Nano-Radiopharmaceuticals in Colon Cancer: Current Applications, Challenges, and Future Directions
Abstract
:1. Introduction
2. Common Properties of Nanoparticles and Radionuclides Used for the Diagnosis and Treatment of Colon Cancer
2.1. Properties of Common Nanoparticles Used in Colon Cancer Management
2.1.1. Properties of Nanocrystal Quantum Dots (QDs)
2.1.2. Properties of Iron Oxide Nano-Formulation Nanoparticles
2.1.3. Properties of Poly Lactic-co-Glycolic Acid (PLGA) Nanoparticles
2.1.4. Properties of Carbon Nanotube (CNT) Nanoparticles
2.1.5. Properties of Dendrimer Nanoparticles
2.1.6. Properties of Liposome Nanoparticles
2.1.7. Properties of Gold Nanoparticles (AuNPs)
2.1.8. Properties of Core/Shell Polymeric Nano-Formulations
2.1.9. Properties of Mesoporous Nanoparticles
2.2. Properties of Radionuclides as Part of Nano-Radiopharmaceuticals
3. Clinical Application of Nano-Radiopharmaceuticals in Colon Cancer
3.1. Nano-Radiopharmaceuticals and Tracers for Colon Cancer Diagnosis
3.1.1. Combination of Nanoparticles and 18F-FDG in Colon Cancer Theranostics
Nanoparticles and 18F-FDG as a Functionalized Multi-Carrier
Nanoparticles and 18F-FDG in Theranostic Applications for Colon Cancer
3.1.2. 99mTc-Labeled Agents: 99mTc-HYNIC-D(TPPE)
99mTc-HYNIC-D(TPPE)
Mechanisms and Considerations for 99mTc-HYNIC-D(TPPE) as a Radiopharmaceutical Agent in Colon Cancer Imaging
3.2. Nano-Targeted Radiopharmaceuticals for Colon Cancer Therapy
3.2.1. Neurotensin (NT) Radiolabeled with 68Ga and 177Lu
3.2.2. Precision Treatment of Colorectal Cancer Through Radiolabeled Antibodies
3.2.3. Imaging Modality Used for Radiolabeled Antibodies for Precise Treatment of Colorectal Cancer
3.2.4. Chemo-Radiotherapy with 177Lu-PLGA(RGF)-CXCR4L for the Targeted Treatment of Colorectal Cancer
3.2.5. Other Available Techniques for Colon Cancer Treatment
- Surgery: The main therapy for resectable CRC is surgical excision. Surgery is the preferred therapy option for patients with early-stage colorectal cancer (CRC), where healing is attained by excising the tumor along with a portion of healthy intestine. In inoperable CRC, standard therapies include chemotherapy, radiotherapy, and immunotherapy. However, these therapies have certain limitations, including their non-specific and cytotoxic effects on normal healthy cells, which result in secondary complications [114,115].
- Chemotherapy: Cytotoxic drugs approved for CRC help to slow disease development and extend individual lifespan. The approved medications include irinotecan, fluoropyrimidines, oxaliplatin, trifluridine-tipiracil, 5-fluorouracil (5-FU), and capecitabine, which are primarily used as chemotherapeutic agents for curing CRC. Fluorouracil (5-Fu) and oxaliplatin are the primary treatment agents utilized for CRC chemotherapy, and 5-Fu-based chemotherapy regimens are commonly used in CRC patients.
- Radiotherapy: Radiotherapy is also a promising option for CRC patients. However, it has some plausible and long-term toxicity effects on vital organs that must be overcome by modifying radiation intensities. Different specialized radiotherapy techniques for colorectal cancer have been suggested for varying tumor stages. While radiotherapy provides effective control locally, concerns persist regarding treatment side effects, local recurrence, and distant metastasis.
- Nano-radiopharmaceuticals represent a promising approach to treating colon cancer, combining nanotechnology and nuclear medicine to deliver radiation therapy selectively to specific organs or tissues. These nanoparticles target specific cancer cells, reducing harm to healthy cells and tissues. They work by integrating radiopharmaceuticals inside their structure, which are then transported to tumor sites in the patient’s body. The benefits of nano-radiopharmaceuticals include targeted therapy, enhanced imaging, improved drug delivery, personalized medicine, and reduced toxicity. Current research focuses on developing new nanomaterials and nanosystems for radiopharmaceutical delivery, investigating different radionuclides and their combinations, and designing targeted nano-radiopharmaceuticals that selectively accumulate in cancer cells. Future prospects include targeted therapy, personalized medicine, and combination therapies. Overall, nano-radiopharmaceuticals offer a promising approach to colon cancer treatment, improving patient outcomes and quality of life [3,12].
3.3. Using of Nanoparticles or Surface-Functionalized Nanoparticles in Both Diagnostic and Therapeutic Purposes
3.3.1. Diagnostic Applications
3.3.2. Therapeutic Uses
3.3.3. Combination Therapies
4. The Development of Colon Cancer Drugs with Radionuclides and Nanostructures
4.1. Radionuclides in the Therapy of Colon Cancer
4.2. Nanostructures as Multifunctional Platforms
4.3. Theranostic Nanostructures: Where Diagnostics Meet Therapy
4.4. Stability and Safety: Challenges Overcome
4.5. Future Directions and Clinical Translation
5. Challenges and Limitations of Nano-Radiopharmaceuticals in Colon Cancer
5.1. Challenges and Prospects of Combining Nanoparticles with 18F-FDG for Colon Cancer Treatment
5.2. Toxicity Concerns
5.3. Manufacturing and Cost
5.4. Biodistribution and Toxicity
5.5. Limited Clinical Data
5.6. Complexity of Tumor Microenvironment
6. Future Direction and Development
6.1. Development of More Targeted Radiopharmaceuticals
6.2. Future Studies for 18F-Fluorodeoxyglucose (18F-FDG) as a Radiopharmaceutical
- Enhancing specificity: Although 18F-FDG is drawn to regions with elevated glucose metabolism, it is not exclusive to cancer cells, which can result in misleading results such as inflammation being mistaken for cancer. Further research could explore new glucose analogs or other metabolic tracers that specifically target the unique metabolic pathways of colon cancer [150,151].
6.3. Future Studies for 99mTc-Labeled Agents: 99mTc-HYNIC-D(TPPE) as a Radiopharmaceutical
- Tumor-targeted delivery: Future research may involve the modification of compounds labeled with 99mTc, such as 99mTc-HYNIC-D(TPPE), to enhance their ability to specifically target receptors or biomarkers associated with colon cancer. Utilizing peptides or antibodies conjugated to 99mTc has the potential to greatly enhance the specificity of tumor targeting, helping to distinguish between cancerous and non-cancerous lesions [65,149,150,151,152].
- Nanoparticle conjugation: The incorporation of 99mTc into nanoparticles shows promise in enhancing the targeted delivery to colon cancer cells. Studies have demonstrated that nanoparticles can boost the concentration of tracers at tumor sites by taking advantage of the enhanced permeability and retention (EPR) effect. This finding opens up new possibilities for enhancing accumulation in tumors through scientific research [149,150,151,152,153,154,155].
