Modulation of the Physical Properties of 3D Spheroids Derived from Human Scleral Stroma Fibroblasts (HSSFs) with Different Axial Lengths Obtained from Surgical Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of 3D Spheroid Cultures of Human Scleral Stroma Fibroblasts (HSSFs)
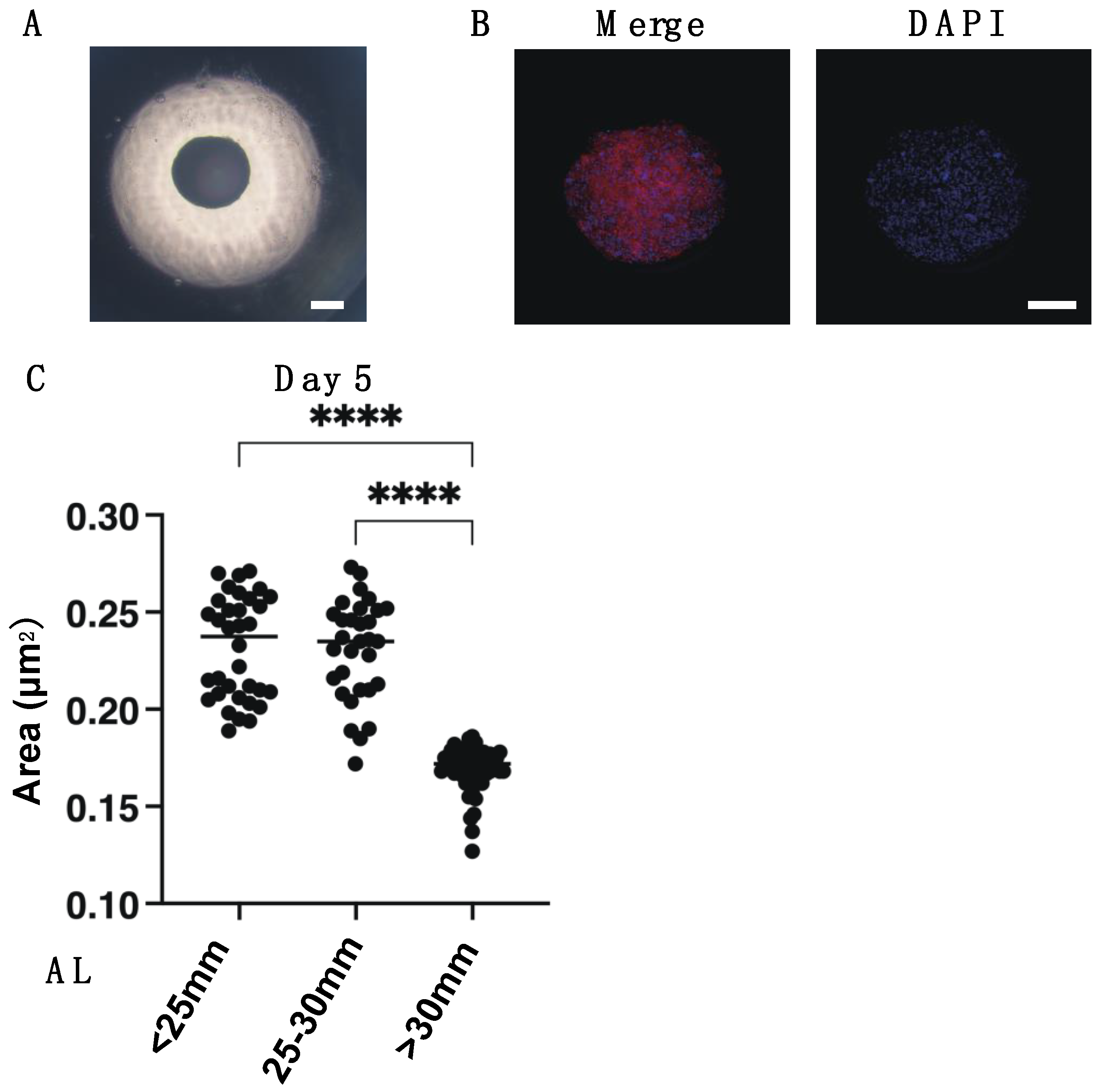
2.2. Measurement of 3D HSSFs Spheroid Sizes
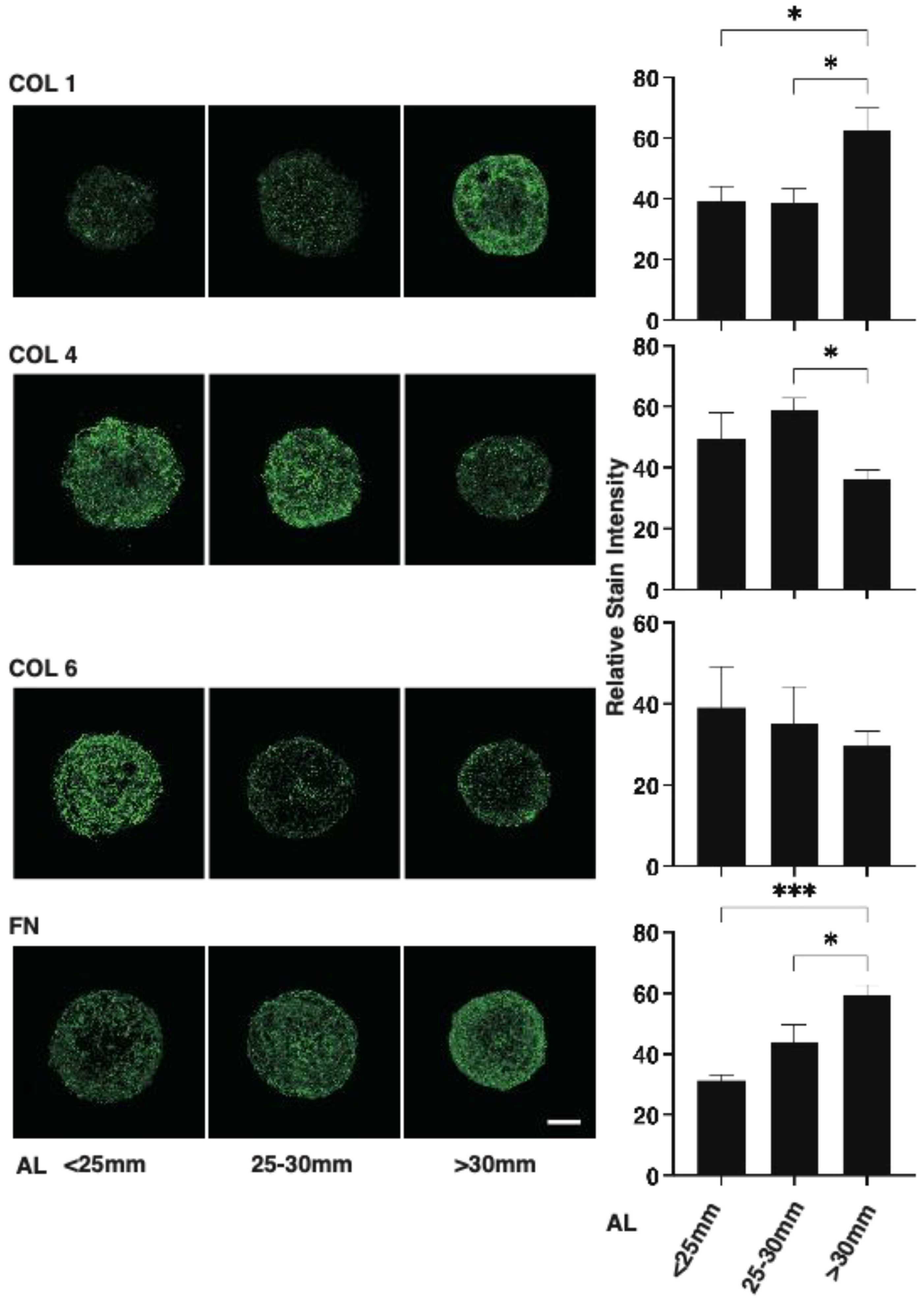
2.3. Immunohistochemistry of 3D HSSFs Spheroids
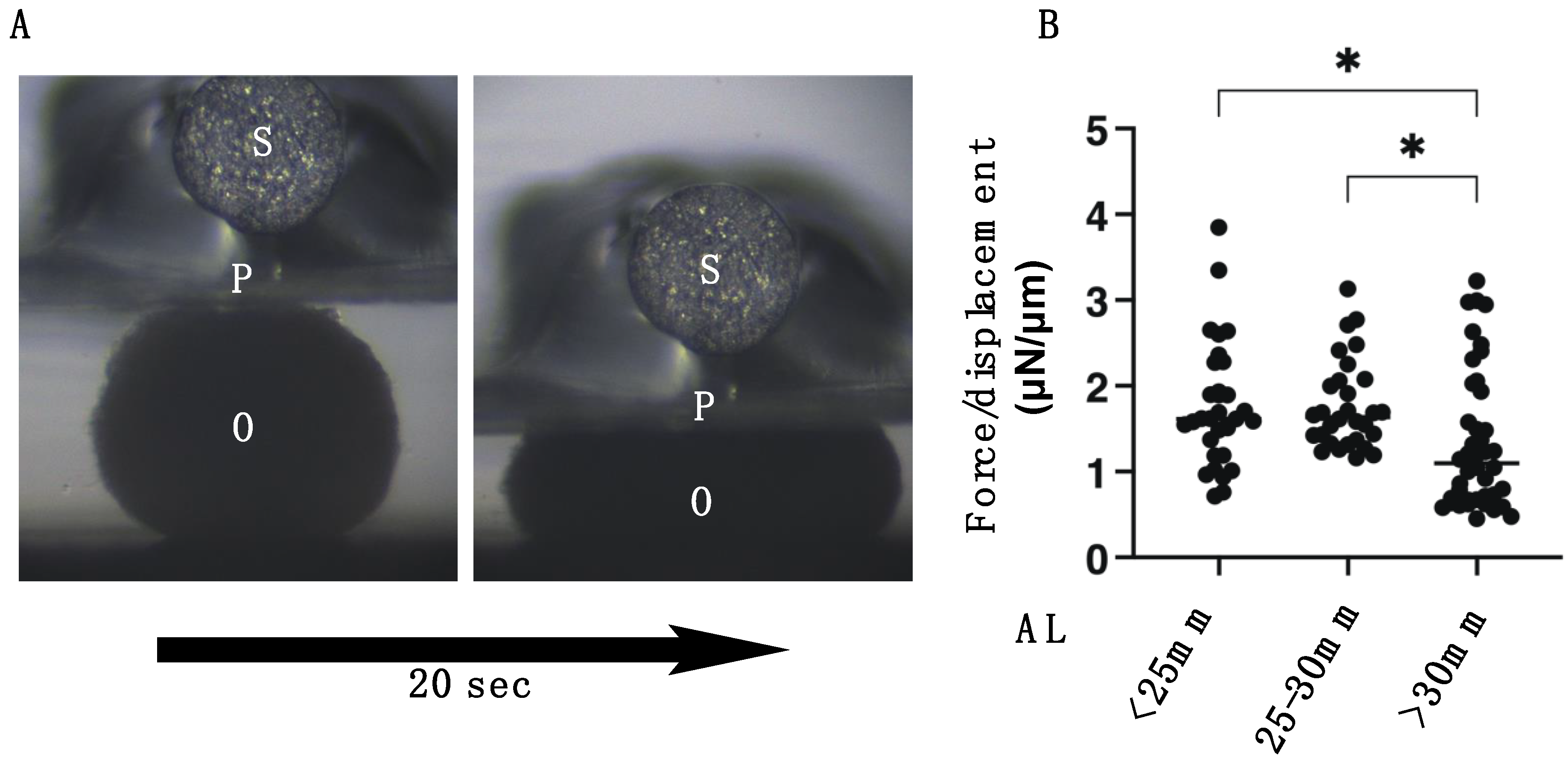
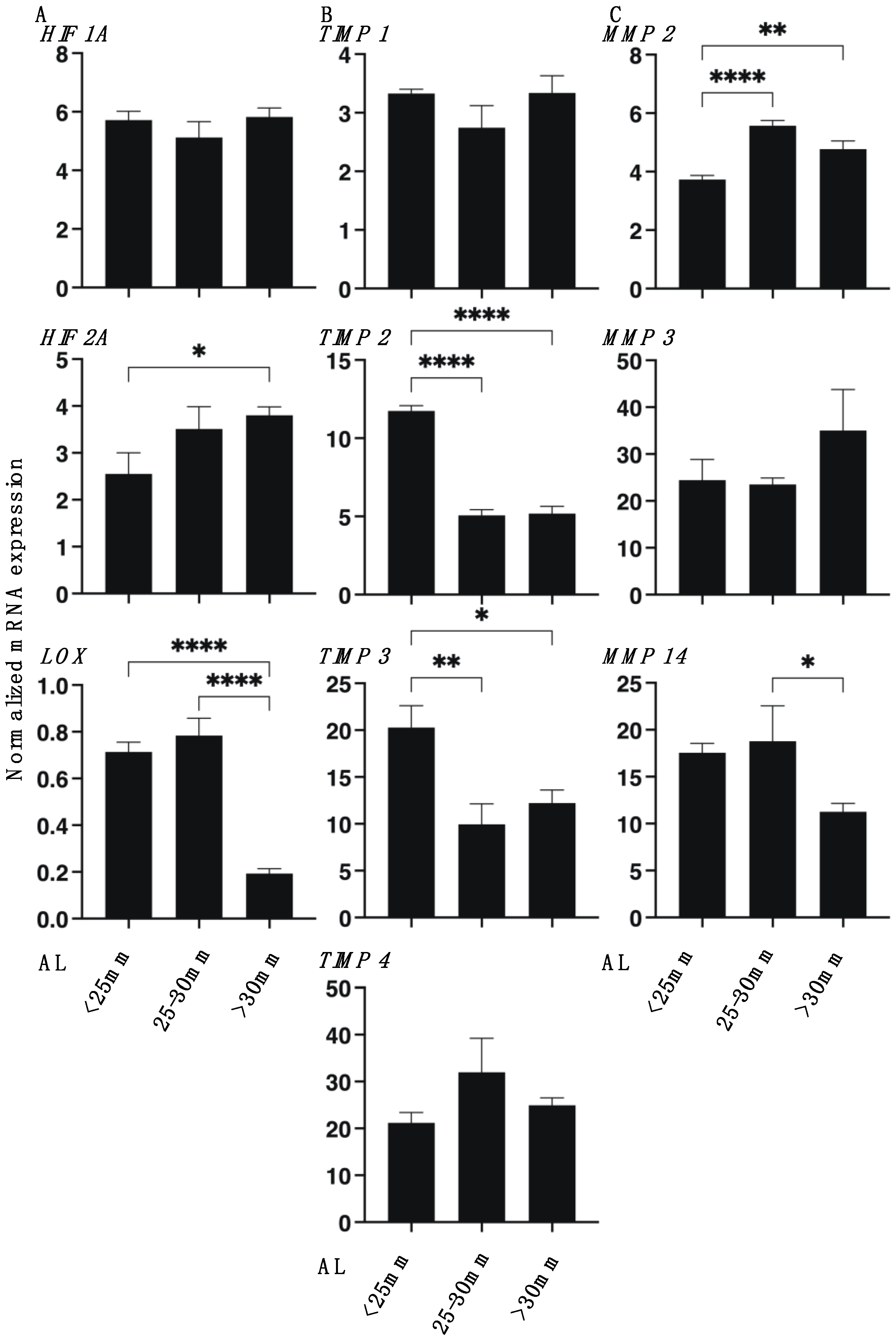
2.4. Quantitative PCR and Solidity Measurement of 3D Spheroids
2.5. Statistical Analysis
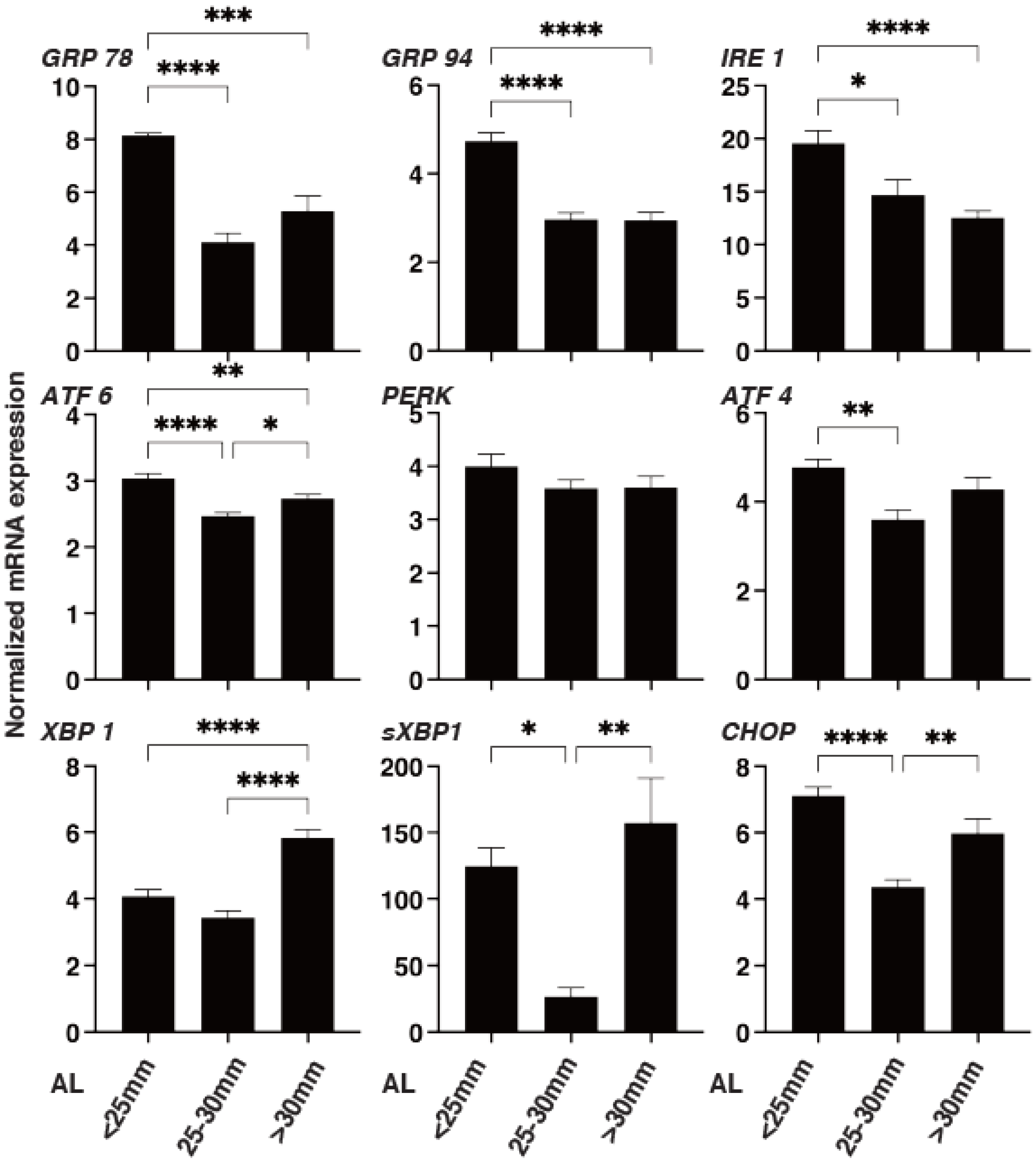
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boote, C.; Sigal, I.A.; Grytz, R.; Hua, Y.; Nguyen, T.D.; Girard, M.J.A. Scleral structure and biomechanics. Prog. Retin. Eye Res. 2020, 74, 100773. [Google Scholar] [CrossRef]
- McBrien, N.A.; Gentle, A. Role of the sclera in the development and pathological complications of myopia. Prog. Retin. Eye Res. 2003, 22, 307–338. [Google Scholar] [CrossRef]
- Metlapally, R.; Wildsoet, C.F. Scleral Mechanisms Underlying Ocular Growth and Myopia. Prog. Mol. Biol. Transl. Sci. 2015, 134, 241–248. [Google Scholar]
- Harper, A.R.; Summers, J.A. The dynamic sclera: Extracellular matrix remodeling in normal ocular growth and myopia development. Exp. Eye Res. 2015, 133, 100–111. [Google Scholar] [CrossRef] [Green Version]
