Computational Insights into the Antioxidant Activity of Luteolin: Density Functional Theory Analysis and Docking in Cytochrome P450 17A1
Abstract
1. Introduction
2. Results and Discussion
2.1. Molecular Structure Analysis
2.2. Electronic Structure Analysis
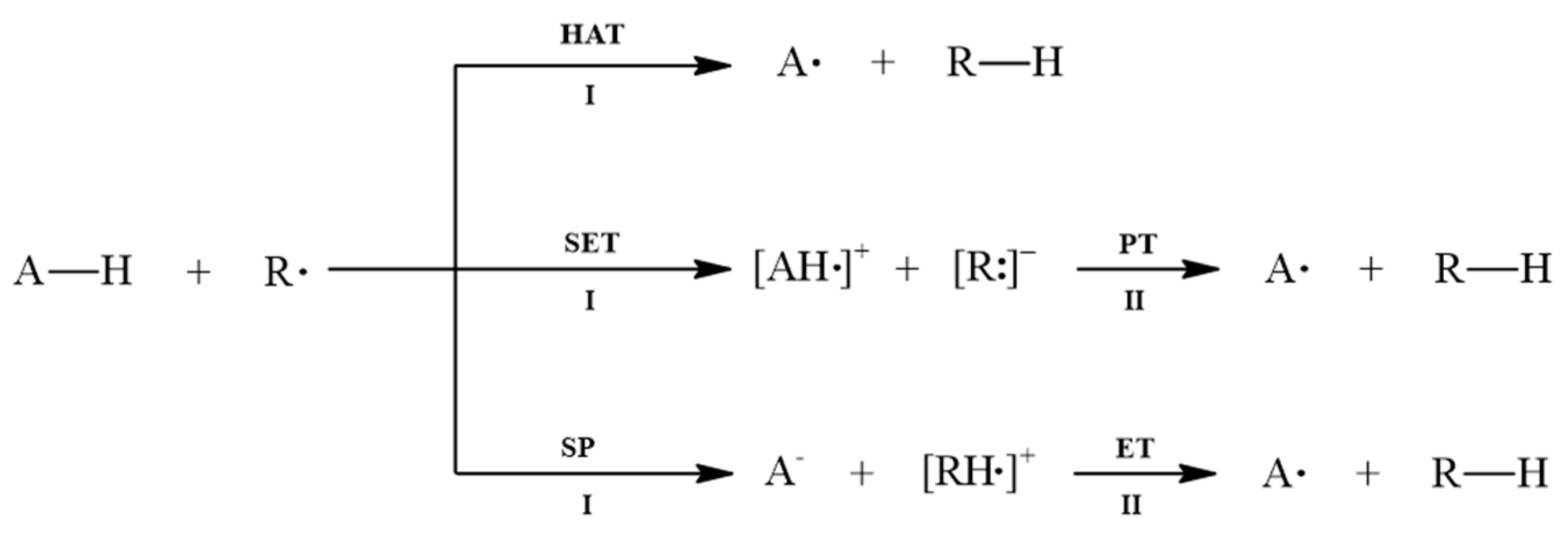
2.3. Antioxidant Potential Analysis
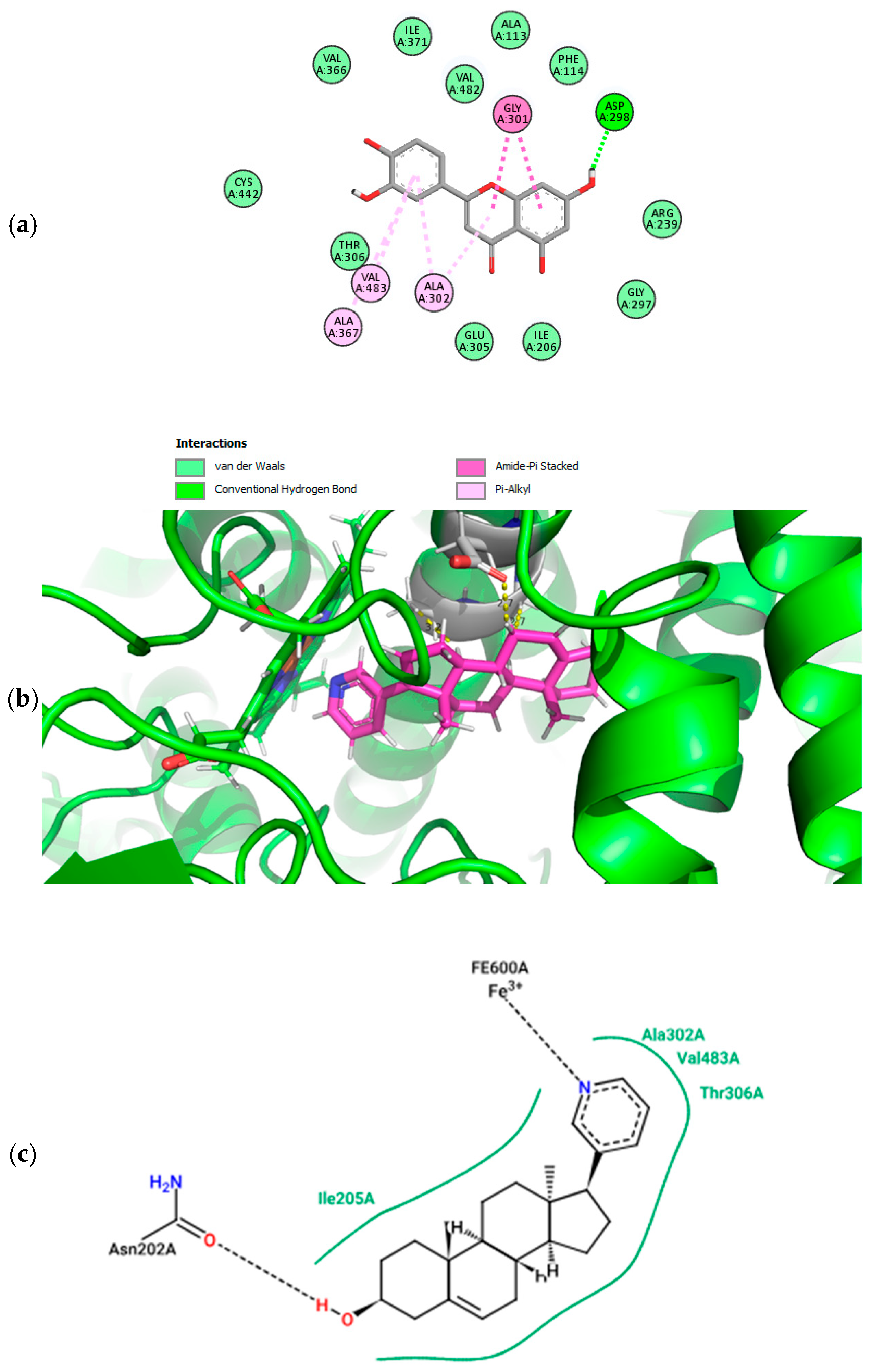
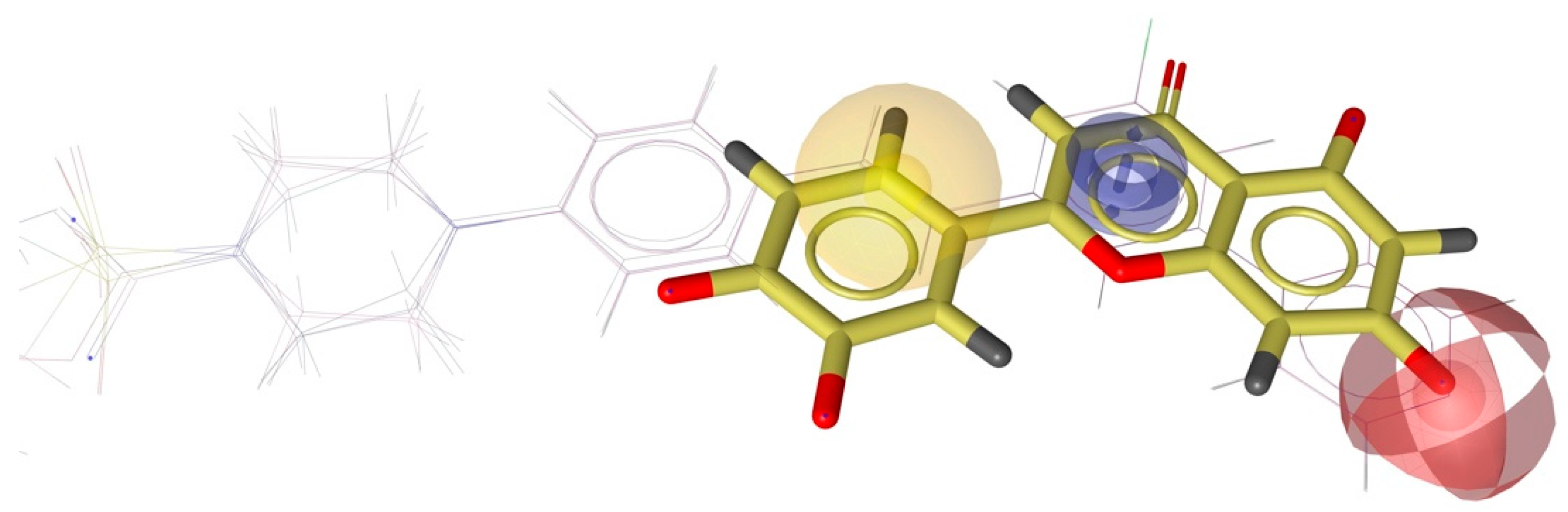
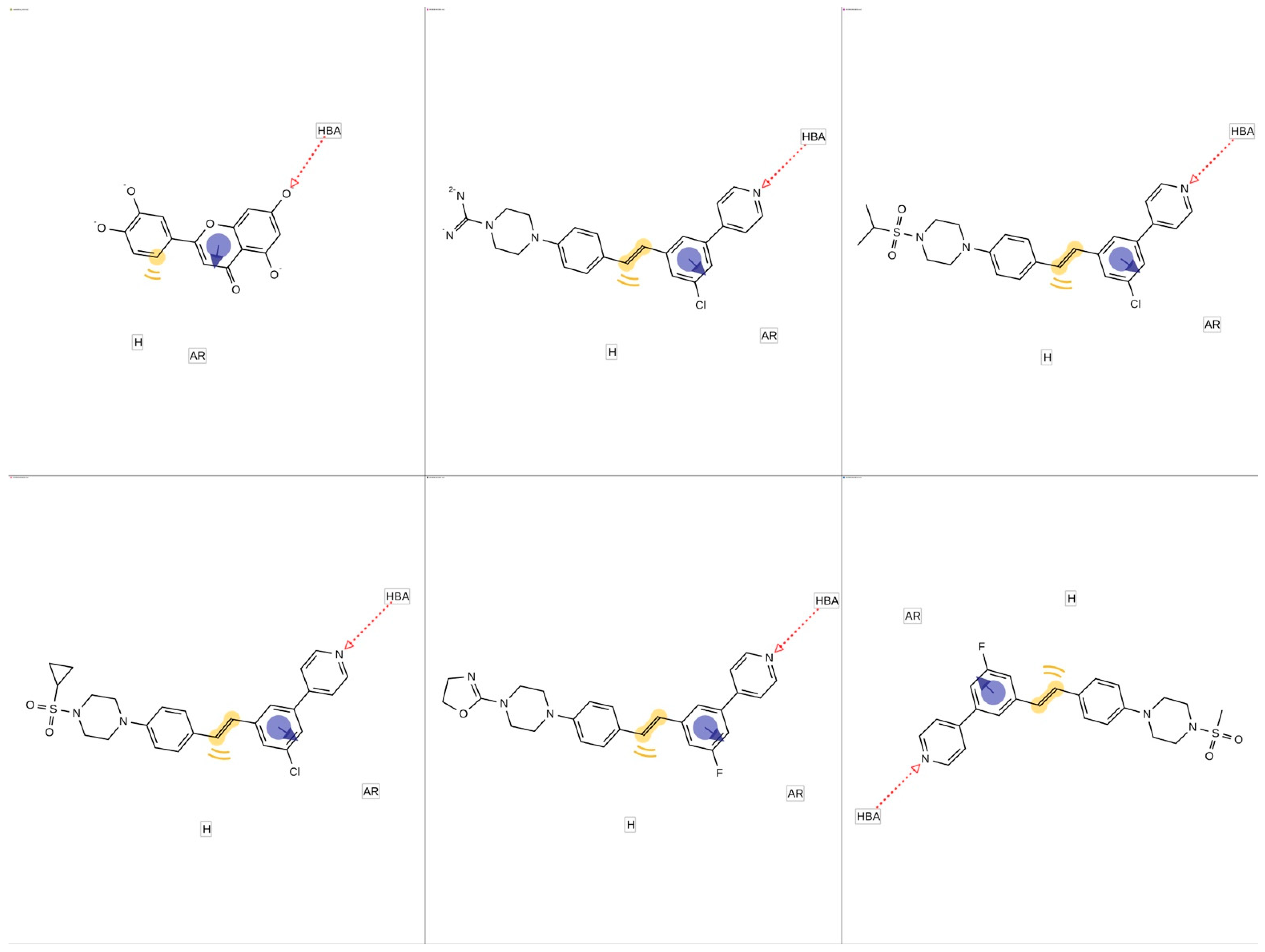
2.4. Molecular Docking and Pharmacophore Modeling
3. Materials and Methods
3.1. Molecular Modeling
3.2. Antioxidant Potential Analysis
3.3. Docking Analysis
3.4. Generation of Shared Feature Pharmacophore Modeling of Ligands
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DFT | Density Functional Theory |
| HOMO | Highest Occupied Molecular Orbital |
| LUMO | Lowest Unoccupied Molecular Orbital |
| MEP | Molecular Electrostatic Potential |
| QTAIMs | Quantum Theory of Atoms in Molecules |
| NBO | Natural Bond Orbital |
References
- Ahmadi, S.M.; Farhoosh, R.; Sharif, A.; Rezaie, M. Structure-Antioxidant Activity Relationships of Luteolin and Catechin. J. Food Sci. 2020, 85, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the Anti-Inflammatory and Antioxidant Activities of Luteolin, Kaempferol, Apigenin and Quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Chen, D.F.; Deng, G.; Guo, R.; Fu, Z.M. The Surrounding Environments on the Structure and Antioxidative Activity of Luteolin. J. Mol. Model. 2018, 24, 149. [Google Scholar] [CrossRef]
- Primikyri, A.; Mazzone, G.; Lekka, C.; Tzakos, A.G.; Russo, N.; Gerothanassis, I.P. Understanding Zinc(II) Chelation with Quercetin and Luteolin: A Combined NMR and Theoretical Study. J. Phys. Chem. B 2015, 119, 83–95. [Google Scholar] [CrossRef]
- Ali, H.M.; Ali, I.H. Structure-Antioxidant Activity Relationships, QSAR, DFT Calculation, and Mechanisms of Flavones and Flavonols. Med. Chem. Res. 2019, 28, 2262–2269. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Sadasivam, K.; Kumaresan, R. Antioxidant Behavior of Mearnsetin and Myricetin Flavonoid Compounds—A DFT Study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Sadasivam, K.; Kumaresan, R. A Comparative DFT Study on the Antioxidant Activity of Apigenin and Scutellarein Flavonoid Compounds. Mol. Phys. 2011, 109, 839–852. [Google Scholar] [CrossRef]
