Usage Frequency and Ecotoxicity of Skin Depigmenting Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Overview of the Use of Depigmenting Agents in Cosmetic Products
2.2. Scientific Evidence Regarding Aquatic Toxicity and Biodegradability of Most-Used Depigmenting Agents
2.2.1. Hydroxy (HA) and Keto Acids
2.2.2. Vitamin C and Derivatives
2.2.3. Niacinamide
2.2.4. Resorcinol Derivatives
2.2.5. Peptides and Amino Acids
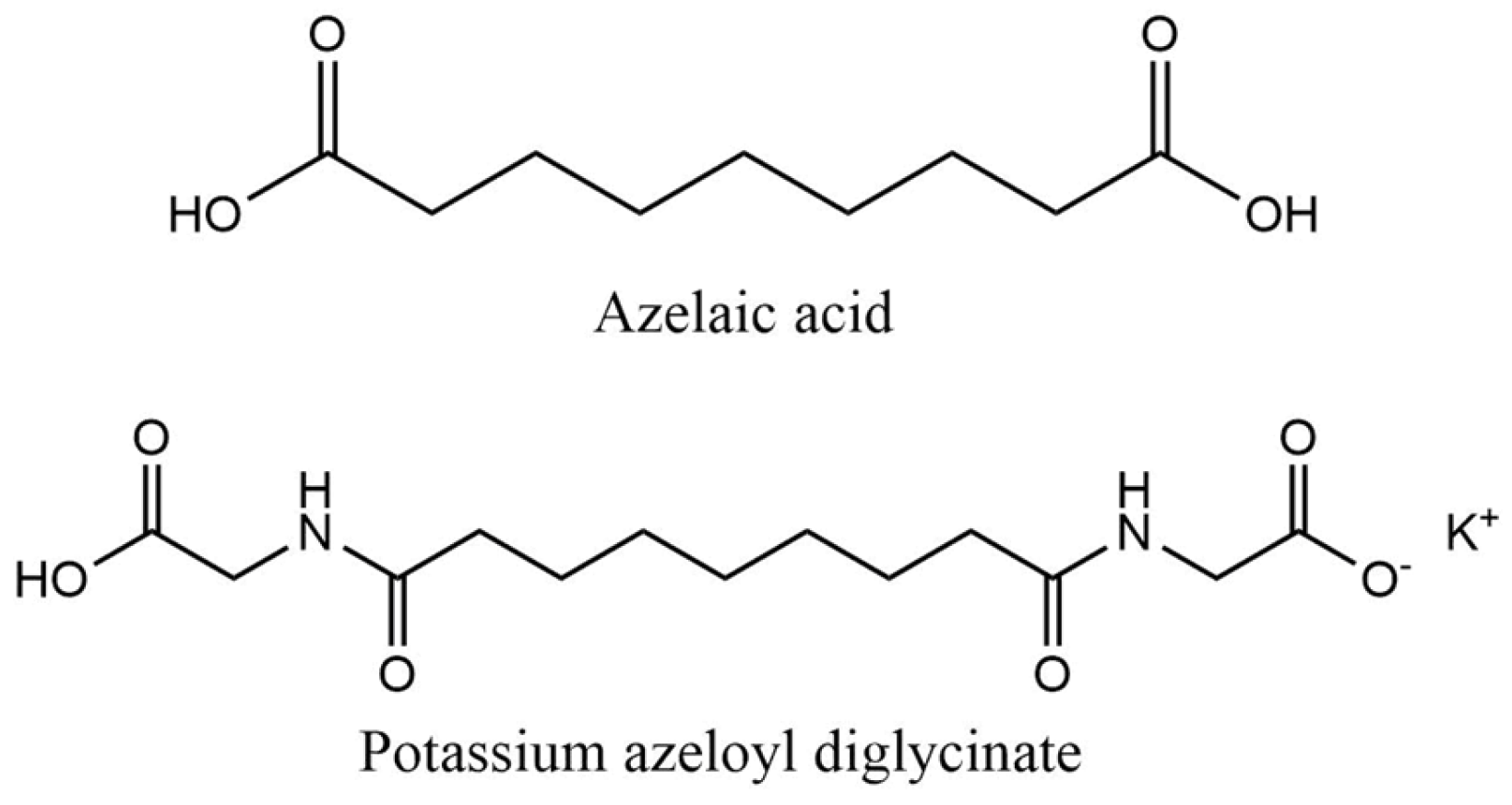
2.2.6. Azelaic Acid and Derivatives
2.2.7. Tranexamic Acid
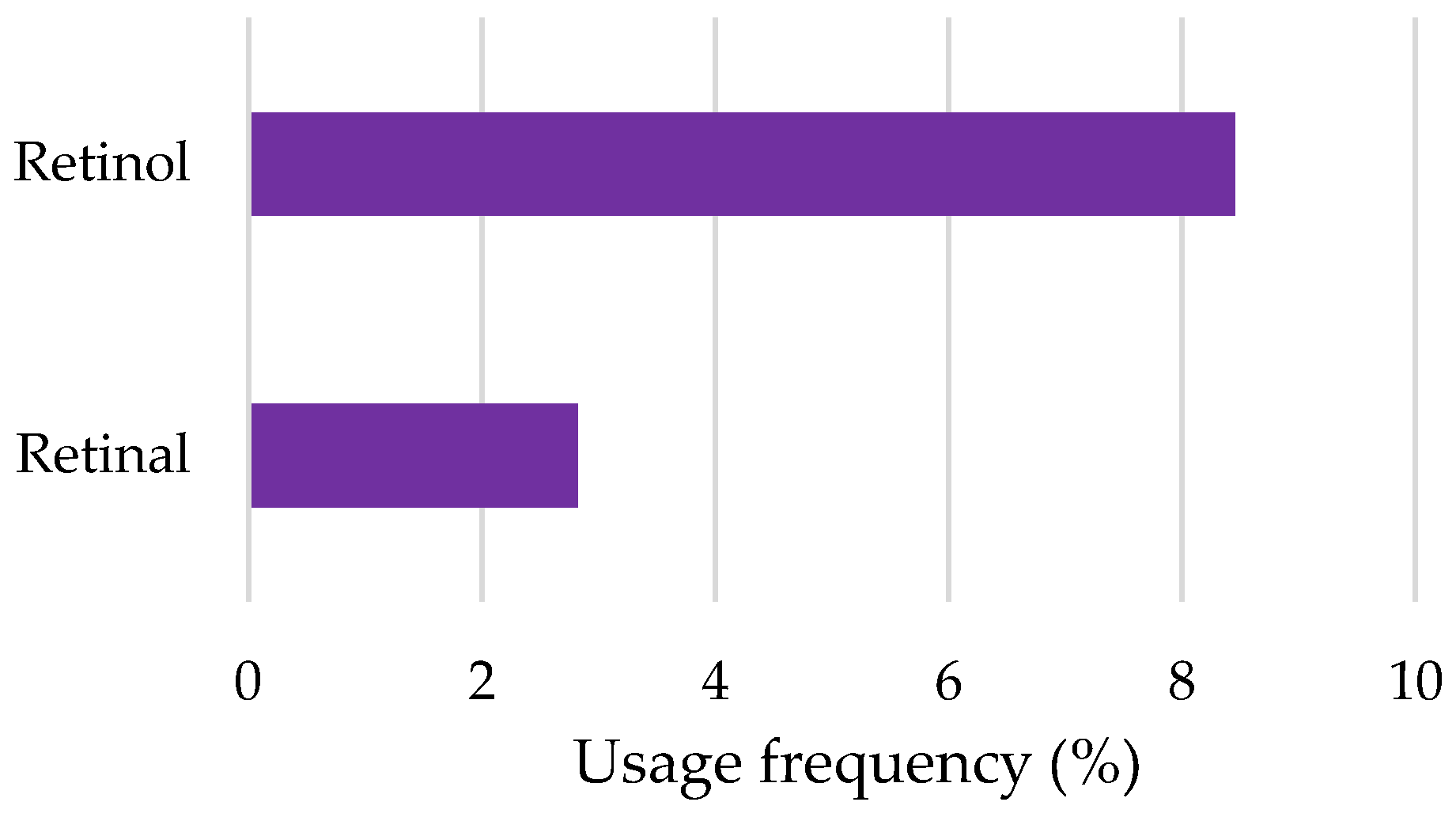
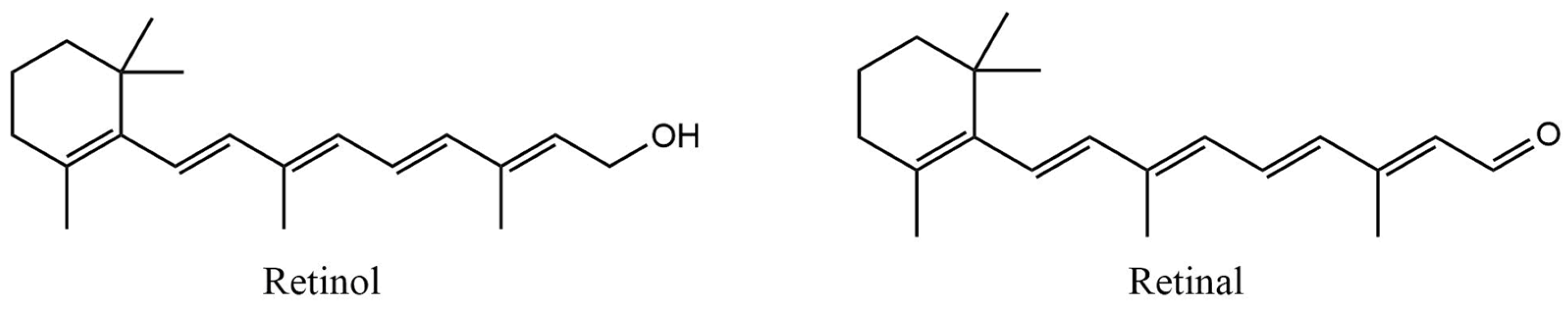
2.2.8. Retinoids
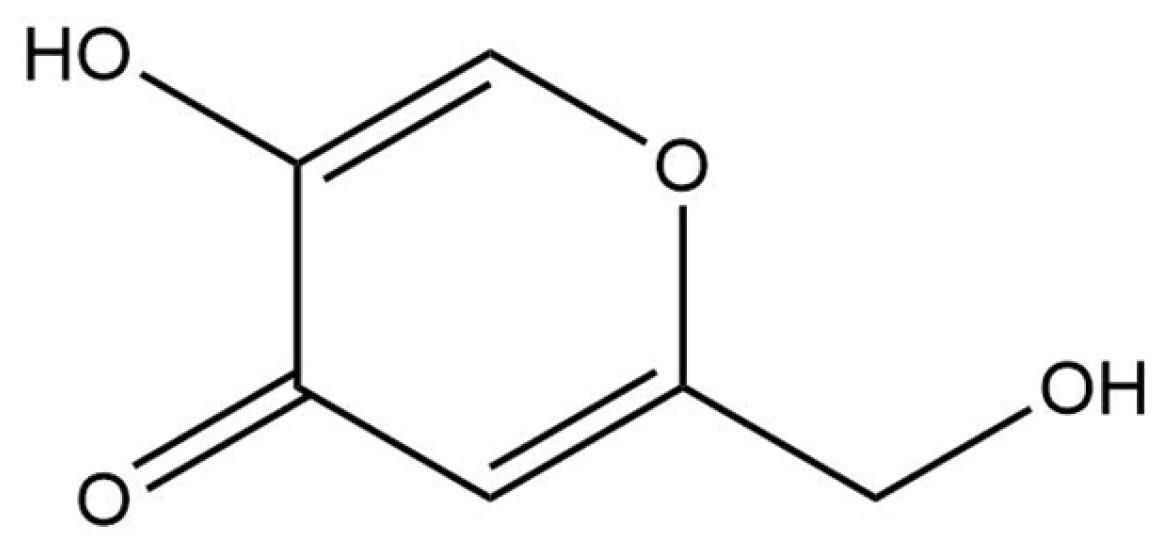
2.2.9. Kojic Acid
2.2.10. N-Acetylglucosamine
3. Materials and Methods
3.1. Data Collection
3.1.1. Product Selection Criteria
3.1.2. Data Sources
3.1.3. Exclusion Criteria
3.1.4. Ecotoxicity Analysis
3.2. Data Analysis
3.2.1. Ingredient Identification and Classification
3.2.2. Analysis Parameters
- Top Depigmenting Agents Used in Cosmetic Products
- Scientific Evidence Regarding Aquatic Toxicity and Biodegradability of Depigmenting Agents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, W.; Yang, A.; Wang, J.; Huang, D.; Deng, Y.; Zhang, X.; Qu, Q.; Ma, W.; Xiong, R.; Zhu, M.; et al. Potential application of natural bioactive compounds as skin-whitening agents: A review. J. Cosmet. Dermatol. 2022, 21, 6669–6687. [Google Scholar] [CrossRef] [PubMed]
- Resende, D.I.S.P.; Ferreira, M.S.; Lobo, J.M.S.; Sousa, E.; Almeida, I.F. Skin depigmenting agents in anti-aging cosmetics: A medicinal perspective on emerging ingredients. Appl. Sci. 2022, 12, 775. [Google Scholar] [CrossRef]
- Smit, N.; Vicanova, J.; Pavel, S. The hunt for natural skin whitening agents. Int. J. Mol. Sci. 2009, 10, 5326–5349. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Kang, M.; Chung, H.S.; Cho, C.; Hong, M.C.; Shin, M.K.; Bae, H. Survey and mechanism of skin depigmenting and lightening agents. Phytother. Res. 2006, 20, 921–934. [Google Scholar] [CrossRef]
- Rendon, M.I.; Gaviria, J.I. Review of skin-lightening agents. Dermatol. Surg. 2005, 31, 886–890. [Google Scholar] [CrossRef]
- Rotava, P.A.; Favero, J.S.; Garcia, K.R.; Angeli, V.W. Profile of depigmenting cosmetics and dermatological products on the market. Rev. Cienc. Farm. Basica Apl. 2020, 41, 1–11. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Taylor, A.; Pawaskar, M.; Taylor, S.L.; Balkrishnan, R.; Feldman, S.R. Prevalence of pigmentary disorders and their impact on quality of life: A prospective cohort study. J. Cosmet. Dermatol. 2008, 7, 164–168. [Google Scholar] [CrossRef]
- Dlova, N.C.; Akintilo, L.O.; Taylor, S.C. Prevalence of pigmentary disorders: A cross-sectional study in public hospitals in Durban, South Africa. Int. J. Womens Dermatol. 2019, 5, 345–348. [Google Scholar] [CrossRef]
- Yener, G.; Secer, A.; Ghazalian, P.L. What factors influence consumers to buy green products? An analysis through the motivation–opportunity–ability framework and consumer awareness. Sustainability 2023, 15, 13872. [Google Scholar] [CrossRef]
- Cosmetics Europe – The Personal Care Association. Environmental Sustainability—The European Cosmetics Industry’s Contribution 2017–2018; Cosmetics Europe–The Personal Care Association: Brussels, Belgium, 2018. [Google Scholar]
- Milosheska, D.; Roškar, R. Use of retinoids in topical antiaging treatments: A focused review of clinical evidence for conventional and nanoformulations. Adv. Ther. 2022, 39, 5351–5375. [Google Scholar] [CrossRef]
- Jafry, M.; Guan, L.L.; Mohammad, T.F. A practical guide to over-the-counter treatments for hyperpigmentation. JEADV Clin. Pract. 2024, 3, 433–447. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Overview of skin whitening agents: Drugs and cosmetic products. Cosmetics 2016, 3, 27. [Google Scholar] [CrossRef]
- Burger, P.; Landreau, A.; Azoulay, S.; Michel, T.; Fernandez, X. Skin whitening cosmetics: Feedback and challenges in the development of natural skin lighteners. Cosmetics 2016, 3, 36. [Google Scholar] [CrossRef]
- Caritá, A.C.; Fonseca-Santos, B.; Shultz, J.D.; Michniak-Kohn, B.; Chorilli, M.; Leonardi, G.R. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102117. [Google Scholar] [CrossRef]
- Jacques, C.; Genies, C.; Bacqueville, D.; Tourette, A.; Borotra, N.; Chaves, F.; Sanches, F.; Gaudry, A.L.; Bessou-Touya, S.; Duplan, H. Ascorbic acid 2-glucoside: An ascorbic acid pro-drug with longer-term antioxidant efficacy in skin. Int. J. Cosmet. Sci. 2021, 43, 691–702. [Google Scholar] [CrossRef]
- Pintea, A.; Manea, A.; Pintea, C.; Vlad, R.-A.; Bîrsan, M.; Antonoaea, P.; Rédai, E.M.; Ciurba, A. Peptides: Emerging candidates for the prevention and treatment of skin senescence: A review. Biomolecules 2025, 15, 88. [Google Scholar] [CrossRef]
- Searle, T.; Ali, F.R.; Al-Niaimi, F. The versatility of azelaic acid in dermatology. J. Dermatol. Treat. 2022, 33, 722–732. [Google Scholar] [CrossRef]
- Zeichner, J. New insights into azelaic acid. Pract. Dermatol. 2013, 45–46. [Google Scholar]
- Kornhauser, A.; Coelho, S.G.; Hearing, V.J. Applications of hydroxy acids: Classification, mechanisms, and photoactivity. Clin. Cosmet. Investig. Dermatol. 2010, 3, 135–142. [Google Scholar] [CrossRef]
- Cosmetic Ingredient Review. Safety Assessment of Alpha Hydroxy Acids as Used in Cosmetics; Cosmetic Ingredient Review: Washington, DC, USA, 2013. [Google Scholar]
