Endocrine Disruptors and Prostate Cancer
Abstract
:1. Introduction
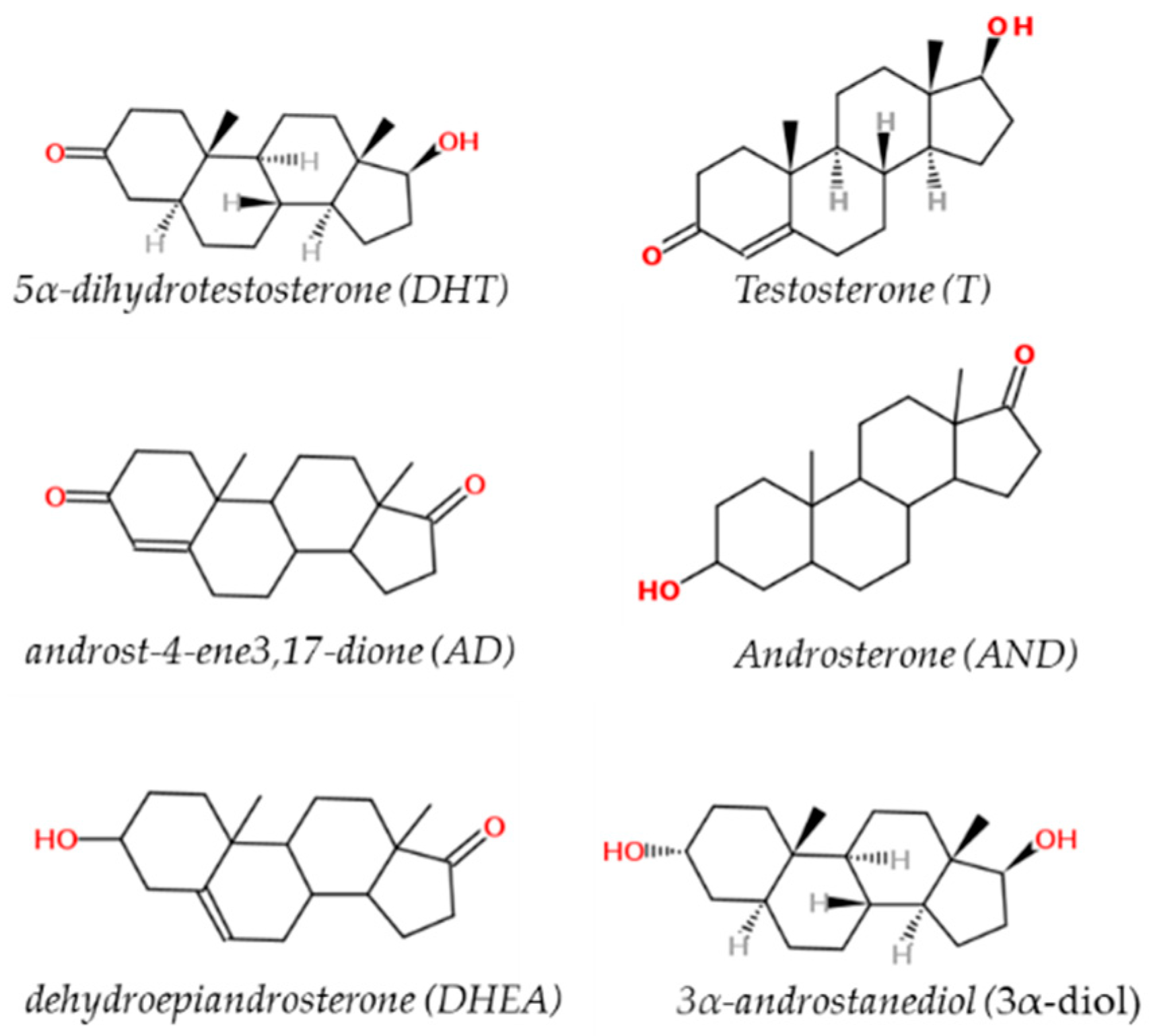
2. Sex Steroid Hormones (Androgens)
3. Mechanisms of Androgen Action in Prostate Gland: The Androgen Receptor
4. Androgen Receptor Mutations and Splice Variants in the Prostate Gland
5. Membrane Androgen Receptors (mARs) and WNT-Pathway in Prostate
6. Pesticides/Biocides and Plasticizers
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verze, P.; Cai, T.; Lorenzetti, S. The role of the prostate in male fertility, health and disease. Nat Rev Urol. 2016, 13, 379–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzetti, S.; Narciso, L.; Marcoccia, D.; Altieri, I. A novel in vitro toxicological approach to identify chemicals with a prostate-mediated effects on male reproduction. J. Biol. Res. 2011, 84, 36–41. [Google Scholar] [CrossRef]
- Duffy, M.J. Biomarkers for prostate cancer: Prostate-specific antigen and beyond. Clin. Chem. Lab. Med. 2020, 58, 326–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Testa, U.; Castelli, G.; Pelosi, E. Cellular and Molecular Mechanisms Underlying Prostate Cancer Development: Therapeutic Implications. Medicines 2019, 6, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.A. Treatment effects in prostate cancer. Mod. Pathol. 2018, 31, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Pippione, A.C.; Boschi, D.; Pors, K.; Oliaro-Basso, S.; Lolli, M.L. Androgen-AR axis in primary and metastatic prostate cancer: Chasing steroidogenic enzymes for therapeutic intervention. J. Cancer Metastasis Treat. 2017, 3, 328–361. [Google Scholar] [CrossRef]
- Lorenzin, F.; Demichelis, F. Evolution of the prostate cancer genome towards resistance. J. Transl. Genet. Genom. 2019, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Girling, J.S.; Whitakerm, H.C.; Millsm, I.G.; Nealm, D.E. Pathogenesis of prostate cancer and hormone refractory prostate cancer. Indian J. Urol. 2007, 23, 35–42. [Google Scholar] [PubMed]
- Tewari, R.; Chhabra, M.; Natu, S.M.; Goel, A.; Dalela, D.; Goel, M.M.; Rajender, S. Significant association of metabolic indices, lipid profile, and androgen levels with prostate cancer. Asian Pac. J. Cancer Prev. 2014, 15, 9841–9846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rise, M.A.; Malhotra, S.V.; Stoyanova, T. Second-Generation Antiandrogens: From Discovery to Standard of Care in Castration Resistant Prostate Cancer. Front Oncol. 2019, 28, 9–801. [Google Scholar]
- Wadosky, K.M.; Koochekpour, S. Molecular mechanisms underlying resistance to androgen deprivation therapy in prostate cancer. Oncotarget 2016, 7, 64447–64470. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P. Membrane Androgen Receptors Unrelated to Nuclear Steroid Receptors. Endocrinology 2019, 160, 772–781. [Google Scholar] [CrossRef]
- D’Arrigo, G.; Gianquinto, E.; Rossetti, G.; Cruciani, G.; Lorenzetti, S.; Spyrakis, F. Binding of Androgen- and Estrogen-Like Flavonoids to Their Cognate (Non)Nuclear Receptors: A Comparison by Computational Prediction. Molecules 2021, 26, 1613. [Google Scholar] [CrossRef]
- Prins, G.S. Endocrine disruptors and prostate cancer risk. Endocr. Relat. Cancer 2008, 15, 649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Zazzo, E.; Galasso, G.; Giovannelli, P.; Di Donato, M.; Castoria, G. Estrogens and Their Receptors in Prostate Cancer: Therapeutic Implications. Front. Oncol. 2018, 8, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Zazzo, E.; Giovannelli, P.; Di Donato, M.; Di Santi, A.; Cernera, G.; Rossi, V.; Abbondanza, C.; Moncharmont, B.; Sinisi, A.A.; Castoria, G.; et al. Prostate cancer stem cells: The role of androgen and estrogen receptors. Oncotarget 2016, 7, 193–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcoccia, D.; Pellegrini, M.; Fiocchetti, M.; Lorenzetti, S.; Marino, M. Food components and contaminants as (anti)androgenic molecules. Genes Nutr. 2017, 16, 12–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hur, J.; Giovannucci, E. Racial differences in prostate cancer: Does timing of puberty play a role? Br. J. Cancer 2020, 123, 349–354. [Google Scholar] [CrossRef]
- Ng, L.K. The Etiology of Prostate Cancer. In Prostate Cancer; Exon Publications: Brisbane, Australia, 2021; Chapter 2. [Google Scholar]
