Abstract
Background/Objectives: The inner blood–retinal barrier (iBRB) is a specialized neurovascular interface essential for retinal homeostasis and visual function and is compromised in several vision-threating conditions. Therefore, the ability to model iBRB function and dysfunction in a controlled, reproducible and scalable manner is crucial for pharmaceutical research. However, the complex anatomy and physiology of the iBRB raise challenges for cell-based in vitro modeling. Methods/Results: This review follows the evolution of iBRB models—from simple monolayers of retinal endothelial cells (ECs) to sophisticated multicellular microphysiological systems (MPs). Advanced diverse microfluidic platforms aim to replicate key structural, biochemical and functional aspects of the iBRB, each incorporating distinct strategies regarding cell sourcing, device design, flow dynamics and functional readouts. Conclusions: Despite their limitations, these models are highly valuable for drug screening and mechanistic studies aimed at preserving or restoring barrier integrity while also helping to bridge the translational gap in ophthalmic drug discovery.
1. Introduction
1.1. The Inner Blood-Retinal Barrier (iBRB in Health and Disease)
The retina, as a direct extension of the central nervous system and functional continuation of the brain, has the highest oxygen demand per unit weight of any tissue in the body. In order to support this intense metabolic activity, its microenvironment requires highly controlled regulation. This is achieved through the blood–retinal barrier (BRB), which separates the retina from the systemic circulation to maintain homeostasis (Figure 1). The BRB plays a critical role in preserving the defined physiological conditions necessary for retinal function. Structurally, it consists of the following two components: the outer BRB (oBRB), formed by the tight junctions between retinal pigment epithelial (RPE) cells, and the iBRB, constituted by the tight junctions of retinal capillary endothelial cells (ECs) [1]. The outer retina, including the photoreceptor and RPE layers, receives its vascular supply from the choriocapillaris [2]. The choroidal endothelium is characterized by fenestrations and, subsequently, by increased endothelial permeability. Around 80% of the neuroretina’s oxygen and nutrient needs are met through passive diffusion from the choroidal blood supply [3]. In contrast, the inner two-thirds of the retina depend on the retinal capillary plexuses, whose non-fenestrated ECs form the iBRB. The iBRB is, therefore, a specialized vascular interface that regulates the exchange of nutrients, ions and metabolic waste between the blood and the neural retina.
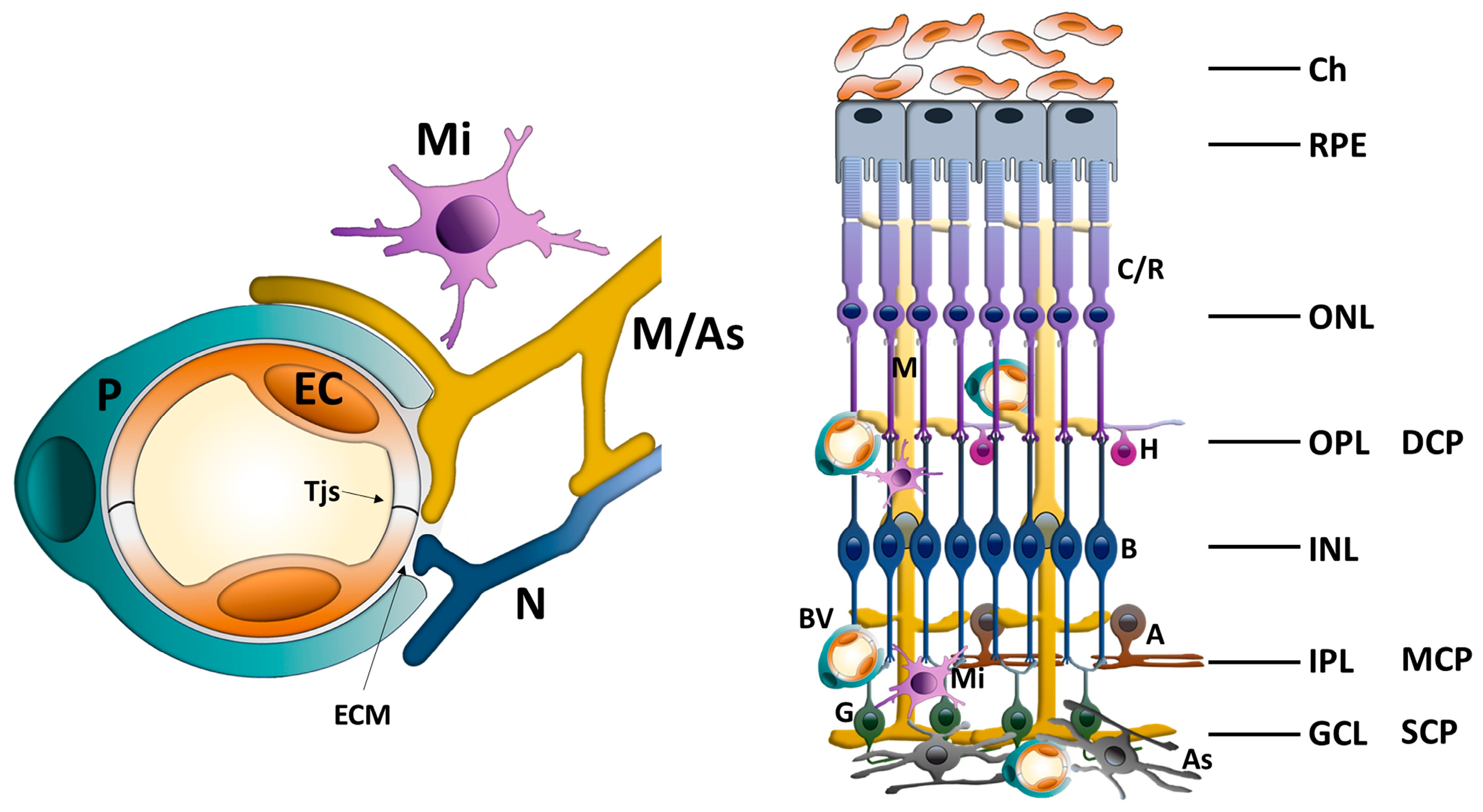
Figure 1.
Schematic illustration of the iBRB as an integral part of the rNVU showing the interactions between ECs, pericytes, glial cells (astrocytes and Mϋller cells), retinal neural cells and microglia. iBRB: inner blood–retinal barrier; rNVU: retinal neurovascular unit; Tjs: tight junctions; ECM: extracellular matrix; P: pericyte; EC: endothelial cell; Mi: microglia; M: Mϋller glial cell; As: astrocyte; N: retinal neuron cell; C/R: cones/rods; H: horizontal cell; B: bipolar cell; A: amacrine; G: ganglion cell; BV: blood vessel; Ch: choroid; RPE: retina pigment epithelium; ONL: outer nuclear layer; OPL: outer plexiform layer; DCP: deep capillary plexus; INL: inner nuclear layer; IPL: inner plexiform layer; GCL: ganglion cell layer; MCP: middle capillary plexus; SCP: superficial capillary plexus.
1.1.1. Beyond a Barrier: The iBRB as a Dynamic Component of the Neurovascular Unit (NVU)
With evolving insights into the dynamic signaling interactions between the retinal vasculature and surrounding neural components, the iBRB is increasingly viewed as part of a functional retinal NVU (rNVU) [4]. The integrated system involves various interacting cell types—including ECs, pericytes, astrocytes, Mϋller cells and microglia—that collectively contribute to the formation, maintenance and regulation of barrier integrity and retinal homeostasis [5] (Figure 1).
ECs form the core of the iBRB and similar to those of the blood–brain barrier (BBB), are highly specialized forming tight junctions (Tjs), adherens junctions and gap junctions [6]. The Tjs consist of transmembrane proteins, such as claudin-5, occludin and junctional adhesion molecules (JAMs-A/B/C) linked to intracellular scaffold proteins ZO-1, ZO-2 and ZO-3 [6]. Dynamic phosphorylation of TJ proteins controls paracellular permeability, while intracellular actin-myosin contractility modulates junctional tension [7]. Retinal ECs, unlike choroidal ECs, lack fenestrations and exhibit extremely low rates of transcytosis with few transcytotic vesicles, thereby regulating the selective transcellular transport [8,9]. In addition retinal ECs express low levels of vesicle transporters and high levels of efflux pumps (e.g., MDR1 and ABCG2), which together further control transcellular transport across the endothelium and contribute to the maintenance of a tightly regulated barrier [10]. The function of the EC junctions is finely tuned by interactions with surrounding rNVU components [4]. In this way, iBRB employs a multifaceted transport system [8]; Tjs and related junctional complexes primarily control paracellular flow, while energy-dependent vesicular pathways regulate transcellular movement. Key modulators, such as caveolin-1, MFSD2A and Wnt/β-catenin signaling, orchestrate the dynamic interplay among these routes, ensuring precise control of the retinal microenvironment and presenting, at the same time, possible therapeutic targets in retinal vascular diseases. This minimizes paracellular leakage, and the low rate of caveolae-mediated transcytosis, regulated by the expression of MFSD2A [11], is reflected in transendothelial electrical resistance (TEER) values of 1500–2000 Ω·cm2 in the healthy retina under physiological shear stress.
The basement membrane and the extracellular matrix (ECM) play key structural and signaling roles in the iBRB. Both ECs and pericytes are ensheathed by the basement membrane facilitating biochemical crosstalk and anchorage. It is composed of ECM proteins, such as collagen IV, nidogen, laminin and heparan sulfate proteoglycans, which support barrier integrity and influence cellular behavior [12]. Dynamic ECM remodeling can impact endothelial junctions and permeability, while integrin-mediated signaling from the ECM regulates survival, polarity and function of NVU cells [13].
Pericytes are embedded within the basement membrane and maintain close physical and functional contact with ECs. They are highly enriched in the retina compared to other vascular beds, with a near 1:1 ratio with ECs [14]. Pericytes regulate vascular stability, angiogenesis and barrier function via platelet-derived growth factor beta (PDGF-β)/PDGF receptor beta (PDGFRβ) signaling and other pathways like transforming growth factor beta (TGF-β) and angiopoietin 1 (Ang1)/Tie2 [15,16], while endothelial-derived PDGF-BB recruits pericytes during angiogenesis. Pericytes control capillary diameter through contractility and modulate endothelial permeability through the release of factors like angiopoietin [17]. They also deposit basement membrane proteins, including collagen IV α1/α2, laminin-511/521, nidogen and perlecan, creating a specialized ECM that supports barrier integrity [18]. Pericyte dysfunction or dropout, as seen in diabetic retinopathy (DR), leads to increased permeability and vascular instability [19].
Glial cells, notably Mϋller cells and astrocytes, provide metabolic and structural support. Astrocytes, predominantly located in the nerve fiber layer and ganglion cell layer, extend end-feet that ensheath vessels, influencing EC behavior through the secretion of Sonic hedgehog (Shh), cytokines and trophic factors [20,21,22]. They contribute to tight junction maintenance and ionic homeostasis, partly through calcium signaling and expression of aquaporin-4 and ion channels. While astrocytes are prominent regulators in the brain, their role in the retina appears more limited to the superficial vascular plexus (SVP) [20,23]. Mϋller cells are the principal macroglia in the retina and span all retinal layers. They intimately associate with vessels, especially in the intermediate and deep plexuses, and help regulate neurovascular coupling [24]. Mϋller cell end-feet ensheath capillaries, secreting neurotrophic factor (BDNF), which upregulates TJ proteins and promote antioxidant defenses. Their foot processes make contact with both neurons and vasculature, releasing gliotransmitters and vasoactive factors such as nitric oxide and prostaglandins [25,26]. In vivo and in vitro models have revealed the essential role of Mϋller cells in barrier maintenance, and their ablation leads to increased permeability [27].
