Repositioning Perindopril for Mitigation of Methotrexate-Induced Hepatotoxicity in Rats
Abstract
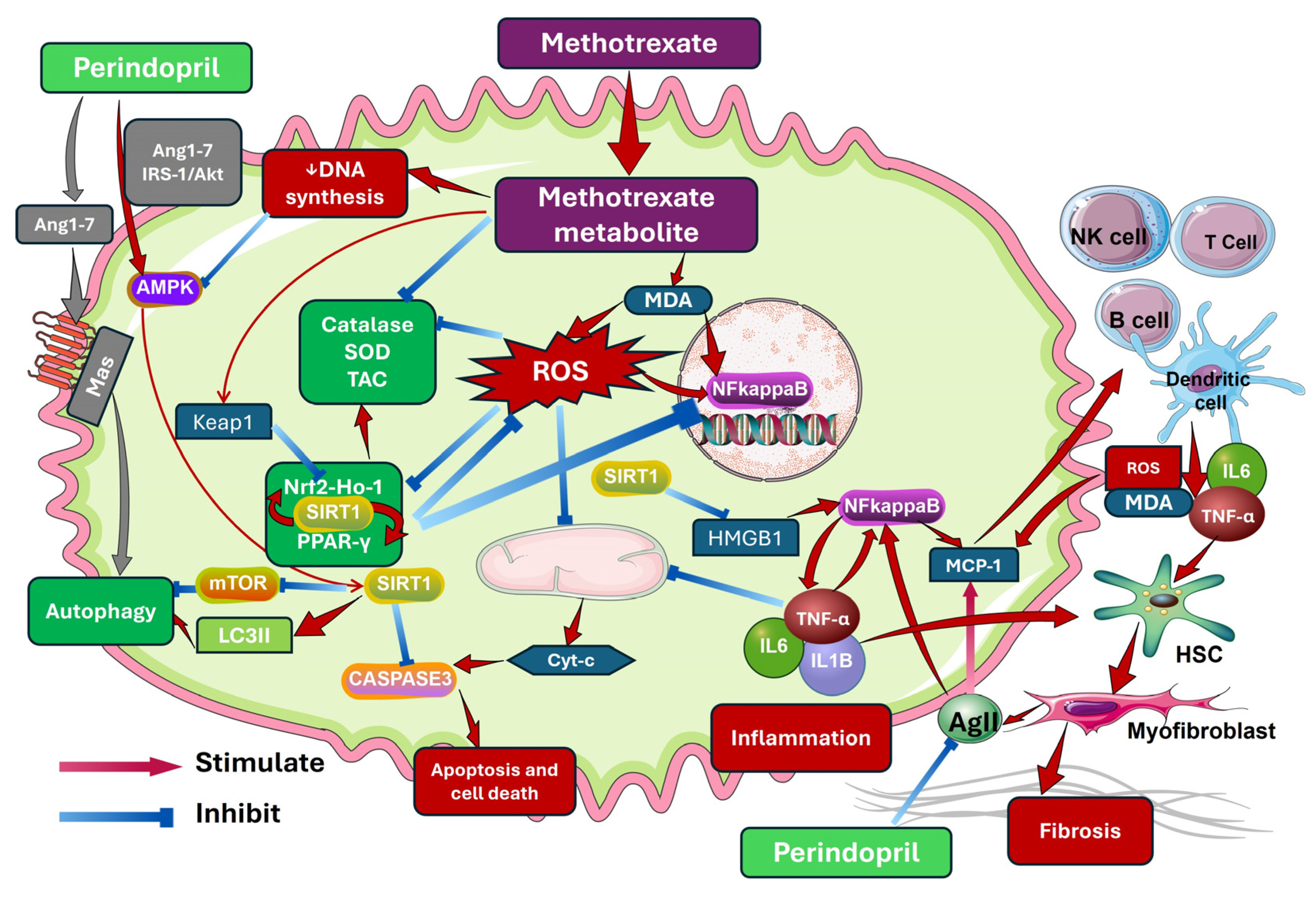
:1. Introduction
2. Results
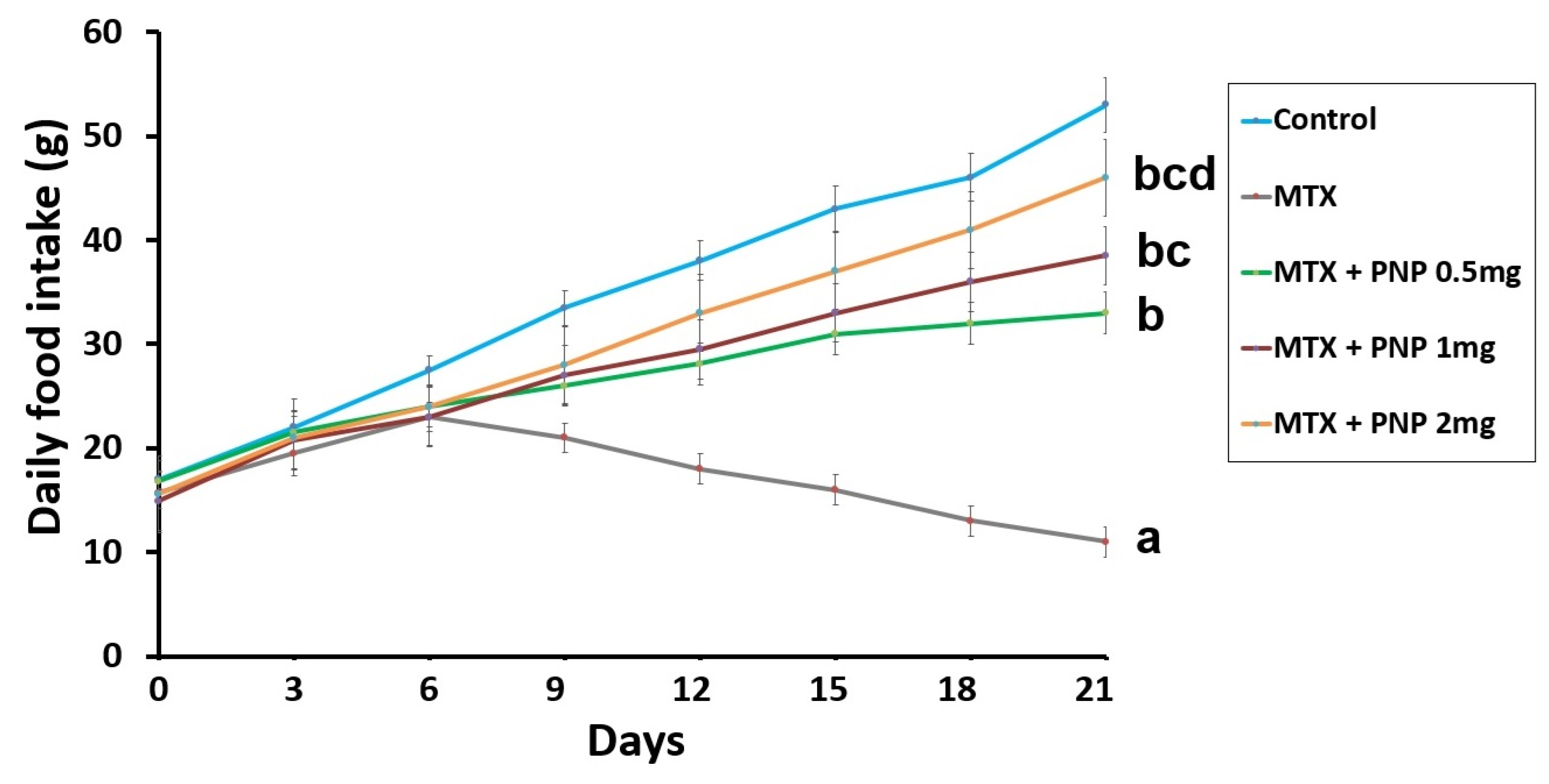
2.1. Perindopril Dose-Dependently Combatted the Effect of Methotrexate on the Daily Food Intake
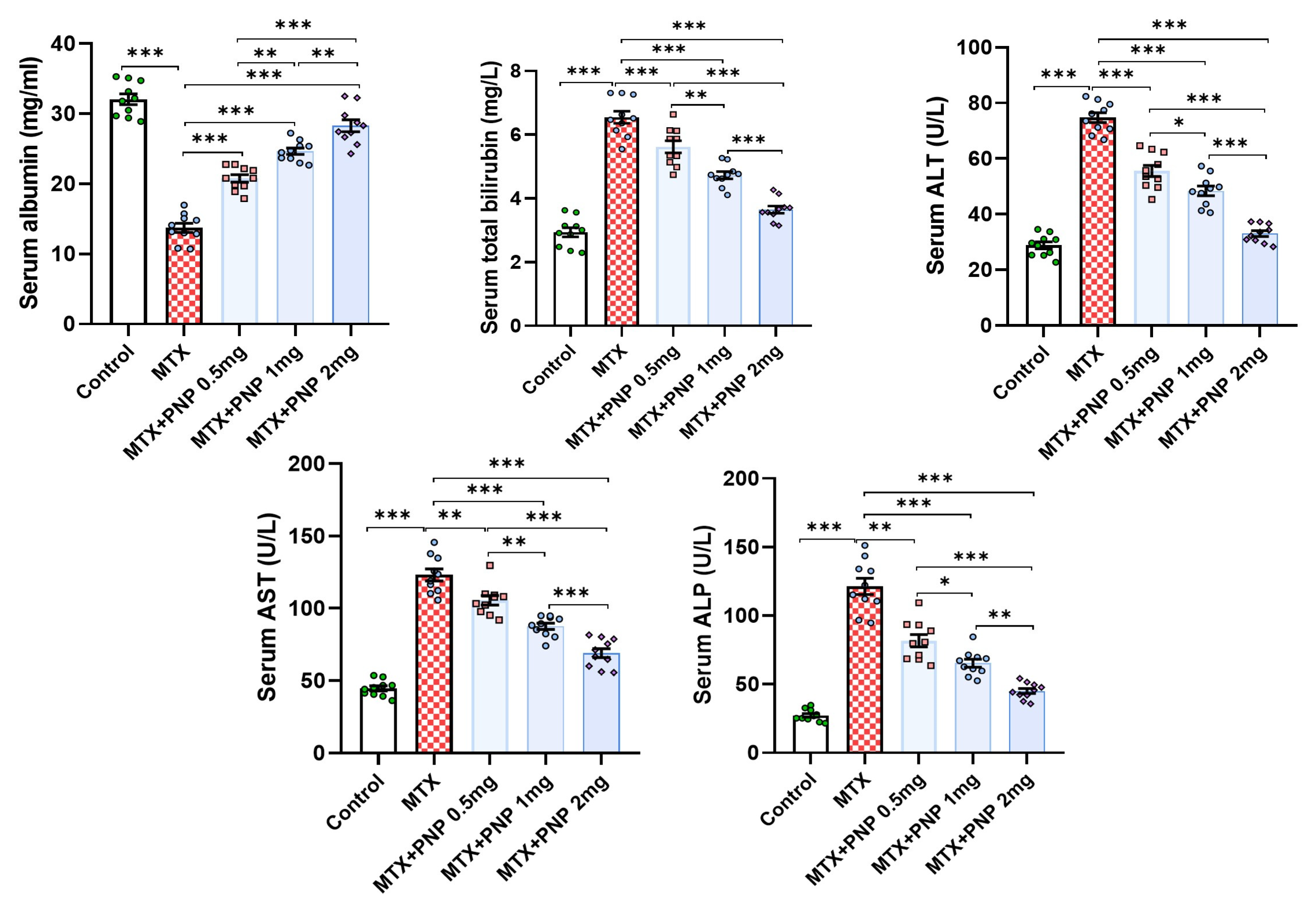
2.2. Perindopril Dose Dependently Ameliorated the Effect of Methotrexate on the Liver Function Tests
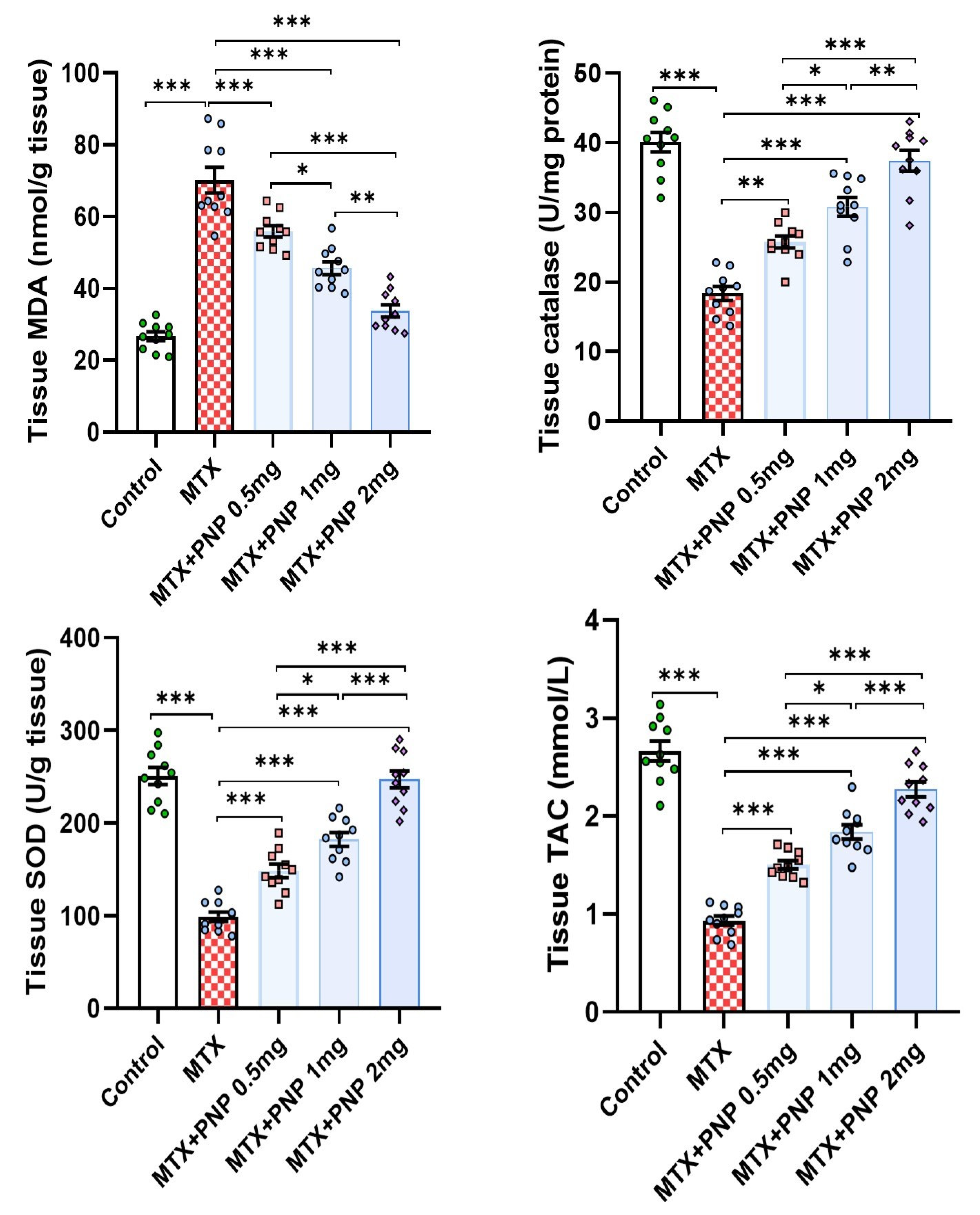
2.3. Perindopril Dose Dependently Mitigated the Effect of Methotrexate on the Redox Status of the Hepatic Tissues
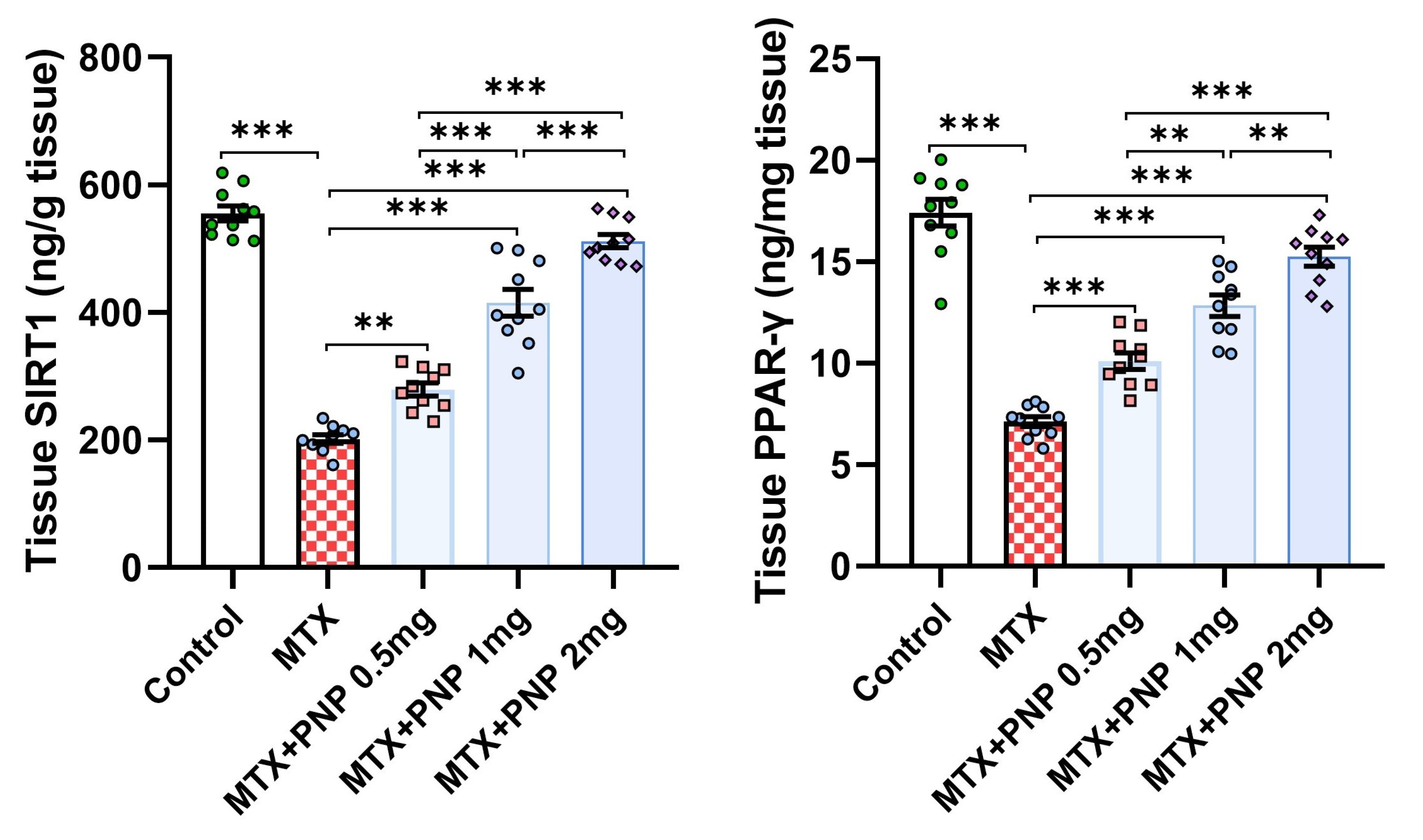
2.4. Perindopril Dose Dependently Combatted the Effect of Methotrexate on SIRT1 and PPAR-γ Content of the Hepatic Tissues
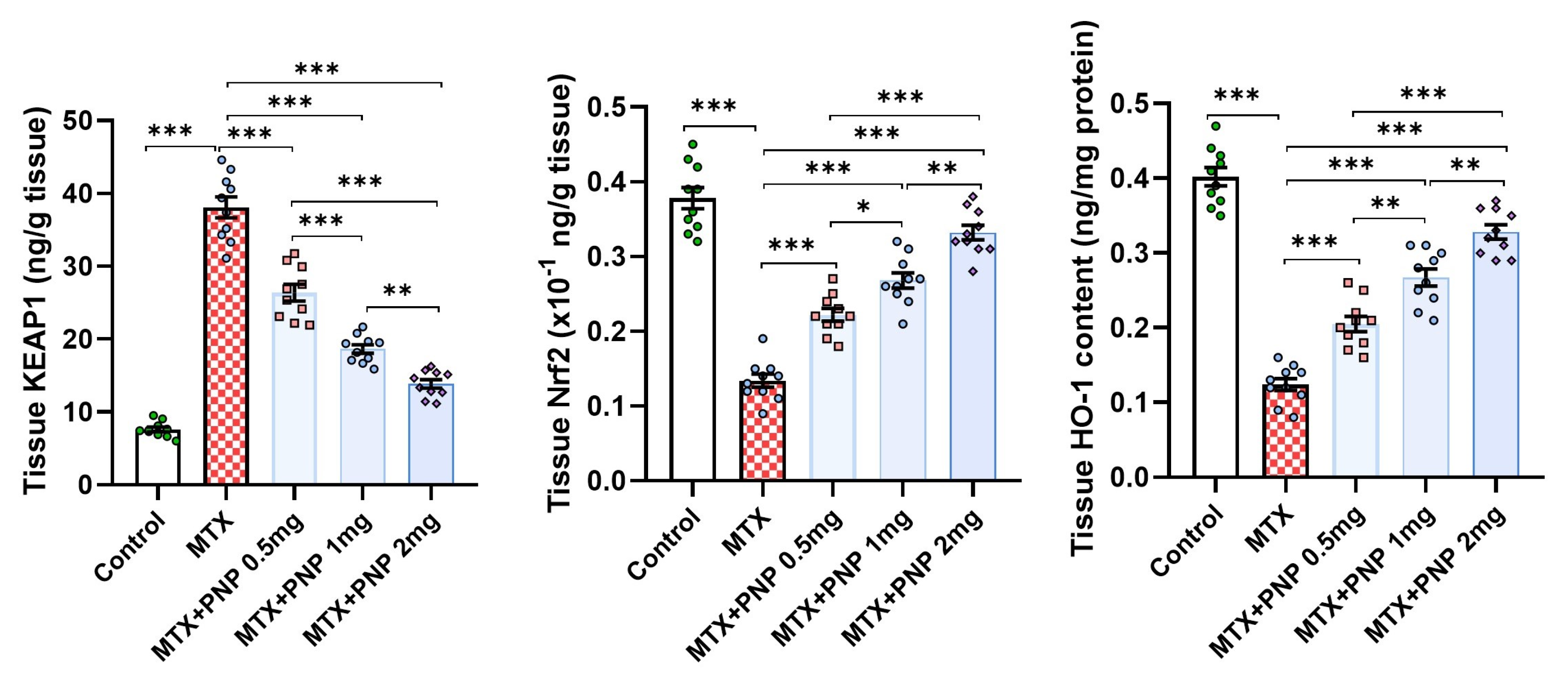
2.5. Perindopril Dose Dependently Abrogated the Effect of Methotrexate on KEAP1, Nrf2, and HO-1 Content of the Hepatic Tissues
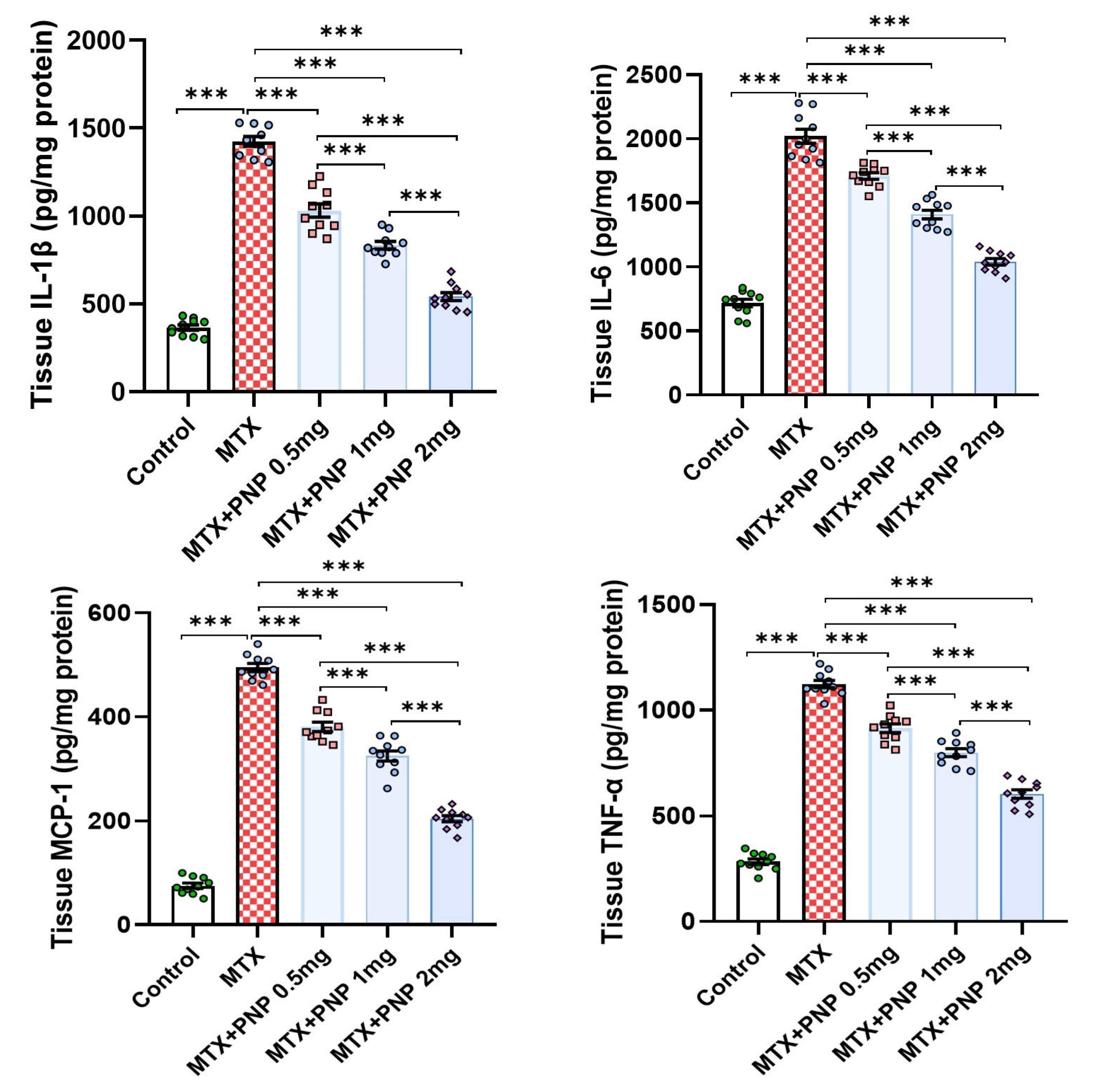
2.6. Perindopril Dose Dependently Decreased the Hepatic Tissue Levels of the Proinflammatory Cytokines in Rats Treated with Methotrexate
2.7. Perindopril Dose Dependently Mitigated the HMGB1/RAGE/Nuclear Factor Kappa B (NF-κB) Axis in the Hepatic Tissues of Rats Treated with Methotrexate
2.8. Perindopril Dose Dependently Reversed the Effect of Methotrexate on Phospho-mTOR, Total AMPK, and LC3-II in the Hepatic Tissues
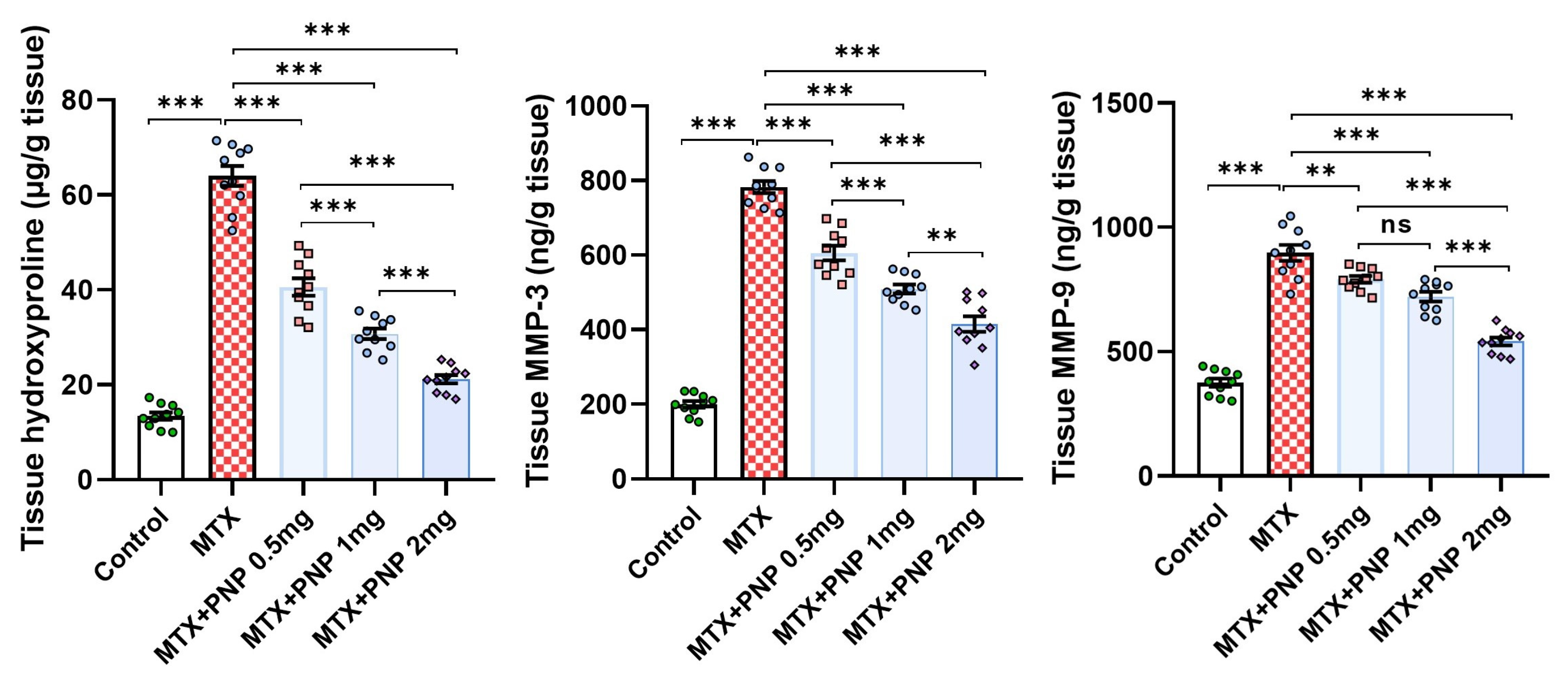
2.9. Perindopril Dose Dependently Abrogated the Fibrogenic Process Induced by Methotrexate in the Hepatic Tissues
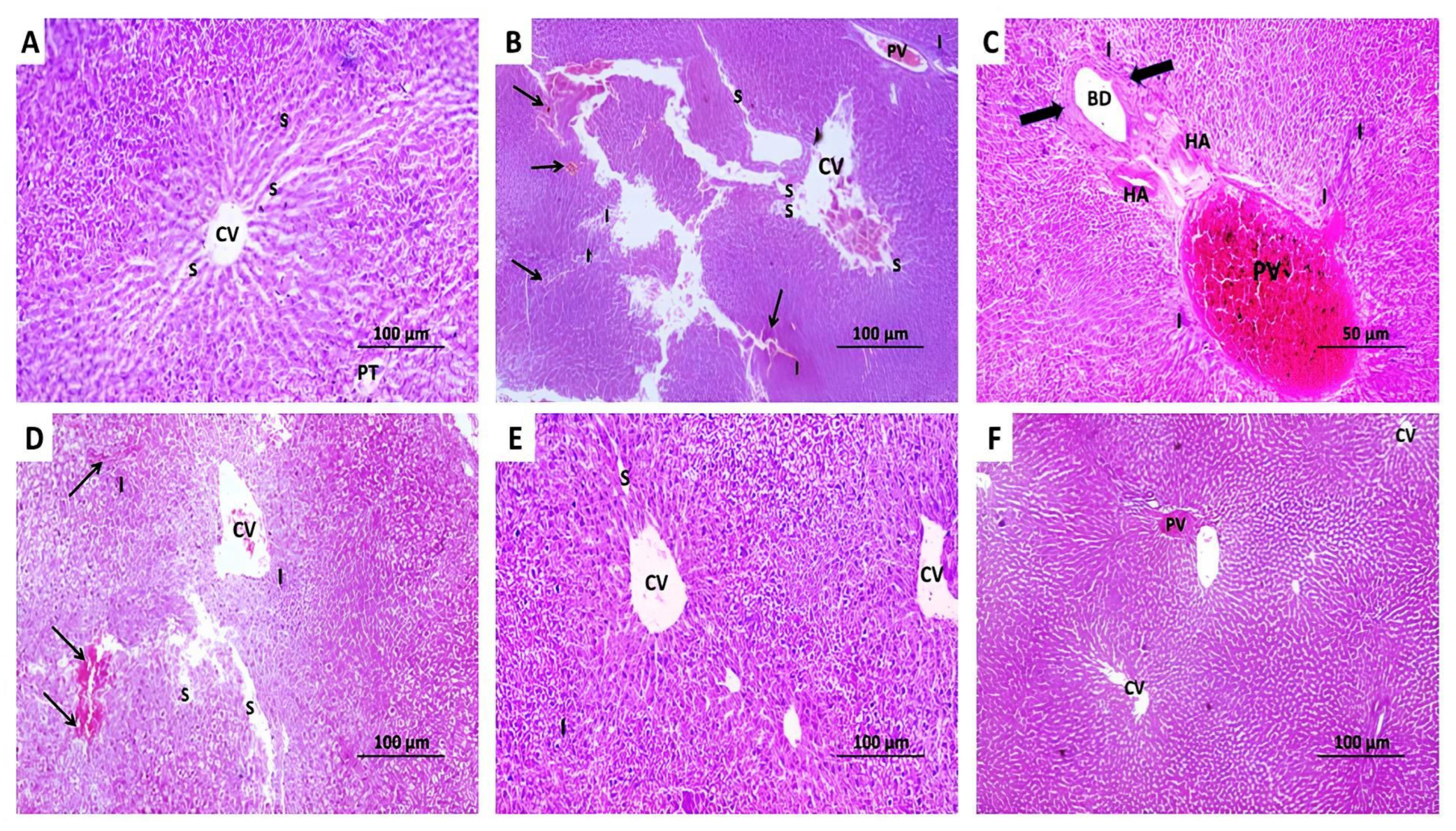
2.10. The Changes Elicited by Administration of Methotrexate With or Without the Different Doses of Perindopril on the Histopathological View of the Hepatic Tissues
2.11. The Impact of Administration of Methotrexate With or Without the Different Doses of Perindopril on the Extent of He Immunohistochemical Positive Expression of Cleaved Caspase 3 in the Hepatic Tissue Specimens
2.12. Perindopril Dose-Dependently Combatted Methotrexate-Elicited Perturbations in the Electron Microscopic Picture of the Hepatic Tissues
3. Discussion
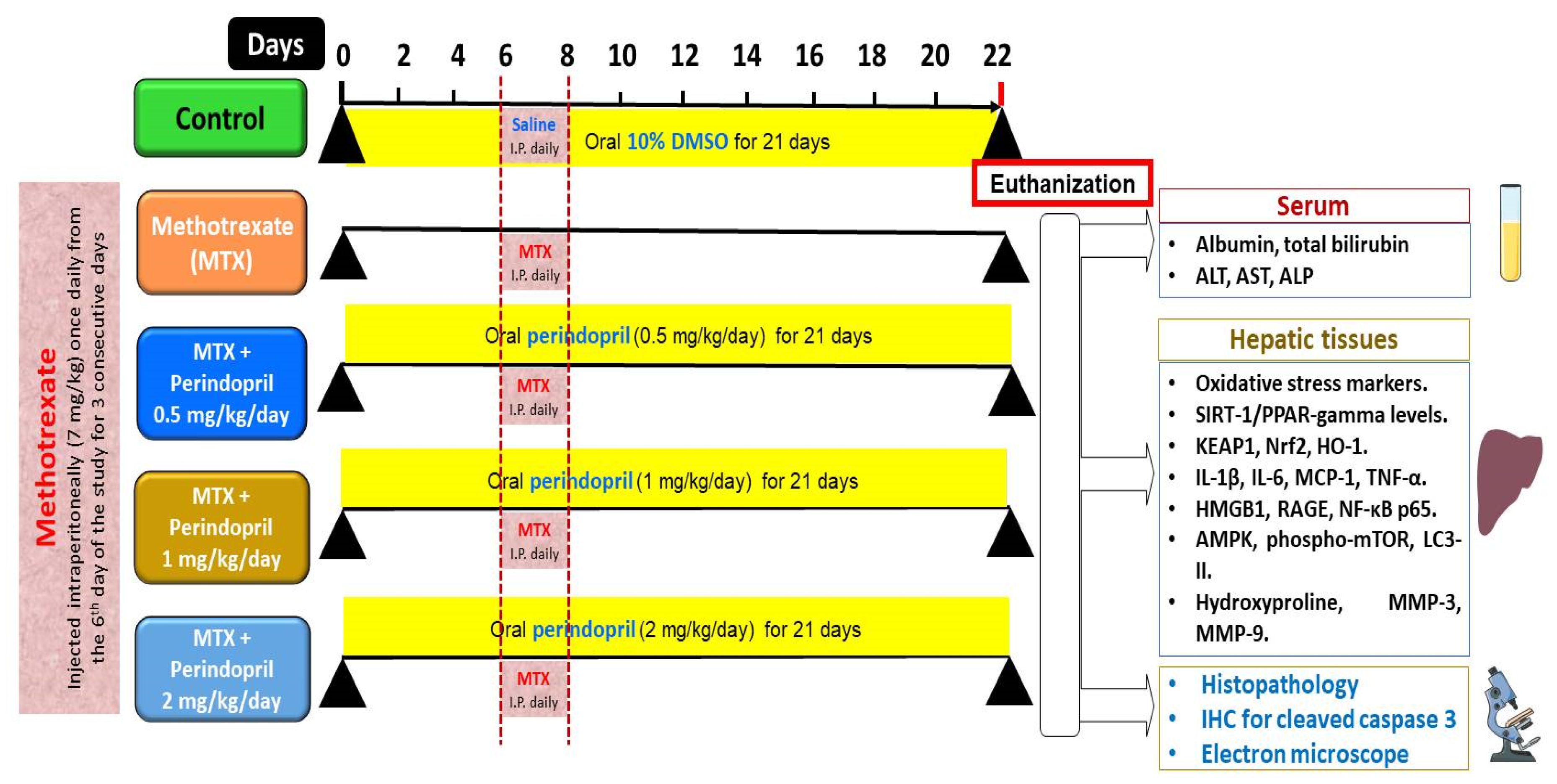
4. Materials and Methods
4.1. Ethical Considerations
4.2. Drugs and Reagents Used
4.3. Grouping of the Experimental Animals and the Study Design
4.4. Determination of the Daily Food Intake in the Different Studied Groups
4.5. Collection of the Blood and the Liver Tissue Samples from the Different Animal Groups
4.6. Determination of the Serum Biochemical Parameters
4.7. Assessment of the Biochemical Measurements in the Supernatant of the Hepatic Tissues’ Homogenates
4.7.1. Evaluation of the Redox Status of the Hepatic Tissues
4.7.2. Quantification of the Hepatic Tissue Content of Sirtuin-1 (SIRT1) and Peroxisome Proliferator-Activated Receptor Gamma (PPAR-γ)
4.7.3. Determination of the Hepatic Tissue Content of Kelch-like ECH-Associated Protein 1 (KEAP1), Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2), and Heme Oxygenase-1 (HO-1)
