Gigantol Improves Cholesterol Metabolism and Progesterone Biosynthesis in MA-10 Leydig Cells
Abstract
:1. Introduction
2. Materials and Methods
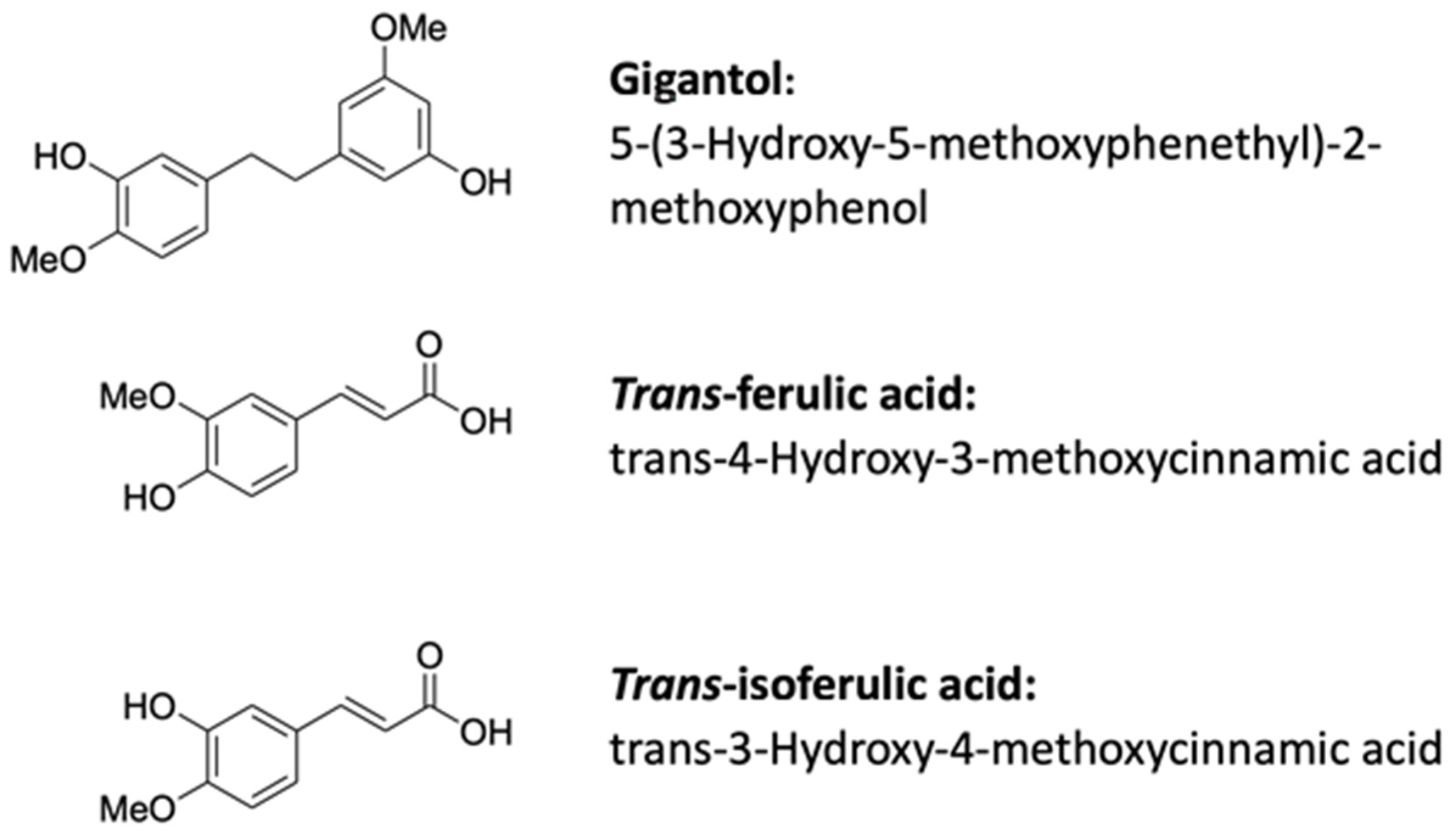
2.1. Chemicals
2.2. Cell Culture and Treatments
2.3. Cell Viability
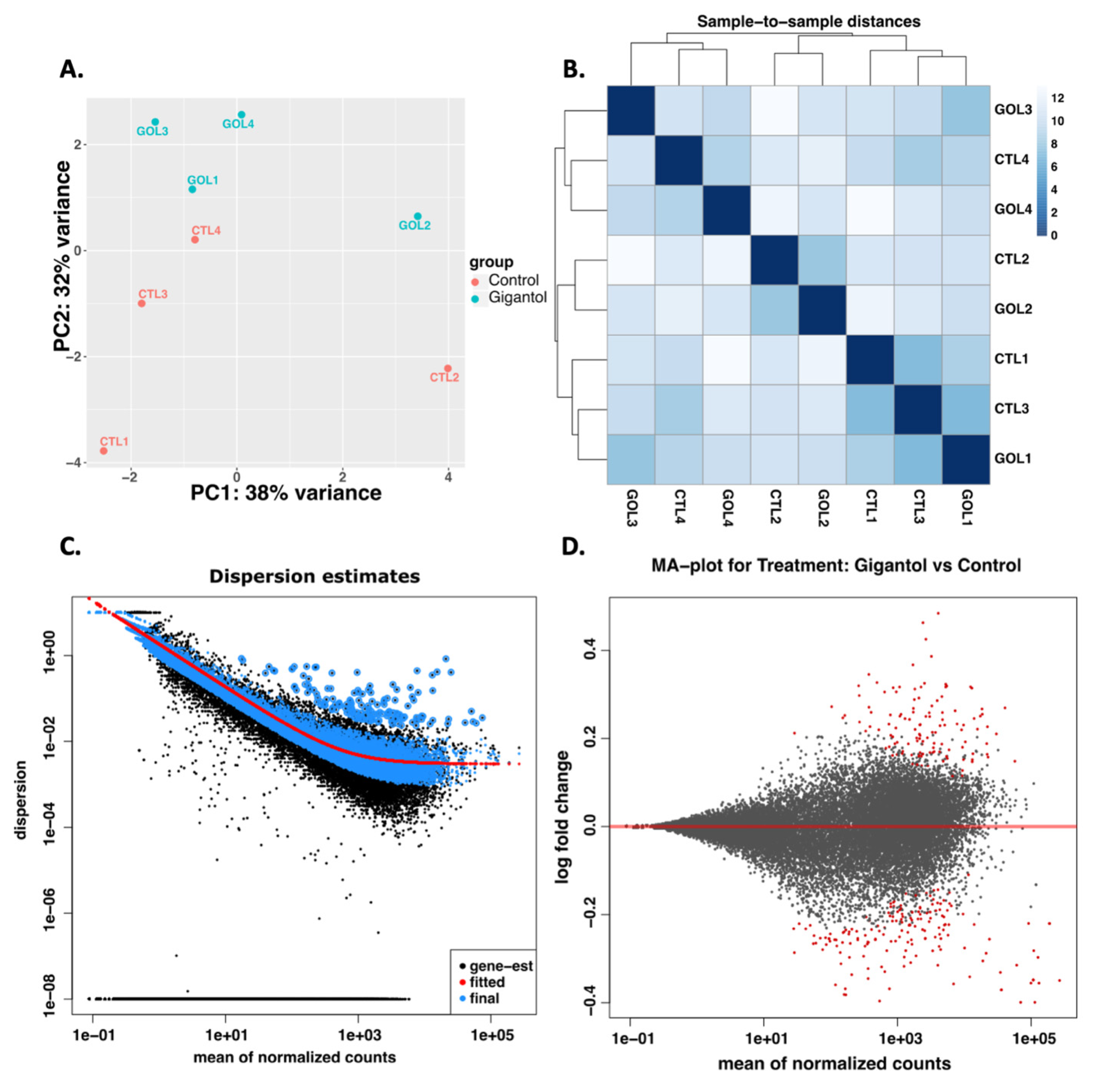
2.4. RNA Extraction and RNA Sequencing Analyses
2.5. Pathways and Networks Analyses
2.6. Quantification of Lipid Droplets
2.7. Western Blot Assays
2.8. Plasmid Constructs and Transfection Assays
2.9. Progesterone Quantification Assays
2.10. Statistical Analyses
3. Results
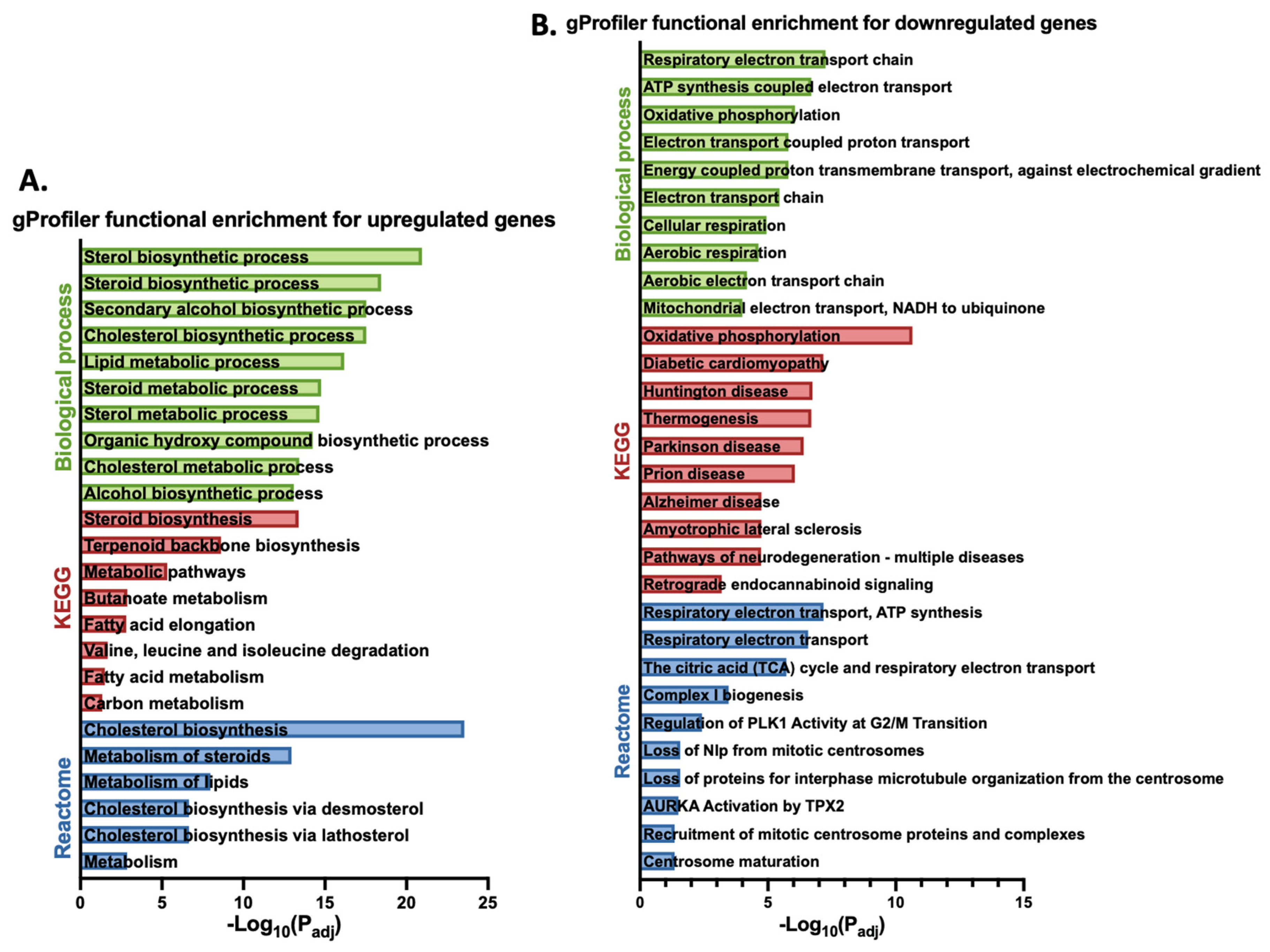
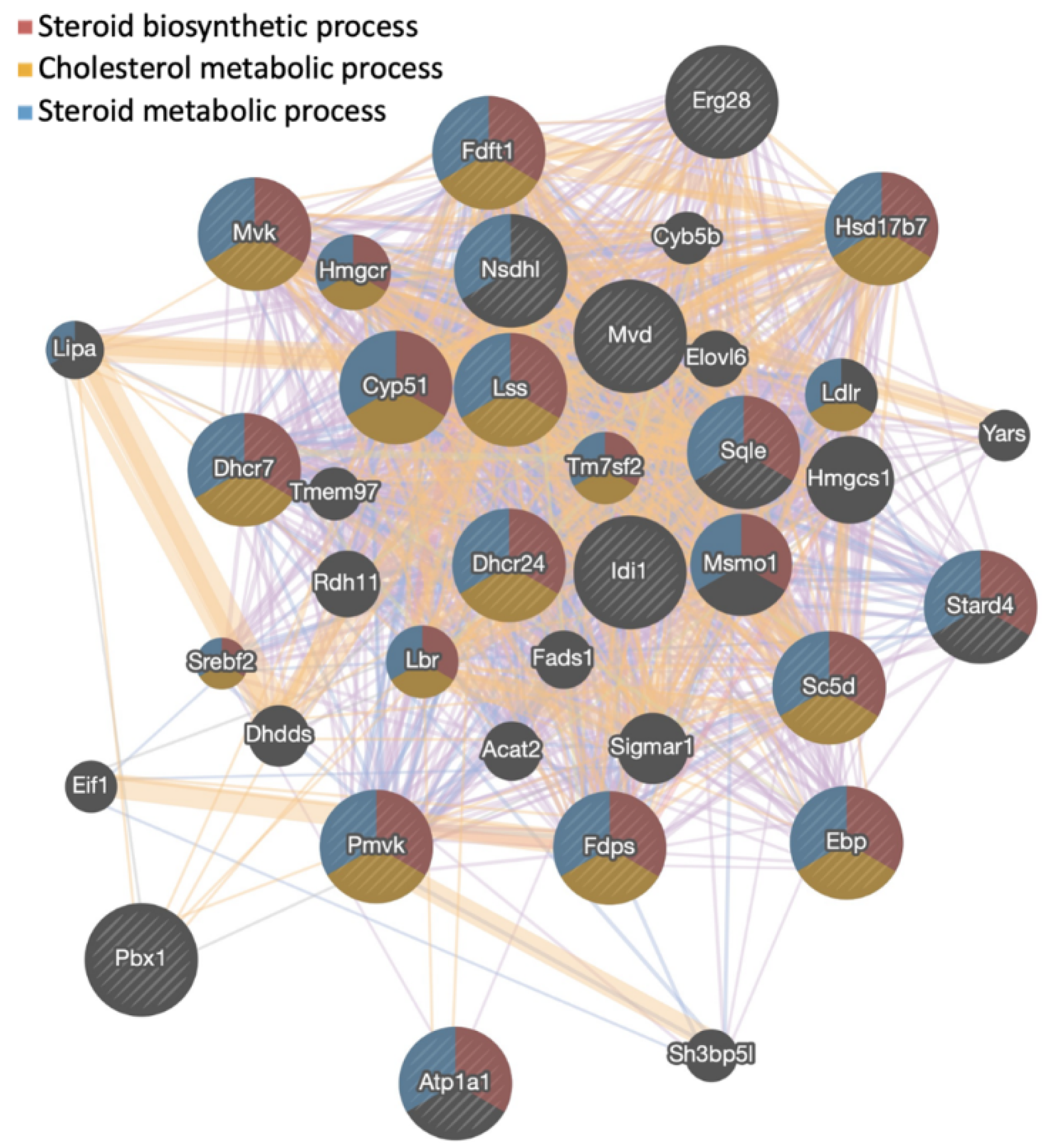
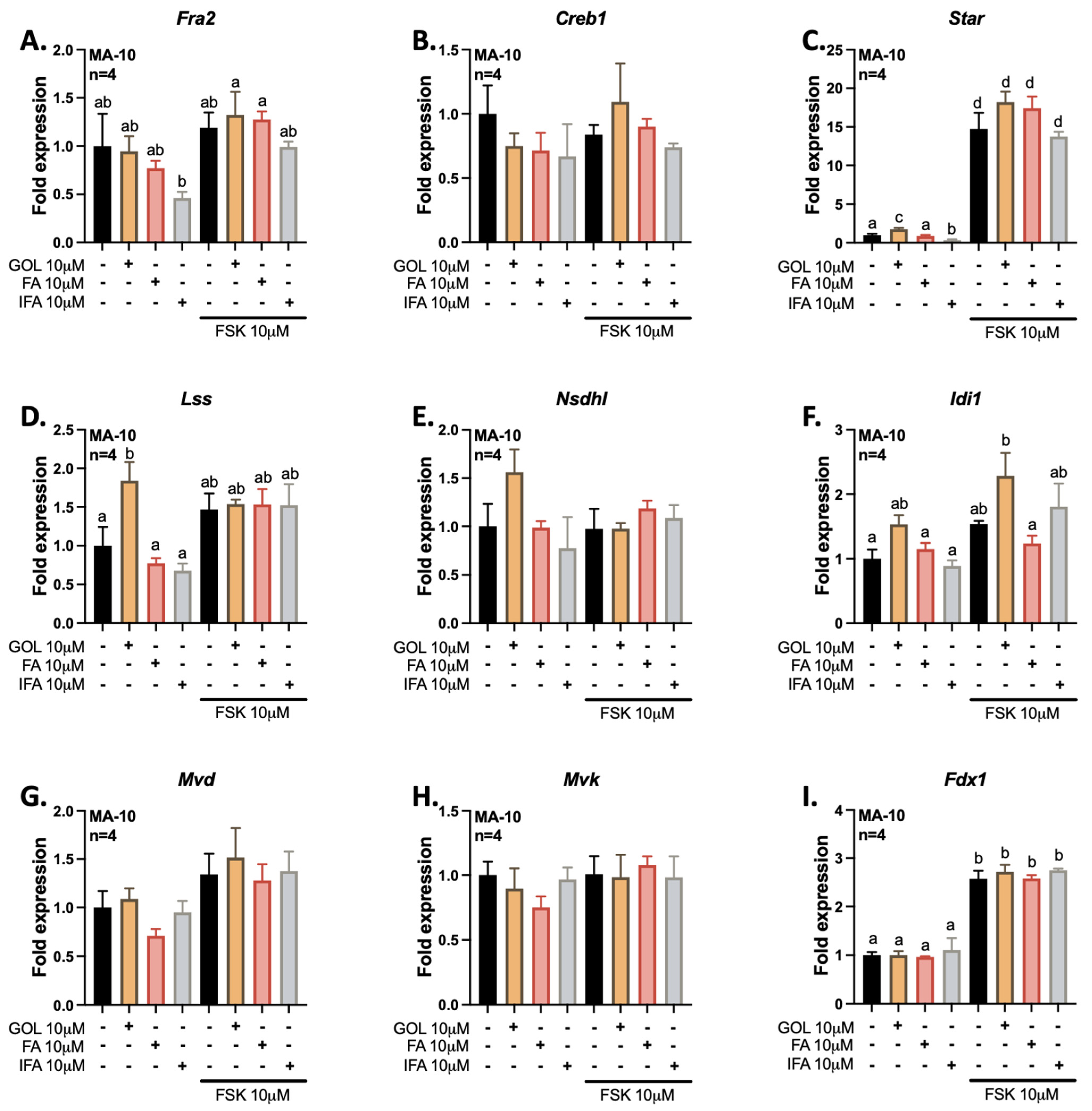
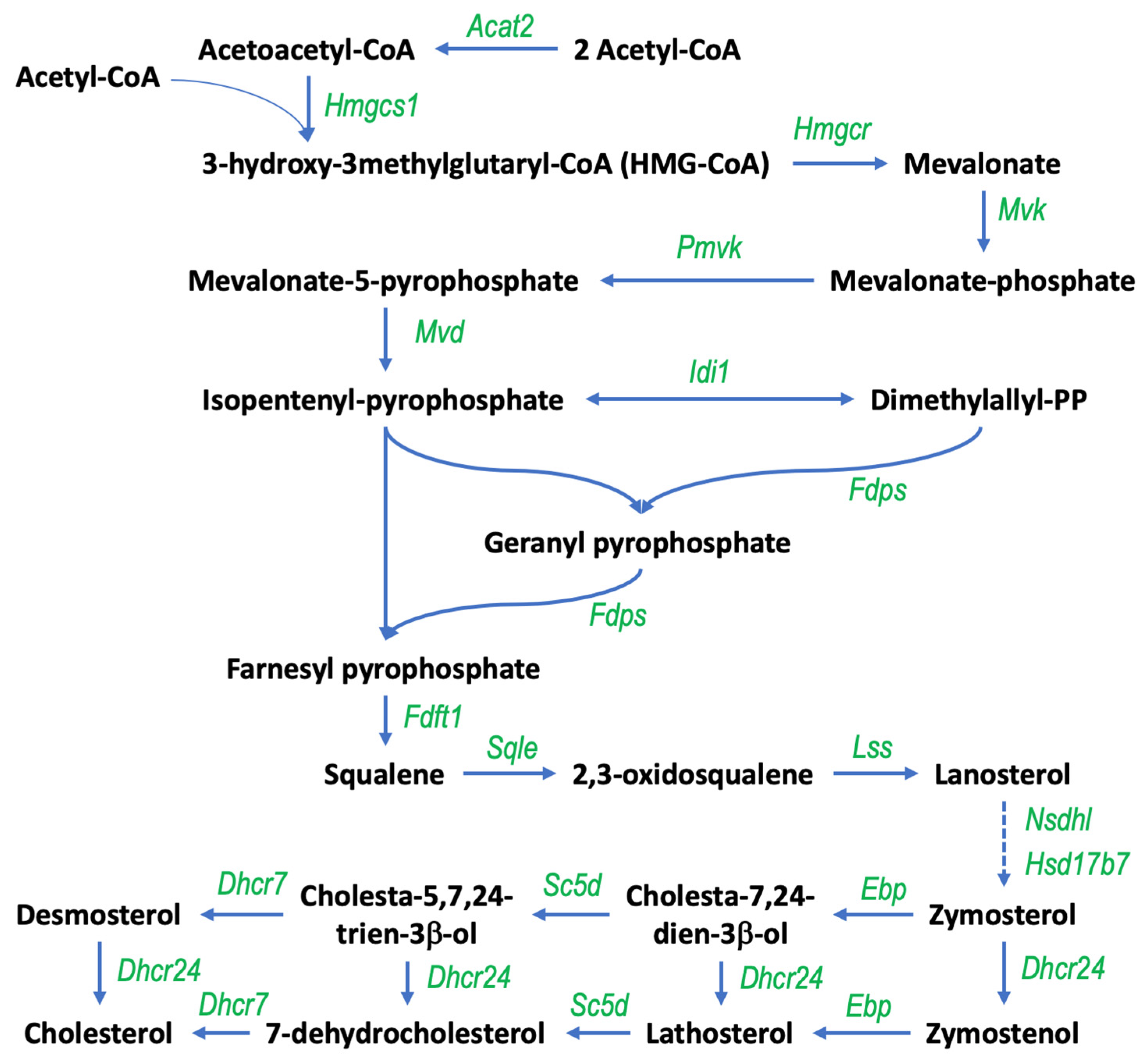
3.1. Gigantol Increases the Expression of Genes Involved in Cholesterol and Steroid Metabolic Processes
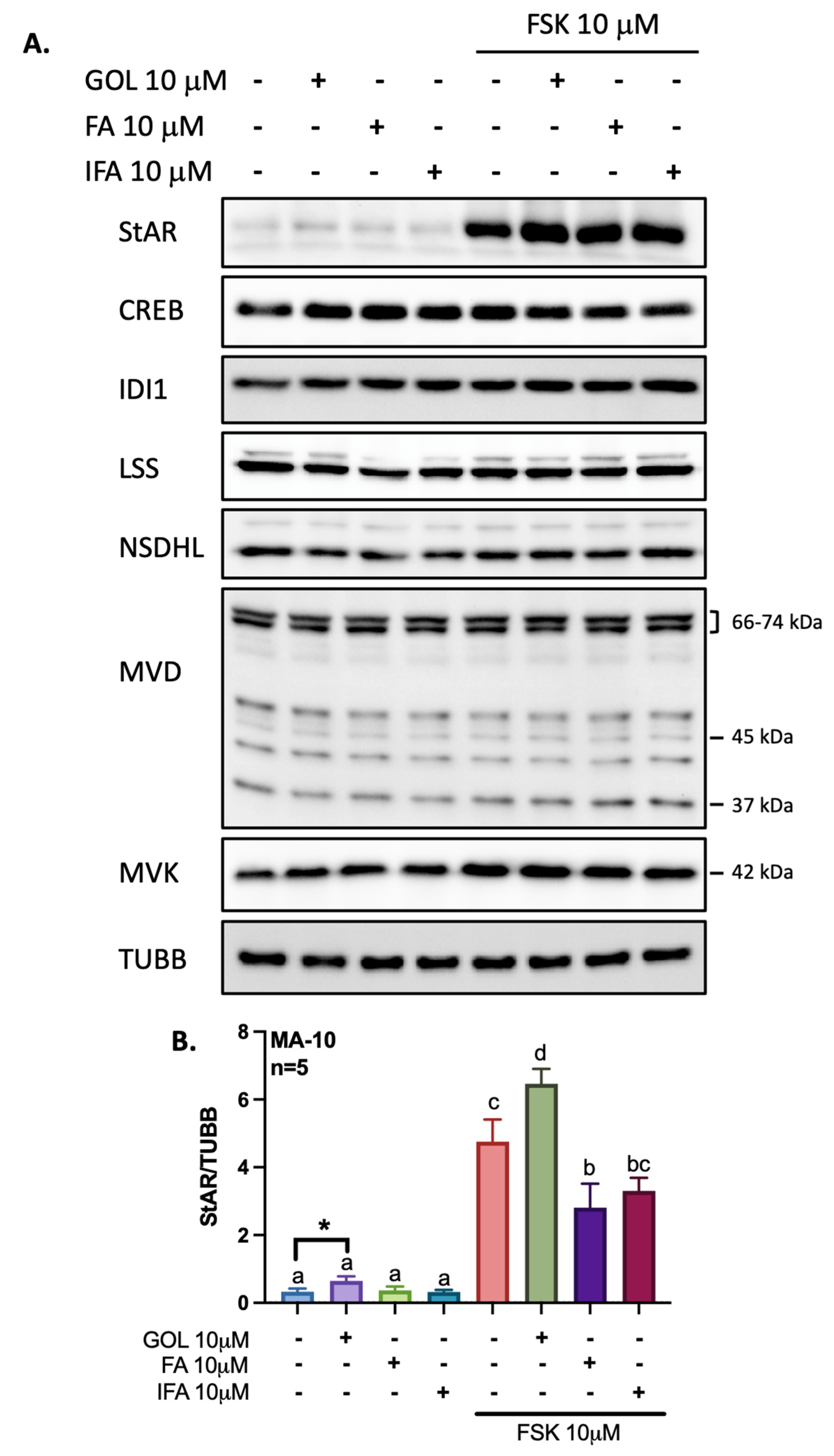
3.2. Gigantol Increases STAR mRNA Expression and Protein Levels
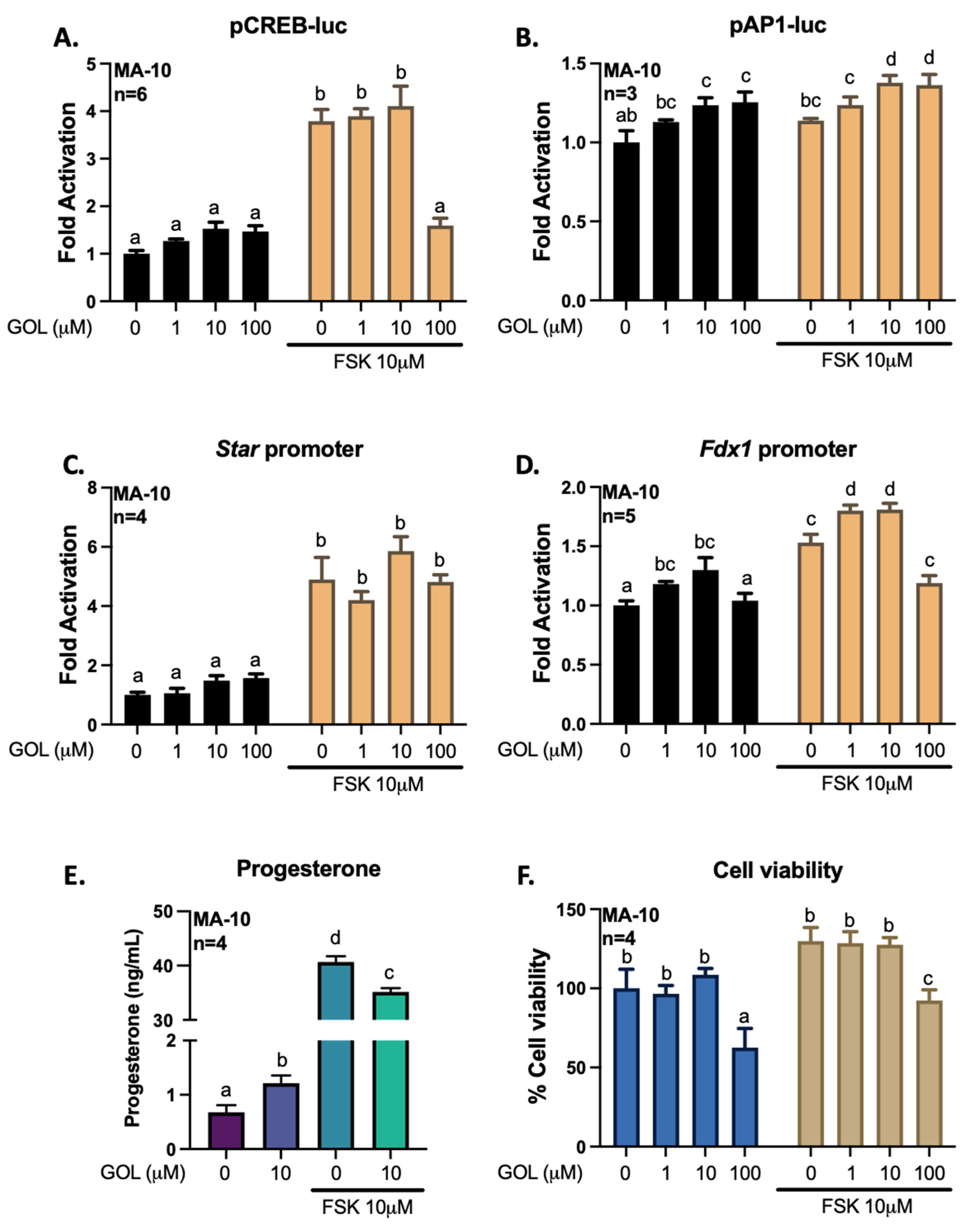
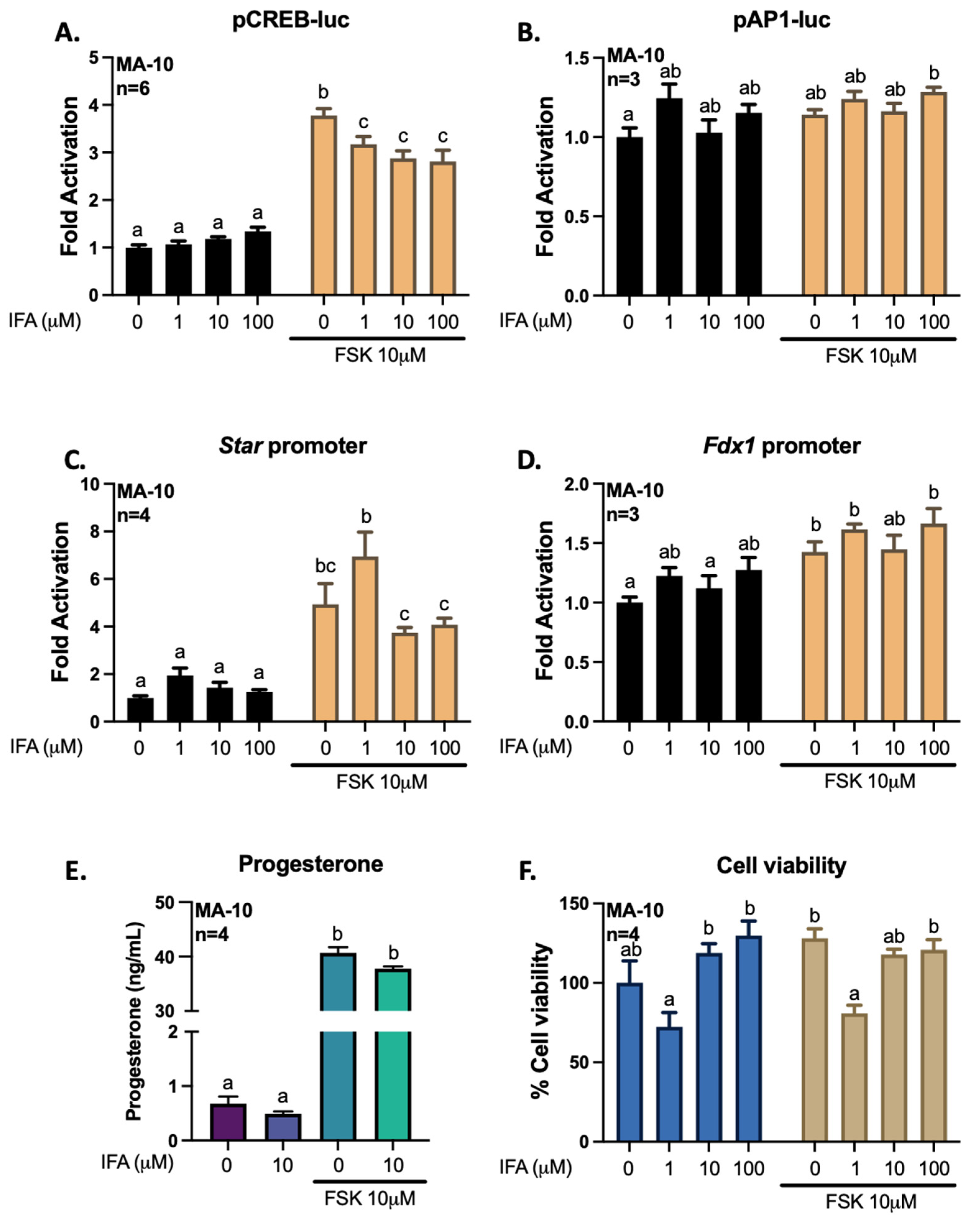
3.3. Gigantol Improves Progesterone Production in MA-10 Leydig Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gray, A.; Feldman, H.A.; McKinlay, J.B.; Longcope, C. Age, Disease, and Changing Sex Hormone Levels in Middle-Aged Men: Results of the Massachusetts Male Aging Study. J. Clin. Endocrinol. Metab. 1991, 73, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Touaibia, M. Improvement of Testicular Steroidogenesis Using Flavonoids and Isoflavonoids for Prevention of Late-Onset Male Hypogonadism. Antioxidants 2020, 9, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, D.; Sugawara, T.; Strauss, J.F.; Clark, B.J.; Stocco, D.M.; Saenger, P.; Rogol, A.; Miller, W.L. Role of Steroidogenic Acute Regulatory Protein in Adrenal and Gonadal Steroidogenesis. Science 1995, 267, 1828–1831. [Google Scholar] [CrossRef] [PubMed]
- Stocco, D.M.; Clark, B.J. Regulation of the Acute Production of Steroids in Steroidogenic Cells. Endocr. Rev. 1996, 17, 221–244. [Google Scholar] [CrossRef] [Green Version]
- Culty, M.; Luo, L.; Yao, Z.-X.; Chen, H.; Papadopoulos, V.; Zirkin, B.R. Cholesterol Transport, Peripheral Benzodiazepine Receptor, and Steroidogenesis in Aging Leydig Cells. J. Androl. 2002, 23, 439–447. [Google Scholar]
