Abstract
Astragali Radix (AR), a traditional Chinese herbal medicine, is derived from the dried roots of Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao (A. membranaceus var. mongholicus, AMM) or Astragalus membranaceus (Fisch.) Bge (A. membranaceus, AM). According to traditional Chinese medicine (TCM) theory, AR is believed to tonify qi, elevate yang, consolidate the body’s surface to reduce sweating, promote diuresis and reduce swelling, generate body fluids, and nourish the blood. It has been widely used to treat general weakness and chronic illnesses and to improve overall vitality. Extensive research has identified various medicinal properties of AR, including anti-tumor, antioxidant, cardiovascular-protective, immunomodulatory, anti-inflammatory, anti-diabetic, and neuroprotective effects. With advancements in technology, methods such as computer-aided drug design (CADD) and artificial intelligence (AI) are increasingly being applied to the development of TCM. This review summarizes the progress of research on AR over the past decades, providing a comprehensive overview of its traditional efficacy, botanical characteristics, drug design and distribution, chemical constituents, and phytochemistry. This review aims to enhance researchers’ understanding of AR and its pharmaceutical potential, thereby facilitating further development and utilization.
1. Introduction
Astragalus Radix (AR), a member of the Astragalus genus within the Fabaceae family, holds a prestigious status in TCM. Classified as a “top-grade” herb in the ancient Shen Nong’s Materia Medica, AR has been valued for its medicinal properties for centuries. According to the 2020 edition of the Pharmacopoeia of the People’s Republic of China, AR refers to the dried roots of AM [1]. It has a sweet flavor and warm nature, with meridian tropism for the lungs and spleen. In TCM theory, AR is believed to tonify qi, raise yang, consolidate the exterior to stop sweating, promote diuresis and reduce swelling, generate body fluids, nourish the blood, alleviate stagnation, relieve arthralgia, expel toxins and pus, and facilitate wound healing. Due to these therapeutic effects, AR is widely used in TCM formulations to treat general weakness and chronic illnesses and enhance overall vitality [2]. Modern pharmacological studies have further validated AR’s diverse bioactive properties. Research has demonstrated its anti-tumor, antioxidant, cardiovascular-protective, immunomodulatory, anti-inflammatory, anti-diabetic, and neuroprotective effects [3,4,5,6,7,8,9]. Additionally, AR has gained attention in the food industry, with its incorporation into products such as biscuits and beverages, thereby expanding its applications beyond traditional medicine [10].
From virtual screening to drug design, computational and artificial intelligence-based methods are increasingly utilized in TCM research. The identification of lead compounds from extensive compound libraries is a critical aspect of TCM research and development [11]. Currently, TCM research methods focus on reverse prediction, protein-protein interaction networks, molecular docking, virtual screening, target fishing, machine learning, and other computational approaches [12,13,14]. These methods are closely associated with the chemical constituents of TCM. The constituent structure of TCM is multi-source and available in online databases [15]. The structural composition of TCM constituents is diverse and supported by online databases [16]. This study provides a more comprehensive structural analysis of AR constituents, facilitating further research on AR and the identification of lead compounds. These findings contribute to the development of novel drugs and the advancement of intelligent drug design for AR, ultimately promoting the integration of traditional and modern medicine and accelerating drug discovery [17].
In this comprehensive review, we aim to provide an updated and detailed overview of AR, covering its traditional efficacy, botanical characteristics, distribution, chemical constituents, phytochemistry, and role in drug design. Given that existing reviews on AR are often fragmented or lack sufficient detail, we seek to present a more thorough and systematic summary of recent advancements in AR research. Specifically, we examine its botanical characteristics, distribution, chemical constituents, phytochemistry, and potential applications in drug design. Furthermore, by systematically reviewing its chemical constituents, this study aims to guide the discovery of lead compounds and the development of novel drugs.
2. Methods
It is crucial to delve deeper into the various studies and advancements made in recent years to further understand the research progress of AR. Data were obtained from resources including CNKI, PubMed, and ScienceDirect. Keyword searches for information included “Astragali Radix”, “Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao”, “Astragalus membranaceus (Fisch.) Bge”, “pharmacologic actions”, “chemical constituents”, “traditional efficacy”, “botany”, “preparation”, “virtual filtering”, “lead compound”. At the same time, the related research of each part was deeply investigated. This review encompasses 332 references spanning September 1983 to 2024.
3. Traditional Efficacy
AR is one of the most renowned herbal medicines and is recognized as the foremost qi-boosting tonic in China. In TCM theory, qi is considered a fundamental substance that constitutes the human body and sustains essential life activities [18]. Due to its extensive clinical applications and well-documented therapeutic effects, AR continues to be widely used. AM is primarily prescribed for qi deficiency and fatigue, whereas AMM is used to address qi and blood deficiencies. AR is often combined with various herbs, such as Angelica sinensis and Glycyrrhiza uralensis, to enhance its medicinal efficacy [19]. For instance, Danggui Buxue Decoction, a formulation consisting of AR and Angelica sinensis, is traditionally used to tonify qi and blood in individuals with blood deficiency. The ratio of AR to Angelica sinensis in this formula is 5:1, reflecting the TCM principle that qi generates blood. A detailed description of this decoction can be found in the ancient medical text, Lan Shi Mi Zang. Modern research has demonstrated that Danggui Buxue Decoction plays a role in immune regulation [20,21], and AR has a long history of application. It is widely used in clinical practice. The relevant classic formulations and listed drugs are presented in Table 1.
TCM processing, which has a long history, represents the essence of TCM applications [22]. According to the different processed methods of AR, it can be categorized into raw AR and processed AR. Raw AR refers to the dried slices of AR root, which are effective in fixing the surface and stopping sweating, supporting sores and generating muscle, promoting diuresis, and detumescence. It is commonly used for general health maintenance and treatment. Honey-processed Astragalus Radix is a representative product of processed AR, typically denoting a processing method in which AR is stir-fried with refined honey. It is prepared by stir-frying sliced AR with honey [23]. Honey-processed Astragalus Radix excels at tonifying qi and generating blood, mainly tonifying middle qi, and is primarily used to treat qi deficiency and fatigue, less food, and loose stool [23,24]. Depending on the specific conditions of different patients, various processed AR products can be selected to optimize treatment effectiveness.
Due to its exceptional tonic properties, AR is widely regarded as both a medicinal and dietary ingredient, highlighting its dual role in healthcare and nutrition. However, wild AR resources are becoming increasingly scarce due to excessive harvesting and environmental degradation. With the rising demand for AR, which has recently exceeded supply, artificially cultivated varieties such as AM and AMM have emerged as the primary medicinal sources. AR is frequently incorporated into daily diets as a condiment and can also be consumed as a tea substitute. Moreover, when combined with Jujubae Fructus, it forms Astragalus tea, which is believed to strengthen the spleen and enhance immunity. These edible applications underscore the significant potential for the further development and utilization of AR in functional foods.

Table 1.
Classic formulations and marketed drugs for AR.
Table 1.
Classic formulations and marketed drugs for AR.
| Classic Formulations | Main Composition | Traditional and Clinical Uses | Marketed Drugs | References |
|---|---|---|---|---|
| Buzhong Yiqi Decoction | Astragali Radix, Ginseng Radix.et Rhizoma, Cimicifugae Rhizoma | spleen asthenia, prolapse of anus, sagging of viscera | Buzhong Yiqi Wan, Buzhong Yiqi Mixture, Buzhong Yiqi granule | [1,25] |
| Yupingfeng san | Astragali Radix, Atractylodis Macrocephalae Rhizoma, Saposhnikoviae Radix | Superficial asthenia, spontaneous sweating | Yupingfeng Wan, Yupingfeng Granule, Yupingfeng oral liquid | [1,25] |
| Danggui Buxue Decoction | Astragali Radix, Angelicae Sinensis Radix | blood-deficiency fever | Dangguibuxue Wan, Danggui buxue capsule, Danggui buxue oral liquid | [1,25] |
| Guipi Decoction | Astragali Radix, Ginseng Radix.et Rhizoma, Atractylodis, Macrocephalae Rhizoma | qi and blood deficiency, morbid forgetfulness insomnia, night-sweat | Guipi Wan, Guipi Ointment | [1,25] |
| Huangqi Jianzhong Decoction | Astragali Radix, Cerealose, Cinnamomi Ramulus | deficiency of vital energy internal cold, Abdominal urgent pain | Huangqi Jianzhong Wan | [1,25] |
| Baoyuan Decoction | Astragali Radix, Ginseng Radix.et Rhizoma, Cinnamomi Cortex | Variola, Qi deficiency subsidence | Baoyuan Wan | [1,25] |
| Bufei Decoction | Astragali Radix, Asteris Radix et Rhizoma, Ginseng Radix.et Rhizoma | pulmonary asthenia, cough with asthma, short breath, spontaneous sweating | Bufei Wan | [1,25] |
| Yuye Decoction | Astragali Radix, Trichosanthis Radix, Puetaaiae Lobatae Radix | thirst | Yuye wan, Yuye Xiaoke Granules, Yuye Xiaoke Granules | [1,25] |
| Buyang Huanwu Decoction | Astragali Radix, Angelicae Sinensis Radix, Chuanxiong Rhizoma | blood stasis, half-length-flabbiness | [1,25] | |
| Shiquandabu Decoction | Astragali Radix, Ginseng Radix.et Rhizoma, Angelicae Sinensis Radix, Cinnamomi Cortex | sallow complexion, Knee weakness, deficiency of qi and blood, Various kinds of weakness | Shiquan Dabu Ointment Shiquan Dabu Wan Shiquan Dabu tabella sze chuan dah boochiew | [1,25] |
| Renshen yangrong wan | Astragali Radix, Cinnamomi Cortex, Ginseng Radix.et Rhizoma, Atractylodis Macrocephalae Rhizoma | deficiency of heart and spleen, deficiency of qi and blood, poor appetite and loose stools, Weakness after illness | Renshen Yangrong Wan | [1,25] |
| Fangji Huangqi Decoction | Astragali Radix, Stephaniae Tetrandorae Radix, Glycyrrhizae Radix et Rhizoma | wind damp syndrome, Limb pain, difficult urination | — | [1,25] |
| Huangqi guizhi wuwu Decoction | Astragali Radix, Paeoniae Radix Alba, Cinnamomi Ramulus | blood arthralgia, flesh benumbed and unresponsive | — | [1,25] |
| Tuolitounong san | Astragali Radix, Ginseng Radix.et Rhizoma, Anglicae Dahuricae Radix | superficial infection invade into cerebral carbuncle pus hard to rupture | — | [1,25] |
4. Botanical Characteristics and Distribution
AM and AMM are the original species and variations of the same species (Figure 1). Both are considered authentic AR in the 2020 edition of the Chinese Pharmacopoeia, and they have close genetic relationships. Some differences have been observed between them in terms of their botanical features. Both species are perennial herbs with stout taproots, woody, upright stems, and pinnately compound leaves. The flower is raceme, and the bracts are linear and lanceolate. The pedicel is about 3–4 mm in length and is densely covered with black pubescence. The calyx is bell-shaped, and the corolla is pale yellow and about 12–20 mm in length, forming a papilionaceous. There are 10 stamens, forming diadelphous stamens. Pods are membranous and ovate-oblong, and the seeds are 5–6, kidney-shaped, and black. The flowering period is from June to July, and the fruiting period is from August to September [19]. Although the two plants have great similarities in botanical characteristics, there are few differences in height of stems, the shapes and size of leaves, the number of seeds and other aspects. The root is the main medicinal part of AR. The taste is slightly sweet. The root is cylindrical, about 40–100 cm long, with few branches, some of which are slightly distorted. The root head is slightly enlarged, the surface is grayish yellow or light brown, the cortex is yellowish white, and the xylem is light yellow, with a radial texture and or fissures. There is a beany smell when chewing. Due to the different colors of the roots, AMM is called “Heipiqi” in the commodity, and AM is called “Baipiqi” [26] (Table 2).

Figure 1.
The botanical of AR including AMM (A), AM (B), URL: http://ppbc.iplant.cn/ (accessed on 1 March 2025), photo A ID: 1649885, photo B ID: 15508236.
AR is mainly produced in Inner Mongolia, Shanxi, Gansu, Heilongjiang, and other places in China, and also grows in Sichuan, Yunnan, Jilin, Hebei, and other places. Due to the influence of climate, temperature, and other environmental factors, the chemical constituents of AR in different regions are not the same [27]. The genuine AR used in clinics is the dry root of AMM or AM. Due to the profit in the sale process, there are many adulterants in AR. After processing, the adulterants are more similar to genuine AR in appearance and shape, and it is difficult to distinguish the true and false, which undoubtedly causes great hidden dangers to the drug efficacy and drug safety of consumers. At present, the common adulterants of AR in the market are mainly Hedysari Radix, Medicago sativa L., A stragalus ernestii Comb., Malva rotundifolia L., Gossypium herbaceum L., etc. The common confusions of AR are listed in Table 3. Therefore, clinical medication needs to do a good job of quality control [28].

Table 2.
The difference between AMM and AM.
Table 2.
The difference between AMM and AM.
| Species | Leaf | Corolla | Ovary | Fruit | Root | References |
|---|---|---|---|---|---|---|
| Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao | 25–37 leaflets, and broadly elliptical small leaves; 5–9 mm long, width 3–5 mm, with short white pubescence | light yellow | glabrous | Ovate oblong; no pubescence | 30–90 cm long, surface brown bitumen | [19,26] |
| Astragalus membranaceus (Fisch.) Bge. | 13–31 leaflets, and oval or oblong ovate, small leaves; 4–10 mm long, width 3–5 mm, with short white pubescence | yellow | puberulous | half the oval; having white or black pubescence | 40–80 cm long, surface brown taupe | [19,26] |

Table 3.
The common adulterants of AR.
Table 3.
The common adulterants of AR.
| Variety | Taxonomic Position | Root Traits | References |
|---|---|---|---|
| Medicago sativa L. | Leguminosae, Medicago L. | Bitter flavor, pungent smell, cylindrical root, crisp texture, easy to break. The section is strong in fiber and the forming ring is not obvious. | [28] |
| Astragalus ernestii Comb. | Leguminosae, Astragalus L. | Weak taste, the root is thick, the texture is loose and flexible. The section has strong fiber and the skin is easy to fall off. | [28,29] |
| Oxytropis coerulea (Pall.) DC. | Leguminosae, Oxytropis DC | Weak taste, cylindrical root with branches, surface brown yellow or brown red, light and tough, strong toughness. | [30,31] |
| Caragana sinica (Buchoz.) Rehd. | Leguminosae, Caragana Fabr. | Weak taste, cylindrical root, surface brown yellow, brittle and easy to break. | [30,32] |
| Melilotus albus Desr. | Leguminosae, Melilotus (L.) Mill. | Weak taste, cylindrical root, with a swollen head, surface brown yellow brown to red brown surface. The root is hard and brittle, and the section is prickly. | [30] |
| Glycyrrhiza pallidiflora Maxim. | Leguminosae, Glycyrrhiza L. | Sweet flavor, cylindrical root, the head has more branches, surface brown grayish yellow to grayish brown. The texture is hard to break, and the section is fibrous. | [33,34] |
| V.gigantea Bge. | Leguminosae, Pisum Linn | Bitter flavor, cylindrical root, hard and crisp, surface brown yellowish white, yellowish yellow in wood. | [35,36] |
| Malva rotundifolia L. | Malvaceae, Malva Linn. | Sweet flavor, cylindrical root, cylindrical root, multi-branched, surface brown earthy yellow, hard and brittle, easy to break. The section is fibrous and flat. | [32] |
| Althaea rosea (L.) Cavan | Malvaceae, Althaea Linn | Sweet flavor, cylindrical root, the root head is coarse. The lower end is fine, surface brown yellowish brown. The texture is hard, and the section is not neat. | [33,37] |
| Malva verticillata L. | Malva verlicillala L. | Sweet flavor, cylindrical root, surface brown light yellowish, with longitudinal stripes. The section is yellowish white. | [28] |
| Hedysarum polybotrys Hand.-Mazz. | Malvaceae, Malva Linn. | Sweet flavor, cylindrical root, surface brown gray reddish brown, and the texture is hard and tough, sectional fiber. | [28] |
| Astragalus tongolensis Ulbr. | Leguminosae, Astragalus L. | Sweet flavor, surface brown epidermis yellowish to brownish brown. The texture is loose and flexible, not easy to break, sectional fiber is weak. | [28] |
| Astragalus floridulus Podlech | Leguminosae, Astragalus L. | Sweet flavor, epidermis dark brown, hard and tough texture. Section fibrous, weak powder, narrow skin. | [32] |
| Astragalus chrysopterus Bunge | Leguminosae, Astragalus L. | Sweet flavor, rhizome is thick, surface brown yellowish brown. The texture is dense and tough. Section fiber, powder-rich. | [28] |
| Sphaerophysa salsula (Pall.) DC. | Leguminosae, Sphaerophysa DC | Bitter flavor, the root is multi-branched, with small poison and semi-shrub. crisp and easy to break. The section is neat. poor fiber, no bean smell. | [38] |
| Gossypium hirsutum Linn. | Malvaceae, Gossypium Linn. | Bitter flavor, the roots are cylindrical, with small branches at the lower part, surface brown yellow or light brown. Hard and light, not easy to break, Sectional fiber. | [26] |
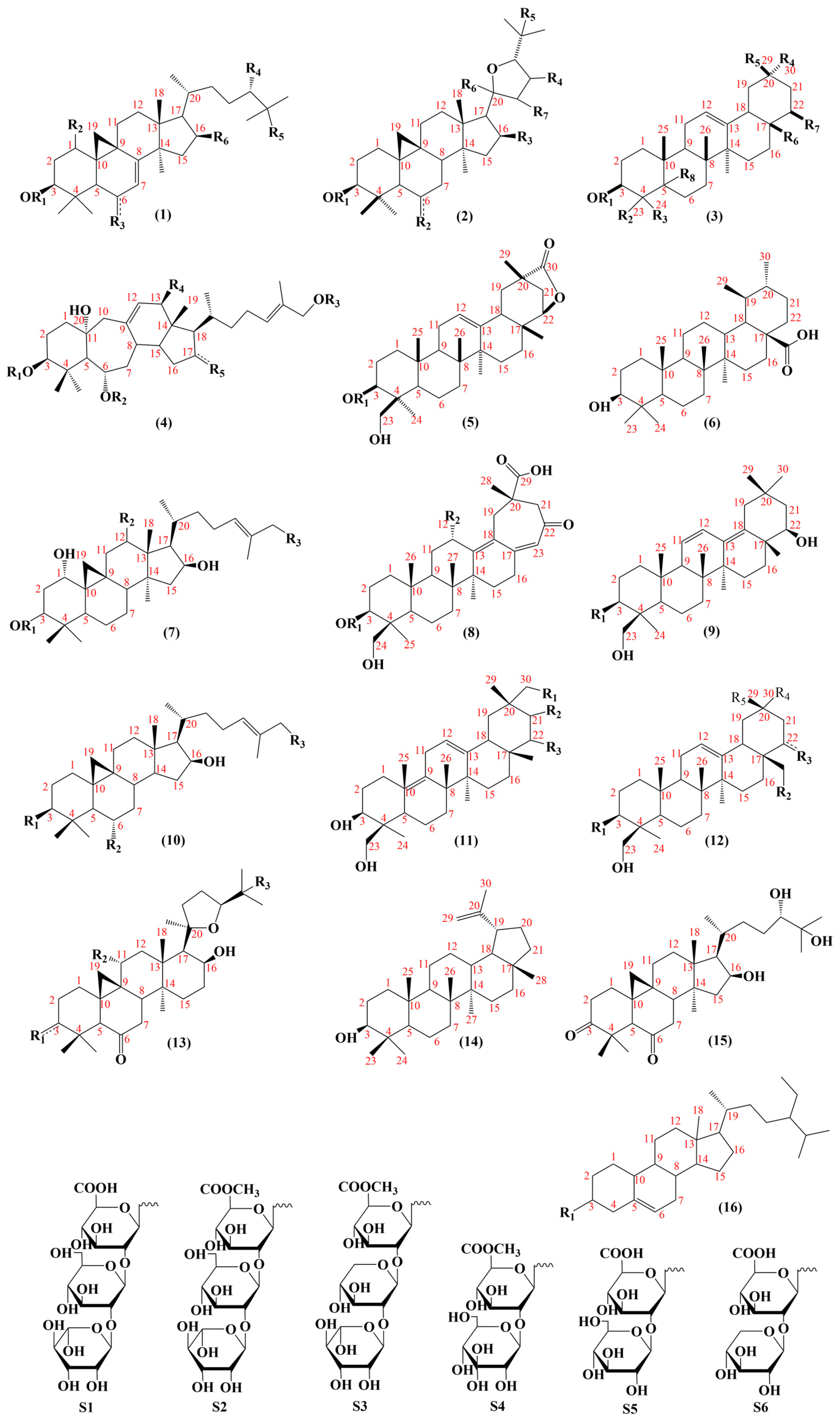
5. Chemical Constituents of AMM and AM
AR is a prominent herbal medicine noted for its diverse constituents and multi-faceted activities, with a wide geographical distribution. Among the various types of Astragalus, AMM and AM are the two most commonly used medicinal applications. Although the primary concentration of chemical constituents is derived from the root, some active constituents can also be extracted from the flowers of AR, which possess certain anti-oxidation effects [39]. To date, researchers have isolated over 300 constituents from AMM and AM, comprising 184 flavonoids, 101 saponins, and 42 other constituents.
This study aims to furnish a more comprehensive understanding of the chemical constituents found in AR through an extensive literature search [40,41,42]. Detailed information is presented in the tables below.
5.1. Flavonoids
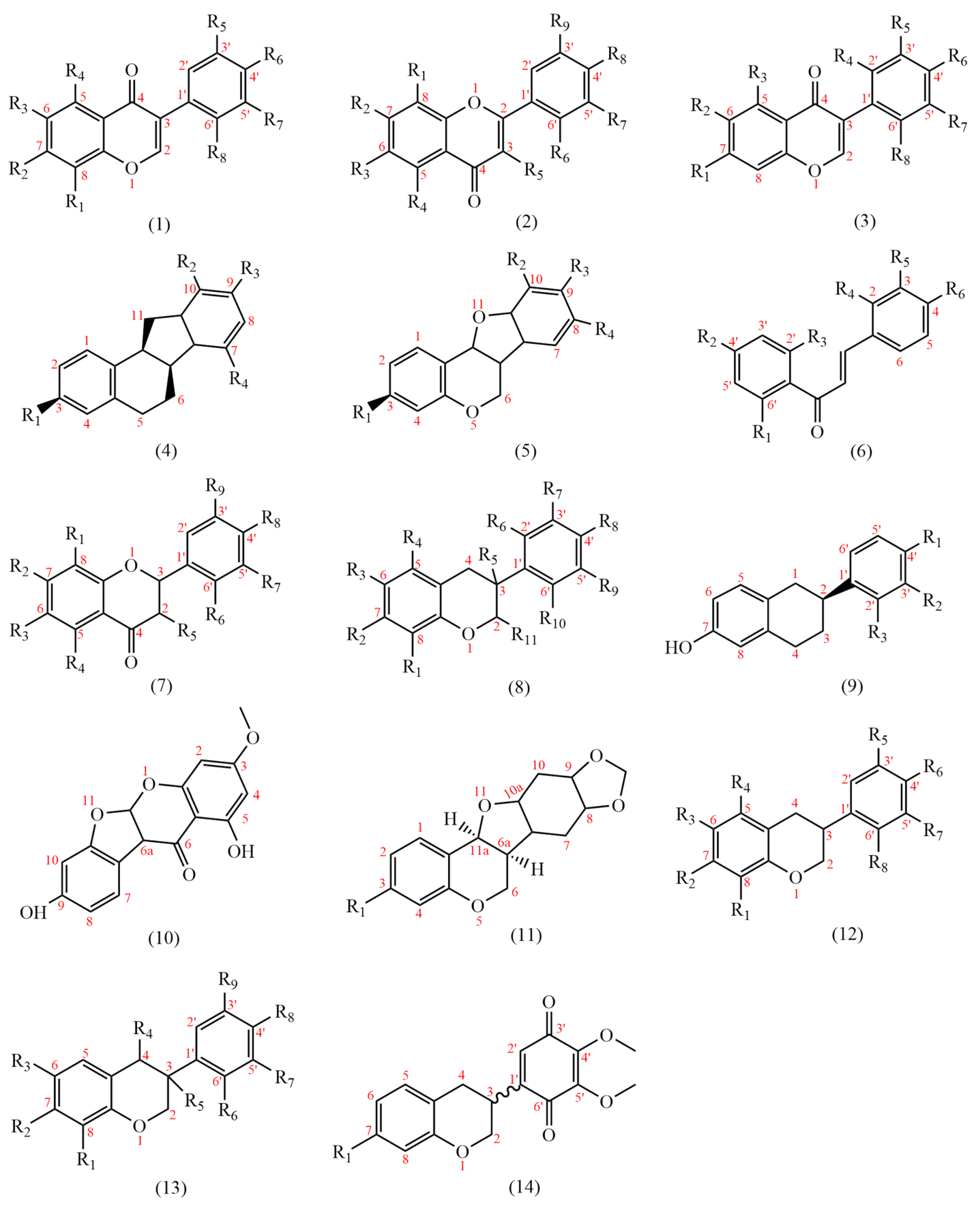
At present, 183 flavonoids have been isolated from AMM and AM (Table 4); AMM contained 153 flavonoids, and AM contained 69 flavonoids, including 38 common flavonoids. The diversity of flavonoids in AMM significantly surpasses that of AM. The structures of the flavonoid skeletons from AM and AMM are shown in Figure 2.
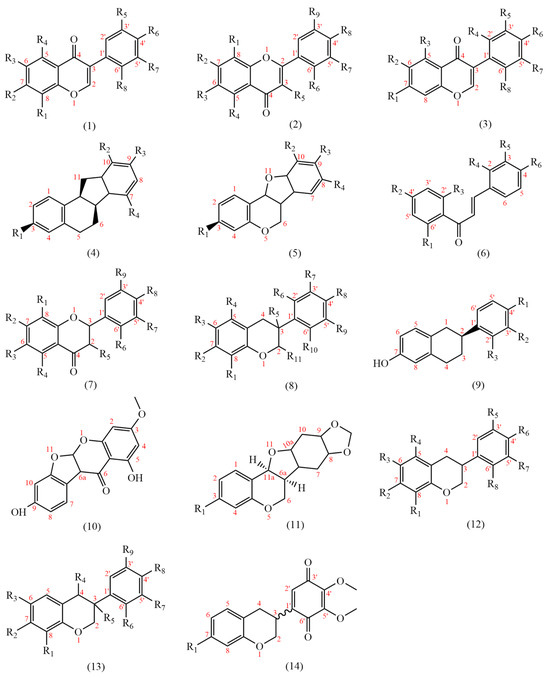
Figure 2.
The structural backbones of flavonoids in AM and AMM.
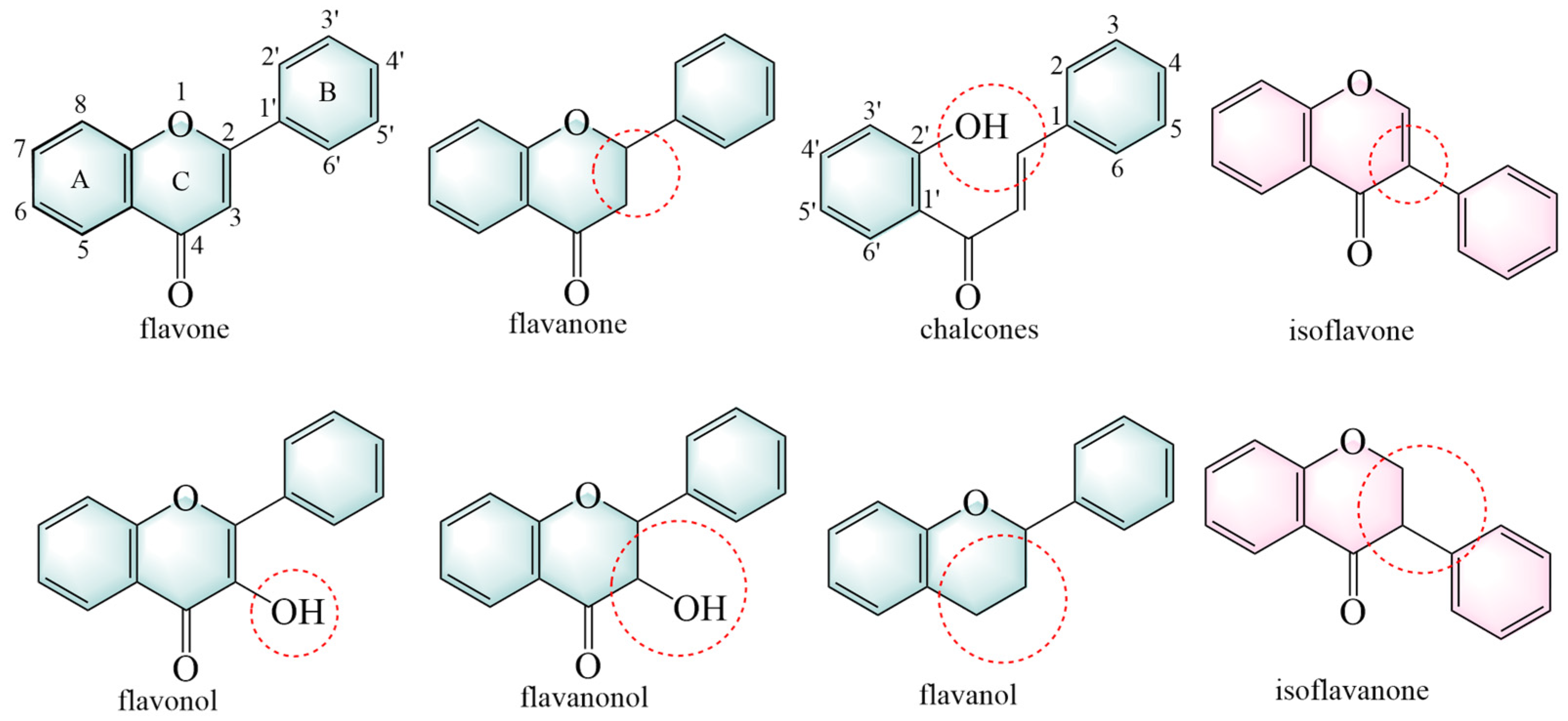
Flavonoids are primarily classified based on their chemical structures into several major subclasses, including flavones, flavanones, chalcones, isoflavones, flavanols, flavanonols, and isoflavanone (Figure 3) [40].