- Zoeller, R.T.; Brown, T.R.; Doan, L.L.; Gore, A.C.; Skakkebaek, N.E.; Soto, A.M.; Woodruff, T.J.; Vom Saal, F.S. Endocrine-Disrupting Chemicals and Public Health Protection: A Statement of Principles from The Endocrine Society. Endocrinology 2012, 153, 4097–4110. [Google Scholar] [CrossRef]
- De Falco, M.; Laforgia, V. Combined Effects of Different Endocrine-Disrupting Chemicals (EDCs) on Prostate Gland. Int. J. Environ. Res. Public Health 2021, 18, 9772. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Ontiveros, Y.; Paez, S.; Monteagudo, C.; Rivas, A. Endocrine Disruptors in Food: Impact on Gut Microbiota and Metabolic Diseases. Nutrients 2020, 4, 1158. [Google Scholar] [CrossRef] [PubMed]
- Bleak, T.C.; Calaf, G.M. Breast and prostate glands affected by environmental substances (Review). Oncol. Rep. 2021, 45, 20. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, M.; Barra, T.; Rosati, L.; Valiante, S.; Capaldo, A.; De Falco, M.; Laforgia, V. Adrenal gland response to endocrine disrupting chemicals in fishes, amphibians and reptiles: A comparative overview. Gen. Comp. Endocrinol. 2020, 297, 113550. [Google Scholar] [CrossRef]
- Silva, J.F.S.; Mattos, I.E.; Luz, L.L.; Carmo, C.N.; Aydos, R.D. Exposure to pesticides and prostate cancer: Systematic review of the literature. Rev. Environ. Health 2016, 1, 311–327. [Google Scholar] [CrossRef] [PubMed]
- De Badajoz, S.E.; Lage-Sanchez, J.M.; Sanchez-Gallegos, P. Endocrine disruptors and prostate cancer. Arch. Esp. Urol. 2017, 70, 331–335. [Google Scholar]
- Rohayem, J.; Zitzmann, M.; Laurentino, S.; Kliesch, S.; Nieschlag, E.; Holterhus, P.M.; Kulle, A. The role of gonadotropins in testicular and adrenal androgen biosynthesis pathways—Insights from males with congenital hypogonadotropic hypogonadism on hCG/rFSH and on testosterone replacement. Clin. Endocrinol. 2021, 94, 90–101. [Google Scholar] [CrossRef]
- Connan-Perrot, S.; Léger, T.; Lelandais, P.; Desdoits-Lethimonier, C.; David, A.; Fowler, P.A.; Mazaud-Guittot, S. Six Decades of Research on Human Fetal Gonadal Steroids. Int. J. Mol. Sci. 2021, 22, 6681. [Google Scholar] [CrossRef]
- Wei, R.; Zhong, S.; Qiao, L.; Guo, M.; Shao, M.; Wang, S.; Jiang, B.; Yang, Y.; Gu, C. Steroid 5α-Reductase Type I Induces Cell Viability and Migration via Nuclear Factor-κB/Vascular Endothelial Growth Factor Signaling Pathway in Colorectal Cancer. Front. Oncol. 2020, 10, 1501. [Google Scholar] [CrossRef] [PubMed]
- Opoku-Acheampong, A.B.; Henningson, J.N.; Beck, A.P.; Lindshield, B.L. 5α-reductase 1 mRNA levels are positively correlated with TRAMP mouse prostate most severe lesion scores. PLoS ONE 2017, 12, 175874. [Google Scholar] [CrossRef] [Green Version]
- McEwan, I.J.; Brinkmann, A.O. Androgen Physiology: Receptor and Metabolic Disorders. In Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2021. [Google Scholar]
- Round, P.; Das, S.; Wu, T.S.; Wähälä, K.; Van Petegem, F.; Hammond, G.L. Molecular interactions between sex hormone-binding globulin and nonsteroidal ligands that enhance androgen activity. J. Biol. Chem. 2020, 31, 1202–1211. [Google Scholar] [CrossRef]
- Goldman, A.L.; Bhasin, S.; Wu, F.C.W.; Krishna, M.; Matsumoto, A.M.; Jasuja, R. A Reappraisal of Testosterone’s Binding in Circulation: Physiological and Clinical Implications. Endocr. Rev. 2017, 38, 302–324. [Google Scholar] [CrossRef] [Green Version]
- Tunn, S.; Hochstrate, H.; Grunwald, I.; Fluchter, S.H.; Krieg, M. Effect of aging on kinetic parameters of 5 alpha-reductase in epithelium and stroma of normal and hyperplastic human prostate. J. Clin. Endocrinol. Metab. 1988, 67, 979–985. [Google Scholar] [CrossRef]
- Izumi, K.; Mizokami, A.; Lin, W.J.; Lai, K.P.; Chang, C. Androgen receptor roles in the development of benign prostate hyperplasia. Am. J. Pathol. 2013, 182, 1942–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estebanez-Perpina, E.; Bevan, C.L.; McEwan, I.J. Eighty Years of Targeting Androgen Receptor Activity in Prostate Cancer: The Fight Goes on. Cancers 2021, 13, 509. [Google Scholar] [CrossRef] [PubMed]
- van de Wijngaart, D.J.; Dubbink, H.J.; van Royen, M.E.; Trapman, J.; Jenster, G. Androgen receptor coregulators: Recruitment via the coactivator binding groove. Mol. Cell Endocrinol. 2012, 352, 57–69. [Google Scholar] [CrossRef]
