Abstract
Liver transplantation (LT) is the standard of care for both end-stage liver failure and hepatocellular carcinoma (HCC). Side effects of the main used immunosuppressive drugs have a noteworthy impact on the long-term outcome of LT recipients. Consequently, to achieve a balance between optimal immunosuppression and minimal side effects is a cornerstone of the post-LT period. Today, there are no validated markers for overimmunosuppression and underimmunosuppression, only a few drugs have therapeutic drug monitoring, and immunosuppression regimens vary from center to center and from country to country. Currently, there are many drugs with different efficacy and safety profiles. Using different agents permits a decrease in the dosage and minimizes the toxicities. A small subset of recipients achieves immunotolerance with the chance to stop immunosuppressive therapy. This article focuses on the side effects of immunosuppressive drugs, which significantly impact long-term outcomes for LT recipients. The primary aim is to highlight the balance between achieving effective immunosuppression and minimizing adverse effects, emphasizing the role of personalized therapeutic strategies. Moreover, this review evaluates the mechanisms of action and specific complications associated with immunosuppressive agents. Finally, special attention is given to strategies for reducing immunosuppressive burdens, improving patient quality of life, and identifying immunotolerant individuals.
1. Introduction
Liver transplantation (LT) represents the standard of care for end-stage liver diseases and hepatocellular carcinoma (HCC). Significant advancements in surgical techniques and the introduction of novel immunosuppressive drugs with tailored therapeutic protocols have substantially improved short-term survival in LT recipients, with a low rate of organ rejection (1.7%) [1] and a 10-year survival rate ranging from 61% to 65% [1,2]. The main causes of mortality are graft failure, infections, metabolic and cardiovascular (CV) complications, and malignancy [3]. Notably, immunosuppression can be partially or entirely responsible for the development of de novo malignancies, graft failure, or infectious risk, with an almost direct association. Conversely, in case of CV and metabolic complications, such as arterial hypertension, insulin resistance or type 2 diabetes mellitus (T2DM), overweight/obesity, dyslipidemia, and more generally, the development of metabolic syndrome (MetS), the role of immunosuppressive drugs—discussed in detail later—can contribute to the de novo onset of these conditions or exacerbate pre-existing ones. It is important to emphasize that recent decades have witnessed a significant increase in the prevalence of obesity [4], T2DM [5], and MetS [6] in the general population to the extent that they are now described as an epidemic. This trend has led to a rise in CV diseases both in the general population and among patients with liver disease. Furthermore, due to advancements in antiviral therapies that have resulted in the eradication of hepatitis C virus and the improved management of hepatitis B virus infection, Metabolic Associated Steatohepatitis (MASH) has become the second-leading cause of placement on the waiting list for LT in the United States [7]. In 2021, 19.3% of patients on the waiting list for LT in the United States had liver disease related to MASH. Moreover, in certain subgroups, such as patients over 65 years of age, MASH was the primary indication for transplantation [8]. Fibrosis in patients with MASH has been demonstrated to be the most important predictor of mortality from both hepatic and extrahepatic causes. Among the extrahepatic causes of death in MASH, CV diseases and extrahepatic malignancies represent the leading causes of mortality [9,10,11,12,13,14].
Therefore, although some cardiometabolic risk factors may be induced de novo by immunosuppression, a significant proportion of patients on the waiting list for LT already present pre-existing cardiometabolic conditions, which can be exacerbated by immunosuppressive therapy. Selecting the most appropriate drug to address the oncological, infectious, cardiometabolic, nephrotoxic, neurotoxic, and myelotoxic complications of LT patients is therefore crucial, emphasizing the need to tailor immunosuppressive therapy to the individual.
This review evaluates the side effects of immunosuppressive drugs used in LT, detailing the risk and underlying mechanisms associated with each drug class in the development of specific complications.
2. Methods
We developed a non-systematic review using the following electronic sources: PubMed, MEDLINE, Google Scholar, Ovid, Scopus, and Web of Science. We used the following search words: “LT”, “immunosuppression”, “basiliximab”, “corticosteroid”, “cyclosporine”, “tacrolimus”, “mycophenolate”, “azathioprine”, “sirolimus”, and “everolimus” alone or in combination with “cardiovascular disease”, “liver transplantation”, “epidemiology”, “neoplasm”, “cancer”, “metabolic syndrome”, “side effect”, “infection”, “graft survival”, “nephrotoxicity”, “neurotoxicity”, “myelosuppression”, “osteoporosis”, “frailty”. We considered all papers reporting human-related data (inclusion criteria), excluding articles with unavailable full text, not in the English language, abstracts, book chapters, and articles published before 1990 (exclusion criteria). We then examined supplementary references/articles among manuscripts considered in the first research round.
3. Immunosuppression After LT: The State of the Art
Notably, the liver is a privileged organ in terms of immunotolerance. Indeed, simpler and lower-dose regimens are necessary, especially in the long-term period, in comparison to other solid organ transplants [15,16].
In clinical practice, immunosuppression after LT is divided into two phases: an induction phase and a maintenance phase. The induction phase is initiated immediately after surgery to prevent rejection and immune-mediated graft damage. Current European and American guidelines [15,17] recommend a triple-drug immunosuppressant regimen consisting of CNIs (preferably TAC over CsA), a short course corticosteroid (3 months), and an antimetabolite (preferably MMF over AZA) as the primary choice to ensure graft survival while minimizing the side effects associated with these drug classes. Alternative drug combinations, including antibodies such as basiliximab, may also be used in patients who require a steroid-sparing or CNI-sparing regimen [18]. The maintenance regimen is initiated 30 days after transplantation, and in the majority of LT recipients, immunosuppression is continued lifelong. This regimen is individualized based on the patient’s comorbidities, the recurrence risk of the pretransplant condition (especially hepatitis C or autoimmune liver disease), the risk of infection or malignancy, and the potential toxicities of the medications. In patients with non-melanoma skin cancer, the use of everolimus (EVR), initiated one week after transplantation, or sirolimus (SRL), started one month post-transplant, is recommended [15]. Some evidence has also shown that immunosuppressive regimens incorporating mTOR inhibitors may reduce the recurrence of HCC, although specific randomized controlled trials (RCTs) have not yet been conducted [19,20,21,22,23].
4. Mechanism of Action of Immunosuppressant Drugs
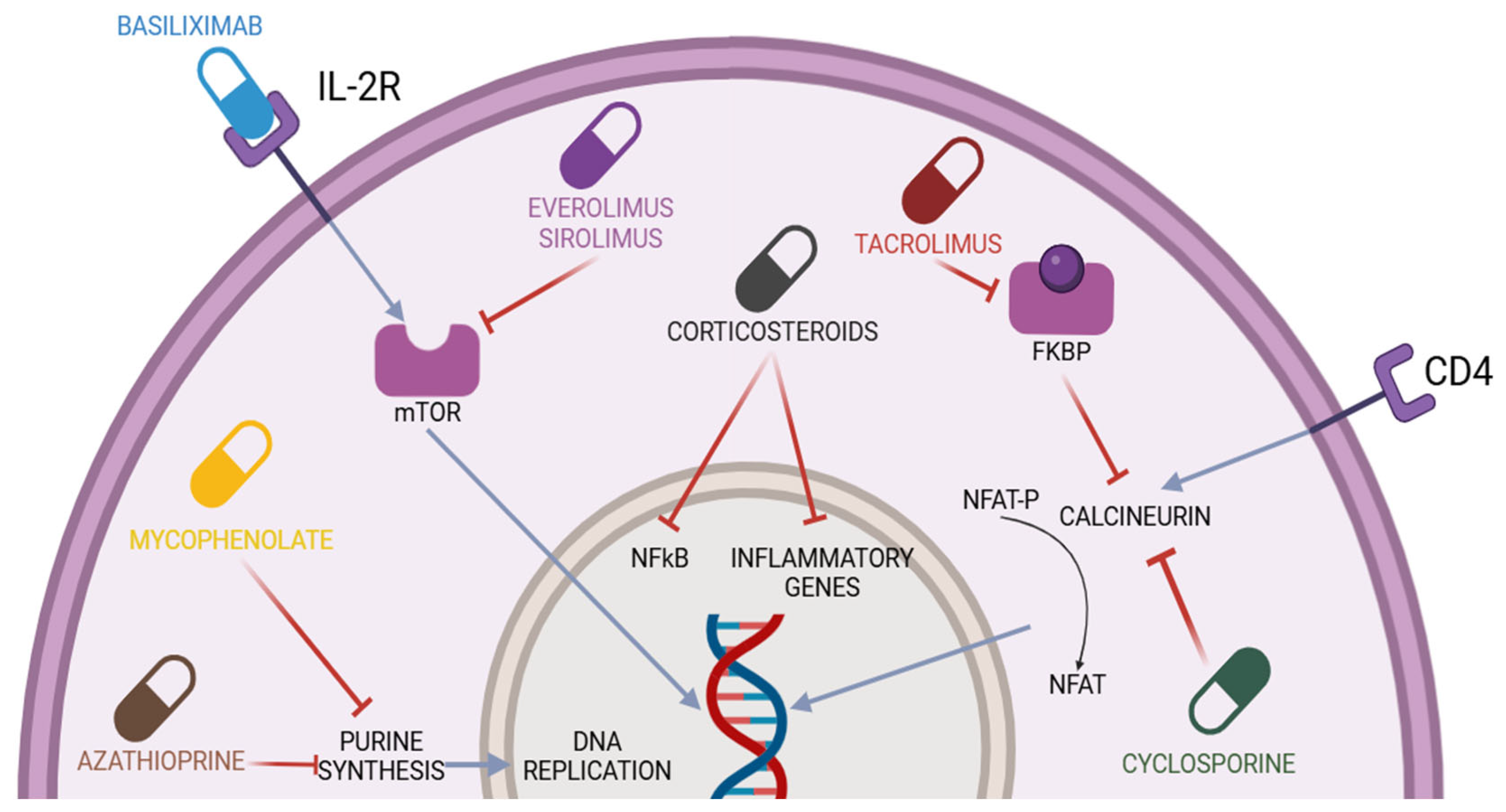
This section will address the mechanisms of action of the main immunosuppressive drugs used in LT. The immunosuppressive drugs typically used in LT belong to the class of calcineurin inhibitors (CNIs), including cyclosporine (CsA) and tacrolimus (TAC), corticosteroids (primarily prednisone), antimetabolite drugs (mycophenolate mofetil (MMF) and azathioprine (AZA)), inhibitors of the mammalian target of rapamycin (mTOR-I, everolimus (EVR), and sirolimus (SRL)), and biologic agents (basiliximab). Figure 1 shows the various molecules used in the context of post-LT immunosuppression and their respective mechanisms of action.
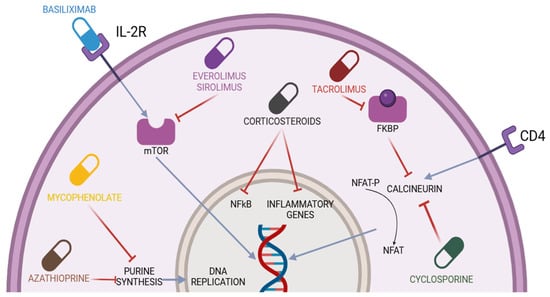
Figure 1.
Immunosuppressive drugs used in liver transplantation and their mechanisms of action. CD4 = cluster differentiation 4; NFAT-P = nuclear factor of activated T-cell precursor; NFAT = nuclear factor of activated T-cells; IL-2R = interleukin-2 receptor; FKBP = FK506 binding proteins; NF-kB = nuclear factor kappa-light-chain-enhancer of activated B-cells; mTOR= mammalian target of rapamycin. The blue lines represent activation mechanisms, while the red lines represent inhibition mechanisms.
4.1. Calcineurin Inhibitors (CNIs)
CNIs are the backbone of most post-LT immunosuppression regimens; it has been estimated that 97% of patients discharged after an LT are on a CNI-based therapy [24]. Tacrolimus (FK506) was isolated from the soil fungus Streptomyces tsukubaensis [25]. Physiologically, calcineurin is a serine/threonine phosphatase composed of a catalytic subunit that binds to calmodulin (calcineurin A) and a regulatory subunit that can bind intracellular calcium (calcineurin B), thereby regulating the activity of calcineurin A [26,27]. At the level of T lymphocytes, the binding between the T-cell receptor (TCR) and an antigen leads to an increase in intracellular calcium concentrations, which activates calcineurin B. As a result, calcineurin A exerts its enzymatic activity and dephosphorylates the nuclear factor of activated T-cells (NFATs). At this point, both calcineurin A and NFATs are able to translocate into the nucleus, where they are involved in the transcriptional activation of genes encoding various cytokines, particularly interleukin-2 (IL-2) [28]. IL-2 is responsible for the growth and differentiation of T lymphocytes. CsA and TAC inhibit calcineurin by binding to intracellular small molecules: cyclophilin for CsA and FKBP12 for TAC. These are low-molecular-weight intracellular proteins that facilitate protein folding, intracellular protein transport, and the stability of multiprotein complexes [18]. While some side effects, such as alopecia, gingival hypertrophy, and hirsutism, may be tolerated by patients, others, such as hypertension, de novo diabetes, and hyperlipidemia, can significantly impact long-term outcomes [29]. However, the greatest concerns are related to the potential nephrotoxicity and neurotoxicity of the drugs.
4.2. Corticosteroids
Corticosteroids are used for the induction and maintenance of immunosuppression and treating acute cellular rejection.
Corticosteroids are steroid hormones, small lipophilic molecules capable of crossing the cell membrane. They exert their effects in different ways depending on the dosage [30]. In the case of low doses (e.g., <30 mg of prednisone), the action is primarily genomic. In this case, an intracellular interaction occurs between the corticosteroid and the cytoplasmic glucocorticoid receptor (GR) [31]. Once this binding occurs, the GR undergoes hyperphosphorylation, enabling it to migrate into the nucleus, where it regulates gene expression. This action can directly affect certain genes but also, and perhaps more importantly, influence various transcription factors, including NF-kB and AP-1. Moreover, the action extends to genes encoding enzymes involved in gluconeogenesis, such as tyrosine aminotransferase, serine dehydrogenase, and phosphoenolpyruvate carboxykinase [32]. NF-kB and AP-1are involved in the regulation of genes related to the synthesis of adhesion molecules and cytokines. The inhibition of NF-kB by the corticosteroid–GR complex leads to a reduction in the production of interleukins such as IL-1, IL-6, interferon (IFN), and nitric oxide [33]. This effect takes more time to manifest but also leads to anti-inflammatory and immunomodulatory actions. It occurs through interactions with antigen-presenting dendritic cells, the modulation of IL-1 transcription, a decrease in the number of circulating CD4+ T-cells, and the inhibition of IL-1-dependent lymphocyte activation [18]. Higher doses of corticosteroids, on the other hand, act directly on the membrane (non-genomic effect), resulting in a faster response to the steroid. This is primarily due to three mechanisms: the release of proteins from the multiprotein complex after binding to the glucocorticoid receptor in the cytosol, interactions with membrane-bound receptors, and non-specific effects on the modification of the cell membrane induced by the steroids [34,35]. Higher doses of corticosteroids lead to the saturation of the receptors responsible for the long-term side effects.
4.3. Antimetabolites Drugs
There are three antimetabolite drugs available: AZA, MMF, and the enteric-coated formulation of mycophenolate sodium (EC-MPS). Only AZA and MMF are approved for LT [15]. Since the introduction of MMF, the use of AZA has declined, although evidence of a significant benefit of MMF over AZA remains limited [36,37,38].
AZA, synthesized in 1957, is a prodrug of 6-mercaptopurine, absorbed in the gastrointestinal tract and converted non-enzymatically into 6-mercaptopurine by intestinal, red blood, and hepatic cells [39,40]. In the liver, it forms thioinosinic acid, a purine analogue that interferes with DNA and RNA synthesis, exerting cytotoxic effects on leukocytes [40]. Enzymes such as hypoxanthine-guanine phosphoribosyltransferase (HGPRT) and other enzymes determine the formation of cytotoxic compounds, while xanthine oxidase and thiopurine S-methyltransferase (TPMT) create inactive compounds. The balance of these enzymes influences AZA’s efficacy and toxicity, with high TPMT activity reducing efficacy and low activity increasing toxicity. 6-Mercaptopurine inhibits leukocyte proliferation by disrupting nucleic acid synthesis and induces apoptosis through alternative pathways, including interactions with RAC-1, a GTPase that modulates T-cell migration and adhesion [41,42,43]. Additionally, 6-thioguanine metabolites downregulate anti-apoptotic molecules like BCL-XL, promoting lymphocyte apoptosis [44].