- Gentle, A.; Liu, Y.; Martin, J.E.; Conti, G.L.; McBrien, N.A. Collagen gene expression and the altered accumulation of scleral collagen during the development of high myopia. J. Biol. Chem. 2003, 278, 16587–16594. [Google Scholar] [CrossRef] [Green Version]
- Siegwart, J.T., Jr.; Norton, T.T. Selective regulation of MMP and TIMP mRNA levels in tree shrew sclera during minus lens compensation and recovery. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3484–3492. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.H.; Kenning, M.S.; Jobling, A.I.; McBrien, N.A.; Gentle, A. Reduced Scleral TIMP-2 Expression Is Associated With Myopia Development: TIMP-2 Supplementation Stabilizes Scleral Biomarkers of Myopia and Limits Myopia Development. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1971–1981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBrien, N.A.; Lawlor, P.; Gentle, A. Scleral remodeling during the development of and recovery from axial myopia in the tree shrew. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3713–3719. [Google Scholar]
- McBrien, N.A.; Cornell, L.M.; Gentle, A. Structural and ultrastructural changes to the sclera in a mammalian model of high myopia. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2179–2187. [Google Scholar]
- Hikage, F.; Atkins, S.; Kahana, A.; Smith, T.J.; Chun, T.H. HIF2A-LOX Pathway Promotes Fibrotic Tissue Remodeling in Thyroid-Associated Orbitopathy. Endocrinology 2019, 160, 20–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, K.; Hikage, F.; Ida, Y.; Ohguro, H. Prostaglandin F2α Agonists Negatively Modulate the Size of 3D Organoids from Primary Human Orbital Fibroblasts. Investig. Ophthalmol. Vis. Sci. 2020, 61, 13. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Ida, Y.; Ohguro, H.; Hikage, F. Prostaglandin F2α agonists induced enhancement in collagen1 expression is involved in the pathogenesis of the deepening of upper eyelid sulcus. Sci. Rep. 2021, 11, 9002. [Google Scholar] [CrossRef] [PubMed]
- Ota, C.; Ida, Y.; Ohguro, H.; Hikage, F. ROCK inhibitors beneficially alter the spatial configuration of TGFβ2-treated 3D organoids from a human trabecular meshwork (HTM). Sci. Rep. 2020, 10, 20292. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, H.; Jalilian, I.; Murphy, C.J.; Thomasy, S.M. Modulation of human corneal stromal cell differentiation by hepatocyte growth factor and substratum compliance. Exp. Eye Res. 2018, 176, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Ida, Y.; Hikage, F.; Itoh, K.; Ida, H.; Ohguro, H. Prostaglandin F2α agonist-induced suppression of 3T3-L1 cell adipogenesis affects spatial formation of extra-cellular matrix. Sci. Rep. 2020, 10, 7958. [Google Scholar] [CrossRef] [PubMed]
- Ida, Y.; Hikage, F.; Ohguro, H. ROCK inhibitors enhance the production of large lipid-enriched 3D organoids of 3T3-L1 cells. Sci. Rep. 2021, 11, 5479. [Google Scholar] [CrossRef] [PubMed]
