The Use of Salivary Levels of Matrix Metalloproteinases as an Adjuvant Method in the Early Diagnosis of Oral Squamous Cell Carcinoma: A Narrative Literature Review
Abstract
:1. Introduction
2. Data Collection
3. Clinical Significance of Salivary Levels of MMPs in OSCC Detection
4. The Role of Saliva in Oral Cancer Detection
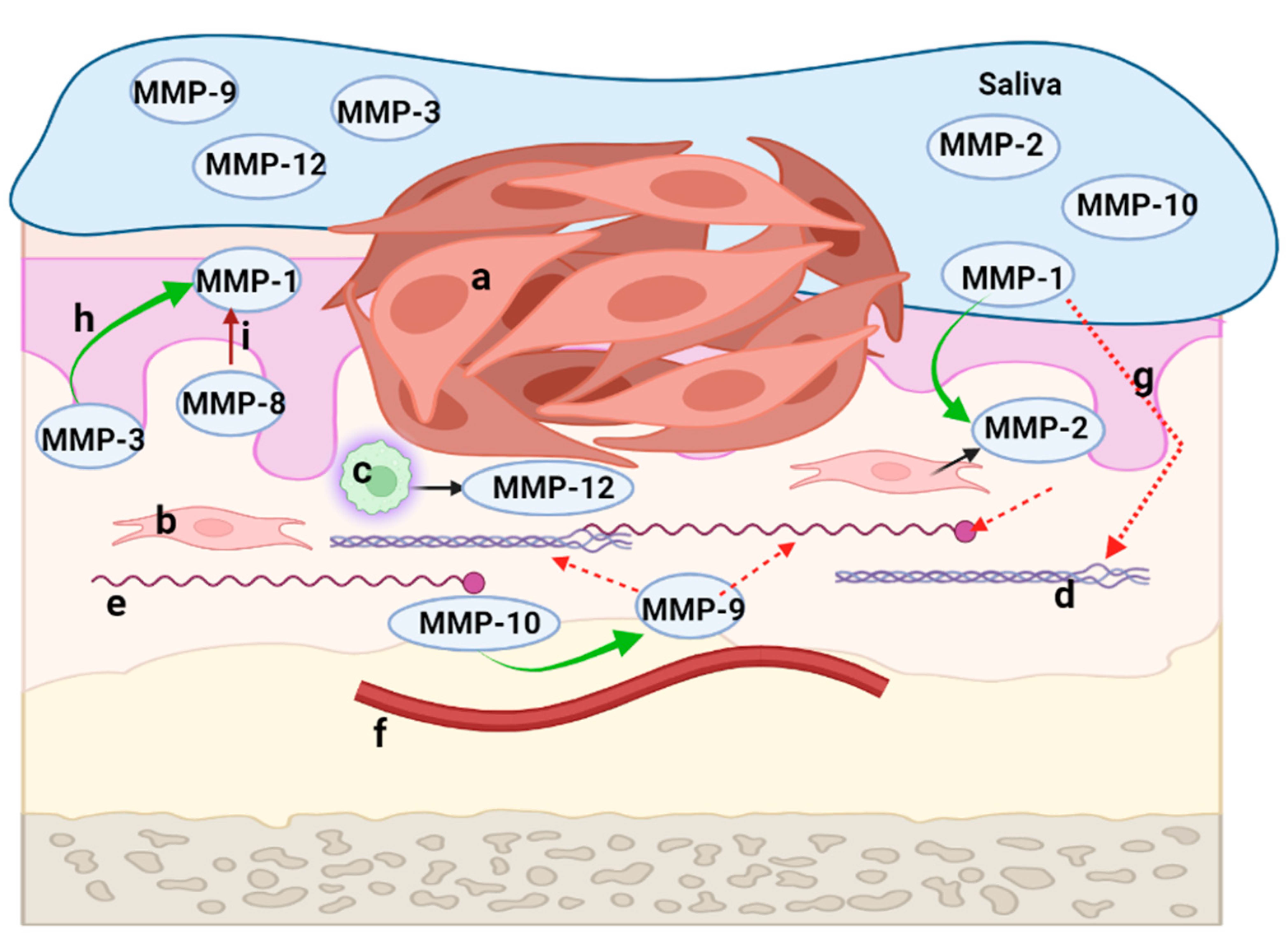
5. The Key Role of MMPs in the Development of the Tumor Microenvironment of OSCC
6. MMPs with Identified Roles in the Development and Progression of OSCC
6.1. Collagenases
6.2. Gelatinases
6.3. Stromelysins
6.4. Matrylisins
6.5. Others
7. The Use of Salivary MMPs as Biomarkers for OSCC
8. Difficulties Associated with the Early Diagnosis of OSCC
9. Strengths and Limitations
10. Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kazmi, F.; Alkait, S.; Alghamdi, H.; Alhussain, G.; Tabassum, A. Assessing Knowledge, Attitude and Practices for Oral Squamous Cell Carcinoma among Health Care Professionals in Princess Nourah University, Riyadh, KSA. Asian Pac. J. Cancer Prev. 2020, 21, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Dhanuthai, K.; Rojanawatsirivej, S.; Thosaporn, W.; Kintarak, S.; Subarnbhesaj, A.; Darling, M.; Kryshtalskyj, E.; Chiang, C.P.; Shin, H.I.; Choi, S.Y.; et al. Oral cancer: A multicenter study. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e23–e29. [Google Scholar] [CrossRef]
- Cicciù, M.; Cervino, G.; Fiorillo, L.; D’Amico, C.; Oteri, G.; Troiano, G.; Zhurakivska, K.; Lo Muzio, L.; Herford, A.S.; Crimi, S.; et al. Early Diagnosis on Oral and Potentially Oral Malignant Lesions: A Systematic Review on the VELscope® Fluorescence Method. Dent. J. 2019, 7, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Porcheri, C.; Meisel, C.T.; Mitsiadis, T. Multifactorial Contribution of Notch Signaling in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 1520. [Google Scholar] [CrossRef] [Green Version]
- Komlós, G.; Csurgay, K.; Horváth, F.; Pelyhe, L.; Németh, Z. Periodontitis as a risk for oral cancer: A case-control study. BMC Oral Health 2021, 21, 640. [Google Scholar] [CrossRef]
- Kavarthapu, A.; Gurumoorthy, K. Linking chronic periodontitis and oral cancer: A review. Oral Oncol. 2021, 121, 105375. [Google Scholar] [CrossRef]
- Irani, S.; Barati, I.; Badiei, M. Periodontitis and oral cancer—Current concepts of the etiopathogenesis. Oncol. Rev. 2020, 14, 465. [Google Scholar] [CrossRef] [Green Version]
- Strome, A.; Kossatz, S.; Zanoni, D.K.; Rajadhyaksha, M.; Patel, S.; Reiner, T. Current Practice and Emerging Molecular Imaging Technologies in Oral Cancer Screening. Mol. Imaging 2018, 17, 1536012118808644. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 2020, 6, 92. [Google Scholar] [CrossRef]
- Lin, N.C.; Hsien, S.I.; Hsu, J.T.; Chen, M.Y.C. Impact on patients with oral squamous cell carcinoma in different anatomical subsites: A single-center study in Taiwan. Sci. Rep. 2021, 11, 15446. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warnakulasuriya, S.; Kerr, A.R. Oral Cancer Screening: Past, Present, and Future. J. Dent. Res. 2021, 100, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, J.; Lee, M.H.; Kim, S.W.; Hwang, S.H. Efficacy of chemiluminescence in the diagnosis and screening of oral cancer and precancer: A systematic review and meta-analysis. Braz. J. Otorhinolaryngol. 2022, 88, 358–364. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bosetti, C.; Carioli, G.; Santucci, C.; Bertuccio, P.; Gallus, S.; Garavello, W.; Negri, E.; La Vecchia, C. Global trends in oral and pharyngeal cancer incidence and mortality. Int. J. Cancer 2020, 147, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.T.; Chen, W.C.; Hsu, C.M.; Tsai, M.S.; Chang, G.H.; Lee, Y.C.; Huang, E.I.; Fang, C.C.; Lai, C.H. Survival-Weighted Health Profiles in Patients Treated for Advanced Oral Cavity Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 754412. [Google Scholar] [CrossRef]
- Mishra, R. Biomarkers of oral premalignant epithelial lesions for clinical application. Oral Oncol. 2012, 48, 578–584. [Google Scholar] [CrossRef]
- Speight, P.M.; Epstein, J.; Kujan, O.; Lingen, M.W.; Nagao, T.; Ranganathan, K.; Vargas, P. Screening for oral cancer-a perspective from the Global Oral Cancer Forum. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, 680–687. [Google Scholar] [CrossRef]
- Pavani, N.P.M.; Srinivas, P.; Kothia, N.R.; Chandu, V.C. Recent advances in the early diagnosis of oral cancer: A systematic review. Int. J. Med. Rev. 2017, 4, 119–125. [Google Scholar] [CrossRef]
- Madhura, M.G.; Rao, R.S.; Patil, S.; Fageeh, H.N.; Alhazmi, A.; Awan, K.H. Advanced diagnostic aids for oral cancer. Dis. Mon. 2020, 66, 101034. [Google Scholar] [CrossRef] [PubMed]