- Green, B.A.; Yu, R.J.; Van Scott, E.J. Clinical and cosmeceutical uses of hydroxyacids. Clin. Dermatol. 2009, 27, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Van Scott, E.J.; Yu, R.J. Control of keratinization with alpha-hydroxy acids and related compounds. I. Topical treatment of ichthyotic disorders. Arch. Dermatol. 1974, 110, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Ditre, C.M.; Griffin, T.D.; Murphy, G.F.; Sueki, H.; Telegan, B.; Johnson, W.C.; Yu, R.J.; Van Scott, E.J. Effects of alpha-hydroxy acids on photoaged skin: A pilot clinical, histologic, and ultrastructural study. J. Am. Acad. Dermatol. 1996, 34, 187–195. [Google Scholar] [CrossRef]
- Karwal, K.; Mukovozov, I. Topical AHA in dermatology: Formulations, mechanisms of action, efficacy, and future perspectives. Cosmetics 2023, 10, 131. [Google Scholar] [CrossRef]
- Almeman, A.A. Evaluating the efficacy and safety of alpha-hydroxy acids in dermatological practice: A comprehensive clinical and legal review. Clin. Cosmet. Investig. Dermatol. 2024, 17, 1661–1685. [Google Scholar] [CrossRef]
- Freelance Formulations. Exploring Common and Uncommon Acids in Skin Care. Available online: https://www.freelanceformulations.com/post/exploring-common-and-uncommon-acids-in-skin-care (accessed on 26 September 2024).
- Arif, T. Salicylic acid as a peeling agent: A comprehensive review. Clin. Cosmet. Investig. Dermatol. 2015, 8, 455–461. [Google Scholar] [CrossRef]
- Davies, M.; Marks, R. Studies on the effect of salicylic acid on normal skin. Br. J. Dermatol. 1976, 95, 187–192. [Google Scholar] [CrossRef]
- Fiume, M.M.; Heldreth, B.A.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Safety assessment of citric acid, inorganic citrate salts, and alkyl citrate esters as used in cosmetics. Int. J. Toxicol. 2014, 33, 16S–46S. [Google Scholar] [CrossRef]
- Williams, R.J.E. The Use and Safety of Hydroxy Acids in Cosmetics; Australian Society of Cosmetic Chemists (ASCC): Moorebank, Australia, 2005. [Google Scholar]
- Bernstein, E.F.; Brown, D.B.; Schwartz, M.D.; Kaidbey, K.; Ksenzenko, S.M. The polyhydroxy acid gluconolactone protects against ultraviolet radiation in an in vitro model of cutaneous photoaging. Dermatol. Surg. 2004, 30, 189–195, discussion 196. [Google Scholar] [CrossRef]
- Wulaningsih, T.I. Gluconolactone in cosmetic. KESANS Int. J. Health Sci. 2023, 2, 730–745. [Google Scholar] [CrossRef]
- Cotellesa, C.; Manunta, T.; Ghersetich, I.; Brazzini, B.; Peris, K. The use of pyruvic acid in the treatment of acne. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 275–278. [Google Scholar] [CrossRef]
- Jankowska, B.; Zujko, M.E. The effectiveness of pyruvic acid peeling in improving the quality of life of patients with acne vulgaris. J. Clin. Med. 2023, 12, 3592. [Google Scholar] [CrossRef]
- Tosson, Z.; Attwa, E.; Al-Mokadem, S. Pyruvic acid as a new therapeutic peeling agent in acne, melasma and warts. Egypt. Dermatol. Online J. 2006, 2, 7. [Google Scholar]
- European Commission. Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labeling and Packaging of Substances and Mixtures; European Commission: Brussels, Belgium, 2008. [Google Scholar]
- European Chemicals Agency (ECHA). Registration Dossier—Citric Acid. Available online: https://chem.echa.europa.eu/100.000.973/dossier-view/891540c8-350a-4291-95ce-9a577d99f6e7/7d3c8b9b-e7a7-4fd6-a232-227918bc8085_7d3c8b9b-e7a7-4fd6-a232-227918bc8085?searchText=citric%20acid (accessed on 26 October 2023).
- European Chemicals Agency (ECHA). Registration Dossier—Salicylic Acid. Available online: https://chem.echa.europa.eu/100.000.648/dossier-view/58350bce-5d00-4254-90f2-fc9bbae79437/04470c3b-cf4a-4b6c-adae-76df0f50e040_04470c3b-cf4a-4b6c-adae-76df0f50e040?searchText=salicylic%20acid (accessed on 26 October 2023).
- European Chemicals Agency (ECHA). Registration Dossier—Glycolic Acid. Available online: https://chem.echa.europa.eu/100.001.073/dossier-list/reach/dossiers/active?searchText=glycolic%20acid (accessed on 26 October 2023).
- European Chemicals Agency (ECHA). Registration Dossier—Lactic Acid. Available online: https://chem.echa.europa.eu/100.000.017/dossier-list/reach/dossiers/active?searchText=lactic%20acid (accessed on 26 October 2023).
- European Chemicals Agency (ECHA). Registration Dossier—Malic Acid. Available online: https://chem.echa.europa.eu/100.027.293/dossier-view/98f2d387-6152-4a27-89e5-7486a5627d63/9517df64-0c6b-4645-b7a2-e5d278aae26f_9517df64-0c6b-4645-b7a2-e5d278aae26f?searchText=malic%20acid (accessed on 23 October 2023).
- European Chemicals Agency (ECHA). Registration Dossier—Tartaric Acid. Available online: https://chem.echa.europa.eu/100.001.606/dossier-view/0cdd8531-8acb-469b-a934-c2de6fc6d515/aa654079-0621-4c29-bc7d-f2411d473f10_aa654079-0621-4c29-bc7d-f2411d473f10?searchText=tartaric%20acid (accessed on 23 October 2023).
- European Chemicals Agency (ECHA). Registration Dossier—Fytic Acid. Available online: https://chem.echa.europa.eu/100.001.369/dossier-list/reach/dossiers/active?searchText=phytic%20acid (accessed on 25 October 2023).
- European Chemicals Agency (ECHA). Registration Dossier—Mandelic Acid. Available online: https://chem.echa.europa.eu/100.001.825/dossier-view/71d849af-b8c7-4f40-95d0-60bb50b876f3/7e2da5cf-69fd-4bff-ba63-3e4ddbf3fa9f_7e2da5cf-69fd-4bff-ba63-3e4ddbf3fa9f?searchText=mandelic%20acid (accessed on 25 October 2023).