Mycophenolic acid (MPA) was identified in 1913 following the isolation of Penicillium stoloniferum, initially noted for its antibiotic, antiviral, and anti-inflammatory activities [45]. Although the compound was initially used for certain autoinflammatory diseases, its gastrointestinal side effects and association with the development of neoplasms led to its near abandonment [46]. Between the 1980s and 1990s, an ester of MPA, MMF, was developed, which demonstrated better bioavailability and fewer gastrointestinal side effects. This led the Food and Drug Administration (FDA) to approve it as a drug for the prevention of transplant rejection [47]. MMF is a prodrug of MPA that is almost completely absorbed in the small intestine and, in the bloodstream, is deesterified to MPA [48]. Only a small fraction of MPA enters the cell; the metabolite is subsequently converted into phenolic glucuronide and excreted via the urinary route [48]. Mycophenolate sodium, unlike MMF, has a slower absorption rate, resulting in a longer plasma concentration duration and a delayed time to reach peak serum concentration compared to MMF [48]. Like AZA, MMF acts on nucleotides by inhibiting guanine formation, specifically targeting the enzyme inosine monophosphate dehydrogenase (IMPDH), thereby effectively blocking the de novo synthesis of guanine [49]. Unlike other cells in the body, lymphocytes rely primarily on this pathway for guanine synthesis. The inhibition caused by MPA results in reduced lymphocyte proliferation and, consequently, a diminished antibody response, without inducing myelotoxicity [49]. MPA also appears to influence the expression of microRNAs in CD4+ T lymphocytes, reducing the expression of CD40 ligand. Additionally, it exerts effects on fibroblasts by inhibiting their proliferation [50,51,52,53]. They are less efficient than CNIs and are often used in combination with lower doses of CNIs to allow for dose reduction or the eventual discontinuation of CNIs [54].
4.4. Inhibitors of the Mammalian Target of Rapamycin (mTOR-I)
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase that regulates a wide range of processes in eukaryotic cells in response to nutrients and growth factors [55]. mTOR is composed by two distinct complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTORC1 promotes cellular anabolism and suppresses catabolism, leading to the increased synthesis of proteins, lipids, and nucleotides, while reducing autophagy. In contrast, mTORC2 is involved in cell survival, cytoskeletal organization, gluconeogenesis, and lipogenesis [55,56]. In the context of LT, sirolimus (SRL) and everolimus (EVR) are used as mTOR inhibitors (mTOR-I). SRL was initially isolated from the fungus Streptomyces hygroscopicus and became the first mTOR-I to receive approval. EVR, which shares a similar structure with SRL, exhibits improved oral bioavailability and was approved by the European Medicines Agency (EMA) in 2003 as an immunosuppressive agent [56]. Other mTOR-I drugs, such as temsirolimus, deforolimus, ridaforolimus, and the novel class of mTOR-I known as TORKinibs, are not used in the context of LT and, therefore, will not be discussed in this article. SRL and EVR primarily inhibit mTOR within the mTORC1 complex by blocking substrate recruitment and subsequently interfering with their sites of action [57]. It has also been demonstrated that prolonged treatment with mTOR-I affects mTORC2 [58]. The effects of mTOR-I are multifaceted, as mTOR is involved in various processes, including protein synthesis, lipid metabolism, energy metabolism, autophagy, angiogenesis, epithelial-to-mesenchymal remodeling, and the development and function of the immune system. Regarding immunosuppression, mTOR-I can affect different cell populations, such as T and B lymphocytes and dendritic cells. mTOR-I are capable of reducing the functionality of T lymphocytes and regulating the synthesis of molecules like SP-1, CD62L, and CCR7, which reduce the ability of T lymphocytes to migrate out of lymphoid tissues [59,60]. They can also reduce the differentiation of CD4+ lymphocytes into Th1 and Th17 subsets without affecting the Th2 population as well as influence the differentiation of Treg cells, inducing apoptosis in activated CD4+ T-cells [61,62,63,64,65,66]. Dendritic cells (DCs) are antigen-presenting cells (APCs) with a critical role in immune response. The presence of mTOR-I can inhibit the maturation of DCs and induce apoptosis in monocyte-derived (mo)-DCs and CD34+-derived DCs but not in other cell lines such as macrophages and myeloid cells [67,68,69,70,71,72]. Mo-DCs in the presence of mTOR-I exhibit a reduced expression of antigen uptake receptors and decreased production of cytokines [70,73]. Additionally, mTOR-I can regulate plasmacytoid dendritic cells (pDCs), reducing the secretion of interferon α/β and impairing their ability to stimulate CD4+ T-cell activation. Regarding B lymphocytes, although further studies are needed for a more comprehensive understanding of this cell population, in vitro studies have demonstrated that mTOR-I can influence the proliferation and maturation of B-cells as well as reduce class switching recombination [60,74,75,76].
4.5. Basiliximab
One of the objectives of induction therapy is to inhibit T lymphocyte activation or block specific receptors (such as CD3 or IL-2R) through the use of monoclonal antibodies. Interleukin-2 (IL-2) is a cytokine responsible for the growth and activation of T and B lymphocytes as well as the activation of natural killer cells, macrophages, and monocytes [77]. The IL-2R is composed of three subunits: α-(CD25), β-(CD122), and γ-(CD132). The α-subunit is specific to IL-2R, and the binding between the α- and β-subunits is crucial for the activation of the IL-2R signaling pathway, which subsequently leads to the proliferation and clonal expansion of antigen-specific T and B lymphocytes involved in rejection [78]. The activation of these lymphocytes leads to further production of IL-2 and the activation of additional lymphocytes, which contribute to the destruction of the transplant’s vasculature [79]. Basiliximab is a chimeric monoclonal antibody, where the murine variable region is fused to a human immunoglobulin, and it inhibits T-cell proliferation by binding to IL-2R on activated T lymphocytes. It is used in induction therapy as a steroid- or CNI-sparing agent and in the treatment of steroid-resistant rejection [18]. The drug is administered in two doses: 20 mg within 2 h prior to LT and another 20 mg on the fourth day post-LT [80].
5. Side Effects
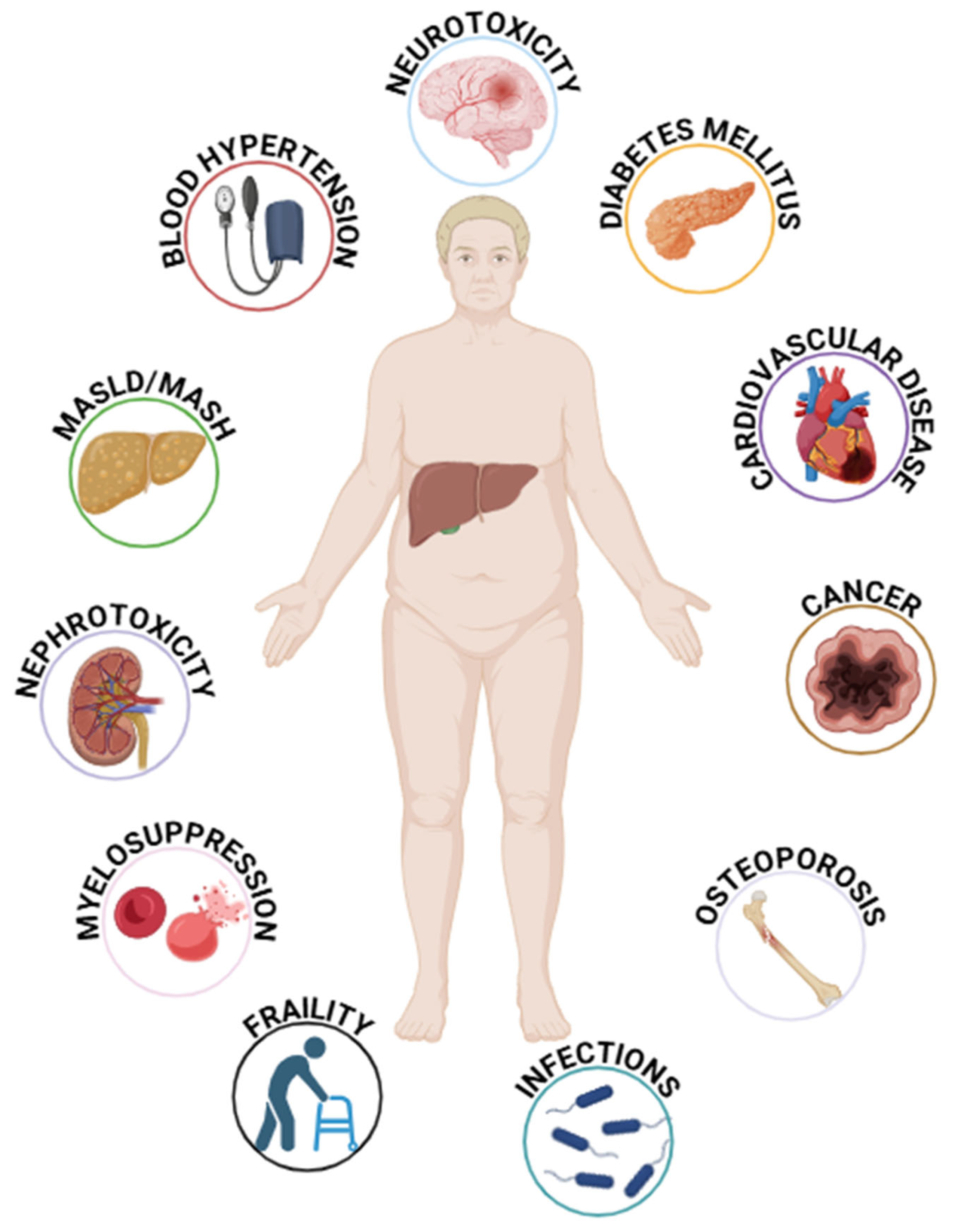
Immunosuppression reduces immune surveillance against malignancies and infections and may have class-specific side effects. For instance, renal dysfunction is common with CNIs, while corticosteroids exacerbate metabolic profiles. Immunosuppression-related malignancy (16.4%) and opportunistic infections (10.5%) are the leading causes of death in long-term survivors after LT, followed by metabolic disorders, CV events, and renal dysfunction [18]. Moreover, a crucial aspect to consider is the significant pharmacological interactions both among immunosuppressive agents themselves and between immunosuppressants and other drugs. In vitro studies have demonstrated that CsA can increase SRL serum concentrations to a greater extent than TAC [81]. Drugs capable of inducing or inhibiting CYP3A4 or P-glycoprotein can alter the serum levels of CNIs and mTOR-I [82]. A review conducted by Van Matre et al. highlighted significant interactions between azathioprine (AZA) and ribavirin, mycophenolate mofetil (MMF) and amoxicillin/clavulanate, ciprofloxacin, and isavuconazole as well as between corticosteroids and ketoconazole, rifampin, isoniazid, erythromycin, ritonavir, and cobicistat [83]. This section discusses the long-term side effects of immunosuppressive therapies used in LT. Figure 2 highlights the potential side effects of immunosuppressive therapy in LT recipients.
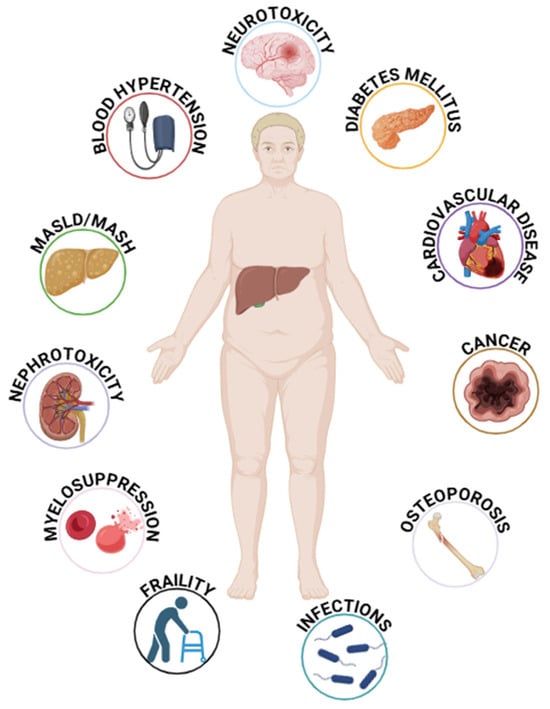
Figure 2.
The main factors associated with immunosuppressive therapy in liver transplant patients. MASLD = Metabolic Associated Steatotic Liver Disease; MASH = Metabolic Associated Steatohepatitis.
5.1. Malignancy
LT recipients have an approximately 11-fold increased risk of developing malignancies compared to the general population [84]. The prevalence of de novo tumors specifically ranges from 3.1% to 14.4% at 5 years and from 10% to 14.6% at 10 years post-LT [85]. Malignancy remains the leading cause of mortality in this population [86]. Post-transplant neoplasms include both solid tumors and hematologic malignancies, which occur more frequently in LT recipients and often present with more aggressive behavior and earlier onset compared to the general population [87].
Skin cancer is the most common de novo malignancy, with squamous cell carcinoma and basal cell carcinoma being the most frequently observed types [86]. The overall incidence of non-melanoma skin cancer ranges between 16% and 22.5%, and it can occur at any time after LT, although it generally does not significantly impact mortality [88]. Established risk factors for skin cancer include advanced age, a history of extensive ultraviolet exposure, a tendency to sunburn easily, infection with human papillomavirus, a history of actinic keratosis, CD4 lymphocytopenia, blue or hazel eyes, and a higher intensity or prolonged duration of immunosuppressive therapy [87,88]. Notably, immunosuppression with CsA has been identified as one of the strongest independent predictors of skin cancer development. This association is likely attributable to CsA’s direct carcinogenic potential via activation of the Ras pathway, the promotion of tumor growth, increased metastatic potential through TGF-β1 activation, and the disruption of angiogenesis and apoptosis [86,89].
Post-LT lymphoproliferative disorder (PTLD) refers to a heterogeneous group of conditions characterized by abnormal lymphoid cell proliferation in immunocompromised individuals following solid organ transplantation [88]. PTLD exhibits a broad clinical spectrum, ranging from infectious mononucleosis with reactive polyclonal hyperplasia to systemic high-grade monoclonal lymphoma involving both nodal and extranodal sites. The latter manifests with greater aggressiveness and poorer clinical outcomes compared to lymphomas in non-transplanted populations [88]. The prevalence of PTLD in adult LT recipients is estimated to be 2–4%, making it the second most commonly diagnosed malignancy in this population [86]. EBV plays a pivotal etiological role in most PTLD cases, as immunosuppression compromises the activity of EBV-specific cytotoxic T lymphocytes, thereby permitting uncontrolled proliferation of EBV-infected B- or T-cells [88]. Management of PTLD typically begins with a 50% reduction in the dose of immunosuppressive therapy, which is often sufficient to induce remission while maintaining protection against graft rejection [88,90]. Among immunosuppressive agents, registry studies have identified a strong association between PTLD development and the use of CNIs and polyclonal T-cell-depleting antibodies, particularly ATG [90]. CNIs have been shown to facilitate PTLD by promoting EBV replication and enhancing the expression of EBV growth factors such as IL-1, IL-6, and transforming growth factor-β (TGF-β). Additionally, CNIs augment the expression of anti-apoptotic genes, which contributes to immune evasion and the persistence of infected cells [91]. The incidence of non-skin solid tumors in LT recipients exhibits significant variability, as most epidemiological data are derived from registry databases or single-center retrospective studies [86]. Although less frequent than skin malignancies, certain solid tumors are associated with extremely high mortality rates, for instance, 100% for lung cancer, 62.5% for esophageal and gastric cancers, 57% for head and neck cancers, and 50% for Kaposi’s sarcoma [87]. As in the general population, tobacco and alcohol consumption are well-established risk factors for oral, pharyngeal, laryngeal, esophageal, and upper airway cancers. These substances have a synergistic effect, increasing the risk of such malignancies by more than seven-fold in individuals who are both heavy drinkers and smokers [89]. Lung cancer, in particular, occurs at a two- to three-fold higher incidence in LT recipients compared to the general population and typically manifests 2–6 years post-transplantation, highlighting the critical need for aggressive smoking cessation strategies [86,88]. The incidence of colorectal cancer also appears to be elevated in the LT population. However, this increased risk is largely attributable to patients transplanted for primary sclerosing cholangitis, often in association with chronic inflammatory bowel disease [87,89]. Kaposi’s sarcoma, though rare in the general population, occurs more frequently in regions where human herpesvirus 8 (HHV-8) is endemic [89]. In individuals infected with HHV-8, the most significant risk factor for developing Kaposi’s sarcoma is the degree of immunosuppression. Management typically involves tapering immunosuppressive therapy, with or without the addition of chemotherapeutic agents [87].
To date, no robust evidence has confirmed an increased post-transplant risk for genitourinary cancers, including bladder and renal cancers, or for non-breast gynecological cancers, such as cervical and ovarian cancer, in LT recipients [87,89]. Nevertheless, the development of these malignancies has been associated with well-established environmental risk factors, such as smoking for bladder cancer and infection with oncogenic HPV types for cervical cancer [89]. In contrast, prostate and breast cancer, which rank among the most common malignancies in the general adult population, do not appear to occur at an increased frequency in LT recipients compared to age-matched controls [87,89].
Cumulative exposure to TAC levels exceeding 10 ng/mL within the first month and 8 ng/mL within the first three months post-LT has been identified as a predictor of immunosuppression-related post-transplant de novo malignancies, particularly skin cancer, colorectal cancer, lung cancer, and HCC recurrence [92].
Notably, the use of SRL or EVR has been associated with a reduced risk of both de novo and recurrent post-transplant malignancies, including HCC, due to their antiproliferative properties [18]. A recent retrospective study conducted by De Simone et al. showed that LT patients with HCC receiving EVR had a reduced risk of HCC recurrence compared to those on TAC [93].
5.2. Infections
Infections remain the leading cause of mortality and morbidity among LT recipients, with reported rates reaching up to 80% [94]. Bacterial infections account for approximately 70% of all infections following LT, followed by fungal and viral infections [95]. These infections generally follow a distinct temporal pattern: during the first month post-transplant, nosocomial infections associated with surgical procedures and invasive post-operative interventions (e.g., mechanical ventilation, indwelling vascular, and urinary catheters) predominate. Between 2- and 6-months post-transplant, when immunosuppression reaches its peak, opportunistic infections and the reactivation of latent infections represent the primary sources of morbidity. Beyond 6 months, the frequency of infections declines, with community-acquired infections becoming the principal concern [15,94].
