Protective Effects of Frankincense Oil on Wound Healing: Downregulating Caspase-3 Expression to Facilitate the Transition from the Inflammatory to Proliferative Phase
Abstract
:1. Introduction
2. Results
2.1. Chemical Characterisation of Essential Oil
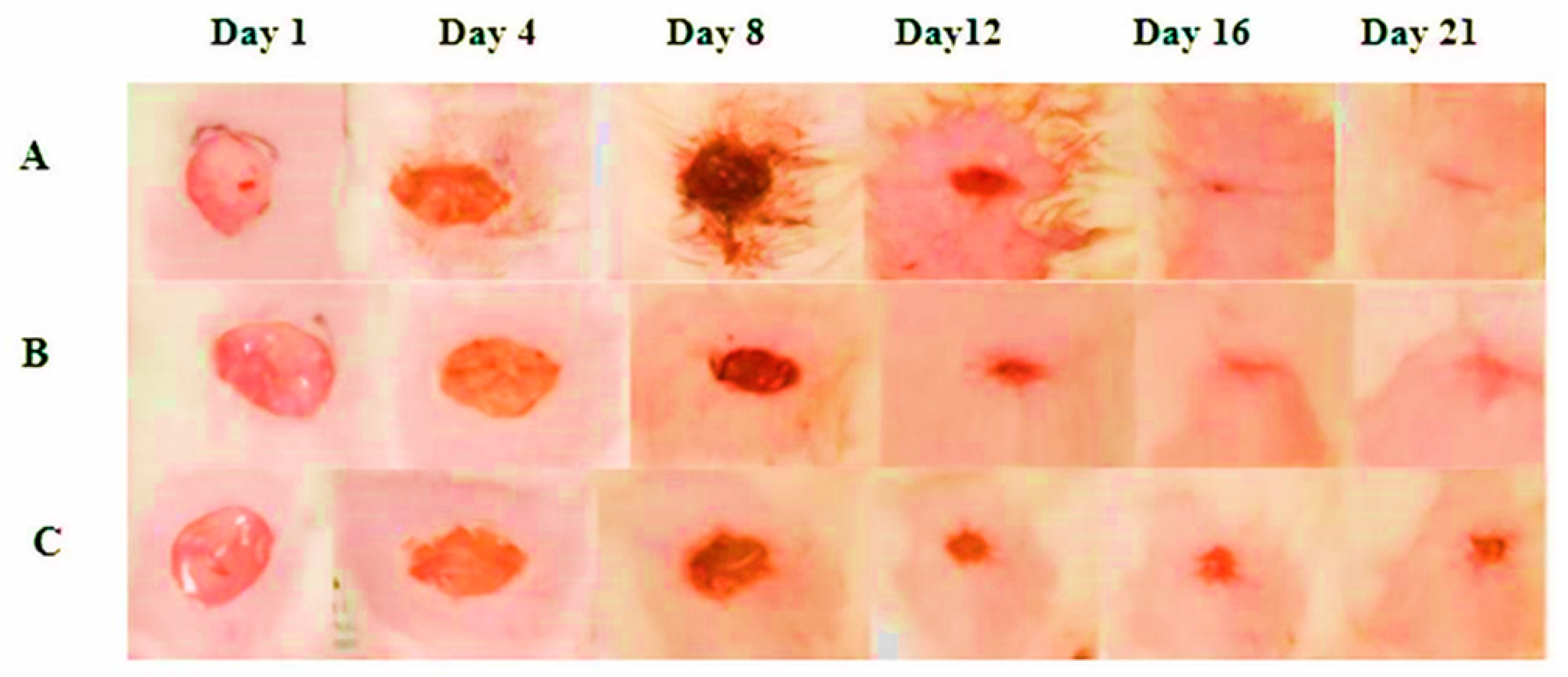
2.2. Percentage Wound Contraction
2.3. Body Weight
2.4. Effect of FEO on Inflammatory Markers and Wound Healing Dynamics
2.5. Effect of FEO on CD68 Level in FEO Treated Rats
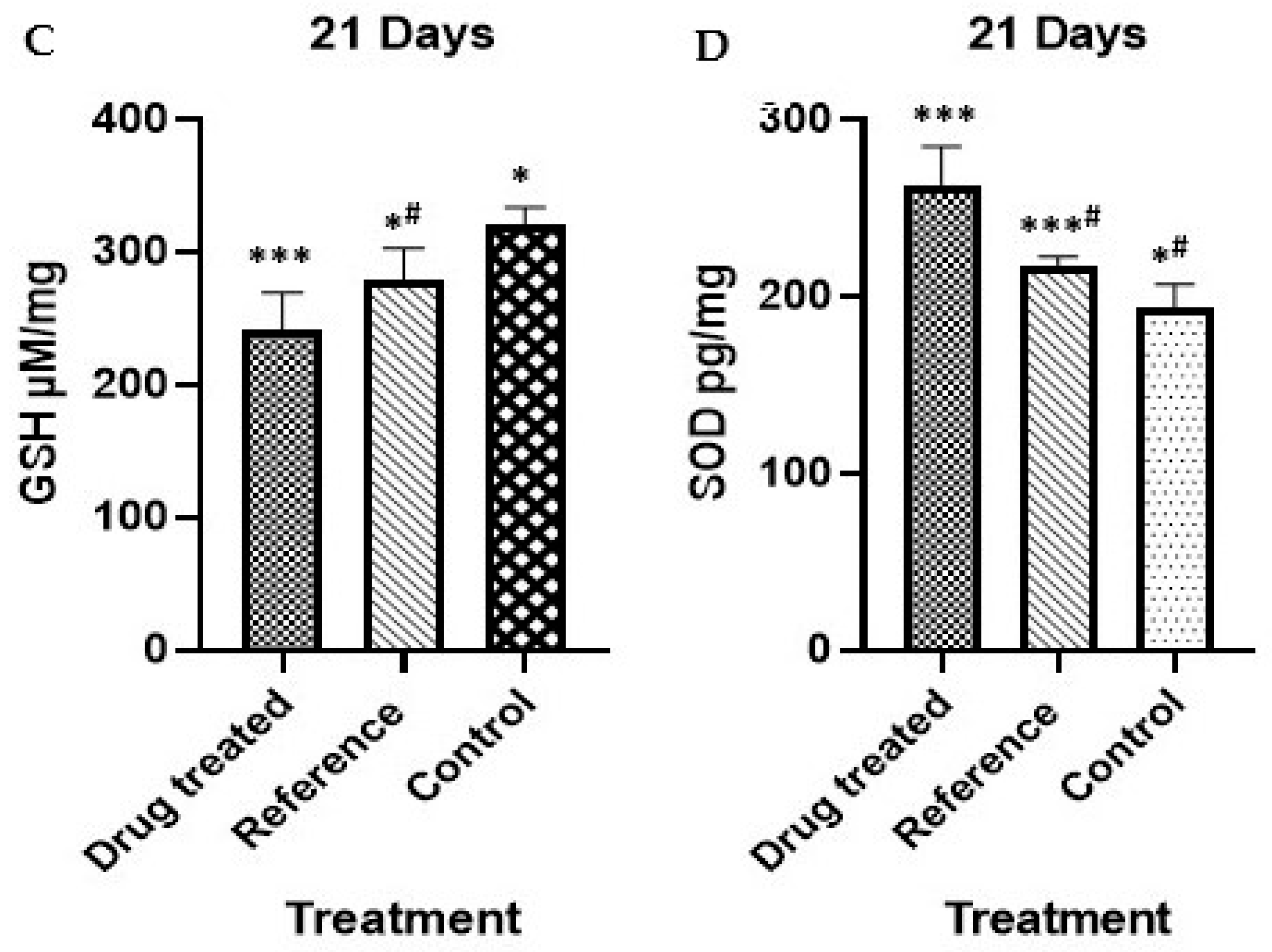
2.6. Effect on Oxidative Stress Markers and Antioxidant Profile
2.7. Histological Results
Measurement of Histopathological Changes
2.8. Immunohistochemical Staining
3. Discussion
4. Materials and Methods
4.1. Albino Rats
4.2. Experimental Design
- Group I—the control group received yellow soft paraffin
- Group II—animals treated with standard (1% w/w silver sulfadiazine)
- Group III—animals treated with test drug (10% w/w FEO)
4.3. Wounding (Morton and Malone Method)
4.4. Source of Oil
4.5. Identification of Compounds in FEO by GCMS
4.6. Preparation of Ointment
4.7. Treatment
4.8. Evaluation Parameters
4.9. Biochemical Evaluation
4.9.1. Sample Collection
4.9.2. Determination of the Rat Serum TNF-α and IL-1β Levels
4.9.3. Estimation of CD68 in the Rat Serum
4.9.4. Antioxidant Activity
4.10. Histopathological Evaluation
4.10.1. Sample Collection
4.10.2. Immunohistochemical Staining
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yazarlu, O.; Iranshahi, M.; Kashani, H.R.; Reshadat, S.; Habtemariam, S.; Iranshahy, M.; Hasanpour, M. Perspective on the application of medicinal plants and natural products in wound healing: A mechanistic review. Pharmacol. Res. 2021, 174, 105841. [Google Scholar] [CrossRef] [PubMed]
- Sujatha, P.S.; Pavithran, S.; Sujatha, P.S. Wound Healing Effect of Furfural and Pentadecanal from Lagerstroemia speciosa (L.) Pers Acetone Flower extracts against Haemadipsa sylvestris Bite. J. Adv. Sci. Res. 2024, 15, 12–15. [Google Scholar]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.; Dudhipala, N. Innovative treatment strategies to accelerate wound healing: Trajectory and recent advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.S.; Chin, G.A.; Moldawer, L.; Diegelmann, R.F. Principles of Wound Healing. Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists [Internet]; University of Adelaide Press: Adelaide, Australia, 2011. [Google Scholar]