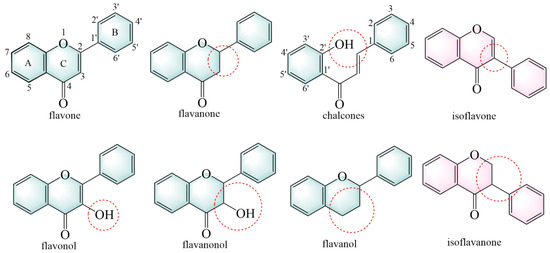
Figure 3.
The structural backbones of flavonoids. The red circle indicates the site of modification in the flavonoid’s parent nucleus.
AR contains many flavones (54–61) based on 2-phenylchromone as the basic structural backbone. Flavones have anti-diabetic [43], neuroprotective [44], and anti-inflammatory properties [45]. Apigenin (58) is a typical flavonoid component that exerts protective effects on the liver [46] and vasculature [47]. It also has a certain curative effect on Alzheimer’s disease [48] and acute lymphoblastic [49].
Flavonols (62–95) represent a class of constituents characterized by the presence of a hydroxyl group at the 3-position of the 2-phenylchromone backbone. It can treat cardiovascular diseases, with anti-tumor [50], anti-oxidation [51], and other effects [50].
AR contains many isoflavones (1–53, 96–100), and 3-phenylchromone is the basic structural backbone of isoflavones. It can be antibacterial [52] and anti-inflammatory [53]. Genistein (4) can anti-cancer [54], anti-neurodegenerative diseases [55], and relieve rheumatoid osteoarthritis [56]. Formononetin (1) is a derivative of isoflavones. It has anti-inflammatory [57] and neuroprotective effects [58] and can ameliorate polycystic ovary syndrome [53].
Isoflavanones are products resulting from the hydrogenation reduction of the 2,3 double bonds in 3-phenylchromone. Isoflavanones (107–126, 164, 166) have anti-tumor effects [59].
Chalcones are constituents formed by the cleavage of the chemical bond between positions 1 and 2 of 2-phenylchromone, resulting in a benzaldehyde-condensing acetophenone structure, and their 2′-hydroxy derivative is an isomer of isoflavanone [40]. Chalcones (127–133) can be converted into dihydroflavones under acidic conditions. Chalcones have anti-oxidatant, antibacterial [60], neuroinflammation-relieving [61], and anti-cancer effects [62].
Flavanones (134–138) are formed by the hydrogenation of the 2, 3 double bonds in 2-phenylchromone. Naringin (135) is a common flavanone, which can protect blood vessels [63] and has anti-atherosclerotic properties [64].

Table 4.
Flavonoids isolated from AM and AMM.
Table 4.
Flavonoids isolated from AM and AMM.
| No. | Name | Substituent | Skeletons | Species | References |
|---|---|---|---|---|---|
| 1 | formononetin | R2=OH R6=OMe | 1 | AMM, AM | [65,66] |
| 2 | calycosin | R2=OH R7=OH R6=OMe | 1 | AMM, AM | [65,66] |
| 3 | calycosin-7-O-β-D-glucopyranoside | R2=β-D-OGlcp R7=OH R6=OMe | 1 | AMM, AM | [65,66] |
| 4 | genistein | R2=R4=R6=OH | 1 | AMM, AM | [66,67] |
| 5 | ononin | R2=β-D-OGlcp R6=OMe | 1 | AMM, AM | [66,68] |
| 6 | 6″-acetylononin | R2=β-D-(6-acetxyl)-OGlcp R6=OMe | 1 | AMM, AM | [68,69] |
| 7 | pratensein | R2=R4=R7=OH R6=OMe | 1 | AMM, AM | [70] |
| 8 | 3′-methoxy-5′-hydroxy-isoflavone-7-O-β-D-glucopyranoside | R1=β-D-OGlcp R7=OMe R5=OH | 1 | AMM, AM | [71] |
| 9 | odoratin-7-O-β-D-glucopyranoside | R2=β-D-OGlcp R4=R6=OMe R7=OH | 1 | AMM, AM | [69,71] |
| 10 | daidzein | R2=R6=OH | 1 | AMM, AM | [72,73] |
| 11 | (3R)-2′-hydroxy-7,3′,4′trimethoxy-isoflavan | R1=R4=R5=OMe R3=OH | 1 | AM | [74] |
| 12 | isomucronulatol | R2=OH R5=R6=OMe R7=OH | 1 | AMM, AM | [68,75] |
| 13 | isomucronulatol-7,2′-di-O-glucoside | R2=R8=OGlcp R5=R6=OMe | 1 | AMM, AM | [73,75] |
| 15 | calycosin7-O-(6-O-acety1-β-D-glucopyranoside) | R2=β-D-O-(6-O-Ac-Glcp) R6=OMe R7=OH | 1 | AMM, AM | [76] |
| 16 | (3R)-7-O-β-glc-isomucronulatol | R2=β-D-OGlcp R8=OH R6=R7=OMe | 1 | AMM, AM | [76] |
| 17 | pratensein-7-O-β-D-glycoside | R2=β-D-OGlc R4=R7=OH R6=OMe | 1 | AMM | [66] |
| 18 | sissotrin | R2=β-D-OGlcp R2=R4=OH R6=OMe | 1 | AMM | [77] |
| 19 | 5′,7-dihydroxy-3′-methoxyisoflavone | R2=R5=OH R7=OMe | 1 | AMM | [78] |
| 20 | 5,7,4′-trihydroxy-3′-methoxyisoflavone | R2=R4=R6=OH R7=OMe | 1 | AMM | [77] |
| 21 | 4′-methoxyiso-flavone-7-O-β-D-glucopyranoside | R2=β-D-OGlcp R6=OMe | 1 | AMM | [79] |
| 22 | 7,3′-diohydroxy-5′-methoxyisoflavone | R2=R5=OH R7=OMe | 1 | AMM | [79] |
| 23 | 3′-hydroxy-4′-methoxy-7-O-(6″-butylene beaser-O)-β-glucopyranoside | R2= [6-(E)-But-2-enoyl]-β-D-OGlcp R7=OH R6=OMe | 1 | AMM | [80] |
| 24 | 5′-hydroxy-3′-methoxy-isoflavone-7-O-β-D-glucoside | R2=β-D-OGlcp R7=OMe R5=OH | 1 | AMM | [78] |
| 25 | sophorabioside | R2=R4=OH R6=β-D-OGlcp-(2→1)-α-L-Rha | 1 | AMM | [69] |
| 26 | odoratin | R2=OH R3=R6=OMe | 1 | AMM | [81] |
| 27 | calycosin 7-O-(6-O-malony1-β-D-glucopyranoside) | R2=O-(6-O-malonyl-β-D-Glcp) R7=OH R6=OMe | 1 | AMM | [76] |
| 28 | formononetin 7-O-(6-O-malony1-β-D-glucopyranoside) | R2=O-(6-O-malonyl-β-D-Glcp) R7=OH | 1 | AMM | [76] |
| 29 | formononetin 7-O-(6-O-acety1-β-D-glucopyranoside) | R2=O-(6-O-Ac-β-D-Glcp) R7=OH | 1 | AMM | [76] |
| 30 | calycosin 7-O-(6-O-butanoyl-β-D-glucopyranoside) | R2=O-(6-O-butanoyl-β-D-Glcp) R6=OMe R7=OH | 1 | AMM | [76] |
| 31 | 5′,7-di-OH-3′-methoxyisoflavone | R2=R5=OH R7=OMe | 1 | AMM | [76] |
| 32 | calycosin-7-O-glc-6″-O-acetate | R2=β-D-OGlcp-Ac R7=OH R6=OMe | 1 | AMM | [82] |
| 33 | dihydroxy-dimethoxy isoflavone | R3=R7=OMe R4=R8=OH | 1 | AMM | [82] |
| 34 | formononetin-7-O-glc-6″-O-acetate | R2=β-D-OGlcp-Ac R7=OH R6=OMe | 1 | AMM | [82] |
| 35 | calycosin-Glc-malonate | R4=β-D-OGlcp-Mal R7=OH R6=OMe | 1 | AMM | [82] |
| 36 | calycosin-7-O-Glc-6″-O-malonate | R2=β-D-OGlcp-Mal R7=OH R6=OMe | 1 | AMM | [82] |
| 37 | odoratin-7-O-Glc-6″-O-malonate | R2=β-D-OGlcp-Mal R3=R6=OMe R7=OH | 1 | AMM | [82] |
| 38 | 6,4′-Dimethoxyisoflavone-7-O-Glc | R1=β-D-OGlcp R3=R6=OMe | 1 | AMM | [82] |
| 39 | pratensein-7-O-Glc-6″-O-malonate | R2=β-D-OGlcp-Mal R4=R7=OH R6=OMe | 1 | AMM | [82] |
| 40 | formononetin-7-O-Glc-6″-O-malonate | R2=β-D-OGlcp-Mal R6=OMe | 1 | AMM | [82] |
| 41 | 7-Hydroxy-6,4-dimethoxyisoflavone | R3=OH R4=R6=OMe | 1 | AMM | [82] |
| 42 | 3′-Hydroxy-6′,4′-dimethoxyisoflavone-7-O-Glc | R2=β-D-OGlcp R7=OH R1=R6=OMe | 1 | AMM | [82] |
| 43 | 2′,3′-dihydroxy-7,4′-dimethoxyisoflavone | R2=R6=OMe R8=R7=OH | 1 | AMM | [82] |
| 44 | 7,3′-dihydroxy-8,4′-dimethoxyisoflavone | R2=R5=OH R1=R6=OMe | 1 | AM | [65] |
| 45 | calycosin 7-O-β-D-(6″-acetyl)-glucoside | R2=(6″-acetxyl)-β-D-OGlcp R6=OMe | 1 | AM | [68] |
| 46 | calycosin 7-O-β-D-{6″-[(E)-But-2-enoyl]}-glucoside | R2=[6-(E)-But-2-enoyl]-β-D-OGlcp R6=OMe | 1 | AM | [68] |
| 47 | 8,3′-dihydroxy-7,4′-dimethoxyisoflavone | R2=R6=OMe R1=R7=OH | 1 | AM | [65] |
| 48 | ammopiptanoside A | R2=[6-(E)-But-2-enoyl]-OGlcp R6=OMe | 1 | AM | [68] |
| 49 | 3′,7,8-trihydroxy-4′-methoxyisoflavone | R1=R2=R7=OH R6=OMe | 1 | AM | [70] |
| 50 | glycitein | R2=R8=OH R4=OMe | 1 | AM | [83] |
| 51 | 4′,7-dihydroxy-3′-methoxy isoflavone | R2=R6=OH R7=OMe | 1 | AM | [83] |
| 52 | genistin | R2=β-D-OGlcp R8=OH | 1 | AM | [83] |
| 53 | glycitin | R2=β-D-OGlcp R3=OMe R8=OH | 1 | AM | [83] |
| 54 | (6aR,11aR)-3-OH-9,10-dimethoxypterocarpan | R1=OH R2=R3=OMe | 2 | AMM | [76] |
| 55 | astrapterocarpan 3-O-(6-O-malony1-β-D-glucopyranoside) | R1=O-(6-O-malonyl-β-D-Glcp) R2=R3=OMe | 2 | AMM | [76] |
| 56 | oroxylin A | R2=R4=OH R3=OMe | 2 | AMM | [84] |
| 57 | wogonin | R1=OMe R2=R4=OH | 2 | AMM | [84] |
| 58 | apigenin | R2=R4=R8=OH | 2 | AMM | [82] |
| 59 | baicalein | R2=R3=R4=OH | 2 | AMM | [82] |
| 60 | baicalin | R2=β-D-OGlcp R3=R4=OH | 2 | AMM | [82] |
| 61 | Oroxylin A | R2=R4=OH R3=OMe | 2 | AMM | [84] |
| 62 | kaempferide | R2=R4=R5=OH R8=OMe | 2 | AMM | [82] |
| 63 | kaempferol | R2=R4=R5=R8=R9=OH | 2 | AMM, AM | [41,69] |
| 64 | rhamnocitrin-3-O-β-D-glucopyranoside | R2=R8=OH R4=OMe R5=β-D-OGlcp | 2 | AMM, AM | [69,83] |
| 65 | rhamnocitrin-3-O-β-neohesperidoside | R2=R4=R8=OH R5=β-D-Rha-(1→2)-OGlcp | 2 | AMM, AM | [69,83] |
| 66 | complanatuside | R2=OMe R4=OH R8=R5=β-D-OGlcp | 2 | AMM, AM | [69,83] |
| 67 | isoquercitrin | R4=OH R2=OMe R8=R5=β-D-OGlcp | 2 | AMM, AM | [41] |
| 68 | quercetin | R3=R4=R5=R8=R7=OH | 2 | AMM, AM | [41,77] |
| 69 | quercetin-3-glucoside | R2=R4=R9=R8=OH R5=β-D-OGlcp | 2 | AMM, AM | [71,77] |
| 70 | isorhamnetin | R2=R4=R5=R8=OH R7=OMe | 2 | AMM, AM | [41,77] |
| 71 | astraflavonoid B | R2=(5′-R)-OApi R4=R8=OH R5=β-D-OGlcp-(2→1)-α-L-Rha | 2 | AMM | [69] |
| 72 | kaempferol-3-O-β-D-glucoside | R2=R4=R8=OH R5=β-D-OGlcp | 2 | AMM | [69] |
| 73 | kaempferol-3,7-di-O-β-D-glucopyranoside | R2=R5=β-D-OGlcp R4=R8=OH | 2 | AMM | [69] |
| 74 | quercetin-3-O-β-D-neospheroside | R2=R4=R7=R8=OH R5=β-D-OGlcp-(2→1)-L-Rha | 2 | AMM | [69] |
| 75 | tamarixin | R2=R4=R7=OH R5=β-D-OGlcp R8=OMe | 2 | AMM | [85] |
| 76 | isorhamnetin-3-β-D-glucoside | R2=R4=R8=OH R5=β-D-OGlcp R9=OMe | 2 | AMM | [81] |
| 77 | 4′-methoxy-kaempferol 3-O-glucoside | R2=R4=OH R8=OMe R5=β-D-OGlcp | 2 | AMM | [81] |
| 78 | kumatakenin | R2=R5=OMe R4=R8=OH | 2 | AMM | [81] |
| 79 | Rhamnocitrin | R4=R5=R8=OH R2=OMe | 2 | AMM | [76] |
| 80 | 3-O-β-D-glc-isorhamnetin | R2=R4=R8=OH R9=OMe R5=β-D-OGlcp | 2 | AMM | [76] |
| 81 | 5,2′,6′-Trihydroxy-6,7,8-trimethoxyflavone | R1=R2=R3=R5=R6=OMe R4=OH | 2 | AMM | [82] |
| 82 | apigenin-Hex | R2=R4=R5=R7=OH R6=OMe | 2 | AMM | [82] |
| 83 | rhamnocitrin-Hex | R2=OMe R4=R8=OH R5=β-D-OGlc | 2 | AMM | [82] |
| 84 | rhamnocitrin-Hex-malonateHex Hex | R2=OMe R4=R8=OH R5=OGlcp | 2 | AMM | [82] |
| 85 | rhamnocitrin-Hex-acetate | R2=OMe R4=R8=OH R5=β-D-OGlcp-Ac | 2 | AMM | [82] |
| 86 | hyperoside | R2=R4=R8=R7=OH R5=β-D-OGlcp | 2 | AMM | [82] |
| 87 | isorhamnetin-3-O-neohespeidoside | R2=R4=R7=OH R5=OH-Neohesperidin R6=OMe | 2 | AMM | [82] |
| 88 | 3-hydroxydihydroisoflavone | R2=R4=R8=OH R5=OMe | 2 | AMM | [82] |
| 89 | Quercetin-3-O-robinobioside | R2=R4=R7=R8=OH R5=OH-Roeinobioside | 2 | AMM | [82] |
| 90 | kaempferol-3-O-rutinoside | R2=R4=R8=OH R5=OH-Rutinoside | 2 | AMM | [82] |
| 91 | kaempferol-3-O-Glucosyl galactoside | R5=OGlcp-Gal R2=R4=R8=OH | 2 | AMM | [82] |
| 92 | kaempferol-4′-methoxy-3-O-glucopyranoside | R2=R4=OH R8=OMe R5=β-D-OGlcp | 2 | AMM | [82] |
| 93 | 7-Methoxy-Kaempferol-3-O-Glc | R2=OMe R4=R8=OH R5=β-D-OGlcp | 2 | AMM | [82] |
| 94 | rhamnocitrin-3-O-β-D-glucopyranoside (1″→2″)-β-D-apiofuranosy | R4=R8=OH R2=OMe R5=β-D-OGlcp-(2→1)-Api | 2 | AM | [83] |
| 95 | tiliroside | R4=R2=R8=OH R5=(6″-p-coumaroyl)-β-D-Glcp | 2 | AM | [83] |
| 96 | dihydroxy-trimethoxy DHIF | R3=R8=OH R2=R7=R6=OMe | 3 | AMM | [82] |
| 97 | dihydroxy-trimethoxy DHIF-Hex | R3=β-D-OGlcp R2=R7=R6=OMe R8=OH | 3 | AMM | [82] |
| 98 | dihydroxy-dimethoxy DHIF-Hex | R3=β-D-OGlcp R2=R7=OMe R8=OH | 3 | AMM | [82] |
| 99 | dihydroxy-trimethoxy DHIF-Pen | R3=β-D-OGlcp R2=R7=R6=OMe R8=OH | 3 | AMM | [82] |
| 100 | trihydroxy-dimethoxy DHIF-Hex | R3=β-D-OGlcp R2=R6=OMe R8=R7=OH | 3 | AMM | [82] |
| 101 | methylnissolin | R1=R2=R3=OMe | 4 | AMM, AM | [68,86] |
| 102 | (-)-methylnissolin3-O-β-D-glucoside | R1=β-D-OGlcp R2=OMe R3=OMe | 4 | AMM, AM | [68,81] |
| 103 | (-)-methylinissolin3-O-β-d-(6′-acetyl)-glucoside | R1= (6″-acetxyl)-β-D-OGlcp R2=R3=OMe | 4 | AMM, AM | [68,77] |
| 104 | maakiain | R1=OH R3=R4=OCH2O | 4 | AMM, AM | [76] |
| 105 | (6aR,11aR)-3,9,10-trimethoxypterocarpan | R1=R2=R3=OMe | 4 | AMM, AM | [76] |
| 106 | (6aR,11aR)-3-OH-9,10-dimethoxypterocarpan-3-O-β-D-glucopyranoside | R1=β-D-OGlcp R2=R3=OMe | 4 | AMM, AM | [76] |
| 107 | 3,9-di-O-methylnissolin | R1=OH R2=R3=OMe | 5 | AMM | [87] |
| 108 | wogonin | R1=OMe R2=R4=OH | 5 | AMM | [78] |
| 109 | astraflavonoids A | R2=R4=R6=OH R8=β-D-OGlcp-(2→1)-(5‘-R)-Api | 5 | AMM | [69] |
| 110 | 3-Hydroxy-9,10-dimethoxy pterocarpan | R1=OH R2=R3=OMe | 5 | AMM | [82] |
| 111 | 9,10-dimethoxypterocarpan-3-O-glucoside | R1=β-D-OGlcp R2=R3=OMe | 5 | AMM | [82] |
| 112 | 10-Hydroxy-3,9-dimethoxypterocarpan | R1=R3=OMe R2=OH | 5 | AMM | [82] |
| 113 | 3-Hydro-9-MP-Hex-Hex | R1=β-D-OGlcp-Glcp R3=OMe | 5 | AMM | [82] |
| 114 | 3-Hydro-9-MP-Hex | R1=β-D-OGlcp R3=OMe | 5 | AMM | [82] |
| 115 | 3-Hydro-9,10-diMP-Pen-HeX | R1=OH-Pen-Glcp R2=R3=OMe | 5 | AMM | [82] |
| 116 | 3-Hydro-9-MP-malonyl-Glc | R1=β-D-OGlcp-Mal R3=OMe | 5 | AMM | [82] |
| 117 | MP | R3=OMe | 5 | AMM | [82] |
| 118 | 9,10-DiMP-3-O-malonyl-Glc | R1=OGlcp-Mal R2=R3=OMe | 5 | AMM | [82] |
| 119 | 9,10-DiMP-3-O-acetyl-Glc | R1=β-D-OGlcp-Ac R2=R3=OMe | 5 | AMM | [82] |
| 120 | 9,10-dimethoxypterocarpan-3-O-glucopyranoside | R1=β-D-OGlcp R2=R3=OMe | 5 | AMM | [82] |
| 121 | (-)-methylinissolin3-O-β-d-(6′-(E)-But-2-enoyl)-glucoside | R1=β-[6-(E)-But-2-enoyl]- D-OGlcp R2=R3=OMe | 5 | AM | [68] |
| 122 | (+)-vesticarpan | R1=R2=OH R3=OMe | 5 | AM | [68] |
| 123 | licoagroside D | R1=β-D-OGlcp R2=OH R3=OMe | 5 | AM | [68] |
| 124 | (6aR,11aR)-10-OH-3,9, -dimethoxypterocarpan | R1=R3=OMe R2=OH | 5 | AM | [76] |
| 125 | (-)-Methylinissolin 3-O-(6-acety1-β-D-glucopyranoside) | R1=O-(6-O-Ac-β-D-Glcp) R2=R3=OMe | 5 | AM | [76] |
| 126 | (-)-Methylinissolin 3-O-[6-O-(E)-but-2-enoyl-β-D-glucopyranoside] | R1=O-[6-O-(E)-but-2-enoyl-β-D-Glc] R2=R3=OMe | 5 | AM | [76] |
| 127 | 2′,4′,4-trihydroxy-chaleone (Isoliquiritigenin) | R1=R2=R3=R6=OH | 6 | AMM, AM | [70,88] |
| 128 | 4,4′,6′-trihydroxychalcone | R2=R3=R6=OH | 6 | AMM | [89] |
| 129 | 2′-methoxyisoliquiritigenin | R1=OMe R2=R6=OH | 6 | AM | [70] |
| 130 | echinatin | R4=OMe R2=R6=OH | 6 | AM | [70] |
| 131 | licochalcone B | R2=R6=R5=OH R4=OMe | 6 | AM | [70] |
| 132 | 4,4′-dimethyl-6′-hydroxychalcone | R2=R4=CH3 R1=H | 6 | AMM | [89] |
| 133 | 4-methoxy-4′,6′-dihydroxychalcone | R2=R1=OH R6=OMe | 6 | AMM | [89] |
| 134 | 4′-hydroxyflavonone-7-O-β-D-glucoside | R2=β-D-OGlcp R8=OH | 7 | AMM | [89] |
| 135 | naringin | R4=R8=OH R2=β-D-O-Glcp-α-L-Rha | 7 | AMM | [82] |
| 136 | 3′,4′,7-trihydroxyflavone | R2=R8=R9=OH | 7 | AM | [70] |
| 137 | liquiritigenin | R2=R8=OH | 7 | AMM, AM | [70,89] |
| 138 | dihydroxyflavone | R1=R2=OH | 7 | AMM | [82] |
| 139 | isomucronulatol-7-O-glycoside | R2=β-D-OGlcp R6=OH R7=R8=OMe | 8 | AMM, AM | [68] |
| 140 | 6″-O-acetyl-(3R)-7,2′-dihydroxy-3′,4′-dimethoxyisoflavan-7-O-β-D-glucopyranoside | R2=(6″-acetxyl)-β-D-OGlcp R9=R8=OMe | 8 | AMM | [77] |
| 141 | 3,2′-dihydroxy-3′,4′-dimethylisoflavan-7-O-β-d-glucoside | R2=β-D-OGlcp R8=OMe R9=OMe R10=OH | 8 | AMM | [89] |
| 142 | astraflavonoids C | R2=R10=OH R9=R7=OMe R8=β-D-OGlcp | 8 | AMM | [69] |
| 143 | 7-O-methylisomucronulatol | R2=R7=R8=OMe | 8 | AMM | [87] |
| 144 | 5-hydroxyisomucronulatol 2′,5′-di-O-glucoside | R2=OH R9=R6=β-D-OGlcp R8=R7=OMe | 8 | AMM | [87] |
| 145 | astraisoflavanin | R2=β-D-OGlcp R5=R6=OMe R7=OH | 8 | AMM | [75] |
| 146 | 3′-OH-2,4′-dimethoxyisoflavane-6-O-glc | R9=OH R8=R11=OMe R3=β-D-OGlcp | 8 | AMM | [90] |
| 147 | (3R)-isomucronulatol | R2=R10=OH R8=R9=OMe | 8 | AMM | [76] |
| 148 | isomucronulatol 5′-OH-2′,5′-di-O-glc | R2=OH R6=R9=β-D-OGlcp R8=R7=OMe | 8 | AMM | [76] |
| 149 | isomucronulatol 7,2′-di-O-β-glucoside | R2=R6=β-D-OGlcp R8=R7=OMe | 8 | AMM | [76] |
| 150 | Astraisoflavan 7-O-(6-malony1-β-D-glucopyranoside) | R2= O-(6-O-malonyl-β-D-Glcp) R10=OH R8=R9=OMe | 8 | AMM | [76] |
| 151 | 7,2′-Dihydroxy-3′4′-dimethoxyisoflavan | R2=R10=OH R9=R8=OMe | 8 | AMM | [82] |
| 152 | 2′-Hydroxy-3′,4′-dimethoxyisoflavan-7-O-Glc | R2=β-D-OGlcp R10=OH R8=R9=OMe | 8 | AMM | [82] |
| 153 | 7-Hydroxy-6,4′-dimethoxyisoflavan | R2=OH R3=R8=OMe | 8 | AMM | [82] |
| 154 | trihydroxy-methoxyisoflavan-Hex-hex | R10=OMe R3=R9=OH R8=OGlcp-Glcp | 8 | AMM | [82] |
| 155 | trihydroxy-dimethoxyisoflavan-HeX | R9=R10=OMe R8=OGlc R3=R7=OH | 8 | AMM | [82] |
| 156 | isomucronulatol-Hex-Hex | R2=β-D-OGlc-Glc R10=OH R5=R9=OMe | 8 | AMM | [82] |
| 157 | dihydroxy-dimethoxyisoflavan | R9=R10=OMe R3=R8=OH | 8 | AMM | [82] |
| 158 | isomucronulatol-acetyl-Glc | R2=β-D-OGlc-Ac R10=OH R8=R9=OMe | 8 | AMM | [82] |
| 159 | 3-Mucronulatol-O-glucopyranoside | R2=β-D-OGlcp R9=OH R3=R8=OMe | 8 | AMM | [82] |
| 160 | 5′-Hydroxy-isomucronulatol-2′,5′-glucoside | R3=R8=β-D-OGlcp | 8 | AMM | [82] |
| 161 | 2′,4′-Dimethoxy-3′-hydroxyisoflavan-6-O-Glc | R10=R8=OMe R9=OH R3=β-D-OGlcp | 8 | AMM | [82] |
| 162 | 3,2′-Dihydroxy-3′,4′-dimethoxyisoflavan-7-O-Glc | R2=β-D-OGlcp R10=OH R8=R9=OMe | 8 | AMM | [82] |
| 163 | sphaerophyside SB | R3=OH R1=R2=OMe R3=β-D-OGlcp | 9 | AM | [67] |
| 164 | sophorophenolone | - | 10 | AM | [91] |
| 165 | (3R)-7,2′,3′-trihydroxy-4′-methoxy-isoflavane | R1=R3=R4=OH R5=OMe | 12 | AM | [92] |
| 166 | Trifolinhizin | R1=β-D-OGlcp | 11 | AMM | [93] |
| 167 | (3R)-(5′-hydroxy-2′,3′,4′-trimethoxyphenyl)-chroman-7-ol | R1=OH R3=R4=R5=OMe | 12 | AM | [68] |
| 168 | (3R)-8,2′-dihydroxy-7,4′-dimethoxyisoflavan | R1=OH R2=R6=OMe R8=OAc | 12 | AMM, AM | [94] |
| 169 | isomucronulatol 7,3′-di-O-glc | R1=R4=β-D-OGlcp R3=OH R5=OMe | 12 | AM | [76] |
| 170 | (3R)-8,2′-Dihydroxy-7,4′-dimethoxyisoflavan | R1=R6=OH R2=R8=OMe | 13 | AMM, AM | [95] |
| 171 | isomucronulatol | R2=R7=R8=OMe R6=OH | 13 | AMM, AM | [87] |
| 172 | 7-O-methylisomucronulatol | R2= R7=R8=OMe R6=OH | 13 | AMM, AM | [96] |
| 174 | (3R)-7,2′,3′-Trihydroxy-4′-methoxy-isoflavane | R2=R6=R7=R9=OH R8=OMe | 13 | AM | [95] |
| 175 | (R)-3-(5-Hydroxy-2,3,4-trimethoxyphenyl)-chroman-7-ol | R2=R9=OH R6=R7=R8=OMe | 13 | AM | [68] |
| 176 | (3R)-(-)-Mucronulatol 7-O-β-D-glucoside | R1=β-D-OGlcp R6=R8=OMe R7=OH | 13 | AMM | [97] |
| 177 | 6″-O-Acetyl-(3 R)-2′-hydroxy-3′,4′-dimethoyl-isoflavan 7-O-β-D- glucopyranoside | R3=β-D-6-O-Ac-Glcp R6=OH R7=R8=OMe | 13 | AMM | [98] |
| 178 | 3′-Hydroxy-2′,4′-dimethoxyisoflavan 6-O-β-D-glucopyranoside | R3=β-D-OGlcp R5=R7=OH R6=R8=OMe | 13 | AMM | [99] |
| 179 | Astraflavonoid C | R2=R6=OH R7=R9=OMe R8=β-D-OGlcp | 13 | AMM | [69] |
| 180 | 3,2′-Dihydroxyl-3′,4′-methoxyisoflavanone 7-O-β-D-glucoside | R2=β-D-OGlcp R4=R6=OH R7=R8=OMe | 13 | AMM | [100] |
| 181 | (3R,4R)-4,7-Hydroxy-2′,3′-dimethoxyisoflavane 4′-O-β-D-glucoside | R2=R4=OH R5=Me R6=β-D-OGlcp R7=R8=OMe | 13 | AMM | [101] |
| 182 | 2′,5′-Dicarbonyl-3′,4′-dimethoxyisoflavanequinone 7-O-β-D-glucoside | R1=β-D-OGlcp | 14 | AMM | [93] |
| 183 | Pendulone | R1=OH | 14 | AM | [68] |
Note: Unmarked in the table: R=H, Glc-glucose, Rha-rhamnose, Me-methyl, Ac-acetyl, Xyl-xylose, Glcp-glucopyranoside.
