Contribution of Neuropilin-1 in Radiation-Survived Subclones of NSCLC Cell Line H1299
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells Culture and Reagents
2.2. Reverse Transcription PCR (RT-PCR)
2.3. Tube Formation Assay
2.4. Enzyme-Linked Immunosorbent Assay (ELISA) for Vascular Endothelial Growth Factor (VEGF)
2.5. Cell Motility Assay
2.6. Immunopreciptation and Western Blotting
2.7. DNA Microarray Analysis
2.8. Statististical Analysis
3. Results
3.1. Expression of NRP-1 in H1299-IR Cells
3.2. Tube Formation of HUVECs
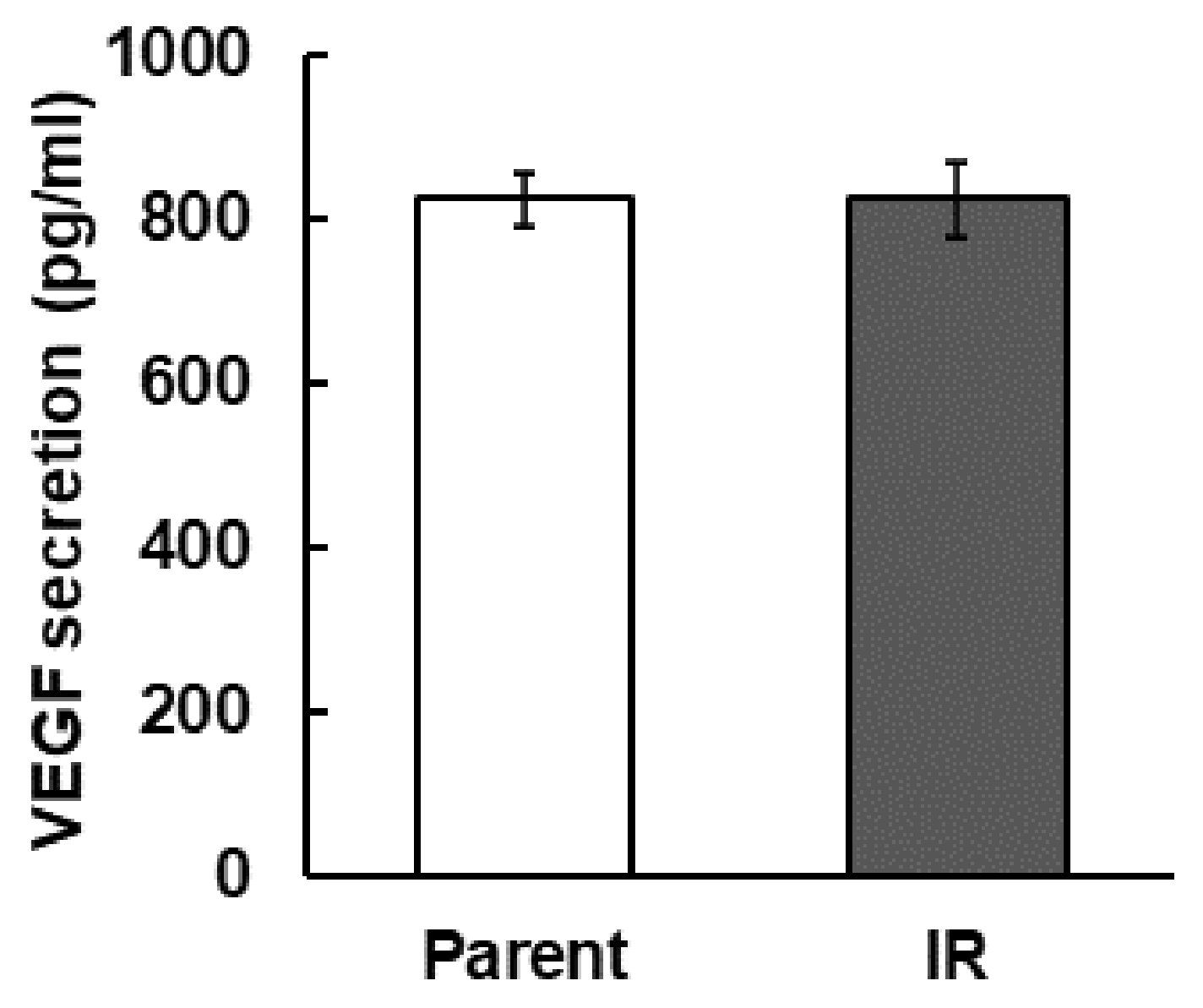
3.3. Secretion of VEGF
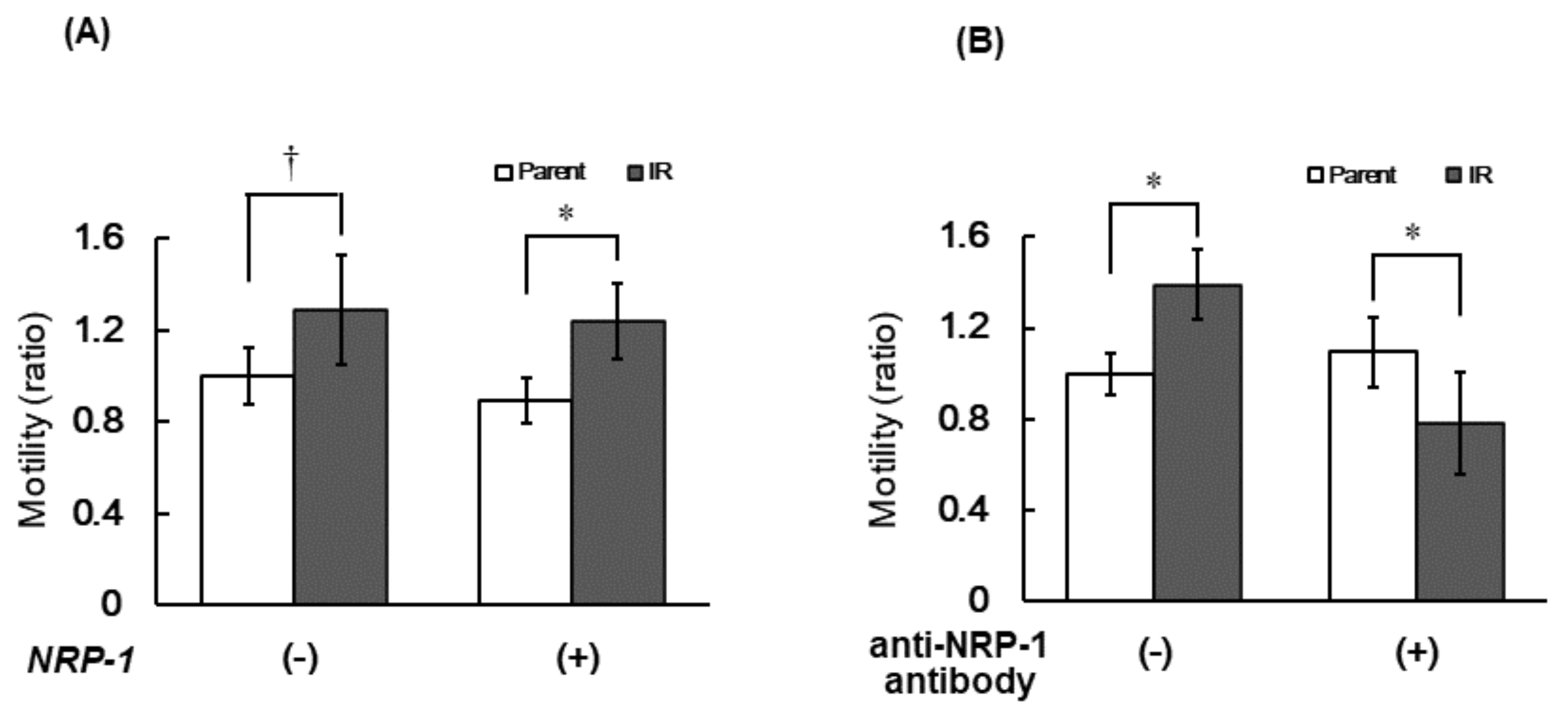
3.4. Cell Motility of IR Cells
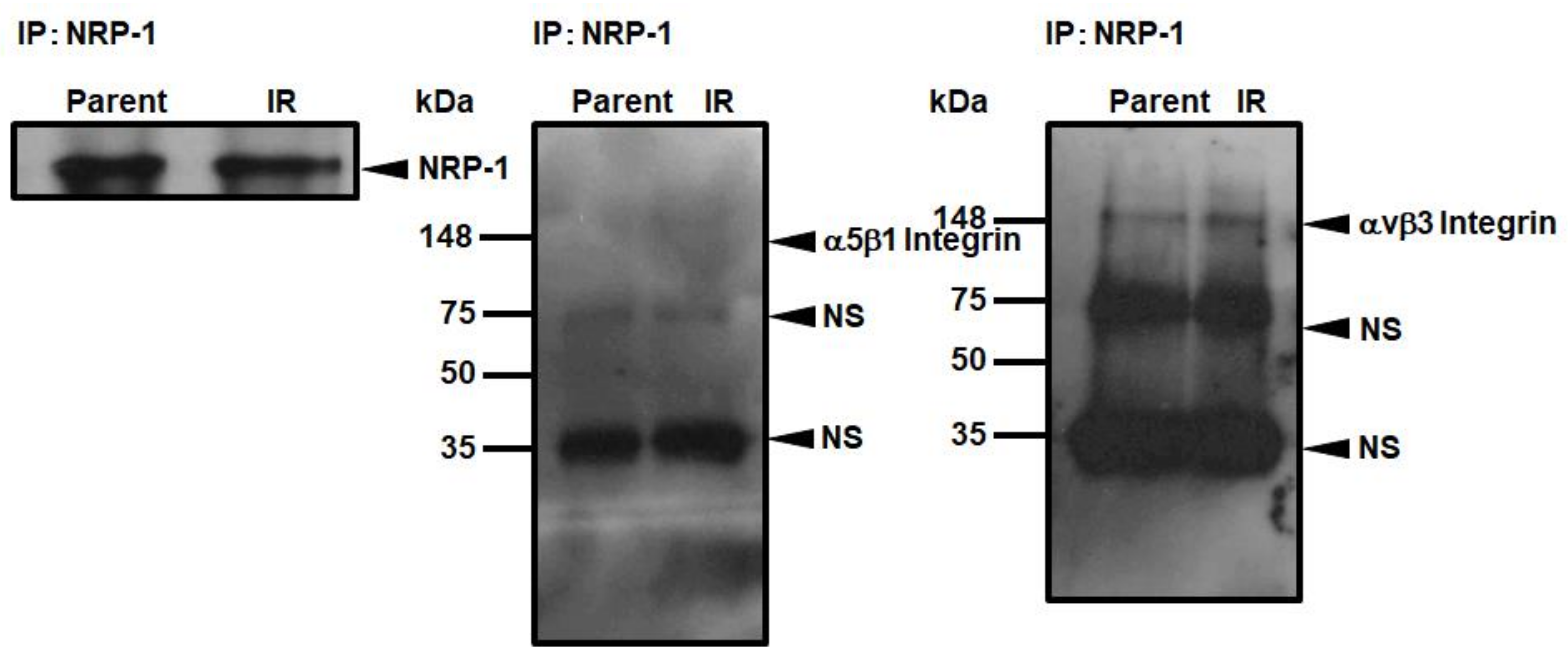
3.5. Interaction of NRP-1 with αVβ3 Integrins
3.6. Changes in NRP-1 Expression Profiles in Three Cell Lines Analyzed by Microarrays
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wood, A.J.J.; Spira, A.; Ettinger, D.S. Multidisciplinary Management of Lung Cancer. N. Engl. J. Med. 2004, 350, 379–392. [Google Scholar]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances since the 2004 Classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Xu, L. Stereotactic Ablative Radiotherapy versus Lobectomy for Operable Stage I Non-Small-Cell Lung Cancer: A Pooled Analysis of Two Randomized Trials—Interpretation of Thoracic Surgeons. Chin. J. Lung Cancer 2015, 18, 321–322. [Google Scholar] [CrossRef]
- Knight, S.B.; Crosbie, P.A.; Balata, H.; Chudziak, J.; Hussell, T.; Dive, C. Progress and Prospects of Early Detection in Lung Cancer. Open Biol. 2017, 7, 170070. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.M.; De Ruysscher, D.; Hoffmann, H.; Reu, S.; Tufman, A. Interdisciplinary Multimodality Management of Stage III Nonsmall Cell Lung Cancer. Eur. Respir. Rev. 2019, 28, 190024. [Google Scholar] [CrossRef] [PubMed]
