Effect of Azithromycin on Mineralized Nodule Formation in MC3T3-E1 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture and Azithromycin Stimulation
2.3. Cell Proliferation and ALPase Activity
2.4. Alizarin Red S Staining
2.5. Real-Time PCR
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Statistical Analyses
3. Results
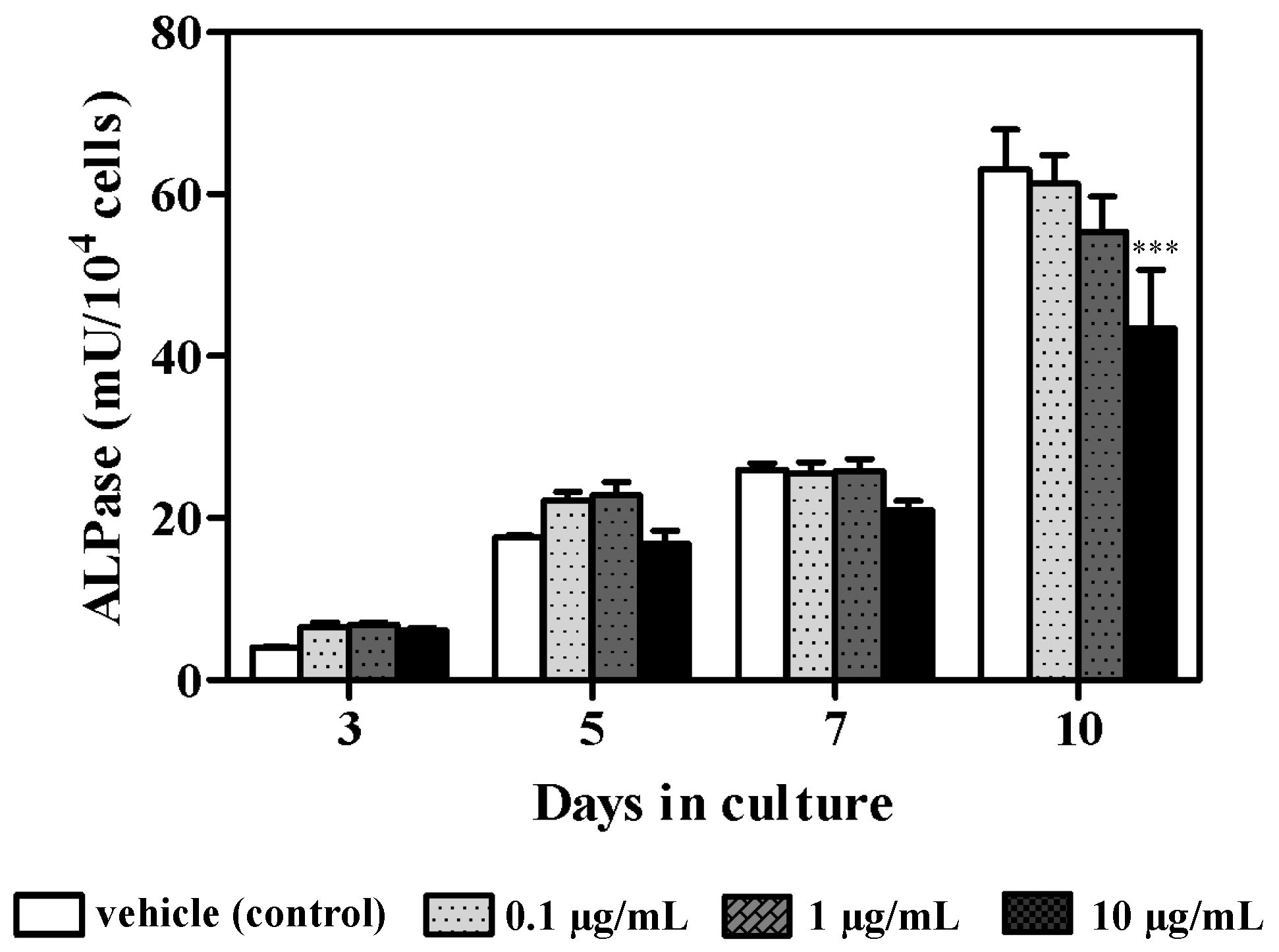
3.1. Effect of Azithromycin on Cellular Proliferation and ALPase Activity
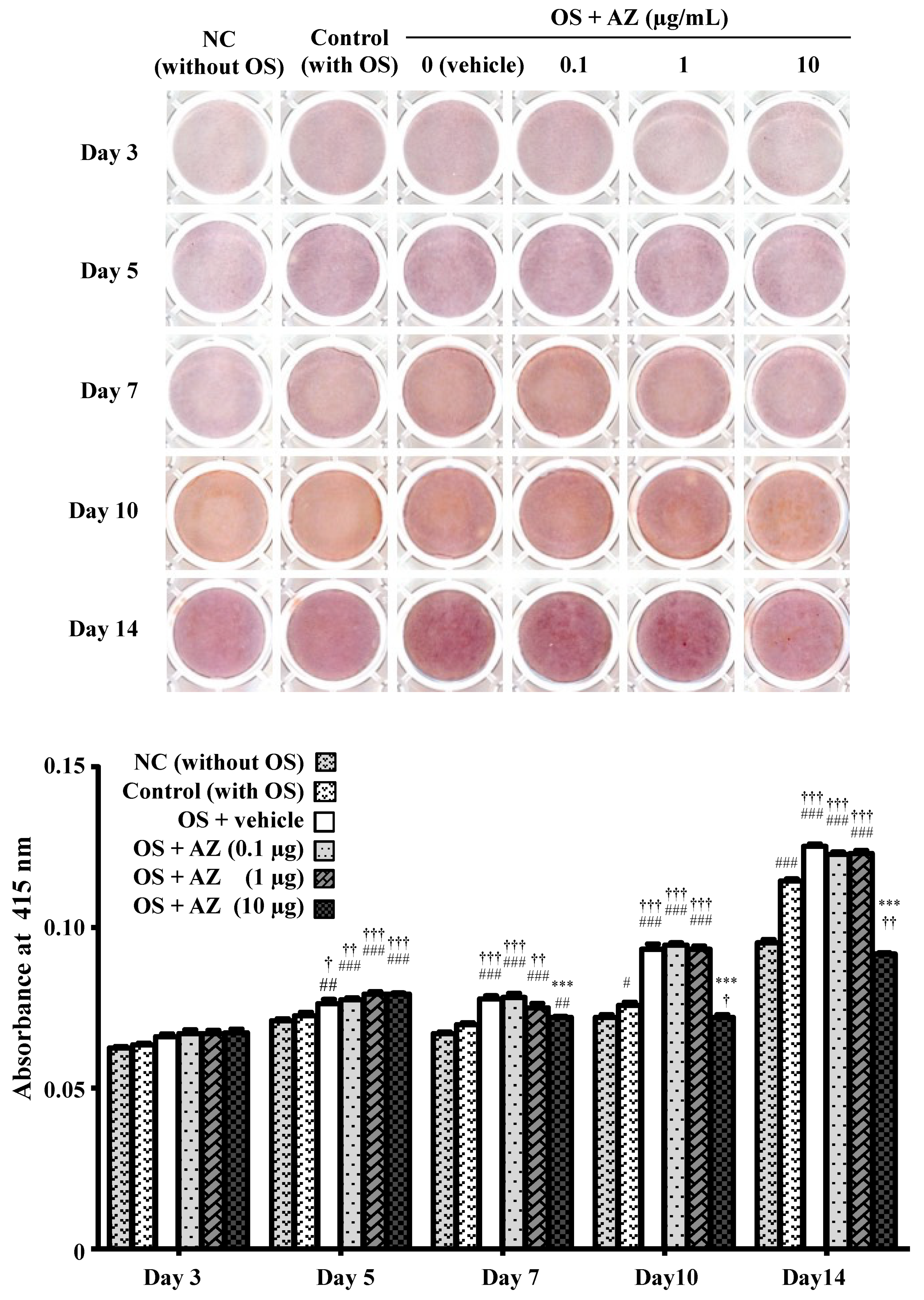
3.2. Effect of Azithromycin on Mineralized Nodule Formation
3.3. Effects of Azithromycin on Bone Matrix Protein mRNA Expression
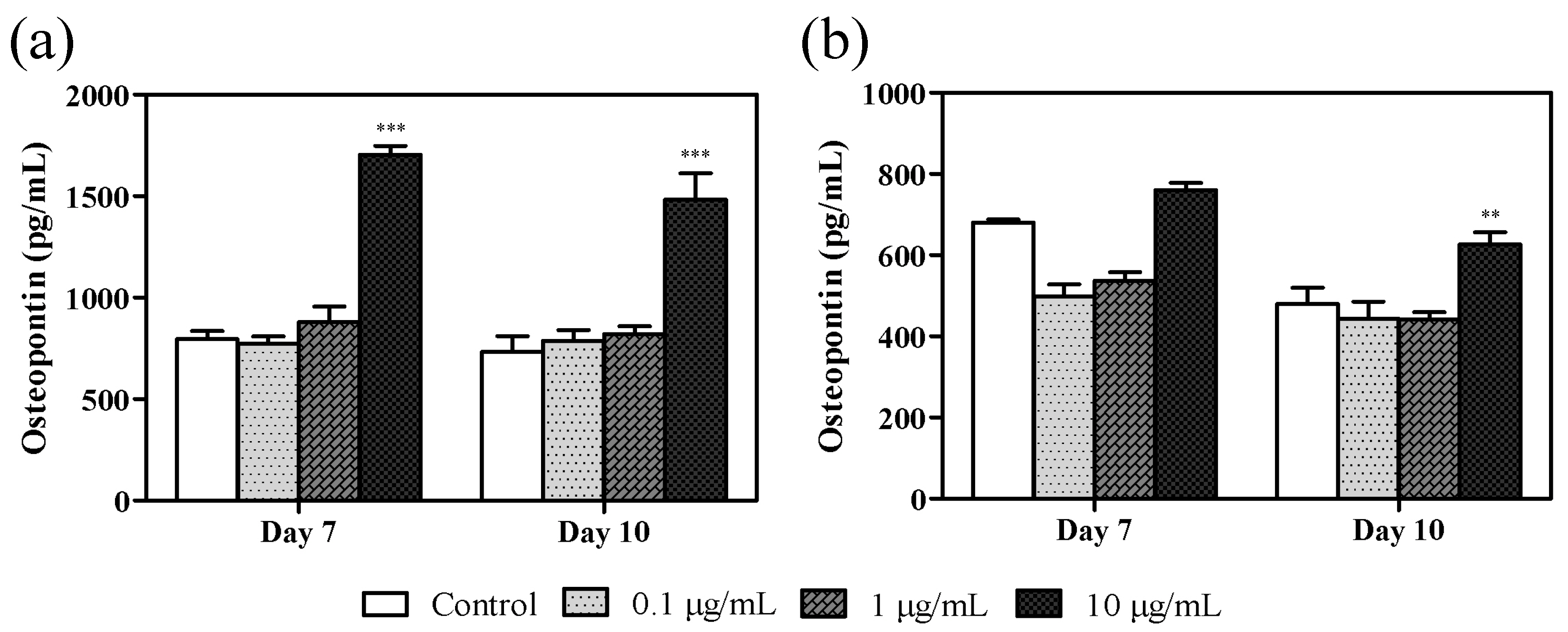
3.4. Effect of Azithromycin on Osteopontin Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakheit, A.H.; Al-Hadiya, B.M.; Abd-Elgalil, A.A. Azithromycin. Profiles Drug Subst. Excip. Relat. Methodol. 2014, 39, 1–40. [Google Scholar] [CrossRef]
- Uzun, S.; Djamin, R.S.; Kluytmans, J.A.; Mulder, P.G.; van’t Veer, N.E.; Ermens, A.A.; Pelle, A.J.; Hoogsteden, H.C.; Aerts, J.G.; van der Eerden, M.M. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): A randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2014, 2, 361–368. [Google Scholar] [CrossRef]
- Parnham, M.J.; Erakovic Haber, V.; Giamarellos-Bourboulis, E.J.; Perletti, G.; Verleden, G.M.; Vos, R. Azithromycin: Mechanisms of action and their relevance for clinical applications. Pharmacol. Ther. 2014, 143, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Keestra, J.A.J.; Grosjean, I.; Coucke, W.; Quirynen, M.; Teughels, W. Non-surgical periodontal therapy with systemic antibiotics in patients with untreated chronic periodontitis: A systematic review and meta-analysis. J. Periodontal. Res. 2015, 50, 294–314. [Google Scholar] [CrossRef] [PubMed]
