A Pilot Clinical Study of Hyperacute Serum Treatment in Osteoarthritic Knee Joint: Cytokine Changes and Clinical Effects
Abstract
1. Introduction
2. Materials and Methods
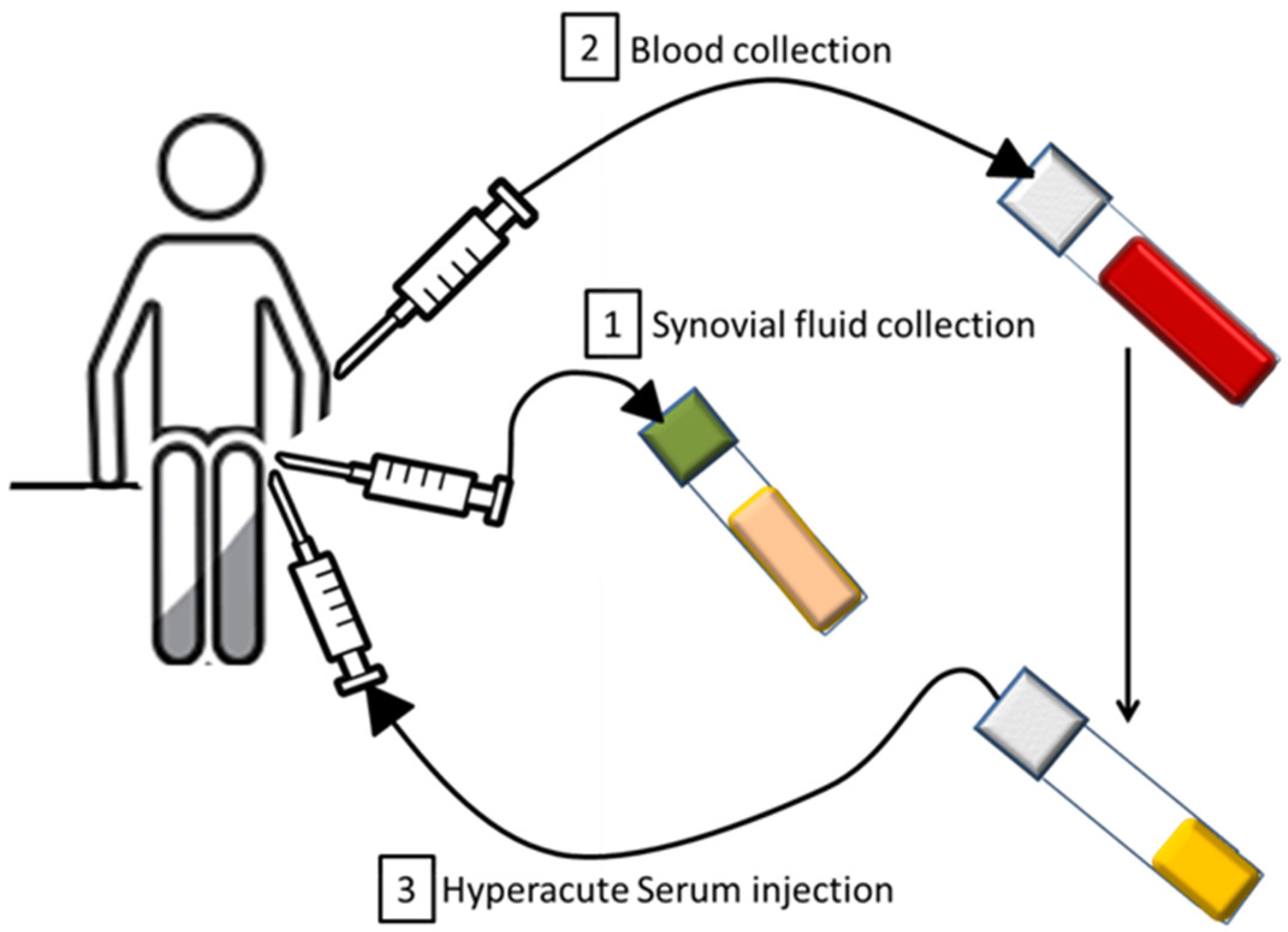
2.1. Hyperacute Serum Isolation
2.2. Intraarticular Serum Treatment
2.3. Cytokine Measurements
2.4. Statistical Analysis
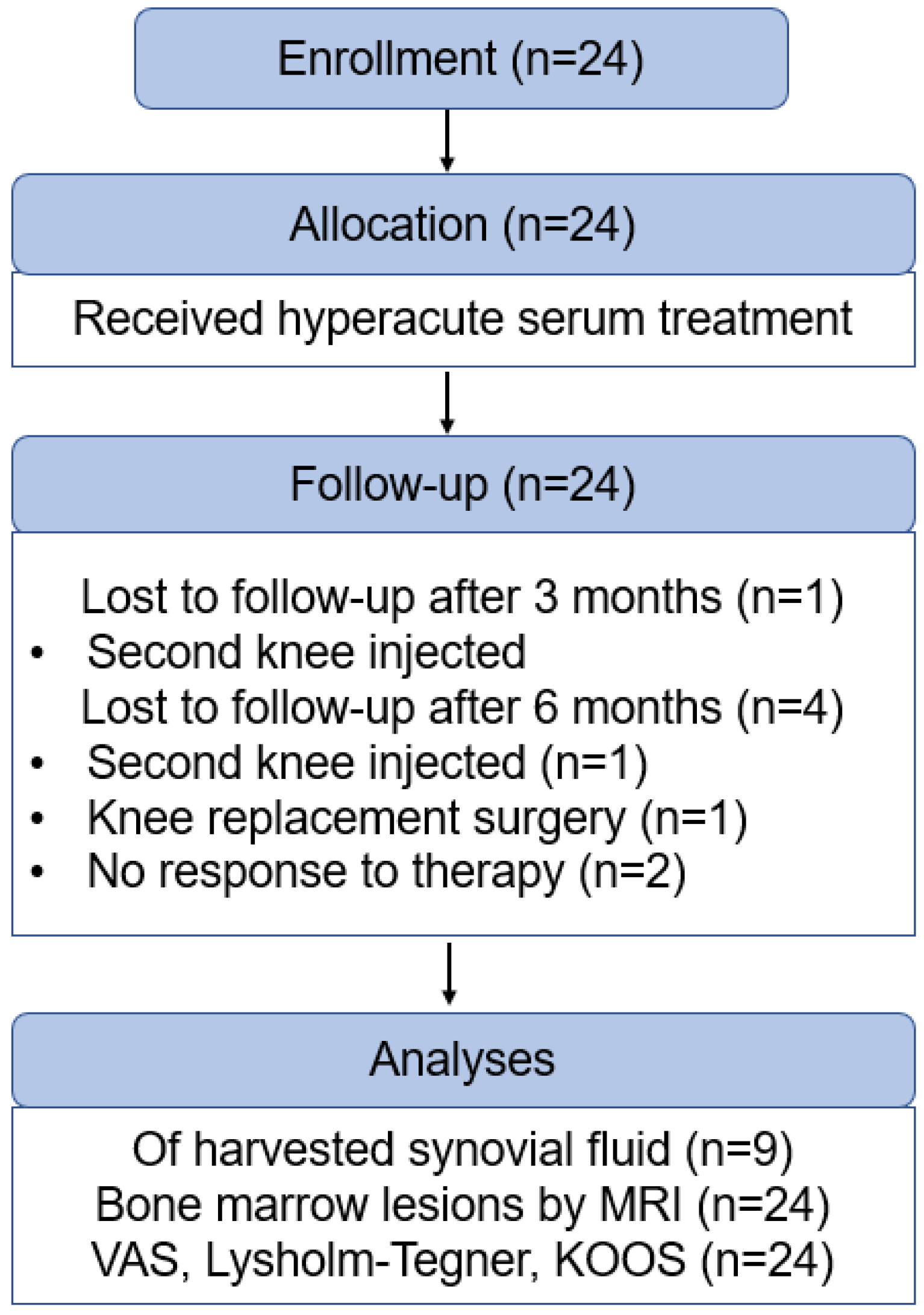
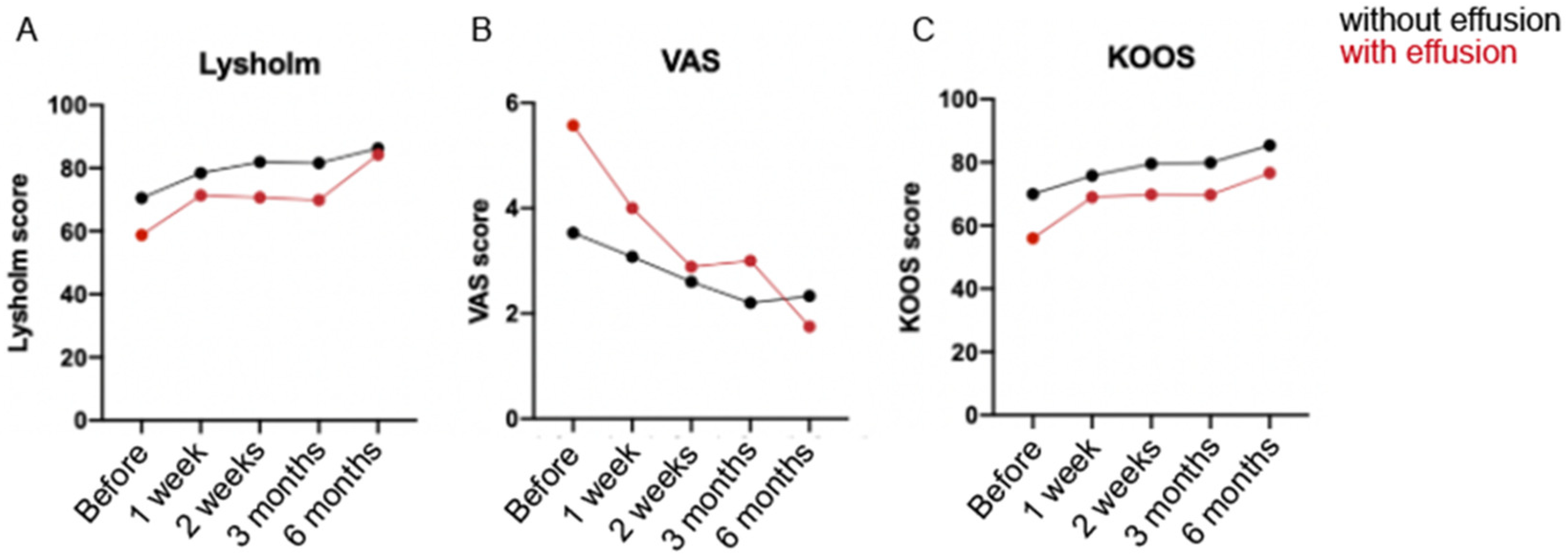
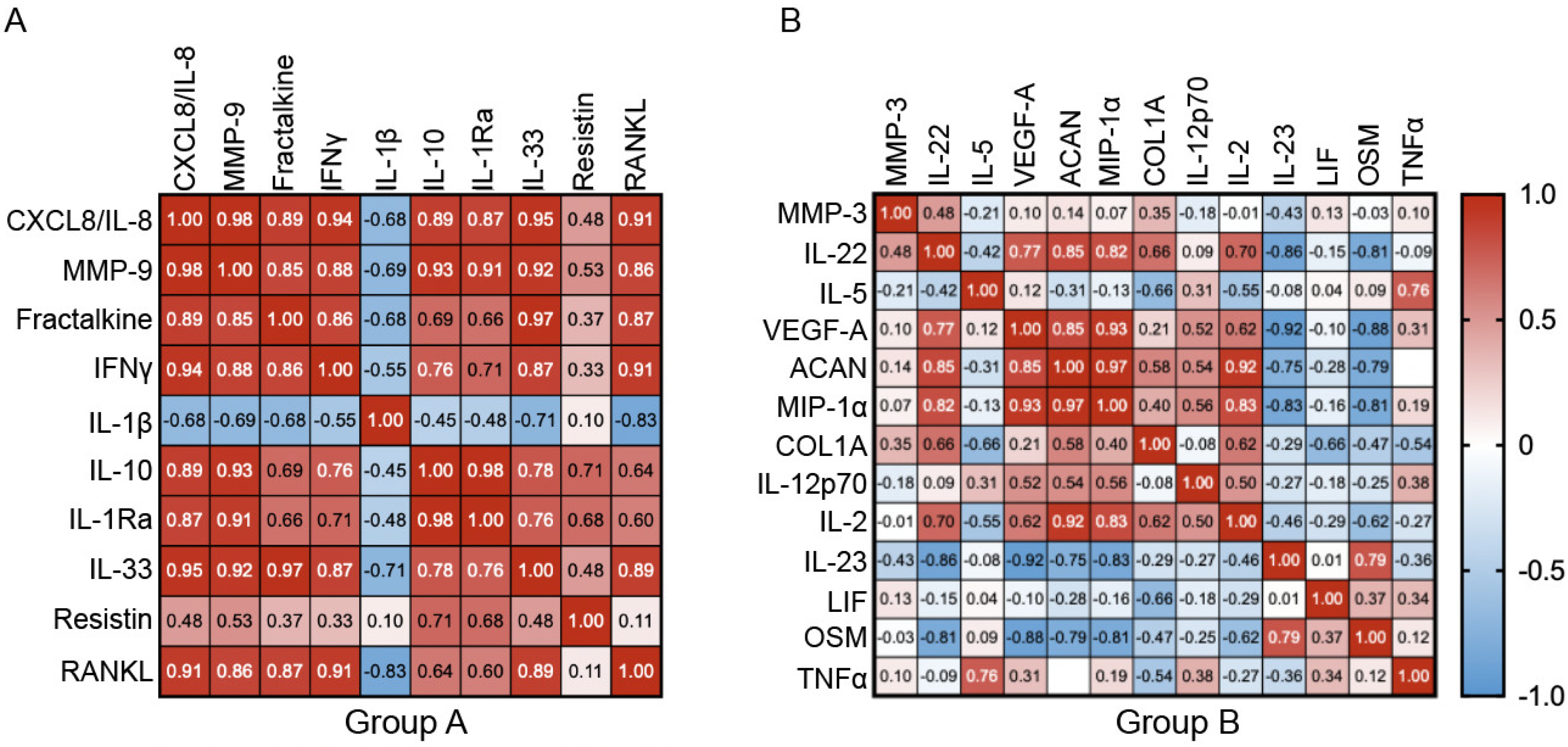
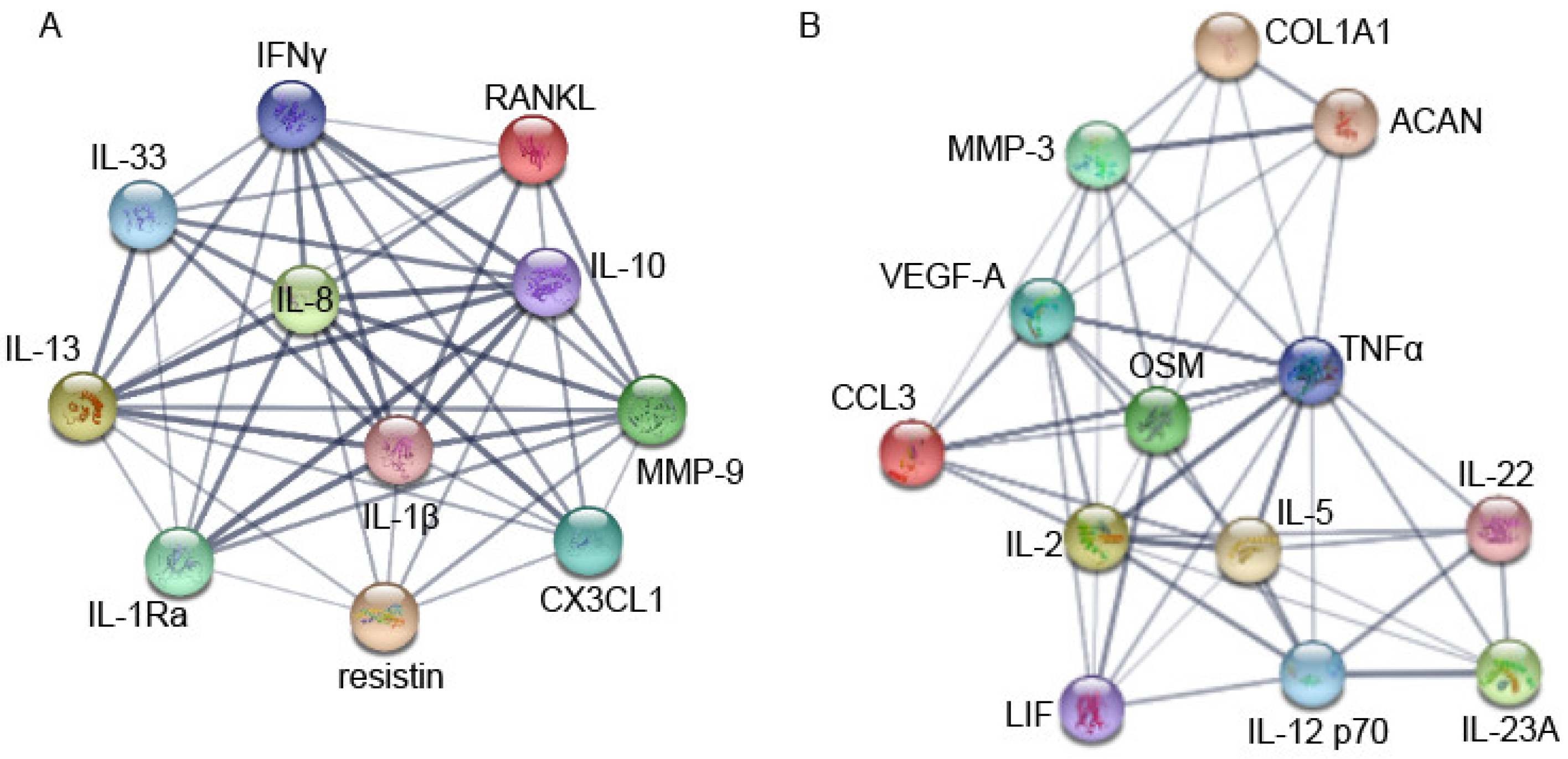
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Yoshioka, T. Intra-articular platelet-rich plasma (PRP) injections for treating knee pain associated with osteoarthritis of the knee in the Japanese population: A phase I and IIa clinical trial. Nagoya J. Med. Sci. 2018, 80, 39–51. [Google Scholar] [PubMed]
- Zhang, W.; Nuki, G. OARSI recommendations for the management of hip and knee osteoarthritis: Part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthr. Cartil. 2010, 18, 476–499. [Google Scholar] [CrossRef] [PubMed]
- De Pascale, M.R.; Sommese, L.; Casamassimi, A.; Napoli, C. Platelet derivatives in regenerative medicine: An update. Transfus. Med. Rev. 2015, 129, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Kon, E.; Di Matteo, B.; Di Marino, A.; Sessa, A.; Merli, M.; Marcacci, M.; Sigascot Cartilage Committee. Leukocyte-poor PRP application for the treatment of knee osteoarthritis. Joints 2014, 1, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, A.; Matthewson, S.; Macdonald, P. Platelet-Rich Plasma and the Knee-Applications in Orthopedic Surgery. Curr. Rev. Musculoskelet. Med. 2018, 11, 607–615. [Google Scholar] [CrossRef]
- Wang-Saegusa, A.; Cugat, R.; Ares, O.; Seijas, R.; Cusco, X.; Garcia-Balletbo, M. Infiltration of plasma rich in growth factors for osteoarthritis of the knee short-term effects on function and quality of life. Arch. Orthop. Trauma Surg. 2013, 131, 311–317. [Google Scholar] [CrossRef]
- Rutgers, M.; Creemers, L.B.; Yang, K.G.A.; Raijmakers, N.J.; Dhert, W.J.; Saris, D.B. Osteoarthritis treatment using autologous conditioned serum after placebo. Acta Orthop. 2015, 86, 114–118. [Google Scholar] [CrossRef]
- Frizziero, A.; Giannotti, E.; Oliva, F.; Masiero, S.; Maffulli, N. Autologous conditioned serum for the treatment of osteoarthritis and other possible applications in musculoskeletal disorders. Br. Med. Bull. 2013, 105, 169–184. [Google Scholar] [CrossRef]
- Kon, E.; Engebretsen, L.; Verdonk, P.; Nehrer, S.; Filardo, G. Clinical outcomes of knee osteoarthritis treated with an autologous protein solution injection: A 1-year pilot double-blinded randomized controlled trial. Am. J. Sports Med. 2018, 46, 171–180. [Google Scholar] [CrossRef]
- van Drumpt, R.A.M.; van der Weegen, W.; King, W.; Toler, K.; Macenski, M.M. Safety and treatment effectiveness of a single autologous protein solution injection in patients with knee osteoarthritis. BioRes. Open Access 2016, 5, 261–268. [Google Scholar] [CrossRef]
- Zarringam, D.; Bekkers, J.E.J.; Saris, D.B.F. Long-term effect of injection treatment for osteoarthritis in the knee by orthokin autologous conditioned serum. Cartilage 2018, 9, 140–145. [Google Scholar] [CrossRef]
