Abstract
2-Chloro-3-methylquinoxaline was selected as a nucleus around which various molecular transformations were performed to obtain new compounds expected to possess optimized antimicrobial activity. As very little work regarding attachment of ether linkages replacing chlorine at C-2 has been reported, it was thought worthwhile to synthesize various quinoxaline derivatives by replacing the C2 chlorine with an ether linkage attached to a benzene ring possessing an aldehyde or a free amino group which can be further reacted with aromatic amines and aromatic aldehydes, respectively, to yield new Schiff bases containing quinoxaline moieties. Thus the compounds 4-(2-methylquinoxalinyloxy) benzaldehyde (4), 2-[4-(substituted-benziminomethyl)-phenoxy]-3-methyl quinoxalines 5a–e, 4-(2-methyl-quinoxaline-3-yloxy)benzamine (6) and 4-(2-methylquinoxalin-3-yloxy)-N-substituted benzylidine benzamines 7a–e were synthesized and tested for their antimicrobial activity. The structures of the compounds were confirmed on the basis of their elemental and spectral data.
1. Introduction
Compounds containing the quinoxaline nucleus exhibit a broad spectrum of biological activity such as antibacterial [1,2,3], antifungal [4,5], antiviral [6], anticancer [7], antituberculosis [8], antimalarial [9] and anti-inflammatory properties [10]. Many researchers have reported the synthesis and biological activity of quinoxaline derivatives [11,12,13,14]. In the light of these facts we decided to synthesize some new quinoxaline derivatives incorporating aromatic aldehyde and aromatic amine moieties attached to a 2-hydroxy-3-methylquinoxaline nucleus with an ether linkage followed by the treatment with aromatic amines or aromatic aldehydes to afford Schiff bases in the hope of obtaining better antimicrobial agents. All the synthesized compounds were screened for their antimicrobial activity.
2. Result and Discussion
2.1. Synthesis
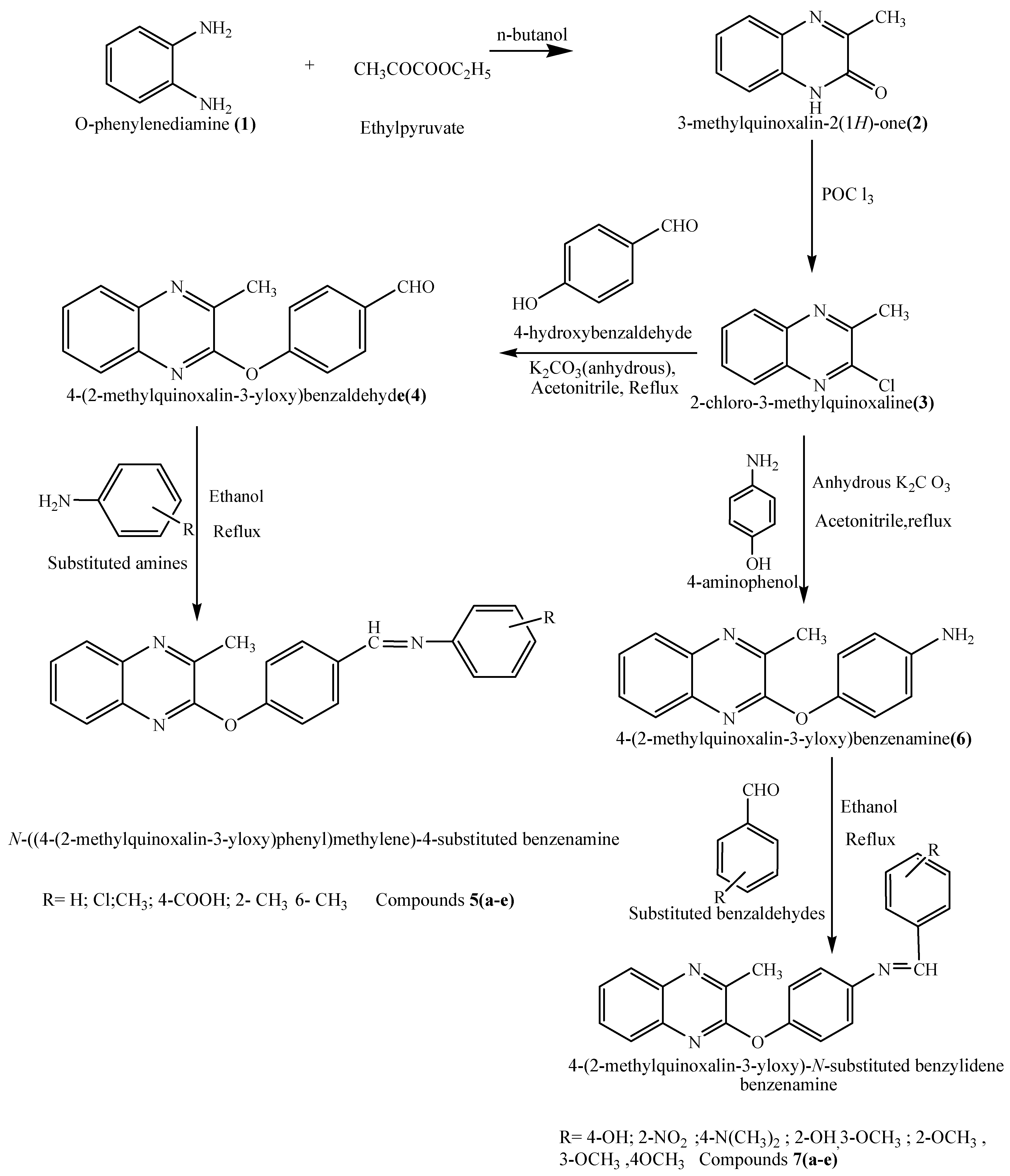