4.7.4. Quantification of the Hepatic Tissue Content of Interleukin 1-β (IL-1β), IL-6, Monocyte Chemoattractant Protein 1 (MCP-1), and Tumor Necrosis Factor Alpha (TNF-α)
4.7.5. Evaluation of HMGB1/RAGE/Nuclear Factor Kappa B (NF-κB) Axis in the Hepatic Tissues
4.7.6. Assay of the Hepatic Tissue Levels of Phosphorylated Mammalian Target of Rapamycin (Phospho-mTOR), Total Adenosine Monophosphate-Activated Protein Kinase (AMPK), and LC3-II
4.7.7. Assessment of the Hepatic Tissue Content of Hydroxyproline, Matrix Metalloproteinase-3 (MMP-3), and MMP-9
4.8. Light Microscopic Evaluation of the Morphologic Changes Induced by the Different Treatments in the Hepatic Tissue Specimens
4.9. Evaluation of the Immunohistochemical Expression of Cleaved Caspase 3 in the Hepatic Tissue Specimens of the Different Studied Groups
4.10. Clarification of the Alterations Induced by the Different Treatments in the Electron Microscopic Picture of the Hepatic Tissues
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamed, K.M.; Dighriri, I.M.; Baomar, A.F.; Alharthy, B.T.; Alenazi, F.E.; Alali, G.H.; Alenazy, R.H.; Alhumaidi, N.T.; Alhulayfi, D.H.; Alotaibi, Y.B.; et al. Overview of methotrexate toxicity: A comprehensive literature review. Cureus 2022, 14, e29518. [Google Scholar] [CrossRef] [PubMed]
- Corazza, V.; Cusano, F.; De Pità, O.; Rossi, L.; Virno, G.G. Methotrexate in the therapeutic pathway of patients with psoriasis. Analysis of clinical practice data and comparison with guidelines. Dermatol. Rep. 2021, 14, 9454. [Google Scholar] [CrossRef] [PubMed]
- Mahil, S.K.; Bechman, K.; Raharja, A.; Domingo-Vila, C.; Baudry, D.; Brown, M.A.; Cope, A.P.; Dasandi, T.; Graham, C.; Lechmere, T.; et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: A cohort study. Lancet Rheumatol. 2021, 3, e627–e637. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.H.; Glynn, R.J.; Karlson, E.W.; Lu, F.; Corrigan, C.; Colls, J.; Xu, C.; MacFadyen, J.; Barbhaiya, M.; Berliner, N.; et al. Adverse effects of low-dose methotrexate: A randomized trial. Ann. Intern. Med. 2020, 172, 369–380. [Google Scholar] [CrossRef]
- Ezhilarasan, D. Hepatotoxic potentials of methotrexate: Understanding the possible toxicological molecular mechanisms. Toxicology 2021, 458, 152840. [Google Scholar] [CrossRef]
- Di Martino, V.; Verhoeven, D.W.; Verhoeven, F.; Aubin, F.; Avouac, J.; Vuitton, L.; Lioté, F.; Thévenot, T.; Wendling, D. Busting the myth of methotrexate chronic hepatotoxicity. Nat. Rev. Rheumatol. 2023, 19, 96–110. [Google Scholar] [CrossRef]
- Olayinka, E.T.; Ore, A.; Adeyemo, O.A.; Ola, O.S. Ameliorative effect of gallic acid on methotrexate-induced hepatotoxicity and nephrotoxicity in rat. J. Xenobiotics 2016, 6, 6092. [Google Scholar] [CrossRef]
- Kanugula, A.K.; Kaur, J.; Batra, J.; Ankireddypalli, A.R.; Velagapudi, R. Renin-Angiotensin System: Updated Understanding and Role in Physiological and Pathophysiological States. Cureus 2023, 15, e40725. [Google Scholar] [CrossRef]