- Filardo, G.; Kon, E.; Longo, U.G.; Madry, H.; Marchettini, P.; Marmotti, A.; Van Assche, D.; Zanon, G.; Peretti, G. Non-surgical treatments for the management of early osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Roffi, A.; di Matteo, B.; Krishnakumar, G.S.; Kon, E.; Filardo, G. Platelet-rich plasma for the treatment of bone defects: From pre-clinical rational to evidence in the clinical practice. A systematic review. Intern. Orthop. 2017, 41, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Canella, V.; Cattini, L.; Kon, E.; Marcacci, M.; Di Matteo, B.; Pulsatelli, L.; Filardo, G. Leukocyte-rich platelet-rich plasma injections do not up-modulate intra-articular pro-inflammatory cytokines in the osteoarthritic knee. PLoS ONE 2016, 11, e0156137. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Kon, E. PRP: Product rich in placebo? Knee Surg. Sports Traumatol. Arthrosc. 2014, 24, 3702–3703. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.A.; Saltzman, B.M.; Mascarenhas, R.; Khair, M.M.; Verma, N.N.; Bach, B.R., Jr.; Cole, B.J. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy 2015, 31, 2213–2221. [Google Scholar] [CrossRef]
- Southworth, T.M.; Naveen, N.B.; Tauro, T.M.; Leong, N.; Cole, B.J. The use of platelet-rich plasma in symptomatic knee osteoarthritis. J. Knee Surg. 2018, 32, 37–45. [Google Scholar] [CrossRef]
- Kardos, D.; Marschall, B.; Simon, M.; Hornyák, I.; Hinsenkamp, A.; Kuten, O.; Gyevnár, Z.; Erdélyi, G.; Bárdos, T.; Paukovits, T.M.; et al. Investigation on cytokine changes in osteoarthritic knee joint tissues in response to hyperacute serum treatment. Cells 2019, 8, 824. [Google Scholar] [CrossRef]
- Otahal, A.; Kramer, K.; Kuten-Pella, O.; Weiss, R.; Stotter, C.; Lacza, Z.; Weber, V.; Nehrer, S.; De Luna, A. Characterization and chondroprotective effects of extracellular vesicles from plasma- and serum-based autologous blood-derived products for osteoarthritis therapy. Front. Bioeng. Biotechnol. 2020, 8, 584050. [Google Scholar] [CrossRef]
- Andia, I.; Perez-Valle, A.; Del Amo, C.; Maffulli, N. Freeze-Drying of Platelet-Rich Plasma: The Quest for Standardization. Int. J. Mol. Sci. 2020, 21, 6904. [Google Scholar] [CrossRef]
- Kuten, O.; Simon, M.; Hornyák, I.; De Luna-Preitschopf, A.; Nehrer, S.; Lacza, Z. The Effects of Hyperacute Serum on Adipogenesis and Cell Proliferation of Mesenchymal Stromal Cells. Tissue Eng. Part A 2018, 24, 1011–1021. [Google Scholar] [CrossRef]
- Jeyakumar, V.; Niculescu-Morzsa, E.; Bauer, C.; Lacza, Z.; Nehrer, S. Platelet-Rich Plasma Supports Proliferation and Redifferentiation of Chondrocytes during In Vitro Expansion. Front. Bioeng. Biotechnol. 2017, 5, 75. [Google Scholar] [CrossRef]
- Simon, M.; Major, B.; Vácz, G.; Kuten, O.; Hornyak, I.; Hinsenkamp, A.; Kardos, D.; Bagó, M.; Cseh, D.; Sarkozi, A.; et al. The Effects of Hyperacute Serum on the Elements of the Human Subchondral Bone Marrow Niche. Stem Cells Int. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kardos, D.; Simon, M.; Vácz, G.; Hinsenkamp, A.; Holczer, T.; Cseh, D.; Sárközi, A.; Szenthe, K.; Bánáti, F.; Szathmary, S.; et al. The Composition of Hyperacute Serum and Platelet-Rich Plasma Is Markedly Different despite the Similar Production Method. Int. J. Mol. Sci. 2019, 20, 721. [Google Scholar] [CrossRef] [PubMed]