The chemical synthesis (Scheme 1) was initiated with the reaction of o-phenylenediamine (1) with ethyl pyruvate in n-butanol to yield 2-hydroxy-3-methylquinoxaline (2), which on treatment with POCl3 yielded 2-chloro-3-methylquinoxaline (3). A mixture of compound 3 and 4-hydroxy benzaldehyde was next refluxed in acetonitrile for 30 hours to afford 2-(p-formylphenoxy)-3-methyl quinoxaline (4) as an intermediate. Mixtures of compound 4 and various substituted aromatic amines were refluxed in ethanol to afford 2-[4-(substituted benziminomethyl)phenoxy]-3-methylquinoxalines 5a–e. In another set of reactions, compound 3 was refluxed with p-aminophenol in acetonitrile for 30 hours to yield 4-(2-methylquinoxalin-3-yloxy) benzamine (6) as a second intermediate. Compound 6 was refluxed with different substituted aromatic aldehydes in order to prepare compounds 7a–e. The structures of all newly synthesized compounds were elucidated on the basis of their spectral and analytical data.
The IR spectrum of compound 4 showed absorption bands at 3,038 cm−1 due to CH3 stretching, at 1,600 cm−1 due to C=N stretching, a strong band at 1,699 cm−1 due to an aldehyde function and a band at 1,222 cm−1 due to the C-O-C aryl ether (C-O stretching). Its 1H-NMR spectrum showed a singlet (3H) at δ 2.870 due to CH3 protons, a broad set of multiplets between δ 6.6 - 8.0 (8H) due to aromatic hydrogens and a sharp singlet at δ 10.07 due to an aldehyde proton. This indicated that a free aldehyde function was present which could be reacted with substituted aromatic amines to form Schiff bases. Similarly the IR spectrum of compound 6 showed two bands at 3,466 cm−1 and 3,425 cm−1 due to primary amine N-H stretching, while other bands at 3,041 cm−1 due to CH3 stretching and a band at 1,220 cm−1 due to (C-O-C) aryl ether were also present. In the case of the intermediate 6, the 1H-NMR spectrum showed a sharp singlet at δ 2.825 due to the protons of a CH3 group attached to the quinoxaline ring, a broad D2O exchangeable singlet at δ 3.742 due to NH2 protons, and a characteristic aromatic proton multiplet between δ 6.77-8.00 ppm. A singlet at around δ 8.40-9.03 due to presence of the (CH=N-) group in the compounds 5a–e and 7a–e clearly suggested the formation of the expected Schiff bases. IR, 1H-NMR spectra and elemental analytical data of compounds 5a–e and 7a–e confirmed the structures of the newly synthesized compounds.