- Oouchi, Y.; Watanabe, M.; Ida, Y.; Ohguro, H.; Hikage, F. Rosiglitasone and ROCK Inhibitors Modulate Fibrogenetic Changes in TGF-β2 Treated Human Conjunctival Fibroblasts (HconF) in Different Manners. Int. J. Mol. Sci. 2021, 22, 7335. [Google Scholar] [CrossRef] [PubMed]
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Rada, J.A.; Shelton, S.; Norton, T.T. The sclera and myopia. Exp. Eye Res. 2006, 82, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Keeley, F.W.; Morin, J.D.; Vesely, S. Characterization of collagen from normal human sclera. Exp. Eye Res. 1984, 39, 533–542. [Google Scholar] [CrossRef]
- Larsen, J.S. The sagittal growth of the eye: IV. Ultrasonic measurement of the axial length of the eye from birth to puberty. Acta Ophthalmol. 1971, 49, 873–886. [Google Scholar]
- Zadnik, K.; Satariano, W.A.; Mutti, D.O.; Sholtz, R.I.; Adams, A.J. The effect of parental history of myopia on children’s eye size. JAMA 1994, 271, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Curtin, B.J. The posterior staphyloma of pathologic myopia. Trans. Am. Ophthalmol. Soc. 1977, 75, 67–86. [Google Scholar] [PubMed]
- Avetisov, E.S.; Savitskaya, N.F.; Vinetskaya, M.I.; Iomdina, E.N. A study of biochemical and biomechanical qualities of normal and myopic eye sclera in humans of different age groups. Metab. Pediatric Syst. Ophthalmol. 1983, 7, 183–188. [Google Scholar]