- Vácz, G.; Major, B.; Gaál, D.; Petrik, L.; Horváthy, D.B.; Han, W.; Holczer, T.; Simon, M.; Muir, J.M.; Hornyák, I.; et al. Hyperacute serum has markedly better regenerative efficacy than platelet-rich plasma in a human bone oxygen–glucose deprivation model. Regen. Med. 2018, 13, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Briggs, K.K.; Lysholm, J.; Tegner, Y.; Rodkey, W.G.; Kocher, M.S.; Steadman, J.R. The reliability, validity, and responsiveness of the lysholm score and tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am. J. Sports Med. 2009, 37, 890–897. [Google Scholar] [CrossRef]
- Collins, N.J.; Misra, D.; Tegner, Y.; Rodkey, W.G.; Kocher, M.S.; Steadman, J.R. Measures of knee function. Arthritis Care Res. 2011, 63, S208–S228. [Google Scholar] [CrossRef]
- Aksekili, M.A.E.; Fidan, F.; Alkan, B.M.; Alemdar, A.; Aksekili, H.; Ardıçoğlu, Ö. Quality of Life in Knee Osteoarthritis; Correlation with Clinical Measures and the Knee Injury and Osteoarthritis Outcome Score. Acta Med. Anatolia 2016, 4, 1–7. [Google Scholar] [CrossRef]
- Bekkers, J.E.J.; Windt, T.S.; Raijmakers, N.J.H.; Dhert, W.J.A.; Saris, D.B.F. Validation of the knee injury and osteoarthritis outcome score (KOOS) for the treatment of focal cartilage lesions. Osteoarthr. Cartil. 2009, 17, 1434–1439. [Google Scholar] [CrossRef]
- Martini, L.I.; Via, A.G.; Fossati, C.; Randelli, F.; Randelli, P.; Cucchi, D. Single Platelet-Rich Plasma Injection for Early Stage of Osteoarthritis of the Knee. Joints 2017, 5, 002–006. [Google Scholar] [CrossRef]
- Vangsness, C.T., Jr.; Burke, W.S.; Narvy, S.J.; Macphee, R.D.; Fedenko, A.N. Human knee synovial fluid cytokines correlated with grade of knee osteoarthritis—A pilot study. Bull. Hosp. J. Dis. 2011, 69, 122–127. [Google Scholar]
- Mabey, T.; Honsawek, S.; Tanavalee, A.; Yuktanandana, P.; Wilairatana, V.; Poovorawan, Y. Plasma and synovial fluid inflammatory cytokine profiles in primary knee osteoarthritis. Biomarkers 2016, 21, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-H.; Hsieh, M.-S.; Liang, Y.-C.; Li, C.-Y.; Sheu, M.-T.; Chou, D.-T.; Chen, T.-F.; Chen, C.-H. Production of the chemokine eotaxin-1 in osteoarthritis and its role in cartilage degradation. J. Cell. Biochem. 2004, 93, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Monibi, F.; Roller, B.L.; Stoker, A.; Garner, B.; Bal, B.S.; Cook, J.L. Identification of Synovial Fluid Biomarkers for Knee Osteoarthritis and Correlation with Radiographic Assessment. J. Knee Surg. 2015, 29, 242–247. [Google Scholar] [CrossRef]
- Chen, J.J.; Huang, J.F.; Du, W.X.; Tong, P.J. Expression and significance of MMP3 in synovium of knee joint at different stage in osteoarthritis pa-tients. Asian Pac. J. Trop. Med. 2014, 7, 297–300. [Google Scholar] [CrossRef]