During the first month after transplantation, bacterial infections are most prevalent and typically involve the surgical site, abdomen, bloodstream, and the urinary and respiratory tracts [94]. Common causative pathogens include Enterococcus spp., Streptococcus viridans, Staphylococcus aureus, and members of the Enterobacteriaceae family [94]. Alarmingly, multidrug-resistant (MDR) infections are on the rise, with a reported prevalence of 18–50%, posing a significant challenge in clinical management. Particularly concerning are infections caused by Methicillin-Resistant Staphylococcus aureus (MRSA), Vancomycin-Resistant Enterococcus (VRE), carbapenem-resistant Enterobacteriales, and extended-spectrum beta-lactamase (ESBL)-producing Pseudomonas aeruginosa and Acinetobacter species [94].
During the period from 1 to 6 months post-LT, recipients are at risk of developing opportunistic infections such as candidiasis, aspergillosis, and toxoplasmosis [96]. Invasive fungal infections are reported in 5% to 42% of LT recipients [96]. Among these, candidiasis (60–80%) and aspergillosis (1–8%) are the most frequently observed mycoses, with high mortality rates of 30–50% and 65–90%, respectively [96,97].
Several risk factors predispose recipients to invasive fungal infections, including the preoperative use of broad-spectrum antibiotics, a pretransplant diagnosis of fulminant hepatic failure, concurrent CMV or HCV infection, serum creatinine levels > 3 mg/dL, prolonged intensive care unit stays, and extended surgical durations exceeding 11 h [96].
Toxoplasmosis, although less common in LT recipients, has an incidence rate ranging from 0.9% to 2.5%. Despite its rarity, toxoplasmosis carries a high morbidity and mortality rate, likely attributable to delays in diagnosis [98].
Infections caused by Pneumocystis jirovecii and herpesviruses (CMV, EBV, HSV, and VZV) have become less frequent in LT recipients due to the routine use of prophylactic therapy [96]. Recent studies indicate that the overall incidence of P. jirovecii infections in LT recipients has decreased significantly, from as high as 10% in the absence of prophylaxis [99] to less than 2% in patients receiving prophylaxis [94]. The risk of P. jirovecii pneumonia in LT recipients remains closely linked to the intense immunosuppressive regimens used to manage acute cellular rejection. Other risk factors include prolonged courses of high-dose corticosteroid therapy, CMV infection, persistent neutropenia, and exacerbations of autoimmune diseases [99].
Regarding HBV, it has been known since the 1980s that viremic patients undergoing transplantation while in a viremic phase are at the highest risk of HBV recurrence. Therefore, all patients on the LT waiting list should be treated with nucleos(t)ide analogues (NA) with the goal of achieving undetectable viral concentrations in plasma [15]. The combination of hepatitis B immunoglobulin (HBIG) and a potent NA is recommended post-transplant to prevent HBV recurrence. Low-risk patients for recurrence may also discontinue HBIG [100]. In patients with latent HBV infection, lifelong prophylaxis with lamivudine is required [100].
It is important to note that in immunosuppressed patients, other viruses can become chronic. One example is the hepatitis E virus (HEV), which accounts for approximately 20 million infections annually and is not routinely tested by organizations involved in blood collection and storage [101,102]. Moreover, the virus can be acquired through the consumption of inadequately cooked pork or wild boar meat. The first-line treatment for patients with chronic HEV involves reducing immunosuppressive therapy, which may lead to viral clearance through the host’s immune system. In other cases, the use of ribavirin, sofosbuvir, or pegylated interferon-alpha may be required [102,103].
CMV infection typically manifests within the first three months following LT and is the most common viral complication, accounting for 54.3% of all viral infections in this population [104]. CMV has been shown to quadruple the risk of acute and chronic allograft rejection by potentiating alloantigens and impairing CD4 T-cell and macrophage functions [104,105]. These indirect effects further predispose patients to invasive fungal infections, bacteremia, EBV-associated PTLD, and CV complications [104,105]. CMV viremia should be monitored at least during the first month post-LT [15]. Proper CMV prophylaxis must be implemented to reduce the incidence of infection [15]. The treatment of choice is intravenous ganciclovir or oral valganciclovir and should be considered for all patients with persistent CMV viremia and those who develop infection-related symptoms [15].
The incidence of VZV infection after LT is reported as 17.83 per 1000 person-years, a rate significantly higher than that in the general population [106]. Older age and exposure to MMF or AZA have been identified as independent risk factors for VZV infections in this context [106,107].
Cryptosporidium spp. infection is caused by protozoa that contaminate food or water and typically induce diarrhea in immunocompetent hosts [108]. In immunosuppressed patients, more severe conditions, such as disseminated disease, may occur [109]. Cryptosporidiosis in immunosuppressed patients can also result in fatal forms [110]. The first-line therapy in these cases is the reduction of immunosuppression. If this is not possible or sufficient, nitazoxanide may be administered alone or in combination with azithromycin [110,111].
Active tuberculosis (TB) in LT recipients typically arises from the reactivation of latent Mycobacterium tuberculosis infection, with most cases occurring within the first year post-LT [96,112]. The prevalence of M. tuberculosis infection in LT recipients varies significantly by geographic region, reported as 0.6% in the United States, 1.4% in European Union countries, and 2.2% in non-US/non-EU countries. Mortality associated with post-LT TB ranges from 18% to 36% [94]. Pulmonary TB is the most common clinical manifestation, accounting for up to 60% of cases in this population [94]. However, extrapulmonary and disseminated TB presentations are significantly more frequent in transplant recipients compared to immunocompetent individuals [112].
TB prophylaxis in liver transplant patients is a critical consideration due to their immunosuppressed condition. Additionally, in organ transplant recipients, the likelihood of developing active TB is between 20 and 74 times higher than in the general population, with the majority of cases resulting from the reactivation of latent TB [113,114]. Screening for latent TB is important both due to the high incidence of the disease and the associated risk of mortality from active TB in solid organ transplant recipients [115]. There are no specific indications from the EASL guidelines regarding the treatment of latent TB; however, the WHO guidelines classify transplant recipients as high-risk individuals for TB and recommend treatment for them [15,116]. The drugs typically used in these contexts are isoniazid and rifampin. Close laboratory monitoring should be conducted in these patients, as these medications can increase transaminase levels and cause interactions with immunosuppressive drugs [117].
EVR has been linked to an increased risk of interstitial pneumonia, which is dose-dependent and resolves upon the discontinuation of EVR; however, it has not been associated with an increased risk of opportunistic infections [18]. Finally, corticosteroids have been identified as a risk factor for invasive aspergillosis [97].
5.3. Metabolic Disorders
MetS, characterized by obesity, dyslipidemia, hypertension, and hyperglycemia, represents one of the most common complications following LT, with reported prevalence rates ranging from 44% to 58% across various studies [118]. Alongside immunosuppression, MetS is recognized as a key risk factor for the development of CV disease in LT recipients, which accounts for 19% to 42% of all non-graft-related deaths in this population [118].
The prevalence and severity of obesity notably increase post-LT, rising from 23.8% at four months to 40.8% by three years after transplantation [119]. Charlton and colleagues observed that patients with obesity prior to transplantation tend to remain obese, while overweight individuals often progress to obesity following the procedure [119]. In their study, individuals with normal weight at baseline who received EVR combined with reduced TAC exhibited less weight gain compared to those treated with TAC alone [119].
This finding aligns with evidence suggesting that mTOR-I, such as EVR, may alter fatty acid uptake and triglyceride synthesis pathways, in addition to reducing adipocyte cell proliferation, thereby contributing to weight modulation [120].
Blood hypertension is a common complication after LT, affecting up to 70% of long-term patients. This condition is attributed not only to post-LT hemodynamic changes but also to immunosuppressive therapy [121]. In a study evaluating the effects of CNIs and EVR two years post-transplantation, blood hypertension was observed in 57.6% of patients treated with TAC, 50.0% of those receiving EVR, and all patients on CsA [122].
Several pathogenic mechanisms underlie CNI-related hypertension, with key contributors including renal vasoconstriction, reduced glomerular filtration rate, and enhanced sodium absorption in the proximal renal tubule. Additionally, CsA increases sympathetic nervous system activity through endothelial dysfunction, further elevating blood pressure [122].
While studies in kidney transplant recipients suggest that mTOR-I may exacerbate hypertension when combined with CNIs [107], research on LT recipients has produced inconsistent findings [123,124,125]. Furthermore, corticosteroids contribute to blood hypertension via their mineralocorticoid effects, increased vascular resistance, and enhanced cardiac contractility [118].
Immunosuppression plays a significant role in the development of dyslipidemia following LT. Findings from three randomized trials evaluating early conversion from CNI therapy to either SRL or EVR revealed elevated levels of total cholesterol and low-density lipoprotein cholesterol (LDLc) in the mTOR-I group [126]. This is consistent with the lipogenic effects of mTOR-I, which inhibit lipid uptake into adipocytes and promote lipolysis [126]. CNIs have also been linked to hypercholesterolemia and hypertriglyceridemia, with CsA showing a higher prevalence of these complications (30% and 33%) compared to TAC (6% and 3%) [127]. CsA contributes to dyslipidemia by reducing biliary cholesterol excretion and inhibiting LDLc receptors, leading to increased cholesterol levels in the bloodstream [118].
Steroids induce lipogenesis by stimulating acetyl-CoA carboxylase activity and impairing LDLc receptor function. However, their impact on lipid metabolism is typically transient due to their limited long-term use in post-transplant regimens [120].
Post-transplant diabetes mellitus (PTDM) is a common metabolic complication that significantly compromises long-term survival by increasing the risk of end-stage renal disease, infection, graft failure, CV events, and mortality. Risk factors associated with the development of PTDM are high recipient age at the time of transplant, male gender, the indication for LT (cirrhosis due to MASH, HCV, or alcohol or cryptogenic cirrhosis), an increase in body mass index after transplantation, smoking, and immunosuppression [118,121]. LT recipients on SRL have the highest incidence of PTDM (27%) at 2–3 years after transplant compared to TAC and CsA (23% and 15%, respectively) [128].
While acute use of SRL has been shown to prevent insulin resistance, chronic use of SRL has been associated with the development of glucose intolerance and hyperlipidemia in animal models, particularly rats [129]. These effects are primarily attributed to a reduction in β-cell mass, decreased hepatic insulin clearance, and increased hepatic gluconeogenesis, all of which are induced by direct mTOR-I [129].
When comparing the two CNIs head-to-head, CsA was found to induce a higher incidence of PTDM one year after LT compared to TAC (18% vs. 11%, respectively). However, after two to three years, the incidence of PTDM was higher among patients treated with TAC compared to those on CsA (23% vs. 15%, respectively) [128]. Both TAC and CsA have similar inhibitory effects on pancreatic β cells, leading to reduced insulin synthesis and increased insulin resistance [130]. The difference between CsA and TAC may be attributed to the potency of calcineurin inhibition. The superior effect of TAC in preventing rejection has been found to be accompanied by an increased rate of diabetes [131]. Additionally, corticosteroids induce insulin resistance in a dose-dependent manner by decreasing β-cell insulin production, enhancing gluconeogenesis, and reducing peripheral glucose utilization. However, corticosteroids are typically not used over the long term, so their impact on the MetS is generally transient [120].
MASLD and MASH are well-established metabolic complications that can affect patients after LT. In a meta-analysis, the mean 1-, 3-, and 5-year incidence rates of recurrent and de novo MASLD were 59%, 57%, and 82% and 67%, 40%, and 78%, respectively [121]. In contrast, the incidence of post-transplant MASH was significantly higher for recurrent MASH (53%, 57.4%, and 38% at 1, 3, and 5 years after LT, respectively) compared to de novo MASH (13%, 16%, and 17%, respectively) [121]. Cirrhosis was reported in patients with recurrent or de novo MASLD at a rate of 11–14% and 1%, respectively, at 5 years post-LT [121]. Despite MASLD being an extremely common post-transplant complication, no significant difference in graft survival between patients with recurrent MASH or MASLD has been observed [132]. However, it is important to note that although long-term survival rates are not significantly different, these patients remain at an increased risk for CV events due to underlying metabolic disease.
5.4. Nephrotoxicity
Renal dysfunction following LT can result from perioperative acute kidney injury (AKI), pre-existing chronic kidney disease (CKD), or the development of CKD after the transplant. AKI after LT is a common complication, with a reported incidence ranging from 30% to 60%, and is caused by acute tubular necrosis in most cases [133]. The incidence of CKD has been reported to range from 4.0% to 27.5% within one year and 30% to 50% within 10 years post-surgery [133]. Independent risk factors for new-onset CKD include higher intraoperative blood loss, blood transfusions leading to renal ischemia-reperfusion injury, postoperative AKI, postoperative hypertension, and elevated average plasma concentrations of CNIs [134].
CNIs are responsible for over 70% of cases of end-stage renal disease (ESRD) after LT [15]. CNIs cause a dose-dependent afferent renal arteriolar vasoconstriction, which is typically reversible. However, prolonged ischemic glomerular and tubular injuries can lead to irreversible tubulointerstitial fibrosis [18]. Additionally, the side effects of CNIs, including blood hypertension and diabetes, further exacerbate renal damage [134]. It has been demonstrated that conversion from CNIs to mTOR-I improves renal function in patients with plasma creatinine levels > 1.5 mg/dL and stabilizes proteinuria and creatinine levels in those with diabetic and hypertensive nephropathy after LT [135]. Specifically, the use of EVR combined with CNI minimization, resulted in a significant improvement in renal function at one year, with an estimated glomerular filtration rate (GFR) increase of 10.2 mL/min (95% CI: 2.75–17.8) in a meta-analysis [136]. Furthermore, the combination of MMF and SRL showed a significantly greater improvement in renal function compared to MMF and CNIs in a randomized trial with a median follow-up of 1.4 years, with a mean percentage change in the GFR of 19.7 ± 40.6 versus 1.2 ± 39.9, respectively (p = 0.0012) [137].
Immunosuppressive regimens using MMF or Basilixumab have been shown to offer a better renal profile compared to conventional CNI-based regimens. However, it is challenging to accurately assess the real impact of both MMF and Basilixumab, as the data supporting their use come from small patient cohorts [138,139,140,141,142].
5.5. Neurotoxicity
The overall prevalence of neurological symptoms or disorders after LT is reported to be around 25%, which is notably higher than that seen in other organ transplants such as the kidneys (0.5%) and heart (3.6%) [143]. Neurological complications are most common during the early period (1–3 months) following LT and can range from mild to severe. Mild complications include tremors, headache, sleep disorders, and peripheral neuropathy, which often resolve spontaneously. Severe complications, such as seizures, stroke, posterior leukoencephalopathy, central pontine myelinolysis syndrome, toxic-metabolic encephalopathy, and central nervous system (CNS) infections, can have significant and lasting consequences [143,144].
Among the immunosuppressive drugs used after LT, MMF is unique in not being associated with neurotoxic side effects [143,144]. In contrast, CNIs such as CsA and TAC are commonly linked to neurotoxicity. The manifestations of CNI-related neurotoxicity can range from tremors and headache to more severe symptoms, such as seizures, altered mental status, stupor, coma, confusion, agitation, and psychosis [145]. Approximately 18% of moderate-to-severe neurological complications occurring within 4 weeks after LT are related to CNI use. Risk factors for the development of CNI-related neurotoxicity include older recipient age, a history of pre-LT hepatic encephalopathy, pre-LT hyponatremia, surgical time longer than 7 h, and post-LT hyponatremia [145]. Neurotoxicity is observed in about 10% to 28% of patients treated with CsA and 21% to 32% of those treated with TAC [146].
The exact neurotoxic mechanism of CNIs remains unclear. However, it is hypothesized that the neurotoxicity may be related to the high-affinity binding of CNIs to immunophilins, which reach elevated levels in central nervous system (CNS) cells [143]. Oligodendrocytes, which contain higher levels of calcineurin compared to astrocytes, may be particularly vulnerable to this binding, explaining the selective toxic effects on these cells and why CsA exposure can lead to demyelination [143]. Both TAC and CsA are known to reduce endothelial nitric oxide production, which can lead to severe vasoconstriction in cerebral vessels and resulting ischemia [143]. Additionally, the reduction in calcineurin phosphorylation caused by CNIs disrupts GABAergic neurotransmission, which can predispose patients to seizures, while altering serotonergic neurotransmission may facilitate tremors [143].
In contrast, mTOR-I binds to a different site on immunophilins and does not cause neurotoxic effects. Studies evaluating the conversion to SRL or EVR following CNI-related neurotoxicity in LT patients have shown that neurological complications improve or resolve in 80–100% of cases [146,147,148].
Corticosteroids are associated with neuropsychiatric side effects in 3–4% of LT patients. The most common side effects include mood changes, behavioral disorders (including psychotic reactions), and neuromyopathy, which manifests as muscular weakness [143,144].