- ChemSpider. Pyruvic Acid. Available online: https://www.chemspider.com/Chemical-Structure.1031.html?rid=58614bec-5164-465c-a3d9-64f8b4f1d6cc (accessed on 3 March 2024).
- ChemSpider. Maltobionic Acid. Available online: https://www.chemspider.com/Chemical-Structure.2300679.html?rid=6d503220-193e-4d4c-8642-f67b23b1c479 (accessed on 3 March 2024).
- ChemSpider. D-Glucono-δ-Lactone. Available online: https://www.chemspider.com/Chemical-Structure.6760.html?rid=9f5f6f11-85e8-450c-9b13-4f5f4589c72a (accessed on 3 March 2024).
- Pinnell, S.R.; Madey, D.L. Topical vitamin C in skin care. Aesthetic Surg. J. 1998, 18, 468–470. [Google Scholar] [CrossRef] [PubMed]
- Takenouchi, K.; Aso, K. The relation between melanin formation and ascorbic acid. J. Vitaminol. 1964, 10, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Pasonen-Seppänen, S.; Suhonen, T.M.; Kirjavainen, M.; Suihko, E.; Urtti, A.; Miettinen, M.; Hyttinen, M.; Tammi, M.; Tammi, R. Vitamin C enhances differentiation of a continuous keratinocyte cell line (REK) into epidermis with normal stratum corneum ultrastructure and functional permeability barrier. Histochem. Cell Biol. 2001, 116, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Shultz, J.D.; Caritá, A.C.; Mohd, H.; Michniak-Kohn, B.; Aiello, L.M.; Leonardi, G.R. Cosmetics formulations containing vitamin C and the instability challenge. Res. J. Top. Cosmet. Sci. 2022, 13, 9–13. [Google Scholar] [CrossRef]
- Enrique Lorente, P. Cosmetic topical use of vitamin C. In Ascorbic Acid; Abdulsamed, K., Volkan, G., Eds.; IntechOpen: Rijeka, Croatia, 2023; Chapter 8. [Google Scholar]
- The Organization for Economic Cooperation and Development (OECD). SIDS (Screening Information Dataset) Initial Assessment Report; OECD: Paris, France, 1994. [Google Scholar]
- European Chemicals Agency (ECHA). Registration Dossier—(1S)-1-{(2R)-3,4-bis[(2-Hexyldecanoyl)oxy]-5-oxo-2,5-Dihydrofuran-2-yl}ethane-1,2-diyl bis(2-Hexyldecanoate). Available online: https://chem.echa.europa.eu/100.102.845/dossier-view/43ee0024-1039-4381-84e9-a62b9d5601f9/IUC5-fcb8148c-8136-4c78-ad25-bb7ac2e9f4b9_d67bee54-61f8-447d-91a9-4b97a569f8d4?searchText=183476-82-6 (accessed on 5 March 2024).
- European Chemicals Agency (ECHA). Registration Dossier—(5R)-5-[(1S)-1,2-Dihydroxyethyl]-4-Ethoxy-3-Hydroxy-5H-Furan-2-One. Available online: https://chem.echa.europa.eu/100.123.448/dossier-view/2eb8df08-c959-4ab3-93a1-463e84584a1d/IUC5-abee2aee-d09c-49d8-8d47-79dd258c6a2f_eeecef67-49b9-4ba9-9d19-df485a8f92b6?searchText=86404-04-8 (accessed on 10 March 2024).
- European Chemicals Agency (ECHA). Registration Dossier—Trisodium (2R)-2-[(1S)-1,2-Dihydroxyethyl]-5-oxo-4-(Phosphonooxy)-2,5-Dihydrofuran-3-Olate. Available online: https://chem.echa.europa.eu/100.102.364/dossier-view/8b7ba0f5-30f1-4002-b819-4151ce81a4be/9a7bc668-26c5-4c64-868c-bd4919670b87_d37217a3-84c3-4541-b9ca-c4474ec47344?searchText=66170-10-3 (accessed on 10 March 2024).
- European Chemicals Agency (ECHA). Registration Dossier—Sodium Ascorbate. Available online: https://chem.echa.europa.eu/100.004.661/dossier-view/102dde58-4e3f-4444-8445-a20a5b0ace9c/16c937f8-24e2-4ad6-b450-8ab19ce8656f_9b3d6fa0-1f83-47d1-93d4-1df408f91f90?searchText=ascorbic%20acid (accessed on 5 March 2024).
- European Chemicals Agency (ECHA). Registration Dossier—(5R)-5-[(1S)-1,2-Dihydroxyethyl]-4-Hydroxy-3-{[(2R,3R,4S,5S,6R)-3,4,5-Trihydroxy-6-(Hydroxymethyl)oxan-2-yl]oxy}-2,5-Dihydrofuran-2-One. Available online: https://chem.echa.europa.eu/100.102.444/dossier-view/1b87bd74-2e9a-4555-902a-857bad881290/IUC5-1e1ee32a-9827-46fc-bc39-f7f5f43683d0_624d83f5-9c49-48ea-b19b-7d1c15ea86f5?searchText=Ascorbyl%20glucoside (accessed on 5 March 2024).
- Hakozaki, T.; Minwalla, L.; Zhuang, J.; Chhoa, M.; Matsubara, A.; Miyamoto, K.; Greatens, A.; Hillebrand, G.G.; Bissett, D.L.; Boissy, R.E. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br. J. Dermatol. 2002, 147, 20–31. [Google Scholar] [CrossRef]
- Berson, D.S.; Osborne, R.; Oblong, J.E.; Hakozaki, T.; Johnson, M.B.; Bissett, D.L. Niacinamide. In Cosmeceuticals and Cosmetic Practice; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 103–112. [Google Scholar]
- Bissett, D.L.; Miyamoto, K.; Sun, P.; Li, J.; Berge, C.A. Topical niacinamide reduces yellowing, wrinkling, red blotchiness, and hyperpigmented spots in aging facial skin. Int. J. Cosmet. Sci. 2004, 26, 231–238. [Google Scholar] [CrossRef]
- Monfrecola, G.; Gaudiello, F.; Cirillo, T.; Fabbrocini, G.; Balato, A.; Lembo, S. Nicotinamide downregulates gene expression of interleukin-6, interleukin-10, monocyte chemoattractant protein-1, and tumour necrosis factor-α gene expression in HaCaT keratinocytes after ultraviolet B irradiation. Clin. Exp. Dermatol. 2013, 38, 185–188. [Google Scholar] [CrossRef]
- Cosmetic Ingredient Review Expert Panel. Final report of the safety assessment of niacinamide and niacin. Int. J. Toxicol. 2005, 24 (Suppl. 5), 1–31. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA). Registration Dossier—Nicotinamide. Available online: https://chem.echa.europa.eu/100.002.467/dossier-view/81331048-f0b1-402b-9a98-a23603b265f8/IUC5-f534cd87-319d-4b2f-bf34-601b8e5d79a1_57b0c2ea-b7ea-4420-bdb7-ee5faf089773?searchText=98-92-0 (accessed on 10 March 2024).
- Searle, T.; Al-Niaimi, F.; Ali, F.R. The top 10 cosmeceuticals for facial hyperpigmentation. Dermatol. Ther. 2020, 33, e14095. [Google Scholar] [CrossRef]
- William, W.A.; Messai, A.M.; Penny, P.G. Phenolic Compounds in Water: Sources, Reactivity, Toxicity and Treatment Methods. In Phenolic Compounds; Marcos, S.-H., Mariana, P.-T., Maria del Rosario, G.-M., Eds.; IntechOpen: Rijeka, Croatia, 2017; Chapter 17. [Google Scholar]
- Yang, L.-H.; Cheng, H.-Y.; Zhu, T.-T.; Wang, H.-C.; Haider, M.R.; Wang, A.-J. Resorcinol as a highly efficient aromatic electron donor in bioelectrochemical system. J. Hazard. Mater. 2021, 408, 124416. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.K.; Kielhorn, J.; Koppenhofer, J.; Wibbertmann, A.; Mangelsdorf, I. International Programme on Chemical Safety; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- European Chemicals Agency (ECHA). Registration Dossier—1,3-Benzenediol, 4-butyl-. Available online: https://echa.europa.eu/pt/registration-dossier/-/registered-dossier/1235/6/2/6/?documentUUID=32c84603-5929-4f70-94a4-93663d50edc6 (accessed on 21 June 2023).
- European Chemicals Agency (ECHA). Registration Dossier—1,3-Benzenediol, 4-(1-Phenylethyl)-. Available online: https://echa.europa.eu/pt/registration-dossier/-/registered-dossier/11024 (accessed on 21 June 2023).
- European Chemicals Agency (ECHA). Registration Dossier—4-Hexylresorcinol. Available online: https://echa.europa.eu/pt/registration-dossier/-/registered-dossier/19033 (accessed on 21 June 2023).
- European Chemicals Agency (ECHA). Registration Dossier—Propanamide, N-[4-(2,4-Dihydroxyphenyl)-2-Thiazolyl]-2-methyl-. Available online: https://echa.europa.eu/pt/registration-dossier/-/registered-dossier/16989 (accessed on 21 June 2023).
- Ngoc, L.T.N.; Moon, J.-Y.; Lee, Y.-C. Insights into bioactive peptides in cosmetics. Cosmetics 2023, 10, 111. [Google Scholar] [CrossRef]
- Noguchi, A.; Djerassi, D. Chapter 15—Amino acids and peptides: Building blocks for skin proteins. In Nutritional Cosmetics; Tabor, A., Blair, R.M., Eds.; William Andrew Publishing: Boston, MA, USA, 2009; pp. 287–317. [Google Scholar]
- Lintner, K. Peptides, amino acids and proteins in skin care. Cosmet. Toilet. 2007, 122, 26. [Google Scholar]
- Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Liceaga, A.M. Potential role of natural bioactive peptides for development of cosmeceutical skin products. Peptides 2019, 122, 170170. [Google Scholar] [CrossRef]
- Farwick, M.; Grether-Beck, S.; Marini, A.; Maczkiewitz, U.; Lange, J.; Köhler, T.; Lersch, P.; Falla, T.; Felsner, I.; Brenden, H.; et al. Bioactive tetrapeptide GEKG boosts extracellular matrix formation: In vitro and in vivo molecular and clinical proof. Exp. Dermatol. 2011, 20, 602–604. [Google Scholar] [CrossRef]
- Falla, T.J.; Zhang, L. Efficacy of hexapeptide-7 on menopausal skin. J. Drugs Dermatol. 2010, 9, 49–54. [Google Scholar]
- Bauza, E.; Oberto, G.; Berghi, A.; Dal, C.F.; Domloge, N. Collagen-like peptide exhibits a remarkable antiwrinkle effect on the skin when topically applied: In vivo study. Int. J. Tissue React. 2004, 26, 105–111. [Google Scholar]
- Fitzpatrick, R.E.; Rostan, E.F. Reversal of photodamage with topical growth factors: A pilot study. J. Cosmet. Laser Ther. 2003, 5, 25–34. [Google Scholar] [CrossRef]
- Karkouch, I.; Tabbene, O.; Gharbi, D.; Ben Mlouka, M.A.; Elkahoui, S.; Rihouey, C.; Coquet, L.; Cosette, P.; Jouenne, T.; Limam, F. Antioxidant, antityrosinase and antibiofilm activities of synthesized peptides derived from Vicia faba protein hydrolysate: A powerful agents in cosmetic application. Ind. Crops Prod. 2017, 109, 310–319. [Google Scholar] [CrossRef]
- Kubglomsong, S.; Theerakulkait, C.; Reed, R.L.; Yang, L.; Maier, C.S.; Stevens, J.F. Isolation and identification of tyrosinase-inhibitory and copper-chelating peptides from hydrolyzed rice-bran-derived albumin. J. Agric. Food Chem. 2018, 66, 8346–8354. [Google Scholar] [CrossRef] [PubMed]
- Nakchum, L.; Kim, S.M. Preparation of squid skin collagen hydrolysate as an antihyaluronidase, antityrosinase, and antioxidant agent. Prep. Biochem. Biotechnol. 2016, 46, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Widlund, H.R.; Fisher, D.E. Microphthalamia-associated transcription factor: A critical regulator of pigment cell development and survival. Oncogene 2003, 22, 3035–3041. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA). Registration Dossier—2-(undec-10-Enoylamino)-3-Phenylpropanoic Acid. Available online: https://chem.echa.europa.eu/100.104.081/dossier-view/cadc1de7-0943-4045-a313-7bf931abad35/IUC5-bc7bcc55-a589-4829-aecc-447ff9ecbfbb_593ba7bf-7097-4842-b33d-bf1db3f6d9c2?searchText=175357-18-3 (accessed on 10 March 2024).
