Behavioral and Biochemical Insights into the Therapeutic Potential of Mitocurcumin in a Zebrafish–Pentylenetetrazole (PTZ) Epilepsy Model
Abstract
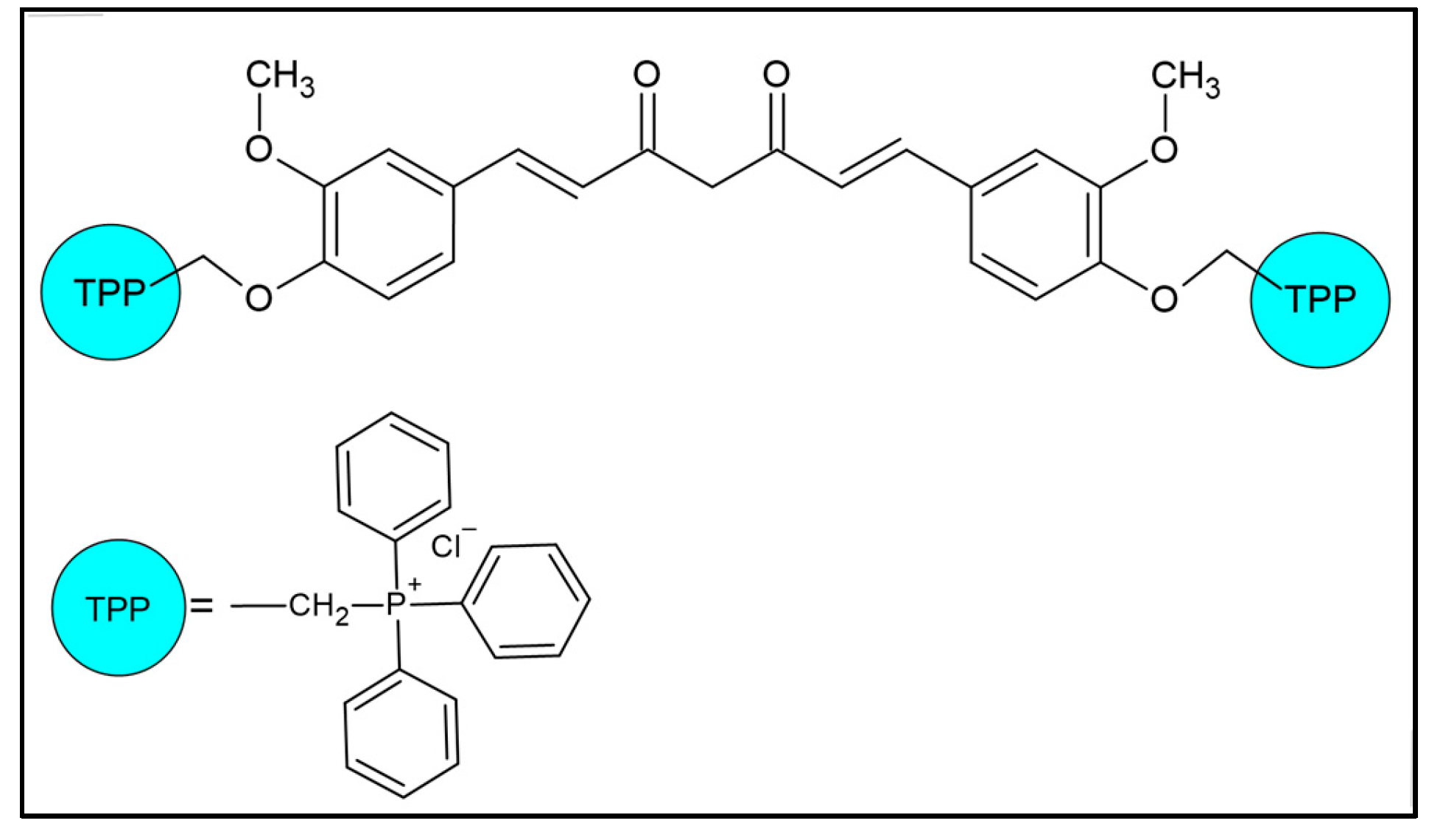
1. Introduction
2. Results
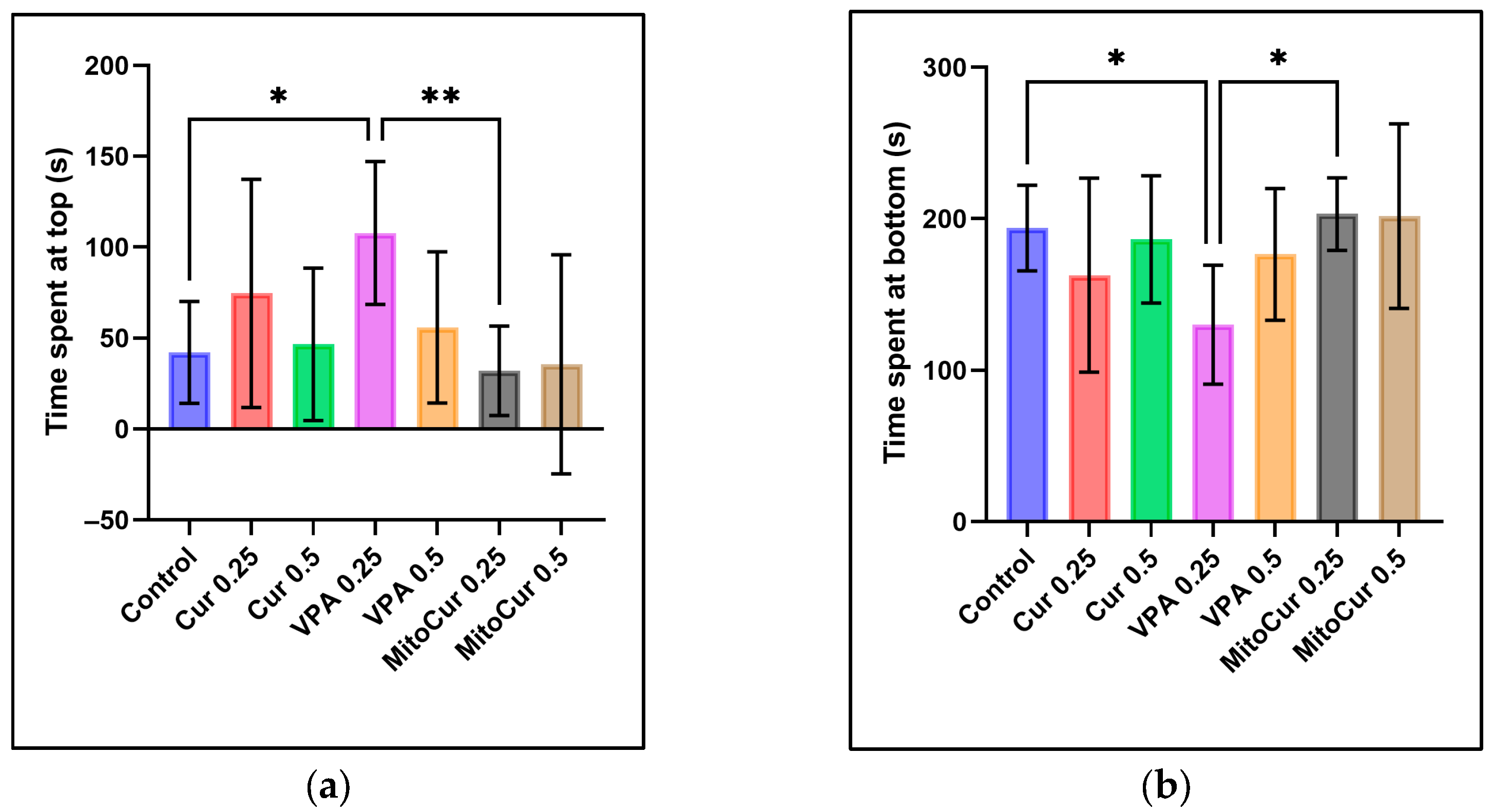
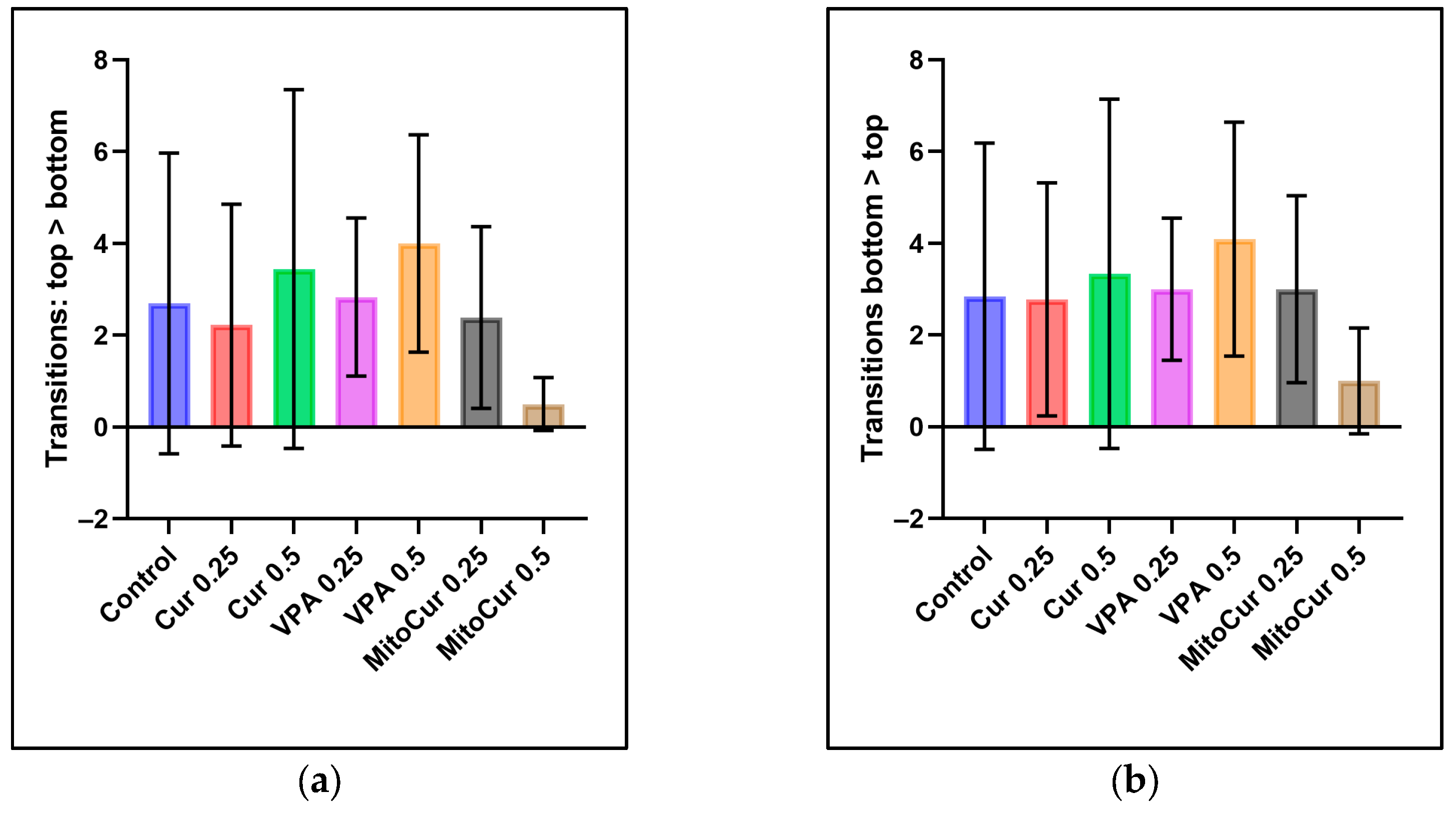
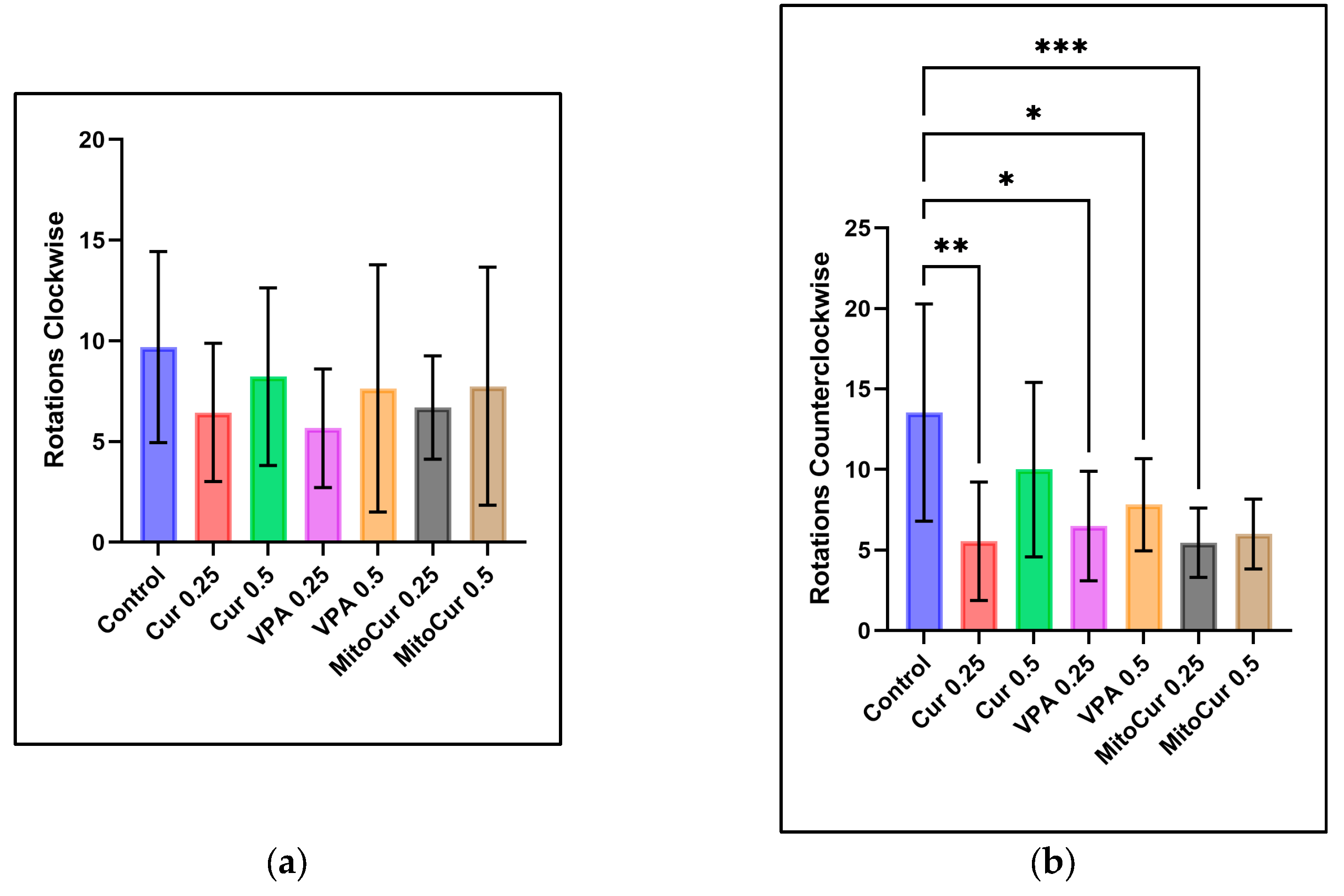
2.1. Behavioral Parameters
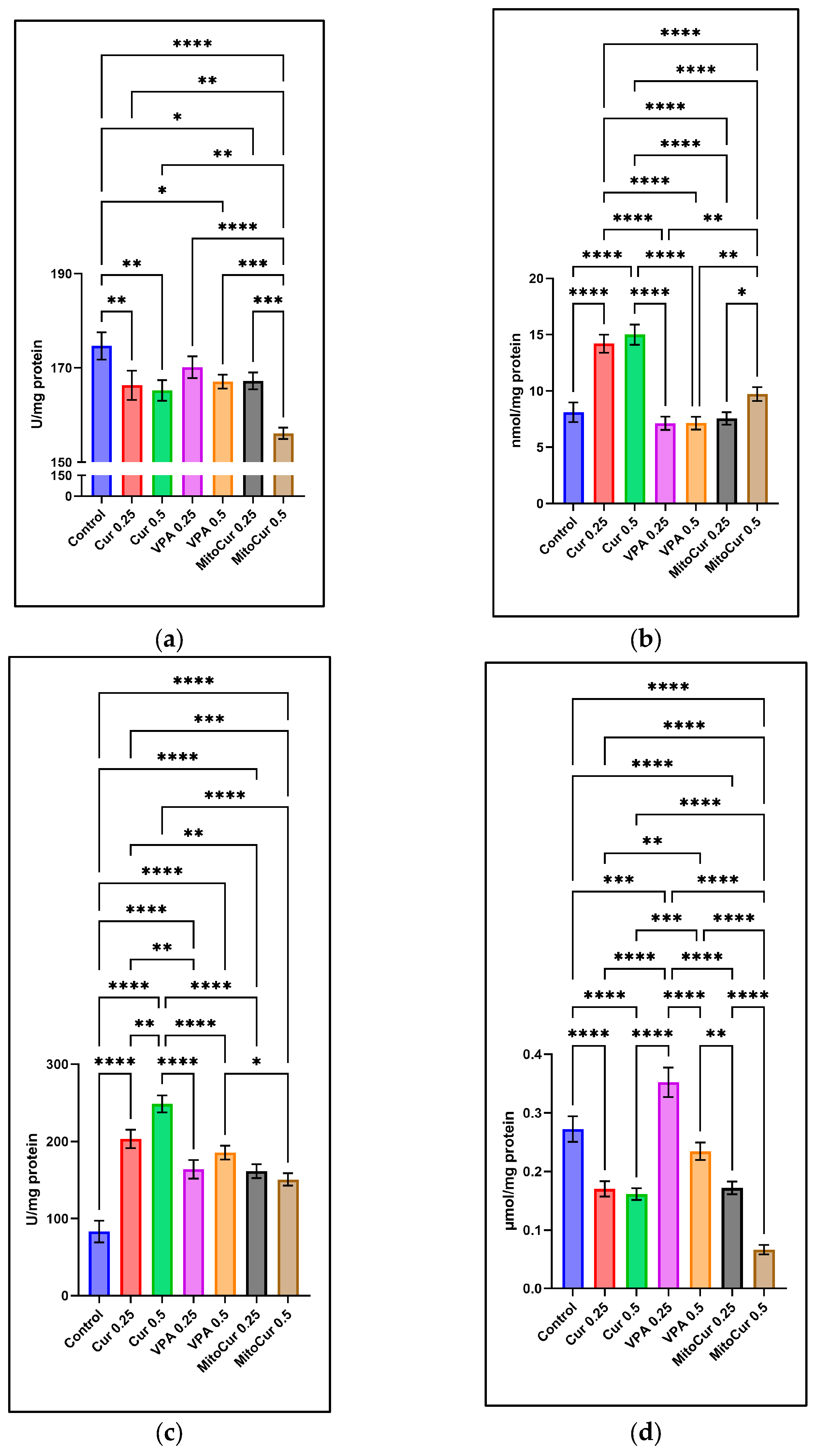
2.2. Oxidative Stress Parameters
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals and Reagents
4.3. Experiment Design
4.4. Behavioral Assessment—Locomotor and Exploratory Behavior
4.5. Biochemical Analyses of Oxidative Stress
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAT | Catalase |
| CCW | Counterclockwise |
| Cur | Curcumin |
| DMSO | Dimethyl sulfoxide |
| GPx | Glutathione peroxidase |
| LSD | Lysergic acid diethylamide |
| MDA | Malondialdehyde |
| MitoCur | Mitocurcumin |
| PTZ | Pentylenetetrazole |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TAS | Total antioxidant status |
| TPP | Triphenylphosphonium |
| VPA | Sodium valproate |
References
- Thomas, S.V.; Nair, A. Confronting the Stigma of Epilepsy. Ann. Indian Acad. Neurol. 2011, 14, 158. [Google Scholar] [CrossRef] [PubMed]
- Vetri, L.; Roccella, M.; Parisi, L.; Smirni, D.; Costanza, C.; Carotenuto, M.; Elia, M. Epilepsy: A Multifaced Spectrum Disorder. Behav. Sci. 2023, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-M.; Hu, W.-B.; Hong, C.-G.; Duan, R.; Chen, M.-L.; Cao, J.; Wang, Z.-X.; Chen, C.-Y.; Yin, F.; Hu, Z.-H.; et al. Recurrent de Novo Single Point Mutation on the Gene Encoding Na+/K+ Pump Results in Epilepsy. Prog. Neurobiol. 2022, 216, 102310. [Google Scholar]