- Yom, S.S. Accelerated Repopulation as a Cause of Radiation Treatment Failure in Non-Small Cell Lung Cancer: Review of Current Data and Future Clinical Strategies. Semin. Radiat. Oncol. 2015, 25, 93–99. [Google Scholar] [CrossRef]
- Tsutsumi, K.; Yasuda, M.; Nishioka, T. X-ray Irradiation Altered Chemosensitivity of a P53-Null Non-Small Cell Lung Cancer Cell Line. Cell Struct. Funct. 2006, 31, 47–52. [Google Scholar] [CrossRef][Green Version]
- Tsutsumi, K.; Tsuda, M.; Yazawa, N.; Nakamura, H.; Ishihara, S.; Haga, H.; Yasuda, M.; Yamazaki, R.; Shirato, H.; Kawaguchi, H.; et al. Increased Motility and Invasiveness in Tumor Cells That Survive 10 Gy Irradiation. CELL Struct. Funct. 2009, 34, 89–96. [Google Scholar] [CrossRef]
- Prud’homme, G.J.; Glinka, Y.; Prud’homme, G.J.; Glinka, Y. Neuropilins Are Multifunctional Coreceptors Involved in Tumor Initiation, Growth, Metastasis and Immunity. Oncotarget 2012, 3, 921–939. [Google Scholar] [CrossRef]
- Chaudhary, B.; Khaled, Y.S.; Ammori, B.J.; Elkord, E.; Chaudhary, B.; Khaled, Y.S.; Ammori, B.J.; Elkord, E.; Elkord, E. Neuropilin 1: Function and Therapeutic Potential in Cancer. Cancer Immunol. Immunother. 2014, 3, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Hansel, D.E.; Wilentz, R.E.; Yeo, C.J.; Schulick, R.D.; Montgomery, E.; Maitra, A. Expression of Neuropilin-1 in High-Grade Dysplasia, Invasive Cancer, and Metastases of the Human Gastrointestinal Tract. Am. J. Surg. Pathol. 2004, 28, 347–356. [Google Scholar] [CrossRef]
- Gray, M.J.; Van Buren, G.; Dallas, N.A.; Xia, L.; Wang, X.; Yang, A.D.; Somcio, R.J.; Lin, Y.G.; Lim, S.; Fan, F.; et al. Therapeutic Targeting of Neuropilin-2 on Colorectal Carcinoma Cells Implanted in the Murine Liver. J. Natl. Cancer Inst. 2008, 100, 109–120. [Google Scholar] [CrossRef]