- Lousada-Fernandez, F.; Rapado-Gonzalez, O.; Lopez-Cedrun, J.L.; Lopez-Lopez, R.; Muinelo-Romay, L.; Suarez-Cunqueiro, M.M. Liquid Biopsy in Oral Cancer. Int. J. Mol. Sci. 2018, 19, 1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaves, A.L.F.; Silva, A.G.; Maia, F.M.; Lopes, G.F.M.; de Paulo, L.F.B.; Muniz, L.V.; Dos Santos, H.B.; Soares, J.M.A.; Souza, A.A.; de Oliveira Barbosa, L.A.; et al. Reduced CD8+ T cells infiltration can be associated to a malignant transformation in potentially malignant oral epithelial lesions. Clin. Oral Investig. 2019, 23, 1913–1919. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.; Macey, R.; Kerr, A.R.; Lingen, M.W.; Ogden, G.R.; Warnakulasuriya, S. Diagnostic tests for oral cancer and potentially malignant disorders in patients presenting with clinically evident lesions. Cochrane Database Syst. Rev. 2021, 7, CD010276. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Zafar, M.S.; Khan, R.S.; Najeeb, S.; Slowey, P.D.; Rehman, I.U. Role of Salivary Biomarkers in Oral Cancer Detection. Adv. Clin. Chem. 2018, 86, 23–70. [Google Scholar] [CrossRef]
- Stott-Miller, M.; Houck, J.R.; Lohavanichbutr, P.; Méndez, E.; Upton, M.P.; Futran, N.D.; Schwartz, S.M.; Chen, C. Tumor and salivary matrix metalloproteinase levels are strong diagnostic markers of oral squamous cell carcinoma. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2628–2636. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.T.; Chu, L.J.; Liu, Y.C.; Chen, C.J.; Wu, S.F.; Chen, C.H.; Chang, I.Y.; Wang, J.S.; Wu, T.Y.; Dash, S.; et al. Verification of Saliva Matrix Metalloproteinase-1 as a Strong Diagnostic Marker of Oral Cavity Cancer. Cancers 2020, 12, 2273. [Google Scholar] [CrossRef]
- Yu, J.S.; Chen, Y.T.; Chiang, W.F.; Hsiao, Y.C.; Chu, L.J.; See, L.C.; Wu, C.S.; Tu, H.T.; Chen, H.W.; Chen, C.C.; et al. Saliva protein biomarkers to detect oral squamous cell carcinoma in a high-risk population in Taiwan. Proc. Natl. Acad. Sci. USA 2016, 113, 11549–11554. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Li, Q.; Chen, J.; Yi, P.; Xu, X.; Fan, Y.; Cui, B.; Yu, Y.; Li, X.; Du, Y.; et al. Salivary protease spectrum biomarkers of oral cancer. Int. J. Oral Sci. 2019, 11, 7. [Google Scholar] [CrossRef]
- Cai, M.; Zheng, Z.; Bai, Z.; Ouyang, K.; Wu, Q.; Xu, S.; Huang, L.; Jiang, Y.; Wang, L.; Gao, J.; et al. Overexpression of angiogenic factors and matrix metalloproteinases in the saliva of oral squamous cell carcinoma patients: Potential non-invasive diagnostic and therapeutic biomarkers. BMC Cancer 2022, 22, 530. [Google Scholar] [CrossRef]
- Agha-Hosseini, F.; Mirzaii-Dizgah, I. Serum and saliva collagenase-3 (MMP-13) in patients with oral lichen planus and oral squamous cell carcinoma. Med. J. Islam. Repub. Iran 2015, 29, 218. [Google Scholar] [PubMed]
- Ghallab, N.A.; Shaker, O.G. Serum and salivary levels of chemerin and MMP-9 in oral squamous cell carcinoma and oral premalignant lesions. Clin. Oral Investig. 2017, 21, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Peisker, A.; Raschke, G.F.; Fahmy, M.D.; Guentsch, A.; Roshanghias, K.; Hennings, J.; Schultze-Mosgau, S. Salivary MMP-9 in the detection of oral squamous cell carcinoma. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e270–e275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, Y.J.; Vu, H.; Lee, J.H.; Kim, H.D. Diagnostic and prognostic ability of salivary MMP-9 for oral squamous cell carcinoma: A pre-/post-surgery case and matched control study. PLoS ONE 2021, 16, e0248167. [Google Scholar] [CrossRef]
- Smriti, K.; Ray, M.; Chatterjee, T.; Shenoy, R.P.; Gadicherla, S.; Pentapati, K.C.; Rustaqi, N. Salivary MMP-9 as a Biomarker for the Diagnosis of Oral Potentially Malignant Disorders and Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2020, 21, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Saleem, Z.; Shaikh, A.H.; Zaman, U.; Ahmed, S.; Majeed, M.M.; Kazmi, A.; Farooqui, W.A. Estimation of salivary matrix metalloproteinases-12 (MMP-12) levels among patients presenting with oral submucous fibrosis and oral squamous cell carcinoma. BMC Oral Health 2021, 21, 205. [Google Scholar] [CrossRef]
- Cristaldi, M.; Mauceri, R.; Di Fede, O.; Giuliana, G.; Campisi, G.; Panzarella, V. Salivary Biomarkers for Oral Squamous Cell Carcinoma Diagnosis and Follow-Up: Current Status and Perspectives. Front. Physiol. 2019, 10, 1476. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Panda, M. Recent trends of saliva omics biomarkers for the diagnosis and treatment of oral cancer. J. Oral Biosci. 2019, 61, 84–94. [Google Scholar] [CrossRef]
- Song, X.; Yang, X.; Narayanan, R.; Shankar, V.; Ethiraj, S.; Wang, X.; Duan, N.; Ni, Y.H.; Hu, Q.; Zare, R.N. Oral squamous cell carcinoma diagnosed from saliva metabolic profiling. Proc. Natl. Acad. Sci. USA 2020, 117, 16167–16173. [Google Scholar] [CrossRef]
- Bonne, N.J.; Wong, D.T. Salivary biomarker development using genomic, proteomic and metabolomic approaches. Genome Med. 2012, 4, 82. [Google Scholar] [CrossRef]
- Papale, F.; Santonocito, S.; Polizzi, A.; Giudice, A.L.; Capodiferro, S.; Favia, G.; Isola, G. The New Era of Salivaomics in Dentistry: Frontiers and Facts in the Early Diagnosis and Prevention of Oral Diseases and Cancer. Metabolites 2022, 12, 638. [Google Scholar] [CrossRef] [PubMed]
- AlAli, A.M.; Walsh, T.; Maranzano, M. CYFRA 21-1 and MMP-9 as salivary biomarkers for the detection of oral squamous cell carcinoma: A systematic review of diagnostic test accuracy. Int. J. Oral Maxillofac. Surg. 2020, 49, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Kaczor-Urbanowicz, K.E.; Martin Carreras-Presas, C.; Aro, K.; Tu, M.; Garcia-Godoy, F.; Wong, D.T. Saliva diagnostics—Current views and directions. Exp. Biol. Med. 2017, 242, 459–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, L.A.; Mussavira, S.; Bindhu, O.S. Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: A systematic review. Biochem. Med. 2015, 25, 177–192. [Google Scholar] [CrossRef]