- Striano, P.; Minassian, B.A. From Genetic Testing to Precision Medicine in Epilepsy. Neurotherapeutics 2020, 17, 609–615. [Google Scholar] [CrossRef]
- Huang, L.-T. Early-Life Stress Impacts the Developing Hippocampus and Primes Seizure Occurrence: Cellular, Molecular, and Epigenetic Mechanisms. Front. Mol. Neurosci. 2014, 7, 8. [Google Scholar] [CrossRef]
- Bauer, J.; Becker, A.J.; Elyaman, W.; Peltola, J.; Rüegg, S.; Titulaer, M.J.; Varley, J.A.; Beghi, E. Innate and Adaptive Immunity in Human Epilepsies. Epilepsia 2017, 58, 57–68. [Google Scholar] [CrossRef]
- Borowicz-Reutt, K.K.; Czuczwar, S.J. Role of Oxidative Stress in Epileptogenesis and Potential Implications for Therapy. Pharmacol. Rep. 2020, 72, 1218–1226. [Google Scholar] [CrossRef]
- Fabisiak, T.; Patel, M. Crosstalk between Neuroinflammation and Oxidative Stress in Epilepsy. Front. Cell Dev. Biol. 2022, 10, 976953. [Google Scholar] [CrossRef]
- Shin, E.-J.; Jeong, J.H.; Chung, Y.H.; Kim, W.-K.; Ko, K.-H.; Bach, J.-H.; Hong, J.-S.; Yoneda, Y.; Kim, H.-C. Role of Oxidative Stress in Epileptic Seizures. Neurochem. Int. 2011, 59, 122–137. [Google Scholar] [CrossRef]
- Olowe, R.; Sandouka, S.; Saadi, A.; Shekh-Ahmad, T. Approaches for Reactive Oxygen Species and Oxidative Stress Quantification in Epilepsy. Antioxidants 2020, 9, 990. [Google Scholar] [CrossRef]
