Combating Antibiotic Resistance: Mechanisms, Multidrug-Resistant Pathogens, and Novel Therapeutic Approaches: An Updated Review
Abstract
:1. Introduction
2. Types and Resistance Mechanisms of Bacteria to Antibiotics
2.1. Intrinsic Resistance
2.2. Acquired Resistance
2.3. Adaptive Resistance
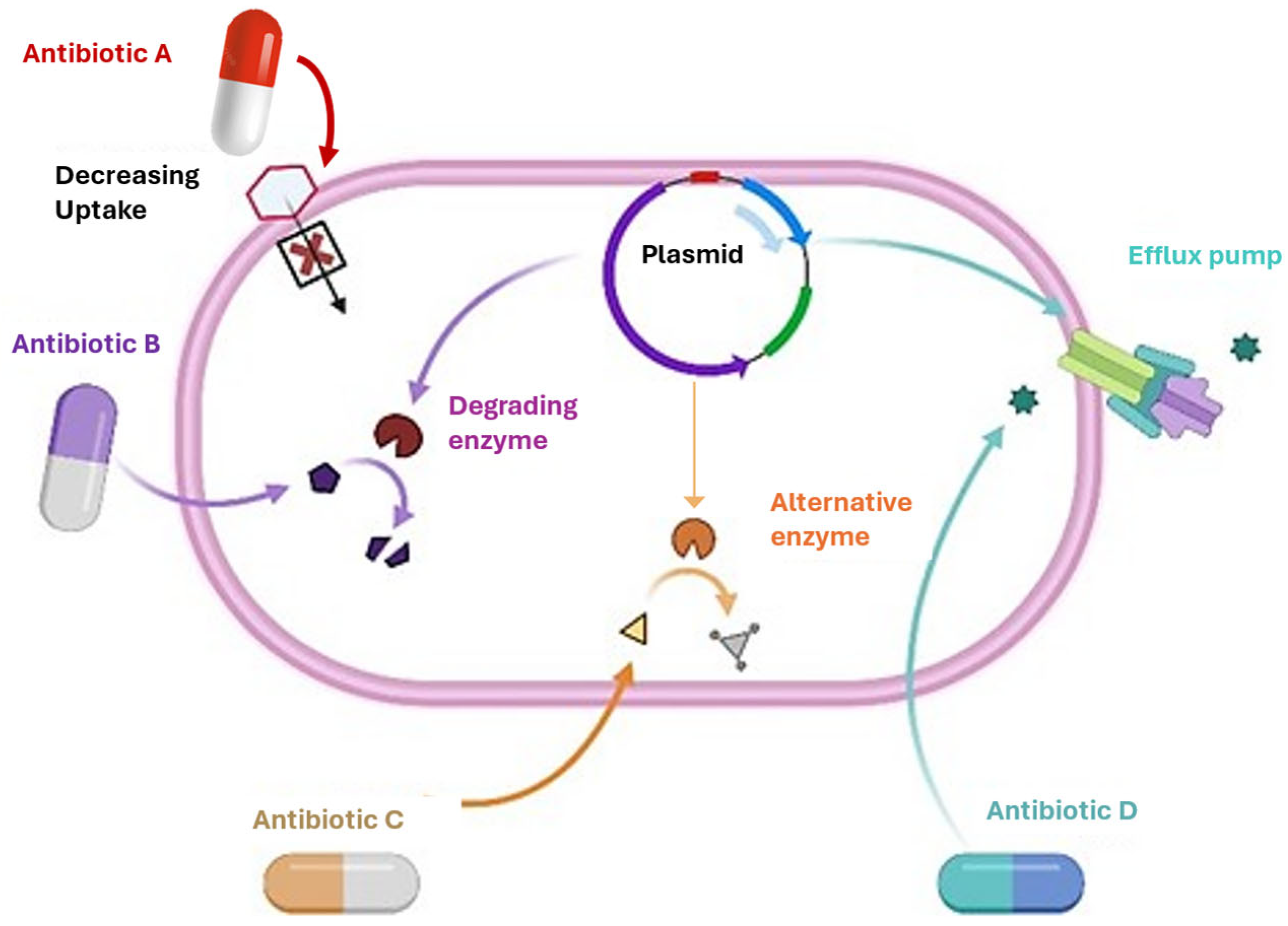
3. Mechanisms of Antibiotic Resistance in Bacteria
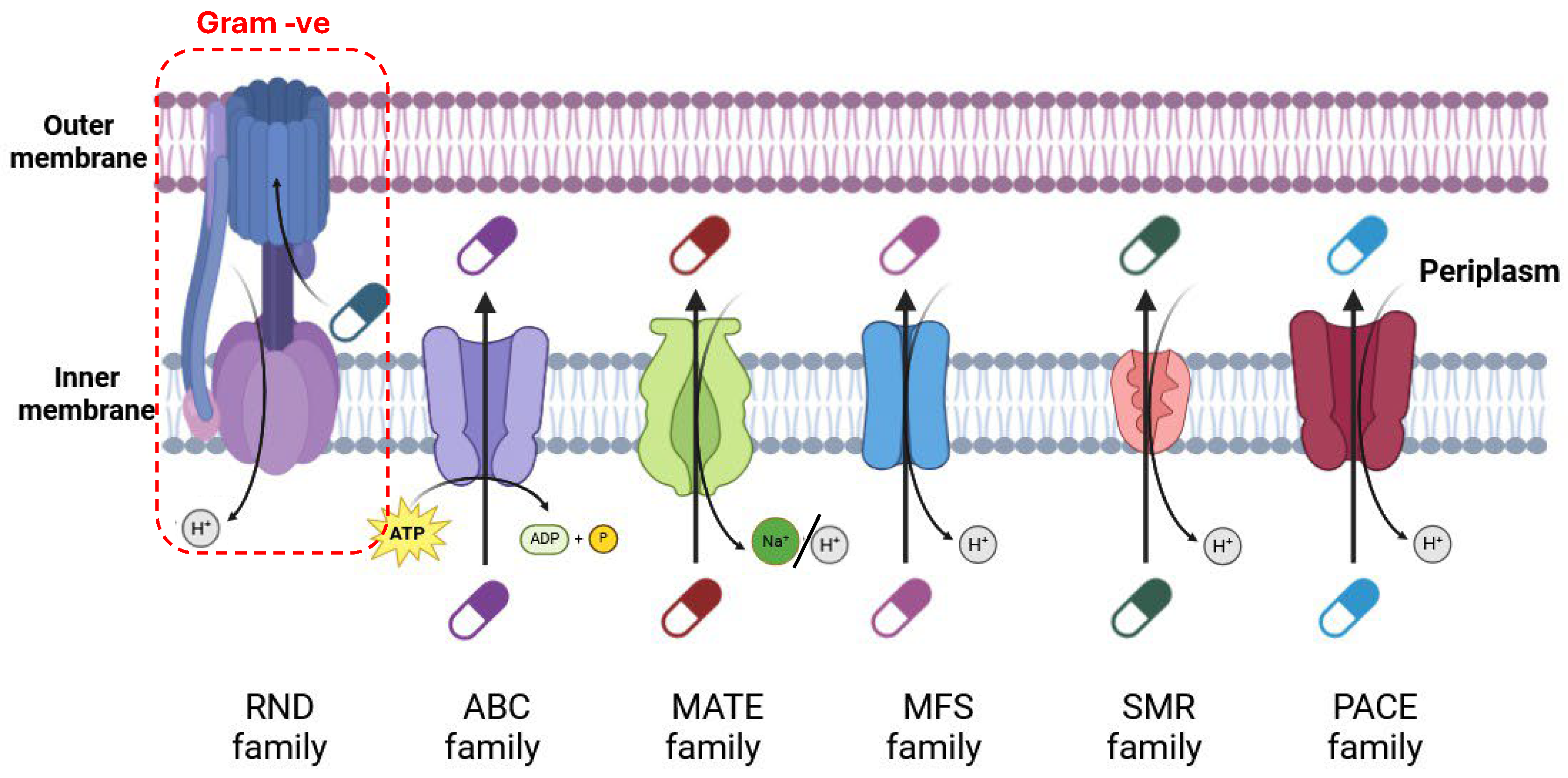
3.1. Membrane Transport Systems in Antibiotic Resistance
3.1.1. ATP-Driven Efflux Pumps (ABC)
3.1.2. Major Facilitator Superfamily (MFS)
3.1.3. Small Multidrug Resistance (SMR) Family
3.1.4. Multidrug and Toxic Compound Extrusion (MATE) Family
3.1.5. Proteobacterial Antimicrobial Compound Efflux (PACE) Family
3.1.6. Resistance Nodulation Cell Division (RND) Superfamily
3.2. Reduced Membrane Permeability
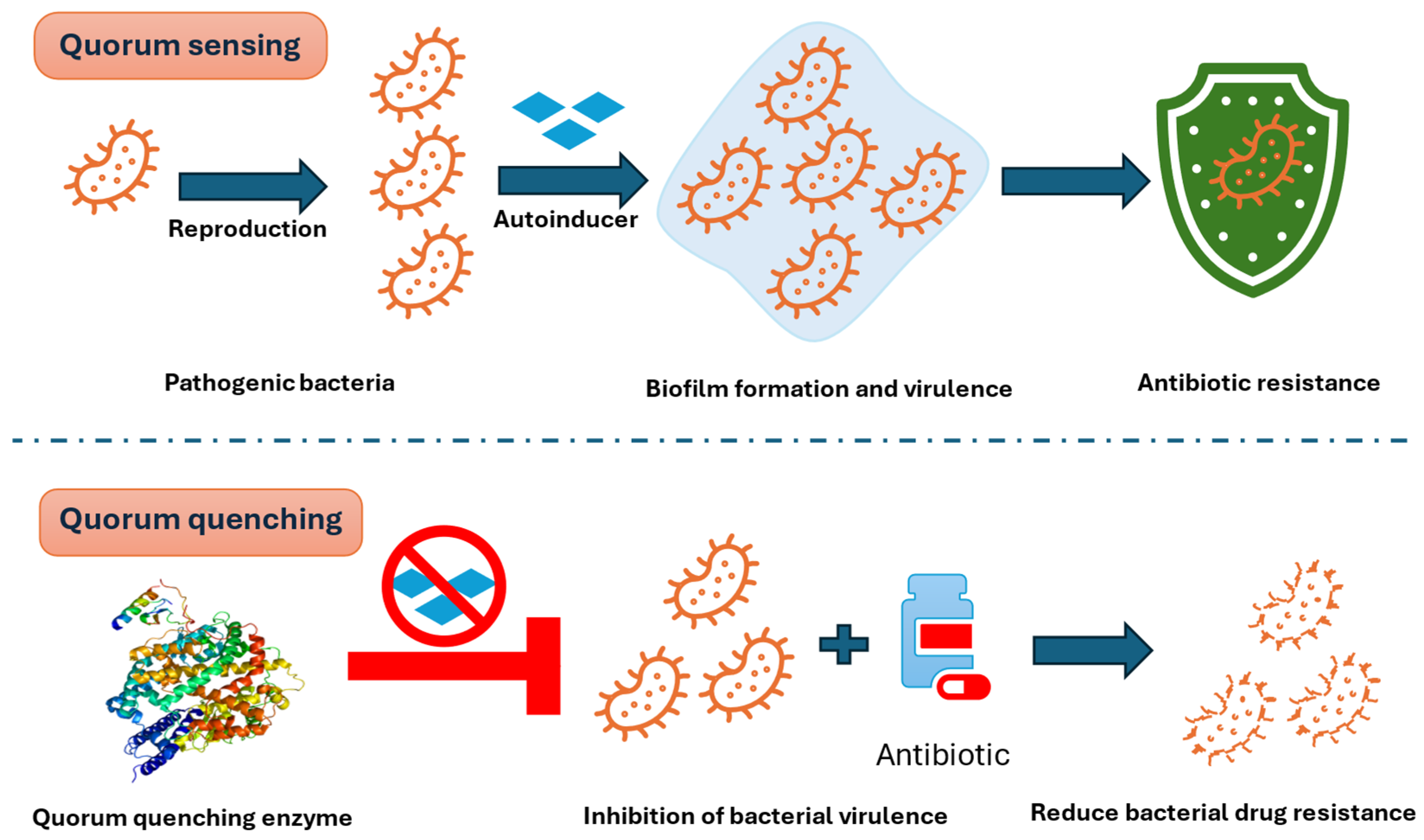
3.3. Biofilm Formation
3.4. Target Site Modifications
3.5. Enzymatic Degradation of Antibiotics
4. Strategies to Combat Antibiotic Resistance
4.1. Quorum Quenching (QQ): Targeting Bacterial Communication
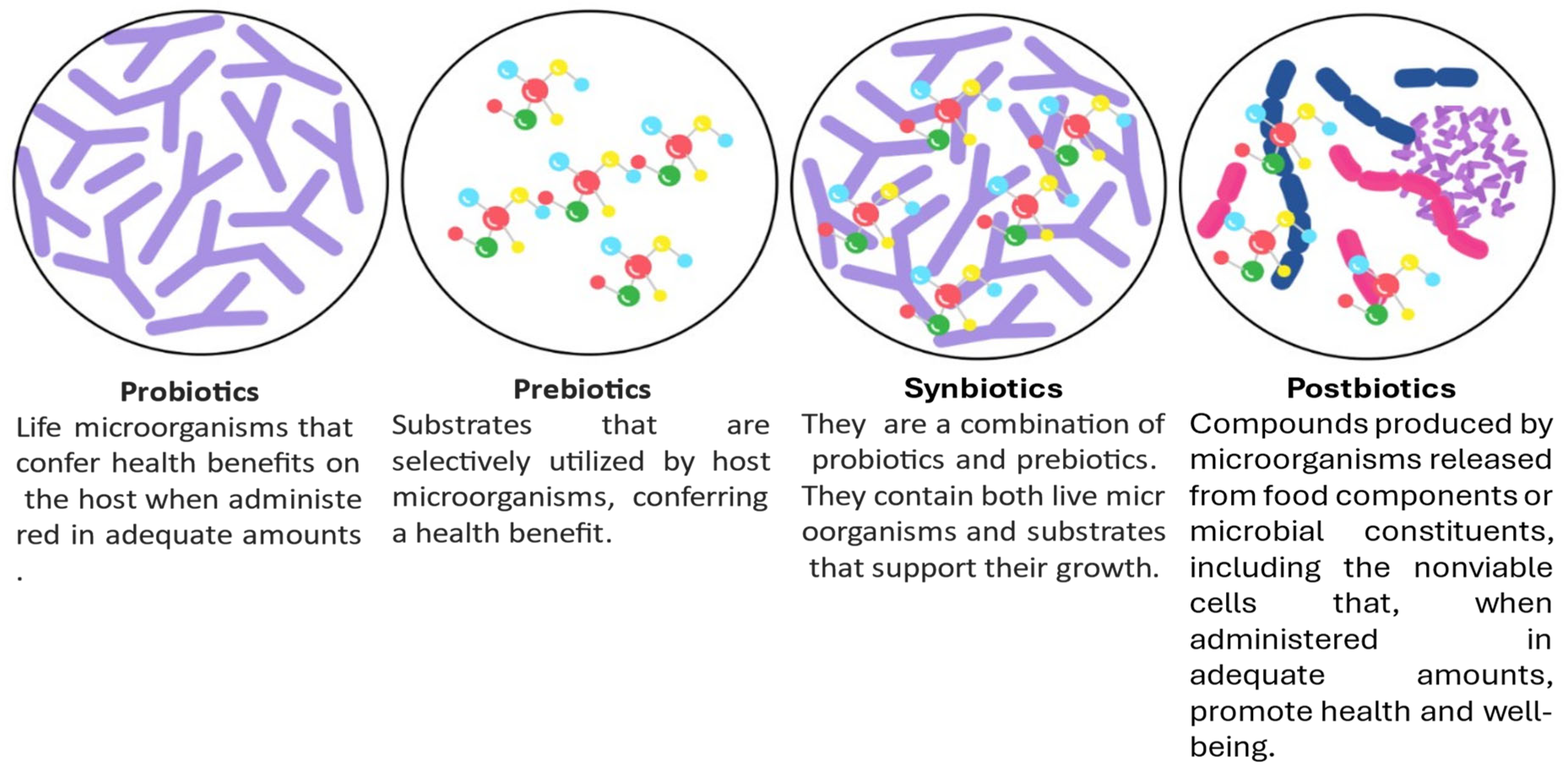
4.2. Probiotics, Postbiotics, Prebiotics, and Synbiotics
| Prebiotics, Postbiotics, Probiotics, and Synbiotics | Action | AMR Bacteria | References |
|---|---|---|---|
| Prebiotics | |||
| Fructooligosaccharides (FOS), Inulin, Galactooligosaccharides (GOS), Polydextrose | Stimulate beneficial microbial growth, improve gut health, enhance immunity, and reduce pathogen colonization | Escherichia coli, Clostridium difficile, Bacteroides fragilis, Streptococcus mutans | [86,87] |
| Postbiotics | |||
| Short-chain fatty acids, enzymes, vitamins, extracellular polysaccharides, cell wall fragments and bacterial lysates | Positively affect immunity, are anti-inflammatory, antibacterial, anti-carcinogenic, and antibiofilm | Fusobacterium, Clostridioides difficile, Escherichia coli, Streptococcus mutans, Porphyromonas gingivalis, Tannerella forsythia, Prevotella loescheii, and Salmonella spp. | [88] |
| Probiotics | |||
| Lactobacillus rhamnosus GG and Lactobacillus acidophilus produce bacteriocins such asrhamnosin and lactocin | Are anticancer and alleviate intestinal damage, mucositis, and antibiofilm | Clostridium difficile, Escherichia coli, and Pseudomonas aeruginosa | [89] |
| Bifidobacterium produce bifidocin and lactic acid | Regulate inflammation and antimicrobial peptides, reduce antibiotic overuse, and improve gut health | Pseudomonas aeruginosa and Clostridium difficile | [90] |
| Leuconostoc mesenteroides MJM60376 and Leuconostoc mesenteroides LVBH107 | Antibiofilm and antimicrobial activities | Porphyromonas gingivalis and Streptococcus mutans KCTC3065 | [91] |
| Synbiotic | |||
| Bifidobacterium lactis BL-99 with Fructooligosaccharide (FOS) | Regulate intestinal microbiota | Bilophila, Escherichia, and Shigella | [92] |
| Lactobacillus paracasei VL8 and Mannan oligosaccharide (MOS) | Positive role in foodborne pathogens | Salmonella Typhimurium | [93] |
| Lactobacillus rhamnosus GG and Fructooligosaccharide (FOS) Produces Bacteriocins like rhamnosin | Coordinating gut microbiota | Shigella sonnei, Salmonella typhimurium, Klebsiella pneumoniae, and Clostridioides difficile | [94] |
| Streptococcus thermophiles and Xylooligosaccharides (XOS) | Positive role in colorectal cancer | Helicobacter hepaticus, Helicobacter pylori, Escherichia coli, Enterococcus faecalis, and Streptococcus bovis | [95] |
| Streptococcus thermophiles and Fructooligosaccharide (FOS) | Overcoming diarrhea | Clostridioides difficile | [96] |
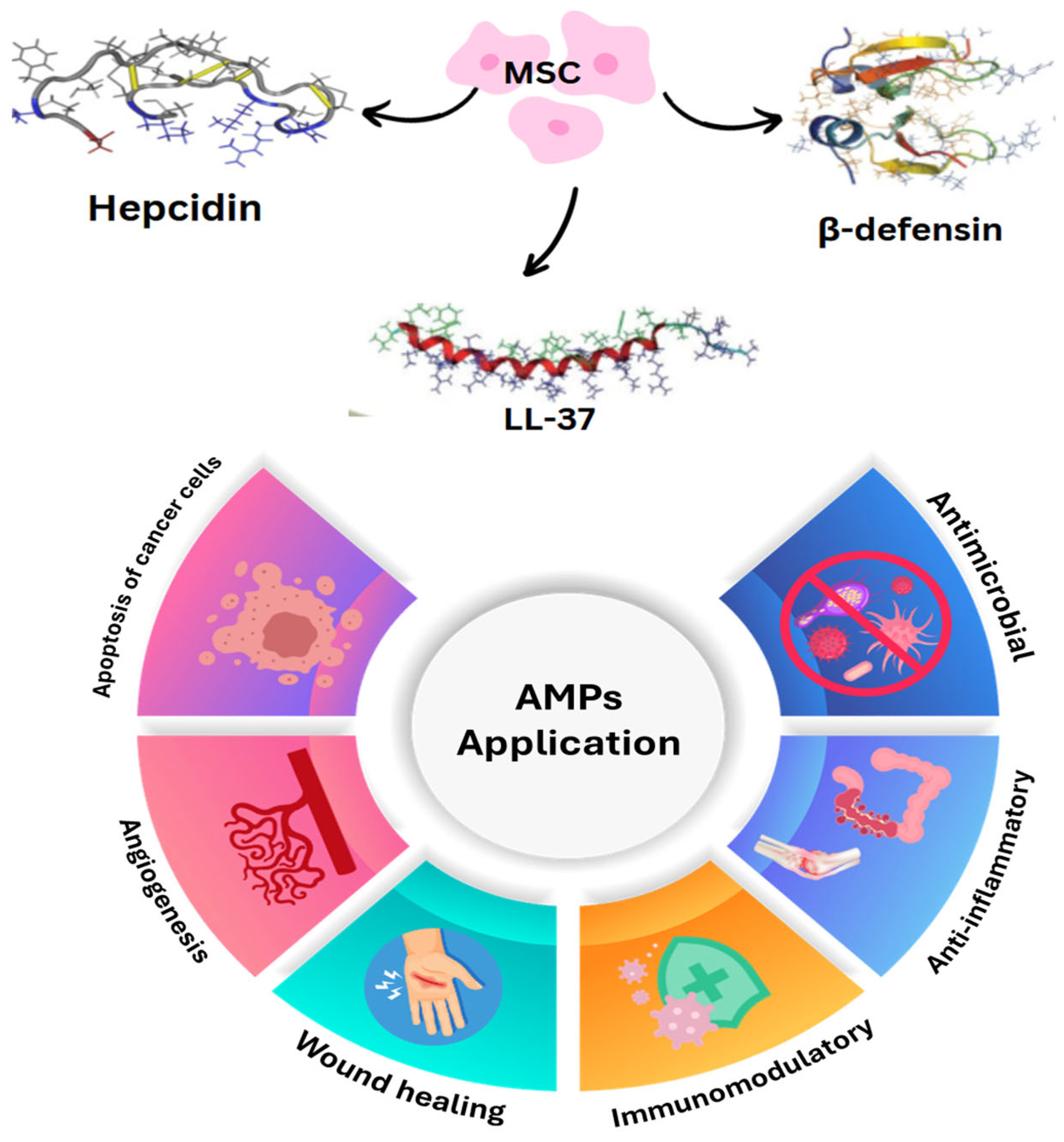
4.3. Stem Cells
4.4. Immunotherapeutic Approaches
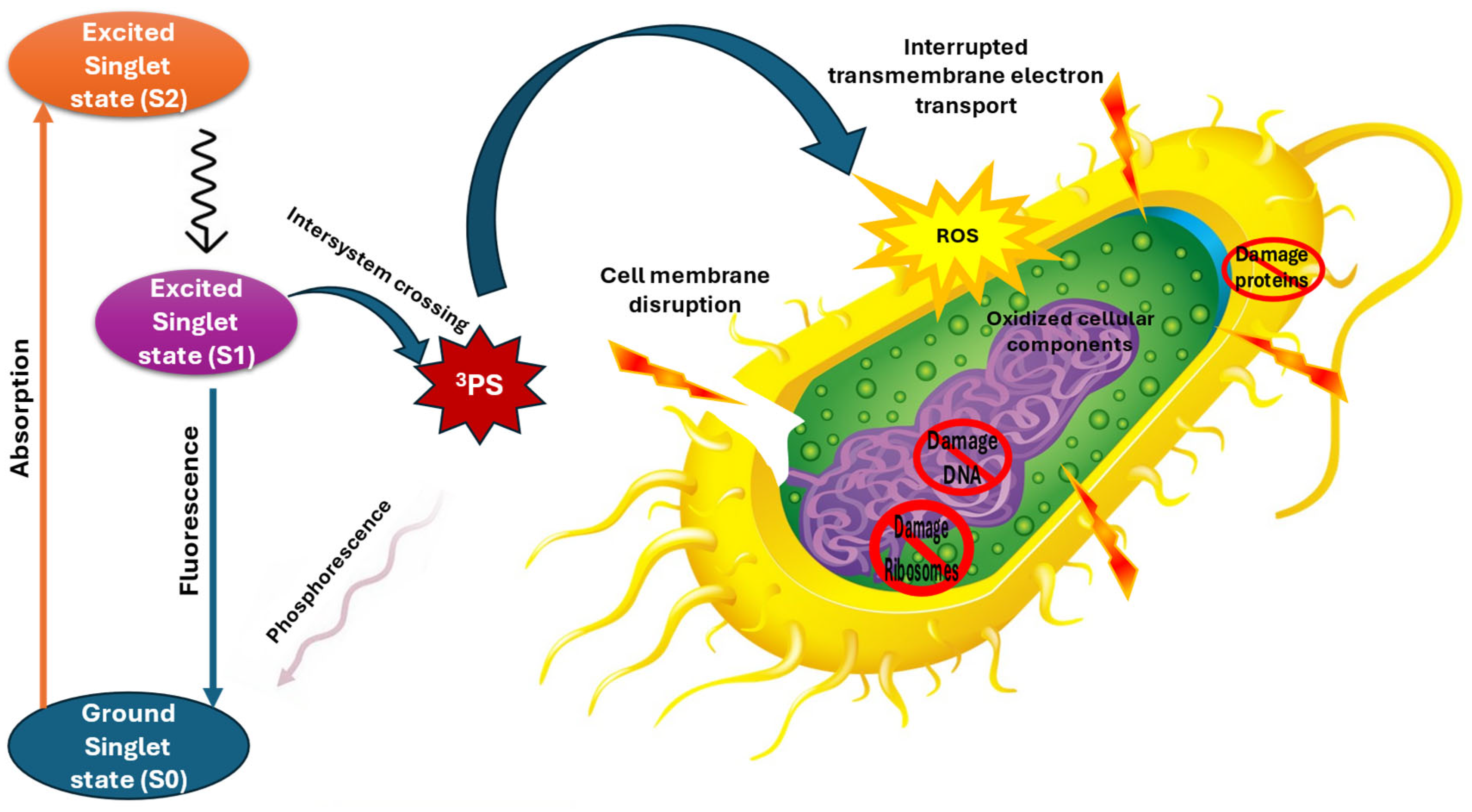
4.5. Antibacterial Photodynamic Therapy
| Photosensitizer | Pathogenic Bacteria | Light Type | Wavelength (NM) | Key Findings | Reference |
|---|---|---|---|---|---|
| Gentamicin | Staphylococcus aureus | Blue Light | 630–660 | Effective against standard and MDR strains | [108] |
| Methylene blue | Escherichia coli | Red Light | 660–670 | Significant membrane damage | [109] |
| Chlorin e6 | Methicillin-Resistant S. aureus | Red Light | 665–685 | Enhanced ROS generation | [110] |
| Phthalocyanine | Vancomycin-Resistant Enterococci | Far-Red Light | 670–690 | Effective against resistant strains | [111] |
| 5-aminolevulinic acid | Carbapenem-Resistant Acinetobacter baumannii | Red Light | 630–635 | Significant bacterial reduction | [112] |
| Aluminum phthalocyanine chloride | Multidrug-Resistant Enterobacter | Near-Infrared | 670–690 | Improved tissue penetration | [113] |
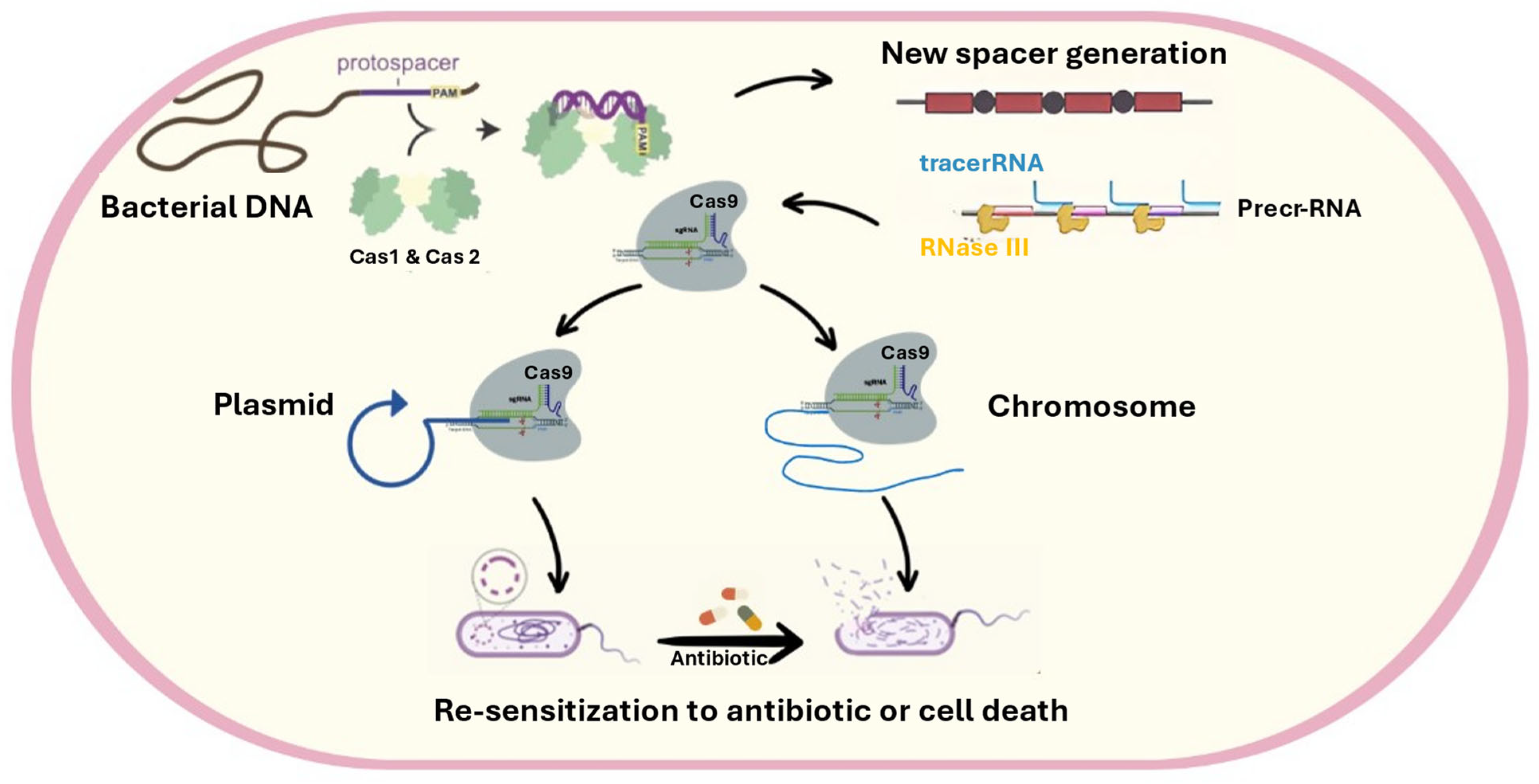
4.6. Relationship Between CRISPR CAS (Adaptive Immunity of Bacteria) and Antibiotic Resistance
4.7. Bacteriophages and Their Role in Antibiotic Resistance and Sensitivity
4.8. Animal Venoms
4.8.1. Scorpion Venom
4.8.2. Bee Venom
4.8.3. Snake Venom
4.8.4. Spider Venom
| Venom Species | Peptides’ Name | Amino Acid Residues | Antimicrobial Activity | MIC | Reference |
|---|---|---|---|---|---|
| Scorpion (Hoffmannihadrurus aztecus) | Hadurin | 41 amino acid-long AMP | Antimicrobial activity was mainly detected against Escherichia coli, Serratia marscencens, and Enterococcus cloacae | Lower than 10 µm | [155] |
| Scorpion (Pandinus imperator) | Pandinin-1 | 44 amino acids | Antimicrobial activity was mainly detected against Enterococcus faecalis, Bacillus subtilis, Staphylococcus aureus, and Staphylococcus epidermidis | 1.3 µm, 5.2 µm, 2.6 µm, and 5.2 µm, respectively, according to those species | [156] |
| Scorpion (Pandinus imperator) | Pandinin-2, | 24 amino acid residues | Antimicrobial activity was mainly detected against Enterococcus faecalis, Bacillus subtilis, Staphylococcus aureus, and Staphylococcus epidermidis strains and Mycobacterium tuberculosis | 2.4 µm, 4.8 µm, 2.4 µm and 4.8 µm, respectively, according to those species | [157] |
| Scorpion (Mesobuthus martensii) | Bmkbpp | 47 amino acid residues | Antimicrobial activity was mainly detected against Gram-negative bacteria | 2.3 to 68.2 µm for different strains | [158] |
| Scorpion (Vaejovis punctatus) | Vpamp1.0 and vpamp2.0 | 19 to 25 amino acid residues | Antimicrobial activity was mainly detected against Gram-positive and Gram-negative bacteria | 2.5 to 15 µm and 2.5 to 24 µm | [159] |
| Scorpion (Tityus serrulatus) | Tsap-2 | 17 amino acid residues | Antimicrobial activity was mainly detected against Staphylococcus aureus | 5 µm | [160] |
| Scorpion (Scorpiops tibetanus) | CT2 | 14 amino acid residues | Inhibits mainly Gram-positive bacteria, especially Staphylococcus aureus Effective against methicillin-resistant bacterial strains | 6.25 μg/mL | [161] |
| Bee (Apis mellifera) | Melittin | 26 amino acids | Broad-spectrum antimicrobial activity against Gram-positive and Gram-negative bacteria, as well as fungi | 0.5–100 μg/mL against various bacteria | [162] |
| Bee (Apis mellifera) | Apidaecin | 18 amino acids | Potent activity against Gram-negative bacteria, particularly Escherichia coli and Salmonella spp. | 1.25–5 μg/mL against E. coli | [163] |
| Snake (Bothrops atrox) | Cathelicidin-NA | 34 amino acids | Antimicrobial activity against Gram-positive and Gram-negative bacteria, as well as fungi | 0.25–128 µg/mL | [164] |
| Snake (Naja atra) | Cathelicidin-NA | 34 amino acids | Bacillus anthracis | 0.29 µg/mL | [165] |
| Snake (Ophiophagus hannah) | Cathelicidin-NA | - | P. Aeruginosa | 3.25 µm | [166] |
| Snake (Crotalus durissus terrificus) | Crotamine | 42 amino acids | Citrobacter freundii, B. Subtilis, and Micrococcus luteus | - | [144] |
| Spider (Acanthoscurria paulensis) | Gomesin | 18 amino acids | Potent antimicrobial activity against Gram-positive and Gram-negative bacteria, as well as fungi | 0.8–6.4 μm against various bacteria | [153] |