- Murugesu, S.; Selamat, J.; Perumal, V. Phytochemistry, pharmacological properties, and recent applications of Ficus benghalensis and Ficus religiosa. Plants 2021, 10, 2749. [Google Scholar] [CrossRef]
- Frank, M.B.; Yang, Q.; Osban, J.; Azzarello, J.T.; Saban, M.R.; Saban, R.; Ashley, R.A.; Welter, J.C.; Fung, K.M.; Lin, H.K. Frankincense oil derived from Boswellia carteri induces tumor cell-specific cytotoxicity. BMC Complement. Altern. Med. 2009, 9, 6. [Google Scholar] [CrossRef]
- Borotová, P.; Čmiková, N.; Galovičová, L.; Vukovic, N.L.; Vukic, M.D.; Tvrdá, E.; Kowalczewski, P.Ł.; Kluz, M.I.; Puchalski, C.; Schwarzová, M.; et al. Antioxidant, antimicrobial, and anti-insect properties of Boswellia carterii essential oil for food preservation improvement. Horticulturae 2023, 9, 333. [Google Scholar] [CrossRef]
- Obiștioiu, D.; Hulea, A.; Cocan, I.; Alexa, E.; Negrea, M.; Popescu, I.; Herman, V.; Imbrea, I.M.; Heghedus-Mindru, G.; Suleiman, M.A.; et al. Boswellia Essential Oil: Natural Antioxidant as an Effective Antimicrobial and Anti-Inflammatory Agent. Antioxidants 2023, 12, 1807. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; Ramakrishna, S.; Berto, F. Antioxidant, antimicrobial and antiviral properties of herbal materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef]
- Al-Yasiry, A.R.; Kiczorowska, B. Frankincense–therapeutic properties. Adv. Hyg. Exp. Med. 2016, 70, 380–391. [Google Scholar] [CrossRef]
- Almutairi, M.B.; Alrouji, M.; Almuhanna, Y.; Asad, M.; Joseph, B. In-Vitro and In-Vivo Antibacterial Effects of Frankincense Oil and Its Interaction with Some Antibiotics against Multidrug-Resistant Pathogens. Antibiotics 2022, 11, 1591. [Google Scholar] [CrossRef]