5.6. Myelosuppression
Immunosuppressive medications can contribute to the development of anemia following LT due to their bone marrow-suppressive effects. Controlled trials comparing different immunosuppressive regimens have reported an incidence ranging from 1% to 53% [149]. In the European FK506 Liver Study comparing CsA and TAC, the incidence of anemia was 1% in the CsA group and 5% in the TAC group [149]. In contrast, the US FK506 Liver Study group reported significantly higher anemia incidences, with 38% of patients in the CsA group and 47% in the TAC group [149]. Anemia in this context is typically mild to moderate (mean hemoglobin 10.3 ± 1.6 g/dL), and in most cases, the discontinuation of CNIs leads to complete hemoglobin recovery within three weeks [150]. Additionally, sporadic cases of thrombotic thrombocytopenic purpura and red cell aplasia have been reported with TAC use [150].
The use of mTOR-I has also been associated with anemia, likely due to iron deficiency secondary to an inflammatory state [151]. The myelosuppressive effects of mTOR-I result from the inhibition of signal transduction pathways shared by several cytokine receptors, including those that stimulate platelet, leukocyte, and erythrocyte production [151]. MMF is generally well tolerated; however, its most common side effects include leukopenia, anemia, and thrombocytopenia [152]. A randomized study comparing MMF and AZA, both in combination with CsA and corticosteroids in LT recipients, reported anemia as an adverse event in 43% of patients in the MMF group and 53% in the AZA group [36]. The incidence of anemia with MMF is dose-dependent and can generally be managed by reducing the MMF dose. Treatment discontinuation is rarely required [152].
5.7. Osteoporosis
Patients with liver cirrhosis exhibit a higher prevalence of metabolic bone disease compared with the general population, with an estimated prevalence ranging from 12% to 55% [153,154]. This condition is associated with a higher propensity for fragility fractures, with an incidence rate of approximately 40% [155,156]. Several factors contribute to the pathogenesis of osteoporosis in transplanted patients: pre-existing conditions prior to transplantation, factors such as the use of immunosuppressive drugs and lifestyle choices, and imbalances in the parathyroid–calcium–vitamin D and pituitary–gonadal axes [157,158].
Furthermore, patients on the transplant waiting list often experience complications associated with organ failure, such as cachexia, nausea, inadequate nutrition, reduced mobility, and hypogonadism, which are particularly common in end-stage liver disease patients [158]. Additionally, certain medications commonly used in liver disease, such as corticosteroids, loop diuretics, ribavirin, and tenofovir, may negatively affect bone balance [158].
In a recent study by Zavatta et al. [159], a 42% prevalence of osteoporotic fractures was identified among LT recipients, most commonly affecting the thoracic vertebrae, femur, humerus, and ribs. An interesting finding from the study is that a 1-point reduction in BMI was associated with a 5.8% increase in the risk of fragility fractures (p < 0.05) [159].
After transplantation, bone mineral density decreases rapidly within the first 3–6 months but tends to recover by two years post-LT [160,161,162].
CNIs have been associated with bone mineral density loss following LT [160], with bone density being inversely correlated with serum CsA levels at one-year post-transplant [163].
Corticosteroids also induce significant bone mineral loss, particularly in the short term. However, steroid therapy in LT patients should generally be limited to the early post-transplant phase or episodes of liver rejection [15,154,158,164]. A proper supplementation of calcium and vitamin D should generally be ensured for patients with deficiencies, and the use of bisphosphonates may be considered for patients at high fracture risk. Denosumab has been shown to significantly reduce osteoporosis in transplanted patients, as demonstrated in a retrospective study [165].
According to a study by Bueno et al., LT recipients appear to have a higher propensity for developing osteonecrosis of the jaw when treated with bisphosphonates compared to those not receiving bisphosphonates [166].
5.8. Frailty
Frailty is a multidimensional concept primarily described in geriatrics. It is defined as a syndrome characterized by weight loss, exhaustion, weakness, reduced gait speed, and decreased physical activity [167]. Frail patients are at a higher risk of accidental falls, loss of independence, reduced quality of life, increased hospitalization rates, and greater mortality [167]. There is limited but growing evidence describing the post-LT progression of frailty. Generally, a worsening of frailty is observed at three months post-LT, followed by an improvement at twelve months [168]. The mechanisms by which individual immunosuppressants contribute to sarcopenia and, more broadly, frailty in post-LT patients remain unclear. However, the deterioration of metabolic, renal, and neurological or psychological profiles may exacerbate frailty in these patients. Additionally, inadequate lifestyle factors and limited social support networks may further contribute to this condition [169,170].
6. Is Immunosuppressive Withdrawal Feasible After LT?
As mentioned earlier, among the various solid organ transplants, the liver is the organ with the best immunotolerance, requiring comparatively lower levels of immunosuppression than other organs [15,16]. The 2024 EASL guidelines for LT recommend a gradual reduction of immunosuppression under expert supervision, emphasizing that long-term survivors are often overly immunosuppressed [15]. Several studies have explored the possibility of discontinuing immunosuppression in LT patients [171,172,173,174,175,176,177,178,179,180,181,182]. Although the EASL guidelines do not recommend the suspension of immunosuppression either as a standard of care or in patients with an extended postoperative course without transplant rejection [15], some estimates suggest that 20–40% of LT patients may maintain immunotolerance after discontinuation of immunosuppression [183]. Additionally, beyond the spontaneous operational immunotolerance observed in these patients, a question currently being explored is the potential contribution of pharmacologically induced immunotolerance, or therapeutic operational immunotolerance [184]. Therefore, in addition to the search for a greater understanding of the underlying mechanisms of immunosuppression, efforts are being made to identify biochemical or histological factors that could define patients at high risk of rejection. The decision to modulate or discontinue immunosuppression may stem from the side effects of immunosuppressive drugs and the risk of rejection, and this decision should be personalized for each patient. Currently, no tools are available to identify which patients are immunotolerant; liver transaminases may be normal despite increased hepatic fibrosis, as characterized histologically in cohorts of patients who have discontinued immunosuppression [185]. Some interesting lessons have been provided by Benitez et al. [186], Shaked et al. [187], and Duizendstra et al. [182]. In the study conducted by Benitez et al. on nearly 100 LT patients followed for 3 years with serial biopsies, it was observed that the likelihood of immunotolerance was associated with older age, longer time since transplantation, and male sex [186]. Specifically, the percentage of immunotolerant patients was 20% in total, with 78% in patients who had undergone LT at least 10.6 years earlier, which decreased to 38% if the transplant had been performed 5.7 years earlier. When the LT was performed less than 5.7 years prior, the percentage dropped to 0% [186]. In the study conducted by Shaked on 77 LT patients, a progressive reduction in immunosuppressive therapy was induced until withdrawal in patients who had undergone transplantation 1–2 years earlier, with biopsy verification at three months and after immunosuppressive withdrawal [187]. In this study, the percentage of immunotolerant patients was 19%, with better outcomes in terms of survival, secondary opportunistic infections, reduced renal function, and secondary malignancies [187]. Duizendstra et al. conducted a retrospective case-control study on the effects of complete immunosuppression withdrawal in patients with a median time since LT of 10.8 ± 5.1 years [182]. Patients who underwent immunosuppressive withdrawal showed improvements in LDL values, a reduction in new infections, and resolution of persistent infections; however, no improvement in renal function, reduction in new diagnoses of T2DM, hypertension, malignancies, or CV events was observed [182]. A smaller-scale study analyzed 15 non-immune, non-viremic LT recipients undergoing SRL therapy for at least three months and at least three years post LT [188]. Of these 15 patients, 8 were found to be immunotolerant one year after discontinuation of SRL therapy. Immunotolerant patients exhibited a higher percentage of HLA-DR+CD11c+ILT3+ILT4+ DCs prior to weaning (p < 0.01) [188]. Further studies are required to confirm these findings. In a recent multinational randomized controlled trial (RCT), a 28% discontinuation rate of immunosuppressive therapy without the development of allograft rejection was reported, with 16% of patients achieving operational immunotolerance at one year [189]. An interesting finding reported by Vionnet et al. is the overexpression of interferon-stimulated genes (ISG15, ISG20, OASL, OAS1, IFI6, IFITM1, IFI35) in immunotolerant patients [189].
The results of these studies raise several considerations regarding immunosuppression withdrawal, namely that only a small, selected portion of patients in these studies were found to be immunotolerant, and the long-term benefits remain uncertain. Moreover, the search for tools capable of predicting which patients may develop immunotolerance is crucial in identifying the small subset of patients who could benefit from a reduction or discontinuation of immunosuppressive therapy. Further studies will be needed to identify ideal markers and timing of immunosuppression withdrawal.
7. Unconventional Strategies in Post-Transplant Immunosuppression
Although the exact mechanisms by which the immune system interacts with the LT remain incompletely understood, the past 15 years have witnessed the discovery and development of novel immunosuppressive molecules that could improve long-term outcomes for LT patients.
7.1. Costimulatory Blockade Agents
Costimulation represents a crucial step in the activation of an antigen-specific immune response, and its inhibition could theoretically lead to clinical operational tolerance. The first study in the context of LT was conducted in the late 1990s using an anti-CD2 monoclonal antibody [190]; in this context, the authors concluded that the incidence of moderate-to-severe rejection was lower in the experimental group compared to the group receiving TAC [190].
Currently, there are two clinical studies involving Siplizumab, an anti-CD2 monoclonal antibody capable of inducing antibody-dependent cell-mediated cytotoxicity among NK cells [NCT06019507, NCT06455280].
Another drug capable of blocking costimulation is belatacept. This is a fusion protein composed of an FC fragment of a human immunoglobulin associated with an extracellular domain of the Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4, also known as CD152) molecule, a receptor expressed on CD4+ and CD8+ T lymphocytes. It can bind to CD80 and CD86 on APCs, resulting in a significant reduction in the activity of effector lymphocytes and Tregs [191,192]. It is currently approved for kidney transplantation with the aim of reducing the dosage of CNIs during the maintenance phase [193]. In LT, the results are limited to a single phase 2 study, which showed disappointing outcomes. In fact, the graft survival at one year was lower compared to the group receiving TAC and MMF. The arm with more intensive belatacept therapy demonstrated higher mortality, often leading to early treatment discontinuation due to a greater number of graft losses [194]. Some case reports and case series on the use of belatacept in LT patients experiencing rejection or in patients with combined kidney and LT have reported the resolution of rejection in specific contexts or the reduction of CNI dosage with relatively few side effects [194,195].
The blockade of the interaction between CD40 and CD40L is another mechanism under investigation; notably, two monoclonal drugs, bleselumab and iscalimab, have already been evaluated in clinical research [192]. However, only iscalimab has been studied in LT patients in a phase 2 RCT. Nonetheless, the study was terminated due to the less favorable efficacy of iscalimab (CFZ533) in LT patients compared to TAC[NCT03781414].
7.2. T-Cell Exhaustion
Exhausted T lymphocytes are dysfunctional T-cells characterized by a progressive loss of cytokine production (interferon-γ and tumor necrosis factor (TNF)-α), an increased expression of inhibitory receptors, and reduced proliferative capacity. T-cell exhaustion can occur as a result of continuous and prolonged exposure to antigens [192]. At present, the mechanisms underlying T-cell exhaustion are not well understood. However, it has been observed that T-cell exhaustion is associated with improved graft function and that the only inhibitory receptor identified is Fcγ RIIb [196,197]. Fcγ RIIb appears to play a role in both innate immunity, by acting on CD8+ T lymphocytes, and in inflammation, by acting on B lymphocytes. Therefore, the development of an Fcγ RIIb agonist could represent a significant step forward in achieving operational clinical tolerance. T-cell exhaustion can be achieved through induction with depleting agents and the progressive reduction of CNIs [198].
The available techniques include the administration of depleting agents capable of upregulating various immune checkpoints [199]. Muromonab-OKT3, a murine-derived monoclonal antibody targeting CD3 on human T lymphocytes, was the first to be used in solid organ transplantation and successfully reversed graft rejection [200,201]. The administration of Muromonab-OKT3 induces T lymphocyte depletion through immune-mediated phagocytosis or via TCR internalization [202]. However, due to the potential for polyclonal expansion, the risk of cytokine storm development, drug sensitization, the increased incidence of de novo neoplasms, and the lack of documented long-term effects, the drug was withdrawn from the market [203]. Another depleting agent used in LT is Alemtuzumab, a monoclonal antibody targeting CD52, which allows for a reduction in acute rejection episodes while using lower doses of immunosuppressive medications [204,205]. However, an increase in complications due to viral reactivations has been observed, leading to its discontinuation [206,207,208].
Calcineurin and NFAT are important in the induction of T-cell exhaustion, as they are capable of increasing the transcription of Thymocyte selection-associated high mobility group box protein (TOX), which leads to T-cell exhaustion [209]. Since CNIs hinder this process, several studies have evaluated the use of antibodies against lymphocytes to minimize CNI doses. However, the results obtained have not yet been confirmed by RCTs [210,211,212].
7.3. Selective Inhibitors of T-Cell Signaling
The Janus tyrosine kinase (JAK)/signal transduction and activators of transcription (STAT) system is responsible for signal transduction mediated by various cytokines and growth factors [213]. Dysregulation and mutations of the JAK/STAT pathway are associated with the development and progression of various diseases, such as solid tumors, autoimmune diseases, and inflammatory disorders [214,215]. Indeed, JAK/STAT upregulation can lead to the activation and expansion of autoreactive T lymphocytes or APCs, playing a role in this system in the development of allograft rejection [216]. There are several JAK/STAT inhibitors, among which tyrphostin AG490, a JAK2 inhibitor, has been shown to inhibit T-cell proliferation and render them hyporesponsive when stimulated by alloantigens or by CD3 or CD28 [217]. Ruxolitinib has been approved by the FDA to improve steroid-refractory graft-versus-host disease in stem cell bone marrow transplantation [218], while Tofacitinib has shown improvements in kidney transplantation [219]. Regarding LT, there are no studies on JAK/STAT inhibitors in LT patients, but in vitro studies have shown a reduction in liver damage induced by the ischemia/reperfusion process in mice [220].
7.4. Monoclonal Antibodies
Monoclonal antibodies can be used in the immediate post-transplant period to prevent acute rejection by inhibiting a fulminant T-cell response before conventional immunosuppressive drugs reach adequate concentrations [221]. Furthermore, the use of these drugs may lead to a reduction in the dose of CNIs and could be employed to replace steroids in certain immunosuppressive protocols, according to some authors [222,223,224]. In addition to muromonab, previously discussed, antibodies against the interleukin-2 receptor (such as basiliximab and daclizimab) induce immunosuppression by reducing T-cell expansion and have been used as prophylaxis for acute organ rejection following transplantation [225,226,227]. A meta-analysis has shown that the use of interleukin-2 receptor inhibitors is associated with a lower risk of acute organ rejection but does not lead to an increase in survival [221].
8. Conclusions
Immunosuppression is a cornerstone of LT therapy. Although the liver is a more immunotolerant organ and requires a lower dosage of immunosuppressants, there are currently no criteria to guide its withdrawal under specific conditions. TAC represents the cornerstone of immunosuppressive therapy, with levels maintained at 6–10 ng/mL during the first month, followed by 4–8 ng/mL thereafter. The combination of TAC with other immunosuppressive drugs (MMF, AZA, or mTORi) is recommended to achieve lower TAC trough levels than those required for monotherapy, thereby helping to preserve renal function. The use of basiliximab for induction therapy (with a delayed introduction of TAC) is advisable in recipients at a high risk of renal dysfunction [15]. The adverse effects of immunosuppression, both general and molecule-specific, in addition to the changing population of transplant recipients—particularly with a significant contribution from MASH-related cirrhosis—have increased focus on the metabolic and CV aspects of LT patients, alongside the usual surveillance for rejection and neoplasms [1,2]. This highlights an even greater need for personalized immunosuppressive therapy, aiming to balance the complications of immunosuppression with the risk of liver rejection. A crucial future objective will be the development of new immunosuppressive strategies with a reduced metabolic impact. These approaches could lower the incidence of metabolic syndrome, post-transplant diabetes mellitus, and dyslipidemia, thereby improving patients’ quality of life and reducing the risk of CV complications. Simultaneously, the introduction of novel molecules or pharmacological combinations may enable more targeted management while minimizing class-specific side effects of currently used drugs.
Another strategic goal is the identification of biomarkers or clinical-laboratory tools capable of identifying immunotolerant patients, who can safely discontinue immunosuppressive therapy. This innovation would further reduce the risks associated with chronic immunosuppression, improve long-term outcomes, and pave the way for a more selective and safer approach to patient management. In this regard, encouraging results have been demonstrated by deep learning and machine learning models in artificial intelligence, both in the pre-LT and post-LT settings [228,229,230]. Machine learning algorithms can be used to predict the outcome of a new observation based on a training set containing previous observations where the outcome is known [231]. Regarding the pre-LT setting, some artificial intelligence models have demonstrated utility in assigning a specific donor to a list of potential recipients without compromising the principles of urgency and utility [230]. Meanwhile, other models have demonstrated excellent diagnostic accuracy in predicting de novo or recurrent liver disease, the development of significant fibrosis, recurrent HCC, the onset of MetS, or renal damage in the post-LT setting [228]. Further studies need to be conducted in this field to enhance personalized medicine for these patients. Finally, it will be essential to continue fostering clinical studies and translational research to deepen our understanding of the immunological mechanisms underlying immunosuppression and immunotolerance.
