Enhanced Anti-Babesia Efficacy of Buparvaquone and Imidocarb When Combined with ELQ-316 In Vitro Culture of Babesia bigemina
Abstract
:1. Introduction
2. Results
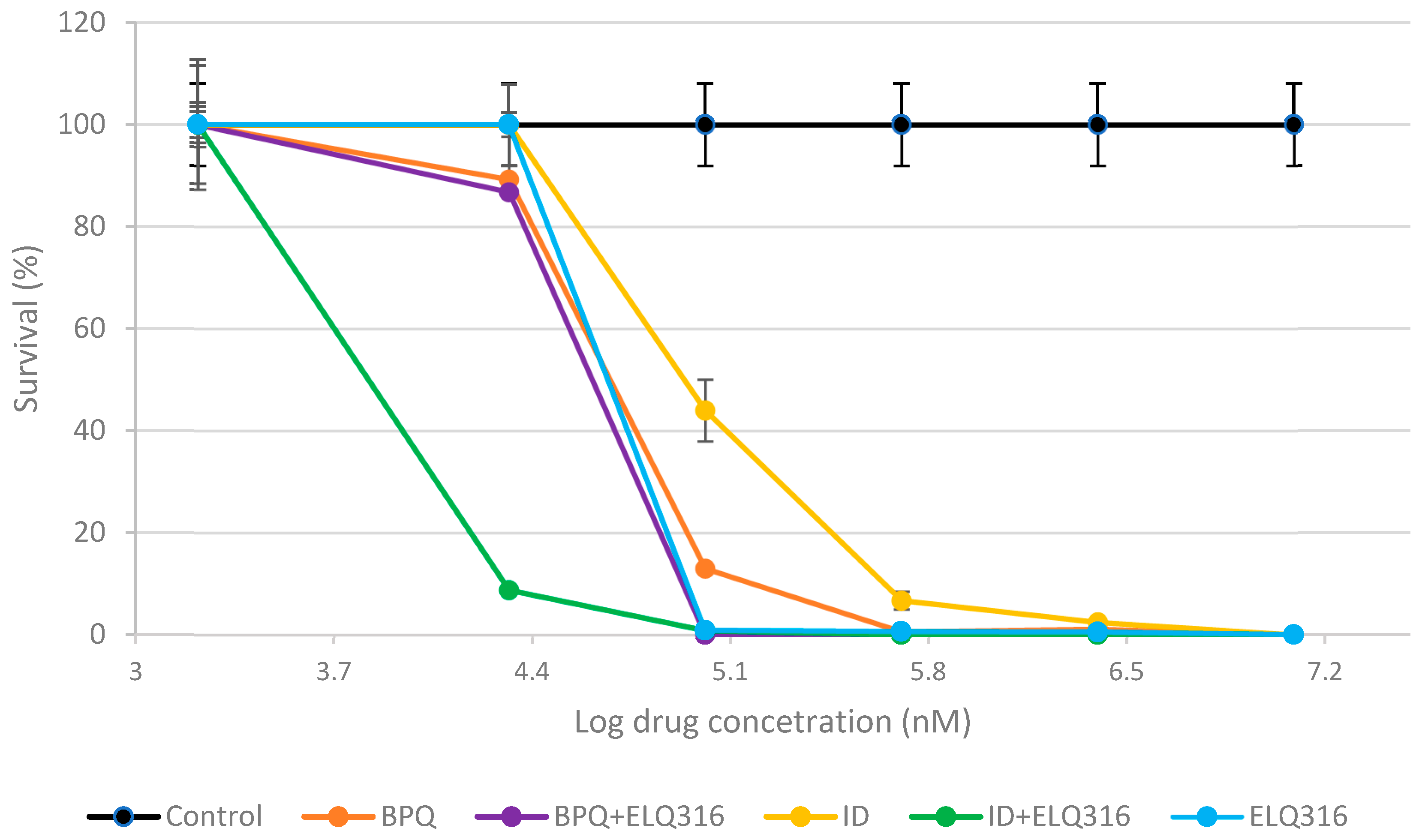
2.1. Comparative Inhibitory Effects of BPQ, ID and ELQ-316 and the Combinations ELQ-316 + BPQ and ELQ-316 + ID Against B. bigemina In Vitro Replication
2.2. Drug Potency
2.3. Time and Drug Concentration Required to Reach 0% Survival After Treatment
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Parasites Culture
4.3. In Vitro Growth of Initial Inhibitory Assay
4.4. Viability After Treatment
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asrar, R.; Farhan, H.R.; Sultan, D.; Ahmad, M.; Hassan, S.; Kalim, F.; Shakoor, A.; Muhammad Taimoor Ihsan, H.; Shahab, A.; Ali, W.; et al. Review Article Bovine Babesiosis; Review on Its Global Prevalence and Anticipatory Control for One Health. Cont. Vet. J. 2022, 2, 42–49. [Google Scholar]
- Bock, R.; Jackson, L.; De Vos, A.; Jorgensen, W. Babesiosis of Cattle. Parasitology 2004, 129, S247–S269. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.S.; Sengupta, P.P.; Paramanandham, K.; Suresh, K.P.; Chamuah, J.K.; Rudramurthy, G.R.; Roy, P. Bovine Babesiosis: An Insight into the Global Perspective on the Disease Distribution by Systematic Review and Meta-Analysis. Vet. Parasitol. 2020, 283, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pipano, E.; Hadani, A. Control of Bovine Babesiosis. In Malaria and Babesiosis; Ristic, M., Ambroise-Thomas, P., Kreier, J.P., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 263–303. [Google Scholar]