Retinal neural cells are specialized neural cell types, including photoreceptors, bipolar cells, amacrine cells, horizontal cells and ganglion cells that together process visual information and maintain retinal function. Recent findings reveal that neural activity, particularly from starburst amacrine cells and cholinergic signaling, plays a crucial role in coordinating both angiogenesis and BRB formation in the retina [28]. Moreover, it has been shown that gaps in the Mϋller sheath, found mainly in the intermediate vascular plexus (IVP), permit diverse neurons, such as bipolar, amacrine and ganglion cells, to contact pericytes and ECs directly [29]. Although the precise nature of these interactions remains unclear due to the absence of presynaptic specializations, it is likely that neurons communicate with perivascular elements via non-synaptic pathways, such as gap junctions, transporter-mediated signaling or diffusible molecules like nitric oxide, while signals may also move in the opposite direction, from blood vessels to neurons and glia [30]. These findings underscore the cellular complexity and diversity of interactions within the NVU, highlighting retinal layer-specific contributions to the integrity of the iBRB [31].
Microglia are resident immune cells distributed throughout the synaptic layers of the retina and are closely associated with small vessels. Once thought to be primarily immune guardians, microglia are now recognized as active rNVU members that regulate vascular function through cytokine secretion, phagocytosis and interaction with neurons and pericytes [32,33,34]. Recent findings suggest that although microglia may not play a central role in maintaining iBRB integrity under normal physiological conditions, they become activated in the ischemic retina releasing pro-inflammatory cytokines like interleukin-1 beta (IL-1β) [35]. This, in turn, stimulates vascular endothelial growth factor (VEGF) secretion from Mϋller cells contributing to the breakdown of the iBRB [35]. Microglia proximity to metabolically active layers (inner and outer plexiform layers) and structural association with capillaries identifies them as potential regulators of blood flow, especially in the deep vascular plexus (DVP) and the SVP, where Mϋller cell regulation is limited [29].
In summary, while the iBRB refers to the unique features of the retinal ECs, the general make-up of the iBRB is an integral part of the rNVU [4], which is similar in structure and function to the brain NVU [36]. As a result, the iBRB relies on a complex interplay of multiple cellular components. ECs form the barrier core, pericytes provide structural and functional support, astrocytes and Mϋller cells regulate vascular behavior and barrier properties, and microglia contribute to immune surveillance and neurovascular signaling, while emerging research findings suggest that neural cells may contribute to rNVU function in a retinal layer-specific manner [29]. The ECM, particularly the basement membrane, is equally essential, serving as a physical scaffold and modulator of cellular communication. Impaired function of one or more of the involved cell types can lead to a wide range of retinal degenerative diseases, ultimately leading to blindness [4,8,10]. Therefore, understanding this multicellular and matrix coordination is crucial for accurate in vitro modeling of the iBRB and developing therapies targeting retinal vascular diseases.
1.1.2. Breaking the Barrier: iBRB Dysfunction in the Retinal Disease
DR, one of the leading causes of blindness, affecting approximately 34% of diabetic patients worldwide [37,38,39,40,41], involves iBRB compromise [5]. Breakdown of the iBRB has also been implicated in retinal vein occlusion [42], retinopathy of prematurity [43], uveitis [44], retinoblastoma [45], Coat’s disease [46] and congenital vascular disorders linked to mutations in the Wnt/β-catenin signaling pathway, such as familial exudative vitreoretinopathy (FEVR) and Norrie disease [47,48,49]. In diabetic macular edema (DME), chronic hyperglycemia triggers oxidative stress, PKC activation and advanced glycation end product (AGE) accumulation, leading to TJ phosphorylation, occludin/VE-cadherin internalization and barrier leakage. Hypoxia-driven hypoxia-inducible factor-1 alpha (HIF-1α) stabilization elevates VEGF-A, activating VEGF receptor2 (VEGFR2)/Src signaling to disrupt junctions and enhance vesicular trafficking (52–54). Pro-inflammatory cytokines (tumor necrosis factor alpha (TNF-α), IL-1β and IL-6) further weaken the barrier via NF-κB signaling (54) and matrix metalloproteinase (MMP)-mediated basement membrane degradation. Mitochondrial fragmentation in ECs and pericytes, along with dysregulated choroidal flow, exacerbates dysfunction, manifesting clinically as macular fluid, cystoid changes and vision loss (55) (Figure 2). In age-related macular degeneration (AMD), affecting over 200 million people worldwide, complement activation and drusen deposition impair RPE, driving chronic inflammation, oxidative stress and secondary iBRB breakdown (57). However, the intricate intercellular dialogues among retinal cell populations under conditions of inflammation and oxidative stress remain poorly understood in the complex retinal milieu. Understanding these interactions highlights the need for advanced in vitro cellular platforms that recapitulate both normal and pathological rNVU states.
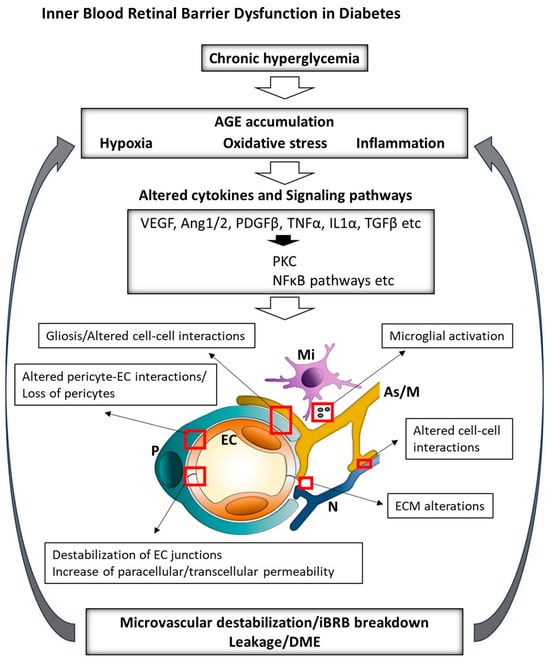
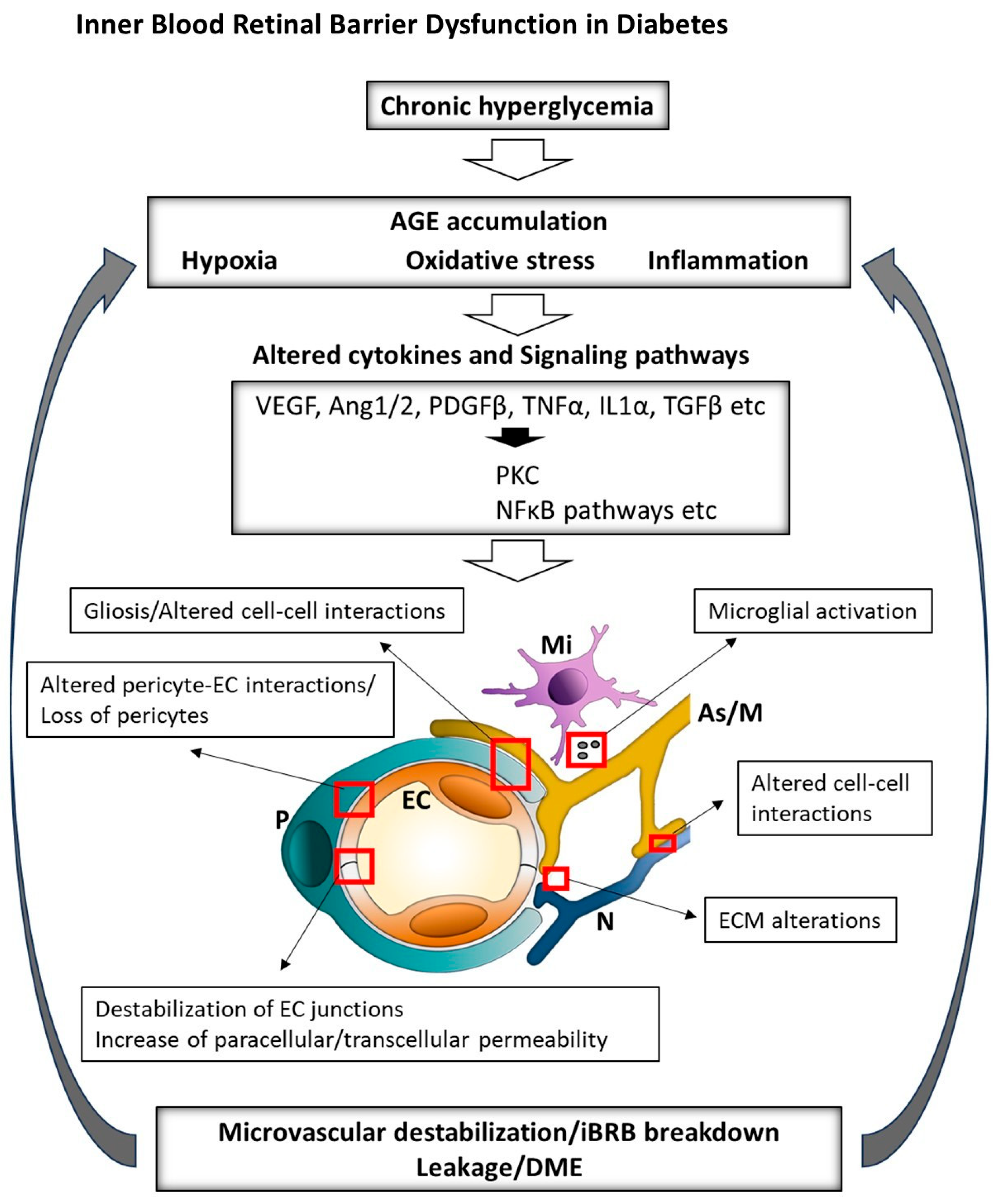
Figure 2.
Overview of the key factors driving the pathogenesis of DME. Chronic hyperglycemia, hypoxia and inflammation alter cytokine expression and associated signaling pathways, leading to ECM damage, disruption of iBRB components and exacerbation of retinal hypoxia and inflammation. iBRB: inner blood–retinal barrier; ECM: extracellular matrix; DME: diabetic macular edema; AGEs: advanced glycation end-products; PKC: protein kinase C; VEGF: vascular endothelial growth factor; Ang: angiopoietin; PDGFβ: platelet-derived growth factor beta; TNFα: tumor necrosis factor alpha; IL1α: interleukin-1 alpha; TGFβ: transforming growth factor beta; NFκB: nuclear factor kappa-light-chain-enhancer of activated B cells; EC: endothelial cell; P: pericyte; As: astrocyte; M: Müller cell; Mi: microglia.
1.2. Rationale for In Vitro Modeling of the iBRB: Bridging the Translational Gap in Retinal Drug Development
The pharmaceutical industry faces a major challenge in translating early discovery findings into clinical success, especially in the field of retinal disease due to the complexity of iBRB physiology and pathology. Although anti-VEGF therapies have transformed DME and wet AMD treatment, 30–40% of patients exhibit incomplete or transient responses and frequent intravitreal injections carry risks of endophthalmitis and patient burden [50]. These challenges highlight the urgent need for identifying more precise therapeutic targets and using next-generation physiologically relevant cellular platforms for drug development and screening.
From Bench to Barrier: The Preclinical Challenge in Drug Development
Traditional cell-based assays utilize static monolayers of retinal microvascular ECs on transwell inserts, which facilitate high-throughput screening but lack the biomechanical cues of blood flow, ECM microenvironment and cellular crosstalk essential for accurate drug permeability, toxicity and metabolic evaluations. The development of physiologically relevant cellular models of the iBRB has also faced obstacles, such as the limited availability of primary retinal cells and the difficulty in preserving their phenotypic stability and proliferative capacity in vitro [51,52]. While immortalized cell lines offer convenience, they often lack the in vivo physiological characteristics of native cells and fail to replicate complex tissue-level interactions [53]. Finally, perfusion-free systems also fail to maintain stable barrier properties over extended periods of time, limiting chronic exposure studies relevant to DME and AMD. Consequently, these platforms show poor predictive accuracy. They are inadequate in understanding retinal physiology and retinal disease, as well as in developing therapeutic strategies, evaluating drug permeability, toxicity and efficacy under clinically relevant conditions.