| Spider (Cupiennius salei) | Cupiennin 1a | 35 amino acids | Antimicrobial activity against Gram-positive and Gram-negative bacteria, as well as fungi | 0.5–4 μm against various bacteria | [167] |
| Spider (Psalmopoeus cambridgei) | Psalmopeotoxin I | 28 amino acids | Antimicrobial activity against Gram-positive and Gram-negative bacteria, as well as fungi | 0.4–12.5 μm against various bacteria | [168] |
4.8.5. Potential Side Effects of Venom-Based Antimicrobial Agents
4.9. Nanobiotics
5. Potential Limitations of Combatting Antibiotic Resistance Strategies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Chen, Z.; Yang, T.; Jiang, M.; Wang, J.; Cheng, Z.; Yang, M.; Zhu, J.; Zhang, T.; Li, H.; et al. Glutamine Promotes Antibiotic Uptake to Kill Multidrug-Resistant Uropathogenic Bacteria. Sci. Transl. Med. 2021, 13, eabj0716. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Islam, M.R.; Khan, R.; Amin, M.B.; Rahman, M.; Hossain, M.I.; Ahmed, D.; Asaduzzaman, M.; Riley, L.W. Prevalence, Etiology and Antibiotic Resistance Patterns of Community-Acquired Urinary Tract Infections in Dhaka, Bangladesh. PLoS ONE 2022, 17, e0274423. [Google Scholar] [CrossRef] [PubMed]
- Safdar, N.; Zahra, A.; Komal, S.; Ul Ain, Q.; Mazhar, M. The Trend of Antibiotic Resistance In the Era of COVID-19 and Its Trailing. SSRN J. 2023. [Google Scholar] [CrossRef]
- El-Sapagh, S.; El-Shenody, R.; Pereira, L.; Elshobary, M. Unveiling the Potential of Algal Extracts as Promising Antibacterial and Antibiofilm Agents against Multidrug-Resistant Pseudomonas aeruginosa: In Vitro and In Silico Studies Including Molecular Docking. Plants 2023, 12, 3324. [Google Scholar] [CrossRef]
- Osei Sekyere, J.; Kerdsin, A.; Chopjitt, P.; Wendling, C.C. Editorial: Community Series—Characterization of Mobile Genetic Elements Associated with Acquired Resistance Mechanisms, Volume II. Front. Microbiol. 2023, 14, 1230730. [Google Scholar] [CrossRef]
- Günther, T.; Kramer-Schadt, S.; Fuhrmann, M.; Belik, V. Environmental Factors Associated with the Prevalence of ESBL/AmpC-Producing Escherichia coli in Wild Boar (Sus scrofa). Front. Vet. Sci. 2022, 9, 980554. [Google Scholar] [CrossRef]
- Lee, A.R.; Park, S.B.; Kim, S.W.; Jung, J.W.; Chun, J.H.; Kim, J.; Kim, Y.R.; Lazarte, J.M.S.; Jang, H.B.; Thompson, K.D.; et al. Membrane Vesicles from Antibiotic-Resistant Staphylococcus aureus Transfer Antibiotic-Resistance to Antibiotic-Susceptible Escherichia coli. J. Appl. Microbiol. 2022, 132, 2746–2759. [Google Scholar] [CrossRef]
- Terefinko, D.; Caban, M.; Motyka-Pomagruk, A.; Babinska, W.; Pohl, P.; Jamroz, P.; Cyganowski, P.; Sledz, W.; Lojkowska, E.; Stepnowski, P.; et al. Removal of Clinically Significant Antibiotics from Aqueous Solutions by Applying Unique High-Throughput Continuous-Flow Plasma Pencil and Plasma Brush Systems. Chem. Eng. J. 2023, 452, 139415. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhong, Z.; Hu, S.; Wang, J.; Deng, Y.; Li, X.; Chen, X.; Li, X.; Tang, Y.; Li, X.; et al. A Survey of Helicobacter pylori Antibiotic-Resistant Genotypes and Strain Lineages by Whole-Genome Sequencing in China. Antimicrob. Agents Chemother. 2022, 66, e02188-21. [Google Scholar] [CrossRef]
- Alam, M.M.; Islam, N.; Hossain Hawlader, M.D.; Ahmed, S.; Wahab, A.; Islam, M.; Uddin, K.R.; Hossain, A. Prevalence of Multidrug Resistance Bacterial Isolates from Infected Wound Patients in Dhaka, Bangladesh: A Cross-Sectional Study. Int. J. Surg. Open 2021, 28, 56–62. [Google Scholar] [CrossRef]
- Nocera, F.P.; Ambrosio, M.; Fiorito, F.; Cortese, L.; De Martino, L. On Gram-Positive- and Gram-Negative-Bacteria-Associated Canine and Feline Skin Infections: A 4-Year Retrospective Study of the University Veterinary Microbiology Diagnostic Laboratory of Naples, Italy. Animals 2021, 11, 1603. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.; Yu, X.; Zhou, H.; Li, B.; Chen, G.; Ye, Z.; Wang, Y.; Cui, X.; Zheng, Y.; et al. Impact of Antimicrobial Stewardship Managed by Clinical Pharmacists on Antibiotic Use and Drug Resistance in a Chinese Hospital, 2010–2016: A Retrospective Observational Study. BMJ Open 2019, 9, e026072. [Google Scholar] [CrossRef] [PubMed]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial Resistance: Prevalence, Economic Burden, Mechanisms of Resistance and Strategies to Overcome. Eur. J. Pharm. Sci. 2021, 170, 106103. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, P.; Cooper, B.S.; Coast, J.; Oppong, R.; Do Thi Thuy, N.; Phodha, T.; Celhay, O.; Guerin, P.J.; Wertheim, H.; Lubell, Y. Enumerating the Economic Cost of Antimicrobial Resistance per Antibiotic Consumed to Inform the Evaluation of Interventions Affecting Their Use. Antimicrob. Resist. Infect. Control 2018, 7, 98. [Google Scholar] [CrossRef]
- Li, T.; Wang, Z.; Guo, J.; de la Fuente-Nunez, C.; Wang, J.; Han, B.; Tao, H.; Liu, J.; Wang, X. Bacterial Resistance to Antibacterial Agents: Mechanisms, Control Strategies, and Implications for Global Health. Sci. Total Environ. 2022, 860, 160461. [Google Scholar] [CrossRef]
- Hosny, S.; Elshobary, M.E.; El-Sheekh, M.M. Unleashing the Power of Microalgae: A Pioneering Path to Sustainability and Achieving the Sustainable Development Goals. Environ. Sci. Pollut. Res. 2025, 1–31. [Google Scholar] [CrossRef]
- Theuretzbacher, U.; Bush, K.; Harbarth, S.; Paul, M.; Rex, J.H.; Tacconelli, E.; Thwaites, G.E. Critical Analysis of Antibacterial Agents in Clinical Development. Nat. Rev. Microbiol. 2020, 18, 286–298. [Google Scholar] [CrossRef]
- Outterson, K. Estimating the Appropriate Size of Global Pull Incentives For Antibacterial Medicines: Study Examines Global Antibacterial Pull Incentives. Health Aff. 2021, 40, 1758–1765. [Google Scholar] [CrossRef]
- Årdal, C.; Balasegaram, M.; Laxminarayan, R.; McAdams, D.; Outterson, K.; Rex, J.H.; Sumpradit, N. Antibiotic Development—Economic, Regulatory and Societal Challenges. Nat. Rev. Microbiol. 2020, 18, 267–274. [Google Scholar] [CrossRef]
- Blind, K. The Overall Impact of Economic, Social and Institutional Regulation on Innovation: An Update. In Handbook of Innovation and Regulation; Edward Elgar Publishing: Northampton, MA, USA, 2023; pp. 230–262. [Google Scholar]
- Pierce, J.D.; Shen, Q.; Cintron, S.A.; Hiebert, J.B. Post-COVID-19 Syndrome. Nurs. Res. 2022, 71, 164–174. [Google Scholar] [CrossRef]
- Smith, W.P.; Wucher, B.R.; Nadell, C.D.; Foster, K.R. Bacterial Defences: Mechanisms, Evolution and Antimicrobial Resistance. Nat. Rev. Microbiol. 2023, 21, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Sakagianni, A.; Koufopoulou, C.; Koufopoulos, P.; Kalantzi, S.; Theodorakis, N.; Nikolaou, M.; Paxinou, E.; Kalles, D.; Verykios, V.S.; Myrianthefs, P. Data-Driven Approaches in Antimicrobial Resistance: Machine Learning Solutions. Antibiotics 2024, 13, 1052. [Google Scholar] [CrossRef] [PubMed]
- Abushaheen, M.A.; Muzaheed; Fatani, A.J.; Alosaimi, M.; Mansy, W.; George, M.; Acharya, S.; Rathod, S.; Divakar, D.D.; Jhugroo, C.; et al. Antimicrobial Resistance, Mechanisms and Its Clinical Significance. Disease-a-Month 2020, 66, 100971. [Google Scholar] [CrossRef] [PubMed]
- Tavares-Carreon, F.; De Anda-Mora, K.; Rojas-Barrera, I.C.; Andrade, A. Serratia marcescens Antibiotic Resistance Mechanisms of an Opportunistic Pathogen: A Literature Review. PeerJ 2023, 11, e14399. [Google Scholar] [CrossRef]
- Lade, H.; Kim, J.-S. Molecular Determinants of β-Lactam Resistance in Methicillin-Resistant Staphylococcus aureus (MRSA): An Updated Review. Antibiotics 2023, 12, 1362. [Google Scholar] [CrossRef]