Author Contributions
Conceptualization, F.G. and S.G.; methodology, F.G. and E.B.; writing—original draft preparation, F.G. and E.B.; writing—review and editing, F.G., E.B., A.C., A.T. and A.A.; visualization, F.G. and E.B.; supervision, S.G. and P.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study received funding from the European Union–NextGenerationEU through the Ministry of University and Research within the framework of the PNRR-M4C2-I1.3 Project PE_00000019 “HEAL ITALIA” to Pietro Andreone CUP E93C22001860006. The views and opinions expressed are those of the authors only and do not necessarily reflect those of the European Union or the European Commission. The European Union or the European Commission cannot be held responsible for these opinions.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created.
Acknowledgments
We would like to thank a friend who is no longer with us. Have a good trip, Ezechielo.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rana, A.; Ackah, R.L.; Webb, G.J.; Halazun, K.J.; Vierling, J.M.; Liu, H.; Wu, M.-F.; Yoeli, D.; Kueht, M.; Mindikoglu, A.L.; et al. No Gains in Long-Term Survival After Liver Transplantation Over the Past Three Decades. Ann. Surg. 2019, 269, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.T.; Sabroso, S.; Esteban, L.M.; Berenguer, M.; Fondevila, C.; Lorente, S.; Cortés, L.; Sanchez-Antolin, G.; Nuño, J.; De la Rosa, G.; et al. Mortality and Causes of Death After Liver Transplantation: Analysis of Sex Differences in a Large Nationwide Cohort. Transpl. Int. 2022, 35, 10263. [Google Scholar] [CrossRef] [PubMed]
- Watt, K.D.S.; Pedersen, R.A.; Kremers, W.K.; Heimbach, J.K.; Charlton, M.R. Evolution of Causes and Risk Factors for Mortality Post-Liver Transplant: Results of the NIDDK Long-Term Follow-up Study. Am. J. Transplant. 2010, 10, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Emmerich, S.D.; Fryar, C.D.; Stierman, B.; Ogden, C.L. Obesity and Severe Obesity Prevalence in Adults: United States, August 2021–August 2023. Available online: https://www.cdc.gov/nchs/products/databriefs/db508.htm (accessed on 16 December 2024).
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Kwong, A.J.; Kim, W.R.; Lake, J.R.; Schladt, D.P.; Schnellinger, E.M.; Gauntt, K.; McDermott, M.; Weiss, S.; Handarova, D.K.; Snyder, J.J.; et al. OPTN/SRTR 2022 Annual Data Report: Liver. Am. J. Transplant. 2024, 24, S176–S265. [Google Scholar] [CrossRef]
- Kwong, A.J.; Ebel, N.H.; Kim, W.R.; Lake, J.R.; Smith, J.M.; Schladt, D.P.; Schnellinger, E.M.; Handarova, D.; Weiss, S.; Cafarella, M.; et al. OPTN/SRTR 2021 Annual Data Report: Liver. Available online: https://srtr.transplant.hrsa.gov/ADR/Chapter?name=Liver&year=2021 (accessed on 22 January 2024).
- Simon, T.G.; Roelstraete, B.; Khalili, H.; Hagström, H.; Ludvigsson, J.F. Mortality in Biopsy-Confirmed Nonalcoholic Fatty Liver Disease: Results from a Nationwide Cohort. Gut 2021, 70, 1375–1382. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P. The NAFLD Fibrosis Score: A Noninvasive System That Identifies Liver Fibrosis in Patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Calzadilla-Bertot, L.; Wai-Sun Wong, V.; Castellanos, M.; Aller-de la Fuente, R.; Metwally, M.; Eslam, M.; Gonzalez-Fabian, L.; Alvarez-Quiñones Sanz, M.; Conde-Martin, A.F.; et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients with Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology 2018, 155, 443–457.e17. [Google Scholar] [CrossRef]
- Haflidadottir, S.; Jonasson, J.G.; Norland, H.; Einarsdottir, S.O.; Kleiner, D.E.; Lund, S.H.; Björnsson, E.S. Long-Term Follow-up and Liver-Related Death Rate in Patients with Non-Alcoholic and Alcoholic Related Fatty Liver Disease. BMC Gastroenterol. 2014, 14, 166. [Google Scholar] [CrossRef]
- Nasr, P.; Ignatova, S.; Kechagias, S.; Ekstedt, M. Natural History of Nonalcoholic Fatty Liver Disease: A Prospective Follow-up Study with Serial Biopsies. Hepatol. Commun. 2018, 2, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Hagström, H.; Nasr, P.; Ekstedt, M.; Hammar, U.; Stål, P.; Hultcrantz, R.; Kechagias, S. Fibrosis Stage but Not NASH Predicts Mortality and Time to Development of Severe Liver Disease in Biopsy-Proven NAFLD. J. Hepatol. 2017, 67, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. Clinical Practice Guideline Panel: Chair; Samuel, D.; Secretary to the Chair; EASL Governing Board Representative; Panel members EASL Clinical Practice Guidelines on Liver Transplantation. J. Hepatol. 2024, 81, 1040–1086. [Google Scholar] [CrossRef]
- Knechtle, S.J.; Kwun, J. Unique Aspects of Rejection and Tolerance in Liver Transplantation. Semin. Liver Dis. 2009, 29, 91–101. [Google Scholar] [CrossRef]
- Martin, P.; DiMartini, A.; Feng, S.; Brown, R.J.; Fallon, M. Evaluation for Liver Transplantation in Adults: 2013 Practice Guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 2014, 59, 1144. [Google Scholar] [CrossRef]
- Panackel, C.; Mathew, J.F.; Fawas, N.M.; Jacob, M. Immunosuppressive Drugs in Liver Transplant: An Insight. J. Clin. Exp. Hepatol. 2022, 12, 1557–1571. [Google Scholar] [CrossRef]
- Liang, W.; Wang, D.; Ling, X.; Kao, A.A.; Kong, Y.; Shang, Y.; Guo, Z.; He, X. Sirolimus-Based Immunosuppression in Liver Transplantation for Hepatocellular Carcinoma: A Meta-Analysis. Liver Transpl. 2012, 18, 62–69. [Google Scholar] [CrossRef]
- Menon, K.V.; Hakeem, A.R.; Heaton, N.D. Meta-Analysis: Recurrence and Survival Following the Use of Sirolimus in Liver Transplantation for Hepatocellular Carcinoma. Aliment. Pharmacol. Ther. 2013, 37, 411–419. [Google Scholar] [CrossRef]
- Cholongitas, E.; Mamou, C.; Rodríguez-Castro, K.I.; Burra, P. Mammalian Target of Rapamycin Inhibitors Are Associated with Lower Rates of Hepatocellular Carcinoma Recurrence after Liver Transplantation: A Systematic Review. Transpl. Int. 2014, 27, 1039–1049. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Li, L.X.; Li, P.; Lv, S.-C.; Pan, B.; He, Q. Sirolimus in Liver Transplant Recipients with Hepatocellular Carcinoma: An Updated Meta-Analysis. J. Investig. Surg. 2019, 32, 632–641. [Google Scholar] [CrossRef]
- Grigg, S.E.; Sarri, G.L.; Gow, P.J.; Yeomans, N.D. Systematic Review with Meta-Analysis: Sirolimus- or Everolimus-Based Immunosuppression Following Liver Transplantation for Hepatocellular Carcinoma. Aliment. Pharmacol. Ther. 2019, 49, 1260–1273. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, R.H.; Fung, J.J. Present State of Immunosuppressive Therapy in Liver Transplant Recipients. Liver Transpl. 2011, 17 (Suppl. S3), S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Azzi, J.R.; Sayegh, M.H.; Mallat, S.G. Calcineurin Inhibitors: 40 Years Later, Can’t Live Without. J. Immunol. 2013, 191, 5785–5791. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, J.; Yuan, J.; Dang, Y.; Yang, C.; Chen, X.; Xu, J.; Yu, L. Characterization of a Human Regulatory Subunit of Protein Phosphatase 3 Gene (PPP3RL) Expressed Specifically in Testis. Mol. Biol. Rep. 2005, 32, 41–45. [Google Scholar] [CrossRef]
- Hurwitz, M.Y.; Putkey, J.A.; Klee, C.B.; Means, A.R. Domain II of Calmodulin Is Involved in Activation of Calcineurin. FEBS Lett. 1988, 238, 82–86. [Google Scholar] [CrossRef]
- Rao, A.; Luo, C.; Hogan, P.G. Transcription Factors of the NFAT Family: Regulation and Function. Annu. Rev. Immunol. 1997, 15, 707–747. [Google Scholar] [CrossRef]
- Matas, A.J. Calcineurin Inhibitors: Short-Term Friend, Long-Term Foe? Clin. Pharmacol. Ther. 2011, 90, 209–211. [Google Scholar] [CrossRef]
- Buttgereit, F.; da Silva, J.A.P.; Boers, M.; Burmester, G.-R.; Cutolo, M.; Jacobs, J.; Kirwan, J.; Köhler, L.; Van Riel, P.; Vischer, T.; et al. Standardised Nomenclature for Glucocorticoid Dosages and Glucocorticoid Treatment Regimens: Current Questions and Tentative Answers in Rheumatology. Ann. Rheum. Dis. 2002, 61, 718–722. [Google Scholar] [CrossRef]
- Meneghini, M.; Bestard, O.; Grinyo, J.M. Immunosuppressive Drugs Modes of Action. Best Pract. Res. Clin. Gastroenterol. 2021, 54–55, 101757. [Google Scholar] [CrossRef]
- Strehl, C.; Ehlers, L.; Gaber, T.; Buttgereit, F. Glucocorticoids-All-Rounders Tackling the Versatile Players of the Immune System. Front. Immunol. 2019, 10, 1744. [Google Scholar] [CrossRef]
- Necela, B.M.; Cidlowski, J.A. Mechanisms of Glucocorticoid Receptor Action in Noninflammatory and Inflammatory Cells. Proc. Am. Thorac. Soc. 2004, 1, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Stahn, C.; Buttgereit, F. Genomic and Nongenomic Effects of Glucocorticoids. Nat. Clin. Pract. Rheumatol. 2008, 4, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Stahn, C.; Löwenberg, M.; Hommes, D.W.; Buttgereit, F. Molecular Mechanisms of Glucocorticoid Action and Selective Glucocorticoid Receptor Agonists. Mol. Cell Endocrinol. 2007, 275, 71–78. [Google Scholar] [CrossRef]
- Wiesner, R.; Rabkin, J.; Klintmalm, G.; McDiarmid, S.; Langnas, A.; Punch, J.; McMaster, P.; Kalayoglu, M.; Levy, G.; Freeman, R.; et al. A Randomized Double-Blind Comparative Study of Mycophenolate Mofetil and Azathioprine in Combination with Cyclosporine and Corticosteroids in Primary Liver Transplant Recipients. Liver Transpl. 2001, 7, 442–450. [Google Scholar] [CrossRef]
- Sterneck, M.; Fischer, L.; Gahlemann, C.; Gundlach, M.; Rogiers, X.; Broelsch, C. Mycophenolate Mofetil for Prevention of Liver Allograft Rejection: Initial Results of a Controlled Clinical Trial. Ann. Transplant. 2000, 5, 43–46. [Google Scholar]
- Fischer, L.; Sterneck, M.; Gahlemann, C.G.; Malago, M.; Rogiers, X.; Broelsch, C.E. A Prospective Study Comparing Safety and Efficacy of Mycophenolate Mofetil versus Azathioprine in Primary Liver Transplant Recipients. Transplant. Proc. 2000, 32, 2125–2127. [Google Scholar] [CrossRef]
- Elion, G.B. The Purine Path to Chemotherapy. Science 1989, 244, 41–47. [Google Scholar] [CrossRef]
- Broen, J.C.A.; van Laar, J.M. Mycophenolate Mofetil, Azathioprine and Tacrolimus: Mechanisms in Rheumatology. Nat. Rev. Rheumatol. 2020, 16, 167–178. [Google Scholar] [CrossRef]
- Van Scoik, K.G.; Johnson, C.A.; Porter, W.R. The Pharmacology and Metabolism of the Thiopurine Drugs 6-Mercaptopurine and Azathioprine. Drug Metab. Rev. 1985, 16, 157–174. [Google Scholar] [CrossRef]
- Van Os, E.C.; Zins, B.J.; Sandborn, W.J.; Mays, D.C.; Tremaine, W.J.; Mahoney, D.W.; Zinsmeister, A.R.; Lipsky, J.J. Azathioprine Pharmacokinetics after Intravenous, Oral, Delayed Release Oral and Rectal Foam Administration. Gut 1996, 39, 63–68. [Google Scholar] [CrossRef]
- Stolk, J.N.; Boerbooms, A.M.; de Abreu, R.A.; de Koning, D.G.; van Beusekom, H.J.; Muller, W.H.; van de Putte, L.B. Reduced Thiopurine Methyltransferase Activity and Development of Side Effects of Azathioprine Treatment in Patients with Rheumatoid Arthritis. Arthritis Rheum. 1998, 41, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Maltzman, J.S.; Koretzky, G.A. Azathioprine: Old Drug, New Actions. J. Clin. Investig. 2003, 111, 1122–1124. [Google Scholar] [CrossRef] [PubMed]
- Alsberg, C.; Black, O.F.; United States. Bureau of Plant Industry. Contributions to the Study of Maize Deterioration: Biochemical and Toxicological Investigations of Penicillium Puberulum and Penicillium Stoloniferum; US Government Printing Office: Washington, DC, USA, 1913.