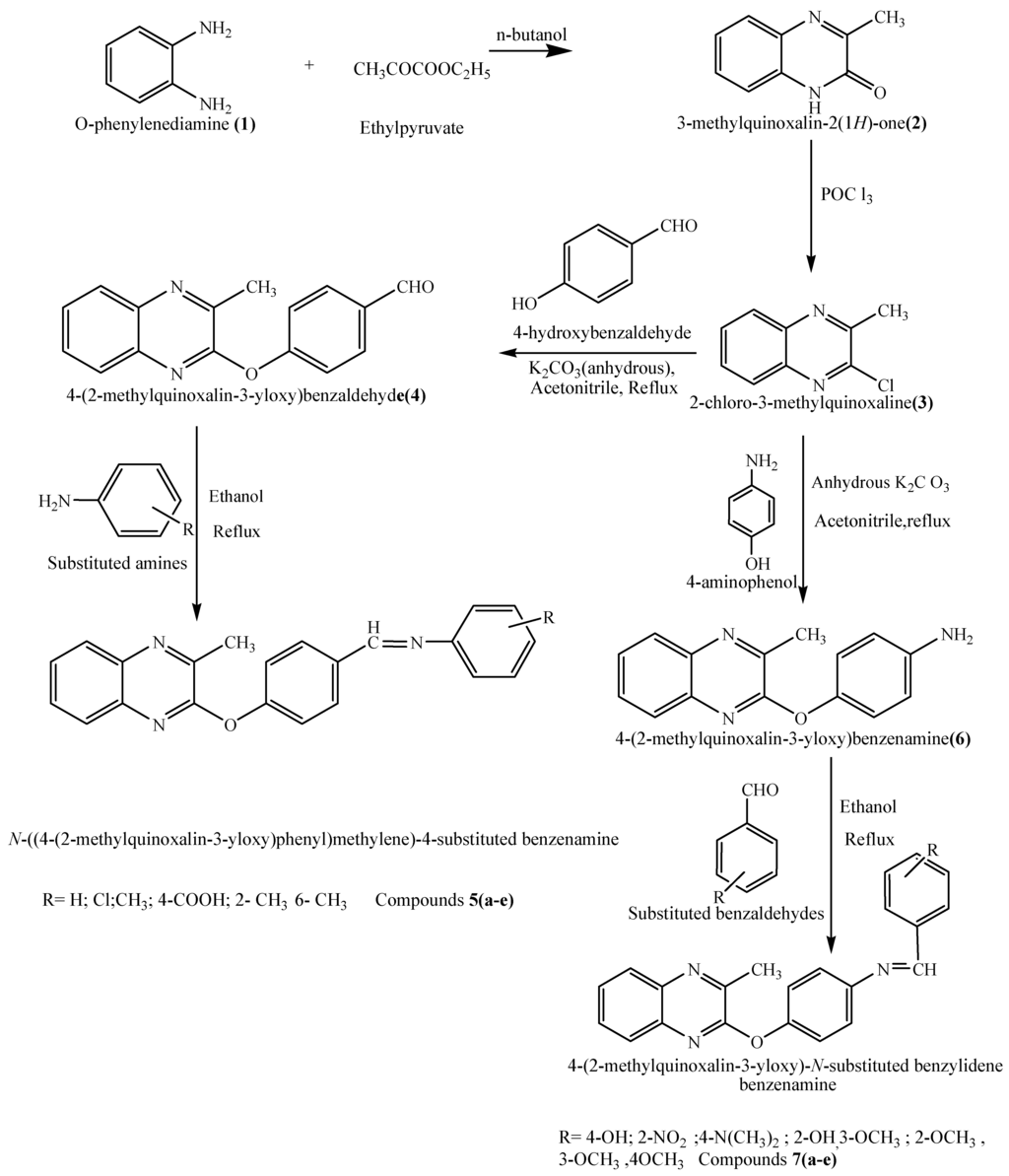
Scheme 1.
Synthesis of some new quinoxaline derivatives.
Scheme 1.
Synthesis of some new quinoxaline derivatives.
2.2. Antibacterial Activity
The antibacterial activity was determined by the disc diffusion method at the concentration of 50 µg per disk. All the synthesized compounds were tested in vitro for their antibacterial activity against microorganisms such as Staphylococcus aureus, Bacillus subtilis (Gram positive), Escherichia coli, Pseudomonas aeruginosa (Gram negative), using ciprofloxacin as standard antibacterial. The results of activity, presented in the Table 1 suggested that compounds 4, 5a, 5c, 5d, 5e, 7a and 7c were highly active against both Gram positive and Gram negative bacteria, among them compounds 5c, 5d, 7a, 7c, and 7e were specifically highly active against E. coli. Compound 5a possessed no activity against Bacillus subtilis, Compounds 5b, and 5c showed no activity against Pseudomonas aeruginosa. Compound 7d showed no activity against Escherichia coli and Pseudomonas aeruginosa. The rest of tested compounds were found to have moderate antibacterial activity. The high activity of compounds 4, 5a, 5c, 5d, 5e and 7c could be explained on the basis of the contributions of incorporated aromatic ring and –CH3 groups, which we know should increase the lipophilicity of the compounds. This increase in lipophilicity would help their permeability through the microbial cell wall resulting in higher activity. Compound 5d may be considered the analogue of benzoic acid (a known antimicrobial) due the presence of the –COOH group.
2.3. Antifungal Activity
The antifungal activity was tested against strain such as A. niger and C. albicans, using fluconazole as standard antifungal. Compounds 4, 6, and 7a showed moderate activity against both strains. Compounds 5a, 5b, 5c, 5d and 5e showed no activity against A. niger. Compounds 7b, 7c, 7d and 7e showed moderate activity against A. niger but no activity against C. albicans.

Table 1.