- Wang, Q.; Li, W.; Liu, X.S.; Carroll, J.S.; Jänne, O.A.; Keeton, E.K.; Chinnaiyan, A.M.; Pienta, K.J.; Brown, M. A Hierarchical Network of Transcription Factors Governs Androgen Receptor-Dependent Prostate Cancer Growth. Mol. Cell. 2007, 27, 380–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Yi, P.; Hamilton, R.A.; Shen, H.; Chen, M.; Foulds, C.E.; Mancini, M.A.; Ludtke, S.J.; Wang, Z.; O’Malley, B.W. Structural Insights of Transcriptionally Active, Full-Length Androgen Receptor Coactivator Complexes. Mol. Cell. 2020, 79, 812–823.e4. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.M.; Christiaens, V.; Voulgaraki, D.; Waxman, J.; Claessens, F.; Bevan, C.L. Mechanisms of Androgen Receptor Sig-nalling Via Steroid Receptor Coactivator-1 in Prostate. Endocr. Relat. Cancer 2004, 11, 117–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangel, N.; Villegas, V.E.; Rondon-Lagos, M. Obesity and Androgen Receptor Signaling: Associations and Potential Crosstalk in Breast Cancer Cells. Cancers 2021, 13, 2218. [Google Scholar] [CrossRef] [PubMed]
- Zamagni, A.; Cortesi, M.; Zanoni, M.; Tesei, A. Non-nuclear AR Signaling in Prostate Cancer. Front. Chem. 2019, 7, 651. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fu, A.; Cao, S.; Li, J.; Xing, A.; Li, D.; Dong, Y.; Cardin, D.; Park, H.W.; Mauvais-Jarvis, F.; et al. Membrane-associated androgen receptor (AR) potentiates its transcriptional activities by activating heat shock protein 27 (HSP27). Cell Biol. 2018, 293, 12719–12729. [Google Scholar] [CrossRef] [Green Version]
- Bennett, N.C.; Hooper, J.D.; Johnson, D.W.; Gobe, G.C. Expression profiles and functional associations of endogenous androgen receptor and caveolin-1 in prostate cancer cell lines. Prostate 2014, 74, 478–487. [Google Scholar] [CrossRef]
- Morova, T.; McNeill, D.R.; Lallous, N.; Gonen, N.; Dalal, K.; Wilson, D.M.; Gursoy, A.; Keskin, O.; Lack, N.A. Androgen receptor-binding sites are highly mutated in prostate cancer. Nat. Commun. 2020, 11, 832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, K.; Nonomura, N. Role of Androgen Receptor in Prostate Cancer: A Review. World J. Mens. Health 2019, 37, 288–295. [Google Scholar] [CrossRef]
- Eisermann, K.; Wang, D.; Jing, Y.; Pascal, L.E.; Wang, Z. Androgen receptor gene mutation, rearrangement, polymorphism. Transl. Androl. Urol. 2013, 2, 137–147. [Google Scholar]
- Tan, M.E.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef] [Green Version]
- Student, S.; Hejmo, T.; Poterala-Hejmo, A.; Lesniak, A.; Buldak, R. Anti-androgen hormonal therapy for cancer and other diseases. Eur. J. Pharmacol. 2020, 866, 172783. [Google Scholar] [CrossRef] [PubMed]
- Heinlein, C.; Chang, C. Androgen Receptor in Prostate Cancer. Endocr. Rev. 2004, 25, 276–308. [Google Scholar] [CrossRef] [Green Version]
- Aurilio, G.; Cimadamore, A.; Mazzucchelli, R.; Lopez-Beltran, A.; Verri, E.; Scarpelli, M.; Massari, F.; Cheng, L.; Santoni, M.; Montironi, R. Androgen Receptor Signaling Pathway in Prostate Cancer: From Genetics to Clinical Applications. Cells 2020, 9, 2653. [Google Scholar] [CrossRef] [PubMed]
- Bungaro, M.; Buttigliero, C.; Tucci, M. Overcoming the mechanisms of primary and acquired resistance to new generation hormonal therapies in advanced prostate cancer: Focus on androgen receptor independent pathways. Cancer Drug Resist. 2020, 3, 726–741. [Google Scholar] [CrossRef]
- Paschalis, A.; Sharp, A.; Welti, J.C.; Neeb, A.; Raj, G.V.; Luo, J.; Plymate, S.R.; de Bono, J.S. Alternative splicing in prostate cancer. Nat. Rev. Clin. Oncol. 2018, 15, 663–675. [Google Scholar] [CrossRef]
- Wadosky, K.M.; Koochekpour, S. Androgen receptor splice variants and prostate cancer: From bench to bedside. Oncotarget 2017, 14, 18550–18576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murillo-Garzón, V.; Kypta, R. WNT signalling in prostate cancer. Nat. Rev. Urol. 2017, 14, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, A.; Merek, S.; Pardyak, L.; Brzoskwinia, M.; Bilinska, B.; Hejmej, A. Crosstalk between Androgen-ZIP9 Signaling and Notch Pathway in Rodent Sertoli Cells. Int. J. Mol. Sci. 2020, 21, 8275. [Google Scholar] [CrossRef]
- Zhang, L.; Barritt, G.J. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 2004, 64, 8365–8373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsavaler, L.; Shapero, M.H.; Morkowski, S.; Laus, R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 2001, 61, 3760–3769. [Google Scholar] [PubMed]