6.4. Future Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gogoi, P.; Kaur, G.; Singh, N.K. Nanotechnology for colorectal cancer detection and treatment. World J. Gastroenterol. 2022, 28, 6497–6511. [Google Scholar] [CrossRef] [PubMed]
- Farzam, O.R.; Mehran, N.; Bilan, F.; Aghajani, E.; Dabbaghipour, R.; Shahgoli, G.A.; Baradaran, B. Nanoparticles for imaging-guided photothermal therapy of colorectal cancer. Heliyon 2023, 9, e21334. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Mackeyev, Y.; Krishnan, S. Radiolabeled nanomaterial for cancer diagnostics and therapeutics: Principles and concepts. Cancer Nanotechnol. 2023, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Yoo, H.S. Inorganic nanoparticle functionalization strategies in immunotherapeutic applications. Biomater. Res. 2024, 28, 0086. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Mitra, A.; Pathak, S.; Zhang, A.S.; Zhang, H.; Sun, X.-F.; Banerjee, A. Recent Advancements, Limitations, and Future Perspectives of the use of Personalized Medicine in Treatment of Colon Cancer. Technol. Cancer Res. Treat. 2023, 22, 15330338231178403. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ye, J.; Kang, Y.; Niu, G.; Shi, J.; Yuan, X.; Li, R.; Han, J.; Ji, X. Biomimetic piezoelectric nanomaterial-modified oral microrobots for targeted catalytic and immunotherapy of colorectal cancer. Sci. Adv. 2024, 10, eadm9561. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Kang, Y.; Dong, J.; Li, R.; Ye, J.; Fan, Y.; Han, J.; Yu, J.; Ni, G.; Ji, X.; et al. Self-triggered thermoelectric nanoheterojunction for cancer catalytic and immunotherapy. Nat. Commun. 2023, 14, 5140. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Roy, P.; Sharma, R.; Kasana, R.; Rathore, P.; Gupta, T.K. Recent nanotheranostic approaches in cancer research. Clin. Exp. Med. 2024, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- Najdian, A.; Beiki, D.; Abbasi, M.; Gholamrezanezhad, A.; Ahmadzadehfar, H.; Amani, A.M.; Ardestani, M.S.; Assadi, M. Exploring innovative strides in radiolabeled nanoparticle progress for multimodality cancer imaging and theranostic applications. Cancer Imaging 2024, 24, 127. [Google Scholar] [CrossRef]
- Feng, S.T.; Li, J.; Luo, Y. pH-Sensitive Nanomicelles for Controlled and Efficient Drug Delivery to Human Colorectal Carcinoma LoVo Cells. PLoS ONE 2014, 9, 100732. [Google Scholar] [CrossRef] [PubMed]
- Fortina, P.; Kricka, L.J.; Graves, D.J.; Park, J.; Hyslop, T.; Tam, F.; Halas, N.; Surrey, S.; Waldman, S.A. Applications of Nanoparticles to Diagnostics and Therapeutics in Colorectal Cancer. Trends. Biotechnol. 2007, 25, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Brar, B.; Ranjan, K.; Palria, A.; Kumar, R.; Ghosh, M.; Sihag, S.; Minakshi, P. Nanotechnology in Colorectal Cancer for Precision Diagnosis and Therapy. Front. Nanotechnol. 2021, 3, 699266. [Google Scholar] [CrossRef]
- Mundekkad, D.; Cho, W.C. Nanoparticles in clinical translation for cancer therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef] [PubMed]
- Dixit, T.; Dave, N.; Basu, K.; Sonawane, P.; Gawas, T.; Ravindran, S. Nano-radiopharmaceuticals as therapeutic agents. Front. Med. 2024, 11, 1355058. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.-J.; Peng, C.-W.; Yuan, J.-P.; Cui, R.; Li, Y. Quantum Dot-Based Multiplexed Imaging in Malignant Ascites: A New Model for Malignant Ascites Classification. Int. J. Nanomed. 2015, 10, 1759–1768. [Google Scholar] [CrossRef]
- Hamidu, A.; Pitt, W.G.; Husseini, G.A. Recent Breakthroughs in Using Quantum Dots for Cancer Imaging and Drug Delivery Purposes. Nanomaterials 2023, 13, 2566. [Google Scholar] [CrossRef]
- Patil, J.; Bhattacharya, S. Exploring the potential of quantum dots and plasmonic nanoparticles for imaging and phototherapy in colorectal neoplasia. Results Chem. 2024, 10, 101689. [Google Scholar] [CrossRef]
- Dirheimer, L.; Pons, T.; Marchal, F.; Bezdetnaya, L. Quantum Dots Mediated Imaging and Phototherapy in Cancer Spheroid Models: State of the Art and Perspectives. Pharmaceutics 2022, 14, 2136. [Google Scholar] [CrossRef]
- Ramezani, Z.; Thompson, M.; Mohammadi, E. Quantum Dots in Imaging, Diagnosis, and Targeted Drug Delivery to Cancer Cells. In Royal Society of Chemistry eBooks; Royal Society of Chemistry: London, UK, 2023; pp. 107–141. [Google Scholar] [CrossRef]
- Sarkar, S.; Srivastava, T.; Sahoo, O.; Shankar, A.; Rai, A.; Pethusamy, K.; Dhar, R.; Karmakar, S. Applications of Quantum Dots in Preventive Oncology. Asian Pac. J. Cancer Prev. 2024, 25, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.Q.; Shi, Y.N.; Zhu, Y.P.; Liu, Y.Q.; Gu, L.W.; Liu, D.D.; Ma, A.; Xia, F.; Guo, Q.Y.; Xu, C.C.; et al. Recent trends in preparation and biomedical applications of iron oxide nanoparticles. J. Nanobiotechnol. 2024, 22, 24. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zafar, H.; Zia, M.; Ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. Available online: https://doaj.org/article/caaf9c49d0bf4a13932ddcbd4bb9fee5 (accessed on 12 December 2024). [CrossRef] [PubMed]
- Baabu, P.R.S.; Kumar, H.K.; Gumpu, M.B.; Babu K, J.; Kulandaisamy, A.J.; Rayappan, J.B.B. Iron Oxide nanoparticles: A review on the province of its compounds, properties and biological applications. Materials 2022, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, S.A.; Thomas, A.; Revi, N.; Ramakrishna, B.; Rengan, A.K. Iron oxide nanoparticles for theranostic applications—Recent advances. J. Drug Deliv. Sci. Technol. 2022, 70, 103196. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, L.; Wang, J.; Zhang, H.; Zhang, Z.; Xing, G.; Wang, X.; Liu, M. Drug-loaded PEG-PLGA nanoparticles for cancer treatment. Front. Pharmacol. 2022, 13, 990505. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Kong, X.; Cheng, L.; Li, X.; Ren, X.; Zhang, Y. Recent advances in functionalized carbon nanotubes for biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2021, 32, 102336. [Google Scholar]
- Mu, Q.; Su, G.; Li, L.; Gilbertson, B.O.; Yu, H.; Zhang, Q.; Yan, B. Mechanism of cellular uptake of genotoxic iron oxide nanoparticles: The influence of surface chemistry on energy-dependent cellular entry pathways. Biomaterials 2020, 32, 965–975. [Google Scholar]
- Naief, M.F.; Mohammed, S.N.; Mayouf, H.J.; Mohammed, A.M. A review of the role of carbon nanotubes for cancer treatment based on photothermal and photodynamic therapy techniques. J. Organomet. Chem. 2023, 999, 122819. [Google Scholar] [CrossRef]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2021, 39, 268–307. [Google Scholar] [CrossRef]
- Patri, A.K.; Majoros, I.J.; Baker, J.R. Dendritic polymer macromolecular carriers for drug delivery. Curr. Opin. Chem. Biol. 2017, 6, 466–471. [Google Scholar] [CrossRef]
- Carvalho, M.R.; Reis, R.L.; Oliveira, J.M. Dendrimer nanoparticles for colorectal cancer applications. J. Mater. Chem. B 2020, 8, 1128–1138. [Google Scholar] [CrossRef]
- Moghassemi, S.; Hadjizadeh, A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. J. Control. Release 2014, 185, 22–36. [Google Scholar] [CrossRef]