Results of antimicrobial activity of the compounds, zones of inhibition (in mm).
| Compounds | Zone of Inhibition | |||||
|---|---|---|---|---|---|---|
| S. aureus (NCIM 2079) | B. subtilis (NCIM 2439) | E. coli (NCIM 2831) | P. aerug. (NCIM 2863) | A. niger (NCIM 618) | C. alb. (NCIM 3557) | |
| 4 | + + + | + + | + + | + + | + + | + + |
| 5a | + + | - | + + + | + + | - | - |
| 5b | + + | + + | + + | - | - | - |
| 5c | + + + | + + | + + + | - | - | + + |
| 5d | + + + | + + + | + + + | + + | - | - |
| 5e | + + + | + + | + + | + + | - | + + |
| 6 | + + | + + | + + | + + | + + | + + |
| 7a | + + + | + + + | +++ | ++ | + + | + + |
| 7b | + + | + + | + + | + + | + + | - |
| 7c | + + + | + + | + + + | + + | + + | - |
| 7d | + + | + + | - | - | + + | - |
| 7e | + + | + + | + + + | + + | + + | - |
| Ciprofloxacin | + + + | + + + | + + + | + + + | ||
| Fluconazole | + + + | + + + | ||||
Key to symbols: - inactive (inhibition zone < 6 mm); slightly active = + (inhibition zone 7–9 mm); moderately active = + + (inhibition zone 10-13 mm); highly active = + + + (inhibition zone > 14 mm).
3. Experimental
3.1. General
All recorded melting points were determined on a laboratory melting point apparatus using the capillary method and are uncorrected. Purity of the compounds was checked by thin layer chromatography using silica gel-G on micro slide glass plates and spots were detected under iodine vapor. IR spectra were recorded in KBr disk on a Shimadzu FTIR-8400 spectrophotometer and 1H-NMR spectra on a JEOL FT-NMR Spectrometer (300 MHz) using TMS as an internal standard. All chemical shift values were recorded as δ (ppm).
3.1.1. Synthesis of 3-methylquinoxalin-2-(1H)-one (2)
o-Phenylenediamine (10.8 g, 0.10 M) was dissolved in n-butanol (300 mL) with warming. Ethyl pyruvate (11.6 g, 15 mL, 0.10 M) was dissolved separately in n-butanol (100 mL) and added to the former solution with constant stirring. The solution was set aside for 30 min, and then it was heated for 1 hour on a water bath. On cooling, the crystals that separated were filtered, washed with n-hexane and purified by recrystallization from ethanol to yield colorless, needle-shaped crystals of 2-hydroxy-3-methylquinoxaline. Yield 80%; m.p. 246 °C ([15,16] m.p. 245 °C); IR (KBr, cm−1): 3,008 (2° amide N–H), 2,968 (methyl group C-H), 1,665 (amide C=O), and 1,610 (aromatic nucleus C=C multiple bond).
3.1.2. Synthesis of 2-chloro-3-methylquinoxaline (3)
2-Hydroxy-3-methylquinoxaline (16.0 g, 0.10 M) in POCl3 (60 mL) was refluxed for 90 min. Then the excess of POCl3 was distilled off and the residue was cooled to room temperature and added to crushed ice taken in a 1 L beaker. The mixture was made alkaline by adding 2% NaOH solution to isolate the product. The crude product was recrystallized from petroleum ether (40–60 °C), to yield the crystals of 2-chloro-3-methylquinoxaline (3). Yield 60%; m.p. 88 °C (lit. [17] m.p. 86–87 °C); IR (KBr) data clearly showed the disappearance of the amide C=O stretching vibration and the appearance of an aryl halide C-Cl stretching vibration at 1,038.52 cm−1.
3.1.3. Synthesis of 4-(2-methylquinoxalinyloxy) benzaldehyde (4)
p-Hydroxybenzaldehyde (0.1.22 g, 0.01 M) was dissolved in acetonitrile (50 mL) taken in a 250 mL round bottomed flask. Anhydrous K2CO3 (2.0 g) was added to the mixture, which was refluxed for 1 hour, then 2-chloro-3-methylquinoxaline (1.785 g, 0.01 M) was added and the mixture was further refluxed for 30 hours. At the end of the reaction time, the mixture was filtered and excess of acetonitrile was distilled off to get the product. The crude product was treated with 2%NaOH solution to dissolve any unreacted p-hydroxybenzaldehyde. The product was filtered, washed with distilled water to remove traces of alkali and recrystallized from ethanol to get crystals of 4-(2-methylquinoxalinyloxy) benzaldehyde (4). Yield 70%; m.p. (116–117 °C); IR (KBr, cm−1): 1,736 (aldehyde C=O), 1,222 (ether C-O); 1H-NMR (CDCl3): 2.880 (s, 3H, CH3 attached to a quinoxaline ring), 7.288–8.047 (m, 8H, aromatic protons); 10.076 (s, 1H, aldehyde proton).
