Neurological Adverse Events Associated with the Use of Janus Kinase Inhibitors: A Pharmacovigilance Study Based on Vigibase
Abstract
:1. Introduction
2. Results
2.1. Demographic Characteristics of Safety Reports
2.2. Disproportionality Analysis and Neurological AE Signals
3. Discussion
4. Materials and Methods
4.1. Data Source
4.2. Data Mining and Signal Detection Criteria
4.3. Hierarchical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| JAK | Janus kinase |
| STAT | Signal transduction and activators of transcription |
| AE | Adverse event |
| PRR | Proportional reporting ratio |
| ROR | Reporting odds ratio |
| IC | Information component |
| MedDRA | Medical dictionary for regulatory activities |
| SOC | System organ class |
| HLGT | High-level group term |
| HLT | High-level term |
| PT | Preferred term |
| LLT | Lowest-level term |
| TNF | Tumor necrosis factor |
| CNS | Central nervous system |
| PML | Progressive multifocal leukoencephalopathy |
| ICSRs | Individual case safety reports |
| PV | Pharmacovigilance |
| CI | Confidence interval |
| NEC | Not elsewhere classified |
References
- Clark, J.D.; Flanagan, M.E.; Telliez, J.-B. Discovery and Development of Janus Kinase (JAK) Inhibitors for Inflammatory Diseases. J. Med. Chem. 2014, 57, 5023–5038. [Google Scholar] [CrossRef]
- Nash, P.; Kerschbaumer, A.; Dörner, T.; Dougados, M.; Fleischmann, R.M.; Geissler, K.; McInnes, I.; E Pope, J.; van der Heijde, D.; Stoffer-Marx, M.; et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: A consensus statement. Ann. Rheum. Dis. 2021, 80, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Harrington, R.; Al Nokhatha, S.A.; Conway, R. JAK Inhibitors in Rheumatoid Arthritis: An Evidence-Based Review on the Emerging Clinical Data. J. Inflamm. Res. 2020, 13, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef]
- Kragstrup, T.W.; Glintborg, B.; Svensson, A.L.; McMaster, C.; Robinson, P.C.; Deleuran, B.; Liew, D.F. Waiting for JAK inhibitor safety data. RMD Open 2022, 8, e002236. [Google Scholar] [CrossRef] [PubMed]
- Wlassits, R.; Müller, M.; Fenzl, K.H.; Lamprecht, T.; Erlacher, L. JAK-Inhibitors—A Story of Success and Adverse Events. Open Access Rheumatol. 2024, 16, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Abboud, H. Iatrogenic CNS demyelination in the era of modern biologics. Mult. Scler. 2019, 25, 1079–1085. [Google Scholar] [CrossRef]
- Massoud, F.; Ismail, I.I.; Al-Hashel, J.Y.; Abboud, H. CNS demyelination during tofacitinib therapy: First report. Mult. Scler. Relat. Disord. 2020, 46, 102568. [Google Scholar] [CrossRef]
- Schwartzmann, Y.; Vaknin-Dembinsky, A.; Gomori, J.M.; Elinav, H.; Berkun, Y.; Levin, N.; Ekstein, D.; Magadle, J.; Gotkine, M. Tofacitinib-induced progressive multifocal leukoencephalopathy-immune reconstitution inflammatory syndrome. Neurol. Sci. 2023, 44, 3737–3739. [Google Scholar] [CrossRef]
- Wathes, R.; Moule, S.; Milojkovic, D. Progressive multifocal leukoencephalopathy associated with ruxolitinib. N. Engl. J. Med. 2013, 369, 197–198. [Google Scholar] [CrossRef]
- Nakayama, K.; Nakamura, M.; Konishi, A.; Kaneko, S.; Nakamichi, K.; Saijo, M.; Yakushiji, Y.; Kusaka, H. JC virus granule cell neuronopathy associated with Ruxolitinib: A case report and review of the literature. eNeurologicalSci 2020, 21, 100269. [Google Scholar] [CrossRef] [PubMed]
- Reoma, L.B.; Trindade, C.J.; Monaco, M.C.; Solis, J.; Montojo, M.G.; Vu, P.; Johnson, K.; Beck, E.; Nair, G.; Khan, O.I.; et al. Fatal encephalopathy with wild-type JC virus and ruxolitinib therapy. Ann. Neurol. 2019, 86, 878–884. [Google Scholar] [CrossRef]