- Sadasivam, K.; Kumaresan, R. Theoretical Investigation on the Antioxidant Behavior of Chrysoeriol and Hispidulin Flavonoid Compounds—A DFT Study. Comput. Theor. Chem. 2011, 963, 227–235. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Dehkordi, M.M.; Asgarshamsi, M.H. Density Functional Theory Studies of the Antioxidants—A Review. J. Mol. Model. 2021, 27, 271. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Cytochrome P450 Enzymes as Drug Targets in Human Disease. Drug Metab. Dispos. 2024, 52, 493–497. [Google Scholar] [CrossRef]
- Messaadia, L.; Bekkar, Y.; Benamira, M.; Lahmar, H. Predicting the Antioxidant Activity of Some Flavonoids of Arbutus Plant: A Theoretical Approach. Chem. Phys. Impact 2020, 1, 100007. [Google Scholar] [CrossRef]
- Liu, B.; Li, W.; Zhao, J.; Liu, Y.; Zhu, X.; Liang, G. Physicochemical Characterisation of the Supramolecular Structure of Luteolin/Cyclodextrin Inclusion Complex. Food Chem. 2013, 141, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W.; Essén, H. The Characterization of Atomic Interactions. J. Chem. Phys. 1983, 80, 1943–1960. [Google Scholar] [CrossRef]
- Nakanishi, W.; Hayashi, S.; Narahara, K. Atoms-in-Molecules Dual Parameter Analysis of Weak to Strong Interactions: Behaviors of Electronic Energy Densities versus Laplacian of Electron Densities at Bond Critical Points. J. Phys. Chem. A 2008, 112, 13593–13599. [Google Scholar] [CrossRef]
- Nakanishi, W.; Hayashi, S.; Narahara, K. Polar Coordinate Representation of Hb(Rc) versus (ℏ2/8m)∇2ρb(Rc) at BCP in AIM Analysis: Classification and Evaluation of Weak to Strong Interactions. J. Phys. Chem. A 2009, 113, 10050–10057. [Google Scholar] [CrossRef]
- Emamian, S.; Lu, T.; Kruse, H.; Emamian, H. Exploring Nature and Predicting Strength of Hydrogen Bonds: A Correlation Analysis Between Atoms-in-Molecules Descriptors, Binding Energies, and Energy Components of Symmetry-Adapted Perturbation Theory. J. Comput. Chem. 2019, 40, 2868–2881. [Google Scholar] [CrossRef]
- van Acker, S.A.B.E.; de Groot, M.J.; van den Berg, D.-J.; Tromp, M.N.J.L.; den Kelder, G.D.-O.; van der Vijgh, W.J.F.; Bast, A. A Quantum Chemical Explanation of the Antioxidant Activity of Flavonoids. Chem. Res. Toxicol. 1996, 9, 1305–1312. [Google Scholar] [CrossRef]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the Hydrogen Bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar] [CrossRef]
- Malik, P.K.; Tripathy, M.; Patel, S. D-π-A Molecular Probe to Unveil the Role of Solute-Solvent Hydrogen Bonding in Solvatochromism, Location Specific Preferential Solvation and Synergistic Effect in Binary Mixtures. ChemistrySelect 2020, 5, 3551–3566. [Google Scholar] [CrossRef]
