Role of Sleep Restriction in Daily Rhythms of Expression of Hypothalamic Core Clock Genes in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animal Treatments and Ethics Statement
2.2. Sample
2.3. Enzyme-Linked Immunosorbent Assay
2.4. Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.5. Western Blotting
2.6. Statistical Analysis
3. Results
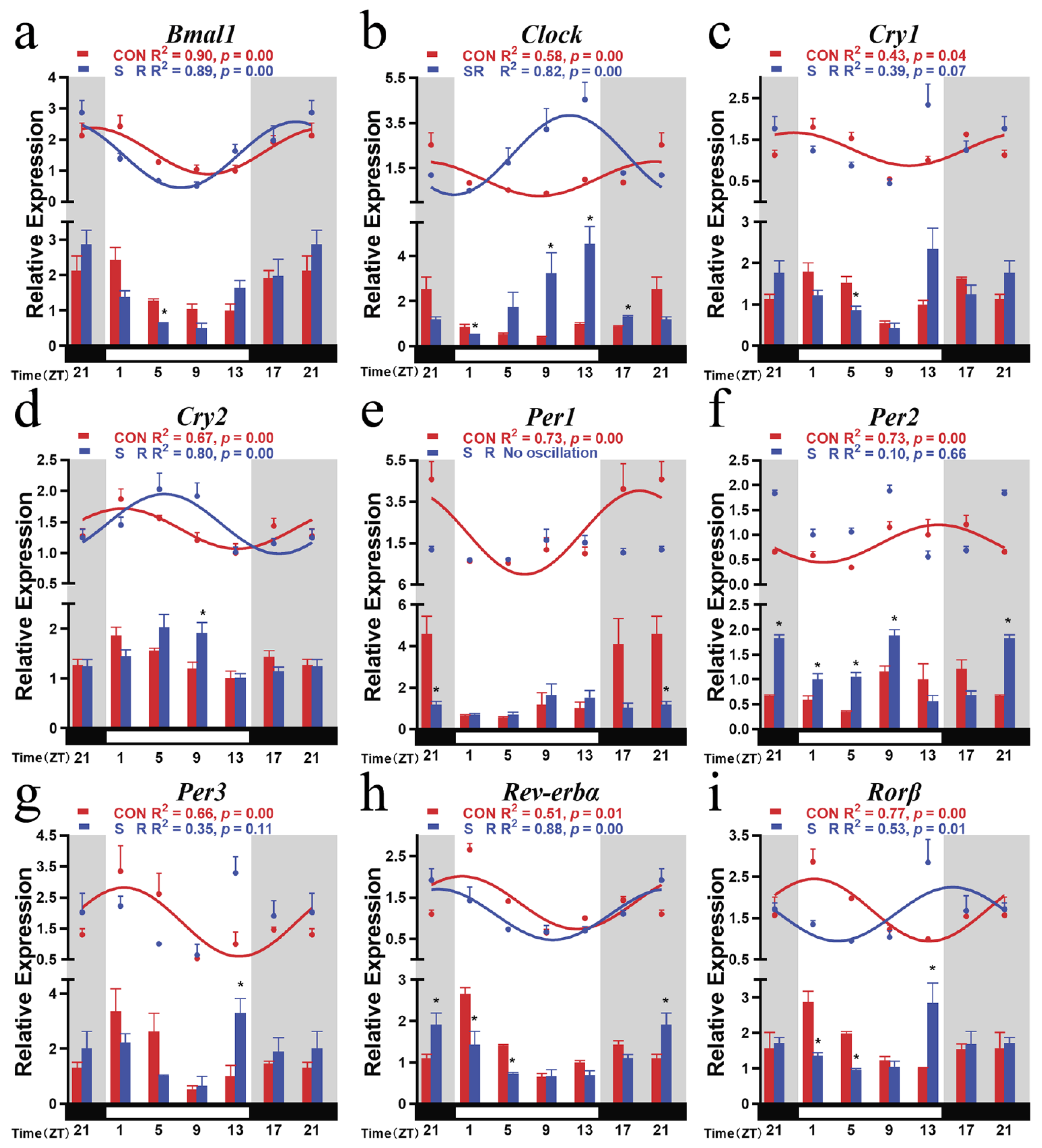
3.1. Circadian Rhythm Expression of Mice Hypothalamic Clock Genes in CON Group
3.2. Circadian Rhythm Changes of Hypothalamic Positive Clock Genes after Sleep Restriction
3.3. Circadian Rhythm Changes of Hypothalamic Negative Clock Genes after Sleep Restriction
3.4. Circadian Rhythm Changes of Hypothalamic Branch Feedback Clock Genes after Sleep Restriction
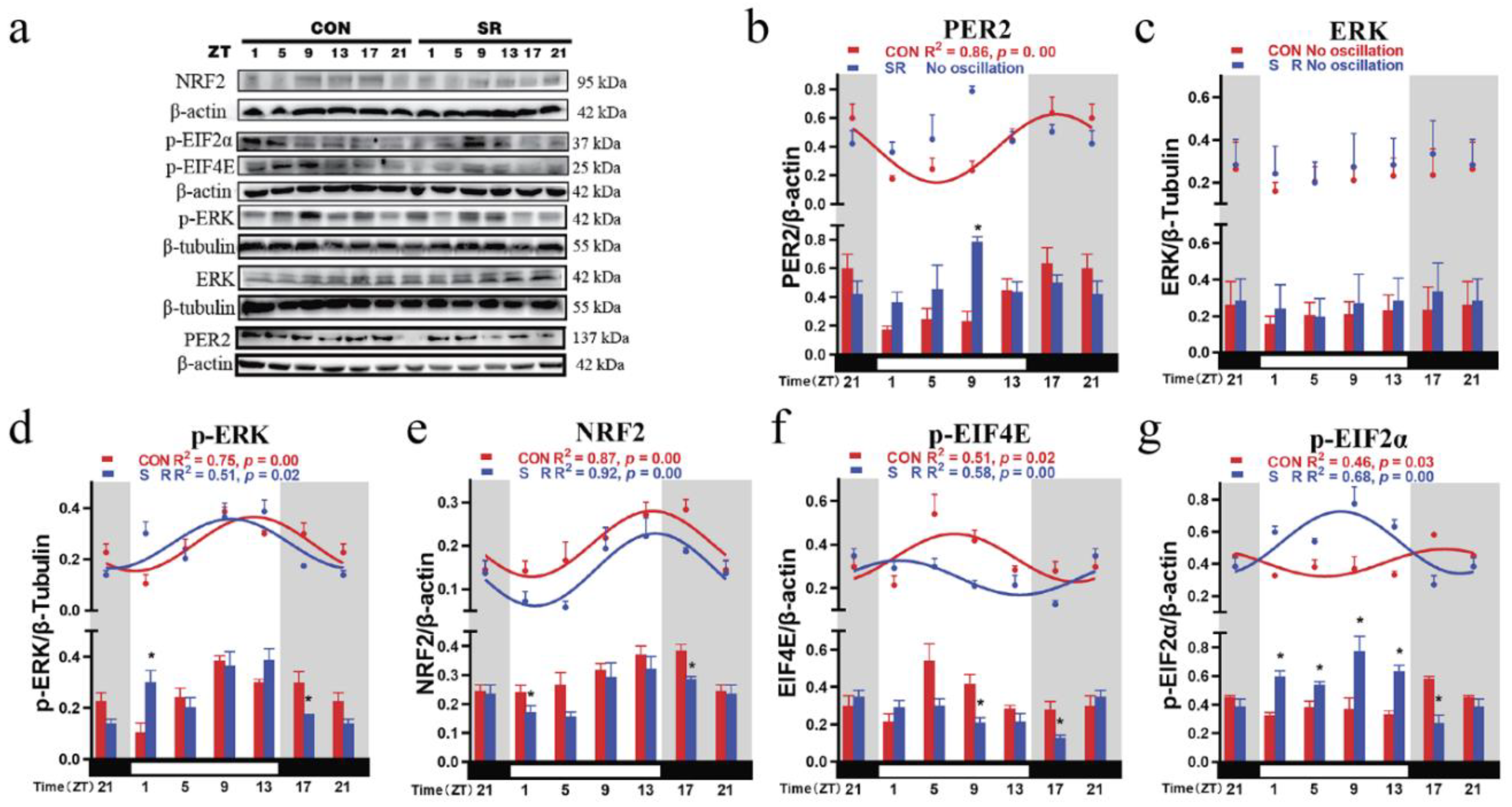
3.5. Circadian Rhythm Changes of Hypothalamic Clock Protein PER2 and Upstream Signals after Sleep Restriction
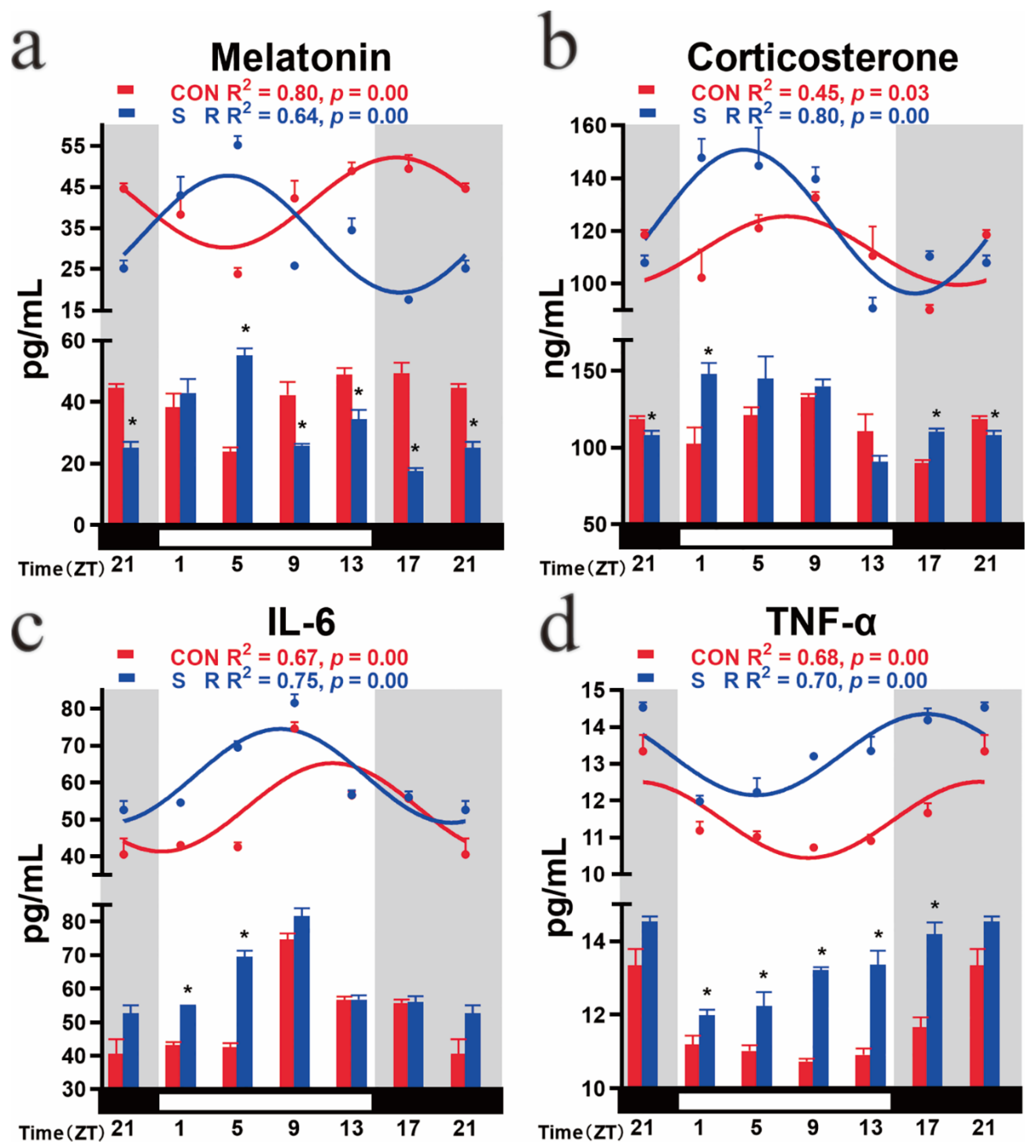
3.6. Circadian Rhythm Changes of Plasma Melatonin, Corticosterone, IL-6, and TNF-α after Sleep Restriction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaput, J.P.; Janssen, I. Sleep duration estimates of Canadian children and adolescents. J. Sleep Res. 2016, 25, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Wheaton, A.G.; Liu, Y.; Perry, G.S.; Croft, J.B. Effect of short sleep duration on daily activities—United States, 2005–2008. Morb. Mortal. Wkly. Rep. 2011, 60, 239–242, reprinted in J. Am. Med. Assoc. 2011, 305, 1956–1958. [Google Scholar]
- Radcliffe, P.N.; Whitney, C.C.; Fagnant, H.S.; Wilson, M.A.; Finlayson, G.; Smith, T.J.; Karl, J.P. Severe sleep restriction suppresses appetite independent of effects on appetite regulating hormones in healthy young men without obesity. Physiol. Behav. 2021, 237, 113438. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Somvanshi, P.; Klerman, E.B.; Marmar, C.; Doyle, F.J., 3rd. Modeling the Influence of Chronic Sleep Restriction on Cortisol Circadian Rhythms, with Implications for Metabolic Disorders. Metabolites 2021, 11, 483. [Google Scholar] [CrossRef]
- Tomaso, C.C.; Johnson, A.B.; Nelson, T.D. The effect of sleep deprivation and restriction on mood, emotion, and emotion regulation: Three meta-analyses in one. Sleep 2021, 44, 289. [Google Scholar] [CrossRef]
- Yamazaki, E.M.; Antler, C.A.; Lasek, C.R.; Goel, N. Residual, differential neurobehavioral deficits linger after multiple recovery nights following chronic sleep restriction or acute total sleep deprivation. Sleep 2021, 44, 224. [Google Scholar] [CrossRef]
- Achermann, P. Technical note: A problem with identifying nonlinear interactions of circadian and homeostatic processes. J. Biol. Rhythm. 1999, 14, 602–603. [Google Scholar] [CrossRef]
- Lee, C.; Etchegaray, J.P.; Cagampang, F.R.; Loudon, A.S.; Reppert, S.M. Posttranslational mechanisms regulate the mammalian circadian clock. Cell 2001, 107, 855–867. [Google Scholar] [CrossRef]
