Ultrasonic-Assisted Synthesis of N-Doped, Multicolor Carbon Dots toward Fluorescent Inks, Fluorescence Sensors, and Logic Gate Operations
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of M-CDs
2.3. Fluorescent Ink Evaluation
2.4. Ion Detection
2.5. Logic Gate Design
2.6. Material Characterization
3. Result and Discussion
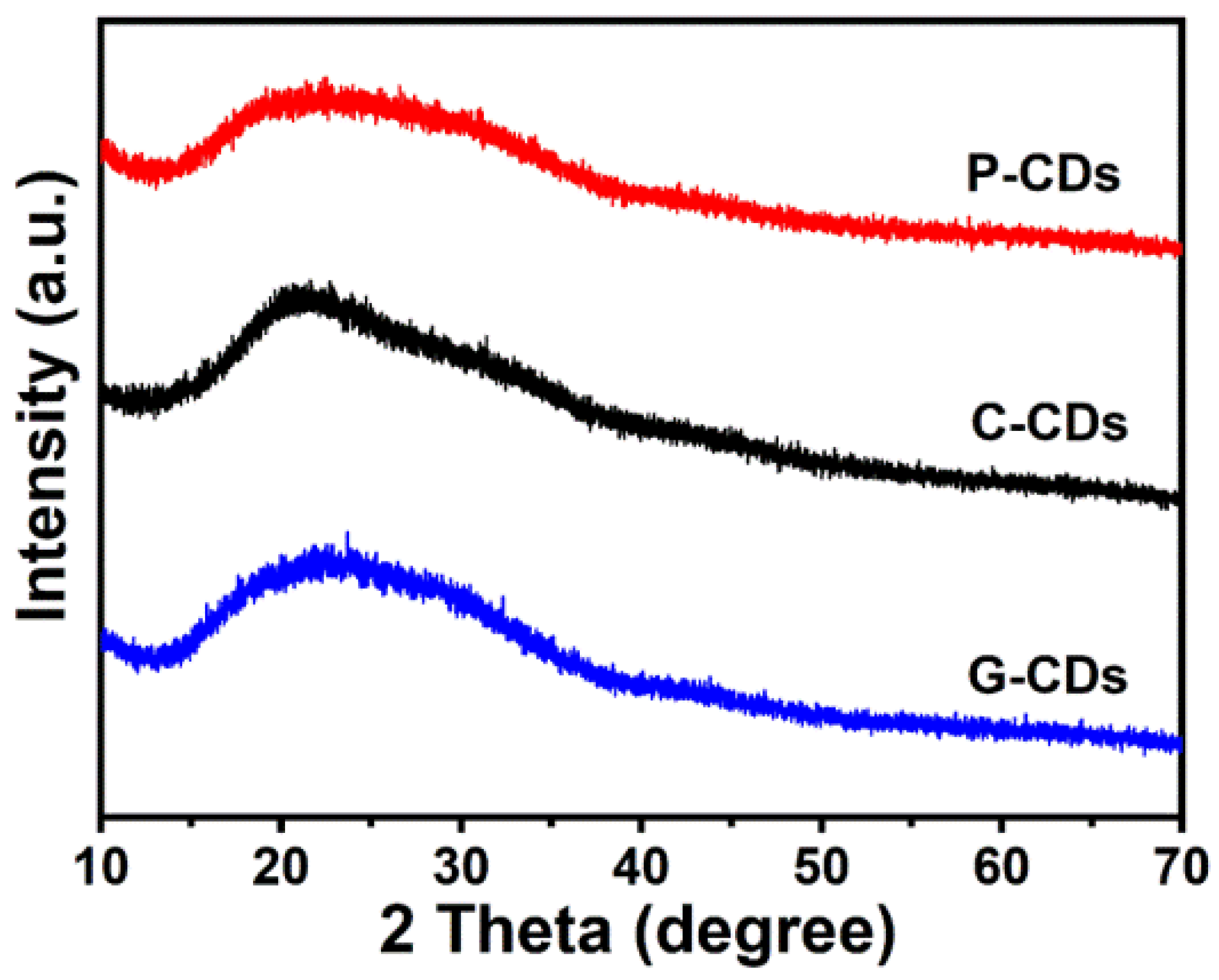
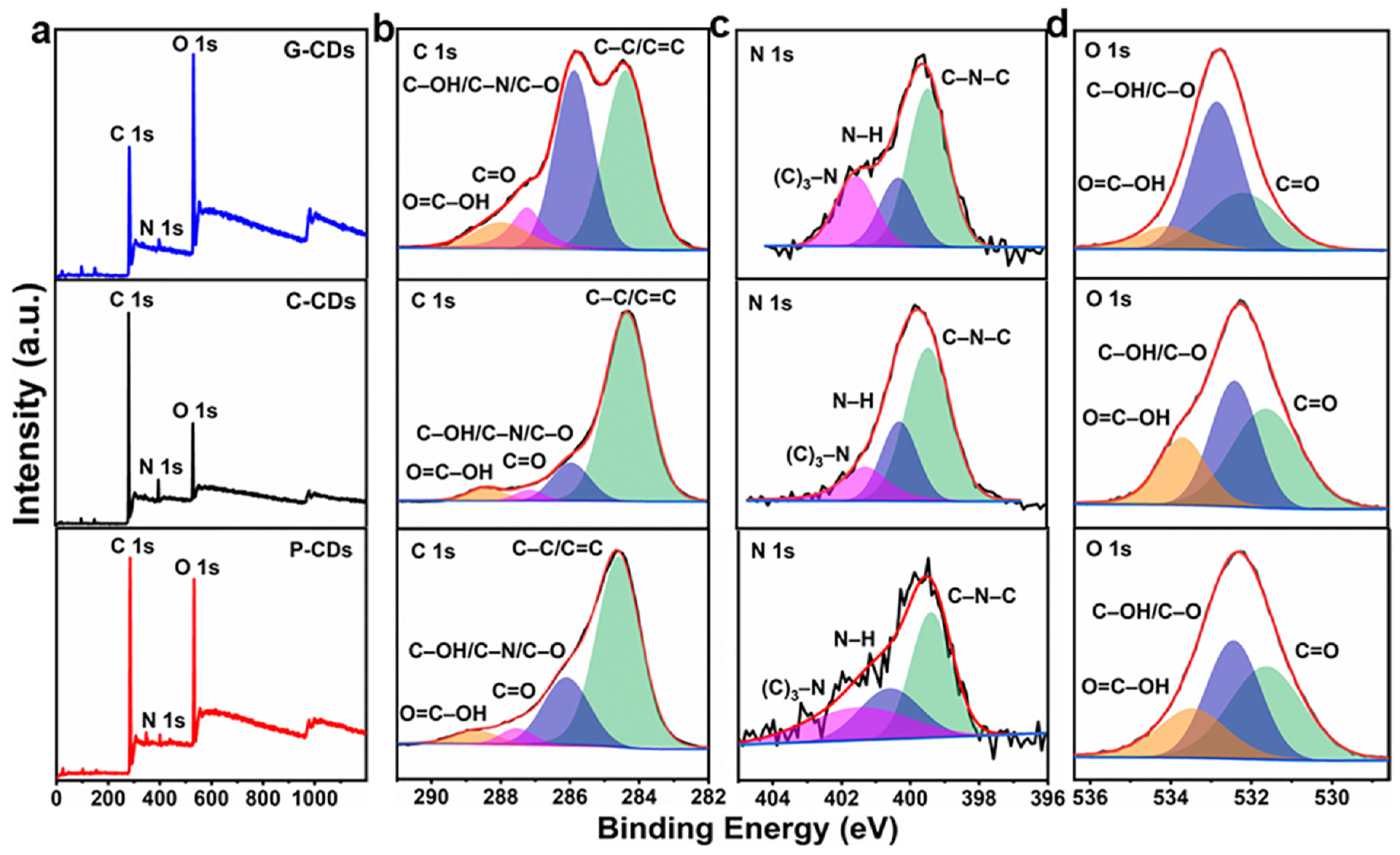
3.1. Physicochemical and Structural Characterizations
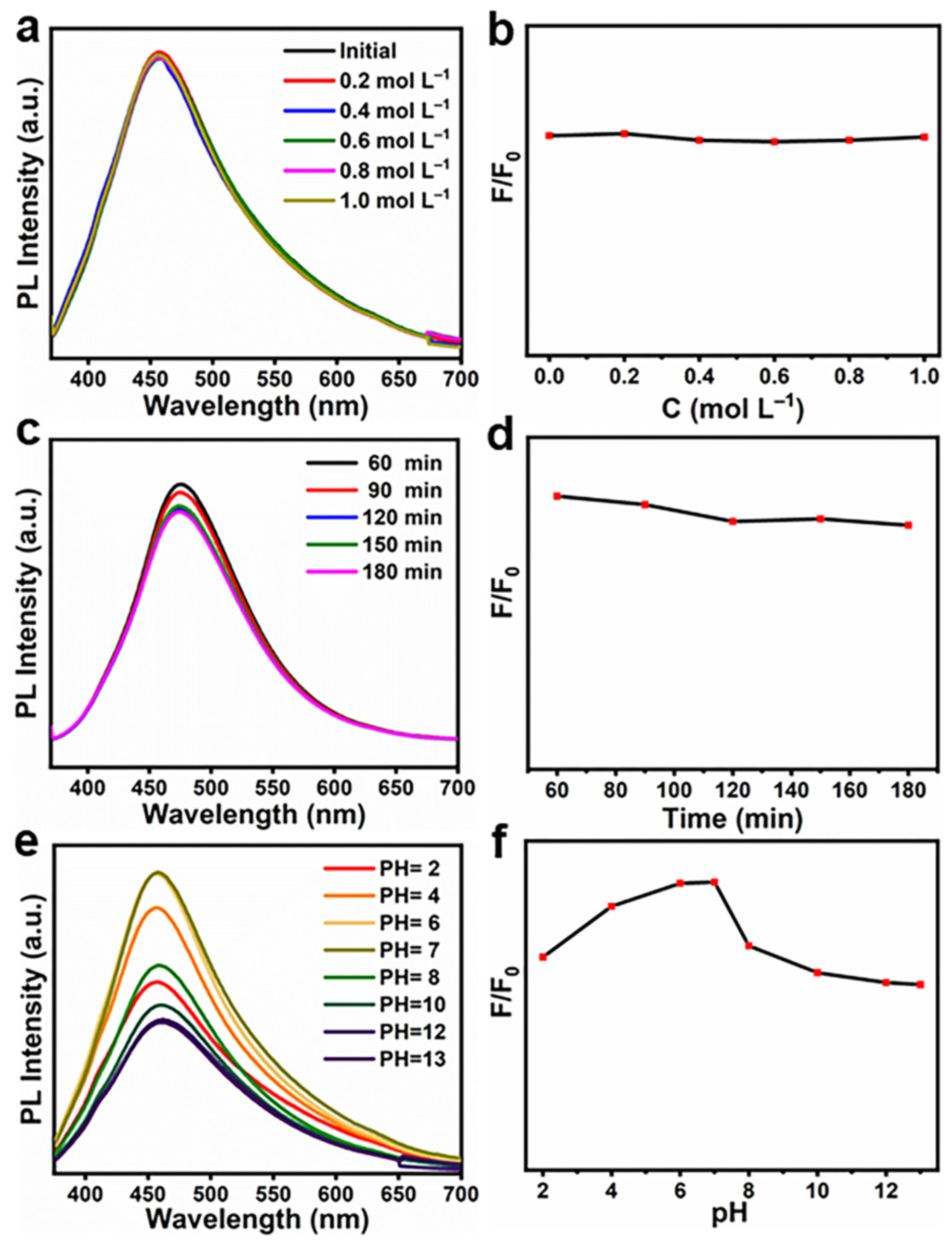
3.2. Optical Studies
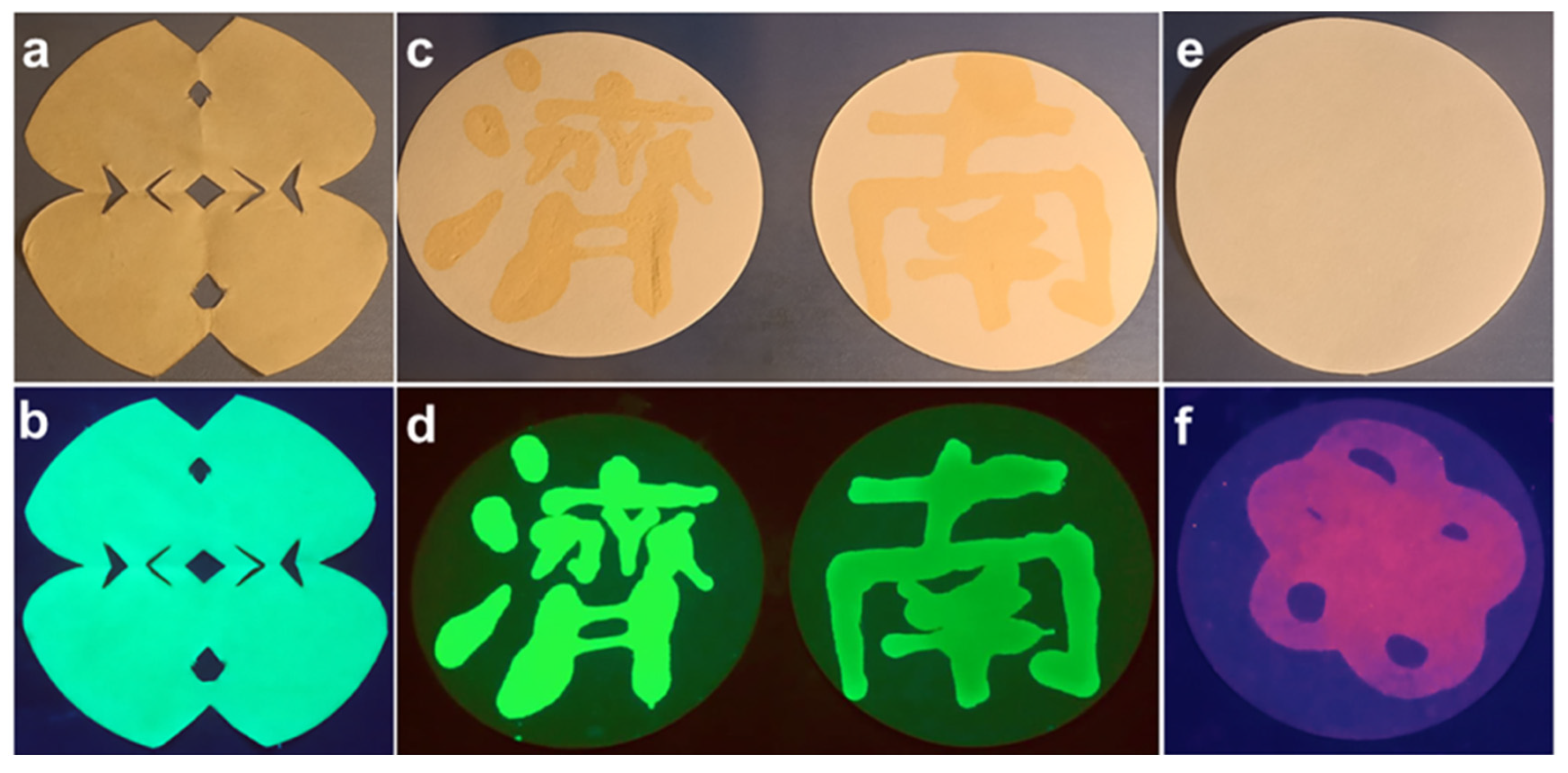
3.3. Fluorescent Ink Properties
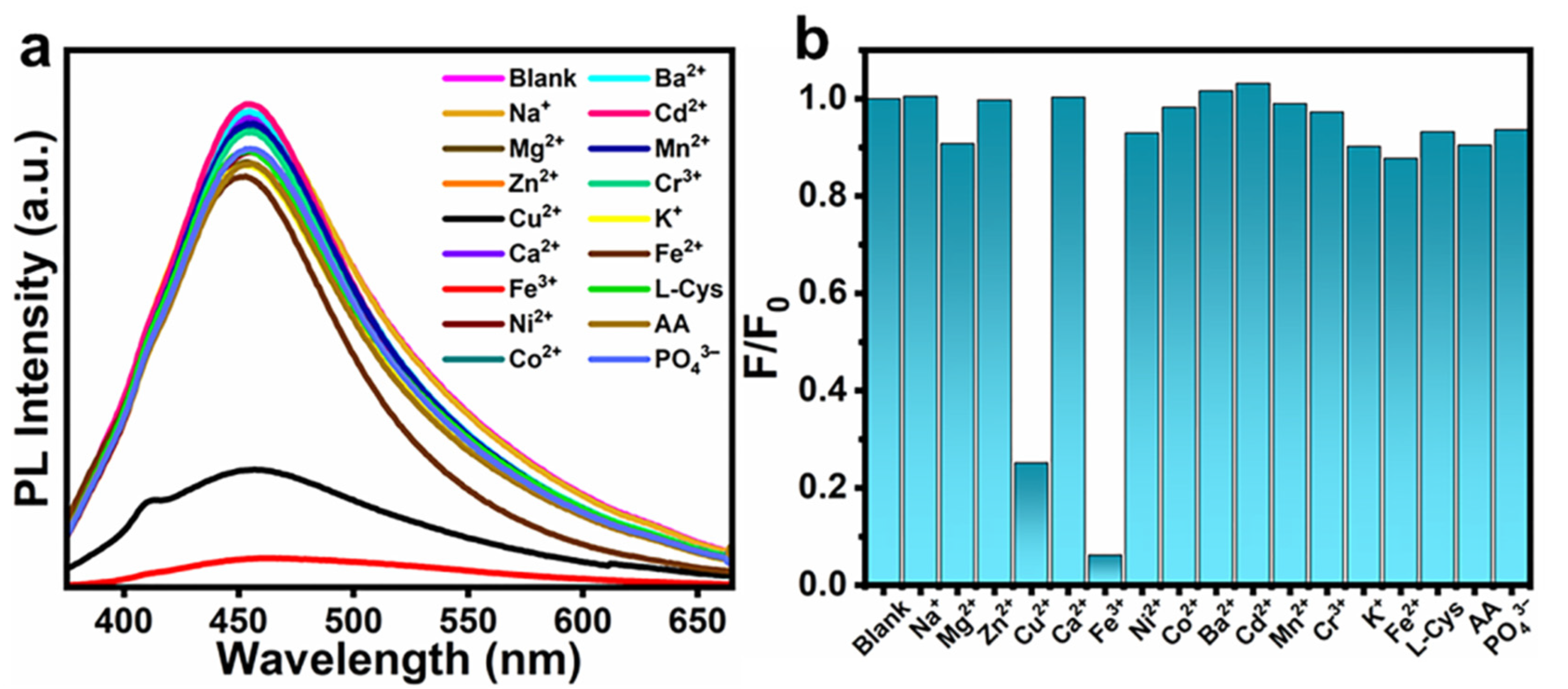
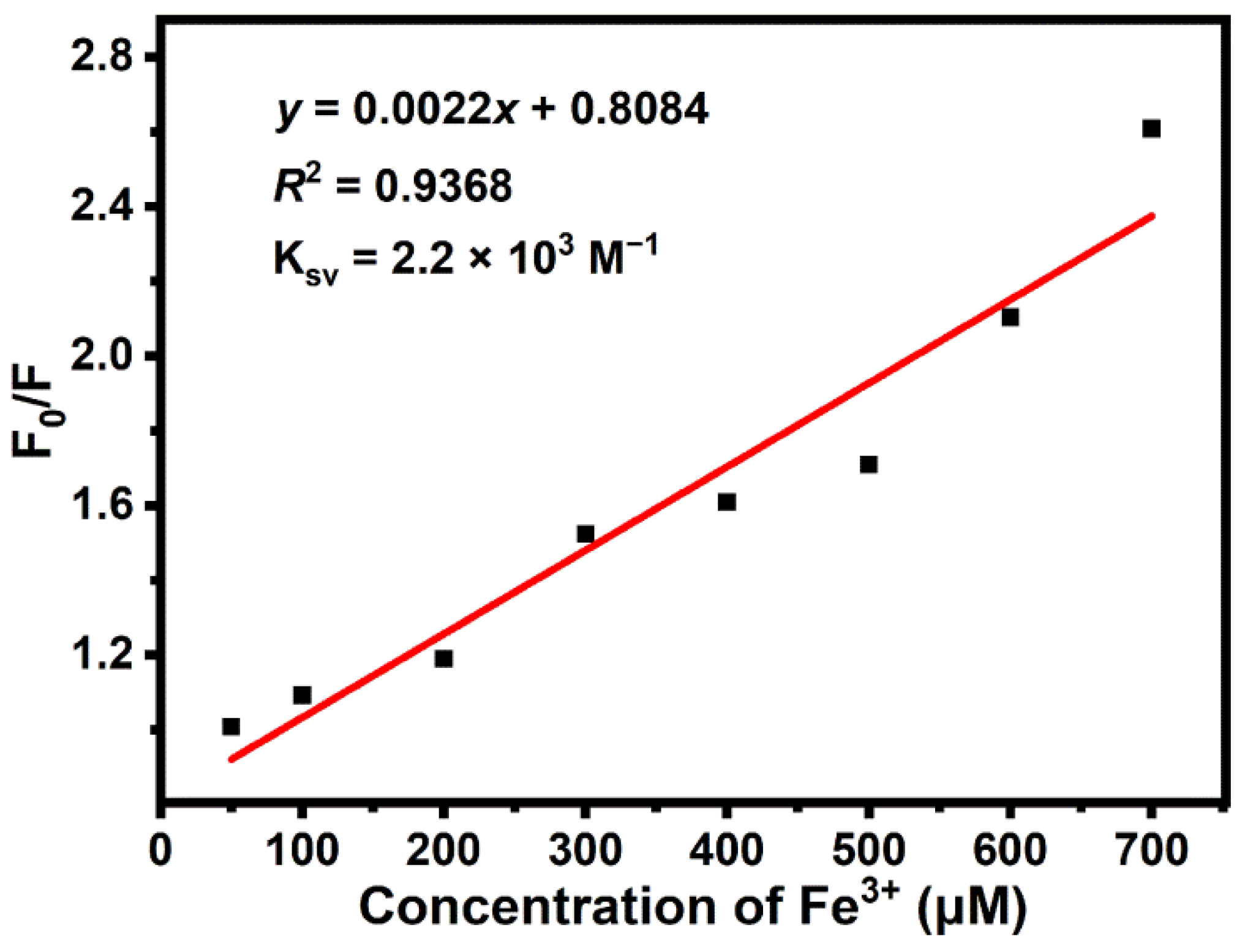
3.4. Sensing Applications
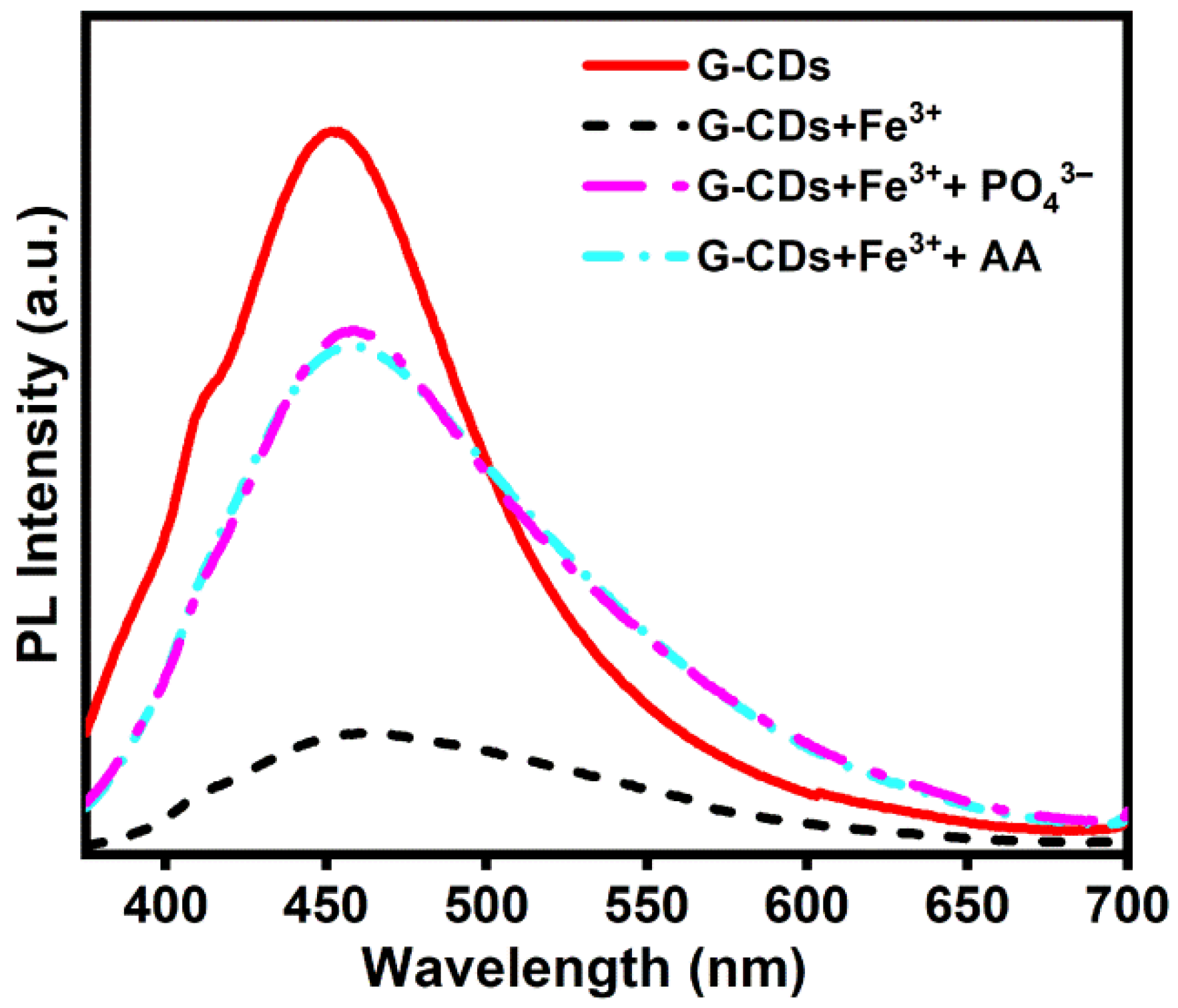
3.5. Quenching Mechanism
3.6. Logic Gate Operation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, J.; Yong, X.; Tang, Z.; Yang, B.; Lu, S. Theoretical understanding of structure–property relationships in luminescence of carbon dots. J. Phys. Chem. Lett. 2021, 12, 7671–7687. [Google Scholar] [CrossRef]
- Javed, N.; O’Carroll, D.M. Carbon dots and stability of their optical properties. Part. Part. Syst. Charact. 2021, 38, 2000271. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon dots: A new type of carbon-based nanomaterial with wide applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Li, L.; Wang, B.; Xiang, Y.; Zou, G.; Zhu, Y.; Hou, H.; Ji, X. Functionalized carbon dots for advanced batteries. Energy Storage Mater. 2021, 37, 8–39. [Google Scholar] [CrossRef]
- Li, X.C.; Zhao, S.J.; Li, B.L.; Yang, K.; Lan, M.H.; Zeng, L.T. Advances and perspectives in carbon dot-based fluorescent probes: Mechanism, and application. Coord. Chem. Rev. 2021, 431, 213686. [Google Scholar] [CrossRef]
- Tian, L.; Li, Z.; Wang, P.; Zhai, X.; Wang, X.; Li, T. Carbon quantum dots for advanced electrocatalysis. J. Energy Chem. 2021, 55, 279–294. [Google Scholar] [CrossRef]
- Wang, B.; Song, H.; Qu, X.; Chang, J.; Yang, B.; Lu, S. Carbon dots as a new class of nanomedicines: Opportunities and challenges. Coord. Chem. Rev. 2021, 442, 214010. [Google Scholar] [CrossRef]