- Instrumentation for Fluorescence Spectroscopy. In Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006; pp. 27–61.
- Jeanmairet, G.; Levesque, M.; Borgis, D. Tackling Solvent Effects by Coupling Electronic and Molecular Density Functional Theory. J. Chem. Theory Comput. 2020, 16, 7123–7134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, T. Efficient Evaluation of Electrostatic Potential with Computerized Optimized Code. Phys. Chem. Chem. Phys. 2021, 23, 20323–20328. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Zhou, Y.; Liang, Q.; Chen, D.-F.; Guo, R. A Theoretical Study on the Hydrogen-Bonding Interactions between Flavonoids and Ethanol/Water. J. Mol. Model. 2016, 22, 95. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Zhou, Y.; Liang, Q.; Chen, D.-F.; Guo, R. Theoretical Studies on the Hydrogen-Bonding Interactions between Luteolin and Water: A DFT Approach. J. Mol. Model. 2016, 22, 257. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-Z.; Zhou, Y.; Liang, Q.; Chen, D.-F.; Guo, R.; Lai, R.-C. Hydrogen-Bonding Interactions between Apigenin and Ethanol/Water: A Theoretical Study. Sci. Rep. 2016, 6, 34647. [Google Scholar] [CrossRef]
- Leopoldini, M.; Pitarch, I.P.; Russo, N.; Toscano, M. Structure, Conformation, and Electronic Properties of Apigenin, Luteolin, and Taxifolin Antioxidants. A First Principle Theoretical Study. J. Phys. Chem. A 2004, 108, 92–96. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Modulation of the Antioxidant Activity of Phenols by Non-Covalent Interactions. Org. Biomol. Chem. 2012, 10, 4147. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, N.; Wen, S.; Wang, X.; Huang, X.; Xia, A. The Mechanism Study of Enhanced Antioxidant Capacity: Intermolecular Hydrogen Bonds between Epigallocatechin Gallate and Theanine in Tea. LWT 2023, 189, 115523. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Zhou, Y.; Liang, Q.; Chen, D.-F.; Guo, R.; Xiong, C.-L.; Xu, X.-J.; Zhang, Z.-N.; Huang, Z.-J. Solvent Effects on the Intramolecular Hydrogen-Bond and Anti-Oxidative Properties of Apigenin: A DFT Approach. Dye. Pigment. 2017, 141, 179–187. [Google Scholar] [CrossRef]
- Sakurai, M.A.; Ozaki, Y.; Okuzaki, D.; Naito, Y.; Sasakura, T.; Okamoto, A.; Tabara, H.; Inoue, T.; Hagiyama, M.; Ito, A.; et al. Gefitinib and Luteolin Cause Growth Arrest of Human Prostate Cancer PC-3 Cells via Inhibition of Cyclin G-Associated Kinase and Induction of MiR-630. PLoS ONE 2014, 9, e100124. [Google Scholar] [CrossRef] [PubMed]
