Diamidines versus Monoamidines as Anti-Pneumocystis Agents: An in Vivo Study
Abstract
:1. Introduction

2. Experimental
2.1. Chemistry
2.2. Determination of the in Vitro Cytotoxicity
2.3. Source of P. Carinii
2.4. Extraction, Purification, and Quantification of P. Carinii
2.5. Determination of the in Vitro Pneumocystis Activity
2.6. Determination of the in Vivo Pneumocystis Activity
3. Results and Discussion
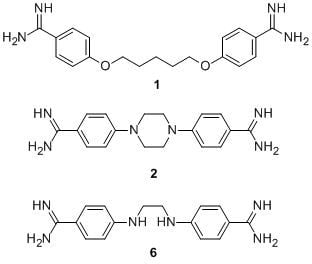
3.1. Chemistry



3.2. In Vitro Screenings
| Compound | Cytotoxicity 3T6 | Anti-Pneumocystis activity % inhibition vs. free-drug control at | ||
|---|---|---|---|---|
| IC50 (µM) | 50 µg/mL | 10 µg/mL | 0.1 µg/mL* | |
| 1 (pentamidine) | 1.98 ± 0.20 | 99.0 ± 0.1 | 99.0 ± 0.1 | 88.9 ± 3.1 |
| 2 | 1.98 ±0.07 | 99.0 ± 0.1 | 99.0 ± 0.1 | 1.7 ± 2.9 |
| 3 | 56.06 ± 0.39 | 99.0 ± 0.1 | 99.0 ± 0.1 | 8.3 ± 14.4 |
| 4 | 40.8 ± 4.4 | inactive | inactive | inactive |
| 5 | 10.2 ± 0.6 | inactive | inactive | inactive |
| 6 | 30.24 ± 1.14 | 98.7 ± 0.6 | 97.7 ± 0.6 | 29.8 ± 19.7 |
| 7 | 40.60 ± 1.30 | 99.5 ± 0.4 | 98.7 ± 0.6 | 42.0 ± 12.5 |
| 8 | 2.48 ± 0.35 | 99.0 ± 0.0 | 99.0 ± 0.0 | 15.0 ± 12.5 |
| 9 | 3.11 ± 0.09 | 88.7 ± 2.5 | 86.3 ± 4.0 | inactive |
3.3. In Vivo Study


4. Conclusions
Acknowledgments
Conflict of Interest
References
- Chabé, M.; Aliouat-Denis, C.; Delhaes, L.; Aliouat, E-M.; Viscogliosi, E.; Dei-Cas, E. Pneumocystis: From a doubtful unique entity to a group of highly diversified fungal species. FEMS Yeast Res. 2011, 11, 2–17. [Google Scholar] [CrossRef]
- Calderon, E.J.; Gutierrez-Rivero, S.; Durand-Joly, I.; Dei-Cas, E. Pneumocystis infection in humans: Diagnosis and treatment. Expert Rev. Anti-Infect. Ther. 2010, 8, 683–701. [Google Scholar] [CrossRef]
- Huang, L. Clinical and translational research in Pneumocystis pneumonia. Parasite 2011, 18, 3–11. [Google Scholar] [CrossRef]
- Larsen, H.H.; von Linstow, M.L.; Lundgren, B.; Hogh, B.; Westh, H.; Lundgren, J.D. Primary Pneumocystis infection in infants hospitalized with acute respiratory tract infection. Emerg. Infect. Dis. 2007, 13, 66–72. [Google Scholar]
- Carmona, E.M.; Limper, A.H. Update on the diagnosis and treatment of Pneumocystis pneumonia. Ther. Adv. Respir. Dis. 2011, 5, 41–59. [Google Scholar] [CrossRef]
- Iliades, P.; Meshnick, S.R.; Macreadie, I.G. Dihydropteroate synthase mutations in Pneumocystis jiroveci can affect sulfamethoxazole resistance in a Saccharomyces cerevisiae model. Antimicrob. Agents Chemother. 2004, 48, 2617–2623. [Google Scholar]
- Friaza, V.; Montes-Cano, M.A.; Respaldiza, N.; Morilla, R.; Calderon, E.J.; de la Horra, C. Prevalence of dihyropteroate synthase mutations in Spanish patients with HIV-associated Pneumocystis pneumonia. Diagn. Microbiol. Infect. Dis. 2009, 64, 104–105. [Google Scholar] [CrossRef]
- Catherinot, F.; Bougnoux, M.E.; Lecuit, M.; Couderc, L.J.; Lortholary, O. Pneumocystis jiroveci pneumonia. Infect. Dis. Clin. North Am. 2010, 24, 107–138. [Google Scholar] [CrossRef]