- Pérez-Varela, M.; Tierney, A.R.; Dawson, E.; Hutcheson, A.R.; Tipton, K.A.; Anderson, S.E.; Haldopoulos, M.E.; Song, S.; Tomlinson, B.R.; Shaw, L.N. Stochastic Activation of a Family of TetR Type Transcriptional Regulators Controls Phenotypic Heterogeneity in Acinetobacter baumannii. PNAS Nexus 2022, 1, pgac231. [Google Scholar] [CrossRef]
- Esau, V.C.-Z. Structure-Activity Relationship Between Klebsiella pneumoniae β-Lactamase CTX-M-15 and Selected β-Lactam Antibiotics: Evaluating the Binding Site Promiscuity. Master’s Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2023. [Google Scholar]
- Miller, W.R.; Murray, B.E.; Rice, L.B.; Arias, C.A. Vancomycin-Resistant Enterococci: Therapeutic Challenges in the 21st Century. Infect. Dis. Clin. 2016, 30, 415–439. [Google Scholar]
- Lewis, K. New Approaches to Antimicrobial Discovery. Biochem. Pharmacol. 2017, 134, 87–98. [Google Scholar] [CrossRef]
- Singh, R.K.; Prasad, A.; Maurya, J.; Prasad, M. Regulation of Small RNA-Mediated High Temperature Stress Responses in Crop Plants. Plant Cell Rep. 2022, 41, 765–773. [Google Scholar] [CrossRef]
- Rodríguez-Núñez, K.; Cortés-Monroy, A.; Serey, M.; Ensari, Y.; Davari, M.D.; Bernal, C.; Martinez, R. Modulating Substrate Specificity of Rhizobium Sp. Histamine Dehydrogenase Through Protein Engineering for Food Quality Applications. Molecules 2023, 28, 3748. [Google Scholar] [CrossRef]
- Kakoullis, L.; Papachristodoulou, E.; Chra, P.; Panos, G. Mechanisms of Antibiotic Resistance in Important Gram-Positive and Gram-Negative Pathogens and Novel Antibiotic Solutions. Antibiotics 2021, 10, 415. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, G.V.; Lentini, G.; Famà, A.; Coppolino, F.; Beninati, C. Antimicrobial Resistance: Two-Component Regulatory Systems and Multidrug Efflux Pumps. Antibiotics 2023, 12, 965. [Google Scholar] [CrossRef]
- Scoffone, V.C.; Trespidi, G.; Barbieri, G.; Arshad, A.; Israyilova, A.; Buroni, S. The Evolution of Antimicrobial Resistance in Acinetobacter baumannii and New Strategies to Fight It. Antibiotics 2025, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, N.F.S.; Yahya, M.F.Z.R.; Jamil, N.M. Multiple Bacterial Strategies to Survive Antibiotic Pressure: A Review. Preprints 2023. [Google Scholar] [CrossRef]
- Belay, W.Y.; Getachew, M.; Tegegne, B.A.; Teffera, Z.H.; Dagne, A.; Zeleke, T.K.; Abebe, R.B.; Gedif, A.A.; Fenta, A.; Yirdaw, G.; et al. Mechanism of Antibacterial Resistance, Strategies and Next-Generation Antimicrobials to Contain Antimicrobial Resistance: A Review. Front. Pharmacol. 2024, 15, 1444781. [Google Scholar] [CrossRef]
- Henderson, R.K.; Fendler, K.; Poolman, B. Coupling Efficiency of Secondary Active Transporters. Curr. Opin. Biotechnol. 2019, 58, 62–71. [Google Scholar] [CrossRef]
- Li, D.; Ge, Y.; Wang, N.; Shi, Y.; Guo, G.; Zou, Q.; Liu, Q. Identification and Characterization of a Novel Major Facilitator Superfamily Efflux Pump, SA09310, Mediating Tetracycline Resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 2023, 67, e01696-22. [Google Scholar] [CrossRef]
- Nag, A.; Mehra, S. A Major Facilitator Superfamily (MFS) Efflux Pump, SCO4121, from Streptomyces coelicolor with Roles in Multidrug Resistance and Oxidative Stress Tolerance and Its Regulation by a MarR Regulator. Appl. Environ. Microbiol. 2021, 87, e02238-20. [Google Scholar] [CrossRef] [PubMed]
- Vrancianu, C.O.; Gheorghe, I.; Czobor, I.B.; Chifiriuc, M.C. Antibiotic Resistance Profiles, Molecular Mechanisms and Innovative Treatment Strategies of Acinetobacter baumannii. Microorganisms 2020, 8, 935. [Google Scholar] [CrossRef]
- Pérez-Varela, M.; Corral, J.; Aranda, J.; Barbé, J. Roles of Efflux Pumps from Different Superfamilies in the Surface-Associated Motility and Virulence of Acinetobacter baumannii ATCC 17978. Antimicrob. Agents Chemother. 2019, 63, e02190-18. [Google Scholar] [CrossRef]
- Thomas, N.E. The Proton/Drug Coupling Mechanism of EmrE; The University of Wisconsin-Madison: Madison, WI, USA, 2021; ISBN 979-8-5442-4690-9. [Google Scholar]
- Teng, D.; Voth, G.A. Ligand Binding by the Small Multidrug-Resistant Transporter EmrE. Biophys. J. 2023, 122, 400a. [Google Scholar] [CrossRef]
- Guérin, F.; Galimand, M.; Tuambilangana, F.; Courvalin, P.; Cattoir, V. Overexpression of the Novel MATE Fluoroquinolone Efflux Pump FepA in Listeria monocytogenes Is Driven by Inactivation of Its Local Repressor FepR. PLoS ONE 2014, 9, e106340. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, H.; Moriyama, S.; Kusakizako, T.; Kumazaki, K.; Nakane, T.; Yamashita, K.; Hirata, K.; Dohmae, N.; Nishizawa, T.; Ito, K.; et al. Structural Basis for Xenobiotic Extrusion by Eukaryotic MATE Transporter. Nat. Commun. 2017, 8, 1633. [Google Scholar] [CrossRef]
- Kim, J.; Cater, R.J.; Choy, B.C.; Mancia, F. Structural Insights into Transporter-Mediated Drug Resistance in Infectious Diseases. J. Mol. Biol. 2021, 433, 167005. [Google Scholar] [CrossRef] [PubMed]
- Stephen, J.; Lekshmi, M.; Ammini, P.; Kumar, S.H.; Varela, M.F. Membrane Efflux Pumps of Pathogenic Vibrio Species: Role in Antimicrobial Resistance and Virulence. Microorganisms 2022, 10, 382. [Google Scholar] [CrossRef]
- Hassan, K.A.; Liu, Q.; Elbourne, L.D.H.; Ahmad, I.; Sharples, D.; Naidu, V.; Chan, C.L.; Li, L.; Harborne, S.P.D.; Pokhrel, A.; et al. Pacing across the Membrane: The Novel PACE Family of Efflux Pumps Is Widespread in Gram-Negative Pathogens. Res. Microbiol. 2018, 169, 450–454. [Google Scholar] [CrossRef]
- Javed, W. Study of the Conformational States of a Bacterial Multidrug ABC Transporter BmrA. Ph.D. Thesis, Université Grenoble Alpes, Saint-Martin-d’Hères, France, 2020. [Google Scholar]
- Migliaccio, A.; Esposito, E.P.; Bagattini, M.; Berisio, R.; Triassi, M.; De Gregorio, E.; Zarrilli, R. Inhibition of AdeB, AceI, and AmvA Efflux Pumps Restores Chlorhexidine and Benzalkonium Susceptibility in Acinetobacter baumannii ATCC 19606. Front. Microbiol. 2022, 12, 790263. [Google Scholar] [CrossRef]
- Garneau-Tsodikova, S.; Labby, K.J. Mechanisms of Resistance to Aminoglycoside Antibiotics: Overview and Perspectives. Medchemcomm 2016, 7, 11–27. [Google Scholar] [CrossRef]
- Nikaido, H. RND Transporters in the Living World. Res. Microbiol. 2018, 169, 363–371. [Google Scholar] [CrossRef]
- Yamasaki, S.; Zwama, M.; Yoneda, T.; Hayashi-Nishino, M.; Nishino, K. Drug Resistance and Physiological Roles of RND Multidrug Efflux Pumps in Salmonella enterica, Escherichia coli and Pseudomonas aeruginosa: This Article Is Part of the Antimicrobial Efflux Collection. Microbiology 2023, 169, 001322. [Google Scholar] [CrossRef]
- Lyu, M.; Ayala, J.C.; Chirakos, I.; Su, C.-C.; Shafer, W.M.; Yu, E.W. Structural Basis of Peptide-Based Antimicrobial Inhibition of a Resistance-Nodulation-Cell Division Multidrug Efflux Pump. Microbiol. Spectr. 2022, 10, e02990-22. [Google Scholar] [CrossRef] [PubMed]
- Ghai, I. Electrophysiological Insights into Antibiotic Translocation and Resistance: The Impact of Outer Membrane Proteins. Membranes 2024, 14, 161. [Google Scholar] [CrossRef] [PubMed]
- Ghai, I. A Barrier to Entry: Examining the Bacterial Outer Membrane and Antibiotic Resistance. Appl. Sci. 2023, 13, 4238. [Google Scholar] [CrossRef]
- Elshobary, M.E.; Ebaid, R.; Alquraishi, M.; Ende, S.S. Synergistic Microalgal Cocultivation: Boosting Flocculation, Biomass Production, and Fatty Acids Profile of Nannochloropsis oculata and Phaeodactylum tricornutum. Biomass Bioenergy 2025, 193, 107595. [Google Scholar] [CrossRef]
- Ahsan, A.; Thomas, N.; Barnes, T.J.; Subramaniam, S.; Loh, T.C.; Joyce, P.; Prestidge, C.A. Lipid Nanocarriers-Enabled Delivery of Antibiotics and Antimicrobial Adjuvants to Overcome Bacterial Biofilms. Pharmaceutics 2024, 16, 396. [Google Scholar] [CrossRef]
