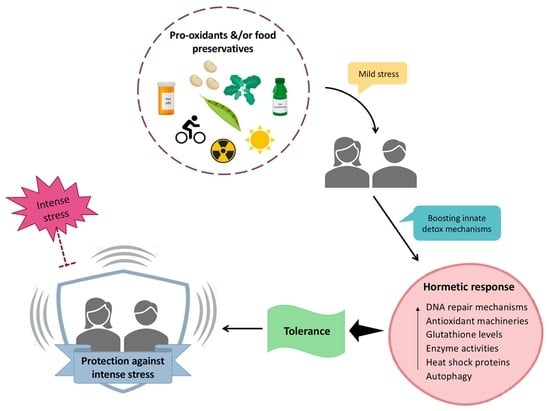
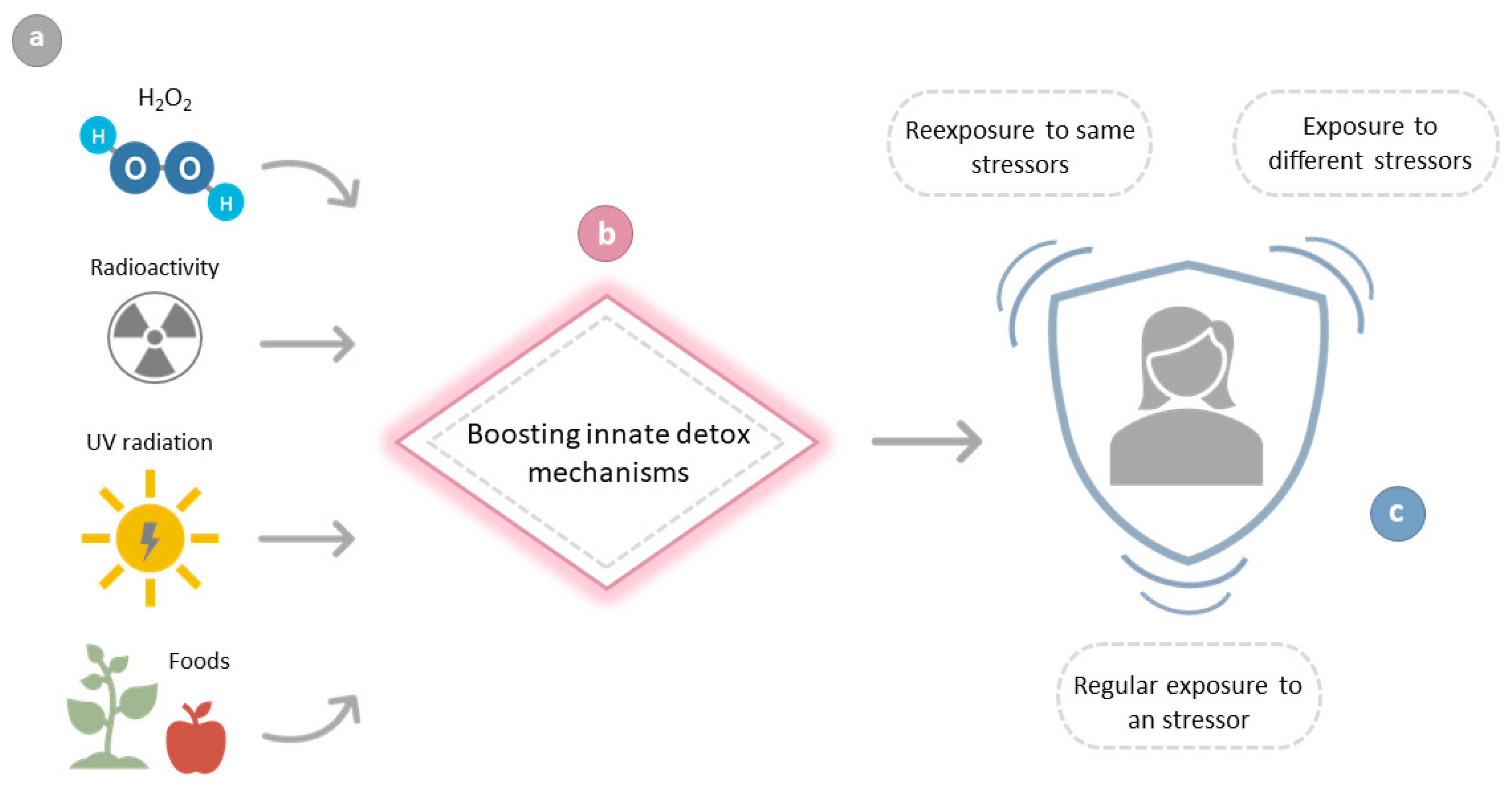
Antioxidant Supplements versus Health Benefits of Brief/Intermittent Exposure to Potentially Toxic Physical or Chemical Agents