- European Chemicals Agency (ECHA). Registration Dossier—3-[(2-Acetamidoacetyl)amino]Propanoic Acid. Available online: https://chem.echa.europa.eu/100.250.335/dossier-view/c8664d17-6d67-4209-b5dd-5885efb9894f/188bc598-81e2-4d82-9d09-57c04ffa2c00_89f2c4ad-d68d-4c4b-9619-c22ab912c445?searchText=1016788-34-3 (accessed on 10 March 2024).
- King, S.; Campbell, J.; Rowe, R.; Daly, M.-L.; Moncrieff, G.; Maybury, C. A systematic review to evaluate the efficacy of azelaic acid in the management of acne, rosacea, melasma and skin aging. J. Cosmet. Dermatol. 2023, 22, 2650–2662. [Google Scholar] [CrossRef]
- Gupta, A.K.; Batra, R.; Bluhm, R.; Faergemann, J. Pityriasis versicolor. Dermatol. Clin. 2003, 21, 413–429. [Google Scholar] [CrossRef]
- Sauer, N.; Oślizło, M.; Brzostek, M.; Wolska, J.; Lubaszka, K.; Karłowicz-Bodalska, K. The multiple uses of azelaic acid in dermatology: Mechanism of action, preparations, and potential therapeutic applications. Postep. Dermatol. Alergol. 2023, 40, 716–724. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Wood, J.M. Azelaic acid as a competitive inhibitor of thioredoxin reductase in human melanoma cells. Cancer Lett. 1987, 36, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Schallreuter, K.U.; Wood, J.W. A possible mechanism of action for azelaic acid in the human epidermis. Arch. Dermatol. Res. 1990, 282, 168–171. [Google Scholar] [CrossRef]
- Gollnick, H. Azelaic acid-pharmacology, toxicology and mechanisms of action on keratinization in vitro and in vivo. J. Dermatol. Treat. 1993, 4, S3–S7. [Google Scholar] [CrossRef]
- Maramaldi, G.; Esposito, M. Potassium azeloyl diglycinate: A multifunctional skin lightener. Cosmet. Toilet. 2002, 117, 43–50. [Google Scholar]
- ChemSpider. Azelaic Acid. Available online: https://www.chemspider.com/Chemical-Structure.2179.html?rid=41b5624d-bc4d-4305-9cbf-aae26f0f40ba (accessed on 10 March 2024).
- European Chemicals Agency (ECHA). Registration Dossier—Azelaic Acid. Available online: https://chem.echa.europa.eu/100.004.246/dossier-view/565ee298-3746-4de4-904b-6aabbfe0f735/IUC5-e2cd19ff-b18a-4efb-8e6a-135efc42aabe_04eb3064-fd5c-48e1-be20-66be321bd0a8?searchText=123-99-9 (accessed on 10 March 2024).
- European Chemicals Agency (ECHA). Registration Dossier—Glycine, N, N’—(1,9-nonandeiyl) bis-, Monopotasium Salt. Available online: https://chem.echa.europa.eu/100.104.938/dossier-view/f52d5fef-aafb-43f3-9a00-6a9c4590231c/7fc6e724-20cb-4e0e-9510-22a56b32860e_91e66f2c-c792-48af-b908-92f54803540d?searchText=Potassium%20azeloyl%20diglycinate (accessed on 10 March 2024).
- Taraz, M.; Niknam, S.; Ehsani, A.H. Tranexamic acid in treatment of melasma: A comprehensive review of clinical studies. Dermatol. Ther. 2017, 30, e12465. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K. Mechanism of action of topical tranexamic acid in the treatment of melasma and sun-induced skin hyperpigmentation. Cosmetics 2022, 9, 108. [Google Scholar] [CrossRef]
- Abiko, Y.; Iwamoto, M. Plasminogen-plasmin system: VII. Potentiation of antifibrinolytic action of a synthetic inhibitor, tranexamic acid, by α2-macroglobulin antiplasmin. Biochim. Biophys. Acta Protein Struct. 1970, 214, 411–418. [Google Scholar] [CrossRef]
- Kim, M.S.; Bang, S.H.; Kim, J.-H.; Shin, H.-J.; Choi, J.-H.; Chang, S.E. Tranexamic acid diminishes laser-induced melanogenesis. Ann. Dermatol. 2015, 27, 250–256. [Google Scholar] [CrossRef]
- Horikoshi, T.; Eguchi, H.; Onodera, H. The effects of tranexamic acid on the growth and melanogenesis of cultured human melanocytes. Jpn. J. Dermatol. 1994, 104, 641–646. [Google Scholar]
- Zhang, X.; Yang, X.; Yang, H.; Yang, Y. Study of inhibitory effect of acidum tranexamicum on melanin synthesis. Chin. J. Dermatovenerol. Integr. Tradit. West. Med. 2003, 2, 227–229. [Google Scholar]
- European Chemicals Agency (ECHA). Registration Dossier—Tranexamic Acid. Available online: https://chem.echa.europa.eu/100.013.471/dossier-view/58598510-a926-4ab7-a54b-87fe23857c4d/a1f3ecb4-2961-4e92-ac77-b8d78c010f4f_ecd9d170-0b37-42a1-861f-0cbc0c117bbf?searchText=1197-18-8 (accessed on 21 March 2024).
- Mambwe, B.; Mellody, K.T.; Kiss, O.; O’Connor, C.; Bell, M.; Watson, R.E.B.; Langton, A.K. Cosmetic retinoid use in photoaged skin: A review of the compounds, their use and mechanisms of action. Int. J. Cosmet. Sci. 2024, 47, 45–57. [Google Scholar] [CrossRef]
- Zasada, M.; Budzisz, E. Retinoids: Active molecules influencing skin structure formation in cosmetic and dermatological treatments. Adv. Dermatol. Allergol. 2019, 36, 392–397. [Google Scholar] [CrossRef]
- Farris, P. Supplement article: Retinol: The ideal retinoid for cosmetic solutions. J. Drugs Dermatol. 2022, 21, s4–s10. [Google Scholar]
- Reichrath, J.; Mittmann, M.; Kamradt, J.; Müller, S.M. Expression of retinoid-X receptors (-alpha,-beta,-gamma) and retinoic acid receptors (-alpha,-beta,-gamma) in normal human skin: An immunohistological evaluation. Histochem. J. 1997, 29, 127–133. [Google Scholar] [CrossRef]
- Kurlandsky, S.B.; Xiao, J.H.; Duell, E.A.; Voorhees, J.J.; Fisher, G.J. Biological activity of all-trans retinol requires metabolic conversion to all-trans retinoic acid and is mediated through activation of nuclear retinoid receptors in human keratinocytes. J. Biol. Chem. 1994, 269, 32821–32827. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Vienne, M.P.; Lauze, C.; Dupuy, P.; Gehring, W.; Gloor, M. Tolerance profile of retinol, retinaldehyde and retinoic acid under maximized and long-term clinical conditions. Dermatology 1999, 199 (Suppl. 1), 57–60. [Google Scholar] [CrossRef] [PubMed]
- Bellemère, G.; Stamatas, G.N.; Bruère, V.; Bertin, C.; Issachar, N.; Oddos, T. Antiaging action of retinol: From molecular to clinical. Skin Pharmacol. Physiol. 2009, 22, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Saurat, J.H.; Didierjean, L.; Masgrau, E.; Piletta, P.A.; Jaconi, S.; Chatellard-Gruaz, D.; Gumowski, D.; Masouyé, I.; Salomon, D.; Siegenthaler, G. Topical retinaldehyde on human skin: Biologic effects and tolerance. J. Investig. Dermatol. 1994, 103, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.W.Y.; Zhou, G.-J.; Hilscherová, K.; Giesy, J.P.; Leung, K.M.Y. Current understanding of potential ecological risks of retinoic acids and their metabolites in aquatic environments. Environ. Int. 2020, 136, 105464. [Google Scholar] [CrossRef]
- ChemSpider. All-Trans-Retinal. Available online: https://www.chemspider.com/Chemical-Structure.553582.html?rid=f9afdfd3-445e-418b-9553-5977e0ddbbfd&page_num=0 (accessed on 5 April 2024).
- European Chemicals Agency (ECHA). Registration Dossier—Retinol. Available online: https://chem.echa.europa.eu/100.000.621/dossier-view/3087b17c-dc0a-4e6b-a512-ada3ff3a689b/IUC5-82e35404-2be7-4891-9b7d-e913fe627b87_a1bbc9de-a626-4e66-a28f-c0e1d7bc9ed1?searchText=retinol (accessed on 5 April 2024).
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharmacother. 2019, 110, 582–593. [Google Scholar] [CrossRef]
- Chaudhary, J.; Pathak, A.; Lakhawat, S. Production technology and applications of kojic acid. Annu. Res. Rev. Biol. 2014, 4, 3165–3196. [Google Scholar] [CrossRef]
- Phasha, V.; Senabe, J.; Ndzotoyi, P.; Okole, B.; Fouche, G.; Chuturgoon, A. Review on the use of kojic acid—A skin-lightening ingredient. Cosmetics 2022, 9, 64. [Google Scholar] [CrossRef]
- Lee, M.; Park, H.Y.; Jung, K.H.; Kim, D.H.; Rho, H.S.; Choi, K. Anti-melanogenic effects of kojic acid and hydroxycinnamic acid derivatives. Biotechnol. Bioprocess. Eng. 2020, 25, 190–196. [Google Scholar] [CrossRef]
- Chen, J.-K.; Shen, C.-R.; Liu, C.-L. N-acetylglucosamine: Production and applications. Mar. Drugs 2010, 8, 2493–2516. [Google Scholar] [CrossRef] [PubMed]
- Bissett, D.L. Glucosamine: An ingredient with skin and other benefits. J. Cosmet. Dermatol. 2006, 5, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Połubinska, A.; Cwalinski, J.; Baum, E.; Bręborowicz, A. N-acetylglucosamine modulates function of the skin fibroblasts. Int. J. Cosmet. Sci. 2013, 35, 472–476. [Google Scholar] [CrossRef]
- Bissett, D.L.; Robinson, L.R.; Raleigh, P.S.; Miyamoto, K.; Hakozaki, T.; Li, J.; Kelm, G.R. Reduction in the appearance of facial hyperpigmentation by topical N-acetyl glucosamine. J. Cosmet. Dermatol. 2007, 6, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Bissett, D.; Robinson, L.; Li, J.; Miyamoto, K. Topical N-acetyl glucosamine reduces the appearance of hyperpigmented spots on human facial skin. J. Am. Acad. Dermatol. 2006, 54, AB43. [Google Scholar]
- European Chemicals Agency (ECHA). Registration Dossier—N-acetyl-β-D-Glucosamine. Available online: https://chem.echa.europa.eu/100.028.517/dossier-view/eda3baba-6480-456f-b565-0066fe63915f/794a3344-c9d8-4bfe-8c3e-3e10c464d786_794a3344-c9d8-4bfe-8c3e-3e10c464d786?searchText=7512-17-6 (accessed on 24 October 2024).
