Abstract
Activation of TRPV1, TRPA1 or TRPM8 channel expressed in the central terminal of dorsal root ganglion (DRG) neuron increases the spontaneous release of l-glutamate onto spinal dorsal horn lamina II (substantia gelatinosa; SG) neurons which play a pivotal role in regulating nociceptive transmission. The TRP channels are activated by various plant-derived chemicals. Although stereoisomers activate or modulate ion channels in a distinct manner, this phenomenon is not fully addressed for TRP channels. By applying the whole-cell patch-clamp technique to SG neurons of adult rat spinal cord slices, we found out that all of plant-derived chemicals, carvacrol, thymol, carvone and cineole, increase the frequency of spontaneous excitatory postsynaptic current, a measure of the spontaneous release of l-glutamate from nerve terminals, by activating TRP channels. The presynaptic activities were different between stereoisomers (carvacrol and thymol; (−)-carvone and (+)-carvone; 1,8-cineole and 1,4-cineole) in the extent or the types of TRP channels activated, indicating that TRP channels in the SG are activated by stereoisomers in a distinct manner. This result could serve to know the properties of the central terminal TRP channels that are targets of drugs for alleviating pain.
Keywords:
TRPV1; TRPA1; TRPM8; plant-derived chemical; l-glutamate release; spinal dorsal horn; patch-clamp; rat 1. TRP Channels Involved in Nociceptive Transmission through Dorsal Root Ganglion Neurons
Cation-permeable transient receptor potential (TRP) channels expressed in dorsal root ganglion (DRG) neurons are involved in nociceptive transmission from the periphery (for a review see [1]). TRP channels, which are synthesized in the cell body of the DRG neuron, are transported to the peripheral and central terminals of the neuron by axonal transport. Among TRP channels involved in the nociceptive transmission, there are TRP vanilloid-1 (TRPV1), TRP ankyrin-1 (TRPA1) and TRP melastatin-8 (TRPM8) channels. The TRPA1 channel is found in a subset of rat DRG neurons in which it is co-expressed with the TRPV1, but not the TRPM8 channel [2,3]. They are activated by chemical substances and temperature (for reviews see [4,5]). For instance, in the peripheral terminal of the DRG neuron, the TRPV1 channel is activated by capsaicin (a natural pungent ingredient contained in red peppers), protons and noxious heat (>43 °C; [6]; for review see [7]); the TRPA1 channel by pungent compounds in mustard, cinnamon and garlic (allyl isothiocyanate (AITC), cinnamaldehyde and allicin, respectively), and noxious cold temperature (<17 °C; [2,8,9,10]); and the TRPM8 channel by menthol (a secondary alcohol contained in peppermint or other mint) and mild temperature (<25 °C; [11,12]). Such an activation depolarizes the membrane of the peripheral terminal, resulting in the production of action potentials. As a result, the nociceptive or temperature information is transferred to the spinal dorsal horn.
On the other hand, TRP channels in the central terminal of the DRG neuron are expressed in the superficial laminae of the dorsal horn, especially the substantia gelatinosa (SG; lamina II of Rexed; [13,14]). In support of this idea about TRP activation in the SG, many of plant-derived chemicals increase in SG neurons the frequency of spontaneous excitatory postsynaptic current (sEPSC), a measure of the spontaneous release of l-glutamate from nerve terminals, by activating TRPV1 channel [15,16,17,18], TRPA1 channel [19,20] and TRPM8 channel [21,22] (for a review see [23]). The SG neurons play a pivotal role in modulating nociceptive transmission from the periphery [24,25,26] and thus TRP channels in the SG are involved in its modulation. Their expressions in the SG have been shown by immunohistochemistry [27].
2. Spinal Substantia Gelatinosa Involved in Regulating Nociceptive Transmission
Nociceptive transmission in the SG is in origin not only monosynaptic from glutamatergic DRG neurons but also polysynaptic from glutamate-, GABA- and/or glycine-containing interneurons [28]. In support of the involvement of the SG in nociceptive transmission, a plastic change in glutamatergic inputs to SG neurons through DRG neurons occurred in hyperalgesic rats that were subject to either an intraplantar injection of complete Freund’s adjuvant [29] or ovariectomy [30]. Endogenous and exogenous analgesics, which exhibit antinociception when administrated intrathecally, hyperpolarize membranes of SG neurons and reduce the release of l-glutamate onto SG neurons from nerve terminals, both of which actions reduce the membrane excitability of the SG neurons [31]. For example, opioids ([32]; for a review see [33]), nociceptin [34,35], a GABAB-receptor agonist baclofen [36,37], a μ-opioid receptor agonist tramadol [38], norepinephrine [39], serotonin [30,40], adenosine ([41,42]; for review see [43]), somatostatin [44,45], dopamine [46,47] and galanin [48] hyperpolarized membranes of rat SG neurons. Inhibition of the release of l-glutamate from nerve terminals onto rat SG neurons was produced by opioids ([49]; for review see [33]), nociceptin [50,51], baclofen [36,52,53], the endocannabinoid anandamide [16,54], norepinephrine [55], serotonin [30,40], adenosine ([41,56,57]; for a review see [43]) and galanin [48,58].
3. TRP Channels in Nociception
Much evidence demonstrates that TRP channels play a role in transferring nociceptive information. For example, neuropathic pain and hyperalgesia developed following excessive or ectopical expression of TRPV1 channel in the central nervous system (CNS) and peripheral nervous system, in both animals and humans [59,60,61,62]. Hyperalgesia in inflammatory pain models was reduced in TRPV1-knockout mice [63]. Nerve growth factor, which mediates inflammatory pain, upregulated the expression of TRPV1 channel in rat DRG neurons by an action of the small GTPase Ras [64]. Excessive expression of TRPV1 channel in primary-afferent fibers occurred in disease states including inflammatory disease and irritable bowel syndrome [65,66]. Peripheral inflammation upregulated TRPV1 channel involved in the enhancement of spontaneous excitatory transmission in rat SG neurons [67]. When intrathecally administrated, a powerful TRPV1 agonist resiniferatoxin (RTX, a component contained in the dried latex of the cactus-like plant; [68]) produced a prolonged antinociceptive response in dogs with bone cancer [69], possibly owing to a desensitization of TRPV1 channel. Selective inhibition of TRPV1 channel attenuated bone cancer pain in the mouse [70]. TRPV1 channel, which is a potential treatment target for cancer pain, was expressed in neurons that transfer information of this type of pain (for a review see [71]). Intrathecal application of a TRPV1 antagonist AS1928370 resulted in an inhibition of mechanical allodynia in a mouse model of neuropathic pain [72].
The TRPA1 agonist cinnamaldehyde evoked spontaneous pain, and induced mechanical hyperalgesia and cold hypoalgesia following its application to the forearm skin of human volunteers [73]. Moreover, TRPA1 channel was over-expressed in rat DRG neurons following peripheral inflammation and nerve injury, and cold hyperalgesia produced by inflammation and nerve injury was accompanied by the activation of the TRPA1 but not the TRPM8 channel [74]. Alternatively, TRPA1 channel was excessively expressed in the mouse spinal cord and DRG after peripheral inflammation occurred as a result of intraplantar injection of complete Freund’s adjuvant; intrathecally-applied TRPA1 antagonist reversed hyperalgesia that occurred in mouse models of neuropathic pain [75].
TRPM8 channel is also involved in nociceptive transmission. Proudfoot et al. [76] have proposed the idea that TRPM8 activation in both peripheral and central terminals of adult rat DRG neurons produces the release of l-glutamate from its central terminal onto dorsal horn neurons expressing group II/III metabotropic glutamate receptors, the activation of which results in antinociception. In chronic constrictive nerve injury rat models, which exhibited cold allodynia in hindlimbs, compared with the sham group, TRPM8-immunoreactive DRG neurons increased in number, menthol-sensitive DRG neurons increased in number in neurons that responded to capsaicin, and membrane currents produced by menthol in DRG neurons enhanced in amplitude [77]. Kono et al. [78] have suggested an involvement of TRPM8 channel in acute peripheral hypersensitivity to cold in oxaliplatin (a third-generation platinum analog)-treated cancer patients. In adult mice, (−)-menthol and its derivative WS-12 (which activated TRPM8 channel in DRG neurons) inhibited acute thermal, capsaicin- and acrolein-induced nociceptive behavior in a manner sensitive to a nonselective opioid-receptor antagonist naloxone, suggesting an involvement of endogenous opioids in antinociception produced by TRPM8 activation [79].
In conclusion, the effects of the activation of TRPV1, TRPA1 or TRPM8 channel on nociceptive transmission appear to depend on the location of the TRP channels activated, i.e., the peripheral or central terminal of DRG neuron, or both of them. When antinociceptive drugs are administrated intrathecally, it would be necessary to consider the activation of TRP channels located in not only primary-afferent central terminal but also neuronal and glial cells in the CNS, because their expressions in the CNS have been reported [80,81].
4. Actions of Plant-Derived Stereoisomers on Spontaneous Excitatory Transmission in Substantia Gelatinosa Neurons
It is well-known that there is a difference among stereoisomers in their actions on voltage-gated ion channels and neurotransmitter receptors (see [82,83] for reviews). Activation of TRPA1 channel expressed in Chinese hamster ovary cells differs in efficacy between stereoisomers such as (+)-menthol and (−)-menthol [84]. In order to know whether such a difference between stereoisomers is seen in the SG, we examined the actions of plant-derived stereoisomers on spontaneous excitatory transmission in SG neurons with a focus on TRP activation by using transverse slice preparations dissected from the adult rat spinal cord [85].