- Foulds, G.; Shepard, R.M.; Johnson, R.B. The pharmacokinetics of azithromycin in human serum and tissues. J. Antimicrob. Chemother. 1990, 25, 73–82. [Google Scholar] [CrossRef]
- Foulds, G.; Johnson, R.B. Selection of dose regimens of azithromycin. J. Antimicrob Chemother 1993, 31, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Gomi, K.; Yashima, A.; Lino, F.; Kanazashi, M.; Nagano, T.; Shibukawa, N.; Ohshima, T.; Maeda, N.; Arai, T. Drug concentration in inflamed periodontal tissues after systemically administered azithromycin. J. Periodontol. 2007, 78, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Katsimbri, P. The biology of normal bone remodelling. Eur. J. Cancer Care (Engl.) 2017, 26, e12740. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-osteoclast interactions. Connect. Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef]
- Reszka, A.A.; Rodan, G.A. Bisphosphonate mechanism of action. Curr. Rheumatol. Rep. 2003, 5, 65–74. [Google Scholar] [CrossRef]
- Nagasaki, M.; Nakai, K.; Tanaka, H.; Ozaki, M.; Kato, K.; Koshi, R.; Maeno, M.; Nishikubo, S.; Kawato, T.; Tonogi, M. Lipopolysaccharide and high concentrations of glucose enhances zoledronate-induced increase in RANKL/OPG ratio by upregulating PGE2 production in osteoblasts. J. Hard. Tissue Biol. 2021, 30, 37–44. [Google Scholar] [CrossRef]
- Kirchgatterer, A.; Aschl, G.; Knoflach, P. Steroid-induced osteoporosis: Pathogenesis and therapeutic consequences. Acta Med. Austriaca 2000, 27, 23–26. [Google Scholar] [CrossRef]
- Hirsch, R. Periodontal healing and bone regeneration in response to azithromycin. Aust. Dent. J. 2010, 55, 193–199. [Google Scholar] [CrossRef]
- Hirsch, R.; Deng, H.; Laohachai, M.N. Azithromycin in periodontal treatment: More than an antibiotic. J. Periodontal. Res. 2012, 47, 137–148. [Google Scholar] [CrossRef]