5.2. Saponins
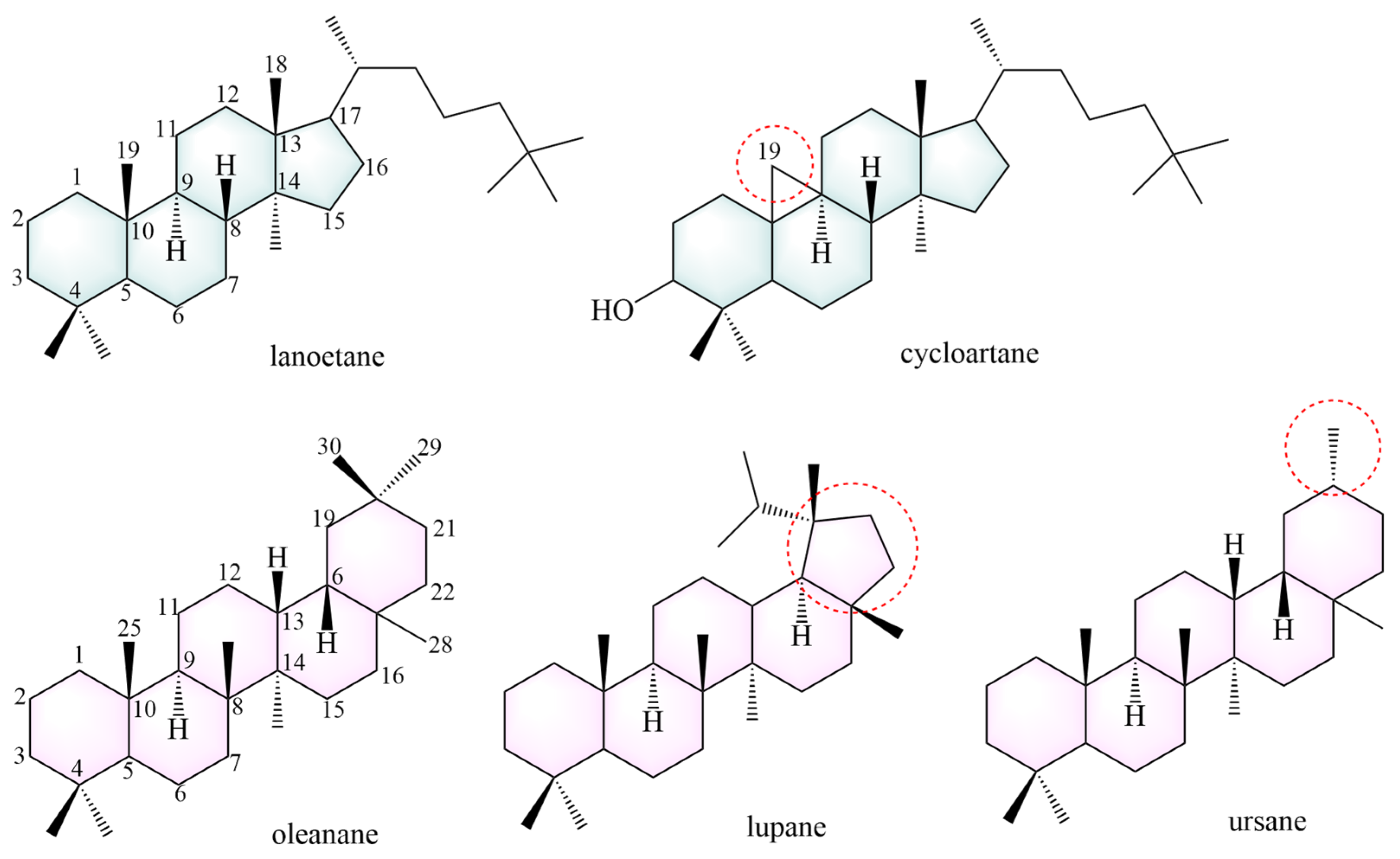
To date, 101 saponins have been isolated from AMM and AM. AMM contains 54 saponins, and AM contains 60 saponins, including 13 common saponins. Tetracyclic triterpenes and pentacyclic triterpenes are included, with tetracyclic triterpenes mainly comprising lanostane and cycloartane (Figure 4).
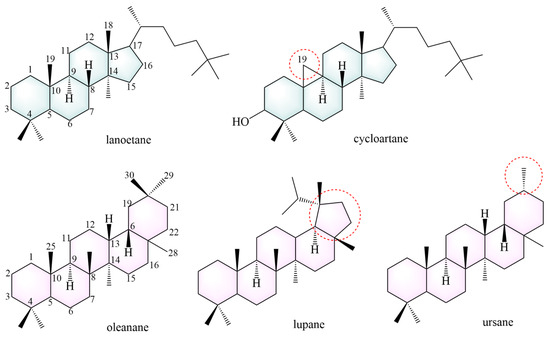
Figure 4.
The structural backbones of saponins. The red circle indicates the site of modification in the saponins’s parent nucleus.
Most tetracyclic triterpenes possess the fundamental skeleton of a cyclopentane-perhydrophenanthrene ring system. Lanostane is formed via a chair-boat-chair-boat conformational cyclization of epoxy squalene. It exhibits anti-diabetic, neuroprotective [102], anti-tumor [103], anti-malarial [104], anti-aging [105], and anti-inflammatory effects [106]. The parent structural backbone of cycloartane is similar to that of lanostane, and dehydrogenation at C19 and C9 of cycloartane results in a three-membered ring. Cycloartane exerts neuroprotective and antioxidant effects [107].
Pentacyclic triterpenoids mainly include oleanane (234–249, 259–261, 269, 270–276), ursane (262) and lupane (281). These constituents exhibit anti-cancer [108] and anti-inflammatory effects [109]. The structures of the saponin skeletons from AM and AMM are illustrated in Figure 5. The saponins were isolated from AMM and AM (Table 5).
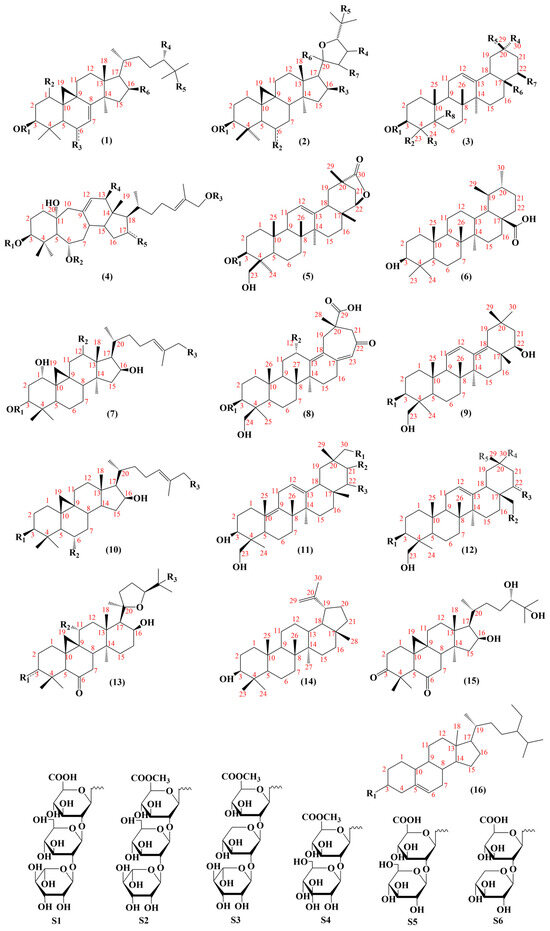
Figure 5.
The structural backbones of saponins in AM and AMM.
Oleanane, also known as β-amyrane, has a basic structural backbone of polyhydropinene [40], and exhibits anti-inflammatory and hepatoprotective effects. The C19 and C21 positions of lupane form a five-membered ring. Lupane possesses antibacterial [110], cardioprotective [111], and anti-inflammatory effects [112]. Ursane, also known as α-amyrin, differs from oleanolic acid in that the C19 and C20 positions each contain a methyl group. Studies have indicated that ursane has anti-tumor [113] and neuroprotective effects [114].

Table 5.
Saponins isolated from AM and AMM.
Table 5.
Saponins isolated from AM and AMM.
| No. | Name | Substituent | Skeletons | Species | References | |
|---|---|---|---|---|---|---|
| 184 | mongholicoside A | R1=β-D-Glcp R2=R3=R4=R5=R6=OH | 1 | AMM | [115] | |
| 185 | mongholicoside B | R1=β-D-Glcp R2=R4=R5=R6=OH R3=O | 1 | AMM | [115] | |
| 186 | alexandroside I | R1=β-D-Glcp R3=R4=R5=R6=OH | 1 | AMM | [81] | |
| 187 | agroastragaloside I | R1=(2′,3′-di-OAc)-β-D-Xyl R3=β-D-Glcp R6=OH | 1 | AM | [116] | |
| 188 | agroastragaloside II | R1=β-(2′-OAc)-D-Xyl R2=β-D-OGlcp R6=OH | 1 | AM | [91] | |
| 189 | agroastragaloside V | R1=β-(2′-OAc)-D-Xyl R2=β-D-OGlcp | 1 | AM | [117] | |
| 190 | astramembranoside B | R1=β-(2′-OAc)-D-Xyl R6=OH | 1 | AM | [91] | |
| 191 | cyclocanthoside A | R1=β-D-Xyl R6=OH | 1 | AM | [91] | |
| 192 | cyclocanthoside E | R1=β-D-Xyl R2=β-D-OGlcp R6=OH | 1 | AM | [70] | |
| 193 | agroastragaloside | R1=2′-O-Ac-β-D-Xyl R3=β-D-OGlcp R4=R5=R6=OH | 1 | AM | [118] | |
| 194 | huangqiyenin II | R3=O R4=R5=R6=OH | 1 | AM | [118] | |
| 195 | huangqiyenin B | R2=β-D-Glcp R3=O R4=R5=R6=OH | 1 | AM | [118] | |
| 196 | isocyclocanthoside E | R1=β-D-Xyl R3=β-D-OGlcp R4=R5=R6=OH | 1 | AM | [118] | |
| 197 | aleksandroside I | R1=β-D-Glcp R2=H R3=R4=R5=R6=OH | 1 | AMM | [119] | |
| 198 | astragaloside I | R1=(2′,3′-di-OAc)-β-D-Xyl R2=β-D-Glcp R3=R5=OH R6=Me | 2 | AMM, AM | [66,71] | |
| 199 | astragaloside II | R1=(2′-OAc)-β-D-Xyl R2=β-D-Glcp R3=R5=OH R6=Me | 2 | AMM AM | [66,71] | |
| 200 | astragaloside III | R1=β-D-Xyl-(2→1)-β-D-Glcp R2=R3=R5=OH R6=Me | 2 | AMM, AM | [66,71] | |
| 201 | astragaloside IV | R1=β-D-Xyl R2=β-D-Glcp R3=R5=OH R6=Me | 2 | AMM, AM | [68,88] | |
| 202 | isoastragaloside I | R1=R2=β-D-Glcp R3=R5=OH | 2 | AMM, AM | [65,90] | |
| 203 | isoastragaloside II | R1=β-D-Xyl R2=β-D-Glcp R3=R5=OH R6=Me | 2 | AMM, AM | [74,120] | |
| 204 | acetylastragaloside Ι | R1=(3′-OAc)-Xyl R2=β-D-Glcp | 2 | AMM, AM | [65,121] | |
| 205 | astragaloside VII | R1=β-D-Xyl R2=R5=β-D-Glcp | 2 | AMM, AM | [65,86] | |
| 206 | isoastragaloside VII | R3=R5=OH R6=Me | 2 | AMM, AM | [118] | |
| 207 | agroastragaloside III | R1=(2′,3′-di-OAc)-β-D-Xyl R2=R5=β-D-OGlcp R3=R5=OH R6=Me | 2 | AM | [122] | |
| 208 | agroastragaloside IV | R1=(2′-OAc)-β-D-Xyl R2=R5=β-D-OGlcp R3=R5=OH R6=Me | 2 | AM | [122] | |
| 209 | astragaloside V | R1=β-D-Xyl-(2→1)-Glc R5=β-D-OGlcp R3=R2=OH R6=Me | 2 | AM | [123] | |
| 210 | astragaloside VI | R1=β-D-Xyl-(2→1)-Glcp R2=β-D-OGlcp R3=R5=OH R6=Me | 2 | AM | [123] | |
| 211 | astramembranoside A | R1=R5=β-D-OGlcp R3=OH R6=Me | 2 | AM | [91] | |
| 212 | brachyoside B | R2=β-D-OGlcp R3=R5=OH R6=Me | 2 | AM | [91] | |
| 213 | cycloastragenol | R2=R3=R5=OH R6=Me | 2 | AM | [123] | |
| 214 | isoastragaloside IV | R1=β-D-Xyl R5=β-D-OGlcp | 2 | AM | [124] | |
| 215 | astramembranin II | R1=β-D-Xyl R2=R3=R5=OH R6=Me | 2 | AM | [125] | |
| 216 | huangqiyiesaponin C | R1=β-D-Glcp | 2 | AM | [126] | |
| 217 | cyclounifolioside B | R1=β-D-Xyl-(2→1)-β-D-Glcp | 2 | AM | [91] | |
| 218 | astraverrucin I | R1=α-L-Rha-(1→4)-β-D-Glcp R2=R3=R5=OH R6=Me | 2 | AM | [118] | |
| 219 | isoastragaloside V | R1=Ara-(1→2)-β-D-Xyl R2=β-D-OXyl R3=R5=OH R6=Me | 2 | AM | [118] | |
| 220 | neoastragaloside I | R1=2′,3′-O-di-Ac-β-D-Xyl R2=OH R3=R5=OH R6=Me | 2 | AM | [118] | |
| 221 | huangqiyenin A | R1=β-D-Glcp R3=R5=OH R6=Me | 2 | AM | [118] | |
| 222 | astrolanosaponin A2 | R1=2-O-Ac-β-D-Glcp R2=H R3=OH R5=β-D-OGlcp R6=Me | 2 | AMM | [119] | |
| 223 | cycloaraloside E | R1=β-D-Glcp R2=O R3=OH R5=β-D-OGlcp R6=Me | 2 | AMM | [119] | |
| 224 | astrolanosaponin A1 | R1=β-D-Glcp R2=β-D-OGlcp R2=OH R3=R5=OH R4=H R6=Me | 2 | AMM | [119] | |
| 225 | astraverrucin II | R1=2-O-Ac-β-D-Glcp R2=OH R3=R5=OH R6=Me | 2 | AMM | [119] | |
| 226 | huangqiyenin K | R1=β-D-Xyl R2=OAc R3=R5=OH R6=Me | 2 | AM | [127] | |
| 227 | astramembrannin II | R1=Glcp R3=R5=OH R6=Me | 2 | AMM, AM | [128] | |
| 228 | agroastragaloside III | R1=β-D-Xyl R3=R5=OH R6=Me | 2 | AM | [41] | |
| 229 | agroastragaloside IV | R1=2-O-Ac-β-D-Xyl R2=R5=β-D-OGlcp R3=OH R6=Me | 2 | AM | [41] | |
| 230 | isoastragaloside I | R1=2,4-O-Ac2-β-D-Xyl R2=β-D-Glcp R3=R5=OH R6=Me | 2 | AMM, AM | [100] | |
| 231 | astrolanosaponin B | R1=β-D-Glcp R2=β-D-OGlcp R2=O R3=OH R6=Me | 2 | AMM | [129] | |
| 232 | astrolanosaponin D | R1=β-D-Glcp R2=OH R3=R5=OH R6=Me | 2 | AMM | [119] | |
| 233 | astrolanosaponin E | R1=β-D-Glcp R2=OH R3=R5=OH R6=Me | 2 | AMM | [119] | |
| 234 | soyasapogenol B | R7=OH R3=Me R3=R4=R5=R6=Me | 3 | AM | [123] | |
| 235 | soyasapogenol B | R7=OH R3=MeOH R3=R4=R5=R6=Me R8=Me | 3 | AM | [118] | |
| 236 | astraisoolesaponins A | R1=S1 R7=O R2=MeOH R3=R4=R5=R6=Me | 3 | AMM | [118] | |
| 237 | astraisoolesaponins B | R1=S1 R2=R5=MeOH R7=OH R6=R3=R4=Me | 3 | AMM | [118] | |
| 238 | astraisoolesaponins Cl | R1=S4 R2=MeOH R7=OH R3=R5=R6=Me R4=COOH | 3 | AMM | [118] | |
| 239 | astraisoolesaponins C2 | R1=S2 R2=MeOH R7=OH R3=R5=R6=Me R4=COOH | 3 | AMM | [118] | |
| 240 | astraisoolesaponins E1 | R1=S5 R2=R6=MeOH R7=O R4=COOH R3=R5=Me | 3 | AMM | [118] | |
| 241 | astraisoolesaponins E2 | R1=S6 R2=R6=MeOH R7=O R4=COOH R3=R5=Me | 3 | AMM | [118] | |
| 242 | azukisaponin V | R1=S1 R2=MeOH R3=R4=R5=R6=Me R7=OH | 3 | AMM | [118] | |
| 243 | astragaloside VIII methyl ester | R1=S3 R2=MeOH R7=OH R3=R4=R5=R6=Me | 3 | AMM | [118] | |
| 244 | robinioside F | R1=S1 R2=MeOH R7=OH R3=R5=R6=Me R4=CH2OH | 3 | AMM | [118] | |
| 245 | robinioside B | R1=S1 R2=MeOH R7=OH R3=R5=R6=Me R4=COOH | 3 | AMM | [118] | |
| 246 | cloversaponin III | R1=S5 R2=MeOH R7=O R3=R5=R6=Me R4=COOH | 3 | AMM | [118] | |
| 247 | soyasaponin I | R1=β-D-GlcA-(2→1)-β-D-Xyl-(2→1)-α-L-Rha R2=CH2OH R3=R4=R5=R6=Me R7=OH | 3 | AMM, AM | [66,71] | |
| 248 | astragaloside VIII | R1=β-D-GlcA-(2→1)-β-D-Xyl-(2→1)-α-L-Rha R2=CH2OH R3=R4=R5=R6=Me R7=OH | 3 | AMM, AM | [71,120] | |
| 249 | robinioside F | R1=β-D-OGlcA-(1→2)-β-D-Glcp-(1→2)-α-L-Rha R2=R4=MeOH R3=R5=R6=MeOH R7=OH | 3 | AMM | [129] | |
| 250 | huangqiyenin E | R1=R2=Ac R4=OAc R5=OH R3=β-D-Glcp | 4 | AM | [130] | |
| 251 | huangqiyenin O | R1=R2=R4=R5=OH R3=β-D-Glcp | 4 | AM | [130] | |
| 252 | huangqiyegenin III | R1=R2=Ac R3=OAc | 4 | AM | [130] | |
| 253 | huangqiyegenin IV | R1=R2=Ac | 4 | AM | [130] | |
| 254 | trideacetylhuangqiyegenin III | R3=OH | 4 | AM | [130] | |
| 255 | huangqiyenin G | R1=H R2=Ac R3=β-D-Glcp R4=O R5=OH | 4 | AM | [131] | |
| 256 | huangqiyenin W | R1=H R2=R4=OAc R3=β-D-Glcp | 4 | AM | [131] | |
| 257 | huangqiyenin R | R1=R2=H R5=OH R4=OAc R3=β-D-Glcp | 4 | AM | [131] | |
| 258 | huangqiyenin Q | R1=Ac R4=R5=OH R3=β-D-Glcp | 4 | AM | [131] | |
| 259 | astraisoolesaponins D | R1=S1 | 5 | AMM | [118] | |
| 260 | astraisoolesaponins F | R1=S2 | 5 | AMM | [118] | |
| 261 | astroolesaponin D | R1=α-L-Rha-(1→2)-β-D-Glcp-(1→2)-β-D-GIcA | 5 | AMM | [41] | |
| 262 | ursolic Acid | - | 6 | AMM | [86] | |
| 263 | Mongholicoside I | R3=β-D-OGlcp | 7 | AMM | [41] | |
| 264 | Mongholicoside II | R1=Ac R2=OH R3=β-D-OGlcp | 7 | AMM | [41] | |
| 265 | astraisoolesaponin A1 | R1=α-L-Rha-(1→2)-β-D-Glcp-(1→+2)-β-D-Glcp R2=OH | 8 | AMM | [41] | |
| 266 | astraisoolesaponin A2 | R1=β-D-Xyl-(1→2)-β-D-GlcA R2=OH | 8 | AMM | [41] | |
| 267 | astraisoolesaponin A3 | R1=β-D-Glcp-(1→2)-β-D-GlcA R2=OH | 8 | AMM | [41] | |
| 268 | astroolesaponin F | R1=β-D-OGlcA-OMe-(1→2)-β-D-Glcp-(1→2)-α-L-Rha | 9 | AMM | [129] | |
| 269 | huangqiyenin L | R1=β-D-OXyl R2=OAc R3=β-D-OGlcp | 10 | AM | [127] | |
| 270 | (3β,21α)-olean-12-ene-3,21,24-triol | R2=OH | 11 | AM | [127] | |
| 271 | (3β,22β)-olean-12-ene- 3,22,24,29-tetro | R1=R3=OH | 11 | AM | [129] | |
| 272 | soyasapogenol E | R3=O | 11 | AM | [127] | |
| 273 | astroolesaponin C1 | R1=OS4 R2=OH R3=OH R4=COOH | 12 | AMM | [129] | |
| 274 | astroolesaponin C2 | R1=β-D-OGlcA-OMe-(1→2)-β-D-Glcp-(1→2)-α-L-Rha R2=OH R3=OH R4=COOH | 12 | AMM | [129] | |
| 275 | robinioside B | R1=OS1 R2=OH R3=OH | 12 | AMM | [129] | |
| 276 | astroolesaponin A | R1=β-D-OGlcp-(1→2)-β-D-Glcp-(1→2)-α-L-Rha R4=R5=Me | 12 | AMM | [129] | |
| 277 | astrolanosaponin C | R1=β-D-OGlcp R3=OH | 13 | AMM | [119] | |
| 278 | cyclocephaloside II | R1=4-O-Ac-β-D-Xyl R3=OH | 13 | AM | [68] | |
| 279 | huangqiyegenin V | R1=O R2=R3=OH | 13 | AM | [127] | |
| 280 | huangqiyegenin I | R1=R3=OH R2=OH | 13 | AM | [127] | |
| 281 | lupeol | - | 14 | AMM | [97] | |
| 282 | huangqiyegenin VI | - | 15 | AM | [127] | |
| 283 | β-daucosterol | R1=OH | 16 | AMM | [66] | |
| 284 | d-3-O-methyl-chiro-inositol | R1=β-D-OGlcp | 16 | AMM | [66] | |
Note: Unmarked in the table: R=H, Glc-glucose, Rha-rhamnose, Me-methyl, Ac-acetyl, Xyl-xylose, OGlcA-glucuronic acid, Glcp-glucopyranoside.
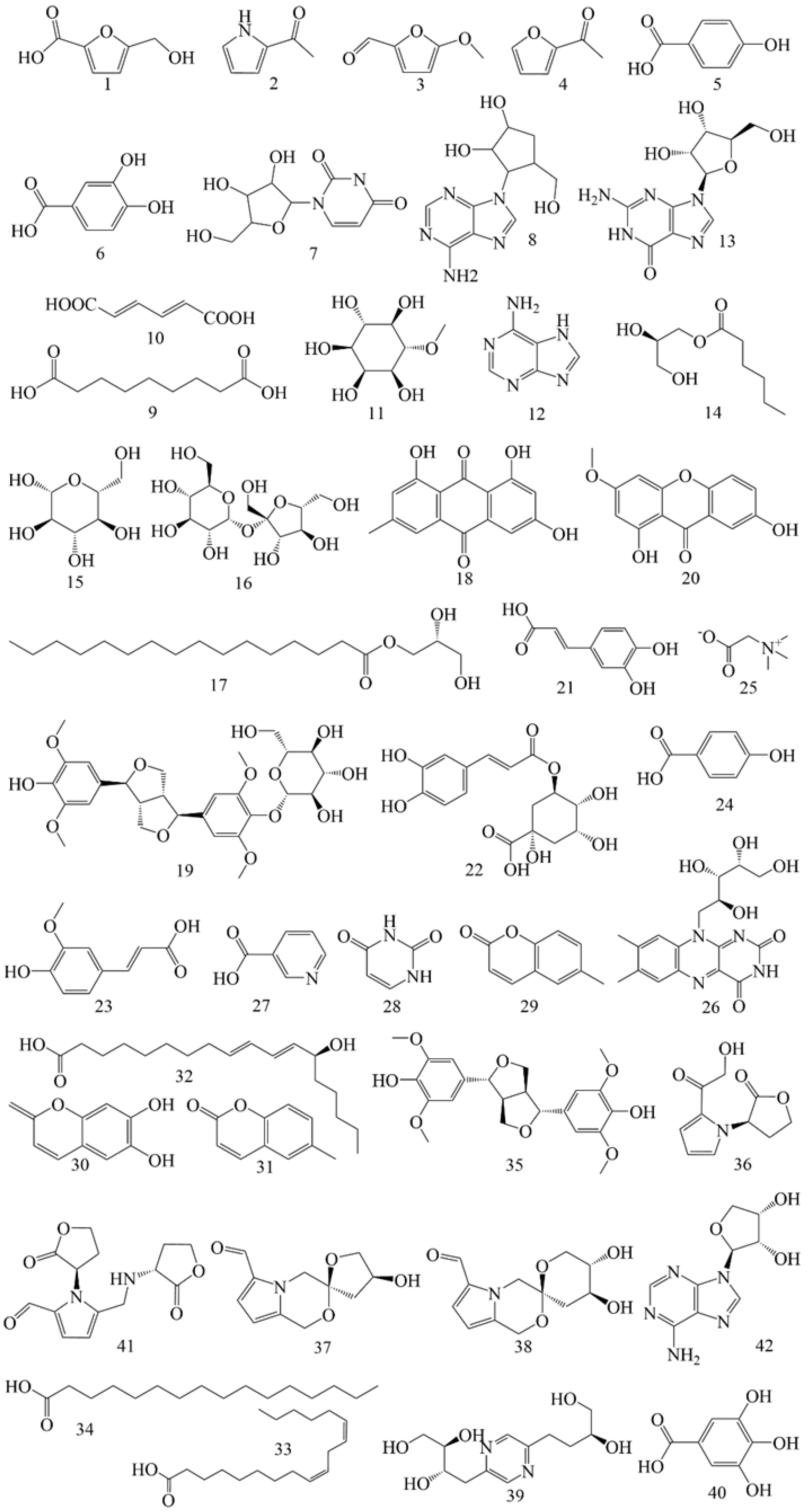
5.3. Others Constituents
In addition to flavonoids and saponins, AR contains numerous other constituents. A total of 40 additional constituents have been reported in AMM and AM (Table 6), including 18 components in AMM and 24 in AM. The structures of these constituents from AM and AMM are shown in Figure 6.