- Verstovsek, S.; Hoffman, R.; Mascarenhas, J.; Soria, J.-C.; Bahleda, R.; McCoon, P.; Tang, W.; Cortes, J.; Kantarjian, H.; Ribrag, V. A phase I, open-label, multi-center study of the JAK2 inhibitor AZD1480 in patients with myelofibrosis. Leuk. Res. 2015, 39, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.L.; Becker, P.S. JAK/STAT Pathway Inhibitors and Neurologic Toxicity: Above All Else Do No Harm? JAMA Oncol. 2015, 1, 651–652. [Google Scholar] [CrossRef]
- Pardanani, A.; Harrison, C.; Cortes, J.E.; Cervantes, F.; Mesa, R.A.; Milligan, D.; Masszi, T.; Mishchenko, E.; Jourdan, E.; Vannucchi, A.M.; et al. Safety and Efficacy of Fedratinib in Patients With Primary or Secondary Myelofibrosis: A Randomized Clinical Trial. JAMA Oncol. 2015, 1, 643–651. [Google Scholar] [CrossRef]
- Pacurariu, A.C.; Coloma, P.M.; van Haren, A.; Genov, G.; Sturkenboom, M.C.; Straus, S.M. A description of signals during the first 18 months of the EMA pharmacovigilance risk assessment committee. Drug Saf. 2014, 37, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Giezen, T.J.; Mantel-Teeuwisse, A.K.; Meyboom, R.H.; Straus, S.M.; Leufkens, H.G.; Egberts, T.C. Mapping the safety profile of biologicals: A disproportionality analysis using the WHO adverse drug reaction database, VigiBase. Drug Saf. 2010, 33, 865–878. [Google Scholar] [CrossRef]
- Sangha, P.S.; Thakur, M.; Akhtar, Z.; Ramani, S.; Gyamfi, R.S. The Link Between Rheumatoid Arthritis and Dementia: A Review. Cureus 2020, 12, e7855. [Google Scholar] [CrossRef]
- Sattui, S.E.; Navarro-Millan, I.; Xie, F.; Rajan, M.; Yun, H.; Curtis, J.R. Incidence of dementia in patients with rheumatoid arthritis and association with disease modifying anti-rheumatic drugs—Analysis of a national claims database. Semin. Arthritis Rheum. 2022, 57, 152083. [Google Scholar] [CrossRef]
- Dahan, L.; Rampon, C.; Florian, C. Age-related memory decline, dysfunction of the hippocampus and therapeutic opportunities. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 102, 109943. [Google Scholar] [CrossRef]
- Taylor, P.C.; Choy, E.; Baraliakos, X.; Szekanecz, Z.; Xavier, R.M.; Isaacs, J.D.; Strengholt, S.; Parmentier, J.M.; Lippe, R.; Tanaka, Y. Differential properties of Janus kinase inhibitors in the treatment of immune-mediated inflammatory diseases. Rheumatology 2024, 63, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, A.; Vrhovac, R.; Verstovsek, S. Ruxolitinib: A new JAK1/2 inhibitor that offers promising options for treatment of myelofibrosis. Future Oncol. 2011, 7, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.H. Clinical significance of Janus Kinase inhibitor selectivity. Rheumatology 2019, 58, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, A.; Spinelli, F.R.; Telliez, J.B.; O’Shea, J.J.; Silvennoinen, O.; Gadina, M. JAK inhibitor selectivity: New opportunities, better drugs? Nat. Rev. Rheumatol. 2024, 20, 649–665. [Google Scholar] [CrossRef]
- Harigai, M.; Honda, S. Selectivity of Janus Kinase Inhibitors in Rheumatoid Arthritis and Other Immune-Mediated Inflammatory Diseases: Is Expectation the Root of All Headache? Drugs 2020, 80, 1183–1201. [Google Scholar] [CrossRef]
- Kavanagh, S.; Bril, V.; Lipton, J.H. Peripheral neuropathy associated with imatinib therapy for chronic myeloid leukemia. Blood Res. 2018, 53, 172–174. [Google Scholar] [CrossRef]
- Chakupurakal, G.; Etti, R.J.; Murray, J.A. Peripheral neuropathy as an adverse effect of imatinib therapy. J. Clin. Pathol. 2011, 64, 456. [Google Scholar] [CrossRef]
- Monge, K.S.; Gálvez-Ruiz, A.; Alvárez-Carrón, A.; Quijada, C.; Matheu, A. Optic neuropathy secondary to dasatinib in the treatment of a chronic myeloid leukemia case. Saudi J. Ophthalmol. 2015, 29, 227–231. [Google Scholar] [CrossRef]