- He, L.; Bastos, R.G.; Sun, Y.; Hua, G.; Guan, G.; Zhao, J.; Suarez, C.E. Babesiosis as a Potential Threat for Bovine Production in China. Parasites Vectors 2021, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Azhar, M.; Gadahi, J.A.; Bhutto, B.; Tunio, S.; Vistro, W.A.; Tunio, H.; Bhutto, S.; Ram, T. Babesiosis: Current Status and Future Perspectives in Pakistan and Chemotherapy Used in Livestock and Pet Animals. Heliyon 2023, 9, e17172. [Google Scholar] [CrossRef] [PubMed]
- Vial, H.J.; Gorenflot, A. Chemotherapy against Babesiosis. Vet. Parasitol. 2006, 138, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-J.; Yamasaki, M.; Nakamura, K.; Sasaki, N.; Murakami, M.; Kumara, B.; Rajapakshage, W.; Ohta, H.; Maede, Y.; Takiguchi, M. Development and Characterization of a Strain of Babesia gibsoni Resistant to Diminazene Aceturate In Vitro. J. Vet. Med. Sci. 2010, 72, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Tuntasuvan, D.; Jarabrum, W.; Viseshakul, N.; Mohkaew, K.; Borisutsuwan, S.; Theeraphan, A.; Kongkanjana, N. Chemotherapy of Surra in Horses and Mules with Diminazene Aceturate. Vet. Parasitol. 2003, 110, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Mosqueda, J.; Olvera-Ramírez, A.; Aguilar-Tipacamú, G.; Cantó, G.J. Current Advances in Detection and Treatment of Babesiosis. Curr. Med. Chem. 2012, 19, 1504–1518. [Google Scholar] [CrossRef] [PubMed]
- Bailey, G. Buparvaquone Tissue Residue Study; Meat and livestock Australia limited: North Sydney, Australia, 2013; pp. 1–152. Available online: https://www.mla.com.au/research-and-development/reports/2013/buparvaquone-tissue-residue-study/ (accessed on 4 February 2025).