- Kumar, V.B.; Sahu, A.K.; Mohsin, A.S.M.; Li, X.; Gedanken, A. Refractive-index tuning of highly fluorescent carbon dots. ACS Appl. Mater. Interfaces 2017, 9, 28930–28938. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Bakandritsos, A.; Kouloumpis, A.; Gournis, D.; Krysmann, M.; Giannelis, E.P.; Polakova, K.; Safarova, K.; Hola, K.; Zboril, R. Gd(III)-doped carbon dots as a dual fluorescent-MRI probe. J. Mater. Chem. 2012, 22, 23327–23330. [Google Scholar] [CrossRef]
- Sun, D.; Ban, R.; Zhang, P.-H.; Wu, G.-H.; Zhang, J.-R.; Zhu, J.-J. Hair fiber as a precursor for synthesizing of sulfur- and nitrogen-co-doped carbon dots with tunable luminescence properties. Carbon 2013, 64, 424–434. [Google Scholar] [CrossRef]
- Hou, X.; Hu, Y.; Wang, P.; Yang, L.; Al Awak, M.M.; Tang, Y.; Twara, F.K.; Qian, H.; Sun, Y.-P. Modified facile synthesis for quantitatively fluorescent carbon dots. Carbon 2017, 122, 389–394. [Google Scholar] [CrossRef]
- Kumar, V.B.; Sheinberger, J.; Porat, Z.; Shav-Tal, Y.; Gedanken, A. A hydrothermal reaction of an aqueous solution of BSA yields highly fluorescent N doped C-dots used for imaging of live mammalian cells. J. Mater. Chem. B 2016, 4, 2913–2920. [Google Scholar] [CrossRef]
- Nissan, I.; Kumar, V.B.; Porat, Z.E.; Makovec, D.; Shefi, O.; Gedanken, A. Sonochemically-fabricated Ga@C-dots@Ga nanoparticle-aided neural growth. J. Mater. Chem. B 2017, 5, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Wang, H.; Yang, A.; Wang, X.; Xu, J. Facile fabrication of highly fluorescent N-Doped carbon quantum dots using an ultrasonic-assisted hydrothermal method: Optical properties and cell Imaging. ACS Omega 2021, 6, 32904–32916. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, M.; Kim, H.Y.; Ding, B.; Park, S.-J. A facile ultrasonic-assisted fabrication of nitrogen-doped carbon dots/BiOBr up-conversion nanocomposites for visible light photocatalytic enhancements. Sci. Rep. 2017, 7, 45086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Ming, H.; Huang, H.; Liu, Y.; Kang, Z. One-step ultrasonic synthesis of fluorescent N-doped carbon dots from glucose and their visible-light sensitive photocatalytic ability. New J. Chem. 2012, 36, 861–864. [Google Scholar] [CrossRef]
- Kumar, V.B.; Perelshtein, I.; Lipovsky, A.; Porat, Z.e.; Gedanken, A. The sonochemical synthesis of Ga@C-dots particles. RSC Adv. 2015, 5, 25533–25540. [Google Scholar] [CrossRef]