- Castagnola, M.; Scarano, E.; Passali, G.C.; Messana, I.; Cabras, T.; Iavarone, F.; Di Cintio, G.; Fiorita, A.; De Corso, E.; Paludetti, G. Salivary biomarkers and proteomics: Future diagnostic and clinical utilities. Acta Otorhinolaryngol. Ital. 2017, 37, 94–101. [Google Scholar] [CrossRef]
- Monea, M.; Olah, P.; Comaneanu, R.M.; Hancu, V.; Ormenisan, A. The Role of Toluidine Blue as a Visual Diagnostic Method in Oral Premalignant Lesions. Rev. Chim. 2016, 67, 1370–1372. [Google Scholar]
- Sannam Khan, R.; Khurshid, Z.; Akhbar, S.; Faraz Moin, S. Advances of Salivary Proteomics in Oral Squamous Cell Carcinoma (OSCC) Detection: An Update. Proteomes 2016, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Patel, S.; Patel, P.; Tanavde, V. Saliva Based Liquid Biopsies in Head and Neck Cancer: How Far Are We from the Clinic? Front. Oncol. 2022, 12, 828434. [Google Scholar] [CrossRef]
- Chundru, V.N.S.; Nirmal, R.M.; Srikanth, B.; Bojji, M.; Midhun, N.; Lakshmi, B.J. Salivaomics for Oral Cancer Detection: An Insight. J. Pharm. Bioallied Sci. 2021, 13, S52–S56. [Google Scholar] [CrossRef]
- Jain, A.; Kotimoole, C.N.; Ghoshal, S.; Bakshi, J.; Chatterjee, A.; Prasad, T.S.K.; Pal, A. Identification of potential salivary biomarker panels for oral squamous cell carcinoma. Sci. Rep. 2021, 11, 3365. [Google Scholar] [CrossRef]
- Bostanci, N.; Mitsakakis, K.; Afacan, B.; Bao, K.; Johannsen, B.; Baumgartner, D.; Müller, L.; Kotolová, H.; Emingil, G.; Karpíšek, M. Validation and verification of predictive salivary biomarkers for oral health. Sci. Rep. 2021, 11, 6406. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Sapna, G.; Gokul, S.; Bagri-Manjrekar, K. Matrix metalloproteinases and periodontal diseases. Oral Dis. 2014, 20, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Mughees, M.; Sengupta, A.; Khowal, S.; Wajid, S. Mechanism of tumour microenvironment in the progression and development of oral cancer. Mol. Biol. Rep. 2021, 48, 1773–1786. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; Del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef] [Green Version]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth factors, matrices, and forces combine and control stem cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef] [Green Version]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Gkouveris, I.; Nikitakis, N.G.; Aseervatham, J.; Rao, N.; Ogbureke, K.E. Matrix metalloproteinases in head and neck cancer: Current perspectives. Met. Med. 2017, 4, 47–61. [Google Scholar] [CrossRef] [Green Version]
- Maciejczyk, M.; Pietrzykowska, A.; Zalewska, A.; Knaś, M.; Daniszewska, I. The Significance of Matrix Metalloproteinases in Oral Diseases. Adv. Clin. Exp. Med. 2016, 25, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Lallemant, B.; Evrard, A.; Combescure, C.; Chapuis, H.; Chambon, G.; Raynal, C.; Reynaud, C.; Sabra, O.; Joubert, D.; Hollande, F.; et al. Clinical relevance of nine transcriptional molecular markers for the diagnosis of head and neck squamous cell carcinoma in tissue and saliva rinse. BMC Cancer 2009, 9, 370. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yan, H.; Huang, L. Salivary matrix metalloproteinase (MMP)-8 as a biomarker for periodontitis: A PRISMA-compliant systematic review and meta-analysis. Medicine 2018, 97, e9642. [Google Scholar] [CrossRef] [PubMed]
- Noack, B.; Kipping, T.; Tervahartiala, T.; Sorsa, T.; Hoffmann, T.; Lorenz, K. Association between serum and oral matrix metalloproteinase-8 levels and periodontal health status. J. Periodontal Res. 2017, 52, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Kushlinskii, N.E.; Solovykh, E.A.; Karaoglanova, T.B.; Bayar, U.; Gershtein, E.S.; Troshin, A.A.; Kostyleva, O.I.; Grinin, V.M.; Maksimovskaya, L.N.; Yanushevitch, O.O. Content of matrix metalloproteinase-8 and matrix metalloproteinase-9 in oral fluid of patients with chronic generalized periodontitis. Bull. Exp. Biol. Med. 2011, 152, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Elebyary, O.; Barbour, A.; Fine, N.; Tenenbaum, H.C.; Glogauer, M. The Crossroads of Periodontitis and Oral Squamous Cell Carcinoma: Immune Implications and Tumor Promoting Capacities. Front. Oral Health 2021, 1, 584705. [Google Scholar] [CrossRef]
- Karmakar, S.; Kar, A.; Thakur, S.; Rao, V. Periodontitis and oral Cancer-a striking link. Oral Oncol. 2020, 106, 104630. [Google Scholar] [CrossRef]
- Gutiérrez-Fernández, A.; Fueyo, A.; Folgueras, A.R.; Garabaya, C.; Pennington, C.J.; Pilgrim, S.; Edwards, D.R.; Holliday, D.L.; Jones, J.L.; Span, P.N.; et al. Matrix metalloproteinase-8 functions as a metastasis suppressor through modulation of tumor cell adhesion and invasion. Cancer Res. 2008, 68, 2755–2763. [Google Scholar] [CrossRef] [Green Version]