- Preussner, M.; Heyd, F. Post-transcriptional control of the mammalian circadian clock: Implications for health and disease. Pflug. Arch. 2016, 468, 983–991. [Google Scholar] [CrossRef]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.K.; Panda, S.; Miraglia, L.J.; Reyes, T.M.; Rudic, R.D.; McNamara, P.; Naik, K.A.; Fitzgerald, G.A.; Kay, S.A.; Hogenesch, J.B. A functional genomics strategy reveals rora as a component of the mammalian circadian clock. Neuron 2004, 43, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, B.; Emmett, M.J.; Damle, M.; Sun, Z.; Feng, D.; Armour, S.M.; Remsberg, J.R.; Jager, J.; Soccio, R.E.; et al. Discrete functions of nuclear receptor Rev-erbα couple metabolism to the clock. Science 2015, 348, 1488–1492. [Google Scholar] [CrossRef] [PubMed]
- Preitner, N.; Damiola, F.; Molina, L.L.; Zakany, J.; Duboule, D.; Albrecht, U.; Schibler, U. The orphan nuclear receptor REV-ERB α controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 2002, 110, 251–260. [Google Scholar] [CrossRef]
- Mitsui, S.; Yamaguchi, S.; Matsuo, T.; Ishida, Y.; Okamura, H. Antagonistic role of E4BP4 and PAR proteins in the circadian oscillatory mechanism. Gene Dev. 2001, 15, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Gachon, F.; Fonjallaz, P.; Damiola, F.; Gos, P.; Kodama, T.; Zakany, J.; Duboule, D.; Petit, B.; Tafti, M.; Schibler, U. The loss of circadian PAR bZip transcription factors results in epilepsy. Gene Dev. 2004, 18, 1397–1412. [Google Scholar] [CrossRef] [PubMed]
- Cedernaes, J.; Osler, M.E.; Voisin, S.; Broman, J.E.; Vogel, H.; Dickson, S.L.; Zierath, J.R.; Schioth, H.B.; Benedict, C. Acute sleep loss induces tissue-specific epigenetic and transcriptional alterations to circadian clock genes in men. J. Clin. Endocrinol. Metab. 2015, 100, E1255–E1261. [Google Scholar] [CrossRef]
- Schmidt, C.; Collette, F.; Leclercq, Y.; Sterpenich, V.; Vandewalle, G.; Berthomier, P.; Berthomier, C.; Phillips, C.; Tinguely, G.; Darsaud, A.; et al. Homeostatic sleep pressure and responses to sustained attention in the suprachiasmatic area. Science 2009, 324, 516–519. [Google Scholar] [CrossRef]
- Wisor, J.P.; O’Hara, B.F.; Terao, A.; Selby, C.P.; Kilduff, T.S.; Sancar, A.; Edgar, D.M.; Franken, P. A role for cryptochromes in sleep regulation. BMC Neurosci. 2002, 3, 20. [Google Scholar] [CrossRef]
- Hor, C.N.; Yeung, J.; Jan, M.; Emmenegger, Y.; Hubbard, J.; Xenarios, I.; Naef, F.; Franken, P. Sleep-wake-driven and circadian contributions to daily rhythms in gene expression and chromatin accessibility in the murine cortex. Proc. Natl. Acad. Sci. USA 2019, 116, 25773–25783. [Google Scholar] [CrossRef]
- Mongrain, V.; La Spada, F.; Curie, T.; Franken, P. Sleep loss reduces the DNA-binding of BMAL1, CLOCK, and NPAS2 to specific clock genes in the mouse cerebral cortex. PLoS ONE 2011, 6, e26622. [Google Scholar] [CrossRef]