- Grolez, G.P.; Gordiendko, D.V.; Clarisse, M.; Hammadi, M.; Desruelles, E.; Fromont, G.; Prevarskaya, N.; Slomianny, C.; Gkika, D. TRPM8-androgen receptor association within lipid rafts promotes prostate cancer cell migration. Cell Death Disease 2019, 10, 652. [Google Scholar] [CrossRef] [Green Version]
- Asuthkar, S.; Velpula, K.K.; Elustondo, P.A.; Demirkhanyan, L.; Zakharian, E. TRPM8 channel as a novel molecular target in androgen-regulated prostate cancer cells. Oncotarget 2015, 6, 17221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henshall, S.M.; Afar, D.E.; Hiller, J.; Horvath, L.G.; Quinn, D.I.; Rasiah, K.K.; Gish, K.; Willhite, D.; Kench, J.G.; Gardiner-Garden, M.; et al. Survival analysis of genome-wide gene expression profiles of prostate cancers identifies new prognostic targets of disease relapse. Cancer Res. 2003, 63, 4196–4203. [Google Scholar]
- Zhang, L.; Barritt, G.J. TRPM8 in prostate cancer cells: A potential diagnostic and prognostic marker with a secretory function? Endocr. Relat. Cancer 2006, 13, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Sarveswaran, S.; Ghosh, J. OXER1, a G protein-coupled oxoeicosatetraenoid receptor, mediates the survival-promoting effects of arachidonate 5-lipoxygenase in prostate cancer cells. Cancer Lett. 2013, 336, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Dattilo, M.; Neuman, I.; Muñoz, M.; Maloberti, P.; Cornejo-Maciel, F. OxeR1 regulates angiotensin II and cAMP-stimulated steroid production in human H295R adrenocortical cells. Mol. Cell Endocrinol. 2015, 408, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Kalyvianaki, K.; Gebhart, V.; Peroulis, N.; Panagiotopoulou, C.; Kiagiadaki, F.; Pediaditakis, I.; Aivaliotis, M.; Moustou, E.; Tzardi, M.; Notas, G. Antagonizing effects of membrane-Acting androgens on the eicosanoid receptor OXER1 in prostate cancer. Sci. Rep. 2017, 7, 44418. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bai, Y.; He, Y.; Zhao, Y.; Chen, J.; Ma, L.; Pan, Y.; Hinten, M.; Zhang, J.; Karnes, R.J.; et al. PTEN loss promotes intra tumoral androgen synthesis and tumor microenvironment remodeling via activation of RUNX2 in castration-resistant prostate cancer. Clin. Cancer Res. 2018, 24, 834–846. [Google Scholar] [CrossRef] [Green Version]
- Pi, M.; Quarles, L.D. Multiligand Specificity and Wide Tissue Expression of GPRC6A Reveals New Endocrine Networks. Endocrinology 2012, 153, 2062–2069. [Google Scholar] [CrossRef] [Green Version]
- Clemmensen, C.; Smajilovic, S.; Wellendorph, P.; Bräuner-Osborne, H. The GPCR, class C, group 6, subtype A (GPRC6A) receptor: From cloning to physiological function. Br. J. Pharmacol. 2014, 171, 1129–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masi, M.; Racchi, M.; Travelli, C.; Corsini, E.; Buoso, E. Molecular Characterization of Membrane Steroid Receptors in Hormone-Sensitive Cancers. Cells 2021, 10, 2999. [Google Scholar] [CrossRef] [PubMed]
- Pi, M.; Nishimoto, S.K.; Quarles, L.D. GPRC6A: Jack of all metabolism (or master of none). Mol. Metab. 2017, 6, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Pi, M.; Nooh, M.M.; Bahout, S.W.; Quarles, L.D. Human GPRC6A Mediates Testosterone-Induced Mitogen-Activated Protein Kinases and mTORC1 Signaling in Prostate Cancer Cells. Mol. Pharmacol. 2019, 95, 563–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, N.; Chen, Y.; Zeng, Y.; Bao, M.; Long, X.; Guo, Y.; Tan, A.; Gao, Y.; Zhang, H.; Yang, X.; et al. rs2274911 polymorphism in GPRC6A associated with serum E2 and PSA in a Southern Chinese male population. Gene 2020, 763, 145067. [Google Scholar] [CrossRef]
- Bulldan, A.; Bartsch, J.W.; Konrad, L.; Scheiner-Bobis, G. ZIP9 but not the androgen receptor mediates testosterone-induced migratory activity of metastatic prostate cancer cells. Biochim. Biophys. Acta Bioenerg. 2018, 1865, 1857–1868. [Google Scholar] [CrossRef]
- Bland, T.; Wang, J.; Yin, L.; Pu, T.; Li, J.; Gao, J.; Lin, T.P.; Gao, A.C.; Wu, B.J. WLS-Wnt signaling promotes neuroendocrine prostate cancer. iScience 2021, 24, 101970. [Google Scholar] [CrossRef] [PubMed]
- Truica, C.I.; Byers, S.; Gelmann, E.P. Beta-catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res. 2000, 60, 4709–4713. [Google Scholar] [PubMed]