- Devore, N.M.; Scott, E.E. Structures of Cytochrome P450 17A1 with Prostate Cancer Drugs Abiraterone and TOK-001. Nature 2012, 482, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Han, J.; Kuang, H.; Sun, Y.; Wang, S.; Yang, B.; Wang, Q. Luteolin: A Promising Multifunctional Natural Flavonoid for Human Diseases. Phytother. Res. 2024, 38, 3417–3443. [Google Scholar] [CrossRef]
- Halder, S.; Brahmachari, G.; Jana, K. Molecular Signaling of Prostate Cancer Chemoprevention by Natural Products: Current Perspectives and Future Directions. In Discovery and Development of Anti-Prostate Cancerous Agents from Natural Products; Elsevier: Amsterdam, The Netherlands, 2025; pp. 9–35. [Google Scholar] [CrossRef]
- Snaterse, G.; Taylor, A.E.; Moll, J.M.; O’Neil, D.M.; Teubel, W.J.; van Weerden, W.M.; Arlt, W.; Hofland, J. Prostate Cancer Androgen Biosynthesis Relies Solely on CYP17A1 Downstream Metabolites. J. Steroid Biochem. Mol. Biol. 2024, 236, 106446. [Google Scholar] [CrossRef]
- Wolber, G.; Langer, T. LigandScout: 3-D Pharmacophores Derived from Protein-Bound Ligands and Their Use as Virtual Screening Filters. J. Chem. Inf. Model. 2005, 45, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Foresman, J.B.; Frisch, A.E. Exploring Chemistry with Electronic Structure Methods, 3rd ed.; Gaussian, Inc.: Wallingford, CT, USA, 2015. [Google Scholar]
- Dennington, R.; Keith, T.A.; Millam, J.M. Millam, GaussView, Version 6.1; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Function. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef]
- Srivastava, R.; Al-Omary, F.A.M.; El-Emam, A.A.; Pathak, S.K.; Karabacak, M.; Narayan, V.; Chand, S.; Prasad, O.; Sinha, L. A Combined Experimental and Theoretical DFT (B3LYP, CAM-B3LYP and M06-2X) Study on Electronic Structure, Hydrogen Bonding, Solvent Effects and Spectral Features of Methyl 1H-Indol-5-Carboxylate. J. Mol. Struct. 2017, 1137, 725–741. [Google Scholar] [CrossRef]
- Zhang, G.; Musgrave, C.B. Comparison of DFT Methods for Molecular Orbital Eigenvalue Calculations. J. Phys. Chem. A 2007, 111, 1554–1561. [Google Scholar] [CrossRef]
- Pearson, R.G. Chemical Hardness and Density Functional Theory. J. Chem. Sci. 2005, 117, 369–377. [Google Scholar] [CrossRef]