- Leers-Sucheta, S.; Stocco, D.M.; Azhar, S. Down-Regulation of Steroidogenic Acute Regulatory (StAR) Protein in Rat Leydig Cells: Implications for Regulation of Testosterone Production during Aging. Mech. Ageing Dev. 1999, 107, 197–203. [Google Scholar] [CrossRef]
- Wang, X.J. Natural Flavonoids in StAR Gene Expression and Testosterone Biosynthesis in Leydig Cell Aging; Basic and Clinical Endocrinology Up-to-Date; IntechOpen Limited: London, UK, 2011. [Google Scholar]
- Cormier, M.; Ghouili, F.; Roumaud, P.; Bauer, W.; Touaibia, M.; Martin, L.J. Influences of Flavones on Cell Viability and CAMP-Dependent Steroidogenic Gene Regulation in MA-10 Leydig Cells. Cell Biol. Toxicol. 2018, 34, 23–38. [Google Scholar] [CrossRef]
- Li, W.; Pandey, A.K.; Yin, X.; Chen, J.-J.; Stocco, D.M.; Grammas, P.; Wang, X. Effects of Apigenin on Steroidogenesis and Steroidogenic Acute Regulatory Gene Expression in Mouse Leydig Cells. J. Nutr. Biochem. 2011, 22, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Shen, C.-L.; Dyson, M.T.; Eimerl, S.; Orly, J.; Hutson, J.C.; Stocco, D.M. Cyclooxygenase-2 Regulation of the Age-Related Decline in Testosterone Biosynthesis. Endocrinology 2005, 146, 4202–4208. [Google Scholar] [CrossRef]
- Manna, P.R.; Soh, J.-W.; Stocco, D.M. The Involvement of Specific PKC Isoenzymes in Phorbol Ester-Mediated Regulation of Steroidogenic Acute Regulatory Protein Expression and Steroid Synthesis in Mouse Leydig Cells. Endocrinology 2011, 152, 313–325. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.T.; Najih, M.; Martin, L.J. The AP-1 Family of Transcription Factors Are Important Regulators of Gene Expression within Leydig Cells. Endocrine 2021, 74, 498–507. [Google Scholar] [CrossRef]
- Cormier, M.; Ghouili, F.; Roumaud, P.; Martin, L.J.; Touaibia, M. Influence of Flavonols and Quercetin Derivative Compounds on MA-10 Leydig Cells Steroidogenic Genes Expressions. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2017, 44, 111–121. [Google Scholar] [CrossRef]
- Hahm, E.-R.; Cheon, G.; Lee, J.; Kim, B.; Park, C.; Yang, C.-H. New and Known Symmetrical Curcumin Derivatives Inhibit the Formation of Fos-Jun-DNA Complex. Cancer Lett. 2002, 184, 89–96. [Google Scholar] [CrossRef]