- Pawlowski, J.E.; Ertel, J.R.; Allen, M.P.; Xu, M.; Butler, C.; Wilson, E.M.; Wierman, M.E. Liganded androgen receptor interaction with beta-catenin: Nuclear co-localization and modulation of transcriptional activity in neuronal cells. J. Biol. Chem. 2002, 277, 20702–20710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Li, X.; Sharma, M.; Sasaki, C.Y.; Longo, D.L.; Lim, B.; Sun, Z. Linking beta-catenin to androgen-signaling pathway. J. Biol. Chem. 2002, 277, 11336–11344. [Google Scholar] [CrossRef] [Green Version]
- Song, L.N.; Herrell, R.; Byers, S.; Shah, S.; Wilson, E.M.; Gelmann, E.P. Beta-catenin binds to the activation function 2 region of the androgen receptor and modulates the effects of the N-terminal domain and TIF2 on ligand-dependent transcription. Mol. Cell Biol. 2003, 23, 1674–1687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masiello, D.; Chen, S.Y.; Xu, Y.; Verhoeven, M.C.; Choi, E.; Hollenberg, A.N.; Balk, S.P. Recruitment of beta-catenin by wildtype or mutant androgen receptors correlates with ligand stimulated growth of prostate cancer cells. Mol. Endocrinol. 2004, 18, 2388–2401. [Google Scholar] [CrossRef] [Green Version]
- Kypta, R.M.; Waxman, J. Wnt/β-catenin signalling in prostate cancer. Nat. Rev. Urol. 2012, 9, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Terry, S.; Yang, X.; Chen, M.-W.; Vacherot, F.; Buttyan, R. Multifaceted interaction between the androgen and Wnt signaling pathways and the implication for prostate cancer. J. Cell Biochem. 2006, 99, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kim, J.H.; Koh, S.S.; Stallcup, M.R. 2004. Synergistic effects of coactivators GRIP1 and beta-catenin on geneactivation: Cross-talk between androgen receptor and Wnt signaling pathways. J. Biol. Chem. 2004, 279, 4212–4220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, D.J.; Dedhar, S.; Wu, H.; Nelson, C.C. PTEN and GSK3b: Key regulators of progression to androgen-independent prostate cancer. Oncogene 2006, 25, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Doumpas, N.; Lampart, F.; Robinson, M.D.; Lentini, A.; Nestor, C.E.; Cantù, C.; Basler, K. TCF/LEF dependent and independent transcriptional regulation of Wnt/β-catenin target genes. EMBO J. 2019, 38, e98873. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, M.W.; Terry, S.; Vacherot, F.; Bemis, D.L.; Capodice, J.; Kitajewski, J.; de la Taille, A.; Benson, M.C.; Guo, Y.; et al. Complex regulation of humanandrogen receptor expression by Wnt signaling inprostate cancer cells. Oncogene 2006, 25, 3436–3444. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Logan, S.K. Revisiting the Role of Wnt/β-catenin Signaling in Prostate Cancer. Mol. Cell Endocrinol. 2018, 462, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Parent, M.E.; Desy, M.; Siemiatycki, J. Does exposure to agricultural chemicals increase the risk of prostate cancer among farmers? Mcgill J. Med. 2009, 12, 70–77. [Google Scholar] [CrossRef]
- Lemarchand, C.; Tual, S.; Boulanger, M. Prostate cancer risk among French farmers in the AGRICAN cohort. Scand. J. Work Environ. Health 2016, 42, 144–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bashir, M.N. Epidemiology of prostate cancer. Asian Pac. J. Cancer Prev. 2015, 16, 5137–5141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerro, C.C.; Koutros, S.; Andreotti, G. Cancer incidence in the agricultural health study after 20 years of follow-up. Cancer Causes Control. 2019, 30, 311–322. [Google Scholar] [CrossRef]
- Pardo, L.A.; Freeman, L.E.B.; Lerro, C.C.; Andreotti, G.; Hofmann, J.N.; Parks, C.G.; Sandler, D.P.; Lubin, J.H.; Blair, A.; Koutros, S. Pesticide exposure and risk of aggressive prostate cancer among private pesticide applicators. Environ. Health 2020, 19, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, J.R.; Fitsanakis, V.; Westerink, R.; Kanthasamy, A.G. Neurotoxicity of Pesticides. Acta Neuropathol. 2019, 138, 343–362. [Google Scholar] [CrossRef]
- Giuliani, A.; Zuccarini, M.; Cichelli, A.; Khan, H.; Reale, M. Critical Review on the Presence of Phthalates in Food and Evidence of Their Biological Impact. Int. J. Environ. Res. Public Health 2020, 17, 5655. [Google Scholar] [CrossRef]
- Legambiente. Available online: https://www.legambiente.it/wp-content/uploads/2020/12/STOP-PESTICIDI-2020.pdf (accessed on 1 December 2021).