- Yasamineh, S.; Yasamineh, P.; Kalajahi, H.G.; Gholizadeh, O.; Yekanipour, Z.; Afkhami, H.; Eslami, M.; Kheirkhah, A.H.; Taghizadeh, M.; Yazdani, Y.; et al. A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system. Int. J. Pharm. 2022, 624, 121878. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.; Rathnanand, M.; Tippavajhala, V.K. Unlocking the potential of bilosomes and modified bilosomes: A comprehensive journey into advanced drug delivery trends. AAPS PharmSciTech 2023, 24, 238. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K.; Ahad, A.; Waheed, A.; Aqil, M.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Bilosomes: A novel platform for drug delivery. In Systems of Nanovesicular Drug Delivery; Academic Press: Cambridge, MA, USA, 2022; pp. 293–309. [Google Scholar]
- Datta, B.; Paul, D.; Pal, U.; Rakshit, T. Intriguing biomedical applications of synthetic and natural cell-derived vesicles: A comparative overview. ACS Appl. Bio Mater. 2021, 4, 2863–2885. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G. Engineering liposomes for drug delivery: Progress and problems. Trends Biotechnol. 2016, 5, 324–330. [Google Scholar] [CrossRef]
- Zhang, J.; Ali, K.; Wang, J. Research Advances of Lipid Nanoparticles in the Treatment of Colorectal Cancer. Int. J. Nanomed. 2024, 19, 6693–6715. [Google Scholar] [CrossRef]
- Chen, J.; Saeki, F.; Wiley, B.J.; Cang, H.; Cobb, M.J.; Li, Z.Y.; Au, L.; Zhang, H.; Kimmey, M.B.; Li, X.; et al. Gold nanocages: Bioconjugation and their potential use as optical imaging contrast agents. Nano Lett. 2008, 5, 473–477. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, N.M.; Khattab, S.M.; Abu-Youssef, M.A.; Badr, A.M.A. Green synthesis of novel stable biogenic gold nanoparticles for breast cancer therapeutics via the induction of extrinsic and intrinsic pathways. Sci. Rep. 2022, 12, 11518. [Google Scholar] [CrossRef] [PubMed]
- Aldahhan, R.; Almohazey, D.; Khan, F.A. Emerging trends in the application of gold nanoformulations in colon cancer diagnosis and treatment. Semin. Cancer Biol. 2021, 86, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Ma, R.; Sang, L.; Fatima, M.; Sheikh, A.; Abourehab, M.A.S.; Gupta, N.; Chen, Z.-S.; Zhou, Y. Gold nanoparticles and gold nanorods in the landscape of cancer therapy. Mol. Cancer 2023, 22, 98. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.; Chow, J.C. Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Lin, Z.; Chou, W.-C.; Cheng, Y.-H.; He, C.; Monteiro-Riviere, N.A.; Riviere, J.E. Predicting Nanoparticle Delivery to Tumors Using Machine Learning and Artificial Intelligence Approaches. Int. J. Nanomed. 2022, 17, 1365–1379. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, X.; Chen, L.; Gong, X.; Yang, H.; Duan, X.; Zhu, Y. Multifunctional Gold Nanoparticles in Cancer Diagnosis and Treatment. Int. J. Nanomed. 2022, 17, 2041–2067. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Lin, M.; Yuan, W.; Xiao, Y.; Wang, Y.; Fan, X. Functionalized gold nanoparticles: Synthesis, characterization, and biological applications. Adv. Sci. 2019, 6, 1900469. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Chaudhuri, R.G.; Paria, S. Core/Shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2011, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Simonet, B.M.; Valcárcel, M. Monitoring nanoparticles in the environment. Anal. Bioanal. Chem. 2009, 393, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric nanoparticles: Production, characterization, toxicology, and ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Kalele, S.; Gosavi, S.; Urban, J.; Kulkarni, S. Nanoshell particles: Synthesis, properties and applications. Curr. Sci. 2006, 91, 1038–1052. [Google Scholar]
- Chatterjee, K.; Sarkar, S.; Rao, K.J.; Paria, S. Core/shell nanoparticles in biomedical applications. Adv. Colloid. Interface Sci. 2014, 209, 8–39. [Google Scholar] [CrossRef] [PubMed]
- Sounderya, N.; Zhang, Y. Use of core/shell structured nanoparticles for biomedical applications. Recent. Pat. Biomed. Eng. 2008, 1, 34–42. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Jin, S.; Xu, L.; Zhao, C. Development of High-Drug-Loading Nanoparticles. ChemPlusChem 2020, 85, 2143–2157. [Google Scholar] [CrossRef] [PubMed]
- Dong, J. Physical Properties of Mesoporous Silica Nanoparticles for Stimuli-Responsive Drug Delivery. Available online: https://escholarship.org/uc/item/8qn138wn (accessed on 12 December 2024).
- Kazemzadeh, P.; Sayadi, K.; Toolabi, A.; Sayadi, J.; Zeraati, M.; Chauhan, N.P.S.; Sargazi, G. Structure-Property Relationship for Different Mesoporous Silica Nanoparticles and its Drug Delivery Applications: A Review. Front. Chem. 2022, 10, 823785. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, J.; John, A.; Lim, Y.C.; Kibria, K.M.K.; Mohiuddin, A.K.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coleman, R.E. Radionuclide Imaging in Cancer Medicine. In Holland-Frei Cancer Medicine, 6th ed.; Kufe, D.W., Pollock, R.E., Weichselbaum, R.R., Bast, R.C., Jr., Gansler, T.S., Holland, J.F., Frei, E., III, Eds.; BC Decker: Hamilton, ON, Canada, 2003; Chapter 36h. Available online: https://www.ncbi.nlm.nih.gov/books/NBK12976/ (accessed on 12 December 2024).
- Shende, P.; Gandhi, S. Current strategies of radiopharmaceuticals in theranostic applications. J. Drug Deliv. Sci. Technol. 2021, 64, 102594. [Google Scholar] [CrossRef]
- Roy, I.; Krishnan, S.; Kabashin, A.V.; Zavestovskaya, I.N.; Prasad, P.N. Transforming Nuclear Medicine with Nanoradiopharmaceuticals. ACS Nano 2022, 16, 5036–5061. [Google Scholar] [CrossRef] [PubMed]
- Crișan, G.; Moldovean-Cioroianu, N.S.; Timaru, D.G.; Andrieș, G.; Căinap, C.; Chiș, V. Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade. Int. J. Mol. Sci. 2022, 23, 5023. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: Clinical advances and challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef]
- Rudd, S.E.; Van Zuylekom, J.K.; Raicevic, A.; Pearce, L.A.; Cullinane, C.; Williams, C.C.; Adams, T.E.; Hicks, R.J.; Donnelly, P.S. Enzyme mediated incorporation of zirconium-89 or copper-64 into a fragment antibody for same day imaging of epidermal growth factor receptor. Chem. Sci. 2021, 12, 9004–9016. [Google Scholar] [CrossRef] [PubMed]
- Yeong, C.H.; Cheng, M.H.; Ng, K.H. Therapeutic radionuclides in nuclear medicine: Current and future prospects. J. Zhejiang Univ. Sci. B 2014, 15, 845–863. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salih, S.; Alkatheeri, A.; Alomaim, W.; Elliyanti, A. Radiopharmaceutical Treatments for Cancer Therapy, Radionuclides Characteristics, Applications, and Challenges. Molecules 2022, 27, 5231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoefnagel, C.A. Radionuclide therapy revisited. Eur. J. Nucl. Med. 1991, 18, 408–431. [Google Scholar] [CrossRef] [PubMed]
- Altıparmak Güleç, B.; Yurt, F. Treatment with Radiopharmaceuticals and Radionuclides in Breast Cancer: Current Options. Eur. J. Breast Health 2021, 17, 214–219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef]