- Gannon, S.C.; Cantley, M.D.; Haynes, D.R.; Hirsch, R.; Bartold, P.M. Azithromycin suppresses human osteoclast formation and activity in vitro. J. Cell Physiol. 2013, 228, 1098–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, C.J.; Fitzsimmons, T.R.; Marchant, C.; Dharmapatni, A.A.; Hirsch, R.; Bartold, P.M. Azithromycin suppresses P. gingivalis LPS-induced pro-inflammatory cytokine and chemokine production by human gingival fibroblasts in vitro. Clin. Oral Investig. 2015, 19, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lee, Y.S.; Choi, E.Y.; Choi, J.I.; Choi, I.S.; Kim, S.J. Subantibiotic dose of azithromycin attenuates alveolar bone destruction and improves trabecular microarchitectures in a rat model of experimental periodontitis: A study using micro-computed tomography. Int. Immunopharmacol. 2017, 47, 212–217. [Google Scholar] [CrossRef]
- Andrada, A.C.; Azuma, M.M.; Furusho, H.; Hirai, K.; Xu, S.; White, R.R.; Sasaki, H. Immunomodulation mediated by azithromycin in experimental periapical inflammation. J. Endod. 2020, 46, 1648–1654. [Google Scholar] [CrossRef]
- Nakai, K.; Kawato, T.; Morita, T.; Iinuma, T.; Kamio, N.; Zhao, N.; Maeno, M. Angiotensin II induces the production of MMP-3 and MMP-13 through the MAPK signaling pathways via the AT1 receptor in osteoblasts. Biochimie 2013, 95, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.M.; Ng, W.W.; Kung, A.W. Dimethyl sulfoxide as an inducer of differentiation in preosteoblast MC3T3-E1 cells. FEBS Lett. 2006, 580, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Malizia, T.; Tejada, M.R.; Ghelardi, E.; Senesi, S.; Gabriele, M.; Giuca, M.R.; Blandizzi, C.; Danesi, R.; Campa, M.; Del Tacca, M. Periodontal tissue disposition of azithromycin. J. Periodontol. 1997, 68, 1206–1209. [Google Scholar] [CrossRef] [PubMed]