- Lipsky, J.J. Mycophenolate Mofetil. Lancet 1996, 348, 1357–1359. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.C.; Eugui, E.M. Immunosuppressive and Long-Acting Anti-Inflammatory Activity of Mycophenolic Acid and Derivative, RS-61443. Br. J. Rheumatol. 1991, 30 (Suppl. S2), 57–61. [Google Scholar]
- Bullingham, R.E.; Nicholls, A.J.; Kamm, B.R. Clinical Pharmacokinetics of Mycophenolate Mofetil. Clin. Pharmacokinet. 1998, 34, 429–455. [Google Scholar] [CrossRef]
- Ransom, J.T. Mechanism of Action of Mycophenolate Mofetil. Ther. Drug Monit. 1995, 17, 681–684. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, Q.; Zhao, M.; Liang, G.; Wu, H.; Li, D.; Xie, Y.; Tan, Y.; Dai, Y.; Yung, S.; et al. The Effect of Mycophenolic Acid on Epigenetic Modifications in Lupus CD4+T Cells. Clin. Immunol. 2015, 158, 67–76. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, T.; Chen, M.; Zhou, Y.; Yi, D.; Guo, R. The CD40/CD40L System: A New Therapeutic Target for Disease. Immunol. Lett. 2013, 153, 58–61. [Google Scholar] [CrossRef]
- Morales-Cárdenas, A.; Pérez-Madrid, C.; Arias, L.; Ojeda, P.; Mahecha, M.P.; Rojas-Villarraga, A.; Carrillo-Bayona, J.A.; Anaya, J.-M. Pulmonary Involvement in Systemic Sclerosis. Autoimmun. Rev. 2016, 15, 1094–1108. [Google Scholar] [CrossRef]
- Morath, C.; Reuter, H.; Simon, V.; Krautkramer, E.; Muranyi, W.; Schwenger, V.; Goulimari, P.; Grosse, R.; Hahn, M.; Lichter, P.; et al. Effects of Mycophenolic Acid on Human Fibroblast Proliferation, Migration and Adhesion in Vitro and in Vivo. Am. J. Transplant. 2008, 8, 1786–1797. [Google Scholar] [CrossRef]
- Tasdogan, B.E.; Ma, M.; Simsek, C.; Saberi, B.; Gurakar, A. Update on Immunosuppression in Liver Transplantation. Euroasian J. Hepatogastroenterol. 2019, 9, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR Signaling in Growth Control and Disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Zaza, G.; Granata, S.; Caletti, C.; Signorini, L.; Stallone, G.; Lupo, A. mTOR Inhibition Role in Cellular Mechanisms. Transplantation 2018, 102, S3–S16. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Rudge, D.G.; Koos, J.D.; Vaidialingam, B.; Yang, H.J.; Pavletich, N.P. mTOR Kinase Structure, Mechanism and Regulation. Nature 2013, 497, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Sarbassov, D.D.; Ali, S.M.; Sengupta, S.; Sheen, J.-H.; Hsu, P.P.; Bagley, A.F.; Markhard, A.L.; Sabatini, D.M. Prolonged Rapamycin Treatment Inhibits mTORC2 Assembly and Akt/PKB. Mol. Cell 2006, 22, 159–168. [Google Scholar] [CrossRef]
- Sinclair, M.; Poltavskiy, E.; Dodge, J.L.; Lai, J.C. Frailty Is Independently Associated with Increased Hospitalisation Days in Patients on the Liver Transplant Waitlist. World J. Gastroenterol. 2017, 23, 899–905. [Google Scholar] [CrossRef]
- Thomson, A.W.; Turnquist, H.R.; Raimondi, G. Immunoregulatory Functions of mTOR Inhibition. Nat. Rev. Immunol. 2009, 9, 324–337. [Google Scholar] [CrossRef]
- Wing, K.; Sakaguchi, S. Regulatory T Cells Exert Checks and Balances on Self Tolerance and Autoimmunity. Nat. Immunol. 2010, 11, 7–13. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, S.; Xie, Q.; Wu, S.; Su, J.; Li, S.; Xu, Y.; Li, X.C. Natural CD8+CD122+ T Cells Are More Potent in Suppression of Allograft Rejection than CD4+CD25+ Regulatory T Cells. Am. J. Transplant. 2014, 14, 39–48. [Google Scholar] [CrossRef]
- Battaglia, M.; Stabilini, A.; Roncarolo, M.-G. Rapamycin Selectively Expands CD4+CD25+FoxP3+ Regulatory T Cells. Blood 2005, 105, 4743–4748. [Google Scholar] [CrossRef]
- Kopf, H.; de la Rosa, G.M.; Howard, O.M.Z.; Chen, X. Rapamycin Inhibits Differentiation of Th17 Cells and Promotes Generation of FoxP3+ T Regulatory Cells. Int. Immunopharmacol. 2007, 7, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Coenen, J.J.A.; Koenen, H.J.P.M.; van Rijssen, E.; Kasran, A.; Boon, L.; Hilbrands, L.B.; Joosten, I. Rapamycin, Not Cyclosporine, Permits Thymic Generation and Peripheral Preservation of CD4+ CD25+ FoxP3+ T Cells. Bone Marrow Transplant. 2007, 39, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Lu, Y.; El Essawy, B.; Oukka, M.; Kuchroo, V.K.; Strom, T.B. Contrasting Effects of Cyclosporine and Rapamycin in de Novo Generation of Alloantigen-Specific Regulatory T Cells. Am. J. Transplant. 2007, 7, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Kapsenberg, M.L. Dendritic-Cell Control of Pathogen-Driven T-Cell Polarization. Nat. Rev. Immunol. 2003, 3, 984–993. [Google Scholar] [CrossRef]
- Taner, T.; Hackstein, H.; Wang, Z.; Morelli, A.E.; Thomson, A.W. Rapamycin-Treated, Alloantigen-Pulsed Host Dendritic Cells Induce Ag-Specific T Cell Regulation and Prolong Graft Survival. Am. J. Transplant. 2005, 5, 228–236. [Google Scholar] [CrossRef]
- Turnquist, H.R.; Raimondi, G.; Zahorchak, A.F.; Fischer, R.T.; Wang, Z.; Thomson, A.W. Rapamycin-Conditioned Dendritic Cells Are Poor Stimulators of Allogeneic CD4+ T Cells, but Enrich for Antigen-Specific Foxp3+ T Regulatory Cells and Promote Organ Transplant Tolerance. J. Immunol. 2007, 178, 7018–7031. [Google Scholar] [CrossRef]
- Hackstein, H.; Taner, T.; Zahorchak, A.F.; Morelli, A.E.; Logar, A.J.; Gessner, A.; Thomson, A.W. Rapamycin Inhibits IL-4--Induced Dendritic Cell Maturation in Vitro and Dendritic Cell Mobilization and Function in Vivo. Blood 2003, 101, 4457–4463. [Google Scholar] [CrossRef]
- Waskow, C.; Liu, K.; Darrasse-Jèze, G.; Guermonprez, P.; Ginhoux, F.; Merad, M.; Shengelia, T.; Yao, K.; Nussenzweig, M. The Receptor Tyrosine Kinase Flt3 Is Required for Dendritic Cell Development in Peripheral Lymphoid Tissues. Nat. Immunol. 2008, 9, 676–683. [Google Scholar] [CrossRef]
- Woltman, A.M.; de Fijter, J.W.; Kamerling, S.W.; van Der Kooij, S.W.; Paul, L.C.; Daha, M.R.; van Kooten, C. Rapamycin Induces Apoptosis in Monocyte- and CD34-Derived Dendritic Cells but Not in Monocytes and Macrophages. Blood 2001, 98, 174–180. [Google Scholar] [CrossRef]
- Monti, P.; Mercalli, A.; Leone, B.E.; Valerio, D.C.; Allavena, P.; Piemonti, L. Rapamycin Impairs Antigen Uptake of Human Dendritic Cells. Transplantation 2003, 75, 137–145. [Google Scholar] [CrossRef]
- Sakata, A.; Kuwahara, K.; Ohmura, T.; Inui, S.; Sakaguchi, N. Involvement of a Rapamycin-Sensitive Pathway in CD40-Mediated Activation of Murine B Cells in Vitro. Immunol. Lett. 1999, 68, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Aagaard-Tillery, K.M.; Jelinek, D.F. Inhibition of Human B Lymphocyte Cell Cycle Progression and Differentiation by Rapamycin. Cell Immunol. 1994, 156, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Heidt, S.; Roelen, D.L.; Eijsink, C.; van Kooten, C.; Claas, F.H.J.; Mulder, A. Effects of Immunosuppressive Drugs on Purified Human B Cells: Evidence Supporting the Use of MMF and Rapamycin. Transplantation 2008, 86, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.A.; Ruscetti, F.W.; Gallo, R. Selective in Vitro Growth of T Lymphocytes from Normal Human Bone Marrows. Science 1976, 193, 1007–1008. [Google Scholar] [CrossRef]
- Robb, R.J.; Greene, W.C.; Rusk, C.M. Low and High Affinity Cellular Receptors for Interleukin 2. Implications for the Level of Tac Antigen. J. Exp. Med. 1984, 160, 1126–1146. [Google Scholar] [CrossRef]
- Feldmann, M.; Basten, A. Cell Interactions in the Immune Response in Vitro. I. Metabolic Activities of T Cells in a Collaborative Antibody Response. Eur. J. Immunol. 1972, 2, 213–224. [Google Scholar] [CrossRef]
- Simulect|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/simulect (accessed on 15 December 2024).
- Emoto, C.; Vinks, A.A.; Fukuda, T. Risk Assessment of Drug-Drug Interactions of Calcineurin Inhibitors Affecting Sirolimus Pharmacokinetics in Renal Transplant Patients. Ther. Drug Monit. 2016, 38, 607–613. [Google Scholar] [CrossRef]
- Jasiak, N.M.; Park, J.M. Immunosuppression in Solid-Organ Transplantation: Essentials and Practical Tips. Crit. Care Nurs. Q. 2016, 39, 227–240. [Google Scholar] [CrossRef]
- Van Matre, E.T.; Satyanarayana, G.; Page, R.L., II; Levi, M.E.; Lindenfeld, J.; Mueller, S.W. Pharmacokinetic Drug-Drug Interactions Between Immunosuppressant and Anti-Infective Agents: Antimetabolites and Corticosteroids. Ann. Transplant. 2018, 23, 66–74. [Google Scholar] [CrossRef]
- Zhou, J.; Hu, Z.; Zhang, Q.; Li, Z.; Xiang, J.; Yan, S.; Wu, J.; Zhang, M.; Zheng, S. Spectrum of De Novo Cancers and Predictors in Liver Transplantation: Analysis of the Scientific Registry of Transplant Recipients Database. PLoS ONE 2016, 11, e0155179. [Google Scholar] [CrossRef]
- Engels, E.A.; Pfeiffer, R.M.; Fraumeni, J.F.; Kasiske, B.L.; Israni, A.K.; Snyder, J.J.; Wolfe, R.A.; Goodrich, N.P.; Bayakly, A.R.; Clarke, C.A.; et al. Spectrum of Cancer Risk among US Solid Organ Transplant Recipients. JAMA 2011, 306, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Manzia, T.M.; Angelico, R.; Gazia, C.; Lenci, I.; Milana, M.; Ademoyero, O.T.; Pedini, D.; Toti, L.; Spada, M.; Tisone, G.; et al. De Novo Malignancies after Liver Transplantation: The Effect of Immunosuppression-Personal Data and Review of Literature. World J. Gastroenterol. 2019, 25, 5356–5375. [Google Scholar] [CrossRef] [PubMed]
- Burra, P.; Rodriguez-Castro, K.I. Neoplastic Disease after Liver Transplantation: Focus on de Novo Neoplasms. World J. Gastroenterol. 2015, 21, 8753–8768. [Google Scholar] [CrossRef] [PubMed]
- Chandok, N.; Watt, K.D. Burden of de Novo Malignancy in the Liver Transplant Recipient. Liver Transpl. 2012, 18, 1277–1289. [Google Scholar] [CrossRef]
- Carenco, C.; Faure, S.; Ursic-Bedoya, J.; Herrero, A.; Pageaux, G.P. Solid, Non-Skin, Post-Liver Transplant Tumors: Key Role of Lifestyle and Immunosuppression Management. World J. Gastroenterol. 2016, 22, 427–434. [Google Scholar] [CrossRef]
- Dierickx, D.; Cardinaels, N. Posttransplant Lymphoproliferative Disorders Following Liver Transplantation: Where Are We Now? World J. Gastroenterol. 2015, 21, 11034–11043. [Google Scholar] [CrossRef]
- Tanner, J.E.; Alfieri, C. The Epstein-Barr Virus and Post-Transplant Lymphoproliferative Disease: Interplay of Immunosuppression, EBV, and the Immune System in Disease Pathogenesis. Transpl. Infect. Dis. 2001, 3, 60–69. [Google Scholar] [CrossRef]
- Rodríguez-Perálvarez, M.; Colmenero, J.; González, A.; Gastaca, M.; Curell, A.; Caballero-Marcos, A.; Sánchez-Martínez, A.; Di Maira, T.; Herrero, J.I.; Almohalla, C.; et al. Cumulative Exposure to Tacrolimus and Incidence of Cancer after Liver Transplantation. Am. J. Transplant. 2022, 22, 1671–1682. [Google Scholar] [CrossRef]
- De Simone, P.; Precisi, A.; Lai, Q.; Ducci, J.; Campani, D.; Marchetti, P.; Gitto, S. Everolimus Mitigates the Risk of Hepatocellular Carcinoma Recurrence after Liver Transplantation. Cancers 2024, 16, 1243. [Google Scholar] [CrossRef]
- Shbaklo, N.; Tandoi, F.; Lupia, T.; Corcione, S.; Romagnoli, R.; De Rosa, F.G. Bacterial and Viral Infections in Liver Transplantation: New Insights from Clinical and Surgical Perspectives. Biomedicines 2022, 10, 1561. [Google Scholar] [CrossRef]
- Fishman, J.A. Infections in Immunocompromised Hosts and Organ Transplant Recipients: Essentials. Liver Transpl. 2011, 17 (Suppl. S3), S34–S37. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.D.P.; Martin, P.; Simkins, J. Infectious Complications After Liver Transplantation. Gastroenterol. Hepatol. 2015, 11, 741–753. [Google Scholar]
- Melenotte, C.; Aimanianda, V.; Slavin, M.; Aguado, J.M.; Armstrong-James, D.; Chen, Y.-C.; Husain, S.; Van Delden, C.; Saliba, F.; Lefort, A.; et al. Invasive Aspergillosis in Liver Transplant Recipients. Transpl. Infect. Dis. 2023, 25, e14049. [Google Scholar] [CrossRef] [PubMed]
- Caner, A.; Döşkaya, M.; Karasu, Z.; Değirmenci, A.; Guy, E.; Kiliç, M.; Zeytunlu, M.; Francis, J.; Bozoklar, A.; Gürüz, Y. Incidence and Diagnosis of Active Toxoplasma Infection among Liver Transplant Recipients in Western Turkey. Liver Transpl. 2008, 14, 1526–1532. [Google Scholar] [CrossRef]
- Choi, Y.-I.; Hwang, S.; Park, G.-C.; Namgoong, J.-M.; Jung, D.-H.; Song, G.-W.; Ha, T.-Y.; Moon, D.-B.; Kim, K.-H.; Ahn, C.-S.; et al. Clinical Outcomes of Pneumocystis Carinii Pneumonia in Adult Liver Transplant Recipients. Transplant. Proc. 2013, 45, 3057–3060. [Google Scholar] [CrossRef]
- Lampertico, P.; Agarwal, K.; Berg, T.; Buti, M.; Janssen, H.L.; Papatheodoridis, G.; Zoulim, F.; Tacke, F. European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the Management of Hepatitis B Virus Infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef]
- Collaborators, G.B.D. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990-2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Gabrielli, F.; Alberti, F.; Russo, C.; Cursaro, C.; Seferi, H.; Margotti, M.; Andreone, P. Treatment Options for Hepatitis A and E: A Non-Systematic Review. Viruses 2023, 15, 1080. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. European Association for the Study of the Liver EASL Clinical Practice Guidelines on Hepatitis E Virus Infection. J. Hepatol. 2018, 68, 1256–1271. [Google Scholar] [CrossRef]
- Busch, C.J.; Siegler, B.H.; Werle, H.; Lichtenstern, C.; Bruckner, T.; Heininger, A.; Mehrabi, A.; Weiss, K.H.; Weigand, M.A.; Hochreiter, M. Risk Factors for Early Viral Infections after Liver Transplantation. Langenbecks Arch. Surg. 2018, 403, 509–519. [Google Scholar] [CrossRef]
- Lizaola-Mayo, B.C.; Rodriguez, E.A. Cytomegalovirus Infection after Liver Transplantation. World J. Transplant. 2020, 10, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, Y.; Mori, A.; Uemura, T.; Ogawa, K.; Fujimoto, Y.; Okajima, H.; Kaido, T.; Uemoto, S. Incidence and Risk Factors for Herpes Zoster in Patients Undergoing Liver Transplantation. Transpl. Infect. Dis. 2015, 17, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Herrero, J.I.; Quiroga, J.; Sangro, B.; Pardo, F.; Rotellar, F.; Alvarez-Cienfuegos, J.; Prieto, J. Herpes Zoster after Liver Transplantation: Incidence, Risk Factors, and Complications. Liver Transpl. 2004, 10, 1140–1143. [Google Scholar] [CrossRef] [PubMed]
- Checkley, W.; White, A.C.; Jaganath, D.; Arrowood, M.J.; Chalmers, R.M.; Chen, X.-M.; Fayer, R.; Griffiths, J.K.; Guerrant, R.L.; Hedstrom, L.; et al. A Review of the Global Burden, Novel Diagnostics, Therapeutics, and Vaccine Targets for Cryptosporidium. Lancet Infect. Dis. 2015, 15, 85–94. [Google Scholar] [CrossRef]
- Petry, F.; Jakobi, V.; Tessema, T.S. Host Immune Response to Cryptosporidium Parvum Infection. Exp. Parasitol. 2010, 126, 304–309. [Google Scholar] [CrossRef]
- Lanternier, F.; Amazzough, K.; Favennec, L.; Mamzer-Bruneel, M.-F.; Abdoul, H.; Tourret, J.; Decramer, S.; Zuber, J.; Scemla, A.; Legendre, C.; et al. Cryptosporidium Spp. Infection in Solid Organ Transplantation: The Nationwide “TRANSCRYPTO” Study. Transplantation 2017, 101, 826–830. [Google Scholar] [CrossRef]
- Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Korzeniewski, K.; Mularczyk, M.; Kabat-Koperska, J.; Ziętek, P.; Marchelek-Myśliwiec, M. Cryptosporidium spp. Infection in Adult Kidney Transplant Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 6395. [Google Scholar] [CrossRef]
- Yehia, B.R.; Blumberg, E.A. Mycobacterium Tuberculosis Infection in Liver Transplantation. Liver Transpl. 2010, 16, 1129–1135. [Google Scholar] [CrossRef]
- Abad, C.L.R.; Razonable, R.R. Mycobacterium Tuberculosis after Solid Organ Transplantation: A Review of More than 2000 Cases. Clin. Transpl. 2018, 32, e13259. [Google Scholar] [CrossRef]
- Subramanian, A.K.; Theodoropoulos, N.M. Infectious Diseases Community of Practice of the American Society of Transplantation Mycobacterium Tuberculosis Infections in Solid Organ Transplantation: Guidelines from the Infectious Diseases Community of Practice of the American Society of Transplantation. Clin. Transpl. 2019, 33, e13513. [Google Scholar] [CrossRef]
- Holty, J.-E.C.; Gould, M.K.; Meinke, L.; Keeffe, E.B.; Ruoss, S.J. Tuberculosis in Liver Transplant Recipients: A Systematic Review and Meta-Analysis of Individual Patient Data. Liver Transpl. 2009, 15, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Latent Tuberculosis Infection: Updated and Consolidated Guidelines for Programmatic Management. Available online: https://www.who.int/publications/i/item/9789241550239 (accessed on 17 February 2025).