4.1. Actions of Thymol and Carvacrol
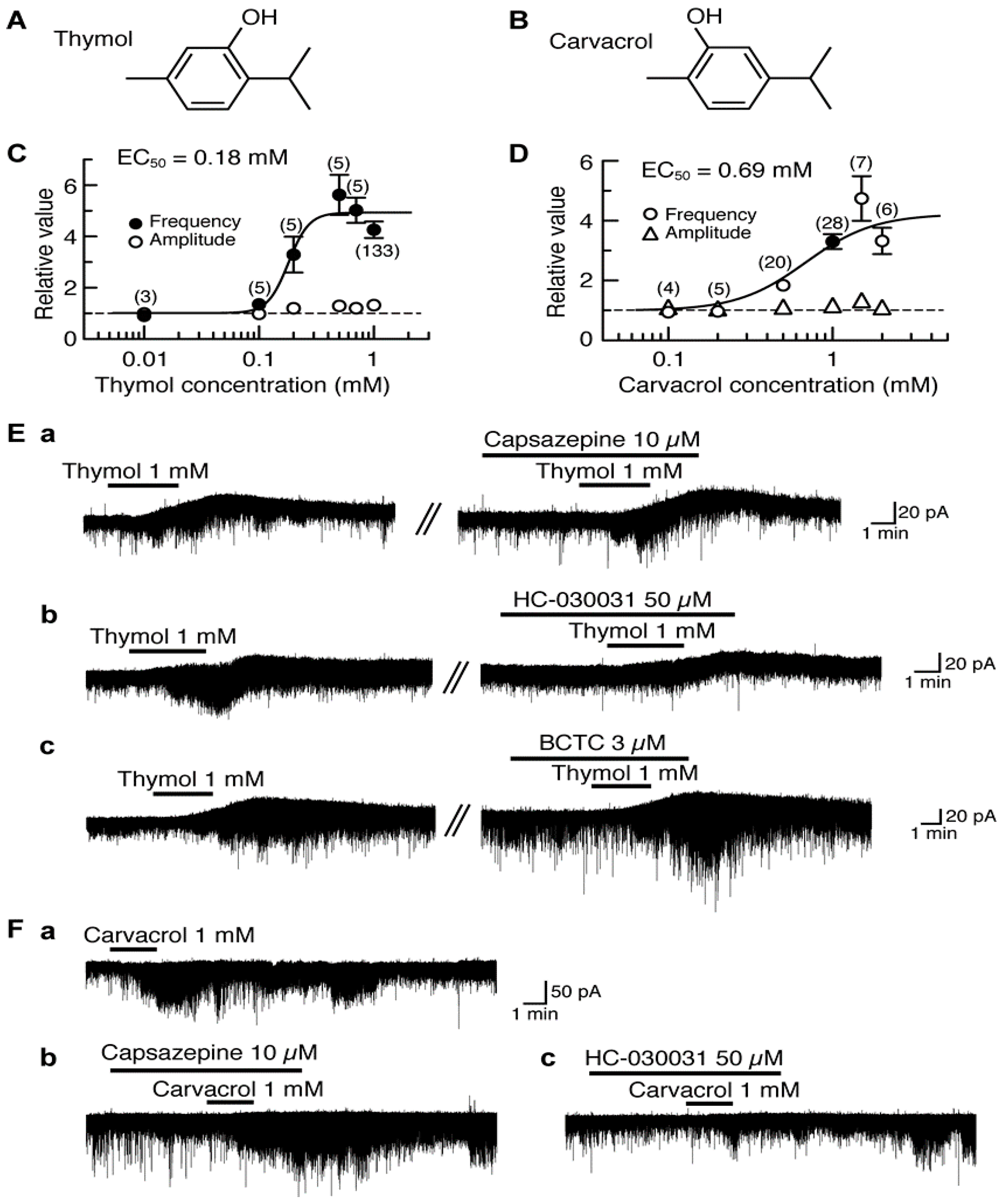
Thymol (5-methyl-2-isopropylphenol, a compound where the cyclohexane ring of menthol is replaced by a benzene ring) differs only in the position of the -OH in the benzene ring from carvacrol (5-isopropyl-2-methylphenol; Figure 1A,B). Thymol and carvacrol are contained in thyme and oregano, respectively, and exhibit antinociception [86,87]. In all SG neurons tested, bath-applied thymol (1 mM) for 3 min increased the frequency of sEPSC with a small increase in its amplitude. The sEPSC frequency increase averaged to be 326% around 5 min (when a maximal effect was obtained) after the onset of thymol superfusion. Such a sEPSC frequency increase was concentration-dependent with the half-maximal effective concentration (EC50) value of 0.18 mM (Figure 1C). In 77% of the neurons tested, thymol (1 mM) produced an outward current having the averaged peak amplitude of 16 pA at the VH of −70 mV (see Figure 1E); remaining neurons had no outward currents [88].
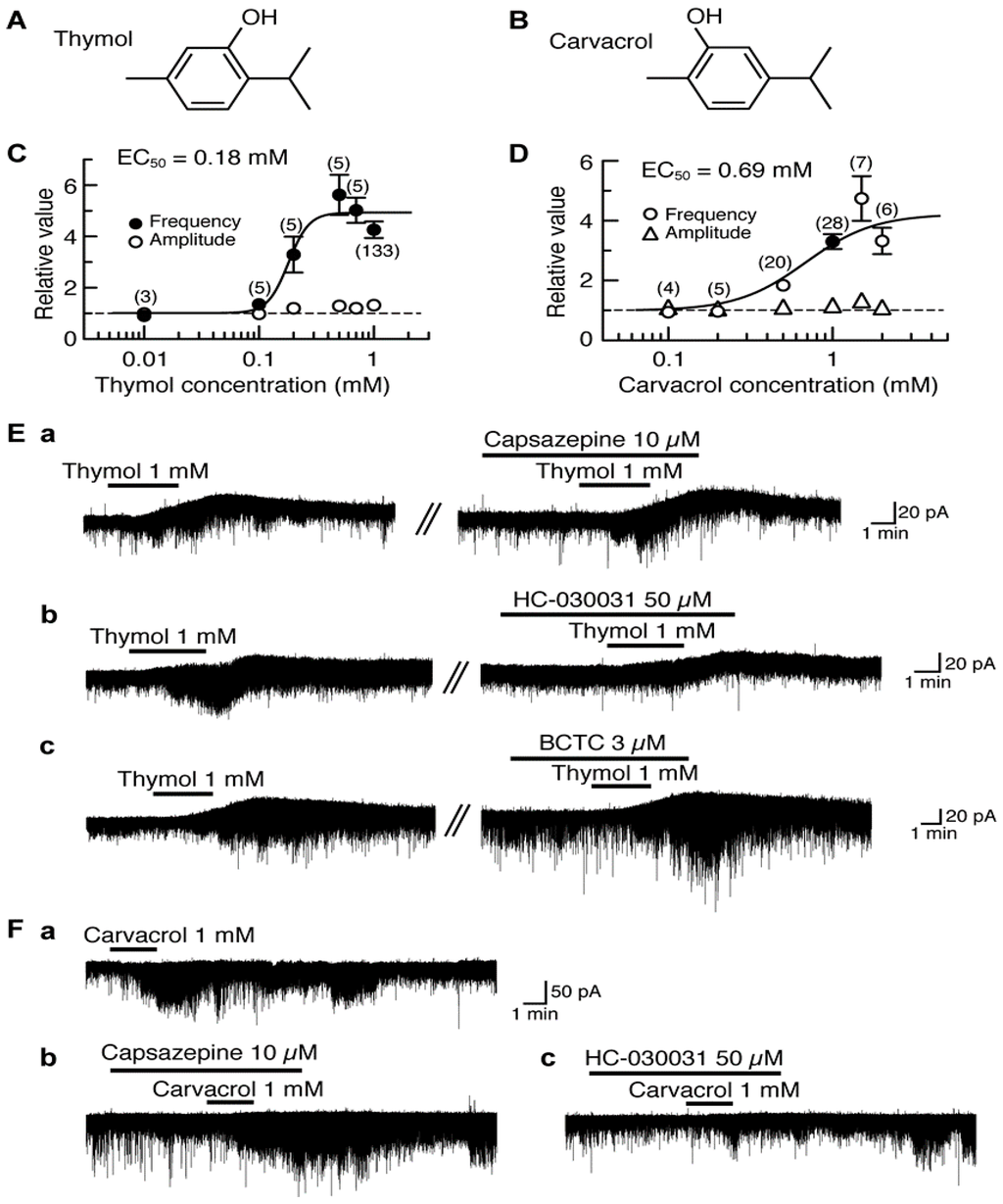
Figure 1.
Effects of thymol and carvacrol on glutamatergic spontaneous excitatory transmission in rat substantia gelatinosa (SG) neurons. (A,B) The chemical structures of thymol (A) and carvacrol (B). (C,D) The frequency and amplitude of sEPSC under the action of thymol (C) or carvacrol (D), relative to those before drug superfusion, which were plotted against the logarithm of drug concentration. This thymol (carvacrol) effect was measured for 0.5 min around 5 min (3.5 min) after the beginning of its superfusion. The results in (C) were obtained from all neurons tested, while those in (D) were obtained from neurons where carvacrol (1 mM) increased sEPSC frequency > 5%. The continuous curves in (C) and (D) were drawn according to the Hill equation [half-maximal effective concentration (EC50) and Hill coefficient (nH) in (C) and (D): 0.18 mM, 4.9 and 0.69 mM, 2.1, respectively]. (Ea–c) Chart recordings showing sEPSCs and holding currents in the absence and presence of thymol in Krebs solution without (left) or with a TRPV1 antagonist capsazepine (Ea), a TRPA1 antagonist HC-030031 (Eb) or a TRPM8 antagonist BCTC (Ec; right). In each of (Ea)–(Ec), the right recording was obtained about 30 min after the left one from the same neuron. (Ea–c) Chart recordings showing sEPSCs in the absence and presence of carvacrol in Krebs solution without (Fa) and with capsazepine (Fb) or HC-030031 (Fc); these recordings were obtained from the same neuron at an interval of 30 min. In this and subsequent figures, value in parentheses indicates the number of neurons tested; each point with vertical bars represents the mean values and standard error of the mean (SEM); if the SEM of the values is less than the size of symbol, the vertical bar is not shown; control level (1) is indicated by horizontal dotted line; the duration of drug superfusion is shown by a horizontal bar above the chart recording. Holding potential (VH) = −70 mV. This research was originally published in [88,89].
Carvacrol exhibited similar actions to those of thymol. In 22% of the SG neurons tested, carvacrol (1 mM) superfused for 2 min produced an outward current, which was not accompanied by a change in sEPSC frequency, at −70 mV. On the other hand, 11% of the SG neurons produced no change in holding currents while exhibiting sEPSC frequency increase (see Figure 1Fa). In 63% of the neurons, both of the outward current and sEPSC frequency increase were produced [89].
sEPSC frequency increase around 3.5 min (when a maximal effect was obtained) after the beginning of carvacrol superfusion averaged to be 262% with a small increase in its amplitude and the outward current had the averaged peak amplitude of 26 pA. Such a sEPSC frequency increase was concentration-dependent with the EC50 value of 0.69 mM (Figure 1D; [89]), a value larger than that of thymol. Thymol has an ability to activate TRP vanilloid-3 (TRPV3; [90]), TRPA1 [91] and TRPM8 channels [92] expressed in heterologous cells. On the other hand, carvacrol has been reported to activate TRPV3 and TRPA1 channels but not TRPV1 channel expressed in human embryonic kidney (HEK) or Xenopus laevis oocyte cells [90,91,93,94]. We next examined what types of TRP channel mediate the sEPSC frequency increases produced by thymol and carvacrol. The thymol activity was inhibited by a TRPA1 antagonist HC-030031 (50 μM; [95]) but not a TRPV1 antagonist capsazepine (10 μM; [96]) and a TRPM8 antagonist (4-(3-chloro-2-pyridinyl)-N-[4-(1,1-dimethyl-ethyl)phenyl]-1-piperazinecarboxamide, BCTC, 3 μM; [97]; Figure 1E). BCTC at this concentration was effective in inhibiting sEPSC frequency increase produced by menthol in adult rat SG neurons [27]. As with thymol, the carvacrol activity was resistant to capsazepine (10 μM) while being depressed by HC-030031 (50 μM; Figure 1F). These results indicate an involvement of TRPA1 channel in the presynaptic activities of thymol and carvacrol. As distinct from these presynaptic actions, the outward currents produced by thymol and carvacrol were insensitive to capsazepine (10 μM), BCTC (3 μM) and HC-030031 (50 μM), indicating no involvement of TRP channels ([88,89]; for example see Figure 1E). The carvacrol current was inhibited in 10 mM-K+ but not K+-channel blockers (5 mM tetraethylammonium and 0.1 mM Ba2+)-containing and 11.0 mM-Cl− Krebs solution, indicating an involvement of tetraethylammonium- and Ba2+-insensitive K+ channels [89]. It remains to be examined what types of ion channel are involved in the thymol current.
