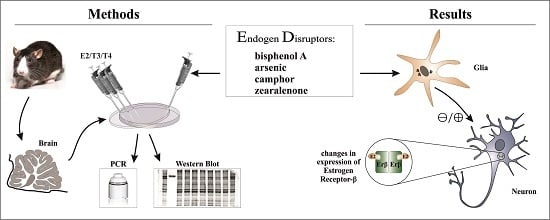
Comparison of Individual and Combined Effects of Four Endocrine Disruptors on Estrogen Receptor Beta Transcription in Cerebellar Cell Culture: The Modulatory Role of Estradiol and Triiodo-Thyronine
Abstract
:1. Introduction
Hypothesis
2. Materials and Methods
2.1. Animals
2.2. Preparation of Primary Granule Cell Cultures
2.3. Treatments
2.4. Real-Time Quantitative PCR Measurements
2.5. Data Analysis
3. Results
3.1. Bisphenol A
3.2. Zearalenone
3.3. Arsenic
3.4. 4-Methylbenzylidene Camphor
3.5. AllEDs
4. Discussion
4.1. Effects of Bisphenol A
4.2. Effects of Zearalenone
4.3. Effects of Arsenic
4.4. Effects of 4-Methylbenzylidene Camphor
4.5. Effects of Treatments with BPA, Zea, As, and MBC Combined (AllEDs)
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AllEDs | all the four Endocrine Disruptors |
| As | Arsenic |
| BPA | Bisphenol A |
| ED | Endocrine Disruptor |
| ER | Estrogen Receptor |
| E2 | 17β-estradiol |
| Glia+ | glia containing cell culture |
| Glia– | glia reduced cell culture |
| MBC | 4-methylbenzylidene camphor |
| ntC | non-treated control |
| P(0) | postnatal day 0 |
| TH | thyroid hormone |
| TR | TH receptors |
| T3 | Triiodo-thyronine |
| ZEA | Zearalenone |
Appendix
| Glia+ | Glia− | |||||||
|---|---|---|---|---|---|---|---|---|
| ntC | ED | E2 + T3 | E2 + T3 + ED | ntC | ED | E2 + T3 | E2 + T3 + ED | |
| BPA | ||||||||
| MEAN | 1000 | 0.023 | 1103 | 36,428 | 7387 | 4141 | 3093 | 6565 |
| SEM | 0.017 | 0.005 | 0.205 | 3087 | 0.182 | 1172 | 1546 | 1770 |
| ZEA | ||||||||
| MEAN | 1000 | 0.389 | 1103 | 0.096 | 7387 | 0.659 | 3093 | 0.421 |
| SEM | 0.017 | 0.073 | 0.205 | 0.190 | 0.182 | 0.182 | 1546 | 0.190 |
| As | ||||||||
| MEAN | 1000 | 0.559 | 1103 | 1428 | 7387 | 0.975 | 3093 | 0.769 |
| SEM | 0.017 | 0.129 | 0.205 | 0.819 | 0.182 | 0.188 | 1546 | 0.039 |
| MBC | ||||||||
| MEAN | 1000 | 2111 | 1103 | 3097 | 7387 | 3623 | 3093 | 1130 |
| SEM | 0.017 | 0.869 | 0.205 | 0.323 | 0.182 | 1436 | 1546 | 0.761 |
| AllEDs | ||||||||
| MEAN | 1000 | 8623 | 1103 | 2351 | 7387 | 6125 | 3093 | 2522 |
| SEM | 0.017 | 4520 | 0.205 | 0.610 | 0.182 | 2468 | 1546 | 0.894 |
References
- Zsarnovszky, A.; Földvári, E.G.; Rónai, Z.; Bartha, T.; Frenyó, L.V. Oestrogens in the mammalian brain: From conception to adulthood—A review. Acta Vet. Hung. 2007, 55, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Jakab, R.L.; Wong, J.K.; Belcher, S.M. Estrogen receptor beta immunoreactivity in differentiating cells of the developing rat cerebellum. J. Comp. Neurol. 2001, 430, 396–409. [Google Scholar] [CrossRef]
- Ikeda, Y. Expression of the two estrogen receptor (ER) subtypes, ERalpha and ERbeta, during postnatal development of the rat cerebellum. Cerebellum 2008, 7, 501–502. [Google Scholar] [PubMed]
- Fan, X.; Xu, H.; Warner, M.; Gustafsson, J.-A. ERbeta in CNS: New roles in development and function. Prog. Brain Res. 2010, 181, 233–250. [Google Scholar] [PubMed]
- Belcher, S.M. Regulated expression of estrogen receptor alpha and beta mRNA in granule cells during development of the rat cerebellum. Brain Res. Dev. Brain Res. 1999, 115, 57–69. [Google Scholar] [CrossRef]
- Price, R.H.; Handa, R.J. Expression of estrogen receptor-beta protein and mRNA in the cerebellum of the rat. Neurosci. Lett. 2000, 288, 115–118. [Google Scholar] [CrossRef]
- Wallis, K.; Dudazy, S.; van Hogerlinden, M.; Nordström, K.; Mittag, J.; Vennström, B. The thyroid hormone receptor alpha1 protein is expressed in embryonic postmitotic neurons and persists in most adult neurons. Mol. Endocrinol. 2010, 24, 1904–1916. [Google Scholar] [CrossRef] [PubMed]