- Juurikka, K.; Butler, G.S.; Salo, T.; Nyberg, P.; Åström, P. The Role of MMP8 in Cancer: A Systematic Review. Int. J. Mol. Sci. 2019, 20, 4506. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, Y.; Kondo, S.; Shirai, A.; Furukawa, M.; Yoshizaki, T. A single nucleotide polymorphism in the matrix metalloproteinase-1 and interleukin-8 gene promoter predicts poor prognosis in tongue cancer. Auris Nasus Larynx 2008, 35, 381–389. [Google Scholar] [CrossRef]
- George, A.; Ranganathan, K.; Rao, U.K. Expression of MMP-1 in histopathological different grades of oral squamous cell carcinoma and in normal buccal mucosa—An immunohistochemical study. Cancer Biomark. 2010, 7, 275–283. [Google Scholar] [CrossRef]
- Ahmed Haji Omar, A.; Haglund, C.; Virolainen, S.; Häyry, V.; Atula, T.; Kontio, R.; Salo, T.; Sorsa, T.; Hagström, J. MMP-7, MMP-8, and MMP-9 in oral and cutaneous squamous cell carcinomas. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, 459–467. [Google Scholar] [CrossRef]
- Lawal, A.; Adisa, A.; Kolude, B.; Adeyemi, B. Immunohistochemical expression of MMP-2 and MMP-8 in oral squamous cell carcinoma. J. Clin. Exp. Dent. 2015, 7, e203–e207. [Google Scholar] [CrossRef] [PubMed]
- De Matos, F.R.; Santos, E.M.; Santos, H.B.P.; Machado, R.A.; Lemos, T.M.A.M.; Coletta, R.D.; Freitas, R.A. Association of polymorphisms in IL-8, MMP-1 and MMP-13 with the risk and prognosis of oral and oropharyngeal squamous cell carcinoma. Arch. Oral Biol. 2019, 108, 104547. [Google Scholar] [CrossRef] [PubMed]
- Vincent-Chong, V.K.; Salahshourifar, I.; Karen-Ng, L.P.; Siow, M.Y.; Kallarakkal, T.G.; Ramanathan, A.; Yang, Y.H.; Khor, G.H.; Rahman, Z.A.; Ismail, S.M.; et al. Overexpression of MMP13 is associated with clinical outcomes and poor prognosis in oral squamous cell carcinoma. Sci. World J. 2014, 2014, 897523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, C.Y.; Lin, T.Y.; Sheu, J.J.; Wu, W.C.; Huang, C.Y. Matrix metalloproteinase-13 is a target gene of high-mobility group box-containing protein 1 in modulating oral cancer cell invasion. J. Cell. Physiol. 2019, 234, 4375–4384. [Google Scholar] [CrossRef]
- Li, S.; Pritchard, D.M.; Yu, L.G. Regulation and Function of Matrix Metalloproteinase-13 in Cancer Progression and Metastasis. Cancers 2022, 14, 3263. [Google Scholar] [CrossRef]
- Jobin, P.G.; Butler, G.S.; Overall, C.M. New intracellular activities of matrix metalloproteinases shine in the moonlight. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2043–2055. [Google Scholar] [CrossRef]
- Hoffmann, C.; Vacher, S.; Sirven, P.; Lecerf, C.; Massenet, L.; Moreira, A.; Surun, A.; Schnitzler, A.; Klijanienko, J.; Mariani, O.; et al. MMP2 as an independent prognostic stratifier in oral cavity cancers. Oncoimmunology 2020, 9, 1754094. [Google Scholar] [CrossRef]
- Matuszczak, E.; Cwalina, I.; Tylicka, M.; Wawrzyn, K.; Nowosielska, M.; Sankiewicz, A.; Ołdak, Ł.; Gorodkiewicz, E.; Hermanowicz, A. Levels of Selected Matrix Metalloproteinases-MMP-1, MMP-2 and Fibronectin in the Saliva of Patients Planned for Endodontic Treatment or Surgical Extraction. J. Clin. Med. 2020, 9, 3971. [Google Scholar] [CrossRef]
- Huang, H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors 2018, 18, 3249. [Google Scholar] [CrossRef] [Green Version]
- Mondal, S.; Adhikari, N.; Banerjee, S.; Amin, S.A.; Jha, T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: A minireview. Eur. J. Med. Chem. 2020, 194, 112260, Erratum in Eur. J. Med. Chem. 2020, 205, 11264. [Google Scholar] [CrossRef]
- Shpitzer, T.; Hamzany, Y.; Bahar, G.; Feinmesser, R.; Savulescu, D.; Borovoi, I.; Gavish, M.; Nagler, R.M. Salivary analysis of oral cancer biomarkers. Br. J. Cancer 2009, 101, 1194–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, B.; Hong, T.; He, X.; Hu, X.; Gao, Y. A circular RNA derived from MMP9 facilitates oral squamous cell carcinoma metastasis through regulation of MMP9 mRNA stability. Cell Transplant. 2019, 28, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Nishio, K.; Motozawa, K.; Omagari, D.; Gojoubori, T.; Ikeda, T.; Asano, M.; Gionhaku, N. Comparison of MMP2 and MMP9 expression levels between primary and metastatic regions of oral squamous cell carcinoma. J. Oral Sci. 2016, 58, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Hema Shree, K.; Ramani, P.; Sherlin, H.; Sukumaran, G.; Jeyaraj, G.; Don, K.R.; Santhanam, A.; Ramasubramanian, A.; Sundar, R. Saliva as a Diagnostic Tool in Oral Squamous Cell Carcinoma—A Systematic Review with Meta Analysis. Pathol. Oncol. Res. 2019, 25, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Okusha, Y.; Eguchi, T.; Sogawa, C.; Okui, T.; Nakano, K.; Okamoto, K.; Kozaki, K.-I. The intranuclear PEX domain of MMP involves proliferation, migration, and metastasis of aggressive adenocarcinoma cells. J. Cell. Biochem. 2018, 119, 7363–7376. [Google Scholar] [CrossRef] [Green Version]
- Okusha, Y.; Eguchi, T.; Tran, M.T.; Sogawa, C.; Yoshida, K.; Itagaki, M.; Taha, E.A.; Ono, K.; Aoyama, E.; Okamura, H.; et al. Extracellular Vesicles Enriched with Moonlighting Metalloproteinase Are Highly Transmissive, Pro-Tumorigenic, and Trans-Activates Cellular Communication Network Factor (CCN2/CTGF): CRISPR against Cancer. Cancers 2020, 12, 881. [Google Scholar] [CrossRef] [Green Version]