- Pearson, R.G. The Electronic Chemical Potential and Chemical Hardness. J. Mol. Struct. 1992, 255, 261–270. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpály, L.V.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Weinstein, H.; Osman, R.; Green, J.P.; Topiol, S. Electrostatic Potentials as Descriptors of Molecular Reactivity: The Basis for Some Successful Predictions of Biological Activity. In Chemical Applications of Atomic and Molecular Electrostatic Potentials; Springer: Boston, MA, USA, 1981; pp. 309–334. [Google Scholar]
- Sjoberg, P.; Politzer, P. Use of the Electrostatic Potential at the Molecular Surface to Interpret and Predict Nucleophilic Processes. J. Phys. Chem. 1990, 94, 3959–3961. [Google Scholar] [CrossRef]
- Chóez-Guaranda, I.; Viteri-Espinoza, R.; Barragán-Lucas, A.; Quijano-Avilés, M.; Manzano, P. Effect of Solvent-Solvent Partition on Antioxidant Activity and GC-MS Profile of Ilex Guayusa Loes. Leaves Extract and Fractions. Nat. Prod. Res. 2022, 36, 1570–1574. [Google Scholar] [CrossRef]
- Weisz, G.M.; Kammerer, D.R.; Carle, R. Identification and Quantification of Phenolic Compounds from Sunflower (Helianthus annuus L.) Kernels and Shells by HPLC-DAD/ESI-MSn. Food Chem. 2009, 115, 758–765. [Google Scholar] [CrossRef]
- Iwatsuki, M.; Tsuchiya, J.; Komuro, E.; Yamamoto, Y.; Niki, E. Effects of Solvents and Media on the Antioxidant Activity of α-Tocopherol. Biochim. Biophys. Acta-Gen. Subj. 1994, 1200, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. Atoms in Molecules. Acc. Chem. Res. 1985, 18, 9–15. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules—A Quantum Theory; Clarendon Press Publication: Oxford, UK, 1994; ISBN 9780198558651. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, F.; Landis, C.R. Discovering Chemistry with Natural Bond Orbitals, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; ISBN 9781118119969. [Google Scholar]
- Weinhold, F.; Landis, C.R. Natural bond orbitals and extensions of localized bonding concepts. Chem. Educ. Res. Pract. 2001, 2, 91–104. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of Antioxidant Activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; Shalaby, E., Ed.; IntechOpen Book Series; IntechOpen: London, UK, 2019; Volume 5, pp. 1–29. [Google Scholar]
- Đorović, J.; Milenković, D.; Joksović, L.; Joksović, M.; Marković, Z. Study of Influence of Free Radical Species on Antioxidant Activity of Selected 1,2,4-Triazole-3-thiones. ChemistrySelect 2019, 4, 7476–7485. [Google Scholar] [CrossRef]
- Markaverich, B.M.; Vijjeswarapu, M.; Shoulars, K.; Rodriguez, M. Luteolin and Gefitinib Regulation of EGF Signaling Pathway and Cell Cycle Pathway Genes in PC-3 Human Prostate Cancer Cells. J. Steroid Biochem. Mol. Biol. 2010, 122, 219–231. [Google Scholar] [CrossRef]
