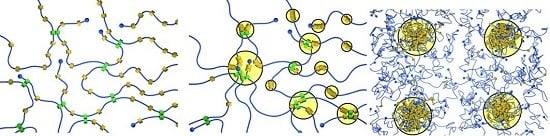
Volume Phase Transitions of Slide-Ring Gels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Carboxylated SR Gels
2.3. Preparation of Sulfonated SR Gels
2.4. Measurements: Swelling-Shrinking Experiments
2.5. SAXS Experiments
3. Results
3.1. Swelling-Shrinking Behaviors of Carboxylated SR Gels
3.2. Swelling-Shrinking Behaviors of Sulfonated SR Gels
3.3. SAXS Profiles of Carboxylated SR Gels in Acetone-Water Mixture.
3.4. Dynamic Swelling-Shrinking Behaviors of SR Gels.
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PEG | Poly(ethylene glycol) |
| CD | Cyclodextrin |
| SR | Slide-ring |
| PR | Polyrotaxane |
| SAXS | Small-angle X-ray scattering |
| CDI | 1,1′-carbonyldiimidazole |
References
- Okumura, Y.; Ito, K. The Polyrotaxane gel: A Topological gel by figure-of-eight cross-links. Adv. Mater. 2001, 13, 485–487. [Google Scholar] [CrossRef]
- Harada, A.; Li, J.; Kamachi, M. The molecular necklace: A rotaxane containing many threaded α-cyclodextrins. Nature 1992, 356, 325–327. [Google Scholar] [CrossRef]
- Harada, A.; Hashidzume, A.; Yamaguchi, H.; Takashima, Y. Polymeric rotaxanes. Chem. Rev. 2009, 109, 5974–6023. [Google Scholar] [CrossRef] [PubMed]
- Fleury, G.; Schlatter, G.; Brochon, C.; Hadziioannou, G. From high molecular weight precursor polyrotaxanes to supramolecular sliding networks. The sliding gels. Polymer 2005, 46, 8494–8501. [Google Scholar] [CrossRef]
- Fleury, G.; Schlatter, G.; Brochon, C.; Hadziioannou, G. Unveiling the sliding motion in topological networks: Influence of the swelling solvent on the relaxation dynamics. Adv. Mater. 2006, 18, 2847–2851. [Google Scholar] [CrossRef]
- Fleury, G.; Schlatter, G.; Brochon, C.; Travelet, C.; Lapp, A.; Lindner, P.; Hadziioannou, G. Topological polymer networks with sliding cross-link points: The “sliding gels”. Relationship between their molecular structure and the viscoelastic as well as the swelling properties. Macromolecules 2007, 40, 535–543. [Google Scholar] [CrossRef]
- Ito, K. Novel cross-linking concept of polymer network: Synthesis, structure, and properties of slide-ring gels with freely movable junctions. Polym. J. 2007, 39, 489–499. [Google Scholar] [CrossRef]
- Karaky, K.; Brochon, C.; Schlatter, G.; Hadziioannou, G. pH-Switchable supramolecular “sliding” gels based on polyrotaxanes of polyethyleneimine-block-poly(ethylene oxide)-block-polyethyleneimineblock copolymer and α-cyclodextrin: Synthesis and swelling behaviour. Soft Matter 2008, 4, 1165–1168. [Google Scholar] [CrossRef]
- Ito, K. Novel entropic elasticity of polymeric materials: Why is slide-ring gel so soft? Polym. J. 2012, 44, 38–41. [Google Scholar] [CrossRef]
- Kato, K.; Ito, K. Dynamic transition between rubber and sliding states attributed to slidable cross-links. Soft Matter 2011, 7, 8737–8740. [Google Scholar] [CrossRef]
- Mayumi, K.; Tezuka, M.; Bando, A.; Ito, K. Mechanics of slide-ring gels: Novel entropic elasticity of a topological network formed by ring and string. Soft Matter 2012, 8, 8179–8183. [Google Scholar] [CrossRef]
- Kato, K.; Okabe, Y.; Okazumi, Y.; Ito, K. A significant impact of host–guest stoichiometry on the extensibility of polyrotaxane gels. Chem. Commun. 2015, 51, 16180–16183. [Google Scholar] [CrossRef] [PubMed]
- Murata, N.; Konda, A.; Urayama, K.; Takigawa, T.; Kidowaki, M.; Ito, K. Anomaly in stretching-induced swelling of slide-ring gels with movable cross-links. Macromolecules 2009, 42, 8485–8491. [Google Scholar] [CrossRef]
- Konda, A.; Mayumi, K.; Urayama, K.; Takigawa, T.; Kidowaki, M.; Ito, K. Influence of structural characteristics on stretching-driven swelling of polyrotaxane gels with movable cross links. Macromolecules 2012, 45, 6733–6740. [Google Scholar] [CrossRef]
- Bitoh, Y.; Akuzawa, N.; Urayama, K.; Takigawa, T.; Kidowaki, M.; Ito, K. Peculiar nonlinear elasticity of polyrotaxane gels with movable cross-links revealed by multiaxial stretching. Macromolecules 2011, 44, 8661–8667. [Google Scholar] [CrossRef]
- Bitoh, Y.; Urayama, K.; Takigawa, T.; Ito, K. Biaxial strain testing of extremely soft polymer gels. Soft Matter 2011, 7, 2632–2638. [Google Scholar]
- Kato, K.; Yasuda, T.; Ito, K. Viscoelastic properties of slide-ring gels reflecting sliding dynamics of partial chains and entropy of ring components. Macromolecules 2012, 46, 310–316. [Google Scholar] [CrossRef]