Table 6.
Others isolated from AM and AMM.
Figure 6.
The structural backbones of s other structures in AM and AMM.
Alkaloids (286, 291–292, 296–297, 340–342) exhibit anti-inflammatory [132], neuroprotective [133] and antiviral properties [134,135].
Glycosides (299–300) are among the primary metabolites of AR, playing a role in immune regulation and exhibiting anti-inflammatory properties [136,137]. Phenolics (5–6, 38) efficiently scavenge free radicals [138] and exhibit antioxidant properties [139].
Quinones (302, 304) are primarily classified into four categories: benzoquinones, naphthoquinones, phenanthraquinones and anthraquinones. These constituents demonstrate cardioprotective [140], anti-inflammatory [141], anti-tumor [142], and anti-oxidation effects [143] while also contributing to kidney protection [144].
Isocoumarins (313–314) are a class of constituents characterized by a benzopyranone structure [145]. Studies have shown that these compounds possess various pharmacological properties. Isocoumarin constituents have demonstrated antioxidant and anti-diabetic effects [136,146]. At the same time, isocoumarins and their glycosyl derivatives exhibit significant therapeutic potential against cancer by influencing several critical cellular processes, such as apoptosis, autophagy, and cell cycle regulation [135,147].
6. Pharmacological Studies
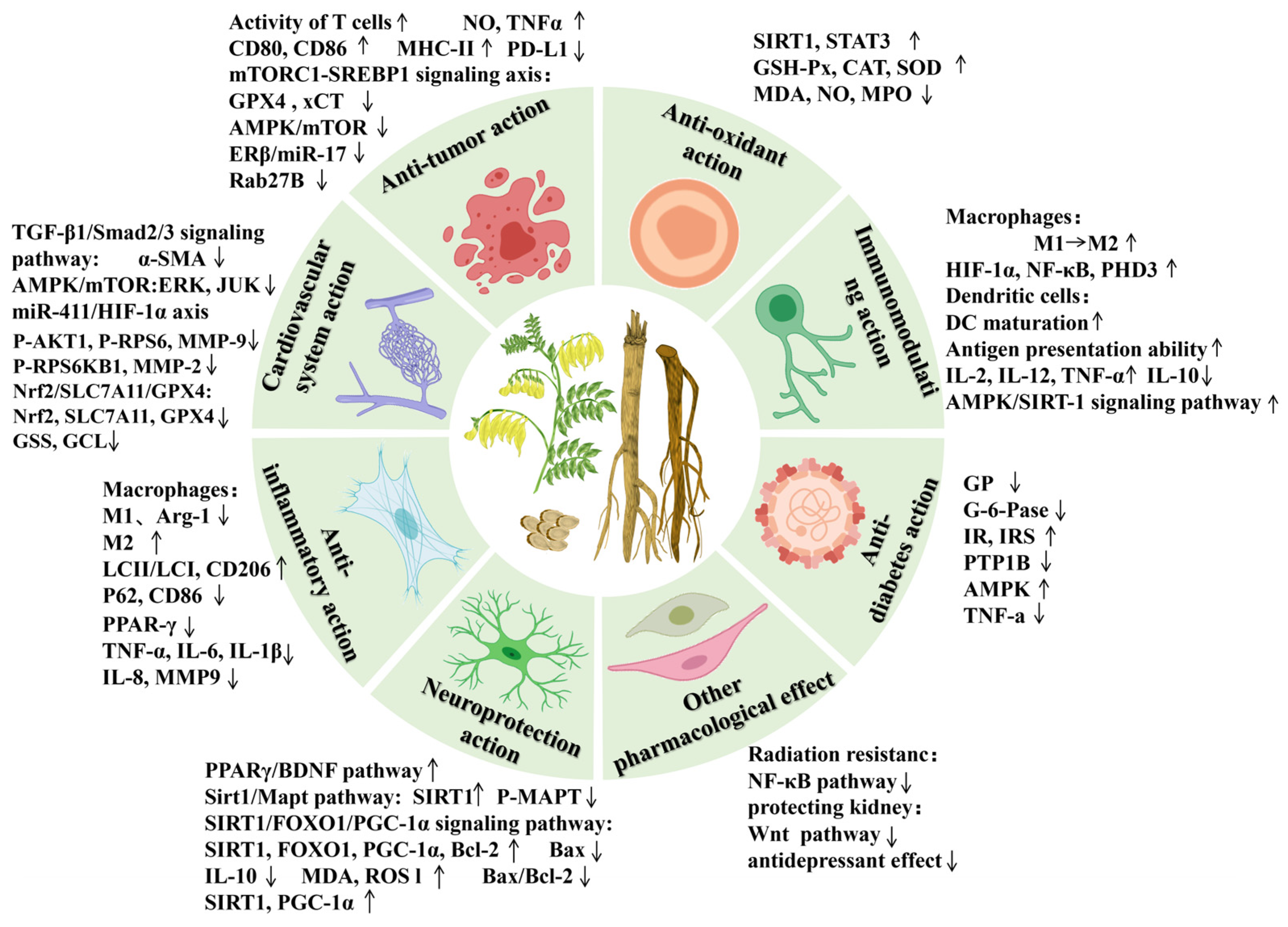
Modern pharmacological studies have demonstrated that AR possesses various pharmacological effects, including anti-tumor, lowering blood glucose, cardiovascular and cerebrovascular protection, immune function improvement, anti-inflammatory, neuroprotection, protecting liver damage, anti-oxidative stress, and other biological activities [3,4,5,6,7,8,9] (Figure 7). The pharmacological effects of the active components of AR are shown in Table 7.
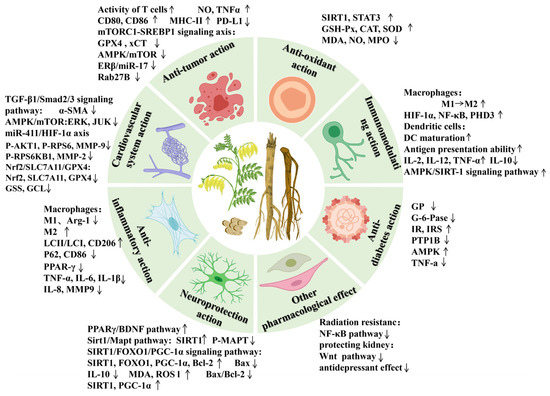
Figure 7.
The modern pharmacologic actions and mechanisms of AR. The upward arrow denotes upregulation of the protein expression and activation of the signaling pathway, whereas the downward arrow represents downregulation of the protein and inhibition of the pathway.
6.1. Anti-Tumor Action
AR, which is rich in Astragalus polysaccharides (APSs), flavonoids, saponins, and other active ingredients, can effectively inhibit tumor metastasis and diffusion.
Chronic inflammation is considered to be the cause of many diseases, such as tumors. APS can alleviate inflammation caused by lipopolysaccharide (LPS) and inhibit the inflammatory reaction of the tumor microenvironment in exosomes [148].
Numerous research studies have shown that dendritic cells (DCs) and T cells are modulators of immune checkpoint therapy and other tumor immunotherapies [149]. APS has gained recognition as an anti-tumor immunomodulator in clinical practice. APS can promote DC and T cell activation by increasing MHC-II, CD80, and CD86 expression [150]. It can regulate immune function and autophagy and enhance the efficacy of chemotherapeutic or targeted drugs by reducing their toxicity [3]. The combination of APS and 5-Fluorouracil can enhance the anti-tumor effect and reduce damage to the immune system [151]. Formononetin restored the activity of T cells and inhibited the growth of tumor xenografts [152].
Promoting autophagy and apoptosis in tumor cells can directly inhibit their growth of tumor cells [153]. Research findings suggest that APS can activate macrophages to release NO and TNF-α, which directly blocks cancer cell growth [154]. APS can also activate macrophages by inducing apoptosis, so that the cell cycle remains in the G2 phase, thereby inhibiting the growth of tumor cells [151]. Calycosin has been found to block the growth cycle of tumor cells, inhibit their proliferation of tumor cells, and induce apoptosis [155]. Formononetin has been shown to inhibit cell proliferation, tube formation, cell migration and promote tumor cell apoptosis by suppressing PD-L1 [152]. Studies have demonstrated that calycosin can induce autophagy and apoptosis in tumor cells by modulating the AMPK/mTOR, ERβ/miR-17, and Rab27B-dependent signaling pathways [156,157,158].
Ferroptosis has emerged as a promising approach for anti-tumor therapy, with targeting ferroptosis to eliminate tumor cells being recognized as a potentially effective strategy [159]. Formononetin triggers ferroptosis in tumor cells by modulating the mTORC1-SREBP1 signaling axis and suppressing the expression of key ferroptosis-related proteins, including GPX4 and xCT [160].
6.2. Antioxidant Action
At present, AR can resist oxidative stress by directly removing free radicals, improving the activity of antioxidant enzymes, inhibiting the activity of promoting enzymes, and regulating signaling pathways [4].
Oxidative stress elevates intracellular levels of reactive oxygen species (ROS), leading to cellular damage. Astragaloside IV (AS-IV) and formononetin have been shown to mitigate oxidative stress by activating the SIRT1 and STAT3 pathways, thereby exerting cytoprotective effects [161,162].
Formononetin, calycosin, and calycosin-7-glucoside demonstrated significant antioxidant activity, as evidenced by their free radical scavenging capabilities assessed through DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activity and oxygen radical absorbance capacity (ORAC) assays [163].
Calycosin mitigates oxidative stress by suppressing the generation of reactive oxygen species (ROS) and enhancing the activity of antioxidant enzymes, including glutathione peroxidase (GSH-Px), catalase (CAT), and superoxide dismutase (SOD) [164]. APS enhanced the antioxidant capacity of tumor-bearing mice, specifically, APS reduced the levels of malondialdehyde (MDA), nitric oxide (NO), and myeloperoxidase (MPO), while increasing the activities of SOD, CAT, and GSH-Px by establishing a mouse ascites tumor model [165]. APS can mitigate oxidative stress and immune injury induced by acute cerebral ischemia-reperfusion injury in rats. This is achieved by enhancing SOD activity, increasing glutathione (GSH) levels, and reducing MDA content [166].
6.3. Cardiovascular System Action
AR has a protective effect on cardiovascular diseases because of its rich bioactive constituents, which exert various pharmacological actions that benefit the cardiovascular system [41,167].
The combination of Astragalus and Angelica has been shown to modulate the TGF-β1/Smad2/3 signaling pathway, thereby suppressing the expression of aortic α-SMA. This inhibition contributes to the prevention of vascular intima proliferation [168]. Calycosin can inhibit vascular calcification by inhibiting AMPK/mTOR [169]. AS-IV has been shown to promote neovascularization and protect cardiac function by modulating the miR-411/HIF-1α signaling axis [170].
Vascular remodeling is a common pathological process [171]. Research indicates that cycloastragenol downregulates the expression of P-AKT1, P-RPS6, and P-RPS6KB1 through the AKT1/RPS6KB1 signaling pathway, thereby enhancing myocardial autophagy. Simultaneously, it inhibits the expression of MMP-2 and MMP-9, improving cardiac insufficiency and remodeling [172]. Additionally, AS-IV has been shown to reduce CDK2 activity and block the G1/S phase transition, thereby mitigating pathological vascular remodeling in atherosclerosis [173].
AS-IV significantly inhibits the phosphorylation of ERK, JNK, and p38 MAPK in endothelial cells, as well as the phosphorylation of the upstream regulator TAK1. This inhibition suppresses the proinflammatory activation of vascular endothelial cells, reduces monocyte migration and adhesion, and ultimately exerts a protective effect on cardiovascular health [174].
Calycosin upregulates the protein expression of Nrf2, SLC7A11, GPX4, GSS, and GCL by activating the Nrf2/SLC7A11/GPX4 signaling pathway. This enhancement strengthens the antioxidant capacity of the myocardial tissue and effectively inhibits ferroptosis in cardiomyocytes [175].
6.4. Immunomodulating Action
According to TCM theory, AR can tonify qi and elevate yang, which is closely associated with immune regulation in humans [6].
DCs are specialized antigen-presenting cells that initiate the primary immune response. APS promote DC maturation, enhance their antigen-presentation capabilities, and reduce their endocytic activity [176].
Macrophages play critical roles in both innate and adaptive immunity [177]. Under the influence of cytokines in the microenvironment, macrophages differentiate into various types of tumor-associated macrophages (TAMs), primarily M1 and M2 phenotypes [178]. AS-IV increases interferon expression and restores suppressed innate immune function by modulating the Cgas/STING signaling pathway [179]. Moreover, AS-IV stimulates the HIF-1α/NF-κB signaling pathway, leading to the upregulation of HIF-1α, NF-κB, and PHD3 protein expression, thereby augmenting macrophage immune function [180].
The immune response is a process in which immune substances generate specific effects. By effectively enhancing the body’s immune response, the stability of the internal environment can be maintained [50]. Treatment with APS promotes the secretion of IL-2, IL-12, and TNF-α in serum, while concurrently decreasing IL-10 levels, thereby enhancing immune responses [181]. Furthermore, APS activates the AMPK/SIRT-1 signaling pathway, thereby alleviating OTA-induced immune stress in both in vitro and in vivo models [182].
6.5. Anti-Inflammatory Action
It has been proven that AR has strong anti-inflammatory activity because the presence of various bioactive constituents in AR that exhibit anti-inflammatory effects.
Some active constituents of AR can directly inhibit the expression of related inflammatory factors. Formononetin inhibits the MAPK signaling pathway, upregulates peroxisome proliferator-activated receptor-γ (PPAR-γ) in the nucleus, and reduces the release of inflammatory factors, thereby exerting anti-inflammatory effects [183]. Research has shown that calycosin has a strong anti-inflammatory effect, which can reduce the levels of TNF-α, interleukin-6 (IL-6), and IL-1β [184]. Quercetin significantly downregulated TNF-α-induced MMP-9 expression in GES-1 cells via the TNFR-c-Src-ERK1/2, c-Fos, and NF-κB signaling pathways, demonstrating its potent anti-inflammatory effects [185,186]. Hou et al. (2019) demonstrated that the production of histamine and proinflammatory cytokines, including IL-6, interleukin-8 (IL-8), IL-1β, and TNF-α, was significantly reduced in KU812 cells treated with quercetin following inflammation. This finding provides evidence that quercetin possesses anti-inflammatory properties [187].
Macrophages are divided into M1 and M2 types [178,188]. M1 macrophages secrete an array of inflammatory mediators, including IL-1β, interferon-γ, and TNF-α, which exacerbate secondary injuries. Conversely, M2 macrophages secrete anti-inflammatory factors to mitigate inflammation in the affected area. Studies have shown that the ethanol extract of AR significantly inhibits the expression of Arginase-1, a marker of the M1 macrophage phenotype [189]. APS suppresses M1 macrophage expression, enhances M2 macrophage expression, facilitates the transition from the M1 to M2 phenotype, and ameliorates the inflammatory microenvironment in experimental autoimmune encephalomyelitis mice [190]. Formononetin increases the expression of LCII/LCI and CD206, decreases the expression of P62 and CD86, and mitigates inflammation by modulating macrophage autophagy and polarization [191,192].
6.6. Anti-Diabetes Action
The bioactive compounds in AR, including polysaccharides, flavonoids, and saponins. These constituents may exert their effects through multiple mechanisms to modulate the blood glucose levels.
One study demonstrated that AS-IV can lower blood glucose levels in high-fat diet plus streptozotocin-induced diabetic mice by inhibiting glycogen phosphorylase (GP) and glucose-6-phosphatase (G-6-Pase) activities, thereby suppressing hepatic glycogenolysis and glucose oxidation in the liver [193].
APS exhibits a potent ability to rectify the abnormally elevated protein tyrosine phosphatase 1B (PTP1B) activity in skeletal muscle during insulin resistance, thereby enhancing insulin receptor (IR) and insulin receptor substrate (IRS) tyrosine phosphorylation, ameliorating insulin signaling, and increasing insulin sensitivity [194]. Concurrently, APS can augment insulin sensitivity by activating protein kinase AMPK and promoting glucose uptake in adipocytes [195]. Additionally, the combination of APS with insulin has been shown to diminish the insulin resistance index by reducing TNF-α expression, which is beneficial for the management of diabetes [196].
6.7. Neuroprotection Action
AR and its active ingredients have been reported to show good curative effects in anti-nerve damage and protection of nerve function [197].
Glial cells and neurons are the two main cell types in the nervous system and play important roles in the repair of nerve injury [198]. AS-IV also protects against neuronal apoptosis by promoting the PPARγ/BDNF signaling pathway [199]. Moreover, it can upregulate SIRT1 through the Sirt1/Mapt pathway and further control MAPT modification to reduce the hyperphosphorylation of MAPT, thereby protecting against neuronal apoptosis [200]. Calycosin-7-O-β-D-glucopyranoside mitigates neuronal injury by modulating the SIRT1/FOXO1/PGC-1α pathway, enhancing the expression of SIRT1, FOXO1, PGC-1α, and Bcl-2, while repressing Bax expression [201].
Nerve injury is closely associated with neuroinflammation in the brain [202]. IL-10 expression in cortical neurons can be activated by formononetin to inhibit neuroinflammation and protect the nerves [203].
Similarly, neuronal injury is associated with oxidative stress in the brain [202]. Formononetin can also affect oxidative stress, reduce MDA and ROS levels, enhance mitochondrial membrane potential and cell viability, and improve nerve injury [58,204]. Additionally, formononetin has been shown to improve neurological deficits by stimulating the PI3K/Akt signaling pathway and downregulating the Bax/Bcl-2 ratio [205]. As a major active constituent of AR, calycosin-7-O-β-D-glucopyranoside alleviates neuronal injury by upregulating SIRT1 and PGC-1α protein expression and reducing excessive mitochondrial fission and overactivation of mitochondrial autophagy [206].
6.8. Other Pharmacological Effect
Moreover, AR can confer resistance to radiation, thereby safeguarding retinal ganglion cells, facilitating osteogenesis, and preserving kidney function [207]. a AR exerts a protective influence on the visceral organs by mitigating oxidative stress, reducing inflammation, and inhibiting visceral fibrosis and apoptosis [208]. Quercetin-3-O-β-d-glucopyranoside can significantly promote cell proliferation and promote osteogenesis [209]. A study indicated that APS elicits a pro-growth effect on bone marrow stromal cells (BMSCs) exposed to 2 Gy12C6+ radiation, which may be associated with the downregulation of NF-κB signaling pathway-related proteins and maintenance of genomic stability in BMSCs [210]. AR has a protective effect on rat retinal ganglion cells. Simultaneously, it inhibits the apoptosis of lower-glucose tubular epithelial cells [211,212]. Tao et al. (2016) developed a mouse model of depression and conducted behavioral experiments related to depression. The results revealed that both liquiritigenin (7.5 mg/kg and 15 mg/kg) and fluoxetine (20 mg/kg) markedly ameliorated depressive symptoms [213].

Table 7.
Pharmacological effects of the active components of AR.
Table 7.
Pharmacological effects of the active components of AR.
| Constituents | Pharmacological Effect | Experimental Model | Concentration | References |
|---|---|---|---|---|
| Calycosin | Nervous system disease | HEK 293 cell | 50 μM | [214] |
| Estrogen-like effect | MCF-7 cell female kunming mice (weight: 18–22 g, age: 12 weeks) | 8 μM in vitro 1, 2, 4 mg/kg in vivo | [215] | |
| Anti-inflammatory effects | HaCaT, NHEK cell male C57BL/6 mice (weight: 22.78 ± 0.85 g; age: 8 weeks) | 0, 2, 5, 10 μM in vitro 5 mg/mL in vivo | [216] | |
| Antiviral effects | HUVEC, MDCK cell | 20 μg/mL | [217] | |
| Anti-Oxidative | male Balb/C mice (weight: 20 ± 2 g, age: 8–10 weeks) | 25, 50 mg/kg | [218] | |
| Breast cancer | MDA-MB-231 cell | 10 μM | [219] | |
| Anti-fatty liver | male ICR mice | 30, 60 mg/kg | [220] | |
| Cervical cancer | SiHa, CaSki, C-33A, HeLa, Etc1/E6E7 cell | 50 μM | [221] | |
| Anti-osteosarcoma | U2OS cell | 0, 10, 20, 40 μM | [222] | |
| Cancers of the liver | HepG2, Hep3, Huh7 cell | 100 μM | [223] | |
| Diabetic nephropathy | NRK-52E cell | 10 μg/mL | [224] | |
| Cardiovascular protection effect | male SD rat (weight: 240–260 g) | 20 mg/kg | [225] | |
| Acute Lung Injury | MLE-12 cell male C57BL/6N mice (weight: 18–22 g, age: 8–10 weeks) | 30 μg/mL in vivo 12.5 mg/kg in vitro | [226] | |
| Pancreatic cancer | PANC1, MIA PaCa-2, RAW 264.7, Pan02 cell | 50 μM | [227] | |
| Calycosin-7-O-β-D-glucopyranoside | Anti-Oxidative | BRL-3A cell | 10, 20, 40 mg/L | [228] |
| Anti-myocardial hypertrophy | male SD rat (weight: 247–250 g, age: 10 weeks) | 26.8 mg/kg | [229] | |
| Neuronal Apoptosis | HT22 cell | 15 μg/mL | [201] | |
| cervical cancer | - | - | [230] | |
| Immunosuppression | Inbred strain male, female Balb/c mice (age:6–8 weeks) | - | [231] | |
| ischemia-reperfusion injury | male Wistar rats | 15, 30 mg/kg | [232] | |
| cervical cancer | HeLa cells | 20, 40, 80 μg/mL | [233] | |
| osteoarthritis | Adolescen New Zealand white rabbit, 9 (weight: 3.0–3.5 kg) | 200 μg/ ml | [234] | |
| Formononetin | Anti-Oxidative | male SD rat (weight: 160–170 g) | 10, 20, 40 mg/kg | [162] |
| Anti-liver damage | male CD-1 mice (age: 7 years) | 50, 100 mg/kg | [235] | |
| Anti-inflammatory effects | HaCaT cell, male BALB/c mice (age: 6–8 weeks) | 0.1, 1, 10 μM in vitro 10 mg/kg in vivo | [236] | |
| Neuroprotection | male SD rat (weight: 160–180 g) | 30 mg/kg | [237] | |
| Immunosuppression | Hep G2 cell | 10, 50 mg/kg· | [238] | |
| Anti-tumor effects | CNE2 cell | 10, 20, 40 μM | [239] | |
| Protecting heart muscle cells | male C57BL/6 mice (weight: 18.9 ± 1.0 g) | 20, 40 mg/kg | [240], | |
| Protective effect on osteoblasts | ROB cell | 10-6, 10-5, 10-4 mol/L | [241] | |
| Ononin | Improve renal injury | male SD rat (weight: 250 ± 00 g) | 50, 200 mg/kg | [242] |
| Anti-inflammatory effects | RAW 264.7 cell | 5, 25, 50, 100 μM | [243] | |
| Isoquercitrin | Anti-Oxidative | PC12 cell | 1, 10, 100 μmol/L | [244] |
| Anti-inflammatory effects | KU812 cell | 12.5, 25, 50 μg/mL | [245] | |
| Promoting osteogenesis | BMSC male Wistar rat (weight: 150 ± 10 g, age: 6 weeks) | 0.1, 1 μM in vitro, 10mg/kg in vivo | [246] | |
| Anti-tumor effects | SD rat | - | [247] | |
| Diuretic effect | SH rat (weight: 250–300 g, age: 3–4 months) | 10 mg/kg | [248] | |
| Anti-hypertension | male Wistar rat (weight: 250–300 g, age: 3–4 months) | 2, 4 mg/kg | [249] | |
| Anti-liver damage | male kunming mice (weight: 20–25 g) | 10, 20, 50 mg/kg | [250] | |
| Isorhamnetin | Anti-tumor effects | AGS, MKN45, HFE-145 cell | 0, 10, 25, 50 μm | [251] |
| Anti-osteoporosis | SD rat (weight: 180 ± 20, age: 11 weeks) | 30 mg/kg | [252] | |
| Anti-Oxidative | H9c2 cell | 0, 3, 6, 12, 25, 50 μM | [253] | |
| Anti-inflammatory effects | HGFs cell | 10, 20, 40 μM | [254] | |
| Kaempferol | breast cancer | SK-BR-3 cell | 30, 60 μmol/L | [255] |
| Anti-inflammatory effects | PC12 cell | 20, 40, 60, 80, 100 μmol/L | [256] | |
| Anti-liver damage | male Kunming mice (weight: 20–22 g) | 6, 18mg/kg | [257] | |
| Quercetin | Anti-Oxidative | Human endometrial stromal cell | 10, 20 μmol/L | [258] |
| Anti-liver damage | male Wistar rat (weight: 240 ± 20 g) | 5, 10, 20 mg/kg | [259] | |
| Anti-inflammatory effects | male SD rat (weight: 250–300 g) | 20 mg/kg | [260], | |
| Neuroprotection | SH-SY5Y cell | 0.1, 1, 10, 25 μmol/L | [261] | |
| Anti-tumor effects | RPMI-8226, NCI-H929 cell | 0, 0.01, 0.1, 1, 10, 50, 100 µM | [262] | |
| Heart protective effect | H9C2 cell male SD rat (weight: 180–200 g) | 50 mg/kg in vivo, 50 μM in vitro | [263] | |
| Anti-aging | - | - | [264] | |
| Immunomodulating effects | male C57BL/6 mice (weight:20–22 g, age: 8 weeks) | 50, 100 mg/kg | [265] | |
| Scavenging free radicals | - | - | [266] | |
| Isoliquiritigenin | Anti-tumor effects | Human lung adenocarcinoma, HCC827, NCI-H1650, NCI-H1975, A549 cell, 293T, NIH3T3 | 10, 20, 40 µM | [267] |
| Anti-Oxidative | female Swiss-Webster mice (age: 9 weeks) | 0, 50, 100, 300 mg/kg | [268] | |
| Anti-inflammatory effects | THP-1 cells | 0, 1, 3, 5, 7, 10 µM | [269] | |
| Liquiritigenin | Anti-inflammatory effects | Swiss albino mice (weight: 180–200 g) | 30, 100, 300 mg/kg | [270] |
| Anti-tumor effects | H1299 cell | 0.1, 0.2, 0.4, 0.8 mmol/L | [271] | |
| Anti-diabetes | male Swiss albino mice (weight: 25–30 g) | 50, 100, 200 mg/kg | [272] | |
| romoting osteogenesis | MC3T3-E1 cell | 0.04, 0.4, 4 µM | [273] | |
| Anti-depression | male ICR mice (weight: 20–22 g) | 20, 7.5, 15 mg/kg | [213] | |
| Anti-liver fibrosis | male C57BL/6 mice (weight: 20–22 g) | 10, 30 mg/kg | [274] | |
| Pratensein | Improving cognitive impairment | male Wistar rat (weight: 300 ± 20 g, age: 10 weeks) | 10, 20 mg/kg | [275] |
| Echinatin | Anti-tumor effects | KYSE 30, KYSE 270 cell, male nude mice (age: 6–8 weeks) | 20, 50 mg/kg in vivo 0, 10, 20, 40 µM in vitro | [276] |
| Anti-inflammatory effects | C57BL/6 mice | 0.4, 0.8 mM | [277] | |
| Licochalcone B | Anti-tumor effects | HepG2 cell | 120 μM | [278] |
| melanoma | B16F0 cell | 5, 7.5, 10, 12.5, 15 mg/L | [279] | |
| Quercetin-3-O-β-D-glucoside | Anti-Oxidative | - | - | [280] |
| Genistein | Anti-Oxidative | Keratinocytes, fibroblasts | 10, 1, 100 μM | [281] |
| Osteoarthritis | Human chondrocytes | 0, 5, 10, 50, 100 μM/mL | [282] | |
| Heart protective effect | H9c2 cell male SD rat (weight: 180–200 g) | 5 μM in vitro 20, 40mg/kg in vivo | [283] | |
| injury of the kidney | male SD rat (weight: 180–210 g) | 30 mg/kg | [284] | |
| Glycitin | Antiallergic | osteoclasts cell | 10 nM | [285] |
| Anti-tumor effects | U87MG cell | 50 μM | [286] | |
| Tiliroside | Anti-tumor effects | BT-549, MDA-MB-46, SK-BR-3, MCF-7, MCF-10A cell | 100, 150 μM | [286] |
| Anti-inflammatory effects | macrophages female C57BL/6 mice, male BALB/c mice (age: 6–8 weeks) | 10, 20, 40 μM in vitro 25, 50 mg/kg in vivo | [287] | |
| Anti-Oxidative | female Wistar rat (weight: 180–200 g) Swiss female mice (weight: 25–30 g) | 50 mg/kg | [288] | |
| Gallic acid | Neuroprotection | male Wistar rat (weight: 250–300 g) | 100 mg/kg | [289] |
| Anti-Oxidative | Male ICR mice (age: 8 weeks) | 100 mg/kg | [290] | |
| Bone Tissue Regeneration | Female SD rat (weight: 200 g) | 1, 5, 25, 100 μM | [291] | |
| Liver Injury | male C57BL/6J mice (age: 8–10 weeks) | 5, 20 mg kg | [292] | |
| Anti-tumor effects | 22 Rv1, DU 145, PWR-1E cell | 25, 50, 75 μM | [18] | |
| Anti-inflammatory effects | Synovial fibroblasts | 40, 60, 80 μM | [293] | |
| Agroastragaloside V | Anti-inflammatory effects | RAW 264.7 macrophages | - | [293] |
| Agroastragalosides I | Anti-inflammatory effects | RAW 264.7 macrophages | - | [293] |
| Agroastragalosides II | Anti-inflammatory effects | RAW 264.7 macrophages | - | [293] |
| Astragaloside IV | Anti-inflammatory effects | male SD rat (weight: 180–200 g) | 40, 80 mg/kg | [294] |
| Anti-tumor effects | RAW264.7 cell | 800, 400, 200, 100, 50, 25, 0 μg/mL | [295] | |
| Immunomodulating effects | PAM cell | 200, 100, 50, 25, 12.5, 6.25 μg/mL | [179] | |
| Anti-tumor effects | RWPE-1, PC3 cell male BALB/c nude mice (age: 4–6 weeks) | 20 μmol/L in vitro, 20 μg/mL in vivo | [296] | |
| ameliorate atherosclerosis | male ApoE-/- mice, male C57BL/6J mice (weight: 20 ± 2 g) | 5 mg/kg | [297] | |
| attenuates renal injury | HK-2 cell, male SD rat (weight: 170 ± 10g) | 20, 40, 80 μM 20, 40, 80 mg/kg in vivo | [298] | |
| Improving cardiac function | male C57BL/6J mice (weight: 22 ± 2 g, age: 4–6 weeks) | 40 mg/kg | [299] | |
| against myocardial fibrosis | male C57BL/6J mice (weight: 20–22 g, age: 7–8 weeks) | 100, 200mg/kg | [300] | |
| Astragaloside II | hepatoma | Hep G2 cell | 20, 40, 80 μmol/L | [301] |
| protect renal | male SD rat (age: 8 weeks) | 3.2, 6.4 mg/kg | [302] | |
| Cycloastragenol | Anti-inflammatory | BMDM cell female C57BL/6 mice (age: 6–8 weeks) | 3, 10, 30 μM in vitro 12.5, 25, 50 mg/kg in vivo | [303] |
| Preventing osteoporosis | MC3T3-E1 cell, male SD rat (age: 9 weeks) | 0.03, 0.1, 0.3 μM | [304] | |
| Neuroprotection | male C57BL/6 mice (weight: 23–26 g) | 5, 10, 20 mg/kg | [305] | |
| Anti-myocardial fibrosis | Cardiac fibroblasts, male BALB/c mice (weight: 24–25 g, age: 10 weeks) | 0, 15.625, 25, 31.25, 50, 62.5 100 μg/mL in vitro 31.25, 62.5, 100, 200 mg/kg in vivo | [306] | |
| gastric cancer | SNU-1, SNU-16 cell | 0, 1, 5, 10, 30, 50 μM | [307] | |
| Anti-aging | famale Kunming mice (weight: 22 ± 2 g) | 2.5, 5.0, 10 mg/kg | [308] | |
| Isoastragaloside II | Anti-inflammatory | - | - | [117] |
| Soyasapogenol B | Liver protection | Male BALB/c mice - | 80mg/kg - | [309] |
| Memory Impairment | BV-2, SH-SY5Y Cell male ICR mice (weight: 25–28 g, age: 6 weeks) | 1, 10, 20 mg/kg in vivo, 5, 10 μM in vitro | [310] | |
| Soyasaponin I | Anti-tumor effects | MCF-7, MDA-MB-231 cell | 50, 70 μM | [311] |
| Anti-inflammatory | IPEC-J2 cell female BALB/c mice | 10 μM in vitro, 20 mg/kg in vivo | [312] |
Note: concetration.