- Rada, J.A.; Nickla, D.L.; Troilo, D. Decreased proteoglycan synthesis associated with form deprivation myopia in mature primate eyes. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2050–2058. [Google Scholar]
- Schaeffel, F.; Feldkaemper, M. Animal models in myopia research. Clin. Exp. Optom. 2015, 98, 507–517. [Google Scholar] [CrossRef] [Green Version]
- Yinon, U.; Koslowe, K.C.; Lobel, D.; Landshman, N.; Barishak, Y.R. Lid suture myopia in developing chicks: Optical and structural considerations. Curr. Eye Res. 1982, 2, 877–882. [Google Scholar] [CrossRef]
- Criswell, M.H.; Goss, D.A. Myopia development in nonhuman primates—A literature review. Am. J. Optom. Physiol. Opt. 1983, 60, 250–268. [Google Scholar] [CrossRef]
- Troilo, D.; Judge, S.J. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus). Vis. Res. 1993, 33, 1311–1324. [Google Scholar] [CrossRef]
- Wiesel, T.N.; Raviola, E. Myopia and eye enlargement after neonatal lid fusion in monkeys. Nature 1977, 266, 66–68. [Google Scholar] [CrossRef]
- Tejedor, J.; de la Villa, P. Refractive changes induced by form deprivation in the mouse eye. Investig. Ophthalmol. Vis. Sci. 2003, 44, 32–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goss, D.A.; Criswell, M.H. Myopia development in experimental animals-a literature review. Am. J. Optom. Physiol. Opt. 1981, 58, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Yeh, L.K.; Liu, C.Y.; Kao, W.W.; Huang, C.J.; Hu, F.R.; Chien, C.L.; Wang, I.J. Knockdown of zebrafish lumican gene (zlum) causes scleral thinning and increased size of scleral coats. J. Biol. Chem. 2010, 285, 28141–28155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schippert, R.; Brand, C.; Schaeffel, F.; Feldkaemper, M.P. Changes in scleral MMP-2, TIMP-2 and TGFbeta-2 mRNA expression after imposed myopic and hyperopic defocus in chickens. Exp. Eye Res. 2006, 82, 710–719. [Google Scholar] [CrossRef] [PubMed]
- McBrien, N.A.; Gentle, A. The role of visual information in the control of scleral matrix biology in myopia. Curr. Eye Res. 2001, 23, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Semenza, G.L. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol. Rev. 2012, 92, 967–1003. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.Y.; Safran, M.; Buckley, M.R.; Ebert, B.L.; Glickman, J.; Bosenberg, M.; Regan, M.; Kaelin, W.G., Jr. Failure to prolyl hydroxylate hypoxia-inducible factor alpha phenocopies VHL inactivation in vivo. EMBO J. 2006, 25, 4650–4662. [Google Scholar] [CrossRef]
- Halberg, N.; Khan, T.; Trujillo, M.E.; Wernstedt-Asterholm, I.; Attie, A.D.; Sherwani, S.; Wang, Z.V.; Landskroner-Eiger, S.; Dineen, S.; Magalang, U.J.; et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell. Biol. 2009, 29, 4467–4483. [Google Scholar] [CrossRef] [Green Version]