4.2. Actions of (−)-Carvone and (+)-Carvone
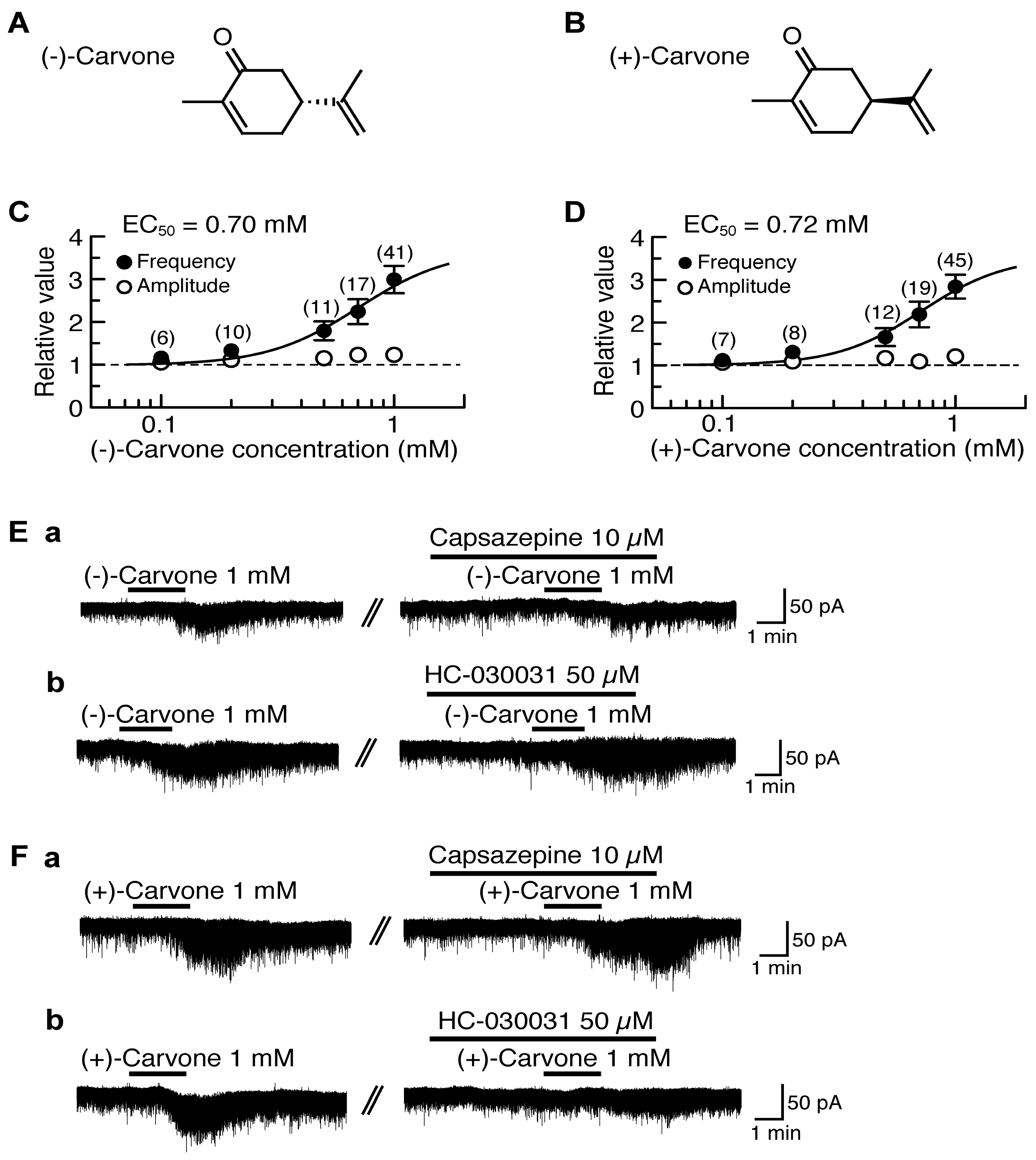
(−)-Carvone [(−)-2-methyl-5-(1-methylethenyl)-2-cyclohexenone; Figure 2A] contained in spearmint increased intracellular Ca2+ concentration in rat DRG neurons in a manner sensitive to capsazepine, indicating an involvement of TRPV1 channel [98]. A similar action of (−)-carvone was observed in HEK293 cells expressing human TRPV1 channel [98]. (+)-Carvone (a stereoisomer of (−)-carvone; Figure 2B) contained in caraway has been shown to have actions different from those of (−)-carvone in mouse locomotive [99] and anticonvulsive activities [100]. We examined the effects of (−)-carvone and (+)-carvone on glutamatergic spontaneous excitatory transmission with a focus on TRP activation. (−)-Carvone and (+)-carvone (each 1 mM) superfused for 2 min increased the frequency of sEPSC with a slight increase in its amplitude. Their sEPSC frequency increases averaged to be 299% and 284%, respectively, around 3 min (when a maximal effect was obtained) after the beginning of its superfusion. Such presynaptic activities of (−)-carvone and (+)-carvone were concentration-dependent with the EC50 values of 0.70 mM and 0.72 mM, respectively (Figure 2C,D).
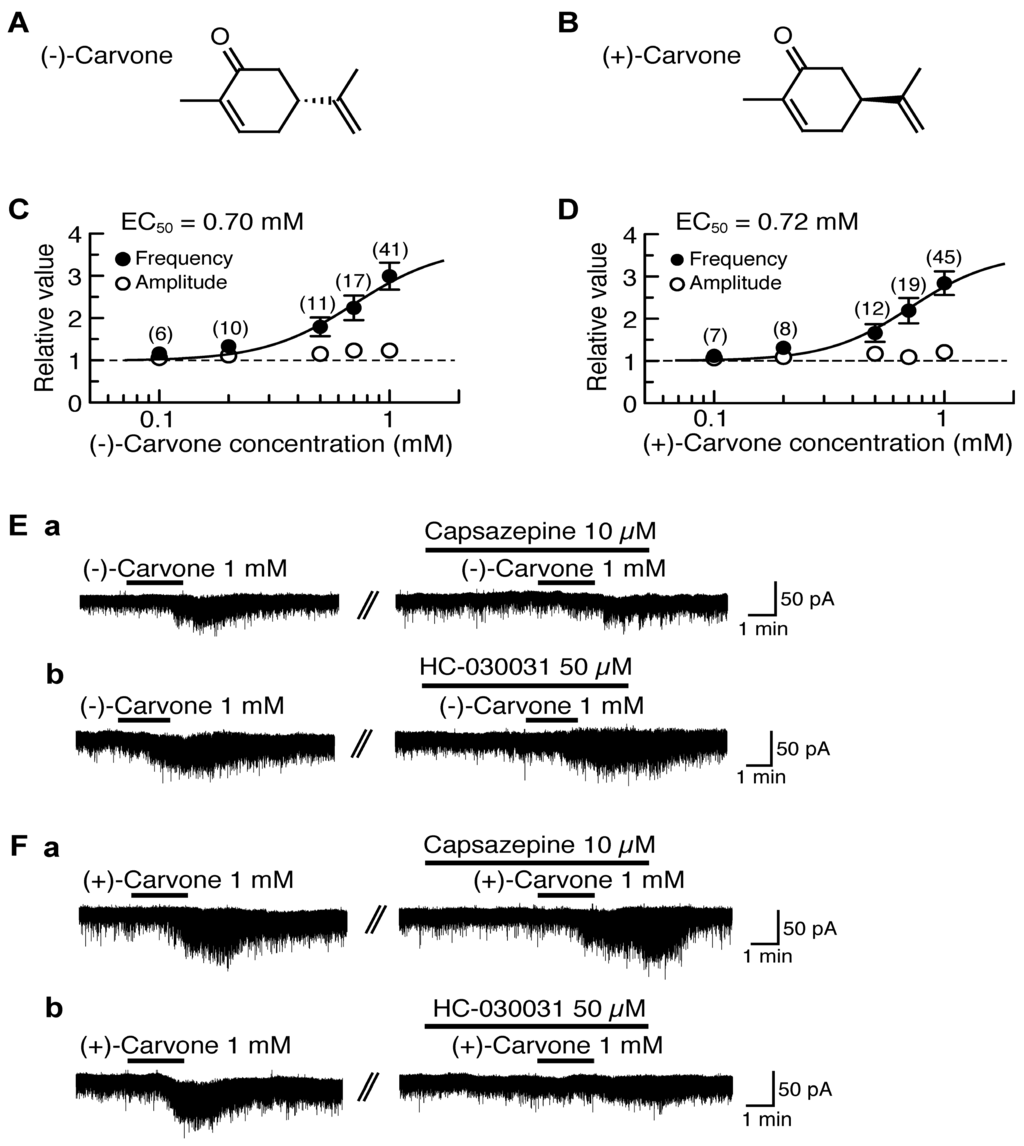
Figure 2.
Effects of (−)-carvone and (+)-carvone on glutamatergic spontaneous excitatory transmission in rat SG neurons. (A,B) The chemical structures of (−)-carvone (A) and (+)-carvone (B). (C,D) The frequency and amplitude of sEPSC under the action of (−)-carvone (C) or (+)-carvone (D), relative to those before drug superfusion, which were plotted against the logarithm of drug concentration. This carvone effect was measured for 0.5 min around 3 min after the beginning of its superfusion. The continuous curves in (C) and (D) were drawn according to the Hill equation (EC50 and nH in (C) and (D): 0.70 mM, 2.2 and 0.72 mM, 2.4, respectively). (Ea,b,Fa,b) Chart recordings showing sEPSCs in the absence and presence of (−)-carvone (E) or (+)-carvone (F) in Krebs solution without (left) and with capsazepine (Ea,Fa) or HC-030031 (Eb,Fb; right). In each of (Ea,b,Fa,b), the right recording was obtained about 20 min after the left one from the same neuron. VH = −70 mV. This research was originally published in [101].
(−)-Carvone and (+)-carvone (each 1 mM) did not produce any outward currents, as different from thymol and carvacrol. About 40% of the SG neurons tested produced a small inward current following the application of (−)-carvone or (+)-carvone [101], as seen by many kinds of TRPV1 agonists (capsaicin and RTX; [15,18]) and TRPA1 agonists (AITC, cinnamaldehyde and allicin; [19]).
The sEPSC frequency increase produced by (−)-carvone was resistant to HC-030031 (50 μM) while being inhibited by capsazepine (10 μM; Figure 2Ea,b). On the other hand, the sEPSC frequency increase produced by (+)-carvone was inhibited by HC-030031 (50 μM) while being resistant to capsazepine (10 μM; Figure 2Fa,b). These results indicate that (−)-carvone and (+)-carvone activate TRPV1 and TRPA1 channels, respectively, in the SG [101].