3.2. General procedure for the synthesis of 2-[4-(substituted benziminomethyl) phenoxy]-3-methyl-quinoxalines 5a–e
Compound 4 (0.01 M) and substituted aromatic amines (0.01 M) in ethanol (25mL) containing a catalytic amount of glacial acetic acid were refluxed for an appropriate time to complete the reaction, as monitored by TLC. After completion of the reactions, the flask contents were cooled and the separated crystalline compound was filtered, washed with a little ethanol and recrystallized from ethanol.
3.2.1. N-[(4-(2-Methylquinoxalin-3-yloxy)-phenyl)-methylenebenzamine (5a)
IR (KBr, cm−1): 3,060 (methyl C-H), 1,578 (-C=N-), 1,222 (ether C-O); 1H-NMR (CDCl3): 2.843 (s, 3H, CH3 attached to quinoxaline ring), 7.226–8.033 (m, 13H, Ar-H), 8.507 (s, 1H, CH=N-); MS: m/z 339. Anal. calc. for C22 H17 N3 O: C 77.86, H 5.05, N 12.38%. Found: C 77.65, H 5.20, N 12.52%.
3.2.2. [(4-(2-Methylquinoxalin-3-yloxy)-phenyl)-methylene)-2-chlorobenzamine (5b)
IR (KBr, cm−1) 3,059, (methyl C-H), 1,583, (-C=N-), 1,222, (ether C-O) and 1,038, (aryl C-Cl). 1H NMR (CDCl3): 2.847, (s, 3H, CH3 attached to quinoxaline ring), 7.044–8.073(12H, Ar-H), 8.437, (s, 1H, CH=N-). MS: m/z 373. Anal. Calculated for C22 H16 Cl N3 O: C 70.68, H 4.31, N 11.24%.Found: C 70.75, H 4.20, N 11.46%.
3.2.3. N-[{4(2-Methylquinoxalin-3-yloxy)-phenyl}-methylyne]-4-methylbenzeneamine (5c)
IR (KBr, cm−1): 1,578 (-C=N), 1,223 (ether C-O). Disappearance of band at 1,735.85 for aldehyde indicated the formation of –CH=N- group.1H NMR (CDCl3): 2.838 (s, 3H, CH3 attached to quinoxaline ring), 2.387 (s, 3H, CH3 attached to benzene), 7.148–8.018 (m, 12H, Ar-H) 8.511, (s, 1H, CH=N-).MS: m/z 353. Anal. Calculated for C23 H19 N3 O: C 78.16, H 5.42, N 11.89%.Found: C 78.08, H 5.54, N 11.78%.
3.2.4. 4-((4-(2-Methylquinoxalin-3yloxy)-phenyl) - methylene-aminobenzoicacid (5d)
IR (KBr, cm−1) 2,927 (methyl C-H), 1,686 (carboxylic C=O), 1,583 (-C=N-), 1,222 (ether C-O). 1H NMR (DMSO): 2.767 (s, 3H, CH3 attached to quinoxaline ring), 7.121–8.094(m, 12H, Ar-H), 8.686, (s, 1H, CH=N-), 12.836 (s, 1H, COOH). MS: m/z 383. Anal. Calculated for C23 H17 N3 O3: C 72.05, H 4.47, N 10.96%.Found: C 71.95, H 4.22, N 11.00%.
3.2.5. N-[(2-methylquinoxalin-3-yloxy)-phenyl)-methylene)-2, 6-dimethylbenzamine (5e)
IR (KBr, cm−1) 2,919 (methyl C-H), 1,577 (-C=N-), 1,224 (ether C-O). 1H NMR (CDCl3): 2.846(s, 3H, CH3 attached to quinoxaline ring), 2.336 and 2.317 (d, 6H, CH3 on aromatic benzene), 6.803–8.042 (m, 11H, Ar-H) 8.390, (s, 1H, CH=N-) group. MS: m/z 367. Anal. Calculated for C24 H21 N3 O: C 78.45, H 5.76, N 11.44%.Found: C 78.62, H 5.58, N 11.52%.