- Yu, R.; Hebbar, V.; Kim, D.W.; Mandlekar, S.; Pezzuto, J.M.; Kong, A.N. Resveratrol Inhibits Phorbol Ester and UV-Induced Activator Protein 1 Activation by Interfering with Mitogen-Activated Protein Kinase Pathways. Mol. Pharmacol. 2001, 60, 217–224. [Google Scholar] [CrossRef]
- Simmler, C.; Antheaume, C.; Lobstein, A. Antioxidant Biomarkers from Vanda Coerulea Stems Reduce Irradiated HaCaT PGE-2 Production as a Result of COX-2 Inhibition. PLoS ONE 2010, 5, e13713. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, R.M.P.; Solis, R.V. Relaxant and Antispasmodic Effects of Extracts of the Orchid Encyclia Michuacana on Isolated Guinea Pig Ileum. J. Nat. Med. 2009, 63, 65–68. [Google Scholar] [CrossRef]
- Déciga-Campos, M.; Palacios-Espinosa, J.F.; Reyes-Ramírez, A.; Mata, R. Antinociceptive and Anti-Inflammatory Effects of Compounds Isolated from Scaphyglottis Livida and Maxillaria Densa. J. Ethnopharmacol. 2007, 114, 161–168. [Google Scholar] [CrossRef]
- Won, J.-H.; Kim, J.-Y.; Yun, K.-J.; Lee, J.-H.; Back, N.-I.; Chung, H.-G.; Chung, S.A.; Jeong, T.-S.; Choi, M.-S.; Lee, K.-T. Gigantol Isolated from the Whole Plants of Cymbidium Goeringii Inhibits the LPS-Induced INOS and COX-2 Expression via NF-KappaB Inactivation in RAW 264.7 Macrophages Cells. Planta Med. 2006, 72, 1181–1187. [Google Scholar] [CrossRef]
- Fan, C.; Wang, W.; Wang, Y.; Qin, G.; Zhao, W. Chemical Constituents from Dendrobium Densiflorum. Phytochemistry 2001, 57, 1255–1258. [Google Scholar] [CrossRef]
- Charoenrungruang, S.; Chanvorachote, P.; Sritularak, B.; Pongrakhananon, V. Gigantol, a Bibenzyl from Dendrobium Draconis, Inhibits the Migratory Behavior of Non-Small Cell Lung Cancer Cells. J. Nat. Prod. 2014, 77, 1359–1366. [Google Scholar] [CrossRef]
- Klongkumnuankarn, P.; Busaranon, K.; Chanvorachote, P.; Sritularak, B.; Jongbunprasert, V.; Likhitwitayawuid, K. Cytotoxic and Antimigratory Activities of Phenolic Compounds from Dendrobium Brymerianum. Evid.-Based Complement. Altern. Med. ECAM 2015, 2015, 350410. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Wang, Z.; Su, Z.; Song, J.; Zhou, L.; Sun, Q.; Liu, S.; Li, S.; Li, Y.; Wang, M.; et al. Gigantol Inhibits Wnt/β-Catenin Signaling and Exhibits Anticancer Activity in Breast Cancer Cells. BMC Complement. Altern. Med. 2018, 18, 59. [Google Scholar] [CrossRef] [Green Version]