4.3. Actions of 1,8-Cineole and 1,4-Cineole
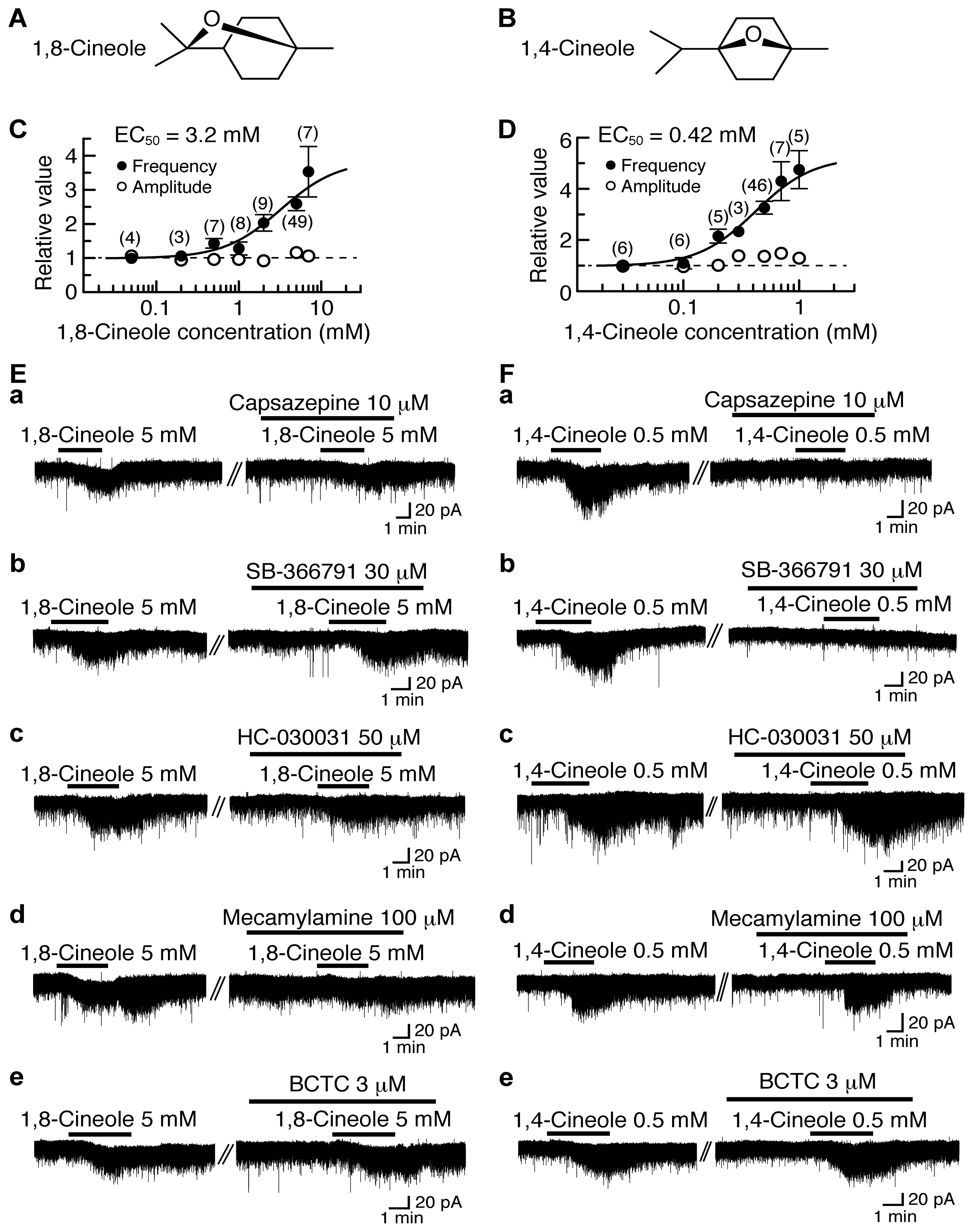
1,8-Cineole (1,3,3-trimethyl-2-oxabicylo[2.2.2]octane; Figure 3A), which is present in eucalyptus and rosemary, has various actions including antinociception [102]. As a minor component of plant extracts containing 1,8-cineole, there is its stereoisomer 1,4-cineole (1-methyl-4-(1-methylethyl)-7-oxabicyclo[2.2.1]heptane; Figure 3B), which has on plant species an action which is different from that of 1,8-cineole [103].
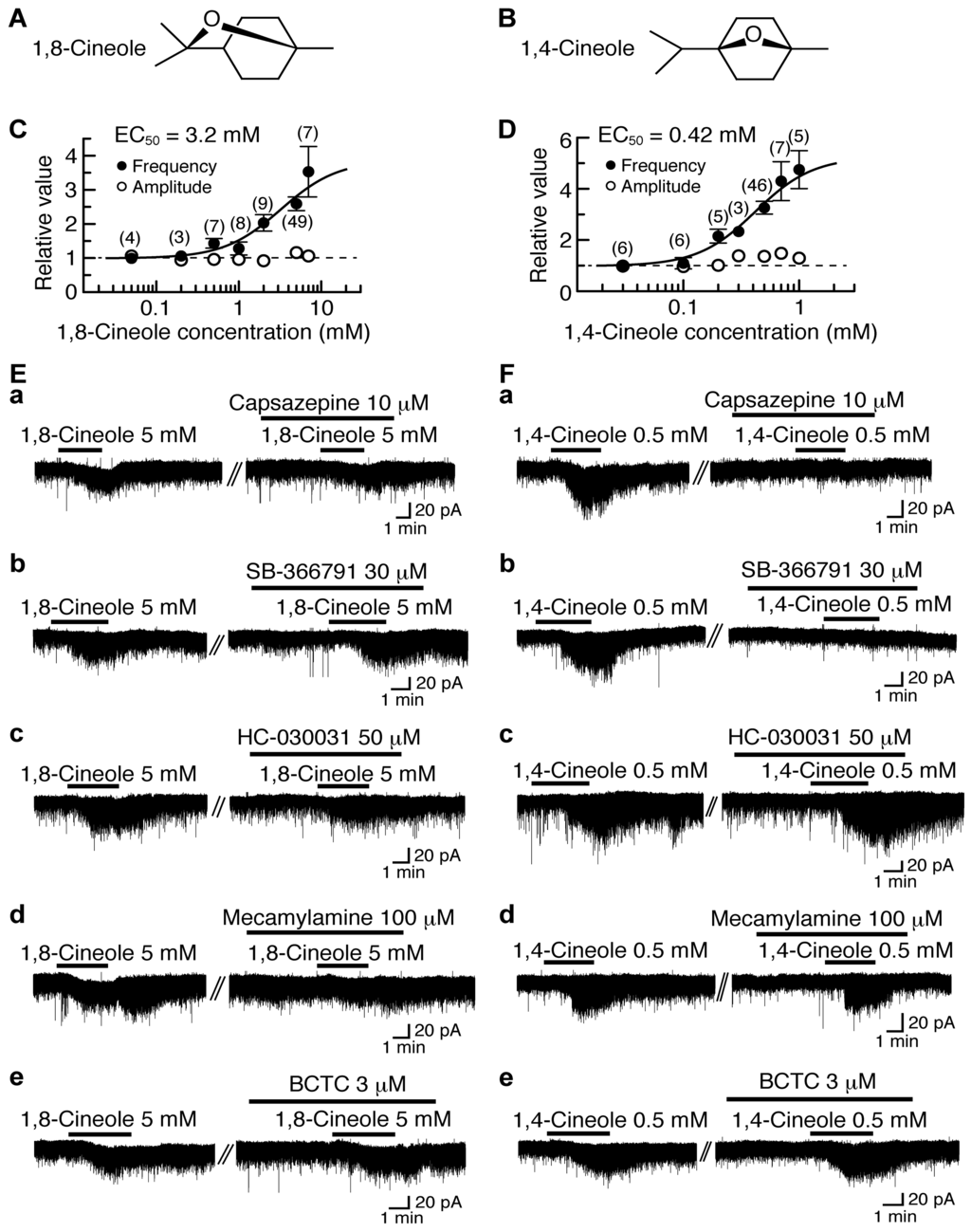
Figure 3.
Effects of 1,8-cineole and 1,4-cineole on glutamatergic spontaneous excitatory transmission in rat SG neurons. (A,B) The chemical structures of 1,8-cineole (A) and 1,4-cineole (B). (C,D) The frequency and amplitude of sEPSC under the action of 1,8-cineole (C) or 1,4-cineole (D), relative to those before drug superfusion, which were plotted against the logarithm of drug concentration. This cineole effect was measured for 0.5 min around 3.5 min after the addition of its drug. The continuous curves in (C) and (D) were drawn according to the Hill equation (EC50 and nH in (C) and (D): 3.2 mM, 1.3 and 0.42 mM, 1.7, respectively). (Ea–e,Fa–e) Chart recordings showing sEPSCs in the absence and presence of 1,8-cineole (E) or 1,4-cineole (F) in Krebs solution without (left) and with capsazepine (a), SB-366791 (b), HC-030031 (c), mecamylamine (d) or BCTC (e; right). In each of (Ea–e,Fa–e), the right recording was obtained about 20 min after the left one from the same neuron. VH = −70 mV. This research was originally published in [27].
1,8-Cineole and 1,4-cineole have an ability to activate TRPM8 channel expressed heterologously in Xenopus oocytes [11] or HEK293 cells [104,105], although their efficacies are much less than that of the TRPM8 agonist menthol. We examined the effects of 1,8-cineole and 1,4-cineole on glutamatergic spontaneous excitatory transmission with a focus on TRP activation. As with (−)-carvone and (+)-carvone, bath-applied 1,8-cineole and 1,4-cineole for 3 min increased the frequency of sEPSC with a small increase in its amplitude. The sEPSC frequency increases produced by 1,8-cineole and 1,4-cineole (5 mM and 0.5 mM, respectively) averaged to be 159% and 226%, respectively, around 3.5 min (when a maximal effect was obtained) after the beginning of its superfusion. Such presynaptic actions of 1,8-cineole and 1,4-cineole were concentration-dependent with the EC50 values of 3.2 mM and 0.42 mM, respectively (Figure 3C,D).
The presynaptic activities of 1,8-cineole and 1,4-cineole were not accompanied by the production of outward current, as different from those of thymol and carvacrol. As with many kinds of TRPV1 and TRPA1 agonists [15,18,19] including (−)-carvone and (+)-carvone, 1,8-cineole and 1,4-cineole produced a small inward current (Figure 3E,F; [27]).
The presynaptic action of 1,8-cineole was inhibited by HC-030031 (50 μM) and another TRPA1 antagonist mecamylamine (100 μM; which is known to be also a nicotinic acetylcholine-receptor antagonist [106]) while being resistant to capsazepine (10 μM) and another TRPV1 antagonist SB-366791 (30 μM; [67]; Figure 3Ea–d). On the contrary, 1,4-cineole’s one was depressed by capsazepine (10 μM) and SB-366791 (30 μM) while being insensitive to HC-030031 (50 μM) and mecamylamine (100 μM; Figure 3Fa–d). BCTC (3 μM) did not affect the activities of 1,8-cineole and 1,4-cineole (Figure 3Ee,Fe). These results indicate that 1,8-cineole and 1,4-cineole activate TRPA1 and TRPV1 channels, respectively, in the SG.
5. Activation by Plant-Derived Stereoisomers of TRP Channels in the Substantia Gelatinosa in a Different Manner
Thymol and carvacrol, which are distinct only in the position of the -OH in the benzene ring (Figure 1A,B), activated the TRPA1 channel with EC50 values which differ four-fold from each other. Optic isomers, (–)-carvone and (+)-carvone (Figure 2A,B), activated TRPV1 and TRPA1 channels, respectively, with almost the same EC50 value. 1,8-Cineole and 1,4-cineole, which are different in the placement of the oxygen bridge (Figure 3A,B; where there is a free dimethyl side chain in 1,4-cineole but not 1,8-cineole [103]), activated TRPA1 and TRPV1 channels, respectively, with EC50 values eight-fold different from each other. The TRPV1 and TRPA1 activations resulted in an increase in spontaneous l-glutamate release from nerve terminals onto SG neurons. These results indicate that TRP channels in the SG have an ability to discriminate plant-derived stereoisomers from each other.