- Banks, S.; Dinges, D.F. Behavioral and physiological consequences of sleep restriction. J. Clin. Sleep Med. 2007, 3, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Yuan, K.; Zhang, W.; Li, S.X.; Gao, G.F.; Lu, L. Regulation of circadian genes by the MAPK pathway: Implications for rapid antidepressant action. Neurosci. Bull. 2020, 36, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Antle, M.C.; Tse, F.; Koke, S.J.; Sterniczuk, R.; Hagel, K. Non-photic phase shifting of the circadian clock: Role of the extracellular signal-responsive kinases I/II/mitogen-activated protein kinase pathway. Eur. J. Neurosci. 2008, 28, 2511–2518. [Google Scholar] [CrossRef] [PubMed]
- Silva-Islas, C.A.; Maldonado, P.D. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol. Res. 2018, 134, 92–99. [Google Scholar] [CrossRef]
- Wible, R.S.; Ramanathan, C.; Sutter, C.H.; Olesen, K.M.; Kensler, T.W.; Liu, A.C.; Sutter, T.R. NRF2 regulates core and stabilizing circadian clock loops, coupling redox and timekeeping in Mus musculus. Elife 2018, 7, e31656. [Google Scholar] [CrossRef]
- Antle, M.C.; Mistlberger, R.E. Circadian clock resetting by sleep deprivation without exercise in the Syrian hamster. J. Neurosci. 2000, 20, 9326–9332. [Google Scholar] [CrossRef]
- Honma, S. The mammalian circadian system: A hierarchical multi-oscillator structure for generating circadian rhythm. J. Physiol. Sci. 2018, 68, 207–219. [Google Scholar] [CrossRef]
- Cao, R.; Gkogkas, C.G.; de Zavalia, N.; Blum, I.D.; Yanagiya, A.; Tsukumo, Y.; Xu, H.; Lee, C.; Storch, K.F.; Liu, A.C.; et al. Light-regulated translational control of circadian behavior by eIF4E phosphorylation. Nat. Neurosci. 2015, 18, 855–862. [Google Scholar] [CrossRef]
- Pathak, S.S.; Liu, D.; Li, T.B.; de Zavalia, N.; Zhu, L.; Li, J.; Karthikeyan, R.; Alain, T.; Liu, A.C.; Storch, K.F.; et al. The eIF2 α kinase GCN2 modulates period and rhythmicity of the circadian clock by translational control of Atf4. Neuron 2019, 104, 724–735. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Perna, S.; Antoniello, N. Update on the role of melatonin in the prevention of cancer tumorigenesis and in the management of cancer correlates, such as sleep-wake and mood disturbances: Review and remarks. Aging Clin. Exp. Res. 2013, 25, 499–510. [Google Scholar] [CrossRef]
- Jha, P.K.; Challet, E.; Kalsbeek, A. Circadian rhythms in glucose and lipid metabolism in nocturnal and diurnal mammals. Mol. Cell Endocrinol. 2015, 418, 74–88. [Google Scholar] [CrossRef]
- Hardeland, R.; Madrid, J.A.; Tan, D.X.; Reiter, R.J. Melatonin, the circadian multioscillator system and health: The need for detailed analyses of peripheral melatonin signaling. J. Pineal Res. 2012, 52, 139–166. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Bixler, E.O.; Lin, H.M.; Prolo, P.; Trakada, G.; Chrousos, G.P. IL-6 and its circadian secretion in humans. Neuroimmunomodulation 2005, 12, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Papanicolaou, D.A.; Bixler, E.O.; Kales, A.; Tyson, K.; Chrousos, G.P. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: Role of sleep disturbance and obesity. J. Clin. Endocrinol. Metab. 1997, 82, 1313–1316. [Google Scholar] [CrossRef]