- Parvin, N.; Joo, S.W.; Mandal, T.K. Nanomaterial-Based Strategies to Combat Antibiotic Resistance: Mechanisms and Applications. Antibiotics 2025, 14, 207. [Google Scholar] [CrossRef]
- Chaudhari, R.; Singh, K.; Kodgire, P. Biochemical and Molecular Mechanisms of Antibiotic Resistance in Salmonella spp. Res. Microbiol. 2022, 174, 103985. [Google Scholar] [CrossRef]
- Mahdally, N.H.; George, R.F.; Kashef, M.T.; Al-Ghobashy, M.; Murad, F.E.; Attia, A.S. Staquorsin: A Novel Staphylococcus aureus Agr-Mediated Quorum Sensing Inhibitor Impairing Virulence In Vivo Without Notable Resistance Development. Front. Microbiol. 2021, 12, 700494. [Google Scholar] [CrossRef]
- Gbian, D.L.; Omri, A. The Impact of an Efflux Pump Inhibitor on the Activity of Free and Liposomal Antibiotics Against Pseudomonas aeruginosa. Pharmaceutics 2021, 13, 577. [Google Scholar] [CrossRef]
- Lopez-Fernandez, M.; Westmeijer, G.; Turner, S.; Broman, E.; Ståhle, M.; Bertilsson, S.; Dopson, M. Thiobacillus as a Key Player for Biofilm Formation in Oligotrophic Groundwaters of the Fennoscandian Shield. npj Biofilms Microbiomes 2023, 9, 41. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Hao, Y.; Liu, Y.; Dong, Z.; Li, K. Biological and Physiochemical Methods of Biofilm Adhesion Resistance Control of Medical-Context Surface. Int. J. Biol. Sci. 2021, 17, 1769–1781. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zeng, C.; Wen, H.; Shi, Q.; Zhao, X.; Meng, Q.; Li, X.; Xiao, J. Discovery of AI-2 Quorum Sensing Inhibitors Targeting the LsrK/HPr Protein–Protein Interaction Site by Molecular Dynamics Simulation, Virtual Screening, and Bioassay Evaluation. Pharmaceuticals 2023, 16, 737. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; Amin, A.; Alharbi, M.; Ishtiaq, S.; Sajjad, W.; Ahmad, F.; Ahmad, S.; Hanif, F.; Faheem, M.; Khalil, A.A.K. Quorum Quenchers from Reynoutria japonica in the Battle against Methicillin-Resistant Staphylococcus aureus (MRSA). Molecules 2023, 28, 2635. [Google Scholar] [CrossRef]
- Shi, Q.; Wen, H.; Xu, Y.; Zhao, X.; Zhang, J.; Li, Y.; Meng, Q.; Yu, F.; Xiao, J.; Li, X. Virtual Screening–Based Discovery of AI-2 Quorum Sensing Inhibitors That Interact with an Allosteric Hydrophobic Site of LsrK and Their Functional Evaluation. Front. Chem. 2023, 11, 1185224. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, Z.; Ding, T. Quorum-Sensing Regulation of Antimicrobial Resistance in Bacteria. Microorganisms 2020, 8, 425. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, P.-P.; Wang, W.; Tang, S.; Deng, S.-M.; Jia, A.-Q. 3-Phenylpropan-1-Amine Enhanced Susceptibility of Serratia Marcescens to Ofloxacin by Occluding Quorum Sensing. Microbiol. Spectr. 2022, 10, e01829-22. [Google Scholar] [CrossRef]
- Křížkovská, B.; Hoang, L.; Brdová, D.; Klementová, K.; Szemerédi, N.; Loučková, A.; Kronusová, O.; Spengler, G.; Kaštánek, P.; Hajšlová, J.; et al. Modulation of the Bacterial Virulence and Resistance by Well-Known European Medicinal Herbs. J. Ethnopharmacol. 2023, 312, 116484. [Google Scholar] [CrossRef]
- Patel, K.; Panchal, R.; Sakariya, B.; Gevariya, M.; Raiyani, R.; Soni, R.; Goswami, D. Combatting Antibiotic Resistance by Exploring the Promise of Quorum Quenching in Targeting Bacterial Virulence. Microbe 2025, 6, 100224. [Google Scholar] [CrossRef]
- Qin, X.; Vila-Sanjurjo, C.; Singh, R.; Philipp, B.; Goycoolea, F.M. Screening of Bacterial Quorum Sensing Inhibitors in a Vibrio Fischeri LuxR-Based Synthetic Fluorescent E. Coli Biosensor. Pharmaceuticals 2020, 13, 263. [Google Scholar] [CrossRef]
- Mirpour, M.; Zahmatkesh, H. Ketoprofen Attenuates Las/Rhl Quorum-Sensing (QS) Systems of Pseudomonas aeruginosa: Molecular and Docking Studies. Mol. Biol. Rep. 2024, 51, 133. [Google Scholar] [CrossRef]
- Saeed, A.; Ali, H.; Yasmin, A.; Baig, M.; Ullah, A.; Kazmi, A.; Ahmed, M.A.; Albadrani, G.M.; El-Demerdash, F.M.; Bibi, M.; et al. Unveiling the Antibiotic Susceptibility and Antimicrobial Potential of Bacteria from Human Breast Milk of Pakistani Women: An Exploratory Study. BioMed Res. Int. 2023, 2023, 6399699. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Chen, E.H.-L.; Chen, R.P.-Y.; Dunny, G.M.; Hu, W.-S.; Lee, K.-T. Probiotic Bacillus Affects Enterococcus faecalis Antibiotic Resistance Transfer by Interfering with Pheromone Signaling Cascades. Appl. Environ. Microbiol. 2021, 87, e00442-21. [Google Scholar] [CrossRef] [PubMed]
- Khalil, T.; Okla, M.K.; Al-Qahtani, W.H.; Ali, F.; Zahra, M.; Shakeela, Q.; Ahmed, S.; Akhtar, N.; AbdElgawad, H.; Asif, R.; et al. Tracing Probiotic Producing Bacterial Species from Gut of Buffalo (Bubalus bubalis), South-East-Asia. Braz. J. Biol. 2024, 84, e259094. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, M.; Yu, L.; Tian, F.; Lu, W.; Wang, G.; Chen, W.; Wang, J.; Zhai, Q. Evaluation of the Potential Protective Effects of Lactobacillus Strains Against Helicobacter pylori Infection: A Randomized, Double-Blinded, Placebo-Controlled Trial. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 6432750. [Google Scholar] [CrossRef]
- Chung The, H.; Nguyen Ngoc Minh, C.; Tran Thi Hong, C.; Nguyen Thi Nguyen, T.; Pike, L.J.; Zellmer, C.; Pham Duc, T.; Tran, T.-A.; Ha Thanh, T.; Van, M.P.; et al. Exploring the Genomic Diversity and Antimicrobial Susceptibility of Bifidobacterium pseudocatenulatum in a Vietnamese Population. Microbiol. Spectr. 2021, 9, e00526-21. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Hu, A.; Shu, X.; Huang, W.; Liu, J.; Wang, B.; Zhang, R.; Yue, M.; Yang, C. Lactobacillus plantarum-Derived Postbiotics Prevent Salmonella-Induced Neurological Dysfunctions by Modulating Gut–Brain Axis in Mice. Front. Nutr. 2022, 9, 946096. [Google Scholar] [CrossRef]
- Badr, H.; Nabil, N.M.; Tawakol, M.M. Effects of the Prebiotic Lactoferrin on Multidrug-Resistant Escherichia coli Infections in Broiler Chickens. Vet. World 2021, 14, 2197–2205. [Google Scholar] [CrossRef]
- Kaczmarczyk, M.; Szulińska, M.; Łoniewski, I.; Kręgielska-Narożna, M.; Skonieczna-Żydecka, K.; Kosciolek, T.; Bezshapkin, V.; Bogdański, P. Treatment with Multi-Species Probiotics Changes the Functions, Not the Composition of Gut Microbiota in Postmenopausal Women with Obesity: A Randomized, Double-Blind, Placebo-Controlled Study. Front. Cell. Infect. Microbiol. 2022, 12, 815798. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, B.; Chouraddi, R.; Bhatia, M.; Rashmi, H.M.; Vishnu Behare, P.; Tyagi, N. In Vitro Screening for Potential Probiotic Properties of Ligilactobacillus salivarius Isolated from Cattle Calves. Curr. Res. Biotechnol. 2022, 4, 275–289. [Google Scholar] [CrossRef]
- Pilakkavil Chirakkara, S.; Abraham, A. Bacillus Strains from the Ovaries of Swiss Albino Mice (Mus musculus): Deciphering of Probiotic Potential through an in Vitro Approach. Biomedicine 2022, 42, 1200–1208. [Google Scholar] [CrossRef]
- Rodríguez-Sorrento, A.; Castillejos, L.; López-Colom, P.; Cifuentes-Orjuela, G.; Rodríguez-Palmero, M.; Moreno-Muñoz, J.A.; Martín-Orúe, S.M. Effects of Bifidobacterium longum Subsp. Infantis CECT 7210 and Lactobacillus rhamnosus HN001, Combined or Not with Oligofructose-Enriched Inulin, on Weaned Pigs Orally Challenged with Salmonella typhimurium. Front. Microbiol. 2020, 11, 2012. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Stanner, S. Prebiotics—An Added Benefit of Some Fibre Types. Nutr. Bull. 2019, 44, 74–91. [Google Scholar] [CrossRef]
- Huang, F.-C.; Huang, S.-C. The Combined Beneficial Effects of Postbiotic Butyrate on Active Vitamin D3-Orchestrated Innate Immunity to Salmonella Colitis. Biomedicines 2021, 9, 1296. [Google Scholar] [CrossRef]
- Salemi, R.; Vivarelli, S.; Ricci, D.; Scillato, M.; Santagati, M.; Gattuso, G.; Falzone, L.; Libra, M. Lactobacillus rhamnosus GG Cell-Free Supernatant as a Novel Anti-Cancer Adjuvant. J. Transl. Med. 2023, 21, 195. [Google Scholar] [CrossRef]