- Vargas, R.A.V.; Millán, J.M.V.; Bonilla, E.F. Diabetes y Nutrición. Renin–angiotensin system: Basic and clinical aspects—A general perspective. Endocrinol. Diabetes Y Nutr. 2022, 69, 52–62. [Google Scholar] [CrossRef]
- Mastoor, Z.; Diz-Chaves, Y.; González-Matías, L.C.; Mallo, F. Renin–angiotensin system in liver metabolism: Gender differences and role of incretins. Metabolites 2022, 12, 411. [Google Scholar] [CrossRef]
- Rajapaksha, I.G.; Gunarathne, L.S.; Angus, P.W.; Herath, C.B. Update on new aspects of the renin-angiotensin system in hepatic fibrosis and portal hypertension: Implications for novel therapeutic options. J. Clin. Med. 2021, 10, 702. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Zhu, Y.; Lee, M.; Nguyen, A.; Ryujin, N.T.; Huang, J.Y.; Pandit, S.K.; Chamseddine, S.; Xiao, L.; Mohamed, Y.I. Angiotensin II receptor inhibition ameliorates liver fibrosis and enhances hepatocellular carcinoma infiltration by effector T cells. Proc. Natl. Acad. Sci. USA 2023, 120, e2300706120. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Pasanisi, G.; Notarstefano, P.; Campo, G.; Gardini, E.; Ceconi, C. Specific properties and effect of perindopril in controlling the renin–angiotensin system. Am. J. Hypertens 2005, 18, 142S–154S. [Google Scholar] [CrossRef]
- Sayed, A.M.; Abdel-Fattah, M.M.; Arab, H.H.; Mohamed, W.R.; Hassanein, E.H. Targeting inflammation and redox aberrations by perindopril attenuates methotrexate-induced intestinal injury in rats: Role of TLR4/NF-κB and c-Fos/c-Jun pro-inflammatory pathways and PPAR-γ/SIRT1 cytoprotective signals. Chem. Biol. Interact. 2022, 351, 109732. [Google Scholar] [CrossRef]
- Ertunç, O.; Erzurumlu, Y.; Savran, M.; Çatakli, D.; Kiran, E.D.; Pekgöz, Ş. Potential Hepatoprotective Effects of Irbesartan, an Accessible Angiotensin II Receptor Blocker, Against Cisplatin-Induced Liver Injury in a Rat Model. Turk. J. Pharm. Sci. 2024, 21, 88. [Google Scholar] [CrossRef]
- McGrath, M.S.; Wentworth, B. The Renin–Angiotensin System in Liver Disease. Int. J. Mol. Sci. 2024, 25, 5807. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Chen, J.; Long, T.; Xu, M.; Luo, T.; Che, Q.; He, Y.; Xu, D. The role of sirtuin1 in liver injury: Molecular mechanisms and novel therapeutic target. PeerJ 2024, 12, e17094. [Google Scholar] [CrossRef]
- Kostakoglu, U.; Mercantepe, T.; Yilmaz, H.K.; Tumkaya, L.; Batcik, S.; Pinarbas, E.; Uydu, H.A. The protective effects of perindopril against acute kidney damage caused by septic shock. Inflammation 2021, 44, 148–159. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and its roles in inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef]
- Ilhan, I.; Asci, H.; Candan, I.A.; Savran, M.; Imeci, O.B.; Sevuk, M.A. Cannabidiol mitigates methotrexate-induced hepatic injury via SIRT-1/p53 signaling and mitochondrial pathways: Reduces oxidative stress and inflammation. Drug Chem. Toxicol. 2024, 48, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Blokker, B.A.; Maijo, M.; Echeandia, M.; Galduroz, M.; Patterson, A.M.; Ten, A.; Philo, M.; Schungel, R.; Gutierrez-de Juan, V.; Halilbasic, E.; et al. Fine-tuning of sirtuin 1 expression is essential to protect the liver from cholestatic liver disease. Hepatology 2019, 69, 699–716. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.F.; Li, J.X.; Liao, H.T.; Liao, M.H.; Luo, L.; Xu, L.; Yuan, K.F.; Zeng, Y. Nicotinamide induces liver regeneration and improves liver function by activating SIRT1. Mol. Med. Rep. 2019, 19, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Rahman, M.A.; Koka, S.; Boini, K.M. High Mobility Group Box 1 (HMGB1): Molecular Signaling and Potential Therapeutic Strategies. Cells 2024, 13, 1946. [Google Scholar] [CrossRef]
- Bedoui, Y.; Guillot, X.; Sélambarom, J.; Guiraud, P.; Giry, C.; Jaffar-Bandjee, M.C.; Ralandison, S.; Gasque, P. Methotrexate an old drug with new tricks. Int. J. Mol. Sci. 2019, 20, 5023. [Google Scholar] [CrossRef]
- Taverna, S.; Tonacci, A.; Ferraro, M.; Cammarata, G.; Cuttitta, G.; Bucchieri, S.; Pace, E.; Gangemi, S. High mobility group box 1: Biological functions and relevance in oxidative stress related chronic diseases. Cells 2022, 11, 849. [Google Scholar] [CrossRef]
- Tan, H.; Hu, J.; Zuo, W.; Huang, Y.; Cui, J.; Gong, F.; Bai, W. Activation of the High Mobility Group Box 1/Receptor for Advanced Glycation Endproducts/NOD-like Receptor Family Pyrin Domain-Containing 3 Axis Under Chronic Intermittent Hypoxia Induction Promotes the Progression of Atherosclerosis in ApoE−/− Mice. J. Am. Heart Assoc. 2023, 12, e024397. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Z.; Zhang, Y.; Peng, L.J.; Hepatology, T. Emerging Roles of High-mobility Group Box-1 in Liver Disease. J. Clin. Transl. Hepatol. 2024, 12, 1043. [Google Scholar] [CrossRef]
- Levêque, D.; Becker, G.; Toussaint, E.; Fornecker, L.-M.; Paillard, C. Clinical pharmacokinetics of methotrexate in oncology. Int. J. Pharmacokinet. 2017, 2, 137–147. [Google Scholar] [CrossRef]
- Rana, R.M.; Rampogu, S.; Abid, N.B.; Zeb, A.; Parate, S.; Lee, G.; Yoon, S.; Kim, Y.; Kim, D.; Lee, K.W. In silico study identified methotrexate analog as potential inhibitor of drug resistant human dihydrofolate reductase for cancer therapeutics. Molecules 2020, 25, 3510. [Google Scholar] [CrossRef]
- Moridi, N.; Najafzadeh, M.; Sayedi, M.; Sajjadi, S.M.; Medicine, C. Astaxanthin Co-treatment with Low Dose Methotrexate Increases the Cell Cycle Arrest and Ameliorates the Methotrexate-induced Inflammatory Response in NALM-6. Int. J. Mol. Cell. Med. 2024, 13, 133. [Google Scholar]
- Meunier, L.; Larrey, D. Drug-induced liver injury: Biomarkers, requirements, candidates, and validation. Front. Pharmacol. 2019, 10, 1482. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Messner, C.J.; Gaiser, C.; Hämmerli, C.; Suter-Dick, L. Methotrexate-induced liver injury is associated with oxidative stress, impaired mitochondrial respiration, and endoplasmic reticulum stress in vitro. Int. J. Mol. Sci. 2022, 23, 15116. [Google Scholar] [CrossRef] [PubMed]
- Alfwuaires, M.A.; Research, P. Galangin mitigates oxidative stress, inflammation, and apoptosis in a rat model of methotrexate hepatotoxicity. Environ. Sci. Pollut. Res. 2022, 29, 20279–20288. [Google Scholar] [CrossRef]