- Ertosun, M.G.; Kocak, G.; Ozes, O.N. The regulation of circadian clock by tumor necrosis factor α. Cytokine Growth Factor Rev. 2019, 46, 10–16. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Z.X.; Dong, Y.L.; Cao, J.; Lin, R.T.; Wang, X.T.; Yu, Z.Q.; Chen, Y.X. Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J. Pineal Res. 2019, 67, e12574. [Google Scholar] [CrossRef]
- Bian, J.; Wang, Z.X.; Dong, Y.L.; Cao, J.; Chen, Y.X. Effect of monochromatic light on the circadian clock of cultured chick retinal tissue. Exp. Eye Res. 2020, 194, 108008. [Google Scholar] [CrossRef]
- Debruyne, J.P.; Noton, E.; Lambert, C.M.; Maywood, E.S.; Weaver, D.R.; Reppert, S.M. A clock shock: Mouse CLOCK is not required for circadian oscillator function. Neuron 2006, 50, 465–477. [Google Scholar] [CrossRef]
- Field, M.D.; Maywood, E.S.; O’Brien, J.A.; Weaver, D.R.; Reppert, S.M.; Hastings, M.H. Analysis of clock proteins in mouse SCN demonstrates phylogenetic divergence of the circadian clockwork and resetting mechanisms. Neuron 2000, 25, 437–447. [Google Scholar] [CrossRef]
- Qi, B.; Li, Q. Effect of light on circadian expression of clock gene in SCN and mam mary gland of mice. J. Northeast Agric. Univ. 2012, 43, 79–82. [Google Scholar]
- Takumi, T.; Taguchi, K.; Miyake, S.; Sakakida, Y.; Takashima, N.; Matsubara, C.; Maebayashi, Y.; Okumura, K.; Takekida, S.; Yamamoto, S.; et al. A light-independent oscillatory gene mPer3 in mouse SCN and OVLT. EMBO J. 1998, 17, 4753–4759. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Sancar, A. Vitamin B2-based blue-light photoreceptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in mammals. Proc. Natl. Acad. Sci. USA 1998, 95, 6097–6102. [Google Scholar] [CrossRef] [PubMed]
- Agez, L.; Laurent, V.; Pevet, P.; Masson-Pevet, M.; Gauer, F. Melatonin affects nuclear orphan receptors mRNA in the rat suprachiasmatic nuclei. Neuroscience 2007, 144, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Comasco, E.; Nordquist, N.; Gokturk, C.; Aslund, C.; Hallman, J.; Oreland, L.; Nilsson, K.W. The clock gene PER2 and sleep problems: Association with alcohol consumption among Swedish adolescents. Upsala J. Med. Sci. 2010, 115, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Hida, A.; Kitamura, S.; Katayose, Y.; Kato, M.; Ono, H.; Kadotani, H.; Uchiyama, M.; Ebisawa, T.; Inoue, Y.; Kamei, Y.; et al. Screening of clock gene polymorphisms demonstrates association of a PER3 polymorphism with morningness-eveningness preference and circadian rhythm sleep disorder. Sci. Rep. 2014, 4, 6309. [Google Scholar] [CrossRef] [PubMed]
- Sato, F.; Wu, Y.Y.; Bhawal, U.K.; Liu, Y.; Imaizumi, T.; Morohashi, S.; Kato, Y.; Kijima, H. PERIOD1 (PER1) has anti-apoptotic effects, and PER3 has pro-apoptotic effects during cisplatin (CDDP) treatment in human gingival cancer CA9-22 cells. Eur. J. Cancer 2011, 47, 1747–1758. [Google Scholar] [CrossRef]
- Yang, X.M.; Wood, P.A.; Ansell, C.M.; Quiton, D.F.T.; Oh, E.Y.; Du-Quiton, J.; Hrushesky, W.J.M. The Circadian clock gene Per1 suppresses cancer cell proliferation and tumor growth at specific times of day. Chronobiol. Int. 2009, 26, 1323–1339. [Google Scholar] [CrossRef]
