A Comparative Analysis of the Altered Levels of Human Seminal Plasma Constituents as Contributing Factors in Different Types of Male Infertility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Consent
2.2. Sample Collection
2.3. Biochemical and Immunological Analysis
2.4. Metabolome Analysis
2.5. Statistical Analysis
3. Results
3.1. Analysis of Semen Parameters among the Fertile and Infertile Groups
3.2. Assessment of Various Biochemical and Immunological Markers between the Fertile and Infertile Subgroups
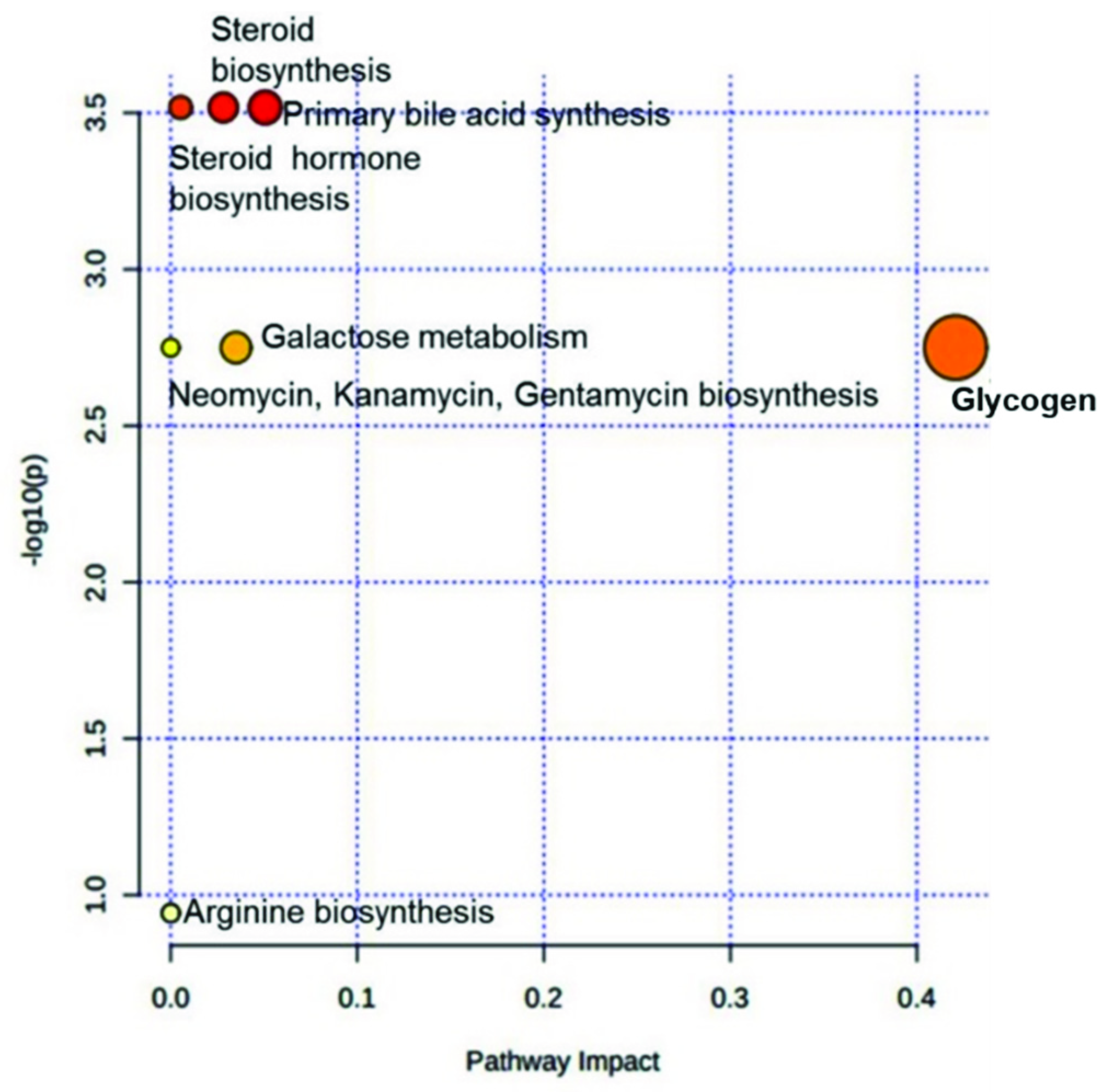
3.3. Assessment of Altered Metabolites in Infertile Subgroups and Their Effect on Metabolic Pathways
3.4. Enzymatic Levels Were Altered in Some Infertile Subgroups
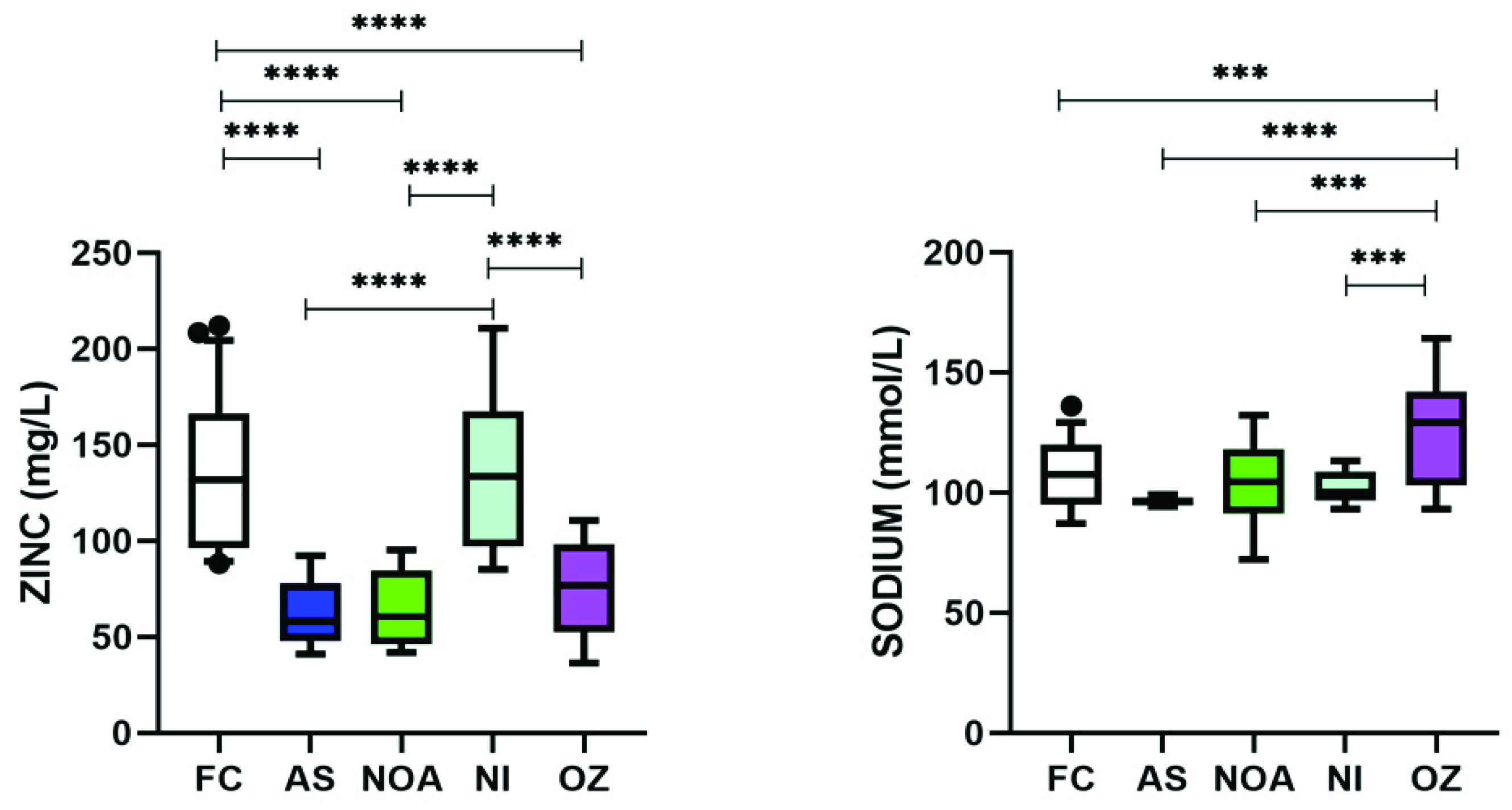
3.5. Varying Levels of Zinc and Sodium Were Found among Trace Elements in Infertile Subgroups
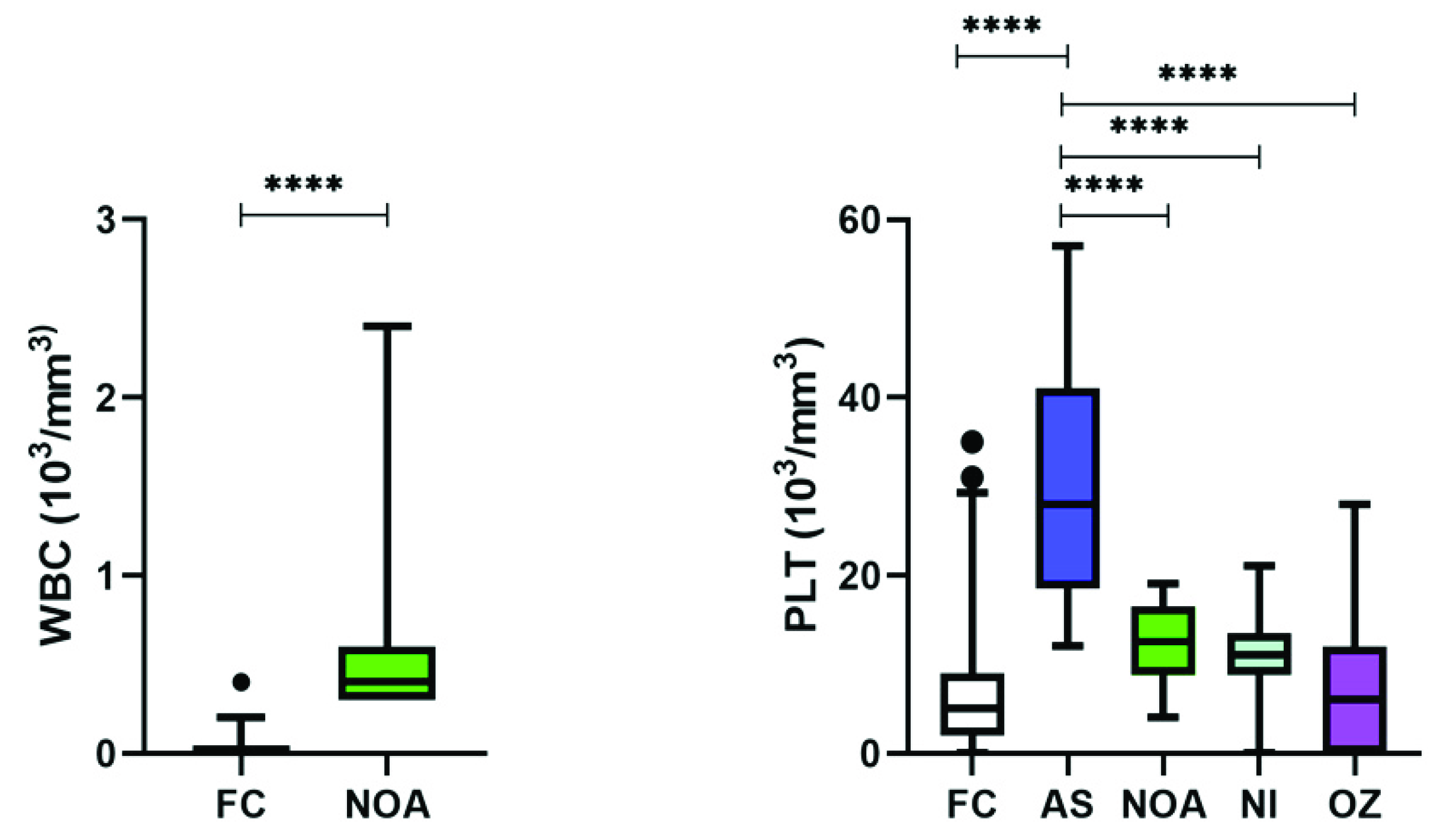
3.6. Immunological Perturbations Were Found Only in NOA Patients
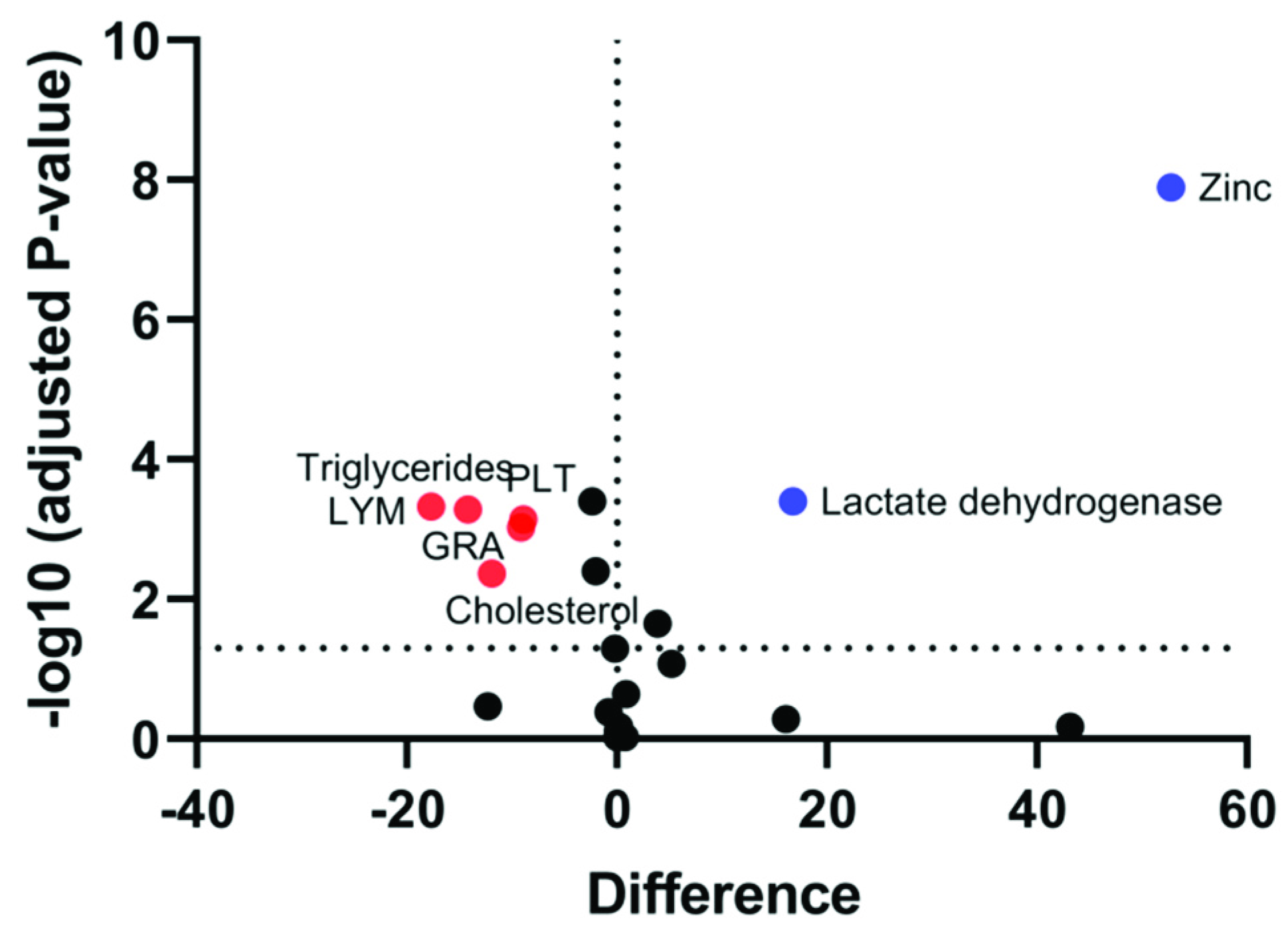