- Almeida-da-Silva, C.L.; Sivakumar, N.; Asadi, H.; Chang-Chien, A.; Qoronfleh, M.W.; Ojcius, D.M.; Essa, M.M. Effects of frankincense compounds on infection, inflammation, and oral health. Molecules 2022, 27, 4174. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Rodriguez, D.; Parker, T.L. Biological activities of frankincense essential oil in human dermal fibroblasts. Biochim. Open 2017, 4, 31–35. [Google Scholar] [CrossRef]
- Orchard, A.; van Vuuren, S. Commercial essential oils as potential antimicrobials to treat skin diseases. Evid. Based Complement. Altern. Med. 2017, 2017, 4517971. [Google Scholar] [CrossRef]
- Romo-Rico, J.; Krishna, S.M.; Bazaka, K.; Golledge, J.; Jacob, M.V. Potential of plant secondary metabolite-based polymers to enhance wound healing. Acta Biomater. 2022, 147, 34–49. [Google Scholar] [CrossRef]
- Nikolic, M.; Andjic, M.; Bradic, J.; Kocovic, A.; Tomovic, M.; Samanovic, A.M.; Jakovljevic, V.; Veselinovic, M.; Capo, I.; Krstonosic, V.; et al. Topical application of siberian pine essential oil formulations enhance diabetic wound healing. Pharmaceutics 2023, 15, 2437. [Google Scholar] [CrossRef]
- Zhang, P.Z.; Li, Y.M.; Xiong, X.M.; Deng, S.; Xiong, W.; Lang, Z.G.; Du, D.Z.; Yu, W.J.; Yue, J.B.; Xiang, Y.; et al. Wound healing potential of the standardized extract of Boswellia serrata on experimental diabetic foot ulcer via inhibition of inflammatory, angiogenetic and apoptotic markers. Planta Med. 2019, 85, 657–669. [Google Scholar]
- Siddiqui, M.Z. Boswellia serrata, a potential antiinflammatory agent: An overview. Indian J. Pharm. Sci. 2011, 73, 255. [Google Scholar]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound healing and the use of medicinal plants. Evid. Based Complement. Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef]
- Jahandideh, M.; Hajimehdipoor, H.; Mortazavi, S.A.; Dehpour, A.; Hassanzadeh, G. Evaluation of the Wound Healing Activity of a Traditional Compound Herbal Product Using Rat Excision Wound Model. Iran. J. Pharm. Res. 2017, 16, 153–163. [Google Scholar]
- Sharma, P.; Kumar, D.; Shri, R.; Dua, K.; Ntie-Kang, F.; Kumar, S. Mechanistic Insights and Therapeutic Potential of Natural Products in Amelioration of Wound Healing. Preprints 2023, 2023030054. [Google Scholar] [CrossRef]