- Huang, F.-C.; Lu, Y.-T.; Liao, Y.-H. Beneficial Effect of Probiotics on Pseudomonas aeruginosa–Infected Intestinal Epithelial Cells through Inflammatory IL-8 and Antimicrobial Peptide Human Beta-Defensin-2 Modulation. Innate Immun. 2020, 26, 592–600. [Google Scholar] [CrossRef]
- Gu, M.; Nguyen, H.T.; Cho, J.; Suh, J.; Cheng, J. Characterization of Leuconostoc mesenteroides MJM60376 as an Oral Probiotic and Its Antibiofilm Activity. Mol. Oral Microbiol. 2023, 38, 145–157. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, W.; Zhao, Y.; Duan, S.; Liu, W.-H.; Zhang, C.; Sun, S.; Wang, T.; Wang, X.; Hung, W.-L.; et al. In Vitro Study of Bifidobacterium lactis BL-99 with Fructooligosaccharide Synbiotics Effected on the Intestinal Microbiota. Front. Nutr. 2022, 9, 890316. [Google Scholar] [CrossRef]
- Geng, S.; Zhang, T.; Gao, J.; Li, X.; Chitrakar, B.; Mao, K.; Sang, Y. In Vitro Screening of Synbiotics Composed of Lactobacillus Paracasei VL8 and Various Prebiotics and Mechanism to Inhibits the Growth of Salmonella typhimurium. LWT 2023, 180, 114666. [Google Scholar] [CrossRef]
- Piatek, J.; Krauss, H.; Ciechelska-Rybarczyk, A.; Bernatek, M.; Wojtyla-Buciora, P.; Sommermeyer, H. In-Vitro Growth Inhibition of Bacterial Pathogens by Probiotics and a Synbiotic: Product Composition Matters. Int. J. Environ. Res. Public Health 2020, 17, 3332. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Daca, A.; Fic, M.; Van De Wetering, T.; Folwarski, M.; Makarewicz, W. Therapeutic Methods of Gut Microbiota Modification in Colorectal Cancer Management—Fecal Microbiota Transplantation, Prebiotics, Probiotics, and Synbiotics. Gut Microbes 2020, 11, 1518–1530. [Google Scholar] [CrossRef]
- Sommermeyer, H.; Pituch, H.M.; Wultanska, D.; Wojtyla-Buciora, P.; Piatek, J.; Bernatek, M. Inhibition of Quinolone- and Multi-Drug-Resistant Clostridioides Difficile Strains by Multi Strain Synbiotics—An Option for Diarrhea Management in Nursing Facilities. Int. J. Environ. Res. Public Health 2021, 18, 5871. [Google Scholar] [CrossRef]
- Gugjoo, M.B.; Sakeena, Q.; Wani, M.Y.; Abdel-Baset Ismail, A.; Ahmad, S.M.; Shah, R.A. Mesenchymal Stem Cells: A Promising Antimicrobial Therapy in Veterinary Medicine. Microb. Pathog. 2023, 182, 106234. [Google Scholar] [CrossRef]
- Silva-Carvalho, A.É.; Cardoso, M.H.; Alencar-Silva, T.; Bogéa, G.M.R.; Carvalho, J.L.; Franco, O.L.; Saldanha-Araujo, F. Dissecting the Relationship between Antimicrobial Peptides and Mesenchymal Stem Cells. Pharmacol. Ther. 2022, 233, 108021. [Google Scholar] [CrossRef]
- Gupta, N.; Mohan, C.D.; Shanmugam, M.K.; Jung, Y.Y.; Chinnathambi, A.; Alharbi, S.A.; Ashrafizadeh, M.; Mahale, M.; Bender, A.; Kumar, A.P.; et al. CXCR4 Expression Is Elevated in TNBC Patient Derived Samples and Z-Guggulsterone Abrogates Tumor Progression by Targeting CXCL12/CXCR4 Signaling Axis in Preclinical Breast Cancer Model. Environ. Res. 2023, 232, 116335. [Google Scholar] [CrossRef]
- Chinipardaz, Z.; Zhong, J.M.; Yang, S. Regulation of LL-37 in Bone and Periodontium Regeneration. Life 2022, 12, 1533. [Google Scholar] [CrossRef]
- McCulloch, T.R.; Wells, T.J.; Souza-Fonseca-Guimaraes, F. Towards Efficient Immunotherapy for Bacterial Infection. Trends Microbiol. 2022, 30, 158–169. [Google Scholar] [CrossRef]
- Ye, Z.; Li, G.; Lei, J. Influencing Immunity: Role of Extracellular Vesicles in Tumor Immune Checkpoint Dynamics. Exp. Mol. Med. 2024, 56, 2365–2381. [Google Scholar] [CrossRef]
- Pang, X.; Liu, X.; Cheng, Y.; Zhang, C.; Ren, E.; Liu, C.; Zhang, Y.; Zhu, J.; Chen, X.; Liu, G. Sono-immunotherapeutic Nanocapturer to Combat Multidrug-resistant Bacterial Infections. Adv. Mater. 2019, 31, 1902530. [Google Scholar] [CrossRef]
- Wang, H.; Wang, D.; Huangfu, H.; Lv, H.; Qin, Q.; Ren, S.; Zhang, Y.; Wang, L.; Zhou, Y. Branched AuAg Nanoparticles Coated by Metal–Phenolic Networks for Treating Bacteria-Induced Periodontitis via Photothermal Antibacterial and Immunotherapy. Mater. Des. 2022, 224, 111401. [Google Scholar] [CrossRef]
- Mitton, D.; Ackroyd, R. A Brief Overview of Photodynamic Therapy in Europe. Photodiagn. Photodyn. Ther. 2008, 5, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-H.; Yu, C.-C.; Chang, K.-C.; Tseng, Y.-H.; Li, P.-J.; Hsia, S.-M.; Chiu, K.-C.; Shieh, T.-M. Synergistic Effect of Combination of a Temoporfin-Based Photodynamic Therapy with Potassium Iodide or Antibacterial Agents on Oral Disease Pathogens In Vitro. Pharmaceuticals 2022, 15, 488. [Google Scholar] [CrossRef]
- Liu, X.; Liu, S.; Mai, B.; Su, X.; Guo, X.; Chang, Y.; Dong, W.; Wang, W.; Feng, X. Synergistic Gentamicin-Photodynamic Therapy against Resistant Bacteria in Burn Wound Infections. Photodiagn. Photodyn. Ther. 2022, 39, 103034. [Google Scholar] [CrossRef]
- Liu, S.; Mai, B.; Jia, M.; Lin, D.; Zhang, J.; Liu, Q.; Wang, P. Synergistic Antimicrobial Effects of Photodynamic Antimicrobial Chemotherapy and Gentamicin on Staphylococcus aureus and Multidrug-Resistant Staphylococcus aureus. Photodiagn. Photodyn. Ther. 2020, 30, 101703. [Google Scholar] [CrossRef]
- Yang, Y.; Chien, H.; Chang, P.; Chen, Y.; Jay, M.; Tsai, T.; Chen, C. Photodynamic Inactivation of Chlorin E6-loaded CTAB-liposomes against Candida albicans. Lasers Surg. Med. 2013, 45, 175–185. [Google Scholar] [CrossRef]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are We Afraid of the Light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef]
- Sperandio, F.F.; Huang, Y.-Y.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Kill Gram-Negative Bacteria. Recent Pat. Anti-Infect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef]
- Li, X.; Guo, H.; Tian, Q.; Zheng, G.; Hu, Y.; Fu, Y.; Tan, H. Effects of 5-Aminolevulinic Acid–Mediated Photodynamic Therapy on Antibiotic-Resistant Staphylococcal Biofilm: An in Vitro Study. J. Surg. Res. 2013, 184, 1013–1021. [Google Scholar] [CrossRef]
- de Moraes, M.; Vasconcelos, R.C.; Longo, J.P.F.; Muehlmann, L.A.; de Azevedo, R.B.; de Araújo Júnior, R.F.; Araujo, A.A.; de Lisboa Lopes Costa, A. Photodynamic Therapy Using. Chloro-Aluminum Phthalocyanine Decreases Inflammatory Response in an Experimental Rat. Periodontal Disease Model. J. Photochem. Photobiol. B Biol. 2017, 167, 208–215. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Iredell, J.R. CRISPR-Cas System in Antibiotic Resistance Plasmids in Klebsiella pneumoniae. Front. Microbiol. 2020, 10, 2934. [Google Scholar] [CrossRef]
- Tao, S.; Chen, H.; Li, N.; Fang, Y.; Xu, Y.; Liang, W. Association of CRISPR-Cas System with the Antibiotic Resistance and Virulence Genes in Nosocomial Isolates of Enterococcus. Infect. Drug Resist. 2022, 15, 6939–6949. [Google Scholar] [CrossRef] [PubMed]
- Greene, A.C. CRISPR-Based Antibacterials: Transforming Bacterial Defense into Offense. Trends Biotechnol. 2018, 36, 127–130. [Google Scholar] [CrossRef]
- Wu, Y.; Battalapalli, D.; Hakeem, M.J.; Selamneni, V.; Zhang, P.; Draz, M.S.; Ruan, Z. Engineered CRISPR-Cas Systems for the Detection and Control of Antibiotic-Resistant Infections. J. Nanobiotechnol. 2021, 19, 401. [Google Scholar] [CrossRef]
- Pursey, E.; Dimitriu, T.; Paganelli, F.L.; Westra, E.R.; Van Houte, S. CRISPR-Cas Is Associated with Fewer Antibiotic Resistance Genes in Bacterial Pathogens. Phil. Trans. R. Soc. B 2022, 377, 20200464. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Sun, X.; Li, M.; Zhang, P.; Zhu, Z.; Jiao, H.; Guo, T.; Li, G. CRISPR-Cas in Acinetobacter baumannii Contributes to Antibiotic Susceptibility by Targeting Endogenous AbaI. Microbiol. Spectr. 2022, 10, e00829-22. [Google Scholar] [CrossRef]