- Taha, E.A.; Sogawa, C.; Okusha, Y.; Kawai, H.; Oo, M.W.; Elseoudi, A.; Lu, Y.; Nagatsuka, H.; Kubota, S.; Satoh, A.; et al. Knockout of MMP3 Weakens Solid Tumor Organoids and Cancer Extracellular Vesicles. Cancers 2020, 12, 1260. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Y.; Wang, X.; Yang, Y. Dysregulation of MiR-519d Affects Oral Squamous Cell Carcinoma Invasion and Metastasis by Targeting MMP3. J. Cancer 2019, 10, 2720–2734. [Google Scholar] [CrossRef] [Green Version]
- Chi, L.M.; Hsiao, Y.C.; Chien, K.Y.; Chen, S.F.; Chuang, Y.N.; Lin, S.Y.; Wang, W.S.; Chang, I.Y.; Yang, C.; Chu, L.J.; et al. Assessment of candidate biomarkers in paired saliva and plasma samples from oral cancer patients by targeted mass spectrometry. J. Proteom. 2020, 211, 103571. [Google Scholar] [CrossRef]
- Lee, L.T.; Wong, Y.K.; Hsiao, H.Y.; Wang, Y.W.; Chan, M.Y.; Chang, K.W. Evaluation of saliva and plasma cytokine biomarkers in patients with oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2018, 47, 699–707. [Google Scholar] [CrossRef]
- Zhang, G.; Miyake, M.; Lawton, A.; Goodison, S.; Rosser, C.J. Matrix metalloproteinase-10 promotes tumor progression through regulation of angiogenic and apoptotic pathways in cervical tumors. BMC Cancer 2014, 14, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deraz, E.M.; Kudo, Y.; Yoshida, M.; Obayashi, M.; Tsunematsu, T.; Tani, H.; Siriwardena, S.B.; Keikhaee, M.R.; Qi, G.; Iizuka, S.; et al. MMP-10/stromelysin-2 promotes invasion of head and neck cancer. PLoS ONE 2011, 6, e25438. [Google Scholar] [CrossRef] [Green Version]
- Kadeh, H.; Saravani, S.; Heydari, F.; Keikha, M.; Rigi, V. Expression of Matrix Metalloproteinase-10 at Invasive Front of Squamous Cell Carcinoma and Verrucous Carcinoma in the Oral Cavity. Asian Pac. J. Cancer Prev. 2015, 16, 6609–6613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashhadiabbas, F.; Mahjour, F.; Mahjour, S.B.; Fereidooni, F.; Hosseini, F.S. The immunohistochemical characterization of MMP-2, MMP-10, TIMP-1, TIMP-2, and podoplanin in oral squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 240–250. [Google Scholar] [CrossRef]
- Yuan, S.; Lin, L.S.; Gan, R.H.; Huang, L.; Wu, X.T.; Zhao, Y.; Su, B.H.; Zheng, D.; Lu, Y.G. Elevated matrix metalloproteinase 7 expression promotes the proliferation, motility and metastasis of tongue squamous cell carcinoma. BMC Cancer 2020, 20, 33. [Google Scholar] [CrossRef] [Green Version]
- Liao, H.Y.; Da, C.M.; Liao, B.; Zhang, H.H. Roles of matrix metalloproteinase-7 (MMP-7) in cancer. Clin. Biochem. 2021, 92, 9–18. [Google Scholar] [CrossRef]
- Li, T.J.; Cui, J. COX-2, MMP-7 expression in oral lichen planus and oral squamous cell carcinoma. Asian Pac. J. Trop. Med. 2013, 6, 640–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Impola, U.; Uitto, V.J.; Hietanen, J.; Hakkinen, L.; Zhang, L.; Larjava, H.; Isaka, K.; Saarialho-Kere, U. Differential expression of matrilysin-1 (MMP-7), 92 kD gelatinase (MMP-9), and metalloelastase (MMP-12) in oral verrucous and squamous cell cancer. J. Pathol. 2004, 202, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, N.; Sarmad, S.; Waheed, N.U.A.; Gondal, A.J. Estimation of serum matrix metalloproteinases among patients of oral squamous cell carcinoma. Pak. J. Med. Sci. 2019, 35, 252–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmström, S.B.; Lira-Junior, R.; Zwicker, S.; Majster, M.; Gustafsson, A.; Åkerman, S.; Klinge, B.; Svensson, M.; Boström, E.A. MMP-12 and S100s in saliva reflect different aspects of periodontal inflammation. Cytokine 2019, 113, 155–161. [Google Scholar] [CrossRef]
- Piyarathne, N.S.; Rasnayake, R.M.S.G.K.; Angammana, R.; Chandrasekera, P.; Ramachandra, S.; Weerasekera, M.; Yasawardene, S.; Abu-Eid, R.; Jayasinghe, J.A.P.; Gupta, E. Diagnostic salivary biomarkers in oral cancer and oral potentially malignant disorders and their relationships to risk factors—A systematic review. Expert Rev. Mol. Diagn. 2021, 21, 789–807. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.W.; Chang, K.P.; Hsu, C.W.; Chang, I.Y.; Liu, H.P.; Chen, Y.T.; Wu, C.C. Identification of Salivary Biomarkers for Oral Cancer Detection with Untargeted and Targeted Quantitative Proteomics Approaches. Mol. Cell. Proteom. 2019, 18, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.H.; Sodnom-Ish, B.; Choi, S.W.; Jung, H.I.; Cho, J.; Hwang, I.; Kim, S.M. Salivary biomarkers in oral squamous cell carcinoma. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Pillai, J.; Chincholkar, T.; Dixit, R.; Pandey, M. A systematic review of proteomic biomarkers in oral squamous cell cancer. World J. Surg. Oncol. 2021, 19, 315. [Google Scholar] [CrossRef]
- Chapman, S.; Mick, M.; Hall, P.; Mejia, C.; Sue, S.; Abdul Wase, B.; Nguyen, M.A.; Whisenant, E.C.; Wilcox, S.H.; Winden, D.; et al. Cigarette smoke extract induces oral squamous cell carcinoma cell invasion in a receptor for advanced glycation end-products-dependent manner. Eur. J. Oral Sci. 2018, 126, 33–40. [Google Scholar] [CrossRef]
- Zimmermann, B.G.; Park, N.J.; Wong, D.T. Genomic targets in saliva. Ann. N. Y. Acad. Sci. 2007, 1098, 184–191. [Google Scholar] [CrossRef]