- Costa, C.; Olivi, P.; Botta, C.; Espíndola, E. Toxicity in aquatic environments: Discussion and evaluation methods. Quim. Nova 2007, 31, 1820–1830. [Google Scholar] [CrossRef]
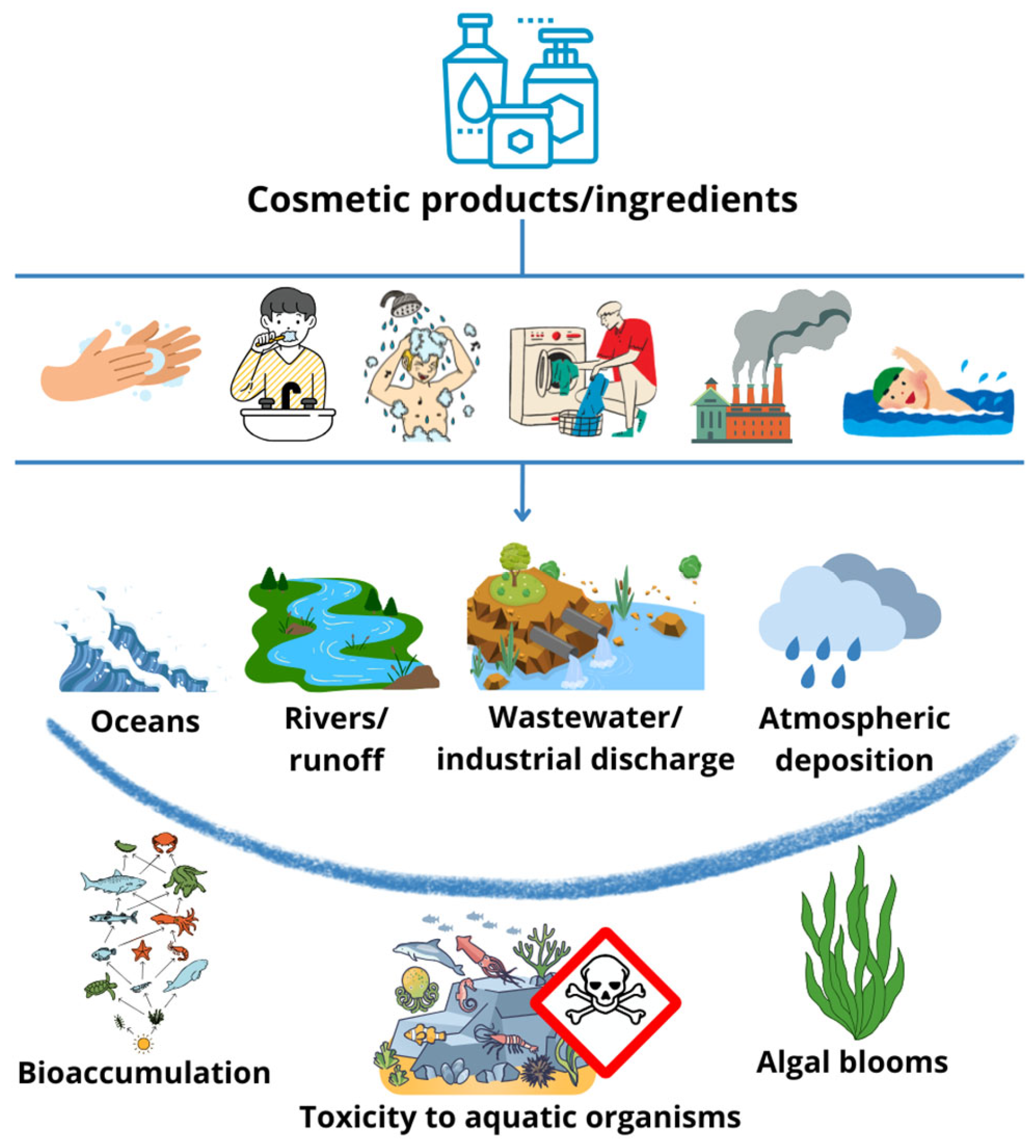

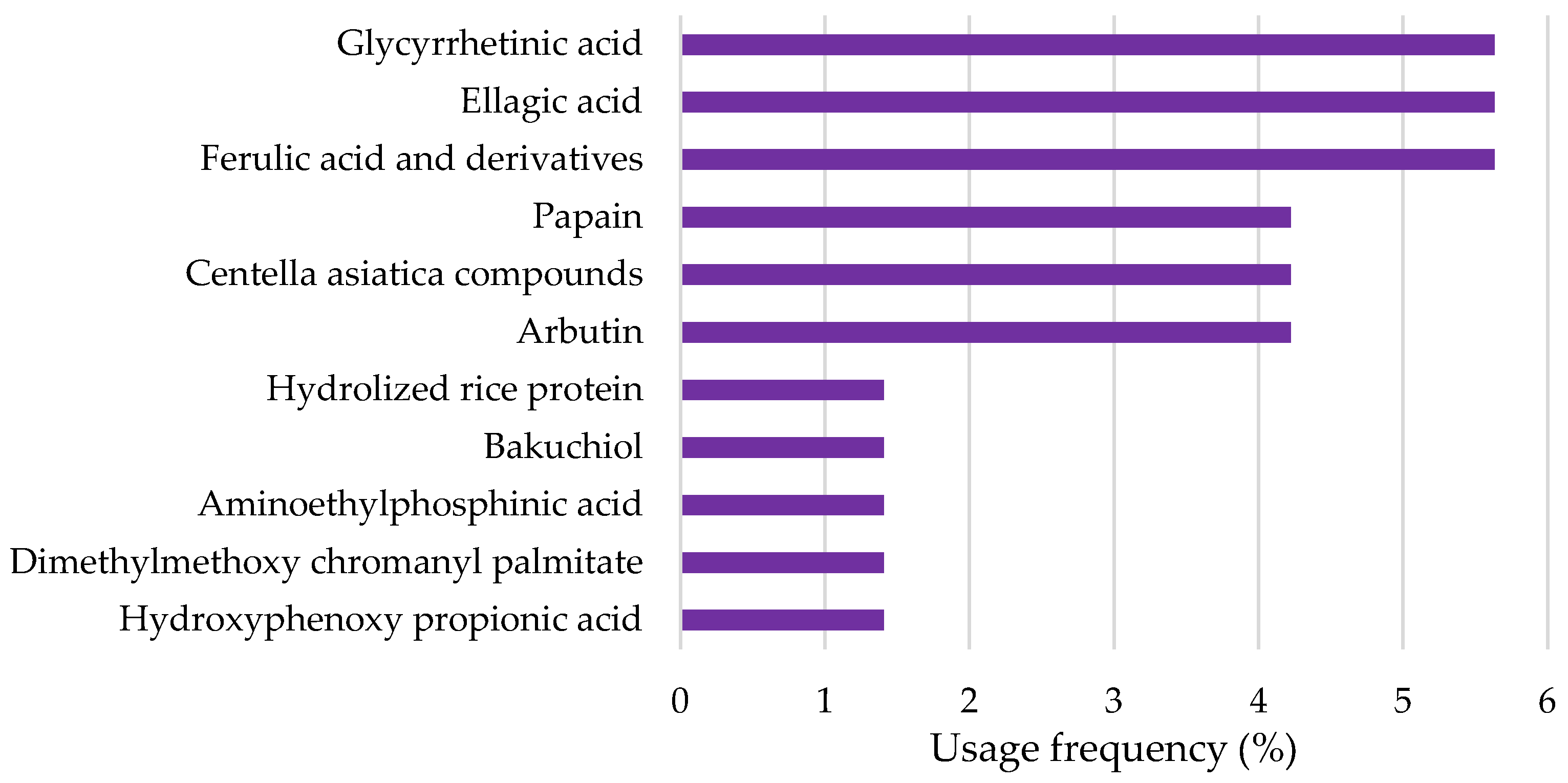
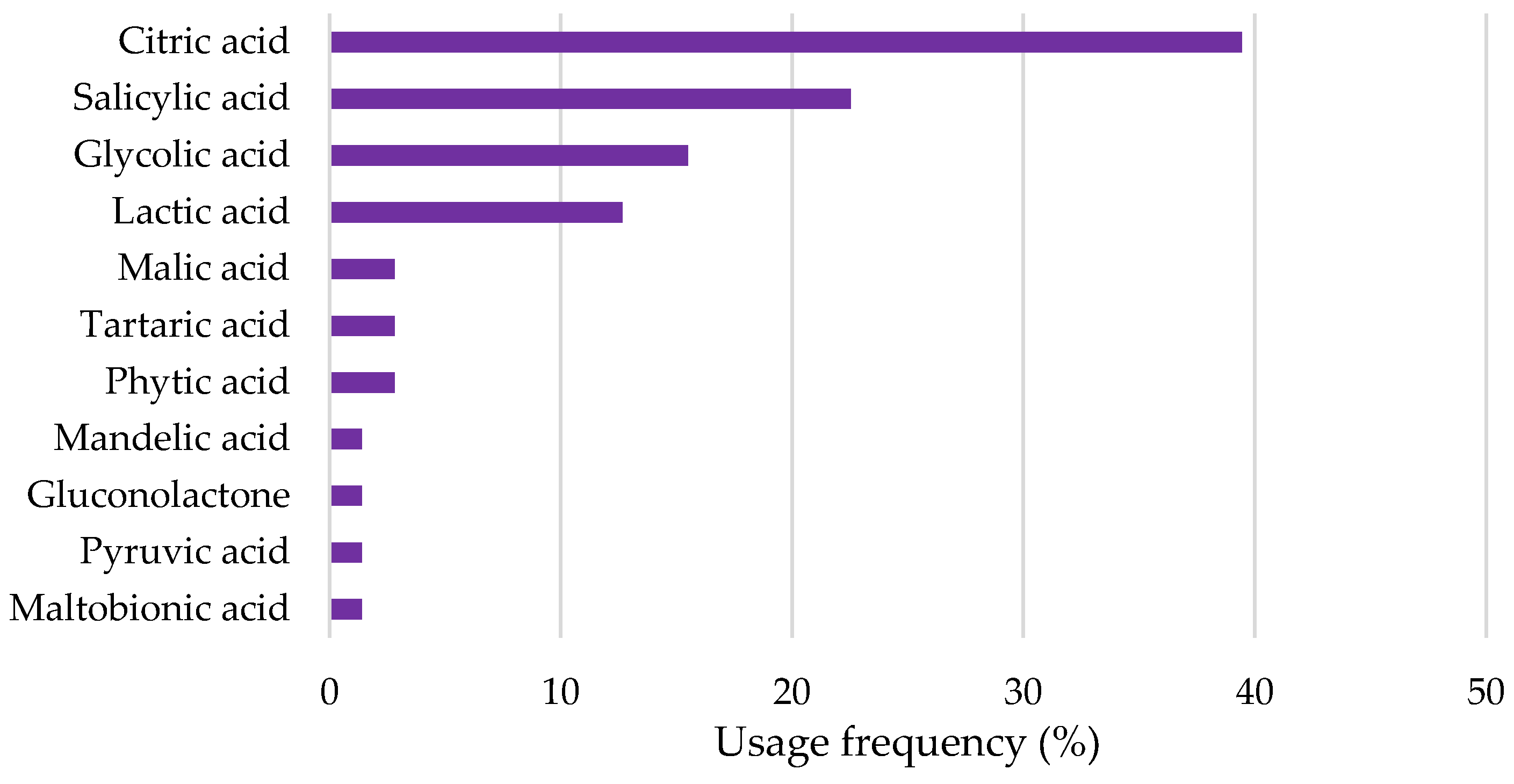
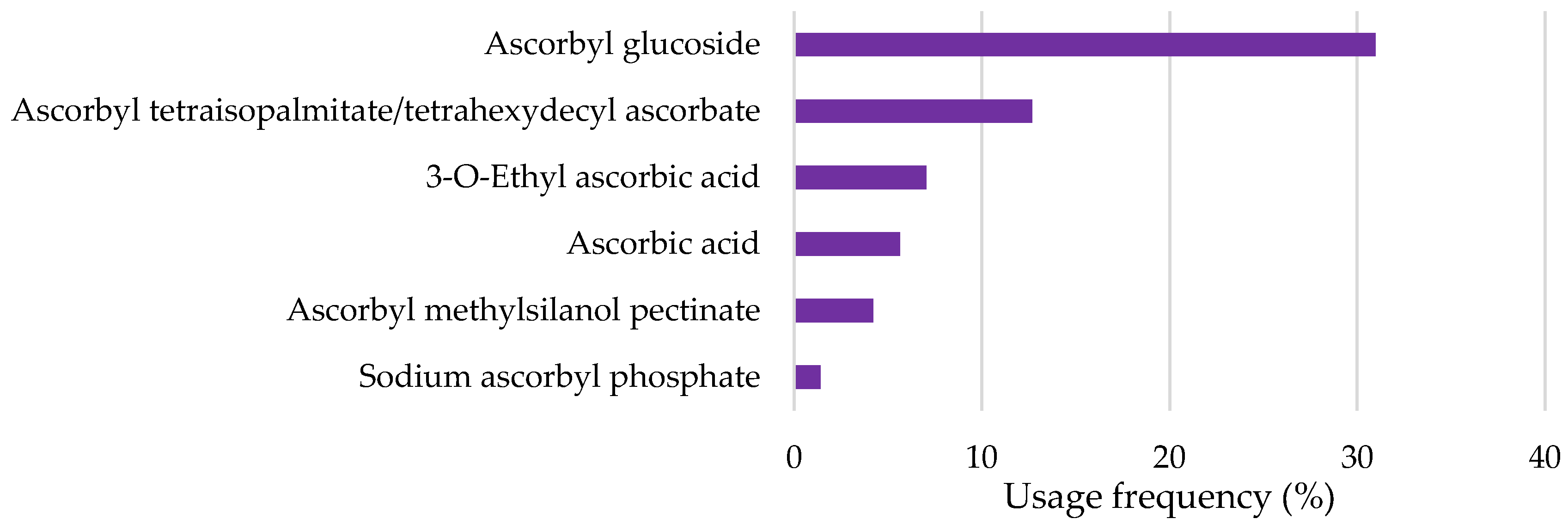
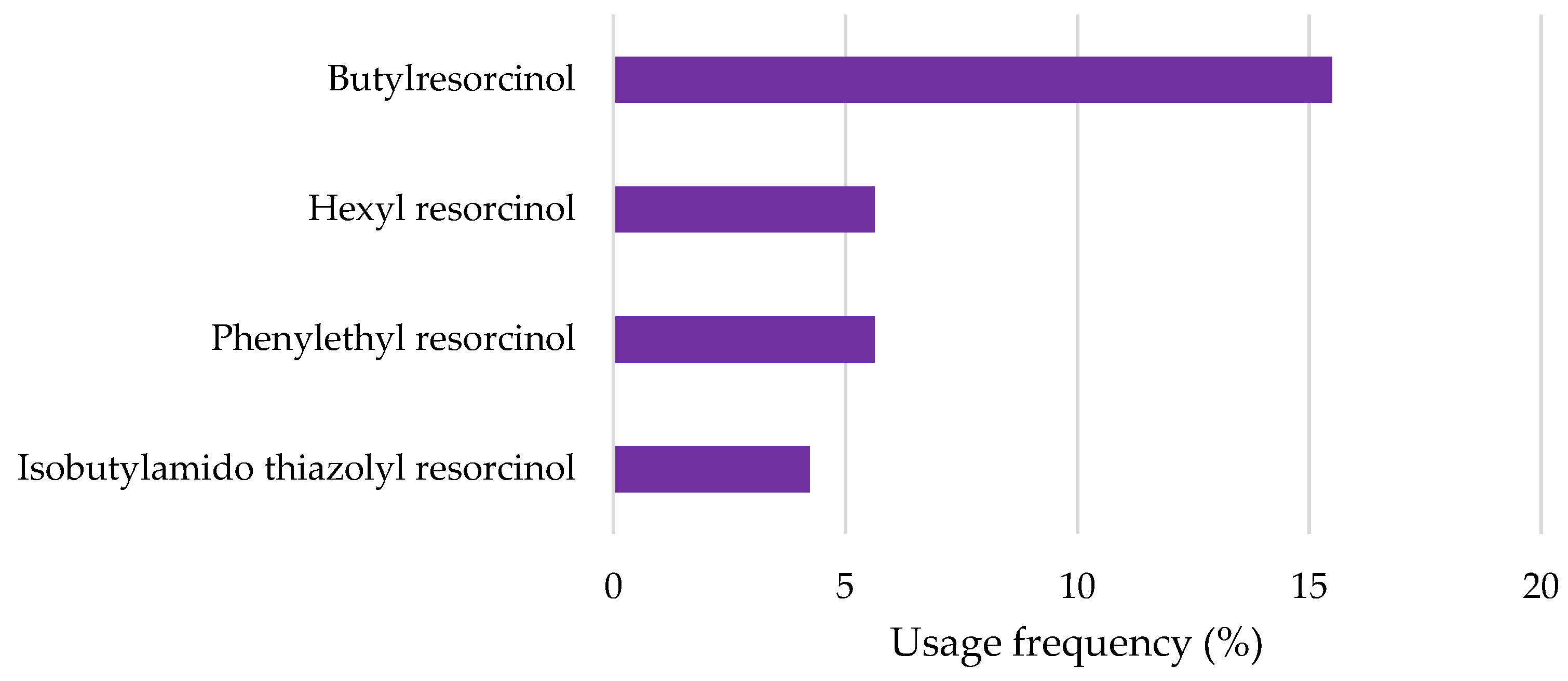
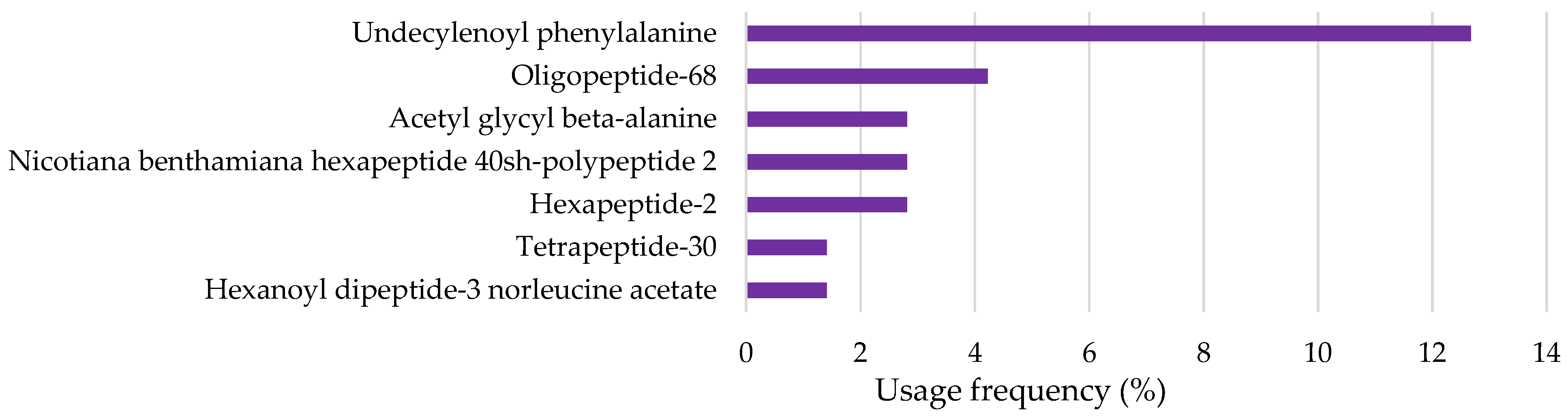
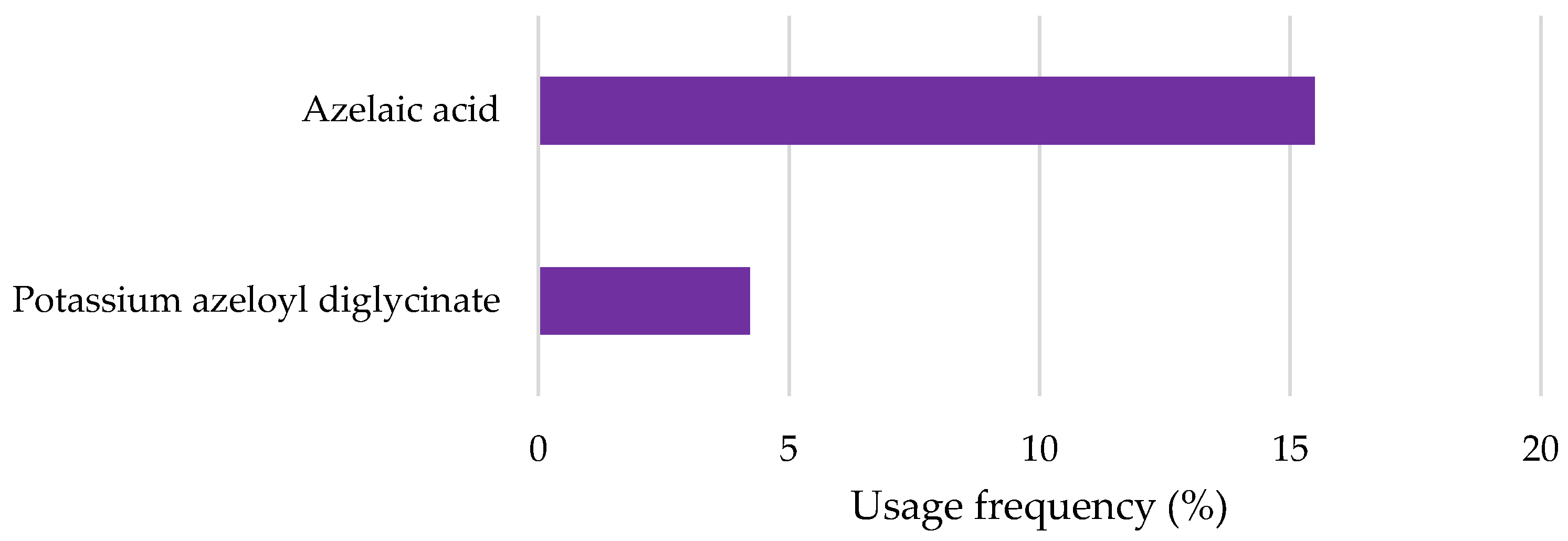
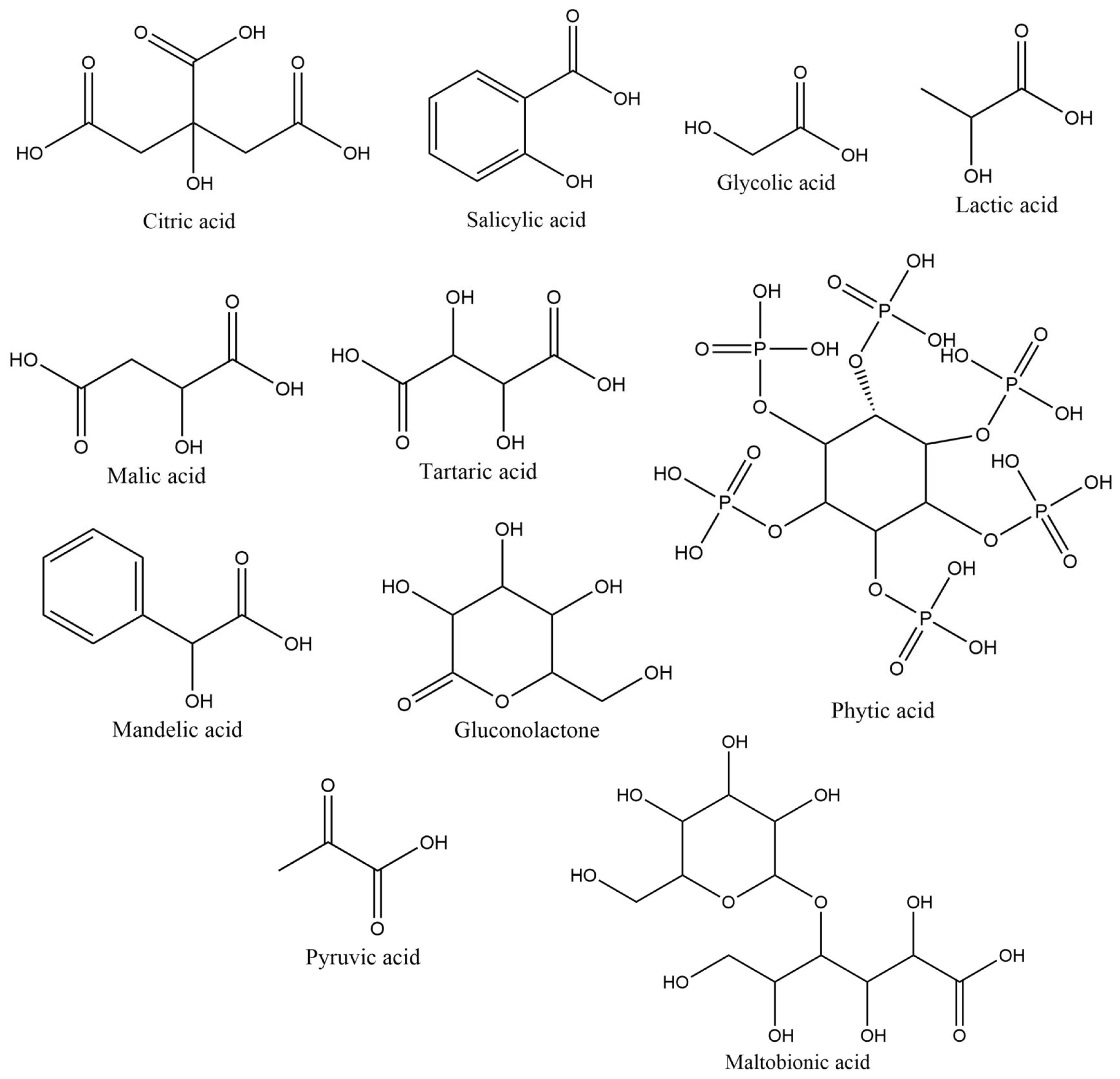
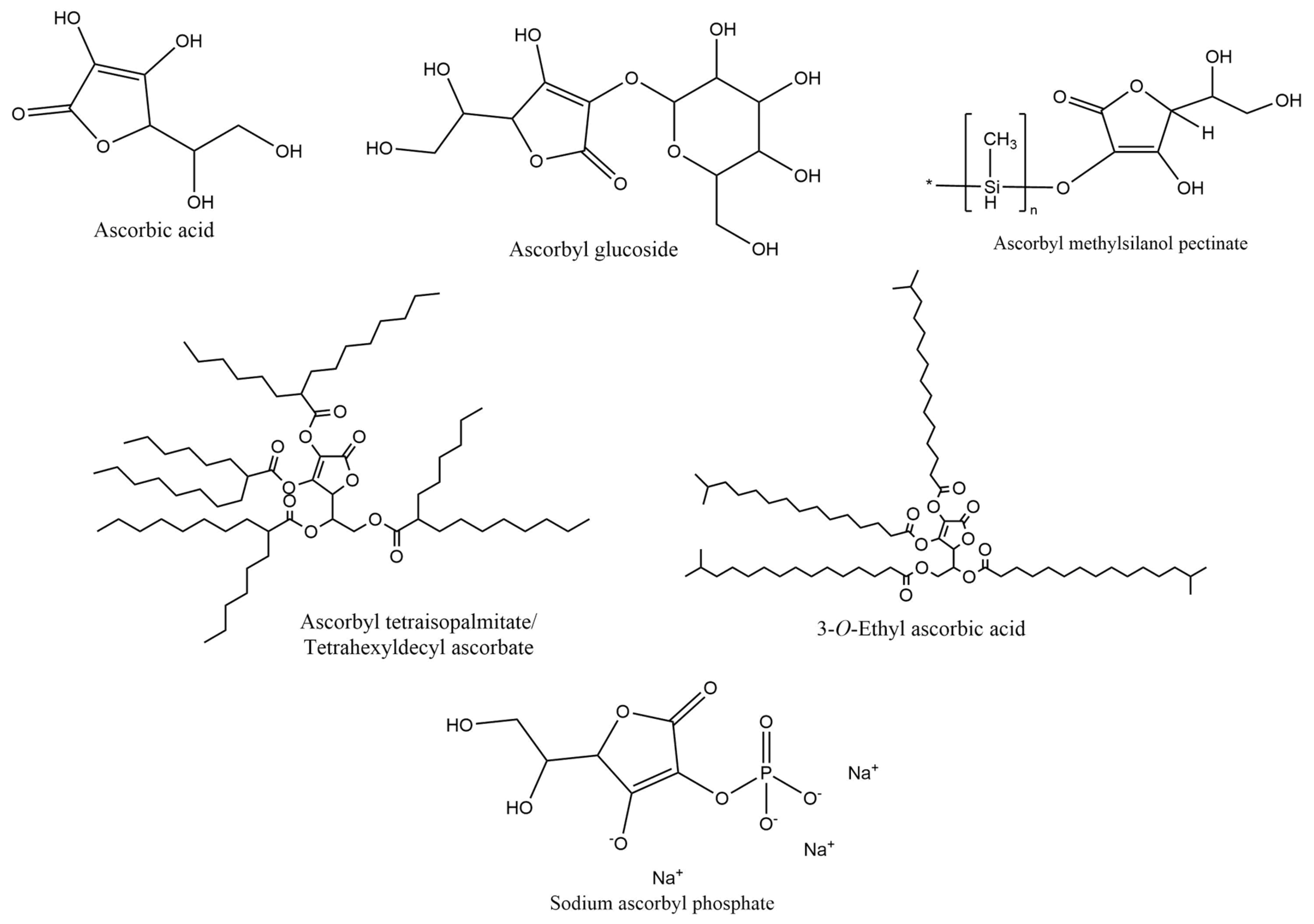
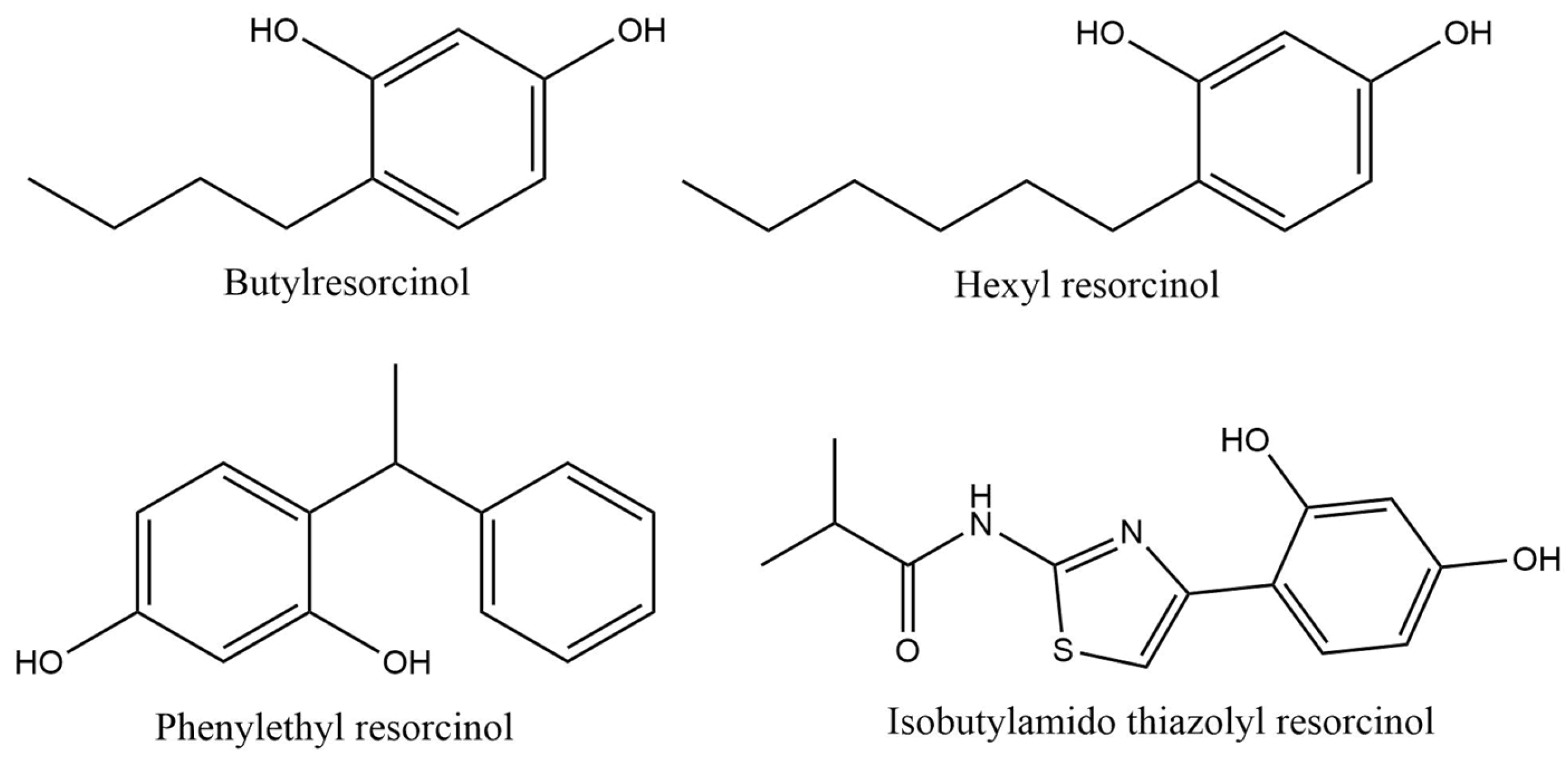
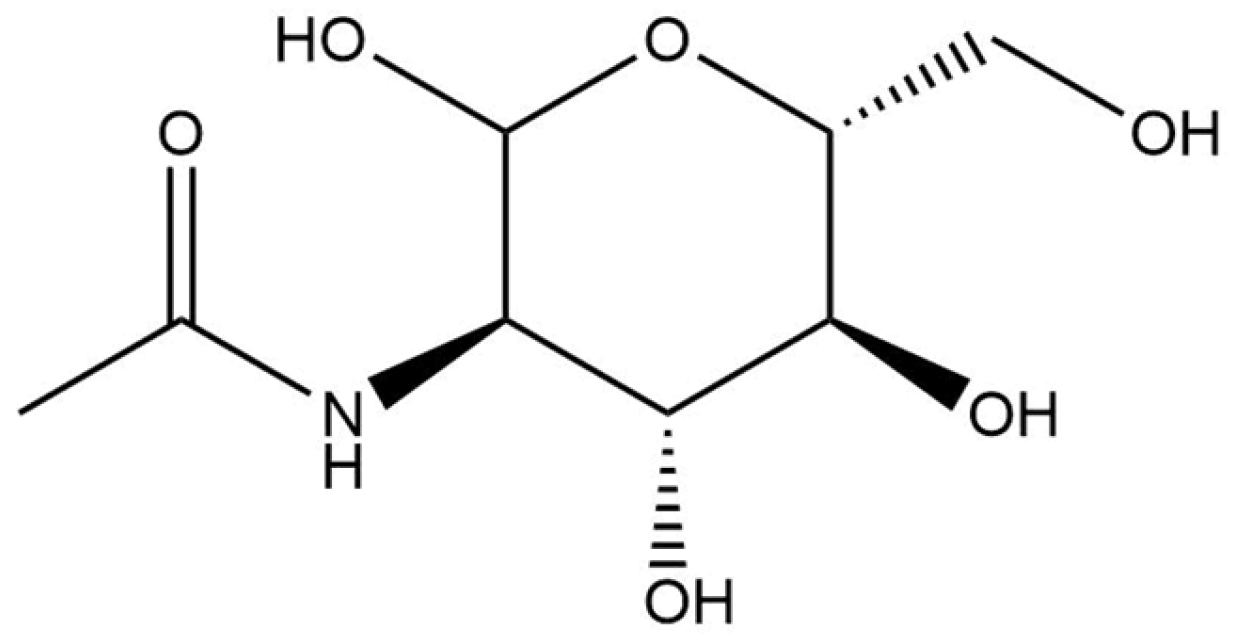
| Hydroxy Acid/Keto Acid | Biodegradation in Water | Aquatic Toxicity | Reference |
|---|---|---|---|
| Citric acid | Modified OECD Guideline 301E Screening Test: 100% degradation in 19 days Readily biodegradable | Immobilization of Daphnia magna (OECD Guideline 202): EC50 = 1535 mg/L after 24 h | [39] |
| Salicylic acid | QSAR prediction (BioWin v4.10): Readily biodegradable | Immobilization of Daphnia magna (OECD Guideline 202): EC50 = 2870 mg/L after 48 h Algae growth inhibition test (OECD guideline 201): EC50 > 100 mg/L after 72 h Inhibition of total respiration in activated sludge: IC50 > 1000 mg/L after 3 h | [40] |
| Glycolic acid | Closed Bottle Test: Degradation after 7 days was 89.6% Readily biodegradable | Mortality of Danio rerio (zebra fish): LC50 > 5000 mg/L No mortality was observed | [41] |
| Lactic acid | QSAR prediction: Probability of ready biodegradability = 0.936 Readily biodegradable | Mortality of Lepomis macrochirus: LC50 = 130 mg/L 96 h Immobilization of Daphnia magna (OECD Guideline 202): EC50 = 130 mg/l after 48 h EC50 ≥ 2.8 g/L after 72 h Inhibition of total respiration in activated sludge: NOEC ≥ 88.2 mg/L | [42] |
| Malic acid | Modified MITI Test conducted according to OECD TG 301 C on dl-malic acid: Readily biodegradable | Immobilization of Daphnia magna (OECD Guideline 202): EC50 >100 mg/L after 48 h exposure to fumaric acid Semi-static acute toxicity test in Juvenile fish: LC50 > 100 mg/L after 96 h Inhibition of total respiration in activated sludge: EC50 > 300 mg fumaric acid/L after 3 h | [43] |
| Tartaric acid | QSAR prediction (BioWin v4.10): Readily biodegradable | QSAR models: LC50 > 100 mg/L | [44] |
| Phytic acid | OECD Guideline 301A test: Degradation after 7 days was over 90% Readily biodegradable | Immobilization of Daphnia magna (OECD Guideline 202): EC50 > 294.6 µg/L after 48 h | [45] |