Abstract
:1. Health and Health Benefits are Human Inventions
2. The Multiple Definitions of Antioxidants
3. The Precise Redox Rules of Chemistry
4. The Conflicting Methods to Determine Antioxidant Action
5. Scientific Evidence in Support of the Need for Boosting Repair Mechanisms
5.1. The Classical Case of DNA Repair after Exposure to Harmful Radiation
5.2. Boosting Repair Mechanisms after Exposure to Foods and Food Supplements
6. Prooxidant Diets with Health Benefits. A Chimera?
7. The Role of the Microbiota
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Declaration
Appendix A
| Gene Symbol | Gene Title | Acute logFC | Low logFC |
|---|---|---|---|
| AEN | Apoptosis-enhancing nuclease | 3.321 | 3.210 |
| TRIOBP | TRIO and F-actin binding protein | 2.793 | 1.197 |
| FDXR | Ferredoxin reductase | 2.774 | 2.637 |
| DDB2 | Damage-specific DNA binding protein 2, 48kDa | 2.071 | 1.901 |
| PAPPA | Pregnancy-associated plasma protein A, Pappalysin 1 | 1.882 | 2.502 |
| CD70 | CD70 molecule | 1.827 | 1.646 |
| BAX | BCL2-associated X protein | 1.698 | 1.592 |
| VWCE | Von Willebrand factor C and EGF domains | 1.660 | 1.528 |
| E2F7 | E2F transcription factor 7 | 1.596 | 1.364 |
| ACTA2 | Actin, alpha 2, smooth muscle, aorta | 1.531 | 1.293 |
| RPS27L | Ribosomal protein S27-like | 1.527 | 1.329 |
| TNFSF4 | Tumor necrosis factor (ligand) superfamily, member 4 | 1.524 | 1.508 |
| CCNG1 | Cyclin G1 | 1.463 | 1.304 |
| TNFRSF10B | Tumor necrosis factor receptor superfamily, member 10b | 1.455 | 1.381 |
| CDKN1A | Cyclin-dependent kinase inhibitor 1A (p21, Cip1) | 1.197 | 1.066 |
| BBC3 | BCL2 binding component 3 | 1.193 | 0.923 |
| OR52N4 | Olfactory receptor, family 52, subfamily N, member 4 | 1.184 | 1.369 |
| GLS2 | Glutaminase 2 (liver, mitochondrial) | 1.172 | 1.117 |
| RBP4 | Retinol binding protein 4, plasma | 1.169 | 1.226 |
| POLH | Polymerase (DNA directed), eta | 1.137 | 0.821 |
| TNFSF9 | Tumor necrosis factor (ligand) superfamily, member 9 | 1.131 | 0.656 |
| PHPT1 | Phosphohistidine phosphatase 1 | 1.123 | 0.847 |
| KLLN | Killin, p53-regulated DNA replication inhibitor | 0.993 | 0.902 |
| XPC | Xeroderma pigmentosum, complementation group C | 0.966 | 0.957 |
| SESN1 | Sestrin 1 | 0.959 | 0.795 |
| TNFSF8 | Tumor necrosis factor (ligand) superfamily, member 8 | 0.925 | 1.063 |
| CST2 | Cystatin SA | 0.924 | 1.067 |
| GDF15 | Growth differentiation factor 15 | 0.832 | 1.153 |
| ACER2 | Alkaline ceramidase 2 | 0.742 | 0.966 |
| KCNN4 | Potassium intermediate/small conductance calcium-activated channel, subfamily N, member 4 | 0.710 | 0.688 |
| PPMD1 | Protein phosphatase, Mg2+/Mn2+ dependent, 1D | 0.692 | 0.707 |
| LIG1 | Ligase I, DNA, ATP-dependent | 0.631 | 0.567 |
| TFAP4 | Transcription factor AP-4 (activating enhancer binding protein 4) | −0.694 | −1.163 |
| C10ORF116 | Chromosome 10 open reading frame 116 | −0.791 | −0.827 |
| TAAR8 | Trace amine associated receptor 8 | −0.807 | −0.740 |
| KRTAP13-2 | Keratin associated protein 13-2 | −0.843 | −0.641 |
| LMX1B | LIM homeobox transcription factor 1, beta | −0.964 | −0.909 |
| DOK5 | Docking protein 5 | −0.976 | −1.534 |
| NR1I3 | Nuclear receptor subfamily 1, group I, member 3 | −1.243 | −0.890 |
| ANKRD34B | Ankyrin repeat domain 34B | −1.248 | −1.538 |
| SCT | Secretin | −1.318 | −1.311 |
| LMOD2 | Leiomodin 2 (cardiac) | −1.639 | −1.845 |
| RXFP2 | Relaxin/insulin-like family peptide receptor 2 | −2.191 | −1.529 |
| Gene Symbol | Gene Title | Acute logFC | Low logFC |
|---|---|---|---|
| APOBEC3H | Apolipoprotein B mRNA editing enzyme. catalytic polypeptide-like 3H | 4.746 | 3.957 |
| VWCE | Von Willebrand factor C and EGF domains | 4.577 | 3.579 |
| FDXR | Ferredoxin reductase | 4.505 | 3.961 |
| E2F7 | E2F transcription factor 7 | 4.108 | 3.581 |
| AEN | Apoptosis enhancing nuclease | 4.001 | 4.155 |
| TNFSF4 | Tumor necrosis factor (ligand) superfamily. member 4 | 3.701 | 2.914 |