- Ishida, T.; Akagawa, N.; Miyata, T.; Tominaga, N.; Iizuka, T.; Higashihara, M.; Suzuki, T.; Miyazaki, K. Dasatinib-associated reversible demyelinating peripheral polyneuropathy in a case of chronic myeloid leukemia. Int. J. Hematol. 2018, 107, 373–377. [Google Scholar] [CrossRef]
- Kaltsonoudis, E.; Voulgari, P.V.; Konitsiotis, S.; Drosos, A.A. Demyelination and other neurological adverse events after anti-TNF therapy. Autoimmun. Rev. 2014, 13, 54–58. [Google Scholar] [CrossRef]
- Voulgari, P.V.; Alamanos, Y.; Nikas, S.N.; Bougias, D.V.; Temekonidis, T.I.; Drosos, A.A. Infliximab therapy in established rheumatoid arthritis: An observational study. Am. J. Med. 2005, 118, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Vivekanandan, G.; Abubacker, A.P.; Myneni, R.; Chawla, H.V.; Iqbal, A.; Grewal, A.; Ndakotsu, A.; Khan, S. Risk of Progressive Multifocal Leukoencephalopathy in Multiple Sclerosis Patient Treated With Natalizumab: A Systematic Review. Cureus 2021, 13, e14764. [Google Scholar] [CrossRef] [PubMed]
- Adas, M.A.; Alveyn, E.; Cook, E.; Dey, M.; Galloway, J.B.; Bechman, K. The infection risks of JAK inhibition. Expert Rev. Clin. Immunol. 2022, 18, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Hoisnard, L.; Lebrun-Vignes, B.; Maury, S.; Mahevas, M.; El Karoui, K.; Roy, L.; Zarour, A.; Michel, M.; Cohen, J.L.; Amiot, A.; et al. Adverse events associated with JAK inhibitors in 126,815 reports from the WHO pharmacovigilance database. Sci. Rep. 2022, 12, 7140. [Google Scholar] [CrossRef]
- Ono, D.; Shishido-Hara, Y.; Mizutani, S.; Mori, Y.; Ichinose, K.; Watanabe, M.; Tanizawa, T.; Yokota, T.; Uchihara, T.; Fujigasaki, H. Development of demyelinating lesions in progressive multifocal leukoencephalopathy (PML): Comparison of magnetic resonance images and neuropathology of post-mortem brain. Neuropathology 2019, 39, 294–306. [Google Scholar] [CrossRef]
- Eng, P.M.; Turnbull, B.R.; Cook, S.F.; Davidson, J.E.; Kurth, T.; Seeger, J.D. Characteristics and antecedents of progressive multifocal leukoencephalopathy in an insured population. Neurology 2006, 67, 884–886. [Google Scholar] [CrossRef]
- Yun, J.; Osehobo, E.; Lawson, E.C.; Harrison, T.; Harrison, A. Tofacitinib-induced Progressive Multifocal Leukoencephalopathy with Immune Reconstitution Inflammatory Syndrome. Clin. Neurol. Neurosurg. 2022, 214, 107143. [Google Scholar] [CrossRef]
- Chiba, T.; Yamada, M.; Sasabe, J.; Terashita, K.; Shimoda, M.; Matsuoka, M.; Aiso, S. Amyloid-beta causes memory impairment by disturbing the JAK2/STAT3 axis in hippocampal neurons. Mol. Psychiatry 2009, 14, 206–222. [Google Scholar] [CrossRef]
- Ota, Y.; Zanetti, A.T.; Hallock, R.M. The role of astrocytes in the regulation of synaptic plasticity and memory formation. Neural Plast. 2013, 2013, 185463. [Google Scholar] [CrossRef]
- Kim, B.K.; Tran, H.Y.; Shin, E.J.; Lee, C.; Chung, Y.H.; Jeong, J.H.; Bach, J.H.; Kim, W.K.; Park, D.H.; Saito, K.; et al. IL-6 attenuates trimethyltin-induced cognitive dysfunction via activation of JAK2/STAT3, M1 mAChR and ERK signaling network. Cell Signal 2013, 25, 1348–1360. [Google Scholar] [CrossRef]
- Migita, K.; Izumi, Y.; Jiuchi, Y.; Kozuru, H.; Kawahara, C.; Izumi, M.; Sakai, T.; Nakamura, M.; Motokawa, S.; Nakamura, T.; et al. Effects of Janus kinase inhibitor tofacitinib on circulating serum amyloid A and interleukin-6 during treatment for rheumatoid arthritis. Clin. Exp. Immunol. 2014, 175, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Reyes Gaido, O.E.; Pavlaki, N.; Granger, J.M.; Mesubi, O.O.; Liu, B.; Lin, B.L.; Long, A.; Walker, D.; Mayourian, J.; Schole, K.L.; et al. An improved reporter identifies ruxolitinib as a potent and cardioprotective CaMKII inhibitor. Sci. Transl. Med. 2023, 15, eabq7839. [Google Scholar] [CrossRef]