- Akman, A.U. Methotrexate induced hepatotoxicity and antioxidants. Sabuncuoglu Serefeddin Health Sci. 2021, 3, 22–35. [Google Scholar]
- Fouad, A.; Hafez, H.; Hamouda, A. Hydrogen sulfide modulates IL-6/STAT3 pathway and inhibits oxidative stress, inflammation, and apoptosis in rat model of methotrexate hepatotoxicity. Hum. Exp. Toxicol. 2020, 39, 77–85. [Google Scholar] [CrossRef]
- Gong, Z.-J.; Song, S.-L.; Huang, Y.-Q.; Ruan, P. Effects of perindopril and valsartan on the expression of TGF beta 1 and TGF beta receptor II mRNA, Smad3 and Smad7 in experimental hepatic fibrotic rats. Chin. J. Hepatol. 2004, 12, 737–740. [Google Scholar]
- Saber, S. Angiotensin II: A key mediator in the development of liver fibrosis and cancer. Bull. Natl. Res. Cent. 2018, 42, 18. [Google Scholar] [CrossRef]
- Silva-Velasco, D.L.; Cervantes-Pérez, L.G.; Sánchez-Mendoza, A. ACE inhibitors and their interaction with systems and molecules involved in metabolism. Heliyon 2024, 10, e24655. [Google Scholar] [CrossRef]
- Al-khawalde, A.A.-m.A.; Abukhalil, M.H.; Jghef, M.M.; Alfwuaires, M.A.; Alaryani, F.S.; Aladaileh, S.H.; Algefare, A.I.; Karimulla, S.; Alasmari, F.; Aldal’in, H.K.; et al. Punicalagin protects against the development of methotrexate-induced hepatotoxicity in mice via activating Nrf2 signaling and decreasing oxidative stress, inflammation, and cell death. Int. J. Mol. Sci. 2022, 23, 12334. [Google Scholar] [CrossRef]
- Chen, F.; Xiao, M.; Feng, J.; Wufur, R.; Liu, K.; Hu, S.; Zhang, Y. Different inhibition of Nrf2 by two Keap1 isoforms α and β to shape malignant behaviour of human hepatocellular carcinoma. Int. J. Mol. Sci. 2022, 23, 10342. [Google Scholar] [CrossRef] [PubMed]
- Sweilam, S.H.; Ali, D.E.; Atwa, A.M.; Elgindy, A.M.; Mustafa, A.M.; Esmail, M.M.; Alkabbani, M.A.; Senna, M.M.; El-Shiekh, R.A. A First Metabolite Analysis of Norfolk Island Pine Resin and Its Hepatoprotective Potential to Alleviate Methotrexate (MTX)-Induced Hepatic Injury. Pharmaceuticals 2024, 17, 970. [Google Scholar] [CrossRef] [PubMed]
- Kabel, A.M.; Atef, A.; Borg, H.M.; El-Sheikh, A.A.; Al Khabbaz, H.J.; Arab, H.H.; Estfanous, R.S. Perindopril/ambrosin combination mitigates dextran sulfate sodium-induced colitis in mice: Crosstalk between toll-like receptor 4, the pro-inflammatory pathways, and SIRT1/PPAR-γ signaling. Pharmaceuticals 2022, 15, 600. [Google Scholar] [CrossRef] [PubMed]
- Ucar, B.I.; Ucar, G.; Saha, S.; Buttari, B.; Profumo, E.; Saso, L. Pharmacological protection against ischemia-reperfusion injury by regulating the Nrf2-Keap1-ARE signaling pathway. Antioxidants 2021, 10, 823. [Google Scholar] [CrossRef]
- Abd-Alhameed, E.K.; Azouz, A.A.; Abo-Youssef, A.M.; Ali, F.E. The enteroprotective effect of nifuroxazide against methotrexate-induced intestinal injury involves co-activation of PPAR-γ, SIRT1, Nrf2, and suppression of NF-κB and JAK1/STAT3 signals. Int. Immunopharmacol. 2024, 127, 111298. [Google Scholar] [CrossRef]