- Shekh-Ahmad, T.; Kovac, S.; Abramov, A.Y.; Walker, M.C. Reactive Oxygen Species in Status Epilepticus. Epilepsy Behav. 2019, 101, 106410. [Google Scholar] [CrossRef] [PubMed]
- Ciubotaru, A.D.; Stoica, L.; Foia, L.G.; Stoica, B.-A.; Toma, V.; Salaru, D.L.; Leferman, C.-E.; Ghiciuc, C.-M. Investigation of the Antioxidant Capacity of Thiol-Containing Compounds Using the Photochemiluminescence Technique. Rom. J. Oral. Rehabil. 2023, 15, 417–425. [Google Scholar]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative Stress and Inflammation in the Pathogenesis of Neurological Disorders: Mechanisms and Implications. Acta Pharm. Sin. B 2024, 15, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef] [PubMed]
- Parsons, A.L.M.; Bucknor, E.M.V.; Castroflorio, E.; Soares, T.R.; Oliver, P.L.; Rial, D. The Interconnected Mechanisms of Oxidative Stress and Neuroinflammation in Epilepsy. Antioxidants 2022, 11, 157. [Google Scholar] [CrossRef]
- Puttachary, S.; Sharma, S.; Stark, S.; Thippeswamy, T. Seizure-Induced Oxidative Stress in Temporal Lobe Epilepsy. BioMed Res. Int. 2015, 2015, 745613. [Google Scholar] [CrossRef]
- Liang, L.P.; Waldbaum, S.; Rowley, S.; Huang, T.T.; Day, B.J.; Patel, M. Mitochondrial Oxidative Stress and Epilepsy in SOD2 Deficient Mice: Attenuation by a Lipophilic Metalloporphyrin. Neurobiol. Dis. 2012, 45, 1068–1076. [Google Scholar] [CrossRef]