| PAPPA | Pregnancy-associated plasma protein A. pappalysin 1 | 3.602 | 3.379 |
| PHLDA3 | Pleckstrin homology-like domain. family A. member 3 | 3.314 | 2.830 |
| NTN1 | Netrin 1 | 3.204 | 2.227 |
| DDB2 | Damage-specific DNA binding protein 2. 48kDa | 3.025 | 2.900 |
| CD70 | CD70 molecule | 2.978 | 2.895 |
| PCNA | Proliferating cell nuclear antigen | 2.895 | 2.787 |
| GDF15 | Growth differentiation factor 15 | 2.872 | 2.751 |
| TMPRSS7 | Transmembrane protease. serine 7 | 2.777 | 2.194 |
| CDKN1A | Cyclin-dependent kinase inhibitor 1A (p21. Cip1) | 2.619 | 2.297 |
| ACTA2 | Actin. alpha 2. smooth muscle. aorta | 2.400 | 1.548 |
| CCNG1 | Cyclin G1 | 2.390 | 1.865 |
| TAC3 | Tachykinin 3 | 2.367 | 1.459 |
| BBC3 | BCL2 binding component 3 | 2.133 | 1.867 |
| POLH | Polymerase (DNA directed). eta | 2.101 | 1.632 |
| BAX | BCL2-associated X protein | 2.074 | 2.134 |
| GRIN3B | Glutamate receptor. ionotropic. N-methyl-D-aspartate 3B | 2.069 | 1.856 |
| GNAT2 | Guanine nucleotide binding protein (G protein). alpha transducing activity polypeptide 2 | 2.056 | 2.409 |
| PHPT1 | Phosphohistidine phosphatase 1 | 2.037 | 1.731 |
| TNFSF9 | Tumor necrosis factor (ligand) superfamily. member 9 | 2.028 | 2.195 |
| FCAMR | Fc receptor. IgA. IgM. high affinity | 1.842 | 1.704 |
| LAMC3 | Laminin. gamma 3 | 1.774 | 1.444 |
| SESN1 | Sestrin 1 | 1.751 | 1.538 |
| GNG4 | Guanine nucleotide binding protein (G protein). gamma 4 | 1.728 | 1.802 |
| PIGR | Polymeric immunoglobulin receptor | 1.639 | 2.238 |
| ACER2 | Alkaline ceramidase 2 | 1.628 | 1.424 |
| TNFRSF10B | Tumor necrosis factor receptor superfamily. member 10b | 1.599 | 1.905 |
| GPR172B | G protein-coupled receptor 172B | 1.574 | 0.966 |
| PPM1D | Protein phosphatase. Mg2+/Mn2+ dependent. 1D | 1.566 | 1.310 |
| TNFSF8 | Tumor necrosis factor (ligand) superfamily. member 8 | 1.559 | 1.449 |
| TYMS | Thymidylate synthetase | 1.491 | 1.579 |
| LMNA | Lamin A/C | 1.438 | 1.780 |
| IER5 | Immediate early response 5 | 1.417 | 1.237 |
| OR52N4 | Olfactory receptor. family 52. subfamily N. member 4 | 1.388 | 1.965 |
| MGAT3 | Mannosyl (beta-1.4-)-glycoprotein beta-1.4-N-acetylglucosaminyltransferase | 1.358 | 0.998 |
| DHDH | Dihydrodiol dehydrogenase (dimeric) | 1.291 | 1.124 |
| LIG1 | Ligase I. DNA. ATP-dependent | 1.235 | 0.885 |
| APOBEC3C | Apolipoprotein B mRNA editing enzyme. catalytic polypeptide-like 3C | 1.163 | 1.144 |
| KCNN4 | Potassium intermediate/small conductance calcium-activated channel. subfamily N. member 4 | 1.148 | 0.913 |
| DRAM1 | DNA-damage regulated autophagy modulator 1 | 1.141 | 0.973 |
| MERTK | C-mer proto-oncogene tyrosine kinase | 1.126 | 1.101 |
| MAP4K4 | Mitogen-activated protein kinase kinase kinase kinase 4 | 1.052 | 1.003 |
| ARHGEF3 | Rho guanine nucleotide exchange factor (GEF) 3 | 1.052 | 1.090 |
| F5 | Coagulation factor V (proaccelerin. labile factor) | 1.049 | 1.150 |
| RGL1 | Ral guanine nucleotide dissociation stimulator-like 1 | 1.027 | 1.078 |
| PTP4A1 | Protein tyrosine phosphatase type IVA. member 1 | 1.006 | 0.883 |
| PRKAB1 | Protein kinase. AMP-activated. beta 1 non-catalytic subunit | 1.002 | 0.872 |
| ABLIM2 | Actin binding LIM protein family. member 2 | −0.929 | −0.967 |
| PPP2R1B | Protein phosphatase 2. regulatory subunit A. beta | −1.091 | −0.900 |
| OSBPL10 | Oxysterol binding protein-like 10 | −1.225 | −0.798 |
| TFAP4 | Transcription factor AP-4 (activating enhancer binding protein 4) | −1.309 | −1.188 |
| CORO2B | Coronin. actin binding protein. 2B | −1.712 | −1.996 |
| FCRL2 | Fc receptor-like 2 | −1.925 | −1.498 |
| CD160 | CD160 molecule | −2.519 | −1.765 |
References
- Horowitz, S. Health Benefits of Meditation: What the Newest Research Shows. Altern. Complement. Ther. 2010, 16, 223–228. [Google Scholar] [CrossRef]