The stereoisomers mentioned in this review article are not endogenous ones that act on TRP channels located in the central terminals of DRG neurons under physiological conditions. There are several candidates for endogenous substances that activate TRP channels. For example, endogenous agonists for TRPV1 channel include endocannabinoids such as anandamide and lipoxygenase metabolites, which have structures similar to that of capsaicin which is not produced endogenously ([107,108]; for reviews see [7,109]). TRPV1 channel in the SG did not appear to be activated by anandamide [54] while anandamide-transport inhibitor AM404 activated the SG TRPV1 channel [110]. As candidates of endogenous TRPA1 activators, there is a potent and systemically active inhibitor of fatty acid amide hydrolase, 3′-carbamoylbiphenyl-3-yl cyclohexylcarbamate (URB597; [111]), a cyclopentane prostaglandin D2 metabolite (15-deoxy-Δ12,14-prostaglandin J2; [112]) or bradykinin [113]. To our knowledge, endogenous agonists for TRPM8 channel do not appear to be surely identified. Testosterone (a steroid hormone from the androgen group) has been recently reported to activate TRPM8 channel [114,115]. Although endogenous stereoisomers for TRP activation do not appear to be available, our findings about stereoisomers could serve to know the properties of the central terminal TRP channels.
Many of the properties of the TRP channels have been examined in the cell body of the primary-afferent neuron and in heterologous cell expressing the TRP channels. We have found out that a local anesthetic lidocaine, which acts on TRPV1 channel [116] and by a less extent on TRPA1 channel [117] in the cell body of primary-afferent neuron, activates TRPA1 but not TRPV1 channel in its central terminal [118]. The central terminal TRPV1 channel was activated by piperine (a pungent component of black pepper; [119]) but not olvanil (the synthetic oleic acid homologue of capsaicin; [120]), both of which compounds activated TRPV1 channel in the cell body of primary-afferent neuron [121,122]. Vanilloid compounds, eugenol (contained in clove) and zingerone (a pungent component of ginger), that reportedly activated TRPV1 channel in the cell body of primary-afferent neuron [121,123] were shown to activate the central terminal TRPA1 but not TRPV1 channel [124,125]. Based on their findings, we have proposed the idea that TRP channels located in the cell body and central terminal of the primary-afferent neuron have properties different from each other [23]. Such a difference may be due to a distinction between cell body and central terminal TRPA1 channels in terms of a functional interaction of TRPA1 channel and toll-like receptor-7 ([126]; see [125] for the other possibilities). It remains to be examined whether TRP channels in the cell body of DRG neuron are activated by plant-derived stereoisomers in a distinct manner.
TRPA1 channel in the central terminal of DRG neuron in the SG is activated by many plant-derived chemicals. Very recently, we have reported that citral, a mixture of geranial and neral, which is contained in lemongrass, activates TRPA1 channel in the SG [127]. Table 1 summarizes available values of EC50 for plant-derived chemicals in activating TRPV1 and TRPA1 channels in the adult rat SG. Their efficacy sequence for the TRPA1 activation was thymol (EC50 = 0.18 mM) > citral (0.58 mM) ≥ carvacrol (0.69 mM) ≥ (+)-carvone (0.72 mM) > zingerone (1.3 mM) > 1,8-cineole (3.2 mM) ≥ eugenol (3.8 mM). This result could serve to know the property of central terminal TRPA1 channel that is a target of drugs for alleviating pain together with the above-mentioned results of stereoisomers.

Table 1.
EC50 values for plant-derived chemicals in activating TRPV1 and TRPA1 channels in the adult rat SG.
TRP activation in the SG is generally thought to be involved in nociception [1], because the enhancement of the spontaneous release of l-glutamate from nerve terminals onto SG neurons as a result of the TRP activation increases an excitability of the SG neurons, an action different from those of analgesic substances (see Section 2). However, antinociception produced by the intrathecal administration of acetaminophen has been attributed to TRPA1 activation in the superficial spinal dorsal horn [128]. AITC inhibited current responses recorded from SG neurons by using the in vivo patch-clamp technique [129] in response to pinch stimuli given to the skin [130]. It remains to be addressed whether TRPA1 activation in the SG results in nociception or antinociception.
Although the present review article mentions the actions of plant-derived stereoisomers on excitatory transmission, the regulation of nociceptive transmission in the SG is due to a modulation of not only excitatory but also GABAergic and/or glycinergic inhibitory transmissions [25,131,132]. It is possible that a modulation of inhibitory transmission by TRP activation is involved in nociceptive transmission. There is much evidence supporting the idea that inhibitory transmission enhancement in the spinal dorsal horn results in antinociception. First, the lack of GABA-synthesizing enzyme [133,134] and also reduction in the expression of K+-Cl− exporter KCC2, which causes inhibitory synaptic response to be excitatory [135], in the rat spinal dorsal horn leaded to nociception. Second, peripheral inflammation resulted in a reduced glycinergic transmission in rat spinal lamina I neurons [136]. Third, an increase in endogenous glycine due to glycine transporter-1 blockade produced an inhibitory effect on spinal nociceptive transmission ([137]; for reviews see [138,139]). Fourth, endogenous analgesics, acetylcholine, norepinephrine and serotonin, enhanced GABAergic and glycinergic inhibitory transmissions [140,141,142,143,144,145]. It appears to depend on the agonists used to activate TRP channels how TRP activation affects spontaneous inhibitory transmission in SG neurons. AITC and zingerone enhanced inhibitory transmission [19,125] while capsaicin and cinnamaldehyde did not [15,20]. It remains to be examined how plant-derived stereoisomers affect spontaneous inhibitory transmission.
Itch is partly similar to pain in the neuronal pathway and receptors involved, although they are distinct from each other in many points of neuronal transmissions. There is much evidence supporting the idea that TRPV1 and TRPA1 channels are involved in itch. For instance, a topical application of capsaicin to the skin produced itch [146,147]. It is thus likely that TRP channels located in the central terminal of a pruriceptive primary-afferent neuron are involved in the modulation of itch sensation. Although proteinase-activated receptor (PAR)-2 in the peripheral terminal of a primary-afferent neuron is involved in the production of itch [148,149], other types of PAR may play a role in modulating pruriceptive transmission in the spinal dorsal horn. Fujita et al. [150] have reported that PAR-1 activation presynaptically increases spontaneous excitatory transmission in adult rat SG neurons. The findings about plant-derived stereoisomers, mentioned in this review article, may serve to know how to modulate itch transmission in the spinal dorsal horn.
There are known to be species differences on the pharmacology of TRP channels. For example, Jordt and Julius [151] have found out that chicken TRPV1 channel has a much less sensitivity to capsaicin than rat one. Rat and human TRPV1 channels were about 100-fold more sensitive to capsaicin than rabbit one [152]. 4-Methyl-N-[2,2,2-trichloro-1-(4-nitro-phenylsulfanyl)-ethyl]-benzamide activated rat TRPA1 channel while blocking human TRPA1 activation by reactive and nonreactive agonists [153]. Caffeine inhibited human TRPA1 channel but activated mouse TRPA1 channel [154]. A967079 blocked mammalian TRPA1 channel [155], but failed to inhibit chicken TRPA1 channel [156]. It remains to be investigated whether a difference in TRP activation among plant-derived stereoisomers is seen in animal species other than rats.
6. Conclusions
Plant-derived chemical stereoisomers, i.e., thymol and carvacrol, (−)-carvone and (+)-carvone, or 1,8-cineole and 1,4-cineole, activated TRP channels in the SG in a manner different from each other in the extent and the types of TRP channels activated. TRP channels expressed in the central terminals of DRG neurons are thus suggested to have an ability to discriminate stereoisomers.
Acknowledgments
This study was partly supported by JSPS KAKENHI Grant Number 15K08673.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Patapoutian, A.; Tate, S.; Woolf, C.J. Transient receptor potential channels: Targeting pain at the source. Nat. Rev. Drug Discov. 2009, 8, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef]
- Kobayashi, K.; Fukuoka, T.; Obata, K.; Yamanaka, H.; Dai, Y.; Tokunaga, A.; Noguchi, K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with Aδ/C-fibers and colocalization with Trk receptors. J. Comp. Neurol. 2005, 493, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M. TRP channels and nociception. In Cellular and Molecular Mechanisms for the Modulation of Nociceptive Transmission in the Peripheral and Central Nervous Systems; Kumamoto, E., Ed.; Research Signpost: Kelara, India, 2007; pp. 23–40. [Google Scholar]
- Julius, D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [PubMed]
- Caterina, M.J.; Julius, D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu. Rev. Neurosci. 2001, 24, 487–517. [Google Scholar] [CrossRef] [PubMed]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef]
- Jordt, S.E.; Bautista, D.M.; Chuang, H.H.; McKemy, D.D.; Zygmunt, P.M.; Högestätt, E.D.; Meng, I.D.; Julius, D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Voets, T. TRP channels: a TR(I)P through a world of multifunctional cation channels. Pflügers Arch. 2005, 451, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP channel that senses cold stimuli and menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef]
- Kumazawa, T.; Perl, E.R. Excitation of marginal and substantia gelatinosa neurons in the primate spinal cord: indications of their place in dorsal horn functional organization. J. Comp. Neurol. 1978, 177, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Lee, C.L.; Perl, E.R. Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science 1986, 234, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Yang, K; Kumamoto, E.; Furue, H.; Yoshimura, M. Capsaicin facilitates excitatory but not inhibitory synaptic transmission in substantia gelatinosa of the rat spinal cord. Neurosci. Lett. 1998, 255, 135–138. [Google Scholar] [CrossRef]
- Morisset, V.; Urbán, L. Cannabinoid-induced presynaptic inhibition of glutamatergic EPSCs in substantia gelatinosa neurons of the rat spinal cord. J. Neurophysiol. 2001, 86, 40–48. [Google Scholar] [PubMed]