- Gholizadeh, P.; Aghazadeh, M.; Ghotaslou, R.; Rezaee, M.A.; Pirzadeh, T.; Cui, L.; Watanabe, S.; Feizi, H.; Kadkhoda, H.; Kafil, H.S. Role of CRISPR-Cas System on Antibiotic Resistance Patterns of Enterococcus faecalis. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 49. [Google Scholar] [CrossRef]
- Murugesan, A.C.; Varughese, H.S. Analysis of CRISPR–Cas System and Antimicrobial Resistance in Staphylococcus Coagulans Isolates. Lett. Appl. Microbiol. 2022, 75, 126–134. [Google Scholar] [CrossRef]
- Alduhaidhawi, A.H.M.; AlHuchaimi, S.N.; Al-Mayah, T.A.; Al-Ouqaili, M.T.; Alkafaas, S.S.; Muthupandian, S.; Saki, M. Prevalence of CRISPR-Cas Systems and Their Possible Association with Antibiotic Resistance in Enterococcus faecalis and Enterococcus faecium Collected from Hospital Wastewater. Infect. Drug Resist. 2022, 15, 1143–1154. [Google Scholar] [CrossRef]
- Sahu, R.; Singh, A.K.; Kumar, A.; Singh, K.; Kumar, P. Bacteriophages Concept and Applications: A Review on Phage Therapy. Curr. Pharm. Biotechnol. 2023, 24, 1245–1264. [Google Scholar]
- Ling, H.; Lou, X.; Luo, Q.; He, Z.; Sun, M.; Sun, J. Recent Advances in Bacteriophage-Based Therapeutics: Insight into the Post-Antibiotic Era. Acta Pharm. Sin. B 2022, 12, 4348–4364. [Google Scholar] [CrossRef]
- Dufour, N.; Delattre, R.; Ricard, J.-D.; Debarbieux, L. The Lysis of Pathogenic Escherichia coli by Bacteriophages Releases Less Endotoxin than by β-Lactams. Clin. Infect. Dis. 2017, 64, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, F.; Piccardi, P.; Mancini, S.; Gabard, J.; Moreillon, P.; Entenza, J.M.; Resch, G.; Que, Y.-A. Synergistic Interaction between Phage Therapy and Antibiotics Clears Pseudomonas aeruginosa Infection in Endocarditis and Reduces Virulence. J. Infect. Dis. 2017, 215, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.Y.J.; Weitz, J.S. Modeling the Synergistic Elimination of Bacteria by Phage and the Innate Immune System. J. Theor. Biol. 2017, 429, 241–252. [Google Scholar] [CrossRef]
- Durbas, I.; Machnik, G. Phage Therapy: An Old Concept with New Perspectives. J. Appl. Pharm. Sci. 2022, 12, 027–038. [Google Scholar] [CrossRef]
- Enault, F.; Briet, A.; Bouteille, L.; Roux, S.; Sullivan, M.B.; Petit, M.-A. Phages Rarely Encode Antibiotic Resistance Genes: A Cautionary Tale for Virome Analyses. ISME J. 2017, 11, 237–247. [Google Scholar] [CrossRef]
- Suhas, R. Structure, Function and Mechanistic Aspects of Scorpion Venom Peptides—A Boon for the Development of Novel Therapeutics. Eur. J. Med. Chem. Rep. 2022, 6, 100068. [Google Scholar] [CrossRef]
- Yacoub, T.; Rima, M.; Karam, M.; Sabatier, J.-M.; Fajloun, Z. Antimicrobials from Venomous Animals: An Overview. Molecules 2020, 25, 2402. [Google Scholar] [CrossRef]
- Ageitos, L.; Torres, M.D.T.; De La Fuente-Nunez, C. Biologically Active Peptides from Venoms: Applications in Antibiotic Resistance, Cancer, and Beyond. Int. J. Mol. Sci. 2022, 23, 15437. [Google Scholar] [CrossRef]
- Li, Z.; Hu, P.; Wu, W.; Wang, Y. Peptides with Therapeutic Potential in the Venom of the Scorpion Buthus martensii Karsch. Peptides 2019, 115, 43–50. [Google Scholar] [CrossRef]
- Zerouti, K.; Khemili, D.; Laraba-Djebari, F.; Hammoudi-Triki, D. Nontoxic Fraction of Scorpion Venom Reduces Bacterial Growth and Inflammatory Response in a Mouse Model of Infection. Toxin Rev. 2019, 40, 1–15. [Google Scholar] [CrossRef]
- Ahmadi, S.; Knerr, J.M.; Argemi, L.; Bordon, K.C.F.; Pucca, M.B.; Cerni, F.A.; Arantes, E.C.; Çalışkan, F.; Laustsen, A.H. Scorpion Venom: Detriments and Benefits. Biomedicines 2020, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Cesa-Luna, C.; Muñoz-Rojas, J.; Saab-Rincon, G.; Baez, A.; Morales-García, Y.E.; Juárez-González, V.R.; Quintero-Hernández, V. Structural Characterization of Scorpion Peptides and Their Bactericidal Activity against Clinical Isolates of Multidrug-Resistant Bacteria. PLoS ONE 2019, 14, e0222438. [Google Scholar] [CrossRef] [PubMed]
- Teerapo, K.; Roytrakul, S.; Sistayanarain, A.; Kunthalert, D. A Scorpion Venom Peptide Derivative BmKn‑22 with Potent Antibiofilm Activity against Pseudomonas aeruginosa. PLoS ONE 2019, 14, e0218479. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Xie, S.; Yang, J.; Zhang, Y.; Han, S.; Su, S.; Yao, H. Pharmacological Effects and Mechanisms of Bee Venom and Its Main Components: Recent Progress and Perspective. Front. Pharmacol. 2022, 13, 1001553. [Google Scholar] [CrossRef]
- Tiwari, R.; Tiwari, G.; Lahiri, A.; Ramachandran, V.; Rai, A. Melittin: A Natural Peptide with Expanded Therapeutic Applications. Nat. Prod. J. 2022, 12, 13–29. [Google Scholar] [CrossRef]
- El-Seedi, H.; El-Wahed, A.A.; Yosri, N.; Musharraf, S.G.; Chen, L.; Moustafa, M.; Zou, X.; Al-Mousawi, S.; Guo, Z.; Khatib, A.; et al. Antimicrobial Properties of Apis mellifera’s Bee Venom. Toxins 2020, 12, 451. [Google Scholar] [CrossRef]
- Hegazi, A.G.; Abdou, A.M.H.; El-Moez, I.A.; Allah, F.M.A. Evaluation of the Antibacterial Activity of Bee Venom from Different Sources. World Appl. Sci. J. 2014, 30, 266–270. [Google Scholar]
- Fadl, A.E. Antibacterial and Antibiofilm Effects of Bee Venom from (Apis mellifera) on Multidrug-Resistant Bacteria (Mdrb). Al-Azhar J. Pharm. Sci. 2018, 58, 60–80. [Google Scholar] [CrossRef]
- Choi, J.H.; Jang, A.-Y.; Lin, S.; Lim, S.; Kim, D.; Park, K.; Han, S.M.; Yeo, J.-H.; Seo, H.S. Melittin, a Honeybee Venom-Derived Antimicrobial Peptide, May Target Methicillin-Resistant Staphylococcus aureus. Mol. Med. Rep. 2015, 12, 6483–6490. [Google Scholar] [CrossRef]
- Oguiura, N.; Corrêa, P.G.; Rosmino, I.L.; de Souza, A.O.; Pasqualoto, K.F.M. Antimicrobial Activity of Snake β-Defensins and Derived Peptides. Toxins 2021, 14, 1. [Google Scholar] [CrossRef]
- De Barros, E.; Gonçalves, R.M.; Cardoso, M.H.; Santos, N.C.; Franco, O.L.; Cândido, E.S. Snake Venom Cathelicidins as Natural Antimicrobial Peptides. Front. Pharmacol. 2019, 10, 489134. [Google Scholar] [CrossRef] [PubMed]
- Blaylock, R.S.M. Antibacterial Properties of KwaZulu Natal Snake Venoms. Toxicon 2000, 38, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Dal Mas, C.; Rossato, L.; Shimizu, T.; Oliveira, E.B.D.; da Silva Júnior, P.I.; Meis, J.F.; Colombo, A.L.; Hayashi, M.A.F. Effects of the Natural Peptide Crotamine from a South American Rattlesnake on Candida auris, an Emergent Multidrug Antifungal Resistant Human Pathogen. Biomolecules 2019, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, I.C.J.; Bandeira-Lima, D.; Mello, C.P.; Pereira, T.P.; de Menezes, R.R.P.P.B.; Sampaio, T.L.; Falcao, C.B.; Rádis-Baptista, G.; Martins, A.M.C. Antichagasic Effect of Crotalicidin, a Cathelicidin-like Vipericidin, Found in Crotalus durissus terrificus Rattlesnake’s Venom Gland. Parasitology 2017, 145, 1059–1064. [Google Scholar] [CrossRef]
- de Oliveira Junior, N.G.; e Silva Cardoso, M.H.; Franco, O.L. Snake Venoms: Attractive Antimicrobial Proteinaceous Compounds for Therapeutic Purposes. Cell. Mol. Life Sci. 2013, 70, 4645–4658. [Google Scholar] [CrossRef]
- Guo, C.; Liu, S.; Yao, Y.; Zhang, Q.; Sun, M.-Z. Past Decade Study of Snake Venom L-Amino Acid Oxidase. Toxicon 2012, 60, 302–311. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Viegas, M.F.; da Silva, S.L.; Soares, A.M.; Ramos, M.J.; Fernandes, P.A. The Chemistry of Snake Venom and Its Medicinal Potential. Nat. Rev. Chem. 2022, 6, 451–469. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Wang, L.; Yang, H.; Xiao, H.; Farooq, A.; Liu, Z.; Hu, M.; Shi, X. The Spider Venom Peptide Lycosin-II Has Potent Antimicrobial Activity against Clinically Isolated Bacteria. Toxins 2016, 8, 119. [Google Scholar] [CrossRef]
- Abreu, T.F.; Sumitomo, B.N.; Nishiyama, M.Y.; de Oliveira, U.C.; Souza, G.H.M.F.; Kitano, E.S.; Zelanis, A.; Serrano, S.M.T.; Junqueira-de-Azevedo, I.L.M.; da Silva, P.I.; et al. Peptidomics of Acanthoscurria gomesiana Spider Venom Reveals New Toxins with Potential Antimicrobial Activity. J. Proteom. 2017, 151, 232–242. [Google Scholar] [CrossRef]