- Wong, T.S.; Gao, W.; Li, Z.H. Matrix Metalloproteinase Family as Molecular Biomarkers in Oral Squamous Cell Carcinoma. In Biomarkers in Cancer. Biomarkers in Disease: Methods, Discoveries and Applications; Preedy, V., Patel, V., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 771–790. [Google Scholar] [CrossRef]
- Seethalakshmi, C. Early Detection of Oral Squamous Cell Carcinoma (OSCC)—Role of Genetics: A Literature Review. J. Clin. Diagn. Res. 2013, 7, 1824–1826. [Google Scholar] [CrossRef]
- Mori, K.; Hamada, T.; Beppu, M.; Tsuchihashi, H.; Goto, Y.; Kume, K.; Hijioka, H.; Nishi, K.; Mishima, Y.; Sugiura, T. Detecting Early-Stage Oral Cancer from Clinically Diagnosed Oral Potentially Malignant Disorders by DNA Methylation Profile. Cancers 2022, 14, 2646. [Google Scholar] [CrossRef]
- Speight, P.M.; Khurram, S.A.; Kujan, O. Oral potentially malignant disorders: Risk of progression to malignancy. Oral Surg Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 612–627. [Google Scholar] [CrossRef]
- Dost, F.; Lê Cao, K.; Ford, P.J.; Ades, C.; Farah, C.S. Malignant transformation of oral epithelial dysplasia: A real-world evaluation of histopathologic grading. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 343–352. [Google Scholar] [CrossRef] [Green Version]
- Shearston, K.; Fateh, B.; Tai, S.; Hove, D.; Farah, C.S. Malignant transformation rate of oral leukoplakia in an Australian population. J. Oral Pathol. Med. 2019, 48, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Reichart, P.A.; Philipsen, H.P. Oral erythroplakia—A review. Oral Oncol. 2005, 41, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.F.; Ho, C.H.; Chen, Y.C.; Wu, L.W.; Chen, Y.L.; Wu, J.H.; Wu, W.S.; Hung, H.K.; Chiang, W.F. Malignant transformation of oral potentially malignant disorders in Taiwan: An observational nationwide population database study. Medicine 2021, 100, e24934. [Google Scholar] [CrossRef]
- Van der Waal, I. Oral potentially malignant disorders: Is malignant transformation predictable and preventable? Med. Oral Patol. Oral Cir. Bucal 2014, 19, e386–e390. [Google Scholar] [CrossRef]
- Rosa, E.A.; Hurtado-Puerto, A.M.; Falcão, D.P.; Brietzke, A.P.; De Almeida Prado Franceschi, L.E.; Cavalcanti Neto, F.F.; Tiziane, V.; Carneiro, F.P.; Kogawa, E.M.; Moreno, H.; et al. Oral lichen planus and malignant transformation: The role of p16, Ki-67, Bub-3 and SOX4 in assessing precancerous potential. Exp. Ther. Med. 2018, 15, 4157–4166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giuliani, M.; Troiano, G.; Cordaro, M.; Corsalini, M.; Gioco, G.; Lo Muzio, L.; Pignatelli, P.; Lajolo, C. Rate of malignant transformation of oral lichen planus: A systematic review. Oral Dis. 2019, 25, 693–709. [Google Scholar] [CrossRef]
- Richards, D. Malignant transformation rates in Oral Lichen Planus. Evid. Based Dent. 2018, 19, 122. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Liu, W.; Feng, J.Q.; Zhou, H.W.; Zhou, Z.T. Squamous cell carcinoma development in previously diagnosed oral lichen planus: De novo or transformation? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, 592–596. [Google Scholar] [CrossRef]
- Altuwaijri, A.A.; Aldrees, T.M.; Alessa, M.A. Prevalence of Metastasis and Involvement of Level IV and V in Oral Squamous Cell Carcinoma: A Systematic Review. Cureus 2021, 13, e20255. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, R.; Wang, C.; Gong, W.; Xue, M.; Liu, L.; Zhang, Y. Central neck lymph node metastasis in oral squamous cell carcinoma at the floor of mouth. BMC Cancer 2021, 21, 225. [Google Scholar] [CrossRef]
- Carreras-Torras, C.; Gay-Escoda, C. Techniques for early diagnosis of oral squamous cell carcinoma: Systematic review. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e305–e315. [Google Scholar] [CrossRef]
- Pop, A.M.; Coroș, R.; Stoica, A.M.; Monea, M. Early Diagnosis of Oral Mucosal Alterations in Smokers and E-Cigarette Users Based on Micronuclei Count: A Cross-Sectional Study among Dental Students. Int. J. Environ. Res. Public Health 2021, 18, 13246. [Google Scholar] [CrossRef] [PubMed]
- Alsarraf, A.H.; Kujan, O.; Farah, C.S. The utility of oral brush cytology in the early detection of oral cancer and oral potentially malignant disorders: A systematic review. J. Oral Pathol. Med. 2018, 47, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Xu, H.; He, M.; Han, Q.; Wang, H.; Sun, C.; Li, J.; Jiang, L.; Zhou, Y.; Dan, H.; et al. Accuracy of autofluorescence in diagnosing oral squamous cell carcinoma and oral potentially malignant disorders: A comparative study with aero-digestive lesions. Sci. Rep. 2016, 6, 29943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagi, R.; Reddy-Kantharaj, Y.B.; Rakesh, N.; Janardhan-Reddy, S.; Sahu, S. Efficacy of light based detection systems for early detection of oral cancer and oral potentially malignant disorders: Systematic review. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e447–e455. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Pezzi, M.E.; Cassi, D.; Pertinhez, T.A.; Spisni, A.; Meleti, M. Salivary Cytokines as Biomarkers for Oral Squamous Cell Carcinoma: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 6795. [Google Scholar] [CrossRef]