Animal models—rodents, rabbits, and non-human primates—have offered valuable insights regarding our understanding of retinal vascular diseases and iBRB dysfunction. These models, including those with human disease gene mutations and targeted disruptions in key pathways such as VEGF and Wnt, helped to elucidate mechanisms of retinal angiogenesis and barrier breakdown (reviewed in [54]). However, they have limitations in translating to human conditions due to significant anatomical, molecular and behavioral differences, such as absence of a macula, differences in photoreceptor density and gene expression profiles [55,56,57]. Moreover, they exhibit species-specific differences in TJ protein expression (e.g., claudin-5 isoforms), pericyte coverage and transporter distribution, complicating translation of pharmacokinetic and pharmacodynamic data. As a result, disease manifestation and response to treatment are affected. Finally, while in vivo techniques enable the study of vascular leakage, they often fail to distinguish among specific transport mechanisms in order to reveal specific alterations of the iBRB in various retinal diseases [54]. Therefore, human-cell-based in vitro models offer a promising alternative by enabling more precise, high-throughput and physiologically relevant investigations of iBRB function and drug response while circumventing ethical issues, the expense of animal studies and variability inherent to animal models. Consequently, these translational shortcomings contribute to low success rates in drug development, with only 13.8% of drug candidates progressing from Phase I clinical trials to approval [58]. It is estimated that bringing a first-in-class drug to the market requires around ten years of development and an investment of approximately USD 2.5 billion [59].
Overall, despite significant advancements, it seems that the primary limitation in accurately replicating in vivo biological processes has been the lack of cellular complexity and native tissue architecture in traditional cell culture systems. Recent progress in stem cell engineering, along with the advent of omics technologies and organoid development, has enabled the creation of three-dimensional (3D) models derived from human embryonic stem cells (ESCs) and human-induced pluripotent stem cells (hiPSCs). HiPSCs have inexhaustible self-renewal and proliferation ability, can differentiate into all three germ layers and can be further directed into specialized cell types. To date, ophthalmic diseases represent a substantial share of hiPSC-based clinical trials, comprising up to 24.4% of all studies [60]. Organoids, 3D multicellular constructs, developed in vitro from ESCs or hiPSCs offer a more physiologically relevant platform by recapitulating the cellular diversity and complex architecture of native tissues, thereby enhancing the study of disease mechanisms at molecular, cellular and transcriptomic levels [61]. Specifically, in retinal research, stem-cell-derived retinal organoids (ROs) have opened new avenues for understanding human retinogenesis and generating patient-specific in vitro models, an essential step toward personalized medicine [62,63]. Often described as “miniature retinas”, ROs display a multilayered, laminated 3D architecture composed of key retinas cell types, including rods and cones, horizontal, bipolar, amacrine, ganglion and Mϋller cells, closely resembling the native neural retina, though they lack astrocytes, ECs and pericytes
The intrinsic complexity of these “mini-retinas” is being utilized by their integration with microphysiological organ-on-chip platforms, which can fine-tune their microenvironment and elevate their physiological relevance. Microphysiological organ-on-chip systems (MPs) are miniature devices which include tissue-specific cells, often in multicellular 3D arrangements, within a microfluidic environment that allows precise control of physical and biochemical conditions [64,65,66]. By replicating the architecture and function of native tissues, organ-on-chip models offer significant advantages over traditional 2D systems, including enhanced physiological relevance and customized microenvironments. Specifically, these advanced 3D cell culture platforms can effectively mimic complex vascular barriers such as the iBRB, oBRB and BBB [67,68,69,70]. Therefore, MPs are emerging as revolutionary platforms, offering human-based models that by integrating key elements of the native rNVU microenvironment, including multicellular ECM organization and perfusion, not only enhance physiological reliability but also enable high-throughput, reproducible drug screening and toxicity testing [70]. Therefore, the development and refinement of iBRB-specific MPs platforms utilizing ROs technology can reduce reliance on animal studies, improve safety and, ultimately, accelerate the delivery of novel therapeutics for retinal diseases, satisfying a pressing and growing public health need [71].
In this review, we focus on the current state and evolution of cellular models used to study the iBRB, with an emphasis on their relevance to drug development. While several reviews have discussed the BRB and its role in ocular health and disease, this work provides a distinct perspective by focusing specifically on the iBRB and its modeling. We highlight the progression from static single-cell and transwell-based co-culture systems to advanced MPs that better recapitulate the rNVU and iBRB, emphasizing how these evolving platforms address key translational challenges in drug discovery. Special emphasis is placed on how these models simulate barrier function, disease pathology and drug transport—underlining their potential to bridge the translational gap in ophthalmic drug discovery and screening. Despite recent advancements, critical challenges remain in retinal MPs development, such as standardization, throughput, cell sourcing and limited physiological relevance. While static mono- and multicellular iBRB models have provided valuable insights into barrier function and cell–cell interactions, they are also characterized by clear and quantifiable readouts that make them powerful tools for drug screening. This feature is particularly important as it informs the design of next-generation microphysiological platforms. Defining future directions—such as incorporating additional NVU components, employing advanced tissue-engineering strategies and integrating dynamic organ-on-chip technologies—is essential for achieving a more accurate representation of the iBRB.
2. Modeling the NVU of the Retina
2.1. Static Single-EC Culture Models: The Foundation
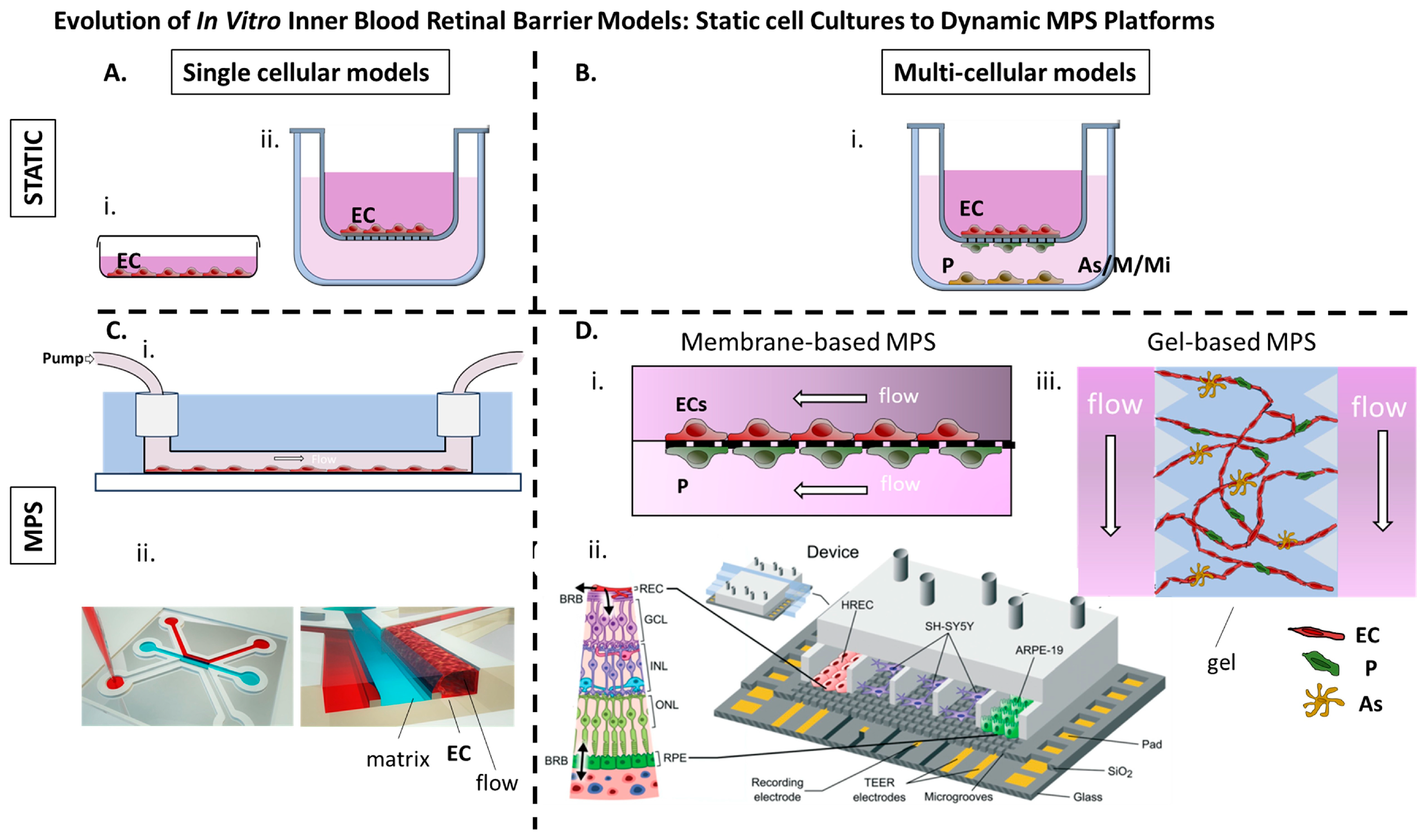
Static single-cell culture models were the earliest in vitro approach for replicating the iBRB (Figure 3).
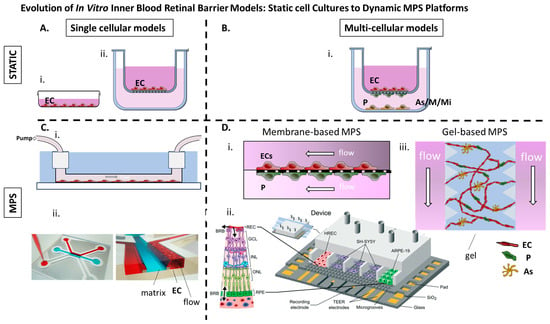
Figure 3.
(A) i. Retinal ECs cultured as a monolayer on a dish. ii. Retinal ECs cultured on the apical side of a porous membrane (transwell insert). (B) i. Retinal ECs are cultured on the apical side of a porous membrane (transwell insert), while pericytes, glial cells, or microglia are cultured according to different setups on the basal side of the membrane or within the lower compartment. (C) i. Retinal ECs cultured on laminin coated microchannel under flow [72]. ii. Immortalized Retinal ECs cultured adjacent to a collagen I matrix under flow (OrganoPlate® platform from MIMETAS) [73]. (D) i. MPs comprising two microchannels separated by a 10 μm microporous membrane, allowing co-culture of retinal microvascular ECs and immortalized human retinal pericytes on opposite sides to mimic the iBRB interface [71] ii. PDMS-based MPs with seven parallel compartments separated by microgrooves allowing for controlled communication between endothelial, epithelial, and neuronal cell types (Reproduced from [74]) with permission from the Royal Society of Chemistry) iii. Gel-based MPs in which human retinal microvascular ECs, pericytes, and astrocytes seeded in a fibrin hydrogel with flanking medium channels and allowed to be self-organized into perfusable microvascular networks [75]. BRB: Blood-retinal barrier, MPs: Microphysiological system, EC: Endothelial cells, P: Pericytes, As: Astrocytes, M: Mϋller cells, Mi: Microglia HREC: human retinal endothelial cells, GCL: ganglion cell layer, INL: inner nuclear layer ONL: outer nuclear layer RPE: retinal pigment epithtelium, TEER transendothelial electrical resistance.
Among these, the TR-iBRB2 cell line—a conditionally immortalized rat retinal-capillary EC line—has been extensively used [76,77,78,79,80,81,82,83,84,85,86,87]. These cells express several essential iBRB transporter and tight junction proteins, including GLUT1, P-glycoprotein (ABCB1), LAT1, CRT, TauT, RFC1 and SR-BI, reflecting in vivo expression patterns and validating their use for transport and barrier function studies [88]. In addition, primary bovine and human retinal microvascular ECs, as well as hiPSC-derived retinal ECs, have been cultured on porous membranes or embedded within hydrogel inserts. These single-cell systems offer key advantages, such as simplicity, cost efficiency and compatibility with high-throughput screening. Standard functional assessments include TEER, which typically reaches quite low values of ~100–200 Ω·cm2, permeability assays with small- and large-molecular tracers and transporter activity assessed using radiolabeled or fluorescent substrates (Table 1).

Table 1.
Static single-endothelial-cell culture models of the iBRB.