- Fauquier, T.; Chatonnet, F.; Picou, F.; Richard, S.; Fossat, N.; Aguilera, N.; Lamonerie, T.; Flamant, F. Purkinje cells and Bergmann glia are primary targets of the TRα1 thyroid hormone receptor during mouse cerebellum postnatal development. Development 2014, 141, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Somogyi, V.; Gyorffy, A.; Scalise, T.J.; Kiss, D.S.; Goszleth, G.; Bartha, T.; Frenyo, V.L.; Zsarnovszky, A. Endocrine factors in the hypothalamic regulation of food intake in females: A review of the physiological roles and interactions of ghrelin, leptin, thyroid hormones, oestrogen and insulin. Nutr. Res. Rev. 2011, 24, 132–154. [Google Scholar] [CrossRef] [PubMed]
- Picou, F.; Fauquier, T.; Chatonnet, F.; Flamant, F. A bimodal influence of thyroid hormone on cerebellum oligodendrocyte differentiation. Mol. Endocrinol. 2012, 26, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.; Eller, C.; Viana, N.B.; Gomes, F.C.A. Thyroid hormone induces cerebellar neuronal migration and Bergmann glia differentiation through epidermal growth factor/mitogen-activated protein kinase pathway. Eur. J. Neurosci. 2011, 33, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Mendes-de-Aguiar, C.B.N.; Alchini, R.; Zucco, J.K.; Costa-Silva, B.; Decker, H.; Alvarez-Silva, M.; Tasca, C.I.; Trentin, A.G. Impaired astrocytic extracellular matrix distribution under congenital hypothyroidism affects neuronal development in vitro. J. Neurosci. Res. 2010, 88, 3350–3360. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, N.; Koibuchi, N.; Chin, W.W.; Pfaff, D.W. Differential crosstalk between estrogen receptor (ER)alpha and ERbeta and the thyroid hormone receptor isoforms results in flexible regulation of the consensus ERE. Brain Res. Mol. Brain Res. 2001, 95, 9–17. [Google Scholar] [CrossRef]
- Zhao, X.; Lorenc, H.; Stephenson, H.; Wang, Y.J.; Witherspoon, D.; Katzenellenbogen, B.; Pfaff, D.; Vasudevan, N. Thyroid hormone can increase estrogen-mediated transcription from a consensus estrogen response element in neuroblastoma cells. Proc. Natl. Acad. Sci. USA 2005, 102, 4890–4895. [Google Scholar] [CrossRef] [PubMed]
- Kirby, M.; Zsarnovszky, A.; Belcher, S.M. Estrogen receptor expression in a human primitive neuroectodermal tumor cell line from the cerebral cortex: estrogen stimulates rapid ERK1/2 activation and receptor-dependent cell migration. Biochem. Biophys. Res. Commun. 2004, 319, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Andersson, S.; Warner, M.; Gustafsson, J.A. Morphological abnormalities in the brains of estrogen receptor beta knockout mice. Proc. Natl. Acad. Sci. USA 2001, 98, 2792–2796. [Google Scholar] [CrossRef] [PubMed]
- Andreescu, C.E.; Milojkovic, B.A.; Haasdijk, E.D.; Kramer, P.; de Jong, F.H.; Krust, A.; de Zeeuw, C.I.; de Jeu, M.T.G. Estradiol improves cerebellar memory formation by activating estrogen receptor beta. J. Neurosci. 2007, 27, 10832–10839. [Google Scholar] [CrossRef] [PubMed]
- Contestabile, A. Cerebellar granule cells as a model to study mechanisms of neuronal apoptosis or survival in vivo and in vitro. Cerebellum 2002, 1, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.W. Thyroid hormone and cerebellar development. Cerebellum 2008, 7, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Patel, E.; Reynolds, M. Methylmercury impairs motor function in early development and induces oxidative stress in cerebellar granule cells. Toxicol. Lett. 2013, 222, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.K.; Kennedy, P.R.; Belcher, S.M. Simplified serum- and steroid-free culture conditions for high-throughput viability analysis of primary cultures of cerebellar granule neurons. J. Neurosci. Methods 2001, 110, 45–55. [Google Scholar] [CrossRef]