- Bunger, M.K.; Wilsbacher, L.D.; Moran, S.M.; Clendenin, C.; Radcliffe, L.A.; Hogenesch, J.B.; Simon, M.C.; Takahashi, J.S.; Bradfield, C.A. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 2000, 103, 1009–1017. [Google Scholar] [CrossRef]
- Laposky, A.; Easton, A.; Dugovic, C.; Walisser, J.; Bradfield, C.; Turek, F. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep 2005, 28, 395–409. [Google Scholar] [CrossRef]
- Ehlen, J.C.; Brager, A.J.; Baggs, J.; Pinckney, L.; Gray, C.L.; DeBruyne, J.P.; Esser, K.A.; Takahashi, J.S.; Paul, K.N. Bmal1 function in skeletal muscle regulates sleep. Elife 2017, 6, e26557. [Google Scholar] [CrossRef]
- Hendricks, J.C.; Lu, S.M.; Kume, K.; Yin, J.C.P.; Yang, Z.H.; Sehgal, A. Gender dimorphism in the role of cycle (BMAL1) in rest, rest regulation, and longevity in Drosophila melanogaster. J. Biol. Rhythm. 2003, 18, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.J.; Tononi, G.; Greenspan, R.J.; Robinson, D.F. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature 2002, 417, 287–291. [Google Scholar] [CrossRef]
- Jones, C.R.; Huang, A.L.; Ptacek, L.J.; Fu, Y.H. Genetic basis of human circadian rhythm disorders. Exp. Neurol. 2013, 243, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Griffin, E.A.; Staknis, D.; Weitz, C.J. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science 1999, 286, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.K.; Fribourgh, J.L.; Van Gelder, R.N.; Partch, C.L. Animal cryptochromes: Divergent roles in light perception, circadian timekeeping and beyond. Photochem. Photobiol. 2017, 93, 128–140. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kanno, S.; Smit, R.; van der Horst, G.T.J.; Takao, M.; Yasui, A. Characterization of photolyase/blue-light receptor homologs in mouse and human cells. Nucleic Acids. Res. 1998, 26, 5086–5092. [Google Scholar] [CrossRef][Green Version]
- Jiang, N.; Wang, Z.X.; Cao, J.; Dong, Y.L.; Chen, Y.X. Role of monochromatic light on daily variation of clock gene expression in the pineal gland of chick. J. Photochem. Photobio. B 2016, 164, 57–64. [Google Scholar] [CrossRef]
- Mang, G.M.; La Spada, F.; Emmenegger, Y.; Chappuis, S.; Ripperger, J.A.; Albrecht, U.; Franken, P. Altered sleep homeostasis in Rev-erb α knockout mice. Sleep 2016, 39, 589–601. [Google Scholar] [CrossRef]
- Mongrain, V.; Hernandez, S.A.; Pradervand, S.; Dorsaz, S.; Curie, T.; Hagiwara, G.; Gip, P.; Heller, H.C.; Franken, P. Separating the contribution of glucocorticoids and wakefulness to the molecular and electrophysiological correlates of sleep homeostasis. Sleep 2010, 33, 1147–1157. [Google Scholar] [CrossRef]
- Andre, E.; Gawlas, K.; Steinmayr, M.; Becker-Andre, M. A novel isoform of the orphan nuclear receptor ROR β is specifically expressed in pineal gland and retina. Gene 1998, 216, 277–283. [Google Scholar] [CrossRef]