- Huang, L.; Morris, A.; Limper, A.H.; Beck, J.M. ATS Pneumocystis Workshop Participants, An Official ATS Workshop Summary: Recent advances and future directions in Pneumocystis pneumonia (PCP). Proc. Am. Thorac. Soc. 2006, 3, 655–664. [Google Scholar] [CrossRef]
- Tidwell, R.R.; Jones, S.K.; Geratz, J.D.; Ohemeng, K.A.; Cory, M.; Hall, J.E. Analogs of 1,5-di(4-amidinophenoxy)pentane (pentamidine) in the treatment of experimental Pneumocystis carinii pneumonia. J. Med. Chem. 1990, 33, 1252–1257. [Google Scholar] [CrossRef]
- Jones, S.K.; Hall, J.E.; Allen, M.A.; Morrison, S.D.; Ohemeng, K.A.; Reddy, V.V.; Geratz, J.E.; Tidwell, R.R. Novel pentamidine analogs in the treatment of experimental Pneumocystis carinii pneumonia. Antimicrob. Agents Chemother. 1990, 34, 1026–1030. [Google Scholar] [CrossRef]
- Patrick, D.A.; Boykin, D.W.; Wilson, W.D.; Tanous, F.A.; Spychala, J.; Bender, B.C.; Hall, J.E.; Dykstra, C.C.; Ohemeng, K.A.; Tidwell, R.R. Anti-Pneumocystis carinii pneumonia activity of dicationic carbazoles. Eur. J. Med. Chem. 1997, 32, 781–793. [Google Scholar] [CrossRef]
- Boykin, D.W.; Kumar, A.; Bajic, M.; Xiao, G.; Wilson, W.D.; Bender, B.C.; McCurdy, D.R.; Hall, J.E.; Tidwell, R.R. Anti-Pneumocystis activity of dicationic diaryl methylpyrimidines. Eur. J. Med. Chem. 1997, 32, 965–972. [Google Scholar]
- Francesconi, I.; Wilson, W.D.; Tanious, F.A.; Hall, J.E.; Bender, B.C.; Tidwell, R.R.; McCurdy, D.; Boykin, D.W. 2,4-Diphenyl furan diamidines as novel anti-Pneumocystis carinii pneumonia agents. J. Med. Chem. 1999, 42, 2260–2265. [Google Scholar] [CrossRef]
- Huang, T.L.; Vanden Eynde, J.J.; Mayence, A.; Collins, M.S.; Cushion, M.T.; Rattendi, D.; Londono, I.; Mazumder, L.; Bacchi, C.J.; Yartlet, N. Synthesis and SAR of alkanediamide-linked bisbenzamidines with anti-trypanosomal and anti-Pneumocystis activity. Bioorg. Med. Chem. Lett. 2009, 19, 5884–5886. [Google Scholar] [CrossRef]
- Bakunov, S.A.; Bakunova, S.M.; Wenzler, T.; Ghebru, M.; Werbovetz, K.A.; Brun, R.; Tidwell, R.R. Synthesis and antiprotozoal activity of cationic 1,4-diphenyl-1H-1,2,3-triazoles. J. Med. Chem. 2010, 53, 254–272. [Google Scholar]
- Farahat, A.A.; Paliakov, E.; Kumar, A.; Barghash, A.E.M.; Goda, F.E.; Eisa, H.M.; Wenzler, T.; Brun, R.; Liu, Y.; Wilson, W.D.; et al. Exploration of larger central ring linker in furamidine analogues: Synthesis and evaluation of their DNA binding, antiparasitic and fluorescence properties. Bioorg. Med. Chem. 2011, 19, 2156–2167. [Google Scholar] [CrossRef]
- Maciejewska, D.; Zabinski, J.; Kazmierczak, P.; Rezler, M.; Krassowska-Swiebocka, B.; Collins, M.S.; Cushion, M.T. Analogs of pentamidine as potential anti-Pneumocystis chemotherapeutics. Eur. J. Med. Chem. 2012, 48, 164–173. [Google Scholar] [CrossRef]
- Cushion, M.T.; Walzer, P.D.; Collins, M.S.; Rebholz, S.; Vanden Eynde, J.J.; Mayence, A.; Huang, T.L. Highly active anti-Pneumocystis carinii compounds in a library of novel piperazine-linked bisbenzamidines and related compounds. Antimicrob. Agents Chemother. 2004, 48, 4209–4216. [Google Scholar]
- Laurent, J.; Stanicki, D.; Huang, T.L.; Dei-Cas, E.; Pottier, M.; Aliouat, E.M.; Vanden Eynde, J.J. Bisbenzamidines as antifungal agents. Are both amidine functions required to observe an anti-Pneumocystis carinii activity? Molecules 2010, 15, 4283–4293. [Google Scholar]