- Cialdai, F.; Risaliti, C.; Monici, M. Role of fibroblasts in wound healing and tissue remodeling on Earth and in space. Front. Bioeng. Biotechnol. 2022, 10, 958381. [Google Scholar] [CrossRef] [PubMed]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.A.; Qazi, G.N.; Taneja, S.C. Boswellic acids: A group of medicinally important compounds. Nat. Prod. Rep. 2009, 26, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Quan, Z.; Xiao, P.; Duan, J.A. New Insights into Antioxidant Peptides: An Overview of Efficient Screening, Evaluation Models, Molecular Mechanisms, and Applications. Antioxidants 2024, 13, 203. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Srivastava, G.K.; Martinez-Rodriguez, S.; Md Fadilah, N.I.; Looi Qi Hao, D.; Markey, G.; Shukla, P.; Fauzi, M.B.; Panetsos, F. Progress in Wound-Healing Products Based on Natural Compounds, Stem Cells, and MicroRNA-Based Biopolymers in the European, United States of America, and Asian Markets: Opportunities, Barriers, and Regulatory Issues. Polymers 2024, 16, 1280. [Google Scholar] [CrossRef]
- Hoch, C.C.; Petry, J.; Griesbaum, L.; Weiser, T.; Werner, K.; Ploch, M.; Verschoor, A.; Multhoff, G.; Dezfouli, A.B.; Wollenberg, B. 1,8-cineole (eucalyptol): A versatile phytochemical with therapeutic applications across multiple diseases. Biomed. Pharmacother. 2023, 167, 115467. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Herbal products and their active constituents for diabetic wound healing—Preclinical and clinical studies: A systematic review. Pharmaceutics 2023, 15, 281. [Google Scholar] [CrossRef]
- Yadav, J.P.; Verma, A.; Pathak, P.; Dwivedi, A.R.; Singh, A.K.; Kumar, P.; Khalilullah, H.; Jaremko, M.; Emwas, A.H.; Patel, D.K. Phytoconstituents as modulators of NF-κB signalling: Investigating therapeutic potential for diabetic wound healing. Biomed. Pharmacother. 2024, 177, 117058. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Brochhausen, C.; Schmitt, V.H.; Mamilos, A.; Schmitt, C.; Planck, C.N.; Rajab, T.K.; Hierlemann, H.; Kirkpatrick, C.J. Expression of CD68 positive macrophages in the use of different barrier materials to prevent peritoneal adhesions-an animal study. J. Mater. Sci. Mater. Med. 2017, 28, 15. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Merecz-Sadowska, A.; Ghorbanpour, M.; Szemraj, J.; Piekarski, J.; Bijak, M.; Śliwiński, T.; Zajdel, R.; Sitarek, P. Enhanced natural strength: Lamiaceae essential oils and nanotechnology in in vitro and in vivo medical research. Int. J. Mol. Sci. 2023, 24, 15279. [Google Scholar] [CrossRef]
- Jimenez, M.T.; Frenis, K.; Hahad, O.; Steven, S.; Cohen, G.; Cuadrado, A.; Muenzel, T.; Daiber, A. Protective actions of nuclear factor erythroid 2-related factor 2 (NRF2) and downstream pathways against environmental stressors. Free Radic. Biol. Med. 2022, 187, 72–91. [Google Scholar] [CrossRef]
- El Kebir, D.; Filep, J.G. Modulation of Neutrophil Apoptosis and the Resolution of Inflammation through β2 Integrins. Front. Immunol. 2013, 4, 60. [Google Scholar] [CrossRef]
- Helmy, N.A.; Abdel Aziz, E.A.; Raouf, M.A.; Korany, R.M.; Mansour, D.A.; Baraka, S.M.; Hassan, A.A.; Gomaa, E.; Faisal, M.M.; Basha, W.A.; et al. Revealing the impact of tadalafil-loaded proniosomal gel against dexamethasone-delayed wound healing via modulating oxido-inflammatory response and TGF-β/Macrophage activation pathway in rabbit model. PLoS ONE 2025, 20, e0315673. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta BBA Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Marques, M.P.; Mendonça, L.; Neves, B.G.; Varela, C.; Oliveira, P.; Cabral, C. Exploring Iberian Peninsula Lamiaceae as potential therapeutic approaches in wound healing. Pharmaceuticals 2023, 16, 347. [Google Scholar] [CrossRef]
- Merecz-Sadowska, A.; Sitarek, P.; Zajdel, K.; Kucharska, E.; Kowalczyk, T.; Zajdel, R. The modulatory influence of plant-derived compounds on human keratinocyte function. Int. J. Mol. Sci. 2021, 22, 12488. [Google Scholar] [CrossRef]
- Fana, S.E.; Ahmadpour, F.; Rasouli, H.R.; Tehrani, S.S.; Maniati, M. The effects of natural compounds on wound healing in Iranian traditional medicine: A comprehensive review. Complement. Ther. Clin. Pract. 2021, 42, 101275. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Ammar, N.M.; Hassan, H.A.; El-Kashak, W.A.; Al-Rejaie, S.S.; Abd-ElGawad, A.M.; Farrag, A.R. Topical wound healing activity of myricetin isolated from Tecomaria capensis v. aurea. Molecules 2020, 25, 4870. [Google Scholar] [CrossRef]