- Prins, G.S.; Hu, W.Y.; Xie, L.; Shi, G.B.; Hu, D.P.; Birch, D.P.; Bosland, M.C. Evaluation of Bisphenol A (BPA) Exposures on Prostate Stem Cell Homeostasis and Prostate Cancer Risk in the NCTR-Sprague-Dawley Rat: An NIEHS/FDA CLARITY-BPA Consortium Study. Environ. Health Perspect. 2018, 126, 117001. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.; Li, Y.; Liu, Y.; Tao, L. Exposure to Dibutyl Phthalate and Reproductive-Related Outcomes in Animal Models: Evidence From Rodents Study. Front. Physiol. 2021, 12, 684532. [Google Scholar] [CrossRef] [PubMed]
- Minotoya, M.; Kishi, R. A reviw of recent studies of bisphenol A and phthalate exposures and child neurodevelopment. Int. J. Environ. Res. Public Health 2021, 18, 3585. [Google Scholar] [CrossRef] [PubMed]
- Kelce, W.R.; Stone, C.R.; Laws, S.C.; Gray, L.E.; Companion, J.A.; Wilson, E.M. Persistent DDT metabolite p,p’-DDE is a potent androgen receptor antagonist. Nature 1995, 375, 581–585. [Google Scholar] [CrossRef]
- Gray, L.E.; Ostby, J.; Monosson, E.; Kelce, W.R. Environmental antiandrogens: Low doses of the fungicide vinclozolin alter sexual differentiation of the male rat. Toxicol. Ind. Health 1999, 15, 48–64. [Google Scholar] [CrossRef]
- Cowin, P.A.; Gold, E.; Aleksova, J.; O’Bryan, M.K.; Foster, P.M.; Scott, H.S.; Risbridger, G.P. Vinclozolin exposure in utero induces postpubertal prostatitis and reduces sperm production via a reversible hormone-regulated mechanism. Endocrinology 2010, 151, 783–792. [Google Scholar] [CrossRef] [Green Version]
- Klukovich, R.; Nilsson, E.; Sadler-Riggleman, I.; Beck, D.; Xie, Y.; Yan, W.; Skinner, M.K. Environmental Toxicant Induced Epigenetic Transgenerational Inheritance of Prostate Pathology and Stromal-Epithelial Cell Epigenome and Transcriptome Alterations: Ancestral Origins of Prostate Disease. Sci. Rep. 2019, 9, 2209. [Google Scholar]
- Kelce, W.R.; Monosson, E.; Gamcsik, M.P.; Laws, S.C.; Gray, L.E. Environmental hormone disruptors: Evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxicol. Appl. Pharmacol. 1994, 126, 275–285. [Google Scholar] [CrossRef]
- Wong, C.; Kelce, W.R.; Sar, M.; Wilson, E.M. Androgen receptor antagonist versus agonist activities of the fungicide vinclozolin relative to hydroxyflutamide. J. Biol. Chem. 1995, 270, 19998–20003. [Google Scholar] [CrossRef] [Green Version]
- Kelce, W.R.; Wilson, E.M. Environmental antiandrogens: Developmental effects, molecular mechanisms, and clinical implications. J. Mol. Med. 1997, 75, 198–207. [Google Scholar] [CrossRef]
- Molina-Molina, J.M.; Hillenweck, A.; Jouanin, I.; Zalko, D.; Cravedi, J.P.; Fernández, M.F. Steroid receptor profiling of vinclozolin and its primary metabolites. Toxicol. Appl. Pharmacol. 2006, 216, 44–54. [Google Scholar] [CrossRef]
- Robitaille, C.N.; Rivest, P.; Sanderson, J.T. Antiandrogenic mechanisms of pesticides in human LNCaP prostate and H295R adrenocortical carcinoma cells. Toxicol. Sci. 2015, 143, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, E.; King, S.E.; McBirney, M.; Kubsad, D.; Pappalardo, M.; Beck, D.; Sadler-Riggleman, I.; Skinner, M.K. Vinclozolin induced epigenetic transgenerational inheritance of pathologies and sperm epimutation biomarkers for specific diseases. PLoS ONE 2018, 29, e0202662. [Google Scholar] [CrossRef]
- Bhatt, P.; Huang, Y.; Zhan, H.; Chen, S. Insight into Microbial Applications for the Biodegradation of Pyrethroid Insecticides. Front. Microbiol. 2019, 10, 1778. [Google Scholar] [CrossRef]
- Alaa-Eldin, E.A.; El-Shafei, D.A.; Abouhashem, N.S. Individual and combined effect of chlorpyrifos and cypermethrin on reproductive system of adult male albino rats. Environ. Sci. Pollut. Res. 2017, 24, 1532–1543. [Google Scholar] [CrossRef]
- Ding, Z.; Shen, J.Y.; Hong, J.W.; Zhang, R.; Li, Z.; Wang, Q.; Zhang, J.P.; Zhiang, M.R.; Xu, L.C. Inhibitory Effects of Cypermethrin on Interactions of the Androgen Receptor with Coactivators ARA70 and ARA55. Biomed. Environ. Sci. 2020, 20, 158–164. [Google Scholar]
- Wang, Q.; Zhou, J.L.; Wang, H.; Lu, Q.; Ding, Z.; Zhou, X.L.; Ge, X.; Shi, Q.M.; Pan, C.; Zhang, J.P.; et al. Inhibition effect of cypermethrin mediated by co-regulators SRC-1 and SMRT in interleukin-6-induced androgen receptor activation. Chemosphere 2016, 158, 24–29. [Google Scholar] [CrossRef]
- Hong, D.; Min, J.Y.; Min, K.B. Association between pyrethroids and prostate endpo-ints; stratified according to renal function. Environ. Int. Aug. 2021, 153, 106489. [Google Scholar] [CrossRef]