- Unahabhokha, T.; Chanvorachote, P.; Pongrakhananon, V. The Attenuation of Epithelial to Mesenchymal Transition and Induction of Anoikis by Gigantol in Human Lung Cancer H460 Cells. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2016, 37, 8633–8641. [Google Scholar] [CrossRef]
- Avila-Carrasco, L.; Majano, P.; Sánchez-Toméro, J.A.; Selgas, R.; López-Cabrera, M.; Aguilera, A.; González Mateo, G. Natural Plants Compounds as Modulators of Epithelial-to-Mesenchymal Transition. Front. Pharmacol. 2019, 10, 715. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Liu, C.; Duan, S.; Lin, J.; Luo, Y.; Tao, S.; Xing, S.; Zhang, X.; Du, H.; Wang, H.; et al. Gigantol Inhibits Proliferation and Enhances DDP-Induced Apoptosis in Breast-Cancer Cells by Downregulating the PI3K/Akt/MTOR Signaling Pathway. Life Sci. 2021, 274, 119354. [Google Scholar] [CrossRef]
- Osaki, M.; Oshimura, M.; Ito, H. PI3K-Akt Pathway: Its Functions and Alterations in Human Cancer. Apoptosis Int. J. Program. Cell Death 2004, 9, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Ouyang, G.; Bao, S. The Activation of Akt/PKB Signaling Pathway and Cell Survival. J. Cell. Mol. Med. 2005, 9, 59–71. [Google Scholar] [CrossRef]
- Estrada, S.; Rojas, A.; Mathison, Y.; Israel, A.; Mata, R. Nitric Oxide/CGMP Mediates the Spasmolytic Action of 3,4′-Dihydroxy-5,5′-Dimethoxybibenzyl from Scaphyglottis Livida. Planta Med. 1999, 65, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Romero, Y.; Rojas, J.-I.; Castillo, R.; Rojas, A.; Mata, R. Spasmolytic Effects, Mode of Action, and Structure-Activity Relationships of Stilbenoids from Nidema Boothii. J. Nat. Prod. 2004, 67, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Hartley, R.D.; Ford, C.W. Phenolic Constituents of Plant Cell Walls and Wall Biodegradability. In Plant Cell Wall Polymers; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1989; Volume 399, pp. 137–145. ISBN 978-0-8412-1658-7. [Google Scholar]
- Liu, I.-M.; Chen, W.-C.; Cheng, J.-T. Mediation of Beta-Endorphin by Isoferulic Acid to Lower Plasma Glucose in Streptozotocin-Induced Diabetic Rats. J. Pharmacol. Exp. Ther. 2003, 307, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, X.; Cheng, W.; Yang, K.; Ma, Y.; Bi, K. RP-LC Determination and Pharmacokinetic Study of Ferulic Acid and Isoferulic Acid in Rat Plasma After Taking Traditional Chinese Medicinal-Preparation: Guanxinning Lyophilizer. Chromatographia 2008, 67, 1007–1011. [Google Scholar] [CrossRef]