- Wu, T.; Wang, M.; Wu, W.; Luo, Q.; Jiang, L.; Tao, H.; Deng, M. Spider Venom Peptides as Potential Drug Candidates Due to Their Anticancer and Antinociceptive Activities. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e146318. [Google Scholar] [CrossRef]
- Torres-Larios, A.; Gurrola, G.B.; Zamudio, F.Z.; Possani, L.D. Hadrurin, a New Antimicrobial Peptide from the Venom of the Scorpion Hadrurus aztecus. Eur. J. Biochem. 2000, 267, 5023–5031. [Google Scholar] [CrossRef] [PubMed]
- Corzo, G.; Escoubas, P.; Villegas, E.; Barnham, K.J.; He, W.; Norton, R.S.; Nakajima, T. Characterization of Unique Amphipathic Antimicrobial Peptides from Venom of the Scorpion Pandinus Imperator. Biochem. J. 2001, 359, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Villegas, E.; Montoya-Rosales, A.; Rivas-Santiago, B.; Corzo, G. Characterization of Antibacterial and Hemolytic Activity of Synthetic Pandinin 2 Variants and Their Inhibition against Mycobacterium tuberculosis. PLoS ONE 2014, 9, e101742. [Google Scholar] [CrossRef]
- Zeng, X.-C.; Wang, S.; Nie, Y.; Zhang, L.; Luo, X. Characterization of BmKbpp, a Multifunctional Peptide from the Chinese Scorpion Mesobuthus martensii Karsch: Gaining Insight into a New Mechanism for the Functional Diversification of Scorpion Venom Peptides. Peptides 2012, 33, 44–51. [Google Scholar] [CrossRef]
- Ramírez-Carreto, S.; Jiménez-Vargas, J.M.; Rivas-Santiago, B.; Corzo, G.; Possani, L.D.; Becerril, B.; Ortiz, E. Peptides from the Scorpion Vaejovis punctatus with Broad Antimicrobial Activity. Peptides 2015, 73, 51–59. [Google Scholar] [CrossRef]
- Guo, X.; Ma, C.; Du, Q.; Wei, R.; Wang, L.; Zhou, M.; Chen, T.; Shaw, C. Two Peptides, TsAP-1 and TsAP-2, from the Venom of the Brazilian Yellow Scorpion, Tityus serrulatus: Evaluation of Their Antimicrobial and Anticancer Activities. Biochimie 2013, 95, 1784–1794. [Google Scholar] [CrossRef]
- Cao, L.; Li, Z.; Zhang, R.; Wu, Y.; Li, W.; Cao, Z. StCT2, a New Antibacterial Peptide Characterized from the Venom of the Scorpion Scorpiops tibetanus. Peptides 2012, 36, 213–220. [Google Scholar] [CrossRef]
- Ratajczak, M.; Kaminska, D.; Matuszewska, E.; Hołderna-Kedzia, E.; Rogacki, J.; Matysiak, J. Promising Antimicrobial Properties of Bioactive Compounds from Different Honeybee Products. Molecules 2021, 26, 4007. [Google Scholar] [CrossRef]
- Pluta, P.; Sokol, R. Changes in the Expression of Antimicrobial Peptide Genes in Honey Bees (Apis mellifera) under the Influence of Various Pathogens. Ann. Parasitol. 2020, 66, 457–465. [Google Scholar]
- Falcao, C.B.; de La Torre, B.; Pérez-Peinado, C.; Barron, A.E.; Andreu, D.; Rádis-Baptista, G. Vipericidins: A Novel Family of Cathelicidin-Related Peptides from the Venom Gland of South American Pit Vipers. Amino Acids 2014, 46, 2561–2571. [Google Scholar] [CrossRef]
- Blower, R.J.; Popov, S.G.; van Hoek, M.L. Cathelicidin Peptide Rescues G. mellonella Infected with B. Anthracis. Virulence 2018, 9, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Creane, S.E.; Carlile, S.R.; Downey, D.; Weldon, S.; Dalton, J.P.; Taggart, C.C. The Impact of Lung Proteases on Snake-Derived Antimicrobial Peptides. Biomolecules 2021, 11, 1106. [Google Scholar] [CrossRef] [PubMed]
- Kuhn-Nentwig, L. Complex Precursor Structures of Cytolytic Cupiennins Identified in Spider Venom Gland Transcriptomes. Sci. Rep. 2021, 11, 4009. [Google Scholar] [CrossRef] [PubMed]
- Salimo, Z.M.; Barros, A.L.; Adrião, A.A.; Rodrigues, A.M.; Sartim, M.A.; de Oliveira, I.S.; Pucca, M.B.; Baia-da-Silva, D.C.; Monteiro, W.M.; de Melo, G.C. Toxins from Animal Venoms as a Potential Source of Antimalarials: A Comprehensive Review. Toxins 2023, 15, 375. [Google Scholar] [CrossRef]
- Rodriguez, C.; Carrasco, J.; Bruner-Montero, G.; Júnior, O.R.P.; Gutiérrez, M.; Díaz-Ferguson, E. Components and Biological Activities of Venom from Lionfishes (Scorpaenidae: Pterois). Mar. Drugs 2025, 23, 55. [Google Scholar] [CrossRef]
- Shin, M.K.; Hwang, I.-W.; Kim, Y.; Kim, S.T.; Jang, W.; Lee, S.; Bang, W.Y.; Bae, C.-H.; Sung, J.-S. Antibacterial and Anti-Inflammatory Effects of Novel Peptide Toxin from the Spider Pardosa astrigera. Antibiotics 2020, 9, 422. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, M.; Lai, R.; Zhang, Z. Chemical Modifications to Increase the Therapeutic Potential of Antimicrobial Peptides. Peptides 2021, 146, 170666. [Google Scholar] [CrossRef]
- Carratalá, J.V.; Serna, N.; Villaverde, A.; Vázquez, E.; Ferrer-Miralles, N. Nanostructured Antimicrobial Peptides: The Last Push towards Clinics. Biotechnol. Adv. 2020, 44, 107603. [Google Scholar] [CrossRef]
- Bellotto, O.; Semeraro, S.; Bandiera, A.; Tramer, F.; Pavan, N.; Marchesan, S. Polymer Conjugates of Antimicrobial Peptides (AMPs) with D-Amino Acids (D-Aa): State of the Art and Future Opportunities. Pharmaceutics 2022, 14, 446. [Google Scholar] [CrossRef]
- Tyagi, R.; Srivastava, M.; Jain, P.; Pandey, R.P.; Asthana, S.; Kumar, D.; Raj, V.S. Development of Potential Proteasome Inhibitors against Mycobacterium tuberculosis. J. Biomol. Struct. Dyn. 2022, 40, 2189–2203. [Google Scholar] [CrossRef]
- Nazir, F.; Tabish, T.A.; Tariq, F.; Iftikhar, S.; Wasim, R.; Shahnaz, G. Stimuli-Sensitive Drug Delivery Systems for Site-Specific Antibiotic Release. Drug Discov. Today 2022, 27, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Kassinger, S.J.; van Hoek, M.L. Biofilm Architecture: An Emerging Synthetic Biology Target. Synth. Syst. Biotechnol. 2020, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Himanshu; Mukherjee, R.; Vidic, J.; Leal, E.; da Costa, A.C.; Prudencio, C.R.; Raj, V.S.; Chang, C.-M.; Pandey, R.P. Nanobiotics and the One Health Approach: Boosting the Fight Against Antimicrobial Resistance at the Nanoscale. Biomolecules 2023, 13, 1182. [Google Scholar] [CrossRef]
- Elfadil, D.; Elkhatib, W.F.; El-Sayyad, G.S. Promising Advances in Nanobiotic-Based Formulations for Drug Specific Targeting Against Multidrug-Resistant Microbes and Biofilm-Associated Infections. Microb. Pathog. 2022, 170, 105721. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Nasr, M.; Elkhatib, W.F.; Eltayeb, W.N.; Elshamy, A.A.; El-Sayyad, G.S. Nanobiotic Formulations as Promising Advances for Combating MRSA Resistance: Susceptibilities and Post-Antibiotic Effects of Clindamycin, Doxycycline, and Linezolid. RSC Adv. 2021, 11, 39696–39706. [Google Scholar] [CrossRef] [PubMed]
- Milewska, S.; Niemirowicz-Laskowska, K.; Siemiaszko, G.; Nowicki, P.; Wilczewska, A.Z.; Car, H. Current Trends and Challenges in Pharmacoeconomic Aspects of Nanocarriers as Drug Delivery Systems for Cancer Treatment. Int. J. Nanomed. 2021, 16, 6593–6644. [Google Scholar] [CrossRef]
- Chakraborty, N.; Jha, D.; Roy, I.; Kumar, P.; Gaurav, S.S.; Marimuthu, K.; Ng, O.-T.; Lakshminarayanan, R.; Verma, N.K.; Gautam, H.K. Nanobiotics against Antimicrobial Resistance: Harnessing the Power of Nanoscale Materials and Technologies. J. Nanobiotechnol. 2022, 20, 375. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.-H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-Based Therapeutics for Antibiotic-Resistant Bacterial Infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef]
- Geersing, A.; de Vries, R.H.; Jansen, G.; Rots, M.G.; Roelfes, G. Folic Acid Conjugates of a Bleomycin Mimic for Selective Targeting of Folate Receptor Positive Cancer Cells. Bioorganic Med. Chem. Lett. 2019, 29, 1922–1927. [Google Scholar] [CrossRef]
- Tripathi, N.; Goshisht, M.K. Recent Advances and Mechanistic Insights into Antibacterial Activity, Antibiofilm Activity, and Cytotoxicity of Silver Nanoparticles. ACS Appl. Bio Mater. 2022, 5, 1391–1463. [Google Scholar] [CrossRef]