7. Computer-Aided Drug Design Research
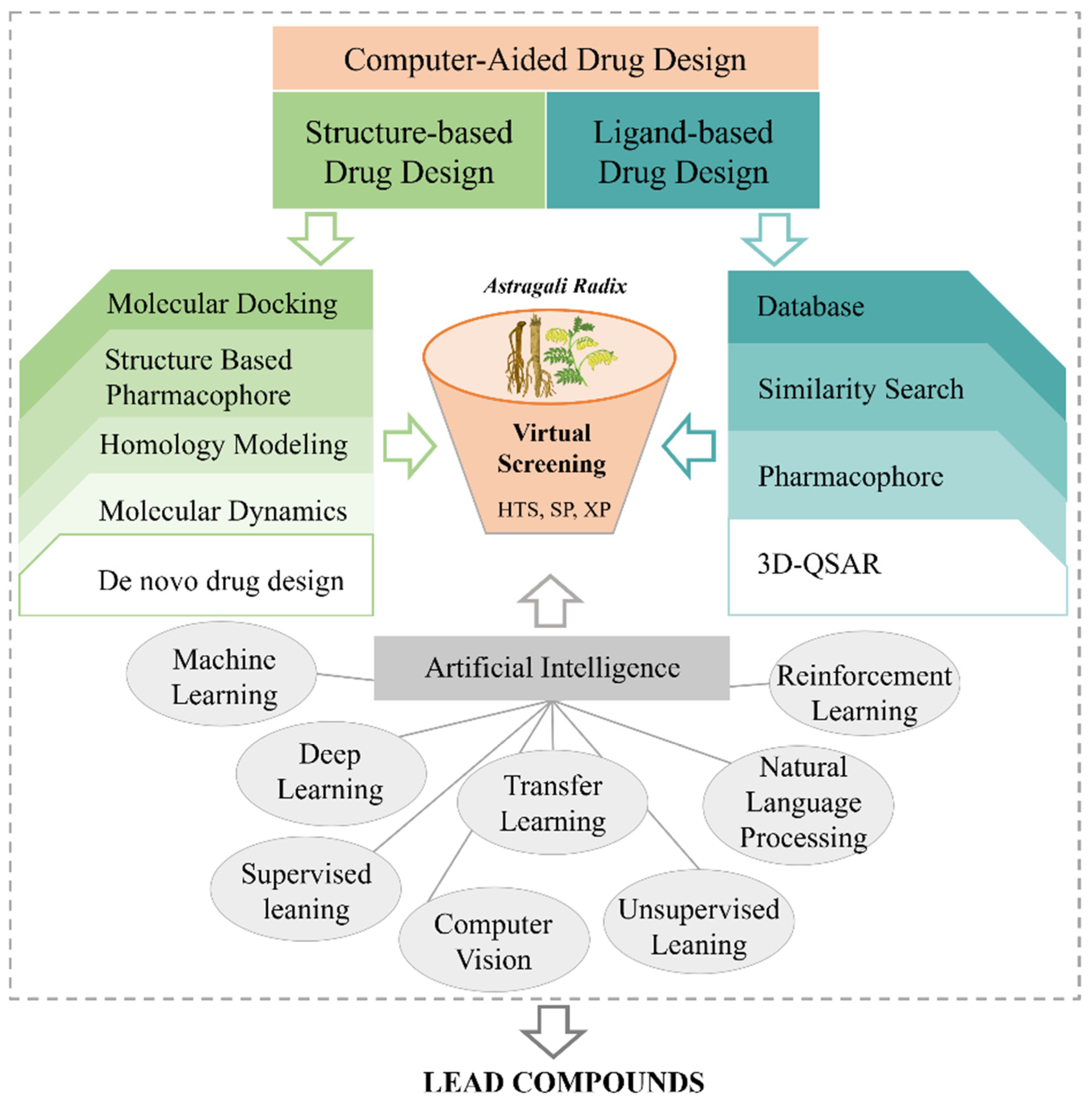
As a renowned practice of natural medicine, TCM employs a personalized and holistic approach to treat diseases through the use of natural medical products. It offers an extensive pool of potential therapeutic candidates, leveraging the vast chemical structure space of its components. However, despite the significant potential of TCM in drug discovery, traditional methods of identifying new drugs from this rich resource have proven to be challenging [313,314]. Recently, advancements in network pharmacology, complex networks, computer-aided drug design (CADD), and other methodologies have revolutionized TCM research, particularly in the discovery and optimization of lead compounds (Figure 8) [315]. These methods not only overcome the limitations of animal pharmacological experiments but also significantly enhance the efficiency and success rate of scientific research [316].
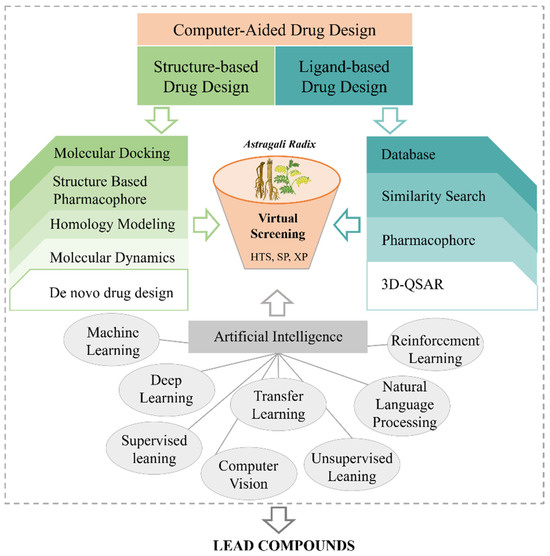
Figure 8.
Computer-aided drug design aided the durg development of AR.
7.1. Discovery of Lead Compounds
Computer-aided drug design and artificial intelligence technology play key roles in the discovery of lead compounds, accelerating the development of new drugs through efficient screening and optimization [11]. The chemical constituents of AR were collected, and reverse docking, target prediction, network pharmacological analysis, molecular docking, and virtual screening were performed to identify the potential active ingredients as lead compounds for further evaluation and optimization.
Network pharmacology explores disease development from a systems biology perspective, elucidates drug-body interactions from a holistic viewpoint, and guides the discovery of new drugs [317]. Recent studies have utilized databases to collect the chemical constituents of AR, constructing networks to predict its material basis and molecular mechanisms for treating colon cancer [318]. Molecular docking, a crucial virtual screening method, predicts the interactions between TCM molecules and target proteins, allowing for the identification of potential lead compounds and facilitating the efficient screening of small molecules in TCM [319]. In 2024, Chen et al. explored the active components and molecular mechanisms of AR in heart failure by integrating component-signal-target networks with molecular docking [320].
In recent years, molecular dynamics simulations have also been incorporated into molecular screening to enhance accuracy [321]. Additionally, Artificial intelligence (AI) methods, such as graph neural networks, have also been applied in TCM research [322]. For instance, Zhang et al. identified the authenticity of more than 160 AR samples using a random-weight neural network, further demonstrating that artificial intelligence algorithms, including graph neural networks, hold significant potential for modernizing TCM [322]. Additionally, by combining machine learning algorithms with molecular simulation techniques, researchers screened the Specs database to identify potential αvβ3 integrin inhibitors. Systematic structural modifications and in vitro validation revealed that compound C19-9 exhibits significant anti-tumor activity [323]. These findings underscore the transformative potential of AI-driven methodologies, particularly machine learning-based approaches, for modern drug discovery and pharmaceutical innovation.
7.2. Discovery Potential Drug Targets of AR
A comprehensive understanding of the active component structures of AR facilitated constituent profiling, reverse docking searches, and Pharmacophore Construction to identify potential pharmacodynamic targets. Reverse docking computationally simulates the binding of small molecules to multiple proteins and predicts their most likely biological targets [324]. This technique has been extensively used to discover new targets for existing drugs and natural constituents [325]. For example, Kichul Park et al. applied reverse docking to ginsenosides, the active ingredients in Korean ginseng, identifying four potential targets and evaluating their potential toxicity and side effects [326]. Thus, reverse docking offers a novel approach for advancing drug research in TCM.
Pharmacophore construction represents the atomic or molecular features of a drug that facilitate non-bond interactions, such as hydrogen bonding, electrostatic interactions, and hydrophobicity with receptor-binding sites, along with their spatial arrangements [327]. Pharmacophore models have been employed to identify targets through reverse screening in target interaction databases [328]. For instance, Tang et al. matched AS-IV with multiple pharmacophore models representing various target proteins to determine their potential targets [329]. These computational approaches not only enhance the efficiency of drug discovery but also provide deeper insights into the molecular mechanisms of compounds from TCM. Future research should focus on refining these computational methods and integrating them with experimental validation to accelerate the development of novel therapeutics and advance the modernization of AR.
8. Conclusions and Future Direction Discussion
As research on Astragali Radix (AR) progresses, its active constituents have long been recognized as pleiotropic agents for treating various acute and chronic diseases. The diverse pharmacological properties of AR components make it a promising therapeutic option for multiple conditions [330]. Modern pharmacological studies have demonstrated that AR exhibits anti-tumor, antioxidant, cardiovascular-protective, immunomodulatory, anti-inflammatory, anti-diabetic, and neuroprotective effects, among others [3,4,5,6,7,8,9]. This review offers a comprehensive analysis of AR, covering its traditional use, botanical characteristics, drug formulation, chemical composition, and modern pharmacological properties. This review serves as a valuable resource for researchers seeking a deeper understanding of AR and its pharmaceutical potential.
AR, a renowned traditional Chinese medicinal herb, has been extensively utilized in the treatment of a wide array of diseases. Despite significant advancements in AR research, some problems remain. AR is composed of a complex array of chemical constituents, with the content of bioactive constituents varying among different varieties of AR. The extraction and processing methods affect the effective constituents of AR. In contrast, after entering the human body, the constituents of TCM are metabolized and distributed, ultimately entering the bloodstream. The identification of the components in the blood is crucial for the efficacy and safety of AR. The identification and quantification of these blood-absorbed constituents are critical for evaluating the pharmacological effects and safety profiles of AR. Therefore, it is important to control the quality of AR and determine its active ingredients. Although the pharmacological effects of AR have been extensively studied, based on the theory of TCM, we need to focus on the main effects of AR. Moreover, TCM has the characteristics of multi-component, multi-target, and multi-pathway, and AR also affects multiple pathways through multiple components and targets. Based on the primary TCM efficacy of AR, such as tonifying qi, elevating yang, and consolidating the body’s surface to reduce sweating, research should focus on its roles in immune regulation, metabolic function, and other related areas. By combining TCM theories with modern scientific approaches, studies can ex-plore the mechanisms of AR in enhancing immune responses, improving metabolic homeostasis, and addressing related disorders, thereby advancing its application in integrative medicine and contributing to the modernization of AR.
Furthermore, the field of TCM has entered a new era of development. CADD and AI have been increasingly integrated into modern TCM research. AR contains complex chemical constituents; it is necessary to build an intelligent database to summarize the constituents of AR. Conversely, there is a lack of in-depth calculation and research on the absolute configuration and medicinal properties of sugar-containing substances in AR. Molecular docking, 3D-QSAR, and similarity search have been widely used to identify effective molecules and potential targets of AR. Advanced techniques such as molecular docking, three-dimensional quantitative structure-activity relationship (3D-QSAR) modeling, and similarity searching have been widely employed to identify bioactive molecules in Astragalus and their potential therapeutic targets. Computational simulation technologies have emerged as powerful tools for elucidating the dynamic interaction mechanisms between AR’s bioactive constituents and their pharmacological targets. These technologies not only predict the binding sites and affinities between active constituents and targets but also simulate the dynamic behavior of drug molecules within biological environments, providing profound insights into their mechanisms of action and facilitating the development of targeted therapies. These advanced technologies provide a pathway for the modernization of AR. By leveraging these methods, researchers will be able to delve deeper into the mechanisms of action of AR in treating diseases, providing a more comprehensive understanding of the intricate molecular interactions between AR constituents and their targets. These efforts will provide valuable insights into the pharmacological mechanisms of AR.
In addition, clinical studies have demonstrated that APS injections effectively enhance the immune response. When used in combination with other chemical drugs, APS has been shown to amplify anti-tumor effects while reducing adverse reactions [331]. Similarly, AS-IV injections have exhibited significant efficacy in treating cardiovascular diseases. However, due to side effects and poor oral bioavailability, its development was discontinued during preclinical trials [332]. These findings suggest that the active compounds derived from Astragalus hold substantial potential for further exploration. Moreover, the integration of advanced methodologies, such as complex network analysis and CADD, has significantly improved the efficiency and success rate of drug development in AR-related research. These cutting-edge approaches offer promising prospects for screening active compounds and elucidating the mechanisms of action of Astragalus. Ongoing advancements in modern science and technology continue to facilitate the exploration and global dissemination of AR-based therapies.
Author Contributions
Conceptualization, writing-original draft, writing-review and editing, X.J.; writing-original draft, writing-review and editing, H.Z.; writing-original draft, Investigation, visualization, X.X., M.Z., R.W. and H.L.; investigation, resources, X.W., J.W. and D.L.; visualization, Supervision, Y.L. (Yaling Li), J.L., W.X. and J.H.; Supervision, investigation, J.Y. and Y.L. (Yongqi Liu); All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Provincial University industry support project in Gansu (2022CYZC-54), National Natural Science Foundation of China (No. 82004202), National Natural Science Foundation of China (No. 82460862), 2021 Gansu Province Youth Science and Technology Talent Lift Project (GXH20210611-02).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AR | Astragali Radix |
| AMM | Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao |
| AM | Astragalus membranaceus (Fisch.) Bge |
| APS | Astragalus Polysaccharide |
| AS-IV | Astragaloside IV |
| CAT | Catalase |
| CD | cluster of differentiation |
| CADD | Computer-aided drug design |
| DCs | Dendritic cells |
| GP | glycogen phosphorylase |
| GSH | glutathione |
| GSH-Px | Glutathione Peroxidase |
| G-6-Pase | glucose-6-phosphatase |
| IL-6 | interleukin-6 |
| IL-1β | interleukin-1β |
| IL-8 | interleukin-8 |
| IFN-γ | interferon-γ |
| IR | insulin receptor |
| IRS | insulin receptor substrate |
| LPS | lipopolysaccharide |
| MPO | Myeloperoxidase |
| MDA | malondialdehyde |
| NF-κB | nuclear factor kappa-B |
| NO | nitric oxide |
| ORAC | absorbance capacity |
| PTP1B | Protein Tyrosine Phosphatase 1B |
| SIRT1 | silent information regulator 1 |
| SOD | superoxide dismutase |
| TAMs | tumor-associated Macrophages |
| TNF-α | tumor necrosis factor-α |
| TCM | Traditional Chinese medicine |
References
- Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2020.
- Shao, C.; Lin, H.; Jin, X.; Li, Y.; Liu, Y.; Yao, J. Historical evolution and modern research progress of Astragali Radix processing. Chin. Tradit. Herb. Drugs 2023, 54, 5057–5074. [Google Scholar] [CrossRef]
- Yang, Q.; Meng, D.; Zhang, Q.; Wang, J. Advances in research on the anti-tumor mechanism of Astragalus polysaccharides. Front. Oncol. 2024, 14, 1334915. [Google Scholar] [CrossRef]
- Yao, J.; Peng, T.; Shao, C.; Liu, Y.; Lin, H.; Liu, Y. The Antioxidant Action of Astragali radix: Its Active Components and Molecular Basis. Molecules 2024, 29, 1691. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Chen, H.; Cui, Z.; Yu, B.; Kou, J.; Li, F. Astragali Radix-Notoginseng Radix et Rhizoma medicine pair prevents cardiac remodeling by improving mitochondrial dynamic balance. Chin. J. Nat. Med. 2025, 23, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Gui, W.Y.; Yin, J.G.; Liao, J.C.; Luo, H.Z.; You, Q.; Gong, J.H.; Xiang, J.; Zou, J.D.; Li, C.Y. Integrated analysis of metabolome, lipidome, and gut microbiome reveals the immunomodulation of Astragali radix in healthy human subjects. Chin. Med. 2024, 19, 174. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Shi, C.; Wei, C.; Wang, C.; Du, H.; Hong, Q.; Chen, X. Fufang shenhua tablet, astragali radix and its active component astragaloside IV: Research progress on anti-inflammatory and immunomodulatory mechanisms in the kidney. Front. Pharmacol. 2023, 14, 1131635. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Xiao, X.; Wang, S.; Shi, F.; Liu, N.; Chen, X.; Yang, W.; Wang, L.; Chen, F. Hypoglycemic effect of astragaloside IV via modulating gut microbiota and regulating AMPK/SIRT1 and PI3K/AKT pathway. J. Ethnopharmacol. 2021, 281, 114558. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Guo, R.; Ma, B.; Miao, L.; Sun, M.; He, L.; Lin, L.; Pan, Y.; Ren, J.; et al. Neuroprotective effect of Astragali Radix on cerebral infarction based on proteomics. Front. Pharmacol. 2023, 14, 1162134. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, L.; Cheng, X.L.; Qin, X.M.; Chai, Z.; Li, Z.Y. The Beneficial Effects of Dietary Astragali Radix Are Related to the Regulation of Gut Microbiota and Its Metabolites. J. Med. Food 2024, 27, 22–34. [Google Scholar] [CrossRef]
- Wang, K.; Huang, Y.; Wang, Y.; You, Q.; Wang, L. Recent advances from computer-aided drug design to artificial intelligence drug design. RSC Med. Chem. 2024, 15, 3978–4000. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, F.; Sun, J.; Zhang, L. Identifying the natural products in the treatment of atherosclerosis by increasing HDL-C level based on bioinformatics analysis, molecular docking, and in vitro experiment. J. Transl. Med. 2023, 21, 920. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Chen, J.; Zhang, P.; Zheng, N.; Ma, L.; Zhang, Y.; Zhang, H. Repurposing Drugs for Inhibition against ALDH2 via a 2D/3D Ligand-Based Similarity Search and Molecular Simulation. Molecules 2023, 28, 7325. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Y.; Deng, W.; Xie, J.; He, Y.; Wang, L.; Zhang, J.; Cui, M. Combining network pharmacology, machine learning, molecular docking and molecular dynamic to explore the mechanism of Chufeng Qingpi decoction in treating schistosomiasis. Front. Cell. Infect. Microbiol. 2024, 14, 1453529. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Liu, C.; Zhang, Z.; Cheng, M.; Mei, Z.; Li, X.; Liu, P.; Diao, L.; Ma, Y.; Jiang, P.; et al. BATMAN-TCM 2.0: An enhanced integrative database for known and predicted interactions between traditional Chinese medicine ingredients and target proteins. Nucleic Acids Res. 2024, 52, D1110–D1120. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef]
- Paul, D.; Sanap, G.; Shenoy, S.; Kalyane, D.; Kalia, K.; Tekade, R.K. Artificial intelligence in drug discovery and development. Drug Discov. Today 2021, 26, 80–93. [Google Scholar] [CrossRef]
- Kaur, M.; Velmurugan, B.; Rajamanickam, S.; Agarwal, R.; Agarwal, C. Gallic acid, an active constituent of grape seed extract, exhibits anti-proliferative, pro-apoptotic and anti-tumorigenic effects against prostate carcinoma xenograft growth in nude mice. Pharm. Res. 2009, 26, 2133–2140. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Z.; Huang, L.; Zheng, S.; Wang, D.; Chen, S.; Zhang, H.; Yang, S. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 2014, 28, 1275–1283. [Google Scholar] [CrossRef]
- Li, C.; Zhu, F.; Wang, S.; Wang, J.; Wu, B. Danggui Buxue Decoction Ameliorates Inflammatory Bowel Disease by Improving Inflammation and Rebuilding Intestinal Mucosal Barrier. Evid. Based Complement Altern. Med. 2021, 2021, 8853141. [Google Scholar] [CrossRef]
- Zhan, X.; Xu, X.; Zhang, P.; Wang, X.; Hu, Z.; Zhao, W.; Hang, T.; Song, M. Crude polysaccharide from Danggui Buxue decoction enhanced the anti-tumor effect of gemcitabine by remodeling tumor-associated macrophages. Int. J. Biol. Macromol. 2023, 242, 125063. [Google Scholar] [CrossRef]
- Li, M.; Jiang, H.; Hao, Y.; Du, K.; Du, H.; Ma, C.; Tu, H.; He, Y. A systematic review on botany, processing, application, phytochemistry and pharmacological action of Radix Rehmnniae. J. Ethnopharmacol. 2022, 285, 114820. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.Y.; Yang, C.M.; Chen, C.H. Effects of Physical Properties and Processing Methods on Astragaloside IV and Flavonoids Content in Astragali radix. Molecules 2022, 27, 575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Niu, J.; Zhang, S.; Si, X.; Bian, T.T.; Wu, H.; Li, D.; Sun, Y.; Jia, J.; Xin, E.; et al. Comparative study on the gastrointestinal- and immune-regulation functions of Hedysari Radix Paeparata Cum Melle and Astragali Radix Praeparata cum Melle in rats with spleen-qi deficiency, based on fuzzy matter-element analysis. Pharm. Biol. 2022, 60, 1237–1254. [Google Scholar] [CrossRef] [PubMed]
- China National Medical Products Administration. Available online: https://www.nmpa.gov.cn/datasearch/home-index.html#category=ylqx (accessed on 1 March 2025).