- Fukahi, K.; Fukasawa, M.; Neufeld, G.; Itakura, J.; Korc, M. Aberrant Expression of Neuropilin-1 and -2 in Human Pancreatic Cancer Cells. Clin. Cancer Res. 2004, 10, 581–590. [Google Scholar] [CrossRef]
- Osada, R.; Horiuchi, A.; Kikuchi, N.; Ohira, S.; Ota, M.; Katsuyama, Y.; Konishi, I. Expression of Semaphorins, Vascular Endothelial Growth Factor, and Their Common Receptor Neuropilins and Alleic Loss of Semaphorin Locus in Epithelial Ovarian Neoplasms: Increased Ratio of Vascular Endothelial Growth Factor to Semaphorin Is a Poor Prognos. Hum. Pathol. 2006, 37, 1414–1425. [Google Scholar] [CrossRef]
- Kofler, N.M.; Simons, M. Angiogenesis versus Arteriogenesis: Neuropilin 1 Modulation of VEGF Signaling. F1000Prime Rep. 2015, 7, 26. [Google Scholar] [CrossRef]
- Brambilla, E.; Constantin, B.; Drabkin, H.; Lle Roche, J. Semaphorin SEMA3F Localization in Malignant Human Lung and Cell Lines A Suggested Role in Cell Adhesion and Cell Migration. Am. J. Pathol. 2000, 156, 939–950. [Google Scholar] [CrossRef]
- Ding, Z.; Du, W.; Lei, Z.; Zhang, Y.; Zhu, J.; Zeng, Y.; Wang, S.; Zheng, Y.; Liu, Z.; Huang, J.A. Neuropilin 1 Modulates TGF-β1-induced Epithelial-mesenchymal Transition in Non-small Cell Lung Cancer. Int. J. Oncol. 2020, 56, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, Y.; Li, L.; Baba, T.; Nakagawa, H.; Shimura, T.; Yamamoto, Y.; Ohkubo, Y.; Fukumoto, M. Clinically Relevant Radioresistant Cells Efficiently Repair DNA Double-Strand Breaks Induced by X-rays. Cancer Sci. 2009, 100, 747–752. [Google Scholar] [CrossRef]
- Ishihara, S.; Haga, H.; Yasuda, M.; Mizutani, T.; Kawabata, K.; Shirato, H.; Nishioka, T. Integrin Β1-Dependent Invasive Migration of Irradiation-Tolerant Human Lung Adenocarcinoma Cells in 3D Collagen Matrix. Biochem. Biophys. Res. Commun. 