- Slifkin, S.C. Light sensitive diazotype materials. US Patent 2632703, 24 March 1953. [Google Scholar]
- Vamecq, J.; Maurois, P.; Pages, N.; Bac, P.; Stables, J.P.; Gressens, P.; Stanicki, D.; Vanden Eynde, J.J. 1,2-Ethane-bis-1-amino-4-benzamidine is active against several brain insult and seizure challenges through anti-NMDA mechanisms targeting the 3H-TCP binding site and antioxidant action. Eur. J. Med. Chem. 2010, 45, 3101–3110. [Google Scholar] [CrossRef]
- Winkelman, E.; Raether, W.; Duewel, W.; Gericke, D.; Hohorst, W.; Rolly, H.; Schrinner, E. Chemotherapeutisch wirksame Nitroverbindungen. Arzneim. Forsch. 1975, 25, 681–708. [Google Scholar]
- Popov, D. Synthesis of 1,4-unsymmetrically substituted piperazines. Preparation of 1-phenyl-4-arylpiperazines. C. R. Acad. Bulg. Sci. 1966, 19, 1663–1666. [Google Scholar]
- Berg, S.S. The search for chemotherapeutic amidines. Part XVII. α,ω-Di-p-amidinoanilino-alkanes. J. Chem. Soc. 1968, 5172–5176. [Google Scholar]
- Fourneau, J.-P.; de Lestrange, Y. Derivatives of N-phenylethylenediamine. Bull. Soc. Chim. France 1947, 185, 827–838. [Google Scholar]
- Crespi, C.I. Xenobiotic-metabolizing human cells as tools for pharmacological and toxicological research. Adv. Drug Res. 1995, 26, 179–235. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 62, 55–63. [Google Scholar]
- Martinez, A.; Aliouat, E.M.; Pottier, M.; Gantois, N.; Pinçon, C.; Standaert-Vitse, A.; Dei-Cas, E.; Aliouat-Denis, C.-M. High-speed cell sorting of infectious trophic and cystic forms of Pneumocystis carinii. J. Eukaryot. Microbiol. 2009, 56, 446–453. [Google Scholar] [CrossRef]
- Dei-Cas, E.; Fleurisse, L.; Aliouat, E.M.; Bahon-Le-Capon, J.; Cailliez, C.; Creusy, C. Morphological and ultrastructural methods for Pneumocystis. FEMS Immunol. Med. Microbiol. 1998, 22, 185–189. [Google Scholar] [CrossRef]
- Garry, S.; Nesslany, F.; Aliouat, E.M.; Haguenoer, J.M.; Marzin, D. Hematite (Fe(2)O(3)) enhances benzo[a]pyrene genotoxicity in endotracheally treated rat, as determined by Comet Assay. Mutation Res. 2003, 538, 19–29. [Google Scholar]
- Ambrose, H.E.; Keely, S.P.; Aliouat, E.M.; Dei-Cas, E.; Wakefield, A.E.; Miller, R.F.; Stringer, J.R. Expression of the MSG and PRT1 multigene families of Pneumocystis carinii are not regulated in the same manner. Microbiology 2004, 150, 293–300. [Google Scholar] [CrossRef]
- Smith, W.; Bartlett, M.S.; Queener, S.F. Development of models and their use to discover new drugs for therapy and prophylaxis of Pneumocystis carinii Pneumonia. In Pneumocystis, Pneumonia, 3rd ed.; Walzer, P.D., Cushion, M.T., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2004; pp. 487–509. [Google Scholar]
- Aviles, P.; Aliouat, E.M.; Martinez, A.; Dei-Cas, E.; Herreros, E.; Dujardin, L.; Gargallo-Viola, D. In vitro pharmacodynamic parameters of sordarin derivatives in comparison with atovaquone, pentamidine and trimethoprim/sulfamethoxazol against rat-derived Pneumocystis carinii. Antimicrob. Agents Chemother. 2000, 44, 1284–1290. [Google Scholar] [CrossRef]