- Cervino, G.; Fiorillo, L.; Herford, A.S.; Romeo, U.; Bianchi, A.; Crimi, S.; D’Amico, C.; De Stefano, R.; Troiano, G.; Santoro, R.; et al. Molecular Biomarkers Related to Oral Carcinoma: Clinical Trial Outcome Evaluation in a Literature Review. Dis. Markers 2019, 2019, 8040361. [Google Scholar] [CrossRef]
| Authors | Evaluated Biomarkers | Study Participants | Laboratory Analysis | Results | Conclusion |
|---|---|---|---|---|---|
| Stott-Miller et al., 2011 [26] | MMP-1, MMP-3 | 60 primary OSCC cases, 15 cases of oral dysplastic lesions and 25 controls | ELISA | Higher salivary concentration of MMPs in OSCC cases compared to controls (6.2 times higher for MMP-1 and 14.8 times higher for MMP-3); Stronger results for both MMP-1 and MMP-3 in oral cavity cancer versus controls, rather than in oropharyngeal cancer versus controls; Salivary concentrations of MMP-1 and MMP-3 correlated with tumor stage. | MMPs may be very useful in monitoring dysplasia progression to OSCC; Salivary MMP-1 can be an important biomarker of OSCC development. |
| Chang et al., 2020 [27] | MMP-1 | 269 primary OSCC cases, 578 PMOLs and 313 healthy controls | ELISA | Higher salivary concentration of MMP-1 in OSCC cases than in non-cancerous patients (PMOLs and healthy controls); Satisfactory reliability of MMP-1 in distinguishing OSCC from non-tumor lesions; Good power of discrimination between OSCC located on the tongue, cheek mucosa, gum and multiple sites of the oral cavity from non-tumor lesions; Higher salivary concentrations of MMP-1 in high-risk PMOLs compared to low-risk PMOLs and healthy controls; Correlation of the salivary levels of MMP-1 with tumor size and lymph node metastasis. | Useful salivary biomarker in evaluating malignant transformation; Promising potential in early-stage screening of patients with increased risk for OSCC development; Important biomarker of poor prognosis. |
| Yu et al., 2016 [28] | MMP-1, MMP-3, MMP-9, annexin-2 (ANXA2), Heat Shock Protein Family A (Hsp70) Member 5 (HSPA5) and other salivary proteins | 131 OSCC cases, 233 PMOLs and 96 healthy controls | Liquid chromatography–multiple reaction monitoring-mass spectrometry | MMP-1 and kininogen 1 (KNG 1) had the highest salivary concentrations in OSCC patients among the analyzed salivary proteins; The sensitivity and specificity of the analyzed MMPs in differentiating OSCC from healthy controls and low-risk PMOLs were: 69.5% and 95% for MMP-1, 62.6% and 76.9% for MMP-3 and 75.6% and 60.3% for MMP-9; | An identified panel of 4 salivary biomarkers (MMP-1, KNG 1, ANXA2 and HSPA5) may represent a promising tool in the diagnosis of OSCC and in the monitoring of malignant transformation occurring in high-risk PMOLs. |
| Feng et al., 2019 [29] | MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, MMP-10, MMP-12, MMP-13 and other salivary proteins | 20 OSCC cases, 20 oral benign masses (OBM), 20 mild chronic periodontal disease (CPD) and 20 healthy controls | ELISA | MMP-1, MMP-2, MMP-3, MMP-10, MMP-12, MMP-13 were detected only in the saliva of patients with OSCC; MMP-1, MMP-2, MMP-10, MMP-12 along with cathepsin V, A disintegrin and a metalloprotease 9 (ADAM9) and kallikrein 5 has higher salivary concentration in OSCC patients compared to OBM, CPD and healthy controls. | By evaluating the combined sensitivity and specificity, the concomitant use of ADAM9, cathepsin V and kallikrein 5 was the most promising candidate for diagnosing OSCC. |
| Cai et al., 2022 [30] | MMP-1, MMP-2, MMP-3, MMP-8, MMP-9, MMP-10, MMP-13, Hepatocyte growth factor (HGF) and other salivary proteins | 8 OSCC cases and 8 healthy controls | Protein chip array | Higher salivary concentrations of MMP-1, MMP-3, MMP-8, MMP-9, MMP-10 and MMP-13 in OSCC patients compared to controls, the most significant increases being observed for MMP-1, MMP-3 and MMP-13; No difference between salivary concentration of MMP-2 in OSCC compared to controls; MMP-1, MMP-3 and MMP-13 were not detected in the saliva of healthy controls. The salivary levels of HGF and MMP-9 significantly differed between OSCC patients and healthy subjects. | Both HGF and MMP-9 can be useful biomarkers for the diagnosis and prognosis of OSCC. |
| Agha-Hosseini et al., 2015 [31] | MMP-13 | 20 OSCC cases and 30 oral lichen planus (OLP) | ELISA | No differences between salivary concentrations of MMP-13 in OSCC patients compared to OLP. | Salivary MMP-13 may not be a valuable adjuvant in the diagnosis and screening of OSCC. |
| Ghallab and Shaker, 2017 [32] | MMP-9 and chemerin | 15 early-stage OSCC cases, 15 PMOLs and 15 healthy controls | ELISA | Higher salivary levels of MMP-9 and chemerin in patients with OSCC compared to those with PMOLs and healthy subjects; Higher salivary levels of MMP-9 and chemerin in PMOLs compared to healthy subjects; Salivary MMP-9 had the greatest accuracy in distinguishing PMOLs from OSCC (sensitivity 100% and specificity 93%). | Salivary MMP-9 and chemerin can be used as adjuvant tools in the early diagnosis of OSCC and PMOLs. |