- Łukawski, K.; Czuczwar, S.J. Oxidative Stress and Neurodegeneration in Animal Models of Seizures and Epilepsy. Antioxidants 2023, 12, 1049. [Google Scholar] [CrossRef]
- Dal-Pizzol, F.; Klamt, F.; Vianna, M.M.; Schröder, N.; Quevedo, J.; Benfato, M.S.; Moreira, J.C.; Walz, R. Lipid Peroxidation in Hippocampus Early and Late after Status Epilepticus Induced by Pilocarpine or Kainic Acid in Wistar Rats. Neurosci. Lett. 2000, 291, 179–182. [Google Scholar] [CrossRef]
- Sidorova, Y.; Domanskyi, A. Detecting Oxidative Stress Biomarkers in Neurodegenerative Disease Models and Patients. Methods Protoc. 2020, 3, 66. [Google Scholar] [CrossRef]
- Chidambaram, S.B.; Anand, N.; Varma, S.R.; Ramamurthy, S.; Vichitra, C.; Sharma, A.; Mahalakshmi, A.M.; Essa, M.M. Superoxide Dismutase and Neurological Disorders. IBRO Neurosci. Rep. 2024, 16, 373–394. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Shin, E.-J.; Sharma, N.; Nah, S.-Y.; Mai, H.N.; Nguyen, B.T.; Jeong, J.H.; Lei, X.G.; Kim, H.-C. Glutathione Peroxidase-1 and Neuromodulation: Novel Potentials of an Old Enzyme. Food Chem. Toxicol. 2021, 148, 111945. [Google Scholar] [CrossRef] [PubMed]
- Bahadır Taşlıdere, F.U. Investigation of Total Antioxidant Status and Total Oxidant Status with Seizure Types in Patients with Epilepsy. Available online: https://cyprusjmedsci.com/articles/investigation-of-total-antioxidant-status-and-total-oxidant-status-with-seizure-types-in-patients-with-epilepsy/doi/cjms.2022.2022-11 (accessed on 22 February 2025).