- Cardillo, N.M.; Lacy, P.A.; Villarino, N.F.; Doggett, J.S.; Riscoe, M.K.; Bastos, R.G.; Laughery, J.M.; Ueti, M.W.; Suarez, C.E. Comparative Efficacy of Buparvaquone and Imidocarb in Inhibiting the in Vitro Growth of Babesia bovis. Front. Pharmacol. 2024, 15, 1407548. [Google Scholar] [CrossRef]
- Carter, P. Assessment of the Efficacy of Buparvaquone for the Treatment of ‘Benign’ Bovine Theileriosis; Meat & Livestock Australia: North Sydney, Australia, 2011; pp. 1–12. Available online: https://www.mla.com.au/research-and-development/reports/2011/assess-the-efficacy-of-buparvaquone-for-the-treatment-of-bovine-theileriosis/ (accessed on 4 February 2025).
- Checa, R.; Montoya, A.; Ortega, N.; González-Fraga, J.L.; Bartolomé, A.; Gálvez, R.; Marino, V.; Miró, G. Efficacy, Safety and Tolerance of Imidocarb Dipropionate versus Atovaquone or Buparvaquone plus Azithromycin Used to Treat Sick Dogs Naturally Infected with the Babesia microti-like Piroplasm. Parasites Vectors 2017, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Goud, S.K.; Vijayakumar, K.; Davis, J.; Tresamol, P.V.; Ravishankar, C.; Devada, K. Efficacy of Different Treatment Regimens against Oriental Theileriosis in Naturally Infected Cattle. Indian J. Vet. Med. 2021, 40, 14–19. [Google Scholar]
- Hudson, A.T.; Randall, A.W.; Fry, M.; Ginger, C.D.; Hill, B.; Latter, V.S.; McHardy, N.; Williams, R.B. Novel Anti-Malarial Hydroxynpahthoquinones with Potent Broad Spectrum Anti-Protozoal Activity. Parasitology 1985, 90, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Gupta, A.K.; Pal, Y.; Dwivedi, S.K. In-Vivo Therapeutic Efficacy Trial with Artemisinin Derivative, Buparvaquone and Imidocarb Dipropionate against Babesia equi Infection in Donkeys. J. Vet. Med. Sci. 2003, 65, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Muraguri, G.R.; Ngumi, P.N.; Wesonga, D.; Ndungu, S.G.; Wanjohi, J.M.; Bang, K.; Fox, A.; Dunne, J.; McHardy, N. Clinical Efficacy and Plasma Concentrations of Two Formulations of Buparvaquone in Cattle Infected with East Coast Fever (Theileria parva Infection). Res. Vet. Sci. 2006, 81, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, G.M.; Kirvar, E.; Thomas, E.M.; Sparagano, O.; Brown, C.G.D. Stage-Specific Activity in Vitro on the Theileria Infection Process of Serum from Calves Treated Prophylactically with Buparvaquone. Vet. Parasitol. 1998, 80, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, A.B.; Tuvshintulga, B.; Guswanto, A.; Tayebwa, D.S.; Rizk, M.A.; Gantuya, S.; El-Saber Batiha, G.; Beshbishy, A.M.; Sivakumar, T.; Yokoyama, N.; et al. Screening the Medicines for Malaria Venture Pathogen Box against Piroplasm Parasites. Int. J. Parasitol. Drugs Drug Resist. 2019, 10, 84–90. [Google Scholar] [CrossRef]
- Müller, J.; Aguado-Martinez, A.; Manser, V.; Balmer, V.; Winzer, P.; Ritler, D.; Hostettler, I.; Solís, D.A.; Ortega-Mora, L.; Hemphill, A. Buparvaquone Is Active against Neospora caninum in Vitro and in Experimentally Infected Mice. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 16–25. [Google Scholar] [CrossRef]