- Kumar, V.B.; Kumar, R.; Gedanken, A.; Shefi, O. Fluorescent metal-doped carbon dots for neuronal manipulations. Ultrason. Sonochem. 2019, 52, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.B.; Porat, Z.e.; Gedanken, A. Facile one-step sonochemical synthesis of ultrafine and stable fluorescent C-dots. Ultrason. Sonochem. 2016, 28, 367–375. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Wang, L.; Luo, Y.; Zhai, J.; Sun, X. Preparation of photoluminescent carbon nitride dots from CCl4 and 1,2-ethylenediamine: A heat-treatment-based strategy. J. Mater. Chem. 2011, 21, 11726–11729. [Google Scholar] [CrossRef]
- Deng, X.; Wu, D. Highly sensitive photoluminescence energy transfer detection for 2,4,6-trinitrophenol using photoluminescent carbon nanodots. RSC Adv. 2014, 4, 42066–42070. [Google Scholar] [CrossRef]
- Jiao, Y.; Liu, Y.; Meng, Y.; Gao, Y.; Lu, W.; Liu, Y.; Gong, X.; Shuang, S.; Dong, C. Novel processing for color-tunable luminescence carbon dots and their advantages in biological systems. ACS Sustain. Chem. Eng. 2020, 8, 8585–8592. [Google Scholar] [CrossRef]
- Huo, F.; Liu, Y.; Zhu, M.; Gao, E.; Zhao, B.; Yang, X. Ultrabright full color carbon dots by fine-tuning crystal morphology controllable synthesis for multicolor bioimaging and sensing. ACS Appl. Mater. Interfaces 2019, 11, 27259–27268. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Sahu, S.K. Effect of solvent-derived highly luminescent multicolor carbon dots for white-light-emitting diodes and water detection. Langmuir 2020, 36, 5287–5295. [Google Scholar] [CrossRef]
- Zheng, Y.; Arkin, K.; Hao, J.; Zhang, S.; Guan, W.; Wang, L.; Guo, Y.; Shang, Q. Multicolor carbon dots prepared by single-factor control of graphitization and surface oxidation for high-quality white light-emitting diodes. Adv. Opt. Mater. 2021, 9, 2100688. [Google Scholar] [CrossRef]
- Ding, H.; Yu, S.-B.; Wei, J.-S.; Xiong, H.-M. Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism. ACS Nano 2016, 10, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Shen, H.; Liu, R.; Shao, J. Solvent effects on fluorescence properties of carbon dots: Implications for multicolor imaging. ACS Omega 2021, 6, 26499–26508. [Google Scholar] [CrossRef]
- Bae, H.J.; Bae, S.; Park, C.; Han, S.; Kim, J.; Kim, L.N.; Kim, K.; Song, S.-H.; Park, W.; Kwon, S. Biomimetic microfingerprints for anti-counterfeiting strategies. Adv. Mater. 2015, 27, 2083–2089. [Google Scholar] [CrossRef]
