Locatelli M, Perrucci M, Balaha M, Acharya T-R, Kaushik N-K, Choi E-H, Rapino M, Perrotti V. Chemical Analysis of Plasma-Activated Culture Media by Ion Chromatography. Pharmaceuticals. 2025; 18(2):199. https://doi.org/10.3390/ph18020199
Chicago/Turabian StyleLocatelli, Marcello, Miryam Perrucci, Marwa Balaha, Tirtha-Raj Acharya, Nagendra-Kumar Kaushik, Eun-Ha Choi, Monica Rapino, and Vittoria Perrotti. 2025. "Chemical Analysis of Plasma-Activated Culture Media by Ion Chromatography" Pharmaceuticals 18, no. 2: 199. https://doi.org/10.3390/ph18020199
APA StyleLocatelli, M., Perrucci, M., Balaha, M., Acharya, T.-R., Kaushik, N.-K., Choi, E.-H., Rapino, M., & Perrotti, V. (2025). Chemical Analysis of Plasma-Activated Culture Media by Ion Chromatography. Pharmaceuticals, 18(2), 199. https://doi.org/10.3390/ph18020199