- Langer, T.; Wolber, G. Pharmacophore Definition and 3D Searches. Drug Discov. Today Technol. 2004, 1, 203–207. [Google Scholar] [CrossRef]
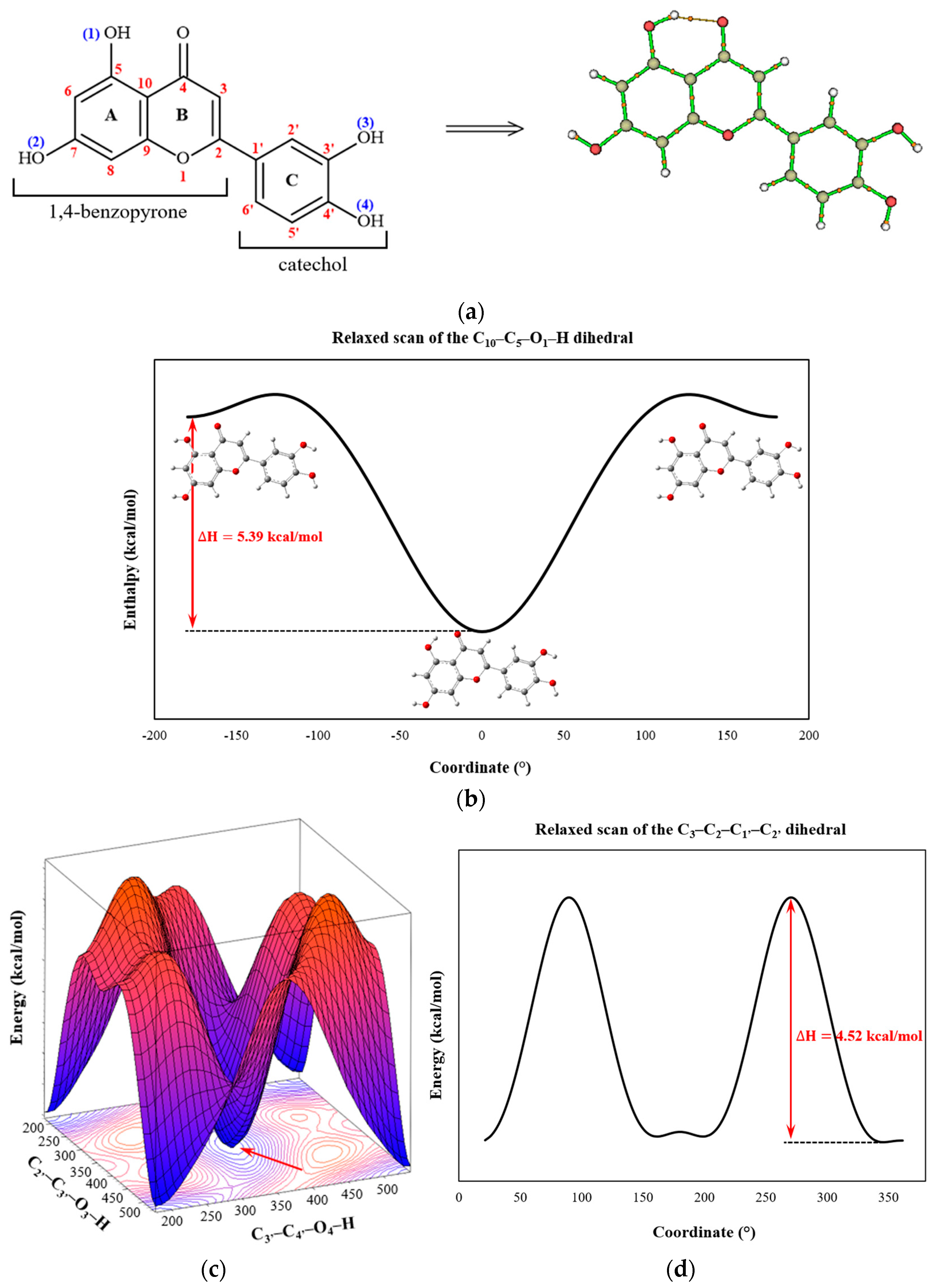
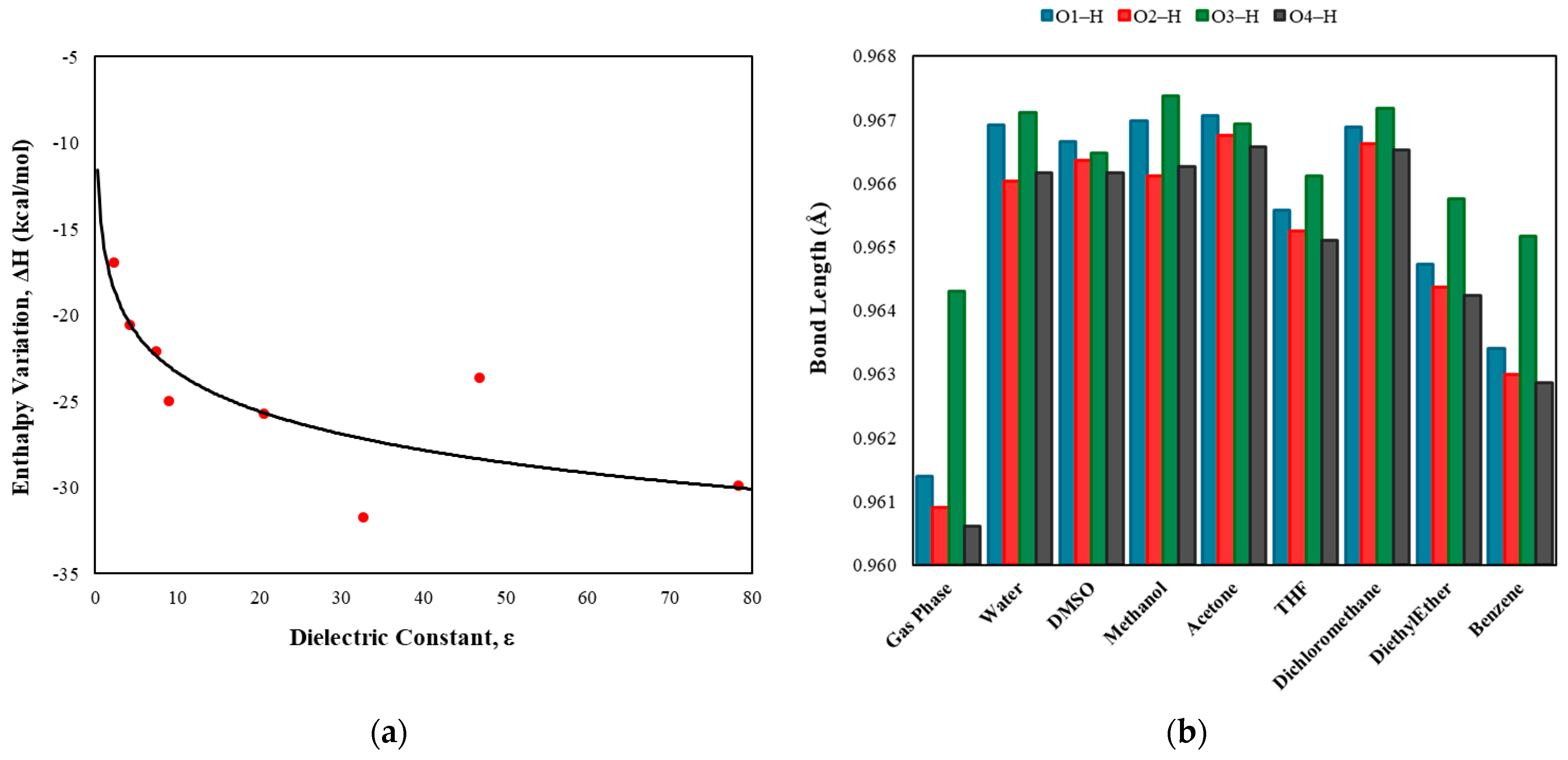
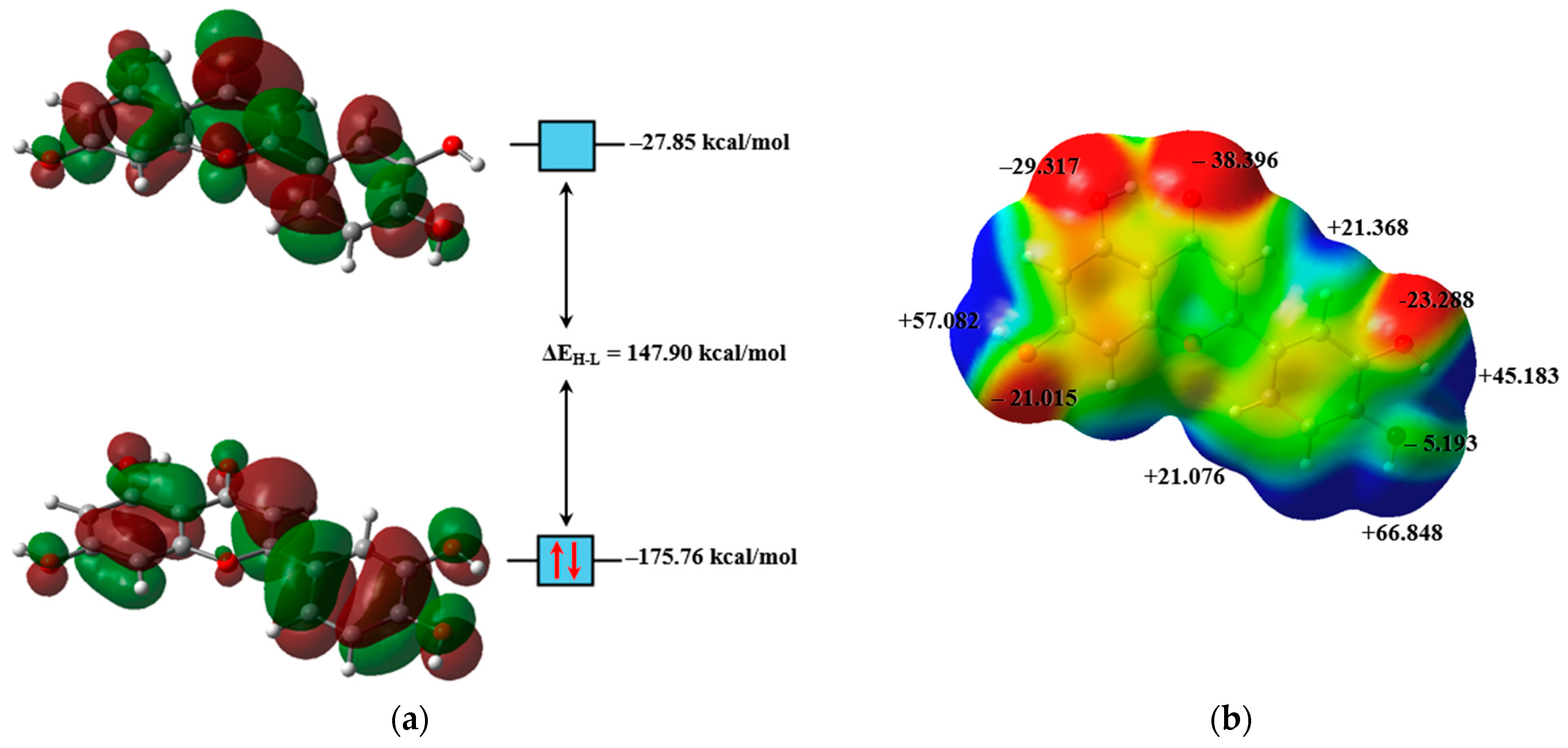
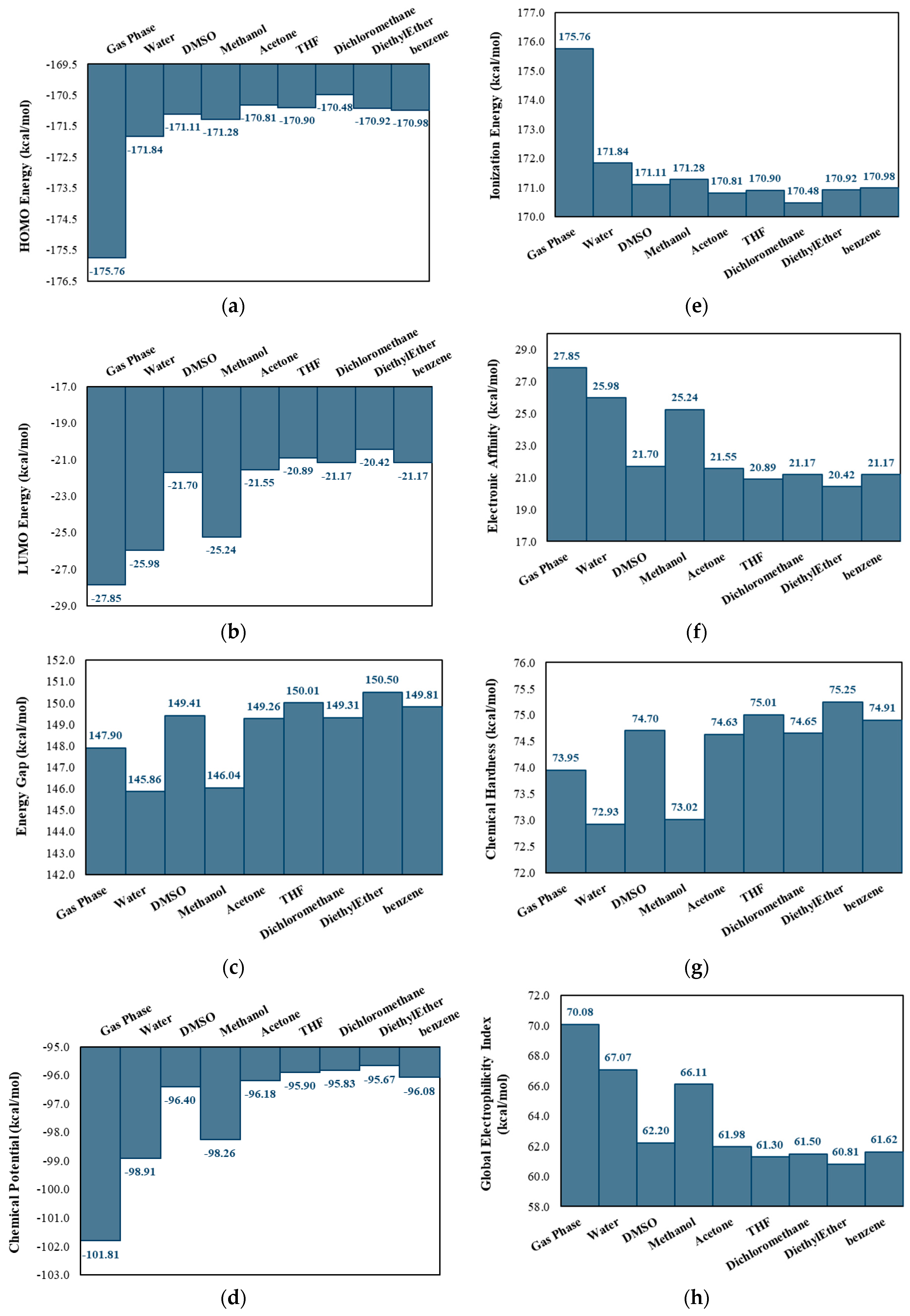

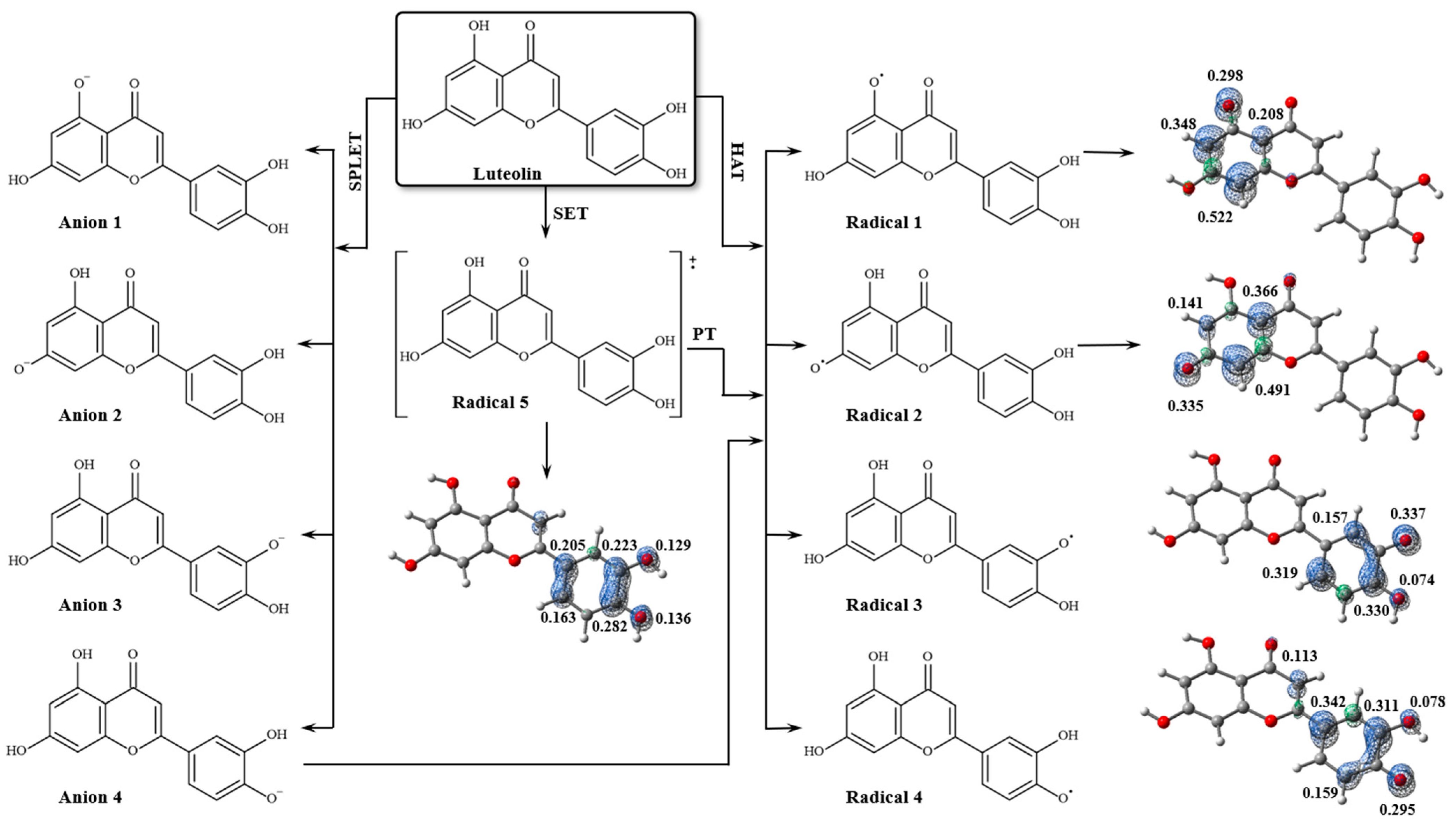
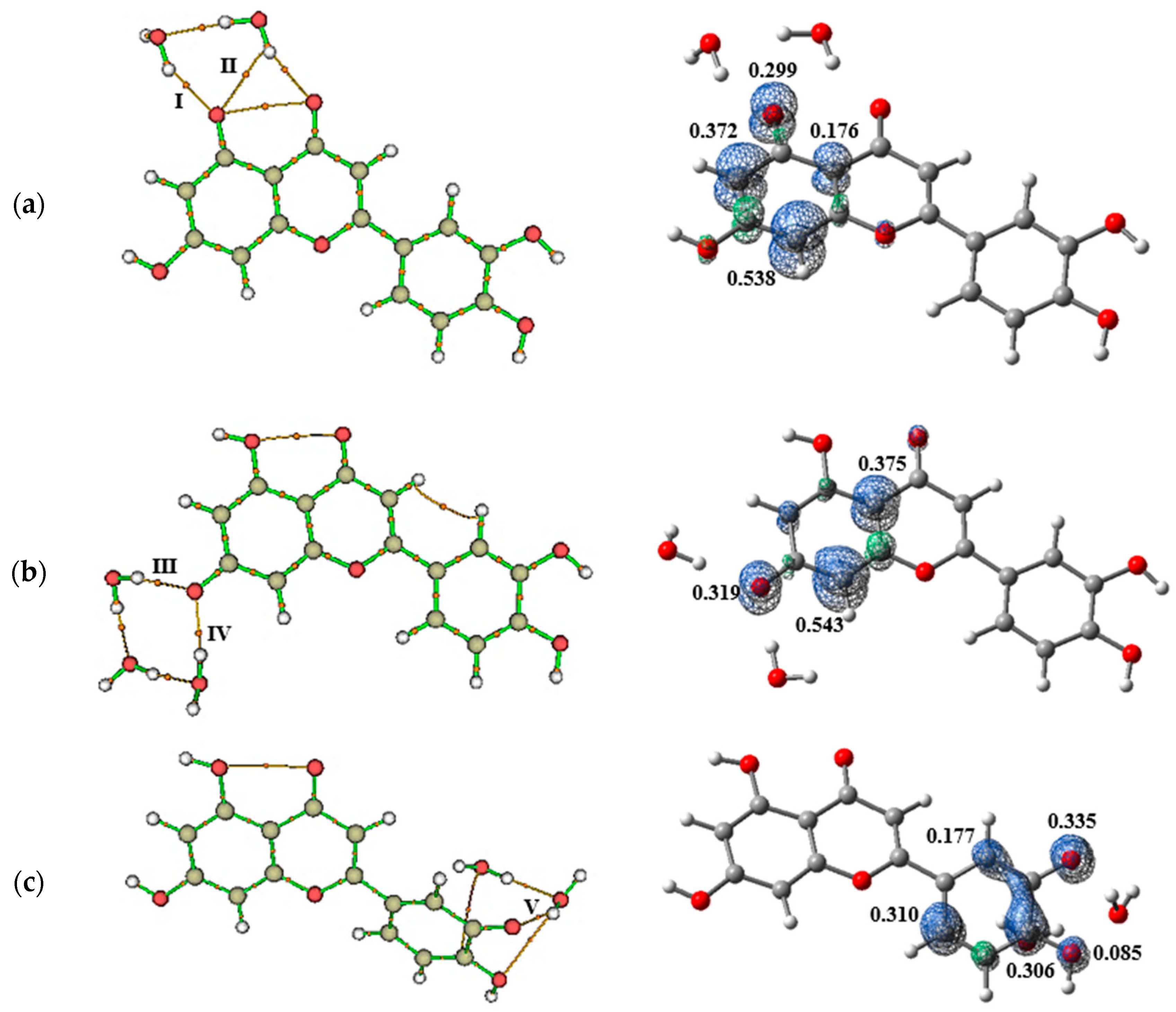

| BCP | Interaction | Length (Å) | Angle (°) | (a.u.) | (a.u.) | (a.u.) | (a.u.) | (a.u.) | (kcal/mol) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Luteolin + Water | ||||||||||
| I | H–Ow | 1.92185 | 176.9067 | 0.0222 | 0.0952 | 0.0208 | −0.0177 | 0.0030 | 0.9 | −8.43 |
| II | Ow | 1.63838 | 171.2233 | 0.0308 | 0.1254 | 0.0296 | −0.0279 | 0.0017 | 0.9 | −11.29 |
| III | H–Ow | 1.88308 | 165.6301 | 0.0230 | 0.0977 | 0.0215 | −0.0186 | 0.0029 | 0.9 | −8.71 |
| VI | Ow | 1.76759 | 163.1268 | 0.0389 | 0.1314 | 0.0346 | −0.0363 | −0.0017 | 1.0 | −14.00 |
| V | H–Ow | 2.15803 | 129.3470 | 0.0207 | 0.0852 | 0.0188 | −0.0162 | 0.0026 | 0.9 | −7.95 |
| VI | Ow | 1.68388 | 170.2508 | 0.0308 | 0.1210 | 0.0289 | −0.0275 | 0.0014 | 1.0 | −11.31 |
| VII | H–Ow | 1.97618 | 156.8234 | 0.0227 | 0.0969 | 0.0216 | −0.0190 | 0.0026 | 0.9 | −8.61 |
| Luteolin + DMSO | ||||||||||
| I | H–Od | 1.81451 | 140.9443 | 0.0313 | 0.1232 | 0.0298 | −0.0287 | 0.0011 | 1.0 | −11.45 |
| II | H–Od | 1.67271 | 161.178 | 0.0478 | 0.1499 | 0.0429 | −0.0484 | −0.0055 | 1.1 | −16.94 |
| III | H–Od | 1.74762 | 167.7532 | 0.0385 | 0.1403 | 0.0363 | −0.0375 | −0.0012 | 1.0 | −13.85 |
| IV | H–Od | 1.75890 | 155.9998 | 0.0396 | 0.1374 | 0.0363 | −0.0383 | −0.0020 | 1.1 | −14.22 |
| Luteolin + Methanol | ||||||||||
| I | H–Om | 2.02554 | 143.9005 | 0.0206 | 0.0838 | 0.0187 | −0.0164 | 0.0023 | 0.9 | −7.92 |
| II | Om | 1.76984 | 160.7465 | 0.0352 | 0.1358 | 0.0336 | −0.0333 | 0.0003 | 1.0 | −12.76 |
| III | H–Om | 1.98083 | 149.8292 | 0.0228 | 0.0920 | 0.0208 | −0.0186 | 0.0022 | 0.9 | −8.63 |
| IV | Om | 1.74293 | 150.5949 | 0.0393 | 0.1349 | 0.0355 | −0.0372 | −0.0018 | 1.0 | −14.13 |
| V | H–Om | 1.99651 | 142.3781 | 0.0210 | 0.0868 | 0.0191 | −0.0166 | 0.0026 | 0.9 | −8.03 |
| VI | Om | 1.77792 | 163.0294 | 0.0345 | 0.1335 | 0.0329 | −0.0324 | 0.0005 | 1.0 | −12.54 |
| VII | H–Om | 2.01683 | 133.3419 | 0.0221 | 0.0904 | 0.0204 | −0.0183 | 0.0021 | 0.9 | −8.42 |