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S.; et al. Towards a Comprehensive Catalog of Zebrafish Behavior 1.0 and Beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef]
- Kalueff, A.V. (Ed.) The Rights and Wrongs of Zebrafish: Behavioral Phenotyping of Zebrafish; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-33773-9. [Google Scholar]
- Stewart, A.M.; Grieco, F.; Tegelenbosch, R.A.J.; Kyzar, E.J.; Nguyen, M.; Kaluyeva, A.; Song, C.; Noldus, L.P.J.J.; Kalueff, A.V. A Novel 3D Method of Locomotor Analysis in Adult Zebrafish: Implications for Automated Detection of CNS Drug-Evoked Phenotypes. J. Neurosci. Methods 2015, 255, 66–74. [Google Scholar] [CrossRef]
- Luca, R.M.; Gerlai, R. In Search of Optimal Fear Inducing Stimuli: Differential Behavioral Responses to Computer Animated Images in Zebrafish. Behav. Brain Res. 2012, 226, 66–76. [Google Scholar] [CrossRef]
- Cachat, J.; Stewart, A.; Grossman, L.; Gaikwad, S.; Kadri, F.; Chung, K.M.; Wu, N.; Wong, K.; Roy, S.; Suciu, C.; et al. Measuring Behavioral and Endocrine Responses to Novelty Stress in Adult Zebrafish. Nat. Protoc. 2010, 5, 1786–1799. [Google Scholar] [CrossRef]
- Grossman, L.; Stewart, A.; Gaikwad, S.; Utterback, E.; Wu, N.; Dileo, J.; Frank, K.; Hart, P.; Howard, H.; Kalueff, A.V. Effects of Piracetam on Behavior and Memory in Adult Zebrafish. Brain Res. Bull. 2011, 85, 58–63. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an Emerging Model for Studying Complex Brain Disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef]
- Bailey, J.M.; Oliveri, A.N.; Levin, E.D. Pharmacological Analyses of Learning and Memory in Zebrafish (Danio Rerio). Pharmacol. Biochem. Behav. 2015, 139, 103–111. [Google Scholar] [CrossRef]
- Baraban, S.C.; Taylor, M.R.; Castro, P.A.; Baier, H. Pentylenetetrazole Induced Changes in Zebrafish Behavior, Neural Activity and c-Fos Expression. Neuroscience 2005, 131, 759–768. [Google Scholar] [CrossRef]
- Mussulini, B.H.M.; Leite, C.E.; Zenki, K.C.; Moro, L.; Baggio, S.; Rico, E.P.; Rosemberg, D.B.; Dias, R.D.; Souza, T.M.; Calcagnotto, M.E.; et al. Seizures Induced by Pentylenetetrazole in the Adult Zebrafish: A Detailed Behavioral Characterization. PLoS ONE 2013, 8, e54515. [Google Scholar] [CrossRef] [PubMed]
- Bachiega, J.C.; Blanco, M.M.; Perez-Mendes, P.; Cinini, S.M.; Covolan, L.; Mello, L.E. Behavioral Characterization of Pentylenetetrazol-Induced Seizures in the Marmoset. Epilepsy Behav. 2008, 13, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Yakmaz, F.; Bozkurt, A.S.; Görücü Yilmaz, Ş. PTZ-Kindled Rat Model; Evaluation of Seizure, Hippocampal EGR-1, and Rev-Erbα Gene Regulation, Behavioral Analysis, and Antioxidant Capacity of Gum Arabic. Mol. Biol. Rep. 2024, 51, 279. [Google Scholar] [CrossRef] [PubMed]
- Milior, G.; Morin-Brureau, M.; Pallud, J.; Miles, R.; Huberfeld, G. Animal Models and Human Tissue Compared to Better Understand and Treat the Epilepsies. Epilepsia 2023, 64, 1175–1189. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, P.; Yan, F.; Luo, Y.; Zhao, G. Animal Models of Epilepsy: A Phenotype-Oriented Review. Aging Dis. 2022, 13, 215–231. [Google Scholar] [CrossRef]
- Kandratavicius, L.; Balista, P.A.; Lopes-Aguiar, C.; Ruggiero, R.N.; Umeoka, E.H.; Garcia-Cairasco, N.; Bueno-Junior, L.S.; Leite, J.P. Animal Models of Epilepsy: Use and Limitations. Neuropsychiatr. Dis. Treat. 2014, 10, 1693–1705. [Google Scholar] [CrossRef]
- Garodia, P.; Hegde, M.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin, Inflammation, and Neurological Disorders: How Are They Linked? Integr. Med. Res. 2023, 12, 100968. [Google Scholar] [CrossRef]
- Azzini, E.; Peña-Corona, S.I.; Hernández-Parra, H.; Chandran, D.; Saleena, L.A.K.; Sawikr, Y.; Peluso, I.; Dhumal, S.; Kumar, M.; Leyva-Gómez, G.; et al. Neuroprotective and Anti-Inflammatory Effects of Curcumin in Alzheimer’s Disease: Targeting Neuroinflammation Strategies. Phytother. Res. 2024, 38, 3169–3189. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Neuroprotective Effects of Curcumin in Neurodegenerative Diseases. Foods 2024, 13, 1774. [Google Scholar] [CrossRef]
- Bhalani, D.V.; Nutan, B.; Kumar, A.; Singh Chandel, A.K. Bioavailability Enhancement Techniques for Poorly Aqueous Soluble Drugs and Therapeutics. Biomedicines 2022, 10, 2055. [Google Scholar] [CrossRef]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving Curcumin Bioavailability: Current Strategies and Future Perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef] [PubMed]
- Bertoncini-Silva, C.; Vlad, A.; Ricciarelli, R.; Giacomo Fassini, P.; Suen, V.M.M.; Zingg, J.-M. Enhancing the Bioavailability and Bioactivity of Curcumin for Disease Prevention and Treatment. Antioxidants 2024, 13, 331. [Google Scholar] [CrossRef]
- Gupta, T.; Singh, J.; Kaur, S.; Sandhu, S.; Singh, G.; Kaur, I.P. Enhancing Bioavailability and Stability of Curcumin Using Solid Lipid Nanoparticles (CLEN): A Covenant for Its Effectiveness. Front. Bioeng. Biotechnol. 2020, 8, 879. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, S.; Patwardhan, R.S.; Pal, D.; Singh, B.; Sharma, D.; Kutala, V.K.; Sandur, S.K. Mitochondrial Targeted Curcumin Exhibits Anticancer Effects through Disruption of Mitochondrial Redox and Modulation of TrxR2 Activity. Free Radic. Biol. Med. 2017, 113, 530–538. [Google Scholar] [CrossRef]
- Gaur, T.; Ali, A.; Sharma, D.; Gupta, S.K.; Gota, V.; Bagal, B.; Platzbeckar, U.; Mishra, R.; Dutt, A.; Khattry, N.; et al. Mitocurcumin Utilizes Oxidative Stress to Upregulate JNK/P38 Signaling and Overcomes Cytarabine Resistance in Acute Myeloid Leukemia. Cell Signal 2024, 114, 111004. [Google Scholar] [CrossRef]