- Tao, W.; Lee, J.; Chen, X.; Díaz-Alonso, J.; Zhou, J.; Pleasure, S.; A Nicoll, R. Synaptic memory requires CaMKII. Elife 2021, 10, e60360. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.P.; Posso-Osorio, I.; Aguirre-Valencia, D.; Naranjo-Escobar, J.; Tobón, G.J. Tofacitinib and Risk of Peripheral Neuropathy? Experience of 2 Cases in Patients With Rheumatoid Arthritis. J. Clin. Rheumatol. 2021, 27, e58–e60. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Singh, M.K.; Shyam, H.; Mishra, A.; Kumar, S.; Kumar, A.; Kushwaha, J. Role of JAK/STAT in the Neuroinflammation and its Association with Neurological Disorders. Ann. Neurosci. 2021, 28, 191–200. [Google Scholar] [CrossRef]
- Choi, M.; Kim, H.; Yang, E.J.; Kim, H.S. Inhibition of STAT3 phosphorylation attenuates impairments in learning and memory in 5XFAD mice, an animal model of Alzheimer’s disease. J. Pharmacol. Sci. 2020, 143, 290–299. [Google Scholar] [CrossRef]
- Sui, S.; Lv, H. Cognitive improving actions of tofacitinib in a mouse model of Alzheimer disease involving TNF-α, IL-6, PI3K-Akt and GSK-3β signalling pathway. Int. J. Neurosci. 2024, 134, 795–803. [Google Scholar] [CrossRef]
- Totoson, P.; Peyronnel, C.; Quirié, A.; Pédard, M.; Cefis, M.; Bermont, L.; Prigent-Tessier, A.; Prati, C.; Tournier, M.; Wendling, D.; et al. Tofacitinib improved peripheral endothelial dysfunction and brain-derived neurotrophic factor levels in the rat adjuvant-induced arthritis model. Fundam. Clin. Pharmacol. 2022, 36, 363–374. [Google Scholar] [CrossRef]
- Matsushita, T.; Otani, K.; Yoshiga, M.; Hirano, M.; Noda, K.; Kurosaka, D. Inhibitory effect of baricitinib on microglia and STAT3 in a region with a weak blood-brain barrier in a mouse model of rheumatoid arthritis. Rheumatology 2023, 62, 2908–2917. [Google Scholar] [CrossRef]
- Gavegnano, C.; Haile, W.B.; Hurwitz, S.; Tao, S.; Jiang, Y.; Schinazi, R.F.; Tyor, W.R. Baricitinib reverses HIV-associated neurocognitive disorders in a SCID mouse model and reservoir seeding in vitro. J. Neuroinflamm. 2019, 16, 182. [Google Scholar] [CrossRef]
- Rodriguez, S.; Hug, C.; Todorov, P.; Moret, N.; Boswell, S.A.; Evans, K.; Zhou, G.; Johnson, N.T.; Hyman, B.T.; Sorger, P.K.; et al. Machine learning identifies candidates for drug repurposing in Alzheimer’s disease. Nat. Commun. 2021, 12, 1033. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Cooles, F.A.; Isaacs, J.D. Basic Mechanisms of JAK Inhibition. Mediterr. J. Rheumatol. 2020, 31 (Suppl. S1), 100–104. [Google Scholar] [CrossRef]
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N. Engl. J. Med. 2021, 384, 795–807. [Google Scholar] [CrossRef]
- Guimarães, P.O.; Quirk, D.; Furtado, R.H.; Maia, L.N.; Saraiva, J.F.; Antunes, M.O.; Filho, R.K.; Junior, V.M.; Soeiro, A.M.; Tognon, A.P.; et al. Tofacitinib in Patients Hospitalized with Covid-19 Pneumonia. N. Engl. J. Med. 2021, 385, 406–415. [Google Scholar] [CrossRef]
- Han, M.K.; Antila, M.; Ficker, J.H.; Gordeev, I.; Guerreros, A.; Bernus, A.L.; Roquilly, A.; Sifuentes-Osornio, J.; Tabak, F.; Teijeiro, R.; et al. Ruxolitinib in addition to standard of care for the treatment of patients admitted to hospital with COVID-19 (RUXCOVID): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Rheumatol. 2022, 4, e351–e361. [Google Scholar] [CrossRef] [PubMed]
- Namazi, S.; Borhani-Haghighi, A.; Karimzadeh, I. Adverse reactions to antiepileptic drugs in epileptic outpatients: A cross-sectional study in iran. Clin. Neuropharmacol. 2011, 34, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Edwards, I.R.; Biriell, C. Harmonisation in pharmacovigilance. Drug Saf. 1994, 10, 93–102. [Google Scholar] [CrossRef]