- Hou, X.; Ke, C.; Bruns, C.J.; McGonigal, P.R.; Pettman, R.B.; Stoddart, J.F. Tunable solid-state fluorescent materials for supramolecular encryption. Nat. Commun. 2015, 6, 6884. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Huang, Z.; Cheung, M.H.C.; Motto-Ros, V.; Chu, P.-C.; Wang, Y.; Zhong, H.; Yuen, R.; Leung, K.S.Y.; Lum, J.T.S.; et al. Elemental analysis of Chinese black inks on xuan paper by ArF laser-excited plume fluorescence. Anal. Chem. 2016, 88, 10971–10978. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, Z.; Wang, Z.; Zhang, Y.; Sun, X.; Hou, L.; Yuan, C. Foxtail millet-derived highly fluorescent multi-heteroatom doped carbon quantum dots towards fluorescent inks and smart nanosensors for selective ion detection. New J. Chem. 2018, 42, 7326–7331. [Google Scholar] [CrossRef]
- Khan, W.U.; Wang, D.; Zhang, W.; Tang, Z.; Ma, X.; Ding, X.; Du, S.; Wang, Y. High quantum yield green-emitting carbon dots for Fe(III) detection, biocompatible fluorescent ink and cellular imaging. Sci. Rep. 2017, 7, 14866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Song, A.; Feng, L.; Ruan, H.; Li, H.; Dong, S.; Hao, J. Tunable amphiphilicity and multifunctional applications of ionic-liquid-modified carbon quantum dots. ACS Appl. Mater. Interfaces 2015, 7, 6919–6925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Y.; Wang, Y.; Xiao, S.; Wang, H.; Wang, J.-H.; Feng, L. Rapid detection of Cr(VI) ions based on cobalt(II)-doped carbon dots. Biosens. Bioelectron. 2017, 87, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, Z.; Ehtesabi, H.; Rahmandoust, M.; Ahadian, M.M.; Hallaji, Z.; Eskandari, F.; Jokar, E. New insight into the concept of carbonization degree in synthesis of carbon dots to achieve facile smartphone based sensing platform. Sci. Rep. 2017, 7, 11013. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Wang, Y.; Liu, J.; Yang, Q.; Zhang, B.; Gao, Y.; Wang, Y.; Lu, G. Dual functional N- and S-co-doped carbon dots as the sensor for temperature and Fe3+ ions. Sens. Actuators B Chem. 2017, 242, 1272–1280. [Google Scholar] [CrossRef]
- Yang, X.-F.; Wang, L.; Xu, H.; Zhao, M. A fluorescein-based fluorogenic and chromogenic chemodosimeter for the sensitive detection of sulfide anion in aqueous solution. Anal. Chim. Acta 2009, 631, 91–95. [Google Scholar] [CrossRef]