- Scanzello, C.; Umoh, E.; Pessler, F.; Diaz-Torne, C.; Miles, T.; DiCarlo, E.; Potter, H.; Mandl, L.; Marx, R.; Rodeo, S.; et al. Local cytokine profiles in knee osteoarthritis: Elevated synovial fluid interleukin-15 differentiates early from end-stage disease. Osteoarthr. Cartil. 2009, 17, 1040–1048. [Google Scholar] [CrossRef]
- Zeng, G.; Chen, A.; Li, W.; Song, J.; Gao, C. High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genet. Mol. Res. 2015, 14, 14811–14822. [Google Scholar] [CrossRef]
- Blair-Levy, J.M.; Watts, C.E.; Fiorientino, N.M.; Dimitriadis, E.K.; Marini, J.C.; Lipsky, P.E.; Blair-Levy, J.M.; Watts, C.E.; Fiorientino, N.M.; Dimitriadis, E.K.; et al. A type I collagen defect leads to rapidly progressive osteoarthritis in a mouse model. Arthritis Rheum. 2008, 58, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Struglics, A.; Larsson, S.; Pratta, M.A.; Kumar, S.; Lark, M.W.; Lohmander, L.S. Human osteoarthritis synovial fluid and joint cartilage contain both aggrecanase- and matrix metal-loproteinase-generated aggrecan fragments. Osteoarthr. Cartil. 2006, 14, 101–111. [Google Scholar] [CrossRef]
- El-Arman, M.M.; El-Fayoumi, G.; El-Shal, E.; El-Boghdady, I.; El-Ghaweet, A. Aggrecan and Cartilage Oligomeric Matrix Protein in Serum and Synovial Fluid of Patients with Knee Osteoarthritis. HSS J. 2010, 6, 171–176. [Google Scholar] [CrossRef]
- Nakamura, S.; Kamihagi, K.; Satakeda, H.; Katayama, M.; Pan, H.; Okamoto, H.; Noshiro, M.; Takahashi, K.; Yoshihara, Y.; Shimmei, M.; et al. Enhancement of sparc (osteonectin) synthesis in arthritic cartilage: Increased levels in synovial fluids from patients with rheumatoid arthritis and regulation by growth factors and cytokines in chondrocyte cultures. Arthritis Rheum. 1996, 39, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Mabey, T.; Honsawek, S. Cytokines as biochemical markers for knee osteoarthritis. World J. Orthop. 2015, 6, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Scanzello, C.R.; Goldring, S.R. The role of synovitis in osteoarthritis pathogenesis. Bone 2012, 51, 249–257. [Google Scholar] [CrossRef]
- Pierzchala, A.W.; Kusz, D.J.; Hajduk, G. CXCL8 and CCL5 expression in synovial fluid and blood serum in patients with osteoarthritis of the knee. Arch. Immunol. Ther. Exp. 2011, 59, 151–155. [Google Scholar] [CrossRef]
- Vernal, R.; Velasquez, E.; Gamonal, J.; Garcia-Sanz, J.A.; Silva, A.; Sanz, M. Expression of proinflammatory cytokines in osteoarthritis of the temporomandibular joint. Arch. Oral. Biol. 2008, 53, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteo-arthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Hermanns, H.M. Oncostatin M and interleukin-31: Cytokines, receptors, signal transduction and physiology. Cytokine Growth Factor Rev. 2015, 26, 545–558. [Google Scholar] [CrossRef]
- Zhang, Q.; Putheti, P.; Zhou, Q.; Liu, Q.; Gao, W. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. 2008, 19, 347–356. [Google Scholar] [CrossRef]
- Jacques, C.; Gosset, M.; Berenbaum, F.; Gabay, C. The Role of IL-1 and IL-1Ra in Joint Inflammation and Cartilage Degradation. Vitam. Horm. 2006, 74, 371–403. [Google Scholar]