- Leferman, C.-E.; Stoica, L.; Stoica, B.A.; Ciubotaru, A.D.; Badescu, A.C.; Bogdanici, C.-M.; Neagu, T.P.; Ghiciuc, C.-M. Mitochondria-Targeted Curcumin: A Potent Antibacterial Agent against Methicillin-Resistant Staphylococcus Aureus with a Possible Intracellular ROS Accumulation as the Mechanism of Action. Antibiotics 2023, 12, 401. [Google Scholar] [CrossRef]
- Patwardhan, R.S.; Gohil, D.; Singh, B.; Kumar, B.K.; Purohit, V.; Thoh, M.; Checker, R.; Gardi, N.; Gota, V.; Kutala, V.K.; et al. Mitochondrial-Targeted Curcumin Inhibits T-Cell Activation via Nrf2 and Inhibits Graft-versus-Host-Disease in a Mouse Model. Phytother. Res. 2024, 38, 1555–1573. [Google Scholar] [CrossRef]
- Ciubotaru, A.D.; Leferman, C.-E.; Ignat, B.-E.; Knieling, A.; Salaru, D.L.; Turliuc, D.M.; Foia, L.G.; Dima, L.; Minea, B.; Hritcu, L.D.; et al. Anti-Epileptic Activity of Mitocurcumin in a Zebrafish–Pentylenetetrazole (PTZ) Epilepsy Model. Pharmaceuticals 2024, 17, 1611. [Google Scholar] [CrossRef]
- Reddy, C.A.; Somepalli, V.; Golakoti, T.; Kanugula, A.K.; Karnewar, S.; Rajendiran, K.; Vasagiri, N.; Prabhakar, S.; Kuppusamy, P.; Kotamraju, S.; et al. Mitochondrial-Targeted Curcuminoids: A Strategy to Enhance Bioavailability and Anticancer Efficacy of Curcumin. PLoS ONE 2014, 9, e89351. [Google Scholar] [CrossRef]
- Uzel, G.; Oylumlu, E.; Durmus, L.; Ciraci, C. Duality of Valproic Acid Effects on Inflammation, Oxidative Stress and Autophagy in Human Eosinophilic Cells. Int. J. Mol. Sci. 2023, 24, 13446. [Google Scholar] [CrossRef]
- Terzïoğlu Bebïtoğlu, B.; Oğuz, E.; Acet, G. Effect of Valproic Acid on Oxidative Stress Parameters of Glutamate-induced Excitotoxicity in SH-SY5Y Cells. Exp. Ther. Med. 2020, 20, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- D’Amora, M.; Galgani, A.; Marchese, M.; Tantussi, F.; Faraguna, U.; De Angelis, F.; Giorgi, F.S. Zebrafish as an Innovative Tool for Epilepsy Modeling: State of the Art and Potential Future Directions. Int. J. Mol. Sci. 2023, 24, 7702. [Google Scholar] [CrossRef] [PubMed]
- Cunliffe, V.T.; Baines, R.A.; Giachello, C.N.G.; Lin, W.-H.; Morgan, A.; Reuber, M.; Russell, C.; Walker, M.C.; Williams, R.S.B. Epilepsy Research Methods Update: Understanding the Causes of Epileptic Seizures and Identifying New Treatments Using Non-Mammalian Model Organisms. Seizure 2015, 24, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Doszyn, O.; Dulski, T.; Zmorzynska, J. Diving into the Zebrafish Brain: Exploring Neuroscience Frontiers with Genetic Tools, Imaging Techniques, and Behavioral Insights. Front. Mol. Neurosci. 2024, 17, 1358844. [Google Scholar] [CrossRef]
- Bertoncello, K.T.; Aguiar, G.P.S.; Oliveira, J.V.; Siebel, A.M. Micronization Potentiates Curcumin’s Anti-Seizure Effect and Brings an Important Advance in Epilepsy Treatment. Sci. Rep. 2018, 8, 2645. [Google Scholar] [CrossRef]
- Idalencio, R.; Kalichak, F.; Rosa, J.G.S.; de Oliveira, T.A.; Koakoski, G.; Gusso, D.; de Abreu, M.S.; Giacomini, A.C.V.; Barcellos, H.H. de A.; Piato, A.L.; et al. Waterborne Risperidone Decreases Stress Response in Zebrafish. PLoS ONE 2015, 10, e0140800. [Google Scholar] [CrossRef]
- Stewart, A.; Gaikwad, S.; Kyzar, E.; Green, J.; Roth, A.; Kalueff, A.V. Modeling Anxiety Using Adult Zebrafish: A Conceptual Review. Neuropharmacology 2012, 62, 135–143. [Google Scholar] [CrossRef]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding Behavioral and Physiological Phenotypes of Stress and Anxiety in Zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef]
- Perdikaris, P.; Dermon, C.R. Behavioral and Neurochemical Profile of MK-801 Adult Zebrafish Model: Forebrain Β2-Adrenoceptors Contribute to Social Withdrawal and Anxiety-like Behavior. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 115, 110494. [Google Scholar] [CrossRef]
- Bencan, Z.; Sledge, D.; Levin, E.D. Buspirone, Chlordiazepoxide and Diazepam Effects in a Zebrafish Model of Anxiety. Pharmacol. Biochem. Behav. 2009, 94, 75–80. [Google Scholar] [CrossRef]
- Gebauer, D.L.; Pagnussat, N.; Piato, Â.L.; Schaefer, I.C.; Bonan, C.D.; Lara, D.R. Effects of Anxiolytics in Zebrafish: Similarities and Differences between Benzodiazepines, Buspirone and Ethanol. Pharmacol. Biochem. Behav. 2011, 99, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Flores-Prieto, B.; Manzo-Denes, J.; Hernández-Aguilar, M.E.; Coria-Avila, G.A.; Herrera-Covarrubias, D.; Aranda-Abreu, G.E.; Rojas-Durán, F.; Pérez-Estudillo, C.A.; Suárez-Medellín, J.; Toledo-Cárdenas, M.R. Effects of Valproic Acid Embryonic Exposure on Zebrafish: A Systematic Review and Meta-Analysis. NeuroSci 2024, 5, 650–665. [Google Scholar] [CrossRef] [PubMed]
- Sachett, A.; Gallas-Lopes, M.; Benvenutti, R.; Marcon, M.; Linazzi, A.M.; Aguiar, G.P.S.; Herrmann, A.P.; Oliveira, J.V.; Siebel, A.M.; Piato, A. Non-Micronized and Micronized Curcumin Do Not Prevent the Behavioral and Neurochemical Effects Induced by Acute Stress in Zebrafish. Pharmacol. Rep. 2022, 74, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Swain, H.A.; Sigstad, C.; Scalzo, F.M. Effects of Dizocilpine (MK-801) on Circling Behavior, Swimming Activity, and Place Preference in Zebrafish (Danio Rerio). Neurotoxicol Teratol. 2004, 26, 725–729. [Google Scholar] [CrossRef]
- McDougall, S.A.; Apodaca, M.G.; Park, G.I.; Teran, A.; Baum, T.J.; Montejano, N.R. MK801-Induced Locomotor Activity in Preweanling and Adolescent Male and Female Rats: Role of the Dopamine and Serotonin Systems. Psychopharmacology 2020, 237, 2469–2483. [Google Scholar] [CrossRef]