- Simões, E.F.C.; da Silva, L.P.; da Silva, J.C.G.E.; Leitão, J.M.M. Hypochlorite fluorescence sensing by phenylboronic acid-alizarin adduct based carbon dots. Talanta 2020, 208, 120447. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Park, S.-J.; Zhang, Y.; Kim, T.; Chae, S.; Park, M.; Kim, H.-Y. One-step synthesis of robust nitrogen-doped carbon dots: Acid-evoked fluorescence enhancement and their application in Fe3+ detection. J. Mater. Chem. A 2015, 3, 17747–17754. [Google Scholar] [CrossRef]
- Du, F.; Gong, X.; Lu, W.; Liu, Y.; Gao, Y.; Shuang, S.; Xian, M.; Dong, C. Bright-green-emissive nitrogen-doped carbon dots as a nanoprobe for bifunctional sensing, its logic gate operation and cellular imaging. Talanta 2018, 179, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Zhou, Q.; Zhang, H.; Zhou, L.; Ma, J.; Yi, C. Logic gate design using multicolor fluorescent carbon nanodots for smartphone-based information extraction. ACS Appl. Nano Mater. 2021, 4, 8184–8191. [Google Scholar] [CrossRef]
- Dhenadhayalan, N.; Lin, K.-C. Chemically induced fluorescence switching of carbon-dots and its multiple logic gate implementation. Sci. Rep. 2015, 5, 10012. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.-S.; Zhao, Q.-C.; Kong, W.-L.; Wang, X.-F.; Chen, X.-M.; Zhang, J.; Wang, Y.-Q. Multi-level fluorescent logic gate based on polyamine coated carbon dots capable of responding to four stimuli. Chem. Eng. J. 2018, 337, 471–479. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, X.; Liu, S.; Li, Y.; Shi, Y.; Yan, J.; Hu, X. A fluorescence switch sensor used for D-Penicillamine sensing and logic gate based on the fluorescence recovery of carbon dots. Sens. Actuators B Chem. 2016, 236, 565–573. [Google Scholar] [CrossRef]
- Liu, Y.; Zhan, G.; Liu, Z.-W.; Bian, Z.-Q.; Huang, C.-H. Room-temperature phosphorescence from purely organic materials. Chin. Chem. Lett. 2016, 27, 1231–1240. [Google Scholar] [CrossRef]
- Gao, Y.; Jiao, Y.; Lu, W.; Liu, Y.; Han, H.; Gong, X.; Xian, M.; Shuang, S.; Dong, C. Carbon dots with red emission as a fluorescent and colorimeteric dual-readout probe for the detection of chromium(VI) and cysteine and its logic gate operation. J. Mater. Chem. B 2018, 6, 6099–6107. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, J.; Yang, Q.; Wei, S.; Yang, R. Cost-effective and facile fluorescent probes for label-free recognition of chlorpromazine hydrochloride and logic gate operation. J. Photochem. Photobiol. A 2019, 382, 111918. [Google Scholar] [CrossRef]