3.7. Each Infertile Subgroup has a Characteristic Panel of Markers That Are Altered as Compared to Fertile Controls
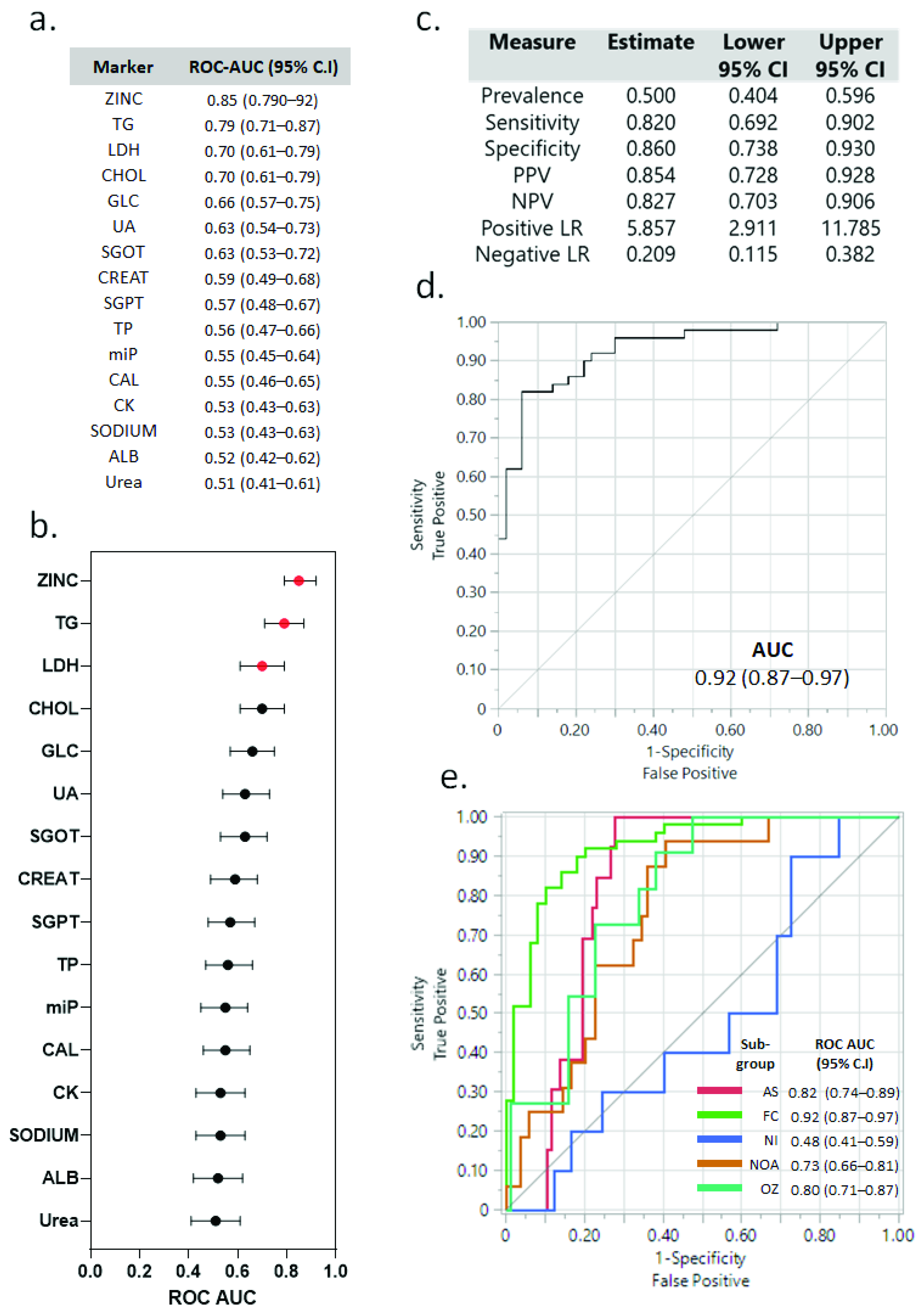
3.8. Correlation between Sperm Parameters and Various Biochemical and Immunological Markers with Fertility
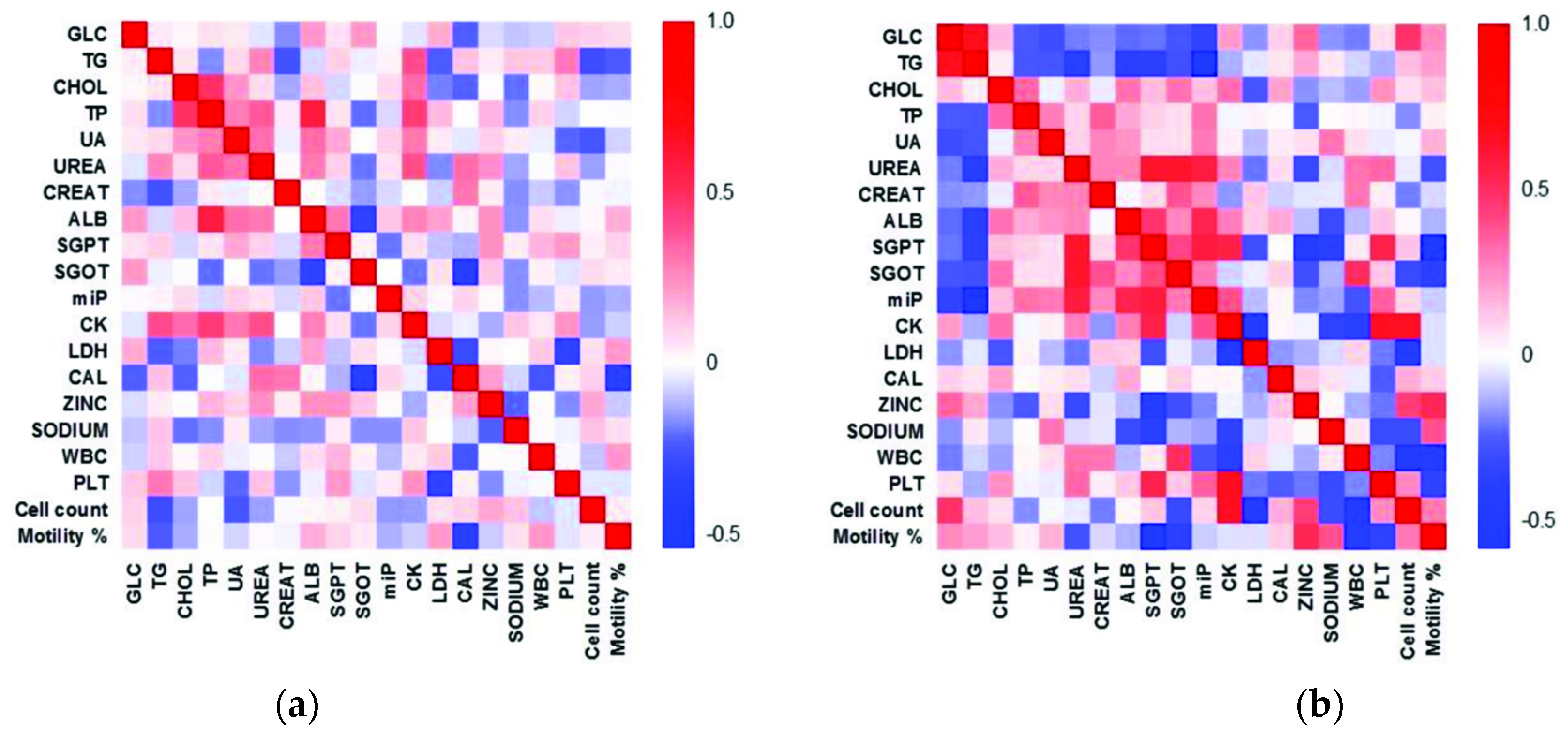
3.9. Heat Maps Depict Altered Correlation between Various Parameters in the Infertile Group
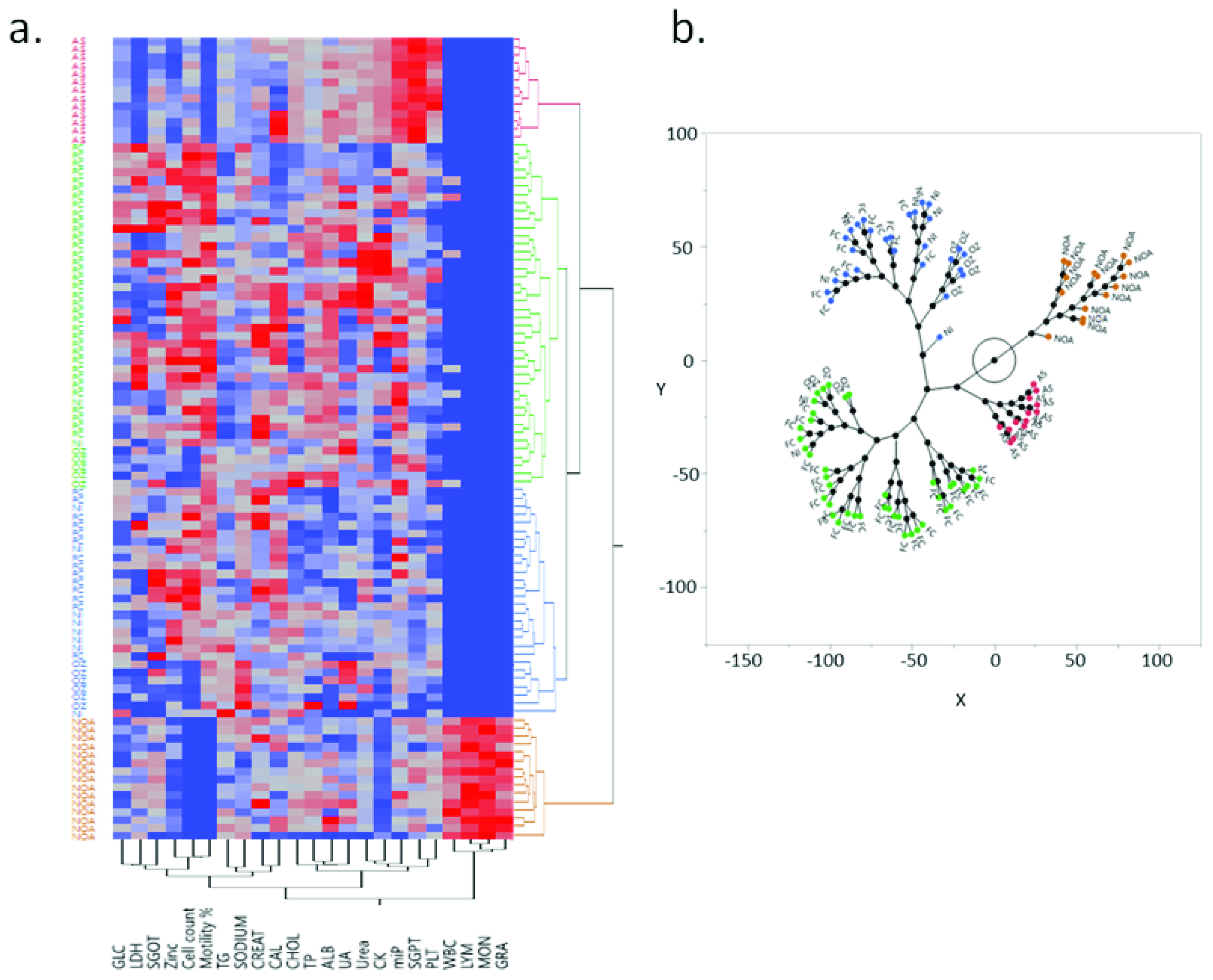
3.10. Hierarchical Clustering Defines Two Distinct Groups among the Infertile
4. Discussion
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharlip, I.D.; Jarow, J.P.; Belker, A.M.; Lipshultz, L.I.; Sigman, M.; Thomas, A.J.; Schlegel, P.N.; Howards, S.S.; Nehra, A.; Damewood, M.D.; et al. Best practice policies for male infertility. Fertil. Steril. 2002, 77, 873–882. [Google Scholar] [CrossRef]
- Winters, B.R.; Walsh, T.J. The epidemiology of male infertility. Urol. Clin. N. Am. [Internet] 2014, 41, 195–204. [Google Scholar] [CrossRef]
- WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; WHO: Geneva, Switzerland, 2010; pp. 1–286.