- Lammers, T.; Hennink, W.E.; Storm, G. Tumourtargeted nanomedicines: Principles and practice. Br. J. Cancer 2008, 99, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Kairemo, K.; Erba, P.; Bergstr, K.; Pauwels, E.K.J. Nanoparticles in cancer. Curr. Radiopharm. 2008, 1, 30–36. [Google Scholar]
- Bawarski, W.E.; Chidlowsky, E.; Bharali, D.J.; Mousa, S.A. Emerging nanopharmaceuticals. Nanomedicine 2008, 4, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef]
- Mitra, A.; Nan, A.; Line, B.R.; Ghandehari, H. Nanocarriers for nuclear imaging and radiotherapy of cancer. Curr. Pharm. Des. 2006, 12, 4729–4749. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Chamarthy, M.R.; Williams, S.C.; Moadel, R.M. Radioimmunotherapy of non-Hodgkin’s lymphoma: From the ‘magic bullets’ to ‘radioactive magic bullets’. Yale J. Biol. Med. 2011, 84, 391–407. [Google Scholar] [PubMed] [PubMed Central]
- Ting, G.; Chang, C.-H.; Wang, H.-E.; Lee, T.-W. Nanotargeted Radionuclides for Cancer Nuclear Imaging and Internal Radiotherapy. BioMed Res. Int. 2010, 2010, 953537. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Mondal, J.; An, J.M.; Park, J.; Lee, Y.K. Advances in Radionuclides and Radiolabelled Peptides for Cancer Therapeutics. Pharmaceutics 2023, 15, 971. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218, Erratum in Trends Biochem. Sci. 2016, 41, 287. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patel, K.; Desai, T. Nanoparticle-based theranostics: Integrating diagnostics and therapy in colorectal cancer. Bioeng. Transl. Med. 2023, 9, e1042. [Google Scholar]
- Kasi, P.B.; Mallela, V.R.; Ambrozkiewicz, F.; Trailin, A.; Liška, V.; Hemminki, K. Theranostics Nanomedicine Applications for Colorectal Cancer and Metastasis: Recent Advances. Int. J. Mol. Sci. 2023, 24, 7922. [Google Scholar] [CrossRef]
- Altammar, K.A. A review on nanoparticles: Characteristics, synthesis, applications, and challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, A. Synergistic effects of nanoparticles and radiopharmaceuticals in cancer therapy. Nucl. Med. Rev. 2023, 45, 208–217. [Google Scholar]
- McNeil, S.E. Challenges in the clinical translation of nanoparticle-based cancer therapies. Pharm. Res. 2023, 40, 879–893. [Google Scholar]
- Zhang, Y.; Han, J.; Li, J.; Cao, J.; Zhou, Y.; Deng, S.; Zhang, B.; Yang, Y. Clinical significance of 18F-FDG-PET/CT for detection of incidental pre-malignant and malignant colonic lesions: Correlation with colonoscopic and histopathological results. J. Cancer Res. Clin. Oncol. 2024, 150, 265. [Google Scholar] [CrossRef]
- Sidiq, S.; Kumar, R.R.; Passi, N.D.; Dhawan, D.K.; Shukla, J.; Mittal, B.R.; Chadha, V.D. Evaluation of 99m Tc-labeled glutathione as a colon cancer targeting probe. J. Radioanal. Nucl. Chem. 2021, 327, 673–689. [Google Scholar] [CrossRef]
- Maleki, F.; Rezazadeh, F.; Varmira, K. MUC1-targeted radiopharmaceuticals in cancer imaging and therapy. Mol. Pharm. 2021, 18, 1842–1861. [Google Scholar] [CrossRef] [PubMed]
- Maleki, F.; Masteri Farahani, A.; Sadeghzadeh, N.; Mardanshahi, A.; Abediankenari, S. Preparation and evaluation of 99m Tc-HYNIC-D (TPPE) as a new targeted imaging probe for detection of colon cancer: Preclinical comparison with 99m Tc-HYNIC-EPPT. Chem. Biol. Drug Des. 2020, 96, 1223–1231. [Google Scholar] [CrossRef]
- Haddock, M.G. Intraoperative radiation therapy for colon and rectal cancers: A clinical review. Radiat. Oncol. 2017, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Sambuceti, G.; Cossu, V.; Bauckneht, M.; Morbelli, S.; Orengo, A.; Carta, S.; Ravera, S.; Bruno, S.; Marini, C. 18 F-fluoro-2-deoxy-d-glucose (FDG) uptake. What are we looking at? Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1278–1286. [Google Scholar] [CrossRef]
- Zhang, T.; Lei, H.; Chen, X.; Dou, Z.; Yu, B.; Su, W.; Wang, W.; Jin, X.; Katsube, T.; Wang, B.; et al. Carrier systems of radiopharmaceuticals and the application in cancer therapy. Cell Death Discov. 2024, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Evers, B.M.; Ishizuka, J.; Chung, D.H.; Townsend, C.M., Jr.; Thompson, J.C. Neurotensin expression and release in human colon cancers. Ann. Surg. 1992, 216, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, R.P.; De Jesus, O. Gallium Scan. StatPearls—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK567748/ (accessed on 12 December 2024).
- Schindler, L.; Moosbauer, J.; Schmidt, D.; Spruss, T.; Grätz, L.; Lüdeke, S.; Hofheinz, F.; Meister, S.; Echtenacher, B.; Bernhardt, G.; et al. Development of a Neurotensin-Derived 68Ga-Labeled PET Ligand with High In Vivo Stability for Imaging of NTS1 Receptor-Expressing Tumors. Cancers 2022, 14, 4922. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leonte, R.A.; Chilug, L.E.; Șerban, R.; Mustăciosu, C.; Raicu, A.; Manda, G.; Niculae, D. Preparation and preliminary evaluation of neurotensin radiolabelled with 68Ga and 177Lu as potential theranostic agent for colon cancer. Pharmaceutics 2021, 13, 506. [Google Scholar] [CrossRef]
- Iyer, M.R.; Kunos, G. Therapeutic approaches targeting the neurotensin receptors. Expert. Opin. Ther. Pat. 2021, 31, 361–386. [Google Scholar] [CrossRef] [PubMed]
- Christou, N.; Blondy, S.; David, V.; Verdier, M.; Lalloué, F.; Jauberteau, M.-O.; Mathonnet, M.; Perraud, A. Neurotensin pathway in digestive cancers and clinical applications: An overview. Cell Death Dis. 2020, 11, 1027. [Google Scholar] [CrossRef]
- Qiu, S.; Pellino, G.; Fiorentino, F.; Rasheed, S.; Darzi, A.; Tekkis, P.; Kontovounisios, C. A Review of the Role of Neurotensin and Its Receptors in Colorectal Cancer. Gastroenterol. Res. Pr. 2017, 2017, 6456257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nikolaou, S.; Qiu, S.; Fiorentino, F.; Simillis, C.; Rasheed, S.; Tekkis, P.; Kontovounisios, C. The role of Neurotensin and its receptors in non-gastrointestinal cancers: A review. Cell Commun. Signal. CCS 2020, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Doerr, R.J.; Abdel-Nabi, H.; Krag, D.; Mitchell, E. Radiolabeled Antibody Imaging in the Management of Colorectal Cancer Results of a Multicenter Clinical Study. Ann. Surg. 1991, 214, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yang, S.; Liu, J.; Jiang, D.; Wei, W. Antibody theranostics in precision medicine. Med 2023, 4, 69–74. [Google Scholar] [CrossRef]
- Goldenberg, D.M. Targeted therapy of cancer with radiolabeled antibodies. PubMed 2002, 43, 693–713. [Google Scholar]
- Afshar-Oromieh, A.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Hadaschik, B.A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Haufe, S.; et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 486–495, Erratum in Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 797–798. [Google Scholar] [CrossRef] [PubMed]
- Parakh, S.; Lee, S.T.; Gan, H.K.; Scott, A.M. Radiolabeled Antibodies for Cancer Imaging and Therapy. Cancers 2022, 14, 1454. [Google Scholar] [CrossRef]
- Monoclonal Antibodies and Their Side Effects. American Cancer Society. Available online: https://www.cancer.org/cancer/managing-cancer/treatment-types/immunotherapy/monoclonal-antibodies.html (accessed on 28 December 2024).