- Pande, S.; Raisuddin, S. Molecular and cellular regulatory roles of sirtuin protein. Crit. Rev. Food Sci. Nutr. 2023, 63, 9895–9913. [Google Scholar] [CrossRef]
- Yan, T.; Huang, J.; Nisar, M.F.; Wan, C.; Huang, W.; Longevity, C. The beneficial roles of SIRT1 in drug-induced liver injury. Oxidative Med. Cell. Longev. 2019, 2019, 8506195. [Google Scholar] [CrossRef]
- Hong, F.; Pan, S.; Guo, Y.; Xu, P.; Zhai, Y. PPARs as nuclear receptors for nutrient and energy metabolism. Molecules 2019, 24, 2545. [Google Scholar] [CrossRef]
- Meng, D.; Zhang, F.; Yu, W.; Zhang, X.; Yin, G.; Liang, P.; Feng, Y.; Chen, S.; Liu, H. Biological role and related natural products of SIRT1 in nonalcoholic fatty liver. Diabetes Metab. Syndr. Obes. Targets Ther. 2023, 16, 4043–4064. [Google Scholar] [CrossRef]
- Alnakhli, A.M.; Saleh, A.; Kabel, A.M.; Estfanous, R.S.; Borg, H.M.; Alsufyani, K.M.; Sabry, N.M.; Gomaa, F.A.M.; Abd Elmaaboud, M.A. Perindopril Ameliorates Sodium Valproate-Induced Rat Model of Autism: Involvement of Sirtuin-1, JAK2/STAT3 Axis, PI3K/Akt/GSK-3β Pathway, and PPAR-Gamma Signaling. Medicina 2024, 60, 1802. [Google Scholar] [CrossRef]
- Wang, C.; Leng, M.; Ding, C.; Zhu, X.; Zhang, Y.; Sun, C.; Lou, P. Ferritinophagy-mediated ferroptosis facilitates methotrexate-induced hepatotoxicity by high-mobility group box 1 (HMGB1). Liver Int. 2024, 44, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.-A.; Chen, H.; Nie, H.; Zheng, B.; Gong, Q. HMGB1: An overview of its roles in the pathogenesis of liver disease. J. Leukoc. Biol. 2021, 110, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Khambu, B.; Yan, S.; Huda, N.; Yin, X.-M. Role of high-mobility group box-1 in liver pathogenesis. Int. J. Mol. Sci. 2019, 20, 5314. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.-j.; Zhou, Y.-h.; Wang, J.-g.; Li, L.; Lu, S. Perindopril improves cardiac function in doxorubicin-induced cardiotoxicity rats. Curr. Sci. 2020, 119, 1838–1845. [Google Scholar] [CrossRef]
- Kamel, E.O.; Hassanein, E.H.; Ahmed, M.A.; Ali, F.E. Perindopril ameliorates hepatic ischemia reperfusion injury via regulation of NF-κB-p65/TLR-4, JAK1/STAT-3, Nrf-2, and PI3K/Akt/mTOR signaling pathways. Anat. Rec. 2020, 303, 1935–1949. [Google Scholar] [CrossRef]
- Arab, H.H.; Abd El-Aal, S.A.; Eid, A.H.; Arafa, E.-S.A.; Mahmoud, A.M.; Ashour, A.M. Targeting inflammation, autophagy, and apoptosis by troxerutin attenuates methotrexate-induced renal injury in rats. Int. Immunopharmacol. 2022, 103, 108284. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Hardie, D.G. New insights into activation and function of the AMPK. Nat. Rev. Mol. Cell Biol. 2023, 24, 255–272. [Google Scholar] [CrossRef]
- Marcondes-de-Castro, I.A.; Reis-Barbosa, P.H.; Marinho, T.S.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. AMPK/mTOR pathway significance in healthy liver and non-alcoholic fatty liver disease and its progression. J. Gastroenterol. Hepatol. 2023, 38, 1868–1876. [Google Scholar] [CrossRef]