- Drabovich, A.P.; Saraon, P.; Jarvi, K.; Diamandis, E.P. Seminal plasma as a diagnostic fluid for male reproductive system disorders. Nat. Rev. Urol. [Internet] 2014, 11, 278–288. [Google Scholar] [CrossRef]
- Camargo, M.; Intasqui, P.; Bertolla, R.P. Understanding the seminal plasma proteome and its role in male fertility. Basic Clin. Androl. 2018, 28, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Mcgraw, L.A.; Suarez, S.S.; Wolfner, M.F. On a matter of seminal importance. BioEssays 2015, 37, 142–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, G.F.; Schust, D.J. Manifestations of immune tolerance in the human female reproductive tract. Front. Immunol. 2013, 4, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Gilany, K.; Moazeni-Pourasil, R.S.; Jafarzadeh, N.; Savadi-Shiraz, E. Metabolomics fingerprinting of the human seminal plasma of asthenozoospermic patients. Mol. Reprod. Dev. 2014, 81, 84–86. [Google Scholar] [CrossRef]
- Murgia, F.; Corda, V.; Serrenti, M.; Usai, V.; Santoru, M.L.; Hurt, K.J.; Passaretti, M.; Monni, M.C.; Atzori, L.; Monni, G. Seminal fluid metabolomic markers of oligozoospermic infertility in humans. Metabolites 2020, 10, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, S.; Wu, W.; Chen, M.; Tang, Q.; Xia, Y.; Jia, W.; Wang, X. Seminal plasma metabolomics approach for the diagnosis of unexplained male infertility. PLoS ONE 2017, 12, e0181115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayaraman, V.; Ghosh, S.; Sengupta, A.; Srivastava, S.; Sonawat, H.M.; Narayan, P.K. Identification of biochemical differences between different forms of male infertility by nuclear magnetic resonance (NMR) spectroscopy. J. Assist. Reprod. Genet. 2014, 31, 1195–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilany, K.; Jafarzadeh, N.; Mani-Varnosfaderani, A.; Minai-Tehrani, A.; Sadeghi, M.R.; Darbandi, M.; Darbandi, S.; Amini, M.; Arjmand, B.; Pahlevanzadeh, Z. Metabolic fingerprinting of seminal plasma from non-obstructive azoospermia patients: Positive versus negative sperm retrieval. J. Reprod. Infertil. 2018, 19, 109–114. [Google Scholar]
- Chen, L.; Wen, C.; Deng, M.J.; Zhang, Z.D.; Zhou, Z.H.; Wang, X. Metabolic and transcriptional changes in seminal plasma of asthenozoospermia patients. Biomed. Chromatogr. 2020, 34, e4769. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.-X.; Lu, J.-C.; Zhang, H.-Y.; Lü, N.-Q. A Pilot Comparative Study of 26 Biochemical Markers in Seminal Plasma and Serum in Infertile Men. Biomed. Res. Int. 2015, 2015, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eroglu, M.; Sahin, S.; Durukan, B.; Ozakpinar, O.B.; Erdinc, N.; Turkgeldi, L.; Sofuoglu, K.; Karateke, A. Blood serum and seminal plasma selenium, total antioxidant capacity and coenzyme Q10 levels in relation to semen parameters in men with idiopathic infertility. Biol. Trace Elem. Res. 2014, 159, 46–51. [Google Scholar] [CrossRef]
- Lu, J.-C.; Jing, J.; Yao, Q.; Fan, K.; Wang, G.-H.; Feng, R.-X.; Liang, Y.-J.; Chen, L.; Ge, Y.-F.; Yao, B. Relationship between lipids levels of serum and seminal plasma and semen parameters in 631 Chinese subfertile men. PLoS ONE 2016, 11, e0146304. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.; Presani, G.; Guaschino, S.; Simeone, R.; Perticarari, S. Leukocyte detection in human semen using flow cytometry. Hum. Reprod. 2000, 15, 1329–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havrylyuk, A.; Chopyak, V.; Boyko, Y.; Kril, I.; Kurpisz, M. Cytokines in the blood and semen of infertile patients. Cent. Eur. J. Immunol. 2015, 40, 337–344. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinforma. 2019, 68, 1–128. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Bellone, S.; Siegel, E.R.; Altwerger, G.; Menderes, G.; Bonazzoli, E.; Egawa-Takata, T.; Pettinella, F.; Bianchi, A.; Riccio, F.; et al. A novel multiple biomarker panel for the early detection of high-grade serous ovarian carcinoma. Gynecol. Oncol. [Internet] 2018, 149, 585–591. [Google Scholar] [CrossRef]
- Hidalgo, D.M.; Romarowski, A.; Gervasi, M.G.; Navarrete, F.; Balbach, M.; Salicioni, A.M.; Levin, L.R.; Buck, J.; Visconti, P.E. Capacitation increases glucose consumption in murine sperm. Mol. Reprod. Dev. 2020, 87, 1037–1047. [Google Scholar] [CrossRef]
- Williams, A.C.; Ford, W.C.L. The role of glucose in supporting motility and capacitation in human spermatozoa. J. Androl. 2001, 22, 680–695. [Google Scholar]