- Evans, S.; Waller, P.; Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 2001, 10, 483–486. [Google Scholar] [CrossRef]
- Rothman, K.; Lanes, S.; Sacks, S. The reporting odds ratio and its advantages over proportional reporting ratio. Pharmacoepidemiol. Drug Saf. 2004, 13, 519–523. [Google Scholar] [CrossRef]
- Bate, A.; Lindquist, M.; Edwards, I.R.; Olsson, S.; Orre, R.; Lansner, A.; De Freitas, R.M. A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 1998, 54, 315–321. [Google Scholar] [CrossRef]
- Cutroneo, P.M.; Sartori, D.; Tuccori, M.; Crisafulli, S.; Battini, V.; Carnovale, C.; Rafaniello, C.; Capuano, A.; Poluzzi, E.; Moretti, U.; et al. Conducting and interpreting disproportionality analyses derived from spontaneous reporting systems. Front. Drug Saf. Regul. 2024, 3, 1323057. [Google Scholar] [CrossRef]
- Bartosik, A.; Whittingham, H. Chapter 7—Evaluating safety and toxicity. In The Era of Artificial Intelligence, Machine Learning, and Data Science in the Pharmaceutical Industry; Ashenden, S.K., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 119–137. [Google Scholar]
- Zink, R.C.; Huang, Q.; Zhang, L.-Y.; Bao, W.-J. Statistical and graphical approaches for disproportionality analysis of spontaneously-reported adverse events in pharmacovigilance. Chin. J. Nat. Med. 2013, 11, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, M.; Jiao, X.; Zhu, Y.; Liu, Y.; Zeng, L.; Wang, H.; Zhang, L.; Zhang, W.; Zhang, L. Using disproportionality analysis to explore the association between periostitis and triazole antifungals in the FDA Adverse Event Reporting System Database. Sci. Rep. 2023, 13, 4475. [Google Scholar] [CrossRef] [PubMed]
- Mozzicato, P. MedDRA: An Overview of the Medical Dictionary for Regulatory Activities. Pharm. Med. 2009, 23, 65–75. [Google Scholar] [CrossRef]
- Edwards, I.R.; Aronson, J.K. Adverse drug reactions: Definitions, diagnosis, and management. Lancet 2000, 356, 1255–1259. [Google Scholar] [CrossRef]
- Bederson, B.B.; Shneiderman, B.E.N.; Wattenberg, M. Ordered and Quantum Treemaps: Making Effective Use of 2D Space to Display Hierarchies. In The Craft of Information Visualization; Bederson, B.B., Shneiderman, B.E.N., Eds.; Morgan Kaufmann: San Francisco, CA, USA, 2003; pp. 257–278. [Google Scholar]
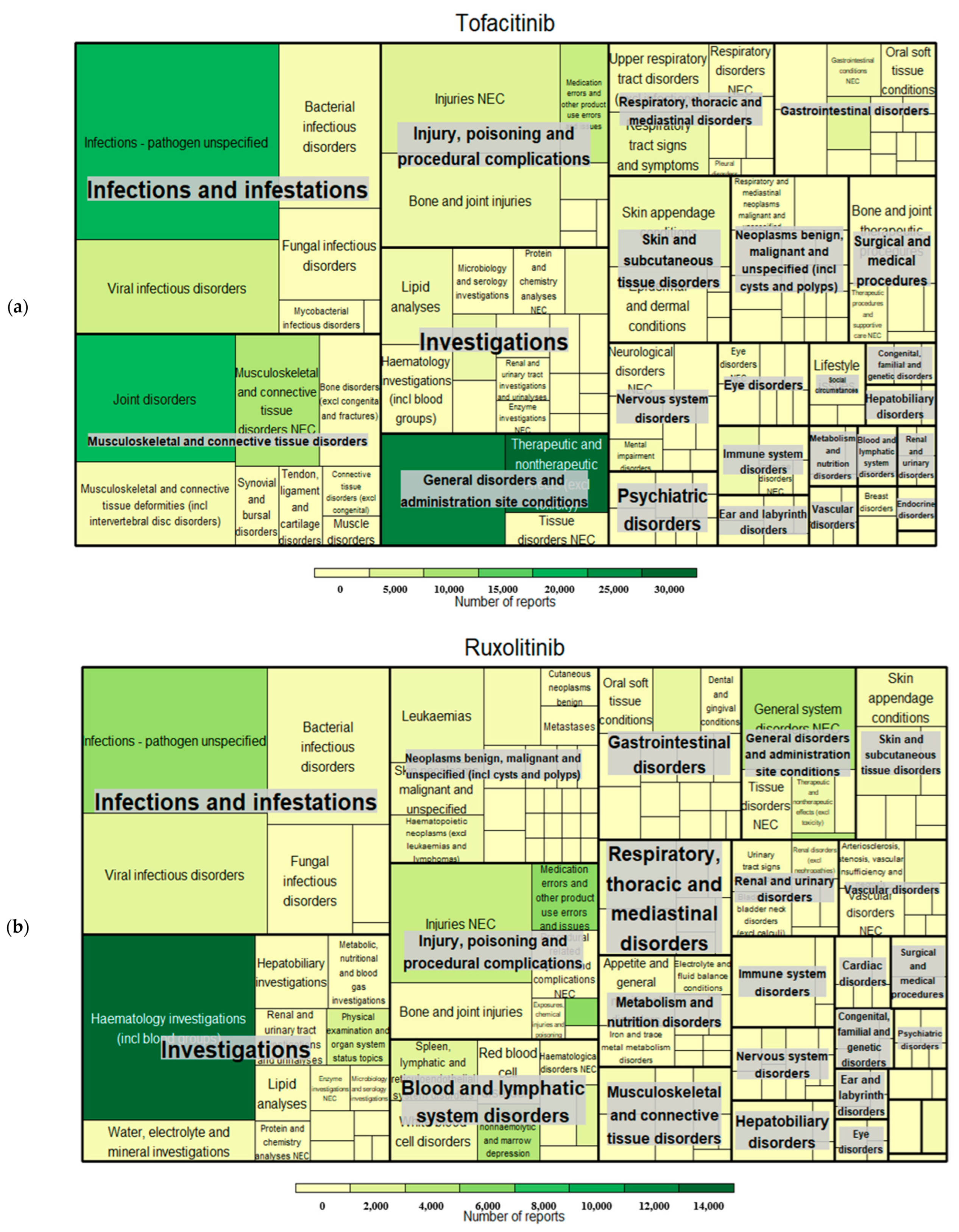
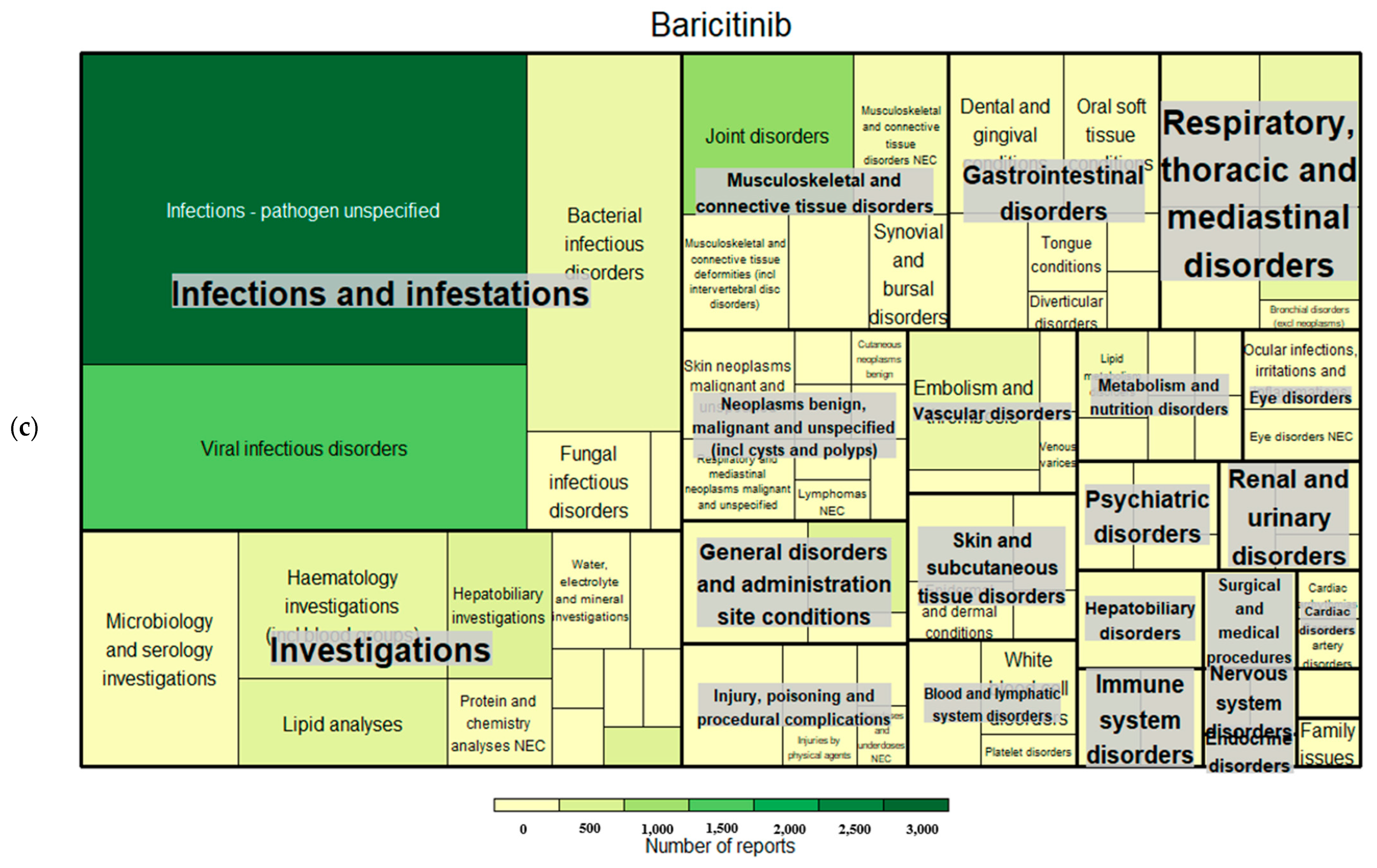
| Tofacitinib | Ruxolitinib | Baricitinib | ||||
|---|---|---|---|---|---|---|
| Total Reports (N = 105,798) | Neurological AE* Reports (N = 14,863) | Total Reports (N = 43,661) | Neurological AE* Reports (N = 6317) | Total Reports (N = 11,963) | Neurological AE* Reports (N = 1216) | |
| Sex | ||||||
| Male | 19,933 (18.8%) | 2297 (15.5%) | 11,856 (27.2%) | 1366 (21.6%) | 2399 (20.1%) | 182 (15.0%) |
| Female | 82,018 (77.5%) | 12,292 (82.7%) | 10,517 (24.1%) | 1675 (26.5%) | 9171 (76.7%) | 1009 (83.0%) |
| Unknown | 3847 (3.7%) | 271 (1.8%) | 21,288 (48.7%) | 3276 (51.9%) | 393 (3.2%) | 25 (2.0%) |
| Age | ||||||
| <18 years | 388 (0.4%) | 32 (0.2%) | 344 (0.8%) | 21 (0.3%) | 42 (0.5%) | 1 (0.1%) |
| 18–44 years | 10,326 (9.8%) | 1437 (9.7%) | 746 (1.7%) | 102 (1.6%) | 930 (7.7%) | 96 (7.9%) |
| 45–64 years | 43,264 (40.9%) | 6810 (45.8%) | 4216 (9.7%) | 644 (10.2%) | 3581 (29.9%) | 417 (34.3%) |
| 65–74 years | 21,802 (20.6%) | 3202 (21.6%) | 5113 (11.7%) | 725 (11.5%) | 1946 (16.3%) | 199 (16.4%) |
| ≥75 years | 10,299 (9.7%) | 1565 (10.5%) | 4670 (10.7%) | 694 (11.0%) | 1022 (8.5%) | 105 (8.6%) |