| Mandelic acid | OECD Guideline 301F test: Degradation after 28 days was 99% Readily biodegradable | Immobilization of Daphnia magna (OECD Guideline 202): EC50 > 100 mg/L after 48 h Algae growth inhibition (OECD Guideline 201): EC50 > 100 mg/L after 72 h | [46] |
| Pyruvic acid | QSAR prediction (BioWin v4.10): Readily biodegradable | No available information | [47] |
| Maltobionic acid | QSAR prediction (BioWin v4.10): Readily biodegradable | No available information | [48] |
| Gluconolactone | QSAR prediction (BioWin v4.10): Readily biodegradable | No available information | [49] |
| Vitamin C Derivative | Biodegradation in Water | Aquatic Toxicity | Reference |
|---|---|---|---|
| Ascorbic acid | OECD Guideline 301A (Ready Biodegradability: DOC Die Away Test): Readily biodegradable after 28 days | Mortality of Oncorhynchus mykiss (OECD Guideline 203): LC50 = 1020 mg/L after 96 h Immobilization of Daphnia magna (OECD Guideline 202): EC50 = 74 mg/L after 48 h Algae growth inhibition (OECD Guideline 201): EC50 > 74 mg/L after 72 h | [59] |
| Ascorbyl glucoside | EU Method C.4-B (Modified OECD Screening Test): Readily biodegradable | Acute Toxicity for Daphnia (EU Method C.2): EC50 > 200 mg/L after 48 h Algal Inhibition test (EU Method C.3): EC50 > 100 mg/L after 72 h | [60] |
| Ascorbyl tetraisopalmitate/tetrahexyldecyl ascorbate | EU Method C.4-C (Carbon Dioxide Evolution Test) and OECD Guideline 301B (CO2 Evolution Test): Under test conditions no biodegradation observed | Immobilization of Daphnia magna (OECD Guideline 202): EC50 < 0.09 mg/L after 48 h Algae growth inhibition (OECD Guideline 201): EC50 > 0.09 mg/L after 72 h | [56] |
| 3-O-Ethyl ascorbic acid | EU Method C.4-C (Carbon Dioxide Evolution Test) and OECD Guideline 301 B (CO2 Evolution Test): Under test conditions no biodegradation observed | Immobilization of Daphnia magna (OECD Guideline 202): EC50 > 78 mg/L after 48 h Algae growth inhibition (OECD Guideline 201): EC50 > 81 mg/L after 72 h | [57] |
| Ascorbyl methylsilanol pectinate | No available information | No available information | - |
| Sodium ascorbyl phosphate | 92/69/EWG, C.4-D (Manom. Respirat.): Not readily biodegradable | Mortality of Danio rerio (zebra fish): LC50 = 5855.8 mg/L after 96 h Immobilization of Daphnia magna (OECD Guideline 202): EC50 > 100 mg/L after 48 h Algae growth inhibition (OECD Guideline 201): EC50 > 100 mg/L after 72 h Pseudomonas putida growth inhibition test: IC50 = 7700 mg/L after 16 h | [58] |
| Resorcinol Derivative | Biodegradation in Water | Aquatic Toxicity | Reference |
|---|---|---|---|