- Caro-Maldonado, A.; Tait, S.W.G.; Ramírez-Peinado, S.; Ricci, J.E.; Fabregat, I.; Green, D.R.; Muñoz-Pinedo, C. Glucose deprivation induces an atypical form of apoptosis mediated by caspase-8 in Bax-, Bak-deficient cells. Cell Death Differ. 2010, 17, 1335–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Alexanian, A.; Ying, R.; Kizhakekuttu, T.J.; Dharmashankar, K.; Vasquez-Vivar, J.; Gutterman, D.D.; Widlansky, M.E. Acute exposure to low glucose rapidly induces endothelial dysfunction and mitochondrial oxidative stress role for AMP kinase. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 712–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ergün, A.; Köse, S.K.; Aydos, K.; Ata, A.; Avci, A. Correlation of seminal parameters with serum lipid profile and sex hormones. Arch. Androl. 2007, 53, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Saz Lancellotti, T.E.; Boarelli, P.V.; Monclus, M.A.; Cabrillana, M.E.; Clementi, M.A.; Espínola, L.S.; Barría, J.L.C.; Vincenti, A.E.; Santi, A.G.; Fornés, M.W. Hypercholesterolemia impaired sperm functionality in rabbits. PLoS ONE 2010, 5, e13457. [Google Scholar] [CrossRef] [Green Version]
- Whitfield, M.; Pollet-Villard, X.; Levy, R.; Drevet, J.R.; Saez, F. Posttesticular sperm maturation, infertility, and hypercholesterolemia. Asian J. Androl. 2015, 17, 742–748. [Google Scholar]
- Kim, N.; Nakamura, H.; Masaki, H.; Kumasawa, K.; Hirano, K.-I.; Kimura, T. Effect of lipid metabolism on male fertility. Biochem. Biophys. Res. Commun. [Internet] 2017, 485, 686–692. [Google Scholar] [CrossRef]
- Saez, F.; Drevet, J.R. Dietary Cholesterol and Lipid Overload: Impact on Male Fertility. Oxid. Med. Cell Longev. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Staudt, A.C.; Wenkel, S. Regulation of protein function by “microProteins”. EMBO Rep. 2011, 12, 35–42. [Google Scholar] [CrossRef]
- Merino-Valverde, I.; Greco, E.; Abad, M. The microproteome of cancer: From invisibility to relevance. Exp. Cell Res. 2020, 392, 111997. [Google Scholar] [CrossRef]
- Durany, N.; Carreras, J.; Valentí, M.; Cámara, J.; Carreras, J. Inactivation of phosphoglycerate mutase and creatine kinase isoenzymes in human serum. J. Clin. Pathol.-Mol. Pathol. 2002, 55, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Tombes, R.M.; Shapiro, B.M. Metabolite channeling: A phosphorylcreatine shuttle to mediate high energy phosphate transport between sperm mitochondrion and tail. Cell 1985, 41, 325–334. [Google Scholar] [CrossRef]
- Krisfalusi, M.; Miki, K.; Magyar, P.L.; O’Brien, D.A. Multiple glycolytic enzymes are tightly bound to the fibrous sheath of mouse spermatozoa. Biol. Reprod. 2006, 75, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.S.L.; O’Brien, D.A.; Hou, E.W.; Versola, J.; Rockett, D.L.; Eddy, E.M. Differential activity and synthesis of lactate dehydrogenase isozymes A (muscle), B (heart), and C (testis) in mouse spermatogenic cells. Biol. Reprod. 1989, 40, 173–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odet, F.; Gabel, S.A.; Williams, J.; London, R.E.; Goldberg, E.; Eddy, E.M. Lactate dehydrogenase C and energy metabolism in mouse sperm. Biol. Reprod. 2011, 85, 556–564. [Google Scholar] [CrossRef] [Green Version]
- Odet, F.; Duan, C.; Willis, W.D.; Goulding, E.H.; Kung, A.; Eddy, E.M.; Goldberg, E. Expression of the gene for mouse lactate dehydrogenase C (Ldhc) is required for male fertility. Biol. Reprod. 2008, 79, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chyra-Jach, D.; Kaletka, Z.; Dobrakowski, M.; Machoń-Grecka, A.; Kasperczyk, S.; Bellanti, F.; Birkner, E.; Kasperczyk, A. Levels of Macro- and Trace Elements and Select Cytokines in the Semen of Infertile Men. Biol. Trace Elem. Res. 2020, 197, 431–439. [Google Scholar] [CrossRef]
- Allouche-Fitoussi, D.; Breitbart, H. The role of zinc in male fertility. Int. J. Mol. Sci. 2020, 21, 7796. [Google Scholar] [CrossRef]
- Nishimura, H.; L’Hernault, S.W. Spermatogenesis. Curr. Biol. 2017, 27, R988–R994. [Google Scholar] [CrossRef] [Green Version]
- Björndahl, L.; Kvist, U. Human sperm chromatin stabilization: A proposed model including zinc bridges. Mol. Hum. Reprod. 2009, 16, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Michailov, Y.; Ickowicz, D.; Breitbart, H. Zn2+-stimulation of sperm capacitation and of the acrosome reaction is mediated by EGFR activation. Dev. Biol. [Internet] 2014, 396, 246–255. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Dong, X.; Hu, X.; Long, Z.; Wang, L.; Liu, Q.; Sun, B.; Wang, Q.; Wu, Q.; Li, L. Zinc levels in seminal plasma and their correlation with male infertility: A systematic review and meta-analysis. Sci. Rep. [Internet] 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Colagar, A.H.; Marzony, E.T.; Chaichi, M.J. Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr. Res. [Internet] 2009, 29, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.; Mohammad-Hasani, A.; Colagar, A.H.; Fallah, A. Zinc is an essential element for male fertility: A review of Zn roles in men’s health, germination, sperm quality, and fertilization. J. Rep. Infertil. 2018, 19, 69–81. [Google Scholar]