- Feng, S.J.; Xu, S.; Wen, Z.Z.; Zhu, Y.L. Retinoic acid-related orphan receptor ROR β, circadian rhythm abnormalities and tumorigenesis. Int. J. Mol. Med. 2015, 35, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.J.; Tsai, S.J.; Kuo, P.H.; Liu, Y.L.; Yang, A.C.; Lin, E.; Lan, T.H. An association study in the Taiwan Biobank reveals RORA as a novel locus for sleep duration in the Taiwanese Population. Sleep Med. 2020, 73, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.L.; Abel, J.H.; Li, Y.; Izumo, M.; Cox, K.H.; Jeong, B.; Yoo, S.H.; Olson, D.P.; Doyle, F.J.; Takahashi, J.S. Dual-color single-cell imaging of the suprachiasmatic nucleus reveals a circadian role in network synchrony. Neuron 2020, 108, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, M.; Edwards, M.D.; Patton, A.P.; Smyllie, N.J.; Chesham, J.E.; Maywood, E.S.; Hastings, M.H. Cell-autonomous clock of astrocytes drives circadian behavior in mammals. Science 2019, 363, 187–192. [Google Scholar] [CrossRef]
- Butcher, G.Q.; Lee, B.; Hsieh, F.; Obrietan, K. Light- and clock-dependent regulation of ribosomal S6 kinase activity in the suprachiasmatic nucleus. Eur. J. Neurosci. 2004, 19, 907–915. [Google Scholar] [CrossRef]
- Travnickova-Bendova, Z.; Cermakian, N.; Reppert, S.M.; Sassone-Corsi, P. Bimodal regulation of mPeriod promoters by CREB-dependent signaling and CLOCK/BMAL1 activity. Proc. Natl. Acad. Sci. USA 2002, 99, 7728–7733. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.L.; Shi, L.; Ko, G.Y. Circadian controls outweigh acute illumination effects on the activity of extracellular signal-regulated kinase (ERK) in the retina. Neurosci. Lett. 2009, 451, 74–78. [Google Scholar] [CrossRef][Green Version]
- Obrietan, K.; Impey, S.; Storm, D.R. Light and circadian rhythmicity regulate MAP kinase activation in the suprachiasmatic nuclei. Nat. Neurosci. 1998, 1, 693–700. [Google Scholar] [CrossRef]
- Pizzio, G.A.; Hainich, E.C.; Ferreyra, G.A.; Coso, O.A.; Golombek, D.A. Circadian and photic regulation of ERK, JNK and p38 in the hamster SCN. NeuroReport 2003, 14, 1417–1419. [Google Scholar] [CrossRef]
- Yu, R.; Lei, W.; Mandlekar, S.; Weber, M.J.; Der, C.J.; Wu, J.; Kong, A.N. Role of a mitogen-activated protein kinase pathway in the induction of phase II detoxifying enzymes by chemicals. J. Biol. Chem. 1999, 274, 27545–27552. [Google Scholar] [CrossRef]
- Yu, R.; Chen, C.; Mo, Y.Y.; Hebbar, V.; Owuor, E.D.; Tan, T.H.; Kong, A.N. Activation of mitogen-activated protein kinase pathways induces antioxidant response element-mediated gene expression via a Nrf2-dependent mechanism. J. Biol. Chem. 2000, 275, 39907–39913. [Google Scholar] [CrossRef] [PubMed]