- Zakaria, S.; Allam, S.; El-Sisi, A.E. Perindopril sensitizes hepatocellular carcinoma to chemotherapy: A possible role of leptin/Wnt/β-catenin axis with subsequent inhibition of liver cancer stem cells. Saudi Pharm. J. 2022, 30, 1170–1180. [Google Scholar] [CrossRef]
- Mahmoud, N.M.; Elshazly, S.M.; El-Shaarawy, F.; Zaitone, S.A.; Aldahish, A.A.; Ahmed, G.A.; Fawzy, M.S.; Aloyouni, S.Y.; Abed, S.Y.; Saeedi, T. Nitazoxanide mitigates methotrexate hepatotoxicity in rats: Role in inhibiting apoptosis and regulating endoplasmic reticulum stress. Front. Pharmacol. 2024, 15, 1491249. [Google Scholar] [CrossRef]
- Dogra, A.; Gupta, D.; Bag, S.; Ahmed, I.; Bhatt, S.; Nehra, E.; Dhiman, S.; Kumar, A.; Singh, G.; Abdullah, S.T. Glabridin ameliorates methotrexate-induced liver injury via attenuation of oxidative stress, inflammation, and apoptosis. Life Sci. 2021, 278, 119583. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, N.; Amer, M.E.; Amer, M.A.; El-Missiry, M.A.; Othman, A.I. Melatonin mitigated methotrexate-induced hepatotoxicity through interrelated biological processes. Mol. Biol. Rep. 2024, 51, 833. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Rahman, S.S.; Fayed, H.M.; Abd El-Rahman, S.S. Page: Targeting AngII/AT1R signaling pathway by Perindopril inhibits ongoing liver fibrosis in rat. J. Tissue Eng. Regen. Med. 2019, 13, 2131–2141. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, Z.; Nie, B.; Chen, D. Perindopril inhibits myocardial apoptosis in mice with acute myocardial infarction through TLR4/NF-κB pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6672–6682. [Google Scholar]
- Ali, N.; Rashid, S.; Nafees, S.; Hasan, S.K.; Sultana, S. Beneficial effects of Chrysin against Methotrexate-induced hepatotoxicity via attenuation of oxidative stress and apoptosis. Mol. Cell. Biochem. 2014, 385, 215–223. [Google Scholar] [CrossRef]
- Elsawy, H.; Algefare, A.I.; Alfwuaires, M.; Khalil, M.; Elmenshawy, O.M.; Sedky, A.; Abdel-Moneim, A.M. Naringin alleviates methotrexate-induced liver injury in male albino rats and enhances its antitumor efficacy in HepG2 cells. Biosci. Rep. 2020, 40, BSR20193686. [Google Scholar] [CrossRef]
- Betto, M.R.; Lazarotto, L.F.; Watanabe, T.T.; Driemeier, D.; Leite, C.E.; Campos, M.M. Effects of treatment with enalapril on hepatotoxicity induced by acetaminophen in mice. Naunyn Schmiedeberg’s Arch. Pharmacol. 2012, 385, 933–943. [Google Scholar] [CrossRef]
- Eid, B.G.; El-Shitany, N.A. Captopril downregulates expression of Bax/cytochrome C/caspase-3 apoptotic pathway, reduces inflammation, and oxidative stress in cisplatin-induced acute hepatic injury. Biomed. Pharmacother. 2021, 139, 111670. [Google Scholar] [CrossRef]
- Xu, W.; Song, S.; Huang, Y.; Gong, Z.J. Effects of perindopril and valsartan on expression of transforming growth factor-β-Smads in experimental hepatic fibrosis in rats. J. Gastroenterol. Hepatol. 2006, 21, 1250–1256. [Google Scholar] [CrossRef]
- Clark, L.T. Safety profile of perindopril. Am. J. Cardiol. 2001, 88, 36–40. [Google Scholar] [CrossRef]