- Chandak, K.K.; Wasule, D.D. Wound healing potential of Launaea pinnati ida Cass leaf juice in rats. Int. J. Res. Pharm. Sci. 2021, 12, 2173–2177. [Google Scholar] [CrossRef]
- Pereira Beserra, F.; Sergio Gushiken, L.F.; Vieira, A.J.; Augusto Bérgamo, D.; Luísa Bérgamo, P.; Oliveira de Souza, M.; Alberto Hussni, C.; Kiomi Takahira, R.; Henrique Nóbrega, R.; Monteiro Martinez, E.R.; et al. From inflammation to cutaneous repair: Topical application of lupeol improves skin wound healing in rats by modulating the cytokine levels, NF-κB, Ki-67, growth factor expression, and distribution of collagen fibers. Int. J. Mol. Sci. 2020, 21, 4952. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Duan, J.; Chen, T.; Huang, X.; Shang, E.; Yu, L.; Wei, K.; Zhu, Y.; Guo, J.; Guo, S.; et al. Frankincense and myrrh suppress inflammation via regulation of the metabolic profiling and the MAPK signaling pathway. Sci. Rep. 2015, 5, 13668. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Borner, F.; Werner, M.; Ertelt, J.; Meins, J.; Abdel-Tawab, M.; Werz, O. Analysis of boswellic acid contents and related pharmacological activities of frankincense-based remedies that modulate inflammation. Pharmaceuticals 2021, 14, 660. [Google Scholar] [CrossRef]
- Al-Salih, M.A.; Al-Jameel, W.H. Inflammatory mediators and inflammatory cells as reliable molecular targets for assessment of wound age and vitality in rats. Iraqi J. Vet. Sci. 2023, 37, 405–411. [Google Scholar] [CrossRef]
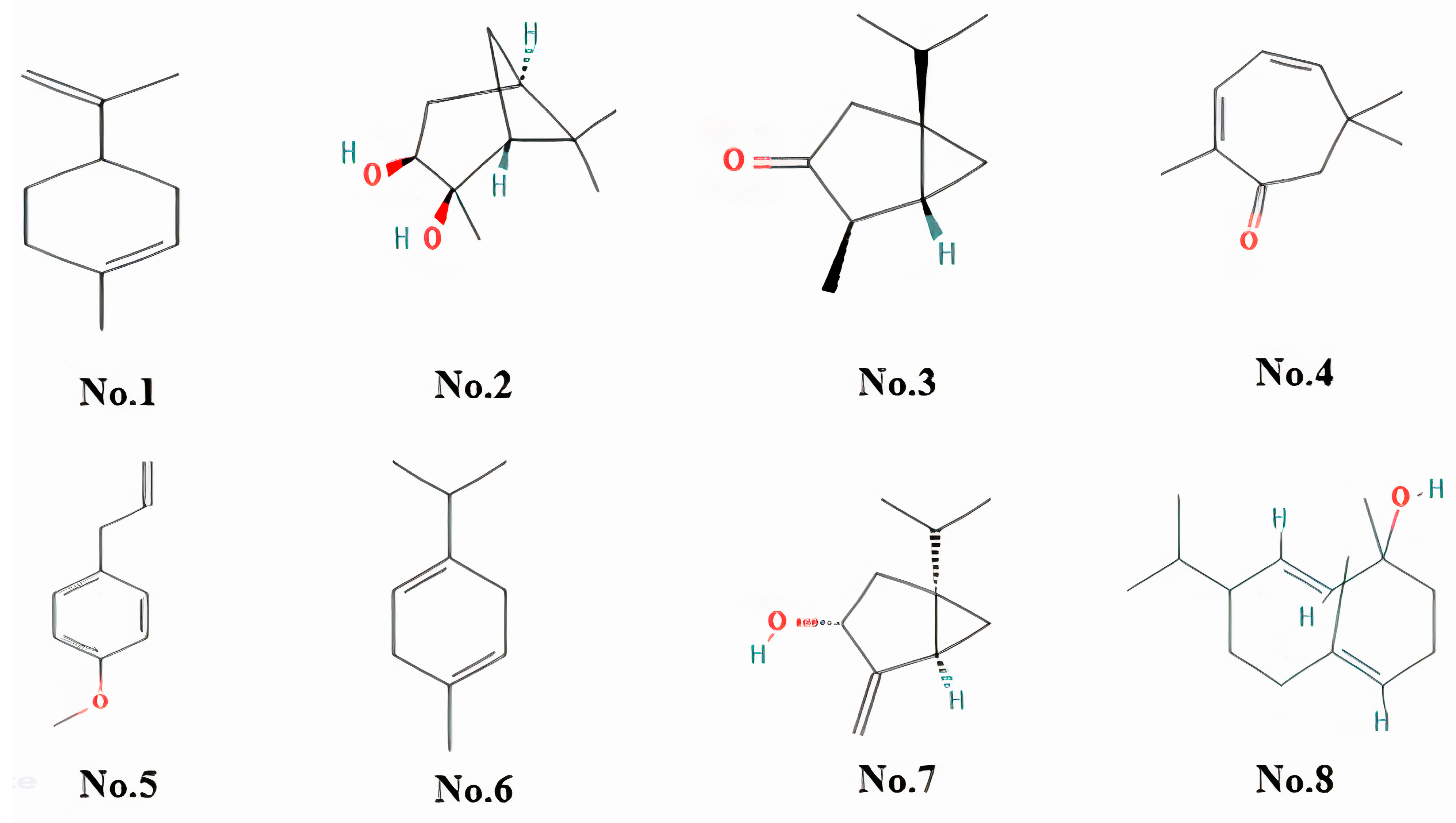



| Retention Time (min) | Compound Name | Peak Area (%) | Molecular Formula | Chemical Class |
|---|---|---|---|---|
| 5.578 | 1,3-cyclohexadiene, 2-methyl-5-(1-methylethyl)- | 10.52 | C10H16 | Alpha-phellandrene |
| 7.347 | Cyclohexene, 1-methyl-4-(1-methylethenyl)- | 7.31 | C10H16 | Limonene/Monoterpene |
| 12.984 | (1s,2s,3r,5s)-(+)-pinanediol | 3.41 | C10H18O2 | Serine Protease |