- Nishigaki, T.; José, O.; González-Cota, A.L.; Romero, F.; Treviño, C.L.; Darszon, A. Intracellular pH in sperm physiology. Biochem. Biophys. Res. Commun. [Internet] 2014, 450, 1149–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Chen, L.; Li, J.; Li, H.; Hong, Z.; Xie, M.; Chen, S.; Yao, B. The semen pH affects sperm motility and capacitation. PLoS ONE 2015, 10, e0132974. [Google Scholar]
- Mah, A.; David, R.; Blumenfeld, Z.; Beti, O.S. Factors affecting sperm motility. VII. Sperm viability as affected by change of pH and osmolarity of semen and urine specimens. Fertil. Steril. 1981, 36, 507–511. [Google Scholar]
- Bonato, M.; Cornwallis, C.K.; Malecki, I.A.; Rybnik-Trzaskowska, P.K.; Cloete, S.W.P. The effect of temperature and pH on the motility and viability of ostrich sperm. Anim. Reprod. Sci. [Internet] 2012, 133, 123–128. [Google Scholar] [CrossRef]
- Henkel, R.R. Leukocytes and oxidative stress: Dilemma for sperm function and male fertility. Asian J. Androl. [Internet] 2011, 13, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.K.; Pasqualotto, F.F.; Nelson, D.R.; Thomas, A.J., Jr.; Agarwal, A. Relationship between seminal white blood cell counts and oxidative stress in men treated at an infertility clinic. J. Androl. 2001, 22, 575–583. [Google Scholar]
- Aziz, N.; Agarwal, A.; Lewis-Jones, I.; Sharma, R.K.; Thomas, A.J. Novel associations between specific sperm morphological defects and leukocytospermia. Fertil. Steril. 2004, 82, 621–627. [Google Scholar] [CrossRef]
- Angle, M.J.; Tom, R.; Khoo, D.; McClure, R.D. Platelet-activating factor in sperm from fertile and subfertile men. Fertil. Steril. 1991, 56, 314–318. [Google Scholar] [CrossRef]
- Lombardo, F.; Sansone, A.; Romanelli, F.; Paoli, D.; Gandini, L.; Lenzi, A. The role of antioxidant therapy in the treatment of male infertility: An overview. Asian J. Androl. [Internet] 2011, 13, 690–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, P.; Shahrokhi, S.Z.; Kamran, T.; Ajami, A.; Taghiyar, S.; Deemeh, M.R. Effect of antioxidant therapy on the sperm DNA integrity improvement; A longitudinal cohort study. Int. J. Reprod. Biomed. 2019, 17, 99–106. [Google Scholar] [CrossRef]
- Fang, Y.; Zhong, R. Effects of Oxidative Stress on Spermatozoa and Male Infertility. Free Radic. Med. Biol. 2020. [Google Scholar] [CrossRef] [Green Version]


| Parameter | Study Groups | p Value | ||||
|---|---|---|---|---|---|---|
| FC (n = 50) | AS (n = 13) | NOA (n = 16) | NI (n = 10) | OZ (n = 11) | ||
| AGE (Years) | 27.94 ± 2.34 | 30.61 ± 2.90 | 29.75 ± 3.15 | 29.3 ± 2.45 | 30.18 ± 3.57 | 0.0056 |
| Sperm Count (Per Million) | 70.32 ± 16.90 | 44.92 ± 12.46 | 0 | 59.50 ± 8.32 | 7.09 ± 2.30 | <0.0001 |
| Sperm Motility (Percentage) | 65.96 ± 14.11 | 7.00 ± 1.83 | 0 | 54.40 ± 8.54 | 53.73 ± 10.39 | <0.0001 |
| Markers | FC | AS | NOA | NI | OZ | p Value |
|---|---|---|---|---|---|---|
| Analytes | ||||||
| GLC (mg/dL) | 8.972 ± 0.859 | 4.337 ± 1.666 | 3.430 ± 1.475 | 9.643 ± 1.855 | 2.704 ± 1.788 | 0.0001 |
| TG (mg/dL) | 20.344 ± 2.221 | 26.711 ± 4.294 | 30.522 ± 3.900 | 50.539 ± 4.770 | 33.241 ± 4.602 | 0.0001 |
| CHOL (mg/dL) | 32.353 ± 2.312 | 49.100 ± 4.485 | 37.724 ± 3.972 | 42.289 ± 4.993 | 49.506 ± 4.812 | 0.003 |
| UA (mg/dL) | 3.374 ± 0.310 | 4.265 ± 0.601 | 3.326 ± 0.533 | 3.391 ± 0.669 | 5.267 ± 0.645 | 0.089 |
| Urea (mg/dL) | 126.967 ± 7.269 | 135.625 ± 14.099 | 112.945 ± 12.485 | 80.598 ± 15.694 | 105.139 ± 15.126 | 0.052 |
| miP (mg/dL) | 763.987 ± 21.739 | 886.422 ± 42.166 | 657.509 ± 37.340 | 595.456 ± 46.938 | 758.115 ± 45.238 | <0.0001 |
| TP (g/dL) | 2.761 ± 0.128 | 2.661 ± 0.248 | 2.631 ± 0.219 | 2.218 ± 0.276 | 2.819 ± 0.266 | 0.475 |
| ALB (mg/dL) | 0.920 ± 0.063 | 1.110 ± 0.122 | 0.867 ± 0.108 | 0.712 ± 0.136 | 0.890 ± 0.131 | 0.285 |
| CREAT (mg/dL) | 5.761 ± 0.289 | 4.595 ± 0.560 | 5.454 ± 0.496 | 4.628 ± 0.624 | 4.819 ± 0.601 | 0.239 |
| Enzymes | ||||||
| SGOT (U/L) | 18.676 ± 1.331 | 11.548 ± 2.582 | 16.063 ± 2.286 | 6.692 ± 2.874 | 10.129 ± 2.77 | 0.001 |
| SGPT (U/L) | 54.363 ± 2.962 | 118.526 ± 5.744 | 65.562 ± 5.087 | 30.511 ± 6.395 | 48.063 ± 6.163 | 0.0001 |
| CK (U/L) | 291.549 ± 26.037 | 413.875 ± 50.504 | 111.383 ± 44.724 | 248.598 ± 56.22 | 147.689 ± 54.183 | <0.0001 |
| LDH (U/L) | 30.968 ± 2.7 | 4.489 ± 5.237 | 20.654 ± 4.638 | 11.462 ± 5.83 | 15.176 ± 5.619 | <0.0001 |
| Trace Elements | ||||||
| CAL (mmol/L) | 4.474 ± 0.058 | 4.431 ± 0.113 | 4.346 ± 0.1 | 4.535 ± 0.126 | 4.415 ± 0.121 | 0.779 |
| Zinc (mg/L) | 134.238 ± 4.848 | 62.678 ± 9.403 | 63.852 ± 8.327 | 137.731 ± 10.467 | 74.671 ± 10.088 | <0.0001 |