- Herzog, H.; Lele, V.R.; Kuwert, T.; Langen, K.J.; Rota Kops, E.; Feinendegen, L.E. Changed pattern of regional glucose metabolism during yoga meditative relaxation. Neuropsychobiology 1990, 23, 182–187. [Google Scholar] [CrossRef]
- Chu, L.-C. The benefits of meditation vis-à-vis emotional intelligence, perceived stress and negative mental health. Stress Health 2010, 26, 169–180. [Google Scholar] [CrossRef]
- Oñatibia-Astibia, A.; Franco, R.; Martínez-Pinilla, E. Health benefits of methylxanthines in neurodegenerative diseases. Mol. Nutr. Food Res. 2017, 61, 1600670. [Google Scholar] [CrossRef] [PubMed]
- Oñatibia-Astibia, A.; Martínez-Pinilla, E.; Franco, R. The potential of methylxanthine-based therapies in pediatric respiratory tract diseases. Respir. Med. 2016, 112, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, R.; Oñatibia-Astibia, A.; Martínez-Pinilla, E. Health benefits of methylxanthines in cacao and chocolate. Nutrients 2013, 5, 4159–4173. [Google Scholar] [CrossRef] [Green Version]
- Franco, R. Café y salud mental. Aten. Primaria 2009, 41, 578–581. [Google Scholar] [CrossRef] [Green Version]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Brambilla, D.; Mancuso, C.; Scuderi, M.R.; Bosco, P.; Cantarella, G.; Lempereur, L.; Di Benedetto, G.; Pezzino, S.; Bernardini, R. The role of antioxidant supplement in immune system, neoplastic, and neurodegenerative disorders: A point of view for an assessment of the risk/benefit profile. Nutr. J. 2008, 7, 29. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2008. [Google Scholar] [CrossRef] [Green Version]
- Bjelakovic, G.; Nikolova, D.; Simonetti, R.G.; Gluud, C. Systematic review: Primary and secondary prevention of gastrointestinal cancers with antioxidant supplements. Aliment. Pharmacol. Ther. 2008, 28, 689–703. [Google Scholar] [CrossRef]
- Halliwell, B.; Aeschbach, R.; Löliger, J.; Aruoma, O.I. The characterization of antioxidants. Food Chem. Toxicol. 1995, 33, 601–617. [Google Scholar] [CrossRef]
- Kaur, C.; Kapoor, H.C. Antioxidants in fruits and vegetables—The millennium’s health. Int. J. Food Sci. Technol. 2008, 36, 703–725. [Google Scholar] [CrossRef]
- Afzal, M.; Armstrong, D. Fractionation of herbal medicine for identifying antioxidant activity. Methods Mol. Biol. 2002, 186, 293–299. [Google Scholar] [PubMed]
- Franco, R.; Navarro, G.; Martínez-Pinilla, E. Hormetic and Mitochondria-Related Mechanisms of Antioxidant Action of Phytochemicals. Antioxidants 2019, 8, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, R.; Martínez-Pinilla, E. Chemical rules on the assessment of antioxidant potential in food and food additives aimed at reducing oxidative stress and neurodegeneration. Food Chem. 2017, 235, 318–323. [Google Scholar] [CrossRef]
- Niki, E.; Traber, M.G. A history of vitamin E. Ann. Nutr. Metab. 2012, 61, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Desmarchelier, C. Genetic variations involved in vitamin E status. Int. J. Mol. Sci. 2016, 17, 2094. [Google Scholar] [CrossRef] [Green Version]
- Franco, R.; Navarro, G.; Martínez-Pinilla, E. Antioxidants versus food antioxidant additives and food preservatives. Antioxidants 2019, 8, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkland, J.B.; Meyer-Ficca, M.L. Niacin. Adv. Food. Nutr. Res. 2018, 83, 83–149. [Google Scholar]
- Ying, W. NAD+ and NADH in brain functions, brain diseases and brain aging. Front. Biosci. 2007, 12, 1863–1888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Sauve, A.A. NAD+ metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim. Biophys. Acta Proteins Proteom. 2016, 1864, 1787–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beya, M.M.; Netzel, M.E.; Sultanbawa, Y.; Smyth, H.; Hoffman, L.C. Plant-based phenolic molecules as natural preservatives in comminuted meats: A review. Antioxidants 2021, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Zehiroglu, C.; Ozturk Sarikaya, S.B. The importance of antioxidants and place in today’s scientific and technological studies. J. Food Sci. Technol. 2019, 56, 4757–4774. [Google Scholar] [CrossRef]