| 9.054 | Thujone | 3.25 | C10H16O | Alpha-Thujone |
| 12.863 | 2,4-cycloheptadien-1-one, 2,6,6-trimethyl- | 2.91 | C10H14O | Eucarvone/Monoterpenoid |
| 10.645 | Estragole | 2.82 | C10H12O | Estragole/Phenylpropanoid |
| 6.96 | 1,4-cyclohexadiene, 1-methyl-4-(1-methylethyl)- | 2.72 | C10H16 | Gamma-Terpinene |
| 10.129 | Bicyclo[3.1.0]hexan-3-ol, 4-methylene-1-(1-methylethyl)-, (1.alpha.,3.alpha.,5.alpha.)- | 2.39 | C10H16O | (-)-Cis-Sabinol |
| 13.102 | Sobrerol 8-acetate | 1.89 | C12H20O3 | Monoterpenoids |
| 14.079 | (2e,4s,7e)-4-isopropyl-1,7-dimethylcyclodeca-2,7-dienol | 1.89 | C15H26O | Germacrene D |
| 5.643 | 1,4-cyclohexadiene, 1-methyl-4-(1-methylethyl)- | 1.85 | C10H16 | Gamma-Terpinene/Cyclohexadiene |
| 10.956 | 3-buten-2-one, 4-(2,6,6-trimethyl-2-cyclohexen-1-yl)- | 1.74 | C13H20O | Alpha-Ionone/Methyl Ketone |
| 9.143 | Trans-verbenol | 1.7 | C10H16O | Trans-Verbenol |
| 14.277 | Isocaucalol | 1.68 | C15H26O3 | Isocaucalol |
| 10.256 | 3-cyclohexen-1-ol, 4-methyl-1-(1-methylethyl)- | 1.5 | C10H18O | Terpinen-4-Ol |
| 12.622 | Phenol, 2-methyl-5-(1-methylethyl)- | 1.5 | C10H14O | Phenol/Carvacrol |
| 7.712 | 5-isopropyl-2-methylbicyclo[3.1.0]hex-3-en-2-ol | 1.2 | C10H16O | Monoterpenoid |
| 11.584 | (1s,2s,3r,5s)-(+)-pinanediol | 1.13 | C10H18O2 | 2,3-Pinanediol |
| 13.9 | 2,7-octadiene-1,6-diol, 2,6-dimethyl- | 1.01 | C10H18O2 | 8-Hydroxylinalool |
| Group | % Wound Closure (Days) | ||||
|---|---|---|---|---|---|
| Day 4 | Day 8 | Day 12 | Day 16 | Day 20 | |
| FEO | 42.02 ± 2.112 ** | 57.21 ± 1.873 ***# | 86.00 ± 1.689 ***# | 98.59 ± 0.2709 *** | 100 ± 0.002 *** |
| Reference | 20.05 ± 3.129 ***# | 44.32 ± 2.192 *** | 87.90 ± 1.620 *** | 95.13 ± 0.7579 *# | 99.12 ± 0.192 **# |
| Control | 26.69 ± 2.334 | 47.98 ± 2.135 *a | 85.79 ± 0.9587 | 91.36 ± 1.203 *a | 98.29 ± 0.226 **a |
| Parameters | Body Weight | Feed Intake |
|---|---|---|
| FEO | 224.5 ± 3.274 *** | 12.46 ± 0.568 *** |
| Reference | 204.7 ± 1.856 ** | 10.79 ± 0.4017 * |
| Control | 202.2 ± 4.110 | 9.138 ± 0.2590 *# |
| Parameters | CD68 (ng/mL) | TNF-α (pg/mg) | IL-1β (pg/mg) |
|---|---|---|---|