| Butylresorcinol | QSAR prediction: Ultimate biodegradation: Weeks Primary biodegradation: Days Anaerobic: Does not biodegrade fast Not readily biodegradable | Immobilization of Daphnia magna (OECD Guideline 202): EC50 = 0.86 mg/L after 48 h Algae growth inhibition (OECD Guideline 201): EC50 = 30 mg/L after 72 h | [71] |
| Hexyl resorcinol | OECD Guideline 301 D (Closed Bottle Test): Degradation after 7, 14, 21, and 28 days was 64.68, 80.85, 85.11, and 80.85% Not readily biodegradable | Immobilization of Daphnia magna (OECD Guideline 202): EC50 = 2.8 mg/L after 48 h Algae growth inhibition (OECD Guideline 201): EC50 = 6.24 mg/L after 72 h | [73] |
| Phenylethyl resorcinol | OECD Guideline 301 D (Closed Bottle Test): Degradation after 28 days was 1% Not readily biodegradable | Mortality of Danio rerio (zebra fish): LC50 ≥ 10, 9.65, 9.65 and 8.94 mg/L after 24, 48, 72 and 96 h Immobilization of Daphnia magna (OECD Guideline 202): EC50 = 1.41 mg/L after 48 h Algae growth inhibition (OECD Guideline 201): EC50 = 4.15 and 2.42 mg/L after 72 h Inhibition of total respiration in activated sludge: EC50 = 33 mg/L after 3 h | [72] |
| Isobutylamido thiazolyl resorcinol | OECD Guideline 301 B (CO2 Evolution Test): Degradation after 29 days was 33.6% Not readily biodegradable | Immobilization of Daphnia magna (OECD Guideline 202) EC50 = 16 mg/L after 48 h Algae growth inhibition (OECD Guideline 201): EC50 = 3.3 and 1 mg/L after 72 h | [74] |
| Peptide or Amino Acid | Biodegradation in Water | Aquatic Toxicity | Reference |
|---|---|---|---|
| Undecylenoyl phenylalanine | OECD Guideline 301 B (CO2 Evolution Test) and EU Method C.4-C (Carbon Dioxide Evolution Test): Degradation after 28 days was 75% Readily biodegradable | Immobilization of Daphnia magna (EU Method C.2 and OECD Guideline 202): EC50 > 110 mg/L after 48 h Algae growth inhibition (EU Method C.3 and OECD Guideline 201): EC50 = 89 mg/L after 72 h | [87] |
| Oligopeptide-68 | QSAR prediction (BioWin v4.10): Not readily biodegradable | No available information | - |
| Acetyl glycyl beta-alanine | OECD Guideline 301 B (CO2 Evolution Test): Degradation after 28 days was 93.7% Readily biodegradable | Immobilization of Daphnia magna (EU Method C.2 and OECD Guideline 202): EC50 > 100 mg/L after 48 h Algae growth inhibition (EU Method C.3 and OECD Guideline 201): EC50 > 100 mg/L after 72 h | [88] |
| Nicotiana benthamiana hexapeptide 40 SH-polypeptide 2 | No available information | No available information | - |
| Hexapeptide-2 | No available information | No available information | |