3.3. Synthesis of 4-(2-methylquinoxalin-3-yloxy)-benzamine (6)
4-Aminophenol (1.09 g, 0.01 M) was dissolved in a mixture of acetonitrile (50 mL) and DMF (10 mL) containing anhydrous K2CO3 (2.0 g). The mixture was refluxed for 1 hour then of 2-chloro-3-methylquinoxaline (1.785 g, 0.01 M) was added and mixture was further refluxed for 30 hour. The mixture was filtered and the excess of acetonitrile was distilled off to get the product. The crude product was treated with 2% NaOH solution to dissolve any unreacted 4-aminophenol. The product was filtered, washed with distilled water to remove traces of alkali and recrystallized from ethanol to give crystals of 4-(2-methylquinoxaline-3yloxy)-benzamine, yield 65%; m.p. 178 °C; IR (KBr, cm−1): 3,466 and 3,426 (1° amine group N-H), 3,041 (methyl group C-H), 1,620 (C=N) and 1,221 (ether C-O); 1H-NMR (CDCl3): 2.825 (s, 3H, CH3 attached to quinoxaline ring), 3.742(s, 2H, NH2 protons, which were D2O exchangeable) 6.77–8.00 (m, 8H, Ar-H)
3.4. General procedure for the synthesis of 4-(2-methylquinoxalin-3-yloxy)-N-substituted benzylidine benzamines 7a–e
A mixture of equalmolar quantities of compound 6 (0.01 M) and aromatic aldehydes (0.01 M) in ethanol (25 mL) containing a catalytic amount of glacial acetic acid were refluxed for sufficient time to complete the reaction, as monitored by TLC. The products so obtained were filtered, washed and recrystallized from appropriate solvents.
3.4.1. 4-[-{4-(2-Methylquinoxalin-3-yloxy)-phenyl}-iminomethyl]-phenol (7a)
IR (KBr, cm−1): 3,400 (phenol O-H), 3,065 (methyl C-H), 1,584, (-N=CH), 1,250 (ether C-O) and at 1,205 (phenol C-O). 1H NMR (CDCl3): 2.840 (s, 3H, CH3 attached to quinoxaline ring), 5.024 (s, 1H, Ar-OH), 6.861–8.052 (m, 12H, Ar-H), 9.031 (s, 1H, CH=N-). MS: m/z 355. Anal. Calculated for C22 H17 N3 O2: C, 74.35; H, 4.82; N, 11.82%.Found: C 74.15, H 5.00, N 12.05%.
3.4.2. 4-(2-methylquinoxalin-3-yloxy)-N-(2-nitrobenzylidene)-benzamine (7b)
IR (KBr, cm−1): 3,060 (methyl C-H), 1,572 (-N=CH), 1,518 and 1,340 (aromatic nitro group -O-N=O & C-N), 1,223 (ether C-O). 1H NMR (CDCl3): 2.842 (s, 3H, CH3 attached to quinoxaline ring), 7.261–8.359 (m, 12H, Ar-H), 9.031, (s, 1H, CH=N-). MS: m/z 384. Anal. Calculated for C22 H16 N4 O3: C 68.74, H 4.20, N 14.58%.Found: C 68.58, H 4.00, N 14.75%.
3.4.3. 4-[-{4-(2-Methylquinoxalin-3-yloxy)-phenyleneimino}-methyl]-N-dimethylbenzamine (7c)
IR (KBr, cm−1): 2,928 (methyl C-H), 1,590 (-N=CH), 1,329 (tertiary amine C-N),1,226 (ether C-O). 1H NMR (CDCl3): 2.835 (s, 3H, CH3 attached to quinoxaline ring), 3.025 (s, 6H, CH3 protons of 3° amine), 7.028–8.212 (m, 12H, Ar-H), 8.516 (s, 1H, CH=N-). MS: m/z 382. Anal. Calculated for C24 H22 N4 O: C 75.37, H 5.80, N 14.65%.Found: C 75.60, H, 5.65, N 14.80%.
3.4.4. [{4-(2-Methylquinoxaline-3-yloxy)-phenyleneimino}-methyl]-6-methoxyphenol (7d)
IR (KBr, cm−1): 3,450 and 1192 (phenol O-H & C-O), 3,060 (methyl C-H), 1,580 (-N=CH), 1,192 and 1,011 (methoxy C-O), 1223 (ether C-O). 1H NMR (CDCl3): 2.841 (s, 3H, CH3 attached to quinoxaline ring), 3.960 (s, 3H, –OCH3), 5.124 (s, 1H, phenolic–OH), 6.887–8.001 (m, 11H, Ar-H), 8.702 (s, 1H, CH=N-). MS: m/z 385. Anal. Calculated for C23 H19 N3 O3: C 71.67, H 4.97, N 10.90%. Found: C 71.56, H 4.80, N 11.05%.
3.4.5. 4-(2-Methylquinoxalin-3-yloxy)-n-(3, 4, 5-trimethoxybenzylidene)-benzamine (7e)
IR (KBr, cm−1): 2,941, (methyl C-H), 1,579 (-N=CH), 1,199 and 999, (methoxy C-O), 1,226 (ether C-O). 1H NMR (CDCl3): 2.838 (s, 3H, CH3 attached to quinoxaline ring), 3.934–3.971 (t, 9H, OCH3), 6.887–7.998(m, 10H, Ar-H), 8.441 (s, 1H, CH=N-) group. MS: m/z 429. Anal. Calculated for C25 H23 N3 O4: C 69.92, H 5.40, N 9.78%.Found: C 70.20, H 5.15, N 9.95%. All the synthesized compounds were purified by recrystallization from appropriate solvent monitored by TLC. The list of all the synthesized compounds is shown in the Table 2.