- Daghestani, H.N.; Pieper, C.F.; Kraus, V.B. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee os-teoarthritis. Arthr. Rheum. 2015, 67, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Saetan, N.; Honsawek, S.; Tanavalee, A.; Tantavisut, S.; Yuktanandana, P.; Parkpian, V. Association of plasma and synovial fluid interferon-gamma inducible protein-10 with radiographic severity in knee osteoarthritis. Clin. Biochem. 2011, 44, 1218–1222. [Google Scholar] [CrossRef]
- Ni, J.; Yuan, X.; Yao, Q.; Peng, L. OSM is overexpressed in knee osteoarthritis and Notch signaling is involved in the effects of OSM on MC3T3-E1 cell proliferation and differentiation. Int. J. Mol. Med. 2015, 35, 1755–1760. [Google Scholar] [CrossRef]
- Huo, L.W.; Ye, Y.L. Fractalkine (CX3CL1): A biomarker reflecting symptomatic severity in patients with knee osteoarthritis. JIM 2015, 63, 626–631. [Google Scholar] [CrossRef]
- Klosowska, K.; Volin, M.V.; Huynh, N.; Chong, K.K.; Halloran, M.M.; Woods, J.M. Fractalkine functions as a chemoattractant for osteoarthritis synovial fibroblasts and stimulates phosphorylation of mitogen-activated protein kinases and Akt. Clin. Exp. Immunol. 2009, 156, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Kokebie, R.; Aggarwal, R.; Lidder, S.; Hakimiyan, A.A.; Rueger, D.C.; Block, J.A.; Chubinskaya, S. The role of synovial fluid markers of catabolism and anabolism in osteoarthritis, rheumatoid arthritis and asymptomatic organ donors. Arthritis Res. 2011, 13, R50. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-Z.; Guan, J.; Wang, H.-J.; Ma, W.; Li, F.; Xu, F.; Ding, L.-B.; Xie, L.; Liu, B.; Liu, K.; et al. Possible Involvement of Serum and Synovial Fluid Resistin in Knee Osteoarthritis: Cartilage Damage, Clinical, and Radiological Links. J. Clin. Lab. Anal. 2015, 30, 437–443. [Google Scholar] [CrossRef]
- Koskinen, A.; Vuolteenaho, K.; Moilanen, T.; Moilanen, E. Resistin as a factor in osteoarthritis: Synovial fluid resistin concentrations correlate positively with interleukin 6 and matrix metalloproteinases MMP-1 and MMP-3. Scand. J. Rheumatol. 2013, 43, 249–253. [Google Scholar] [CrossRef]
- Kahle, P.; Saal, J.G.; Schaudt, K.; Zacher, J.; Fritz, P.; Pawelec, G. Determination of cytokines in synovial fluids: Correlation with diagnosis and histomorphological charac-teristics of synovial tissue. Ann. Rheum. Dis. 1992, 51, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liao, Q.; Ming, J.-H.; Hu, G.-L.; Chen, Q.; Liu, S.-Q.; Li, Y.-M. The effects of chitosan oligosaccharides on OPG and RANKL expression in a rat osteoarthritis model. Acta Cir. Bras. 2017, 32, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Deligne, C.; Casulli, S.; Pigenet, A.; Bougault, C.; Campillo-Gimenez, L.; Nourissat, G.; Berenbaum, F.; Elbim, C.; Houard, X. Differential expression of interleukin-17 and interleukin-22 in inflamed and non-inflamed synovium from osteoarthritis patients. Osteoarthr. Cartil. 2015, 23, 1843–1852. [Google Scholar] [CrossRef]