| Unknown | 19,719 (18.6%) | 1817 (12.2%) | 28,572 (65.4%) | 4131 (65.4%) | 4442 (37.1%) | 398 (32.7%) |
| Serious | ||||||
| Yes | 33,052 (31.2%) | 5865 (38.2%) | 21,573 (49.4%) | 3023 (47.8%) | 3285 (27.5%) | 392 (32.3%) |
| No | 72,405 (68.4%) | 9137 (61.5%) | 21,497 (49.2%) | 3225 (51.1%) | 8673 (72.5%) | 823 (67.7%) |
| Unknown | 341 (0.4%) | 41 (0.3%) | 591 (1.4%) | 69 (1.1%) | 5 (0.0%) | 1 (0.1%) |
| Notifier | ||||||
| Physician | 24,507 (23.2%) | 2613 (17.6%) | 12,668 (29.0%) | 1583 (25.1%) | 2900 (24.2%) | 231 (19.0%) |
| Pharmacist | 3639 (3.4%) | 606 (4.1%) | 1810 (4.2%) | 294 (4.6%) | 800 (7.4%) | 59 (4.8%) |
| Other Health Professional | 22,534 (21.3%) | 3357 (22.6%) | 5370 (12.3%) | 697 (11.0%) | 824 (6.9%) | 70 (5.8%) |
| Consumer/Non-Health Professional | 53,971 (51.0%) | 8162 (54.9%) | 22,904 (52.5%) | 3675 (58.2%) | 7211 (60.3%) | 844 (69.4%) |
| Lawyer | 66 (0.1%) | 8 (0.1%) | 19 (0.0%) | 5 (0.1%) | 1 (0.0%) | 1 (0.1%) |
| Unknown | 1081 (1.0%) | 117 (0.8%) | 890 (2.0%) | 63 (1.0%) | 147 (1.2%) | 11 (0.9%) |
| UN continent | ||||||
| Americas | 97,351 (92.0%) | 14,046 (94.5%) | 33,382 (76.5%) | 5375 (85.1%) | 1780 (14.9%) | 150 (12.3%) |
| Europe | 5700 (5.4%) | 613 (4.1%) | 6614 (15.1%) | 766 (12.1%) | 9455 (79.0%) | 1004 (82.6%) |
| Asia | 2155 (2.1%) | 126 (0.9%) | 3284 (7.5%) | 160 (2.5%) | 624 (5.2%) | 48 (4.0%) |
| Oceania | 559 (0.5%) | 76 (0.5%) | 348 (0.8%) | 14 (0.2%) | 67 (0.6%) | 9 (0.7%) |
| Africa | 33 (0.0%) | 2 (0.0%) | 33 (0.1%) | 2 (0.0%) | 37 (0.3%) | 5 (0.4%) |
| First Date Database | ||||||
| 2010~2014 | 2323 (2.2%) | 394 (2.6%) | 3127 (7.2%) | 442 (7.0%) | 5 (0.0%) | 0 (0.0%) |
| 2015 | 5093 (4.8%) | 916 (6.2%) | 3345 (7.6%) | 485 (7.7%) | 4 (0.0%) | 0 (0.0%) |
| 2016 | 5769 (5.5%) | 939 (6.3%) | 3366 (7.7%) | 525 (8.3%) | 5 (0.0%) | 1 (0.1%) |
| 2017 | 11,717 (11.1%) | 1940 (13.0%) | 5786 (13.3%) | 1014 (16.1%) | 91 (0.8%) | 13 (1.1%) |
| 2018 | 20,083 (19.0%) | 2495 (16.8%) | 7154 (16.4%) | 1156 (18.3%) | 1369 (11.4%) | 161 (13.2%) |
| 2019 | 25,297 (23.9%) | 3191 (21.5%) | 7793 (17.8%) | 1180 (18.7%) | 3411 (28.5%) | 357 (29.4%) |
| 2020 | 17,261 (16.3%) | 2447 (16.5%) | 5883 (13.5%) | 696 (11.0%) | 3001 (25.1%) | 384 (23.3%) |
| 2021 | 17,293 (16.3%) | 2470 (16.6%) | 6978 (16.0%) | 804 (12.7%) | 3465 (29.0%) | 353 (29.0%) |
| 2022.4 | 962 (0.9%) | 71 (0.5%) | 229 (0.5%) | 15 (0.2%) | 612 (5.1%) | 47 (3.9%) |
| Report Type | ||||||
| Spontaneous | 101,750 (96.2%) | 14,443 (97.2%) | 31,923 (73.1%) | 4647 (73.6%) | 5501 (46.0%) | 520 (42.8%) |
| From study | 3650 (3.5%) | 375 (2.5%) | 11,175 (25.6%) | 1592 (25.2%) | 6429 (53.7%) | 520 (56.7%) |
| Indication § | ||||||
| Rheumatoid Arthritis | 55,028 (52.0%) | 8828 (59.4%) | 3 (0.0%) | 2 (0.0%) | 8661 (72.4%) | 920 (75.7%) |
| Other §§ | 9462 (8.9%) | 1228 (8.3%) | 35,601 (81.6%) | 5560 (88.0%) | 1211 (10.1%) | 100 (8.2%) |
| Missing | 41,308 (39.0%) | 4807 (32.3%) | 8007 (18.4%) | 755 (12.0%) | 2091 (17.5%) | 196 (16.1%) |
| Drugs | HLGT * | PT * | Number of Reports | PRR * (95% CI *) | ROR * (95% CI *) | IC * (95% CI *) |
|---|---|---|---|---|---|---|
| Tofacitinib | Mental impairment disorders | Memory impairment | 1275 | 3.42 (3.23–3.61) | 3.44 (3.26–3.64) | 1.76 (1.68–1.84) |
| Dementia | 159 | 2.63 (2.25–3.07) | 2.63 (2.25–3.08) | 1.38 (1.15–1.60) | ||
| Dementia Alzheimer’s type | 50 | 2.88 (2.18–3.80) | 2.88 (2.18–3.80) | 1.49 (1.06–1.86) | ||
| Neurological disorders NEC | Dysstasia | 232 | 2.56 (2.25–2.91) | 2.56 (2.25–2.92) | 1.34 (1.15–1.52) | |
| Dysgraphia | 80 | 4.54 (3.64–5.67) | 4.55 (3.65–5.67) | 2.14 (1.80–2.43) | ||
| Cerebral disorder | 40 | 2.68 (1.97–3.66) | 2.68 (1.97–3.66) | 1.39 (0.90–1.80) | ||