3.5. Antibacterial Activity
The antibacterial activity was assayed by agar plate disc diffusion method [18] at the concentration of 50 µg per disk. All the synthesized compounds were tested in vitro for their antibacterial activity against microorganisms such as Staphylococcus aureus, Bacillus subtilis (gram positive), Escherichia coli, and Pseudomonas aerugenosa (gram negative) strains. Each test compounds were dissolved in dimethylsulphoxide (DMSO) to get a concentration of 10 mg/mL. The disc (6 mm in diameter) was impregnated with 5 µL of each test solution to get 50 µg/disc, air dried and placed on the agar medium, previously seeded with 0.2 mL of broth culture of each organism for 18 hours. The plates were incubated at 37 °C for 24 hours and the inhibition zones measured in mm. Discs impregnated with DMSO were used as a control and ciprofloxacin discs as antibacterial reference standard.

Table 2.
Reaction conditions and physical data of synthesized compounds.
| Compounds | R | Reaction Time | Crystallization Solvents | M.P. (°C) | Mobile phase | Rf value |
|---|---|---|---|---|---|---|
| 4 | - | 30 hours | Ethanol | 116–117 | Ethyl acetate: n-Hexane (1:1) | 0.72 |
| 5a | H | 10 hours | Ethanol | 140 | Ethyl acetate: n-Hexane (1:1) | 0.80 |
| 5b | 2-Cl | 17 hours | Ethanol | 142 | Ethyl acetate: n-Hexane (1:1) | 0.78 |
| 5c | 4-CH3 | 9 hours | Ethanol | 159–160 | Ethyl acetate | 0.75 |
| 5d | 4-COOH | 16 hours | Ethanol | 221–222 | Ethyl acetate: n-Hexane (1:1) | 0.50 |
| 5e | 2-CH3,6-CH3 | 5 hours | Ethanol | 131–132 | Ethyl acetate | 0.87 |
| 6 | - | 30 hours | Ethanol | 178 | Ethyl acetate: n-Hexane (1:1) | 0.70 |
| 7a | 4-OH | 6 hours | Ethanol | 220 | Ethyl acetate: n-Hexane (1:1) | 0.60 |
| 7b | 2-NO2 | 1 hours | Ethanol | 171–172 | Ethyl acetate: n-Hexane (1:1) | 0.77 |
| 7c | 4-N(CH3)2 | 15 hours | Ethanol | 220 | Ethyl acetate: n-Hexane (1:1) | 0.70 |
| 7d | 2-OH,3-OCH3 | 1 hours | Ethanol | 190 | Ethyl acetate: n-Hexane (1:1) | 0.85 |
| 7e | 3,4,5(OCH3)3 | 21 hours | Ethanol | 135 | Ethyl acetate: n-Hexane (1:1) | 0.65 |
3.6. Antifungal Activity
The antifungal activity [19] was assayed by the Sabouraud dextrose agar media plate disc diffusion method at a concentration of 50 µg per disk. All the synthesized compounds were tested in vitro for their antifungal activity against microorganisms such as Aspergillus niger and Candida albicans. Each test compound was dissolved in dimethylsulphoxide (DMSO) to get a concentration of 10 mg/mL. The disc (6 mm in diameter) was impregnated with 5 µL of each test solution to get 50 µg/disc; air dried and placed on the Sabouraud dextrose agar media, previously seeded with 0.2 mL of broth culture of each organism for 18 hours. The plates were incubated at 22 °C for 48 hours and the inhibition zones measured in mm. Discs impregnated with DMSO were used as a negative control and fluconazole discs as antifungal reference standard. The results have already been shown are in Table 1.
Acknowledgements
The authors are thankful to the Director, College of Pharmacy and the Managing Director, I.F.T.M. Moradabad (India) for providing research facilities. We also extend our thanks to the Director, National Collection of Industrial Microorganisms, National Chemical Laboratory, Pune (India) for providing the microbial strains.