- Márton, K.; Tamás, S.B.; Orsolya, N.; Béla, C.; Ferenc, D.; Péter, N.; Csaba, D.-N.; Lajos, C.; Zsombor, L.; Eitan, M.; et al. Microarchitecture of the Augmented Bone Following Sinus Elevation with an Albumin Impregnated Demineralized Freeze-Dried Bone Allograft (BoneAlbumin) versus Anorganic Bovine Bone Mineral: A Randomized Prospective Clinical, Histomorphometric, and Micro-Computed Tomography Study. Materials 2018, 11, 202. [Google Scholar]
- Li, C.; Chen, K.; Kang, H.; Yan, Y.; Liu, K.; Guo, C.; Qi, J.; Yang, K.; Wang, F.; Guo, L.; et al. Double-stranded RNA released from damaged articular chondrocytes promotes cartilage degeneration via Toll-like receptor 3-interleukin-33 pathway. Cell Death Dis. 2017, 8, e3165. [Google Scholar] [CrossRef] [PubMed]
- Hinsenkamp, A.; Kardos, D.; Lacza, Z.; Hornyák, I. A Practical Guide to Class IIa Medical Device Development. Appl. Sci. 2020, 10, 3638. [Google Scholar] [CrossRef]
- Sánchez, M.; Fiz, N.; Azofra, J.; Usabiaga, J.; Recalde, E.A.; Gutierrez, A.G.; Albillos, J.; Gárate, R.; Aguirre, J.J.; Padilla, S.; et al. A Randomized Clinical Trial Evaluating Plasma Rich in Growth Factors (PRGF-Endoret) Versus Hyaluronic Acid in the Short-Term Treatment of Symptomatic Knee Osteoarthritis. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Gato-Calvo, L.; Magalhães, J.; Ruiz-Romero, C.; Blanco, F.J.; Burguera, E.F. Platelet-rich plasma in osteoarthritis treatment: Review of current evidence. Ther. Adv. Chronic Dis. 2019, 10, 2040622319825567. [Google Scholar] [CrossRef]
- Tuakli-Wosornu, Y.A.; Terry, A.; Boachie-Adjei, K.; Harrison, J.R.; Gribbin, C.K.; LaSalle, E.E.; Nguyen, J.T.; Solomon, J.L.; Lutz, G.E. Lumbar Intradiskal Platelet-Rich Plasma (PRP) Injections: A Prospective, Double-Blind, Randomized Controlled Study. PM&R 2016, 8, 1–10. [Google Scholar]
- Meheux, C.J.; McCulloch, P.C.; Lintner, D.M.; Varner, K.E.; Harris, J.D. Efficacy of intra-articular PRP-injections in knee osteoarthritis: A systemic review. Arthroscopy 2016, 32, 495–505. [Google Scholar] [CrossRef]
- di Martino, A.; di Matteo, B.; Papio, T.; Tentoni, F.; Selleri, F.; Cenacchi, A.; Kon, E.; Filardo, G. Platelet-rich plasma versus hyaluronic acid injections for the treatment of knee osteoarthritis. Am. J. Sports Med. 2019, 47, 347–354. [Google Scholar] [CrossRef]
- Gobezie, R.; Kho, A.; Krastins, B.; Sarracino, D.A.; Thornhill, T.S.; Chase, M.; Millett, P.J.; Lee, D.M. High abundance synovial fluid proteome: Distinct profiles in health and osteoarthritis. Arthritis Res. Ther. 2007, 9, R36. [Google Scholar] [CrossRef]
- Westacott, C.I.; Whicher, J.T.; Barnes, I.C.; Thompson, D.; Swan, A.J.; Dieppe, P.A. Synovial fluid concentration of five different cytokines in rheumatic diseases. Ann. Rheum. Dis. 1990, 49, 676–681. [Google Scholar] [CrossRef]
- Scanzello, C.R. Chemokines and inflammation in osteoarthritis: Insights from patients and animal models. J. Orthop Res. 2017, 35, 735–739. [Google Scholar] [CrossRef]
- Jacquemier, J.; Ginestier, C.; Rougemont, J.; Bardou, V.-J.; Charafe-Jauffret, E.; Geneix, J.; Adélaïde, J.; Koki, A.; Houvenaeghel, G.; Hassoun, J.; et al. Protein expression profiling identifies subclasses of breast cancer and predicts prognosis. Cancer Res. 2005, 65, 767–779. [Google Scholar]