- Fray, M.; Pudney, M. Site of Action of the Antimalarial Hydroxynaphthoquinone,2-[Trans-4-(40chlorophenyl)Cyclohexyl]-3-Hydroxy-1,4,-Naphthoquinone (566c80). Biochem. Pharmacol. 1992, 40, 914–919. [Google Scholar] [CrossRef]
- Mhadhbi, M.; Chaouch, M.; Ajroud, K.; Darghouth, M.A.; BenAbderrazak, S. Sequence Polymorphism of Cytochrome b Gene in Theileria annulata Tunisian Isolates and Its Association with Buparvaquone Treatment Failure. PLoS ONE 2015, 10, e0129678. [Google Scholar] [CrossRef]
- Sharifiyazdi, H.; Namazi, F.; Oryan, A.; Shahriari, R.; Razavi, M. Point Mutations in the Theileria annulata Cytochrome b Gene Is Associated with Buparvaquone Treatment Failure. Vet. Parasitol. 2012, 187, 431–435. [Google Scholar] [CrossRef]
- McConnell, E.V.; Bruzual, I.; Pou, S.; Winter, R.; Dodean, R.A.; Smilkstein, M.J.; Krollenbrock, A.; Nilsen, A.; Zakharov, L.N.; Riscoe, M.K.; et al. Targeted Structure-Activity Analysis of Endochin-like Quinolones Reveals Potent Qi and Qo Site Inhibitors of Toxoplasma gondii and Plasmodium falciparum Cytochrome Bc1 and Identifies ELQ-400 as a Remarkably Effective Compound against Acute Experimental Toxoplasmosis. ACS Infect. Dis. 2018, 4, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Lawres, L.A.; Garg, A.; Kumar, V.; Bruzual, I.; Forquer, I.P.; Renard, I.; Virji, A.Z.; Boulard, P.; Rodriguez, E.X.; Allen, A.J.; et al. Radical Cure of Experimental Babesiosis in Immunodeficient Mice Using a Combination of an Endochin-like Quinolone and Atovaquone. J. Exp. Med. 2016, 213, 1307–1318. [Google Scholar] [CrossRef]
- Pritchard, J.R.; Bruno, P.M.; Gilbert, L.A.; Capron, K.L.; Lauffenburger, D.A.; Hemann, M.T. Defining Principles of Combination Drug Mechanisms of Action. Proc. Natl. Acad. Sci. USA 2012, 110, E170–E179. [Google Scholar] [CrossRef]
- Silva, M.G.; Bastos, R.G.; Stone Doggett, J.; Riscoe, M.K.; Pou, S.; Winter, R.; Dodean, R.A.; Nilsen, A.; Suarez, C.E. Endochin-like Quinolone-300 and ELQ-316 Inhibit Babesia bovis, B. Bigemina, B. Caballi and Theileria Equi. Parasites Vectors 2020, 13, 1–11. [Google Scholar] [CrossRef]
- Eberhard, N.; Balmer, V.; Müller, J.; Müller, N.; Winter, R.; Pou, S.; Nilsen, A.; Riscoe, M.; Francisco, S.; Leitao, A.; et al. Activities of Endochin-Like Quinolones Against In Vitro Cultured Besnoitia besnoiti Tachyzoites. Front. Vet. Sci. 2020, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, N.M.; Villarino, N.F.; Lacy, P.A.; Riscoe, M.K.; Doggett, J.S.; Ueti, M.W.; Chung, C.J.; Suarez, C.E. The Combination of Buparvaquone and ELQ316 Exhibit a Stronger Effect than ELQ316 and Imidocarb Against Babesia bovis In Vitro. Pharmaceutics 2024, 16, 1402. [Google Scholar] [CrossRef]