References
- Badran, M.M.; Abonzid, K.A.; Hussein, M.H. Synthesis of certain substituted quinoxalines as antimicrobials agents. Part II. Arch. Pharm. Res. 2003, 26, 107–113. [Google Scholar]
- Griffith, R.K.; Chittur, S.V.; Chen, Y.C. Inhibition of glucosamine-6-Phosphate synthase from candida albicans by quinoxaline-2, 3-diones. Med. Chem. Res. 1992, 2, 467–473. [Google Scholar]
- E-lGendy, A.A.; El-Meligie, S.; El-Ansry, A.; Ahmedy, A.M. Synthesis of some quinoxaline derivatives containing Indoline-2, 3-dione or, thiazolidine residue as potential antimicrobials agents. Arch. Pharm. Res. 1995, 18, 44–47. [Google Scholar]
- Reddy-Sastry, C.V.; Shrinivas-Rao, K.; Krishanan, V.S.H.; Rastogi, K.; Jain, M.L.; Narayanan, G. Synthesis and biological activity of some new tetrazolobenzoxazines as bis-tetrazoloquinoxalines. Indian J. Chem. 1990, 29, 396–403. [Google Scholar]
- El-Hawash, S.A.; Habib, N.S.; Franki, N.H. Synthesis and antimicrobial testing of 1,2,4-triazolo[4,3-a] quinoxalines,1,2,4-triazino[4,3-a] quinoxalines and 2-pyrazolylquinoxalines. Pharmazie 1999, 54, 808–815. [Google Scholar]
- Westphal, G.; Wasiki, H.; Zielinski, U.; Weberr, F.G.; Tonew, M.; Tonew, E. Potentielle virostatica. Pharmazie 1977, 35, 570–571. [Google Scholar]
- Monge, A.; Martinez-Crespo, F.J.; Cerai, A.L.; Palop, J.A.; Narro, S.; Senador, V.; Marin, A.; Sainz, Y.; Gonzalez, M.; Hamilton, E.; Barker, A.J. Hypoxia selective agents derived from 2-quinoxalinecarbonitrile 1, 2-di-N-oxides. J. Med. Chem. 1995, 38, 4488–4495. [Google Scholar]
- Michael, J.W.; Ben-Hadda, T.; Kotchevan, A.T.; Ramdani, A.; Touzani, R.; Elkadiri, S.; Hakkou, A.; Boukka, M.; Elli, T. 2, 3-bifunctionalized quinoxalines: Synthesis, DNA Interactions and Evaluation of Anticancer, Anti-tuberculosis and Antifungal Activity. Molecules 2002, 7, 641–656. [Google Scholar]
- Rangisetty, J.B.; Gupta, C.N.; Prasad, A.L.; Srinavas, P.; Sridhar, N.; Perimoo, P.; Veeranjaneyulu, A. Synthesis of new arylaminoquinoxalines and their antimalarial activity in mice. J. Pharm. Pharmacol. 2001, 53, 1409–1413. [Google Scholar] [PubMed]
- Wagle, S.; Adhikari, A.V.; Kumari, N.S. Synthesis of some new 2-(3-methyl-7-substituted-2-oxoquinoxalinyl)-5-(aryl)-1,3,4-oxadiazoles as potential non-steroidal anti-inflammatory and analgesic agents. Ind. J. Chem. 2008, 47, 439–448. [Google Scholar]
- Hong, Y.S.; Kim, H.M.; Park, Y.T.; Kirn, H.S. Synthesis of 1-arenesulphonyl-2-quinoxalinones. Bull. Korean Chem. Soc. 2000, 21, 133. [Google Scholar]
- Ali, M.M.; Ismail, M.M.F.; Elgamy, M.S.A.; Zahran, M.A.; Ammar, Y.A. Synthesis and antimicrobial activity of some novel quinoxalinones derivatives. Molecules 2000, 5, 864–873. [Google Scholar] [CrossRef]
- Dubey, P.K.; Naidu, A.; Vayas, S.; Vineel, B.G. Facile ring opening of 2-aryl[1,2,4]oxadiazino[5,6-b]quinoxalines with sodium alkoxides. Ind. J. Chem. 2005, 44B, 573–576. [Google Scholar]
- Vayas, D.A.; Chauhan, N.A.; Parikh, A.R. Synthesis and antimicrobial activity of quinoxaline based thiazolidinones and azetidinones. Ind. J. Chem. 2007, 46, 1699–1702. [Google Scholar]
- L’Italien, J.; Banks, C.K. 2-Hydroxy-3-alkylquinoxalines. J. Am. Chem. Soc. 1951, 73, 3246. [Google Scholar]
- Leese, C.L.; Rydon, H.N. Polyazanaphthalenes. Part I. Some derivatives of 1:4:5-Triazanaphthalenes and Quinoxaline. J. Chem. Soc. 1955, 303–308. [Google Scholar]
- Platt, B.C.; Sharp, T.M. N1-sulphanilamides derived from aminoquinoxalines and aminomethylquinoxalines. J. Chem. Soc. 1948, 2129. [Google Scholar]
- Collin, C.H. Microbiological Methods; Butterworths: London, UK, 1964; p. 92. [Google Scholar]
- Gravestock, M.B.; Ryley, J.F. Antifungal Chemotherapy. Ann. Rep. Med. Chem. 1984, 19, 127. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