- Aliouat, E.M.; Dujardin, L.; Martínez, A.; Duriez, T.; Dei-Cas, E. Pneumocystis carinii growth kinetics in culture systems and in hosts: involvement of each life cycle parasite stage. J. Eukaryot. Microbiol. 1999, 46, 116S–117S. [Google Scholar] [CrossRef]
- Dei-Cas, E.; Chabé, M.; Moukhlis, R.; Durand-Joly, I.; Aliouat, E.M.; Stringer, J.R.; Cushion, M.T.; Noel, C.; de Hoog, G.S.; Guillot, J.; Viscogliosi, E. Pneumocystis oryctolagi sp. nov., an uncultured fungus causing pneumonia in rabbits at weaning: Review of current knowledge, and description of a new taxon on genotypic, phylogenetic and phenotypic bases. FEMS Microbiol. Rev. 2006, 30, 853–871. [Google Scholar]
- Dei-Cas, E.; Cailliez, J.C. European Concerted Action on Pneumocystis carinii. In vitro systems in Pneumocystis research. Parasitol. Today 1996, 12, 245–249. [Google Scholar] [CrossRef]
- Dei-Cas, E.; Aliouat, E.M.; Cailliez, J.C. Cellular Structure. In Pneumocystis Pneumonia, 3rd ed.; Walzer, P.D., Cushion, M.T., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2004; pp. 61–94. [Google Scholar]
- Tao, B.; Huang, T.L.; Zhang, Q.; Jackson, L.; Queener, S.F.; Donkor, I.O. Synthesis and anti-Pneumocystis carinii activity of conformationally restricted analogues of pentamidine. Eur. J. Med. Chem. 1999, 34, 531–538. [Google Scholar] [CrossRef]
- Hambye, S.; Stanicki, D.; Colet, J.M.; Aliouat, E.M.; Vanden Eynde, J.J.; Blankert, B. Three optimized and validated (using accuracy profiles) LC methods for the determination of pentamidine and new analogs in rat plasma. Talanta 2011, 83, 832–839. [Google Scholar] [CrossRef]
- Hughes, W.T.; Killmar, J.T.; Oz, H.S. Relative potency of 10 drugs with anti-Pneumocystis carinii activity in an animal model. J. Infect. Dis. 1994, 170, 906–911. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Stanicki, D.; Pottier, M.; Gantois, N.; Pinçon, C.; Forge, D.; Mahieu, I.; Boutry, S.; Vanden Eynde, J.J.; Martinez, A.; Dei-Cas, E.; et al. Diamidines versus Monoamidines as Anti-Pneumocystis Agents: An in Vivo Study. Pharmaceuticals 2013, 6, 837-850. https://doi.org/10.3390/ph6070837
Stanicki D, Pottier M, Gantois N, Pinçon C, Forge D, Mahieu I, Boutry S, Vanden Eynde JJ, Martinez A, Dei-Cas E, et al. Diamidines versus Monoamidines as Anti-Pneumocystis Agents: An in Vivo Study. Pharmaceuticals. 2013; 6(7):837-850. https://doi.org/10.3390/ph6070837
Chicago/Turabian StyleStanicki, Dimitri, Muriel Pottier, Nausicaa Gantois, Claire Pinçon, Delphine Forge, Isabelle Mahieu, Sébastien Boutry, Jean Jacques Vanden Eynde, Anna Martinez, Eduardo Dei-Cas, and et al. 2013. "Diamidines versus Monoamidines as Anti-Pneumocystis Agents: An in Vivo Study" Pharmaceuticals 6, no. 7: 837-850. https://doi.org/10.3390/ph6070837
APA StyleStanicki, D., Pottier, M., Gantois, N., Pinçon, C., Forge, D., Mahieu, I., Boutry, S., Vanden Eynde, J. J., Martinez, A., Dei-Cas, E., & Aliouat, E.-M. (2013). Diamidines versus Monoamidines as Anti-Pneumocystis Agents: An in Vivo Study. Pharmaceuticals, 6(7), 837-850. https://doi.org/10.3390/ph6070837