- Scalise, T.J.; Győrffy, A.; Tóth, I.; Kiss, D.S.; Somogyi, V.; Goszleth, G.; Bartha, T.; Frenyó, L.V.; Zsarnovszky, A. Ligand-induced changes in oestrogen and thyroid hormone receptor expression in the developing rat cerebellum: A comparative quantitative PCR and western blot study. Acta Vet. Hung. 2012, 60, 263–284. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.K.; Le, H.H.; Zsarnovszky, A.; Belcher, S.M. Estrogens and ICI182,780 (Faslodex) modulate mitosis and cell death in immature cerebellar neurons via rapid activation of p44/p42 mitogen-activated protein kinase. J. Neurosci. 2003, 23, 4984–4995. [Google Scholar] [PubMed]
- Sayed-Ahmed, A.; Kulcsár, M.; Rudas, P.; Bartha, T. Expression and localisation of leptin and leptin receptor in the mammary gland of the dry and lactating non-pregnant cow. Acta Vet. Hung. 2004, 52, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, C.; Chesnel, F.; Schausi, D.; Tiffoche, C.; Thieulant, M.L. Expression of estrogen receptor subtypes in rat pituitary gland during pregnancy and lactation. Endocrinology 2002, 143, 4249–4258. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST (c)) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef] [PubMed]
- Zsarnovszky, A.; Le, H.H.; Wang, H.S.; Belcher, S.M. Ontogeny of rapid estrogen-mediated extracellular signal-regulated kinase signaling in the rat cerebellar cortex: Potent nongenomic agonist and endocrine disrupting activity of the xenoestrogen bisphenol A. Endocrinology 2005, 146, 5388–5396. [Google Scholar] [CrossRef] [PubMed]
- Withanage, G.; Murata, H.; Koyama, T.; Ishiwata, I. Agonistic and antagonistic effects of zearalenone, an etrogenic mycotoxin, on SKN, HHUA, HepG2 human cancer cell lines. Vet. Hum. Toxicol. 2001, 43, 6–10. [Google Scholar] [PubMed]
- Pillay, D.; Churturgoon, A.A.; Nevines, E.; Manickum, T.; Deppe, W.; Dutton, M.F. The quantitative analysis of zearalenone and its derivatives in plasma of patients with breast and cervical cancer. Clin. Chem. Lab. Med. 2002, 40, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Parveen, M.; Zhu, Y.; Kiyama, R. Expression profiling of the genes responding to zearalenone and its analogues using estrogen-responsive genes. FEBS Lett. 2009, 583, 2377–2384. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S.; Obremski, K.; Calka, J. The Influence of Low Doses of Zearalenone on Distribution of Selected Active Substances in Nerve Fibers Within the Circular Muscle Layer of Porcine Ileum. J. Mol. Neurosci. 2015, 56, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, J.C.; Hunt, P.J.B.; Blaustein, J.D. Estrogenic effects of zearalenone on the expression of progestin receptors and sexual behavior in female rats. Horm. Behav. 2005, 47, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Greenman, D.L.; Mehta, R.G.; Wittliff, J.L. Nuclear interaction of Fusarium mycotoxins with estradiol binding sites in the mouse uterus. J. Toxicol. Environ. Health 1979, 5, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Takemura, H.; Shim, J.Y.; Sayama, K.; Tsubura, A.; Zhu, B.T.; Shimoi, K. Characterization of the estrogenic activities of zearalenone and zeranol in vivo and in vitro. J. Steroid Biochem. Mol. Biol. 2007, 103, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Frizzell, C.; Ndossi, D.; Verhaegen, S.; Dahl, E.; Eriksen, G.; Sørlie, M.; Ropstad, E.; Muller, M.; Elliott, C.T.; Connolly, L. Endocrine disrupting effects of zearalenone, alpha- and beta-zearalenol at the level of nuclear receptor binding and steroidogenesis. Toxicol. Lett. 2011, 206, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Mastri, C.; Mistry, P.; Lucier, G.W. In vivo oestrogenicity and binding characteristics of alpha-zearalanol (P-1496) to different classes of oestrogen binding proteins in rat liver. J. Steroid Biochem. 1985, 23, 279–289. [Google Scholar] [CrossRef]
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.Å. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [PubMed]