- Roy, S.; Metya, S.K.; Rahaman, N.; Sannigrahi, S.; Ahmed, F. Ferulic Acid in the Treatment of Post-Diabetes Testicular Damage: Relevance to the down Regulation of Apoptosis Correlates with Antioxidant Status via Modulation of TGF-Β1, IL-1β and Akt Signalling. Cell Biochem. Funct. 2014, 32, 115–124. [Google Scholar] [CrossRef]
- Zheng, R.L.; Zhang, H. Effects of Ferulic Acid on Fertile and Asthenozoospermic Infertile Human Sperm Motility, Viability, Lipid Peroxidation, and Cyclic Nucleotides. Free Radic. Biol. Med. 1997, 22, 581–586. [Google Scholar] [CrossRef]
- Ghouili, F.; Martin, L.J. Cooperative Regulation of Gja1 Expression by Members of the AP-1 Family CJun and CFos in TM3 Leydig and TM4 Sertoli Cells. Gene 2017, 635, 24–32. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinform. Oxf. Engl. 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinforma. Oxf. Engl. 2014, 30, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Reimand, J.; Arak, T.; Adler, P.; Kolberg, L.; Reisberg, S.; Peterson, H.; Vilo, J. G:Profiler-a Web Server for Functional Interpretation of Gene Lists (2016 Update). Nucleic Acids Res. 2016, 44, W83–W89. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. G:Profiler: A Web Server for Functional Enrichment Analysis and Conversions of Gene Lists (2019 Update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [Green Version]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T.; et al. The GeneMANIA Prediction Server: Biological Network Integration for Gene Prioritization and Predicting Gene Function. Nucleic Acids Res. 2010, 38, W214–W220. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Martin, L.J.; Boucher, N.; Brousseau, C.; Tremblay, J.J. The Orphan Nuclear Receptor NUR77 Regulates Hormone-Induced StAR Transcription in Leydig Cells through Cooperation with Ca2+/Calmodulin-Dependent Protein Kinase I. Mol. Endocrinol. Baltim. Md 2008, 22, 2021–2037. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Villarroel, R.E.; Robert, N.M.; Martin, L.J.; Brousseau, C.; Tremblay, J.J. The Nuclear Receptor NR2F2 Activates Star Expression and Steroidogenesis in Mouse MA-10 and MLTC-1 Leydig Cells. Biol. Reprod. 2014, 91, 26. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Su, Q.; Bai, L.; Li, M.; Liu, J.; Liu, X.; Zhang, C.; Jiang, Z.; He, J.; Shi, J.; et al. Recent Research Progress on Natural Small Molecule Bibenzyls and Its Derivatives in Dendrobium Species. Eur. J. Med. Chem. 2020, 204, 112530. [Google Scholar] [CrossRef]