- Scandalios, J.G. Oxidative stress: Molecular perception and transduction of signals triggering antioxidant gene defenses. Braz. J. Med. Biol. Res. 2005, 38, 995–1014. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Navarro, G.; Martínez-Pinilla, E. Antioxidant Defense Mechanisms in Erythrocytes and in the Central Nervous System. Antioxidants 2019, 8, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, X.; Cueto, R.; Effi, C.; Zhang, Y.; Tan, H.; Qin, X.; Ji, Y.; Yang, X.; Wang, H. Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 2019, 26, 101284. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonenn, A.L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebman, S.E.; Le, T.H. Eat your broccoli: Oxidative stress, nrf2, and sulforaphane in chronic kidney disease. Nutrients 2021, 13, 266. [Google Scholar] [CrossRef] [PubMed]
- McWalter, G.K.; Higgins, L.G.; McLellan, L.I.; Henderson, C.J.; Song, L.; Thornalley, P.J.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Transcription factor Nrf2 is essential for induction of NAD(P)H:quinone oxidoreductase 1, glutathione S-transferases, and glutamate cysteine ligase by broccoli seeds and isothiocyanates. J. Nutr. 2004, 134, 3499S–3506S. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Talalay, P.; Cho, C.G.; Posner, G.H. A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403. [Google Scholar] [CrossRef] [Green Version]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician’s Expectation Be Matched by the Reality? Oxid. Med. Cell. Longev. 2016, 2016, 7857186. [Google Scholar] [CrossRef] [Green Version]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Kostov, R.V.; Kensler, T.W. KEAP1 and done? Targeting the NRF2 pathway with sulforaphane. Trends Food Sci. Technol. 2017, 69, 257–269. [Google Scholar] [CrossRef] [Green Version]
- Eggler, A.L.; Savinov, S.N. Chemical and Biological Mechanisms of Phytochemical Activation of NRF2 and Importance in Disease Prevention. In 50 Years of Phytochemistry Research; Springer International Publishing: Berlin/Heidelberg, Germany, 2013; Volume 43, pp. 121–155. [Google Scholar]
- Cintra, E.; Silva, D.D.O.; Estevanato, L.L.C.; Simioni, A.R.; De Andrade Rodrigues, M.M.; Lacava, B.M.; Lacava, Z.G.M.; Tedesco, A.C.; Morais, P.C.; Báo, S.N. Successful strategy for targeting the central nervous system using magnetic albumin nanospheres. J. Biomed. Nanotechnol. 2012, 8, 182–189. [Google Scholar]
- Schreck, R.; Rieber, P.; Baeuerle, P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO J. 1991, 10, 2247–2258. [Google Scholar] [CrossRef]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef] [Green Version]
- Halvey, P.J.; Hansen, J.M.; Johnson, J.M.; Go, Y.M.; Samali, A.; Jones, D.P. Selective oxidative stress in cell nuclei by nuclear-targeted D-amino acid oxidase. Antioxid. Redox Signal. 2007, 9, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.M.; Dick, T.P. Redox Biology on the rise. Biol. Chem. 2012, 393, 999–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naidu, A.S. Redox Life; Bio-Rep Network: Pomona, CA, USA, 2013. [Google Scholar]
- Gitler, C.; Danon, A. Cellular Implications of Redox Signaling; Gitler, C., Danon, A., Eds.; Imperial College Press: London, UK, 2003. [Google Scholar]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Zwieten, R.; Verhoeven, A.J.; Roos, D. Inborn defects in the antioxidant systems of human red blood cells. Free Radic. Biol. Med. 2014, 67, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.; Cheng, M.; Chiu, D.T. Glucose-6-phosphate dehydrogenase—From oxidative stress to cellular functions and degenerative diseases. Redox Rep. 2007, 12, 109–118. [Google Scholar] [CrossRef]