- Blandizzi, C.; Malizia, T.; Lupetti, A.; Pesce, D.; Gabriele, M.; Giuca, M.R.; Campa, M.; Del Tacca, M.; Senesi, S. Periodontal tissue disposition of azithromycin in patients affected by chronic inflammatory periodontal diseases. J. Periodontol. 1999, 70, 960–966. [Google Scholar] [CrossRef]
- Malizia, T.; Batoni, G.; Ghelardi, E.; Baschiera, F.; Graziani, F.; Blandizzi, C.; Gabriele, M.; Campa, M.; Del Tacca, M.; Senesi, S. Interaction between piroxicam and azithromycin during distribution to human periodontal tissues. J. Periodontol. 2001, 72, 1151–1156. [Google Scholar] [CrossRef]
- Gordon, J.A.; Tye, C.E.; Sampaio, A.V.; Underhill, T.M.; Hunter, G.K.; Goldberg, H.A. Bone sialoprotein expression enhances osteoblast differentiation and matrix mineralization in vitro. Bone 2007, 41, 462–473. [Google Scholar] [CrossRef]
- Baht, G.S.; Hunter, G.K.; Goldberg, H.A. Bone sialoprotein-collagen interaction promotes hydroxyapatite nucleation. Matrix Biol. 2008, 27, 600–608. [Google Scholar] [CrossRef]
- Rammelt, S.; Neumann, M.; Hanisch, U.; Reinstorf, A.; Pompe, W.; Zwipp, H.; Biewener, A. Osteocalcin enhances bone remodeling around hydroxyapatite/collagen composites. J. Biomed. Mater. Res. A 2005, 73, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.C. Mechanism of mineral formation in bone. Lab. Investig. 1989, 60, 320–330. [Google Scholar]
- Addison, W.N.; Azari, F.; Sørensen, E.S.; Kaartinen, M.T.; McKee, M.D. Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J. Biol. Chem. 2007, 282, 15872–15883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jono, S.; Peinado, C.; Giachelli, C.M. Phosphorylation of osteopontin is required for inhibition of vascular smooth muscle cell calcification. J. Biol. Chem. 2000, 275, 20197–20203. [Google Scholar] [CrossRef] [Green Version]