| Radical + Water | ||||||||||
| I | O1...H–Ow | 1.89589 | 150.992 | 0.0249 | 0.1082 | 0.0241 | −0.0211 | 0.0029 | 0.9 | −9.34 |
| II | O1...H–Ow | 2.33842 | 121.682 | 0.0121 | 0.0457 | 0.0102 | −0.0090 | 0.0012 | 0.9 | −5.08 |
| III | O2...H–Ow | 1.77020 | 174.843 | 0.0240 | 0.0970 | 0.0218 | −0.0193 | 0.0025 | 0.9 | −9.04 |
| IV | O2...H–Ow | 1.66756 | 172.960 | 0.0275 | 0.1112 | 0.0255 | −0.0233 | 0.0023 | 0.9 | −10.22 |
| V | O3...H–Ow | 1.96877 | 155.187 | 0.0217 | 0.0871 | 0.0193 | −0.0168 | 0.0025 | 0.9 | −8.28 |
| VI | O4...H–Ow | 2.23303 | 118.390 | 0.0152 | 0.0598 | 0.0133 | −0.0116 | 0.0016 | 0.9 | −6.10 |
| VII | O4...H–Ow | 1.82504 | 177.968 | 0.0312 | 0.1184 | 0.0284 | −0.0272 | 0.0012 | 1.0 | −11.43 |
| Interaction | Hyperconjugation | (kcal/mol) | Donor | Acceptor | ||
|---|---|---|---|---|---|---|
| Occupancy | Hybrid | Occupancy | Hybrid | |||
| Luteolin + Water | ||||||
| H–Ow | (Ow–H) | 5.09 | 1.96714 | O1: sp1.52 | 0.01157 | Ow: sp2.85 H: s |
| H–Ow | (Ow–H) | 5.87 | 1.96837 | O2: sp1.57 | 0.01279 | Ow: sp2.82 H: s |
| Ow | (O2–H) | 13.81 | 1.97236 | Ow: sp1.06 | 0.02861 | O2: sp2.97 H: s |
| H–Ow | (Ow–H) | 2.56 | 1.87545 | O3: p | 0.01088 | Ow: sp2.86 H: s |
| Ow | (O3–H) | 21.0 | 1.95287 | Ow: sp4.56 | 0.04652 | O3: sp2.83 H: s |
| H–Ow | (Ow–H) | 3.46 | 1.97141 | O4: sp1.63 | 0.00902 | Ow: sp2.87 H: s |
| Ow | (O4–H) | 13.46 | 1.97099 | Ow: sp1.69 | 0.02908 | O4: sp3.10 H: s |
| Luteolin + DMSO | ||||||
| Od | (O1–H) | 4.49 | 1.98584 | Od: sp0.30 | 0.04233 | O1: sp2.66 H: s |
| Od | (O2–H) | 20.57 | 1.89740 | Od: p | 0.05544 | O2: sp2.89 H: s |
| Od | (O3–H) | 12.72 | 1.86769 | Od: p | 0.03811 | O3: sp2.86 H: s |
| Od | (O4–H) | 14.74 | 1.91064 | Od: p | 0.04176 | O4: sp2.90 H: s |
| Luteolin + Methanol | ||||||
| H–Om | (Om–H) | 3.59 | 1.96886 | O1: sp1.52 | 0.01371 | Om: sp2.85 H: s |
| H–Om | (Om–H) | 4.43 | 1.96914 | O2: sp2.19 | 0.01613 | Om: sp3.39 H: s |
| Om | (O2–H) | 13.70 | 1.95560 | Om: sp2.84 | 0.03314 | O2: sp2.96 H: s |
| H–Om | (Om–H) | 2.07 | 1.87565 | O3: p | 0.01572 | Om: sp3.35 H: s |
| Om | (O3–H) | 18.63 | 1.93513 | Om: sp6.17 | 0.04631 | O3: sp2.83 H: s |
| H–Om | (Om–H) | 3.22 | 1.97194 | O4: sp1.56 | 0.01320 | Om: sp3.42 H: s |
| Om | (O4–H) | 13.40 | 1.95535 | Om: sp3.15 | 0.03252 | O4: sp2.97 H: s |
| Radical + Water | ||||||
| [O1∙]H–Ow | (O1∙) (Ow–H) | 0.31 | 0.95732 | O1∙: p | 0.00906 | Ow: sp2.72 H: s |
| [O2∙]H–Ow | (O2∙) (Ow–H) | 1.18 | 0.98396 | O2∙: sp0.69 | 0.01036 | Ow: sp2.67 H: s |