- Baccei, M.L.; Bardoni, R.; Fitzgerald, M. Development of nociceptive synaptic inputs to the neonatal rat dorsal horn: glutamate release by capsaicin and menthol. J. Physiol. 2003, 549, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.-Y.; Fujita, T.; Yue, H.-Y.; Piao, L.-H.; Liu, T.; Nakatsuka, T.; Kumamoto, E. Effect of resiniferatoxin on glutamatergic spontaneous excitatory synaptic transmission in substantia gelatinosa neurons of the adult rat spinal cord. Neuroscience 2009, 164, 1833–1844. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, M.; Nakatsuka, T.; Fujita, T.; Kuroda, Y.; Kumamoto, E. Activation of TRPA1 channel facilitates excitatory synaptic transmission in substantia gelatinosa neurons of the adult rat spinal cord. J. Neurosci. 2007, 27, 4443–4451. [Google Scholar] [CrossRef] [PubMed]
- Uta, D.; Furue, H.; Pickering, A.E.; Rashid, M.H.; Mizuguchi-Takase, H.; Katafuchi, T.; Imoto, K.; Yoshimura, M. TRPA1-expressing primary afferents synapse with a morphologically identified subclass of substantia gelatinosa neurons in the adult rat spinal cord. Eur. J. Neurosci. 2010, 31, 1960–1973. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.C.; Furue, H.; Koga, K.; Jiang, N.; Nohmi, M.; Shimazaki, Y.; Katoh-Fukui, Y.; Yokoyama, M.; Yoshimura, M.; Takeichi, M. Cadherin-8 is required for the first relay synapses to receive functional inputs from primary sensory afferents for cold sensation. J. Neurosci. 2007, 27, 3466–3476. [Google Scholar] [CrossRef] [PubMed]
- Wrigley, P.J.; Jeong, H.-J.; Vaughan, C.W. Primary afferents with TRPM8 and TRPA1 profiles target distinct subpopulations of rat superficial dorsal horn neurones. Br. J. Pharmacol. 2009, 157, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, E.; Fujita, T.; Jiang, C.-Y. TRP channels involved in spontaneous l-glutamate release enhancement in the adult rat spinal substantia gelatinosa. Cells 2014, 3, 331–362. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R.; Wall, P.D. Pain mechanisms: a new theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Willis, W.D., Jr.; Coggeshall, R.E. Sensory Mechanisms of the Spinal Cord, 2nd ed.; Plenum: New York, NY, USA, 1991. [Google Scholar]
- Todd, A.J. Neuronal circuitry for pain processing in the dorsal horn. Nat. Rev. Neurosci. 2010, 11, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.-Y.; Wang, C.; Xu, N.-X.; Fujita, T.; Murata, Y.; Kumamoto, E. 1,8- and 1,4-cineole enhance spontaneous excitatory transmission by activating different types of transient receptor potential channels in the rat spinal substantia gelatinosa. J. Neurochem. 2016, 136, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Nishi, S. Blind patch-clamp recordings from substantia gelatinosa neurons in adult rat spinal cord slices: pharmacological properties of synaptic currents. Neuroscience 1993, 53, 519–526. [Google Scholar] [CrossRef]
- Nakatsuka, T.; Park, J.-S.; Kumamoto, E.; Tamaki, T.; Yoshimura, M. Plastic changes in sensory inputs to rat substantia gelatinosa neurons following peripheral inflammation. Pain 1999, 82, 39–47. [Google Scholar] [CrossRef]
- Ito, A.; Kumamoto, E.; Takeda, M.; Takeda, M.; Shibata, K.; Sagai, H.; Yoshimura, M. Mechanisms for ovariectomy-induced hyperalgesia and its relief by calcitonin: participation of 5-HT1A-like receptor on C-afferent terminals in substantia gelatinosa of the rat spinal cord. J. Neurosci. 2000, 20, 6302–6308. [Google Scholar] [PubMed]
- Fürst, S. Transmitters involved in antinociception in the spinal cord. Brain Res. Bull. 1999, 48, 129–141. [Google Scholar] [CrossRef]
- Yoshimura, M.; North, R.A. Substantia gelatinosa neurones hyperpolarized in vitro by enkephalin. Nature 1983, 305, 529–530. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Nakatsuka, T.; Kumamoto, E. Opioid receptor activation in spinal dorsal horn. In Cellular and Molecular Mechanisms for the Modulation of Nociceptive Transmission in the Peripheral and Central Nervous Systems; Kumamoto, E., Ed.; Research Signpost: Kelara, India, 2007; pp. 87–111. [Google Scholar]
- Lai, C.C.; Wu, S.Y.; Dun, S.L.; Dun, N.J. Nociceptin-like immunoreactivity in the rat dorsal horn and inhibition of substantia gelatinosa neurons. Neuroscience 1997, 81, 887–891. [Google Scholar] [CrossRef]
- Luo, C.; Kumamoto, E.; Furue, H.; Yoshimura, M. Nociceptin-induced outward current in substantia gelatinosa neurones of the adult rat spinal cord. Neuroscience 2001, 108, 323–330. [Google Scholar] [CrossRef]
- Kangrga, I.; Jiang, M.; Randić, M. Actions of (−)-baclofen on rat dorsal horn neurons. Brain Res. 1991, 562, 265–275. [Google Scholar] [CrossRef]
- Yang, K.; Kumamoto, E.; Furue, H.; Li, Y.-Q.; Yoshimura, M. Capsaicin induces a slow inward current which is not mediated by substance P in substantia gelatinosa neurons of the rat spinal cord. Neuropharmacology 2000, 39, 2185–2194. [Google Scholar] [CrossRef]
- Koga, A.; Fujita, T.; Totoki, T.; Kumamoto, E. Tramadol produces outward currents by activating μ-opioid receptors in adult rat substantia gelatinosa neurones. Br. J. Pharmacol. 2005, 145, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Sonohata, M.; Furue, H.; Katafuchi, T.; Yasaka, T.; Doi, A.; Kumamoto, E.; Yoshimura, M. Actions of noradrenaline on substantia gelatinosa neurones in the rat spinal cord revealed by in vivo patch recording. J. Physiol. 2004, 555, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Kato, G.; Katafuchi, T.; Tamae, A.; Furue, H.; Yoshimura, M. Responses to 5-HT in morphologically identified neurons in the rat substantia gelatinosa in vitro. Neuroscience 2009, 159, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Perl, E.R. Adenosine inhibition of synaptic transmission in the substantia gelatinosa. J. Neurophysiol. 1994, 72, 1611–1621. [Google Scholar] [PubMed]
- Liu, T.; Fujita, T.; Kawasaki, Y.; Kumamoto, E. Regulation by equilibrative nucleoside transporter of adenosine outward currents in adult rat spinal dorsal horn neurons. Brain Res. Bull. 2004, 64, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, E.; Fujita, T. Role of adenosine in regulating nociceptive transmission in the spinal dorsal horn. In Recent Research Developments in Physiology; Pandalai, S.G., Ed.; Research Signpost: Kerala, India, 2005; Volume 3, pp. 39–57. [Google Scholar]
- Jiang, N.; Furue, H.; Katafuchi, T.; Yoshimura, M. Somatostatin directly inhibits substantia gelatinosa neurons in adult rat spinal dorsal horn in vitro. Neurosci. Res. 2003, 47, 97–107. [Google Scholar] [CrossRef]
- Nakatsuka, T.; Fujita, T.; Inoue, K.; Kumamoto, E. Activation of GIRK channels in substantia gelatinosa neurones of the adult rat spinal cord: a possible involvement of somatostatin. J. Physiol. 2008, 586, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Tamae, A.; Nakatsuka, T.; Koga, K.; Kato, G.; Furue, H.; Katafuchi, T.; Yoshimura, M. Direct inhibition of substantia gelatinosa neurones in the rat spinal cord by activation of dopamine D2-like receptors. J. Physiol. 2005, 568, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, W.; Nakatsuka, T.; Miyazaki, N.; Yamada, H.; Takeda, D.; Fujita, T.; Kumamoto, E.; Yoshida, M. In vivo patch-clamp analysis of dopaminergic antinociceptive actions on substantia gelatinosa neurons in the spinal cord. Pain 2011, 152, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.-Y.; Fujita, T.; Kumamoto, E. Biphasic modulation by galanin of excitatory synaptic transmission in substantia gelatinosa neurons of adult rat spinal cord slices. J. Neurophysiol. 2011, 105, 2337–2349. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T.; Kumamoto, E.; Higashi, H.; Shimoji, K.; Yoshimura, M. Actions of opioids on excitatory and inhibitory transmission in substantia gelatinosa of adult rat spinal cord. J. Physiol. 1999, 518, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Liebel, J.T.; Swandulla, D.; Zeilhofer, H.U. Modulation of excitatory synaptic transmission by nociceptin in superficial dorsal horn neurones of the neonatal rat spinal cord. Br. J. Pharmacol. 1997, 121, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Kumamoto, E.; Furue, H.; Chen, J.; Yoshimura, M. Nociceptin inhibits excitatory but not inhibitory transmission to substantia gelatinosa neurones of adult rat spinal cord. Neuroscience 2002, 109, 349–358. [Google Scholar] [CrossRef]
- Ataka, T.; Kumamoto, E.; Shimoji, K.; Yoshimura, M. Baclofen inhibits more effectively C-afferent than Aδ-afferent glutamatergic transmission in substantia gelatinosa neurons of adult rat spinal cord slices. Pain 2000, 86, 273–282. [Google Scholar] [CrossRef]
- Iyadomi, M.; Iyadomi, I.; Kumamoto, E.; Tomokuni, K.; Yoshimura, M. Presynaptic inhibition by baclofen of miniature EPSCs and IPSCs in substantia gelatinosa neurons of the adult rat spinal dorsal horn. Pain 2000, 85, 385–393. [Google Scholar] [CrossRef]
- Luo, C.; Kumamoto, E.; Furue, H.; Chen, J.; Yoshimura, M. Anandamide inhibits excitatory transmission to rat substantia gelatinosa neurones in a manner different from that of capsaicin. Neurosci. Lett. 2002, 321, 17–20. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Kumamoto, E.; Furue, H.; Yoshimura, M. α2 Adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantia gelatinosa neurons. Anesthesiology 2003, 98, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Lao, L.-J.; Kumamoto, E.; Luo, C.; Furue, H.; Yoshimura, M. Adenosine inhibits excitatory transmission to substantia gelatinosa neurons of the adult rat spinal cord through the activation of presynaptic A1 adenosine receptor. Pain 2001, 94, 315–324. [Google Scholar] [CrossRef]
- Lao, L.-J.; Kawasaki, Y.; Yang, K.; Fujita, T.; Kumamoto, E. Modulation by adenosine of Aδ and C primary-afferent glutamatergic transmission in adult rat substantia gelatinosa neurons. Neuroscience 2004, 125, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Alier, K.A.; Chen, Y.; Sollenberg, U.E.; Langel, Ü.; Smith, P.A. Selective stimulation of GalR1 and GalR2 in rat substantia gelatinosa reveals a cellular basis for the anti- and pro-nociceptive actions of galanin. Pain 2008, 137, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Hudson, L.J.; Bevan, S.; Wotherspoon, G.; Gentry, C.; Fox, A.; Winter, J. VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. Eur. J. Neurosci. 2001, 13, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.M.; Urbán, L.; Medhurst, S.J.; Patel, S.; Panesar, M.; Fox, A.J.; McIntyre, P. The VR1 antagonist capsazepine reverses mechanical hyperalgesia in models of inflammatory and neuropathic pain. J. Pharmacol. Exp. Ther. 2003, 304, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Gavva, N.R.; Tamir, R.; Qu, Y.; Klionsky, L.; Zhang, T.J.; Immke, D.; Wang, J.; Zhu, D.; Vanderah, T.W.; Porreca, F.; et al. AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J. Pharmacol. Exp. Ther. 2005, 313, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Culshaw, A.J.; Bevan, S.; Christiansen, M.; Copp, P.; Davis, A.; Davis, C.; Dyson, A.; Dziadulewicz, E.K.; Edwards, L.; Eggelte, H.; et al. Identification and biological characterization of 6-aryl-7-isopropylquinazolinones as novel TRPV1 antagonists that are effective in models of chronic pain. J. Med. Chem. 2006, 49, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.B.; Gray, J.; Gunthorpe, M.J.; Hatcher, J.P.; Davey, P.T.; Overend, P.; Harries, M.H.; Latcham, J.; Clapham, C.; Atkinson, K.; et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 2000, 405, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Bron, R.; Klesse, L.J.; Shah, K.; Parada, L.F.; Winter, J. Activation of Ras is necessary and sufficient for upregulation of vanilloid receptor type 1 in sensory neurons by neurotrophic factors. Mol. Cell. Neurosci. 2003, 22, 118–132. [Google Scholar] [CrossRef]