- Boskey, A.L.; Maresca, M.; Ullrich, W.; Doty, S.B.; Butler, W.T.; Prince, C.W. Osteopontin-hydroxyapatite interactions in vitro: Inhibition of hydroxyapatite formation and growth in a gelatin-gel. Bone Miner. 1993, 22, 147–159. [Google Scholar] [CrossRef]
- Hunter, G.K.; Kyle, C.L.; Goldberg, H.A. Modulation of crystal formation by bone phosphoproteins: Structural specificity of the osteopontin-mediated inhibition of hydroxyapatite formation. Biochem. J. 1994, 300, 723–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, K.; Ozaki, M.; Nakai, K.; Nagasaki, M.; Nakajima, J.; Koshi, R.; Tanaka, H.; Kawato, T.; Tonogi, M. Effect of Azithromycin on Mineralized Nodule Formation in MC3T3-E1 Cells. Curr. Issues Mol. Biol. 2021, 43, 1451-1459. https://doi.org/10.3390/cimb43030102
Kato K, Ozaki M, Nakai K, Nagasaki M, Nakajima J, Koshi R, Tanaka H, Kawato T, Tonogi M. Effect of Azithromycin on Mineralized Nodule Formation in MC3T3-E1 Cells. Current Issues in Molecular Biology. 2021; 43(3):1451-1459. https://doi.org/10.3390/cimb43030102
Chicago/Turabian StyleKato, Kengo, Manami Ozaki, Kumiko Nakai, Maki Nagasaki, Junya Nakajima, Ryosuke Koshi, Hideki Tanaka, Takayuki Kawato, and Morio Tonogi. 2021. "Effect of Azithromycin on Mineralized Nodule Formation in MC3T3-E1 Cells" Current Issues in Molecular Biology 43, no. 3: 1451-1459. https://doi.org/10.3390/cimb43030102
APA StyleKato, K., Ozaki, M., Nakai, K., Nagasaki, M., Nakajima, J., Koshi, R., Tanaka, H., Kawato, T., & Tonogi, M. (2021). Effect of Azithromycin on Mineralized Nodule Formation in MC3T3-E1 Cells. Current Issues in Molecular Biology, 43(3), 1451-1459. https://doi.org/10.3390/cimb43030102