- Wronikowska, O.; Michalak, A.; Skalicka-Woźniak, K.; Crawford, A.D.; Budzyńska, B. Fishing for a Deeper Understanding of Nicotine Effects Using Zebrafish Behavioural Models. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 98, 109826. [Google Scholar] [CrossRef]
- Wong, K.; Elegante, M.; Bartels, B.; Elkhayat, S.; Tien, D.; Roy, S.; Goodspeed, J.; Suciu, C.; Tan, J.; Grimes, C.; et al. Analyzing Habituation Responses to Novelty in Zebrafish (Danio Rerio). Behav. Brain Res. 2010, 208, 450–457. [Google Scholar] [CrossRef]
- Ilie, O.-D.; Duta, R.; Jijie, R.; Nita, I.-B.; Nicoara, M.; Faggio, C.; Dobrin, R.; Mavroudis, I.; Ciobica, A.; Doroftei, B. Assessing Anti-Social and Aggressive Behavior in a Zebrafish (Danio Rerio) Model of Parkinson’s Disease Chronically Exposed to Rotenone. Brain Sci. 2022, 12, 898. [Google Scholar] [CrossRef]
- Robea, M.A.; Petrovici, A.; Ureche, D.; Nicoara, M.; Ciobica, A.S. Histopathological and Behavioral Impairments in Zebrafish (Danio Rerio) Chronically Exposed to a Cocktail of Fipronil and Pyriproxyfen. Life 2023, 13, 1874. [Google Scholar] [CrossRef]
- Desland, F.A.; Afzal, A.; Warraich, Z.; Mocco, J. Manual versus Automated Rodent Behavioral Assessment: Comparing Efficacy and Ease of Bederson and Garcia Neurological Deficit Scores to an Open Field Video-Tracking System. J. Cent. Nerv. Syst. Dis. 2014, 6, 7–14. [Google Scholar] [CrossRef]
- Gabriel, J.P.; Mahmood, R.; Kyriakatos, A.; Söll, I.; Hauptmann, G.; Calabrese, R.L.; El Manira, A. Serotonergic Modulation of Locomotion in Zebrafish—Endogenous Release and Synaptic Mechanisms. J. Neurosci. 2009, 29, 10387–10395. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Pedroni, A.; Bertuzzi, M.; Kizil, C.; Simon, A.; Ampatzis, K. Locomotion Dependent Neuron-Glia Interactions Control Neurogenesis and Regeneration in the Adult Zebrafish Spinal Cord. Nat. Commun. 2021, 12, 4857. [Google Scholar] [CrossRef] [PubMed]
- Choo, B.K.M.; Kundap, U.P.; Faudzi, S.M.M.; Abas, F.; Shaikh, M.F.; Samarut, É. Identification of Curcumin Analogues with Anti-Seizure Potential in Vivo Using Chemical and Genetic Zebrafish Larva Seizure Models. Biomed. Pharmacother. 2021, 142, 112035. [Google Scholar] [CrossRef]
- Grossman, L.; Utterback, E.; Stewart, A.; Gaikwad, S.; Chung, K.M.; Suciu, C.; Wong, K.; Elegante, M.; Elkhayat, S.; Tan, J.; et al. Characterization of Behavioral and Endocrine Effects of LSD on Zebrafish. Behav. Brain Res. 2010, 214, 277–284. [Google Scholar] [CrossRef]
- Johnson, A.; Loh, E.; Verbitsky, R.; Slessor, J.; Franczak, B.C.; Schalomon, M.; Hamilton, T.J. Examining Behavioural Test Sensitivity and Locomotor Proxies of Anxiety-like Behaviour in Zebrafish. Sci. Rep. 2023, 13, 3768. [Google Scholar] [CrossRef]
- Sharma, V.; Nehru, B.; Munshi, A.; Jyothy, A. Antioxidant Potential of Curcumin against Oxidative Insult Induced by Pentylenetetrazol in Epileptic Rats. Methods Find. Exp. Clin. Pharmacol. 2010, 32, 227–232. [Google Scholar] [CrossRef]
- Jantawong, C.; Priprem, A.; Intuyod, K.; Pairojkul, C.; Pinlaor, P.; Waraasawapati, S.; Mongkon, I.; Chamgramol, Y.; Pinlaor, S. Curcumin-Loaded Nanocomplexes: Acute and Chronic Toxicity Studies in Mice and Hamsters. Toxicol. Rep. 2021, 8, 1346–1357. [Google Scholar] [CrossRef]
- Aggarwal, M.L.; Chacko, K.M.; Kuruvilla, B.T. Systematic and Comprehensive Investigation of the Toxicity of Curcuminoid-Essential Oil Complex: A Bioavailable Turmeric Formulation. Mol. Med. Rep. 2016, 13, 592–604. [Google Scholar] [CrossRef]
- Malik, P.; Mukherjee, T.K. Structure-Function Elucidation of Antioxidative and Prooxidative Activities of the Polyphenolic Compound Curcumin. Chin. J. Biol. 2014, 2014, 396708. [Google Scholar] [CrossRef]
- Leferman, C.-E.; Stoica, L.; Tiglis, M.; Stoica, B.A.; Hancianu, M.; Ciubotaru, A.D.; Salaru, D.L.; Badescu, A.C.; Bogdanici, C.-M.; Ciureanu, I.-A.; et al. Overcoming Drug Resistance in a Clinical C. Albicans Strain Using Photoactivated Curcumin as an Adjuvant. Antibiotics 2023, 12, 1230. [Google Scholar] [CrossRef]
- Stoica, L.; Stoica, B.A.; Olinici, D.; Onofrei, P.; Botez, E.A.; Cotrutz, C.E. Correlations between Morphological Changes Induced by Curcumin and Its Biological Activities. Rom. J. Morphol. Embryol. 2018, 59, 65–69. [Google Scholar] [PubMed]
- Stoica, L.; Stoica, B.; Ciobîcă, A.; Zlei, M.; Timofte, D.; Filip, N.; Olinici, D.; Cotrutz, C. Photocytotoxicity of Some Curcumin Derivatives. Rom. Biotechnol. Lett. 2018, 23, 13862. [Google Scholar]
- Shen, S.-Q.; Zhang, Y.; Xiang, J.-J.; Xiong, C.-L. Protective Effect of Curcumin against Liver Warm Ischemia/Reperfusion Injury in Rat Model Is Associated with Regulation of Heat Shock Protein and Antioxidant Enzymes. World J. Gastroenterol. 2007, 13, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in Liver Diseases: A Systematic Review of the Cellular Mechanisms of Oxidative Stress and Clinical Perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef]
- Das, K.C.; Das, C.K. Curcumin (Diferuloylmethane), a Singlet Oxygen (1O2) Quencher. Biochem. Biophys. Res. Commun. 2002, 295, 62–66. [Google Scholar] [CrossRef]
- Yoshino, M.; Haneda, M.; Naruse, M.; Htay, H.H.; Tsubouchi, R.; Qiao, S.L.; Li, W.H.; Murakami, K.; Yokochi, T. Prooxidant Activity of Curcumin: Copper-Dependent Formation of 8-Hydroxy-2′-Deoxyguanosine in DNA and Induction of Apoptotic Cell Death. Toxicol. In Vitro 2004, 18, 783–789. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, B.; Duan, D.; Wu, J.; Fang, J. Curcumin Targeting the Thioredoxin System Elevates Oxidative Stress in HeLa Cells. Toxicol. Appl. Pharmacol. 2012, 262, 341–348. [Google Scholar] [CrossRef]