The use of single-retinal-EC cultures enables the study of isolated endothelial-specific functions, particularly in transport kinetics and barrier regulation. TR-iBRB2 and similar lines retain transporter expression patterns and junctional features seen in vivo, allowing for a detailed mechanistic understanding. They are especially valuable for evaluating carrier-mediated drug delivery, understanding disease-associated transporter dysregulation and testing molecular interventions that target endothelial function (Table 1). However, they lack multicellular interactions and ECM complexity, leading to premature barrier breakdown and limited relevance for multi-factorial pathologies [103]. hRMVECs cultured on transwell inserts, for instance, showed low TEER values and were also insensitive to VEGFA treatment [73]. Finally, these single-retinal-EC models do not emulate flow-induced shear stress, which critically shapes EC morphology, alignment and barrier function through mechanosensitive pathways (e.g., PECAM-1, VE-cadherin and Notch signaling) [104]. Nonetheless, these models serve as valuable initial screens to identify compounds that modulate EC junctional integrity before further evaluation in complex platforms.
2.2. Static Multicellular Models: Toward Structural Complexity
The retina is composed of several specialized cell types, including retinal microvascular ECs, pericytes, astrocytes, Müller glia, microglia and neurons. Single-cell models—though useful—fail to account for the intricate cellular interactions that govern barrier function. Therefore, static multicellular co-culture and tri-culture systems have been developed using combinations of retinal cell types, often assembled in transwell systems to replicate the anatomical layering of the retina (Figure 2).
In these configurations, ECs are seeded on the apical side of a porous membrane, while pericytes, glial cells or microglia are cultured on the basal side or within the lower compartment. This setup not only separates the vascular and retinal compartments, mimicking physiological architecture, but also enables controlled paracrine signaling and direct cell–cell contact. When compared to monocultures, these co- and tri-culture models consistently show elevated TEER and reduced permeability, indicating improved barrier integrity. Adding pericytes and glia to static transwell enhanced NVU reliability. In co-culture setups, pericytes seeded on the basal side of a transwell membrane increased TEER by 2- to 3-fold [105]. Moreover, the highest TEER was observed in triple co-cultures, when glial cells (Mϋller or astrocytes) were added in the culture [53] (Table 2).

Table 2.
Static multicellular models of the iBRB.
Basement membrane proteins coated on inserts, such as collagen IV and fibronectin, have been shown to support more robust junctional protein expression and long-term cell viability. As a result, these static multicellular models have allowed investigation of cell–cell signaling axes, such as EC-pericyte PDGF-BB/TGF-β loops and EC–glia VEGF/TNF-α responses, within a controlled environment, albeit without dynamic perfusion [112,114].
The assessment of the barrier in these multicellular static models includes basically TEER evaluation and permeability. While TEER primarily reflects paracellular barrier integrity, transwell transcytosis assays assess transcellular transport via clathrin- or caveolae-mediated pathways. In these assays, ECs alone or co-cultured with pericytes or astrocytes are grown on permeable membranes. Tracer molecules (e.g., radiolabeled sucrose, FITC-dextran, HRP or fluorescently tagged transferrin) are added to one compartment, and their movement across the monolayer is measured to evaluate active transport. Careful washing is essential to exclude paracellular leakage and isolate vesicular transport. These assays offer insights into the functional transcytotic capacity of the iBRB. In addition to TEER and permeability assays, immunofluorescence and qPCR have been employed to examine the expression of tight junction proteins (e.g., ZO-1, occludin and claudin-5) and key influx and efflux transporters (e.g., GLUT1 and P-gp) (Table 2). Such analyses confirm the enhanced morphological and functional features of the iBRB in multicellular systems.
Importantly, these models have been employed to simulate disease conditions relevant to drug development, including DR (via high glucose or AGEs), ischemia (via hypoxia) [35] and even viral infections (e.g., Ebola-like particles) [115]. Many of the observed responses, such as VEGF-mediated tight junction disruption or altered transporter expression, align closely with in vivo data, validating their relevance for translational research [111].
From a pharmaceutical perspective, these systems offer valuable platforms for evaluating drug permeability, toxicity and neurovascular protection. By using primary human retinal cells, interspecies variability can be reduced while improving the predictive value of preclinical studies. For instance, human retinal pericytes have been shown to respond differently than bovine pericytes under diabetic-like conditions, exhibiting increased susceptibility to apoptosis in response to fluctuating glucose levels [109,113]. Additionally, primary retinal ECs typically exhibit higher TEER and junctional protein expression than immortalized cell lines, though the latter are characterized by extended proliferative capacity and ease of use [53]. However, primary retinal cells are a limited resource because these cells can only be obtained from deceased donors, they can undergo replicative senescence, few cell lots are available from vendors, and donor information is limited, making it difficult to interpret factors that might affect their phenotypes [51,52]. Primary Müller cell cultures, as well as other primary retinal cell types, might also experience phenotypic instability during extended in vitro expansion. These changes challenge the use of primary Müller cells for long-term or high-throughput in vitro applications, especially in drug screening, as they may no longer represent the physiology of in vivo retinal glia [118]. HiPSC technology offers a promising solution to these challenges. HiPSC-derived microvascular ECs, astrocytes and retinal ganglion cells (RGCs) have been successfully incorporated into transwell models, demonstrating key barrier characteristics comparable to those observed in primary-cell-based systems [116].
In conclusion, static multicellular iBRB models are increasingly refined and have shown utility in drug development pipelines. They serve not only as tools for mechanistic investigation but also as scalable platforms for evaluating the safety, efficacy and delivery of retinal therapeutics in a controlled and physiologically relevant setting. However, caution must be taken in interpreting results from 2D static co-cultures. While they allow for complex signaling and junctional maturation, they lack the ECM components, 3D architecture and flow critical for replicating true vascular morphogenesis, such as lumen formation. These limitations highlight the need for further refinement of in vitro cellular models, including the integration of basement membrane components or transition to dynamic 3D systems.
2.3. Microphysiological Models of the iBRB: Bridging Physiology and Pharmacology
MPs constitute the current pinnacle of in vitro modeling for the iBRB, merging microfluidic design, advanced biomaterials and multicellular co-cultures to replicate the architecture and function of the rNVU under dynamic flow conditions. Transwell rNVU models—while capable of reaching TEER values on the order of 500 Ω·cm2 and suitable for acute permeability assays—fall short for studies requiring chronic exposure or physiologic shear. Fluid flow has been shown to assist the formation of tight endothelial or epithelial barriers [119]. Therefore, organ-on-chip platforms employing continuous perfusion have achieved TEER levels comparable to those measured in vivo and can maintain stable barrier function for a few weeks, thereby enabling longitudinal investigation of disease progression and pharmaceutical effects within a single device.
The following two main approaches have emerged: membrane-based microfluidic chips and gel-based self-assembled vascular networks (Table 3, Figure 2).

Table 3.
Microphysiological models of the iBRB.
One of the earliest approaches involved the use of immortalized human retinal microvascular ECs (hTERT hRMVECs) cultured in a dual-lane OrganoPlate platform, which enabled the formation of tubular vascular structures adjacent to a collagen I matrix [73]. Notably, this model did not rely on a synthetic membrane but instead used ECM confinement and bidirectional perfusion to guide tubulogenesis and promote tight junction formation. Under flow conditions, endothelial monolayers exhibited markedly improved barrier function compared to static cultures, as evidenced by reduced permeability to 20 kDa and 70 kDa dextran tracers. The system proved responsive to cytokines, such as VEGFA and IL-1β, which increased permeability in a dose-dependent manner, thus validating the model for pharmacological screening of vascular-stabilizing agents. Automated imaging pipelines allowed for quantitative measurement of leakage and apparent permeability, making the platform particularly attractive for mid-throughput drug discovery. However, this model was limited by the absence of mural or supporting cells, such as pericytes and astrocytes, which are essential for the full replication of iBRB physiology.
To further increase accessibility and customization, another approach leveraged 3D printing technologies to fabricate molds for PDMS-based microfluidic devices, termed glial line (gLL) platforms [72]. These systems consisted of two reservoirs connected by microchannels of ~190 μm in hydraulic diameter, simulating the scale of retinal microenvironments. Devices were cast from molds created using fused deposition modeling (FDM) or stereolithography (SLA), with SLA yielding smoother and more consistent microchannel geometries. Flow was introduced using a syringe pump and cell behavior was assessed through live/dead staining, morphological quantification and cell shape index calculations. ECs tolerated shear stress better than neural cells, with increased elongation and structural alignment in flow conditions. Although the gLL system lacks vascular network formation and tight junction quantification, it serves as a valuable platform for studying basic flow-related cellular responses and for early-phase phenotypic screens.
A high-throughput alternative was presented with the PREDICT96 platform, a thermoplastic microfluidic system comprising 96 bilayer devices, each featuring two microchannels separated by a 10 μm microporous membrane [71]. This configuration enabled the co-culture of hRMVECs and immortalized human retinal pericytes on opposing sides of the membrane, supporting cell–cell communication and allowing for differential access to readouts, such as gene expression and cytokine secretion. The platform was designed to be compatible with scalable assays, including FITC-dextran permeability testing, qPCR and Luminex cytokine profiling. Notably, endothelial monolayers co-cultured with pericytes exhibited enhanced resistance to inflammatory stimulation, reduced IL-6 secretion and greater structural stability under flow. The capacity to monitor channel-specific responses significantly improved the interpretability of drug-screening data. Nevertheless, this model lacked the anatomical specificity and 3D architecture of the native iBRB, which may limit its predictive power for ophthalmic drug discovery.
A tri-culture strategy using a PDMS-based microfluidic device with seven parallel compartments separated by microgrooves has been also employed allowing for controlled communication between endothelial, epithelial and neuronal cell [74] types (Yeste et al., 2018). In this system, human retinal ECs, ARRPE-19 epithelial cells and SH-SY5Y neuroblastoma cells were cultured in discrete but interconnected compartments. The device incorporated platinum electrodes beneath the culture chambers to allow for real-time measurement of TEER, avoiding the need for complex electrode embedding. Fluorescent dextran assays, immunostaining for tight junction proteins such as ZO-1 and electrical impedance measurements confirmed the formation of functional barrier layers. This setup allowed for simultaneous assessment of multiple cell types and their contributions to barrier integrity, providing a multifaceted platform for analyzing drug-induced perturbations. However, direct heterotypic contact among cell populations was limited to paracrine signaling, the use of tumor-derived neuronal cells may not accurately reflect retinal neurophysiology and critical components of the iBRB such as pericytes and glial cells were missing.
The most comprehensive iBRB model to date was developed to specifically recapitulate the early pathological features of DR [75]. This platform combined primary hRMVECs, pericytes and astrocytes in a tri-culture system embedded in fibrin hydrogels. Under VEGF-guided conditions, the cells self-organized into perfusable microvascular networks with basement membrane deposition and functional junction formation. When subjected to chronic exposure to high glucose, TNF-α and IL-6, the system developed hallmark features of non-proliferative DR, including pericyte dropout, vascular regression, ghost vessel formation and increased expression of inflammatory cytokines. High-content imaging, RNA sequencing and cytokine profiling enable detailed analysis of disease mechanisms and identification of therapeutic targets. Targeted pharmacological interventions, such as inhibition of PDGFRβ, NOTCH and TIE2 signaling, produced phenotype-specific responses, demonstrating the utility of the platform for mechanism-based drug testing. This model provided insights into pericyte–endothelial interactions and mimicked aspects of basement membrane thickening, glycocalyx degradation and astrocyte loss observed in clinical DR. Although Müller cells, key cell mediators of iBRB, were not incorporated, the model offers a robust human-relevant system for translational drug development.
Collectively, these diverse microfluidic platforms reflect the growing sophistication of in vitro iBRB modeling. While some prioritize throughput and compatibility with standardized assays, others emphasize biological reliability through complex co-culture or hiPSC-derived systems. Perfusion plays a critical role in enhancing barrier integrity, yet flow strategies vary widely—from passive hydrostatic gradients [73] to actively pumped channels [71,72,75]. Similarly, strategies differ in their inclusion of supporting cell types, use of synthetic membranes versus self-assembled networks and degree of 3D architecture. From a pharmaceutical perspective, the choice of model depends on the intended application: high-throughput screens might benefit from robust bilayer systems, while mechanistic studies and precision medicine may favor patient-derived, biologically reliable platforms. Future directions include integration of additional rNVU components, continuous flow regimes and real-time biosensors for enhanced functional readouts. As these platforms continue to evolve, their impact on drug discovery and therapeutic development for retinal diseases is expected to grow rapidly.