- Dalaklioglu, S.; Genc, G.; Aksoy, N.; Akçit, F.; Gumuslu, S. Resveratrol ameliorates methotrexate-induced hepatotoxicity in rats via inhibition of lipid peroxidation. Hum. Exp. Toxicol. 2013, 32, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, Y.M.; Sattar, M.A.A.A.; Kamel, F.O.; Ramadan, W.S.; Alzahrani, Y.A. Possible combined effect of perindopril and Azilsartan in an experimental model of dementia in rats. Saudi Pharm. J. 2020, 28, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Mashhoody, T.; Rastegar, K.; Zal, F. Perindopril may improve the hippocampal reduced glutathione content in rats. Adv. Pharm. bulletin 2013, 4, 155. [Google Scholar]
- Křížova, E.; Imek, V.S.; Abelenda, M.; Puerta, M. Food intake and body weight in rats with daily food-availability restrictions. Physiol. Behav. 1996, 60, 791–794. [Google Scholar] [CrossRef]
- Moura, E.L.; Dos Santos, H.; Celes, A.P.M.; Bassani, T.B.; Souza, L.C.; Vital, M.A. Effects of a nutritional formulation containing caprylic and capric acid, phosphatidylserine, and docosahexaenoic acid in streptozotocin-lesioned rats. J. Alzheimer’s Dis. Rep. 2020, 4, 353–363. [Google Scholar] [CrossRef]
- Han, Q.-J.; Mu, Y.-L.; Zhao, H.-J.; Zhao, R.-R.; Guo, Q.-J.; Su, Y.-H.; Zhang, J. Fasudil prevents liver fibrosis via activating natural killer cells and suppressing hepatic stellate cells. World J. Gastroenterol. 2021, 27, 3581. [Google Scholar] [CrossRef]
- Lu, W.; Kang, J.; Hu, K.; Tang, S.; Zhou, X.; Xu, L.; Li, Y.; Yu, S. The role of the Nox4-derived ROS-mediated RhoA/Rho kinase pathway in rat hypertension induced by chronic intermittent hypoxia. Sleep Breath. 2017, 21, 667–677. [Google Scholar] [CrossRef]
- Eckle, V.-S.; Buchmann, A.; Bursch, W.; Schulte-Hermann, R.; Schwarz, M. Immunohistochemical detection of activated caspases in apoptotic hepatocytes in rat liver. Toxicol. Pathol. 2004, 32, 9–15. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atia, H.A.; Elariny, H.A.; Abdallah, M.H.; Khalifa, A.M.; Estfanous, R.S.; Abd Elmaaboud, M.A.; Kabel, A.M. Repositioning Perindopril for Mitigation of Methotrexate-Induced Hepatotoxicity in Rats. Pharmaceuticals 2025, 18, 358. https://doi.org/10.3390/ph18030358
Atia HA, Elariny HA, Abdallah MH, Khalifa AM, Estfanous RS, Abd Elmaaboud MA, Kabel AM. Repositioning Perindopril for Mitigation of Methotrexate-Induced Hepatotoxicity in Rats. Pharmaceuticals. 2025; 18(3):358. https://doi.org/10.3390/ph18030358
Chicago/Turabian StyleAtia, Hanan Abdelmawgoud, Hemat A. Elariny, Marwa H. Abdallah, Amany M. Khalifa, Remon S. Estfanous, Maaly A. Abd Elmaaboud, and Ahmed M. Kabel. 2025. "Repositioning Perindopril for Mitigation of Methotrexate-Induced Hepatotoxicity in Rats" Pharmaceuticals 18, no. 3: 358. https://doi.org/10.3390/ph18030358
APA StyleAtia, H. A., Elariny, H. A., Abdallah, M. H., Khalifa, A. M., Estfanous, R. S., Abd Elmaaboud, M. A., & Kabel, A. M. (2025). Repositioning Perindopril for Mitigation of Methotrexate-Induced Hepatotoxicity in Rats. Pharmaceuticals, 18(3), 358. https://doi.org/10.3390/ph18030358