- Zhang, B.; Verberkmoes, N.C.; Langston, M.A.; Uberbacher, E.; Hettich, R.L.; Samatova, N.F. Detecting Differential and Correlated Protein Expression in Label-Free Shotgun Proteomics. J. Proteome Res. 2006, 5, 2909–2918. [Google Scholar] [CrossRef] [PubMed]
- Medina, T.S.; Costa, S.P.T.; Oliveira, M.D.; Ventura, A.M.; Souza, J.M.; Gomes, T.F.; Vallinoto, A.C.R.; Póvoa, M.M.; Silva, J.S.; Cunha, M.G. Increased interleukin-10 and interferon-gamma levels in Plasmodium vivax malaria suggest a re-ciprocal regulation which is not altered by IL-10 gene promoter polymorphism. Malar. J. 2011, 10, 264. [Google Scholar] [CrossRef] [PubMed]
- Kopach, P.; Lockatell, V.; Pickering, E.M.; Haskell, R.E.; Anderson, R.D.; Hasday, J.D.; Todd, N.W.; Luzina, I.G.; Atamas, S.P. IFN-gamma directly controls IL-33 protein level through a STAT1- and LMP2- dependent mechanism. J. Biol. Chem. 2014, 289, 11829–11843. [Google Scholar] [CrossRef] [PubMed]
- Lettner, T.; Lang, R.; Klausegger, A.; Hainzl, S.; Bauer, J.W.; Wally, V. MMP-9 and CXCL8/IL-8 Are Potential Therapeutic Targets in Epidermolysis Bullosa Simplex. PLoS ONE 2013, 8, e70123. [Google Scholar] [CrossRef]
- Daheshia, M.; Yao, J.Q. The Interleukin 1β Pathway in the Pathogenesis of Osteoarthritis. J. Rheumatol. 2008, 35, 2306–2312. [Google Scholar] [CrossRef]
- Vincent, T.L. IL-1 in osteoarthritis: Time for a critical review of the literature. F1000Research 2019, 8, 934. [Google Scholar] [CrossRef]
- Kloppenburg, M.; Peterfy, C.; Haugen, I.K.; Kroon, F.; Chen, S.; Wang, L.; Liu, W.; Levy, G.; Fleischmann, R.M.; Berenbaum, F.; et al. Phase IIa, placebo-controlled, randomised study of lutikizumab, an anti-interleukin-1α and an-ti-interleukin-1β dual variable domain immunoglobulin, in patients with erosive hand osteoarthritis. Ann. Rheum. Dis. 2019, 78, 413–420. [Google Scholar] [CrossRef]
- Fleischmann, R.M.; Bliddal, H.; Blanco, F.J.; Schnitzer, T.J.; Peterfy, C.; Wang, S.C.L.; Feng, S.; Conaghan, P.G.; Berenbaum, F.; Pelletier, J.-P.; et al. A phase II trial of lutikizumab, an anti-interleukin 1α/β dual variable domain immunoglobulin, in knee osteoarthritis patients with synovitis. Arthritis Rheum. 2019, 71, 1056–1069. [Google Scholar] [CrossRef] [PubMed]
- Zupan, J.; Vrtačnik, P.; Cör, A.; Haring, G.; Weryha, G.; Visvikis-Siest, S.; Marc, J. VEGF-A is associated with early degenerative changes in cartilage and subchondral bone. Growth Factors 2018, 36, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Hao, Y.R.; Wang, Z.; Zhou, J.L.; Jia, Q.X.; Qiu, B. The effect of vascular endothelial growth factor on aggrecan and type II collagen expression in rat ar-ticular chondrocytes. Rheumatol. Int. 2012, 32, 3359–3364. [Google Scholar] [CrossRef] [PubMed]
- Von Delwig, A.; Locke, J.; Robinson, J.H.; Ng, W.-F. Response of Th17 cells to a citrullinated arthritogenic aggrecan peptide in patients with rheumatoid arthritis. Arthritis Rheum. 2010, 62, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Botta, C.; di Martino, M.T.; Ciliberto, D.; Cucè, M.; Correale, P.; Rossi, M.; Tagliaferri, P.; Tassone, P. A gene expression inflammatory signature specifically predicts multiple myeloma evolution and patients survival. Blood Cancer J. 2016, 6, e511. [Google Scholar] [CrossRef] [PubMed]