- Sandur, S.K.; Ichikawa, H.; Pandey, M.K.; Kunnumakkara, A.B.; Sung, B.; Sethi, G.; Aggarwal, B.B. Role of Pro-Oxidants and Antioxidants in the Anti-Inflammatory and Apoptotic Effects of Curcumin (Diferuloylmethane). Free Radic. Biol. Med. 2007, 43, 568–580. [Google Scholar] [CrossRef]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef]
- Stoica, B.A.; Petreus, T.; Cioanca, O.; Hancianu, M. ANtiproliferative Effects of a Novel Manganese Mitochondrial Targeted Complex. Farmacia 2015, 63, 886–889. [Google Scholar]
- Smith, R.A.J.; Porteous, C.M.; Gane, A.M.; Murphy, M.P. Delivery of Bioactive Molecules to Mitochondria in Vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 5407–5412. [Google Scholar] [CrossRef] [PubMed]
- Gane, E.J.; Weilert, F.; Orr, D.W.; Keogh, G.F.; Gibson, M.; Lockhart, M.M.; Frampton, C.M.; Taylor, K.M.; Smith, R.A.J.; Murphy, M.P. The Mitochondria-Targeted Anti-Oxidant Mitoquinone Decreases Liver Damage in a Phase II Study of Hepatitis C Patients. Liver Int. 2010, 30, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Skulachev, V.P.; Antonenko, Y.N.; Cherepanov, D.A.; Chernyak, B.V.; Izyumov, D.S.; Khailova, L.S.; Klishin, S.S.; Korshunova, G.A.; Lyamzaev, K.G.; Pletjushkina, O.Y.; et al. Prevention of Cardiolipin Oxidation and Fatty Acid Cycling as Two Antioxidant Mechanisms of Cationic Derivatives of Plastoquinone (SkQs). Biochim. Biophys. Acta 2010, 1797, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, W.; Su, Z.; Kong, A.-N.T. The Complexity of the Nrf2 Pathway: Beyond the Antioxidant Response. J Nutr Biochem 2015, 26, 1401–1413. [Google Scholar] [CrossRef]
- Demyashkin, G.; Blinova, E.; Grigoryan, M.; Parshenkov, M.; Skovorodko, P.; Ius, V.; Lebed, A.; Shegay, P.; Kaprin, A. Neuroprotective Effects of Myricetin on PTZ-Induced Seizures in Mice: Evaluation of Oxidation, Neuroinflammation and Metabolism, and Apoptosis in the Hippocampus. Curr. Issues Mol. Biol. 2024, 46, 8914–8944. [Google Scholar] [CrossRef]
- Lv, S.; Liu, H.; Wang, H. The Interplay between Autophagy and NLRP3 Inflammasome in Ischemia/Reperfusion Injury. Int. J. Mol. Sci. 2021, 22, 8773. [Google Scholar] [CrossRef]
- Orellana-Paucar, A.M.; Serruys, A.-S.K.; Afrikanova, T.; Maes, J.; Borggraeve, W.D.; Alen, J.; León-Tamariz, F.; Wilches-Arizábala, I.M.; Crawford, A.D.; de Witte, P.A.M.; et al. Anticonvulsant Activity of Bisabolene Sesquiterpenoids of Curcuma Longa in Zebrafish and Mouse Seizure Models. Epilepsy Behav. 2012, 24, 14–22. [Google Scholar] [CrossRef]
- Gawel, K.; Langlois, M.; Martins, T.; van der Ent, W.; Tiraboschi, E.; Jacmin, M.; Crawford, A.D.; Esguerra, C.V. Seizing the Moment: Zebrafish Epilepsy Models. Neurosci. Biobehav. Rev. 2020, 116, 1–20. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, D.; Kim, Y.-H.; Lee, H.; Lee, C.-J. Improvement of Pentylenetetrazol-Induced Learning Deficits by Valproic Acid in the Adult Zebrafish. Eur. J. Pharmacol. 2010, 643, 225–231. [Google Scholar] [CrossRef]
- Gupta, P.; Khobragade, S.B.; Shingatgeri, V.M. Effect of Various Antiepileptic Drugs in Zebrafish PTZ-Seizure Model. Indian J. Pharm. Sci. 2014, 76, 157. [Google Scholar]
- Singh, H.; Ramon, A.; Finore, D.; Burnham, K.; McRobert, S.; Lippman-Bell, J. Learning Deficits and Attenuated Adaptive Stress Response After Early-Life Seizures in Zebrafish. Front. Neurosci. 2022, 16, 869671. [Google Scholar] [CrossRef] [PubMed]
- DePasquale, C.; Franklin, K.; Jia, Z.; Jhaveri, K.; Buderman, F.E. The Effects of Exploratory Behavior on Physical Activity in a Common Animal Model of Human Disease, Zebrafish (Danio Rerio). Front. Behav. Neurosci. 2022, 16, 1020837. [Google Scholar] [CrossRef] [PubMed]
- Afrikanova, T.; Serruys, A.-S.K.; Buenafe, O.E.M.; Clinckers, R.; Smolders, I.; de Witte, P.A.M.; Crawford, A.D.; Esguerra, C.V. Validation of the Zebrafish Pentylenetetrazol Seizure Model: Locomotor versus Electrographic Responses to Antiepileptic Drugs. PLoS ONE 2013, 8, e54166. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciubotaru, A.D.; Leferman, C.-E.; Ignat, B.-E.; Knieling, A.; Esanu, I.M.; Salaru, D.L.; Foia, L.G.; Minea, B.; Hritcu, L.D.; Dimitriu, C.D.; et al. Behavioral and Biochemical Insights into the Therapeutic Potential of Mitocurcumin in a Zebrafish–Pentylenetetrazole (PTZ) Epilepsy Model. Pharmaceuticals 2025, 18, 382. https://doi.org/10.3390/ph18030382
Ciubotaru AD, Leferman C-E, Ignat B-E, Knieling A, Esanu IM, Salaru DL, Foia LG, Minea B, Hritcu LD, Dimitriu CD, et al. Behavioral and Biochemical Insights into the Therapeutic Potential of Mitocurcumin in a Zebrafish–Pentylenetetrazole (PTZ) Epilepsy Model. Pharmaceuticals. 2025; 18(3):382. https://doi.org/10.3390/ph18030382
Chicago/Turabian StyleCiubotaru, Alin Dumitru, Carmen-Ecaterina Leferman, Bogdan-Emilian Ignat, Anton Knieling, Irina Mihaela Esanu, Delia Lidia Salaru, Liliana Georgeta Foia, Bogdan Minea, Luminita Diana Hritcu, Cristina Daniela Dimitriu, and et al. 2025. "Behavioral and Biochemical Insights into the Therapeutic Potential of Mitocurcumin in a Zebrafish–Pentylenetetrazole (PTZ) Epilepsy Model" Pharmaceuticals 18, no. 3: 382. https://doi.org/10.3390/ph18030382
APA StyleCiubotaru, A. D., Leferman, C.-E., Ignat, B.-E., Knieling, A., Esanu, I. M., Salaru, D. L., Foia, L. G., Minea, B., Hritcu, L. D., Dimitriu, C. D., Stoica, L., Ciureanu, I.-A., Ciobica, A. S., Neamtu, A., Stoica, B. A., & Ghiciuc, C. M. (2025). Behavioral and Biochemical Insights into the Therapeutic Potential of Mitocurcumin in a Zebrafish–Pentylenetetrazole (PTZ) Epilepsy Model. Pharmaceuticals, 18(3), 382. https://doi.org/10.3390/ph18030382