| Peisker et al., 2017 [33] | MMP-9 | 30 OSCC cases and 30 healthy controls | ELISA | Higher salivary levels of MMP-9 in OSCC patients compared to healthy controls. Salivary MMP-9 had 100% sensitivity and 26.7% specificity in diagnosing OSCC. | Salivary MMP-9 may be a complementary tool for the early diagnosis of OSCC. |
| Shin et al., 2021 [34] | MMP-9 and 8-hydroxydeoxy- guanosine (8-OHdG) | 106 OSCC cases and 212 healthy controls | ELISA | The salivary levels of MMP-9 in OSCC patients were 17 times higher than those of healthy controls; After surgical excision of the tumor, salivary MMP-9 decreased by 80% in the first nine months but was still higher in comparison with the value in healthy controls; The diagnostic ability of salivary MMP-9 after adjusting covariates showed 97.2% sensitivity and 94.2% specificity; | Salivary MMP-9 may be used in the early diagnosis and screening of OSCC. |
| Smriti et al., 2020 [35] | MMP-9 | 24 OSCC cases, 20 PMOLs, 22 subjects consuming tobacco and 22 healthy controls | ELISA | Higher levels of MMP-9 in the saliva of patients with OSCC and PMOLs compared to tobacco users and healthy controls; Salivary levels of MMP-9 increased according to tumor stage and were higher in poorly differentiated tumors; | Salivary MMP-9 can aid in the diagnosis of OSCC and PMOLs. |
| Saleem et al., 2021 [36] | MMP-12 | 30 OSCC cases, 30 patients with oral submucous fibrosis (OSF) and 30 healthy controls | ELISA | Higher levels of MMP-12 in the saliva of patients with OSCC and OSF compared to healthy controls and also in OSCC group compared to OSF group; Salivary MMP-12 had 100% sensitivity and 100% specificity in detecting OSF and OSCC. | Salivary MMP-12 can be an adjuvant method in the early diagnosis of OSF and OSCC. |
| MMPs | Study Findings and Clinical Relevance | |
|---|---|---|
| Collagenases | MMP-1 | Higher salivary levels in OSCC patients compared to healthy controls [26,29]; Higher levels in the saliva of patients with OSCC compared to that of patients with OBM and CPD [29]; Significant ability to discriminate OSCC from non-tumor lesions [27]; Correlation of the salivary levels with OSCC tumor stage and nodal metastasis [26,27]; Higher salivary levels in HPV-negative OSCC and higher differences between cases and controls in OSCC compared to oropharyngeal tumors [26]. |
| MMP-8 | Identified upregulation in the saliva of patients with OSCC [30]. | |
| MMP-13 | Identified upregulation in the saliva of patients with OSCC [30]; Lack of difference between the salivary levels detected in patients with OSCC and OLP [31]. | |
| Gelatinases | MMP-2 | Higher levels in the saliva of patients with OSCC compared to that of patients with OBM, CPD and healthy controls [29]. |
| MMP-9 | Higher salivary levels in patients with OSCC compared to patients with PMOLs and healthy controls; higher salivary levels in patients with PMOLs compared to healthy controls [32,33]; Greater sensitivity and specificity compared to serum [32]; Salivary levels significantly decrease (up to 80%) after surgical excision of OSCC [34]; Salivary levels increased in poorly differentiated OSCC in comparison with moderate and well-differentiated tumors [35]. | |
| Stromelysins | MMP-3 | Identified upregulation in the saliva of patients with OSCC [30]; Increasing salivary levels from the reticular to the erosive form of OLP and then to early and advanced stages of OSCC [31]; Higher salivary levels in patients with OSCC compared to healthy controls and correlation with disease stage [26]; Higher salivary levels in HPV-negative OSCC and higher differences between cases and controls in OSCC compared to oropharyngeal tumors [26]. |
| MMP-10 | Higher levels in the saliva of patients with OSCC compared to that of patients with OBM, CPD and healthy controls [29]; Identified upregulation in the saliva of patients with OSCC [30]. | |
| Others | MMP-12 | Higher levels in the saliva of patients with OSCC compared to that of patients with OBM, CPD and healthy controls [29]; Higher salivary levels among patients with OSCC, patients with OSF and healthy controls [36]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monea, M.; Pop, A.M. The Use of Salivary Levels of Matrix Metalloproteinases as an Adjuvant Method in the Early Diagnosis of Oral Squamous Cell Carcinoma: A Narrative Literature Review. Curr. Issues Mol. Biol. 2022, 44, 6306-6322. https://doi.org/10.3390/cimb44120430
Monea M, Pop AM. The Use of Salivary Levels of Matrix Metalloproteinases as an Adjuvant Method in the Early Diagnosis of Oral Squamous Cell Carcinoma: A Narrative Literature Review. Current Issues in Molecular Biology. 2022; 44(12):6306-6322. https://doi.org/10.3390/cimb44120430
Chicago/Turabian StyleMonea, Monica, and Anca Maria Pop. 2022. "The Use of Salivary Levels of Matrix Metalloproteinases as an Adjuvant Method in the Early Diagnosis of Oral Squamous Cell Carcinoma: A Narrative Literature Review" Current Issues in Molecular Biology 44, no. 12: 6306-6322. https://doi.org/10.3390/cimb44120430
APA StyleMonea, M., & Pop, A. M. (2022). The Use of Salivary Levels of Matrix Metalloproteinases as an Adjuvant Method in the Early Diagnosis of Oral Squamous Cell Carcinoma: A Narrative Literature Review. Current Issues in Molecular Biology, 44(12), 6306-6322. https://doi.org/10.3390/cimb44120430