- Katsuno, C.; Konda, A.; Urayama, K.; Takigawa, T.; Kidowaki, M.; Ito, K. Pressure-responsive polymer membranes of slide-ring gels with movable cross-links. Adv. Mater. 2013, 25, 4636–4640. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T. Collapse of gels and the critical endpoint. Phys. Rev. Lett. 1978, 40, 820–823. [Google Scholar] [CrossRef]
- Tanaka, T.; Fillmore, D.; Sun, S.-T.; Nishio, I.; Swislow, G.; Shah, A. Phase transitions in ionic gels. Phys. Rev. Lett. 1980, 45, 1636–1639. [Google Scholar] [CrossRef]
- Ilavsky, M. Phase transition in swollen gels. 2. Effect of charge concentration on the collapse and mechanical behavior of polyacrylamide networks. Macromolecules 1982, 15, 782–788. [Google Scholar] [CrossRef]
- Hirokawa, Y.; Tanaka, T. Volume phase transition in a nonionic gel. J. Chem. Phys. 1984, 81, 6379–6380. [Google Scholar] [CrossRef]
- Ilavsky, M.; Hrouz, J.; Ulbrich, K. Phase transition in swollen gels 3. The temperature collapse and mechanical behaviour of poly(N,N-diethylacrylamide) networks in water. Polym. Bull. 1982, 7, 107–113. [Google Scholar]
- Hirotsu, S.; Hirokawa, Y.; Tanaka, T. Volume-phase transitions of ionized N-isopropylacrylamide gels. J. Chem. Phys. 1987, 87, 1392–1395. [Google Scholar] [CrossRef]
- Ohmine, I.; Tanaka, T. Salt effects on the phase transition of ionic gels. J. Chem. Phys. 1982, 77, 5725–5729. [Google Scholar] [CrossRef]
- Tanaka, T.; Nishio, I.; Sun, S.-T.; Ueno-Nishio, S. Collapse of gels in an electric field. Science 1982, 218, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Mamada, A.; Tanaka, T.; Kungwatchakun, D.; Irie, M. Photoinduced phase transition of gels. Macromolecules 1990, 23, 1517–1519. [Google Scholar] [CrossRef]
- Suzuki, A.; Tanaka, T. Phase transition in polymer gels induced by visible light. Nature 1990, 346, 345–347. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell Univ. Press: New York, NY, USA, 1953. [Google Scholar]
- Harada, A.; Li, J.; Kamachi, M. Preparation and properties of inclusion complexes of polyethylene glycol with α-cyclodextrin. Macromolecules 1993, 26, 5698–5703. [Google Scholar] [CrossRef]
- Amemiya, Y.; Wakabayashi, K.; Hamanaka, T.; Wakabayashi, T.; Matsushita, T.; Hashizume, H. Design of small-angle X-ray diffractometer using synchrotron radiation at the photon factory. Nucl. Instrum. Methods Phys. Res. 1983, 208, 471–477. [Google Scholar] [CrossRef]
- Amemiya, Y.; Ito, K.; Yagi, N.; Asano, Y.; Wakabayashi, K.; Ueki, T.; Endo, T. Large-aperture TV detector with a beryllium-windowed image intensifier for X-ray diffraction. Rev. Sci. Instrum. 1995, 66, 2290–2294. [Google Scholar] [CrossRef]
- Glatter, O.; Kratky, O. Small-Angle X-ray Scattering; Academic Press: London, UK, 1982. [Google Scholar]
- Bando, A.; Mayumi, K.; Yokoyama, H.; Ito, K. Theory of volume phase transition of slide-ring gels. React. Funct. Polym. 2013, 73, 904–910. [Google Scholar] [CrossRef]
- Shinohara, Y.; Kayashima, K.; Okumura, Y.; Zhao, C.; Ito, K.; Amemiya, Y. Small-angle X-ray scattering study of the pulley effect of slide-ring gels. Macromolecules 2006, 39, 7386–7391. [Google Scholar] [CrossRef]
















© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bando, A.; Kasahara, R.; Kayashima, K.; Okumura, Y.; Kato, K.; Sakai, Y.; Yokoyama, H.; Shinohara, Y.; Amemiya, Y.; Ito, K. Volume Phase Transitions of Slide-Ring Gels. Polymers 2016, 8, 217. https://doi.org/10.3390/polym8060217
Bando A, Kasahara R, Kayashima K, Okumura Y, Kato K, Sakai Y, Yokoyama H, Shinohara Y, Amemiya Y, Ito K. Volume Phase Transitions of Slide-Ring Gels. Polymers. 2016; 8(6):217. https://doi.org/10.3390/polym8060217
Chicago/Turabian StyleBando, Akinori, Rumiko Kasahara, Kentaro Kayashima, Yasushi Okumura, Kazuaki Kato, Yasuhiro Sakai, Hideaki Yokoyama, Yuya Shinohara, Yoshiyuki Amemiya, and Kohzo Ito. 2016. "Volume Phase Transitions of Slide-Ring Gels" Polymers 8, no. 6: 217. https://doi.org/10.3390/polym8060217
APA StyleBando, A., Kasahara, R., Kayashima, K., Okumura, Y., Kato, K., Sakai, Y., Yokoyama, H., Shinohara, Y., Amemiya, Y., & Ito, K. (2016). Volume Phase Transitions of Slide-Ring Gels. Polymers, 8(6), 217. https://doi.org/10.3390/polym8060217