| Post-herpetic neuralgia | 35 | 4.31 (3.09–6.02) | 4.32 (3.09–6.03) | 2.03 (1.51–2.47) | ||
| Dyslexia | 18 | 6.53 (4.09–10.42) | 6.53 (4.09–10.42) | 2.48 (1.74–3.07) | ||
| Tinel’s sign | 3 | 23.59 (7.26–76.59) | 23.59 (7.26–76.60) | 2.46 (0.41–3.65) | ||
| Movement disorders (incl. parkinsonism) | Movement disorder | 315 | 3.07 (2.75–3.43) | 3.08 (2.75–3.44) | 1.60 (1.44–1.76) | |
| Fine motor skill dysfunction | 28 | 3.75 (2.58–5.44) | 3.75 (2.5–5.44) | 1.82 (1.24–2.31) | ||
| Bradykinesia | 27 | 2.01 (1.37–2.93) | 2.01 (1.37–2.93) | 0.97 (0.38–1.47) | ||
| Essential tremor | 8 | 2.69 (1.34–5.40) | 2.69 (1.34–5.40) | 1.28 (0.11–2.11) | ||
| Peripheral neuropathies | Carpal tunnel syndrome | 179 | 5.15 (4.45–5.97) | 5.16 (4.45–5.98) | 2.33 (2.11–2.53) | |
| Nerve compression | 134 | 6.21 (5.24–7.37) | 6.22 (5.24–7.38) | 2.58 (2.33–2.82) | ||
| Sciatic nerve neuropathy | 10 | 10.14 (5.40–19.06) | 10.15 (5.40–19.07) | 2.79 (1.76–3.55) | ||
| Spinal cord and nerve root disorders | Sciatica | 172 | 4.42 (3.80–5.14) | 4.42 (3.81–5.14) | 2.11 (1.89–2.32) | |
| Spinal cord compression | 18 | 2.45 (1.54–3.90) | 2.45 (1.54–3.90) | 1.23 (0.49–1.82) | ||
| Spinal cord disorder | 9 | 2.32 (1.21–4.48) | 2.32 (1.21–4.48) | 1.11 (0.02–1.90) | ||
| Radicular pain | 9 | 8.14 (4.20–15.79) | 8.14 (4.20–15.79) | 2.54 (1.45–3.33) | ||
| Headaches | Sinus headache | 71 | 2.77 (2.19–3.50) | 2.77 (2.20–3.50) | 1.44 (1.09–1.76) | |
| Cold-stimulus headache | 6 | 4.17 (1.86–9.34) | 4.17 (1.86–9.34) | 1.73 (0.36–2.66) | ||
| Central nervous system vascular disorders | Intracranial aneurysm | 21 | 2.10 (1.36–3.22) | 2.10 (1.36–3.22) | 1.03 (0.34–1.58) | |
| Neuromuscular disorders | Amyotrophic lateral sclerosis | 12 | 2.51 (1.42–4.43) | 2.51 (1.42–4.43) | 1.24 (0.31–1.94) | |
| Ruxolitinib | Mental impairment disorders | Memory impairment | 696 | 4.50 (4.18–4.85) | 4.56 (4.23–4.91) | 2.16 (2.05–2.26) |
| Dementia | 67 | 2.68 (2.11–3.40) | 2.68 (2.11–3.41) | 1.40 (1.03–1.72) | ||
| Sleep disturbances (incl. subtypes) | Hypersomnia | 132 | 3.15 (2.65–3.73) | 3.15 (2.66–3.74) | 1.64 (1.38–1.87) | |
| Neurological disorders NEC | Postural dizziness | 54 | 2.77 (2.12–3.62) | 2.77 (2.12–3.62) | 1.44 (1.03–1.80) | |
| Post-herpetic neuralgia | 36 | 10.78 (7.76–14.98) | 10.79 (7.76–15.00) | 3.23 (2.72–3.66) | ||
| Spinal cord and nerve root disorders | Sciatica | 60 | 3.71 (2.88–4.77) | 3.71 (2.88–4.78) | 1.85 (1.46–2.19) | |
| Lumbar radiculopathy | 5 | 3.70 (1.54–8.92) | 3.70 (1.54–8.92) | 1.57 (0.04–2.56) | ||
| Peripheral neuropathies | Polyneuropathy | 34 | 2.34 (1.67–3.28) | 2.34 (1.67–3.28) | 1.20 (0.67–1.64) | |
| Nerve compression | 21 | 2.32 (1.51–3.56) | 2.32 (1.51–3.56) | 1.17 (0.49–1.72) | ||
| Axonal neuropathy | 5 | 5.78 (2.40–13.93) | 5.78 (2.40–13.93) | 2.00 (0.48–3.00) | ||
| Neuromuscular disorders | Neuromuscular blockade | 22 | 10.78 (7.08–16.43) | 10.79 (7.08–16.44) | 3.13 (2.46–3.67) | |
| Encephalopathies | Leukoencephalopathy | 8 | 3.02 (1.51–6.04) | 3.02 (1.51–6.04) | 1.43 (0.26–2.26) | |
| Metabolic encephalopathy | 8 | 3.02 (1.51–6.05) | 3.02 (1.51–6.05) | 1.43 (0.26–2.26) | ||
| Movement disorders (incl. parkinsonism) | Clumsiness | 14 | 3.69 (2.18–6.23) | 3.69 (2.18–6.24) | 1.75 (0.90–2.41) | |
| Structural brain disorders | Cerebral atrophy | 10 | 2.91 (1.56–5.42) | 2.91 (1.56–5.42) | 1.41 (0.38–2.17) | |
| Cranial nerve disorders (excl. neoplasms) | Vocal cord paralysis | 8 | 2.76 (1.38–5.52) | 2.76 (1.38–5.52) | 1.32 (0.15–2.15) | |
| Central nervous system vascular disorders | Carotid arteriosclerosis | 4 | 4.93 (1.84–13.17) | 4.93 (1.84–13.17) | 1.77 (0.04–2.85) | |