- Daisley, B.; Trinder, M.; Mcdowell, T.; Welle, H.; Dube, J.; Ali, S. Neonicotinoid-induced pathogen susceptibility is mitigated by Lactobacillus plantarum immune stimulation in a Drosophila melanogaster model. Sci. Rep. 2017, 7, 2703. [Google Scholar] [CrossRef]
- Mikolić, A.; Karačonji, I. Imidacloprid as reproductive toxicant and endocrine disruptor: Investigations in laboratory animals. Arh. Hig. Rada. Toksikol. 2018, 69, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Chi-Hsuan, C.; David, M.; Bernardo, L.; Quan, Z.; Alex, L. Characterization of daily dietary intake and the health risk of neonicotinoid insecticides for the U.S. population. J. Agric. Food Chem. 2018, 66, 10097–10105. [Google Scholar]
- Zhang, Q.; Lua, Z.; Chang, C.; Yu, C.; Wang, X.; Lu, C. Dietary risk of neonicotinoid insecticides through fruit and vegetable consumption in school-age children. Environ. Int. 2019, 126, 672–681. [Google Scholar] [CrossRef]
- Arfat, Y.; Mahmood, N.; Tahir, M.; Rashid, M.; Anjum, S.; Zhao, F. Effect of imidacloprid on hepatotoxicity and nephrotoxicity in male albino mice. Toxicol. Rep. 2014, 1, 554–561. [Google Scholar] [CrossRef] [Green Version]
- Lonare, M.; Kumar, M.; Raut, S.; Badgujar, P.; Doltade, S.; Telang, A. Evaluation of imidacloprid-induced neurotoxicity in male rats: A protective effect of curcumin. Neurochem. Int. 2014, 78, 122–129. [Google Scholar] [CrossRef]
- Abdel-Halim, K.Y.; Osman, S.R. Cytotoxicity and Oxidative Stress Responses of Imidacloprid and Glyphosate in Human Prostate Epithelial WPM-Y.1 Cell Line. J. Toxicol. 2020, 8, 4364650. [Google Scholar] [CrossRef]
- Hafez, E.M.; Issa, S.Y.; Al-Mazroua, M.K.; Ibrahim, K.T.; Rahman, S.M. The neonicotinoid insecticide imidacloprid: A male reproductive system toxicity inducer-human and experimental study. Toxicol. Open Access. 2016, 2, 109. [Google Scholar] [CrossRef] [Green Version]
- Saber, T.M.; Arisha, A.H.; Abo-Elmaaty, A.; Elsayed, A.; Metwally, M.M.M.; Aber, T.; Mansour, M.F. Thymol alleviates imidacloprid-induced testicular toxicity by modulating oxidative stress and expression of steroidogenesis and apoptosis-related genes in adult male rats. Ecotoxicol. Environ. Saf. 2021, 221, 112435. [Google Scholar] [CrossRef]
- Tetsatsi, A.C.; Nkeng-Effouet, P.A.; Alumeti, D.M.; Romeo, G.; Bonsou, F.; Kamanyi, A.; Watcho, P. Colibri® insecticide induces male reproductive toxicity: Alleviating effects of Lannea acida (Anacardiaceae) in rats. Basic Clin. Androl. 2019, 29, 16. [Google Scholar] [CrossRef]
- Yuan, X.; Shen, J.; Zhang, X.; Tu, W.; Fu, Z.; Jin, Y. Imidacloprid disrupts the endocrine system by interacting with androgen receptor in male mice. Sci. Total Environ. 2020, 15, 708–135163. [Google Scholar] [CrossRef]
- Natia, E.A.; Kada, A.S.; Manfo, F.P.; Tangu, N.N.; Mbifung, M.; Mbouobda, D.H.; Kenfack, A. Parastar insecticide induced changes in reproductive parameters and testicular oxidative stress biomarkers in Wistar male rats. Toxicol. Ind. Health 2018, 34, 499–506. [Google Scholar] [CrossRef]
- Maffini, M.V.; Geueke, B.; Groh, K.; Almroth, C.B.; Muncke, J. Role of epidemiology in risk assessment: A case study of five ortho-phthalates. Environ. Health 2021, 20, 114. [Google Scholar] [CrossRef]
- Scarano, W.R.; Bedrat, A.; Alonso-Costa, L.G.; Aquino, A.M.; Fantinatti, B.; Justulin, L.A.; Barbisan, L.F.; Freire, P.P.; Flaws, J.A.; Bernardo, L. Exposure to an environmentally relevant phthalate mixture during prostate development induces microRNA upregulation and transcriptome modulation in rats. Toxicol. Sci. 2019, 171, 84–97. [Google Scholar] [CrossRef]
- De Mello, T.S.; da Silveira, L.T.R.; Rinaldi, J.C.; Scarano, W.R.; Domeniconi, R.F. Alterations in prostate morphogenesis in male rat offspring after maternal exposure to Di-n-butyl-phthalate (DBP). Reprod. Toxicol. 2017, 69, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Wolf, C.; Lambright, C.; Mann, P.; Price, M.; Cooper, R.L.; Ostby, J.; Gray, L.E. Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozolinate, p,p’-DDE, and ketoconazole) and toxic substances (dibutyl- and diethylhexyl phthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicol. Ind. Health 1999, 15, 94–118. [Google Scholar]
- Roy, P.; Salminen, H.; Koskimies, P.; Simola, J.; Smeds, A.; Saukko, P.; Huhtaniemi, I.T. Screening of some anti-androgenic endocrine disruptors using a recombinant cell-based in vitro bioassay. J. Steroid Biochem. Mol. Biol. 2004, 88, 157–166. [Google Scholar] [CrossRef]