- Lu, N. Identification of Astragalus and its adulterants. J. Intern. Med. Tradit. Chin. Med. 2010, 24, 99–101. [Google Scholar] [CrossRef]
- Lei, L.; Ou, Y.; Liu, Y.; Huang, N.; Wang, J. Distribution characteristics and correlation analysis of elements in Astragalus membranaceus from different producing areas. China J. Chin. Mater. Medica 2008, 33, 255–258. [Google Scholar] [CrossRef]
- Pei, W.; He, F.; Cheng, C.; Zhou, H. Research progress on quality evaluation methods of Astragalus membranaceus. Chin. J. Mod. Appl. Pharm. 2020, 37, 620–628. [Google Scholar] [CrossRef]
- Tian, X.; Han, B.; Xiao, C.; Jaing, B. Study on the chemical constituents and biological activities of Astragalus fusiformis. Nat. Prod. Res. Dev. 2014, 26, 188–192+259. [Google Scholar] [CrossRef]
- Li, L. Identification of Astragalus and its adulterants. Strait Pharm. J. 2005, 4, 111–112. [Google Scholar] [CrossRef]
- Yu, J.; Jia, X.; Wang, X. Study on the traits and microscopic identification of Oxytropis coerulea. Chin. Wild Plant Resour. 2022, 41, 33–36. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Zhang, J. Identification comparison of Astragalus and its adulterants. Agric. Sci. Technol. Equip. 2018, 52–53+56. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Ma, Y.; Li, S.; Liang, Y. Differential application of Astragalus and its adulterants Abelmoschus manihot and Glycyrrhiza uralensis. Mod. Distance Educ. Chin. Med. 2013, 11, 128–129. [Google Scholar] [CrossRef]
- Huang, D.; Sun, S.; Xu, y.; Chen, X. Two-dimensional correlation infrared spectroscopy and analysis and identification of Astragalus membranaceus and its counterfeit Licorice. Spectrosc. Spectr. Anal. 2009, 29, 2396–2400. [Google Scholar] [CrossRef]
- Yang, S.; Yang, J.; Xin, J.; Gao, L. Gel electrophoresis identification of Mongolian medicine vetch, multi-stem vetch and vetch. Chin. J. Ethn. Med. 2002, 8, 32. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y. Study on the classification of Vicia plants in Mongolian Plateau. J. Inn. Mong. Univ. Nat. Sci. Ed. 2001, 32, 66–73. [Google Scholar]
- Gu, M. Pharmacognostic identification of Astragalus membranaceus and its counterfeit drug Abelmoschus manihot root. Chin. Pharm. Aff. 2006, 20, 574–575. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, C.; Yao, T. Identification of Astragalus counterfeit Sophora alopecuroides. Shandong J. Tradit. Chin. Med. 1987, 36–37. [Google Scholar]
- Yao, T. Isolation, Identification and the Antioxidant Activity of the Chemical Constituents from the Flower of Astragalus membranaceus var. mongholicus. Master’s Thesis, Inner Mongolia University, Hohhot, China, 2018. [Google Scholar] [CrossRef]
- Kuang, X.H.; Feng, W.S.; Yang, X.W.; Dong, X.P.; Feng, C.D.; Dou, D.Q.; Lu, R.M.; Lu, J.T.; Tian, S.G. Traditional Chinese Medicine Chemistry; China Press of Chinese Medicine: Beijing, China, 2018. [Google Scholar]
- Su, H.F.; Shaker, S.; Kuang, Y.; Zhang, M.; Ye, M.; Qiao, X. Phytochemistry and cardiovascular protective effects of Huang-Qi (Astragali Radix). Med. Res. Rev. 2021, 41, 1999–2038. [Google Scholar] [CrossRef]
- Li, M.; Han, B.; Zhao, H.; Xu, C.; Xu, D.; Sieniawska, E.; Lin, X.; Kai, G. Biological active ingredients of Astragali Radix and its mechanisms in treating cardiovascular and cerebrovascular diseases. Phytomedicine 2022, 98, 153918. [Google Scholar] [CrossRef]
- Kim, M.H.; Yeon, S.W.; Ryu, S.H.; Lee, H.H.; Turk, A.; Jeong, S.Y.; Kim, Y.J.; Lee, K.Y.; Hwang, B.Y.; Lee, M.K. Structural Diversity and Anti-Diabetic Potential of Flavonoids and Phenolic Compounds in Eriobotrya japonica Leaves. Molecules 2025, 30, 736. [Google Scholar] [CrossRef]
- Ogunro, O.B.; Karigidi, M.E.; Gyebi, G.A.; Turkistani, A.; Almehmadi, A.H. Tangeretin offers neuroprotection against colchicine-induced memory impairment in Wistar rats by modulating the antioxidant milieu, inflammatory mediators and oxidative stress in the brain tissue. BMC Complement. Med. Ther. 2025, 25, 40. [Google Scholar] [CrossRef]
- Cao, T.; Li, A.Q.; Zhang, Y.; Xie, T.T.; Weng, D.Z.; Pan, C.S.; Yan, L.; Sun, K.; Wang, D.; Han, J.Y.; et al. Norwogonin attenuates LPS-induced acute lung injury through inhibiting Src/AKT1/NF-κB signaling pathway. Phytomedicine 2025, 139, 156432. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Feng, X.; Li, W.; Chen, L.; Wang, X.; Lan, Y.; Tang, R.; Jiang, T.; Zheng, L.; Liu, G. Apigenin as an emerging hepatoprotective agent: Current status and future perspectives. Front. Pharmacol. 2024, 15, 1508060. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Huang, H. The therapeutic potential of apigenin against atherosclerosis. Heliyon 2025, 11, e41272. [Google Scholar] [CrossRef]
- Hussain, A.; Jairajpuri, D.S.; Anwar, S.; Choudhury, A.; Hawwal, M.F.; Firdous, A.; Alajmi, M.F.; Hassan, M.I. Apigenin-mediated MARK4 inhibition: A novel approach in advancing Alzheimer’s disease therapeutics. Mol. Divers. 2025, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zhang, T.; Xiong, W.; Wang, S.; Sun, X. Apigenin facilitates apoptosis of acute lymphoblastic leukemia cells via AMP-activated protein kinase-mediated ferroptosis. Oncol. Res. 2025, 33, 421–429. [Google Scholar] [CrossRef]
- Wein, T.; Sorek, R. Bacterial origins of human cell-autonomous innate immune mechanisms. Nat. Rev. Immunol. 2022, 22, 629–638. [Google Scholar] [CrossRef]
- Sun, Y.J.; Bai, H.Y.; Han, R.J.; Zhao, Q.L.; Li, M.; Chen, H.; Si, Y.Y.; Xue, G.M.; Zhao, Z.Z.; Feng, W.S. Dysosmaflavonoid A-F, new flavonols with potent DPPH radical scavenging activity from Dysosma versipellis. Fitoterapia 2023, 166, 105440. [Google Scholar] [CrossRef]
- Mughal, N.; Zhang, X.; Shoaib, N.; Deng, J.; Guo, J.; Zhang, J.; Yang, W.; Liu, J. Screening of soybean antifungal isoflavones based on targeted metabolomics analysis. Food Chem. X 2025, 25, 102195. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, R.H.; Wang, K.H. Formononetin ameliorates polycystic ovary syndrome through suppressing NLRP3 inflammasome. Mol. Med. 2025, 31, 27. [Google Scholar] [CrossRef]
- Naponelli, V.; Piscazzi, A.; Mangieri, D. Cellular and Molecular Mechanisms Modulated by Genistein in Cancer. Int. J. Mol. Sci. 2025, 26, 1114. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, V.; Kumar, A.; Singh, T.G. Genistein: A promising ally in combating neurodegenerative disorders. Eur. J. Pharmacol. 2025, 991, 177273. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, J.; Niu, S.; Liu, Z.; Cui, X.; Song, Y.; Tang, X.; Fan, J.; Xu, H.; Yu, W.; et al. Genistein alleviates rheumatoid arthritis by inhibiting fibroblast-like synovial exosome secretion regulated by the Rab27/nSMase2/Mfge8 pathway. Food Funct. 2025, 16, 1407–1422. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, X.; Yang, S.; Jiang, L.; Yu, H.; Li, Y. Formononetin Alleviates the Inflammatory Response Induced by Carotid Balloon Injury in Rats via the PP2A/MAPK Axis. Immunol. Investig. 2025, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kang, N.; Liu, Y.; Xu, G. Formononetin Exerts Neuroprotection in Parkinson’s Disease via the Activation of the Nrf2 Signaling Pathway. Molecules 2024, 29, 5364. [Google Scholar] [CrossRef] [PubMed]
- Benhabrou, H.; Bitam, F.; Cristino, L.; Nicois, A.; Carbone, M.; Ammar, D.; Gavagnin, M.; Ciavatta, M.L. Prenyl Pterocarpans from Algerian Bituminaria bituminosa and Their Effects on Neuroblastoma. Molecules 2024, 29, 3678. [Google Scholar] [CrossRef]
- Ahsan, R.; Paul, S.; Alam, M.S.; Rahman, A. Synthesis, Biological Properties, In Silico ADME, Molecular Docking Studies, and FMO Analysis of Chalcone Derivatives as Promising Antioxidant and Antimicrobial Agents. ACS Omega 2025, 10, 4367–4387. [Google Scholar] [CrossRef]
- Carrasco, M.; Guzman, L.; Olloquequi, J.; Cano, A.; Fortuna, A.; Vazquez-Carrera, M.; Verdaguer, E.; Auladell, C.; Ettcheto, M.; Camins, A. Licochalcone A prevents cognitive decline in a lipopolysaccharide-induced neuroinflammation mice model. Mol. Med. 2025, 31, 54. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.R.; Ahmed, W.U.; Al-Wabli, R.I.; Al-Mutairi, M.S.; Rahman, A. Synthesis and Anticancer Evaluation of O-Alkylated (E)-Chalcone Derivatives: A Focus on Estrogen Receptor Inhibition. Int. J. Mol. Sci. 2025, 26, 833. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, S.; Ye, C.; Li, P.; Xu, B.; Wang, Y.; Yang, Z.; Chen, X.; Chen, J. In Vivo Metabolic Effects of Naringin in Reducing Oxidative Stress and Protecting the Vascular Endothelium in Dyslipidemic Mice. J. Nutr. Biochem. 2025, 139, 109866. [Google Scholar] [CrossRef]
- Kaźmierczak, T.; Cyboran-Mikołajczyk, S.; Trochanowska-Pauk, N.; Walski, T.; Nowicka, P.; Bonarska-Kujawa, D. Insights on the Mechanisms of the Protective Action of Naringenin, Naringin and Naringin Dihydrochalcone on Blood Cells in Terms of Their Potential Anti-Atherosclerotic Activity. Molecules 2025, 30, 547. [Google Scholar] [CrossRef]
- Song, C.; Zheng, Z.; Liu, D.; Hu, Z.; Sheng, W. Isoflavones from Astragalus membranaceus. Acta Bot. Sin. 1997, 39, 764–768. [Google Scholar]
- Ma, X. Studies on the Chemical Constituents of Astragalus membranaceus Bge. var. Mongholicus (Bge.) Hsiao. Ph.D. Thesis, Shenyang Pharmaceutical University, Shenyang, China, 2003. [Google Scholar]
- Wang, X.; Tang, S.; Duan, H. Studies on flavonoids and related constituents from Astragalus membranaceus (Fisch) Bge. J. Tianjin Med. Univ. 2016, 22, 409–411. [Google Scholar] [CrossRef]
- Zhang, L.J.; Liu, H.K.; Hsiao, P.C.; Kuo, L.M.; Lee, I.J.; Wu, T.S.; Chiou, W.F.; Kuo, Y.H. New isoflavonoid glycosides and related constituents from astragali radix (Astragalus membranaceus) and their inhibitory activity on nitric oxide production. J. Agric. Food Chem. 2011, 59, 1131–1137. [Google Scholar] [CrossRef]
- Hao, J.; Li, J.; Li, X.; Liu, Y.; Ruan, J.; Dong, Y.; Zhang, Y.; Wang, T. Aromatic Constituents from the Stems of Astragalus membranaceus (Fisch.) Bge. var. Mongholicus (Bge.) Hsiao. Molecules 2016, 21, 354. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Yan, S.; Su, Y. Chemical constituents in roots of Astragalus membranaceus. J. Chin. Med. Mater. 2017, 48, 2601–2607. [Google Scholar] [CrossRef]
- Cao, Z.; Yu, J.; Zhang, Q. Study on Chemical constituents of Astragalus membranaceus. Chin. Tradit. Herb. Drugs 1985, 16, 38–39. [Google Scholar]
- Tu, T.; Shen, J.; Jiang, J. Study on constituents from the roots of Astragalus membranaceus (Fisch.) Bge.Hsiao. West China J. Pharm. Sci. 2009, 24, 466–468. [Google Scholar] [CrossRef]
- Jung, H.S.; Lee, E.J.; Lee, J.H.; Kim, J.S.; Kang, S.S. Phytochemical studies on Astragalus root (3)—Triterpenoids and sterols. Korean J. Pharmacogn. 2008, 39, 186–193. Available online: https://api.semanticscholar.org/CorpusID:103541570 (accessed on 10 February 2025).
- Song, C.; Zheng, Z.; Liu, D.; Hu, Z.; Sheng, W. Pterocarpns and isoflavans from Astragalus membranaceus (Fisch.) bunge. Acta Bot. Sin. 1997, 39, 1169–1171. [Google Scholar]
- He, Z.; Wang, B. Isolation and identification of chemical constituents from Astragalus mongholicus. Acta Pharm. Sin. 1990, 25, 694–698. [Google Scholar] [CrossRef]
- Su, Y.; Chen, G. Research progress of flavonoids in Astragalus membranaceus (Fisch.) Bge. J. Food Saf. Qual. 2021, 12, 849–857. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, F.; Liang, J.; Tang, J.; Shang, M.; Wang, X.; Cai, S. Study on the chemical composition of isoflavones in Astragalus membranaceus var. mongolicus. China J. Chin. Mater. Medica 2012, 37, 3243–3248. [Google Scholar] [CrossRef]
- Wen, Y.; Cnen, L.; Zheng, D.; Huang, X.; Han, L. Chemical constituents of Astragalus membranaceus var. mongholicus. Pract. Pharm. Clin. Remedies 2010, 13, 115–119. [Google Scholar] [CrossRef]
- Wen, Y. Study on the Chemical Constituents in Astragalus membranaceus Bunge var. mongolicus (Bunge) Hsiao. Master’s Thesis, China Pharmaceutical University, Nanjing, China, 2008. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Y.; Qiao, L.; Fu, H.; Pei, Y. Isolation and identification of the chemical components of Astragalus membranaceus var. mongholicus. J. Shenyang Pharm. Univ. 2007, 20–22. [Google Scholar] [CrossRef]
- Bi, Z.; Yu, Q.; Li, P.; Lin, Y.; Gao, X. Flavonoids From the Aebrial Parts of Astragalus mongholicus. Chin. J. Nat. Med. 2007, 263–265. [Google Scholar]
- Li, R. Investigation of Chemo Markers of Hengshan Astragali Radix by LC-Ms Metabolomics. Master’s Thesis, Shanxi University, Taiyuan, China, 2021. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, W.; Chrn, Y.; Yu, J.; Ma, Z.; Wu, G.; Yang, B.; Kuang, H. Flavonoids from the leaves of Astragalus membranaceus. Chin. Tradit. Pat. Med. 2017, 39, 1634–1638. [Google Scholar] [CrossRef]
- Tian, H.; Deng, Y.; Zhou, K.; Cong, H. Chemical composition of Astragalus membranaceus var mongholicus. Chin. J. Exp. Tradit. Med. Formulae 2016, 22, 70–73. [Google Scholar] [CrossRef]
- Lv, S.; Zhu, Y.; Wu, S. Study on flavonoids of the aboveground part of Astragalus membranaceus var. mongholicus. Chin. Tradit. Herb. Drugs 1990, 21, 9–10+25. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, Z. Study on the Chemical Constituents in Astragalus membranaceus Bunge var. mongolicus (Bunge) Hsiao. J. Shanghai Univ. Tradit. Chin. Med. 2011, 25, 89–94. [Google Scholar] [CrossRef]
- Subarnas, A.; Oshima, Y.; Hikino, H. Isoflavans and a pterocarpan from Astragalus mongholicus. Phytochemistry 1991, 30, 2777–2780. [Google Scholar] [CrossRef]
- Li, R. Study on the Chemical Composition of Dried Root of Astragalus membranaceus Bunge var. mongolicus (Bunge) Hsiao. Master’s Thesis, Shenyang Pharmaceutical University, Shenyang, China, 2006. [Google Scholar]
- Wang, Q.; Wang, X.; Ao, W.; Dainayintai, N. Chemical Constituents of Roots of Astragalus membranaceus (Fisch) Bge. var. mongholicus (Bge) Hsiao. Chin. Pharm. J. 2014, 49, 357–359. [Google Scholar] [CrossRef]
- Bian, Y.; Guan, J.; Bi, Z.; Song, Y.; Li, P. Study on chemical constituents of Astragalus mongholicus. Chin. Pharm. J. 2006, 41, 1217–1221. [Google Scholar]
- Kim, J.-S.; Yean, M.-H.; Lee, E.-J.; Kang, S.-S. Phytochemical studies on Astragalus root (1)-Saponins. Nat. Prod. Sci. 2008, 14, 37–46. Available online: https://api.semanticscholar.org/CorpusID:99039435 (accessed on 10 February 2025).
- Cui, B.; Nakamura, M.; Kinjo, J.; Nohara, T. Chemical Constituents of Astragali Semen. Chem. Pharm. Bull. 1993, 41, 178–182. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Han, N.-R.; Dai, N.-Y.; Wang, X.-L.; Ao, W.-L. The structural elucidation and antimicrobial activities of two isoflavane glycosides from Astragalus membranaceus (Fisch) Bge. var. mongholicus (Bge) Hsiao. J. Mol. Struct. 2014, 1076, 535–538. [Google Scholar] [CrossRef]
- Sing, C.; Zheng, Z.; Liu, T.; Hu, Z. Two new antibacterial isoflavanoids in Astragalus membranaceus. Acta Bot. Sin. 1997, 39, 486–488. [Google Scholar]
- Song, C.; Zheng, Z.; Liu, D.; Hu, Z. Antimicrobial isoflavans from Astragalus membranaceus (Fisch.) Bunge. Acta Bot. Sin. 1997, 39, 486–488. Available online: http://europepmc.org/abstract/CBA/304959 (accessed on 10 February 2025).
- Ma, X.; Tu, P.; Chen, Y.; Zhang, T.; Wei, Y.; Ito, Y. Preparative isolation and purification of isoflavan and pterocarpan glycosides from Astragalus membranaceus Bge. var. mongholicus (Bge.) Hsiao by high-speed counter-current chromatography. J. Chromatogr. A 2004, 1023, 311–315. [Google Scholar] [CrossRef]
- He, Z.Q.; Wang, B.Q. Isolation and identification of chemical constituents of Astragalus root. Yao Xue Xue Bao 1990, 25, 694–698. [Google Scholar]
- Zhang, Y.Z.; Xu, F.; Liang, J.; Tang, J.S.; Shang, M.Y.; Wang, X.; Cai, S.Q. Isoflavonoids from roots of Astragalus membranaceus var. mongholicus. Zhongguo Zhong Yao Za Zhi 2012, 37, 3243–3248. [Google Scholar]
- Liu, R.H.; Yu, B.Y.; Qiu, S.X.; Bai, G.C. Study on scavenging activities for superoxide anion radicals (O2−) and structure-activity relationship of polyphenolic compounds from leaves of Crataegus. Chin. Pharm. J. 2005, 40, 1066–1069. [Google Scholar]
- Li, X.; Qu, L.; Dong, Y.; Han, L.; Liu, E.; Fang, S.; Zhang, Y.; Wang, T. A review of recent research progress on the Astragalus genus. Molecules 2014, 19, 18850–18880. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-H.; Han, N.-R.; Dai, N.-Y.; Wang, X.-L.; Ao, W.-L. Anti-inflammatory effects and structure elucidation of two new compounds from Astragalus membranaceus (Fisch) Bge. var. mongholicus (Bge) Hsiao. J. Mol. Struct. 2014, 1074, 284–288. [Google Scholar] [CrossRef]
- Guo, J.; Yang, L.; Dai, L.; Ma, Q.; Yan, J.; Xie, Q.; Wu, Y.; Dai, H.; Zhao, Y. Neuroprotective and antidiabetic lanostane-type triterpenoids from the fruiting bodies of Ganoderma theaecolum. Chin. J. Nat. Med. 2025, 23, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Chanphen, R.; Pruksatrakul, T.; Choowong, W.; Choeyklin, R.; Surawatanawong, P.; Isaka, M. Ganopyrone A, a highly rearranged lanostane triterpenoid with antimalarial activity from artificially cultivated fruiting bodies of Ganoderma colossus. Phytochemistry 2024, 224, 114168. [Google Scholar] [CrossRef]
- Chen, C.; Xu, R.; Guo, C.; Li, X.; Zhao, Y.; Luo, D. Lanostane triterpenoids from Ganoderma calidophilum exhibit potent anti-tumor activity by inhibiting PTP1B. Chem. Biol. Interact. 2024, 403, 111253. [Google Scholar] [CrossRef]
- Chao, C.L.; Kuo, H.P.; Huang, H.W.; Cheng, M.Y.; Chao, H.F.; Lu, S.M.; Lin, H.C.; Wang, C.J.; Chang, T.C.; Wu, C.R. Poria cocos Lanostane Triterpenoids Extract Promotes Collagen and Hyaluronic Acid Production in D-Galactose-Induced Aging Rats. Life 2023, 13, 2130. [Google Scholar] [CrossRef]
- Teng, L.; Wang, C.; Cui, B.; Zhang, J.; Zhou, S.; Pan, X.; Pan, F.; Dai, Y.; Feng, N. Lanostane triterpenoids from mycelia-associated Ganoderma sinense and their anti-inflammatory activity. Phytochemistry 2023, 215, 113870. [Google Scholar] [CrossRef]
- Stambolov, I.; Shkondrov, A.; Kunert, O.; Bucar, F.; Kondeva-Burdina, M.; Krasteva, I. Cycloartane Saponins from Astragalus glycyphyllos and Their In Vitro Neuroprotective, Antioxidant, and hMAO-B-Inhibiting Effects. Metabolites 2023, 13, 857. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kwon, R.J.; Lee, H.S.; Chung, J.H.; Kim, Y.S.; Jeong, H.S.; Park, S.J.; Lee, S.Y.; Kim, T.; Yoon, S.H. The Role of Pentacyclic Triterpenoids in Non-Small Cell Lung Cancer: The Mechanisms of Action and Therapeutic Potential. Pharmaceutics 2024, 17, 22. [Google Scholar] [CrossRef]
- Peng, Y.; Demidchik, V.; Li, Y.; Shen, Z. Comparison of terpenoids in Nauclea officinalis and Paederia scandens and their anti-inflammatory effects on RAW264.7 macrophages. Fitoterapia 2025, 182, 106411. [Google Scholar] [CrossRef]
- Shakurova, E.R.; Efimova, S.S.; Ostroumova, O.S.; Parfenova, L.V. One-pot synthesis of quaternary pyridinium salts of lupane triterpenoids and their antimicrobial properties. New J. Chem. 2023, 47, 3347–3355. [Google Scholar] [CrossRef]
- Guo, B.; Cao, J.; Liu, Y.; Wang, Y.; Qian, Y.; Chen, G.; Zhu, W. Cardiac Protection of a Novel Lupane-Type Triterpenoid from Injuries Induced by Hypoxia–Reperfusion. Int. J. Mol. Sci. 2022, 16, 473. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Acankoreagenin and acankoreosides, a family of lupane triterpenoids with anti-inflammatory properties: An overview. Ann. N. Y. Acad. Sci. 2021, 1502, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Dubinin, M.V.; Nedopekina, D.A.; Ilzorkina, A.I.; Semenova, A.A.; Sharapov, V.A.; Davletshin, E.V.; Mikina, N.V.; Belsky, Y.P.; Spivak, A.Y.; Akatov, V.S.; et al. Conjugation of Triterpenic Acids of Ursane and Oleanane Types with Mitochondria-Targeting Cation F16 Synergistically Enhanced Their Cytotoxicity against Tumor Cells. Membranes 2023, 13, 563. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, S.L.; Li, X.; Zhang, Q.R.; Tian, M.Y.; Wang, X.L.; Wang, S.J. Structures and neuroprotective activities of triterpenoids from Cynomorium coccineum subsp. songaricum (Rupr.) J. Leonard. Phytochemistry 2022, 198, 113155. [Google Scholar] [CrossRef]
- Yu, Q.T.; Li, P.; Bi, Z.M.; Luo, J.; Gao, X.D. Two new saponins from the aerial part of Astragalus membranaceus var. mongholicus. Chin. Chem. Lett. 2007, 18, 554–556. [Google Scholar] [CrossRef]
- Masao, H.; Yu, Z.; Hekai, L.; Tsutomu, F. Astragalosides from hairy root cultures of Astragalus membranaceus. Phytochemistry 1994, 36, 665–670. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Noh, H.-J.; Choi, J.; Lee, K.-H.; Lee, M.-H.; Lee, J.-H.; Hong, Y.; Lee, S.-E.; Kim, S.-Y.; Kim, G.-S. Anti-Inflammatory Cycloartane-Type Saponins of Astragalus membranaceus. Molecules 2013, 18, 3725–3732. [Google Scholar] [CrossRef]
- Tang, M.; Xu, X. Advances in Studies on Chemical Constituents a ind Pharmacological Effects of Medicinal Astragalus. Guid. J. Tradit. Chin. Med. Pharm. 2018, 24, 117–122. [Google Scholar] [CrossRef]
- Wang, T.; Ruan, J.; Li, X.; Chao, L.; Shi, P.; Han, L.; Zhang, Y.; Wang, T. Bioactive cyclolanstane-type saponins from the stems of Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao. J. Nat. Med. 2016, 70, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Su, M.; Yan, M.; Shi, G.; Zhao, Q. Chemical constituents of Astragalus membranaceus var. mongholicus. Chin. Tradit. Herb. Drugs 2012, 43, 458–462. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Zhang, L.; Yan, R.; Chai, Y.; Wang, Y. Chemical constituents from Astragalus membranaceus var. mongholicus. Drugs Clin. 2013, 28, 138–143. [Google Scholar] [CrossRef]
- Zhou, Y.; Hirotani, M.; Rui, H.; Furuya, T. Two triglycosidic triterpene astragalosides from hairy root cultures of Astragalus membranaceus. Phytochemistry 1995, 38, 1407–1410. [Google Scholar] [CrossRef]
- Kitagawa, I.; Wang, H.; Saito, M.; Takagi, A.; Yoshikawa, M. Saponin and Sapogenol. XXXV. Chemical Constituents of Astragali Radix, the Root of Astragalus membranaceus Bunge. (2). Astragalosides I, II and IV, Acetylastragaloside I and Isoastragalosides I and II. Chem. Pharm. Bull. 1983, 31, 698–708. [Google Scholar] [CrossRef]
- Motomura, K.; Fujiwara, Y.; Kiyota, N.; Tsurushima, K.; Takeya, M.; Nohara, T.; Nagai, R.; Ikeda, T. Astragalosides isolated from the root of Astragalus radix inhibit the formation of advanced glycation end products. J. Agric. Food Chem. 2009, 57, 7666–7672. [Google Scholar] [CrossRef]
- Azimova, S.S. (Ed.) Cyclosieversigenin 3-O-β-D-Xylopyranoside (astramembranin II). In Natural Compounds: Cycloartane Triterpenoids and Glycosides; Springer: New York, NY, USA, 2013; p. 254. [Google Scholar] [CrossRef]
- Ma, Y.; Tian, Z.; Kuang, H.; Yuan, C.; Liu, L. A Study on the constituents of stems anf leaves of Astragalus membranaceus aceus. J. Integr. Plant Biol. 1993, 35, 480–482. [Google Scholar]
- Wang, Z.B.; Zhai, Y.D.; Ma, Z.P.; Yang, C.J.; Pan, R.; Yu, J.L.; Wang, Q.H.; Yang, B.Y.; Kuang, H.X. Triterpenoids and Flavonoids from the Leaves of Astragalus membranaceus and Their Inhibitory Effects on Nitric Oxide Production. Chem. Biodivers. 2015, 12, 1575–1584. [Google Scholar] [CrossRef]
- Kim, J.S.; Yean, M.H.; Lee, E.J.; Jung, H.S.; Lee, J.Y.; Kim, Y.J.; Kang, S.S. Two new cycloartane saponins from the roots of Astragalus membranaceus. Chem. Pharm. Bull. 2008, 56, 105–108. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Ruan, J.; Wang, T.; Dong, Y.; Hao, J.; Liu, E.; Han, L.; Gao, X.; Wang, T. Oleanane type saponins from the stems of Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao. Fitoterapia 2016, 109, 99–105. [Google Scholar] [CrossRef]
- Kuang, H.; Okada, Y.; Yang, B.; Tian, Z.; Okuyama, T. Secocycloartane Triterpenoidal Saponins from the Leaves of Astragalus membranaceus Bunge. Helv. Chim. Acta 2009, 92, 950–958. [Google Scholar] [CrossRef]
- Kuang, H.; Wang, Q.H.; Yang, B.Y.; Wang, Z.B.; Okada, Y.; Okuyama, T. Huangqiyenins G–J, Four New 9, 10-Secocycloartane (=9,19-Cyclo-9,10-secolanostane) Triterpenoidal Saponins from Astragalus membranaceus Bunge Leaves. Helv. Chim. Acta 2011, 94, 2239–2247. [Google Scholar] [CrossRef]
- Li, Y.L.; Wang, T.; Wang, H.; Wang, Q.; Cai, C.H.; Zhu, G.P.; Mei, W.L.; Xu, F.Q.; Dai, H.F.; Huang, S.Z. Nauclofficines A and B, Two Novel Monoterpenoid Indole Alkaloids from the Li Folk Herb Nauclea officinalis with Anti-allergic Inflammatory Effects on RBL-2H3 Cells. J. Ethnopharmacol. 2025, 344, 119533. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Zhu, Z.; Yang, M. Biosynthesis of plant neuroactive alkaloids treating Alzheimer’s disease. Front. Pharmacol. 2025, 16, 1500955. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, H.; Zhang, J.; Wang, J.; Li, C.; Ma, J.; Ye, Z.; Zheng, Y.; Liu, H.; Xiao, G.; et al. Screening of Botanical Drugs Reveals the Potential Anti-human Adenovirus Activity of Berberine. Antivir. Res. 2025, 237, 106105. [Google Scholar] [CrossRef]
- Wang, W.; He, L.; Lin, T.; Xiang, F.; Wu, Y.; Zhou, F.; He, Y. Homoharringtonine: Mechanisms, clinical applications and research progress. Front. Oncol. 2025, 15, 1522273. [Google Scholar] [CrossRef] [PubMed]
- Koopklang, K.; Choodej, S.; Hantanong, S.; Intayot, R.; Jungsuttiwong, S.; Insumran, Y.; Ngamrojanavanich, N.; Pudhom, K. Anti-Inflammatory Properties of Oxygenated Isocoumarins and Xanthone from Thai Mangrove-Associated Endophytic Fungus Setosphaeria rostrata. Molecules 2024, 29, 603. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, Y.; Hou, Z.; Wang, H.; Li, Q. Polysaccharides from Astragalus membranaceus Bunge alleviate LPS-induced neuroinflammation in mice by modulating microbe-metabolite-brain axis and MAPK/NF-κB signaling pathway. Int. J. Biol. Macromol. 2025, 304, 140885. [Google Scholar] [CrossRef]
- Hou, M.; Lin, C.; Zhu, L.; Bian, Z. Phenolics from Chaenomeles speciosa leaves: Ionic liquid-based ultrasound-assisted extraction, adsorptive purification, UPLC-QqQ-MS/MS quantification, and bioactivity assessment. Ultrason. Sonochem. 2025, 114, 107282. [Google Scholar] [CrossRef]
- Bezerra, M.L.R.; Gouveia-Nhanca, M.; da Veiga Dutra, M.L.; Batista, K.S.; de Araújo, A.N.V.; Dos Santos Lima, M.; Ribeiro, M.D.; Silva, A.S.; Alves, A.F.; Pimentel, T.C.; et al. Malícia honey (Mimosa quadrivalvis L.) produced by the jandaíra bee (Melipona subnitida D.) shows antioxidant activity via phenolic compound action in obese rats. Front. Nutr. 2025, 12, 1524642. [Google Scholar] [CrossRef]
- Guo, S.; Qi, X.; Zhang, L.; Lu, K.; Li, X.; Zhu, J.; Lian, F. Plumbagin improves myocardial fibrosis after myocardial infarction by inhibiting the AKT/mTOR pathway to upregulate autophagy levels. Int. Immunopharmacol. 2025, 148, 114086. [Google Scholar] [CrossRef]
- Lee, S.K.; Keng, J.W.; Yon, J.A.; Mai, C.W.; Lim, H.C.; Chow, S.C.; Akowuah, G.A.; Liew, K.B.; Lee, S.K.; Marriott, P.J.; et al. Phytochemical Analysis and Biological Activities of Flavonoids and Anthraquinones from Cassia alata (Linnaeus) Roxburgh and Their Implications for Atopic Dermatitis Management. Plants 2025, 14, 362. [Google Scholar] [CrossRef] [PubMed]
- Ai, Z.; Liu, B.; Chen, J.; Zeng, X.; Wang, K.; Tao, C.; Chen, J.; Yang, L.; Ding, Q.; Zhou, M. Advances in nano drug delivery systems for enhanced efficacy of emodin in cancer therapy. Int. J. Pharm. X 2025, 9, 100314. [Google Scholar] [CrossRef] [PubMed]
- Milko, P.; Roithová, J. Redox processes in the iron(III)/9,10-phenanthraquinone system. Inorg. Chem. 2009, 48, 11734–11742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Y.; Dong, Y.; Li, G.; Wang, S. Thymoquinone: An Effective Natural Compound for Kidney Protection. Am. J. Chin. Med. 2024, 52, 775–797. [Google Scholar] [CrossRef]
- Sudarshan, K.; Aidhen, I.S. Convenient Synthesis of 3-Glycosylated Isocoumarins. Eur. J. Org. Chem. 2017, 2017, 34–38. [Google Scholar] [CrossRef]
- Li, H.; Yao, Y.; Li, L. Coumarins as potential antidiabetic agents. J. Pharm. Pharmacol. 2017, 69, 1253–1264. [Google Scholar] [CrossRef]