- Pan, M.; Jiang, T.S.; Pan, J.L. Antioxidant Activities of Rapeseed Protein Hydrolysates. Food Bioprocess. Technol. 2011, 4, 1144–1152. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC-Fluorescein) Assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Blois, M. Antioxidant determinations by the use of a stable free. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherma, J. Review of the determination of the antioxidant activity of foods, food ingredients, and dietary supplements by thin layer chromatography-direct bioautography, spectrometry, and the dot-blot procedure. J. AOAC Int. 2018, 101, 1285–1294. [Google Scholar] [CrossRef]
- Pohl, F.; Lin, P.K.T. The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: In vitro, in vivo and clinical trials. Molecules 2018, 23, 3283. [Google Scholar] [CrossRef] [Green Version]
- Cruciani, S.; Trenta, M.; Rassu, G.; Garroni, G.; Petretto, G.L.; Ventura, C.; Maioli, M.; Pintore, G. Identifying a role of red and white wine extracts in counteracting skin aging: Effects of antioxidants on fibroblast behavior. Antioxidants 2021, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Boix, N.; Piqué, E.; Gómez-Catalan, J.; Medina-Remon, A.; Sasot, G.; Mercader-Martí, M.; Llobet, J.M.; Lamuela-Raventos, R.M. Identification of phenolic compounds in red wine extract samples and zebrafish embryos by HPLC-ESI-LTQ-Orbitrap-MS. Food Chem. 2015, 181, 146–151. [Google Scholar] [CrossRef]
- Tai, A.; Sawano, T.; Yazama, F.; Ito, H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim. Biophys. Acta Gen. Subj. 2011, 1810, 170–177. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Silva, T.C.C.; Caviglione, C.V.; Bottura, C.; Fonseca, M.J.V.; Vicentini, F.T.M.C.; Vignoli, J.A.; Baracat, M.M.; et al. Topical formulation containing naringenin: Efficacy against ultraviolet B irradiation-induced skin inflammation and oxidative stress in mice. PLoS ONE 2016, 11, e0146296. [Google Scholar] [CrossRef] [Green Version]
- Wulff, D.L.; Rupert, C.S. Disappearance of thymine photodimer in ultraviolet irradiated DNA upon treatment with a photoreactivating enzyme from Baker’s yeast. Biochem. Biophys. Res. Commun. 1962, 7, 237–240. [Google Scholar] [CrossRef]
- Hart, R.W.; Setlow, R.B. Direct evidence that pyrimidine dimers in DNA result in neoplastic transformation. Basic Life Sci. 1975, 5B, 719–724. [Google Scholar]
- Sancar, A.; Sancar, G.B. Escherichia coli DNA photolyase is a flavoprotein. J. Mol. Biol. 1984, 172, 223–227. [Google Scholar] [CrossRef]
- Radman, M. Phenomenology of an inducible mutagenic DNA repair pathway in Escherichia coli: SOS repair hypothesis. Basic. Life. Sci. 1974, 5A, 355–367. [Google Scholar]
- Witkin, E.M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol. Rev. 1976, 40, 869–907. [Google Scholar] [CrossRef] [PubMed]
- Hanawalt, P.C.; Cooper, P.K.; Ganesan, A.K.; Smith, C.A. DNA Repair in Bacteria and Mammalian Cells. Annu. Rev. Biochem. 1979, 48, 783–836. [Google Scholar] [CrossRef] [PubMed]
- Southan, C.; Ehrlich, J. Effects of extract of western red-cedar heartwood on certain wood-decaying fungi in culture. Phytopathology 1943, 33, 517–524. [Google Scholar]
- Boxenbaum, H.; Neafsey, P.J.; Fournier, D.J. Hormesis, gompertz functions, and risk assessment. Drug Metab. Rev. 1988, 19, 195–229. [Google Scholar] [CrossRef] [PubMed]
- Sutou, S. Black rain in Hiroshima: A critique to the Life Span Study of A-bomb survivors, basis of the linear no-threshold model. Genes Environ. 2019, 42, 1. [Google Scholar] [CrossRef]
- Jargin, S.V. Hormesis and radiation safety norms: Comments for an update. Hum. Exp. Toxicol. 2018, 37, 1233–1243. [Google Scholar] [CrossRef]
- Kudryasheva, N.S.; Kovel, E.S. Monitoring of low-intensity exposures via luminescent bioassays of different complexity: Cells, enzyme reactions, and fluorescent proteins. Int. J. Mol. Sci. 2019, 20, 4451. [Google Scholar] [CrossRef] [Green Version]
- Cuttler, J.M. Application of Low Doses of Ionizing Radiation in Medical Therapies. Dose-Response 2020, 18. [Google Scholar] [CrossRef]