3. Challenges & Future Directions
Modeling the iBRB has long been a challenge, evolving from single-cell culture systems to multicellular static platforms and, more recently, to MPs. Each of these approaches offers unique strengths and faces specific limitations (Table 4), but together they have provided critical insights into iBRB biology.

Table 4.
Comparative summary of iBRB models, outlining their main characteristics, advantages and limitations, with emphasis on their applicability for mechanistic studies and drug discovery.
MPs, however, have transformed iBRB modeling approaches by recapitulating key aspects of retinal microarchitecture within perfusable, micrometer-scale devices. The implementation of MPs for ocular drug screening offers numerous advantages. By reducing dependence on animal models and incorporating human-specific NVU components, these systems enhance the physiological relevance of preclinical testing. Their capacity for high-content, phenotypic readouts allows for detailed assessments of drug effects, while the use of patient-derived cells opens the door to personalized medicine strategies. The rich datasets generated from microphysiological platforms can support pharmacokinetic and pharmacodynamic modeling, inform formulation strategies for both intravitreal and systemic delivery and enable early identification of off-target toxicities during the drug development process.
Yet, realizing their routine use in research and drug development hinges on overcoming several interrelated challenges as discussed below.
3.1. Key Areas for Addressing Current Challenges
3.1.1. ECM
Maintaining a tight barrier requires a balance between cell–cell and cell–matrix adhesions and intracellular contractile forces transmitted via the cytoskeleton; disruption of this balance, such as on stiff substrates, can create paracellular gaps and increase permeability [120,121]. Therefore, the ECM plays a crucial role in supporting the structural organization and functional maturation of ECs and pericytes within MPs modeling the iBRB, as it provides the necessary biochemical signals and mechanical stiffness for tight junction formation and barrier integrity [122].
In iBRB-on-a-chip platforms, incorporating ECM components, such as collagen type IV, laminin, and fibronectin, helps recreate the native basement membrane, thereby enhancing cell adhesion, polarization, and intercellular communication among retinal microvascular cells. Moreover, ECM composition and architecture directly influence vascular morphogenesis and modulate the iBRB’s response to pathological stimuli, underscoring the importance of ECM optimization for accurate disease modeling and therapeutic screening [123].
Current challenges in selecting the appropriate ECM for MPs modeling the iBRB include accurately replicating the composition, mechanical properties and spatial organization of the native retinal microenvironment. The iBRB relies on a specialized basement membrane rich in collagen IV, nidogen, laminin and perlecan, yet many existing microphysiological platforms utilize simplified ECMs like collagen I or Matrigel, which may lack the biochemical cues needed for faithful EC and pericyte behavior [12]. Furthermore, batch variability and non-human origin of commonly used ECMs’ present issues for reproducibility and translational relevance [124].
Future directions include the use of tunable, defined ECM hydrogels that better mimic the stiffness and molecular composition of the retinal basement membrane. Advances in synthetic ECMs and decellularized tissue-derived matrices offer promise for greater control and biological relevance. Additionally, engineering ECMs to incorporate dynamic remodeling and cell-responsive degradation could better support long-term studies of iBRB function, maturation and disease progression, enabling more accurate modeling of pathological states like DR.
3.1.2. Cellular Components
Equally critical is the establishment of well-characterized, renewable human cell sources. HiPSC-derived retinal ECs, pericytes and microglia offer an inexhaustible supply. In addition, hiPSC-derived ROs exhibit a multilayered organization recapitulating the 3D architecture and cellular complexity of the neural part of the human retina, comprising essential retinal cell types, such as Müller glia. Overall, it is possible to generate all cellular components of the rNVU from human hiPSCs, enabling the development of sophisticated multicellular models. However, several inherent limitations, such as biological variability among differentiation protocols and the variable maturation states of the derived cells, necessitate thorough benchmarking against primary retinal tissue [125]. Identifying tissue-specific markers of retinal ECs and pericytes is challenging due to limited human data and cell identity loss in culture [126]. Similarly, pericyte plasticity also complicates their characterization [127]. Recognition of tissue-specific markers is critical for validating retinal cells derived from iPSCs. RO heterogeneity, both across protocols and among individual organoids, is likely driven by epigenetic memory retained from the donor somatic cells, influencing lineage-specific differentiation of hiPSCs [128]. Moreover, differences in cell source and empirical differentiation methods contribute to inconsistent results between laboratories and experimental batches. Inadequate electrophysiological and omics-based characterization also hampers the functional validation of ROs as robust drug-screening tools.
Given the layer-specific diversity of the rNVU cellular components, such as astrocytes predominating in the SVP and Müller cells in the IVP, along with regional variation in neuronal populations interactions with the retinal vasculature [29], modeling layer-specific NVUs of the retina may be crucial for effective drug discovery and screening.
To address these issues, systematic improvements in differentiation protocols, tissue engineering, and co-culture are being pursued. Introducing ECs and microglia to ROs can enhance tissue maturity and mimic native retinal microenvironments. The beneficial synergy between ECs and stromal or mural cells in co-culture systems is well documented, leading to enhanced maturation [129]. Furthermore, evidence from oBRB studies demonstrated that co-culturing ECs with RPE, fibroblasts and pericytes enhances vascular specialization and cellular maturation. These cocultures promote choroidal fate in ECs, upregulate capillary and ECM markers and induce gene expression profiles that more closely resemble the native tissue. Importantly, the maturation benefits extend to supporting cells as well. Translating this strategy to iBRB modeling, co-culturing ECs with glial cells (e.g., astrocytes and Müller cells) and pericytes, all key regulators of iBRB, holds strong promise to promote a more physiologically relevant iBRB. Moreover, using technologies such as CRISPR/Cas9 to introduce reporter constructs, dynamic monitoring of growth factor expression and staged induction strategies can refine and standardize RO differentiation [130]. Multimodal pipelines that integrate single-cell RNA sequencing, mass-spectrometry-based proteomics and functional transporter assays are now being used validate differentiation protocols [131]. Together, these innovations aim to increase the maturity, reproducibility and functional reliability of the derived retinal cells, paving the way for their broader use in therapeutic discovery and disease modeling.
Furthermore, to facilitate broad distribution, advanced cryopreservation methods are being optimized to preserve post-thaw viability, barrier function and intercellular signaling competence, thereby enabling a multi-site banking approach for both academic and industrial laboratories.
3.1.3. Reproducibility
Reproducibility remains a critical challenge for both stem-cell-derived models and organ-on-chip platforms. Variability in stem-cell differentiation efficiency, donor-to-donor heterogeneity, culture conditions and microfluidic device fabrication often leads to inconsistent outcomes (also mentioned above). The absence of a unified standard and quality-control framework, including parameters such as size, structural organization and biomarker expression, leads to variability in organoid quality across laboratories. To overcome these issues, strategies include the generation and following of guidelines in cell culture practice [132] and the adoption of fully defined, xeno-free media formulations to avoid variability associated with animal-derived supplements [133], as well as standardized protocols for iPSC differentiation with rigorous quality control checkpoints. Use of isogenic controls is also crucial, as this would allow researchers to isolate the specific effects of a gene mutation without the confounding influence of genetic background variables. In this context of iBRB and MPs studies, they provide a robust framework for distinguishing disease-related phenotypes from background noise, thereby enhancing the reliability of experimental outcomes. Incorporating tissue-engineering techniques that enable precise control over environmental factors could secure standardized production. Automation of culture handling and fluid delivery, combined with omics studies and integrated biosensors for continuous monitoring of TEER, oxygen, pH and cytokine secretion, could further enhance robustness while minimizing operator bias. In parallel, multi-institutional consortia, such as the IQ Consortium, the Organoids for Drug Screening initiative, and platforms like the MPs Development and Qualification Consortium, are driving the development of harmonized reporting standards, shared data repositories and open-source device designs [134,135]. These collaborative efforts collectively improve comparability and validation across laboratories, paving the way for more reproducible and translationally relevant iBRB models that can significantly advance mechanistic research and preclinical drug development.
3.1.4. Monitoring Throughput
Real-time monitoring of culture parameters within retina-on-chip models provides both feedback for dynamic control and a non-invasive means to assess tissue integrity and specific responses, complementing endpoint analyses such as immunofluorescence microscopy. MPs developed in 3D gel-based systems, especially those involving mixed cell populations, can be technically challenging, low-throughput and difficult to image effectively. Quantifying permeability in 3D environments also presents significant obstacles. A 2D “flattened” MPs capillary model offers an alternative, simplifying the evaluation of endothelial barrier function and enabling more accessible high-throughput, imaging-based assays. However, imaging and assessment of such heterogeneous systems characterized by structural complexity and stratification of diverse cell types remain a major challenge. In addition, challenges, such as replicating physiological microenvironments managing drug diffusion and accurately analyzing multicellular drug responses, have limited broader adoption. Emerging technologies, such as microfluidics, bioprinting and fluorescence-based screening, are helping to improve drug testing within complex 3D cultures. Imaging advances like light-sheet and mainly lattice light-sheet microscopy now offer detailed 3D visualization of organoids, shedding light on cellular organization and guiding the optimization of culture conditions. Microfluidic systems with built-in sensors further enhance physiological relevance and allow for precise drug delivery and testing. For example, continuous TEER monitoring provides a reliable means to assess the integrity and maturation of vascular barriers in retina-on-chip models. Coupled with live imaging and other real-time readouts, this integrated approach enables non-invasive tracking and enhances the reliability of endpoint analyses in drug-screening studies.
3.1.5. Scaling
Scaling MPs from PDMS prototypes to industrial-grade platforms under good manufacturing practice (GMP) requires both material innovation and process standardization. Injection molding of biocompatible thermoplastics, precision laser structuring of glass substrates and continuous roll-to-roll polymer film fabrication can deliver sub-micron pattering precision at scale [136]. High-throughput coating with ECM proteins and microtopographic patterning are now achievable via automated inkjet bioprinting and microcontact printing. To ensure consistency across large batches, non-destructive in-line quality-control techniques—optical coherence tomography for structural verification, Raman spectroscopy for biochemical assessment and contact angle measurements for surface wettability—have been implemented directly on production lines [137].
3.1.6. Standardization and Accessibility
A key step toward widespread adoption of MPs, including iBRB models, is the harmonization of regulatory frameworks. Collaborative efforts across academia, industry and regulatory bodies are establishing standardized performance metrics—such as TEER thresholds, permeability assays and transporter activity test—along with transparent reposting practices to support their use in drug development submissions. Advances in automation and AI are increasing MPs throughput and reproducibility by enabling hands-free operation and predictive analytics for experimental optimization. Finally, economic feasibility and equitable access are being addressed through cost–benefit analyses, shared infrastructure models and partnerships that aim to make MPs platforms more assessable to smaller laboratories.
3.2. Conclusions
Looking ahead, the field of iBRB modeling must prioritize strategies to improve reproducibility, including the use of isogenic iPSC-derived cell lines, standardized ECM formulations and harmonized culture protocols. Incorporating additional retinal cell types, such as Müller glia, microglia and neurons, will further enhance physiological relevance and allow for modeling of complex disease mechanisms. The convergence of personalized MPs with immunocompetence and modular multi-organ integration promises to transform ophthalmic research and precision medicine. Patient-specific hiPSC-derived rNVU cells will enable individualized disease modeling and therapeutic screening. Advances in microfabrication, biosensors and automated analysis pipelines will be essential to enable high-throughput, standardized drug screening. Finally, closer collaboration between academia, industry and regulatory bodies is needed to establish shared benchmarks and qualification standards, ensuring that iBRB models can bridge the translational gap in ophthalmic drug discovery.