- Fang, H.; Hu, X.; Wang, M.; Wan, W.; Yang, Q.; Sun, X.; Gu, Q.; Gao, X.; Wang, Z.; Gu, L.; et al. Anti-Osmotic and Antioxidant Activities of Gigantol from Dendrobium Aurantiacum Var. Denneanum against Cataractogenesis in Galactosemic Rats. J. Ethnopharmacol. 2015, 172, 238–246. [Google Scholar] [CrossRef]
- Labunskyy, V.M.; Gladyshev, V.N. Role of Reactive Oxygen Species-Mediated Signaling in Aging. Antioxid. Redox Signal. 2013, 19, 1362–1372. [Google Scholar] [CrossRef] [Green Version]
- Sohal, R.S. Role of Oxidative Stress and Protein Oxidation in the Aging Process. Free Radic. Biol. Med. 2002, 33, 37–44. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; Ye, L.; Zirkin, B.; Chen, H. Steroidogenesis in Leydig Cells: Effects of Aging and Environmental Factors. Reprod. Camb. Engl. 2017, 154, R111–R122. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Leers-Sucheta, S.; Azhar, S. Aging Alters the Functional Expression of Enzymatic and Non-Enzymatic Anti-Oxidant Defense Systems in Testicular Rat Leydig Cells. J. Steroid Biochem. Mol. Biol. 2004, 88, 61–67. [Google Scholar] [CrossRef]
- Couture, R.; Mora, N.; Al Bittar, S.; Najih, M.; Touaibia, M.; Martin, L.J. Luteolin Modulates Gene Expression Related to Steroidogenesis, Apoptosis, and Stress Response in Rat LC540 Tumor Leydig Cells. Cell Biol. Toxicol. 2020, 36, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Guvvala, P.R.; Ravindra, J.P.; Selvaraju, S.; Arangasamy, A.; Venkata, K.M. Ellagic and Ferulic Acids Protect Arsenic-Induced Male Reproductive Toxicity via Regulating Nfe2l2, Ppargc1a and StAR Expressions in Testis. Toxicology 2019, 413, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hasanein, P.; Fazeli, F.; Parviz, M.; Roghani, M. Ferulic Acid Prevents Lead-Induced Testicular Oxidative Stress and Suppressed Spermatogenesis in Rats. Andrologia 2018, 50, e12798. [Google Scholar] [CrossRef] [PubMed]
- Banihani, S.A. Mechanisms of Honey on Testosterone Levels. Heliyon 2019, 5, e02029. [Google Scholar] [CrossRef]