- Lee, Y.W.; Chung, H.; Kim, S.-H.; Sung, H.; Ha, S.-M.; Jwa, E.-K.; Jung, D.-H.; Moon, D.-B.; Lee, S.-G.; Lee, S.-O. Safety and Outcome of Treatment of Latent Tuberculosis Infection in Liver Transplant Recipients. Infection 2024, 52, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Pérez, M.; González-Grande, R.; Omonte Guzmán, E.; Amo Trillo, V.; Rodrigo López, J.M. Metabolic Complications in Liver Transplant Recipients. World J. Gastroenterol. 2016, 22, 6416–6423. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M.; Rinella, M.; Patel, D.; McCague, K.; Heimbach, J.; Watt, K. Everolimus Is Associated with Less Weight Gain Than Tacrolimus 2 Years After Liver Transplantation: Results of a Randomized Multicenter Study. Transplantation 2017, 101, 2873–2882. [Google Scholar] [CrossRef]
- Watt, K.D. Metabolic Syndrome: Is Immunosuppression to Blame? Liver Transpl. 2011, 17 (Suppl. S3), S38–S42. [Google Scholar] [CrossRef]
- Burra, P.; Becchetti, C.; Germani, G. NAFLD and Liver Transplantation: Disease Burden, Current Management and Future Challenges. JHEP Rep. 2020, 2, 100192. [Google Scholar] [CrossRef]
- Gojowy, D.; Adamczak, M.; Dudzicz, S.; Gazda, M.; Karkoszka, H.; Wiecek, A. High Frequency of Arterial Hypertension in Patients After Liver Transplantation. Transplant. Proc. 2016, 48, 1721–1724. [Google Scholar] [CrossRef]
- Gonwa, T.; Mendez, R.; Yang, H.C.; Weinstein, S.; Jensik, S.; Steinberg, S. Prograf Study Group Randomized Trial of Tacrolimus in Combination with Sirolimus or Mycophenolate Mofetil in Kidney Transplantation: Results at 6 Months. Transplantation 2003, 75, 1213–1220. [Google Scholar] [CrossRef]
- Trotter, J.F. Sirolimus in Liver Transplantation. Transplant. Proc. 2003, 35, 193S–200S. [Google Scholar] [CrossRef]
- McKenna, G.J.; Trotter, J.F.; Klintmalm, E.; Ruiz, R.; Onaca, N.; Testa, G.; Saracino, G.; Levy, M.F.; Goldstein, R.M.; Klintmalm, G.B. Sirolimus and Cardiovascular Disease Risk in Liver Transplantation. Transplantation 2013, 95, 215–221. [Google Scholar] [CrossRef]
- Holdaas, H.; Potena, L.; Saliba, F. mTOR Inhibitors and Dyslipidemia in Transplant Recipients: A Cause for Concern? Transplant. Rev. 2015, 29, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Trotter, J.F.; Wachs, M.E.; Trouillot, T.E.; Bak, T.; Kugelmas, M.; Kam, I.; Everson, G. Dyslipidemia during Sirolimus Therapy in Liver Transplant Recipients Occurs with Concomitant Cyclosporine but Not Tacrolimus. Liver Transpl. 2001, 7, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Lawendy, B.; Srinathan, S.; Kotha, S.; Gomes, C.; Misra, S.; Yu, J.; Orchanian-Cheff, A.; Tomlinson, G.; Bhat, M. Systematic Review and Meta-Analysis of Post-Transplant Diabetes Mellitus in Liver Transplant Recipients. Clin. Transplant. 2021, 35, e14340. [Google Scholar] [CrossRef] [PubMed]
- Houde, V.P.; Brûlé, S.; Festuccia, W.T.; Blanchard, P.-G.; Bellmann, K.; Deshaies, Y.; Marette, A. Chronic Rapamycin Treatment Causes Glucose Intolerance and Hyperlipidemia by Upregulating Hepatic Gluconeogenesis and Impairing Lipid Deposition in Adipose Tissue. Diabetes 2010, 59, 1338–1348. [Google Scholar] [CrossRef]
- Øzbay, L.A.; Smidt, K.; Mortensen, D.M.; Carstens, J.; Jørgensen, K.A.; Rungby, J. Cyclosporin and Tacrolimus Impair Insulin Secretion and Transcriptional Regulation in INS-1E Beta-Cells. Br. J. Pharmacol. 2011, 162, 136–146. [Google Scholar] [CrossRef]
- Haddad, E.M.; McAlister, V.C.; Renouf, E.; Malthaner, R.; Kjaer, M.S.; Gluud, L.L. Cyclosporin versus Tacrolimus for Liver Transplanted Patients. Cochrane Database Syst. Rev. 2006, 2006, CD005161. [Google Scholar] [CrossRef]
- Taneja, S.; Roy, A.; Duseja, A. NASH After Liver Transplantation: Impact of Immunosuppression. J. Clin. Exp. Hepatol. 2023, 13, 835–840. [Google Scholar] [CrossRef]
- Weber, M.L.; Ibrahim, H.N.; Lake, J.R. Renal Dysfunction in Liver Transplant Recipients: Evaluation of the Critical Issues. Liver Transplant. 2012, 18, 1290–1301. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.; Wang, W.; Lv, J. Risk Factors for New-Onset Chronic Kidney Disease in Patients Who Have Received a Liver Transplant. Exp. Ther. Med. 2018, 15, 3589–3595. [Google Scholar] [CrossRef]
- Álamo, J.M.; Olivares, C.; Barrera, L.; Marín, L.M.; Suarez, G.; Bernal, C.; Serrano, J.; Muntané, J.; Padillo, F.J.; Gómez, M.A. Conversion from Calcineurin Inhibitors to mTOR Inhibitors Stabilizes Diabetic and Hypertensive Nephropathy after Liver Transplant. World J. Transplant. 2015, 5, 19–25. [Google Scholar] [CrossRef][Green Version]
- Lin, M.; Mittal, S.; Sahebjam, F.; Rana, A.; Sood, G.K. Everolimus with Early Withdrawal or Reduced-Dose Calcineurin Inhibitors Improves Renal Function in Liver Transplant Recipients: A Systematic Review and Meta-Analysis. Clin. Transplant. 2017, 31, e12872. [Google Scholar] [CrossRef] [PubMed]
- Teperman, L.; Moonka, D.; Sebastian, A.; Sher, L.; Marotta, P.; Marsh, C.; Koneru, B.; Goss, J.; Preston, D.; Roberts, J.P.; et al. Calcineurin Inhibitor-Free Mycophenolate Mofetil/Sirolimus Maintenance in Liver Transplantation: The Randomized Spare-the-Nephron Trial. Liver Transplant. 2013, 19, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Haywood, S.; Abecassis, M.; Levitsky, J. The Renal Benefit of Mycophenolate Mofetil after Liver Transplantation. Clin. Transplant. 2011, 25, E88–E95. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Vekatramanan, R.; Eghtesad, B.; Gadomski, M.; Mohanka, R.; Marcos, A.; Fung, J. Long-Term Outcome of Adding Mycophenolate Mofetil to Tacrolimus for Nephrotoxicity Following Liver Transplantation. Transplantation 2005, 80, 859–864. [Google Scholar] [CrossRef]
- Koch, R.O.; Graziadei, I.W.; Schulz, F.; Nachbaur, K.; Königsrainer, A.; Margreiter, R.; Vogel, W. Long-Term Efficacy and Safety of Mycophenolate Mofetil in Liver Transplant Recipients with Calcineurin Inhibitor-Induced Renal Dysfunction. Transpl. Int. 2004, 17, 518–524. [Google Scholar] [CrossRef]
- Mouzaki, M.; Yap, J.; Avinashi, V.; Babu, A.; Fu, A.; Deangelis, M.; Van Roestel, K.; Ghanekar, A.; Kamath, B.; Avitzur, Y.; et al. Basiliximab with Delayed Introduction of Calcineurin Inhibitors as a Renal-Sparing Protocol Following Liver Transplantation in Children with Renal Impairment. Pediatr. Transplant. 2013, 17, 751–756. [Google Scholar] [CrossRef]
- Gibelli, N.E.M.; Pinho-Apezzato, M.L.; Miyatani, H.T.; Maksoud-Filho, J.G.; Silva, M.M.; Ayoub, A.A.R.; Santos, M.M.; Velhote, M.C.P.; Tannuri, U.; Maksoud, J.G. Basiliximab-Chimeric Anti-IL2-R Monoclonal Antibody in Pediatric Liver Transplantation: Comparative Study. Transplant. Proc. 2004, 36, 956–957. [Google Scholar] [CrossRef]
- Amodio, P.; Biancardi, A.; Montagnese, S.; Angeli, P.; Iannizzi, P.; Cillo, U.; D’Amico, D.; Gatta, A. Neurological Complications after Orthotopic Liver Transplantation. Dig. Liver Dis. 2007, 39, 740–747. [Google Scholar] [CrossRef]
- Zivković, S.A. Neurologic Complications after Liver Transplantation. World J. Hepatol. 2013, 5, 409–416. [Google Scholar] [CrossRef]
- Balderramo, D.; Prieto, J.; Cárdenas, A.; Navasa, M. Hepatic Encephalopathy and Post-Transplant Hyponatremia Predict Early Calcineurin Inhibitor-Induced Neurotoxicity after Liver Transplantation. Transpl. Int. 2011, 24, 812–819. [Google Scholar] [CrossRef]
- Forgacs, B.; Merhav, H.J.; Lappin, J.; Mieles, L. Successful Conversion to Rapamycin for Calcineurin Inhibitor-Related Neurotoxicity Following Liver Transplantation. Transplant. Proc. 2005, 37, 1912–1914. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, M.; Dazzi, A.; Cucchetti, A.; Gasbarrini, A.; Zanello, M.; Di Gioia, P.; Bianchi, G.; Tamè, M.R.; Gaudio, M.D.; Ravaioli, M.; et al. Sirolimus in Liver Transplant Recipients: A Large Single-Center Experience. Transplant. Proc. 2010, 42, 2579–2584. [Google Scholar] [CrossRef] [PubMed]
- Bilbao, I.; Dopazo, C.; Castells, L.; Lazaro, J.; Caralt, M.; Sapisochin, G.; Charco, R. Immunosuppression Based on Everolimus in Liver Transplant Recipients with Severe Early Post-Transplantation Neurotoxicity. Transplant. Proc. 2014, 46, 3104–3107. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, A.; Mishra, R.; Thuluvath, P.J. Post-Liver-Transplant Anemia: Etiology and Management. Liver Transpl. 2004, 10, 165–173. [Google Scholar] [CrossRef]
- Nosari, A.; Marbello, L.; De Carlis, L.G.; De Gasperi, A.; Muti, G.; Mancini, V.; Morra, E. Bone Marrow Hypoplasia Complicating Tacrolimus (FK506) Therapy. Int. J. Hematol. 2004, 79, 130–132. [Google Scholar] [CrossRef]
- Masetti, M.; Rompianesi, G.; Montalti, R.; Romano, A.; Spaggiari, M.; Ballarin, R.; Guerrini, G.P.; Gerunda, G.E. Effects of Everolimus Monotherapy on Hematological Parameters and Iron Homeostasis in de Novo Liver Transplant Recipients: Preliminary Results. Transplant. Proc. 2008, 40, 1947–1949. [Google Scholar] [CrossRef]
- Dumortier, J.; Guillaud, O.; Pittau, G.; Salandre, J.; Adham, M.; Scoazec, J.-Y.; Boillot, O. Introduction of Mycophenolate Mofetil in Maintenance Liver Transplant Recipients: What Can We Expect? Results of a 10-Year Experience. Transplant. Proc. 2010, 42, 2602–2606. [Google Scholar] [CrossRef]
- Patel, N.; Muñoz, S.J. Bone Disease in Cirrhosis. Clin. Liver Dis. 2015, 6, 96–99. [Google Scholar] [CrossRef]
- Guañabens, N.; Parés, A. Liver and Bone. Arch. Biochem. Biophys. 2010, 503, 84–94. [Google Scholar] [CrossRef]
- Nakchbandi, I.A. Osteoporosis and Fractures in Liver Disease: Relevance, Pathogenesis and Therapeutic Implications. World J. Gastroenterol. 2014, 20, 9427–9438. [Google Scholar] [CrossRef]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, N.M.; Shane, E. Osteoporosis after Solid Organ Transplantation. J. Clin. Endocrinol. Metab. 2005, 90, 2456–2465. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Ha, J.; Kim, S.W.; Kim, J.-E.; Lee, S.; Choi, H.S.; Hong, N.; Kong, S.H.; Ahn, S.H.; Park, S.Y.; et al. Bone Loss after Solid Organ Transplantation: A Review of Organ-Specific Considerations. Endocrinol. Metab. 2024, 39, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Zavatta, G.; Vitale, G.; Morelli, M.C.; Pianta, P.; Turco, L.; Cappa, F.M.; Ravaioli, M.; Cescon, M.; Piscaglia, F.; Altieri, P.; et al. High Bone Fracture Risk in a Large Modern Cohort of Liver Transplant Recipients. Intern. Emerg. Med. 2024. [CrossRef]
- Lan, G.; Xie, X.; Peng, L.; Liu, L.; Song, L.; Dai, H. Current Status of Research on Osteoporosis after Solid Organ Transplantation: Pathogenesis and Management. Biomed. Res. Int. 2015, 2015, 413169. [Google Scholar] [CrossRef]
- Guichelaar, M.M.J.; Kendall, R.; Malinchoc, M.; Hay, J.E. Bone Mineral Density before and after OLT: Long-Term Follow-up and Predictive Factors. Liver Transpl. 2006, 12, 1390–1402. [Google Scholar] [CrossRef]
- Monegal, A.; Navasa, M.; Guañabens, N.; Peris, P.; Pons, F.; Martinez de Osaba, M.J.; Ordi, J.; Rimola, A.; Rodés, J.; Muñoz-Gómez, J. Bone Disease after Liver Transplantation: A Long-Term Prospective Study of Bone Mass Changes, Hormonal Status and Histomorphometric Characteristics. Osteoporos. Int. 2001, 12, 484–492. [Google Scholar] [CrossRef]
- Giannini, S.; Nobile, M.; Ciuffreda, M.; Iemmolo, R.M.; Dalle Carbonare, L.; Minicuci, N.; Casagrande, F.; Destro, C.; Gerunda, G.E.; Sartori, L.; et al. Long-Term Persistence of Low Bone Density in Orthotopic Liver Transplantation. Osteoporos. Int. 2000, 11, 417–424. [Google Scholar] [CrossRef]
- Pimentel, A.; Ureña-Torres, P.; Zillikens, M.C.; Bover, J.; Cohen-Solal, M. Fractures in Patients with CKD-Diagnosis, Treatment, and Prevention: A Review by Members of the European Calcified Tissue Society and the European Renal Association of Nephrology Dialysis and Transplantation. Kidney Int. 2017, 92, 1343–1355. [Google Scholar] [CrossRef]
- Brunova, J.; Kratochvilova, S.; Stepankova, J. Osteoporosis Therapy with Denosumab in Organ Transplant Recipients. Front. Endocrinol. 2018, 9, 162. [Google Scholar] [CrossRef]
- Bueno, M.V.; Munhoz, L.; Ortega, K.L.; Peres, M.P.S.D.M.; Franco, J.B. Bone Pattern Changes in Post Liver Transplant Patients Using Bisphosphonates. Spec. Care Dent. 2024, 44, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Segev, D.L.; McCulloch, C.E.; Covinsky, K.E.; Dodge, J.L.; Feng, S. Physical Frailty after Liver Transplantation. Am. J. Transplant. 2018, 18, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, F.; Biagi, F.; Avossa, A.; Falcini, M.; Nascimbeni, F.; Andreone, P.; Gitto, S. Frailty after Liver Transplantation: A Complex Unexplored Issue. J. Clin. Med. 2024, 13, 4537. [Google Scholar] [CrossRef]
- Tandon, P.; Zanetto, A.; Piano, S.; Heimbach, J.K.; Dasarathy, S. Liver Transplantation in the Patient with Physical Frailty. J. Hepatol. 2023, 78, 1105–1117. [Google Scholar] [CrossRef]
- Assy, N.; Adams, P.C.; Myers, P.; Simon, V.; Minuk, G.Y.; Wall, W.; Ghent, C.N. Randomized Controlled Trial of Total Immunosuppression Withdrawal in Liver Transplant Recipients: Role of Ursodeoxycholic Acid. Transplantation 2007, 83, 1571–1576. [Google Scholar] [CrossRef]
- Devlin, J.; Doherty, D.; Thomson, L.; Wong, T.; Donaldson, P.; Portmann, B.; Williams, R. Defining the Outcome of Immunosuppression Withdrawal after Liver Transplantation. Hepatology 1998, 27, 926–933. [Google Scholar] [CrossRef]
- Eason, J.D.; Cohen, A.J.; Nair, S.; Alcantera, T.; Loss, G.E. Tolerance: Is It Worth the Risk? Transplantation 2005, 79, 1157–1159. [Google Scholar] [CrossRef]
- Feng, S.; Ekong, U.D.; Lobritto, S.J.; Demetris, A.J.; Roberts, J.P.; Rosenthal, P.; Alonso, E.M.; Philogene, M.C.; Ikle, D.; Poole, K.M.; et al. Complete Immunosuppression Withdrawal and Subsequent Allograft Function among Pediatric Recipients of Parental Living Donor Liver Transplants. JAMA 2012, 307, 283–293. [Google Scholar] [CrossRef]
- Girlanda, R.; Rela, M.; Williams, R.; O’Grady, J.G.; Heaton, N.D. Long-Term Outcome of Immunosuppression Withdrawal after Liver Transplantation. Transplant. Proc. 2005, 37, 1708–1709. [Google Scholar] [CrossRef]
- Mazariegos, G.V.; Reyes, J.; Marino, I.R.; Demetris, A.J.; Flynn, B.; Irish, W.; McMichael, J.; Fung, J.J.; Starzl, T.E. Weaning of Immunosuppression in Liver Transplant Recipients. Transplantation 1997, 63, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Oike, F.; Yokoi, A.; Nishimura, E.; Ogura, Y.; Fujimoto, Y.; Kasahara, M.; Kaihara, S.; Kiuchi, T.; Egawa, H.; Uemoto, S.; et al. Complete Withdrawal of Immunosuppression in Living Donor Liver Transplantation. Transplant. Proc. 2002, 34, 1521. [Google Scholar] [CrossRef] [PubMed]