- Touchefeu, Y.; Bailly, C.; Frampas, E.; Eugène, T.; Rousseau, C.; Bourgeois, M.; Bossard, C.; Faivre-Chauvet, A.; Rauscher, A.; Masson, D.; et al. Promising clinical performance of pretargeted immuno-PET with anti-CEA bispecific antibody and gallium-68-labelled IMP-288 peptide for imaging colorectal cancer metastases: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 874–882. [Google Scholar] [CrossRef]
- Jung, K.H.; Kim, M.; Jung, H.J.; Koo, H.J.; Kim, J.-L.; Lee, H.; Lee, K.-H. PET imaging of colon cancer CD73 expression using cysteine site-specific 89Zr-labeled anti-CD73 antibody. Sci. Rep. 2024, 14, 17994. [Google Scholar] [CrossRef]
- Wang, J.; Zhuo, L.; Zhao, P.; Liao, W.; Wei, H.; Yang, Y.; Peng, S.; Yang, X. Screening for a 177Lu-labeled CA19–9 monoclonal antibody via PET imaging for colorectal cancer therapy. Chin. Chem. Lett. 2022, 33, 3502–3506. [Google Scholar] [CrossRef]
- Xia, D.; Hu, C.; Hou, Y. Regorafenib loaded self-assembled lipid-based nanocarrier for colorectal cancer treatment via lymphatic absorption. Eur. J. Pharm. Biopharm. 2023, 185, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Hjazi, A.; Nasir, F.; Noor, R.; Alsalamy, A.; Zabibah, R.S.; Romero-Parra, R.M.; Ullah, M.I.; Mustafa, Y.F.; Qasim, M.T.; Akram, S.V. The pathological role of C-X-C chemokine receptor type 4 (CXCR4) in colorectal cancer (CRC) progression; special focus on molecular mechanisms and possible therapeutics. Pathol. Res. Pract. 2023, 248, 154616. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gautam, V.; Sandhu, A.; Rawat, K.; Sharma, A.; Saha, L. Current and emerging therapeutic approaches for colorectal cancer: A comprehensive review. World J. Gastrointest. Surg. 2023, 15, 495–519. [Google Scholar] [CrossRef]
- Merchant, J.; McArthur, D.; Ferguson, H.; Ramcharan, S. Concepts and prospects of minimally invasive colorectal cancer surgery. Clin. Radiol. 2021, 76, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Kaltenbach, T.; Dominitz, J.A.; Robertson, D.J.; Anderson, J.C.; Cruise, M.; Burke, C.A.; Gupta, S.; Lieberman, D.; Syngal, S.; et al. Endoscopic Recognition and Management Strategies for Malignant Colorectal Polyps: Recommendations of the US Multi-Society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2020, 115, 1751–1767. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, M.; Zou, Y.; Jin, L.; Zhao, Z.; Liu, Q.; Wang, S.; Li, J. Mechanisms of chemotherapeutic resistance and the application of targeted nanoparticles for enhanced chemotherapy in colorectal cancer. J. Nanobiotechnol. 2022, 20, 371. [Google Scholar] [CrossRef]
- Tam, S.Y.; Wu, V.W.C. A Review on the Special Radiotherapy Techniques of Colorectal Cancer. Front. Oncol. 2019, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Kouklidis, G.; Nikolopoulos, M.; Ahmed, O.; Eskander, B.; Masters, B. A Retrospective Comparison of Toxicity, Response and Survival of Intensity-Modulated Radiotherapy Versus Three-Dimensional Conformal Radiation Therapy in the Treatment of Rectal Carcinoma. Cureus 2023, 15, e48128. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Brown, R.; Taylor, H. Nanoparticle-based radiopharmaceuticals for cancer imaging: A review of the recent advances. J. Nucl. Med. Res. 2019, 62, 145–158. [Google Scholar]
- Kumar, S.; Gupta, R.; Mehta, P. Theranostic applications of functionalized nanoparticles in cancer. Cancer Nanomed. Rev. 2022, 5, 89–112. [Google Scholar]
- Smith, D.; Lee, J.; Wang, Q. Functionalized nanoparticles for molecular imaging of colon cancer. Mol. Imaging Biol. 2020, 22, 556–567. [Google Scholar]
- Zhao, X.; Chen, Y.; Lin, Z. Tumour-targeting radiopharmaceutical nanoparticles in precision oncology. Nanomedicine 2021, 8, 234–247. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, K.; Wang, H.; Jeremy Tan, P.K.; Fan, W.; Venkatraman, S.S.; Li, L.; Yang, Y.Y. Self-assembled cationic peptide nanoparticles as an efficient antimicrobial agent. Nat. Nanotechnol. 2009, 4, 457–463. [Google Scholar] [CrossRef]
- Orive, G.; Gascon, A.R.; Hernández, R.M.; Domínguez-Gil, A.; Pedraz, J.L. Techniques: New approaches to the delivery of biopharmaceuticals. Trends Pharmacol. Sci. 2004, 25, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baum, R.P.; Singh, A.; Kulkarni, H.R. Theranostics: From molecular imaging using Ga-68 labeled tracers and PET/CT to personalized radionuclide therapy—The Bad Berka experience. Theranostics 2021, 11, 731–755. [Google Scholar] [CrossRef]