- Li, M.; Hu, C.; Yu, C.; Wang, S.; Zhang, P.; Qiu, J. Organic amine-grafted carbon quantum dots with tailored surface and enhanced photoluminescence properties. Carbon 2015, 91, 291–297. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, J.; Xu, B.; Jiang, Q.; Ming, S.; Yan, L.; Gao, Z.; Wang, X.; Zhu, C.; Meng, X. Thermally driven amorphous-crystalline phase transition of carbonized polymer dots for multicolor room-temperature phosphorescence. Adv. Opt. Mater. 2021, 9, 2100421. [Google Scholar] [CrossRef]
- Ai, L.; Yang, Y.; Wang, B.; Chang, J.; Tang, Z.; Yang, B.; Lu, S. Insights into photoluminescence mechanisms of carbon dots: Advances and perspectives. Sci. Bull. 2021, 66, 839–856. [Google Scholar] [CrossRef]
- Gharat, P.M.; Chethodil, J.M.; Srivastava, A.P.; Praseetha, P.K.; Pal, H.; Choudhury, S.D. An insight into the molecular and surface state photoluminescence of carbon dots revealed through solvent-induced modulations in their excitation wavelength dependent emission properties. Photochem. Photobiol. Sci. 2019, 18, 110–119. [Google Scholar] [CrossRef]
- Jia, J.; Lin, B.; Gao, Y.; Jiao, Y.; Li, L.; Dong, C.; Shuang, S. Highly luminescent N-doped carbon dots from black soya beans for free radical scavenging, Fe3+ sensing and cellular imaging. Spectrochim. Acta Part A 2019, 211, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Atchudan, R.; Edison, T.N.; Perumal, S.; Vinodh, R.; Sundramoorthy, A.K.; Babu, R.S.; Lee, Y.R. Leftover kiwi fruit peel-derived carbon dots as a highly selective fluorescent sensor for detection of ferric ion. Chemosensors 2021, 9, 166. [Google Scholar] [CrossRef]
- Ding, H.; Ji, Y.; Wei, J.-S.; Gao, Q.-Y.; Zhou, Z.-Y.; Xiong, H.-M. Facile synthesis of red-emitting carbon dots from pulp-free lemon juice for bioimaging. J. Mater. Chem. B 2017, 5, 5272–5277. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.; Yang, M.; Yan, Y.; Liu, E.; Ji, Z.; Fan, J. Facile synthesis of novel carbon quantum dots from biomass waste for highly sensitive detection of iron ions. Mater. Res. Bull. 2020, 124, 110730. [Google Scholar] [CrossRef]