- Huang, H.; Hong, Q.; Tan, H.-L.; Xiao, C.-R.; Gao, Y. Ferulic Acid Prevents LPS-Induced up-Regulation of PDE4B and Stimulates the CAMP/CREB Signaling Pathway in PC12 Cells. Acta Pharmacol. Sin. 2016, 37, 1543–1554. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.-X.; Liang, G.; Chu, Y.; Li, X.; Lian, Q.-Q.; Lin, H.; He, Y.; Huang, Y.; Hardy, D.O.; Ge, R.-S. Curcumin Derivatives Inhibit Testicular 17beta-Hydroxysteroid Dehydrogenase 3. Bioorg. Med. Chem. Lett. 2010, 20, 2549–2551. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chiu, C.-H.; Liu, H.-C.; Wang, J.-Y. Curcumin Downregulates 8-Br-CAMP-Induced Steroidogenesis in Mouse Leydig Cells by Suppressing the Expression of Cyp11a1 and StAR Independently of the PKA-CREB Pathway. Endocr. J. 2018, 65, 833–840. [Google Scholar] [CrossRef] [Green Version]
- Engeli, R.T.; Fürstenberger, C.; Kratschmar, D.V.; Odermatt, A. Currently Available Murine Leydig Cell Lines Can Be Applied to Study Early Steps of Steroidogenesis but Not Testosterone Synthesis. Heliyon 2018, 4, e00527. [Google Scholar] [CrossRef] [Green Version]
- Mellon, S.H.; Vaisse, C. CAMP Regulates P450scc Gene Expression by a Cycloheximide-Insensitive Mechanism in Cultured Mouse Leydig MA-10 Cells. Proc. Natl. Acad. Sci. USA 1989, 86, 7775–7779. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Zhang, Z.; Shen, W.-J.; Azhar, S. Cellular Cholesterol Delivery, Intracellular Processing and Utilization for Biosynthesis of Steroid Hormones. Nutr. Metab. 2010, 7, 47. [Google Scholar] [CrossRef] [Green Version]



| Description | Gene Set | Ontology | NES | p Value | FDR |
|---|---|---|---|---|---|
| Steroid biosynthetic process | GO:0006694 | BP | 2.66 | 0 | 0 |
| Sterol metabolic process | GO:0016125 | BP | 2.65 | 0 | 0 |
| Alcohol biosynthetic process | GO:0046165 | BP | 2.64 | 0 | 0 |
| Secondary alcohol metabolic process | GO:1902652 | BP | 2.63 | 0 | 0 |
| Small molecule biosynthetic process | GO:0044283 | BP | 2.62 | 0 | 0 |
| Organic hydroxy compound biosynthetic process | GO:1901617 | BP | 2.60 | 0 | 0 |
| Cholesterol metabolic process | GO:0008203 | BP | 2.60 | 0 | 0 |
| Steroid metabolic process | GO:0008202 | BP | 2.60 | 0 | 0 |
| Sterol biosynthetic process | GO:0016126 | BP | 2.59 | 0 | 0 |
| Isoprenoid metabolic process | GO:0006720 | BP | 2.56 | 0 | 0 |