Author Contributions
A.A. and G.S. contributed equally to this work. Conceptualization, A.A., G.S., C.M. and E.B.; methodology, A.A., G.S. and E.B.; data curation, A.A. and G.S.; writing—original draft preparation, A.A. and G.S.; writing—review and editing, S.B., M.M., T.F., C.M. and E.B.; supervision, E.B.; funding acquisition, T.F. and E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hellenic Foundation for Research and Innovation (HFRI) under the 3rd Call for HFRI PhD Fellowships (fellowship number: 6498).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cunha-Vaz, J.; Bernardes, R.; Lobo, C. Blood-retinal barrier. Eur. J. Ophthalmol. 2011, 21 (Suppl. 6), S3–S9. [Google Scholar] [CrossRef]
- Guymer, R.H.; Bird, A.C.; Hageman, G.S. Cytoarchitecture of choroidal capillary endothelial cells. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Gensheimer, T.; Veerman, D.; van Oosten, E.M.; Segerink, L.; Garanto, A.; van der Meer, A.D. Retina-on-chip: Engineering functional in vitro models of the human retina using organ-on-chip technology. Lab. A Chip 2025, 25, 996–1014. [Google Scholar] [CrossRef]
- Nian, S.; Lo, A.C.Y.; Mi, Y.; Ren, K.; Yang, D. Neurovascular unit in diabetic retinopathy: Pathophysiological roles and potential therapeutical targets. Eye Vis. 2021, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.W.; Davila, J.R. The neurovascular unit and the pathophysiologic basis of diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1–6. [Google Scholar] [CrossRef]
- O’Leary, F.; Campbell, M. The blood-retina barrier in health and disease. FEBS J. 2023, 290, 878–891. [Google Scholar] [CrossRef] [PubMed]
- Günzel, D.; Yu, A.S. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef]
- Klaassen, I.; Van Noorden, C.J.; Schlingemann, R.O. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog. Retin. Eye Res. 2013, 34, 19–48. [Google Scholar] [CrossRef]
- van Meer, G.; Simons, K. The function of tight junctions in maintaining differences in lipid composition between the apical and the basolateral cell surface domains of MDCK cells. EMBO J. 1986, 5, 1455–1464. [Google Scholar] [CrossRef]
- Díaz-Coránguez, M.; Ramos, C.; Antonetti, D.A. The inner blood-retinal barrier: Cellular basis and development. Vis. Res. 2017, 139, 123–137. [Google Scholar] [CrossRef]
- Ben-Zvi, A.; Lacoste, B.; Kur, E.; Andreone, B.J.; Mayshar, Y.; Yan, H.; Gu, C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 2014, 509, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.S.; Routhe, L.J.; Moos, T. The vascular basement membrane in the healthy and pathological brain. J. Cereb. Blood Flow. Metab. 2017, 37, 3300–3317. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, D.; Franco, C.A.; Estrach, S.; Mettouchi, A.; Sauvaget, D.; Rosewell, I.; Schertel, A.; Armer, H.; Domogatskaya, A.; Rodin, S.; et al. Endothelial basement membrane limits tip cell formation by inducing Dll4/Notch signalling in vivo. EMBO Rep. 2011, 12, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.N.; Turczyn, T.J.; Das, A. Pericyte coverage of retinal and cerebral capillaries. Investig. Ophthalmol. Vis. Sci. 1990, 31, 999–1007. [Google Scholar]
- Lindblom, P.; Gerhardt, H.; Liebner, S.; Abramsson, A.; Enge, M.; Hellstrom, M.; Backstrom, G.; Fredriksson, S.; Landegren, U.; Nystrom, H.C.; et al. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003, 17, 1835–1840. [Google Scholar] [CrossRef]
- Park, D.Y.; Lee, J.; Kim, J.; Kim, K.; Hong, S.; Han, S.; Kubota, Y.; Augustin, H.G.; Ding, L.; Kim, J.W.; et al. Plastic roles of pericytes in the blood-retinal barrier. Nat. Commun. 2017, 8, 15296. [Google Scholar] [CrossRef]
- Gerhardt, H.; Betsholtz, C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003, 314, 15–23. [Google Scholar] [CrossRef]
- Trost, A.; Lange, S.; Schroedl, F.; Bruckner, D.; Motloch, K.A.; Bogner, B.; Kaser-Eichberger, A.; Strohmaier, C.; Runge, C.; Aigner, L.; et al. Brain and Retinal Pericytes: Origin, Function and Role. Front. Cell. Neurosci. 2016, 10, 20. [Google Scholar] [CrossRef]
- Santos, G.S.P.; Prazeres, P.; Mintz, A.; Birbrair, A. Role of pericytes in the retina. Eye 2018, 32, 483–486. [Google Scholar] [CrossRef]
- Newman, E.A. Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140195. [Google Scholar] [CrossRef]
- Dorrell, M.I.; Aguilar, E.; Friedlander, M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3500–3510. [Google Scholar]
- Holden, J.M.; Wareham, L.K.; Calkins, D.J. Retinal astrocyte morphology predicts integration of vascular and neuronal architecture. Front. Neurosci. 2023, 17, 1244679. [Google Scholar] [CrossRef]
- Abbott, N.J.; Ronnback, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Vecino, E.; Rodriguez, F.D.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia-neuron interactions in the mammalian retina. Prog. Retin. Eye Res. 2016, 51, 1–40. [Google Scholar] [CrossRef]
- Metea, M.R.; Newman, E.A. Glial cells dilate and constrict blood vessels: A mechanism of neurovascular coupling. J. Neurosci. 2006, 26, 2862–2870. [Google Scholar] [CrossRef]
- Biesecker, K.R.; Srienc, A.I.; Shimoda, A.M.; Agarwal, A.; Bergles, D.E.; Kofuji, P.; Newman, E.A. Glial Cell Calcium Signaling Mediates Capillary Regulation of Blood Flow in the Retina. J. Neurosci. 2016, 36, 9435–9445. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Fruttiger, M.; Zhu, L.; Chung, S.H.; Barnett, N.L.; Kirk, J.K.; Lee, S.; Coorey, N.J.; Killingsworth, M.; Sherman, L.S.; et al. Conditional Mullercell ablation causes independent neuronal and vascular pathologies in a novel transgenic model. J. Neurosci. 2012, 32, 15715–15727. [Google Scholar] [CrossRef]
- Weiner, G.A.; Shah, S.H.; Angelopoulos, C.M.; Bartakova, A.B.; Pulido, R.S.; Murphy, A.; Nudleman, E.; Daneman, R.; Goldberg, J.L. Cholinergic neural activity directs retinal layer-specific angiogenesis and blood retinal barrier formation. Nat. Commun. 2019, 10, 2477. [Google Scholar] [CrossRef] [PubMed]
- Grimes, W.N.; Berson, D.M.; Sabnis, A.; Hoon, M.; Sinha, R.; Tian, H.; Diamond, J.S. Layer-specific anatomical and physiological features of the retina’s neurovascular unit. Curr. Biol. 2025, 35, 109–120.e104. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Y.; Liu, Z.G.; Sun, Y.Q.; Li, Y.Z.; Teng, Z.Q.; Liu, C.M. Preserving blood-retinal barrier integrity: A path to retinal ganglion cell protection in glaucoma and traumatic optic neuropathy. Cell Regen. 2025, 14, 13. [Google Scholar] [CrossRef]
- Westenskow, P.D. Neurovascular signaling: Irrigating the retina. Curr. Biol. 2023, 33, R1193–R1194. [Google Scholar] [CrossRef]
- Checchin, D.; Sennlaub, F.; Levavasseur, E.; Leduc, M.; Chemtob, S. Potential role of microglia in retinal blood vessel formation. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3595–3602. [Google Scholar] [CrossRef]
- Morris, G.P.; Foster, C.G. Microglia directly associate with pericytes in the central nervous system. Glia 2023, 71, 1847–1869. [Google Scholar] [CrossRef]
- Jobling, A.I.; Greferath, U.; Dixon, M.A.; Quiriconi, P.; Eyar, B.; van Koeverden, A.K.; Mills, S.A.; Vessey, K.A.; Bui, B.V.; Fletcher, E.L. Microglial regulation of the retinal vasculature in health and during the pathology associated with diabetes. Prog. Retin. Eye Res. 2025, 106, 101349. [Google Scholar] [CrossRef]
- Inada, M.; Xu, H.; Takeuchi, M.; Ito, M.; Chen, M. Microglia increase tight-junction permeability in coordination with Muller cells under hypoxic condition in an in vitro model of inner blood-retinal barrier. Exp. Eye Res. 2021, 205, 108490. [Google Scholar] [CrossRef]
- Kugler, E.C.; Greenwood, J.; MacDonald, R.B. The “Neuro-Glial-Vascular” Unit: The Role of Glia in Neurovascular Unit Formation and Dysfunction. Front. Cell Dev. Biol. 2021, 9, 732820. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Tonade, D.; Kern, T.S. Photoreceptor cells and RPE contribute to the development of diabetic retinopathy. Prog. Retin. Eye Res. 2021, 83, 100919. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Silva, P.S.; Stitt, A.W. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat. Rev. Endocrinol. 2021, 17, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Augustine, J.; Troendle, E.P.; Barabas, P.; McAleese, C.A.; Friedel, T.; Stitt, A.W.; Curtis, T.M. The Role of Lipoxidation in the Pathogenesis of Diabetic Retinopathy. Front. Endocrinol. 2021, 11, 621938. [Google Scholar] [CrossRef]
- Altmann, C.; Schmidt, M.H.H. The Role of Microglia in Diabetic Retinopathy: Inflammation, Microvasculature Defects and Neurodegeneration. Int. J. Mol. Sci. 2018, 19, 110. [Google Scholar] [CrossRef]
- Karia, N. Retinal vein occlusion: Pathophysiology and treatment options. Clin. Ophthalmol. 2010, 4, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xiao, W.; Zhu, X.; Mao, Y.; Liu, X.; Chen, X.; Huang, J.; Tang, S.; Rizzolo, L.J. Differential expression of claudins in retinas during normal development and the angiogenesis of oxygen-induced retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7556–7564. [Google Scholar] [CrossRef]
- Kim, J.; Chun, J.; Ahn, M.; Jung, K.; Moon, C.; Shin, T. Blood-retina barrier dysfunction in experimental autoimmune uveitis: The pathogenesis and therapeutic targets. Anat. Cell Biol. 2022, 55, 20–27. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kim, J.H.; Kim, W.J.; Lee, H.Y.; Park, J.A.; Lee, S.W.; Yoon, D.K.; Kim, H.H.; Chung, H.; Yu, Y.S.; et al. AKAP12 regulates human blood-retinal barrier formation by downregulation of hypoxia-inducible factor-1alpha. J. Neurosci. 2007, 27, 4472–4481. [Google Scholar] [CrossRef]
- Tripathi, R.; Ashton, N. Electron microscopical study of Coat’s disease. Br. J. Ophthalmol. 1971, 55, 289–301. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, C.H.; Huang, S.; Chen, J. Wnt Signaling in vascular eye diseases. Prog. Retin. Eye Res. 2019, 70, 110–133. [Google Scholar] [CrossRef]
- Yang, M.; Li, S.; Huang, L.; Zhao, R.; Dai, E.; Jiang, X.; He, Y.; Lu, J.; Peng, L.; Liu, W.; et al. CTNND1 variants cause familial exudative vitreoretinopathy through the Wnt/cadherin axis. JCI Insight 2022, 7, e158428. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Tischfield, M.; Williams, J.; Smallwood, P.M.; Rattner, A.; Taketo, M.M.; Nathans, J. Canonical WNT signaling components in vascular development and barrier formation. J. Clin. Investig. 2014, 124, 3825–3846. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Y.; Wu, X.; Wang, H.; Jin, Z.; Wang, P.; Feng, J.; Chen, S.; Zhou, W. Efficacy and prognostic factors of anti-VEGF treatment for neovascular age-related macular degeneration: An OCTA imaging-based deep learning analysis. Photodiagn. Photodyn. Ther. 2025, 55, 104701. [Google Scholar] [CrossRef] [PubMed]