- Rizk, M.A.; El-Sayed, S.A.E.-S.; Igarashi, I. Diminazene Aceturate and Imidocarb Dipropionate-Based Combination Therapy for Babesiosis—A New Paradigm. Ticks Tick-Borne Dis. 2023, 14, 102145. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, R.A.; Viscaino, O.G.; Gonzalez, E.F.; Adams, L.G. Chemoprophylaxis (Imidocarb) against Babesia bigemina and Babesia argentina Infections. Am. J. Vet. Res. 1973, 34, 1153–1161. [Google Scholar] [PubMed]
- Silva, M.G.; Villarino, N.F.; Knowles, D.P.; Suarez, C.E. Assessment of Draxxin® (Tulathromycin) as an Inhibitor of in Vitro Growth of Babesia bovis, Babesia bigemina and Theileria equi. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 265–270. [Google Scholar] [CrossRef]
- Mazuz, M.L.; Golenser, J.; Fish, L.; Haynes, R.K.; Wollkomirsky, R.; Leibovich, B.; Shkap, V. Artemisone Inhibits in Vitro and in Vivo Propagation of Babesia bovis and B. bigemina Parasites. Exp. Parasitol. 2013, 135, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Winter, R.; Kelly, J.X.; Smilkstein, M.J.; Hinrichs, D.; Koop, D.R.; Riscoe, M.K. Optimization of Endochin-like Quinolones for Antimalarial Activity. Exp. Parasitol. 2011, 127, 545–551. [Google Scholar] [CrossRef]
- Rizk, M.A.; El-Sayed, S.A.E.S.; El-Khodery, S.; Yokoyama, N.; Igarashi, I. Discovering the in Vitro Potent Inhibitors against Babesia and Theileria Parasites by Repurposing the Malaria Box: A Review. Vet. Parasitol. 2019, 274, 108895. [Google Scholar] [CrossRef] [PubMed]
- Rizk, M.A.; El-Sayed, S.A.E.S.; Nassif, M.; Mosqueda, J.; Xuan, X.; Igarashi, I. Assay Methods for in Vitro and in Vivo Anti-Babesia Drug Efficacy Testing: Current Progress, Outlook, and Challenges. Vet. Parasitol. 2020, 279, 109013. [Google Scholar] [CrossRef]
- Rizk, M.A.; El-Sayed, S.A.E.S.; AbouLaila, M.; Tuvshintulga, B.; Yokoyama, N.; Igarashi, I. Large-Scale Drug Screening against Babesia divergens Parasite Using a Fluorescence-Based High-Throughput Screening Assay. Vet. Parasitol. 2016, 227, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Rizk, M.A.; El-Sayed, S.A.E.-S.; Terkawi, M.A.; Youssef, M.A.; El Said, E.S.E.S.; Elsayed, G.; El-Khodery, S.; El-Ashker, M.; Elsify, A.; Omar, M.; et al. Optimization of a Fluorescence-Based Assay for Large-Scale Drug Screening against Babesia and Theileria Parasites. PLoS ONE 2015, 10, e0125276. [Google Scholar] [CrossRef] [PubMed]
- Mehlhorn, H. Babesiacidal Drugs. In Encyclopedia of Parasitology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–11. [Google Scholar]
- Nilsen, A.; Miley, G.P.; Forquer, I.P.; Mather, M.W.; Katneni, K.; Li, Y.; Pou, S.; Pershing, A.M.; Stickles, A.M.; Ryan, E.; et al. Discovery, Synthesis, and Optimization of Antimalarial 4(1H)-Quinolone-3-Diarylethers. J. Med. Chem. 2014, 57, 3818. [Google Scholar] [CrossRef]
- Levy, M.G.; Ristic, M. Babesia bovis: Continuous Cultivation in a Microaerophilous Stationary Phase Culture. Science 1980, 207, 1218–1220. [Google Scholar] [CrossRef]
- Bennett, T.N.; Paguio, M.; Gligorijevic, B.; Seudieu, C.; Kosar, A.D.; Davidson, E.; Roepe, P.D. Novel, Rapid, and Inexpensive Cell-Based Quantification of Antimalarial Drug Efficacy. Antimicrob. Agents Chemother. 2004, 48, 1807. [Google Scholar] [CrossRef]