- Beekhuizen, M.; Van Osch, G.; Bot, A.; Hoekstra, M.; Saris, D.; Dhert, W.; Creemers, L. Inhibition of oncostatin M in osteoarthritic synovial fluid enhances GAG production in osteoarthritic cartilage repair. Eur. Cells Mater. 2013, 26, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.-C.; Zhang, Z.; Achuthan, A.; Fleetwood, A.J.; Smith, J.E.; Hamilton, J.A.; Cook, A.D. IL-23 in arthritic and inflammatory pain development in mice. Arthritis Res. 2020, 22, 1–13. [Google Scholar] [CrossRef]
- Maricar, N.; Callaghan, M.; Parkes, M.J.; Felson, D.; O’Neill, T. Clinical assessment of effusion in knee osteoarthritis—A systematic review. Semin. Arthritis Rheum. 2016, 45, 556–563. [Google Scholar] [CrossRef]
- Berthelot, J.M. Placebo effect in osteoarthritis. World J. Orthop. 2015, 6, 416–420. [Google Scholar]
- Zhang, W.; Robertson, J.; Jones, A.C.; Dieppe, P.A.; Doherty, M. The placebo effect and its determinants in osteoarthritis: Meta-analysis of randomised controlled trials. Ann. Rheum. Dis. 2008, 67, 1716–1723. [Google Scholar] [CrossRef]
- Bannuru, R.; Schmid, C.; Sullivan, M.; Kent, D.; Wong, J.; McAlindon, T. Differential response of placebo treatments in osteoarthritis trials: A systematic review and network meta-analysis. Osteoarthr. Cartil. 2014, 22, S24–S25. [Google Scholar] [CrossRef][Green Version]
- Previtali, D.; Merli, G. The long-lasting effects of “placebo injections” in knee osteoarthritis: A meta-analysis. Cartilage 2020, 1947603520906597. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olmos Calvo, I.; Fodor, E.; Kardos, D.; Hornyák, I.; Hinsenkamp, A.; Kuten-Pella, O.; Gyevnár, Z.; Erdélyi, G.; Bárdos, T.; Paukovits, T.M.; et al. A Pilot Clinical Study of Hyperacute Serum Treatment in Osteoarthritic Knee Joint: Cytokine Changes and Clinical Effects. Curr. Issues Mol. Biol. 2021, 43, 637-649. https://doi.org/10.3390/cimb43020046
Olmos Calvo I, Fodor E, Kardos D, Hornyák I, Hinsenkamp A, Kuten-Pella O, Gyevnár Z, Erdélyi G, Bárdos T, Paukovits TM, et al. A Pilot Clinical Study of Hyperacute Serum Treatment in Osteoarthritic Knee Joint: Cytokine Changes and Clinical Effects. Current Issues in Molecular Biology. 2021; 43(2):637-649. https://doi.org/10.3390/cimb43020046
Chicago/Turabian StyleOlmos Calvo, Isabel, Eszter Fodor, Dorottya Kardos, István Hornyák, Adél Hinsenkamp, Olga Kuten-Pella, Zsuzsanna Gyevnár, Gábor Erdélyi, Tamás Bárdos, Tamás Mirkó Paukovits, and et al. 2021. "A Pilot Clinical Study of Hyperacute Serum Treatment in Osteoarthritic Knee Joint: Cytokine Changes and Clinical Effects" Current Issues in Molecular Biology 43, no. 2: 637-649. https://doi.org/10.3390/cimb43020046
APA StyleOlmos Calvo, I., Fodor, E., Kardos, D., Hornyák, I., Hinsenkamp, A., Kuten-Pella, O., Gyevnár, Z., Erdélyi, G., Bárdos, T., Paukovits, T. M., Magos, K., Béres, G., Nehrer, S., & Lacza, Z. (2021). A Pilot Clinical Study of Hyperacute Serum Treatment in Osteoarthritic Knee Joint: Cytokine Changes and Clinical Effects. Current Issues in Molecular Biology, 43(2), 637-649. https://doi.org/10.3390/cimb43020046