- Aqib, M.; Khatoon, S.; Ali, M.; Sajid, S.; Assiri, M.A.; Ahamad, S.; Saquib, M.; Hussain, M.K. Exploring the anticancer potential and mechanisms of action of natural coumarins and isocoumarins. Eur. J. Med. Chem. 2025, 282, 117088. [Google Scholar] [CrossRef]
- Hu, K.-D.; Yang, K.-G.; Soumia, C.; Wu, M.-Y.; Yan, C.; Li, X.-Y.; Wang, Y. Proteomics analysis of APS on TLR4-activated lung cancer cell- derived exosomes. China J. Chin. Mater. Medica 2022, 613, 707. [Google Scholar] [CrossRef]
- Perez, C.R.; De Palma, M. Engineering dendritic cell vaccines to improve cancer immunotherapy. Nat. Commun. 2019, 10, 5408. [Google Scholar] [CrossRef]
- Lim, S.M.; Park, H.B.; Jin, J.O. Polysaccharide from Astragalus membranaceus promotes the activation of human peripheral blood and mouse spleen dendritic cells. Chin. J. Nat. Med. 2021, 19, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hu, X.; Wang, S.; Jiao, Z.; Sun, T.; Liu, T.; Song, K. Characterization and anti-tumor bioactivity of Astragalus polysaccharides by immunomodulation. Int. J. Biol. Macromol. 2020, 145, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Jiang, M.W.; Li, M.Y.; Zhang, Z.H.; Xing, Y.; Ri, M.; Jin, C.H.; Xu, G.H.; Piao, L.X.; Jin, H.L.; et al. Formononetin represses cervical tumorigenesis by interfering with the activation of PD-L1 through MYC and STAT3 downregulation. J. Nutr. Biochem. 2022, 100, 108899. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Wang, S.; Wang, B.; Hong, X.; Liu, X.; Li, M.; Shen, R.; Dong, Q. The role of interaction between autophagy and apoptosis in tumorigenesis (Review). Oncol. Rep. 2022, 48, 208. [Google Scholar] [CrossRef]
- Li, W.; Song, K.; Wang, S.; Zhang, C.; Zhuang, M.; Wang, Y.; Liu, T. Anti-tumor potential of Astragalus polysaccharides on breast cancer cell line mediated by macrophage activation. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 685–695. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T.; Wang, S.; Yannan, L.I.; Xue, H.; Zuo, W.; Jin, C. Study on Pharmacological Effects of Calycosin in Astragali Radix. Med. Plant. 2020, 11, 4. [Google Scholar]
- Chen, J.; Zhao, X.; Li, X.; Wu, Y. Calycosin induces apoptosis by the regulation of ERβ/miR-17 signaling pathway in human colorectal cancer cells. Food Funct. 2015, 6, 3091–3097. [Google Scholar] [CrossRef]
- Qu, N.; Qu, J.; Huang, N.; Zhang, K.; Ye, T.; Shi, J.; Chen, B.; Kan, C.; Zhang, J.; Han, F.; et al. Calycosin induces autophagy and apoptosis via Sestrin2/AMPK/mTOR in human papillary thyroid cancer cells. Front. Pharmacol. 2022, 13, 1056687. [Google Scholar] [CrossRef]
- Wu, G.; Niu, M.; Qin, J.; Wang, Y.; Tian, J. Inactivation of Rab27B-dependent signaling pathway by calycosin inhibits migration and invasion of ER-negative breast cancer cells. Gene 2019, 709, 48–55. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, L.; Guo, J.; Ma, J. The crosstalk between ferroptosis and anti-tumor immunity in the tumor microenvironment: Molecular mechanisms and therapeutic controversy. Cancer Commun. 2023, 43, 1071–1096. [Google Scholar] [CrossRef]
- Xie, D.; Jiang, Y.; Wang, H.; Zhu, L.; Huang, S.; Liu, S.; Zhang, W.; Li, T. Formononetin triggers ferroptosis in triple-negative breast cancer cells by regulating the mTORC1/SREBP1/SCD1 pathway. Front. Pharmacol. 2024, 15, 1441105. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.A.; Tran, G.V.; Velic, S.; Xiong, H.M.; Kaur, J.; Moosavi, Z.; Nguyen, P.; Duong, N.; Luu, V.T.; Singh, G.; et al. Effects of Astragaloside IV and Formononetin on Oxidative Stress and Mitochondrial Biogenesis in Hepatocytes. Int. J. Mol. Sci. 2025, 26, 774. [Google Scholar] [CrossRef]
- Oza, M.; Kulkarni, Y. Formononetin alleviates diabetic cardiomyopathy by inhibiting oxidative stress and upregulating SIRT1 in rats. Asian Pac. J. Trop. Biomed. 2020, 10, 254–262. [Google Scholar] [CrossRef]
- Lu, X.Q.; Qin, S.; Li, J. Radical Scavenging Capability and Mechanism of Three Isoflavonoids Extracted from Radix Astragali: A Theoretical Study. Molecules 2023, 28, 39. [Google Scholar] [CrossRef]
- Zhai, J.; Tao, L.; Zhang, S.; Gao, H.; Zhang, Y.; Sun, J.; Song, Y.; Qu, X. Calycosin ameliorates doxorubicin-induced cardiotoxicity by suppressing oxidative stress and inflammation via the sirtuin 1-NOD-like receptor protein 3 pathway. Phytother. Res. 2020, 34, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Chen, H.; Zhanhai, Y.; BaoPing, Z. Effect of Astragalus polysaccharide on oxidative stress in mice bearing ascites tumor. Chin. J. Clin. Pharmacol. 2022, 38, 970–973. [Google Scholar] [CrossRef]
- He, J.; Yan, B.; Song, X.; Wu, X. Astragalus polysaccharide alleviates oxidative stress response and immune dysfunction of acute cerebral ischemia reperfusion injury. Chin. J. Immunol. 2019, 35, 1443–1447. [Google Scholar] [CrossRef]
- Li, A.P.; Li, Z.Y.; Sun, H.F.; Li, K.; Qin, X.M.; Du, G.H. Comparison of Two Different Astragali Radix by a ¹H NMR-Based Metabolomic Approach. J. Proteome Res. 2015, 14, 2005–2016. [Google Scholar] [CrossRef]
- Li, W.; Xu, S.; Chen, L.; Tan, W.; Deng, N.; Li, Y.; Zhang, W.; Deng, C. Astragali Radix-Angelicae Sinensis Radix inhibits the activation of vascular adventitial fibroblasts and vascular intimal proliferation by regulating the TGF-β1/Smad2/3 pathway. J. Ethnopharmacol. 2025, 340, 119302. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Y.; Jiang, W.; Wang, Z. Molecular Mechanism of Calycosin Inhibited Vascular Calcification. Nutrients 2023, 16, 99. [Google Scholar] [CrossRef]
- Yang, L.; Liu, N.; Yang, Y. Astragaloside IV-induced BMSC exosomes promote neovascularization and protect cardiac function in myocardial infarction mice via the miR-411/HIF-1α axis. J. Liposome Res. 2024, 34, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Huang, K.; Yao, Q.-P.; Jiang, Z.-L. Mechanobiology in vascular remodeling. Natl. Sci. Rev. 2017, 5, 933–946. [Google Scholar] [CrossRef]
- Wang, J.; Wu, M.L.; Cao, S.P.; Cai, H.; Zhao, Z.M.; Song, Y.H. Cycloastragenol ameliorates experimental heart damage in rats by promoting myocardial autophagy via inhibition of AKT1-RPS6KB1 signaling. Biomed. Pharmacother. 2018, 107, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Q.; Li, J.S.; Zhang, Y.M.; Gao, F.; Dai, R.Z. Astragaloside IV inhibits Angiotensin II-stimulated proliferation of rat vascular smooth muscle cells via the regulation of CDK2 activity. Life Sci. 2018, 200, 105–109. [Google Scholar] [CrossRef]
- Hua, S.; Zhang, H.; Li, J.; Zhou, X.; Zhang, S.; Zhu, Y.; Yan, X.; Gu, P.; Huang, Z.; Jiang, W. Astragaloside IV ameliorates atherosclerosis by targeting TAK1 to suppress endothelial cell proinflammatory activation. Int. Immunopharmacol. 2025, 146, 113842. [Google Scholar] [CrossRef]
- Han, Q.; Shi, J.; Yu, Y.; Yuan, H.; Guo, Y.; Liu, X.; Xue, Y.; Li, Y. Calycosin alleviates ferroptosis and attenuates doxorubicin-induced myocardial injury via the Nrf2/SLC7A11/GPX4 signaling pathway. Front. Pharmacol. 2024, 15, 1497733. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Zhao, L.-H.; Chen, Z.; Pan, J.-P. Regulation on maturation and function of dendritic cells by Astragalus mongholicus polysaccharides. Int. Immunopharmacol. 2006, 6, 1161–1166. [Google Scholar] [CrossRef]
- Poupot, R.; Goursat, C.; Séverine, F. Multivalent nanosystems: Targeting monocytes/macrophages. Int. J. Nanomed. 2018, 13, 5511–5521. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Sun, Q.; Zhou, X.; Ma, W.; Wu, J.; Zhuang, J.; Sun, C. New insights from the single-cell level: Tumor associated macrophages heterogeneity and personalized therapy. Biomed. Pharmacother. 2022, 153, 113343. [Google Scholar] [CrossRef]
- Song, K.; Yu, J.Y.; Li, J.; Li, M.; Peng, L.Y.; Yi, P.F. Astragaloside IV Regulates cGAS-STING Signaling Pathway to Alleviate Immunosuppression Caused by PRRSV Infection. Viruses 2023, 15, 1586. [Google Scholar] [CrossRef]
- Yao, J.; Liu, J.; He, Y.; Liu, L.; Xu, Z.; Lin, X.; Liu, N.; Kai, G. Systems pharmacology reveals the mechanism of Astragaloside IV in improving immune activity on cyclophosphamide-induced immunosuppressed mice. J. Ethnopharmacol. 2023, 313, 116533. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xiao, B.; Sun, T. Antitumor and immunomodulatory activity of Astragalus membranaceus polysaccharides in H22 tumor-bearing mice. Int. J. Biol. Macromol. 2013, 62, 287–290. [Google Scholar] [CrossRef]
- Liu, D.; Su, J.; Lin, J.; Qian, G.; Chen, X.; Song, S.; Huang, K. Activation of AMPK-dependent SIRT-1 by Astragalus polysaccharide protects against ochratoxin A-induced immune stress in vitro and in vivo. Int. J. Biol. Macromol. 2018, 120, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Lv, B.; Tai, Y.; Zuo, H.X.; Xing, Y.; Surh, Y.J.; Li, M.Y.; Ma, J.; Jin, X. Formononetin ameliorates DSS-induced colitis by inhibiting the MAPK/PPAR-γ/NF-κB/ROS signaling pathways. Toxicol. Appl. Pharmacol. 2025, 496, 117239. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, X.; Ding, W. Effect of Calycosin on Acinar Cell lnjury in Severe Acute Pancreatitis by Regulating TREM-1 Expression. J. Nat. Chin. Med. 2021, 53, 1–6. [Google Scholar] [CrossRef]
- Hsieh, H.L.; Yu, M.C.; Cheng, L.C.; Chu, M.Y.; Huang, T.H.; Yeh, T.S.; Tsai, M.M. Quercetin exerts anti-inflammatory effects via inhibiting tumor necrosis factor-α-induced matrix metalloproteinase-9 expression in normal human gastric epithelial cells. World J. Gastroenterol. 2022, 28, 1139–1158. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Hou, D.D.; Zhang, W.; Gao, Y.L.; Sun, Y.Z.; Wang, H.X.; Qi, R.Q.; Chen, H.D.; Gao, X.H. Anti-inflammatory effects of quercetin in a mouse model of MC903-induced atopic dermatitis. Int. Immunopharmacol. 2019, 74, 105676. [Google Scholar] [CrossRef]
- Saijo, K.; Crotti, A.; Glass, C.K. Regulation of microglia activation and deactivation by nuclear receptors. Glia 2013, 61, 104–111. [Google Scholar] [CrossRef]
- Jiang, Y.X.; Chen, Y.; Yang, Y.; Chen, X.X.; Zhang, D.D. Screening Five Qi-Tonifying Herbs on M2 Phenotype Macrophages. Evid. Based Complement. Altern. Med. 2019, 2019, 9549315. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.; Li, J.; Fan, H.; Chai, Z.; Yu, J.; Yu, J.; Xiao, B.; Ma, C. Immunomodulatory effect of Astragalus polysaccharide on spleen cell of experimental autoimmune encephalomyelitis mice. Chin. J. Immunol. 2021, 37, 1701–1705. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, S.; Li, J.; Yu, W.; Gao, W.; Luo, H.; Fang, X. The Anti-Inflammatory Effects of Formononetin, an Active Constituent of Pueraria montana var. Lobata, via Modulation of Macrophage Autophagy and Polarization. Molecules 2025, 30, 196. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Huang, J.; Zhu, X.; Shi, M.; Chen, L.; Chen, L.; Liu, X.; Liu, R.; Zhong, Y. Formononetin ameliorates dextran sulfate sodium-induced colitis via enhancing antioxidant capacity, promoting tight junction protein expression and reshaping M1/M2 macrophage polarization balance. Int. Immunopharmacol. 2024, 142, 113174. [Google Scholar] [CrossRef]
- Lv, L. Effect and Mechanisms of Astragaloside IV in Diabetic Mice Induced by High Fat Diet and Streptozotocin. Ph.D. Thesis, Southern Medical University, Guangdong, China, 2010. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, B.; Li, K.; Wu, K.; Mao, X.; Zhou, F.; Liu, J.; Yang, H.; Ouyang, J. Insulin-Sensitization of Astragalus Polysaccharide and Its Effect on Protein Tyrosine Phosphatase 1B. Med. J. Wuhan Univ. 2010, 31, 288–291. [Google Scholar] [CrossRef]
- Chen, T.; Jian, G.; Wang, N. Research Progress of Astragalus in Treatment of Diabetic Nephropathy. Chin. J. Integr. Tradit. West. Nephrop. 2017, 18, 462–464. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Yang, W. Effect and mechanism of Astragalus polysaccharide combined with insulin on insulin resistance in diabetic rats. Chin. J. Clin. Pharmacol. 2020, 36, 1830–1832. [Google Scholar] [CrossRef]
- Xu, N.; Wu, X. Research advance of pharmacological effects of astragalosides on nervous system diseases. China J. Chin. Mater. Medica 2021, 46, 4674–4682. [Google Scholar] [CrossRef]
- Jessen, K.R. Glial cells. Int. J. Biochem. Cell Biol. 2004, 36, 1861–1867. [Google Scholar] [CrossRef]
- Wang, X.; Xu, W.; Chen, H.; Li, W.; Li, W.; Zhu, G. Astragaloside IV prevents Aβ(1-42) oligomers-induced memory impairment and hippocampal cell apoptosis by promoting PPARγ/BDNF signaling pathway. Brain Res. 2020, 1747, 147041. [Google Scholar] [CrossRef]
- Shi, Y.H.; Zhang, X.L.; Ying, P.J.; Wu, Z.Q.; Lin, L.L.; Chen, W.; Zheng, G.Q.; Zhu, W.Z. Neuroprotective Effect of Astragaloside IV on Cerebral Ischemia/Reperfusion Injury Rats Through Sirt1/Mapt Pathway. Front. Pharmacol. 2021, 12, 639898. [Google Scholar] [CrossRef]
- Yan, X.; Yu, A.; Zheng, H.; Wang, S.; He, Y.; Wang, L. Calycosin-7-O-β-D-glucoside Attenuates OGD/R-Induced Damage by Preventing Oxidative Stress and Neuronal Apoptosis via the SIRT1/FOXO1/PGC-1α Pathway in HT22 Cells. Neural Plast. 2019, 2019, 8798069. [Google Scholar] [CrossRef] [PubMed]
- Scarian, E.; Viola, C.; Dragoni, F.; Di Gerlando, R.; Rizzo, B.; Diamanti, L.; Gagliardi, S.; Bordoni, M.; Pansarasa, O. New Insights into Oxidative Stress and Inflammatory Response in Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 2698. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, G.; Zheng, X.; Wang, W.; Ling, Y.; Tang, H.; Zhang, J. Neuroprotective effect of formononetin against TBI in rats via suppressing inflammatory reaction in cortical neurons. Biomed. Pharmacother. 2018, 106, 349–354. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Lv, S.; Wang, F.; Shan, T.; Wang, J.; Liu, Z.; Zhang, L.; Cui, H.; Tian, J. Mechanism of Formononetin in Improving Energy Metabolism and Alleviating Neuronal Injury in CIRI Based on Nontargeted Metabolomics Research. J. Cell. Mol. Med. 2025, 29, e70340. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Ye, Y.; Wang, Y.; Zhang, J.; Li, C. Formononetin mediates neuroprotection against cerebral ischemia/reperfusion in rats via downregulation of the Bax/Bcl-2 ratio and upregulation PI3K/Akt signaling pathway. J. Neurol. Sci. 2014, 344, 100–104. [Google Scholar] [CrossRef]
- Yan, X.; Quan, S.; Guo, R.; Li, Z.; Bai, M.; Wang, B.; Su, P.; Xu, E.; Li, Y. Calycosin-7-O-β-D-glucoside downregulates mitophagy by mitigating mitochondrial fission to protect HT22 cells from oxygen-glucose deprivation/reperfusion-induced injury. Mol. Med. Rep. 2025, 31, 13436. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Zhang, X. Research Progresson Chemical Constituents and Pharmacological Effects of Astragalus membranaceus. Inf. TCM 2021, 38, 76–82. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, J.; Wu, W.; Duan, Y.; Fan, C.; Feng, T.T.; Wang, X.; Wu, X. Research Progresson Chemical Constituents and Pharmacological Effects of Astragalus membranaceus. Acta Chin. Med. Pharmacol. 2022, 50, 92–95. [Google Scholar] [CrossRef]
- Su, J. Neuroprotective Effect and Mechanism of Astragalus Injection on Multiple Sclerosis. Ph.D. Thesis, Shandong University of Traditional Chinese Medicine, Jinan, China, 2009. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Zhang, L.; Zhang, Y.; Xu, X.; Ding, N.; Hua, J.; Liu, L. Protective effect of Astragalus Polysaccharide on heavy ionizing radiation on BMSCs and its mechanism related with NF-κB. China J. Tradit. Chin. Med. Pharm. 2018, 33, 5576–5580. [Google Scholar]
- Zhang, S.; Xiang, J. Effect of Astragalus on retinal ganglion cells in rats with high intraocular pressure. J. Pract. Prevenying Blind 2020, 15, 97–99+139. [Google Scholar] [CrossRef]
- Bao, F.; Song, J.; Dai, Z.; Liu, J. APS inhibited high glucose-induced apoptosis in lower renal tubular epithelial cells via inactivating the Wnt signaling pathway. J. Chin. Med. Mater. 2019, 42, 414–417. [Google Scholar] [CrossRef]
- Tao, W.; Dong, Y.; Su, Q.; Wang, H.; Chen, Y.; Xue, W.; Chen, C.; Xia, B.; Duan, J.; Chen, G. Liquiritigenin reverses depression-like behavior in unpredictable chronic mild stress-induced mice by regulating PI3K/Akt/mTOR mediated BDNF/TrkB pathway. Behav. Brain Res. 2016, 308, 177–186. [Google Scholar] [CrossRef]
- Zhang, Z.A.; Lin, M.A.; Yang, P.A.; Chen, Z.A.; Liu, Y.A.; Wang, J.B.; Yang, F.C.; Zheng, Y.A. Inhibition of tau aggregation and associated cytotoxicity on neuron-like cells by calycosin. Int. J. Biol. Macromol. 2020, 171, 74–81. [Google Scholar] [CrossRef]
- Chen, J.; Liu, L.; Hou, R.; Shao, Z.; Wu, Y.; Chen, X.; Zhou, L. Calycosin promotes proliferation of estrogen receptor-positive cells via estrogen receptors and ERK1/2 activation in vitro and in vivo. Cancer Lett. 2011, 308, 144–151. [Google Scholar] [CrossRef]
- Ma, X.; Deng, G.; Tian, N.; Wang, H.; Zhao, H.; Kuai, L.; Luo, Y.; Gao, C.; Ding, X.; Li, B.; et al. Calycosin enhances Treg differentiation for alleviating skin inflammation in atopic dermatitis. J. Ethnopharmacol. 2024, 326, 117883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhong, J.; Zhang, S.; Dong, R.; Ge, D.; Xuan, Z.; Chen, X.; Wu, Y. Effects of Calycosinon Permeability and MLC Phosphrylation of HUVEC Infected with Influenza Virus. Chin. Rchines Tradit. Chin. Med. 2019, 37, 1368–1372+1547–1548. [Google Scholar] [CrossRef]
- Ma, R.; Yuan, F.; Wang, S.; Liu, Y.; Fan, T.; Wang, F. Calycosin alleviates cerulein-induced acute pancreatitis by inhibiting the inflammatory response and oxidative stress via the p38 MAPK and NF-κB signal pathways in mice. Biomed. Pharmacother. 2018, 105, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, S.; Chen, Y.; Zhang, X.; Gao, H.; Tian, J.; Chen, J. Calycosin inhibits triple-negative breast cancer progression through down-regulation of the novel estrogen receptor-α splice variant ER-α30-mediated PI3K/AKT signaling pathway. Phytomedicine 2023, 118, 154924. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Liu, N.; Liu, H.; Huang, N.; Sun, X.; Zhang, G. Bioinformatic and biochemical findings disclosed anti-hepatic steatosis mechanism of calycosin. Bioorg. Chem. 2020, 100, 103914. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, G.; Peng, L.; Tian, J.; Zhang, H. Calycosin inhibits viability, induces apoptosis, and suppresses invasion of cervical cancer cells by upregulating tumor suppressor miR-375. Arch. Biochem. Biophys. 2020, 691, 108478. [Google Scholar] [CrossRef]
- Tan, J.; Qin, X.; Liu, B.; Mo, H.; Wu, Z.; Yuan, Z. Integrative findings indicate anti-tumor biotargets and molecular mechanisms of calycosin against osteosarcoma. Biomed. Pharmacother. 2020, 126, 110096. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Piao, X.J.; Xu, W.T.; Zhang, Y.; Zhang, T.; Xue, H.; Li, Y.N.; Zuo, W.B.; Sun, G.; Fu, Z.R.; et al. Calycosin induces mitochondrial-dependent apoptosis and cell cycle arrest, and inhibits cell migration through a ROS-mediated signaling pathway in HepG2 hepatocellular carcinoma cells. Toxicol. In Vitro 2021, 70, 105052. [Google Scholar] [CrossRef] [PubMed]
- Ruifeng, J.; Jun, G.; Juanru, Z.; Xiaohui, Y. The protective effects of calycosin against diabetic nephropathy through Sirt3/SOD2/caspase-3 signaling pathway: In vitro. Arab. J. Chem. 2021, 14, 102988. [Google Scholar] [CrossRef]
- Yan, J.; Guo, J.; Wang, Y.; Xing, X.; Zhang, X.; Zhang, G.; Dong, Z. Acute myocardial infarction therapy using calycosin and tanshinone co-loaded mitochondria targeted lipid-polymer hybrid nano-system: Preparation, characterization, and anti myocardial infarction activity assessment. Biomed. Pharmacother. 2022, 155, 113650. [Google Scholar] [CrossRef]
- Yao, J.; Cheng, M.; Yang, F. Calycosin Attenuates Lipopolysaccharide-Induced Acute Lung Injury in Mice through the miR-375-3p/ROCK2 Axis. J. Investig. Surg. 2023, 36, 2211166. [Google Scholar] [CrossRef]
- Zhang, Z.; Auyeung, K.K.; Sze, S.C.; Zhang, S.; Yung, K.K.; Ko, J.K. The dual roles of calycosin in growth inhibition and metastatic progression during pancreatic cancer development: A “TGF-β paradox”. Phytomedicine 2020, 68, 153177. [Google Scholar] [CrossRef]
- Jian, L.; Xin, L.; Yufang, M.; Yifan, H. Protective effect of calycosin-7-O-β-D-glucopyranoside against oxidative stress of BRL-3A cells induced by thioacetamide. Pharmacogn. Mag. 2015, 11, 524–532. [Google Scholar] [CrossRef]
- Tsai, C.C.; Wu, H.H.; Chang, C.P.; Lin, C.H.; Yang, H.H. Calycosin-7-O-β-D-glucoside reduces myocardial injury in heat stroke rats. J. Formos. Med. Assoc. 2019, 118, 730–738. [Google Scholar] [CrossRef]
- Chen, L.; Li, Z.; Tang, Y.; Cui, X.; Luo, R.; Guo, S.; Zheng, Y.; Huang, C. Isolation, identification and antiviral activities of metabolites of calycosin-7-O-β-D-glucopyranoside. J. Pharm. Biomed. Anal. 2011, 56, 382–389. [Google Scholar] [CrossRef]
- Wang, J.; Tong, X.; Li, P.; Liu, M.; Peng, W.; Cao, H.; Su, W. Bioactive components on immuno-enhancement effects in the traditional Chinese medicine Shenqi Fuzheng Injection based on relevance analysis between chemical HPLC fingerprints and in vivo biological effects. J. Ethnopharmacol. 2014, 155, 405–415. [Google Scholar] [CrossRef]
- Ren, M.; Wang, X.; Du, G.; Tian, J.; Liu, Y. Calycosin-7-O-β-D-glucoside attenuates ischemia-reperfusion injury in vivo via activation of the PI3K/Akt pathway. Mol. Med. Rep. 2016, 13, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.M. Effects of calycosin-7-O-β-D-glucoside on cell apoptosis in cervical cancer HeLa cells and expression of Bcl-2/Bax. Chin. Tradit. Herb. Drugs 2015, 46, 1498–1502. [Google Scholar] [CrossRef]
- Choi, S.I.; Heo, T.R.; Min, B.H.; Cui, J.H.; Choi, B.H.; Park, S.R. Alleviation of osteoarthritis by calycosin-7-O-beta-D-glucopyranoside (CG) isolated from Astragali radix (AR) in rabbit osteoarthritis (OA) model. Osteoarthr. Cartil. 2007, 15, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Huang, L.; Wei, X.; Yin, L.; Wei, X.; Li, T. Bioinformatic and biochemical studies of formononetin against liver injure. Life Sci. 2021, 272, 119229. [Google Scholar] [CrossRef]
- Yuan, W.; Chen, Y.; Zhou, Y.; Bao, K.; Yu, X.; Xu, Y.; Zhang, Y.; Zheng, J.; Jiang, G.; Hong, M. Formononetin attenuates atopic dermatitis by upregulating A20 expression via activation of G protein-coupled estrogen receptor. J. Ethnopharmacol. 2021, 266, 113397. [Google Scholar] [CrossRef]
- Wu, Q.L.; Cheng, Y.Q.; Liu, A.J.; Zhang, W.D. Formononetin recovered injured nerve functions by enhancing synaptic plasticity in ischemic stroke rats. Biochem. Biophys. Res. Commun. 2020, 525, 67–72. [Google Scholar] [CrossRef]
- Li, M.; Jiang, C.; Chen, J.; Wang, J. Formononetin inhibits tumor immune escape in liver cancer-bearing mice through TLR4/NF-κB pathway. Chin. J. Immunol. 2023, 39, 1872–1877. [Google Scholar] [CrossRef]
- Ying, K.; Liu, Y.; Zhang, C.; Shangguan, M. Medical findings of nasopharyngeal carcinoma patients and anti-tumor benefits of formononetin. Eur. J. Pharmacol. 2019, 861, 172619. [Google Scholar] [CrossRef]
- Cheng, S.; He, X.; Huang, J.; Yang, P.; Fan, Z. Formononetin Attenuates Myocardial Injury in Diabetic Mice through AKT/FoxO1 Signaling. Tradit. Chin. Drug Res. Clin. Pharmacol. 2020, 31, 1165–1172. [Google Scholar] [CrossRef]
- Han, G.; Wang, M.; Chen, K.; Ge, B.; Ma, H.; Zhou, J.; Ming, L.; Zhu, R. Protective effect of formononetin against hypoxic injury in cultured osteoblasts in vitro. Chin. Pharmacol. Bull. 2011, 27, 671–677. [Google Scholar] [CrossRef]
- Yao, Y.; Li, Y.; Ding, H.; Chen, S. The improvement effect of ononin on renal injury in type 2 diabetic nephropathy rats through AMPK/SIRT1/FoxO1 pathway. Hebei Med. 2022, 28, 1785–1790. [Google Scholar] [CrossRef]
- Dong, L.; Yin, L.; Zhang, Y.; Fu, X.; Lu, J. Anti-inflammatory effects of ononin on lipopolysaccharide-stimulated RAW 264.7 cells. Mol. Immunol. 2017, 83, 46–51. [Google Scholar] [CrossRef]
- Zheng, C.; Zhou, X.; Gao, J. Protective effect and mechanism of isoquercitrin on Aβ25–35 uced PC12 cell injury. Nat. Prod. Res. Dev. 2021, 33, 73–78+136. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.H.; Liu, G.R.; Liu, C.; Dong, Y.M. Isoquercitrin suppresses the expression of histamine and pro-inflammatory cytokines by inhibiting the activation of MAP Kinases and NF-κB in human KU812 cells. Chin. J. Nat. Med. 2016, 14, 407–412. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Wang, Y.; Lu, C.; Zheng, D.; Zhang, J. Isoquercitrin, a flavonoid glucoside, exerts a positive effect on osteogenesis in vitro and in vivo. Chem. Biol. Interact. 2019, 297, 85–94. [Google Scholar] [CrossRef]
- Meng, Y.; Lu, S.; Chen, M.; Wang, S.; Cao, G.; Yang, P. Study on pharmacokinetics of rutin, quercitrin and isoquercitrin in Hedyotis diffusa in normal rats and tumor-bearing rats. Chin. Med. Herb 2018, 49, 1345–1350. [Google Scholar] [CrossRef]
- Gasparotto Junior, A.; Prando, T.B.; Leme Tdos, S.; Gasparotto, F.M.; Lourenço, E.L.; Rattmann, Y.D.; Da Silva-Santos, J.E.; Kassuya, C.A.; Marques, M.C. Mechanisms underlying the diuretic effects of Tropaeolum majus L. extracts and its main component isoquercitrin. J. Ethnopharmacol. 2012, 141, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Junior, A.G.; Gasparotto, F.M.; Lourenço, E.L.B.; Crestani, S.; Stefanello, M.E.A.; Salvador, M.J.; da Silva-Santos, J.E.; Marques, M.C.A.; Kassuya, C.A.L. Antihypertensive effects of isoquercitrin and extracts from Tropaeolum majus L.: Evidence for the inhibition of angiotensin converting enzyme. J. Ethnopharmacol. 2011, 134, 363–372. [Google Scholar] [CrossRef]
- Xie, W.; Wang, M.; Chen, C.; Zhang, X.; Melzig, M.F. Hepatoprotective effect of isoquercitrin against acetaminophen-induced liver injury. Life Sci. 2016, 152, 180–189. [Google Scholar] [CrossRef]
- Ramachandran, L.; Manu, K.A.; Shanmugam, M.K.; Li, F.; Siveen, K.S.; Vali, S.; Kapoor, S.; Abbasi, T.; Surana, R.; Smoot, D.T. Isorhamnetin inhibits proliferation and invasion and induces apoptosis through the modulation of peroxisome proliferator-activated receptor γ activation pathway in gastric cancer. J. Biol. Chem. 2013, 288, 18777. [Google Scholar] [CrossRef]
- Chao, G.; Liao, S.; Zhou, J.; Zhong, S.; Hong, Z. Antiosteoporosis effect and mechanism of isorhamnetin against ovariectomy-induced osteoporosis in rats. Chin. J. Hosp. Pharm. 2016, 36, 1456–1460. [Google Scholar] [CrossRef]
- Zhao, T.T.; Yang, T.L.; Gong, L.; Wu, P. Isorhamnetin protects against hypoxia/reoxygenation-induced injure by attenuating apoptosis and oxidative stress in H9C2 cardiomyocytes. Gene 2018, 666, 92–99. [Google Scholar] [CrossRef]
- Qi, F.; Sun, J.H.; Yan, J.Q.; Li, C.M.; Lv, X.C. Anti-inflammatory effects of isorhamnetin on LPS-stimulated human gingival fibroblasts by activating Nrf2 signaling pathway. Microb. Pathog. 2018, 120, 37–41. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, S.; Qiu, H.; Huang, L. Effect and mechanism of kaempferol on proliferation and apoptosis of breast cancer cells. J. Clin. Pharmacol. 2020, 36, 3679–3682. [Google Scholar] [CrossRef]
- Cai, M.; Zhuang, W.; Lv, E.; Fu, W. Kaempferol attenuates 6-OHDA-induced inflammation in PC12 cells via inhibiting P38 MAPK signaling pathway. Chin. J. Cell. Mol. Immunolo. 2020, 36, 583–589. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, C.; Zhang, H. Hepatoprotective effects of kaempferol 3-O-rutinoside and kaempferol 3-O-glucoside from Carthamus tinctorius L. on CCl(4)-induced oxidative liver injury in mice. J. Food Drug Anal. 2015, 23, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, R.; Shan, H.; Zhou, P.; Li, R. Quercetin attenuates H2O2-induced oxidative stress injury in human endometrial stromal cells by inhibiting p38 MAPK / NOX4 signaling pathway. J. Sichuan Univ. 2024, 55, 552–558. Available online: https://link.cnki.net/urlid/51.1644.R.20240530.0928.002 (accessed on 10 February 2025).