- Hauck, S.M.; Suppmann, S.; Ueffing, M. Proteomic profiling of primary retinal Muller glia cells reveals a shift in expression patterns upon adaptation to in vitro conditions. Glia 2003, 44, 251–263. [Google Scholar] [CrossRef]
- Chavkin, N.W.; Walsh, K.; Hirschi, K.K. Isolation of Highly Purified and Viable Retinal Endothelial Cells. J. Vasc. Res. 2021, 58, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska-Kruk, J.; Hoeben, K.A.; Vogels, I.M.; Gaillard, P.J.; Van Noorden, C.J.; Schlingemann, R.O.; Klaassen, I. A novel co-culture model of the blood-retinal barrier based on primary retinal endothelial cells, pericytes and astrocytes. Exp. Eye Res. 2012, 96, 181–190. [Google Scholar] [CrossRef]
- Bora, K.; Kushwah, N.; Maurya, M.; Pavlovich, M.C.; Wang, Z.; Chen, J. Assessment of Inner Blood-Retinal Barrier: Animal Models and Methods. Cells 2023, 12, 2443. [Google Scholar] [CrossRef]
- Charbel Issa, P.; Barnard, A.R.; Singh, M.S.; Carter, E.; Jiang, Z.; Radu, R.A.; Schraermeyer, U.; MacLaren, R.E. Fundus autofluorescence in the Abca4(-/-) mouse model of Stargardt disease—Correlation with accumulation of A2E, retinal function, and histology. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5602–5612. [Google Scholar] [CrossRef]
- Volland, S.; Esteve-Rudd, J.; Hoo, J.; Yee, C.; Williams, D.S. A comparison of some organizational characteristics of the mouse central retina and the human macula. PLoS ONE 2015, 10, e0125631. [Google Scholar] [CrossRef]
- Bennis, A.; Gorgels, T.G.; Ten Brink, J.B.; van der Spek, P.J.; Bossers, K.; Heine, V.M.; Bergen, A.A. Comparison of Mouse and Human Retinal Pigment Epithelium Gene Expression Profiles: Potential Implications for Age-Related Macular Degeneration. PLoS ONE 2015, 10, e0141597. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.H.; Siah, K.W.; Lo, A.W. Estimation of clinical trial success rates and related parameters. Biostatistics 2019, 20, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Balijepalli, A.; Sivaramakrishan, V. Organs-on-chips: Research and commercial perspectives. Drug Discov. Today 2017, 22, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Deinsberger, J.; Reisinger, D.; Weber, B. Global trends in clinical trials involving pluripotent stem cells: A systematic multi-database analysis. NPJ Regen. Med. 2020, 5, 15. [Google Scholar] [CrossRef]
- Bell, C.M.; Zack, D.J.; Berlinicke, C.A. Human Organoids for the Study of Retinal Development and Disease. Annu. Rev. Vis. Sci. 2020, 6, 91–114. [Google Scholar] [CrossRef]
- Jin, Z.B.; Gao, M.L.; Deng, W.L.; Wu, K.C.; Sugita, S.; Mandai, M.; Takahashi, M. Stemming retinal regeneration with pluripotent stem cells. Prog. Retin. Eye Res. 2019, 69, 38–56. [Google Scholar] [CrossRef]
- Kruczek, K.; Swaroop, A. Pluripotent stem cell-derived retinal organoids for disease modeling and development of therapies. Stem Cells 2020, 38, 1206–1215. [Google Scholar] [CrossRef]
- Fernandez, C.E.; Yen, R.W.; Perez, S.M.; Bedell, H.W.; Povsic, T.J.; Reichert, W.M.; Truskey, G.A. Human Vascular Microphysiological System for in vitro Drug Screening. Sci. Rep. 2016, 6, 21579. [Google Scholar] [CrossRef]
- Kim, S.; Kim, W.; Lim, S.; Jeon, J.S. Vasculature-On-A-Chip for In Vitro Disease Models. Bioengineering 2017, 4, 8. [Google Scholar] [CrossRef]
- Hoganson, D.M.; Finkelstein, E.B.; Owens, G.E.; Hsiao, J.C.; Eng, K.Y.; Kulig, K.M.; Kim, E.S.; Kniazeva, T.; Pomerantseva, I.; Neville, C.M.; et al. A bilayer small diameter in vitro vascular model for evaluation of drug induced vascular injury. Biomicrofluidics 2016, 10, 054116. [Google Scholar] [CrossRef]
- Haderspeck, J.C.; Chuchuy, J.; Kustermann, S.; Liebau, S.; Loskill, P. Organ-on-a-chip technologies that can transform ophthalmic drug discovery and disease modeling. Expert. Opin. Drug Discov. 2019, 14, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Arik, Y.B.; Buijsman, W.; Loessberg-Zahl, J.; Cuartas-Velez, C.; Veenstra, C.; Logtenberg, S.; Grobbink, A.M.; Bergveld, P.; Gagliardi, G.; den Hollander, A.I.; et al. Microfluidic organ-on-a-chip model of the outer blood-retinal barrier with clinically relevant read-outs for tissue permeability and vascular structure. Lab. A Chip 2021, 21, 272–283. [Google Scholar] [CrossRef]
- Maoz, B.M.; Herland, A.; FitzGerald, E.A.; Grevesse, T.; Vidoudez, C.; Pacheco, A.R.; Sheehy, S.P.; Park, T.E.; Dauth, S.; Mannix, R.; et al. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat. Biotechnol. 2018, 36, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Maurissen, T.L.; Pavlou, G.; Bichsel, C.; Villasenor, R.; Kamm, R.D.; Ragelle, H. Microphysiological Neurovascular Barriers to Model the Inner Retinal Microvasculature. J. Pers. Med. 2022, 12, 148. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.T.; Gard, A.L.; Gaibler, R.; Mulhern, T.J.; Strelnikov, R.; Azizgolshani, H.; Cain, B.P.; Isenberg, B.C.; Haroutunian, N.J.; Raustad, N.E.; et al. A high-throughput microfluidic bilayer co-culture platform to study endothelial-pericyte interactions. Sci. Rep. 2021, 11, 12225. [Google Scholar] [CrossRef]
- Leverant, A.; Oprysk, L.; Dabrowski, A.; Kyker-Snowman, K.; Vazquez, M. Three-Dimensionally Printed Microsystems to Facilitate Flow-Based Study of Cells from Neurovascular Barriers of the Retina. Micromachines 2024, 15, 1103. [Google Scholar] [CrossRef] [PubMed]
- Ragelle, H.; Goncalves, A.; Kustermann, S.; Antonetti, D.A.; Jayagopal, A. Organ-On-A-Chip Technologies for Advanced Blood-Retinal Barrier Models. J. Ocul. Pharmacol. Ther. 2020, 36, 30–41. [Google Scholar] [CrossRef]
- Yeste, J.; García-Ramírez, M.; Illa, X.; Guimerà, A.; Hernández, C.; Simó, R.; Villa, R. A compartmentalized microfluidic chip with crisscross microgrooves and electrophysiological electrodes for modeling the blood-retinal barrier. Lab. A Chip 2017, 18, 95–105. [Google Scholar] [CrossRef]
- Maurissen, T.L.; Spielmann, A.J. Modeling early pathophysiological phenotypes of diabetic retinopathy in a human inner blood-retinal barrier-on-a-chip. Nat. Commun. 2024, 15, 1372. [Google Scholar] [CrossRef]
- Tega, Y.; Kubo, Y.; Miura, H.; Ri, K.; Tomise, A.; Akanuma, S.I.; Hosoya, K.I. Carrier-Mediated Process of Putrescine Elimination at the Rat Blood-Retinal Barrier. Int. J. Mol. Sci. 2023, 24, 9003. [Google Scholar] [CrossRef]
- Tun, T.; Kang, Y.S. Effects of simvastatin on CAT-1-mediated arginine transport and NO level under high glucose conditions in conditionally immortalized rat inner blood-retinal barrier cell lines (TR-iBRB). Microvasc. Res. 2017, 111, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Tachikawa, M.; Murakami, K.; Martin, P.M.; Hosoya, K.; Ganapathy, V. Retinal transfer of nicotinate by H+ -monocarboxylate transporter at the inner blood-retinal barrier. Microvasc. Res. 2011, 82, 385–390. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Akanuma, S.I. Comprehensive Evidence of Carrier-Mediated Distribution of Amantadine to the Retina across the Blood-Retinal Barrier in Rats. Pharmaceutics 2021, 13, 1339. [Google Scholar] [CrossRef]
- Ohkura, Y.; Akanuma, S.; Tachikawa, M.; Hosoya, K. Blood-to-retina transport of biotin via Na+-dependent multivitamin transporter (SMVT) at the inner blood-retinal barrier. Exp. Eye Res. 2010, 91, 387–392. [Google Scholar] [CrossRef]
- Yoneyama, D.; Shinozaki, Y.; Lu, W.L.; Tomi, M.; Tachikawa, M.; Hosoya, K. Involvement of system A in the retina-to-blood transport of l-proline across the inner blood-retinal barrier. Exp. Eye Res. 2010, 90, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Akanuma, S.; Tachikawa, M.; Hosoya, K. Characteristics of glycine transport across the inner blood-retinal barrier. Neurochem. Int. 2009, 55, 789–795. [Google Scholar] [CrossRef]
- Hosoya, K.; Fujita, K.; Tachikawa, M. Involvement of reduced folate carrier 1 in the inner blood-retinal barrier transport of methyltetrahydrofolate. Drug Metab. Pharmacokinet. 2008, 23, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Nagase, K.; Tomi, M.; Tachikawa, M.; Hosoya, K. Functional and molecular characterization of adenosine transport at the rat inner blood-retinal barrier. Biochim. Biophys. Acta 2006, 1758, 13–19. [Google Scholar] [CrossRef]
- Nakashima, T.; Tomi, M.; Katayama, K.; Tachikawa, M.; Watanabe, M.; Terasaki, T.; Hosoya, K. Blood-to-retina transport of creatine via creatine transporter (CRT) at the rat inner blood-retinal barrier. J. Neurochem. 2004, 89, 1454–1461. [Google Scholar] [CrossRef]
- Gyawali, A.; Kim, M.H.; Kang, Y.S. A novel organic cation transporter involved in paeonol transport across the inner blood-retinal barrier and changes in uptake in high glucose conditions. Exp. Eye Res. 2021, 202, 108387. [Google Scholar] [CrossRef]
- Tega, Y.; Kubo, Y.; Yuzurihara, C.; Akanuma, S.; Hosoya, K. Carrier-Mediated Transport of Nicotine Across the Inner Blood-Retinal Barrier: Involvement of a Novel Organic Cation Transporter Driven by an Outward H(+) Gradient. J. Pharm. Sci. 2015, 104, 3069–3075. [Google Scholar] [CrossRef]
- Tachikawa, M.; Takeda, Y.; Tomi, M.; Hosoya, K. Involvement of OCTN2 in the transport of acetyl-L-carnitine across the inner blood-retinal barrier. Investig. Ophthalmol. Vis. Sci. 2010, 51, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Kubo, Y.; Obata, A.; Akanuma, S.; Hosoya, K. Impact of Cationic Amino Acid Transporter 1 on Blood-Retinal Barrier Transport of L-Ornithine. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5925–5932. [Google Scholar] [CrossRef] [PubMed]
- Kubo, Y.; Kusagawa, Y.; Tachikawa, M.; Akanuma, S.; Hosoya, K. Involvement of a novel organic cation transporter in verapamil transport across the inner blood-retinal barrier. Pharm. Res. 2013, 30, 847–856. [Google Scholar] [CrossRef]
- Hosoya, K.; Minamizono, A.; Katayama, K.; Terasaki, T.; Tomi, M. Vitamin C transport in oxidized form across the rat blood-retinal barrier. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1232–1239. [Google Scholar] [CrossRef]
- Hosoya, K.; Kondo, T.; Tomi, M.; Takanaga, H.; Ohtsuki, S.; Terasaki, T. MCT1-mediated transport of L-lactic acid at the inner blood-retinal barrier: A possible route for delivery of monocarboxylic acid drugs to the retina. Pharm. Res. 2001, 18, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; Humphries, P. Size-selective and in vitro assessment of inner blood retina barrier permeability. Methods Mol. Biol. 2011, 763, 355–367. [Google Scholar] [CrossRef]