- Salgueiro, M.J.; Portillo, M.; Tesán, F.; Nicoud, M.; Medina, V.; Moretton, M.; Chiappetta, D.; Zubillaga, M. Design and development of nanoprobes radiolabelled with 99mTc for the diagnosis and monitoring of therapeutic interventions in oncology preclinical research. EJNMMI Radiopharm. Chem. 2024, 9, 74. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2020, 53, 283–318. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, F.; Zhu, L.; Choi, K.Y. Mesoporous silica nanoparticles for advanced drug delivery and imaging in oncology. Adv. Ther. 2020, 3, 2000032. [Google Scholar]
- Almeida Junior, J.C.; Helal-Neto, E.; Pinto, S.R.; Dos Santos, S.N.; Bernardes, E.S.; Al-Qahtani, M.; Nigro, F.; Alencar, L.M.R.; Ricci-Junior, E.; Santos-Oliveira, R. Colorectal Adenocarcinoma: Imaging using 5-Fluoracil Nanoparticles Labeled with Technetium 99 Metastable. Curr. Pharm. Des. 2019, 25, 3282–3288. [Google Scholar] [CrossRef] [PubMed]
- Borra, R.; Mäkelä, M.; Jalava, J. Novel theranostic approaches for cancer imaging and treatment: Focus on colon cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 845–856. [Google Scholar]
- Sun, X.; Yu, J.; Jiang, W. Theranostic quantum dots for dual-modality PET and fluorescence imaging in cancer treatment. Small 2021, 17, e2101883. [Google Scholar]
- Ligiero, T.B.; Cerqueira-Coutinho, C.; de Souza Albernaz, M.; Szwed, M.; Bernardes, E.S.; Wasserman, M.A.; Santos-Oliveira, R. Diagnosing gastrointestinal stromal tumours by single photon emission computed tomography using nano-radiopharmaceuticals based on bevacizumab monoclonal antibody. Biomed. Phys. Eng. Express 2016, 2, 045017. [Google Scholar] [CrossRef]
- Cai, W.; Chen, X.; Zhu, Z. Peptide-labeled nanoparticles for imaging and therapy in cancer research. Nat. Mater. 2008, 7, 927–930. [Google Scholar]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Li, M. AI-driven nanoparticle design for cancer treatment: From bench to bedside. J. Control. Release 2022, 344, 275–292. [Google Scholar]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Shrestha, S.; Shrestha, A.; Kim, J.; Thapa, R.K.; Kim, J.O. Recent advances in albumin nanoparticle-based cancer therapies. J. Pharm. Investig. 2024, 55, 1–14. [Google Scholar] [CrossRef]
- Naeimi, R.; Najafi, R.; Molaei, P.; Amini, R.; Pecic, S. Nanoparticles: The future of effective diagnosis and treatment of colorectal cancer? Eur. J. Pharmacol. 2022, 936, 175350. [Google Scholar] [CrossRef] [PubMed]
- Dhoundiyal, S.; Srivastava, S.; Kumar, S.; Singh, G.; Ashique, S.; Pal, R.; Mishra, N.; Taghizadeh-Hesary, F. Radiopharmaceuticals: Navigating the frontier of precision medicine and therapeutic innovation. Eur. J. Med. Res. 2024, 29, 26. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Soman, S.; Navti, P.D.; Roy, A.A.; Nikam, A.N.; Vineeth, P.; Kulkarni, J.; Shirur, K.S.; Pandey, A.; George, S.D.; et al. Nano-Innovations in Cancer Therapy: The Unparalleled Potential of MXene Conjugates. Materials 2024, 17, 1423. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, D.; Jangra, P.; Verma, R.; Purohit, D.; Pandey, P.; Sharma, S.; Sharma, R.K. Radiopharmaceuticals: An insight into the latest advances in medical uses and regulatory perspectives. J. Biosci. 2021, 46, 27. [Google Scholar] [CrossRef]
- Egbuna, C.; Parmar, V.K.; Jeevanandam, J.; Ezzat, S.M.; Patrick-Iwuanyanwu, K.C.; Adetunji, C.O.; Khan, J.; Onyeike, E.N.; Uche, C.Z.; Akram, M.; et al. Toxicity of Nanoparticles in Biomedical Application: Nanotoxicology. J. Toxicol. 2021, 2021, 9954443. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [PubMed]
- Arua, S.; Mitragotri, S. Challenges associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pulumati, A.; Pulumati, A.; Dwarakanath, B.S.; Verma, A.; Papineni, R.V.L. Technological advancements in cancer diagnostics: Improvements and limitations. Cancer Rep. 2023, 6, e1764. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Lee, J.H.; Lee, H.S.; Cho, E.S.; Park, E.J.; Baik, S.H.; Lee, K.Y.; Park, C.; Yeu, Y.; Clemenceau, J.R.; et al. Radiomics Features of 18F-Fluorodeoxyglucose Positron-Emission Tomography as a Novel Prognostic Signature in Colorectal Cancer. Cancers 2021, 13, 392. [Google Scholar] [CrossRef]