- Huang, Q.; Li, Q.; Chen, Y.; Tong, L.; Lin, X.; Zhu, J.; Tong, Q. High quantum yield nitrogen-doped carbon dots: Green synthesis and application as “off-on” fluorescent sensors for the determination of Fe3+ and adenosine triphosphate in biological samples. Sens. Actuators B Chem. 2018, 276, 82–88. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, X.; Wei, C.; Qu, Y.; Xiao, X.; Cheng, H. One-step synthesis of red-emitting carbon dots via a solvothermal method and its application in the detection of methylene blue. RSC Adv. 2019, 9, 29533–29540. [Google Scholar] [CrossRef] [Green Version]
- Khan, Z.M.S.H.; Rahman, R.S.; Shumaila; Islam, S.; Zulfequar, M. Hydrothermal treatment of red lentils for the synthesis of fluorescent carbon quantum dots and its application for sensing Fe3+. Opt. Mater. 2019, 91, 386–395. [Google Scholar] [CrossRef]
- Singh, B.; Bahadur, R.; Rangara, M.; Gandhi, M.N.; Srivastava, R. Influence of surface states on the optical and cellular property of thermally stable red emissive graphitic carbon dots. ACS Appl. Bio Mater. 2021, 4, 4641–4651. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, L.; Li, Y.; Deng, R.; Zhang, H. Highly fluorescent nitrogen-doped carbon dots with excellent thermal and photo stability applied as invisible ink for loading important information and anti-counterfeiting. Nanoscale 2017, 9, 491–496. [Google Scholar] [CrossRef] [Green Version]
- Yuan, F.; Wang, Y.-K.; Sharma, G.; Dong, Y.; Zheng, X.; Li, P.; Johnston, A.; Bappi, G.; Fan, J.Z.; Kung, H.; et al. Bright high-colour-purity deep-blue carbon dot light-emitting diodes via efficient edge amination. Nat. Photonics 2020, 14, 171–176. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Park, M.; Park, S.-J.; Zhang, Y.; Akanda, M.R.; Park, B.-Y.; Kim, H.Y. Green synthesis of fluorescent carbon dots from carrot juice for in vitro cellular imaging. Carbon Lett. 2017, 21, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Picard, M.; Thakur, S.; Misra, M.; Mohanty, A.K. Miscanthus grass-derived carbon dots to selectively detect Fe3+ ions. RSC Adv. 2019, 9, 8628–8637. [Google Scholar] [CrossRef] [Green Version]
- Shangguan, J.; Huang, J.; He, D.; He, X.; Wang, K.; Ye, R.; Yang, X.; Qing, T.; Tang, J. Highly Fe3+-selective fluorescent nanoprobe based on ultrabright N/P codoped carbon dots and its application in biological samples. Anal. Chem. 2017, 89, 7477–7484. [Google Scholar] [CrossRef]