- Rada, P.; Rojo, A.I.; Evrard-Todeschi, N.; Innamorato, N.G.; Cotte, A.; Jaworski, T.; Tobon-Velasco, J.C.; Devijver, H.; Garcia-Mayoral, M.F.; Van Leuven, F.; et al. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/β-TrCP axis. Mol. Cell Biol. 2012, 32, 3486–3499. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, M.; Wood, S.A.; Mellick, G.D. Nrf2: A modulator of Parkinson’s disease? J. Neural. Transm. 2016, 123, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Palam, L.R.; Baird, T.D.; Wek, R.C. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J. Biol. Chem. 2011, 286, 10939–10949. [Google Scholar] [CrossRef] [PubMed]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Harding, H.P.; Novoa, I.; Zhang, Y.; Zeng, H.; Wek, R.; Schapira, M.; Ron, D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 2000, 6, 1099–1108. [Google Scholar] [CrossRef]
- Challet, E. Minireview: Entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology 2007, 148, 5648–5655. [Google Scholar] [CrossRef]
- Casiraghi, L.P.; Plano, S.A.; Fernandez-Duque, E.; Valeggia, C.; Golombek, D.A.; de la Iglesia, H.O. Access to electric light is associated with delays of the dim-light melatonin onset in a traditionally hunter-gatherer Toba/Qom community. J. Pineal Res. 2020, 69, e12689. [Google Scholar] [CrossRef]
- Watson, L.A.; McGlashan, E.M.; Hosken, I.T.; Anderson, C.; Phillips, A.J.K.; Cain, S.W. Sleep and circadian instability in delayed sleep-wake phase disorder. J. Clin. Sleep Med. 2020, 16, 1431–1436. [Google Scholar] [CrossRef]
- Cho, H.; Zhao, X.; Hatori, M.; Yu, R.T.; Barish, G.D.; Lam, M.T.; Chong, L.W.; DiTacchio, L.; Atkins, A.R.; Glass, C.K.; et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 2012, 485, 123–127. [Google Scholar] [CrossRef]
- Reinhardt, E.L.; Fernandes, P.A.C.M.; Markus, R.P.; Fischer, F.M. Short sleep duration increases salivary IL-6 production. Chronobiol Int. 2016, 33, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Rahman, S.U.; Hao, Q.; Li, W.F.; Liu, Z.Z.; Shah, F.A.; Murtaza, I.; Zhang, Z.J.; Yang, X.F.; Liu, G.P.; et al. Melatonin prevents neuroinflammation and relieves depression by attenuating autophagy impairment through FOXO3a regulation. J. Pineal Res. 2020, 69, e12667. [Google Scholar] [CrossRef] [PubMed]
- Sacco, S.; Aquilini, L.; Ghezzi, P.; Pinza, M.; Guglielmotti, A. Mechanism of the inhibitory effect of melatonin on tumor necrosis factor production in vivo and in vitro. Eur. J. Pharmacol. 1998, 343, 249–255. [Google Scholar] [CrossRef]

| Gene | Accession No. | Primer Sequence (5′ to 3′) | Length (bp) | |
|---|---|---|---|---|
| Bmal1 | NM_001374642.1 | F:CAGAGCCGGAGCAGGAAAAATAGGT | R:CAGGGGGAGGCGTACTTGTGATGT | 128 |
| Clock | XM_011249402.2 | F: ATGGTGTTTACCGTAAGCTGTAG | R: CTCGCGTTACCAGGAAGCAT | 197 |
| Cry1 | NM_007771.3 | F: CACTGGTTCCGAAAGGGACTC | R: CTGAAGCAAAAATCGCCACCT | 153 |
| Cry2 | NM_009963.4 | F: CACTGGTTCCGCAAAGGACTA | R: CCACGGGTCGAGGATGTAGA | 102 |
| Per1 | NM_001159367.2 | F: CGGATTGTCTATATTTCGGAGCA | R: TGGGCAGTCGAGATGGTGTA | 142 |
| Per2 | NM_011066.3 | F: GAAAGCTGTCACCACCATAGAA | R: AACTCGCACTTCCTTTTCAGG | 186 |
| Per3 | NM_001289878.1 | F: TCAAGACGTGAGGGCGTTCTA | R: CATTCATACTGCGAGGCTCTTT | 90 |
| Rorβ | NM_001289921.1 | F: GCAGCATTAGCAATGGCCTC | R: GACGGCTGACCGGAATCTATG | 121 |
| Reverbα | NM_145434.4 | F: TACATTGGCTCTAGTGGCTCC | R: CAGTAGGTGATGGTGGGAAGTA | 127 |