- Jargin, S.V. Radiation Safety and Hormesis. Front. Public Health 2020, 8. [Google Scholar] [CrossRef]
- Fritz-Niggli, H. 100 years of radiobiology: Implications for biomedicine and future perspectives. Experientia 1995, 51, 652–664. [Google Scholar] [CrossRef]
- Hattori, S. Current status and perspectives of research on radiation hormesis in Japan. Chin. Med. J. 1994, 107, 420–424. [Google Scholar] [PubMed]
- Ghandhi, S.A.; Smilenov, L.B.; Elliston, C.D.; Chowdhury, M.; Amundson, S.A. Radiation dose-rate effects on gene expression for human biodosimetry Functional and structural genomics. BMC Med. Genom. 2015, 8, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morifuji, M.; Sakai, K.; Sanbongi, C.; Sugiura, K. Dietary whey protein downregulates fatty acid synthesis in the liver, but upregulates it in skeletal muscle of exercise-trained rats. Nutrition 2005, 21, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Jamurtas, A.Z.; Fatouros, I.G.; Koukosias, N.; Manthou, E.; Tofas, T.; Yfanti, C.; Nikolaidis, M.G.; Koutedakis, Y. Effect of exercise on oxidative stress in individuals with glucose-6-phosphate dehydrogenase deficiency. In Vivo 2006, 20, 875–880. [Google Scholar]
- Georgakouli, K.; Fatouros, I.G.; Draganidis, D.; Papanikolaou, K.; Tsimeas, P.; Deli, C.K.; Jamurtas, A.Z. Exercise in glucose-6-phosphate dehydrogenase deficiency: Harmful or harmless? A narrative review. Oxid. Med. Cell. Longev. 2019, 2019, 8060193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sodeinde, O. Glucose-6-phosphate dehydrogenase deficiency. Baillieres. Clin. Haematol. 1992, 5, 367–382. [Google Scholar] [CrossRef]
- Baker, M.A.; Bosia, A.; Pescarmona, G.; Turrini, F.; Arese, P. Mechanism of Action of Divicine in a Cell-free System and in Glucose-6-phosphate Dehydrogenase-deficient Red Cells. Toxicol. Pathol. 1984, 12, 331–336. [Google Scholar] [CrossRef]
- Vural, N.; Sardas, S. Biological activities of broad bean (Vicia faba L.) extracts cultivated in South Anatolia in favism sensitive subjects. Toxicology 1984, 31, 175–179. [Google Scholar] [CrossRef]
- Luzzatto, L.; Nannelli, C.; Notaro, R. Glucose-6-Phosphate Dehydrogenase Deficiency. Hematol. Oncol. Clin. N. Am. 2016, 30, 373–393. [Google Scholar] [CrossRef]
- Lessire, M.; Gallo, V.; Prato, M.; Akide-Ndunge, O.; Mandili, G.; Marget, P.; Arese, P.; Duc, G. Effects of faba beans with different concentrations of vicine and convicine on egg production, egg quality and red blood cells in laying hens. Animal 2017, 11, 1270–1278. [Google Scholar] [CrossRef] [Green Version]
- La Marca, M.; Beffy, P.; Della Croce, C.; Gervasi, P.G.; Iori, R.; Puccinelli, E.; Longo, V. Structural influence of isothiocyanates on expression of cytochrome P450, phase II enzymes, and activation of Nrf2 in primary rat hepatocytes. Food Chem. Toxicol. 2012, 50, 2822–2830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adegbeye, M.J.; Reddy, P.R.K.; Chilaka, C.A.; Balogun, O.B.; Elghandour, M.M.M.Y.; Rivas-Caceres, R.R.; Salem, A.Z.M. Mycotoxin toxicity and residue in animal products: Prevalence, consumer exposure and reduction strategies—A review. Toxicon 2020, 177, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Saleh, D.O.; Mansour, D.F.; Hashad, I.M.; Bakeer, R.M. Effects of sulforaphane on D-galactose-induced liver aging in rats: Role of keap-1/nrf-2 pathway. Eur. J. Pharmacol. 2019, 855, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.L.; Chang, J.L.; Peterson, S.; Chen, C.; King, I.B.; Schwarz, Y.; Li, S.S.; Li, L.; Potter, J.D.; Lampe, J.W. Modulation of human serum glutathione S-transferase A1/2 concentration by cruciferous vegetables in a controlled feeding study is influenced by GSTM1 and GSTT1 genotypes. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2974–2978. [Google Scholar] [CrossRef] [Green Version]