- Yiangou, Y.; Facer, P.; Dyer, N.H.C.; Chan, C.L.H.; Knowles, C.; Williams, N.S.; Anand, P. Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet 2001, 357, 1338–1339. [Google Scholar] [CrossRef]
- Chan, C.L.H.; Facer, P.; Davis, J.B.; Smith, G.D.; Egerton, J.; Bountra, C.; Williams, N.S.; Anand, P. Sensory fibres expressing capsaicin receptor TRPV1 in patients with rectal hypersensitivity and faecal urgency. Lancet 2003, 361, 385–391. [Google Scholar] [CrossRef]
- Lappin, S.C.; Randall, A.D.; Gunthorpe, M.J.; Morisset, V. TRPV1 antagonist, SB-366791, inhibits glutamatergic synaptic transmission in rat spinal dorsal horn following peripheral inflammation. Eur. J. Pharmacol. 2006, 540, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A.; Blumberg, P.M. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience 1989, 30, 515–520. [Google Scholar] [CrossRef]
- Brown, D.C.; Iadarola, M.J.; Perkowski, S.Z.; Erin, H.; Shofer, F.; Laszlo, K.J.; Olah, Z.; Mannes, A.J. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology 2005, 103, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Ghilardi, J.R.; Röhrich, H.; Lindsay, T.H.; Sevcik, M.A.; Schwei, M.J.; Kubota, K.; Halvorson, K.G.; Poblete, J.; Chaplan, S.R.; Dubin, A.E.; et al. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J. Neurosci. 2005, 25, 3126–3131. [Google Scholar] [CrossRef] [PubMed]
- Prevarskaya, N.; Zhang, L.; Barritt, G. TRP channels in cancer. Biochim. Biophys. Acta 2007, 1772, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Watabiki, T.; Kiso, T.; Tsukamoto, M.; Aoki, T.; Matsuoka, N. Intrathecal administration of AS1928370, a transient receptor potential vanilloid 1 antagonist, attenuates mechanical allodynia in a mouse model of neuropathic pain. Biol. Pharm. Bull. 2011, 34, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Namer, B.; Seifert, F.; Handwerker, H.O.; Maihöfner, C. TRPA1 and TRPM8 activation in humans: effects of cinnamaldehyde and menthol. NeuroReport 2005, 16, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Obata, K.; Katsura, H.; Mizushima, T.; Yamanaka, H.; Kobayashi, K.; Dai, Y.; Fukuoka, T.; Tokunaga, A.; Tominaga, M.; Noguchi, K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J. Clin. Investig. 2005, 115, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- da Costa, D.S.M.; Meotti, F.C.; Andrade, E.L.; Leal, P.C.; Motta, E.M.; Calixto, J.B. The involvement of the transient receptor potential A1 (TRPA1) in the maintenance of mechanical and cold hyperalgesia in persistent inflammation. Pain 2010, 148, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, C.J.; Garry, E.M.; Cottrell, D.F.; Rosie, R.; Anderson, H.; Robertson, D.C.; Fleetwood-Walker, S.M.; Mitchell, R. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr. Biol. 2006, 16, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Chen, M.; Ling, J.; Tan, W.; Gu, J.G. TRPM8 mechanism of cold allodynia after chronic nerve injury. J. Neurosci. 2007, 27, 13680–13690. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Satomi, M.; Suno, M.; Kimura, N.; Yamazaki, H.; Furukawa, H.; Matsubara, K. Oxaliplatin-induced neurotoxicity involves TRPM8 in the mechanism of acute hypersensitivity to cold sensation. Brain Behav. 2012, 2, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fan, L.; Balakrishna, S.; Sui, A.; Morris, J.B.; Jordt, S.-E. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain 2013, 154, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.G. TRPV1 in the central nervous system: synaptic plasticity, function, and pharmacological implications. Prog. Drug Res. 2014, 68, 77–104. [Google Scholar] [PubMed]
- Zygmunt, P.M.; Högestätt, E.D. TRPA1. Handb. Exp. Pharmacol. 2014, 222, 583–630. [Google Scholar] [PubMed]
- Soudijn, W.; van Wijngaarden, I.; IJzerman, A.P. Stereoselectivity of drug-receptor interactions. IDrugs 2003, 6, 43–56. [Google Scholar]
- Valenzuela, C.; Moreno, C.; de la Cruz, A.; Macías, Á.; Prieto, Á.; González, T. Stereoselective interactions between local anesthetics and ion channels. Chirality 2012, 24, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Karashima, Y.; Damann, N.; Prenen, J.; Talavera, K.; Segal, A.; Voets, T.; Nilius, B. Bimodal action of menthol on the transient receptor potential channel TRPA1. J. Neurosci. 2007, 27, 9874–9884. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Li, Y.-Q.; Kumamoto, E.; Furue, H.; Yoshimura, M. Voltage-clamp recordings of postsynaptic currents in substantia gelatinosa neurons in vitro and its applications to assess synaptic transmission. Brain Res. Protoc. 2001, 7, 235–240. [Google Scholar] [CrossRef]
- Baser, K.H.C. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des. 2008, 14, 3106–3119. [Google Scholar] [CrossRef] [PubMed]
- Angeles-López, G.; Pérez-Vásquez, A.; Hernández-Luis, F.; Déciga-Campos, M.; Bye, R.; Linares, E.; Mata, R. Antinociceptive effect of extracts and compounds from Hofmeisteria schaffneri. J. Ethnopharmacol. 2010, 131, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-H.; Wang, C.; Fujita, T.; Jiang, C.-Y.; Kumamoto, E. Action of thymol on spontaneous excitatory transmission in adult rat spinal substantia gelatinosa neurons. Neurosci. Lett. 2015, 606, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.-T.; Fujita, T.; Jiang, C.-Y.; Kumamoto, E. Carvacrol presynaptically enhances spontaneous excitatory transmission and produces outward current in adult rat spinal substantia gelatinosa neurons. Brain Res. 2014, 1592, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Delling, M.; Jun, J.C.; Clapham, D.E. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 2006, 9, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P.; Buber, M.T.; Yang, Q.; Cerne, R.; Cortés, R.Y.; Sprous, D.G.; Bryant, R.W. Thymol and related alkyl phenols activate the hTRPA1 channel. Br. J. Pharmacol. 2008, 153, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Ortar, G.; Morera, L.; Moriello, A.S.; Morera, E.; Nalli, M.; Di Marzo, V.; De Petrocellis, L. Modulation of thermo-transient receptor potential (thermo-TRP) channels by thymol-based compounds. Bioorg. Med. Chem. Lett. 2012, 22, 3535–3539. [Google Scholar] [CrossRef] [PubMed]
- Vogt-Eisele, A.K.; Weber, K.; Sherkheli, M.A.; Vielhaber, G.; Panten, J.; Gisselmann, G.; Hatt, H. Monoterpenoid agonists of TRPV3. Br. J. Pharmacol. 2007, 151, 530–540. [Google Scholar] [CrossRef] [PubMed]
- de la Roche, J.; Eberhardt, M.J.; Klinger, A.B.; Stanslowsky, N.; Wegner, F.; Koppert, W.; Reeh, P.W.; Lampert, A.; Fischer, M.J.M.; Leffler, A. The molecular basis for species-specific activation of human TRPA1 protein by protons involves poorly conserved residues within transmembrane domains 5 and 6. J. Biol. Chem. 2013, 288, 20280–20292. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef] [PubMed]
- Urban, L.; Dray, A. Capsazepine, a novel capsaicin antagonist, selectively antagonises the effects of capsaicin in the mouse spinal cord in vitro. Neurosci. Lett. 1991, 134, 9–11. [Google Scholar] [CrossRef]
- Madrid, R.; Donovan-Rodríguez, T.; Meseguer, V.; Acosta, M.C.; Belmonte, C.; Viana, F. Contribution of TRPM8 channels to cold transduction in primary sensory neurons and peripheral nerve terminals. J. Neurosci. 2006, 26, 12512–12525. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.C.R.; Silveira, A.L.; de Souza, H.D.N.; Nery, A.A.; Prado, V.F.; Prado, M.A.M.; Ulrich, H.; Araújo, D.A.M. The monoterpene (−)-carvone: a novel agonist of TRPV1 channels. Cytometry Part A 2013, 83, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Buchbauer, G.; Jäger, W.; Gruber, A.; Dietrich, H. R-(+)- and S-(−)-carvone: influence of chirality on locomotion activity in mice. Flavour Fragr. J. 2005, 20, 686–689. [Google Scholar] [CrossRef]
- de Sousa, D.P.; de Farias Nóbrega, F.F.; de Almeida, R.N. Influence of the chirality of (R)-(−)- and (S)-(+)-carvone in the central nervous system: a comparative study. Chirality 2007, 19, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Jiang, C.-Y.; Fujita, T.; Kumamoto, E. Spontaneous l-glutamate release enhancement in rat substantia gelatinosa neurons by (−)-carvone and (+)-carvone which activate different types of TRP channel. Biochem. Biophys. Res. Commun. 2015, 459, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Liapi, C.; Anifantis, G.; Chinou, I.; Kourounakis, A.P.; Theodosopoulos, S.; Galanopoulou, P. Antinociceptive properties of 1,8-cineole and β-pinene, from the essential oil of Eucalyptus camaldulensis leaves, in rodents. Planta Med. 2007, 73, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Romagni, J.G.; Allen, S.N.; Dayan, F.E. Allelopathic effects of volatile cineoles on two weedy plant species. J. Chem. Ecol. 2000, 26, 303–313. [Google Scholar] [CrossRef]
- Behrendt, H.-J.; Germann, T.; Gillen, C.; Hatt, H.; Jostock, R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br. J. Pharmacol. 2004, 141, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, M.; Fujita, F.; Uchida, K.; Yamamoto, S.; Sawada Shimizu, M.; Hatai Uotsu, C.; Shimizu, M.; Tominaga, M. 1,8-Cineole, a TRPM8 agonist, is a novel natural antagonist of human TRPA1. Mol. Pain 2012, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Talavera, K.; Gees, M.; Karashima, Y.; Meseguer, V.M.; Vanoirbeek, J.A.J.; Damann, N.; Everaerts, W.; Benoit, M.; Janssens, A.; Vennekens, R.; et al. Nicotine activates the chemosensory cation channel TRPA1. Nat. Neurosci. 2009, 12, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, P.M.; Petersson, J.; Andersson, D.A.; Chuang, H.-h.; Sørgård, M.; Di Marzo, V.; Julius, D.; Högestätt, E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 1999, 400, 452–457. [Google Scholar] [PubMed]