| Gapdh | NM_001289726.1 | F: CCGAGAATGGGAAGCTTGTC | R: TTCTCGTGGTTCACACCCATC | 232 |
| Mesor | Amplitude | Acrophase (ZT) | ||||
|---|---|---|---|---|---|---|
| SR | CON | SR | CON | SR | CON | |
| Bmal1 | 1.51 ± 0.23 | 1.63 ± 0.21 | 1.07 ± 0.20 | 0.75 ± 0.14 | 19.47 ± 0.23 * | 22.28 ± 0.44 |
| Clock | 2.08 ± 0.40 | 1.03 ± 0.13 | 1.85 ± 0.34 * | 0.77 ± 0.16 | 11.80 ± 0.82 * | 20.02 ± 0.41 |
| Cry1 | - | 1.27 ± 0.11 | - | 0.41 ± 0.041 | - | 22.89 ± 0.58 |
| Cry2 | 1.47 ± 0.10 | 1.391 ± 0.04 | 0.49 ± 0.11 | 0.33 ± 0.05 | 5.62 ± 0.36 * | 1.32 ± 0.39 |
| Per1 | - | 2.01 ± 0.48 | - | 2.06 ± 0.41 | - | 19.05 ± 0.55 |
| Per2 | - | 0.83 ± 0.08 | - | 0.40 ± 0.09 | - | 14.09 ± 0.75 |
| Per3 | - | 1.71 ± 0.36 | - | 1.14 ± 0.31 | - | 1.38 ± 0.59 |
| Rorβ | 1.60 ±0.03 | 1.70 ± 0.15 | 0.72 ± 0.06 | 0.79 ± 0.13 | 15.71 ± 1.00 * | 1.49 ± 0.73 |
| Reverbα | 1.09 ± 0.06 * | 1.38 ± 0.01 | 0.66 ± 0.06 | 0.64 ± 0.11 | 21.49 ± 0.78 * | 0.13 ± 0.21 |
| BMAL1 | 0.56 ± 0.06 | 0.58 ± 0.07 | 0.24 ± 0.01 * | 0.27 ± 0.00 | 8.15 ± 0.42 * | 20.31 ± 0.62 |
| CLOCK | 0.22 ± 0.01 * | 0.33 ± 0.04 | 0.09 ± 0.02 | 0.18 ± 0.02 | 3.44 ± 0.67 * | 6.19 ± 0.32 |
| PER2 | - | 0.39 ± 0.04 | - | 0.25 ± 0.07 | - | 18.35 ± 1.10 |
| NRF2 | 0.24 ± 0.02 * | 0.30 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 | 13.98 ± 0.68 | 14.15 ± 1.19 |
| p-ERK | 0.26 ± 0.03 | 0.26 ± 0.03 | 0.10 ± 0.02 | 0.11 ± 0.01 | 9.81 ± 0.70 * | 11.92 ± 0.19 |
| p-EIF4E | 0.25 ± 0.03 | 0.34 ± 0.05 | 0.08 ± 0.00 * | 0.12 ± 0.01 | 1.52 ± 0.49 * | 7.12 ± 0.72 |
| p-EIF2α | 0.53 ± 0.04 * | 0.41 ± 0.02 | 0.20 ± 0.02 * | 0.10 ± 0.02 | 7.54 ± 0.70 * | 14.71 ± 0.78 |
| Melatonin | 33.55 ± 1.23 * | 41.25 ± 0.85 | 14.42 ± 1.35 | 12.61 ± 0.17 | 4.48 ± 0.34 * | 16.06 ± 1.14 |
| Corticosterone | 123.58 ± 1.86 * | 112.56 ± 2.00 | 28.11 ± 3.50 | 16.49 ± 4.56 | 3.94 ± 0.58 | 6.86 ± 1.26 |
| IL-6 | 61.85 ± 1.56 * | 52.19 ± 1.61 | 12.72 ± 0.22 | 13.78 ± 1.09 | 7.99 ± 0.10 * | 11.29 ± 0.11 |
| TNF-α | 13.25 ± 0.23 * | 11.48 ± 0.17 | 1.10 ± 0.05 | 1.03 ± 0.10 | 16.96 ± 0.16 * | 20.61 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Role of Sleep Restriction in Daily Rhythms of Expression of Hypothalamic Core Clock Genes in Mice. Curr. Issues Mol. Biol. 2022, 44, 609-625. https://doi.org/10.3390/cimb44020042
Li W, Wang Z, Cao J, Dong Y, Chen Y. Role of Sleep Restriction in Daily Rhythms of Expression of Hypothalamic Core Clock Genes in Mice. Current Issues in Molecular Biology. 2022; 44(2):609-625. https://doi.org/10.3390/cimb44020042
Chicago/Turabian StyleLi, Weitian, Zixu Wang, Jing Cao, Yulan Dong, and Yaoxing Chen. 2022. "Role of Sleep Restriction in Daily Rhythms of Expression of Hypothalamic Core Clock Genes in Mice" Current Issues in Molecular Biology 44, no. 2: 609-625. https://doi.org/10.3390/cimb44020042
APA StyleLi, W., Wang, Z., Cao, J., Dong, Y., & Chen, Y. (2022). Role of Sleep Restriction in Daily Rhythms of Expression of Hypothalamic Core Clock Genes in Mice. Current Issues in Molecular Biology, 44(2), 609-625. https://doi.org/10.3390/cimb44020042