- Gray, L.E.; Ostby, J.; Furr, J.; Price, M.; Veeramachaneni, D.N.; Parks, L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol. Sci. 2000, 58, 350–365. [Google Scholar] [CrossRef] [Green Version]
- Parks, L.G.; Ostby, J.S.; Lambright, C.R.; Abbott, B.D.; Klinefelter, G.R.; Barlow, N.J.; Gray, L.E. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol. Sci. 2000, 58, 339–349. [Google Scholar] [CrossRef]
- Casati, L.; Sendra, R.; Colciago, A.; Negri-Cesi, P.; Berdasco, M.; Esteller, M.; Celotti, F. Polychlorinated biphenyls affect histone modification pattern in early development of rats: A role for androgen receptor-dependent modulation? Epigenomics 2012, 4, 101–112. [Google Scholar] [CrossRef]
- Basak, S.; Das, M.K.; Duttaroy, A.K. Plastics derived endocrine-disrupting compounds and their effects on early development. Birth Defects Res. 2020, 112, 1308–1325. [Google Scholar] [CrossRef]
- Beg, M.A.; Sheikh, I.A. Endocrine Disruption: Structural Interactions of Androgen Receptor against Di(2-ethylhexyl) Phthalate and Its Metabolites. Toxics 2020, 8, 115. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, J.; Zhang, X.; Zhou, X.; Wang, S.; Wang, Z. Pharmacophore based in si-lico study with laboratory verification—environmental explanation of prostate cancer re-currence. Environ. Sci. Pollut. Res. 2021, 28, 61581–61591. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, C.; Ma, X.; Wu, R.; Zhu, W.; Li, X.; Liang, Z.; Deng, F.; Wu, J.; Geng, S.; et al. Phthalates promote prostate cancer cell proliferation through activation of ERK5 and p38. Environ. Toxicol. Pharmacol. 2018, 63, 29–33. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Forte, M.; Valiante, S.; Laforgia, V.; De Falco, M. Interference of dibutylphthalate on human prostate cell viability. Ecotoxicol. Environ. Saf. 2018, 147, 565–573. [Google Scholar] [CrossRef]
- Prins, G.S.; Korach, K.S. The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids 2008, 73, 233–244. [Google Scholar] [CrossRef] [Green Version]
- Liquori, F.; Moreno-Marrodan, C.; Barbaro, P. Biomass-derived chemical substitutes for bisphenol A: Recent advancements in catalytic synthesis. Chem. Soc. Rev. 2020, 49, 6329–6363. [Google Scholar] [CrossRef]
- Bao, W.; Liu, B.; Rong, S. Association between Bisphenol A Exposure and Risk of All-Cause and Cause-Specific Mortality in US Adults. JAMA Netw Open 2020, 3, e2011620. [Google Scholar] [CrossRef]
- Geens, T.; Aerts, D.; Berthot, C.; Bourguignon, J.P.; Goeyens, L.; Lecomte, P. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 2012, 50, 3725–3740. [Google Scholar] [CrossRef]
- Olusayo, E.; Rahman, M.S.; Pang, M.G. Bisphenols Threaten Male Reproductive Health via Testicular Cells. Front. Endocrinol. 2020, 11, 624. [Google Scholar]
- Wang, H.; Ding, Z.; Shi, Q.M.; Ge, X.; Wang, H.X.; Li, M.X.; Chen, G.; Wang, Q.; Ju, Q.; Zhang, J.P.; et al. Anti-androgenic mechanisms of Bisphenol A involve androgen receptor signaling pathway. Toxicology 2017, 387, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Lacouture, A.; Lafront, C.; Peillex, C.; Pelletier, M.; Audet-Walsh, E. Impacts of endocrine-disrupting chemicals on prostate function and cancer. Environ. Res. 2022, 204, 112085. [Google Scholar] [CrossRef] [PubMed]
- Acconcia, F.; Pallottini, V.; Marino, M. Molecular Mechanisms of Action of BPA. Dose Response 2015, 13, 1559325815610582. [Google Scholar] [CrossRef] [Green Version]
- Renaud, L.; Huff, M.; da Silveira, W.A.; Angert, M.; Haas, M.; Hardiman, G. Genome-Wide Analysis of Low Dose Bisphenol-A (BPA) Exposure in Human Prostate Cells. Curr. Genom. 2019, 20, 260–274. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corti, M.; Lorenzetti, S.; Ubaldi, A.; Zilli, R.; Marcoccia, D. Endocrine Disruptors and Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 1216. https://doi.org/10.3390/ijms23031216
Corti M, Lorenzetti S, Ubaldi A, Zilli R, Marcoccia D. Endocrine Disruptors and Prostate Cancer. International Journal of Molecular Sciences. 2022; 23(3):1216. https://doi.org/10.3390/ijms23031216
Chicago/Turabian StyleCorti, Margherita, Stefano Lorenzetti, Alessandro Ubaldi, Romano Zilli, and Daniele Marcoccia. 2022. "Endocrine Disruptors and Prostate Cancer" International Journal of Molecular Sciences 23, no. 3: 1216. https://doi.org/10.3390/ijms23031216
APA StyleCorti, M., Lorenzetti, S., Ubaldi, A., Zilli, R., & Marcoccia, D. (2022). Endocrine Disruptors and Prostate Cancer. International Journal of Molecular Sciences, 23(3), 1216. https://doi.org/10.3390/ijms23031216