- Suleimanov, A.; Saduakassova, A.; Vinnikov, D.; Pokrovsky, V.; Mamyrbekova, S.; Daniyarova, A.; Kozhabek, L. Predictive value of 18F-fluorodeoxyglucose accumulation in visceral fat activity to detect colorectal cancer metastases (prospective observational cohort study). F1000Research 2022, 11, 1158. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Myers, C.R.; Wang, Y.; You, M. Mitochondria as a Novel Target for Cancer Chemoprevention: Emergence of Mitochondrial-targeting Agents. Cancer Prev. Res. 2021, 14, 285–306. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.X.; Xie, M.Z.; Liang, X.Q.; Ye, M.L.; Li, J.L.; Hu, B.L. Clinical Significance and Prognostic Value of the Maximum Standardized Uptake Value of 18F-Flurodeoxyglucose Positron Emission Tomography–Computed Tomography in Colorectal Cancer. Front. Oncol. 2021, 11, 741612. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Pen, R.; Zuo, W.; Chen, Y.; Sun, X.; Gou, J.; Guo, Q.; Wen, M.; Li, W.; et al. Targeted delivery of irinotecan to colon cancer cells using epidermal growth factor receptor-conjugated liposomes. BioMedical Eng. OnLine 2022, 21, 53. [Google Scholar] [CrossRef]
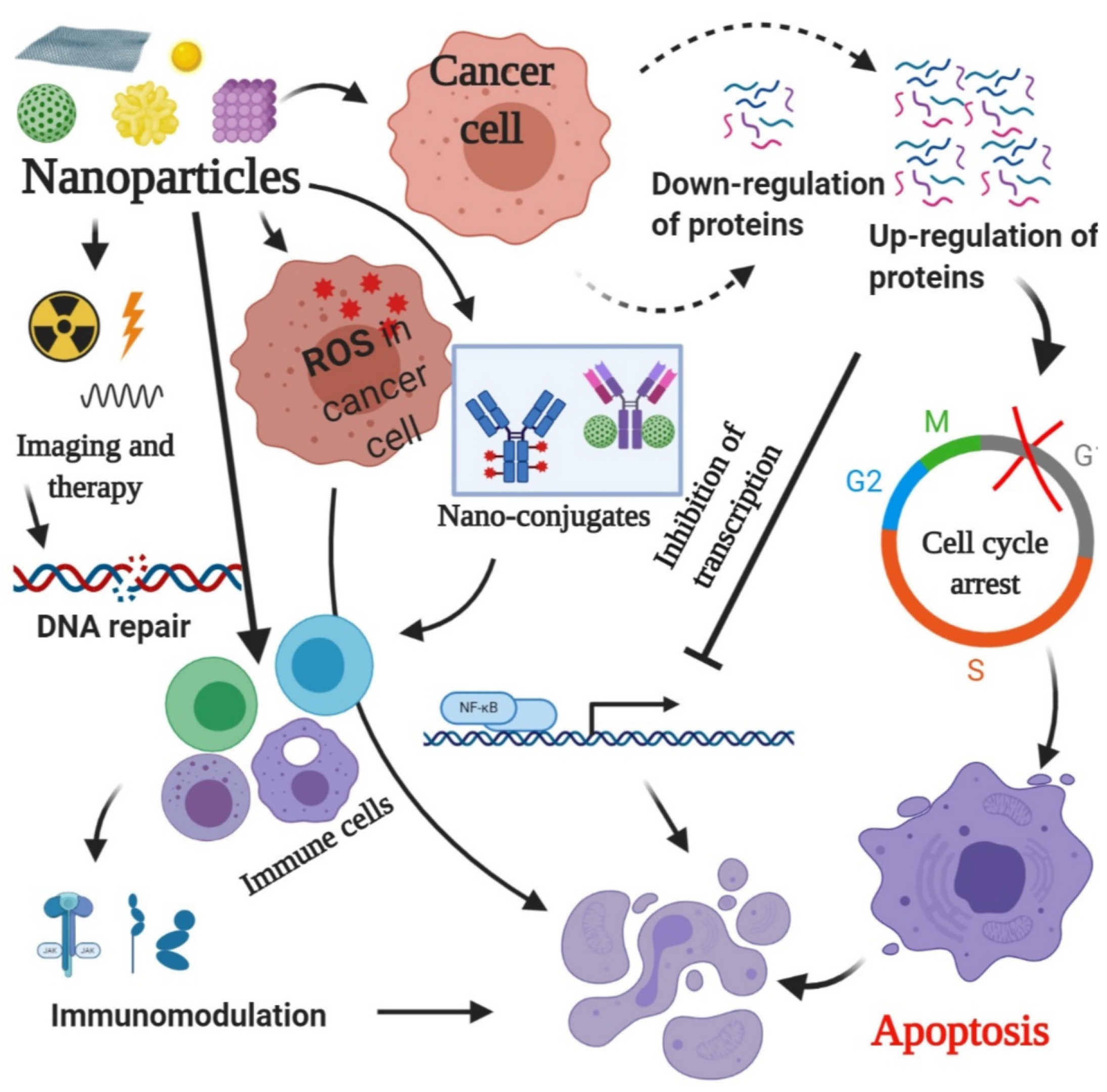
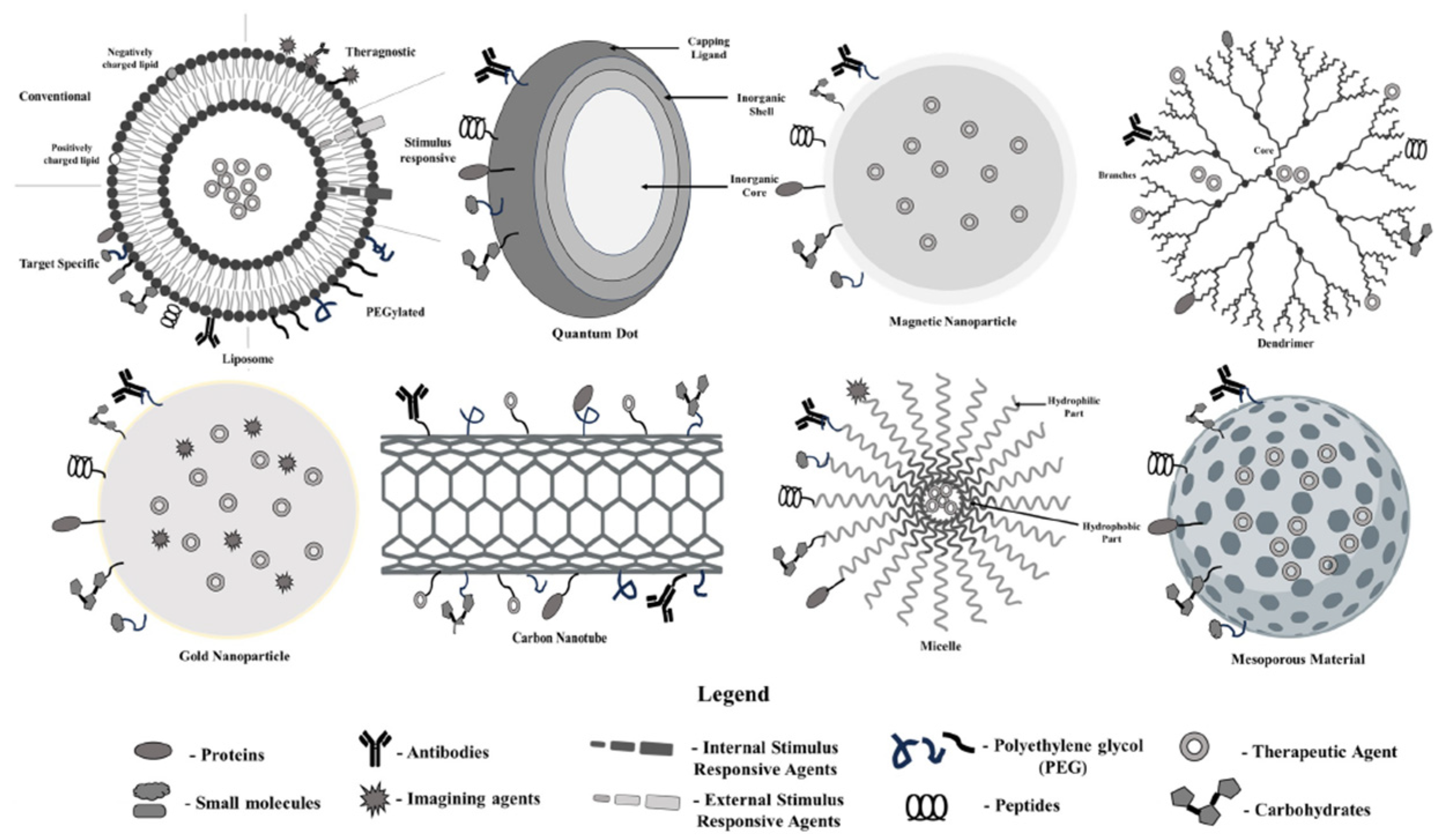
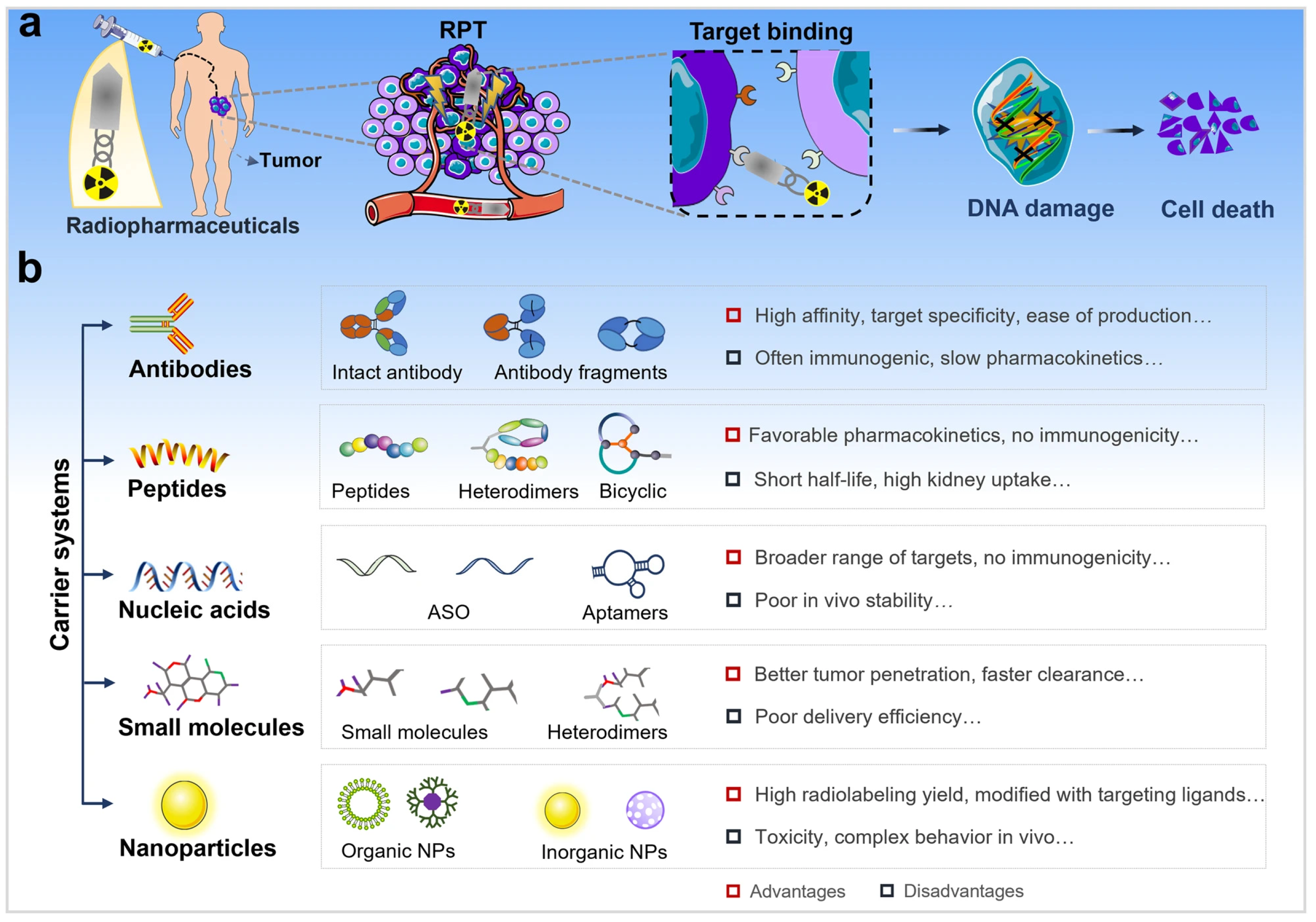


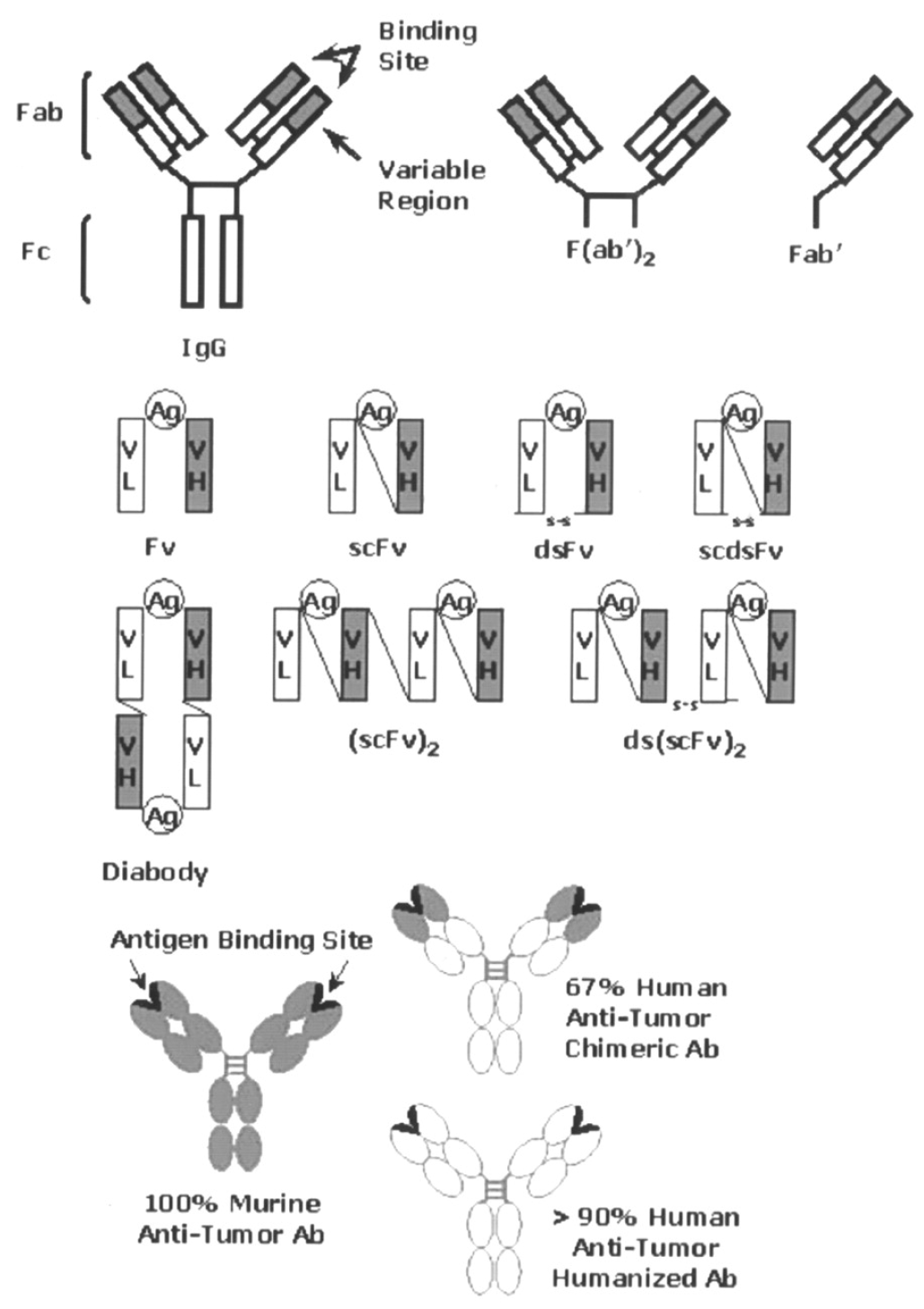
| Nanoparticles Type | Advantages | Disadvantages |
|---|---|---|
| Liposome | Liposomes exhibit better properties, including site-targeting, sustained or controlled release, protection of drugs from degradation and clearance, superior therapeutic effects, and lower toxic side effects. | Liposomes can be sensitive to environmental conditions, leading to instability, synthesis of liposomes can be complex, materials and processes involved in producing high-quality liposomes can be expensive, poor stability, could crystallize after prolonged storage conditions. |
| Quantum Dot | Ideal for long-term applications in imaging and sensing, can be made from various semiconductor materials for diverse applications, biocompatible. | Many contain heavy metals, synthesis of high-quality quantum dots can be complex and expensive, may degrade or release toxic components in biological settings. |
| Magnetic nanoparticles | Targeted drug delivery, used in imaging, drug delivery, and remediation, increased loading capacity for drugs, biocompatible. | Potential toxicity from heavy metals or degradation, can agglomerate or degrade over time, production can be complex and costly, precision targeting can be challenging. |
| Dendrimer | Precise, repetitive branching allows for uniformity, increased functional groups enhance drug loading and reactivity, can be easily modified for various applications, targeted delivery | Complex synthesis, synthesis and purification can be expensive, may degrade under certain conditions, affecting performance, low loading capacity |
| Gold nanoparticle | Generally non-toxic and well tolerated in biological systems, strong optical properties, efficiently encapsulates and delivers therapeutic agents, used in photothermal therapy to destroy cancer cells. | Synthesis and purification can be expensive, may aggregate over time, affecting performance, potential toxicity |
| Carbon Nanotube | Water-soluble, less toxic, provide a large surface area for enhanced interactions, excellent conductivity, useful in electronic and photonic applications, high thermal stability allows for use in extreme conditions. | Can clump together, reducing effectiveness in applications, complex synthesis, limited functionalization |
| Micelle | Enhanced drug solubilization, targeted delivery, capable of controlled and sustained release of therapeutics, improve the solubility of hydrophobic drugs | Stability issues, limited drug loading capacity, size may restrict penetration into certain tissues, complex formulation |
| Mesoporous Nanoparticle | High drug and gene loading capacity, tuneable pore size, large surface area, biocompatible and biodegradable, controlled porosity | Expensive, not enough information about cytotoxicity, biodistribution, biocompatibility, low stability, formation of aggregates, hemolysis |
| Type | Radionuclide Used in Nano-Radiopharmaceuticals | Half-Life | Reference |
|---|---|---|---|
| Diagnostic Radionuclides |
|
| [65] |
| Therapeutic Radionuclides |
|
| [68] |
| Diagnostic And Therapeutic Radionuclides |
|
| [67] |
| Aspect | Description | References |
|---|---|---|
| 68Ga-NT in PET Imaging for Colon Cancer | In order to radiolabel NT with 68Ga, the radionuclide is bonded to NT using a chelator such as DOTA (1,4,7,10-tetraazacyclododecane-N,N′,N″,N′′′-tetraacetic acid). The 68Ga-DOTA-NT compound is subsequently synthesized for therapeutic use. | [95] |
| Imaging Application | Using PET imaging with 68Ga-NT, it is possible to visualize lesions in the colon that express neurotensin receptors, providing valuable insights into colon cancer. Through meticulous localization and evaluation, the technology enables the identification of primary tumors, metastases, and any remaining disease, leading to improved detection. | [96,97] |