| Cytosolic ribosome | GO:0022626 | CC | 2.65 | 0 | 0 |
| Ribosomal subunit | GO:0044391 | CC | 2.58 | 0 | 0 |
| Cytosolic large ribosomal subunit | GO:0022625 | CC | 2.54 | 0 | 0 |
| Ribosome | GO:0005840 | CC | 2.51 | 0 | 0 |
| Large ribosome subunit | GO:0015934 | CC | 2.48 | 0 | 0 |
| Mitochondrial matrix | GO:0005759 | CC | 2.37 | 0 | 0 |
| Cytoplasmic part | GO:0044445 | CC | 2.30 | 0 | 0 |
| Microbody | GO:0042579 | CC | 2.30 | 0 | 0 |
| Peroxisome | GO:0005777 | CC | 2.29 | 0 | 0 |
| Cytosolic small ribosomal subunit | GO:0022627 | CC | 2.26 | 0 | 0 |
| Structural constituent of ribosome | GO:0003735 | MF | 2.57 | 0 | 0 |
| rRNA Binding | GO:0019843 | MF | 2.38 | 0 | 0 |
| Intramolecular oxidoreductase activity | GO:0016860 | MF | 2.18 | 0 | 0.001 |
| Ligase activity | GO:0016874 | MF | 2.16 | 0 | 0.002 |
| Lyase activity | GO:0016829 | MF | 2.15 | 0 | 0.002 |
| Carbon-carbon lyase activity | GO:0016830 | MF | 2.14 | 0.001 | 0.002 |
| snoRNA binding | GO:0030515 | MF | 2.13 | 0 | 0.003 |
| Oxidoreductase activity, acting on the ch-ch group of donors | GO:0016627 | MF | 2.12 | 0 | 0.003 |
| Oxidoreductase activity, acting on ch-oh group of donors | GO:0016614 | MF | 2.09 | 0 | 0.005 |
| Isomerase activity | GO:0016853 | MF | 2.08 | 0 | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basque, A.; Nguyen, H.T.; Touaibia, M.; Martin, L.J. Gigantol Improves Cholesterol Metabolism and Progesterone Biosynthesis in MA-10 Leydig Cells. Curr. Issues Mol. Biol. 2022, 44, 73-93. https://doi.org/10.3390/cimb44010006
Basque A, Nguyen HT, Touaibia M, Martin LJ. Gigantol Improves Cholesterol Metabolism and Progesterone Biosynthesis in MA-10 Leydig Cells. Current Issues in Molecular Biology. 2022; 44(1):73-93. https://doi.org/10.3390/cimb44010006
Chicago/Turabian StyleBasque, Audrey, Ha Tuyen Nguyen, Mohamed Touaibia, and Luc J. Martin. 2022. "Gigantol Improves Cholesterol Metabolism and Progesterone Biosynthesis in MA-10 Leydig Cells" Current Issues in Molecular Biology 44, no. 1: 73-93. https://doi.org/10.3390/cimb44010006
APA StyleBasque, A., Nguyen, H. T., Touaibia, M., & Martin, L. J. (2022). Gigantol Improves Cholesterol Metabolism and Progesterone Biosynthesis in MA-10 Leydig Cells. Current Issues in Molecular Biology, 44(1), 73-93. https://doi.org/10.3390/cimb44010006