| Sodium (mmol/L) | 107.300 ± 2.103 | 96.178 ± 4.08 | 103.938 ± 3.613 | 101.973 ± 4.542 | 125.995 ± 4.377 | <0.0001 |
| Immunological Markers | ||||||
| WBC (103/mm3) | 0.042 ± 0.03 | 0 | 0.580 ± 0.052 | 0 | 0.42 ± 0.028 | <0.001 |
| LYM (%) | 0 | 0 | 55.575 ± 1.983 | 0 | 0 | - |
| MON (%) | 0 | 0 | 7.395 ± 0.234 | 0 | 0 | - |
| GRAN (%) | 0 | 0 | 28.825 ± 1.345 | 0 | 0 | - |
| PLT (103/mm3) | 7.006 ± 1.229 | 30.585 ± 2.384 | 12.199 ± 2.111 | 10.978 ± 2.653 | 7.921 ± 2.557 | <0.0001 |
| Pathway | Total Compounds | Hits | Raw p | -log(10)p | Holm p | FDR | Impact |
|---|---|---|---|---|---|---|---|
| Primary bile acid biosynthesis | 46 | 1 | 3.04 × 10−4 | 3.52 | 2.43 × 10−3 | 8.10 × 10−4 | 0.05 |
| Steroid biosynthesis | 42 | 1 | 3.04 × 10−4 | 3.52 | 2.43 × 10−3 | 8.10 × 10−4 | 0.03 |
| Steroid hormone biosynthesis | 85 | 1 | 3.04 × 10−4 | 3.52 | 2.43 × 10−3 | 8.10 × 10−4 | 0.01 |
| Glycogen metabolism | 18 | 1 | 1.78 × 10−3 | 2.75 | 8.90 × 10−3 | 2.37 × 10−3 | 0.42 |
| Galactose metabolism | 27 | 1 | 1.78 × 10−3 | 2.75 | 8.90 × 10−3 | 2.37 × 10−3 | 0.03 |
| Neomycin, kanamycin, and gentamicin biosynthesis | 2 | 1 | 1.78 × 10−3 | 2.75 | 8.90 × 10−3 | 2.37 × 10−3 | 0.00 |
| Purine metabolism | 65 | 2 | 1.14 × 10−1 | 9.43 × 10−1 | 2.28 × 10−1 | 1.14 × 10−1 | 0.00 |
| Arginine biosynthesis | 14 | 1 | 1.14 × 10−1 | 9.43 × 10−1 | 2.28 × 10−1 | 1.14 × 10−1 | 0.00 |
| Seminal Plasma Markers | Infertility Groups | |||
|---|---|---|---|---|
| AS | NOA | NI | OZ | |
| GLC | - | S↓ | - | S↓ |
| TG | - | S↑ | S↑ | |
| CHOL | S↑ | - | - | S↑ |
| miP | - | - | S↓ | - |
| SGPT | S↑ | - | S↓ | - |
| SGOT | - | - | S↓ | - |
| CK | - | S↓ | - | - |
| LDH | S↓ | - | S↓ | - |
| Zinc | S↓ | S↓ | - | S↓ |
| Sodium | - | - | - | S↑ |
| WBC | - | S↑ | - | - |
| PLT | S↑ | - | - | - |
| Variable 1 | Variable 2 | Correlation (r) | Lower 95% | Upper 95% | Probability (P) |
|---|---|---|---|---|---|
| Fertile Controls | |||||
| ALB | TP | 0.5796 | 0.3591 | 0.7387 | <0.0001 |
| TP | CHOL | 0.4878 | 0.2423 | 0.6746 | 0.0003 |
| CK | TP | 0.4469 | 0.1926 | 0.6450 | 0.0011 |
| CK | UREA | 0.3967 | 0.1331 | 0.6079 | 0.0043 |
| CK | TG | 0.3977 | 0.1312 | 0.6106 | 0.0047 |
| UREA | TP | 0.3679 | 0.0998 | 0.5862 | 0.0086 |
| CAL | SGOT | −0.3604 | −0.5805 | −0.0912 | 0.0101 |
| % Motility | CAL | −0.3523 | −0.5743 | −0.0820 | 0.0121 |
| Infertile Patients | |||||
| MON | LYM | 0.9539 | 0.9203 | 0.9739 | <0.0001 |
| GRA | MON | 0.9039 | 0.8358 | 0.9446 | <0.0001 |
| GRA | LYM | 0.8244 | 0.7086 | 0.8969 | <0.0001 |
| PLT | CK | 0.6622 | 0.4706 | 0.7942 | <0.0001 |
| TG | GLC | 0.6665 | 0.4742 | 0.7981 | <0.0001 |
| Cell count | CK | 0.6554 | 0.4612 | 0.7897 | <0.0001 |
| Cell count | MON | −0.6266 | −0.7706 | −0.4219 | <0.0001 |
| SGOT | UREA | 0.6217 | 0.4152 | 0.7673 | <0.0001 |
| SGPT | UREA | 0.6213 | 0.4147 | 0.7670 | <0.0001 |
| Cell count | LYM | −0.6178 | −0.7647 | −0.4099 | <0.0001 |
| % Motility | MON | −0.6060 | −0.7567 | −0.3941 | <0.0001 |
| % Motility | LYM | −0.5974 | −0.7509 | −0.3827 | <0.0001 |
| Cell count | GRA | −0.5863 | −0.7434 | −0.3681 | <0.0001 |
| miP | TG | −0.5859 | −0.7444 | −0.3648 | <0.0001 |
| miP | SGPT | 0.5756 | 0.3540 | 0.7360 | <0.0001 |
| miP | UREA | 0.5736 | 0.3513 | 0.7346 | <0.0001 |
| % Motility | GRA | −0.5670 | −0.7301 | −0.3428 | <0.0001 |
| CK | SGPT | 0.5533 | 0.3251 | 0.7207 | <0.0001 |
| % Motility | SGPT | −0.5530 | −0.7204 | −0.3246 | <0.0001 |
| PLT | SGPT | 0.5437 | 0.3127 | 0.7140 | <0.0001 |
| miP | ALB | 0.5372 | 0.3044 | 0.7095 | <0.0001 |
| LYM | CK | −0.5334 | −0.7068 | 0.2995 | <0.0001 |
| MON | CK | −0.5255 | −0.7013 | −0.2895 | <0.0001 |
| % Motility | Zinc | 0.5219 | 0.2849 | 0.6987 | 0.0001 |
| LYM | LDH | 0.5131 | 0.2739 | 0.6925 | 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vashisht, A.; Ahluwalia, P.K.; Gahlay, G.K. A Comparative Analysis of the Altered Levels of Human Seminal Plasma Constituents as Contributing Factors in Different Types of Male Infertility. Curr. Issues Mol. Biol. 2021, 43, 1307-1324. https://doi.org/10.3390/cimb43030093
Vashisht A, Ahluwalia PK, Gahlay GK. A Comparative Analysis of the Altered Levels of Human Seminal Plasma Constituents as Contributing Factors in Different Types of Male Infertility. Current Issues in Molecular Biology. 2021; 43(3):1307-1324. https://doi.org/10.3390/cimb43030093
Chicago/Turabian StyleVashisht, Ashutosh, Pankaj Kumar Ahluwalia, and Gagandeep Kaur Gahlay. 2021. "A Comparative Analysis of the Altered Levels of Human Seminal Plasma Constituents as Contributing Factors in Different Types of Male Infertility" Current Issues in Molecular Biology 43, no. 3: 1307-1324. https://doi.org/10.3390/cimb43030093