- Zhang, H.; Zhou, Y.; Liu, J.; Pang, Q. Exploring protective effect and mechanism of quercetin on liver injury based on NF-κB and Nrf2 signaling pathway. Acta Agric. Boreali-Occident. Sin. 2020, 29, 143–149. Available online: https://kns.cnki.net/kcms/detail/61.1220.S.20200108.1601.007.html (accessed on 10 February 2025).
- Wang, Y.; Li, G.; Wang, M.; Li, W.; Xu, W.; Zhou, Z.; Zhang, H. Quercetin attenuates spinal cord injury by inhibiting TLR4/NF-κB-mediated inflammation response. Chin J. Orthop. 2020, 28, 1311–1316. [Google Scholar] [CrossRef]
- Liu, Y.W.; Liu, X.L.; Kong, L.; Zhang, M.Y.; Chen, Y.J.; Zhu, X.; Hao, Y.C. Neuroprotection of quercetin on central neurons against chronic high glucose through enhancement of Nrf2/ARE/glyoxalase-1 pathway mediated by phosphorylation regulation. Biomed. Pharmacother. 2019, 109, 2145–2154. [Google Scholar] [CrossRef]
- Xu, Y.-w.; Zou, L.-F.; Li, F. Effect of Quercetin on Proliferation and Apoptosis of Multiple Myeloma Cells and Its Related Mechanism. J. Exp. Hematol. 2020, 28, 1234–1239. [Google Scholar] [CrossRef]
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.S. Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 2018, 155, 889–904. [Google Scholar] [CrossRef]
- Chondrogianni, N.; Kapeta, S.; Chinou, I.; Vassilatou, K.; Papassideri, I.; Gonos, E.S. Anti-ageing and rejuvenating effects of quercetin. Exp. Gerontol. 2010, 45, 763–771. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Wang, J.; Yu, Y.; Gu, Y. The effect of quercetin on immune function and anti-inflammatory effect in mice with viral respiratory tract infection based on TLR4-NF-κB pathway. Pharmacol. Clin. Chin. Mater. Medica 2023, 39, 53–57. [Google Scholar] [CrossRef]
- Hu, X.; Yu, Y.; Li, H.; Cao, S.; Yin, W. Free radical scavenging activity of genistein and quercetin and their derivatives. Nat. Prod. Res. Dev. 2008, 20, 203–206. [Google Scholar] [CrossRef]
- Jung, S.K.; Lee, M.H.; Lim, D.Y.; Kim, J.E.; Singh, P.; Lee, S.Y.; Jeong, C.H.; Lim, T.G.; Chen, H.; Chi, Y.I.; et al. Isoliquiritigenin induces apoptosis and inhibits xenograft tumor growth of human lung cancer cells by targeting both wild type and L858R/T790M mutant EGFR. J. Biol. Chem. 2014, 289, 35839–35848. [Google Scholar] [CrossRef]
- Chin, Y.W.; Jung, H.A.; Liu, Y.; Su, B.N.; Castoro, J.A.; Keller, W.J.; Pereira, M.A.; Kinghorn, A.D. Anti-oxidant constituents of the roots and stolons of licorice (Glycyrrhiza glabra). J. Agric. Food Chem. 2007, 55, 4691–4697. [Google Scholar] [CrossRef]
- Usui-Kawanishi, F.; Kani, K.; Karasawa, T.; Honda, H.; Takayama, N.; Takahashi, M.; Takatsu, K.; Nagai, Y. Isoliquiritigenin inhibits NLRP3 inflammasome activation with CAPS mutations by suppressing caspase-1 activation and mutated NLRP3 aggregation. Genes Cells 2024, 29, 423–431. [Google Scholar] [CrossRef]
- Du, Y.; Luo, M.; Feng, M.; Wang, K.; He, G. The protective effect of liquritigenin on Alzheimer’s disease by inhibiting inflammation response. Immunol. J. 2019, 35, 327–333. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, D.; Shen, X.; Zhang, F.; Zhang, H. The effect of liquiritigenin on the proliferation, apoptosis and radiosensitivity of lung cancer cells by regulating WIG-1 gene. Chin. J. Immunol. 2020, 36, 1194–1200. [Google Scholar] [CrossRef]
- Gaur, R.; Yadav, K.S.; Verma, R.K.; Yadav, N.P.; Bhakuni, R.S. In vivo anti-diabetic activity of derivatives of isoliquiritigenin and liquiritigenin. Phytomedicine 2014, 21, 415–422. [Google Scholar] [CrossRef]
- Choi, E.M. Liquiritigenin isolated from Glycyrrhiza uralensis stimulates osteoblast function in osteoblastic MC3T3-E1 cells. Int. Immunopharmacol. 2011, 12, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Park, K.-I.; Kim, K.-Y.; Lee, J.-H.; Jang, E.J.; Ku, S.K.; Kim, S.C.; Suk, H.Y.; Park, J.Y.; Baek, S.Y.; et al. Liquiritigenin inhibits hepatic fibrogenesis and TGF-β1/Smad with Hippo/YAP signal. Phytomedicine 2019, 62, 152780. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Tan, S.; Huang, Q.; Lin, J.; Lu, Z.; Lin, X. Pratensein ameliorates β-amyloid-induced cognitive impairment in rats via reducing oxidative damage and restoring synapse and BDNF levels. Neurosci. Lett. 2015, 592, 48–53. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, X.F.; Wei, W.S.; Zhang, J.; Li, Z.Z. The cardiovascular protective effect and mechanism of calycosin and its derivatives. Chin. J. Nat. Med. 2020, 18, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Guang, X.; Shubin, F.; Xiaoyan, Z.; Zhilei, W.; Ping, Z.; Wei, S.; Nan, Q.; Yuanyuan, C.; Chunyu, W.; Ming, N.; et al. Echinatin effectively protects against NLRP3 inflammasome-driven diseases by targeting HSP90. JCI Insight 2020, 6, e134601. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.Y. Integrated miRNA and mRNA omics reveal the anti-cancerous mechanism of Licochalcone B on Human Hepatoma Cell HepG2. Food Chem. Toxicol. 2021, 150, 112096. [Google Scholar] [CrossRef]
- Ran, F.; Wang, A.; Yuan, X.; Jiang, J.; Yang, F.; Wang, Z.; Zhang, B.; Zheng, Q. Apoptosis-inducing Effect of Licochalcone B on Mouse Melanoma and its Mechanism. Chin. J. Exp. Tradit. Med. Formulae 2013, 19, 220–224. [Google Scholar] [CrossRef]
- Moon, J.-H.; Tsushida, T.; Nakahara, K.; Terao, J. Identification of quercetin 3-O-β-D-glucuronide as an antioxidative metabolite in rat plasma after oral administration of quercetin. Free Radic. Biol. Med. 2001, 30, 1274–1285. [Google Scholar] [CrossRef]
- Savoia, P.; Raina, G.; Camillo, L.; Farruggio, S.; Mary, D.; Veronese, F.; Graziola, F.; Zavattaro, E.; Tiberio, R.; Grossini, E. Anti-oxidative effects of 17 β-estradiol and genistein in human skin fibroblasts and keratinocytes. J. Dermatol. Sci. 2018, 92, 62–77. [Google Scholar] [CrossRef]
- Liu, F.C.; Wang, C.C.; Lu, J.W.; Lee, C.H.; Chen, S.C.; Ho, Y.J.; Peng, Y.J. Chondroprotective Effects of Genistein against Osteoarthritis Induced Joint Inflammation. Nutrients 2019, 11, 1180. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Feng, A.; Liu, C.; Zhou, W.; Li, K.; Liu, Y.; Shi, Y.; Adu-Amankwaah, J.; Yu, H.; Pan, X.; et al. Genistein alleviates doxorubicin-induced cardiomyocyte autophagy and apoptosis via ERK/STAT3/c-Myc signaling pathway in rat model. Phytother. Res. 2024, 38, 3921–3934. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.; Hao, X.; He, Q.; Hou, S. To investigate the effect and mechanism of genistein on renal injury in diabetic nephropathy rats based on Nrf2/HO-1 pathway. Mod. J. Integr. Med. 2023, 32, 2241–2248. [Google Scholar] [CrossRef]
- Winzer, M.; Rauner, M.; Pietschmann, P. Glycitein decreases the generation of murine osteoclasts and increases apoptosis. Wien. Med. Wochenschr. 2010, 160, 446–451. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, S.Y.; Hyun, J.W.; Min, S.W.; Kim, D.H.; Kim, H.S. Glycitein inhibits glioma cell invasion through down-regulation of MMP-3 and MMP-9 gene expression. Chem. Biol. Interact. 2010, 185, 18–24. [Google Scholar] [CrossRef]
- Zhuang, H.; Lv, Q.; Zhong, C.; Cui, Y.; He, L.; Zhang, C.; Yu, J. Tiliroside Ameliorates Ulcerative Colitis by Restoring the M1/M2 Macrophage Balance via the HIF-1α/glycolysis Pathway. Front. Immunol. 2021, 12, 649463. [Google Scholar] [CrossRef]
- Sala, A.; Recio, M.C.; Schinella, G.R.; Máñez, S.; Giner, R.M.; Cerdá-Nicolás, M.; Rosí, J.L. Assessment of the anti-inflammatory activity and free radical scavenger activity of tiliroside. Eur. J. Pharmacol. 2003, 461, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Thong-Asa, W.; Wassana, C.; Sukkasem, K.; Innoi, P.; Dechakul, M.; Timda, P. Neuroprotective effect of gallic acid in mice with rotenone-induced neurodegeneration. Exp. Anim. 2024, 73, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Sheikhpour, E.; Mard, S.A.; Farbood, Y.; Bavarsad, K.; Sarkaki, A. The effects of gallic acid and vagotomy on motor function, intestinal transit, brain electrophysiology and oxidative stress alterations in a rat model of Parkinson’s disease induced by rotenone. Life Sci. 2023, 315, 121356. [Google Scholar] [CrossRef]
- Wan, J.; Wu, L.; Liu, H.; Zhao, J.; Xie, T.; Li, X.; Huang, S.; Yu, F. Incorporation of Zinc-Strontium Phosphate into Gallic Acid-Gelatin Composite Hydrogel with Multiple Biological Functions for Bone Tissue Regeneration. ACS Biomater. Sci. Eng. 2024, 10, 5057–5067. [Google Scholar] [CrossRef]
- Cai, Y.; Zhao, D.; Pan, Y.; Chen, B.; Cao, Y.; Han, S.; Lian, F.; Zhang, Y.; Yan, X. Gallic Acid Attenuates Sepsis-Induced Liver Injury through C/EBPβ-Dependent MAPK Signaling Pathway. Mol. Nutr. Food Res. 2024, 68, e2400123. [Google Scholar] [CrossRef]
- Kang, J.; Jie, L.; Lu, G.; Fu, H.; Liao, T.; Liu, D.; Shi, L.; Yin, S.; Zhang, L.; Wang, P. Gallic acid ameliorates synovial inflammation and fibrosis by regulating the intestinal flora and its metabolites. Toxicol. Appl. Pharmacol. 2024, 490, 117033. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Hu, M.; Yuan, C.; Ren, B.; Zhong, M.; Zhou, S.; Wang, X.; Gao, Q.; Zeng, M.; Cai, X.; et al. Astragaloside IV ameliorates indomethacin-induced intestinal inflammation in rats through inhibiting the activation of NLRP3 inflammasome. Int. Immunopharmacol. 2024, 135, 112281. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Sun, C.B.; Zhang, S.Q.; Chen, B.J.; Yu, J.Z.; Liu, F.Y.; Wen, J.; Hou, J.; Han, S.S.; Yan, J.Y.; et al. Induction of autophagy via the TLR4/NF-κB signaling pathway by astragaloside IV contributes to the amelioration of inflammation in RAW264.7 cells. Biomed. Pharmacother. 2021, 137, 111271. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Lu, X.; Tian, W.; Chen, Y.; He, X. Astragaloside IV mediates the effect and mechanism of KPNB1 on biological behavior and tumor growth in prostate cancer. Heliyon 2024, 10, e33904. [Google Scholar] [CrossRef]
- Qin, H.W.; Sun, M.Y.; Wang, M.N.; Liu, D.D.; Gao, Y. [Mechanism of astragaloside IV modulation of Nrf2/HO-1/GPX4 pathway to inhibit ferroptosis and ameliorate atherosclerosis in ApoE~(−/−) mice]. Zhongguo Zhong Yao Za Zhi 2024, 49, 3619–3626. [Google Scholar] [CrossRef]
- Li, X.; Dong, X.; Zhang, L.; Zhang, S.; Huang, W.; Wang, C.; Huo, Z.; Li, X.; Zhang, X.; Jia, X.; et al. Astragaloside IV attenuates renal tubule injury in DKD rats via suppression of CD36-mediated NLRP3 inflammasome activation. Front. Pharmacol. 2024, 15, 1285797. [Google Scholar] [CrossRef]
- Chen, X.; Tian, C.; Zhang, Z.; Qin, Y.; Meng, R.; Dai, X.; Zhong, Y.; Wei, X.; Zhang, J.; Shen, C. Astragaloside IV Inhibits NLRP3 Inflammasome-Mediated Pyroptosis via Activation of Nrf-2/HO-1 Signaling Pathway and Protects against Doxorubicin-Induced Cardiac Dysfunction. Front. Biosci. 2023, 28, 45. [Google Scholar] [CrossRef]
- Shi, L.; Deng, J.; He, J.; Zhu, F.; Jin, Y.; Zhang, X.; Ren, Y.; Du, X. Integrative transcriptomics and proteomics analysis reveal the protection of Astragaloside IV against myocardial fibrosis by regulating senescence. Eur. J. Pharmacol. 2024, 975, 176632. [Google Scholar] [CrossRef]
- Wu, C.; Xu, D.; Yang, C.; Xia, Q.; Zhang, Y. Astragaloside II increased 5-fluorouracil to inhibit the proliferation of human hepatocellular carcinoma cell line HepG2. Acta Univ. Med. Anhui 2016, 51, 78–82. [Google Scholar]
- Gao, C. Study on the Renal Protective Effect of Astragaloside II in Diabetic Rats. Master’s Thesis, Shanghai Jiaotong University, Shanghai, China, 2020. [Google Scholar] [CrossRef]
- Deng, G.; Chen, W.; Wang, P.; Zhan, T.; Zheng, W.; Gu, Z.; Wang, X.; Ji, X.; Sun, Y. Inhibition of NLRP3 inflammasome-mediated pyroptosis in macrophage by cycloastragenol contributes to amelioration of imiquimod-induced psoriasis-like skin inflammation in mice. Int. Immunopharmacol. 2019, 74, 105682. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, J.; Li, J.; Liu, Y.; Zheng, X.; Du, M.; Zhou, L.; Yang, Y.; Luo, S.; Hu, W.; et al. Cycloastragenol prevents age-related bone loss: Evidence in d -galactose-treated and aged rats. Biomed. Pharmacother. 2020, 128, 110304. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, S.C.; Dou, B.K.; Zou, Y.X.; Han, H.Z.; Liu, D.X.; Ke, Z.J.; Wang, Z.F. Cycloastragenol upregulates SIRT1 expression, attenuates apoptosis and suppresses neuroinflammation after brain ischemia. Acta Pharmacol. Sin. 2020, 41, 1025–1032. [Google Scholar] [CrossRef]
- Wan, Y.; Xu, L.; Wang, Y.; Tuerdi, N.; Ye, M.; Qi, R. Preventive effects of astragaloside IV and its active sapogenin cycloastragenol on cardiac fibrosis of mice by inhibiting the NLRP3 inflammasome. Eur. J. Pharmacol. 2018, 833, 545–554. [Google Scholar] [CrossRef]
- Hwang, S.T.; Kim, C.; Lee, J.H.; Chinnathambi, A.; Alharbi, S.A.; Shair, O.H.M.; Sethi, G.; Ahn, K.S. Cycloastragenol can negate constitutive STAT3 activation and promote paclitaxel-induced apoptosis in human gastric cancer cells. Phytomedicine 2019, 59, 152907. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, W.; Wei, L.; Liu, X.; Li, Z.; Zhang, Y. Anti-aging Function of Cycloastragenolin Aging MiceInducedby D-galactose. Chin. J. Exp. Tradit. Med. Formulae 2012, 18, 208–211. [Google Scholar]
- Sasaki, K.; Minowa, N.; Kuzuhara, H.; Nishiyama, S. Preventive effects of soyasapogenol B derivatives on liver injury in a concanavalin A-induced hepatitis model. Bioorg. Med. Chem. 2005, 13, 4900–4911. [Google Scholar] [CrossRef]
- Lee, H.J.; Lim, S.M.; Ko, D.B.; Jeong, J.J.; Hwang, Y.H.; Kim, D.H. Soyasapogenol B and Genistein Attenuate Lipopolysaccharide-Induced Memory Impairment in Mice by the Modulation of NF-κB-Mediated BDNF Expression. J. Agric. Food Chem. 2017, 65, 6877–6885. [Google Scholar] [CrossRef]
- Hsu, C.C.; Lin, T.W.; Chang, W.W.; Wu, C.Y.; Lo, W.H.; Wang, P.H.; Tsai, Y.C. Soyasaponin-I-modified invasive behavior of cancer by changing cell surface sialic acids. Gynecol. Oncol. 2005, 96, 415–422. [Google Scholar] [CrossRef]
- Li, M.; Zhao, D.; Meng, J.; Pan, T.; Li, J.; Guo, J.; Huang, H.; Wang, N.; Zhang, D.; Wang, C.; et al. Bacillus halotolerans attenuates inflammation induced by enterotoxigenic Escherichia coli infection in vivo and in vitro based on its metabolite soyasaponin I regulating the p105-Tpl2-ERK pathway. Food Funct. 2024, 15, 6743–6758. [Google Scholar] [CrossRef]
- Baig, M.H.; Ahmad, K.; Rabbani, G.; Danishuddin, M.; Choi, I. Computer Aided Drug Design and its Application to the Development of Potential Drugs for Neurodegenerative Disorders. Curr. Neuropharmacol. 2018, 16, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Liu, X.; Tan, X.; Li, X.; Jiang, J.; Xiong, Z.; Xu, T.; Jiang, H.; Qiao, N.; Zheng, M. Generative Models for De Novo Drug Design. J. Med. Chem. 2021, 64, 14011–14027. [Google Scholar] [CrossRef] [PubMed]
- Mullowney, M.W.; Duncan, K.R.; Elsayed, S.S.; Garg, N.; van der Hooft, J.J.J.; Martin, N.I.; Meijer, D.; Terlouw, B.R.; Biermann, F.; Blin, K.; et al. Artificial intelligence for natural product drug discovery. Nat. Rev. Drug Discov. 2023, 22, 895–916. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, Y.; Wang, D.; Yang, B.; Shen, Y.Q. Computer especially AI-assisted drug virtual screening and design in traditional Chinese medicine. Phytomedicine 2022, 107, 154481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, D.; Zhou, W.; Wang, L.; Wang, B.; Zhang, T.; Li, S. Network pharmacology: Towards the artificial intelligence-based precision traditional Chinese medicine. Brief Bioinform. 2023, 25, bbad518. [Google Scholar] [CrossRef]
- Hu, Y.; Zhai, W.; Tan, D.; Chen, H.; Zhang, G.; Tan, X.; Zheng, Y.; Gao, W.; Wei, Y.; Wu, J.; et al. Uncovering the effects and molecular mechanism of Astragalus membranaceus (Fisch.) Bunge and its bioactive ingredients formononetin and calycosin against colon cancer: An integrated approach based on network pharmacology analysis coupled with experimental validation and molecular docking. Front. Pharmacol. 2023, 14, 1111912. [Google Scholar] [CrossRef]
- Paggi, J.M.; Pandit, A.; Dror, R.O. The Art and Science of Molecular Docking. Annu. Rev. Biochem. 2024, 93, 389–410. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, J.; Sun, L.; Ba, B.; Shen, D. Mechanism of Astragalus membranaceus (Huangqi, HQ) for treatment of heart failure based on network pharmacology and molecular docking. J. Cell Mol. Med. 2024, 28, e18331. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, X.; Yan, D.; Dang, X.; Guo, L.; Jia, T.; Wang, Q. Molecular Dynamics Simulation-Driven Focused Virtual Screening and Experimental Validation of Inhibitors for MTDH-SND1 Protein-Protein Interaction. J. Chem. Inf. Model. 2023, 63, 3614–3627. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, H.; Wang, H.; Chen, Z.; Zhang, Z.; Chen, X.; Li, Y.; Qi, Y.; Wang, R. PLANET: A Multi-objective Graph Neural Network Model for Protein-Ligand Binding Affinity Prediction. J. Chem. Inf. Model. 2024, 64, 2205–2220. [Google Scholar] [CrossRef]
- Pang, X.; Sun, X.; Gu, Y.; He, X.; Gong, K.; Song, S.; Zhang, J.; Xia, J.; Liu, Z.; Cui, Y. Discovery of C19-9 as a novel non-RGD inhibitor of αvβ3 to overcome enzalutamide resistance in castration-resistant prostate cancer. Signal Transduct. Target. Ther. 2023, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wang, S.; Li, H.Y.; Zheng, L.; Mu, Y.; Guo, J. Benchmarking reverse docking through AlphaFold2 human proteome. Protein Sci. 2024, 33, e5167. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Lee, K.; Kim, D. Using reverse docking for target identification and its applications for drug discovery. Expert Opin. Drug Discov. 2016, 11, 707–715. [Google Scholar] [CrossRef]
- Park, K.; Cho, A.E. Using reverse docking to identify potential targets for ginsenosides. J. Ginseng Res. 2017, 41, 534–539. [Google Scholar] [CrossRef]
- Dai, J.; Zhou, Z.; Zhao, Y.; Kong, F.; Zhai, Z.; Zhu, Z.; Cai, J.; Huang, S.; Xu, Y.; Sun, T. Combined usage of ligand- and structure-based virtual screening in the artificial intelligence era. Eur. J. Med. Chem. 2025, 283, 117162. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Liu, H.; Peng, Y.; Xiao, P. In silico target fishing and pharmacological profiling for the isoquinoline alkaloids of Macleaya cordata (Bo Luo Hui). Chin. Med. 2015, 10, 37. [Google Scholar] [CrossRef]
- Tang, W.; Lu, M.; Tang, B. Study on the effect and mechanism of astragaloside IV on improving insulin resistance in HepG2 cells based on pharmacophore and molecular docking. Chin. Med. Herb 2020, 51, 163–168. [Google Scholar]
- Abd Elrahim Abd Elkader, H.T.; Essawy, A.E.; Al-Shami, A.S. Astragalus species: Phytochemistry, biological actions and molecular mechanisms underlying their potential neuroprotective effects on neurological diseases. Phytochemistry 2022, 202, 113293. [Google Scholar] [CrossRef]
- Yang, H.; Su, M. Clinical effect of Astragalus polysaccharide for injection combined with Xindi Limab injection and paclitaxel combined with cisplatin in the treatment of advanced non-small cell lung cancer. Proper Clin. Appl. 2024, 17, 65–68. [Google Scholar] [CrossRef]
- Jin, B.; Zhang, J.; Kang, W.; Wang, L.; Xiong, S.; Shen, X. Study on animal allergy of astragaloside IV injection. Drug Eval. Study 2017, 40, 1086–1089+1097. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).