- Wagi, S.; Ahmed, A. Bacterial Nanobiotic Potential. Green Process. Synth. 2020, 9, 203–211. [Google Scholar] [CrossRef]
- Brown, A.N.; Smith, K.; Samuels, T.A.; Lu, J.; Obare, S.O.; Scott, M.E. Nanoparticles Functionalized with Ampicillin Destroy Multiple-Antibiotic-Resistant Isolates of Pseudomonas aeruginosa and Enterobacter aerogenes and Methicillin-Resistant Staphylococcus aureus. Appl. Environ. Microbiol. 2012, 78, 2768–2774. [Google Scholar] [CrossRef] [PubMed]
- Okkeh, M.; Bloise, N.; Restivo, E.; De Vita, L.; Pallavicini, P.; Visai, L. Gold Nanoparticles: Can. They Be the Next Magic. Bullet for Multidrug-Resistant Bacteria? Nanomaterials 2021, 11, 312. [Google Scholar] [CrossRef]
- Cheng, X.; Pei, X.; Xie, W.; Chen, J.; Li, Y.; Wang, J.; Gao, H.; Wan, Q. pH-Triggered Size-Tunable Silver Nanoparticles: Targeted Aggregation for Effective Bacterial Infection Therapy. Small 2022, 18, 2200915. [Google Scholar] [CrossRef]
- Mamun, M.M.; Sorinolu, A.J.; Munir, M.; Vejerano, E.P. Nanoantibiotics: Functions and Properties at the Nanoscale to Combat Antibiotic Resistance. Front. Chem. 2021, 9, 687660. [Google Scholar] [CrossRef]
- Chung, H.J.; Castro, C.M.; Im, H.; Lee, H.; Weissleder, R. A Magneto-DNA Nanoparticle System for Rapid Detection and Phenotyping of Bacteria. Nat. Nanotechnol. 2013, 8, 369–375. [Google Scholar] [CrossRef]
- Kulshrestha, S.; Khan, S.; Hasan, S.; Khan, M.E.; Misba, L.; Khan, A.U. Calcium Fluoride Nanoparticles Induced Suppression of Streptococcus Mutans Biofilm: An In Vitro and In Vivo Approach. Appl. Microbiol. Biotechnol. 2016, 100, 1901–1914. [Google Scholar] [CrossRef]
- Wang, Y. Liposome as a Delivery System for the Treatment of Biofilm-mediated Infections. J. Appl. Microbiol. 2021, 131, 2626–2639. [Google Scholar] [CrossRef]
- Cano, A.; Ettcheto, M.; Espina, M.; López-Machado, A.; Cajal, Y.; Rabanal, F.; Sánchez-López, E.; Camins, A.; García, M.L.; Souto, E.B. State-of-the-Art Polymeric Nanoparticles as Promising Therapeutic Tools against Human Bacterial Infections. J. Nanobiotechnol. 2020, 18, 156. [Google Scholar] [CrossRef]
- Forier, K.; Raemdonck, K.; De Smedt, S.C.; Demeester, J.; Coenye, T.; Braeckmans, K. Lipid and Polymer Nanoparticles for Drug Delivery to Bacterial Biofilms. J. Control. Release 2014, 190, 607–623. [Google Scholar] [CrossRef]
- Rajendiran, K.; Zhao, Z.; Pei, D.-S.; Fu, A. Antimicrobial Activity and Mechanism of Functionalized Quantum Dots. Polymers 2019, 11, 1670. [Google Scholar] [CrossRef] [PubMed]
- Courtney, C.M.; Goodman, S.M.; McDaniel, J.A.; Madinger, N.E.; Chatterjee, A.; Nagpal, P. Photoexcited Quantum Dots for Killing Multidrug-Resistant Bacteria. Nature Mater. 2016, 15, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Kalhapure, R.S.; Suleman, N.; Mocktar, C.; Seedat, N.; Govender, T. Nanoengineered Drug Delivery Systems for Enhancing Antibiotic Therapy. J. Pharm. Sci. 2015, 104, 872–905. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Malviya, R.; Srivastava, S.; Ahmad, I.; Rab, S.O.; Uniyal, P. Construction, Features and Regulatory Aspects of Organ-Chip for Drug Delivery Applications: Advances and Prospective. Curr. Pharm. Des. 2024, 30, 1952–1965. [Google Scholar] [CrossRef]
- Lazar, V.; Oprea, E.; Ditu, L. Resistance, Tolerance, Virulence and Bacterial Pathogen Fitness—Current State and Envisioned Solutions for the near Future. Pathogens 2023, 12, 746. [Google Scholar] [CrossRef]
- Kulchar, R.J.; Singh, R.; Ding, S.; Alexander, E.; Leong, K.W.; Daniell, H. Delivery of Biologics: Topical Administration. Biomaterials 2023, 302, 122312. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic Resistance: A Rundown of a Global Crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
| Type of Resistance | Bacterial Species | Specific Mechanism | Antibiotic Class | Resistance Strategy | Clinical Implications | References |
|---|---|---|---|---|---|---|
| Intrinsic | Pseudomonas aeruginosa | Efflux Pump Overexpression | Carbapenems | Reduced Antibiotic Accumulation | High Treatment Failure Rates | [25] |
| Acquired | Staphylococcus aureus | mecA Gene Horizontal | β-Lactam Antibiotics | Transfer Penicillin-Binding Protein Modification | MRSA Infections | [26] |
| Adaptive | Acinetobacter baumannii | Biofilm Formation | Multiple Antibiotics | Phenotypic Heterogeneity | Persistent Infections | [27] |
| Intrinsic | Klebsiella pneumoniae | β-Lactamase Production | Cephalosporins | Enzymatic Antibiotic Degradation | Extended-Spectrum Resistance | [28] |
| Acquired | Enterococcus faecium | Vancomycin Resistance | Gene (vanA) Glycopeptide | Antibiotics Target Site Modification | VRE Nosocomial Infections | [29] |
| Adaptive | Multiple Bacterial Species | Metabolic Dormancy | Broad-Spectrum | Reduced Metabolic Activity | Antibiotic Tolerance | [30] |
| Intrinsic | Multiple Bacterial Species | ABC Transporter Regulation | Broad-Spectrum Antibiotics | Complex Efflux Mechanism Therapeutic | Targeting Challenges | [31,32] |
| Advantage | Description | Ref. |
|---|---|---|
| Reduced Toxicity and Enhanced Stability | Encapsulating antibiotics in nanoparticles can reduce their overall toxicity and enhance their stability in vivo, preventing premature degradation. | [173] |
| Targeted Delivery to Sites of Infection | Nanoparticles can be designed to target specific sites of infection, either passively or actively, allowing for higher antibiotic concentrations at the infected site while minimizing systemic exposure and adverse effects. | [174] |
| Stimuli-Sensitive Drug Release | Nanoparticles can be engineered to release antibiotics in response to specific stimuli (e.g., pH, enzymes, reactive oxygen species) present in the infected tissues, enabling targeted and controlled drug release. | [175] |
| Directed towards Biofilm Microenvironments | Nanoparticles can be tailored to target and disrupt biofilms, which are a significant contributor to antimicrobial resistance, by exploiting the unique microenvironment of biofilms. | [176] |
| Combined Physical Therapy | Nanoparticles can be combined with other physical therapies, such as photothermal therapy (PTT) and antibacterial photodynamic therapy (aPDT), to enhance their antimicrobial efficacy through synergistic mechanisms. | [177] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elshobary, M.E.; Badawy, N.K.; Ashraf, Y.; Zatioun, A.A.; Masriya, H.H.; Ammar, M.M.; Mohamed, N.A.; Mourad, S.; Assy, A.M. Combating Antibiotic Resistance: Mechanisms, Multidrug-Resistant Pathogens, and Novel Therapeutic Approaches: An Updated Review. Pharmaceuticals 2025, 18, 402. https://doi.org/10.3390/ph18030402
Elshobary ME, Badawy NK, Ashraf Y, Zatioun AA, Masriya HH, Ammar MM, Mohamed NA, Mourad S, Assy AM. Combating Antibiotic Resistance: Mechanisms, Multidrug-Resistant Pathogens, and Novel Therapeutic Approaches: An Updated Review. Pharmaceuticals. 2025; 18(3):402. https://doi.org/10.3390/ph18030402
Chicago/Turabian StyleElshobary, Mostafa E., Nadia K. Badawy, Yara Ashraf, Asmaa A. Zatioun, Hagar H. Masriya, Mohamed M. Ammar, Nourhan A. Mohamed, Sohaila Mourad, and Abdelrahman M. Assy. 2025. "Combating Antibiotic Resistance: Mechanisms, Multidrug-Resistant Pathogens, and Novel Therapeutic Approaches: An Updated Review" Pharmaceuticals 18, no. 3: 402. https://doi.org/10.3390/ph18030402
APA StyleElshobary, M. E., Badawy, N. K., Ashraf, Y., Zatioun, A. A., Masriya, H. H., Ammar, M. M., Mohamed, N. A., Mourad, S., & Assy, A. M. (2025). Combating Antibiotic Resistance: Mechanisms, Multidrug-Resistant Pathogens, and Novel Therapeutic Approaches: An Updated Review. Pharmaceuticals, 18(3), 402. https://doi.org/10.3390/ph18030402