| Drug Treatment (nM) | BPQ | ID | ELQ-316 | ID + ELQ-316 | BPQ + ELQ-316 |
|---|---|---|---|---|---|
| Median Range (%) | |||||
| 25 | 89.26 (84.30–92.98) | 100 (88–100) | 100 (89.26–100) | 8.80 (6.94–11.9) | 86.78 (79.34–100) |
| 75 | 13.02 (8.68–13.64) a,b | 44.01 (38.43–49.59) b | 0.87 (0.62–0.99) a,b | 0.87 (0.62–0.99) a,c | 0 c |
| 150 | 0.62 (0.37–0.74) a | 6.76 (2.48–11.03) a | 0.65 (0.5–1.12) a | 0 c | 0 c |
| 300 | 1.12 (0.99–1.86) a | 2.48 (1.24–3.72) a | 0.53 (0.37–0.87) a,b | 0 c | 0 c |
| Drugs | IC50% (nM) | IC99% (nM) | ||
|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |
| ID + ELQ-316 | 9.248 | (8.667–9.887) | 65.52 | (61.35–69.81) |
| BPQ + ELQ-316 | 27.59 | N/A | 34.23 | N/A |
| BPQ | 44.66 | (43.56–45.81) | 156.4 | (148.9–164.4) |
| ELQ-316 | 48.10 | (42.76–58.83) | 133 | (102.2–155.6) |
| ID | 61.49 | (59.54–63.46) | 441.4 | (392.3–498) |
| Single Drugs and Combination Treatments | Time Post-Treatment with Drug (h) | Time Post-Treatment Without Drug (h) | ||||||
|---|---|---|---|---|---|---|---|---|
| 48 | 72 | 96 | 24 | 48 | 72 | 96 | 120 | |
| BPQ | - | 1200 | 600 to 1200 | (--------------- 150 to 1200 ------------) | ||||
| BPQ + ELQ-316 | 1200 | 300 to 1200 | (------------------------- 75 to 1200 ----------------------) | |||||
| ELQ-316 | - | 1200 | 600 to 1200 | (--------------- 150 to 1200 ------------) | ||||
| ID + ELQ-316 | - | 600 to 1200 | (--------- 150 to 1200 -------) | (------ 75 to 1200 -----) | ||||
| ID | - | - | 600–1200 | 300–1200 | (----- 150 to 1200 ----) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardillo, N.M.; Villarino, N.F.; Lacy, P.A.; Doggett, J.S.; Riscoe, M.K.; Suarez, C.E.; Ueti, M.W.; Chung, C.J. Enhanced Anti-Babesia Efficacy of Buparvaquone and Imidocarb When Combined with ELQ-316 In Vitro Culture of Babesia bigemina. Pharmaceuticals 2025, 18, 218. https://doi.org/10.3390/ph18020218
Cardillo NM, Villarino NF, Lacy PA, Doggett JS, Riscoe MK, Suarez CE, Ueti MW, Chung CJ. Enhanced Anti-Babesia Efficacy of Buparvaquone and Imidocarb When Combined with ELQ-316 In Vitro Culture of Babesia bigemina. Pharmaceuticals. 2025; 18(2):218. https://doi.org/10.3390/ph18020218
Chicago/Turabian StyleCardillo, Natalia M., Nicolas F. Villarino, Paul A. Lacy, Joseph S. Doggett, Michael K. Riscoe, Carlos E. Suarez, Massaro W. Ueti, and Chungwon J. Chung. 2025. "Enhanced Anti-Babesia Efficacy of Buparvaquone and Imidocarb When Combined with ELQ-316 In Vitro Culture of Babesia bigemina" Pharmaceuticals 18, no. 2: 218. https://doi.org/10.3390/ph18020218
APA StyleCardillo, N. M., Villarino, N. F., Lacy, P. A., Doggett, J. S., Riscoe, M. K., Suarez, C. E., Ueti, M. W., & Chung, C. J. (2025). Enhanced Anti-Babesia Efficacy of Buparvaquone and Imidocarb When Combined with ELQ-316 In Vitro Culture of Babesia bigemina. Pharmaceuticals, 18(2), 218. https://doi.org/10.3390/ph18020218