| FEO | 23.33 ± 1.054 *** | 358.3 ± 20.07 *** | 666.7 ± 44.10 ** |
| Reference | 26.83 ± 0.6009 **# | 491.7 ± 37.45 * | 858.3 ± 41.67 * |
| Control | 31.83 ± 1.014 *# | 650 ± 42.82 *# | 983.3 ± 60.09 |
| Histopathological Changes | Average Score | ||
|---|---|---|---|
| FEO | Reference | Control | |
| Inflammatory response | 1 | 1 | 1 |
| Granulation tissue formation | 2 | 1 | 1 |
| Re-epithelization | 1 | 2 | 1 |
| Angiogenesis | 1 | 2 | 3 |
| Collagen deposition | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatesan, K.; Sivadasan, D.; Abderrahmen Al Weslati, M.; Gayasuddin Mouid, M.; Goyal, M.; Bansal, M.; Salama, M.E.-D.M.; Azizullah Ghori, S.; Ahmad, F. Protective Effects of Frankincense Oil on Wound Healing: Downregulating Caspase-3 Expression to Facilitate the Transition from the Inflammatory to Proliferative Phase. Pharmaceuticals 2025, 18, 407. https://doi.org/10.3390/ph18030407
Venkatesan K, Sivadasan D, Abderrahmen Al Weslati M, Gayasuddin Mouid M, Goyal M, Bansal M, Salama ME-DM, Azizullah Ghori S, Ahmad F. Protective Effects of Frankincense Oil on Wound Healing: Downregulating Caspase-3 Expression to Facilitate the Transition from the Inflammatory to Proliferative Phase. Pharmaceuticals. 2025; 18(3):407. https://doi.org/10.3390/ph18030407
Chicago/Turabian StyleVenkatesan, Krishnaraju, Durgaramani Sivadasan, Moufida Abderrahmen Al Weslati, Mohammed Gayasuddin Mouid, Manoj Goyal, Monika Bansal, Mohamed EL-Dosoky Mohamed Salama, Syed Azizullah Ghori, and Fazil Ahmad. 2025. "Protective Effects of Frankincense Oil on Wound Healing: Downregulating Caspase-3 Expression to Facilitate the Transition from the Inflammatory to Proliferative Phase" Pharmaceuticals 18, no. 3: 407. https://doi.org/10.3390/ph18030407
APA StyleVenkatesan, K., Sivadasan, D., Abderrahmen Al Weslati, M., Gayasuddin Mouid, M., Goyal, M., Bansal, M., Salama, M. E.-D. M., Azizullah Ghori, S., & Ahmad, F. (2025). Protective Effects of Frankincense Oil on Wound Healing: Downregulating Caspase-3 Expression to Facilitate the Transition from the Inflammatory to Proliferative Phase. Pharmaceuticals, 18(3), 407. https://doi.org/10.3390/ph18030407