- Herchenhan, A.; Uhlenbrock, F.; Eliasson, P.; Weis, M.; Eyre, D.; Kadler, K.E.; Magnusson, S.P.; Kjaer, M. Lysyl Oxidase Activity Is Required for Ordered Collagen Fibrillogenesis by Tendon Cells. J. Biol. Chem. 2015, 290, 16440–16450. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Zhang, D.; Zhou, Q.; Zhao, F.; He, M.; Yang, Z.; Su, Y.; Zhai, Y.; Yan, J.; Zhang, G.; et al. Scleral HIF-1α is a prominent regulatory candidate for genetic and environmental interactions in human myopia pathogenesis. EBioMedicine 2020, 57, 102878. [Google Scholar] [CrossRef]
- Wu, H.; Chen, W.; Zhao, F.; Zhou, Q.; Reinach, P.S.; Deng, L.; Ma, L.; Luo, S.; Srinivasalu, N.; Pan, M.; et al. Scleral hypoxia is a target for myopia control. Proc. Natl. Acad. Sci. USA 2018, 115, E7091–E7100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasalu, N.; Zhang, S.; Xu, R.; Reinach, P.S.; Su, Y.; Zhu, Y.; Qu, J.; Zhou, X. Crosstalk between EP2 and PPARα Modulates Hypoxic Signaling and Myopia Development in Guinea Pigs. Investig. Ophthalmol. Vis. Sci. 2020, 61, 44. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Guan, Z.; Reinach, P.S.; Kang, L.; Cao, Y.; Zhou, D.; Srinivasalu, N.; Zhao, F.; Qu, J.; Zhou, X. PPARγ modulates refractive development and form deprivation myopia in Guinea pigs. Exp. Eye Res. 2021, 202, 108332. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katayama, H.; Furuhashi, M.; Umetsu, A.; Hikage, F.; Watanabe, M.; Ohguro, H.; Ida, Y. Modulation of the Physical Properties of 3D Spheroids Derived from Human Scleral Stroma Fibroblasts (HSSFs) with Different Axial Lengths Obtained from Surgical Patients. Curr. Issues Mol. Biol. 2021, 43, 1715-1725. https://doi.org/10.3390/cimb43030121
Katayama H, Furuhashi M, Umetsu A, Hikage F, Watanabe M, Ohguro H, Ida Y. Modulation of the Physical Properties of 3D Spheroids Derived from Human Scleral Stroma Fibroblasts (HSSFs) with Different Axial Lengths Obtained from Surgical Patients. Current Issues in Molecular Biology. 2021; 43(3):1715-1725. https://doi.org/10.3390/cimb43030121
Chicago/Turabian StyleKatayama, Hiroyasu, Masato Furuhashi, Araya Umetsu, Fumihito Hikage, Megumi Watanabe, Hiroshi Ohguro, and Yosuke Ida. 2021. "Modulation of the Physical Properties of 3D Spheroids Derived from Human Scleral Stroma Fibroblasts (HSSFs) with Different Axial Lengths Obtained from Surgical Patients" Current Issues in Molecular Biology 43, no. 3: 1715-1725. https://doi.org/10.3390/cimb43030121
APA StyleKatayama, H., Furuhashi, M., Umetsu, A., Hikage, F., Watanabe, M., Ohguro, H., & Ida, Y. (2021). Modulation of the Physical Properties of 3D Spheroids Derived from Human Scleral Stroma Fibroblasts (HSSFs) with Different Axial Lengths Obtained from Surgical Patients. Current Issues in Molecular Biology, 43(3), 1715-1725. https://doi.org/10.3390/cimb43030121