- Hwang, S.W.; Cho, H.; Kwak, J.; Lee, S.-Y.; Kang, C.-J.; Jung, J.; Cho, S.; Min, K.H.; Suh, Y.-G.; Kim, D.; et al. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. USA 2000, 97, 6155–6160. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, K.; Niga, S.; Di Marzo, V. Biochemistry and pharmacology of endovanilloids. Pharmacol. Ther. 2007, 114, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.-Y.; Fujita, T.; Kawasaki, Y.; Kumamoto, E. AM404 enhances the spontaneous release of l-glutamate in a manner sensitive to capsazepine in adult rat substantia gelatinosa neurones. Brain Res. 2004, 1018, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Niforatos, W.; Zhang, X.-F.; Lake, M.R.; Walter, K.A.; Neelands, T.; Holzman, T.F.; Scott, V.E.; Faltynek, C.R.; Moreland, R.B.; Chen, J. Activation of TRPA1 channels by the fatty acid amide hydrolase inhibitor 3′-carbamoylbiphenyl-3-yl cyclohexylcarbamate (URB597). Mol. Pharmacol. 2007, 71, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Orengo, L.; Dhaka, A.; Heuermann, R.J.; Young, T.J.; Montana, M.C.; Cavanaugh, E.J.; Kim, D.; Story, G.M. Cutaneous nociception evoked by 15-delta PGJ2 via activation of ion channel TRPA1. Mol. Pain 2008, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kohno, T.; Amaya, F.; Brenner, G.J.; Ito, N.; Allchorne, A.; Ji, R.-R.; Woolf, C.J. Bradykinin produces pain hypersensitivity by potentiating spinal cord glutamatergic synaptic transmission. J. Neurosci. 2005, 25, 7986–7992. [Google Scholar] [CrossRef] [PubMed]
- Asuthkar, S.; Elustondo, P.A.; Demirkhanyan, L.; Sun, X.; Baskaran, P.; Velpula, K.K.; Thyagarajan, B.; Pavlov, E.V.; Zakharian, E. The TRPM8 protein is a testosterone receptor: I. Biochemical evidence for direct TRPM8-testosterone interactions. J. Biol. Chem. 2015, 290, 2659–2669. [Google Scholar] [CrossRef] [PubMed]
- Asuthkar, S.; Demirkhanyan, L.; Sun, X.; Elustondo, P.A.; Krishnan, V.; Baskaran, P.; Velpula, K.K.; Thyagarajan, B.; Pavlov, E.V.; Zakharian, E. The TRPM8 protein is a testosterone receptor: II. Functional evidence for an ionotropic effect of testosterone on TRPM8. J. Biol. Chem. 2015, 290, 2670–2688. [Google Scholar] [CrossRef] [PubMed]
- Leffler, A.; Fischer, M.J.; Rehner, D.; Kienel, S.; Kistner, K.; Sauer, S.K.; Gavva, N.R.; Reeh, P.W.; Nau, C. The vanilloid receptor TRPV1 is activated and sensitized by local anesthetics in rodent sensory neurons. J. Clin. Invest. 2008, 118, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Leffler, A.; Lattrell, A.; Kronewald, S.; Niedermirtl, F.; Nau, C. Activation of TRPA1 by membrane permeable local anesthetics. Mol. Pain 2011, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Piao, L.-H.; Fujita, T.; Jiang, C.-Y.; Liu, T.; Yue, H.-Y.; Nakatsuka, T.; Kumamoto, E. TRPA1 activation by lidocaine in nerve terminals results in glutamate release increase. Biochem. Biophys. Res. Commun. 2009, 379, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A. Piperine: researchers discover new flavor in an ancient spice. Trends Pharmacol. Sci. 2005, 26, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fujita, T.; Jiang, C.-Y.; Piao, L.-H.; Yue, H.-Y.; Mizuta, K.; Kumamoto, E. TRPV1 agonist piperine but not olvanil enhances glutamatergic spontaneous excitatory transmission in rat spinal substantia gelatinosa neurons. Biochem. Biophys. Res. Commun. 2011, 410, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Simon, S.A. Similarities and differences in the currents activated by capsaicin, piperine, and zingerone in rat trigeminal ganglion cells. J. Neurophysiol. 1996, 76, 1858–1869. [Google Scholar] [PubMed]
- Liu, L.; Lo, Y.-C.; Chen, I.-J.; Simon, S.A. The responses of rat trigeminal ganglion neurons to capsaicin and two nonpungent vanilloid receptor agonists, olvanil and glyceryl nonamide. J. Neurosci. 1997, 17, 4101–4111. [Google Scholar] [PubMed]
- Yang, B.H.; Piao, Z.G.; Kim, Y.-B.; Lee, C.-H.; Lee, J.K.; Park, K.; Kim, J.S.; Oh, S.B. Activation of vanilloid receptor 1 (VR1) by eugenol. J. Dent. Res. 2003, 82, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Fujita, T.; Goto, M.; Kumamoto, E. Presynaptic enhancement by eugenol of spontaneous excitatory transmission in rat spinal substantia gelatinosa neurons is mediated by transient receptor potential A1 channels. Neuroscience 2012, 210, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.-Y.; Jiang, C.-Y.; Fujita, T.; Kumamoto, E. Zingerone enhances glutamatergic spontaneous excitatory transmission by activating TRPA1 but not TRPV1 channels in the adult rat substantia gelatinosa. J. Neurophysiol. 2013, 110, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-K.; Xu, Z.-Z.; Berta, T.; Han, Q.; Chen, G.; Liu, X.-J.; Ji, R.-R. Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron 2014, 82, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Fujita, T.; Jiang, C.-Y.; Kumamoto, E. Enhancement by citral of glutamatergic spontaneous excitatory transmission in adult rat substantia gelatinosa neurons. NeuroReport 2016, 27, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.A.; Gentry, C.; Alenmyr, L.; Killander, D.; Lewis, S.E.; Andersson, A.; Bucher, B.; Galzi, J.-L.; Sterner, O.; Bevan, S.; et al. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Δ9-tetrahydrocannabiorcol. Nat. Commun. 2011, 2, 551. [Google Scholar] [CrossRef] [PubMed]
- Furue, H.; Narikawa, K.; Kumamoto, E.; Yoshimura, M. Responsiveness of rat substantia gelatinosa neurones to mechanical but not thermal stimuli revealed by in vivo patch-clamp recording. J. Physiol. 1999, 521, 529–535. [Google Scholar]
- Yamanaka, M.; Taniguchi, W.; Nishio, N.; Hashizume, H.; Yamada, H.; Yoshida, M.; Nakatsuka, T. In vivo patch-clamp analysis of the antinociceptive actions of TRPA1 activation in the spinal dorsal horn. Mol. Pain 2015, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Todd, A.J.; Watt, C.; Spike, R.C.; Sieghart, W. Colocalization of GABA, glycine and their receptors at synapses in the rat spinal cord. J. Neurosci. 1996, 16, 974–982. [Google Scholar] [PubMed]
- Coggeshall, R.E.; Carlton, S.M. Receptor localization in the mammalian dorsal horn and primary afferent neurons. Brain Res. Rev. 1997, 24, 28–66. [Google Scholar] [CrossRef]
- Moore, K.A.; Kohno, T.; Karchewski, L.A.; Scholz, J.; Baba, H.; Woolf, C.J. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J. Neurosci. 2002, 22, 6724–6731. [Google Scholar] [PubMed]
- Kohno, T. A role of spinal inhibition in neuropathic pain. In Cellular and Molecular Mechanisms for the Modulation of Nociceptive Transmission in the Peripheral and Central Nervous Systems; Kumamoto, E., Ed.; Research Signpost: Kelara, India, 2007; pp. 131–145. [Google Scholar]
- Coull, J.A.M.; Boudreau, D.; Bachand, K.; Prescott, S.A.; Nault, F.; Sik, A.; de Koninck, P.; de Koninck, Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 2003, 424, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; Heinke, B.; Sandkühler, J. Reduction of glycine receptor-mediated miniature inhibitory postsynaptic currents in rat spinal lamina I neurons after peripheral inflammation. Neuroscience 2003, 122, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, M.; Takasu, K.; Yamaguchi, S.; Kodama, D.; Ono, H. Glycine transporter inhibitors as a potential therapeutic strategy for chronic pain with memory impairment. Anesthesiology 2008, 108, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Sandkühler, J. Models and mechanisms of hyperalgesia and allodynia. Physiol. Rev. 2009, 89, 707–758. [Google Scholar] [CrossRef] [PubMed]
- Zeilhofer, H.U.; Wildner, H.; Yévenes, G.E. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol. Rev. 2012, 92, 193–235. [Google Scholar] [CrossRef] [PubMed]
- Baba, H.; Kohno, T.; Okamoto, M.; Goldstein, P.A.; Shimoji, K.; Yoshimura, M. Muscarinic facilitation of GABA release in substantia gelatinosa of the rat spinal dorsal horn. J. Physiol. 1998, 508, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Baba, H.; Goldstein, P.A.; Okamoto, M.; Kohno, T.; Ataka, T.; Yoshimura, M.; Shimoji, K. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 2): effects on somatodendritic sites of GABAergic neurons. Anesthesiology 2000, 92, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Baba, H.; Shimoji, K.; Yoshimura, M. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 1): effects on axon terminals of GABAergic and glycinergic neurons. Anesthesiology 2000, 92, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Takeda, D.; Nakatsuka, T.; Papke, R.; Gu, J.G. Modulation of inhibitory synaptic activity by a non-α4β2, non-α7 subtype of nicotinic receptors in the substantia gelatinosa of adult rat spinal cord. Pain 2003, 101, 13–23. [Google Scholar] [CrossRef]
- Fukushima, T.; Ohtsubo, T.; Tsuda, M.; Yanagawa, Y.; Hori, Y. Facilitatory actions of serotonin type 3 receptors on GABAergic inhibitory synaptic transmission in the spinal superficial dorsal horn. J. Neurophysiol. 2009, 102, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Fujita, T.; Kumamoto, E. Acetylcholine and norepinephrine mediate GABAergic but not glycinergic transmission enhancement by melittin in adult rat substantia gelatinosa neurons. J. Neurophysiol. 2011, 106, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Bíró, T.; Tóth, B.I.; Marincsák, R.; Dobrosi, N.; Géczy, T.; Paus, R. TRP channels as novel players in the pathogenesis and therapy of itch. Biochim. Biophys. Acta 2007, 1772, 1004–1021. [Google Scholar] [CrossRef] [PubMed]
- Sikand, P.; Shimada, S.G.; Green, B.G.; LaMotte, R.H. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain 2009, 144, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Carstens, E. Neural processing of itch. Neuroscience 2013, 250, 697–714. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ji, R.-R. New insights into the mechanisms of itch: are pain and itch controlled by distinct mechanisms? Pflügers Arch. 2013, 465, 1671–1685. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Liu, T.; Nakatsuka, T.; Kumamoto, E. Proteinase-activated receptor-1 activation presynaptically enhances spontaneous glutamatergic excitatory transmission in adult rat substantia gelatinosa neurons. J. Neurophysiol. 2009, 102, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Jordt, S.-E.; Julius, D. Molecular basis for species-specific sensitivity to "hot" chili peppers. Cell 2002, 108, 421–430. [Google Scholar] [CrossRef]
- Gavva, N.R.; Klionsky, L.; Qu, Y.; Shi, L.; Tamir, R.; Edenson, S.; Zhang, T.J.; Viswanadhan, V.N.; Toth, A.; Pearce, L.V.; et al. Molecular determinants of vanilloid sensitivity in TRPV1. J. Biol. Chem. 2004, 279, 20283–20295. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.-F.; Kort, M.E.; Huth, J.R.; Sun, C.; Miesbauer, L.J.; Cassar, S.C.; Neelands, T.; Scott, V.E.; Moreland, R.B.; et al. Molecular determinants of species-specific activation or blockade of TRPA1 channels. J. Neurosci. 2008, 28, 5063–5071. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, K.; Kubo, Y. Caffeine activates mouse TRPA1 channels but suppresses human TRPA1 channels. Proc. Natl. Acad. Sci. USA 2008, 105, 17373–17378. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Joshi, S.K.; DiDomenico, S.; Perner, R.J.; Mikusa, J.P.; Gauvin, D.M.; Segreti, J.A.; Han, P.; Zhang, X.-F.; Niforatos, W.; et al. Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain 2011, 152, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Banzawa, N.; Saito, S.; Imagawa, T.; Kashio, M.; Takahashi, K.; Tominaga, M.; Ohta, T. Molecular basis determining inhibition/activation of nociceptive receptor TRPA1 protein: a single amino acid dictates species-specific actions of the most potent mammalian TRPA1 antagonist. J. Biol. Chem. 2014, 289, 31927–31939. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).