- Rodríguez-Barranco, M.; Lacasaña, M.; Aguilar-Garduño, C.; Alguacil, J.; Gil, F.; González-Alzaga, B.; Rojas-García, A. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: A systematic review and meta-analysis. Sci. Total Environ. 2013, 454, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Amador, D.; Navarro, M.E.; Carrizales, L.; Morales, R.; Calderón, J. Decreased intelligence in children and exposure to fluoride and arsenic in drinking water. Cad. Saude Publica 2007, 23, S579–S587. [Google Scholar] [CrossRef] [PubMed]
- Calderón, J.; Navarro, M.E.; Jimenez-Capdeville, M.E.; Santos-Diaz, M.A.; Golden, A.; Rodriguez-Leyva, I.; Borja-Aburto, V.; Dı́az-Barriga, F. Exposure to Arsenic and Lead and Neuropsychological Development in Mexican Children. Environ. Res. 2001, 85, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, G.A.; Liu, X.; Parvez, F.; Factor-Litvak, P.; Ahsan, H.; Levy, D.; Kline, J.; van Geen, A.; Mey, J.; Slavkovich, V.; et al. Arsenic and manganese exposure and children’s intellectual function. Neurotoxicology 2011, 32, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Rodrı́guez, V.; Carrizales, L.; Jiménez-Capdeville, M.; Dufour, L.; Giordano, M. The effects of sodium arsenite exposure on behavioral parameters in the rat. Brain Res. Bull. 2001, 55, 301–308. [Google Scholar] [CrossRef]
- Yamanaka, K.; Okada, S. Induction of lung-specific DNA damage by metabolically methylated arsenics via the production of free radicals. Environ. Health Perspect. 1994, 102, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Sundari, P.N.; Wilfred, G.; Ramakrishna, B. Does oxidative protein damage play a role in the pathogenesis of carbon tetrachloride-induced liver injury in the rat? Biochim. Biophys. Acta 1997, 1362, 169–176. [Google Scholar] [CrossRef]
- Zhong, C.X.; Mass, M.J. Both hypomethylation and hypermethylation of DNA associated with arsenite exposure in cultures of human cells identified by methylation-sensitive arbitrarily-primed PCR. Toxicol. Lett. 2001, 122, 223–234. [Google Scholar] [CrossRef]
- Bodwell, J.E.; Kingsley, L.A.; Hamilton, J.W. Arsenic at very low concentrations alters glucocorticoid receptor (GR)-mediated gene activation but not GR-mediated gene repression: Complex dose-response effects are closely correlated with levels of activated GR and require a functional GR DNA binding d. Chem. Res. Toxicol. 2004, 17, 1064–1076. [Google Scholar] [CrossRef] [PubMed]
- Bodwell, J.E.; Gosse, J.A.; Nomikos, A.P.; Hamilton, J.W. Arsenic disruption of steroid receptor gene activation: Complex dose-response effects are shared by several steroid receptors. Chem. Res. Toxicol. 2006, 19, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Cimino-Reale, G.; Ferrario, D.; Casati, B.; Brustio, R.; Diodovich, C.; Collotta, A.; Vahter, M.; Gribaldo, L. Combined in utero and juvenile exposure of mice to arsenate and atrazine in drinking water modulates gene expression and clonogenicity of myeloid progenitors. Toxicol. Lett. 2008, 180, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-C.; Guan, L.-S.; Hu, W.-L.; Wang, Z.-Y. Functional repression of estrogen receptor a by arsenic trioxide in human breast cancer cells. Anticancer Res. 2002, 22, 633–638. [Google Scholar] [PubMed]
- Schmutzler, C.; Hamann, I.; Hofmann, P.J.; Kovacs, G.; Stemmler, L.; Mentrup, B.; Schomburg, L.; Ambrugger, P.; Grüters, A.; Seidlova-Wuttke, D.; et al. Endocrine active compounds affect thyrotropin and thyroid hormone levels in serum as well as endpoints of thyroid hormone action in liver, heart and kidney. Toxicology 2004, 205, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Schlumpf, M.; Jarry, H.; Wuttke, W.; Ma, R.; Lichtensteiger, W. Estrogenic activity and estrogen receptor beta binding of the UV filter 3-benzylidene camphor. Comparison with 4-methylbenzylidene camphor. Toxicology 2004, 199, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Seidlová-Wuttke, D.; Christoffel, J.; Rimoldi, G.; Jarry, H.; Wuttke, W. Comparison of effects of estradiol with those of octylmethoxycinnamate and 4-methylbenzylidene camphor on fat tissue, lipids and pituitary hormones. Toxicol. Appl. Pharmacol. 