- Pons, J.A.; Yélamos, J.; Ramírez, P.; Oliver-Bonet, M.; Sánchez, A.; Rodríguez-Gago, M.; Navarro, J.; Bermejo, J.; Robles, R.; Parrilla, P. Endothelial Cell Chimerism Does Not Influence Allograft Tolerance in Liver Transplant Patients after Withdrawal of Immunosuppression. Transplantation 2003, 75, 1045–1047. [Google Scholar] [CrossRef] [PubMed]
- Takatsuki, M.; Uemoto, S.; Inomata, Y.; Egawa, H.; Kiuchi, T.; Fujita, S.; Hayashi, M.; Kanematsu, T.; Tanaka, K. Weaning of Immunosuppression in Living Donor Liver Transplant Recipients. Transplantation 2001, 72, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Tisone, G.; Orlando, G.; Cardillo, A.; Palmieri, G.; Manzia, T.M.; Baiocchi, L.; Lionetti, R.; Anselmo, A.; Toti, L.; Angelico, M. Complete Weaning off Immunosuppression in HCV Liver Transplant Recipients Is Feasible and Favourably Impacts on the Progression of Disease Recurrence. J. Hepatol. 2006, 44, 702–709. [Google Scholar] [CrossRef]
- Tryphonopoulos, P.; Tzakis, A.G.; Weppler, D.; Garcia-Morales, R.; Kato, T.; Madariaga, J.R.; Levi, D.M.; Nishida, S.; Moon, J.; Selvaggi, G.; et al. The Role of Donor Bone Marrow Infusions in Withdrawal of Immunosuppression in Adult Liver Allotransplantation. Am. J. Transplant. 2005, 5, 608–613. [Google Scholar] [CrossRef]
- Duizendstra, A.A.; de Knegt, R.J.; Betjes, M.G.H.; Coenen, S.; Murad, S.D.; de Man, R.A.; Metselaar, H.J.; Sprengers, D.; Litjens, N.H.R.; Kwekkeboom, J. Immunosuppressive Drug Withdrawal Late after Liver Transplantation Improves the Lipid Profile and Reduces Infections. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1444–1451. [Google Scholar] [CrossRef]
- Feng, S.; Bucuvalas, J. Tolerance after Liver Transplantation: Where Are We? Liver Transpl. 2017, 23, 1601–1614. [Google Scholar] [CrossRef]
- Chandrasekharan, D.; Issa, F.; Wood, K.J. Achieving Operational Tolerance in Transplantation: How Can Lessons from the Clinic Inform Research Directions? Transpl. Int. 2013, 26, 576–589. [Google Scholar] [CrossRef]
- Yoshitomi, M.; Koshiba, T.; Haga, H.; Li, Y.; Zhao, X.; Cheng, D.; Miyagawa, A.; Sakashita, H.; Tsuruyama, T.; Ohe, H.; et al. Requirement of Protocol Biopsy before and after Complete Cessation of Immunosuppression after Liver Transplantation. Transplantation 2009, 87, 606–614. [Google Scholar] [CrossRef]
- Benítez, C.; Londoño, M.-C.; Miquel, R.; Manzia, T.-M.; Abraldes, J.G.; Lozano, J.-J.; Martínez-Llordella, M.; López, M.; Angelico, R.; Bohne, F.; et al. Prospective Multicenter Clinical Trial of Immunosuppressive Drug Withdrawal in Stable Adult Liver Transplant Recipients. Hepatology 2013, 58, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Shaked, A.; DesMarais, M.R.; Kopetskie, H.; Feng, S.; Punch, J.D.; Levitsky, J.; Reyes, J.; Klintmalm, G.B.; Demetris, A.J.; Burrell, B.E.; et al. Outcomes of Immunosuppression Minimization and Withdrawal Early after Liver Transplantation. Am. J. Transplant. 2019, 19, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Levitsky, J.; Burrell, B.E.; Kanaparthi, S.; Turka, L.A.; Kurian, S.; Sanchez-Fueyo, A.; Lozano, J.J.; Demetris, A.; Lesniak, A.; Kirk, A.D.; et al. Immunosuppression Withdrawal in Liver Transplant Recipients on Sirolimus. Hepatology 2020, 72, 569–583. [Google Scholar] [CrossRef]
- Vionnet, J.; Torres-Yaguana, J.; Miquel, R.; Abraldes, J.G.; Wall, J.; Kodela, E.; Lozano, J.-J.; Ruiz, P.; Navasa, M.; Marshall, A.; et al. Randomized Trial Investigating the Utility of a Liver Tissue Transcriptional Biomarker in Identifying Adult Liver Transplant Recipients Not Requiring Maintenance Immunosuppression. Am. J. Transplant. 2024, in press. [Google Scholar] [CrossRef]
- Lerut, J.; Van Thuyne, V.; Mathijs, J.; Lemaire, J.; Talpe, S.; Roggen, F.; Ciccarelli, O.; Zuckermann, M.; Goffette, P.; Hope, J.; et al. Anti-CD2 Monoclonal Antibody and Tacrolimus in Adult Liver Transplantation. Transplantation 2005, 80, 1186. [Google Scholar] [CrossRef]
- Salvadori, M.; Tsalouchos, A. Therapeutic Apheresis in Kidney Transplantation: An Updated Review. World J. Transplant. 2019, 9, 103–122. [Google Scholar] [CrossRef]
- Iesari, S.; Nava, F.L.; Zais, I.E.; Coubeau, L.; Ferraresso, M.; Favi, E.; Lerut, J. Advancing Immunosuppression in Liver Transplantation: A Narrative Review. Hepatobiliary Pancreat. Dis. Int. 2024, 23, 441–448. [Google Scholar] [CrossRef]
- Vincenti, F. Belatacept and Long-Term Outcomes in Kidney Transplantation. N. Engl. J. Med. 2016, 374, 2600–2601. [Google Scholar] [CrossRef]
- Klintmalm, G.B.; Feng, S.; Lake, J.R.; Vargas, H.E.; Wekerle, T.; Agnes, S.; Brown, K.A.; Nashan, B.; Rostaing, L.; Meadows-Shropshire, S.; et al. Belatacept-Based Immunosuppression in de Novo Liver Transplant Recipients: 1-Year Experience from a Phase II Randomized Study. Am. J. Transplant. 2014, 14, 1817–1827. [Google Scholar] [CrossRef]
- Cristea, O.; Karadkhele, G.; Kitchens, W.H.; Vasanth, P.; Larsen, C.P.; Badell, I.R. Belatacept Conversion in Kidney After Liver Transplantation. Transplant. Direct 2021, 7, e780. [Google Scholar] [CrossRef]
- Verbeek, J.S.; Hirose, S.; Nishimura, H. The Complex Association of FcγRIIb with Autoimmune Susceptibility. Front. Immunol. 2019, 10, 2061. [Google Scholar] [CrossRef] [PubMed]
- Fribourg, M.; Anderson, L.; Fischman, C.; Cantarelli, C.; Perin, L.; La Manna, G.; Rahman, A.; Burrell, B.E.; Heeger, P.S.; Cravedi, P. T-Cell Exhaustion Correlates with Improved Outcomes in Kidney Transplant Recipients. Kidney Int. 2019, 96, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Angeletti, A.; Cantarelli, C.; Riella, L.V.; Fribourg, M.; Cravedi, P. T-Cell Exhaustion in Organ Transplantation. Transplantation 2022, 106, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Arruda, L.C.M.; Lima-Júnior, J.R.; Clave, E.; Moraes, D.A.; Douay, C.; Fournier, I.; Moins-Teisserenc, H.; Covas, D.T.; Simões, B.P.; Farge, D.; et al. Homeostatic Proliferation Leads to Telomere Attrition and Increased PD-1 Expression after Autologous Hematopoietic SCT for Systemic Sclerosis. Bone Marrow Transplant. 2018, 53, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Kung, P.; Goldstein, G.; Reinherz, E.L.; Schlossman, S.F. Monoclonal Antibodies Defining Distinctive Human T Cell Surface Antigens. Science 1979, 206, 347–349. [Google Scholar] [CrossRef]
- Wilde, M.I.; Goa, K.L. Muromonab CD3: A Reappraisal of Its Pharmacology and Use as Prophylaxis of Solid Organ Transplant Rejection. Drugs 1996, 51, 865–894. [Google Scholar] [CrossRef]
- Norman, D.J. Mechanisms of Action and Overview of OKT3. Ther. Drug Monit. 1995, 17, 615–620. [Google Scholar] [CrossRef]
- Cvetkovski, F.; Hexham, J.M.; Berglund, E. Strategies for Liver Transplantation Tolerance. Int. J. Mol. Sci. 2021, 22, 2253. [Google Scholar] [CrossRef]
- Morris, P.J.; Russell, N.K. Alemtuzumab (Campath-1H): A Systematic Review in Organ Transplantation. Transplantation 2006, 81, 1361–1367. [Google Scholar] [CrossRef]
- Magliocca, J.F.; Knechtle, S.J. The Evolving Role of Alemtuzumab (Campath-1H) for Immunosuppressive Therapy in Organ Transplantation. Transpl. Int. 2006, 19, 705–714. [Google Scholar] [CrossRef]
- Tzakis, A.G.; Tryphonopoulos, P.; Kato, T.; Nishida, S.; Levi, D.M.; Madariaga, J.R.; Gaynor, J.J.; De Faria, W.; Regev, A.; Esquenazi, V.; et al. Preliminary Experience with Alemtuzumab (Campath-1H) and Low-Dose Tacrolimus Immunosuppression in Adult Liver Transplantation. Transplantation 2004, 77, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Tzakis, A.G.; Kato, T.; Nishida, S.; Levi, D.M.; Madariaga, J.R.; Nery, J.R.; Mittal, N.; Regev, A.; Cantwell, P.; Gyamfi, A.; et al. Preliminary Experience with Campath 1H (C1H) in Intestinal and Liver Transplantation. Transplantation 2003, 75, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Tryphonopoulos, P.; Madariaga, J.R.; Kato, T.; Nishida, S.; Levi, D.M.; Moon, J.; Selvaggi, G.; De Faria, W.; Regev, A.; Bejarano, P.; et al. The Impact of Campath 1H Induction in Adult Liver Allotransplantation. Transplant. Proc. 2005, 37, 1203–1204. [Google Scholar] [CrossRef]
- Khan, O.; Giles, J.R.; McDonald, S.; Manne, S.; Ngiow, S.F.; Patel, K.P.; Werner, M.T.; Huang, A.C.; Alexander, K.A.; Wu, J.E.; et al. TOX Transcriptionally and Epigenetically Programs CD8+ T Cell Exhaustion. Nature 2019, 571, 211–218. [Google Scholar] [CrossRef]
- Soliman, T.; Hetz, H.; Burghuber, C.; Györi, G.; Silberhumer, G.; Steininger, R.; Mühlbacher, F.; Berlakovich, G.A. Short-Term Induction Therapy with Anti-Thymocyte Globulin and Delayed Use of Calcineurin Inhibitors in Orthotopic Liver Transplantation. Liver Transpl. 2007, 13, 1039–1044. [Google Scholar] [CrossRef]
- Tchervenkov, J.I.; Tzimas, G.N.; Cantarovich, M.; Barkun, J.S.; Metrakos, P. The Impact of Thymoglobulin on Renal Function and Calcineurin Inhibitor Initiation in Recipients of Orthotopic Liver Transplant: A Retrospective Analysis of 298 Consecutive Patients. Transplant. Proc. 2004, 36, 1747–1752. [Google Scholar] [CrossRef]
- Nair, A.; Coromina Hernandez, L.; Shah, S.; Zervos, X.; Zimmerman, M.; Sasaki, K.; Diago, T.; Hashimoto, K.; Fujiki, M.; Aucejo, F.; et al. Induction Therapy with Antithymocyte Globulin and Delayed Calcineurin Inhibitor Initiation for Renal Protection in Liver Transplantation: A Multicenter Randomized Controlled Phase II-B Trial. Transplantation 2022, 106, 997–1003. [Google Scholar] [CrossRef]
- Rawlings, J.S.; Rosler, K.M.; Harrison, D.A. The JAK/STAT Signaling Pathway. J. Cell Sci. 2004, 117, 1281–1283. [Google Scholar] [CrossRef]
- Qureshy, Z.; Johnson, D.E.; Grandis, J.R. Targeting the JAK/STAT Pathway in Solid Tumors. J. Cancer Metastasis Treat. 2020, 6, 27. [Google Scholar] [CrossRef]
- Jamilloux, Y.; El Jammal, T.; Vuitton, L.; Gerfaud-Valentin, M.; Kerever, S.; Sève, P. JAK Inhibitors for the Treatment of Autoimmune and Inflammatory Diseases. Autoimmun. Rev. 2019, 18, 102390. [Google Scholar] [CrossRef]
- Baan, C.C.; Kannegieter, N.M.; Felipe, C.R.; Tedesco Silva, H. Targeting JAK/STAT Signaling to Prevent Rejection After Kidney Transplantation: A Reappraisal. Transplantation 2016, 100, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Säemann, M.D.; Böhmig, G.A.; Osterreicher, C.H.; Staffler, G.; Diakos, C.; Krieger, P.M.; Hörl, W.H.; Stockinger, H.; Zlabinger, G.J. Suppression of Primary T-Cell Responses and Induction of Alloantigen-Specific Hyporesponsiveness in Vitro by the Janus Kinase Inhibitor Tyrphostin AG490. Transplantation 2000, 70, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhu, G.; Qin, M.; Li, Z.; Wang, B.; Yang, J.; Wang, T. The Effectiveness of Ruxolitinib for Acute/Chronic Graft-versus-Host Disease in Children: A Retrospective Study. Drug Des. Devel Ther. 2021, 15, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Zand, M.S. Tofacitinab in Renal Transplantation. Transplant. Rev. 2013, 27, 85–89. [Google Scholar] [CrossRef]
- Freitas, M.C.S.; Uchida, Y.; Zhao, D.; Ke, B.; Busuttil, R.W.; Kupiec-Weglinski, J.W. Blockade of Janus Kinase-2 Signaling Ameliorates Mouse Liver Damage Due to Ischemia and Reperfusion. Liver Transpl. 2010, 16, 600–610. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, W.; Cai, X. Anti-Interleukin-2 Receptor Antibodies for the Prevention of Rejection in Liver Transplant Recipients: A Systematic Review and Meta-Analysis. Ann. Med. 2017, 49, 365–376. [Google Scholar] [CrossRef]
- Becker, T.; Foltys, D.; Bilbao, I.; D’Amico, D.; Colledan, M.; Bernardos, A.; Beckebaum, S.; Isoniemi, H.; Pirenne, J.; Jaray, J.; et al. Patient Outcomes in Two Steroid-Free Regimens Using Tacrolimus Monotherapy after Daclizumab Induction and Tacrolimus with Mycophenolate Mofetil in Liver Transplantation. Transplantation 2008, 86, 1689–1694. [Google Scholar] [CrossRef]
- Boillot, O.; Mayer, D.A.; Boudjema, K.; Salizzoni, M.; Gridelli, B.; Filipponi, F.; Trunecka, P.; Krawczyk, M.; Clavien, P.-A.; Ducerf, C.; et al. Corticosteroid-Free Immunosuppression with Tacrolimus Following Induction with Daclizumab: A Large Randomized Clinical Study. Liver Transpl. 2005, 11, 61–67. [Google Scholar] [CrossRef]
- TruneČka, P.; Klempnauer, J.; Bechstein, W.O.; Pirenne, J.; Friman, S.; Zhao, A.; Isoniemi, H.; Rostaing, L.; Settmacher, U.; Mönch, C.; et al. Renal Function in De Novo Liver Transplant Recipients Receiving Different Prolonged-Release Tacrolimus Regimens-The DIAMOND Study. Am. J. Transplant. 2015, 15, 1843–1854. [Google Scholar] [CrossRef]
- Moini, M.; Schilsky, M.L.; Tichy, E.M. Review on Immunosuppression in Liver Transplantation. World J. Hepatol. 2015, 7, 1355–1368. [Google Scholar] [CrossRef]
- Bugelski, P.J.; Martin, P.L. Concordance of Preclinical and Clinical Pharmacology and Toxicology of Therapeutic Monoclonal Antibodies and Fusion Proteins: Cell Surface Targets. Br. J. Pharmacol. 2012, 166, 823–846. [Google Scholar] [CrossRef] [PubMed]
- Penninga, L.; Wettergren, A.; Wilson, C.H.; Chan, A.-W.; Steinbrüchel, D.A.; Gluud, C. Antibody Induction versus Placebo, No Induction, or Another Type of Antibody Induction for Liver Transplant Recipients. Cochrane Database Syst. Rev. 2014, 2014, CD010253. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Rabindranath, M.; Chara, B.S.; Simonetto, D.A. Artificial Intelligence, Machine Learning, and Deep Learning in Liver Transplantation. J. Hepatol. 2023, 78, 1216–1233. [Google Scholar] [CrossRef] [PubMed]
- Gulla, A.; Jakiunaite, I.; Juchneviciute, I.; Dzemyda, G. A Narrative Review: Predicting Liver Transplant Graft Survival Using Artificial Intelligence Modeling. Front. Transplant. 2024, 3, 1378378. [Google Scholar] [CrossRef]
- Briceño, J.; Calleja, R.; Hervás, C. Artificial Intelligence and Liver Transplantation: Looking for the Best Donor-Recipient Pairing. Hepatobiliary Pancreat. Dis. Int. 2022, 21, 347–353. [Google Scholar] [CrossRef]
- Lau, L.; Kankanige, Y.; Rubinstein, B.; Jones, R.; Christophi, C.; Muralidharan, V.; Bailey, J. Machine-Learning Algorithms Predict Graft Failure After Liver Transplantation. Transplantation 2017, 101, e125–e132. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).