| Aspect | Description | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| Precision treatment of colorectal cancer through radiolabeled antibodies | Pretargeted immunological Positron Emission Tomography (immuno-PET) using the following: an anti-carcinoembryonic antigen (CEA) recombinant bispecific monoclonal antibody (BsMAb); TF2 and the [68Ga]Ga-labeled HSG peptide IMP288 in patients with metastatic colorectal carcinoma (CRC). | This technology allows enhanced imaging accuracy and targeted treatment approaches, thereby minimizing harm to surrounding healthy tissue. | Potential side effects, such as allergic reactions, infusion-related complications, and long-term immunosuppression, may increase the likelihood of infections. | [108] |
| Imaging application | Pretargeted immuno-PET using anti-CEA/anti-IMP288 BsMAb and a [68Ga]Ga-labeled hapten: safe and feasible. Promising diagnostic performance. | Provides a reliable and effective diagnostic approach, demonstrating encouraging precision in identifying tumor locations and tracking the spread of metastasis. | Possible adverse effects could encompass allergic reactions, symptoms associated with the infusion (such as fever and chills), and a likelihood of immune suppression, which may elevate the risk of infections. | [108] |
| Precision treatment of colorectal cancer through radiolabeled antibodies | Cysteine site-specific 89Zr-labeled anti-CD73 (89Zr-CD73) IgG immuno-PET technique: can image tumor CD73 expression in living bodies. | This aspect allows accurate visualization of tumor-related CD73 expression, enhancing the precision of diagnosis and monitoring in colorectal cancer. | Higher production costs and possible radiation exposure risks could make it hard to use on a larger scale; more clinical testing is needed to prove long-term safety and effectiveness. | [109] |
| Imaging application | 89Zr-CD73 IgG: showed CD73-dependent specific binding to cancer cells; provided high-contrast PET imaging of CD73 expressing tumors; 89Zr-CD73 IgG PET may be useful for the non-invasive assessment of tumor CD73 expression in living subjects. | Delivers enhanced PET imaging of tumors that express CD73, facilitating the early identification and tracking of colorectal cancer metastases. | Radiation exposure hazards require specific facilities for manufacturing and meticulous management, as well as limited accessibility for broad clinical application. | [109] |
| 177Lu-labeled CA19–9 monoclonal antibody via PET imaging for colorectal cancer therapy | A 177Lu-labeled CA19–9 monoclonal antibody was screened via PET imaging for colorectal cancer therapy. | Combines treatment and diagnostic operations, allowing for accurate targeting of cancer cells and immediate observation of treatment effectiveness. | There is a chance that radioactive substances will be harmful. Because of this, PET imaging facilities are specialized, and strict safety rules must be followed when handling radiolabeled antibodies. | [110] |
| Imaging application | Radiolabeled CA19–9 mAb for CRC treatment: both 89Zr-DFO-C003 for CRC immune-PET imaging and 177Lu-DOTA-C003 for radiotherapy against CRC exhibit good potential in clinical application. | It provides versatile applications for both imaging and treatment, effectively targeting CRC cells. | It requires advanced imaging technology and incorporates radioactive substances, potentially leading to safety issues and regulatory hurdles. | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkatheeri, A.; Salih, S.; Kamil, N.; Alnuaimi, S.; Abuzar, M.; Abdelrahman, S.S. Nano-Radiopharmaceuticals in Colon Cancer: Current Applications, Challenges, and Future Directions. Pharmaceuticals 2025, 18, 257. https://doi.org/10.3390/ph18020257
Alkatheeri A, Salih S, Kamil N, Alnuaimi S, Abuzar M, Abdelrahman SS. Nano-Radiopharmaceuticals in Colon Cancer: Current Applications, Challenges, and Future Directions. Pharmaceuticals. 2025; 18(2):257. https://doi.org/10.3390/ph18020257
Chicago/Turabian StyleAlkatheeri, Ajnas, Suliman Salih, Noon Kamil, Sara Alnuaimi, Memona Abuzar, and Shahd Shehadeh Abdelrahman. 2025. "Nano-Radiopharmaceuticals in Colon Cancer: Current Applications, Challenges, and Future Directions" Pharmaceuticals 18, no. 2: 257. https://doi.org/10.3390/ph18020257
APA StyleAlkatheeri, A., Salih, S., Kamil, N., Alnuaimi, S., Abuzar, M., & Abdelrahman, S. S. (2025). Nano-Radiopharmaceuticals in Colon Cancer: Current Applications, Challenges, and Future Directions. Pharmaceuticals, 18(2), 257. https://doi.org/10.3390/ph18020257