- Sharma, D.; Kaur, G.; Bisen, S.; Sharma, A.; Ibrahim, A.S.; Singh, N.K. IL-33 via PKCmu/PRKD1 Mediated alpha-Catenin Phosphorylation Regulates Endothelial Cell-Barrier Integrity and Ischemia-Induced Vascular Leakage. Cells 2023, 12, 703. [Google Scholar] [CrossRef]
- Phuong, T.T.T.; Redmon, S.N.; Yarishkin, O.; Winter, J.M.; Li, D.Y.; Krizaj, D. Calcium influx through TRPV4 channels modulates the adherens contacts between retinal microvascular endothelial cells. J. Physiol. 2017, 595, 6869–6885. [Google Scholar] [CrossRef] [PubMed]
- Brankin, B.; Campbell, M.; Canning, P.; Gardiner, T.A.; Stitt, A.W. Endostatin modulates VEGF-mediated barrier dysfunction in the retinal microvascular endothelium. Exp. Eye Res. 2005, 81, 22–31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qin, Y.J.; Xiao, K.; Zhong, Z.; Zhao, Y.; Yu, T.; Sun, X.F. LECT2 Ameliorates Blood-Retinal Barrier Impairment Secondary to Diabetes Via Activation of the Tie2/Akt/mTOR Signaling Pathway. Investig. Ophthalmol. Vis. Sci. 2022, 63, 7. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, C.; Meng, Z.; Gan, L.; Guo, R.; Liu, J.; Bond Lau, W.; Xie, D.; Zhao, J.; Lopez, B.L.; et al. C1q/TNF-Related Protein 3 Prevents Diabetic Retinopathy via AMPK-Dependent Stabilization of Blood-Retinal Barrier Tight Junctions. Cells 2022, 11, 779. [Google Scholar] [CrossRef]
- Mesquida, M.; Drawnel, F.; Lait, P.J.; Copland, D.A.; Stimpson, M.L.; Llorenc, V.; Sainz de la Maza, M.; Adan, A.; Widmer, G.; Strassburger, P.; et al. Modelling Macular Edema: The Effect of IL-6 and IL-6R Blockade on Human Blood-Retinal Barrier Integrity In Vitro. Transl. Vis. Sci. Technol. 2019, 8, 32. [Google Scholar] [CrossRef]
- Tomi, M.; Terayama, T.; Isobe, T.; Egami, F.; Morito, A.; Kurachi, M.; Ohtsuki, S.; Kang, Y.S.; Terasaki, T.; Hosoya, K. Function and regulation of taurine transport at the inner blood-retinal barrier. Microvasc. Res. 2007, 73, 100–106. [Google Scholar] [CrossRef]
- Tomi, M.; Tajima, A.; Tachikawa, M.; Hosoya, K. Function of taurine transporter (Slc6a6/TauT) as a GABA transporting protein and its relevance to GABA transport in rat retinal capillary endothelial cells. Biochim. Biophys. Acta 2008, 1778, 2138–2142. [Google Scholar] [CrossRef]
- Tomi, M.; Hosoya, K.; Takanaga, H.; Ohtsuki, S.; Terasaki, T. Induction of xCT gene expression and L-cystine transport activity by diethyl maleate at the inner blood-retinal barrier. Investig. Ophthalmol. Vis. Sci. 2002, 43, 774–779. [Google Scholar]
- Bharti, K.; den Hollander, A.I.; Lakkaraju, A.; Sinha, D.; Williams, D.S.; Finnemann, S.C.; Bowes-Rickman, C.; Malek, G.; D’Amore, P.A. Cell culture models to study retinal pigment epithelium-related pathogenesis in age-related macular degeneration. Exp. Eye Res. 2022, 222, 109170. [Google Scholar] [CrossRef] [PubMed]
- Dessalles, C.A.; Leclech, C.; Castagnino, A.; Barakat, A.I. Integration of substrate- and flow-derived stresses in endothelial cell mechanobiology. Commun. Biol. 2021, 4, 764. [Google Scholar] [CrossRef]
- Yang, T.; Guo, L.; Fang, Y.; Liang, M.; Zheng, Y.; Pan, M.; Meng, C.; Liu, G. Pericytes of Indirect Contact Coculture Decrease Integrity of Inner Blood-Retina Barrier Model In Vitro by Upgrading MMP-2/9 Activity. Dis. Markers 2021, 2021, 7124835. [Google Scholar] [CrossRef]
- Abukawa, H.; Tomi, M.; Kiyokawa, J.; Hori, S.; Kondo, T.; Terasaki, T.; Hosoya, K. Modulation of retinal capillary endothelial cells by Müller glial cell-derived factors. Mol. Vis. 2009, 15, 451–457. [Google Scholar] [PubMed]
- Sánchez-Palencia, D.M.; Bigger-Allen, A.; Saint-Geniez, M.; Arboleda-Velásquez, J.F.; D’Amore, P.A. Coculture Assays for Endothelial Cells-Mural Cells Interactions. Methods Mol. Biol. 2016, 1464, 35–47. [Google Scholar] [CrossRef]
- Bryan, B.A.; D’Amore, P.A. Pericyte isolation and use in endothelial/pericyte coculture models. Methods Enzymol. 2008, 443, 315–331. [Google Scholar] [CrossRef]
- Tarallo, S.; Beltramo, E.; Berrone, E.; Porta, M. Human pericyte-endothelial cell interactions in co-culture models mimicking the diabetic retinal microvascular environment. Acta Diabetol. 2012, 49 (Suppl. 1), S141–S151. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, C.; Liu, D.; Yang, Q.; Tang, L.; Wang, T.; Tian, H.; Lu, L.; Xu, J.Y.; Gao, F.; et al. Erythropoietin protects the inner blood-retinal barrier by inhibiting microglia phagocytosis via Src/Akt/cofilin signalling in experimental diabetic retinopathy. Diabetologia 2021, 64, 211–225. [Google Scholar] [CrossRef]
- Yuan, C.; Mo, Y.; Yang, J.; Zhang, M.; Xie, X. Influences of advanced glycosylation end products on the inner blood-retinal barrier in a co-culture cell model in vitro. Open Life Sci. 2020, 15, 619–628. [Google Scholar] [CrossRef]
- Castro, N.; Pena, J.S.; Cliver, R.; Berthiaume, F.; Vazquez, M. Estradiol impacts Muller glia and endothelial cell responses in hyperglycemic microenvironments with advanced glycation end products. Exp. Eye Res. 2025, 251, 110185. [Google Scholar] [CrossRef]
- Beltramo, E.; Mazzeo, A.; Lopatina, T.; Trento, M.; Porta, M. Thiamine transporter 2 is involved in high glucose-induced damage and altered thiamine availability in cell models of diabetic retinopathy. Diabetes Vasc. Dis. Res. 2020, 17, 1479164119878427. [Google Scholar] [CrossRef]
- Sun, P.; Xu, N.; Li, Y.; Han, Y. Destruction of the blood-retina barrier in diabetic retinopathy depends on angiotensin-converting enzyme-mediated TGF-beta1/Smad signaling pathway activation. Int. Immunopharmacol. 2020, 85, 106686. [Google Scholar] [CrossRef] [PubMed]
- Gao, V.; Briano, J.A.; Komer, L.E.; Burré, J. Functional and Pathological Effects of α-Synuclein on Synaptic SNARE Complexes. J. Mol. Biol. 2023, 435, 167714. [Google Scholar] [CrossRef]
- Lavekar, S.S.; Hughes, J.M.; Gomes, C.; Huang, K.C.; Harkin, J.; Canfield, S.G.; Meyer, J.S. Exploring dysfunctional barrier phenotypes associated with glaucoma using a human pluripotent stem cell-based model of the neurovascular unit. Fluids Barriers CNS 2024, 21, 90. [Google Scholar] [CrossRef]
- Pena, J.S.; Berthiaume, F.; Vazquez, M. Muller Glia Co-Regulate Barrier Permeability with Endothelial Cells in an Vitro Model of Hyperglycemia. Int. J. Mol. Sci. 2024, 25, 12271. [Google Scholar] [CrossRef] [PubMed]
- Guidry, C. Isolation and characterization of porcine Muller cells. Myofibroblastic dedifferentiation in culture. Investig. Ophthalmol. Vis. Sci. 1996, 37, 740–752. [Google Scholar]
- Price, G.M.; Wong, K.H.; Truslow, J.G.; Leung, A.D.; Acharya, C.; Tien, J. Effect of mechanical factors on the function of engineered human blood microvessels in microfluidic collagen gels. Biomaterials 2010, 31, 6182–6189. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef]
- Dudek, S.M.; Garcia, J.G. Cytoskeletal regulation of pulmonary vascular permeability. J. Appl. Physiol. 2001, 91, 1487–1500. [Google Scholar] [CrossRef]
- Abbott, N.J. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J. Inherit. Metab. Dis. 2013, 36, 437–449. [Google Scholar] [CrossRef]
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R.D. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 2018, 180, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Fathi, M.; Ross, C.T.; Hosseinzadeh, Z. Functional 3-Dimensional Retinal Organoids: Technological Progress and Existing Challenges. Front. Neurosci. 2021, 15, 668857. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.; Lin, Y.Y.; Gerecht, S. The next generation of endothelial differentiation: Tissue-specific ECs. Cell Stem Cell 2021, 28, 1188–1204. [Google Scholar] [CrossRef] [PubMed]
- Holm, A.; Heumann, T.; Augustin, H.G. Microvascular Mural Cell Organotypic Heterogeneity and Functional Plasticity. Trends Cell Biol. 2018, 28, 302–316. [Google Scholar] [CrossRef]
- Hiler, D.; Chen, X.; Hazen, J.; Kupriyanov, S.; Carroll, P.A.; Qu, C.; Xu, B.; Johnson, D.; Griffiths, L.; Frase, S.; et al. Quantification of Retinogenesis in 3D Cultures Reveals Epigenetic Memory and Higher Efficiency in iPSCs Derived from Rod Photoreceptors. Cell Stem Cell 2015, 17, 101–115. [Google Scholar] [CrossRef]
- Kosyakova, N.; Kao, D.D.; Figetakis, M.; Lopez-Giraldez, F.; Spindler, S.; Graham, M.; James, K.J.; Won Shin, J.; Liu, X.; Tietjen, G.T.; et al. Differential functional roles of fibroblasts and pericytes in the formation of tissue-engineered microvascular networks in vitro. NPJ Regen. Med. 2020, 5, 1. [Google Scholar] [CrossRef]
- Zhong, X.; Gutierrez, C.; Xue, T.; Hampton, C.; Vergara, M.N.; Cao, L.H.; Peters, A.; Park, T.S.; Zambidis, E.T.; Meyer, J.S.; et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat. Commun. 2014, 5, 4047. [Google Scholar] [CrossRef]
- Zhao, H.; Yan, F. Retinal Organoids: A Next-Generation Platform for High-Throughput Drug Discovery. Stem Cell Rev. Rep. 2024, 20, 495–508. [Google Scholar] [CrossRef]
- Pamies, D.; Leist, M.; Coecke, S.; Bowe, G.; Allen, D.G.; Gstraunthaler, G.; Bal-Price, A.; Pistollato, F.; de Vries, R.B.M.; Hogberg, H.T.; et al. Guidance document on Good Cell and Tissue Culture Practice 2.0 (GCCP 2.0). Altex 2022, 39, 30–70. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.C.; Costa, E.C.; Filipe, H.A.L.; Saraiva, S.M.; Jacinto, T.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P. Animal-derived products in science and current alternatives. Biomater. Adv. 2023, 151, 213428. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, L.; Ramsden, D.; Leite, S.B.; Beken, S.; Bonzo, J.A.; Brown, P.; Candarlioglu, P.L.; Chan, T.S.; Chen, E.; Choi, C.K.; et al. Considerations from an International Regulatory and Pharmaceutical Industry (IQ MPS Affiliate) Workshop on the Standardization of Complex In Vitro Models in Drug Development. Adv. Biol. 2024, 8, 2300131. [Google Scholar] [CrossRef]
- Papamichail, L.; Koch, L.S.; Veerman, D.; Broersen, K.; van der Meer, A.D. Organoids-on-a-chip: Microfluidic technology enables culture of organoids with enhanced tissue function and potential for disease modeling. Front. Bioeng. Biotechnol. 2025, 13, 1515340. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, X.; Cai, Z.; Tu, M.; Wang, Y.; Ouyang, Q.; Yan, X.; Jing, G.; Yang, G. Transformation gap from research findings to large-scale commercialized products in microfluidic field. Mater. Today Bio 2024, 29, 101373. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Studts, J.; Wang, G. In-line product quality monitoring during biopharmaceutical manufacturing using computational Raman spectroscopy. mAbs 2023, 15, 2220149. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).