- Wark, P.A.; Grubben, M.J.A.L.; Peters, W.H.M.; Nagengast, F.M.; Kampman, E.; Kok, F.J.; van’t Veer, P. Habitual consumption of fruits and vegetables: Associations with human rectal glutathione S-transferase. Carcinogenesis 2004, 25, 2135–2142. [Google Scholar] [CrossRef] [Green Version]
- Tomar, S.; Hogan, S.P. Recent advances in mechanisms of food allergy and anaphylaxis. F1000Research 2020, 9, 863. [Google Scholar] [CrossRef]
- Imlay, J.A.; Linn, S. Mutagenesis and stress responses induced in Escherichia coli by hydrogen peroxide. J. Bacteriol. 1987, 169, 2967–2976. [Google Scholar] [CrossRef] [Green Version]
- Dalton, T.; Palmiter, R.D.; Andrews, G.K. Transcriptional induction of the mouse metallothionein-I gene in hydrogen peroxide-treated hepa cells involves a composite major late transcription factor/antioxidant response element and metal response promoter elements. Nucleic Acids Res. 1994, 22, 5016–5023. [Google Scholar] [CrossRef] [Green Version]
- Röhrdanz, E.; Kahl, R. Alterations of antioxidant enzyme expression in response to hydrogen peroxide. Free Radic. Biol. Med. 1998, 24, 27–38. [Google Scholar] [CrossRef]
- Nelson, S.K.; Bose, S.K.; Grunwald, G.K.; Myhill, P.; McCord, J.M. The induction of human superoxide dismutase and catalase in vivo: A fundamentally new approach to antioxidant therapy. Free Radic. Biol. Med. 2006, 40, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Hagag, A.A.; Badraia, I.M.; Elfarargy, M.S.; Abd Elmageed, M.M.; Abo-Ali, E.A. Study of Glucose-6-Phosphate Dehydrogenase Deficiency: 5 Years Retrospective Egyptian Study. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Jost, W.H.; Schimrigk, K. Constipation in Parkinson’s disease. Klin. Wochenschr. 1991, 69, 906–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, A.; Biswas, A.; Das, S.K. Gut dysfunction in Parkinson’s disease. World J. Gastroenterol. 2016, 22, 5742–5752. [Google Scholar] [CrossRef]
- Skjærbæk, C.; Knudsen, K.; Horsager, J.; Borghammer, P. Gastrointestinal Dysfunction in Parkinson’s Disease. J. Clin. Med. 2021, 10, 493. [Google Scholar] [CrossRef]
- Parashar, A.; Udayabanu, M. Gut microbiota: Implications in Parkinson’s disease. Park. Relat. Disord. 2017, 38, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mulak, A.; Bonaz, B. Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol. 2015, 21, 10609–10620. [Google Scholar] [CrossRef]
- Huang, Y.; Liao, J.; Liu, X.; Zhong, Y.; Cai, X.; Long, L. Review: The Role of Intestinal Dysbiosis in Parkinson’s Disease. Front. Cell. Infect. Microbiol. 2021, 11. [Google Scholar] [CrossRef]
- Munoz-Pinto, M.F.; Empadinhas, N.; Cardoso, S.M. The neuromicrobiology of Parkinson’s disease: A unifying theory. Ageing Res. Rev. 2021, 70, 101396. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, R.; Casanovas, B.; Camps, J.; Navarro, G.; Martínez-Pinilla, E. Antioxidant Supplements versus Health Benefits of Brief/Intermittent Exposure to Potentially Toxic Physical or Chemical Agents. Curr. Issues Mol. Biol. 2021, 43, 650-664. https://doi.org/10.3390/cimb43020047
Franco R, Casanovas B, Camps J, Navarro G, Martínez-Pinilla E. Antioxidant Supplements versus Health Benefits of Brief/Intermittent Exposure to Potentially Toxic Physical or Chemical Agents. Current Issues in Molecular Biology. 2021; 43(2):650-664. https://doi.org/10.3390/cimb43020047
Chicago/Turabian StyleFranco, Rafael, Berta Casanovas, Jordi Camps, Gemma Navarro, and Eva Martínez-Pinilla. 2021. "Antioxidant Supplements versus Health Benefits of Brief/Intermittent Exposure to Potentially Toxic Physical or Chemical Agents" Current Issues in Molecular Biology 43, no. 2: 650-664. https://doi.org/10.3390/cimb43020047
APA StyleFranco, R., Casanovas, B., Camps, J., Navarro, G., & Martínez-Pinilla, E. (2021). Antioxidant Supplements versus Health Benefits of Brief/Intermittent Exposure to Potentially Toxic Physical or Chemical Agents. Current Issues in Molecular Biology, 43(2), 650-664. https://doi.org/10.3390/cimb43020047