| Baricitinib | Spinal cord and nerve root disorders | Sciatica | 13 | 2.92 (1.70–5.03) | 2.92 (1.70–5.04) | 1.45 (0.56–2.12) |
| Neurological disorders NEC * | Post-herpetic neuralgia | 10 | 10.82 (5.81–20.13) | 10.83 (5.82–20.15) | 2.88 (1.85–3.63) |
| Drugs | Stratum | Number of Reports | PRR * (95% CI *) | ROR * (95% CI *) | IC * (95% CI *) | |
|---|---|---|---|---|---|---|
| Tofacitinib | All § | 1275 | 3.42 (3.23–3.61) | 3.44 (3.26–3.64) | 1.76 (1.68–1.84) | |
| Age group | 12–17 years | 1 | 1.63 (0.23–11.54) | 1.63 (0.23–11.64) | 0.43 (−3.37–2.07) | |
| 18–44 years § | 131 | 4.68 (3.94–5.55) | 4.73 (3.98–5.62) | 2.20 (1.94–2.43) | ||
| 45–64 years § | 556 | 3.45 (3.18–3.75) | 3.48 (3.20–3.79) | 1.76 (1.64–1.88) | ||
| 65–74 years § | 347 | 4.94 (4.44–5.49) | 5.00 (4.49–5.57) | 2.26 (2.10–2.41) | ||
| ≥75 years § | 191 | 5.55 (4.81–6.39) | 5.63 (4.87–6.51) | 2.43 (2.22–2.63) | ||
| Sex | Male § | 204 | 3.43 (2.99–3.93) | 3.45 (3.01–3.96) | 1.76 (1.56–1.95) | |
| Female § | 1110 | 3.15 (2.97–3.34) | 3.18 (3.20–3.79) | 1.64 (1.55–1.72) | ||
| Ruxolitinib | All § | 696 | 4.50 (4.18–1.85) | 4.56 (4.23–4.91) | 2.16 (2.05–2.26) | |
| Age group | 18–44 years | 3 | 1.38 (0.45–4.26) | 1.38 (0.44–4.28) | 0.39 (−1.66–1.58) | |
| 45–64 years § | 45 | 2.81 (2.10–3.76) | 2.83 (2.11–3.79) | 1.46 (1.01–1.85) | ||
| 65–74 years § | 35 | 2.04 (1.47–2.84) | 2.05 (1.47–2.86) | 1.01 (0.49–1.44) | ||
| ≥ 75 years § | 39 | 2.45 (1.79–3.35) | 2.46 (1.79–3.37) | 1.26 (0.77–1.68) | ||
| Sex | Male | 61 | 1.71 (1.33–2.19) | 1.71 (1.33–2.20) | 0.76 (0.38–1.10) | |
| Female § | 108 | 2.36 (1.95–2.85) | 2.37 (1.96–2.87) | 1.23 (0.94–1.49) | ||
| Baricitinib | All | 48 | 1.13 (0.85–1.50) | 1.13 (0.85–1.50) | 0.17 (−0.27–0.55) | |
| Age group | 18–44 years | 5 | 1.89 (0.79–4.53) | 1.89 (0.79–4.56) | 0.81 (−0.72–1.80) | |
| 45–64 years | 19 | 1.40 (0.89–2.19) | 1.40 (0.89–2.20) | 0.47 (−0.25–1.04) | ||
| 65–74 years | 6 | 0.93 (0.42–2.07) | 0.93 (0.42–2.07) | −0.10 (−1.47–0.83) | ||
| ≥ 75 years | 3 | 0.86 (0.28–2.66) | 0.86 (0.28–2.67) | −0.19 (−2.24–1.00) | ||
| Sex | Male | 4 | 0.54 (0.20–1.43) | 0.54 (0.20–1.43) | −0.82 (−2.55–0.26) | |
| Female | 44 | 1.12 (0.84–1.51) | 1.12 (0.84–1.51) | 0.17 (−0.29–0.56) | ||
| Number of Reports | AE*s of Interest | All Other AE*s |
|---|---|---|
| Drug of interest | A | B |
| All other drugs | C | D |
| Indices | Formula | Positive Signal Criteria |
|---|---|---|
| PRR * | [A/(A+B)]/[C/(C+D)] | PRR ≥ 2 |
| ROR * | (A/B)/(C/D) | ROR ≥ 2 |
| IC * | IC = log2P*(AE, Drug)/P(AE)P(Drug) | Lower limit of 95% CI *≥ 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Kim, M.K.; Park, S.B.; Kim, D.H.; Byun, Y.J.; Choi, S.A. Neurological Adverse Events Associated with the Use of Janus Kinase Inhibitors: A Pharmacovigilance Study Based on Vigibase. Pharmaceuticals 2025, 18, 394. https://doi.org/10.3390/ph18030394
Park S, Kim MK, Park SB, Kim DH, Byun YJ, Choi SA. Neurological Adverse Events Associated with the Use of Janus Kinase Inhibitors: A Pharmacovigilance Study Based on Vigibase. Pharmaceuticals. 2025; 18(3):394. https://doi.org/10.3390/ph18030394
Chicago/Turabian StylePark, Sunny, Min Kyu Kim, Sung Bin Park, Dong Hyeok Kim, Young Joo Byun, and Soo An Choi. 2025. "Neurological Adverse Events Associated with the Use of Janus Kinase Inhibitors: A Pharmacovigilance Study Based on Vigibase" Pharmaceuticals 18, no. 3: 394. https://doi.org/10.3390/ph18030394
APA StylePark, S., Kim, M. K., Park, S. B., Kim, D. H., Byun, Y. J., & Choi, S. A. (2025). Neurological Adverse Events Associated with the Use of Janus Kinase Inhibitors: A Pharmacovigilance Study Based on Vigibase. Pharmaceuticals, 18(3), 394. https://doi.org/10.3390/ph18030394