- Qi, H.; Teng, M.; Liu, M.; Liu, S.; Li, J.; Yu, H.; Teng, C.; Huang, Z.; Liu, H.; Shao, Q.; et al. Biomass-derived nitrogen-doped carbon quantum dots: Highly selective fluorescent probe for detecting Fe3+ ions and tetracyclines. J. Colloid Interface Sci. 2019, 539, 332–341. [Google Scholar] [CrossRef]
- Cheng, C.; Xing, M.; Wu, Q. A universal facile synthesis of nitrogen and sulfur co-doped carbon dots from cellulose-based biowaste for fluorescent detection of Fe3+ ions and intracellular bioimaging. Mater. Sci. Eng. C 2019, 99, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.; Afkhami, A.; Hosseinzadeh, L.; Madrakian, T. Green and cost-effective synthesis of carbon dots from date kernel and their application as a novel switchable fluorescence probe for sensitive assay of Zoledronic acid drug in human serum and cellular imaging. Anal. Chim. Acta 2018, 1030, 183–193. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, X.; Yu, H.; Hou, F.; Gao, S.; Zhong, L.; Xu, H.; Yu, Y.; Meng, J.; Wang, R. Continuous response fluorescence sensor for three small molecules based on nitrogen-doped carbon quantum dots from prunus lannesiana and their logic gate operation. Spectrochim. Acta Part A 2021, 257, 119774. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Cui, K.; Gong, T.; Zhang, J.; Zhai, Z.; Hou, L.; Zaman, F.u.; Yuan, C. Ultrasonic-Assisted Synthesis of N-Doped, Multicolor Carbon Dots toward Fluorescent Inks, Fluorescence Sensors, and Logic Gate Operations. Nanomaterials 2022, 12, 312. https://doi.org/10.3390/nano12030312
Xu J, Cui K, Gong T, Zhang J, Zhai Z, Hou L, Zaman Fu, Yuan C. Ultrasonic-Assisted Synthesis of N-Doped, Multicolor Carbon Dots toward Fluorescent Inks, Fluorescence Sensors, and Logic Gate Operations. Nanomaterials. 2022; 12(3):312. https://doi.org/10.3390/nano12030312
Chicago/Turabian StyleXu, Jiali, Kai Cui, Tianyu Gong, Jinyang Zhang, Zhirou Zhai, Linrui Hou, Fakhr uz Zaman, and Changzhou Yuan. 2022. "Ultrasonic-Assisted Synthesis of N-Doped, Multicolor Carbon Dots toward Fluorescent Inks, Fluorescence Sensors, and Logic Gate Operations" Nanomaterials 12, no. 3: 312. https://doi.org/10.3390/nano12030312
APA StyleXu, J., Cui, K., Gong, T., Zhang, J., Zhai, Z., Hou, L., Zaman, F. u., & Yuan, C. (2022). Ultrasonic-Assisted Synthesis of N-Doped, Multicolor Carbon Dots toward Fluorescent Inks, Fluorescence Sensors, and Logic Gate Operations. Nanomaterials, 12(3), 312. https://doi.org/10.3390/nano12030312