2010, 396, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Aoki, K.; Mizutani, T.; Amano, M.; Nishimura, S.I.; Haga, H. Glycosphingolipid GM2 Induces Invasiveness in Irradiation-Tolerant Lung Cancer Cells. Cell Struct. Funct. 2018, 43, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Yasuda, M.; Nishioka, T.; Mizutani, T.; Kawabata, K.; Shirato, H.; Haga, H. Irradiation-Tolerant Lung Cancer Cells Acquire Invasive Ability Dependent on Dephosphorylation of the Myosin Regulatory Light Chain. FEBS Lett. 2013, 587, 732–736. [Google Scholar] [CrossRef]
- Tyrsina, E.G.; Slanina, S.V.; Kakpakova, E.S.; Kalendo, G.S.; Kan, N.G.; Tyrsin, O.Y.; Ryskov, A.P. Isolation and Characterization of Highly Radioresistant Malignant Hamster Fibroblasts That Survive Acute γ Irradiation with 20 Gy. Radiat. Res. 2005, 164, 745–754. [Google Scholar] [CrossRef]
- Deng, X.; Chen, C.; Wu, F.; Qiu, L.; Ke, Q.; Sun, R.; Duan, Q.; Luo, M.; Luo, Z. Curcumin Inhibits the Migration and Invasion of Non-Small-Cell Lung Cancer Cells Through Radiation-Induced Suppression of Epithelial-Mesenchymal Transition and Soluble E-Cadherin Expression. Technol. Cancer Res. Treat. 2020, 19. [Google Scholar] [CrossRef]
- Mahabir, R.; Tanino, M.; Elmansuri, A.; Wang, L.; Kimura, T.; Itoh, T.; Ohba, Y.; Nishihara, H.; Shirato, H.; Tsuda, M.; et al. Sustained Elevation of Snail Promotes Glial-Mesenchymal Transition after Irradiation in Malignant Glioma. Neuro Oncol. 2014, 16, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Pouysségur, J.; Dayan, F.; Mazure, N.M. Hypoxia Signalling in Cancer and Approaches to Enforce Tumour Regression. Nature 2006, 25, 437–443. [Google Scholar] [CrossRef]
- Hirota, K.; Semenza, G.L. Regulation of Angiogenesis by Hypoxia-Inducible Factor 1. Crit Rev. Oncol. Hematol. 2006, 59, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Moeller, B.J.; Cao, Y.; Li, C.Y.; Dewhirst, M.W. Radiation Activates HIF-1 to Regulate Vascular Radiosensitivity in Tumors: Role of Reoxygenation, Free Radicals, and Stress Granules. Cancer Cell 2004, 5, 429–441. [Google Scholar] [CrossRef]