| Tetrapeptide-30 | QSAR prediction (BioWin v4.10): Not readily biodegradable | No available information | |
| Hexanoyl dipeptide-3 norleucine acetate | No available information | No available information |
| Azelaic Acid Derivative | Biodegradation in Water | Aquatic Toxicity | Reference |
|---|---|---|---|
| Azelaic acid | QSAR prediction and read across approach: Readily biodegradable | Algae growth inhibition (OECD Guideline 201): EC50 > 67 mg/L after 72 h Mortality of Oryzias latipes (read across approach): LC50 > 16 mg/L after 96 h Immobilization of Daphnia magna (read across approach): EC50 > 21 mg/L after 48 h | [96,97] |
| Potassium azeloyl diglycinate | OECD Guideline 301 B (CO2 Evolution Test) and EU Method C.4-C (Carbon Dioxide Evolution Test): Degradation after 28 days was between 53.7 and 65.9% Readily biodegradable | Immobilization of Daphnia magna (QSAR prediction): EC50 = 141.25 mg/L after 48 h Algae growth inhibition (QSAR prediction): EC50 = 102.32 mg/L after 72 h | [98] |
| Retinoid | Biodegradation in Water | Aquatic Toxicity | Reference |
|---|---|---|---|
| Retinol | OECD Guideline 301 B (CO2 Evolution Test): Degradation after 28 days was 81% Readily biodegradable | Mortality of Leuciscus idus (German national standard DIN 38 412, part L15): LC50 > 10,000 mg/L after 96 h Mortality of Danio rerio (OECD Guideline 203): LC50 = 316.23 mg/L after 96 h Immobilization of Daphnia magna (OECD Guideline 202): EC50 > 100 mg/L after 48 h Algae growth inhibition (German standard test guideline, DIN 38 412 part 9): EC50 = 152.94 mg/L after 72 h | [116] |
| Retinal | QSAR prediction: Not readily biodegradable | No available information | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, S.; Rego, L.; Sousa, E.; Cruz, M.T.; Almeida, I.M.d. Usage Frequency and Ecotoxicity of Skin Depigmenting Agents. Pharmaceuticals 2025, 18, 368. https://doi.org/10.3390/ph18030368
Mota S, Rego L, Sousa E, Cruz MT, Almeida IMd. Usage Frequency and Ecotoxicity of Skin Depigmenting Agents. Pharmaceuticals. 2025; 18(3):368. https://doi.org/10.3390/ph18030368
Chicago/Turabian StyleMota, Sandra, Liliana Rego, Emília Sousa, Maria Teresa Cruz, and Isabel Martins de Almeida. 2025. "Usage Frequency and Ecotoxicity of Skin Depigmenting Agents" Pharmaceuticals 18, no. 3: 368. https://doi.org/10.3390/ph18030368
APA StyleMota, S., Rego, L., Sousa, E., Cruz, M. T., & Almeida, I. M. d. (2025). Usage Frequency and Ecotoxicity of Skin Depigmenting Agents. Pharmaceuticals, 18(3), 368. https://doi.org/10.3390/ph18030368