2006, 214, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Boas, M.; Feldt-Rasmussen, U.; Main, K.M. Thyroid effects of endocrine disrupting chemicals. Mol. Cell. Endocrinol. 2012, 355, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.O.; Kling, M.; Firzani, P.A.; Mecky, A.; Duranti, E.; Shields-Botella, J.; Delansorne, R.; Broschard, T.; Kramer, P.J. Activation of estrogen receptor alpha and ER beta by 4-methylbenzylidene-camphor in human and rat cells: Comparison with phyto- and xenoestrogens. Toxicol. Lett. 2003, 142, 89–101. [Google Scholar] [CrossRef]
- Durrer, S.; Maerkel, K.; Schlumpf, M.; Lichtensteiger, W. Estrogen target gene regulation and coactivator expression in rat uterus after developmental exposure to the ultraviolet filter 4-methylbenzylidene camphor. Endocrinology 2005, 146, 2130–2139. [Google Scholar] [CrossRef] [PubMed]
- Schlumpf, M.; Schmid, P.; Durrer, S.; Conscience, M.; Maerkel, K.; Henseler, M.; Gruetter, M.; Herzog, I.; Reolon, S.; Ceccatelli, R.; et al. Endocrine activity and developmental toxicity of cosmetic UV filters—An update. Toxicology 2004, 205, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Maerkel, K.; Durrer, S.; Henseler, M.; Schlumpf, M.; Lichtensteiger, W. Sexually dimorphic gene regulation in brain as a target for endocrine disrupters: Developmental exposure of rats to 4-methylbenzylidene camphor. Toxicol. Appl. Pharmacol. 2007, 218, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Carou, M.E.; Deguiz, M.L.; Reynoso, R.; Szwarcfarb, B.; Carbone, S.; Moguilevsky, J.A.; Scacchi, P.; Ponzo, O.J. Impact of the UV-B filter 4-(Methylbenzylidene)-camphor (4-MBC) during prenatal development in the neuroendocrine regulation of gonadal axis in male and female adult rats. Environ. Toxicol. Pharmacol. 2009, 27, 410–414. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jocsak, G.; Kiss, D.S.; Toth, I.; Goszleth, G.; Bartha, T.; Frenyo, L.V.; Horvath, T.L.; Zsarnovszky, A. Comparison of Individual and Combined Effects of Four Endocrine Disruptors on Estrogen Receptor Beta Transcription in Cerebellar Cell Culture: The Modulatory Role of Estradiol and Triiodo-Thyronine. Int. J. Environ. Res. Public Health 2016, 13, 619. https://doi.org/10.3390/ijerph13060619
Jocsak G, Kiss DS, Toth I, Goszleth G, Bartha T, Frenyo LV, Horvath TL, Zsarnovszky A. Comparison of Individual and Combined Effects of Four Endocrine Disruptors on Estrogen Receptor Beta Transcription in Cerebellar Cell Culture: The Modulatory Role of Estradiol and Triiodo-Thyronine. International Journal of Environmental Research and Public Health. 2016; 13(6):619. https://doi.org/10.3390/ijerph13060619
Chicago/Turabian StyleJocsak, Gergely, David Sandor Kiss, Istvan Toth, Greta Goszleth, Tibor Bartha, Laszlo V. Frenyo, Tamas L. Horvath, and Attila Zsarnovszky. 2016. "Comparison of Individual and Combined Effects of Four Endocrine Disruptors on Estrogen Receptor Beta Transcription in Cerebellar Cell Culture: The Modulatory Role of Estradiol and Triiodo-Thyronine" International Journal of Environmental Research and Public Health 13, no. 6: 619. https://doi.org/10.3390/ijerph13060619
APA StyleJocsak, G., Kiss, D. S., Toth, I., Goszleth, G., Bartha, T., Frenyo, L. V., Horvath, T. L., & Zsarnovszky, A. (2016). Comparison of Individual and Combined Effects of Four Endocrine Disruptors on Estrogen Receptor Beta Transcription in Cerebellar Cell Culture: The Modulatory Role of Estradiol and Triiodo-Thyronine. International Journal of Environmental Research and Public Health, 13(6), 619. https://doi.org/10.3390/ijerph13060619