- Shi, M.; Guo, X.T.; Shu, M.G.; Chen, F.L.; Li, L.W. Cell-Permeable Hypoxia-Inducible Factor-1 (HIF-1) Antagonists Function as Tumor Radiosensitizers. Med. Hypotheses 2007, 69, 33–35. [Google Scholar] [CrossRef]


| Cell Line | Species | Tissue | Case History | Fold-Change |
|---|---|---|---|---|
| H1299 | Homo sapiens | lung | non-small cell lung cancer canceradenocarcinoma | 2.44 |
| A549 | Homo sapiens | lung | non-small cell lung cancer | 1.35 |
| MCF7 | Homo sapiens | breast | breast cancer | 2.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsutsumi, K.; Chiba, A.; Tadaki, Y.; Minaki, S.; Ooshima, T.; Takahashi, H. Contribution of Neuropilin-1 in Radiation-Survived Subclones of NSCLC Cell Line H1299. Curr. Issues Mol. Biol. 2021, 43, 1203-1211. https://doi.org/10.3390/cimb43030085
Tsutsumi K, Chiba A, Tadaki Y, Minaki S, Ooshima T, Takahashi H. Contribution of Neuropilin-1 in Radiation-Survived Subclones of NSCLC Cell Line H1299. Current Issues in Molecular Biology. 2021; 43(3):1203-1211. https://doi.org/10.3390/cimb43030085
Chicago/Turabian StyleTsutsumi, Kaori, Ayaka Chiba, Yuta Tadaki, Shima Minaki, Takahito Ooshima, and Haruka Takahashi. 2021. "Contribution of Neuropilin-1 in Radiation-Survived Subclones of NSCLC Cell Line H1299" Current Issues in Molecular Biology 43, no. 3: 1203-1211. https://doi.org/10.3390/cimb43030085
APA StyleTsutsumi, K., Chiba, A., Tadaki, Y., Minaki, S., Ooshima, T., & Takahashi, H. (2021). Contribution of Neuropilin-1 in Radiation-Survived Subclones of NSCLC Cell Line H1299. Current Issues in Molecular Biology, 43(3), 1203-1211. https://doi.org/10.3390/cimb43030085