| [O2∙]H–Ow | (O2∙) (Ow–H) | 3.35 | 0.95440 | O2∙: p | 0.01071 | Ow: sp2.66 H: s |
| [O3∙]H–Ow | (O3∙) (Ow–H) | 1.64 | 0.88284 | O3∙: p | 0.00857 | Ow: sp2.76 H: s |
| [O4∙]H–Ow | (O4∙) (Ow–H) | 5.12 | 0.95218 | O4∙: p | 0.01359 | Ow: sp2.58 H: s |
| Compound | BDE (kcal/mol) | IP + PDE (kcal/mol) | PA + ETE (kcal/mol) | |
|---|---|---|---|---|
| Luteolin | ||||
| No Water | Water | |||
| O1–H | 88.072 | 88.681 | 91.133 | 91.061 |
| O2–H | 92.153 | 93.544 | 95.213 | 95.142 |
| O3–H | 80.662 | 82.757 | 83.722 | 83.651 |
| O4–H | 83.605 | 82.096 | 86.665 | 86.594 |
| Butylhydroxyanisole (BHA) | ||||
| O1–H | 77.161 | 80.213 | 80.150 | |
| Butylhydroxytoluene (BHT) | ||||
| O1–H | 76.690 | 85.515 | 79.679 | |
| Gallic acid (GA) | ||||
| O1–H | 84.056 | 87.118 | 87.045 | |
| O2–H | 81.193 | 84.255 | 84.182 | |
| Propyl gallate (PG) | ||||
| O1–H | 82.836 | 85.897 | 85.825 | |
| O2–H | 78.963 | 82.025 | 81.952 | |
| Pyrogallol (PY) | ||||
| O1–H | 81.331 | 84.388 | 84.320 | |
| O2–H | 78.040 | 81.098 | 81.029 | |
| Tert-butylhydroquinone (TBHQ) | ||||
| O1–H | 77.459 | 91.776 | 80.447 | |
| O2–H | 78.548 | 92.866 | 81.537 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Aguiar, A.S.N.; de Carvalho, L.B.R.; Gomes, C.M.; Castro, M.M.; Martins, F.S.; Borges, L.L. Computational Insights into the Antioxidant Activity of Luteolin: Density Functional Theory Analysis and Docking in Cytochrome P450 17A1. Pharmaceuticals 2025, 18, 410. https://doi.org/10.3390/ph18030410
de Aguiar ASN, de Carvalho LBR, Gomes CM, Castro MM, Martins FS, Borges LL. Computational Insights into the Antioxidant Activity of Luteolin: Density Functional Theory Analysis and Docking in Cytochrome P450 17A1. Pharmaceuticals. 2025; 18(3):410. https://doi.org/10.3390/ph18030410
Chicago/Turabian Stylede Aguiar, Antônio Sérgio Nakao, Lucas Barbosa Ribeiro de Carvalho, Clayson Moura Gomes, Murillo Moraes Castro, Frederico Severino Martins, and Leonardo Luiz Borges. 2025. "Computational Insights into the Antioxidant Activity of Luteolin: Density Functional Theory Analysis and Docking in Cytochrome P450 17A1" Pharmaceuticals 18, no. 3: 410. https://doi.org/10.3390/ph18030410
APA Stylede Aguiar, A. S. N., de Carvalho, L. B. R., Gomes, C. M., Castro, M. M., Martins, F. S., & Borges, L. L. (2025). Computational Insights into the Antioxidant Activity of Luteolin: Density Functional Theory Analysis and Docking in Cytochrome P450 17A1. Pharmaceuticals, 18(3), 410. https://doi.org/10.3390/ph18030410








